Abstract
We have identified three distinct groups of mitochondria in normal living pancreatic acinar cells, located (i) in the peripheral basolateral region close to the plasma membrane, (ii) around the nucleus and (iii) in the periphery of the granular region separating the granules from the basolateral area. Three-dimensional reconstruction of confocal slices showed that the perigranular mitochondria form a barrier surrounding the whole of the granular region. Cytosolic Ca2+ oscillations initiated in the granular area triggered mitochondrial Ca2+ uptake mainly in the perigranular area. The most intensive uptake occurred in the mitochondria close to the apical plasma membrane. Store-operated Ca2+ influx through the basolateral membrane caused preferential Ca2+ uptake into sub-plasmalemmal mitochondria. The perinuclear mitochondria were activated specifically by local uncaging of Ca2+ in the nucleus. These mitochondria could isolate nuclear and cytosolic Ca2+ signalling. Photobleaching experiments indicated that different groups of mitochondria were not luminally connected. The three mitochondrial groups are activated independently by specific spatiotemporal patterns of cytosolic Ca2+ signals and can therefore participate in the local regulation of Ca2+ homeostasis and energy supply.
Keywords: Ca2+ transport/mitochondria/perigranular/perinuclear/sub-plasmalemmal
Introduction
Much recent Ca2+ signalling work has dealt with the importance of the mitochondria. Contrary to conclusions from earlier work, it is now clear that mitochondria can and do take up Ca2+ during normal Ca2+ signalling events and then release it much more slowly (Pozzan et al., 1994, 2000; Duchen, 2000; Rutter and Rizzuto, 2000). Ca2+ in the mitochondria is of importance for the regulation of metabolism, since three dehydrogenases of the Krebs cycle are modulated by the Ca2+ concentration in the micromolar range (Denton and McCormack, 1990; McCormack et al., 1990). Repetitive cytosolic Ca2+ spikes induce mitochondrial Ca2+ spikes. Each spike is sufficient to cause a maximal transient activation of the Ca2+-sensitive mitochondrial dehydrogenases and above a certain frequency there is sustained activation of mitochondrial metabolism (Hajnoczky et al., 1995). Agonist stimulation, evoking cytosolic and mitochondrial Ca2+ signals, elicits increases in both the mitochondrial and cytosolic ATP concentrations that depend on both the amplitude of the Ca2+ rise inside the mitochondria and the availability of mitochondrial substrates. Thus, mitochondrial Ca2+ plays a direct role in driving ATP production (Jouaville et al., 1999). Mitochondrial Ca2+ uptake is also important for the regulation of other processes. In chromaffin cells, stimulation triggers fast millimolar mitochondrial Ca2+ transients and these can modulate secretion (Montero et al., 2000). In pancreatic acinar cells, active mitochondria localized in a belt surrounding the granular region are important for confining cytosolic Ca2+ signals initiated in this region to this part of the cell, where secretion is regulated (Tinel et al., 1999; Straub et al., 2000). It has been shown recently that respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ inflow that is of importance for refilling endoplasmic reticulum (ER) stores with Ca2+ lost during agonist stimulation (Gilabert and Parekh, 2000).
It has been proposed that the localization of mitochondria with respect to Ca2+ sources can be a determinant of their Ca2+ uptake kinetics (Lawrie et al., 1996). It would be desirable to test this critically in intact polarized cells, where different groups of mitochondria could be clearly identified in relation to other subcellular structures and in particular in relation to specific subcellular Ca2+ release sites. Here we have investigated, in normal polarized pancreatic acinar cells, how mitochondria positioned in specific subcellular locations respond to various patterns of cytosolic Ca2+ ([Ca2+]i) signals. We identified three distinct groups of mitochondria located (i) in the peripheral basolateral region very close to the plasma membrane, (i) around the nucleus and (iii) in the periphery of the granular region separating the apical granular area from the basolateral part of the cell. When [Ca2+]i was increased uniformly throughout the cell, by global uncaging of caged Ca2+, we found no regional differences in mitochondrial Ca2+ uptake. In experiments where we used UV laser-induced local bleaching of an intra-mitochondrial dye, we saw no evidence of luminal connectivity between mitochondria in different regions. [Ca2+]i oscillations initiated in the granular area triggered mitochondrial Ca2+ uptake mainly in the perigranular area. Ca2+ influx through the basolateral membrane, generated by emptying intracellular Ca2+ stores, elicited Ca2+ uptake into nearby peripheral basolateral mitochondria. Perinuclear mitochondria were specifically activated by local Ca2+ release in the nucleus. Distinct groups of separate mitochondria, due to their strategic positioning within the cell, can therefore sense and shape [Ca2+]i signals locally to help the cell regulate activities in specific subcellular compartments and ensure adequate local energy supply.
Results
The relative positions of granules, mitochondria, ER and nucleus in the living pancreatic acinar cells
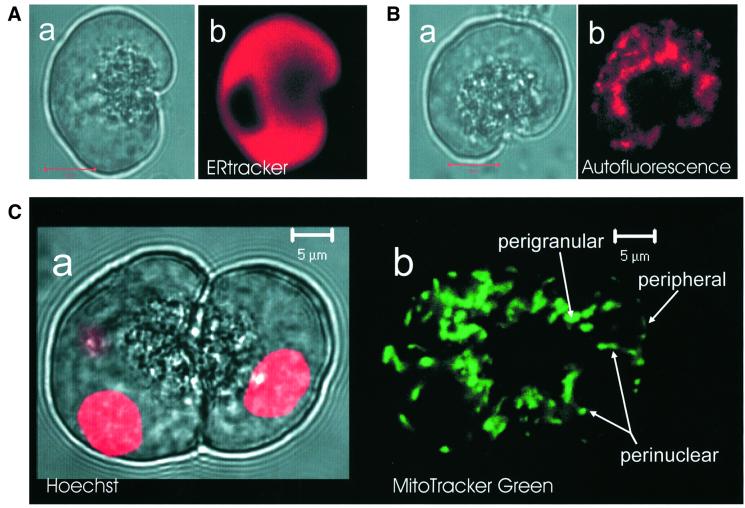
The polarization of pancreatic acinar cells with the ER and the nucleus in the basolateral part and the granules in the apical pole was well maintained even after isolation of single cells, as shown in Figure 1. There was heavy staining with ERtracker in the basolateral areas surrounding the granular region, except in the nucleus (n = 6) (Figure 1A). The granules were localized in the apical pole close to the tiny apical (luminal) membrane. High resolution confocal images of NADH autofluorescence showed the locations of the mitochondria, which were predominantly grouped in a central perigranular area and in a peripheral area close to the plasma membrane (n = 26) (Figure 1B). This is similar to what we have shown previously with the help of MitoTracker Green (Tinel et al., 1999) and tetramethyl rhodamine ethyl ester (Raraty et al., 2000) fluorescence. In some experiments we used both MitoTracker Green FM, to check the mitochondrial distribution, and Hoechst 33342, to visualize the nuclei, simultaneously. In these cells we saw that there was also a group of mitochondria close to and sometimes surrounding the nucleus (Figure 1C) (n = 10).
