Abstract
Background
As a novel selective anticholinergic drug, penehyclidine hydrochloride (PHC) provided the potential to protect organs by inhibiting the inflammatory response, attenuating oxidative stress, and alleviating ischemia / reperfusion injury. This study aimed to evaluate the organ protective effects of PHC in patients undergoing cardiac surgery.
Methods
Six electronic databases were searched systematically for randomized-controlled trials (RCTs) published April 30th 2025 that explored the application of PHC on cardiac surgical patients. Primary outcomes of interest included the biomarkers and variables of major organs (e.g. heart, lung, gastrointestinal tract and immune system) injury. Secondary outcomes of interest included the mechanical ventilation duration and hospital length of stay (LOS). Mean difference (MD) with 95% confidence interval (CI) or odds ratios (OR) with 95% CI were employed to analyze the data.
Results
A total of 37 RCTs with 1929 cardiac surgical patients (PHC group, 1043 patients; Control group, 886 patients) were included. The current study demonstrated that the adult patients in PHC group had lower cardiac troponin I (cTnI) [MD: -1.70, 95%CI: -2.63 to -0.77, P = 0.0003, with heterogeneity (P < 0.00001)] and creatine kinase (CK)-MB levels on post-operative day (POD)-1 after cardiac surgery, while the pediatric patients had lower cardiac troponin T (cTnT) (MD: -0.10, 95%CI: -0.12 to -0.09, P < 0.00001, without heterogeneity) in PHC group on POD-1. The levels of interleukin (IL)-6 and tumor necrosis factor (TNF)-α were significantly lower in both adult and pediatric patients of PHC group on POD-1. The incidence of postoperative pulmonary infection was significantly reduced in the PHC group, and the duration of mechanical ventilation and hospital LOS were shortened in adult patients.
Conclusions
This meta-analysis demonstrated that PHC could provide myocardial protection and suppress the inflammatory response in patients undergoing cardiac surgery, thereby potentially facilitating rapid recovery.
Clinical trial number
PROSPERO registration number CRD42020183260.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12871-025-03396-1.
Keywords: Penehyclidine hydrochloride, PHC, Anticholinergic drug, Organ protection, Cardiac surgery
Introduction
Approximately 40% of patients undergoing cardiac surgery experience organ damage that affects vital organs such as the heart, lungs, brain, or kidneys, which can significantly increase the risk of mortality [1–4]. Surgical trauma and cardiopulmonary bypass during cardiac surgery initiates significant systemic inflammatory response due to tissue damage, hemodynamic disorders, coagulation dysfunction, and so on [5]. It is characterized by massive proinflammatory cytokines release, and subsequent vital organ dysfunction, such as myocardial dysfunction, respiratory failure, acute kidney injury, neurocognitive disorders, coagulopathy, hepatic dysfunction, even multiple organ failure [6]. Acute and persistent organ dysfunction commonly followed cardiovascular surgery, impairing patient prognosis and substantially increasing healthcare-related economic burden.
Anticholinergic drugs (e.g., scopolamine, atropine) have been used as preoperative medications due to antisialogogue, vagolytic, antiemetic, sedative and amnesic effects [7, 8]. In contrast to scopolamine and atropine, penehyclidine hydrochloride (PHC) selectively blocks M1, and M3 receptors distributed in the airway smooth muscle, submucosal glands and alveolar walls, and has no M2 receptor-associated cardiovascular side effects [9]. In recent years, with the exploration of organ-protective effects in ischemia/reperfusion injury, PHC has attracted much attention. Accumulative experimental evidences have demonstrated that, PHC could also provide vital organ protection [10–12]. Most of them have reported that administration of PHC could inhibit inflammation and provide myocardial and pulmonary protection [12, 13]. It has been shown that both pre- and post-conditioning with PHC attenuated myocardial injury by decreasing the release of inflammatory cytokines and mediators, lowering nuclear factor-κB (NF-κB) signaling and reducing Ca2+ overload [11]. Shen and his colleagues have reported that PHC pre-conditioning significantly alleviated lipopolysaccharide-induced inflammatory cell infiltration and alveolar hemorrhage in rat lung pathological changes [14].
Currently, only few relevant meta-analyses have been published showing that PHC has multiple organ protection in the perioperative treatment. Therefore, this study was designed to systemically evaluate the organ protective effects of PHC in cardiac surgical patients.
Methods
Ethics
The ethics approval was not required for meta-analysis.
Search strategy
This systematic review followed the methodology outlined in the Preferred Instrument for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [15]. The protocol of current meta-analysis has been registered on the International Prospective Systematic Reviews Registry database (CRD42020183260). Relevant randomized controlled trials (RCTs) were identified by computerized searches of PubMed, Ovid, EMBASE, the Cochrane Central Register of Controlled Trials (CENTRAL), China National Knowledge Infrastructure (CNKI) database, and Wanfang Data till April 30th 2025, using different combination of search words as follows: (penehyclidine) AND (cardiopulmonary bypass OR heart OR cardiac surgery OR coronary artery bypass surgery OR valve surgery OR congenital heart surgery) AND (randomized controlled trial OR controlled clinical trial OR randomly OR clinical trial) (in Supplemental Table 1). No language restriction was used. To ensure the comprehensiveness of the literature review, two authors (LJT and YTY) conducted a non-blind standardized method to independently evaluate all retrieved records by examining the titles, abstracts, and keywords.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) the target population comprised patients undergoing cardiovascular surgery; (2) intravenous PHC administration versus Control (saline); (3) RCTs; (4) at least one of the predetermined outcomes listed in the following reported. Primary outcomes of interest included the biomarkers and variables of major organs (e.g. heart, lung, gastrointestinal tract and immune system) injury. Secondary outcomes of interest included the mechanical ventilation duration, intensive care unit (ICU) stay and hospital length of stay (LOS). The following exclusion criteria were applied: (1) studies published in the form of review articles, case reports, or abstracts; (2) retrospective or observational studies; (3) animal studies; (4) studies lacking information about outcomes of interest; (5) Duplicate publications.
Study quality assessment
The risk of bias in the studies which were included was assessed independently via the revised Cochrane risk-of-bias tool (RoB 2.0) [16] by two investigators (LJT and YTY). Using RoB 2.0, two investigators independently assessed each study for bias arising from (1) the randomization process, (2) deviations from the intended intervention, (3) missing outcome data, (4) outcome measurement, and (5) selective reporting of results. LJT and JFG independently assessed the methodological quality of each included trial using the modified Jadad score (ranging from 0 to 5) [17]. This scale evaluated the generation of random sequences, randomized concealment, blinding, and reporting of withdrawals. Each item was evaluated based on associated criteria and scores, with ≤ 3 points indicating low-quality research and 4–7 points indicating high-quality research.
Data abstraction
Data were extracted independently by investigators LJT and YTY, and compared to ensure accuracy. Data consisted of research title, first author, year of publication, journal, country, number of patients, gender, age, type of surgical procedure and data regarding outcomes of interest. Disagreements were resolved by discussion among all authors at the end of assessment.
Statistical analysis
All data were analyzed by utilizing RevMan 5.4 (Cochrane Collaboration, Oxford, UK). Pooled odds ratio (OR) and 95% confidence interval (CI) were estimated for dichotomous data, and mean difference (MD) and 95% CI for continuous data, respectively. To account for methodological and clinical heterogeneity, the random-effect model was employed when substantial heterogeneity was detected (I2 >50% or P < 0.05). Conversely, the fixed-effect model was utilized for analysis that there was no significant heterogeneity. In accordance with the Cochrane Handbook, I2 values ranging from 0% to 25%, 25% to 50%, and 75% to 100% indicated low, medium, and high heterogeneity, respectively. The results showed significant heterogeneity when I2 ≥ 50%, in which case a sensitivity analysis and subgroup analyses would be conducted to identify the source of heterogeneity. Subgroup analysis in adult patients was employed to investigate the associations among CPB time (< 120 min vs. ≥ 120 min) [18], surgical technique, and clinical outcomes (myocardial injury and inflammatory biomarkers). Sensitivity analyses were performed to evaluate the influence of individual study on the overall effects. Publication bias was explored through visual inspection of funnel plots of the outcomes. All P-values were two-sided and statistical significance was defined as P < 0.05.
