Abstract
Disrupted bone morphogenetic protein type 2 receptor (BMPR2) signaling in endothelial cells drives pulmonary arterial hypertension (PAH). However, targeted recovery of this signaling pathway by lipid nanoparticles (LNPs) has not been explored as a therapy. Here, we employed Design of Experiments to optimize the delivery efficiency of LNPs targeting pulmonary endothelial cells developed by our laboratory, resulting in a remarkable 35-fold increase in a simplified three-component formulation without helper lipids. Administration of BMPR2 mRNA LNPs effectively reversed established PAH in two experimental rat models (monocrotaline or SU5416-hypoxia) by reversing pulmonary vascular remodeling. Specifically, BMPR2 mRNA LNPs replenished the expression of BMPR2 protein and subsequently activated downstream pathways, as confirmed by elevated levels of p-SMAD1/5/9 and ID1 proteins. The relief of pulmonary arterial occlusion was demonstrated by thinned pulmonary arterial media and decreased proportion of full muscularized vessels. Alleviation of right ventricular hypertrophy was indicated by declined Fulton index, the cross-sectional area of right ventricular cardiomyocytes as well as collagen deposition. Effective recovery of right ventricular function was evidenced by increased pulmonary artery flow acceleration time/pulmonary artery flow ejection time ratio. These findings underscore the potential of restoring BMPR2 signaling through pulmonary endothelial cell-specific LNPs for treating PAH.
Key words: Pulmonary arterial hypertension, BMPR2 restoration, Pulmonary endothelial targeting, mRNA delivery, Lipid nanoparticles, Protein replacement therapy, Design of Experiments, Helper lipid-free LNPs
Graphical abstract
This paper demonstrated the therapeutic potential of pulmonary endothelial cell-targeted LNPs in restoring BMPR2 signaling for the treatment of PAH.
1. Introduction
Pulmonary arterial hypertension (PAH) is a rare and fatal vascular disorder, which are characterized by pulmonary vascular remodeling secondary to proliferation and apoptosis resistance of endothelial cells, smooth muscle cells and fibroblasts1. This will ultimately result in increased pulmonary vascular resistance, right ventricular dysfunction, heart failure, and premature death2. The development of clinical drugs such as phosphodiesterase 5 inhibitors, etc. has improved the outcome of patients to a certain extent by vasodilatation3,4. However, these drugs cannot reverse the remodeling of pulmonary artery vessels, thus failing to halt disease progression.
The identification of heterozygous germline mutations in bone morphogenetic protein receptor 2 (BMPR2) has provided significant insights into the pathobiology of PAH5,6. Subsequent studies identified BMPR2 mutations in 14%–35% of idiopathic PAH cases and 53%–86% of familial PAH cases7. Reduced BMPR2 expression is also a characteristic of nongenetic forms of PAH, further highlighting its critical role in pathogenesis8. The expression of BMPR2 is notably high in the pulmonary arterial endothelium (PAECs), with comparatively lower levels observed in pulmonary arterial smooth muscle cells (PASMCs)8,9. BMPR2 forms a dimer with a family of activin receptor-like kinases (ALKs)10. Specifically, the BMPR2/ALK1 heterodimer mediates signaling in PAECs in response to BMP9 and BMP1011. Upon binding to BMP ligands, it induces phosphorylation of mothers against decapentaplegic homologs (SMAD) proteins, which form a complex and translocate to the nucleus for regulating target gene expression such as inhibitors of DNA binding (ID) proteins12, 13, 14. A shift from impaired BMPR2 pathway towards other homologous receptor pathways including transforming growth factor-β (TGF-β), activins, and anti-Mullerian hormone (AMH) leads to SMAD2/3 protein phosphorylation and subsequent pulmonary vascular remodeling.
Based on these evidences, restoring BMPR2 signaling pathway may offer great potential for effective PAH therapy. Currently, there are mainly three classes of drugs targeting BMPR2 including small-molecule drugs (e.g., tacrolimus, ataluren, sodium 4-phenylbutyrate, and olaparib), large-molecule drugs (e.g., recombinant human BMP9 protein, and sotatercept), as well as adenovirus-based gene therapy15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28. Despite the initiation of clinical trials for certain small-molecule drugs, their lack of lung-specific distribution and durability of action necessitate continuous administration, thereby potentially leading to inevitable systemic toxicity. Moreover, some drugs are applicable only in patients with specific BMPR2 mutations, such as ataluren and sodium 4-phenylbutyrate. Additionally, the strong immunogenicity and the pre-existing antibodies present formidable obstacles to adenovirus-based gene therapy. Recently approved by the FDA as a pioneering biologic, sotatercept is a fusion protein comprising the Fc domain of human IgG and the extracellular domain of ActRIIA, which effectively rebalances the activin and BMP pathway. However, with annual treatment costs exceeding $240,000, it presents an affordability issue for patients outside the US market. Therefore, it remains crucial to develop more BMPR2-targeted therapies to address the unmet needs.
Since the extraordinary success of lipid nanoparticles (LNPs) in the delivery of mRNA COVID-19 vaccine29, LNPs have been considered as promising delivery vehicles for mRNA drugs30, 31, 32. Employing pulmonary endothelial cells targeted LNPs for intravenous delivery of BMPR2 mRNA as a protein replacement therapy could potentially circumvent issues related to systemic distribution toxicity, pre-existing antibodies, and high costs, thereby rendering it suitable for diverse cases of BMPR2 mutations and potentially paving the way for mRNA-based therapies against PAH. Previously, we have developed a novel lipid library and discovered that lipids bearing N-methyl and secondary amine groups in the heads, along with three epoxyalkane-derived tails, demonstrated superior selectivity and efficiency in pulmonary delivery33. In this study, our aim is to develop LNPs containing ionizable lipids that specifically target pulmonary endothelial cells for the purpose of BMPR2 mRNA-based protein replacement therapy against PAH (Fig. 1). Initially, we employed Design of Experiments (DOE) to optimize the formulation based on polydispersity index (PDI), Z-Ave, and lung mRNA expression efficiency, resulting in a remarkable 35-fold increase in efficiency while simplifying the components to ionizable lipid, cholesterol, and PEGylated lipid. Subsequently, we assessed the delivery efficiency of ten representative ionizable lipids in rats using the optimized formulation; among them, A5-3O14 exhibited superior performance with high transfection efficiency and minimal toxicity. Finally, we evaluated the therapeutic potential of BMPR2 mRNA supplementation using A5-3O14 LNPs in both monocrotaline (MCT) and semaxinib (SU5416)-hypoxia PAH rat models. Following intravenous administration, BMPR2 mRNA LNPs coated with protein coronas specifically target pulmonary endothelial cells and triggered an increase in BMPR2 protein levels after endosomal escape. Successful remission of PAH was demonstrated by restored signaling pathways, relieved pulmonary arterial occlusion, alleviated right ventricular hypertrophy as well as increased ratio of pulmonary artery flow acceleration time (PAT)/pulmonary artery flow ejection time (PET).
Figure 1.
Schematic overview of the main contents. Our therapeutic development process encompasses three pivotal phases: 1) Formulation optimization: via three iterative cycles of DOE optimization—a systematic and statistically robust methodology for designing, executing, and analyzing experiments to identify critical variables, optimize formulation parameters, and establish quantitative relationships—we successfully refined the LNP composition from an initial four-component system (ionizable lipid, cholesterol, DOPE, and DMG-PEG) to an optimized three-component formulation (ionizable lipid, cholesterol, and DMG-PEG). This refinement resulted in a significant 35-fold improvement in pulmonary delivery efficiency in ICR mice using the A2-3O14 ionizable lipid. 2) Potential application in large animals: acknowledging interspecies variability, we conducted a systematic evaluation of ten representative ionizable lipids from our prior library (including A2-3O14) in SD rats utilizing the optimized formulation. This comparative analysis identified A5-3O14 as the most suitable ionizable lipid for rats. 3) Therapeutic validation: following comprehensive safety assessments, we evaluated A5-3O14 LNPs encapsulating BMPR2 mRNA in two well-established PAH rat models. The A5-3O14 LNPs restore canonical BMPR2 signaling through a multi-step mechanism involving pulmonary endothelial-specific targeting, cellular internalization, lysosomal escape, BMPR2 protein expression, phosphorylation of Smad1/5/9 proteins, and subsequent transcriptional modulation of downstream effector genes (e.g., ID1).
2. Materials and methods
2.1. Materials
1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) and 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC) were purchased from Avanti Polar Lipids (Birmingham, AL, USA). Cholesterol and d-luciferin was purchased from Adamas-beta (Shanghai, China). Polyethylene glycol (PEG) 2000 dimyristoyl glycerol (DMG) (DMG-PEG) was purchased from Macklin (Shanghai, China). Firefly luciferase mRNA (N1-Me-Pseudo UTP) and Firefly luciferase mRNA were purchased from novoproteins (Suzhou, China). Micro Glutamic-oxaloacetic Transaminase (GOT) Assay Kit and Micro Glutamic-pyruvic Transaminase (GPT) Assay Kit were purchased from Solarbio (Beijing, China). Urea Nitrogen Assay Kit and Creatinine (Cr) Assay Kit were purchased from Sangon Biotech (Shanghai, China). Rat IL-6 and TNF-α Elisa kits were purchased from Jinmei (Chengyan, China). Dialysis membranes (WMCO, 3.5 kDa) were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. Monocrotaline was purchased from ABCONE (Shanghai, China). 4% Paraformaldehyde (PFA) solution was purchased from SenBeijia (Nanjing, China). Beta Actin Monoclonal Antibody (15G5A11/E2), and GOXRBT IGG (H + L) HRP were purchased from Thermo (Waltham, MA). Anti-ID1 antibody and Anti-SMAD1/SMAD5/SMAD9 (phospho S463 + S465 + S467) antibody (ab92698) were purchased from Abcam (Cambridge, UK). BMPR-II (clone 18/BMPR-II) were purchased from BD Transduction Laboratories (Franklin Lakes, NJ, USA).