Fig. 1. Localization of endoplasmic reticulum (ER), nuclei and mitochondria in living pancreatic acinar cells. (A) Endoplasmic reticulum. The transmitted light picture of a single isolated acinar cell is shown in (a). In (b), the fluorescence image obtained with ERtracker is shown. The ER is densely packed outside the granular and nuclear areas. (B) Mitochondria. Transmitted light picture in (a) and autofluorescence (NADH) image in (b). The mitochondria are localized principally as a belt surrounding the granular region and as a peripheral ring just under the plasma membrane. Lengths of red bars in (A, a) and (B, a) represent 10 µm. (C) Nuclei and mitochondria. (a) Transmitted light picture showing the typical structure of two connected acinar cells with the secretory (zymogen) granules in the central part surrounding what in the intact organ would be the lumen. The nuclei are stained red with Hoechst 33342 and the fluorescence image is superimposed on the transmitted light picture. In the cell on the left, the focal plane goes through one nucleus, but it is just possible to detect the presence of a second nucleus. (b) MitoTracker Green fluorescence image. The strongest staining is localized as a ring surrounding the granular area, with some staining at the periphery as well as around the three nuclei (two in the left and one in the right cell). The mitochondria surrounding the nuclei seem to be aligned along the surfaces of these organelles.
Mitochondrial Ca2+ uptake following acetylcholine (ACh)-elicited release of Ca2+ from the endoplasmic reticulum
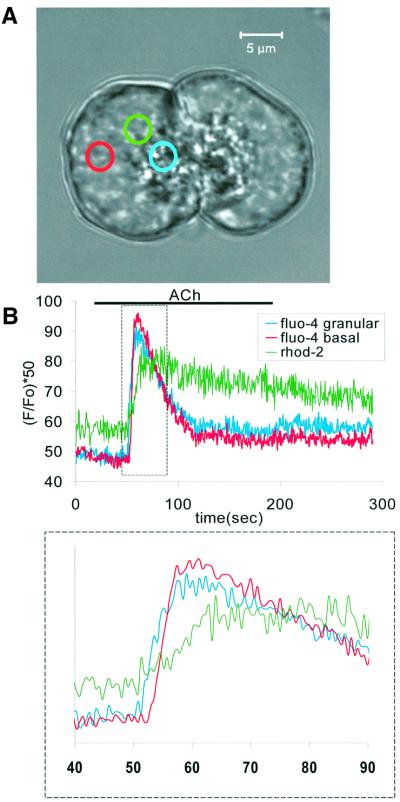
To image mitochondria and measure the mitochondrial Ca2+ concentration ([Ca2+]m), we used the Ca2+-sensitive dye Rhod-2, which is concentrated in compartments with a highly negative voltage (Pinton et al., 2001). The transmitted light picture of a cell under investigation is shown in Figure 2A. The mitochondrial distribution is disclosed in Figure 2B. After exposing the cell to Rhod-2-AM, the fluorescence intensity was generally weak, in the absence of stimulation, as shown in Figure 2C1. However, after ACh (10 µM) application the characteristic localization of mitochondria was revealed gradually and finally the Rhod-2 fluorescence image (Figure 2C6) was similar to the picture obtained imaging the NADH autofluorescence (Figure 2B). This suggests that the initial low Rhod-2 fluorescence intensity was due to the relatively low resting [Ca2+]m and not caused by insufficient mitochondrial dye loading. Mitochondrial Ca2+ uptake occurred, at first, in the lateral area close to the apical membrane and thereafter spread to the basolateral part of the cell (Figure 2C and D). Mitochondrial Ca2+ uptake was rapid and the maximal [Ca2+]m was reached within ∼5 s as seen in Figure 2D, which shows the changes of fluorescence intensity measured in three differently located mitochondria marked in Figure 2A (black, red and green circles). The average time lag between the mitochondrial Ca2+ rise in the lateral area close to the apical membrane (black trace) and at the base of the cell (green trace) was 2.4 ± 0.2 s (n = 7).
Fig. 2. Mitochondrial Ca2+ uptake, measured with Rhod-2, following maximal ACh (10 µM)-elicited Ca2+ release from the endoplasmic reticulum. (A) Transmitted light picture showing the cell under investigation, with the three areas of interest identified by the three coloured circles. The length of the red bar represents 10 µm. (B) Autofluorescence image showing mitochondrial localization. (C) Six images showing mitochondrial Ca2+ concentration. Image 1 shows the situation just before the start of stimulation. Image 2 shows that immediately after ACh application, there was Ca2+ uptake into mitochondria very close to the apical membrane. A little later, the whole perigranular mitochondrial belt was revealed (images 3 and 4) and finally (images 5 and 6) all mitochondria in the cell had taken up Ca2+. (D) The time course of the mitochondrial Ca2+ uptake in the three regions identified in (A) using the appropriate colour coding. The main part of the figure represents a short time segment of the more complete traces shown in the inset. The time scale relates to the expanded traces labeled with the numbers 1–6, corresponding to the images in (C).
In general, the mitochondrial Ca2+ uptake in response to an ER-derived rise in the cytosolic Ca2+ concentration has been shown to occur immediately (Rizzuto et al., 2000). However, in a recent study on pancreatic acinar cells, it has been reported that there is a substantial delay of ∼10 s, after the initiation of the cytosolic Ca2+ signal, before a measurable rise in the mitochondrial Ca2+ concentration is seen (Gonzalez et al., 2000). In view of these surprising results, we carried out experiments in which changes in the Ca2+ concentration in the cytosol and the mitochondria were monitored simultaneously, by measurement of fluo-4 and Rhod-2 fluorescence, in order to evaluate how quickly the mitochondrial Ca2+ uptake mechanism responded to the cytosolic Ca2+ signal. We saw no evidence of a substantial delay between the initiation of the cytosolic and mitochondrial Ca2+ signals. Figure 3 shows the result from one of these experiments. ACh elicited a cytosolic Ca2+ wave, which was initiated in the granular pole and then moved towards the base and reached the basal region within ∼2 s. As seen in Figure 3, the initial rise in [Ca2+]m coincided with the initial rise in [Ca2+]i, although the peak [Ca2+]m was attained later than the peak [Ca2+]i. Six experiments of this type gave similar results.
Fig. 3. Simultaneous measurements of cytosolic (fluo-4) and mitochondrial (Rhod-2) Ca2+ concentration changes after ACh stimulation. (A) The transmitted light picture shows the structure of the acinar doublet with the three colour-coded circles indicating the regions of interest. (B) The time courses of the ACh-elicited changes in the cytosolic Ca2+ concentrations in the granular (blue) and basal (red) regions, as well as the mitochondrial Ca2+ concentration in the perigranular region (green). In the inset below, showing the initial part of the three curves on an expanded time scale, it is seen that the mitochondrial Ca2+ uptake started immediately after the initial rise in the cytosolic Ca2+ concentration in the granular area.
