Abstract
DNA methylation is necessary for normal embryogenesis in animals. Here we show that loss of the maintenance methyltransferase, xDnmt1p, triggers an apoptotic response during Xenopus development, which accounts for the loss of specific cell populations in hypomethylated embryos. Hypomethylation-induced apoptosis is accompanied by a stabilization in xp53 protein levels after the mid-blastula transition. Ectopic expression of HPV-E6, which promotes xp53 degradation, prevents cell death, implying that the apoptotic signal is mediated by xp53. In addition, inhibition of caspase activation by overexpression of Bcl-2 results in the development of cellular masses that resemble embryonic blastomas. Embryonic tissue explant experiments suggest that hypomethylation alters the developmental potential of early embryo cells and that apoptosis is triggered by differentiation. Our results imply that loss of DNA methylation in differentiated somatic cells provides a signal via p53 that activates cell death pathways.
Keywords: apoptosis/differentiation/DNA hypomethylation/p53/Xenopus
Introduction
Vertebrate cells have the capacity to modify their genomes epigenetically via the covalent addition of a methyl group to the 5-position of the cytosine (m5C) within the context of the CpG dinucleotide (Colot and Rossignol, 1999). DNA methylation is essential for normal development in mice (Li et al., 1992; Walsh and Bestor, 1999), zebrafish (Martin et al., 1999) and Xenopus (Stancheva and Meehan, 2000). In mammals, it also has specific roles in regulating X-chromosome inactivation and genomic imprinting (Li et al., 1993; Panning and Jaenisch, 1996). Biochemical and genetic analyses of hypomethylated embryos in different species suggest that the maintenance methyltransferase (Dnmt1) is not essential for the survival of embryonic cells during early cleavage stages. The effect of disrupting pre-existing methylation patterns only becomes apparent during and after gastrulation when the pluripotent embryonic cells begin to differentiate. In Xenopus, methylation contributes to the maintenance of transcriptional silencing during the first 12 cleavages of the zygote (Stancheva and Meehan, 2000). Transcription normally initiates at the mid-blastula transition (MBT) but in hypomethylated blastulae many developmentally regulated genes are activated two cell cycles earlier probably due to a chromatin reorganization. This results in an altered developmental program of gene expression and the appearance of microcephalic and axis-truncated phenotypes in the maternal xDnmt1-depleted embryos. Similar studies in mouse are consistent with a role for DNA methylation in transcriptional silencing during animal development (Li et al., 1993; Walsh and Bestor, 1999). The importance of DNA methylation in the maintenance of cellular gene expression patterns is highlighted by the observation that many tumour cells exhibit aberrant patterns of DNA hypermethylation, leading to the specific silencing of tumour-suppressor genes (Baylin and Herman, 2000). In addition, the same tumour cells show a genome-wide hypomethylation, which is thought to contribute to chromosome instability and tumour progression.
In order to characterize the cellular response to low levels of DNA methylation, we have depleted xDnmt1 from Xenopus laevis embryos by antisense RNA either before or after the MBT. In both cases, xDnmt1p-depleted embryos displayed severe, but distinct, phenotypic abnormalities that correlated with the appearance of apoptotic cells. Apoptosis was either evident subsequent to the depletion of maternal xDnmt1 before the MBT or coincided with a substantial hypomethylation of the genome in post-MBT-depleted siblings. We also observed an increase in the levels of Xenopus p53 (xp53) and 14-3-3 proteins in both types of xDnmt1-depleted embryos. Apoptosis could be inhibited by inactivation of xp53 function, leading to the accumulation of what appeared to be undifferentiated or transformed embryonic cells. Our data suggest that there are cellular death pathways mediated by p53, which are triggered by low levels of genomic DNA methylation in differentiated cells.
Results
Depletion of xDnmt1 before or after MBT results in hypomethylation of the genome and developmental abnormalities
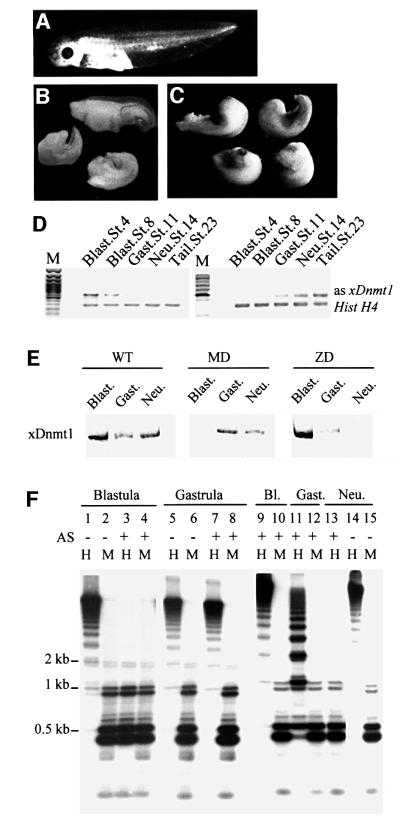
We have generated two types of DNA methyltransferase-deficient Xenopus embryos with reduced levels of xDnmt1 RNA and protein. In one set the maternal form of xDnmt1 RNA was depleted before the MBT by antisense RNA injection and in the second set the zygotic form of xDnmt1 enzyme was eliminated after MBT by injection of plasmid DNA that drives the antisense RNA transcription from a ubiquitously expressed cytomegalovirus (CMV) promoter. Hereafter, the two types of embryos will be referred to as maternally (MD) or zygotically (ZD) xDnmt1 depleted. We have shown previously that loss of maternal xDnmt1p following injection of antisense RNA results in multiple phenotypic abnormalities including microcephaly and axis truncation (Stancheva and Meehan, 2000; see also Table I and Figure 1B). Post-MBT depletion of xDnmt1 produced a different spectrum of phenotypes (Figure 1C; Table I). No microcephaly was observed but instead 34% of the embryos developed with a significantly reduced dorsal axis; 30% also had open neural tubes and 36% had milder phenotypic defects (see Table I). Control embryos injected with the vector alone did not show any phenotypic abnormalities (Table I). Histological analysis of cryosectioned embryos indicated that notochord, neural and muscle tissue were incompletely formed in both types of mutants (data not shown).
Table I. Summary of injection experiments.
| Injection | Number injected | Phenotype | TUNEL-positive embryos |
|---|---|---|---|
| as xDnmt1 RNA 520 pg (MD) | 100 | 55% microcephaly, axis truncation 45% milder defects | 68% ++++30% ++2% + |
| pCMV as xDnmt1 1.5 pg (ZD) | 100 | 34% axis truncation 30% open neural tube 36% milder defects | 71% ++++25% +++4% + |
| pSC-2 plasmid 1.5 pg | 60 | 100% normal | 3.3% +96.7% – |
| as xDnmt1 RNA 520 pg + HPV-18E6 RNA 250 pg (MD/18E6) | 40 | 98.4% no axis1.6% dead | 5% +95% – |
| as xDnmt1 RNA 520 pg + HPV-6E6 RNA 250 pg (MD/6E6) | 40 | 85% microcephaly, axis truncation 15% milder defects | 67.5% ++++32.5% +++ |
| hBcl-2 RNA 250 pg | 84 | 100% normal | 100% – |
| as xDnmt1 RNA 520 pg + hBcl-2 RNA 250 pg (MD/Bcl-2) | 100 | 21% microcephaly, axis truncation 68% no axis 11% dead | 2% +98% – |
Two-cell embryos were injected with the indicated RNA or plasmids and scored independently for phenotypic appearance and for the presence of TUNEL-positive staining at stage 35. The relative number of TUNEL-positive cells after each type of treatment is indicated by +. A high number of TUNEL staining is indicated by ++++ and a lower level by +, see Figure 2 for examples. Embryos that were depleted of the maternal form of xDnmt1 are indicated by MD and those depleted of the zygotic form by ZD.