Results
Literature search and study characteristics
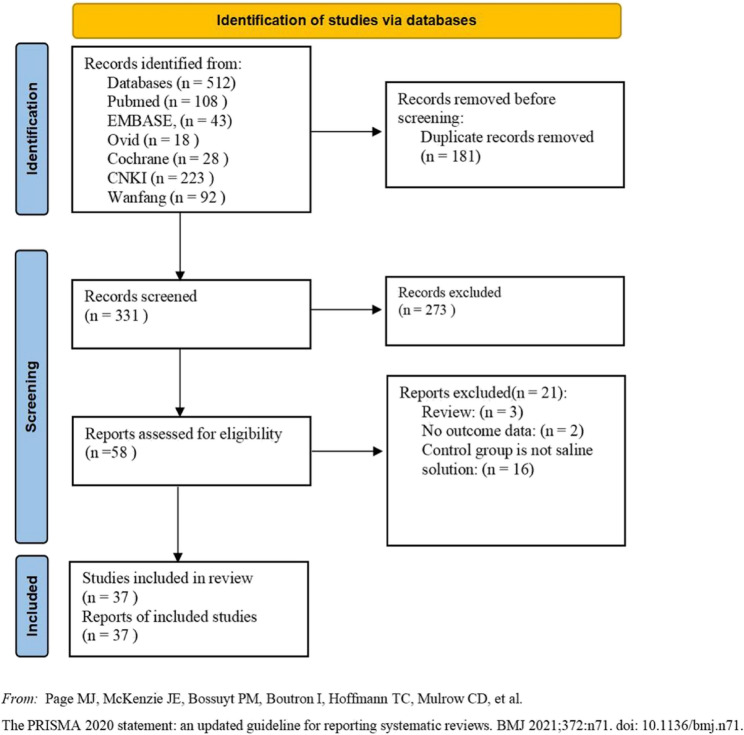
As depicted in the flow chart (Fig. 1), database search identified 58 RCTs for complete evaluation. Finally, 37 eligible RCTs were included in the meta-analysis. The baseline characteristics and perioperative data of the patients were presented in Table 1 and Supplemental Table 2. Among the 37 included RCTs, 29 included only adult cardiac surgical patients, the other 8 enrolled only pediatric cardiac surgical patients.
Fig. 1.
Flowchart of the study search and selection process
Table 1.
PHC administration scheme and reported outcomes
| Trials | Patients | Surgery | Group PHC | Group Control | Outcomes | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Timing | Dose | Route | n | Comparator | Dose | n | ① | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | |||
| Sun 2013[19] | Adults | VR | 10 min pre-CPB | 0.05 mg/kg | iv | 20 | Saline | Same volume | 20 | √ | √ | √ | |||||
| Shi 2019[20] | Adults |
VR VR |
AI | 0.05 mg/kg | iv | 20 | Saline | Same volume | 20 | √ | √ | √ | √ | ||||
| AI | 0.10 mg/kg | iv | 20 | ||||||||||||||
| Zhou 2010[21] | Adults | VR | CPB | 3 mg | CPB prime | 12 | Saline | Same volume | 12 | √ | √ | ||||||
| Wei 2007[22] | Adults | VR | 10 min pre-CPB | 3 mg | iv | 10 | Saline | Same volume | 10 | √ | √ | √ | √ | ||||
| Li 2017[23] | Adults | OPCAB | AI | 0.05 mg/kg | iv | 20 | Saline | Same volume | 20 | √ | √ | √ | √ | ||||
| Shu 2012(1)[24] | Adults | VR | AI and CPB |
0.01 mg/kg 0.015 mg/kg |
iv CPB prime |
92 | Saline | Same volume | 92 | √ | √ | √ | √ | ||||
| Duan 2009[25] | Adults | CABG | 10 min pre-CPB | 3 mg | iv | 20 | Saline | Same volume | 20 | √ | √ | √ | √ | ||||
| Wu 2014[26] | Adults | VR | AI | 0.02 mg/kg | iv | 42 | Saline | Same volume | 42 | √ | √ | √ | √ | ||||
| Li 2009[27] | Adults | CPB-CS |
BA pre-CPB |
0.02 mg/kg 0.02 mg/kg |
iv iv |
12 | Saline | Same volume | 12 | √ | √ | ||||||
| Shu 2012(2)[28] | Adults | VR | BA and CPB |
0.01 mg/kg 0.015 mg/kg |
iv CPB prime |
20 | Saline | Same volume | 20 | √ | √ | √ | √ | ||||
| Zhang 2015[29] | Adults | VR | During induction and CPB |
0.02 mg/kg 0.04 mg/kg |
iv CPB prime |
50 | Saline | Same volume | 50 | √ | √ | √ | √ | ||||
| Yu 2010[30] | Adults |
VR CABG |
30 min pre-CPB | 0.02 mg/kg | iv | 12 | Saline | 5 ml | 12 | √ | √ | ||||||
| Li 2016[31] | Adults | CPB-CS | BA | 0.03 mg/kg | iv | 20 | Saline | Same volume | 19 | √ | √ | √ | |||||
| Zhang 2013[32] | Adults | VR | AI | 0.02 mg/kg | iv | 20 | Saline | Same volume | 20 | √ | √ | ||||||
| Peng 2012[33] | Adults | VR | 10 min pre-CPB | 0.06 mg/kg | iv | 25 | Saline | Same volume | 25 | √ | √ | ||||||
| Liu 2010[34] | Adults | VR | CPB | 0.03 mg/kg | CPB prime | 20 | Saline | Same volume | 20 | √ | |||||||
| Sun 2011[35] | Adults | VR | 10 min pre-CPB | 0.05 mg/kg | iv | 20 | Saline | Same volume | 20 | √ | √ | √ | |||||
| Ai 2009[36] | Adults | VR | AI | 0.05 mg/kg | iv | 10 | Saline | Same volume | 10 | √ | |||||||
| 0.10 mg/kg | iv | 10 | |||||||||||||||
| Sun 2012[37] | Adults | VR | 10 min pre-CPB | 0.05 mg/kg | iv | 20 | Saline | Same volume | 20 | √ | √ | √ | |||||
| Zheng 2014[38] | Adults | VR | AI | 0.05 mg/kg | iv | 20 | Saline | Same volume | 20 | √ | √ | ||||||
| Li 2010[39] | Adults | VR | CPB | 0.05 mg/kg | iv | 15 | Saline | Same volume | 15 | √ | √ | √ | |||||
| He 2007[40] | Adults | VR | CPB | 2 mg | CPB prime | 30 | Saline | Same volume | 30 | √ | |||||||
| Gao 2018[41] | Adults | OPCAB | AI | 0.01 mg/kg | iv | 30 | Saline | Same volume | 30 | √ | √ | √ | √ | ||||
| AI | 0.03 mg/kg | iv | 30 | ||||||||||||||
| AI | 0.05 mg/kg | iv | 30 | ||||||||||||||
| Ai 2010[42] | Adults | VR | 10 min pre-CPB | 0.04 mg/kg | iv | 15 | Saline | Same volume | 15 | √ | |||||||
| 10 min pre-CPB | 0.08 mg/kg | iv | 15 | ||||||||||||||
| Dai 2012[43] | Adults | VR | 10 min pre-CPB | 0.04 mg/kg | iv | 15 | Saline | Same volume | 15 | √ | |||||||
| Wang 2010[44] | Adults | VR and CABG | CPB | 0.05 mg/kg | CPB prime | 20 | Saline | Same volume | 20 | √ | √ | √ | |||||
| Hu 2008[45] | Adults | VR | 10 min pre-CPB | 3 mg | iv | 20 | Saline | Same volume | 20 | √ | |||||||
| Wei 2018[46] | Adults | AD | BA | 0.05 mg/kg | iv | 30 | Saline | Same volume | 30 | √ | √ | √ | |||||
| He 2023[47] | Adults | VR | BA and CPB | 0.02 mg/kg of PHC before anesthesia and with a total dose of 0.04 mg/kg during CPB | iv and CPB prime | 45 | Saline | Same volume | 43 | √ | √ | √ | √ | ||||
| Zhang 2014[48] | Pediatrics |
ASD-R VSD-R |
BA | 0.02 mg/kg | iv | 20 | Saline | Same volume | 20 | √ | √ | ||||||
| AI | 0.04 mg/kg | iv | 20 | ||||||||||||||
| Lang 2017[49] | Pediatrics | TOF-R | AI | 0.04 mg/kg | iv | 50 | Saline | Same volume | 50 | √ | √ | ||||||
| Qin 2013[50] | Pediatrics |
ASD-R VSD-R |
CPB | 0.05 mg/kg | CPB prime | 20 | Saline | Same volume | 20 | √ | √ | √ | |||||
| Fang 2010[51] | Pediatrics |
ASD-R VSD-R |
AI | 0.02 mg/kg | iv | 18 | Saline | Same volume | 12 | √ | √ | ||||||
| AI | 0.04 mg/kg | iv | 20 | ||||||||||||||
| Lu 2011[52] | Pediatrics |
ASD-R VSD-R |
AI | 0.02 mg/kg | iv | 15 | Saline | Same volume | 12 | √ | √ | √ | |||||
| Li 2012[53] | Pediatrics |
ASD-R VSD-R |
During induction | 0.05 mg/kg | iv | 10 | Saline | Same volume | 10 | √ | |||||||
| Wu 2017[54] | Pediatrics | TOF-R | AI | 0.04 mg/kg | iv | 50 | Saline | Same volume | 50 | √ | √ | √ | √ | ||||
| Wei 2023[55] | Pediatrics | CPB-CS | CPB | 0.02 mg/kg | CPB prime | 10 | Saline | Same volume | 10 | √ | √ | √ | |||||