The ER is the main Ca2+ storage organelle as well as a main supplier of Ca2+ to the mitochondria. Therefore, we measured [Ca2+]m, the Ca2+ concentration in the ER ([Ca2+]ER) and [Ca2+]i simultaneously in the same cell. To do this, we exposed cells to Rhod-2-AM and the low affinity Ca2+-sensitive dye Mag-fluo-4-AM (Kd = 22 µM). Thereafter, we introduced a patch pipette (whole-cell recording configuration) to wash out the cytosolic dye components. The time course of changes in [Ca2+]i was assessed by measurement of the Ca2+-dependent current (Petersen, 1992). Since the ER is mainly located in the basolateral part of the cell (Figure 1A), the decrease in [Ca2+]ER was observed mainly in the basolateral region (Figure 4), as demonstrated previously (Park et al., 2000). As discussed extensively (Park et al., 2000; Petersen et al., 2001), this is entirely consistent with the well established finding that the [Ca2+]i rise is initiated in, and often confined to, the apical granular pole. The Ca2+ release occurs from very tiny extensions of the basolateral ER into the apical pole, which is dominated by granules. The [Ca2+]m change was mainly in the perigranular (particularly marked in the lateral area close to the apical membrane) and peripheral areas (Figure 4A, a). The Mag-fluo-4 fluorescence intensity in the perigranular (mitochondrial) area was initially slightly increased after application of ACh, which could be due to some of the dye being trapped in the mitochondria. This phenomenon was abolished by application of mitochondrial metabolic blockers (1 µM oligomycin and 1 µM rotenone; n = 6) or dialysis of a 10 mM BAPTA/2 mM Ca2+ mixture through the patch pipette (n = 17). We plotted the time course of the ACh-elicited changes in [Ca2+]i (Ca2+-dependent Cl– current), [Ca2+]m (Rhod-2 fluorescence) and [Ca2+]ER (Mag-fluo-4 fluorescence) together in Figure 4B. After ACh stimulation, [Ca2+]i and [Ca2+]m increased immediately, even before there was a measurable decrease in [Ca2+]ER. After washout of ACh, [Ca2+]i returned much more quickly to the resting level than [Ca2+]ER and [Ca2+]m. The mitochondria released Ca2+ and the ER refilled with Ca2+ without provoking any Ca2+-dependent current, indicating the possibility of Ca2+ recycling between mitochondria and nearby ER elements. The mitochondrial Ca2+ release following the Ca2+ uptake was very slow (see Figure 4). This phenomenon was similar in intact Rhod-2-loaded cells (n = 12) and cells dialysed via a patch pipette (n = 6). The time course was somewhat variable from cell to cell, but usually it took >10 min to restore [Ca2+]m to the pre-stimulation level after a period of supramaximal ACh (10 µM) stimulation. The mitochondrial Ca2+ uptake was particularly marked in the lateral area close to the apical membrane (Figure 4A, a), which is very close to the primary ER Ca2+ release site (trigger zone) (Kasai et al., 1993; Mogami et al., 1997; Cancela et al., 2000). Also, after washout of ACh, the Ca2+ extrusion was particularly slow in these mitochondria.
Fig. 4. Simultaneous measurements of the ACh-elicted Ca2+ concentration changes in the ER and the mitochondria. (A) Images from a single cell. (a) Rhod-2 fluorescence images taken at 132.6 s intervals showing the accumulation and subsequent loss of Ca2+ from the mitochondria following a short period of ACh stimulation. The first image was obtained just before the start of stimulation with ACh. (b) Mag-fluo-4 fluorescence images showing the gradual loss of Ca2+ from the ER in response to ACh stimulation and the subsequent re-accumulation. The first image was taken just before the start of stimulation. The interval between images was 132.6 s. (B) Time course of ACh-elicited changes in the Ca2+ concentrations in the mitochondria (Rhod-2, red), the ER (Mag-fluo-4, blue) and the cytosol (whole-cell Ca2+-dependent current, black).
In the pancreatic acinar cell, it is well known that low doses of ACh or cholecystokinin (CCK) generate [Ca2+]i oscillations, which always start close to the apical membrane and are mostly confined to the granular area (Thorn et al., 1993). It has been suggested that the perigranular mitochondria provide a Ca2+ buffer barrier, which prevents or reduces the degree of Ca2+ signal spreading to the basolateral region (Tinel et al., 1999). However, so far there has been no direct demonstration of Ca2+ uptake into the perigranular mitochondria during Ca2+ spiking. We therefore evoked [Ca2+]i oscillations with a relatively low dose of ACh and observed [Ca2+]m changes in different parts of the cell. To monitor the [Ca2+]i oscillations we measured the Ca2+-dependent current. In the experiment illustrated in Figure 5, ACh (50 nM) elicited repetitive [Ca2+]i spikes. The individual spikes were initially short-lasting and largely confined to the apical granular area (i.e. large cytosolic Ca2+ concentration gradient between apical and basolateral regions during the spike), but gradually became broader and global. The initial cytosolic Ca2+ spike was associated with a marked Ca2+ uptake into the perigranular mitochondria. In the Rhod-2 fluorescence images shown in Figure 5, the mitochondrial perigranular belt was invisible before the first ACh-elicited Ca2+ spike (image 1), but at the height of the spike the perigranular mitochondrial belt was clearly delineated (image 2). After the spike, the image of the perigranular belt disappeared (image 3). At the height of the first spike there was little sign of Ca2+ uptake into the mitochondria in other parts of the cell. Later, when the cytosolic Ca2+ spikes became broader and global, Ca2+ accumulated both in the perigranular belt and in the peripheral sub-plasmalemmal region (image 4). This was due to the relatively slow Ca2+ liberation from the mitochondria. During each spike, Ca2+ was taken up and then subsequently released, but the release was so slow that when the next spike occurred, all Ca2+ taken up during the previous spike had not yet been liberated. In this phase, the cytosolic Ca2+ spike was associated with mitochondrial Ca2+ uptake both in the perigranular belt and in the peripheral region (image 5). There was a visible, but minor, reduction in the mitochondrial Ca2+ concentration following the spike (image 6). Finally, a high concentration of ACh elicited a marked increase in the fluorescence intensity in the perigranular belt, the peripheral region and near the nucleus (image 7). Five experiments of this type all gave similar results.
Fig. 5. Region-specific mitochondrial Ca2+ uptake following cytosolic Ca2+ oscillations. The coloured traces in the main panel show the time courses of the Ca2+ concentration changes in mitochondria localized as shown in the transmitted light image. The colour coding of the traces corresponds to the coloured circles in the transmitted light picture. The length of the red bar in the transmitted light picture corresponds to 10 µm. The black trace in the main panel represents the Ca2+-dependent whole-cell current, which shows the time course of the changes in the cytosolic Ca2+ concentration. Stimulation with a low ACh concentration elicited repetitive cytosolic Ca2+ spikes. The Rhod-2 fluorescence images taken before, during and after the first ACh-elicited cytosolic Ca2+ spike (images 1–3) show that the main mitochondrial Ca2+ uptake during this spike occurred in the perigranular region. Since the release of Ca2+ taken up into the mitochondria is relatively slow, the mitochondria did not liberate all the Ca2+ accumulated during a single spike before the next spike occurred. Therefore, during this experiment, Ca2+ accumulated gradually in the mitochondria. In relation to the last spike in the series, the corresponding Rhod-2 fluorescence images are shown (images 4–6). It can be seen that even before the spike started there was considerable fluorescence both from the most apically located mitochondria in the perigranular ring and also from the peripheral ring close to the plasma membrane. During the spike there was increased fluorescence in all mitochondrial regions, which then declined after the spike. Finally, the cell was stimulated maximally by a high ACh concentration, causing a marked increase in the cytosolic Ca2+ concentration, as seen in the electrophysiological trace and futher mitochondrial Ca2+ accumulation (image 7).