2.2. LNPs formation and characterization
The lipid mixture containing ionizable lipids, phospholipid, cholesterol, and DMG-PEG2000 was prepared in ethanol, and the mRNA was diluted in buffer. The two phases were rapidly mixed by pipette with a volumetric ratio of ethanol: aqueous = 1:3. In library A of Fig. 2, the mixture was dialyzed against phosphate-buffered saline (PBS) (1 ×, pH = 7.4) using a 3500 MWCO dialysis membrane at 4 °C for 2 h. For other data, the mixture was immediately diluted with 1 × PBS (pH = 7.4). The nanoparticles were diluted into 800 μL 1 × PBS, then particle size, polydispersity (PDI), and zeta potential were determined in PBS (0.4 μg mRNA dissolved in 20 μL) using a NanoZS Zetasizer (Malvern Instruments, Malvern, UK). The zeta potential in water was measured by the same diluted method.
Figure 2.
Formulation optimization of lung-targeting LNPs. (A) Schematic of formulation optimization: A2-3O14, cholesterol, helper lipid, and DMG-PEG were used to form LNPs for the encapsulation of Luc mRNA. The DOE approach was utilized to optimize the formulation with maximized pulmonary delivery efficiency. Optimization was conducted in ICR mice, resulting in a highly effective lung-targeted three-element formulation. n = 3. (B) Parameter setting for different rounds. The combined molar percentage of cholesterol, phospholipid, ionizable lipid, and PEG was fixed at 100%. CBS: sodium citrate buffer. (C) Particle size, PDI, and zeta potential of 22 LNPs in round A. (D) Factors with the most significant influence on particle size in round A. (E) Pulmonary luciferase expression efficiency of various LNPs in round B after intravenous injection to ICR mice with a dose of 0.1 mg/kg of Luc mRNA. Data are plotted as the mean ± SEM. (F) Factors with the greatest influence on the efficiency of Luc mRNA expression in the DOPE group of round B. (G) Pulmonary luciferase expression efficiency of various LNPs in round C after intravenous injection of 0.1 mg/kg of Luc mRNA to ICR mice. Data are plotted as the mean ± SEM. (H) Pie chart illustrating the components of three elements in the DSPC-C6 formulation. (I) Factors with the greatest impact on Luc mRNA expression efficiency in the DSPC group of round C.
2.3. Animal experiments
All animal experiments were approved by the Institutional Animal Care and Use Committee of Shanghai Tech University (Approval of Animal Ethical and Welfare Number: 20230527001) and were consistent with the governmental regulations of China for the care and use of animals. Male ICR (15–20 g) mice and Sprague–Dawley (SD) (60–200 g) rats were purchased from Shanghai Jihui Experimental Animal Breeding Co., Ltd.
2.4. In vivo luc mRNA delivery
Six hours after the i.v. injection of LNPs to male ICR mice (n = 3 per group) or SD rats (n = 3 or 6 per group), the mice or rats were injected with d-luciferin potassium (150 mg/kg, i.p.) and imaged using an IVIS Lumina system (PerkinElmer). Then, the organs were isolated and imaged with the same method.
2.5. Cell-specific assessment of A2-3O14 LNPs
A2-3O14 LNPs were prepared according to the original formulation as shown in Fig. 2B. Then, they were intravenously injected into Ai9 mice which express robust tdTomato fluorescence following Cre-mediated recombination at a dose of 0.3 mg/kg Cre mRNA. After 3 days, the lungs were collected, minced digested, filtered, and lysed with red blood cell lysis buffer to get single-cell suspension. Then antibodies were added and incubated for 30 min on room temperature in the dark. The stained cells were washed twice with PBS+2% fetal bovine serum (FBS)+1% P/S, resuspended in PBS+2% FBS+1% P/S. Ghost Dye Red 780 (Cell Signaling Technology, 18452S), Alexa Fluor 488 anti-mouse CD 31 (BioLegend, 102414), Pacific Blue anti-mouse CD 45 (BioLegend, 157212), and Alexa Fluor 647 anti-mouse CD 326 (Ep-CAM) (BioLegend, 118212) were used here. Ultimately, the lung cells were analyzed with CytoFLEX S (Beckman). Data were analyzed with FLOWJO software version X 10.0.7 r 2 (FLOWJO). The dilution rates of all antibodies were used as per manufacturers’ suggestions.
2.6. Formulation optimization
Formulation optimization was designed and analyzed through JMP Pro 16. In JMP Pro 16 software, select either Definitive Screening Design or Taguchi design from the DOE module. Add design factors in the “Factors” section. Use the Response hierarchy to specify one or more responses (e.g., PDI, Z-Ave, Zeta, and the expression of Luc mRNA), along with their optimization objectives (maximization, target value matching, or minimization). Upon configuration completion, the system automatically generates a design matrix containing multiple experimental runs (e.g., Supporting Information Table S1). After executing experiments according to this matrix, feed critical experimental datasets back into the responses. Process the data through the “Fit Definitive Screening” and “Analytical Tool” modules to derive “Predictive Profiler” illustrating relationships between response variables and design factors.
2.7. TEM imaging of A5-3O14 (DSPC-C6) LNPs
To prepare the sample for TEM measurement, 3 μL of A5-3O14 LNPs solution was applied to a 300 mesh ultra-thin carbon support film with hydrophilic treatment, then volatilized overnight. The image was collected on a JEM-1400 plus Transmission Electron Microscope.
2.8. In vivo toxicity assay
Male SD rats weighing 200–220 g were randomly divided into six groups with three mice in each group. A5-3O14 (DSPC-C6) LNPs was selected to study the in vivo toxicity. The LNPs carrying firefly luciferase mRNA (100% N1-Me-Pseudo UTP) were administered via the tail veins at a dose of 0.1 and 0.2 mg/kg. The negative control group was i.v. injected with PBS. After 6 and 72 h, whole blood was collected and serum was separated by centrifugation. Next, liver (AST and ALT) functions, kidney (BUN and CREA) functions, and inflammatory factors (IL-6 and TNF-α) were measured by kits.
2.9. MCT model
Male SD rats (60–80 g) were randomized into five groups. Control rats were given a single-dose s.c. injections of normal saline. Other rats were given 60 mg/kg of MCT by s.c. injection, and allowed to develop pulmonary hypertension. The first injection was given on Day 6 and then every three days. Dexamethasone was injected intraperitoneally at a dose of 1 mg/kg 1 h before LNPs injection. On Days 7, 13, and 22, rats were anesthetized with 2% isoflurane, and the PAT/PET value was assessed using ultrasound with Vevo3100 (Visual Sonics, US). Tissue harvesting was carried out at the time of sacrifice.
2.10. SU5416 model
Male SD rats (60–80 g) were randomized into four groups. At Day 0, the male SD rats of model group were injected with a VEGF receptor inhibitor SU-5416 (20 mg/kg, s.c.), then immediately placed into a 10% O2 hypoxia chamber and maintained for 3 weeks, followed by a normoxic environment to develop pulmonary hypertension. The rats in the control group were injected with saline (s.c.) and maintained in a normoxic environment. The first injection was given on Day 11 and then every five days until Day 36. During the treatment, dexamethasone was injected intraperitoneally at a dose of 1 mg/kg 1 h before LNPs. On Days (17, 37, and 57), rats were anesthetized with 2% isoflurane, and the PAT/PET value was assessed using ultrasound with Vevo3100 (Visual Sonics, USA). Tissue harvesting was carried out at the time of sacrifice. SU5416 was synthesized as described previously34. 3,5-Dimethyl-1H-pyrrole-2-carbaldehyde (600 mg, 4.87 mmol) and oxindole (778.43 mg, 5.85 mmol) were added into a 50 mL round bottom flask. EtOH (10 mL) and piperidine (82.97 mg, 974.38 mmol) were then added. The flask was equipped with a condenser and heated to reflux for 6 h. The reaction mixture was then cooled to room temperature and concentrated under vacuum to afford an orange solid. The precipitate was collected by filtration and washed with chloroform. Then recrystallization was performed. 1H NMR (500 MHz, Chloroform-d) δ 13.11 (s, 1H), 8.03 (s, 1H), 7.48 (d, J = 7.5 Hz, 1H), 7.40 (s, 1H), 7.15–7.10 (m, 1H), 7.05 (t, J = 7.5 Hz, 1H), 6.89 (d, J = 7.6 Hz, 1H), 5.98 (s, 1H), 2.39 (s, 3H), 2.33 (s, 3H). MALDI-TOF MS m/z of M+ calculated for C15H14N2O: 238.110; Found: 238.000.
2.11. The synthesis of BMPR2 mRNA
The full-length rat BMPR2 gene was cloned into an in vitro transcription vector for mRNA driven by a T7 promotor. The BMPR2 mRNA was capped with cap1, modified by 100% N1-Methylpseudouridine UTP, and purified by Oligo dT (Genscript). The gene sequence transcribed into BMPR2 mRNA was designed as [T7-5′ UTR-KOZAK-ORF (Rat BMPR2)-3′ UTR-polyA (31 A+10 nt+71 A)].