Outcomes: ①=Myocardial biomarkers, ②=Gastrointestinal variables, ③=Inflammatory biomarkers/endotoxin/catecholamine, ④=Blood glucose/L-lactate/coagulation variables, ⑤=Respiratory/oxygenation variables, ⑥=Intraoperative surgical variables/fluid balance, ⑦=Postoperative recovery profiles, ⑧ =Postoperative mortality
CPB-CS Cardiopulmonary bypass-cardiac surgery, AD Aortic Dissection, VR Valve replacement, OPCAB Off-pump coronary artery bypass graft, CABG Coronary artery bypass grafting, ASD-R Repair of atrial septal defect, VSD-R Repair of ventricular septal defect, TOF-R Radical correction of tetralogy of Fallot, CPB Cardiopulmonary bypass, BA Before anesthesia, AI After induction, PHC Penehyclidine hydrochloride, iv Intravenously
As shown in Table 1, the 37 eligible RCTs involved totally 1929 patients, 1043 of whom were allocated into PHC group, and the other 886 into Control group. PHC administration regimens (dosage, timing of administration and route) was not uniform due to differences in the design endpoints of the included trials. The dosages of PHC ranged from 0.01 mg/kg to 0.10 mg/kg, or from 2 mg to 3 mg. PHC was administered before or after anesthesia induction intravenously, or added to the cardiopulmonary bypass (CPB) prime.
Quality assessment
Out of 37 RCTs, 4 (10.8%) had a high risk of bias in the randomization process [25, 35, 43, 45] and 1 (2.3%) had a high risk of bias in measurement of the outcome [27]. Most RCTs were assessed as low risk of bias due to deviations from intended intervention [19, 22, 24–35, 37] and missing outcome data. Overall, a total of 13 trials (35.1%) had an overall low risk of bias [22, 24, 30, 33, 37, 38, 41, 44, 47, 48, 52, 54, 55] 19 trials (51.4%) had some concerns [19, 23, 26, 28, 29, 31, 32, 34, 36, 39, 40, 42, 46, 49–51, 53] and 5 trials (13.5%) had a high risk of bias [25, 27, 35, 43, 45] (Fig. 2). Of the 37 included trials, 16 trials had Jadad scores ≥ 4 and were considered as high-quality RCTs, shown in Supplemental Table 3.
Fig. 2.

Risk of bias traffic light plot for RCT articles
Outcomes
Cardiac function
Due to heterogeneity in outcome definition, measurement, and reporting, we were not able to perform meta-analysis on all endpoints. The summary of all outcomes is shown in Table 1.
As shown in Tables 1 and 2 , 9 RCTs reported perioperative levels of myocardial injury biomarkers [cardiac troponin (cTn), creatine kinase (CK), CK-MB, myoglobin (MYO) and aspartate transaminase(AST)]. As shown in Table 2, meta-analysis demonstrated that, cTnI levels were significantly lower in adult patients of PHC group on post-operative day(POD)−1 [MD: −1.70, 95%CI: −2.63 to −0.77, P = 0.0003, with heterogeneity (I2 = 90%, P < 0.00001)], as compared to those of Control group. CK-MB levels were significantly lower in adult patients of PHC group on POD-1 [MD: −11.32, 95%CI: −20.34 to −2.30, P = 0.0003, with heterogeneity (I2 = 79%, P = 0.0002)], as compared to those of Control group. Similarly, meta-analysis demonstrated that cTnT levels were significantly lower in pediatric patients of PHC group on POD-1 (MD: −0.10, 95%CI: −0.12 to −0.09, P < 0.00001, without heterogeneity), as compared to those of Control group. One study [41] including 120 patients reported on the occurrence of post-operative myocardial infarction (MI) with an overall incidence was 2.5% (PHC, 2.2%; Saline, 3.3%). There was no significant difference with respect to the incidence of post-operative MI in PHC group relative to Control Group (Fig. 3). One trial reported that there was no difference between the PHC group and the Control group in terms of heart failure [1/92 (1.1%) vs. 4/92 (4.3%), P = 0.21].
Table 2.
Myocardial injury biomarkers.
| Variables | Patients | Comparisons (N) |
PHC (n) |
Control (n) |
Heterogeneity | Analysis model | MD | 95%CI | Overall effect P | |
|---|---|---|---|---|---|---|---|---|---|---|
| I2 | P | |||||||||
| cTnI | Adults | |||||||||
| Baseline | 10 | 230 | 135 | 0% | 0.88 | Fixed | −0.00 | −0.01 to 0.01 | 0.91 | |
| POD-1 | 10 | 230 | 135 | 85% | < 0.0001 | Random | −1.70 | −2.63 to −0.77 | 0.0003 | |
| CK | Adults | |||||||||
| Baseline | 3 | 90 | 30 | 0% | 0.40 | Fixed | 1.01 | −11.56 to 13.58 | 0.88 | |
| POD-1 | 3 | 90 | 30 | 0% | 0.67 | Fixed | −106.38 | −211.33 to −1.42 | 0.05 | |
| CK-MB | Adults | |||||||||
| Baseline | 5 | 120 | 45 | 0% | 0.98 | Fixed | 0.14 | −0.13 to 0.42 | 0.29 | |
| POD-1 | 6 | 150 | 75 | 79% | 0.0002 | Random | −11.32 | −20.34 to −2.30 | 0.01 | |
| MYO | Adults | |||||||||
| Baseline | 3 | 90 | 30 | 0% | 0.43 | Fixed | 0.03 | −2.86 to 2.92 | 0.98 | |
| POD-1 | 3 | 90 | 30 | 38% | 0.20 | Fixed | −38.51 | −92.34 to 15.33 | 0.16 | |
| cTnT | Pediatrics | |||||||||
| Baseline | 2 | 100 | 100 | 0% | 1.00 | Fixed | −0.00 | −0.00 to 0.00 | 0.16 | |
| POD-1 | 2 | 100 | 100 | 0% | 0.95 | Fixed | −0.10 | −0.12 to −0.09 | < 0.00001 | |
| CK | Pediatrics | |||||||||
| Baseline | 1 | 10 | 10 | NA | NA | Random | −149.00 | −276.53 to −21.47 | 0.02 | |
| POD-1 | 1 | 10 | 10 | NA | NA | Random | −621.00 | −1151.11 to −90.89 | 0.02 | |
| CK-MB | Pediatrics | |||||||||
| Baseline | 1 | 10 | 10 | NA | NA | Random | −5.00 | −16.64 to 6.64 | 0.40 | |
| POD-1 | 1 | 10 | 10 | NA | NA | Random | −43.00 | −59.00 to −27.00 | < 0.0001 | |
| AST | Pediatrics | |||||||||
| Baseline | 1 | 10 | 10 | NA | NA | Random | −3.00 | −15.40 to 9.40 | 0.64 | |
| POD-1 | 1 | 10 | 10 | NA | NA | Random | −40.00 | −66.48 to −13.52 | 0.003 | |
PHC Penehyclidine hydrochloride, MD Mean difference, CI Confidence interval, cTn Cardiac troponin, CK Creatine kinase, MYO Myoglobin, AST Aspartate transaminase, POD-1 Postoperative day-1, NA Not applicable
Fig. 3.