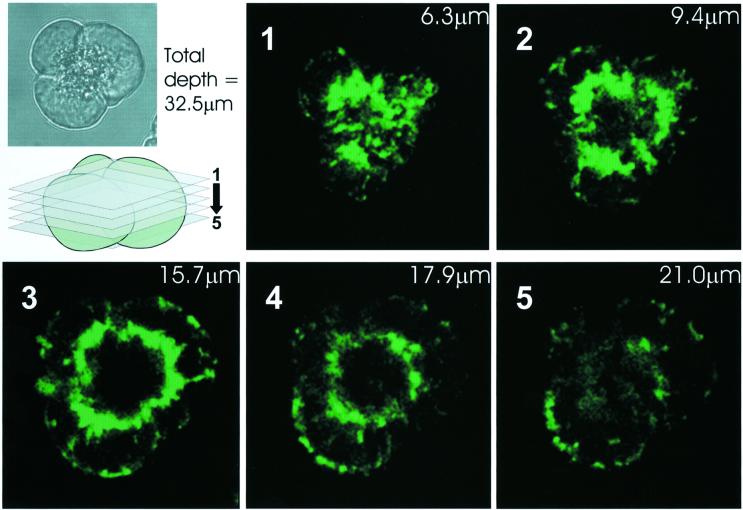
In view of the functional importance of the perigranular mitochondrial belt, we investigated to what extent these mitochondria form a complete barrier around the granules. Using serial confocal slicing through Rhod-2-stained cells, after a maximal ACh-elicited Ca2+ signal had caused substantial Ca2+ uptake into the mitochondria, it was possible to see that the mitochondria essentially cover the whole surface of the granular area, like the skin on a football (Figure 6).
Fig. 6. Serial confocal sectioning reveals essentially complete perigranular mitochondrial belt. An acinar cell triplet (inset transmitted light image) was loaded with Rhod-2 and stimulated maximally with ACh. After the peak response, several confocal sections (see schematic illustration) revealed the almost complete coverage of the granular region by mitochondria. The thickness of the confocal sections was 3.5 µm.
Mitochondrial Ca2+ uptake in response to uncaging of caged Ca2+ in the cytosol and the nucleus
In order to investigate the time course of mitochondrial Ca2+ uptake in different regions, we tried to abruptly increase [Ca2+]i globally or locally by uncaging of Ca2+ from the caged Ca2+ compound o-nitrophenyl (NP)-EGTA. To prevent Ca2+ induction of Ca2+ release from the ER, we incubated the cells in Ca2+-free solution containing 1 µM thapsigargin for 10 min. Thereafter, we introduced a patch pipette (whole-cell configuration) containing 1.2–1.5 mM NP-EGTA. Figure 7 shows an example of such an experiment (n = 5). The transmitted light picture of the cell with the three regions of interest identified by circles in different colours and the outline of the experimental arrangement is shown in Figure 7A, a and b. When Ca2+ was uncaged throughout all regions of the cell, to produce a uniform global rise in [Ca2+]i, we observed that mitochondrial Ca2+ uptake occurred simultaneously everywhere and reached a peak after ∼4 s (Figure 7A, c). [Ca2+]i was monitored by measurement of the Ca2+-sensitive whole-cell current. The initial part of Figure 7A, c is expanded in Figure 7A, d, where it can be seen more clearly that there was no detectable difference in the kinetics of the mitochondrial Ca2+ uptake in the different parts of the cell. The regions of interest are marked in Figure 7A, a with colours corresponding to the traces in Figure 7A, c and d. These experiments demonstrate the high rate of mitochondrial Ca2+ uptake and the absence of a significant delay to the start of Ca2+ accumulation in the mitochondria. The peak of the Ca2+-dependent current did, however, occur earlier than the peak of the mitochondrial Ca2+ concentration. Although it has been proposed that mitochondria possess a Ca2+-induced Ca2+ release (CICR) mechanism in vitro (Ichas et al., 1997), the second uncaging of NP-EGTA consistently failed to evoke signs of CICR from the mitochondria in our intact cells. In all the 11 cells tested, the second uncaging of caged Ca2+ resulted in further mitochondrial Ca2+ uptake, rather than release (Figure 7A, c).
Fig. 7. Local and global uncaging of caged Ca2+ causes local and global mitochondrial Ca2+ uptake. (A) Global uncaging. (a) Transmitted light picture showing the three colour-coded regions of interest. Length of red bar corresponds to 10 µm. (b) Schematic diagram indicating recording configuration and experimental arrangement. (c) Time course of mitochondrial Ca2+ uptake, in the three colour-coded regions identified in (A, a), following two separate global cytosolic Ca2+ uncagings (arrowheads). The uncagings cause large increases in the cytosolic Ca2+ concentration (monitored as Ca2+-dependent current). It is seen that this is associated with a uniform increase in the Rhod-2 fluorescence in all regions. (d) An expanded time scale showing the first part of the response to the first uncaging of Ca2+. (B) The effect of local Ca2+ uncaging in the basal region. (a) Transmitted light picture with the three colour-coded regions of interest identified. (b) The mitochondrial Ca2+ concentration changes, in response to the local basal (green area) Ca2+ uncaging, in the three regions (colour coded as in the transmitted light picture). It can be seen that only the mitochondria near the basal plasma membrane react initially, but later there are rather small increases also in the perigranular region.
We also uncaged Ca2+ locally in the basal region, outlined in green in Figure 7B, a, and measured the mitochondrial Ca2+ uptake in three different areas (green, red and black). In this case the mitochondrial Ca2+ uptake was localized to the basal area (green) in which the uncaging had occurred. In the two other areas there were initially no signs of mitochondrial Ca2+ uptake, although subsequent Ca2+ uncagings induced small rises in [Ca2+]m in the perigranular region (red) (Figure 7B, b) (n = 6).