2.12. Evaluation of the BMPR2 mRNA expression capacity in vitro
HEK 293T cells were cultured in DMEM containing 10% FBS and 1% penicillin/streptomycin at 37 °C/5% CO2. Briefly, HEK 293T cells were seeded into 24-well plates at a cell density of 1.0 × 105 cells per well to allow the confluent to reach 70% at transfection. BMPR2 mRNA was transfected into HEK 293T cells by using the Messenger Max following Invitrogen's standard protocol. The dosage of BMPR2 mRNA was 0.5 μg/well. Cells were incubated for 24 h at 37 °C. Then, the cell samples were collected for Western blot to measure the expression of BMPR2 protein. The cell samples were lysed by RIPA buffer following Beyotime's standard protocol.
2.13. Lung tissue lysis
Lung tissues were lysed in a RIPA buffer (Beyotime Biotechnology, Cat# P0013B, China) containing 1% Triton X-100, 0.1% SDS, 1% sodium deoxycholate, a protease inhibitor cocktail (MedChem Express, Cat# HY-K0010), and a phosphatase inhibitor cocktail (Beyotime Biotechnology, Cat# P0045, China).
2.14. Western blot
The protein extracts were separated by SDS-PAGE electrophoresis and transferred to a PVDF membrane. 4%–12% SDS-PAGE gels were purchased from Genscript. Triple color pre-stained marker (13–180 kDa) was purchased from Shandong Sparkjade Biotechnology Co., Ltd. The membranes were incubated overnight at 4 °C with antibodies against BMPR2, p-SMAD1/5/9, ID1, and β-actin. The PVDF membrane was washed with TBST (Epizyme, Cat#PS103, China). HRP-conjugated secondary antibodies were applied at 1:20,000 dilution. The bands were visualized with an ECL kit (Beyotime Biotechnology, Cat# P0018AS, China), recorded on Amersham™ ImageQuant™ 800 (Cytiva, JP), and quantified by Image J.
2.15. Histology
For pathology analysis, lung tissues were analyzed by H&E staining and anti-α-SMA immunohistochemistry staining. Heart tissues were analyzed by Masson staining and WGA staining to evaluate the therapeutic effect of different treatment groups. Briefly, tissues were immobilized in 4% paraformaldehyde for 24 h. Then the paraffin embedding, sectioning, and staining were done by Servicebio in Wuhan, China. The sections were scanned by Leica Aperio VERSA 8 and analyzed by Image J Fiji and ImageScope x64.
2.16. Immunofluorescence assay
SD rats were randomly divided into two groups with three mice in each group. A5-3O14 (DSPC-C6) LNPs carrying firefly luciferase mRNA were administered via the tail veins at a dose of 0.1 mg/kg. The control group received an equivalent volume of PBS. After 6 h, the rat lungs were perfused and collected. Subsequently, tissues were immobilized in 4% paraformaldehyde for 24 h. Then the paraffin embedding and sectioning were done by Servicebio in Wuhan, China. For the staining, the slides were deparaffinized in xylene and rehydrated through a graded ethanol series. Antigen retrieval was performed by heating the slides in EDTA solution (pH 9.0) in a microwave oven for 15 min. After cooling, tissue autofluorescence quencher (Servicebio, Cat# G1221-5 ML) was applied according to the manufacturer’s instructions. The sections were then blocked with 5% BSA in PBS for 1 h at room temperature and incubated with primary antibody (Goat anti-luciferase, Novus Biologicals, Cat# NB600-307, 1:250; Rabbit anti-CD31, Abcam, 1:200) diluted in the blocking solution overnight at 4 °C. Next, the slides were washed and incubated with secondary antibody (Alexa Fluor 488-conjugated Donkey Anti-Goat IgG, Servicebio, CAT# GB25404; Alexa Fluor Cy3-conjugated Donkey Anti-Rabbit IgG, Servicebio, CAT# GB21403, 1:200) diluted in the blocking solution at room temperature for 1 h. The slides were then washed and incubated with Dapi (Beyotime, Cat# C1002, 1:10,000) in PBS, followed by washing and mounting with anti-fade mounting medium (Servicebio, CAT# 1401-5 ML).
2.17. Statistical analysis
Statistical analyses were performed using GraphPad Prism. Normal distribution and homogeneity of variance were tested. Multiple datasets in Fig. 3H and J, Fig. 4B and C, G and H, J, P, Fig. 5, Supporting Information Fig. S16B and S16D, S16E–S16H, S16J–S16L and other Figure in Supporting Information were analyzed by ordinary one-way ANOVA. Significance was computed according to Tukey's multiple comparison test, with a single pooled variance. Multiple datasets in Fig. 4D and N were analyzed by Brown-Forsythe and Welch ANOVA tests. Significance was computed according to Dunnett's T3 multiple comparison test, with individual variances computed for each comparison. Multiple datasets in Fig. 4K were analyzed by ordinary two-way ANOVA and Tukey's multiple comparisons test, with a single pooled variance. Multiple datasets in Fig. 4L, Fig. S16A, S16C and S16I were analyzed by Kruskal–Wallis test. Significance was computed according to Dunn's multiple comparison test. All data are reported as mean ± standard error of the mean (SEM).
Figure 3.
A5-3O14 LNPs exhibited high pulmonary delivery efficiency and biocompatibility in SD rats. (A) Leading ionizable lipids with pulmonary mRNA delivery capacity in ICR mice were evaluated in rats. The LNPs were formulated according to DSPC-C6 formulation and i.v. injected to rats at a dose of 0.1 mg/kg Luc mRNA. Presented here were the ex vivo multi-organ bioluminescence images of the three most efficient ionizable lipids in rats. (B) A statistical plot of the Luc mRNA expression efficiency in the lung for the three ionizable lipids in A (n = 6). (C) The Z-Ave and PDI of the top three LNPs in B. (D) TEM images of the A5-3O14 LNPs. The dot indicated by the black arrow represented mRNA. (E) BMPR2 expression in healthy rats after injecting varying doses (mg/kg) of BMPR2 mRNA LNPs for 6 h (n = 3). (F) The quantification of BMPR2 expression in E (fold change relative to β-actin). (G) BMPR2 expression in healthy rats after injecting varying doses (mg/kg) of Luc mRNA LNPs for 6 h (n = 3). (H) The quantification of BMPR2 expression in G (fold change relative to β-actin). (I) BMPR2 expression in 1-week-MCT-challenged rats that were treated with BMPR2 mRNA LNPs (0.2 mg/kg) or dexamethasone (1 mg/kg) for 6 h (n = 3). (J) The quantification of BMPR2 expression in I (fold change relative to β-actin). Data are expressed as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Figure 4.
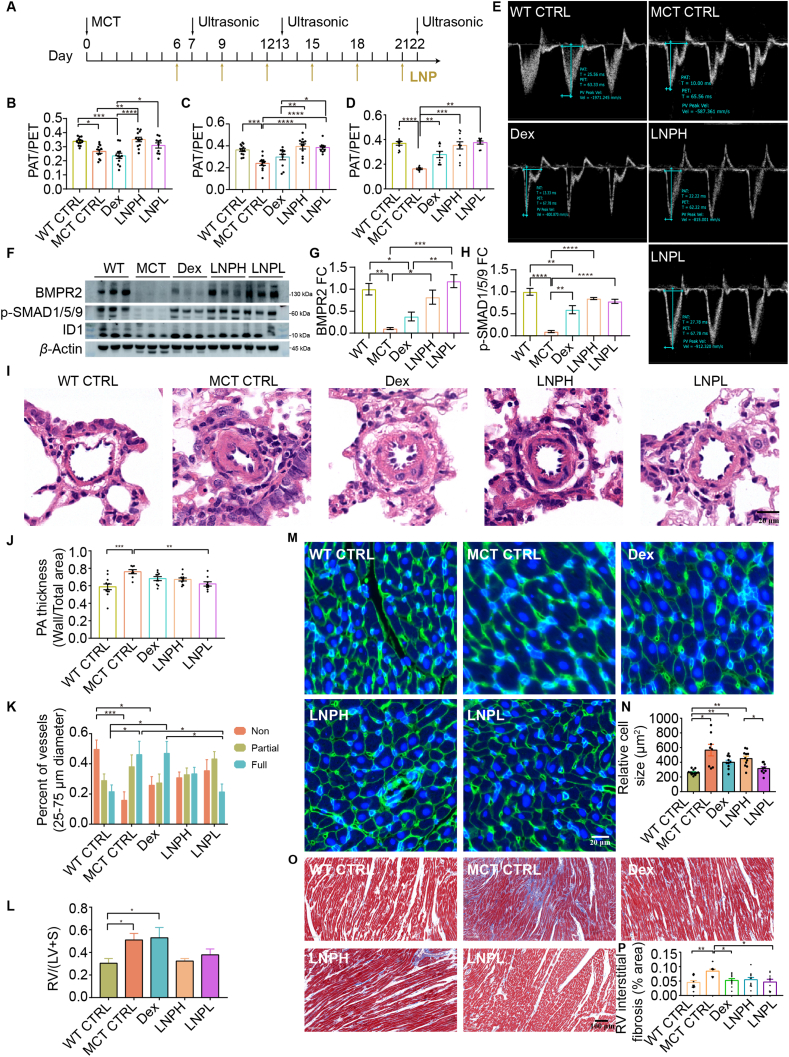
BMPR2 protein replacement therapy prevents the disease progression of MCT rats. (A) Schematic illustration of the experimental design. On Day 0, SD rats were given a vehicle injection (wild type control, n = 12) or treated with MCT (n = 48, 60 mg/kg, s.c.). MCT injected rats were randomly separated into four groups, including LNPH (n = 12), LNPL (n = 12), DEX (n = 12), and MCT CTRL (n = 12). (B–E) Echocardiography was performed to measure the ratio of PAT/PET in rats (n = 8–12 per group). B, PAT/PET on Day 7. C, PAT/PET on Day 13. D, PAT/PET on Day 22. E, Representative echocardiography images on Day 22. (F) Representative Western blot for BMPR2, phosphorylated SMAD1/5/9 (p-SMAD1/5/9), ID1 and β-actin in whole lung lysate (n = 3). (G) The quantification of BMPR2 expression in F (fold change relative to β-actin). (H) The quantification of p-SMAD1/5/9 expression in F (fold change relative to β-actin). (I) Representative hematoxylin and eosin (H&E) images of pulmonary arterioles. Scale bars, 20 μm. (J) The summarized data of (I). The pulmonary arteriole thickness (PA) was expressed as the ratio of wall area to total vessel area. (K) Statistical chart of the proportion of pulmonary arteriole muscularization. Non-muscularized arteries: α-SMA positive length <5% vessel circumference. Fully-muscularized arteries: α-SMA positive length >75% vessel circumference. Partially-muscularized arteries: 5% vessel circumference ≤ α-SMA positive length ≤75% vessel circumference. (L) Assessment of right ventricular hypertrophy, Fulton index [RV/(LV + S)]. (M) Representative images of wheat germ agglutinin (WGA) staining of the right ventricular cardiomyocyte. Scale bars, 20 μm. (N) Quantification of right ventricular cardiomyocyte cross-sectional area in (M). (O) Representative images of Masson staining of right ventricular. Scale bars, 100 μm. (P) Quantification of collagen deposition ratio in (O). Blue indicates collagen deposition. The right ventricular (RV) interstitial fibrosis was expressed as the ratio of blue tissue area to total tissue area. All the diameters of the pulmonary arterioles in Fig. I–K were 25–75 μm. n = 8–12 per group for (I–P). Data are expressed as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
Figure 5.