Incidence of postoperative myocardial infarction, heart failure, pulmonary infection/pneumonia, and systemic inflammatory response syndrome
Gastrointestinal function
As shown in Tables 1 and 3 , 3 RCTs [19, 35] reported perioperative gastrointestinal variables and biomarkers [pH value of gastric mucosa (pHi), intestinal fatty acid-binding protein (I-FABP), D-lactate and diamine oxidase (DAO)] in adult patients. As shown in Table 3, meta-analysis demonstrated that, D-lactate levels were significantly lower in adult patients of PHC group at end of surgery (EOS) (MD: −1.11, 95%CI: −1.29 to −0.94, P < 0.00001, without heterogeneity). The study by Peng et al. [33] suggested that, pHi values in adult patients of PHC group were significantly higher than those of Control group at EOS (MD: 0.07, 95%CI: 0.06 to 0.08, P < 0.00001). The study by Sun et al. [19] suggested that, I-FABP values were significantly lower in adult patients of PHC group at EOS (MD: −234.40, 95%CI: −382.25 to −86.55, P = 0.002). The study by Peng et al. [33] suggested that, DAO values were significantly lower in adult patients of PHC group at EOS (MD: −0.58, 95%CI: −0.69 to −0.47, P < 0.00001).
Table 3.
Gastrointestinal variables.
| Variables | Patients | Comparisons (N) |
PHC (n) |
Control (n) |
Heterogeneity | Analysis model | MD | 95%CI | Overall effect P | |
|---|---|---|---|---|---|---|---|---|---|---|
| I2 | P | |||||||||
| PHi | ||||||||||
| Baseline | Adults | 1 | 25 | 25 | NA | NA | Random | −0.01 | −0.02 to 0.00 | 0.19 |
| EOS | 1 | 25 | 25 | NA | NA | Random | 0.07 | 0.06 to 0.08 | < 0.00001 | |
| I-FABP | ||||||||||
| Baseline | Adults | 1 | 20 | 20 | NA | NA | Random | 2.90 | −73.24 to 79.04 | 0.94 |
| EOS | 1 | 20 | 20 | NA | NA | Random | −234.40 | −382.25 to −86.55 | 0.002 | |
| D-Lactate | ||||||||||
| Baseline | Adults | 2 | 45 | 45 | 0% | 0.86 | Fixed | 0.04 | −0.13 to 0.21 | 0.67 |
| EOS | 2 | 45 | 45 | 0% | 0.76 | Fixed | −1.11 | −1.29 to −0.94 | < 0.00001 | |
| DAO | ||||||||||
| Baseline | Adults | 1 | 25 | 25 | NA | NA | Random | −0.02 | −0.15 to 0.11 | 0.76 |
| EOS | 1 | 25 | 25 | NA | NA | Random | −0.58 | −0.69 to −0.47 | < 0.00001 | |
PHC Penehyclidine hydrochloride, MD Mean difference, CI Confidence interval, PHi pH value of gastric mucosa, I-FABP Intestinal fatty acid-binding protein, DAO Diamine oxidase, EOS End of surgery, NA Not applicable
Pulmonary function
As shown in Tables 1 and 4 , 13 RCTs reported perioperative respiratory and oxygenation parameters [compliance, respiratory index (RI), oxygenation index (OI) and mixed venous oxygen saturation (SmVO2)] in adult patients. As shown in Table 2, meta-analysis demonstrated that the compliance and OI variables were significantly higher in adult patients of PHC group at EOS [MD: 8.35, 95%CI: 2.47 to 14.23, P = 0.005, with heterogeneity (I2 = 88%, P = 0.0003); MD: 64.88, 95%CI: 53.50 to 76.26, P < 0.00001, without heterogeneity]. RI was significantly lower in adult patients of PHC group at EOS [MD: −0.28, 95%CI: −0.52 to −0.03, P = 0.03, with heterogeneity (I2 = 95%, P < 0.00001)], as compared to those of Control group. SmVO2 was significantly higher in adult patients of PHC group at POD-1 (MD: 7.93, 95%CI: 7 0.06 to 8.80, P < 0.00001, without heterogeneity). The occurrence of pulmonary infection/pneumonia was reported in three trials including 372 adult patients with an overall incidence of 13.2% (PHC: 8.0%; Control: 18.4%), there was a significant reduction in pulmonary infection after cardiac surgery in the PHC group (Fig. 3; OR: 0.40; 95% CI: 0.21 to 0.78; P = 0.007; I2 = 0%).
Table 4.
Respiratory and oxygenation parameters
| Variables | Patients | Comparisons (N) |
PHC (n) |
Control (n) |
Heterogeneity | Analysis model | MD | 95%CI | Overall effect P | |
|---|---|---|---|---|---|---|---|---|---|---|
| I2 | P | |||||||||
| Compliance | Adults | |||||||||
| Baseline | 2 | 47 | 47 | 0% | 0.73 | Fixed | 0.04 | −2.29 to 2.36 | 0.97 | |
| EOS | 2 | 47 | 47 | 88% | 0.0003 | Random | 8.35 | 2.47 to 14.23 | 0.005 | |
| Respiratory index | Adults | |||||||||
| Baseline | 5 | 139 | 137 | 0% | 0.97 | Fixed | 0.02 | −0.01 to 0.04 | 0.17 | |
| EOS | 5 | 139 | 137 | 95% | < 0.00001 | Random | −0.28 | −0.52 to −0.03 | 0.03 | |
| Oxygenation index | Adults | |||||||||
| Baseline | 5 | 122 | 120 | 0% | 0.95 | Fixed | 3.97 | −10.53 to18.48 | 0.59 | |
| EOS | 5 | 122 | 120 | 21% | 0.28 | Fixed | 64.88 | 53.50 to 76.26 | < 0.00001 | |
| S m VO 2 | Adults | |||||||||
| Baseline | 3 | 60 | 40 | 85% | 0.001 | Random | −1.13 | −2.92 to 0.66 | 0.22 | |
| POD-1 | 3 | 60 | 40 | 0% | 0.76 | Fixed | 7.93 | 7.06 to 8.80 | < 0.00001 | |
| L-Lactate | Adults | |||||||||
| Baseline | 5 | 100 | 80 | 0% | 0.68 | Fixed | 0.01 | −0.03 to 0.05 | 0.66 | |
| POD-1 | 4 | 80 | 60 | 77% | 0.005 | Random | −0.81 | −0.98 to −0.65 | < 0.00001 | |
PHC Penehyclidine hydrochloride, MD Weighted mean difference, CI Confidence interval, SmVO2 Mixed venous oxygen saturation, EOS End of surgery, POD-1 Postoperative day-1
Inflammatory reaction
As shown in Tables 1, and 5 , 24 RCTs reported perioperative change of inflammatory biomarkers. Meta-analysis demonstrated that, the levels of interleukin (IL)−6 and tumor necrosis factor (TNF)-α were significantly lower in both adult [IL-6: MD: −4.75, 95%CI: −7.23 to −2.27, P = 0.0002, with heterogeneity (I2 = 84%, P < 0.00001); TNF-α: MD: −2.52, 95%CI: −4.31 to −0.27, P = 0.006, with heterogeneity (I2 = 74%, P = 0.0003)] and pediatric patients of PHC group on POD-1 [IL-6: MD: −5.44, 95%CI: −8.84 to −2.04, P = 0.002, with heterogeneity (I2 = 92%, P < 0.00001); TNF-α: MD: −0.99, 95%CI: −1.63 to −0.35, P = 0.002, with heterogeneity (I2 = 94%, P < 0.00001)]. The levels of IL-10 were significantly higher in adult patients of PHC group on POD-1 [MD: 11.50, 95%CI: 2.63 to 20.38, P = 0.01, with heterogeneity (I2 = 76%, P = 0.006)]. The study by Wei et al. [46] suggested that, the levels of IL-1 in adult patients of PHC group were significantly lower than those of Control group on POD-1. The levels of IL-2 and IL-8 were significantly lower in pediatric patients of PHC group on POD-1. The occurrence of systemic inflammatory response syndrome (SIRS) was reported in two trials, including 204 adult patients, with an overall incidence of 17.2% (PHC: 12.7%; Control: 21.6%). There was a reduction in SIRS after cardiac surgery in the PHC group, but this reduction was not statistically significant (Fig. 3. OR: 0.52; 95% CI: 0.25 to 1.12; P = 0.09).