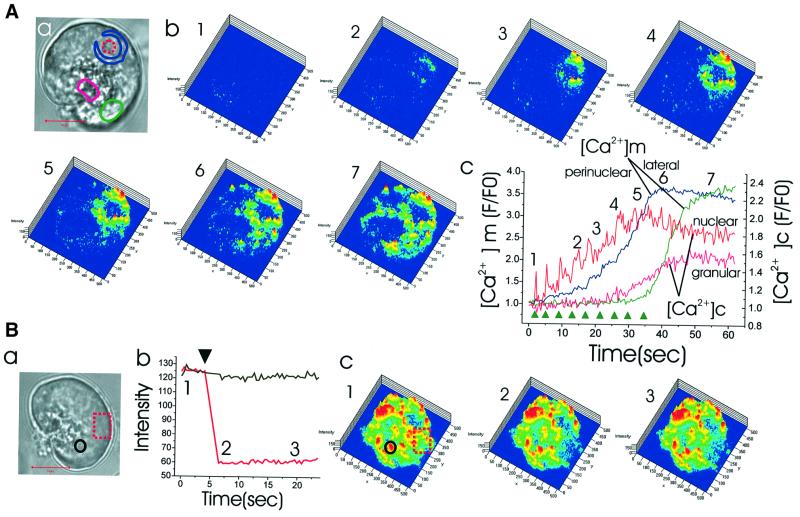
Next, we loaded Rhod-2-AM and NP-EGTA-AM into the same cell and uncaged Ca2+ in a very small area within the nucleus. As shown in Figure 8A, a and b, after repetitive Ca2+ uncaging in a small area within the nucleus (red dotted circle), the perinuclear mitochondria (blue area) were the first to take up Ca2+. Later, mitochondria in the lateral area of the cell (green circle) took up Ca2+. Figure 8A, c shows simultaneous measurements of the Ca2+ concentration changes in the nucleoplasm and the cytosol in the granular area as well as in the mitochondria in the perinuclear and lateral regions. The [Ca2+]m rise in the lateral region was considerably delayed as compared with the [Ca2+]m rise in the perinuclear area, demonstrating the buffering power of the perinuclear mitochondria. Similar results were obtained from six cells. These data explain why the nucleus is not easily affected by short-lasting cytosolic Ca2+ changes (Gerasimenko et al, 1996; Cancela et al., 2000), whereas the steady-state Ca2+ concentration in the nucleoplasm reflects the cytosolic Ca2+ concentration reasonably well (Brini et al., 1993; Mogami et al., 1998).
Fig. 8. Local Ca2+ uncaging in the nucleus results in specific Ca2+ uptake into the mitochondria located just around the nucleus and local bleaching experiment indicates that different mitochondrial groups may not communicate directly. (A) Repetitive nuclear Ca2+ uncagings result in increasing Ca2+ uptake exclusively into the perinuclear mitochondrial ring, until suddenly towards the end of the experiment, all mitochondria take up Ca2+. (a) The transmitted light picture shows the site of Ca2+ uncaging (red broken circle) and the colour-coded regions of interest. Length of red bar corresponds to 10 µm. (b) Series of Rhod-2 fluorescence images showing increasing Ca2+ concentration in the perinuclear mitochondrial ring (images 1–5). The last couple of uncagings result in a generalized increase in the cytosolic Ca2+ concentration, which causes major Ca2+ uptake into the mitochondria, also in other regions (images 6 and 7). (c) Graphs representing the time courses of the Ca2+ concentration changes in the cytosol and the mitochondria in different regions following the repetitive Ca2+ uncagings in the nucleus (arrowheads). (B) An area of a cell represented by the broken red rectangle in (a) was bleached and the changes in the Rhod-2 fluorescence intensity, which had just been increased by uncaging of caged Ca2+, were monitored. Length of red bar represents 10 µm. (b) Graph showing that there is a dramatic decrease in the fluorescence intensity in the bleached area (red), which is not transmitted to the neighbouring region (black). (c) Fluorescence images obtained at the times indicated in (b).
Dye bleaching experiments
The results presented so far show that mitochondria in distinct regions can react to local changes in the cytosolic Ca2+ concentration without necessarily influencing [Ca2+]m in other regions. We therefore tested the possible luminal connection between mitochondria in different areas. Figure 8B, a shows the transmitted light picture of a cell under investigation. We observed a marked rise in [Ca2+]m in all the regions after global cytosolic Ca2+ uncaging. Thereafter, we bleached rapidly one area of the cell (red dotted rectangle in Figure 8B, a and c) using the maximum power of our UV laser (351, 364 nm line). This resulted in complete bleaching of mitochondrial fluorescence in this part of the cell. There was no restoration of fluorescence intensity and the fluorescence intensities in the neighbouring peripheral basal as well as perigranular mitochondria were not changed at all (Figure 8B, b and c, similar data obtained in seven cells). Although the possibility cannot be excluded that UV laser-induced damage could in some manner isolate otherwise communicating organelles, the simplest interpretation of our data indicates that in normal pancreatic acinar cells the mitochondria are not luminally connected.
Mitochondrial Ca2+ uptake specifically due to Ca2+ entry from external solution
The result shown in Figure 7B, demonstrating that local Ca2+ uncaging in the basal area causes selective Ca2+ uptake in mitochondria in that region, might indicate that Ca2+ entry across the basal membrane could selectively affect the peripheral basolaterally located mitochondria. Ca2+ entry through the basal membrane can refill the intracellular ER Ca2+ store after agonist-induced depletion (Mogami et al., 1997) and it has been shown recently that respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current (Gilabert and Parekh, 2000). We therefore investigated directly whether mitochondria in the peripheral basolateral area, which is very close to the plasma membrane, could preferentially respond to store-operated Ca2+ influx. Ca2+ was liberated from the ER store by exposing the cells to a Ca2+-free external solution containing a high concentration (0.5 µM) of the ER Ca2+ pump inhibitor thapsigargin for a relatively short period (3 min). This results in a substantial reduction in the ER Ca2+ concentration (Mogami et al., 1998). When the external Ca2+-free solution is then replaced by a solution containing a high Ca2+ concentration (10 mM), Ca2+ entry should occur through store-operated channels (Parekh and Penner, 1997). The result of such an experiment is illustrated in Figure 9. Admission of the external solution with the high Ca2+ concentration resulted initially in a selective rise in [Ca2+]m in the basolateral region of the cell very close to the plasma membrane (Figure 9). Later, there was also a more modest rise in [Ca2+]m in the perigranular region. At the end of the experiment, a supramaximal concentration of ACh was applied. This resulted in a rise in [Ca2+]m that was most marked near the apical (luminal) membrane. The relatively minor effect of ACh stimulation indicates that refilling of the ER store had not occurred during the period of Ca2+ entry, since the effect of thapsigargin is irreversible. On the other hand, the fact that ACh did induce a rise in [Ca2+]m near the apical membrane (green circle, green curve, image 5) indicates that the relatively short period of thapsigargin exposure had not completely depleted the ER store of Ca2+. Complete depletion of the ER store takes 10–15 min. We performed five separate experiments of the type shown in Figure 9, all with similar results.
Fig. 9. Basolateral mitochondrial Ca2+ uptake due to store-operated Ca2+ entry. In this series of experiments, cells were exposed to a Ca2+-free solution containing 0.5 µM thapsigargin for 3 min to partially deplete internal Ca2+ stores. A high external Ca2+ concentration (10 mM) was then introduced, allowing Ca2+ entry through store-operated Ca2+ channels. (A) Series of Rhod-2 fluorescence images showing increasing Ca2+ accumulation in the peripheral mitochondria during the period of Ca2+ entry (images 1–4) and finally the increase in the Ca2+ concentration in the apically located mitochondria after ACh stimulation (image 5). The numbers correspond in time to those shown in (C). (B) Transmitted light image of the cell under investigation with the three colour-coded regions identified. Length of red bar corresponds to 10 µm. (C) The three coloured traces represent mitochondrial Ca2+ measurements (Rhod-2) in the three correspondingly colour-coded regions identified in (B). It is seen that by far the most marked rise in the mitochondrial Ca2+ concentration occurred in the region very close to the basal membrane. At the end of the experiment, a high dose of ACh is applied, causing further increase in the mitochondrial Ca2+ concentration, but this time most marked in the area very close to the apical membrane [see also image 5 in (A)].