Fig. 1. Post-MBT depletion of xDnmt1 results in phenotypic abnormalities and hypomethylation. (A) Wild-type control at stage 35. (B) Antisense xDnmt1 RNA injection at the 2-cell stage depletes the maternal form of xDnmt1 (MD) and results in headless and axis-truncated phenotypes (55%), equivalent to stage 35. (C) Embryos depleted of the zygotic form of xDnmt1 RNA by antisense RNA expressed from a CMV-driven promoter (ZD) that is activated after MBT develop axis truncation (34%) and open neural tubes (30%), equivalent to stage 35. (D) RT–PCR showing the level of antisense xDnmt1 and endogenous histone H4 (Hist H4) RNAs in maternal (left set) and zygotic (right set) depleted embryos. M is a size marker; stages are indicated on top of each lane. (E) Western blot showing that xDnmt1p is absent at different stages in maternal (MD) and zygotic (ZD) xDnmt1-depleted embryos. (F) Southern blots of genomic DNA from MD and ZD embryos. The DNA was digested with either HpaII (methylation sensitive) or MspI (methylation insensitive), and probed with a 750 bp satellite I probe. This sequence is 750 bp in length, contains two HpaII sites and is present in ∼2–4 × 104 copies in the genome (Lam and Carroll, 1983). In MD embryos (lanes 3 and 4), satellite I DNA is hypomethylated at blastula and remethylated at gastrula stages (lanes 7 and 8). In the ZD embryos, loss of methylation occurs later during gastrula (lanes 11 and 12) and neurula (lane 13) stages. Embryos that received the antisense RNAs are marked with +, wild type is indicated by –.
Both types of embryos showed reduced levels of xDnmt1 protein (Figure 1E), albeit at different stages of development when compared with each other and with wild-type (WT) siblings. The xDnmt1 protein is missing from blastula stage extracts in embryos injected with antisense RNA, but the levels of the enzyme are restored during gastrula and neural stages, due to the short half-life of the antisense RNA (Figure 1D and E). In contrast, embryos injected with the plasmid DNA have normal levels of xDnmt1p during the blastula cleavages but the enzyme is reduced during gastrula and absent from neurula stages when the antisense RNA is synthesized at high levels (Figure 1D and E).
The satellite I repeat is normally methylated at HpaII sites at all stages examined, as shown by the high molecular weight fragments upon digestion of genomic DNA with this restriction enzyme (Figure 1F, lanes 1, 5 and 14). MspI, which is insensitive to CpG methylation at the same site, produced a series of low molecular weight fragments, most of them ∼500 bp (Figure 1F, lanes 2, 6 and 15). In contrast, HpaII and MspI digests of late blastulae DNA from maternally depleted embryos resulted in an almost identical pattern of satellite I fragments (Figure 1F, lanes 3 and 4), indicating that transient loss of xDnmt1 mRNA has resulted in substantial hypomethylation of satellite I sequences at this stage of development. During gastrulation (stage 12.5), methylation of satellite I sequences was restored in the antisense RNA-injected embryos, as HpaII digestion results in the reappearance of high molecular weight fragments compared with MspI digestion (Figure 1F, lanes 7 and 8). Similar patterns can be seen in the control gastrulae DNA (Figure 1F, lanes 5 and 6). These observations are in agreement with our previous results using a monoclonal antibody raised against m5C to monitor methylation changes (Stancheva and Meehan, 2000), and imply that de novo methyltransferase activities are present in early Xenopus embryos. In contrast, the embryos that received the CMV promoter-driven xDnmt1 antisense RNA had a normal methylation pattern for satellite I DNA during the blastula stage (Figure 1F, lanes 9 and 10), but gradually lost DNA methylation during gastrula and neurula stages (Figure 1F, lanes 11 and 13). The alteration in the methylation patterns of satellite I sequences that we observed in both types of xDnmt1-depleted embryos correlated with the changes in xDnmt1 protein levels and the presence of the antisense RNA (Figure 1D and E).
Hypomethylation induces apoptosis after MBT in developing embryos
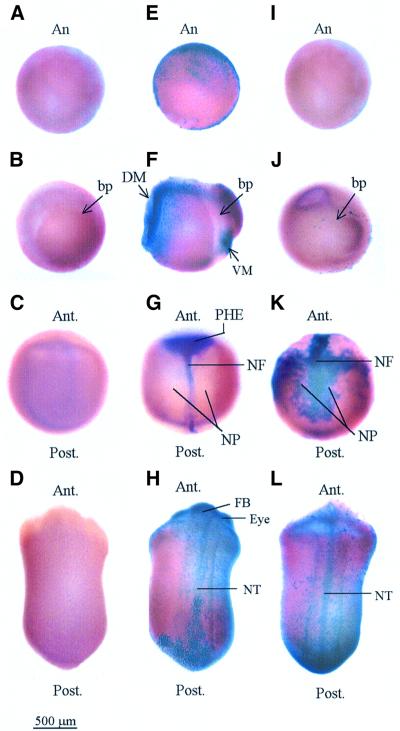
We employed two independent assays to test whether the phenotypic defects that accompany the depletion of xDnmt1 are due to the loss of specific subsets of cells by apoptosis. Whole-mount TUNEL (terminal deoxynucleotidyl transferase-mediated nicked-end labelling) staining, a highly sensitive indicator of DNA fragmentation in situ (Gavrieli et al., 1992; Hensey and Gautier, 1997, 1998), detected the presence of apoptotic cells at high frequency in both MD and ZD embryos (Figure 2). We found, in agreement with previous work (Hensey and Gautier, 1998), that only 3.3% of WT embryos are TUNEL positive after gastrula stages, when apoptosis is restricted mainly to the developing nervous system (Table I). In >95% of the MD embryos TUNEL-positive cells appear after the MBT in late (stage 9) blastula (Figure 2E and Table I), subsequent to the peak of hypomethylation (stage 7.5) in these mutants. Apoptotic cells persist through later stages of development (Figure 2F–H) but initially localize to ecto- and meso-dermal layers of MD gastrulae (Figure 2F) and the presumptive head ectoderm and neural fold (Figure 2G). This pattern corresponds to the regions where xDnmt1 is expressed at high levels in WT embryos (Stancheva and Meehan, 2000). Significantly, re-expression of xDnmt1p (due to degradation of the antisense RNA) in MD embryos was unable to prevent apoptosis occurring in later stage embryos (gastrula onwards; Figure 2G and H). The first appearance of TUNEL-positive cells in ZD embryos occurred at later stages relative to the MD embryos and was coincident with the hypomethylation of DNA (Figure 2J–L). There were no signs of apoptosis in the ZD stage 10 blastulae but a few apoptotic cells are present at stage 12.5 gastrula around the blastopore (Figure 2I and J). The pattern is much more extensive during neurulation of ZD embryos where apoptotic cells could be detected throughout the neural plate (Figure 2K). At later stages, >95% of MD and ZD embryos were TUNEL positive over all regions of the embryo (Figure 2H and L; Table I).
Fig. 2. Whole-mount TUNEL staining of normal and hypomethylated Xenopus embryos. Normal and methylation-deficient embryos were assayed by TUNEL for the appearance of apoptotic cells. Late blastula (A, E and I), gastrula (B, F and J), neurula (C, G and K) and tailbud (D, H and L) were assayed in WT (A–D), MD (E–H) and ZD (I–L) embryos. An MD gastrula embryo with a high number of TUNEL-positive cells, corresponding to +++ in Table I, is shown in (F). A ZD gastrula with a few TUNEL-positive cells, + in Table I, is shown in (J). A ZD neurula with the highest levels of TUNEL-positive cells, ++++, is shown in (K). Embryos in (A), (B), (C), (D) and (I) are TUNEL negative. Note that the positive staining (purple) is subsequent to, or coincident with loss of DNA methylation (Figure 1F) in MD and ZD embryos, respectively. Abbreviations: An, animal pole; bp, blastopore; DM, dorsal mesoderm; VM, ventral mesoderm; PHE, presumptive head ectoderm; NF, neural fold; NP, neural plate; FB, forebrain; NT, neural tube; Ant., anterior; and Post., posterior.