BMPR2 protein replacement therapy prevents SU5416-hypoxia-induced PAH in rats. (A) Schematic illustration of the experimental design. On Day 0, SD rats were given vehicle injections and maintained in normoxia (WT CTRL, n = 10) or challenged with SU-5416 (20 mg/kg, s.c.) and exposing them to 3 weeks of hypoxia (10% O2) before normoxia (n = 30). SU-5416-hypoxia rats were randomly separated into three groups, including untreated group (SU CTRL, n = 10), group treated with 1 mg/kg dexamethasone (Dex, n = 10), and group treated with 0.1 mg/kg BMPR2 mRNA-LNP and 1 mg/kg dexamethasone (LNP, n = 10). The prevention treatment started from Day 11 and was treated every 5 days until Day 36, and the ultrasound monitoring was carried out on Days 17, 37 and 57. On Day 37, half of the mice from each group were sacrificed for therapeutic assessment after the ultrasound monitoring, while the remaining mice were kept in normoxia until Day 57 to evaluate the duration of therapeutic benefit. (B) Representative echocardiography images on Days 37 and 57. (C) PAT/PET on Day 17 (n = 9–10). (D) PAT/PET on Day 37 (n = 8–10). (E) PAT/PET on Day 57 (n = 5). (F) Fulton index on Day 37 (n = 3–5). (G) Fulton index on Day 57 (n = 5). (H) Representative images of WGA staining of right ventricular cardiomyocyte. Scale bars, 20 μm. (I) Quantification of right ventricular cardiomyocyte cross-sectional area in (H). (J) Representative H&E staining images of pulmonary arterioles. Scale bars, 20 μm. (K) The quantification of (J). n = 3–5 for H–K. All the diameter of the pulmonary arterioles in Fig. J–K were 25–75 μm. (L) Representative Western blot for BMPR2, p-SMAD1/5/9, ID1 and β-actin in whole lung lysate. (M) The quantification of BMPR2 expression in (L) (fold change relative to β-actin). (N) The quantification of p-SMAD1/5/9 expression in (L) (fold change relative to β-actin). (O) The quantification of ID1 expression in (L) (fold change relative to β-actin). n = 3 for L–O. Data are expressed as mean ± SEM. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
3. Results
3.1. Formulation optimization to maximize pulmonary expression efficiency
In our previous work, LNPs composed of ionizable lipid A2-3O14 demonstrated exceptionally high efficiency in lung-specific delivery of mRNA33. To identify the targeted cell subpopulations within the lung, we administered the A2-3O14 LNPs carrying Cre mRNA into Ai9 reporter mice. Upon internalization by the targeted cells, expressed Cre recombinase excised the stop cassette, thereby activating tdTomato expression (Supporting Information Fig. S1A). As shown in Fig. S1B–S1C, 75.93% of tdTomato+ populations were identified as endothelial cells, indicating precise targeting of pulmonary endothelial cells by A2-3O14 LNPs.
To maximize the pulmonary delivery efficiency, we employed a DOE35, 36, 37 approach to simultaneously optimize various factors (Fig. 2A and B). Ensuring appropriate particle size with uniform distribution was crucial for superior LNPs. Therefore, our primary focus during round A optimization was on eliminating factors that could increase particle size and PDI. Based on the original formulation, the weight ratio of total lipids to mRNA was expanded to 10:1–30:1. The molar ratio of A2-3O14, phospholipids, and PEG were adjusted to 20%–50%, 5%–25%, and 1%–5%, respectively. Additionally, phospholipid DSPC and NaAc buffer were included in library A for optimization. The buffer concentration was broadened to 10–50 mmol/L (Fig. 2B). A total of 22 formulations were generated in round A through a definitive screening design (Table S1, Fig. 2C). The NaAc buffer was crucial for the LNPs to obtain a small particle size (Fig. 2D, Supporting Information Fig. S2A and S2B). As the buffer concentration increased, the PDI values gradually decreased and reached a plateau at 40 mmol/L. Subsequently, an incremental increase was observed (Fig. S2C–S2E). Therefore, a range of 10–40 mmol/L NaAc was employed as the buffer in round B. Since incorporating DSPC resulted in higher zeta potential compared to DOPE but also led to higher PDI values, both were retained for the next optimization round (Fig. S2E, Supporting Information Fig. S3). Considering that excessive PEG did not significantly reduce the particle size but did affect the zeta potential, the median value of 3% from round A was set as the upper limit for PEG molar ratio in round B (Figs. S2 and S3). The molar ratio of ionizable lipids was closely related to mRNA expression efficiency. However, results of round A were insufficient to support adjustments for subsequent rounds of optimization. Hence, both the molar ratio of ionizable lipids and the weight ratio of total lipids to mRNA remained unchanged in round B. According to the parameters obtained from round A, 13 new formulations were generated through definitive screening design for each phospholipid (Supporting Information Table S2).
Luc mRNA was encapsulated in LNPs according to formulations designed in round B and then injected into ICR mice at a dose of 0.1 mg/kg. The expression of luc mRNA was generally higher in the DOPE group compared to DSPC group (Fig. 2E). Increasing the PEG molar ratio from 1% to 3% resulted in a decrease in both luc mRNA expression (Fig. 2F and Supporting Information Fig. S4) and zeta potential (Supporting Information Fig. S5). Therefore, the preferred ratio was fixed at 1% for the next round of screening. Adjusting the molar ratio of A2-3O14 from 20% to 50% showed a positive correlation with luc mRNA expression (Fig. 2F), while PDI decreased until reaching a plateau around 30% (Supporting Information Fig. S6). In round C, we further optimized the ratio of A2-3O14 to be between 42.5% and 57.5%, incorporating the optimal ratio of 50% from round B as an intermediate value. Gradual increases in both Luc mRNA expression (Fig. 2F) and zeta potential (Fig. S5) were observed with higher weight ratios of total lipid to mRNA. Consequently, we expanded the weight ratio range from 25 to 35 in the next round. Since the PDI began to increase when the molar ratio of phospholipids exceeded 8%, it was deemed appropriate to set the molar ratio to 0%–8% in round C (Fig. S6). The lowest buffer concentration of 10 mmol/L was selected for round C as higher concentrations led to larger particle sizes (Supporting Information Fig. S7).
For the DSPC group, both the molar ratio of A2-3O14 and the weight ratio of total lipids to mRNA were found to be crucial parameters affecting Luc mRNA expression (Supporting Information Fig. S8), which was consistent with the DOPE group. The same range was selected for these two parameters in round C. Despite a decrease in particle size (Supporting Information Fig. S9) and PDI (Supporting Information Fig. S10) with an increase in PEG ratio, exceptional mRNA expression was achieved in formulations B1, B2, and B11 that contained a lower amount of PEG. Therefore, the median value of the percentage of PEG was set at 1%, and a range of 0.5%–1.5% was selected for round C. Increasing DSPC concentration resulted in larger particle size (Fig. S9), but smaller PDI (Fig. S10) and zeta potential (Supporting Information Fig. S11), posing further optimization challenges. Formulations B1 and B11 containing 5% DSPC exhibited high potential for achieving excellent Luc mRNA expression. Considering that 5% fell within the range of 0%–8%, the percentage of phospholipids for the DSPC group was set the same as that for the DOPE group in round C. As indicated by the DOPE group in round B, the higher buffer concentration also led to a larger particle size in the DSPC group (Fig. S9). Therefore, a buffer concentration of 10 mmol/L was selected for round C.