Discussion
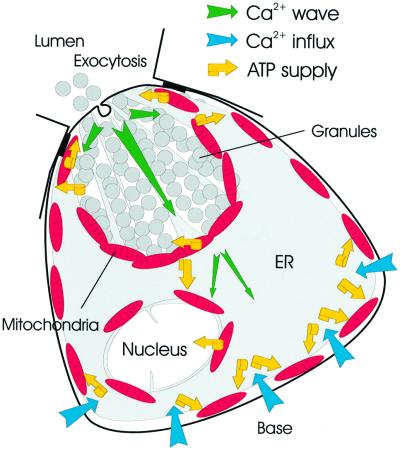
Figure 10 summarizes our findings. In addition to the perigranular mitochondria described previously (Tinel et al., 1999; Straub et al., 2000), we have identified two separate groups of mitochondria located in the peripheral basolateral area close to the plasma membrane and in the perinuclar area. We have now shown that each of these three groups of mitochondria responds specifically to cytosolic Ca2+ signals in their immediate environment. It is not entirely clear to what extent electrical and chemical coupling between mitochondria occurs in different cell types. A recent study of COS-7 cells indicates that as far as electrical coupling is concerned, the situation may be dynamic, with mitochondrial units spontaneously connecting and disconnecting (De Giorgi et al., 2000). Our local bleaching experiments (Figure 8) indicate that the Ca2+-sensitive dye Rhod-2 may not move between mitochondrial areas. At least in normal pancreatic acinar cells, the mitochondria in different regions are apparently not sufficiently luminally connected to allow passage of small molecules along chemical gradients. It is therefore convenient to discuss the function of each of the three mitochondrial groups separately.
Fig. 10. Schematic drawing illustrating the locations of the different mitochondrial groups together with directions of Ca2+ transport and indications of ATP supply. For further details see text.
The perigranular mitochondria
The perigranular mitochondrial belt was described by Tinel et al. (1999), but here we have for the first time shown directly that cytosolic Ca2+ spiking leads to Ca2+ uptake into these mitochondria (Figure 5), thereby directly demonstrating their ability to perform a barrier function. We have now also identified a specific subgroup of perigranular mitochondria situated very close to the apical plasma membrane. These mitochondria are close to the primary Ca2+ release sites in the granular area (trigger zone) (Kasai et al., 1993; Thorn et al., 1993; Mogami et al., 1997; Cancela et al., 2000) and are particularly sensitive to the early stages of agonist-induced Ca2+ release (Figure 2). This is consistent with data showing that even in the case of supramaximal stimulation, when the [Ca2+]i signal spreads rapidly all over the cell, the [Ca2+]i rise is largest in the apical region (Ito et al., 1997).
Under physiological stimulation, the amounts of Ca2+ released from the ER during each spike are so small that the fall in [Ca2+]ER is normally undetectable (Park et al., 2000). Nevertheless, our new results show that these short-lasting cytosolic Ca2+ spikes, which are largely confined to the granular region, cause clearly measurable Ca2+ uptake in the mitochondria located most apically in the perigranular belt (Figure 5). This indicates close contacts between the Ca2+ release sites in the ER and the apical mitochondria, as already described in other systems (Rizzuto et al., 1998). During each local [Ca2+]i spike in the granular region, there is an exocytotic response (capacitance increase) (Maruyama and Petersen, 1994). The apical mitochondria are therefore likely to be most important for stimulus–metabolism coupling under physiological conditions. This makes sense, since exocytotic secretion, which requires a high ATP concentration (Baker and Knight, 1978), is exclusively taking place across the apical membrane (Palade, 1975).
The peripheral mitochondria near the basal plasma membrane
We have demonstrated that store-operated Ca2+ entry, which is important for refilling empty ER stores with Ca2+ (Parekh and Penner, 1997), is sensed by the peripheral basolateral mitochondria. This is in agreement with the recent demonstration (Gilabert and Parekh, 2000) that respiring mitochondria play a role both in the activation and inactivation phases of the store-operated Ca2+ entry. This requires Ca2+ uptake into mitochondria placed very close to the Ca2+ entry channels (Figure 9). This process would increase ATP production, which would be helpful locally, as Ca2+ entering the cell is taken up into the ER by powerful Ca2+-activated ATPases (Mogami et al., 1997). Ca2+ uptake into both the ER and the mitochondria would tend to reduce [Ca2+]i near the Ca2+ entry sites and therefore help diminish the strong negative feedback effect of Ca2+ on the open state probability of the store-operated Ca2+ channels (Parekh and Penner, 1997), which would otherwise effectively stop entry.
The perinuclear mitochondria
To what extent the Ca2+ concentrations in the nucleoplasm and the cytosol can be regulated independently is still controversial (Rogue and Malviya, 1999). The nuclear pore complexes are normally permeable to Ca2+ (Brini et al., 1993; Gerasimenko et al., 1995; Lipp et al., 1997) but, at least temporarily, discrepancies between the Ca2+ levels inside and outside the nuclear envelope can occur (Rogue and Malviya, 1999). Intermediate doses of ACh can initiate cytosolic Ca2+ signals in the granular area that spread to the basal part of the cell, except the nucleus (Gerasimenko et al., 1996). The perinuclear ring of mitochondria (Figure 1C), which takes up Ca2+ specifically when Ca2+ is uncaged in the nucleus (Figure 8A), could play a role in protecting the nucleus against invasion of [Ca2+]i signals. The nucleus has potential for generating its own Ca2+ signals (Gerasimenko et al., 1995; Malviya and Rogue, 1998). The perinuclear mitochondrial barrier could play a role by helping to confine such signals, initiated by release through the inner nuclear membrane, to the nucleoplasm and at the same time supply ATP locally. Ca2+ signals in the perinuclear mitochondria could also be important for the regulation of the energetics of nuclear transport and possibly play a role in triggering release of activators of apoptosis from perinuclear mitochondria.
Conclusion
The spatio-temporal pattern of [Ca2+]i signals depends on agonist type and concentration (Petersen et al., 1991) and this is important for understanding differential physiological and pathophysiological regulation (Petersen et al., 1994; Parekh, 2000). Our new results show that different groups of functionally unconnected regional mitochondria can sense [Ca2+]i signals in their immediate environment (Figure 10). The most restricted [Ca2+]i signals in space and time are the repetitive short-lasting Ca2+ spikes that are confined to the granular region (Thorn et al., 1993), because of the perigranular mitochondrial Ca2+ buffer barrier (Figures 5 and 10). These spikes control exocytotic secretion (Maruyama and Petersen, 1994) through the apical membrane (Palade, 1975), and the local ATP supply from the apical part of the perigranular ring is therefore most likely to be of major physiological significance. Ca2+ for stimulus–secretion coupling is ultimately derived from the external solution and enters the acinar cell across the basal membrane. We have shown previously (Mogami et al., 1997; Park et al., 2000) that Ca2+ is transported across the cell, shielded from the cytosol, by an operational ER tunnel. Ca2+ is therefore always available for release from the ER extensions in the granular area. Ca2+ entry into the basal part of the ER tunnel depends on thapsigargin-sensitive Ca2+ pumps in the ER (Mogami et al., 1997). The peripheral mitochondria, which take up Ca2+ specifically during store-operated Ca2+ entry (Figures 9 and 10), must therefore play an important role in supplying the ER Ca2+ uptake machinery with the necessary ATP. Finally, the nucleus may have special problems in relation to Ca2+ signalling. During normal secretory events, it is not desirable to involve the nucleus. Indeed, we have shown previously that when the Ca2+ signal escapes from the granular region, the nuclear area is still somewhat protected against invasion by Ca2+ waves (Gerasimenko et al., 1996). The perinuclear mitochondrial ring (Figures 8 and 10) is likely to play an important role in this protection. The perinuclear mitochondria could also play an important role in confining signals generated inside the nucleus to this organelle.