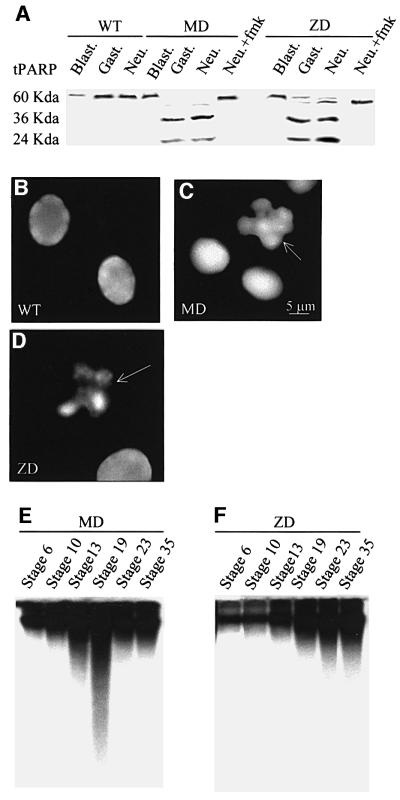
To confirm that the positive TUNEL staining indicates apoptosis, we also assayed MD and ZD embryos for activation of caspases, cysteine proteases that are specifically activated in response to both intracellular and extracellular apoptotic signals (Cohen, 1997; Hensey and Gautier, 1997). We found that protein extracts from gastrula and neurula stage hypomethylated embryos (but not WT, MD and ZD blastula extracts) contain active caspases that cleave in vitro translated poly(ADP) ribose polymerase (tPARP) protein, a well characterized caspase substrate (de Murcia and Menessier de Murcia, 1994; Schreiber et al., 1995), to the predicted fragments of 36 and 24 kDa, respectively (Figure 3A). The caspase 9 inhibitor, z-DEVD-fmk, prevented cleavage of tPARP in both MD and ZD neurula extracts (Figure 3A). Chromatin condensation, DNA fragmentation into nucleosomal fragments, nuclear membrane breakdown and the formation of apoptotic bodies are direct consequences of caspase activity (Chao and Korsmeyer, 1998). Consistent with activation of caspases in the embryo, we could detect fragmented nuclei in cytological preparations from neurula stage MD and ZD embryos with a relative frequency that corresponded to the extent of apoptosis (Figure 3C and D). We also determined the integrity of DNA from different stages of MD and ZD embryos by electrophoresis in native agarose gels (Figure 3E and F). The amount of fragmentation was coincident with the level of apoptosis revealed by TUNEL assay (Figure 2). We did not observe fragmentation to the oligonucleosomal ladder of DNA that typically results from γ-irradiation or cycloheximide treatment of Xenopus embryos, instead the pattern was similar to the effect of α-amanatin treatment (Hensey and Gautier, 1997).
Fig. 3. Hypomethylated embryos show evidence of nuclear fragmentation and activation of caspases. (A) In vitro translated tPARP protein undergoes cleavage in extracts derived from maternal (MD) and zygotic (ZD) xDnmt1-depleted embryos but not in wild-type (WT) extracts. Note that xDnmt1 depletion results in tPARP cleavage during gastrula (Gast.) and neurula (Neu.) but not blastula (Blast.) stages. Note that ZD extracts have a lower caspase activity. Caspase activity can be inhibited by z-DEVD-fmk in MD and ZD neurula extracts (Neu + fmk). (B) Nuclei from wild-type neurula stage embryos stained with 4′-6-diamidine-2-phenylindole (DAPI). (C) Nuclei isolated from MD neurula stage embryos stained with DAPI. Arrow indicates a fragmented nucleus. (D) Nuclei isolated from ZD neurula stage embryos stained with DAPI. Arrow indicates a fragmented nucleus. (E) DNA isolated from MD staged embryos and separated in native agarose gels does not show fragmentation at blastula stages 7 and 10. Low molecular weight fragments appear during and after gastrulation at stages 13 and 19, and are less prominent at 23 and 35. (F) DNA from ZD embryos shows increasing fragmentation beginning at neurula stage 19 and at later stages of development (23 and 35).
In summary, in both types of xDnmt1-depleted embryos, hypomethylation of DNA is associated with induction of cell death, albeit at different times of development. The MD mutants that lack maternal xDnmt1 are unable to initiate apoptotic responses before MBT and show a lag between the peak of hypomethylation and the appearance of TUNEL-positive cells. This is consistent with late activation of the maternal apoptotic pathway and the differentiation state of the embryo (Hensey and Gautier, 1997). In contrast, in ZD embryos, cell death may be triggered upon reaching a critical level of hypomethylation. Since we did not observe DNA fragmentation in MD embryos before MBT when the DNA was already hypomethylated, it is possible that hypomethylation itself does not lead to general DNA breakage in these relatively undifferentiated cells. Moreover, in the two types of xDnmt1-depleted embryos, hypomethylation-induced cell death affects only some tissues at the early stages of development, implying that there are cells in the embryo that are less sensitive to the loss of methylation.
p53 protein accumulates in hypomethylated Xenopus embryos
Several proteins, including p53, play a key role in mediating the response of somatic cells to environmental stress and DNA damage (Larkin and Jackson, 1999). Depending on the cell type and the type of insult, p53 stabilization results in either growth arrest at one of the cell cycle checkpoints, or in apoptosis. With this in mind, we monitored the levels of xp53 protein at different stages in normal and xDnmt1-depleted embryo extracts. Previous work has shown that Xenopus oocytes contain an abundant maternal stockpile of xp53 proteins (Cox et al., 1994; see Figure 4A, WT). We could detect two forms of xp53 protein, at 60 and 46 kDa, which were present after fertilization. In normal embryos, the levels of both forms of xp53 decrease as development proceeds but the 60 kDa form remains prominent at later stages (Figure 4A, WT). In contrast to the WT embryos, the level of the 46 kDa form of xp53, which corresponds to the active form of the protein, remained high after the MBT in the MD embryos (Figure 4A, MD; Bessard et al., 1998). In the ZD embryos we observed stabilization of the 46 kDa form of xp53 during early neurula stages (Figure 4A, ZD). We did not detect any increase in xp53 transcripts at any stage both in MD and ZD embryos (data not shown). The levels of proliferating cell nuclear antigen (PCNA), which serves as a loading control, are relatively constant during development of both normal and xDnmt1-depleted embryos (Figure 4B).
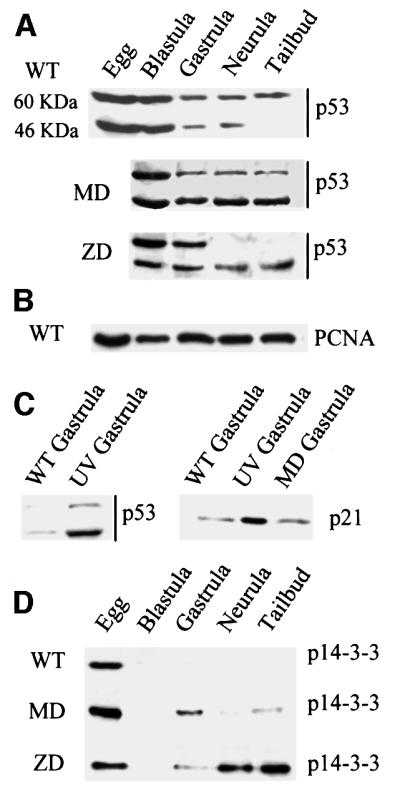
Fig. 4. Hypomethylated Xenopus embryos accumulate stable forms of p53. (A) Western blotting of staged extracts from wild-type (WT), maternally depleted xDnmt1 (MD) and post-MBT-depleted (ZD) embryos. The p53 antibody identifies two forms of xp53 at 60 and 46 kDa; note that both forms decrease as normal development proceeds. In the MD and ZD embryos the active 46 kDa form is preferentially stabilized. (B) As a loading control, all western blots were re-incubated with anti-PCNA antibodies; only the wild type is shown (WT). (C) The 46 kDa form of xp53 is stabilized in UV-treated gastrula embryos, which also leads to the induction of p21. In contrast to UV treatment, MD embryos (MD gastrula) do not induce p21 at gastrulation. (D) 14-3-3 proteins accumulate after MBT in extracts from maternally (MD) and zygotically depleted (ZD) xDnmt1 embryos but not in the wild-type (WT) embryos.
Activation and stabilization of p53 can result in the induction of downstream p53 target genes such as p21, an inhibitor of DNA synthesis and cyclin-dependent kinases (Spitkovsky et al., 1996), and 14-3-3 chaperone proteins (Chan et al., 1999). In Xenopus cell lines, the 46 kDa form of xp53 is stabilized in response to UV irradiation (Cox et al., 1994). We obtained similar results with gastrula stage UV-irradiated embryos (Figure 4C, p53), which accumulate high levels of p21 compared with untreated controls (Figure 4C, p21). We did not detect any changes in the levels of p21 protein in MD embryos compared with the WT and UV-irradiated controls (Figure 4C). In contrast, we could detect induction of 14-3-3 proteins (compared with WT) in gastrula, neural and tailbud extracts derived from MD and ZD embryos (Figure 4D). Induction of 14-3-3 is associated with arrest in the G2 phase of the cell cycle (Chan et al., 1999).