To refine the factors impacting in vivo mRNA expression and minimize the interference of noise in the experimental design, the Taguchi method was applied in round C to generate 9 formulations each for DOPE and DSPC (Supporting Information Tables S3 and S4). The mRNA expression of the DSPC-C6 group was the strongest in round C, with a 35-fold increase compared to the original formulation (Fig. 2G). This optimal formulation consisted of three components (Fig. 2H), namely A2-3O14: cholesterol: DMG-PEG at a ratio of 57.5%: 41.5%: 1%, with a total lipid to mRNA ratio of 30, which aligned perfectly with the predicted optimal formulation (Fig. 2I).
3.2. Leading ionizable lipid A5-3O14 demonstrated high pulmonary delivery efficiency and biocompatibility in SD rats
To explore the potential application of ionizable lipids in large animals, SD rats were used as a model. The most ten effective ionizable lipids in mice from our previous work33 and the optimal formulations obtained from DOE were adopted to evaluate the pulmonary delivery efficiency in rats. The chemical structures of the ten ionizable lipids was shown in Supporting Information Fig. S12. For the three-component formulation (DSPC-C6), A5-3O14, A1-3O14, and A2-3O16 emerged as the top three ionizable lipids in terms of pulmonary expression efficiency (Fig. 3A and B, Supporting Information Fig. S13). Among these three ionizable lipids, A5-3O14 demonstrated better lung-targeting specificity. The Z-Ave and PDI of A5-3O14 LNPs were 125.5 ± 0.96 nm and 0.078 ± 0.025 respectively (Fig. 3C). The representative TEM images of A5-3O14 LNPs were shown in Fig. 3D, where a distinct mRNA encapsulation can be observed. Long-term stability profiling of A5-3O14 LNPs with DSPC-C6 formulation at 4 °C was shown in Supporting Information Fig. S14. Critical quality attributes including Z-Ave (maintained at 98–108 nm), PDI (maintained at 0.16–0.20), and zeta potential (maintained at 4.2–8.5 mV) remained stable throughout the 28-day monitoring period. The endothelial cell-targeting capability of A5-3O14 LNPs in rats was demonstrated in Supporting Information Fig. S15, where immunofluorescence analysis revealed strong colocalization between luciferase (luc) protein and the endothelial marker CD31.
The levels of blood urea nitrogen (BUN), creatinine (CREA), aspartate aminotransferase (AST), alanine aminotransferase (ALT), inflammatory factors IL-6 and TNF-α were measured following intravenous administration of A5-3O14 LNPs to assess their potential toxicity in rats (Supporting Information Fig. S16). Although some fluctuations were observed, no statistically significant differences were found in the levels of CRE, BUN, AST, and ALT between the PBS control group and the groups treated with 0.1 or 0.2 mg/kg A5-3O14 LNPs. Notably, a transient increase in IL-6 was detected at 6 h post-injection in the group receiving 0.2 mg/kg A5-3O14 LNPs, which returned to baseline levels within 72 h after injection. Therefore, it was feasible to administer the three-component A5-3O14 LNPs once every three days to evaluate its efficacy in protein replacement therapy for PAH in rats.
Prior to conducting the assessment of therapeutic efficacy, we firstly synthesized a codon-optimized BMPR2 mRNA and validated its expression in HEK 293T cells. The Western blot analysis clearly demonstrated an elevated expression of BMPR2 compared to the control group (Supporting Information Fig. S17). Subsequently, the in vivo expression of BMPR2 mRNA delivered by A5-3O14 LNPs was further evaluated using normal rats, where different doses of BMPR2 mRNA ranging from 0.1 to 0.6 mg/kg were administered (Fig. 3E and F). There was a modest increase in BMPR2 protein levels following the treatment with 0.1 mg/kg of BMPR2 mRNA LNPs, but this enhancement decreased as the dosage increased to 0.3 mg/kg. The expression of BMPR2 decreased even further compared to the CTRL group at a dose of 0.6 mg/kg, possibly due to inflammatory cytokines such as IL-6 and TNF-α leading to degradation of BMPR238,39. To confirm our assumption that innate inflammatory properties of LNPs contributed to decreased BMPR2 protein levels at high doses, we replaced the BMPR2 mRNA with Luc mRNA to investigate the effect on pulmonary expression of BMPR2. Administration of luc mRNA LNPs (0.1–0.6 mg/kg) to normal rats resulted in a clear down-regulation of BMPR2 in a dose-dependent manner (Fig. 3G and H). This was consistent with the results of toxicity evaluation, which displayed a spontaneous increase in IL-6 at 6 h followed by a return to normal levels at 72 h with administration of 0.2 mg/kg LNPs. These results suggested that suppressing inflammation is essential for restoring BMPR2 in PAH models. Dexamethasone is used as a premedication for Patisiran, an FDA-approved siRNA drug encapsulated with LNPs, to mitigate the risk of inflammation40,41. In the following study, we also included dexamethasone at a dose of 1 mg/kg. The results showed that dexamethasone alone could partially restore the expression of BMPR2, while the combination of BMPR2 mRNA LNPs with dexamethasone induced significant overexpression of BMPR2 in 1-week-MCT-challenged rats (Fig. 3I and J).
3.3. A5-3O14 LNPs encapsulating BMPR2 mRNA prevented PAH induced by MCT
Next, a synergistic administration of dexamethasone and BMPR2 mRNA LNPs was applied for the treatment of PAH. Rats were randomly divided into five groups: high dose of BMPR2 mRNA LNPs (0.2 mg/kg) combined with dexamethasone (LNPH), low dose of BMPR2 mRNA LNPs (0.1 mg/kg) combined with dexamethasone (LNPL), dexamethasone control (DEX), MCT control (MCT CTRL), and wild type control (WT CTRL). The first injection was given on Day 6 and then every three days after the initial administration (Fig. 4A). Ultrasound measurements were conducted on Days 7, 13, and 22 (Fig. 4E and Supporting Information Fig. S18). As indicated in Fig. 4B–E, a significant difference in PAT/PET value between the WT CTRL group and the MCT CTRL was observed on Day 7. The value for the MCT group steadily declined over a period of 22 days, indicating the successful establishment of the PAH model. Treatment with dexamethasone alone slightly relieved the PAH progression. Both LNPH and LNPL groups demonstrated rapid restoration to normal PAT/PET values within one day post-treatment initiation which remained stable throughout the entire experiment period, surpassing those observed in DEX-treated animals during all three ultrasonic assessments conducted. Notably, the treatment outcome of LNPL group was more favorable than that of the LNPH group by Day 22, as evidenced by a higher mean PAT/PET.
To substantiate the regulatory role of BMPR2 and/or its downstream pathways in alleviating PAH, we conducted Western blot analysis to examine the expression levels of BMPR2, p-SMAD1/5/9, and ID1 in lung tissues (Fig. 4F–H, Supporting Information Fig. S19). MCT treatment significantly reduced the expression of BMPR2, p-SMAD1/5/9, and ID1. Dexamethasone treatment significantly increased the levels of p-SMAD1/5/9, and ID1, while no significant augmentation was observed in BMPR2 expression compared to the MCT CTRL group. In the LNPH-treated group, both BMPR2 and p-SMAD1/5/9 were significantly elevated, whereas ID1 did not reach a statistical significance when compared to the MCT CTRL group. In contrast, LNPL treatment significantly restored the expression of all three proteins (BMPR2, p-SMAD1/5/9, and ID1).
The pathology of the lung and right ventricle was further accessed to evaluate the potential of protein replacement therapy. Compared to the WT CTRL group, the MCT CTRL group showed a significant increase in the thickness of the pulmonary arterial wall and the percentage of fully muscularized arteries, as well as a decrease in non-muscularized arteries (Fig. 4I–K, Supporting Information Fig. S20). Following synergetic therapy with LNPL, the thickness of the pulmonary arterial wall returned to normal and the number of fully muscularized pulmonary arteries was significantly reduced. However, both LNPH and DEX groups demonstrated limited therapeutic effects. The right ventricular hypertrophy of the LNPH and LNPL groups was improved, although no statistical significance was observed compared to the MCT CTRL group (Fig. 4L). The cross-sectional area of the cardiomyocyte and collagen deposition in the right ventricle were significantly increased in the MCT CTRL compared to the WT CTRL group (Fig. 4M–P). Treatment with LNPL not only reduced cardiomyocyte cross-sectional area but also significantly decreased collagen deposition. Although treatment with dexamethasone reduced cardiomyocyte cross-sectional area and collagen deposition, it did not reverse the Fulton index. The alleviation of some symptoms of PAH by dexamethasone may be attributed to its anti-inflammatory properties. LNPH treatment did not lead to better outcomes, suggesting that a higher concentration might enhance the inflammation due to LNP's inherent pro-inflammatory properties. Overall, the optimal therapeutic effects were achieved through synergistic treatment with 0.1 mg/kg BMPR2 mRNA LNPs and dexamethasone.