Table 5.
Inflammatory biomarkers and endotoxin
| Variables | Patients | Comparisons (N) |
PHC (n) |
Control (n) |
Heterogeneity | Analysis model | WMD | 95%CI | Overall effect P | |
|---|---|---|---|---|---|---|---|---|---|---|
| I2 | P | |||||||||
| IL-1 | ||||||||||
| Baseline | Adults | 1 | 30 | 30 | NA | NA | Random | −0.01 | −0.09 to 0.07 | 0.81 |
| POD-1 | 1 | 30 | 30 | NA | NA | Random | −0.60 | −0.82 to −0.38 | < 0.00001 | |
| IL-6 | ||||||||||
| Baseline | Adults | 11 | 263 | 263 | 71% | 0.0002 | Random | −0.21 | −0.53 to 0.11 | 0.21 |
| POD-1 | 8 | 139 | 139 | 84% | < 0.00001 | Random | −4.75 | −7.23 to −2.27 | 0.0002 | |
| IL-8 | ||||||||||
| Baseline | Adults | 3 | 47 | 47 | 0% | 0.97 | Fixed | −0.60 | −1.21 to 0.00 | 0.05 |
| POD-1 | 2 | 35 | 35 | 85% | 0.03 | Random | −65.30 | −207.45 to 76.85 | 0.37 | |
| IL-10 | ||||||||||
| Baseline | Adults | 4 | 65 | 65 | 0% | 0.89 | Fixed | 1.39 | −2.00 to 4.78 | 0.42 |
| POD-1 | 4 | 65 | 65 | 76% | 0.006 | Random | 11.50 | 2.63 to 20.38 | 0.01 | |
| TNF-α | ||||||||||
| Baseline | Adults | 10 | 213 | 203 | 0% | 0.95 | Fixed | −0.01 | −0.14 to 0.12 | 0.88 |
| POD-1 | 8 | 109 | 99 | 74% | 0.0003 | Random | −2.52 | −4.31 to −0.27 | 0.006 | |
| IL-2 | ||||||||||
| Baseline | Pediatrics | 2 | 40 | 20 | 0% | 0.55 | Fixed | −0.29 | −0.85 to 0.27 | 0.31 |
| POD-1 | 2 | 40 | 20 | 0% | 0.74 | Fixed | −1.03 | −1.54 to −0.52 | < 0.0001 | |
| IL-6 | ||||||||||
| Baseline | Pediatrics | 6 | 165 | 142 | 97% | < 0.00001 | Random | −2.90 | −6.10 to 0.30 | 0.08 |
| POD-1 | 6 | 165 | 142 | 92% | < 0.00001 | Random | −5.44 | −8.84 to −2.04 | 0.002 | |
| IL-8 | Pediatrics | |||||||||
| Baseline | 6 | 165 | 142 | 57% | 0.04 | Random | −0.29 | −0.39 to −0.19 | < 0.00001 | |
| POD-1 | 6 | 165 | 142 | 95% | < 0.00001 | Random | −0.37 | −0.72 to −0.03 | 0.04 | |
| TNF-α | Pediatrics | |||||||||
| Baseline | 8 | 203 | 154 | 0% | 0.98 | Fixed | −0.50 | −0.56 to −0.43 | < 0.00001 | |
| POD-1 | 8 | 203 | 154 | 94% | < 0.00001 | Random | −0.99 | −1.63 to −0.35 | 0.002 | |
| MMP-9 | ||||||||||
| Baseline | Pediatrics | 3 | 53 | 24 | 0% | 0.99 | Fixed | 6.24 | −10.23 to 22.72 | 0.46 |
| POD-1 | 3 | 53 | 24 | 0% | 0.81 | Fixed | 20.82 | 8.60 to 33.03 | 0.0008 | |
PHC Penehyclidine hydrochloride, MD Mean difference, CI Confidence interval, IL Interleukin, TNF Tumor necrosis factor, MMP-9 Matrix metalloprotein-9, POD-1 Postoperative day-1, NA Not applicable
Post-operative recovery
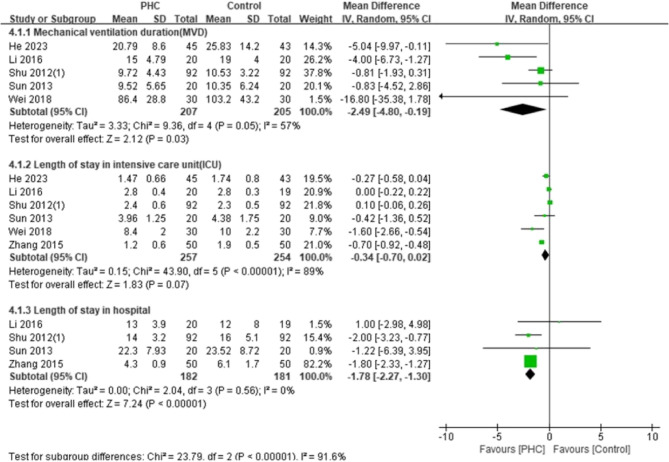
The ICU stay was examined in five trials with 511 adult patients. The lengths of ICU stay were less in the PHC group with a marginal statistical significance (Fig. 4; MD: −0.34 days; 95% CI: −0.70 to 0.02; P = 0.07; I2 = 89%). The pooled analysis found significant difference in hospital LOS between the groups (Fig. 4; MD: −1.78 days; 95% CI: −2.27 to −1.30; P < 0.00001; I2 = 0%). The mechanical ventilation duration was explored in five trials with 412 adult patients and the effect of PHC was significant on reduction of ventilation time (Fig. 4; MD: −2.46 h; 95% CI: −4.80 to −0.19; P = 0.03; I2 = 57%). The lengths of ICU and mechanical ventilation duration were recorded in 2 studies including 124 pediatric patients. The results showed that there was no significant difference between the two groups (Fig. 5). As shown in Tables 1 and 11 studies included 628 patients reported in-hospital mortality, but no deaths occurred.
Fig. 4.
Forest plot of mechanical ventilation duration, length of stay in the intensive care unit, and length of hospital stay in adult patients
Fig. 5.
Forest plot of mechanical ventilation duration, and length of stay in the intensive care unit in pediatric patients
Subgroup analyses for potential sources of heterogeneity
Subgroup analyses showed that PHC administration significantly reduced IL-6 levels in patients with CPB < 120 min [(MD: −3.10; 95% CI: −4.28 to −1.92) vs. (MD: −13.73; 95% CI: −38.76 to 11.30); P = 0.006 for the subgroup difference], whereas no significant reduction was observed in patients with CPB ≥ 120 min. Compared with patients undergoing off-pump surgery, PHC administration significantly reduced cTnI levels in patients undergoing on-pump surgery [(MD: −0.42; 95% CI: −0.63 to −0.21) vs. (MD: −2.60; 95% CI: −3.80 to −1.40); P = 0.0004 for subgroup difference] (in Table 6).
Table 6.