Induction of apoptosis by hypomethylation is dependent upon differentiation
It has been reported that methylation of DNA is not essential for the survival of undifferentiated embryonic mouse stem cells (Li et al., 1992), although Dnmt1–/– cells die when induced to differentiate in culture. This may account for the lag we observe between the appearance of hypomethylated DNA in early embryos and the onset of apoptosis in post-MBT MD Xenopus embryos. In Xenopus, ectodermal tissue explants derived from stage 7–8 blastulae (animal caps) can be cultured in vitro and induced to differentiate towards alternative fates by the addition of growth factors such as TGF-β2 (activin) and FGF (Green and Smith, 1990; Green et al., 1992). Activin can either be used, in a dose-dependent manner, to produce mesoderm (in concentrations >2 U/ml), or under the right conditions can result in a secondary formation of neural tissue (Green et al., 1992; Gurdon et al., 1994) in the overlaying epithelial cells. We monitored apoptosis in animal cap explants derived from WT and MD mutants that were cultured for 6, 12, 24 and 48 h with or without the addition of activin to the culture medium. In no case could we detect the appearance of TUNEL-positive cells in untreated WT or MD explants (Figure 5A and data not shown). WT explants grown either with low (2 U/ml) or high (10 U/ml) doses of TGF-β2 were also TUNEL negative (data not shown). However, activin treatment of MD explants resulted in the appearance of TUNEL-positive cells, which correlated with the dose of TGF-β2 used (Figure 5B and C). When the low dose of activin was applied, some TUNEL-positive cells were observed after only 4 h. At the higher concentration, the staining indicated that up to 50% of the cells in the MD explants were undergoing apoptosis at the same time point.
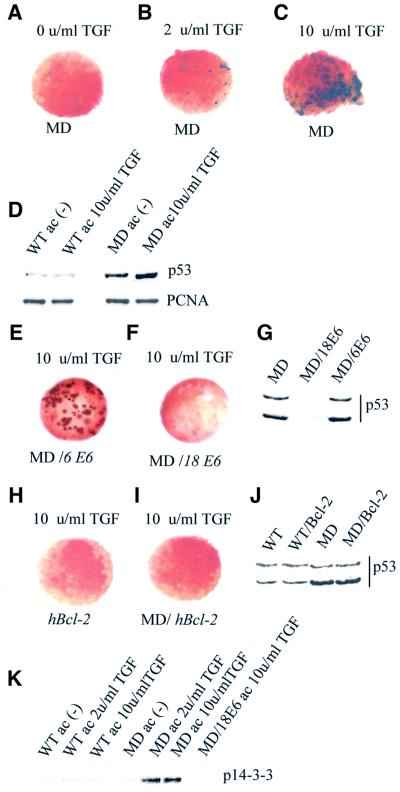
Fig. 5. The apoptotic phenotype of xDnmt1-deficient explants is dependent upon differentiation and can be rescued by co-injection of HPV-E6 and Bcl-2 mRNAs. (A) Animal cap explants from either antisense MD or MD/Bcl-2 co-injected blastulae are TUNEL negative in the absence of activin. An explant derived from an MD embryo is shown. (B) Animal cap explants from MD blastulae treated with a low dose of activin (2 U/ml TGF) exhibit a low level of TUNEL staining (blue). (C) High dose of activin (10 U/ml) causes death of >50% of MD animal cap explant cells as detected by the intensity of TUNEL staining (blue). (D) p53 levels are elevated compared with wild type (WT) in explants derived from antisense xDnmt1 RNA injected embryos (MD) non-induced (–) or induced (MD ac 10 U/ml TGF) with activin. A blot was reprobed for PCNA as a loading control. (E) Co-injection of a defective HPVE6 (MD/HPV-6E6) is unable to prevent hypomethylation-induced apoptosis as shown by TUNEL-positive staining (dark brown) in activin (10 U/ml TGF)-treated MD animal cap explants. (F) Co-injection of a wild-type HPVE6 (MD/HPV-18E6) is able to prevent hypomethylation-induced apoptosis in activin (10 U/ml TGF)-treated MD animal cap explants. (G) Co-injection of a wild-type HPV-E6 (MD/18E6) mRNA promotes p53 degradation in gastrula embryos whilst an N-terminal deletion of HPV-E6 (MD/6E6) does not. (H) Injection of a human Bcl-2 (hBcl-2) mRNA by itself does not lead to apoptosis in activin-treated (10 U/ml TGF) WT animal cap explants. (I) Co-injection of a human Bcl-2 mRNA (MD/hBcl-2) is able to prevent hypomethylation-induced apoptosis in activin (10 U/ml TGF)-treated MD animal caps. (J) Co-injection of a Bcl-2 mRNA does not interfere with p53 stability in wild-type (WT/Bcl-2) or maternal MD gastrula embryos. (K) 14-3-3 proteins accumulate in maternally depleted (MD), but not in the wild-type (WT) cap extracts upon activin induction. Co-injection of functional HPV-18E6 (MD/18E6 ac 10 U/ml TGF) prevents the accumulation of 14-3-3 in activin-treated MD animal cap explants. All the explants were collected 6 h after treatment and TUNEL stained for apoptosis or used for preparation of extracts.
To test whether xp53 is stabilized in hypomethylated ectodermal explants in a similar manner to that observed in whole embryos, we analysed the levels of xp53 in extracts from animal caps derived from WT and MD blastulae (Figure 5D). In this case we could only detect the 46 kDa band due to the small amount of extract. No change was detected in xp53 levels in WT explants before and after activin induction [Figure 5D, WTac (–) and WTac 10 U/ml TGF]. In the extracts from the MD explants, xp53 levels were slightly higher than in WT explants and this level increased slightly upon addition of activin [Figure 5D, compare MD ac (–) to MD ac 10 U/ml TGF]. In all four animal cap extracts, the level of PCNA remained the same before and after activin treatment. We conclude that hypomethylated embryonic explants behave in a similar way to the whole embryos. Thus, loss of methylation is not essential for the survival of embryonic ectodermal cells, but results in stabilization of xp53 and rapid activation of apoptosis upon differentiation to mesodermal and neural tissues. We could also detect induction of 14-3-3 proteins (compared with WT) in MD explants upon treatment with activin (Figure 5K).
Rescue of hypomethylation-induced apoptosis by ectopic expression of HPV-E6 and Bcl-2
Human papilloma virus E6 protein (HPV-18E6) can bind p53 directly and target it for degradation (Spitkovsky et al., 1996; Mantovani and Banks, 1999). Since Xenopus embryos contain abundant amounts (50 ng/embryo) of p53 (Tchang and Mechali, 1999) we decided to test whether ablation of p53 function by expression of E6 protein can prevent apoptosis in differentiating MD explants. We introduced two forms of HPV-E6 into MD explants: a mutant form of the protein (HPV-6E6) lacking the N-terminal p53 binding domain that is unable to reduce p53 levels, and the full-length version (HPV-18E6), which can bind to p53 and target it for ubiquitin-dependent proteolysis (Spitkovsky et al., 1996; Mantovani and Banks, 1999; C.Hensey and J.-P.Gautier, unpublished observations). The TUNEL assay of activin-stimulated MD animal caps showed that HPV-18E6, but not HPV-6E6, is able to decrease the number of TUNEL-positive cells in the activin-treated MD explants (Figure 5E and F). In the case of MD embryos, co-injection of HPV-18E6 RNA results in a dramatic degradation of xp53 (Figure 5G, MD/18E6) as compared with xp53 levels in the control (Figure 5G, MD) and MD/HPV-6E6 co-injected embryo extracts (Figure 5G, MD/6E6). In addition, the presence of HPV-18E6 prevented the induction of 14-3-3 proteins in activin-treated MD explants (Figure 5K). However, the viral protein did not rescue the phenotypes of MD embryos (see Table I), but rather enhanced the developmental defects as compared with the siblings co-injected with antisense xDnmt1 and HPV-6E6 RNA. When monitored at the equivalent of stage 35, HPV-18E6 co-injected hypomethylated embryos do not form head and axis structures, but rather resemble a mass of undifferentiated cells in contrast to the control embryos, which were injected with the same dose of HPV-18E6 or HPV-6E6 and did not exhibit detectable developmental defects (data not shown).