Materials and methods
Cell preparation
Fresh mouse pancreatic acinar cells were isolated after collagenase treatment, as previously described (Osipchuk et al., 1990) and used within 2 h. All experiments were performed at room temperature (22–24°C). Mag-fluo-4-AM, fluo-4-AM, MitoTracker Green FM, Hoechst 33342, Rhod-2-AM and NP-EGTA-AM were purchased from Molecular Probes, and the other chemicals were from Sigma Co.
Solutions
The extracellular bathing solution contained (in mM): NaCl 140, KCl 4.7, MgCl2 1.13, CaCl2 1, glucose 10 and HEPES 10. pH was adjusted to 7.3 by NaOH. We used two different intracellular pipette solutions. One contained (in mM): KCl 135, MgCl2 1.13, NaCl 20, HEPES 10, Na2ATP 2, EGTA 0.1; pH was 7.2, adjusted by KOH. In the other solution we replaced 0.1 mM EGTA with 10 mM BAPTA + 2 mM Ca2+.
Dye-loading procedures
For visualization of mitochondria, isolated cells were incubated with 10 µM MitoTracker Green FM for 30 min at 37°C. For visualization of nuclei, Hoechst 33342 was added to the bathing solution on the microscope stage to a concentration of ∼100 µg/ml. To stain the ER, we incubated cells in a solution containing 0.3–1 µM ERtracker (Molecular Probes) for 15–30 min at 37°C. For imaging of Ca2+ in intracellular stores, cells were incubated with 4–6 µM Mag-fluo-4-AM and 0.01% pluronic acid for 20–30 min. For mitochondrial Ca2+ measurements, cells were incubated with Rhod-2-AM and 0.01% pluronic acid for 20–30 min at 37°C. For combined mitochondrial and cytosolic Ca2+ measurements, the cells were washed after loading of Rhod-2 and then incubated with 2.5 µM fluo-4-AM for 20 min at 22°C, For experiments with uncaging of caged Ca2+, NP-EGTA was loaded into the cells by incubation in a solution containing 10 µM NP-EGTA-AM for 30 min. To remove cytosolic indicators, we used the patch–clamp whole-cell configuration (Mogami et al., 1998). The same pipette was used to introduce calcium buffer into the cell. The patch pipette contained 0.1 mM EGTA or a 10 mM BAPTA/2 mM Ca2+ mixture. Some experiments with Mag-fluo-4 and/or Rhod-2 were performed on intact cells, without unloading the cytosolic calcium indicator.
Confocal imaging, photobleaching and uncaging
Confocal imaging was performed using a Zeiss LSM510 confocal system. This system allows rapidly alternating excitation of multiple fluorophores, thus reducing cross-talk. Also, accurate spatially restricted exposure of laser light can be achieved and this was used for the uncaging and bleaching experiments. Rhod-2 was excited with a 543 nm laser line and emission collected through a BP560-615 or LP560 filter. Fluo-4, Mag-fluo-4 and MitoTracker Green-FM were excited using 488 nm laser light. Emitted light was collected using a BP505-550 or LP505 filter. Hoechst 33342 was excited with UV laser light (351 nm/364 nm) and emission collected through a BP385-470 filter. We used near-maximal power of UV laser lines (351 nm/364 nm) to induce fast bleaching of Rhod-2 and adequate lower power for uncaging of NP-EGTA. Bleaching/uncaging were combined with simultaneous confocal imaging. For the image analysis, we used Zeiss confocal 510 image software as well as software developed by ourselves.
Electrophysiology
Standard patch–clamp whole-cell current recording (Hamill et al., 1981) was used. The electrophysiological recording of Ca2+-dependent current (Petersen, 1992) was made using the EPC-8 amplifier and Pulse software (HEKA). The pipette resistance was 2–3 MΩ. The detailed procedure has been described previously (Thorn and Petersen, 1992).
Acknowledgments
Acknowledgements
We thank Nina Burdakova and Mark Houghton for technical assistance. This work was supported by a Medical Research Council (MRC) Programme Grant. M.C.A. is a Wellcome Trust Prize PhD student. O.H.P. is an MRC Research Professor.
References
- Baker P.F. and Knight,D.E. (1978) Calcium-dependent exocytosis in bovine adrenal medullary cells with leaky plasma membranes. Nature, 276, 620–622. [DOI] [PubMed] [Google Scholar]
- Brini M., Murgia,M., Pasti,L., Picard,D., Pozzan,T. and Rizzuto,R. (1993) Nuclear Ca2+ concentration measured with specifically targeted recombinant aequorin. EMBO J., 12, 4813–4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela J.M., Gerasimenko,O.V., Gerasimenko,J.V., Tepikin,A.V. and Petersen,O.H. (2000) Two different but converging messenger pathways to intracellular Ca2+ release: the roles of nicotinic acid adenine dinucleotide phosphate, cyclic ADP-ribose and inositol trisphosphate. EMBO J., 19, 2549–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Giorgi F., Lartigue,L. and Ichas,F. (2000) Electrical coupling and plasticity of the mitochondrial network. Cell Calcium, 28, 365–370. [DOI] [PubMed] [Google Scholar]
- Denton R.M. and McCormack,J.G. (1990) Ca2+ as a second messenger within mitochondria of the heart and other tissues. Annu. Rev. Physiol., 52, 451–466. [DOI] [PubMed] [Google Scholar]
- Duchen M.R. (2000) Mitochondria and Ca2+ in cell physiology and pathophysiology. Cell Calcium, 28, 339–348. [DOI] [PubMed] [Google Scholar]
- Gerasimenko O.V., Gerasimenko,J.V., Tepikin,A.V. and Petersen,O.H. (1995) ATP-dependent accumulation and inositol trisphosphate- or cyclic ADP-ribose-mediated release of Ca2+ from the nuclear envelope. Cell, 80, 439–444. [DOI] [PubMed] [Google Scholar]
- Gerasimenko O.V., Gerasimenko,J.V., Petersen,O.H. and Tepikin,A.V. (1996) Short pulses of acetylcholine stimulation induce cytosolic Ca2+ signals that are excluded from the nuclear region in pancreatic acinar cells. Pflugers Arch., 432, 1055–1061. [DOI] [PubMed] [Google Scholar]
- Gilabert J.A. and Parekh,A.B. (2000) Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current ICRAC. EMBO J., 19, 6401–6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A., Schulz,I. and Schmid,A. (2000) Agonist-evoked mitochondrial Ca2+ signals in mouse pancreatic acinar cells. J. Biol. Chem., 275, 38680–38686. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G., Robb-Gaspers,L.D., Seitz,M.B. and Thomas,A.P. (1995) Decoding of cytosolic calcium oscillations in the mitochondria. Cell, 82, 415–424. [DOI] [PubMed] [Google Scholar]
- Hamill O.P., Marty,A., Neher,E., Sakmann,B. and Sigworth,F.J. (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch., 391, 85–100. [DOI] [PubMed] [Google Scholar]
- Ichas F., Jouaville,L.S. and Mazat,J.P. (1997) Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell, 89, 1145–1153. [DOI] [PubMed] [Google Scholar]