Subgroup analyses for the potential sources of heterogeneity
| Variables | Patients | Outcomes | Comparisons (N) |
WMD | 95% CI | Heterogeneity P | I2 | Overall effect P | P Difference |
|---|---|---|---|---|---|---|---|---|---|
| CPB duration | Adults | IL-6 | 8 | 0.006 | |||||
| CPB time < 120 min | 6 | −3.10 | −4.28, −1.92 | 0.08 | 50% | < 0.00001 | |||
| CPB time ≥ 120 min | 2 | −13.73 | −38.76, 11.30 | < 0.00001 | 96% | 0.28 | |||
| CPB duration | Adults | TNF-α | 8 | 0.003 | |||||
| CPB time < 120 min | 7 | −1.60 | −3.05, −0.15 | 0.009 | 65% | 0.03 | |||
| CPB time ≥ 120 min | 1 | −11.00 | −17.09, −4.91 | - | - | 0.0004 | |||
| CPB | Adults | cTnI | 10 | 0.0004 | |||||
| Off-pump | 4 | −0.42 | −0.63, −0.21 | 0.99 | 0 | 0.0001 | |||
| On-pump | 6 | −2.60 | −3.80, −1.40 | 0.0005 | 77% | < 0.0001 | |||
| CPB | Adults | CK-MB | 6 | 0.02 | |||||
| Off-pump | 3 | −1.67 | −6.81, 3.47 | 0.99 | 0 | 0.52 | |||
| On-pump | 3 | −27.69 | −49.03, −6.36 | 0.006 | 80% | 0.01 | |||
CPB Cardiopulmonary bypass, IL Interleukin, TNF Tumor necrosis factor, cTn Cardiac troponin, CK Creatine kinase, WMD Weighted mean difference, CI Confidence interval
Publication bias assessment and sensitivity analysis
As for the publication bias, slight asymmetry can be found in the funnel plot for the incidence of postoperative complications. There was an insufficient number of trials providing data (less than 10 studies identified for each outcome), we concluded that there was possibility of publication bias. Sensitivity analysis was performed by excluding some studies to analyze the influence of the overall treatment effect on high heterogeneity outcomes (Supplemental Table 4) but no contradictory results were found.
Discussion
To our best knowledge, this is the first meta-analysis dedicated to evaluating the organ protective effects of PHC in patients undergoing cardiac surgery. The current study demonstrated that, PHC administration in cardiac surgical patients was associated with a significantly decreased incidence of postoperative pulmonary infection, as well as a reduction in the duration of mechanical ventilation and length of hospital stay in adult patients. The adult patients in PHC group had significantly lower cTnI and CK-MB levels on POD-1 after cardiac surgery, while the pediatric patients had lower cTnT levels on POD-1 in PHC group. The levels of IL-6 and TNF-α were significantly lower in both adult and pediatric patients of PHC group on POD-1. The levels of IL-10 were significantly higher in adult patients of PHC group on POD-1. PHC administration was associated with lower levels of MI biomarkers and inflammatory biomarkers, suggesting that PHC could attenuate myocardial injury and inflammatory response.
PHC is a novel anticholinergic drug derived from scopolamine that has anti-muscarinic and anti-nicotinic properties while retaining potent central and peripheral anticholinergic effects [7, 8, 10]. Based on clinical data, PHC has been shown to improve microcirculation, reduce microvascular permeability, inhibit the release of lysomes, prevent lipid peroxidation, and avoid organ injury [7, 14]. The result of this meta-analysis showed that PHC administration could signifcantly reduce the levels of CK-MB and cTn-I within 24 h after cardiac surgery, suggesting a pronounced early myocardial protective effect. Subgroup analyses showed that compared with patients undergoing off-pump surgery, PHC administration significantly reduced cTnI levels in patients undergoing on-pump surgery. The level of CK-MB and cTn-I were recommended for assessing myocardial injury, and its dynamic variations offer a more appropriate reference [56, 57]. Reversible postoperative cardiac dysfunction is predominantly attributable to ischemia–reperfusion injury, an inherent sequela of cardiac surgery performed with cardiopulmonary bypass [4]. Lin et al. demonstrated that preconditioning PHC conferred sustained cardioprotection, manifesting as preserved ventricular performance, smaller infarct size, attenuated oxidative stress, diminished cardiomyocyte apoptosis, and reduced release of inflammatory cytokines. Administration of PHC significantly decreased serum TNF-a, IL-1b, IL-6 and prostaglandin E2 levels [58]. Except for its influence on biomarkers associated with myocardial injury, PHC also reduces the severity of inflammation. Several studies have demonstrated that PHC may reduce ischemia/reperfusion injury, suppress inflammatory responses, and decrease cytokine release, all of which contribute to protect organs [8, 10]. The results showed that PHC decreased the levels of IL-6 and TNF-α within 24 h after cardiac surgery in adult and pediatric patients. In addition, the anti-inflammatory cytokine IL-10 was significantly increased in PHC group. The overall incidence of postoperative SIRS was 17.2%, which was numerically lower in the PHC group than in the control group; however, the difference was not statistically significant. Adequately powered, randomized trials focusing on high-risk cohorts are warranted to clarify the clinical relevance of PHC in attenuating SIRS after cardiac surgery.
PHC has been shown to improve pulmonary function by reducing airway resistance, relaxing airway smooth muscle, dilating bronchioles, and increasing lung compliance [7].Cardiac surgery with cardiopulmonary bypass can cause related atelectasis and pulmonary edema, which aggravates the related hazards of perioperative mechanical ventilation [59]. He et al. reported that the administration of PHC during anesthesia reduced the occurrence of postoperative pulmonary dysfunction, and improved the prognosis of patients undergoing cardiac surgery with CPB [47]. Li Baiqiang and his colleagues have shown that PHC significantly prevents the progression of acute lung injury (ALI) by down regulating the expression of Toll like receptor (TLR) 4 and inhibiting inflammatory cytokines, improving arterial oxygen pressure in patients with ALI [60]. OI and RI values were often used as indicators to evaluate lung function and gas exchange, and were associated with ICU mechanical ventilation time, ICU stay, and hospital stay. Postoperative OI can predict changes in pulmonary function and extubation time following heart valve surgery [61]. The results of this meta-analysis showed that adult patients in the PHC group had significantly lower RI, higher OI and SmVO2 after cardiac surgery, along with a reduced incidence of postoperative pulmonary infection. Evidence indicated that postoperative IL-6 levels serve as an independent prognostic marker and that patients exhibiting IL-6 elevation after CPB might benefit from intensified anti-inflammatory therapy to preserve pulmonary function [62]. In the present study, PHC significantly reduced both IL-6 and TNF-a in both adult and pediatric cohorts. Subgroup analyses showed that PHC administration significantly reduced IL-6 levels in patients with CPB < 120 min, whereas no significant reduction was observed in patients with CPB ≥ 120 min. These findings were consistent with previous reports of PHC-mediated pulmonary protection during cardiac surgery. Moreover, PHC shortened both ICU and hospital length of stay, suggesting that it might facilitate rapid recovery in cardiac surgical patients.
This study has the weakness inherent in meta-analysis. First, there were concerns with quality and heterogeneity of included studies, as well as heterogeneity in the various PHC administration regimens (dosage, route, and timing), surgical procedures, outcome definitions, and patient comorbidities. Second, the meta-analysis included 37 RCTs with comparatively small sample sizes, particularly for several key parameters, which may increase the risk of publication bias. Various postoperative complication events were reported, but heterogeneity limited the pooling of data. The incidence of several complications was low, and most reports were limited to alternative parameters of a single organ system. We contacted the corresponding authors for missing data, but not much reply was received. Although the present meta-analysis confirmed the safety of PHC in adult cardiac surgical patients, the included trials were not powered to detect uncommon or delayed adverse effects. Consequently, the incidence of anticholinergic-related events (e.g., dry mouth, delirium) remained uncertain, and standardized adverse event monitoring in future investigations was warranted.
Conclusions
In conclusion, the available evidence indicated that PHC administration could provide myocardial protection, attenuate inflammatory responses, and decrease the incidence of postoperative pulmonary infection, which may be beneficial to the rapid recovery in cardiac surgical patients. However, further RCTs should focus on the more vulnerable cardiac patients to explore PHC on the protection of single- or multiorgan function.
Supplementary Information
Acknowledgements
None.
Authors’ contributions
LJT, YTY: Substantial contribution to the conception and design of the work, and manuscript drafting.LJT, YTY: Acquisition, analysis, and interpretation of the data.JFG: Formal analysis and manuscript drafting.Evidence in Cardiovascular Anesthesia (EICA) Group: Providing resources and software.All authors were involved in drafting and revision of the manuscript for important intellectual content and approved the final version to be published.
Funding
None.