Regulation of caspase activation can occur at the level of cytochrome c release from mitochondria to cytosol, which is controlled by the Bcl-2 family of proteins (Chao and Korsmeyer, 1998). We decided to try and rescue hypomethylation-induced apoptosis by co-injection of human anti-apoptotic Bcl-2 mRNA to rule out the possibility that xp53 might be essential for the differentiation of specific cell lineages during development (Wallingford et al., 1997). Like HPV-18E6 protein, Bcl-2 was able to prevent apoptosis in differentiating xDnmt1-depleted ectodermal explants (Figure 5I) but did not rescue the phenotype of xDnmt1-deficient embryos (Table I). As in the case of HPV-18E6, the embryos that were co-injected with xDnmt1 antisense and Bcl-2 RNA developed with a ‘no axis’ phenotype resembling over-growing cellular masses of undifferentiated cells in post-gastrula stages (data not shown, but see Figure 7). Injection of the same dose of Bcl-2 mRNA into WT embryos or explants did not induce apoptosis or lead to phenotypic abnormalities (Figure 5H; Table I). Ectopic expression of Bcl-2 does not affect the levels of xp53 in either WT (Figure 5J, WT and WT + Bcl-2) or MD embryos (Figure 5J, MD and MD + Bcl-2).
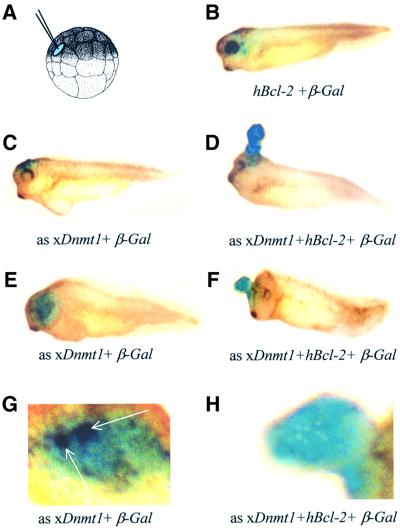
Fig. 7. Ectopic hypomethylation in cells rescued from apoptosis results in embryonic blastomas. (A) Scheme for single-cell injection into a dorsal blastomere of 32- to 64-cell embryos. (B) β-galactosidase and Bcl-2 sense RNAs were co-injected into a single dorsal animal blastomere of a 32- to 64-cell blastula (as indicated on the drawing). The injected embryos were allowed to develop until stage 35 and assayed for β-galactosidase activity. Note the normal appearance of the embryos and that the eye stains blue. (C) Antisense xDnmt1 RNA and β-galactosidase sense RNA were co-injected into a single dorsal animal blastomere of a 32- to 64-cell blastula. When assayed for β-galactosidase activity at stage 35 this resulted in staining on the forehead of the embryos amidst patches of dead cells that colocalize with β-galactosidase staining (blue). (D) Antisense xDnmt1 RNA, Bcl-2 and β-galactosidase sense RNA were co-injected into a single dorsal animal blastomere of a 32- to 64-cell blastula, which was allowed to develop until stage 35 and assayed for β-galactosidase activity. This resulted in the appearance of a large outgrowth of cells that grew out of the forehead and were β-galactosidase positive (blue). (E) Antisense xDnmt1 RNA and β-galactosidase sense RNA were co-injected into a single dorsal animal blastomere of a 32- to 64-cell blastula. When assayed for β-galactosidase activity at stage 35 this resulted in the absence of an eye amidst patches of dead cells (see G) that colocalize with β-galactosidase staining (blue). (F) Antisense xDnmt1 RNA, Bcl-2 and β-galactosidase sense RNA were co-injected into a single dorsal animal blastomere of a 32- to 64-cell blastula, which was allowed to develop until stage 35 and assayed for β-galactosidase activity. This resulted in the appearance of a large outgrowth of cells that grew in place of the eye and were β-galactosidase positive (blue). (G) The embryo in (E) was also assayed for apoptosis by TUNEL staining. A region corresponding to the β-galactosidase staining (blue) is shown under higher magnification to show the presence of TUNEL-positive cells (purple) highlighted by arrows. (H) The embryo in (F) was also assayed for apoptosis by TUNEL staining. A region corresponding to the β-galactosidase staining (blue) is shown under higher magnification to show the absence of TUNEL-positive cells (purple).
These experiments indicate that xp53 is involved in an apoptotic response by xDnmt1 depletion in differentiated Xenopus cells and embryos. Inhibition of apoptosis by elimination of p53 is, however, unable to restore the normal phenotype of MD embryos, suggesting that transiently hypomethylated cells rescued from apoptosis have an altered developmental capacity.
The developmental competence of xDnmt1-depleted explants
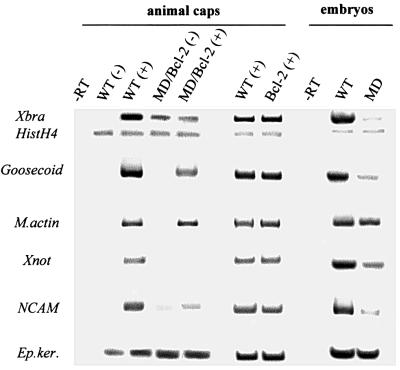
To investigate whether transiently hypomethylated cells maintain their ability to form specific cell lineages when rescued from apoptosis, we monitored the expression of specific mesodermal and tissue-specific molecular markers by RT–PCR, to determine the extent of differentiation by WT and rescued MD/Bcl-2 explants in response to activin (Green et al., 1992; Harland and Gerhart, 1997). In the absence of activin, MD/Bcl-2 explants contained transcripts for the transcription factor Xbra and the neural-specific marker NCAM, both of which are absent from WT explants [Figure 6, compare WT(–) with MD/Bcl-2(–)]. Under these conditions neither WT nor MD/Bcl-2 explants expressed Goosecoid, M-actin or Xnot marker genes, while the maternal transcripts for histone H4 and epidermal keratin (E-ker) were present in equal amounts in all samples tested (Figure 6 and data not shown). However, activin treatment of MD/Bcl-2 explants failed to increase the expression of Xbra to that observed for WT explants and also failed to induce Xnot [Figure 6, MD/Bcl-2(+)]. By contrast, MD/Bcl-2 explants did show activin-dependent expression of Goosecoid and NCAM (Figure 6), albeit to a lower extent than in the WT animal caps and embryos. The expression levels of muscle actin, E-ker and histone H4 were not substantially different between WT and MD/Bcl-2 explants, nor did expression of Bcl-2 by itself interfere with the induction of any of the analysed transcripts after activin treatment [Figure 6, compare WT(+) and Bcl-2(+)]. These experiments imply that transient DNA hypomethylation changes the transcriptional competence and the developmental properties of the early blastula embryo cells. Although they are not restricted to differentiate into epidermis and muscle tissue, their ability to form notochord and neural lineage cells is very much reduced.
Fig. 6. Developmental competence of xDnmt1-depleted ectodermal explants. The expression of specific transcripts was monitored by RT–PCR analysis of untreated and treated explants derived from wild-type embryos (WT) and embryos co-injected with antisense xDnmt1 RNA and Bcl-2 sense RNA (MD/Bcl-2). Note that the pattern of expression for differentiation-specific markers is disrupted in the xDnmt1-depleted explants in the absence (–) and presence (+) of activin. Histone H4 (HistH4) serves as a control as its levels do not change in response to xDnmt1 depletion or activin (+) treatment. Developmental competence was monitored by expression of Xbra, Goosecoid, muscle-specific actin (M.actin), Xnot, NCAM and epidermal keratin (Ep.ker.). A further control shows the expression of these markers in normal (WT) and maternal xDnmt1-depleted (MD) gastrula embryos. Note the expression of differentiation-specific markers is also disrupted in the xDnmt1-depleted gastrula embryos.
Inhibition of hypomethylation-induced apoptosis generates embryonic blastomas
The embryonic tissue explant experiments suggest that the developmental potentials of differentiated MD/Bcl-2 animal caps are altered by hypomethylation. We tested how inhibition of apoptosis would affect the development of hypomethylated cells in vivo by depleting xDnmt1 in a small population of cells of the embryo and following their fate through development using β-galactosidase RNA as a lineage tracer (Wallingford, 1999). For this purpose, we co-injected single dorsal blastomeres of 32- to 64-cell embryos with either (i) Bcl-2 and β-galactosidase RNAs, (ii) antisense xDnmt1 and β-galactosidase RNAs, or (iii) xDnmt1 antisense, Bcl-2 and β-galactosidase RNAs (Figure 7A). The injected embryos were allowed to develop to tadpole stages, fixed and stained for β-galactosidase activity. Co-injection of Bcl-2 and β-galactosidase mRNA alone had no effect on injected blastomere-derived tissues, and β-galactosidase-stained cells were found incorporated into the developing eye or forehead and surrounding tissue (Figure 7B). If xDnmt1 antisense RNA was injected, the eye or forehead did not develop, probably due to the induction of apoptosis in the β-galactosidase-positive cells (Figure 7C, E and G). The co-injection of Bcl-2 together with the antisense xDnmt1 RNA led to the dramatic overgrowth of β-galactosidase-positive cells in place of the eye or on the top of the head (Figure 7D, F and H). These large cellular growths did not show any signs of apoptosis, they were disorganized in appearance and persisted long after the injected RNA, leading to their formation, had been degraded. This indicates that transiently hypomethylated, but rescued from apoptosis, cells (Figure 7H) are unable to respond to the differentiation signals. They remain in the embryo as large undifferentiated cell masses, which may correspond to undifferentiated embryonic tumours or blastomas (Wallingford, 1999).