- Ito K., Miyashita,Y. and Kasai,H. (1997) Micromolar and submicro molar Ca2+ spikes regulating distinct cellular functions in pancreatic acinar cells. EMBO J., 16, 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouaville L.S., Pinton,P., Bastianutto,C., Rutter,G.A. and Rizzuto,R. (1999) Regulation of mitochondrial ATP synthesis by calcium: Evidence for a long-term metabolic priming. Proc. Natl Acad. Sci. USA, 96, 13807–13812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Li,Y.X. and Miyashita,Y. (1993) Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell, 74, 669–677. [DOI] [PubMed] [Google Scholar]
- Lawrie A.M., Rizzuto,R., Pozzan,T. and Simpson,A.W.M. (1996) A role for calcium influx in the regulation of mitochondrial calcium in endothelial cells. J. Biol. Chem., 271, 10753–10759. [DOI] [PubMed] [Google Scholar]
- Lipp P., Thomas,D., Berridge,M.J. and Bootman,M.D. (1997) Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J., 16, 7166–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malviya A.N. and Rogue,P.J. (1998) ‘Tell me where is calcium bred’: clarifying the roles of nuclear calcium. Cell, 92, 17–23. [DOI] [PubMed] [Google Scholar]
- Maruyama Y. and Petersen,O.H. (1994) Delay in granular fusion evoked by repetitive cytosolic Ca2+ spikes in mouse pancreatic acinar cells. Cell Calcium, 16, 419–430. [DOI] [PubMed] [Google Scholar]
- McCormack J.G., Halestrap,A.P. and Denton,R.M. (1990) Role of calcium-ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev., 70, 391–425. [DOI] [PubMed] [Google Scholar]
- Mogami H., Nakano,K., Tepikin,A.V. and Petersen,O.H. (1997) Ca2+ flow via tunnels in polarized cells: recharging of apical Ca2+ stores by focal Ca2+ entry through basal membrane patch. Cell, 88, 49–55. [DOI] [PubMed] [Google Scholar]
- Mogami H., Tepikin,A.V. and Petersen,O.H. (1998) Termination of cytosolic Ca2+ signals: Ca2+ reuptake into intracellular stores is regulated by the free Ca2+ concentration in the store lumen. EMBO J., 17, 435–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero M., Alonso,M.T., Carnicero,E., Cuchillo-Ibanez,I., Albillos,A., Garcia,A.G., Garcia-Sancho,J. and Alvarez,J. (2000) Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nature Cell Biol., 2, 57–61. [DOI] [PubMed] [Google Scholar]
- Osipchuk Y.V., Wakui,M., Yule,D.I., Gallacher,D.V. and Petersen,O.H. (1990) Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+-dependent Cl– current recording. EMBO J., 9, 697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G.E. (1975) Intracellular aspects of the process of protein secretion. Science, 189, 347–358. [DOI] [PubMed] [Google Scholar]
- Parekh A. (2000) Calcium signaling and acute pancreatitis: specific response to a promiscuous messenger. Proc. Natl Acad. Sci. USA, 97, 12933–12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh A. and Penner,R. (1997) Store depletion and calcium influx. Physiol. Rev., 77, 901–930. [DOI] [PubMed] [Google Scholar]
- Park M.K., Petersen,O.H. and Tepikin,A.V. (2000) The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movements and equilibration. EMBO J., 19, 5729–5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C.C.H., Toescu,E.C. and Petersen,O.H. (1991) Different patterns of receptor-activated cytoplasmic Ca2+ oscillations in single pancreatic acinar cells: dependence on receptor type, agonist concentration and intracellular Ca2+ buffering. EMBO J., 10, 527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O.H. (1992) Stimulus–secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J. Physiol., 448, 1–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O.H., Petersen,C.C.H. and Kasai,H. (1994) Calcium and hormone action. Annu. Rev. Physiol., 56, 297–319. [DOI] [PubMed] [Google Scholar]
- Petersen O.H., Tepikin,A.V. and Park,M.K. (2001) The endoplasmic reticulum: one continuous or several separate Ca2+ stores. Trends Neurosci., 24, 271–276. [DOI] [PubMed] [Google Scholar]
- Pinton P., Drummond,R., Magalhaes,P., Brini,M., Chiesa,A., Pozzan,T. and Rizzuto,R. (2001) Ca2+ measurements in mitochondria. In Petersen,O.H. (ed.), Measuring Calcium and Calmodulin Inside and Outside Cells. Springer Laboratory Manual, Heidelberg, Germany, pp. 185–210.
- Pozzan T., Rizzuto,R., Volpe,P. and Meldolesi,J. (1994) Molecular and cellular physiology of intracellular Ca2+ stores. Physiol. Rev., 74, 595–636. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Magalhaes,P. and Rizzuto,R. (2000) The comeback of mitochondria to calcium signalling. Cell Calcium, 28, 279–283. [DOI] [PubMed] [Google Scholar]
- Raraty M., Ward,J., Erdemli,G., Vaillant,C., Neoptolemos,J.P., Sutton,R. and Petersen,O.H. (2000) Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc. Natl Acad. Sci. USA, 97, 13126–13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Pinton,P., Carrington,W., Fay,F.S., Fogarty,K.E., Lifshitz,L.S., Tuft,R.A. and Pozzan,T. (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science, 280, 1763–1766. [DOI] [PubMed] [Google Scholar]
- Rizzuto R., Bernadi,P. and Pozzan,T. (2000) Mitochondria as all-round players of the calcium game. J. Physiol., 529, 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogue P.J. and Malviya,A.N. (1999) Calcium signals in the cell nucleus. EMBO J., 18, 5147–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter G.A. and Rizzuto,R. (2000) Regulation of mitochondrial metabolism by ER Ca2+ release: an intimate connection. Trends Biochem. Sci., 25, 215–221. [DOI] [PubMed] [Google Scholar]
- Straub S.V., Giovannucci,D.R. and Yule,D.I. (2000) Calcium wave propagation in pancreatic acinar cells. Functional interaction of inositol 1,4,5-trisphosphate receptors, ryanodine receptors and mitochondria. J. Gen. Physiol., 116, 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P. and Petersen,O.H. (1992) Activation of nonselective cation channels by physiological cholecystokinin concentrations in mouse pancreatic acinar cells. J. Gen. Physiol., 100, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn P., Lawrie,A.M., Smith,P.M., Gallacher,D.V. and Petersen,O.H. (1993) Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell, 74, 661–668. [DOI] [PubMed] [Google Scholar]
- Tinel H., Cancela,J.M., Mogami,H., Gerasimenko,J.V., Gerasimenko,O.V., Tepikin,A.V. and Petersen,O.H. (1999) Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J., 18, 4999–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]