Data availability
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval was not needed because this is a meta-analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hall R. Identification of inflammatory mediators and their modulation by strategies for the management of the systemic inflammatory response during cardiac surgery. J Cardiothorac Vasc Anesth. 2013;27(5):983–1033. 10.1053/j.jvca.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Hausenloy DJ, Candilio L, Evans R, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373(15):1408–17. 10.1056/NEJMoa1413534. Epub 2015 Oct 5. PMID: 26436207. [DOI] [PubMed] [Google Scholar]
- 3.Landis RC, Brown JR, Fitzgerald D, et al. Attenuating the systemic inflammatory response to adult cardiopulmonary bypass: a critical review of the evidence base. J Extra Corpor Technol. 2014;46(3):197–211 (PMID: 26357785; PMCID: PMC4566828). [PMC free article] [PubMed] [Google Scholar]
- 4.Stoppe C, McDonald B, Benstoem C, et al. Evaluation of persistent organ dysfunction plus death as a novel composite outcome in cardiac surgical patients. J Cardiothorac Vasc Anesth. 2016;30(1):30–8. 10.1053/j.jvca.2015.07.035. Epub 2015 Jul 29. PMID: 26847748. [DOI] [PubMed] [Google Scholar]
- 5.Hill A, Clasen KC, Wendt S et al. Effects of Vitamin C on Organ Function in Cardiac Surgery Patients: A Systematic Review and Meta-Analysis. Nutrients. 2019;11(9):2103. 10.3390/nu11092103. Erratum in: Nutrients. 2020;12(12):E3910. doi: 10.3390/nu12123910. PMID: 31487905; PMCID: PMC6769534. [DOI] [PMC free article] [PubMed]
- 6.Abbasciano RG, Lai FY, Roman MA, et al. Activation of the innate immune response and organ injury after cardiac surgery: a systematic review and meta-analysis of randomised trials and analysis of individual patient data from randomised and non-randomised studies. Br J Anaesth. 2021;127(3):365–75. 10.1016/j.bja.2021.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Gao Y, Ma J. Pleiotropic effects and pharmacological properties of penehyclidine hydrochloride. Drug Des Devel Ther. 2018;12:3289–99. 10.2147/DDDT.S177435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Liu F, Xu M, et al. Penehyclidine hydrochloride alleviates lipopolysaccharide-induced acute respiratory distress syndrome in cells via regulating autophagy–related pathway. Mol Med Rep. 2021;23(2):100. 10.3892/mmr.2020.11739. Epub 2020 Dec 10. PMID: 33300058; PMCID: PMC7723159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma TF, Zhou L, Wang Y, et al. A selective M1 and M3 receptor antagonist, penehyclidine hydrochloride, prevents postischemic LTP: involvement of NMDA receptors. Synapse. 2013;67(12):865–74. 10.1002/syn.21693. [DOI] [PubMed] [Google Scholar]
- 10.Lin D, Cui B, Qi Z, et al. A new aspect of penehyclidine hydrochloride in alleviating myocardial ischemia-reperfusion injury: ferroptosis. J Cardiovasc Transl Res. 2023;16(6):1373–82. 10.1007/s12265-023-10420-7. [DOI] [PubMed] [Google Scholar]
- 11.Tan H, Lin D, Wang Z, et al. Cardioprotective time-window of Penehyclidine hydrochloride postconditioning: a rat study. Eur J Pharmacol. 2017;812:48–56. 10.1016/j.ejphar.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Zheng F, Xiao F, Yuan QH, et al. Penehyclidine hydrochloride decreases pulmonary microvascular endothelial inflammatory injury through a beta-arrestin-1-dependent mechanism. Inflammation. 2018;41(5):1610–20. 10.1007/s10753-018-0804-9. [DOI] [PubMed] [Google Scholar]
- 13.Ren JY, Lin DM, Wang CB, et al. Postconditioning protection against myocardiocyte anoxia/reoxygenation injury from penehyclidine hydrochloride. Drug Des Devel Ther. 2019;13:3977–88. 10.2147/DDDT.S224282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen W, Gan J, Xu S, et al. Penehyclidine hydrochloride attenuates LPS-induced acute lung injury involvement of NF-κB pathway. Pharmacol Res. 2009;60(4):296–302. 10.1016/j.phrs.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JPT, Thomas J, Chandler J et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.1. (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook
- 16.Sterne JAC, Savović J, Page MJ et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898. PMID: 31462531. [DOI] [PubMed]
- 17.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12. 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Wei C, Peng B, et al. Association between cardiopulmonary bypass duration and early major adverse cardiovascular events after surgical repair of supravalvular aortic stenosis. Front Cardiovasc Med. 2025;12:1519251. 10.3389/fcvm.2025.1519251. PMID: 39906758; PMCID: PMC11790573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun YJ,Song DD, Diao YG, et al. Penehyclidine hydrochloride preserves the intestinal barrier function inpatients undergoing cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2013;146(1):179-85. 10.1016/j.jtcvs.2013.01.042. Epub 2013 Feb 21. PMID: 23434449. [DOI] [PubMed]
- 20.Shi L, Ma Y, Dong T, et al. Effects of different doses of penehyclidine hydrochloride on microcirculation in patientsundergoing cardiac valve replacement with cardiopulmonary bypass. J Medical Forum. 2019;40(11):83-590.
- 21.Zhou X, Luo Y, Xi J, et al. Protective effect of penehyclidine hydrochloride onlung injury during cardiopulmonary bypass. J Practical Med. 2010;26(21):3901-3. 10.3969/j.issn.1006-5725.2010.21.017.
- 22.Wei X, Gao Y, Qin H, et al. The effect ofpenehyclidine hydrochloride on interleukins and tumor necrosis factor in patients undergoingvalve replacement surgery before and after cardiopulmonary bypass. Acta UniversitatisMedicinalis Anhui. 2007;42(02):208-10. 10.19405/j.cnki-issn1000-1492.2007.02.026.
- 23.Li S, Yang X. Effect of penehyclidine hydrochloride on microcirculation and myocardial injury inpatients underwent off-pump coronary artery bypass grafting. Journal of Xinxiang Medical University. 2017;34(08):697-9.
- 24.Shu L, Wei X. Effect of Penehyclidine hydrochloride in systemic inflammatory response syndrome caused by cardiopulmonary bypass. Chin J ECC. 2012;10:32-35. 10.13498/j.cnki.chin.j.ecc.2012.01.016. [PubMed]
- 25.Duan W. Effects of penehyclidine hydrochloride on cytokines during extra corporeal circulation in patients receiving coronary artery bypass grafting. J Chin Pract Diagn Ther. 2009;23(06):563-5.
- 26.Wu C. Effect of penehyclidine hydrochloride on micro circulation in elderly patients receiving heart valve replacement surgery. Pract Geriatr. 2014;28(12):1039-42.
- 27.LiK, Cao Q, Guo W. The protection effect of penehyclidine hydrochloride on cardio pulmonary bypass induced lung injury. Chin J Pract Med. 2009;36(9):27-29. 10.3760/cma.j.issn.1674-4756.2009.09.012.
- 28.Shu L, Wei X. The effect of penehyclidine hydrochloride on the inflammatory response of the body during cardiopulmonary bypass. J Sichuan Univ (Med Sci Edi). 2012;43(04):543-6. 10.13464/j.scuxbyxb.2012.04.040. [PubMed]
- 29.Zhang Y, Tu J, Deng J, et al. Protective effects of hydrochloric acid penehyclidine on lung function in heart valve replacement surgery under cardiopulmonary bypass. Hainan Med J. 2015;26(11):1601-3. 10.3969/j.issn.1003-6350.2015.11.0573.
- 30.Yu K, Pan X, Li Y. Effect of penehyclidine hydrochloride on lung function in patients undergoing cardiac surgery with cardiopulmonary bypass. J HBUM. 2010;29(06):500-2.
- 31.Li B, Zhu S, Yin G, et al. Effect of penehyclidine hydrochloride on systemic inflammatory response in patients undergoing cardiacsurgery with cardiopulmonary bypass. Chin J ECC. 2016;14(04):222-5. 10.13498/j.cnki.chin.j.ecc.2016.04.08.
- 32.Zhang X, Zhang D, Zhao N, et al. Effects of penehyclidine hydrochloride on Lactic Acid Metabolism in Patients Undergoing cardiac surgery with cardiopulmonary bypass. J Ningxia Med Univ. 2013;35(03):305-7. 10.16050/j.cnki.issn1674-6309.2013.034.
- 33.Peng H, Jiang W, Cheng P, et al. Protection of Penehyclidine Hydrochloride on the gastrointestinal mucosal injury in patients during cardio pulmonary bypass. J HBUM. 2012;31(01):29-31.