3.4. A5-3O14 LNPs encapsulating BMPR2 mRNA prevented PAH induced by SU5416-hypoxia
To further investigate the therapeutic potential of BMPR2 protein replacement therapy, a second model of PAH was established by combining SU5416 with hypoxia. The preventive treatment started on Day 11 and continued every 5 days until Day 36. Ultrasound monitoring was conducted on Days 17, 37, and 57 (Fig. 5A and Supporting Information Fig. S21). After the treatment with BMPR2 mRNA LNPs (0.1 mg/kg), the PAT/PET value recovered to normal levels (Fig. 5B–E), indicating a significant improvement in all three ultrasonic measurements between the LNP and SU CTRL groups. A significant improvement in Fulton index was also observed between the LNP and SU CRTL groups on Day 37 (Fig. 5F and G). No significant differences were found in PAT/PET and Fulton index between the DEX group and SU CTRL group on Days 37 and 57.
The cross-sectional area of cardiomyocytes and the thickness of the pulmonary arterial wall increased in the SU CRTL group on Day 37, while they returned to normal levels in the LNP group (Fig. 5H–K). Treatment with dexamethasone alone reduced the thickness of the pulmonary arterial wall, but did not significantly affect the cross-sectional area of cardiomyocytes as compared to SU CRTL group. Following six treatments, BMPR2 expression was significantly higher in both the DEX and LNP groups compared to the SU group (Fig. 5L and M). The downstream proteins p-SMAD1/5/9 and ID1 exhibited a slight increasing trend, although no statistically significant differences were observed in the DEX and LNP groups compared to SU group (Fig. 5N and O). Overall, the administration of 0.1 mg/kg of mBMPR2 mRNA LNPs and dexamethasone could effectively prevent the progression of PAH. Notably, even after treatment withdrawal, the PAT/PET values between the LNP and SU CTRL groups remained statistically significant on Day 57, indicating a sustained therapeutic effect of mBMPR2 mRNA LNPs combined with dexamethasone.
4. Discussion
Pulmonary vascular remodeling constitutes a pivotal pathological process in patients with PAH. In the absence of effective interventions, this process can lead to significant morbidity and mortality among affected individuals. The intima, composed of endothelial cells, serve as the interface where the blood vessel directly interacts with circulating blood. Impairment of BMPR2 function in endothelial cells leads to apoptosis and proliferation, endothelial-to-mesenchymal transition, infiltration of inflammatory factors, and loss of vascular barrier function, which may initiate vascular remodeling42. Given the inherently conservative nature of the BMPR2 pathway, targeted delivery of BMPR2 mRNA to pulmonary endothelial cells is of great importance for treating PAH while minimizing potential side effects.
Inhalation has been recognized as a direct route of administration for treating respiratory diseases, offering numerous advantages such as circumventing first-pass metabolism, minimizing systemic exposure, and improving patient adherence43, 44, 45, 46. However, inhalation of LNPs necessitates traversing multiple layers of physical barriers, including mucociliary clearance, pulmonary surfactants, phagocytosis by pulmonary macrophages, the epithelial cell layer, and the basement membrane prior to reaching their target cells predominantly comprising endothelial cells45. Therefore, intravenous injection emerges as a more rational and efficacious delivery approach for targeting pulmonary endothelial cells. Currently, approved LNPs have demonstrated significant efficacy in transporting mRNA to the liver and muscles via intravenous and local injections, while challenges persist in achieving effective systemic administration of mRNA therapeutics for targeting tissues beyond the liver. With continuous efforts, several delivery systems have been developed that can achieve pulmonary targeting at the animal level via intravenous administration. These include the SORT delivery system47, N-serial LNPs with an amide bond in the tail48, one-component ionizable amphiphilic Janus dendrimers49, 50, 51, poly (β-amino esters)35,52, 53, 54, and so on55, 56, 57. However, there is still a relative lack of rational design principles for ionizable lipids targeting the pulmonary system. Consequently, our laboratory has been dedicated to investigating these rational design rules. In our previous work, we discovered that intravenous administration of lipids containing a N-methyl group and a secondary amine in the head region, along with three tails derived from epoxyalkane, resulted in high lung-selectivity due to specific protein fingerprints being absorbed33. In this study, we have successfully demonstrated that the leading LNPs from our previous work exhibit remarkable specificity towards pulmonary endothelial cells at 75.93%, making them suitable for precise restoration of the BMPR2 pathway.
It is widely acknowledged that inflammation plays a crucial role in the degradation of BMPR2 protein, exacerbation of vascular remodeling, and increased susceptibility to PAH58,59. Compelling clinical evidence indeed demonstrated significant elevation in levels of multiple inflammatory factors such as IL-6, TNF-α, and IL-1β among PAH patients60. Transgenic mice overexpressing IL-6 or TNF-α spontaneously developed PAH61, 62, 63, 64, 65. In our experiment, we observed that high dose of LNPs induced the upregulation of IL-6 due to its innate immunogenicity, resulting in a reduction of BMPR2 proteins. To mitigate the potential adverse impact of LNPs’ immunogenicity on PAH recovery, we implemented pretreatment with glucocorticoid dexamethasone in a manner similar to that employed for Patisiran. Several studies demonstrated that dexamethasone alone exhibited some therapeutic effects when administered earlier, at higher doses, and more frequent intervals66,67. Given the potential side effects associated with dexamethasone, the practicality of employing high doses and frequent administration of this drug in clinical settings is limited, which may account for the paucity of clinical trials investigating its efficacy in treating PAH. Attempts to lower the dose (1.25 mg/kg) and frequency (once every two days) of dexamethasone treatment demonstrated limited therapeutic efficacy in rats, which was consistent to our findings. Specifically, the reduction of dexamethasone dosage (1 mg/kg) and treatment frequency (MCT model: once every three days, Su5416 model: once every five days) proved ineffective in reducing the Fulton index and the cross-sectional area of cardiomyocyte cells in both models. In contrast, LNPL treatment exhibited superior efficacy across various aspects including the restoration of BMPR2 signaling pathway, alleviation of pulmonary arterial occlusion, mitigation of right ventricular hypertrophy, and improvement in right ventricular function. These findings emphasized the essentiality of supplementing BMPR2 in PAH therapy.
Both MCT-induced and SU5416/hypoxia-induced PAH models are well-established preclinical systems with distinct pathophysiological mechanisms68,69. The MCT model, utilizing a pyrrolizidine alkaloid, induces PAH through direct endothelial injury accompanied by mononuclear cell infiltration and significant hepatic/renal damage70,71. While demonstrating high pharmacological sensitivity, this model fails to replicate characteristic plexiform lesions observed in human PAH68,72. In contrast, the SU5416 model employs a vascular endothelial growth factor receptor-2 inhibitor under chronic hypoxia conditions, generating severe irreversible endothelial proliferation with pathognomonic plexiform lesions72. This model exhibits organ-specific pulmonary effects without systemic involvement, minimal vascular inflammation, and superior animal survival rates compared to the MCT model73. As demonstrated in Fig. 4F and 5L, the SU5416 model group exhibited higher baseline protein levels of BMPR2 and p-SMAD1/5/9 compared to the MCT group, potentially attributable to its relatively preserved endothelial integrity. The attenuated inflammatory response in the SU5416 model may further explain the enhanced therapeutic efficacy of Dex observed in this system, as evidenced by improvements in critical pathological indicators, including BMPR2 signaling (p-SMAD1/5/9, ID1) and pulmonary arterial wall thickening (PA thickness). Nevertheless, the precise regulatory mechanisms underlying these model-specific therapeutic outcomes still demand in-depth exploration through dedicated molecular studies.
5. Conclusions
This study demonstrated for the first time that targeted delivery of BMPR2 mRNA by LNPs successfully attenuated PAH through the reversal of pulmonary vascular remodeling. This approach holds promise as an alternative treatment for PAH. While our current findings indicate promising progress, several key challenges must be systematically addressed to advance this delivery platform toward clinical implementation. First, the anatomical and biochemical differences between rodent models and humans, particularly regarding vascular architecture, plasma protein profiles, and receptor expression patterns, necessitate validation in large animal models and non-human primates to ensure translational relevance of organ targeting outcomes, with parallel mechanistic studies to decipher interspecies variations in biodistribution. Furthermore, to enhance clinical practicality, we propose exploring the delivery of epigenetic regulators through our platform to achieve sustained BMPR2 upregulation for PAH treatment. This approach could significantly reduce dosing frequency compared to the current regimen of administration every three days. Simultaneously, for inflammatory pathologies like pulmonary hypertension, strategic formulation innovations could be pursued, such as engineering LNPs with structurally integrated dexamethasone to enable combinatorial mRNA/anti-inflammatory therapy, or developing novel lipid compositions with inherent anti-inflammatory properties.
Author contributions
Yan Cao, Runyuan Wang and Xiaoyan He designed the research and collected the data. Yan Cao drew the graphical abstract and Fig. 1. Yan Ding, Yan Chang, and Runyue Yang conducted the cell-specific assessment of LNPs. Yan Cao, Runyuan Wang and Xiaoyan He wrote the manuscript. Guisheng Zhong, Huiying Yang and Jianfeng Li revised the manuscript. All the authors have read and approved the final manuscript.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was sponsored by Science and Technology Commission of Shanghai Municipality (YDZX20223100001002, China), the Natural Science Foundation of Shanghai (25ZR1401251), and the ShanghaiTech University Startup Grant. We thank the Molecular Imaging Core Facility, the Molecular and Cell Biology Core Facility, and the Animal Core Facility at the School of Life Science and Technology for providing technical support. We thank Lin Xiong in the Electron Microscopy Center at the School of Physical Science and Technology for providing technical support.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2025.07.004.
Contributor Information
Huiying Yang, Email: huiying_@fudan.edu.cn.