Discussion
In this paper we have demonstrated that depletion of xDnmt1 protein induces apoptosis in developing Xenopus embryos. Mutants depleted of either the maternal or zygotic form of xDnmt1 exhibited characteristic features of programmed cell death such as DNA fragmentation, nuclear blebbing, TUNEL-positive staining, protease activation and cell death rescue by overexpression of Bcl-2 or HPV-E6. As with other genotoxic insults, the apoptotic response cannot be activated prior to the equivalent of MBT in normal Xenopus embryos. The earliest point at which TUNEL-positive cells could be detected was during late blastula (stage 9), which was subsequent to the peak of hypomethylation (Stancheva and Meehan, 2000; Figure 1F). The same embryos express developmentally regulated genes prior to the MBT, suggesting that the transcriptional response to hypomethylation via xDnmt1 depletion is distinct from the apoptotic response in early blastula. By contrast, in ZD embryos, where xDnmt1 depletion was delayed until late gastrula (stage 13), the onset of cell death correlated with extensive demethylation of the genome. The maternal cell death program in Xenopus is set up at fertilization and can be activated at the onset of gastrulation following DNA damage (Hensey and Gautier, 1997). In this case apoptosis is induced synchronously and embryos die at stage 10.5, and apoptosis is thought to be activated during the MBT in order to eliminate damaged cells in the embryo. Hypomethylation-induced apoptosis is different in that, although TUNEL-positive cells are apparent during late blastula stages, no synchronous wave of embryo death is observed at the onset of gastrulation. This may be because transient hypomethylation does not lead to generalized DNA damage (Figure 3E) and requires differentiation signals to induce apoptosis, which is in contrast to the response to insults used to define the maternal apoptotic pathway. In essence, this suggests that induction of apoptosis in MD and ZD embryos occurs by an indirect mechanism.
We have demonstrated that activation of apoptosis in MD embryos is dependent on (i) differentiation and (ii) xp53 function. Hypomethylated ectodermal explants stabilize xp53 and initiate an apoptotic response only after induction of differentiation by TGF-β2. The ectopic expression of HPV-E6 protein, which promotes xp53 degradation, prevents the activation of the cell death pathway. xp53 is biochemically similar to human p53 in terms of its tetramerization and transactivation properties (Bessard et al., 1998; Tchang and Mechali, 1999). Elimination of xp53 activity in Xenopus by single cell injection of a dominant-negative form of p53 into a 32-cell blastomere results in a block to differentiation in derived cell lineages later in development (Wallingford et al., 1997). Together with our data this implies that p53 function (as in mammals) is operative only in somatic cells and is dispensable during early embryonic development.
What are the signals that trigger apoptosis in xDnmt1-depleted embryos? In MD and ZD embryos, xp53 protein levels are stabilized without altering p53 transcript levels (R.R.Meehan and I.Stancheva, unpublished data). It is possible that pro-apoptotic genes are activated in hypomethylated embryos, which leads to the stabilization of p53. Elucidation of this potential mechanism awaits a comprehensive survey and functional analysis of the genes that are activated in hypomethylated explants before and after differentiation. Another possibility is that hypomethylation leads to genome instability and consequent DNA damage, which triggers an apoptotic response via activation of DNA damage kinases (ATM and ATR), which are known to stabilize p53 levels by phosphorylation of specific residues (Larkin and Jackson, 1999). In this context it is worth noting that loss of Dnmt1 function in mouse embryonic stem (ES) cells leads to hyper-recombination and chromosomal deletions (Chen et al., 1998). Mutation of the human de novo methyltransferase DNMT3B in lymphoblastoid cells has been shown to cause centromeric instability, probably by converting a repressive chromatin domain to a more open chromatin structure by loss of methylation, which causes aberrant centromeric separation during mitosis (Xu et al., 1999). Once activated by a DNA damage pathway, p53 can induce apoptosis via its ability to act as a transcription activator or repressor. We did not observe an induction of p21 in hypomethylated embryos, but did see an increase in the level of 14-3-3 isoforms, which may be indicative of activation of a G2/M checkpoint. Models have been proposed whereby 14-3-3 isoforms (including 14-3-3σ) sequester cdc2 and cdc25 in the cytoplasm, thus preventing initiation of mitosis and contributing to the subsequent induction of apoptosis (Chan et al., 1999). In addition, p53-dependent transcriptional repression of cdc2 and cyclin B genes may also contribute to G2 arrest (Taylor et al., 1999). How the repression function of p53 is targeted to genes is unknown at present as many of them do not contain consensus p53 binding sites. However, p53 has been found to associate with a transcription repressor complex containing histone deacetylase (HDAC) activity, and inhibition of deacetylation can prevent p53-dependent apoptosis in a mouse cell line (Murphy et al., 1999). This raises the possibility that there is cross-talk between methyl-CpG binding proteins (MeCP) and checkpoint kinases (ATR and ATM), all of which are found in similar chromatin remodelling complexes (Jones et al., 1998; Nan et al., 1998; Kim et al., 1999; Ng et al., 1999; Schmidt and Schreiber, 1999). It is possible that in certain cell types, release of MeCP-containing complexes from hypomethylated chromatin may also be instrumental in activating p53 (and hence apoptosis) by checkpoint kinases. A fourth possibility is that xDnmt1p serves as a structural component of a complex that recruits transcriptional repression factors such as HDAC1 and Rb to their appropriate chromosomal sites (Robertson et al., 2000; Rountree et al., 2000). Ablation of these complexes may lead to disruption of growth regulatory pathways, leading to apoptosis in tissues where xDnmt1p is highest. Future experiments will address these possibilities.
The importance of transient DNA hypomethylation and p53-mediated checkpoints is highlighted when apoptosis is inhibited in MD embryos and explants. This results in the formation of large cellular masses in late stage embryos comprised of undifferentiated cells that do not respond properly to normal developmental cues. RT–PCR analysis of ectodermal tissue explants indicates that developmental expression patterns are altered in MD embryos and animal caps, as is evident from the reduced expression of neural (NCAM) and mesodermal (Xnot, Xbra and Goosecoid) markers in MD/Bcl-2 explants treated with TGF-β2 (activin). In MD embryos, remethylation after transient depletion of xDnmt1 results in abnormal methylation of the Xbra promoter (Stancheva and Meehan, 2000). A comparable phenomenon at additional loci may lead to the silencing of other genes required for the control of differentiation and cell proliferation. Similar epigenetic events have been observed in some pre-cancerous cells, where global hypomethylation of the genome is subsequently followed by region-specific hyper-methylation of gene promoter regions during neoplasia progression (Goelz et al., 1985; Baylin and Herman, 2000). Inactivation of p53 is also a feature of many of these tumours. It is possible that loss of p53 function allows cells to survive the alterations in DNA methylation patterns that contribute to genome instability (Baylin and Herman, 2000). It will be of interest to determine whether the cell masses that we observe in Bcl-2-rescued xDnmt1-depleted tissues are true tumours. It is possible, after inhibition of apoptosis, that epigenetic silencing by de novo methylation is the mechanism that allows rapid proliferation and escape of these cells from developmental cues.
The response of early Xenopus embryos and explants to hypomethylation is very similar to that observed for Dnmt1-deficient mouse ES cells that proliferate in culture but rapidly die upon differentiation, with evidence of apoptosis (Panning and Jaenisch, 1996). This was originally correlated with aberrant X-inactivation and suggested that the pluripotent embryonic cells do not utilize DNA methylation as a silencing mechanism to the same extent as somatic cells. p53 also becomes active only after differentiation of mouse ES cells, which may account for the death of Dnmt1-deficient cells (Sabapathy et al., 1997). Indeed inactivation of the P53 gene partially rescues demethylated cultured fibroblasts from cell death, which implies that apoptosis represents a general response by animal cells to low levels of DNA methylation (Jackson-Grusby et al., 2001).