- 34.Liu T, Zhao P, Zhou S, et al. The effect of penehyclidine hydrochloride on thromboxane A2 and prostacyclin during cardio pulmonary bypass. Chin J Lab Diagn. 2010;14(03):445-6.
- 35.Sun Y, Zhang L, Song D, et al. The effect of penehyclidine hydrochloride on concentration of blood intestinal fatty acid binding protein and D-lactate during cardiopulmonary bypass in patients undergoing open heart surgery. Chin J Extracorporeal Circ. 2011;9(02):78-81. 10.13498/j.cnki.chin.j.ecc.2011.02.010.
- 36.Ai Y, Xie X, Zhang W, et al. Effects of penehyclidine hydrochloride on the level of myocardium NF-kB and plasma TNF-a in patients undergoing heart valve replacement. J Zhengzhou Univ (Med Sci). 2009;44(05):996-9. 10.13705/j.issn.1671-6825.2009.05.026.
- 37.Sun Y, Zhang L, Diao Y, et al. Effect of penehyclidine Hydrochloride on Perioperative Stress reaction during cardiopulmonary bypass in patients undergoing open heart surgery. J Clin Anesthesiol. 2012;28(03):219-21.
- 38.Zheng G, An L, Su Z, et al. The effect of pentachloroquine hydrochloride combined with glutathione on lung injury in patients undergoing cardiopulmonary bypass heart valve replacement surgery. J Nantong Univ (Medical Edition). 2014;34(5):386-9.
- 39.Li J, Pan J, Kang F, et al. Effect of penehychdine hydrochloride combined with ulinastatin on lung injury in patients undergoing cardiac valve replacement with cardiopulmonary bypass. Chin J Anesthesiol. 2010;30(12):1420-3.10.3760/cma/j.issn.0254-1416.2010.12.004.
- 40.He Y, He D, Luo Y, et al. Research of protecting cardiac muscle pre-treated with penehyclidine hydrochloride during open heart surgery with cardiopulmonary bypass. Chongqing Med. 2007;36(7):597-8.
- 41.Gao Y, Lin D, Wang Y, et al. Effects of penehyclidine hydrochloride preconditioning on post operative myocardial enzymes in patients undergoing off-pump coronary artery bypass grafting. J Cardiovasc Pulmon Dis. 2018;37(10):935-9.
- 42.Ai Y, Li N,Zhang W, et al. Effect of penehyclidine hydrochloride pre-treatment on plasma cTN-I and CK-MB during heart valve replacement with cardiopulmonary bypass. J Zhengzhou Univ (Med Sci). 2010;45 (05):803-6. 10.13705/j.issn.1671-6825.2010.05.031.
- 43.Dai P, Wang H, Jia Q, et al. Prevention of intestinal mucosal barrier function damage during cardiopulmonary bypass during cardiac surgery with penehyclidine hydrochloride. Med Innov Chin. 2012;9(33):15-6.
- 44.Wang L, Luan Y, Zhao F. Penehyclidine hydrochloride in cardiopulmonary bypass. J Dalian Med Univ. 2010;32(02):195-7.
- 45.Hu Y, Ye F, Huang A, et al. The influence of penehyclidine hydrochloride injection on cTnI of patients with cardiopulmonary bypass. Chin Prac Med. 2008;3(26):43-5.
- 46.Wei H, Dong T, Yang X. Effect of penehyclidine hydrochloride injection on pulmonary ischemia-reperfusion in aortic dissection surgery. Natl Med J China. 2018; 98(10):777-80. 10.3760/cmA.j.issn.0376-2491.2018.013. [DOI] [PubMed]
- 47.He F, Lu Y, Mao Q, et al. Effects of penehyclidine hydrochloride combined with dexmedetomidine on pulmonary function in patients undergoing heart valve surgery: a double-blind, randomized trial. BMC Anesthesiol. 2023;23(1):237. 10.1186/s12871-023-02176-z. PMID: 37442959; PMCID: PMC10339561. [DOI] [PMC free article] [PubMed]
- 48.Zhang Y, Zeng Z, Wang X. The Effects of Different Doses of Penehyclidine Hydrochloride on the Cytokine Release and Pulmonary Protection in Children with Congenital Heart Disease and Pulmonary Arterial Hypertension after Cardiopulmonary Bypass. Chin Foreign Med Treat. 2014;33(21):117-9. 10.16662/j.cnki.1674-0742.204.21.21.084.
- 49.Lang Z, Qiu L, Zhao L, et al. Effect of Penehyclidine Hydrochloride TLR4/NF-KB signaling pathway in myocardium of pediatric patients undergoing radical correction of tetralogy of Fallot with cardio pulmonary bypass. Chin J Anesthesiol. 2017;37(4):6. 10.3760/cma/j.issn.0254-1416.2017.04.008.
- 50.Qin L, Zhang B, Tu J, et al. The effect of penehyclidine hydrochloride on microcirculation in children undergoing open heart surgery without cardiac arrest. Guangxi Med J. 2013;35(07):862-4.
- 51.Fang Y, Su X. Study on the lung protective effect of penehyclidine hydrochloride on children after cardiopulmonary bypass. Chin Mod Med. 2010;17(19):97-8.
- 52.Lu B, Wu A, Li J. The effect of penehyclidine hydrochloride on inflammatory response and lung injury after cardio pulmonary bypass in children. J Pract Med. 2011;27(06):1061-3.
- 53.Li L, Wei X, Jiang L, et al. Protective effect of penehyclidine hydrochloride on ischemia-reperfusion myocardium during cardiopulmonary bypass heart surgery in infants. Shanghai Med J. 2012;35 (12):1021-3981.
- 54.Wu X. Effects of Penehyclidine hydrochloride preconditioning on myocardial injury in pediatric patients undergoing radical correction of tetralogy of Fallot with cardiopulmonary bypass. Med J Chin People’s Health. 2017;29(04):18-940.
- 55.Wei X, You Z. The effect of PGE1 combined with PHC on perioperative inflammatory factors in children with congenital heart disease undergoing CPB surgery. Med Sci J Cent South Chin. 2023;51(2):229-33.
- 56.Koh TW, Carr-White GS, DeSouza AC, et al. Intraoperative cardiac troponin T release and lactate metabolism during coronary artery surgery: comparison of beating heart with conventional coronary artery surgery with cardiopulmonary bypass. Heart. 1999;81(5):495–500. PMID: 10212167; PMCID: PMC1729023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen M, Li X, Mu G. Myocardial protective and anti-inflammatory effects of Dexmedetomidine in patients undergoing cardiovascular surgery with cardiopulmonary bypass: a systematic review and meta-analysis. J Anesth. 2022;36(1):5–16. 10.1007/s00540-021-02982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin D, Ma J, Xue Y, Wang Z. Penehyclidine hydrochloride preconditioning provides cardioprotection in a rat model of myocardial ischemia/reperfusion injury. PLoS ONE. 2015;10(12):e0138051. 10.1371/journal.pone.0138051. (PMID: 26632817; PMCID: PMC4668996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costa Leme A, Hajjar LA, Volpe MS, et al. Effect of intensive vs moderate alveolar recruitment strategies added to lung-protective ventilation on postoperative pulmonary complications: a randomized clinical trial. JAMA. 2017;317(14):1422–32. 10.1001/jama.2017.2297. [DOI] [PubMed] [Google Scholar]
- 60.Li BQ, Sun HC, Nie SN, et al. Effect of penehyclidine hydrochloride on patients with acute lung injury and its mechanisms. Chin J Traumatol. 2010;13(6):329–35. [PubMed] [Google Scholar]
- 61.Murata T, Maeda M, Amitani R, et al. Postoperative changes in pulmonary function after valve surgery: oxygenation index early after cardiopulmonary is a predictor of postoperative course. J Clin Med. 2021;10(15):3262. 10.3390/jcm10153262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Halter J, Steinberg J, Fink G, Lutz C, Picone A, Maybury R, Fedors N, DiRocco J, Lee HM, Nieman G. Evidence of systemic cytokine release in patients undergoing cardiopulmonary bypass. J Extra Corpor Technol. 2005;37(3):272–7. PMID: 16350379; PMCID: PMC4680784. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.