Jianfeng Li, Email: lijf1@shanghaitech.edu.cn.
Appendix A. Supporting information
The following is the Supporting information to this article.
References
- 1.Southgate L., Machado R.D., Gräf S., Morrell N.W. Molecular genetic framework underlying pulmonary arterial hypertension. Nat Rev Cardiol. 2020;17:85–95. doi: 10.1038/s41569-019-0242-x. [DOI] [PubMed] [Google Scholar]
- 2.Vachiéry J.L., Gaine S. Challenges in the diagnosis and treatment of pulmonary arterial hypertension. Eur Respir Rev. 2012;21:313–320. doi: 10.1183/09059180.00005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thenappan T., Ormiston M.L., Ryan J.J., Archer S.L. Pulmonary arterial hypertension: pathogenesis and clinical management. BMJ. 2018;360 doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montani D., Chaumais M.C., Guignabert C., Günther S., Girerd B., Jaïs X., et al. Targeted therapies in pulmonary arterial hypertension. Pharmacol Ther. 2014;141:172–191. doi: 10.1016/j.pharmthera.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Deng Z., Morse J.H., Slager S.L., Cuervo N., Moore K.J., Venetos G., et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737–744. doi: 10.1086/303059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lane K.B., Machado R.D., Pauciulo M.W., Thomson J.R., Phillips J.A., Loyd J.E., et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Nat Genet. 2000;26:81–84. doi: 10.1038/79226. [DOI] [PubMed] [Google Scholar]
- 7.Machado R.D., Southgate L., Eichstaedt C.A., Aldred M.A., Austin E.D., Best D.H., et al. Pulmonary arterial hypertension: a current perspective on established and emerging molecular genetic defects. Hum Mutat. 2015;36:1113–1127. doi: 10.1002/humu.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atkinson C., Stewart S., Upton P.D., Machado R., Thomson J.R., Trembath R.C., et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- 9.Southwood M., Jeffery T.K., Yang X., Upton P.D., Hall S.M., Atkinson C., et al. Regulation of bone morphogenetic protein signalling in human pulmonary vascular development. J Pathol. 2008;214:85–95. doi: 10.1002/path.2261. [DOI] [PubMed] [Google Scholar]
- 10.Rosenzweig B.L., Imamura T., Okadome T., Cox G.N., Yamashita H., ten Dijke P., et al. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci U S A. 1995;92:7632–7636. doi: 10.1073/pnas.92.17.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orriols M., Gomez-Puerto M.C., Ten Dijke P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell Mol Life Sci : CMLS. 2017;74:2979–2995. doi: 10.1007/s00018-017-2510-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heldin C.H., Miyazono K., ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 13.Yang J., Davies R.J., Southwood M., Long L., Yang X., Sobolewski A., et al. Mutations in bone morphogenetic protein type II receptor cause dysregulation of Id gene expression in pulmonary artery smooth muscle cells: implications for familial pulmonary arterial hypertension. Circ Res. 2008;102:1212–1221. doi: 10.1161/CIRCRESAHA.108.173567. [DOI] [PubMed] [Google Scholar]
- 14.Yang J., Li X., Li Y., Southwood M., Ye L., Long L., et al. Id proteins are critical downstream effectors of BMP signaling in human pulmonary arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2013;305:L312–L321. doi: 10.1152/ajplung.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long L., Ormiston M.L., Yang X., Southwood M., Gräf S., Machado R.D., et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21:777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunmore B.J., Jones R.J., Toshner M.R., Upton P.D., Morrell N.W. Approaches to treat pulmonary arterial hypertension by targeting BMPR2: from cell membrane to nucleus. Cardiovasc Res. 2021;117:2309–2325. doi: 10.1093/cvr/cvaa350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drake K.M., Dunmore B.J., McNelly L.N., Morrell N.W., Aldred M.A. Correction of nonsense BMPR2 and SMAD9 mutations by ataluren in pulmonary arterial hypertension. Am J Respir Cell Mol Biol. 2013;49:403–409. doi: 10.1165/rcmb.2013-0100OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sobolewski A., Rudarakanchana N., Upton P.D., Yang J., Crilley T.K., Trembath R.C., et al. Failure of bone morphogenetic protein receptor trafficking in pulmonary arterial hypertension: potential for rescue. Hum Mol Genet. 2008;17:3180–3190. doi: 10.1093/hmg/ddn214. [DOI] [PubMed] [Google Scholar]
- 19.Spiekerkoetter E., Sung Y.K., Sudheendra D., Bill M., Aldred M.A., van de Veerdonk M.C., et al. Low-dose FK506 (tacrolimus) in end-stage pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:254–257. doi: 10.1164/rccm.201411-2061LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiekerkoetter E., Sung Y.K., Sudheendra D., Scott V., Del Rosario P., Bill M., et al. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur Respir J. 2017;50 doi: 10.1183/13993003.02449-2016. [DOI] [PubMed] [Google Scholar]
- 21.Humbert M., McLaughlin V., Gibbs J.S.R., Gomberg-Maitland M., Hoeper M.M., Preston I.R., et al. Sotatercept for the treatment of pulmonary arterial hypertension. N Engl J Med. 2021;384:1204–1215. doi: 10.1056/NEJMoa2024277. [DOI] [PubMed] [Google Scholar]
- 22.Humbert M., McLaughlin V., Gibbs J.S.R., Gomberg-Maitland M., Hoeper M.M., Preston I.R., et al. Sotatercept for the treatment of pulmonary arterial hypertension: PULSAR open-label extension. Eur Respir J. 2023;61 doi: 10.1183/13993003.01347-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoeper M.M., Badesch D.B., Ghofrani H.A., Gibbs J.S.R., Gomberg-Maitland M., McLaughlin V.V., et al. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388:1478–1490. doi: 10.1056/NEJMoa2213558. [DOI] [PubMed] [Google Scholar]
- 24.McMurtry M.S., Moudgil R., Hashimoto K., Bonnet S., Michelakis E.D., Archer S.L. Overexpression of human bone morphogenetic protein receptor 2 does not ameliorate monocrotaline pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;292:L872–L878. doi: 10.1152/ajplung.00309.2006. [DOI] [PubMed] [Google Scholar]
- 25.Reynolds A.M., Xia W., Holmes M.D., Hodge S.J., Danilov S., Curiel D.T., et al. Bone morphogenetic protein type 2 receptor gene therapy attenuates hypoxic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1182–L1192. doi: 10.1152/ajplung.00020.2006. [DOI] [PubMed] [Google Scholar]
- 26.Reynolds A.M., Holmes M.D., Danilov S.M., Reynolds P.N. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J. 2012;39:329–343. doi: 10.1183/09031936.00187310. [DOI] [PubMed] [Google Scholar]
- 27.Harper R.L., Reynolds A.M., Bonder C.S., Reynolds P.N. BMPR2 gene therapy for PAH acts via Smad and non-Smad signalling. Respirology. 2016;21:727–733. doi: 10.1111/resp.12729. [DOI] [PubMed] [Google Scholar]
- 28.Feng F., Harper R.L., Reynolds P.N. BMPR2 gene delivery reduces mutation-related PAH and counteracts TGF-β-mediated pulmonary cell signalling. Respirology. 2016;21:526–532. doi: 10.1111/resp.12712. [DOI] [PubMed] [Google Scholar]
- 29.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rohner E., Yang R., Foo K.S., Goedel A., Chien K.R. Unlocking the promise of mRNA therapeutics. Nat Biotechnol. 2022;40:1586–1600. doi: 10.1038/s41587-022-01491-z. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Sun C., Wang C., Jankovic K.E., Dong Y. Lipids and lipid derivatives for RNA delivery. Chem Rev. 2021;121:12181–12277. doi: 10.1021/acs.chemrev.1c00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong Y., Anderson D.G. Opportunities and challenges in mRNA therapeutics. Acc Chem Res. 2022;55:1. doi: 10.1021/acs.accounts.1c00739. [DOI] [PubMed] [Google Scholar]
- 33.He X., Wang R., Cao Y., Ding Y., Chang Y., Dong H., Xie R., et al. Lung-specific mRNA delivery by ionizable lipids with defined structure-function relationship and unique protein corona feature. Adv Sci (Weinh) 2025;12 doi: 10.1002/advs.202416525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seath C.P., Fyfe J.W.B., Molloy J.J., Watson A.J.B. Synthesis of oxindoles and benzofuranones via oxidation of 2-heterocyclic BMIDAs. Synth Met. 2017;49:891–898. [Google Scholar]
- 35.Kaczmarek J.C., Kauffman K.J., Fenton O.S., Sadtler K., Patel A.K., Heartlein M.W., et al. Optimization of a degradable polymer-lipid nanoparticle for potent systemic delivery of mRNA to the lung endothelium and immune cells. Nano Lett. 2018;18:6449–6454. doi: 10.1021/acs.nanolett.8b02917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kauffman K.J., Dorkin J.R., Yang J.H., Heartlein M.W., DeRosa F., Mir F.F., et al. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15:7300–7306. doi: 10.1021/acs.nanolett.5b02497. [DOI] [PubMed] [Google Scholar]