Materials and methods
Embryos and microinjections
Xenopus embryos were obtained by natural mating from wild-type and albino parents, grown, staged and microinjected according to standard procedures (Stancheva and Meehan, 2000). Two-cell blastulae were injected with 520 pg per cell of a 1.4 kb xDnmt1 antisense RNA synthesized in vitro from plasmid xDnmt p9/19 as described (Stancheva and Meehan, 2000) or with 3 pg of pCMV-as 9/19 where the 3′ 1.4 kb xDnmt1 antisense transcript is under the control of a minimal CMV promoter. The expression of antisense xDnmt1 RNA in ZD embryos was monitored by whole-mount in situ hybridization. Eighty-six percent of these embryos stained ubiquitously for the antisense RNA and only 14% showed mosaic expression. Human Bcl-2 RNA was synthesized as described by Hensey and Gautier (1997). HPV-18E6 and HPV-6E6 RNAs were transcribed in vitro from linearized plasmids with T7 RNA polymerase (T3/T7 Cap-Scribe kit, Boehringer) and co-injected with antisense xDnmt1 RNA or injected alone at a concentration of 250 pg per cell. Microinjection experiments are summarized in Table I. For the lineage-restricted ectopic hypomethylation of embryo cells, the antisense xDnmt1 RNA was co-injected with 250 pg of β-Gal RNA (Stancheva and Meehan, 2000) or with 250 pg of β-Gal and 100 pg of hBcl-2 RNA into a single cell of 32- or 64-cell blastulae. Embryos were grown until stage 35 and assayed for TUNEL-positive cells and β-galactosidase activity as described (Steinbach et al., 1997).
Differentiation of ectodermal tissue explants in vitro
Wild-type or microinjected albino Xenopus embryos were allowed to develop to stage 8, and ectodermal tissue explants (animal caps) were excised with an eyebrow-knife (Green and Smith, 1990). The caps were transferred to 1× CMF (88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 7.5 mM Tris pH 7.6) to prevent them from folding. The media contained 0, 2 or 10 U/ml synthetic TGF-β2 (activin) obtained from in vitro cultured (24–48 h) mature oocytes injected with activin RNA (McDowell et al., 1997). The growth factor activity was determined according to Green and Smith (1990). Animal caps were incubated in activin/CMF media for 3 h, transferred to 0.1× MMR and cultured for various periods of time from 3 h to overnight (12 h) or to the equivalent of neurula stage embryos (48 h). Uninduced or induced caps were collected and processed either for TUNEL staining or for preparation of total RNA and protein extracts.
DNA isolation and Southern blots
DNA from wild-type and microinjected staged embryos was isolated and 10 µg of each sample were digested to completion with HpaII or MspI (MBI-Fermentas) and electrophoresed in 1.2% native agarose gels. DNA digests were transferred to Pall B membranes and hybridized according to manufacturer’s protocols with a labelled 750 bp Xenopus satellite I probe (Lam and Carroll, 1983).
Western blots
Protein extracts from wild-type, UV-treated or microinjected staged embryos and animal caps were prepared as described (Stancheva and Meehan, 2000). The equivalent of five embryos per lane was run in 10% SDS–PAGE gels and transferred to nitrocellulose membrane (MCI). xDnmt1p was detected by a polyclonal antibody raised against the conserved C-terminal domain of the mouse xDnmt1 (Liu et al., 1998). xp53 was detected by a rabbit polyclonal antibody generated against recombinant xp53 (Cox et al., 1994). The polyclonal antibody against human p21 was described by Zhang et al. (1993). Pan 14-3-3 antibody was a gift from Dr Alistair Aitken’s laboratory.
Whole-mount TUNEL staining
Published procedures for the whole-mount TUNEL staining of embryos were followed (Hensey and Gautier, 1997). The same protocol was used to detect apoptotic cells in cultured animal caps.
tPARP cleavage assay
Detection of proteolytic cleavage of an in vitro translated N-terminal truncated PARP was as described (Hensey and Gautier, 1997). Aliquots (5 µl) of the translation reaction were incubated for 1 h with 20 µl of protein extracts from WT, MD or ZD staged embryos with or without z-DEVD-fmk inhibitor (10 µM final concentration). All reactions were separated in 10% SDS–PAGE gels, which were dried and exposed overnight.
Cytology
Activin (10 U/ml)-treated WT, MD or ZD embryonic tissue explant (animal caps) derived late blastulae embryos were dissociated to single cells in CMF medium by gentle shaking. The cells were washed with phosphate-buffered saline (PBS) and the nuclei attached to glass coverslips by centrifugation (1000 g) at room temperature through a 30% glycerol/PBS cushion. The nuclei were briefly fixed with 0.25% formaldehyde in PBS, stained for 5 min with 25 µg/ml DAPI and mounted in 90% glycerol/PBS to microscope slides. Images were collected using a Zeiss Axioskop (Carl Zeiss, Oberkochen, Germany) with Plan-Apochromat 100×/1.4 lens and Photometrics Sensys cooled CCD camera (Tucson, AZ) controlled by Quips Imaging Software (Vysis, Downers Grove, IL).
RT–PCR
RNA isolation from staged embryos and animal caps, RT–PCR analysis and the primers for Xbra, muscle actin and histone H4 were previously described (Stancheva and Meehan, 2000). Additional primer pairs were: Goosecoid: f CACAGGACCATCTTCACC, r CCAATCAACTGTCAGAGTCC; Xnot: f AGAACAGACAGACCTGCC, r GCGGATTCTCTTCATCTT; NCAM: f ATGCTACCTTCTGTCACCACC, r AGATGGATTGTTE-ker.: f TATTGGCTGAGAAGAACCGCC; r ACTCTGTGTTCTGGTGTTCC.
Acknowledgments
Acknowledgements
We would like to thank D.Caroll for the Xenopus satellite I probe, S.Silverstein for HPV-E6 plasmids, E.Warbrick, D.Beach and T.Dubois for antibodies, A.Zorn for TGF-β2 cDNA plasmid and protocols, W.C.Earnshaw for z-DEVD-fmk, A.Merdes for help with microscopy, and S.Pennings, A.Bird, M.Hooper, S.Maciver, C.Davey and K.Sawin for reading and helpful comments during the manuscript preparation. This work was supported by grants from the Wellcome Trust to R.R.M. and C.H.
References
- Baylin S.B. and Herman,J.G. (2000) DNA hyper-methylation in tumorigenesis: epigenetics joins genetics. Trends Genet., 16, 168–174. [DOI] [PubMed] [Google Scholar]
- Bessard A.C., Garay,E., Lacronique,V., Legros,Y., Demarquay,C., Houque,A., Portefaix,J.M., Granier,C. and Soussi,T. (1998) Regulation of the specific DNA binding activity of Xenopus laevis p53: evidence for conserved regulation through the carboxy-terminus of the protein. Oncogene, 16, 883–890. [DOI] [PubMed] [Google Scholar]
- Chan T.A., Hermeking,H., Lengauer,C., Kinzler,K.W. and Vogelstein,B. (1999) 14-3-3σ is required to prevent mitotic catastrophe after DNA damage. Nature, 401, 616–620. [DOI] [PubMed] [Google Scholar]
- Chao D.T. and Korsmeyer,S.J. (1998) BCL-2 family: regulators of cell death. Annu. Rev. Immunol., 16, 395–419. [DOI] [PubMed] [Google Scholar]
- Chen R.Z., Pettersson,U., Beard,C., Jackson-Grusby,L. and Jaenisch,R. (1998) DNA hypomethylation leads to elevated mutation rates. Nature, 395, 89–93. [DOI] [PubMed] [Google Scholar]
- Cohen J.M. (1997) Caspases: the executioners of apoptosis. Biochem. J., 326, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colot V. and Rossignol,J.L. (1999) Eukaryotic DNA methylation as an evolutionary device. BioEssays, 21, 402–411. [DOI] [PubMed] [Google Scholar]
- Cox L.S., Midgley,C.A. and Lane,D.P. (1994) Xenopus p53 is biochemically similar to the human tumour suppressor protein p53 and is induced upon DNA damage in somatic cells. Oncogene, 9, 2951–2959. [PubMed] [Google Scholar]
- de Murcia G. and Menissier de Murcia,J. (1994) Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem. Sci., 19, 172–176. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman,Y. and Ben-Sasson,S.A. (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol., 119, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz S.E., Vogelstein,B., Hamilton,S.R. and Feinberg,A.P. (1985) Hypomethylation of DNA from benign and malignant human colon neoplasms. Science, 228, 187–190. [DOI] [PubMed] [Google Scholar]
- Green J.B. and Smith,J.C. (1990) Graded changes in dose of a Xenopus activin A homologue elicit stepwise transitions in embryonic cell fate. Nature, 347, 391–394. [DOI] [PubMed] [Google Scholar]
- Green J.B., New,H.V. and Smith,J.C. (1992) Responses of embryonic Xenopus cells to activin and FGF are separated by multiple dose thresholds and correspond to distinct axes of the mesoderm. Cell, 71, 731–739. [DOI] [PubMed] [Google Scholar]
- Gurdon J.B., Harger,P., Mitchell,A. and Lemaire,P. (1994) Activin signalling and response to a morphogen gradient. Nature, 371, 487–492. [DOI] [PubMed] [Google Scholar]
- Harland R. and Gerhart,J. (1997). Formation and function of Spemann’s organizer. Annu. Rev. Cell Dev. Biol., 13, 611–667. [DOI] [PubMed] [Google Scholar]
- Hensey C. and Gautier,J. (1997) A developmental timer that regulates apoptosis at the onset of gastrulation. Mech. Dev., 69, 183–195. [DOI] [PubMed] [Google Scholar]
- Hensey C. and Gautier,J. (1998) Programmed cell death during Xenopus development: a spatio-temporal analysis. Dev. Biol., 203, 36–48. [DOI] [PubMed] [Google Scholar]
- Jackson-Grusby L. et al. (2001) Loss of genomic methylation causes p53-dependent apoptosis and epigenetic deregulation. Nature Genet., 27, 31–39. [DOI] [PubMed] [Google Scholar]
- Jones P.L., Veenstra,G.J., Wade,P.A., Vermaak,D., Kass,S.U., Landsberger,N., Strouboulis,J. and Wolffe,A.P. (1998) Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nature Genet., 19, 187–191. [DOI] [PubMed] [Google Scholar]
- Kim G.D., Choi,Y.H., Dimtchev,A., Jeong,S.J., Dritschilo,A. and Jung,M. (1999) Sensing of ionizing radiation-induced DNA damage by ATM through interaction with histone deacetylase. J. Biol. Chem., 274, 31127–31130. [DOI] [PubMed] [Google Scholar]
- Lam B.S. and Carroll,D. (1983) Tandemly repeated DNA sequences from Xenopus laevis. I. Studies on sequence organization and variation in satellite 1 DNA (741 base-pair repeat). J. Mol. Biol., 165, 567–585. [DOI] [PubMed] [Google Scholar]
- Larkin N.D. and Jackson,S.P. (1999) Regulation of p53 in response to DNA damage. Oncogene, 18, 7644–7655. [DOI] [PubMed] [Google Scholar]
- Li E., Bestor,T.H. and Jaenisch,R. (1992) Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell, 69, 915–926. [DOI] [PubMed] [Google Scholar]
- Li E., Beard,C. and Jaenisch,R. (1993) Role for DNA methylation in genomic imprinting. Nature, 366, 362–365. [DOI] [PubMed] [Google Scholar]
- Liu Y., Oakeley,E.J., Sun,L. and Jost,J.P. (1998) Multiple domains are involved in the targeting of the mouse DNA methyltransferase to the DNA replication foci. Nucleic Acids Res., 26, 1038–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani F. and Banks,L. (1999) The interaction between p53 and papillomaviruses. Semin. Cancer Biol., 9, 387–395. [DOI] [PubMed] [Google Scholar]
- Martin C.C., Laforest,L., Akimenko,M.A. and Ekker,M. (1999) A role for DNA methylation in gastrulation and somite patterning. Dev. Biol., 206, 189–205. [DOI] [PubMed] [Google Scholar]
- McDowell N., Zorn,A.M., Crease,D.J. and Gurdon,J.B. (1997) Activin has direct long-range signalling activity and can form a concentration gradient by diffusion. Curr. Biol., 7, 671–681. [DOI] [PubMed] [Google Scholar]
- Murphy M., Ahn,J., Walker,K.K., Hoffman,W.H., Evans,R.M., Levine,A.J. and George,D.L. (1999) Transcriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev., 13, 2490–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X., Ng,H.H., Johnson,C.A., Laherty,C.D., Turner,B.M., Eisenman,R.N. and Bird,A. (1998) Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature, 393, 386–389. [DOI] [PubMed] [Google Scholar]
- Ng H.H., Zhang,Y., Hendrich,B., Johnson,C.A., Turner,B.M., Erdjument-Bromage,H., Tempst,P., Reinberg,D. and Bird,A. (1999) MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nature Genet., 23, 58–61. [DOI] [PubMed] [Google Scholar]
- Panning B. and Jaenisch,R. (1996) DNA hypomethylation can activate Xist expression and silence X-linked genes. Genes Dev., 10, 1991–2002. [DOI] [PubMed] [Google Scholar]
- Robertson K.D., Ait-Si-Ali,S., Yokochi,T., Wade,P.A., Jones,P.L. and Wolffe,A.P. (2000) DNMT1 forms a complex with Rb, E2F1 and HDAC1 and represses transcription from E2F-responsive promoters. Nature Genet., 25, 338–342. [DOI] [PubMed] [Google Scholar]
- Rountree M.R., Bachman,K.E. and Baylin,S.B. (2000) DNMT1 binds HDAC2 and a new co-repressor, DMAP1, to form a complex at replication foci. Nature Genet., 25, 269–277. [DOI] [PubMed] [Google Scholar]
- Sabapathy K., Klemm,M., Jaenisch,R. and Wagner,E.F. (1997) Regulation of ES cell differentiation by functional and conformational modulation of p53. EMBO J., 16, 6217–6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt D.R. and Schreiber,S.L. (1999) Molecular association between ATR and two components of the nucleosome remodeling and deacetylating complex, HDAC2 and CHD4. Biochemistry, 38, 14711–14717. [DOI] [PubMed] [Google Scholar]
- Schreiber V., Hunting,D., Trucco,C., Gowans,B., Grunwald,D., de Murcia,G. and de Murcia,J.M. (1995) A dominant-negative mutant of human poly(ADP-ribose) polymerase affects cell recovery, apoptosis and sister chromatid exchange following DNA damage. Proc. Natl Acad. Sci. USA, 92, 4753–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitkovsky D., Aengeneyndt,F., Braspenning,J. and von Knebel Doeberitz,M. (1996) p53-independent growth regulation of cervical cancer cells by the papillomavirus E6 oncogene. Oncogene, 13, 1027–1035. [PubMed] [Google Scholar]
- Stancheva I. and Meehan,R.R. (2000) Transient depletion of xDnmt1 leads to premature gene activation in Xenopus embryos. Genes Dev., 14, 313–327. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Steinbach O.C., Wolffe,A.P. and Rupp,R.A. (1997) Somatic linker histones cause loss of mesodermal competence in Xenopus. Nature, 389, 395–399. [DOI] [PubMed] [Google Scholar]
- Taylor W.R., DePrimo,S.E., Agarwal,A., Agarwal,M.L., Schonthal,A.H., Katula,K.S. and Stark,G.R. (1999) Mechanisms of G2 arrest in response to overexpression of p53. Mol. Biol. Cell, 10, 3607–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchang F. and Mechali,M. (1999) Nuclear import of p53 during Xenopus laevis early development in relation to DNA replication and DNA repair. Exp. Cell Res., 251, 46–56. [DOI] [PubMed] [Google Scholar]
- Wallingford J.B. (1999) Tumors in tadpoles: the Xenopus embryo as a model system for the study of tumorigenesis. Trends Genet., 15, 385–388. [DOI] [PubMed] [Google Scholar]
- Wallingford J.B., Seufert,D.W., Virta,V.C. and Vize,P.D. (1997) p53 activity is essential for normal development in Xenopus. Curr. Biol., 7, 747–757. [DOI] [PubMed] [Google Scholar]
- Walsh C.P. and Bestor,T.H. (1999) Cytosine methylation and mammalian development. Genes Dev., 13, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G.L. et al. (1999) Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature, 402, 187–191. [DOI] [PubMed] [Google Scholar]
- Zhang H., Xiong,Y. and Beach,D. (1993) Proliferating cell nuclear antigen and p21 are components of multiple cell cycle kinase complexes. Mol. Biol. Cell, 4, 897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]