- 37.Billingsley M.M., Hamilton A.G., Mai D., Patel S.K., Swingle K.L., Sheppard N.C., et al. Orthogonal design of experiments for optimization of lipid nanoparticles for mRNA engineering of CAR T cells. Nano Lett. 2022;22:533–542. doi: 10.1021/acs.nanolett.1c02503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishibashi T., Inagaki T., Okazawa M., Yamagishi A., Ohta-Ogo K., Asano R., et al. IL-6/gp130 signaling in CD4+ T cells drives the pathogenesis of pulmonary hypertension. Proc Natl Acad Sci U S A. 2024;121 doi: 10.1073/pnas.2315123121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dorfmüller P., Perros F., Balabanian K., Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- 40.Kon E., Ad-El N., Hazan-Halevy I., Stotsky-Oterin L., Peer D. Targeting cancer with mRNA-lipid nanoparticles: key considerations and future prospects. Nat Rev Clin Oncol. 2023;20:739–754. doi: 10.1038/s41571-023-00811-9. [DOI] [PubMed] [Google Scholar]
- 41.Suhr O.B., Coelho T., Buades J., Pouget J., Conceicao I., Berk J., et al. Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis. 2015;10:109. doi: 10.1186/s13023-015-0326-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans C.E., Cober N.D., Dai Z., Stewart D.J., Zhao Y.Y. Endothelial cells in the pathogenesis of pulmonary arterial hypertension. Eur Respir J. 2021;58 doi: 10.1183/13993003.03957-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y.B., Xu D., Bai L., Zhou Y.M., Zhang H., Cui Y.L. A review of non-invasive drug delivery through respiratory routes. Pharmaceutics. 2022;14:1974. doi: 10.3390/pharmaceutics14091974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson S., Atkins P., Bäckman P., Cipolla D., Clark A., Daviskas E., et al. Inhaled medicines: past, present, and future. Pharmacol Rev. 2022;74:48–118. doi: 10.1124/pharmrev.120.000108. [DOI] [PubMed] [Google Scholar]
- 45.Borghardt J.M., Kloft C., Sharma A. Inhaled therapy in respiratory disease: the complex interplay of pulmonary kinetic processes. Can Respir J. 2018;2018 doi: 10.1155/2018/2732017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bai X., Chen Q., Li F., Teng Y., Tang M., Huang J., et al. Optimized inhaled LNP formulation for enhanced treatment of idiopathic pulmonary fibrosis via mRNA-mediated antibody therapy. Nat Commun. 2024;15:6844. doi: 10.1038/s41467-024-51056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng Q., Wei T., Farbiak L., Johnson L.T., Dilliard S.A., Siegwart D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15:313–320. doi: 10.1038/s41565-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qiu M., Tang Y., Chen J., Muriph R., Ye Z., Huang C., et al. Lung-selective mRNA delivery of synthetic lipid nanoparticles for the treatment of pulmonary lymphangioleiomyomatosis. Proc Natl Acad Sci U S A. 2022;119 doi: 10.1073/pnas.2116271119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang D., Atochina-Vasserman E.N., Lu J., Maurya D.S., Xiao Q., Liu M., et al. The unexpected importance of the primary structure of the hydrophobic part of one-component ionizable amphiphilic janus dendrimers in targeted mRNA delivery activity. J Am Chem Soc. 2022;144:4746–4753. doi: 10.1021/jacs.2c00273. [DOI] [PubMed] [Google Scholar]
- 50.Zhang D., Atochina-Vasserman E.N., Maurya D.S., Huang N., Xiao Q., Ona N., et al. One-component multifunctional sequence-defined ionizable amphiphilic janus dendrimer delivery systems for mRNA. J Am Chem Soc. 2021;143:12315–12327. doi: 10.1021/jacs.1c05813. [DOI] [PubMed] [Google Scholar]
- 51.Zhang D., Atochina-Vasserman E.N., Maurya D.S., Liu M., Xiao Q., Lu J., et al. Targeted delivery of mRNA with one-component ionizable amphiphilic janus dendrimers. J Am Chem Soc. 2021;143:17975–17982. doi: 10.1021/jacs.1c09585. [DOI] [PubMed] [Google Scholar]
- 52.Kaczmarek J.C., Patel A.K., Kauffman K.J., Fenton O.S., Webber M.J., Heartlein M.W., et al. Polymer-lipid nanoparticles for systemic delivery of mRNA to the lungs. Angew Chem Int Ed Engl. 2016;55:13808–13812. doi: 10.1002/anie.201608450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaczmarek J.C., Patel A.K., Rhym L.H., Palmiero U.C., Bhat B., Heartlein M.W., et al. Systemic delivery of mRNA and DNA to the lung using polymer-lipid nanoparticles. Biomaterials. 2021;275 doi: 10.1016/j.biomaterials.2021.120966. [DOI] [PubMed] [Google Scholar]
- 54.Chen Q., Chang Y., He X., Ding Y., Wang R., Luo R., et al. Targeted delivery of mRNA with polymer-lipid nanoparticles for in vivo base editing. ACS Nano. 2025;19:7835–7850. doi: 10.1021/acsnano.4c14041. [DOI] [PubMed] [Google Scholar]
- 55.Xue L., Hamilton A.G., Zhao G., Xiao Z., El-Mayta R., Han X., et al. High-throughput barcoding of nanoparticles identifies cationic, degradable lipid-like materials for mRNA delivery to the lungs in female preclinical models. Nat Commun. 2024;15:1884. doi: 10.1038/s41467-024-45422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eygeris Y., Gupta M., Kim J., Jozic A., Gautam M., Renner J., et al. Thiophene-based lipids for mRNA delivery to pulmonary and retinal tissues. Proc Natl Acad Sci U S A. 2024;121 doi: 10.1073/pnas.2307813120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Z., Yang Y., Qiu X., Zhou H., Wang R., Xiong H. Crown-like biodegradable lipids enable lung-selective mRNA delivery and dual-modal tumor imaging in vivo. J Am Chem Soc. 2024;146:34209–34220. doi: 10.1021/jacs.4c14500. [DOI] [PubMed] [Google Scholar]
- 58.Rabinovitch M., Guignabert C., Humbert M., Nicolls M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spiekerkoetter E., Kawut S.M., de Jesus Perez V.A. New and emerging therapies for pulmonary arterial hypertension. Annu Rev Med. 2019;70:45–59. doi: 10.1146/annurev-med-041717-085955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soon E., Holmes A.M., Treacy C.M., Doughty N.J., Southgate L., Machado R.D., et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation. 2010;122:920–927. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 61.Hurst L.A., Dunmore B.J., Long L., Crosby A., Al-Lamki R., Deighton J., et al. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat Commun. 2017;8 doi: 10.1038/ncomms14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fujita M., Shannon J.M., Irvin C.G., Fagan K.A., Cool C., Augustin A., et al. Overexpression of tumor necrosis factor-alpha produces an increase in lung volumes and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2001;280:L39–L49. doi: 10.1152/ajplung.2001.280.1.L39. [DOI] [PubMed] [Google Scholar]
- 63.Kim C.W., Song H., Kumar S., Nam D., Kwon H.S., Chang K.H., et al. Anti-inflammatory and antiatherogenic role of BMP receptor II in endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1350–1359. doi: 10.1161/ATVBAHA.112.300287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steiner M.K., Syrkina O.L., Kolliputi N., Mark E.J., Hales C.A., Waxman A.B. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. 28pp. following 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Savale L., Tu L., Rideau D., Izziki M., Maitre B., Adnot S., et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res. 2009;10:6. doi: 10.1186/1465-9921-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang W., Wang Y.L., Chen X.Y., Li Y.T., Hao W., Jin Y.P., et al. Dexamethasone attenuates development of monocrotaline-induced pulmonary arterial hypertension. Mol Biol Rep. 2011;38:3277–3284. doi: 10.1007/s11033-010-0390-x. [DOI] [PubMed] [Google Scholar]
- 67.Price L.C., Montani D., Tcherakian C., Dorfmüller P., Souza R., Gambaryan N., et al. Dexamethasone reverses monocrotaline-induced pulmonary arterial hypertension in rats. Eur Respir J. 2011;37:813–822. doi: 10.1183/09031936.00028310. [DOI] [PubMed] [Google Scholar]
- 68.Sakao S., Tatsumi K. The effects of antiangiogenic compound SU5416 in a rat model of pulmonary arterial hypertension. Respiration. 2011;81:253–261. doi: 10.1159/000322011. [DOI] [PubMed] [Google Scholar]
- 69.Nogueira-Ferreira R., Vitorino R., Ferreira R., Henriques-Coelho T. Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: a network approach. Pulm Pharmacol Ther. 2015;35:8–16. doi: 10.1016/j.pupt.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 70.Campian M.E., Hardziyenka M., Michel M.C., Tan H.L. How valid are animal models to evaluate treatments for pulmonary hypertension? Naunyn-Schmiedebergs Arch Pharmacol. 2006;373:391–400. doi: 10.1007/s00210-006-0087-9. [DOI] [PubMed] [Google Scholar]
- 71.Gomez-Arroyo J.G., Farkas L., Alhussaini A.A., Farkas D., Kraskauskas D., Voelkel N.F., et al. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012;302:L363–L369. doi: 10.1152/ajplung.00212.2011. [DOI] [PubMed] [Google Scholar]
- 72.Abe K., Toba M., Alzoubi A., Ito M., Fagan K.A., Cool C.D., et al. Formation of plexiform lesions in experimental severe pulmonary arterial hypertension. Circulation. 2010;121:2747–2754. doi: 10.1161/CIRCULATIONAHA.109.927681. [DOI] [PubMed] [Google Scholar]
- 73.Taraseviciene-Stewart L., Kasahara Y., Alger L., Hirth P., Mc Mahon G., Waltenberger J., et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–438. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.