Abstract
Sortilin belongs to a growing family of multiligand type-1 receptors with homology to the yeast receptor Vps10p. Based on structural features and sortilin’s intracellular predominance, we have proposed it to be a sorting receptor for ligands in the synthetic pathway as well as on the cell membrane. To test this hypothesis we examine here the cellular trafficking of chimeric receptors containing constructs of the sortilin tail. We report that sorting signals conforming to YXXΦ and dileucine motifs mediate rapid endocytosis of sortilin chimeras, which subsequently travel to the trans-Golgi network, showing little or no recycling. Furthermore, we found that cation-independent mannose 6-phosphate receptor (MPR300)–sortilin chimeras, expressed in mannose 6-phosphate receptor knockout cells, were almost as efficient as MPR300 itself for transport of newly synthesized β-hexosaminidase and β-glucuronidase to lysosomes, and established that the sortilin tail contains potent signals for Golgi–endosome sorting. Finally, we provide evidence suggesting that sortilin is the first example of a mammalian receptor targeted by the recently described GGA family of cytosolic sorting proteins, which condition the Vps10p-mediated sorting of yeast carboxypeptidase Y.
Keywords: chimeric receptors/GGA/sortilin/sorting/Vps10p
Introduction
Sortilin (∼100 kDa) is a type-1 membrane receptor which is expressed in a number of tissues, notably brain, spinal cord, testis and skeletal muscle (Petersen et al., 1997; Hermans-Borgmeyer et al., 1999). Like the related receptor sorLA (250 kDa), sortilin binds the endoplasmic reticulum-resident receptor-associated protein (RAP), a putative chaperone for members of the low density lipoprotein receptor (LDLR) family (Bu and Schwartz, 1998; Willnow, 1998), and the two were originally co-purified from human brain by RAP affinity chromatography (Jacobsen et al., 1996; Petersen et al., 1997). Along with a third, recently described brain receptor designated sorCS (∼130 kDa) (Hermey et al., 1999) and at least two other proteins (DDBJ/EMBL/GenBank accession Nos AB028982 and AB037750) (Kikuno et al., 1999; Nagase et al., 2000), sortilin and sorLA constitute a new family of receptors sharing the characteristic structural feature of an ∼600-amino-acid N-terminal domain with a strong resemblance to each of the two related domains in the luminal portion of the yeast sorting receptor Vps10p (Marcusson et al., 1994). Information is still limited, but previous findings, in particular regarding sortilin and sorLA, suggest that the receptors are neuropeptide binding proteins with multiple and possibly overlapping functions (Yamazaki et al., 1996; Mazella et al., 1998; Hampe et al., 1999; Nielsen et al., 1999; Petersen et al., 1999).
Unlike in sorLA and sorCS, which both incorporate additional domain types (Jacobsen et al., 1996; Hermey et al., 1999), the Vps10p domain makes up the entire luminal part of sortilin (Petersen et al., 1997). The domain contains two distinctive features: a C-terminal conserved segment of 10 cysteines (10CC) and a 44-amino-acid N-terminal propeptide. The role of the 10CC segment is unknown, but we have recently demonstrated that the propeptide plays an important part in the functional activation of sortilin by preventing ligands, in the early part of the synthetic pathway, from gaining access to the receptor binding site(s). Thus, the receptor is produced as an inert precursor which is converted to its mature binding-active form upon furin-mediated propeptide cleavage in the trans-Golgi network (TGN) (Petersen et al., 1999).
Although its physiological role remains unclarified, sortilin is the best described of the Vps10p-domain receptors and different lines of evidence have given hints towards a possible function. First, sortilin binds a variety of unrelated ligands, including lipoprotein lipase (LpL) (Nielsen et al., 1999) and neurotensin (NT) (Mazella et al., 1998; Petersen et al., 1999). These ligands have two things in common: they each bind to one or more alternative receptor(s) and they are candidates for regulated transport not accounted for by these receptors (Braun and Severson, 1992; Barbero et al., 1998). Thus, members of the LDLR family that bind LpL are primarily endocytic receptors, which are also implicated in signalling (Gliemann, 1998; Cooper and Howell, 1999), and the two seven-transmembrane receptors for neurotensin (NTR-1 and -2) are signalling receptors with a capacity for endocytosis (Vincent, 1995). Therefore, even though sortilin may contribute to endocytosis and perhaps even signalling, it seems plausible that its main concern is functions not covered by the alternative receptors, e.g. regulated transport. Secondly, sortilin has striking (and indicative) structural similarities to receptors involved in intracellular sorting and transport. Thus, Vps10p is the sorting receptor for carboxypeptidase Y (CPY) in yeast (Marcusson et al., 1994) and the C-terminal segment in sortilin’s cytoplasmic tail is closely related to the corresponding and functionally important segment of the cation-independent mannose 6-phosphate receptor (MPR300) (Johnson and Kornfeld, 1992a,b). Moreover, the sortilin cytoplasmic domain contains several potential signal sequences that conform to established consensus motifs, known to be involved in adaptor protein binding, endocytosis, basolateral targeting and Golgi–endosome sorting (Ktistakis and Roth, 1996). Thirdly, previous findings have demonstrated that sortilin predominates in intracellular compartments. Only a minor fraction of the receptors is expressed on the cell surface, whereas ∼90% are found in Golgi and vesicles, showing extensive co-localization with MPR300 (Petersen et al., 1997; Morris et al., 1998).
It would appear from the above that sortilin is likely to serve functions inside the cell and that the combined sum of available evidence suggests it to be a candidate sorting receptor, targeted for transport by ligands in the synthetic pathway as well as on the surface membrane.
In the present study we have pursued this concept. We have probed for interactions between sortilin and cytosolic sorting proteins, and we have examined the cellular trafficking of chimeric receptors to determine and characterize active sorting signals in sortilin’s cyto plasmic domain. In particular, sortilin’s capacity for Golgi–endosome sorting was tested by examining the transport of lysosomal enzymes in mannose 6-phosphate receptor knockout (mpr–) cells expressing MPR300– sortilin chimeras. Our findings provide evidence that sortilin has the potential for intracellular sorting and may well be involved in a hitherto unrecognized type of Golgi–endosome transport.
Results
Expression of chimeric receptors
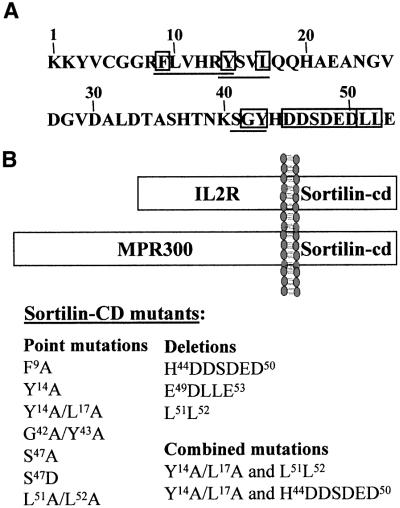
For selection of chimeric receptors likely to hold information on sortilin trafficking, we first examined the sortilin cytoplasmic domain (sortilin-cd) to identify segments conforming to known sorting motifs. The primary sequence of the 53-amino-acid domain and the chosen segments are shown in Figure 1A. We next generated a series of tail constructs in which potentially critical residues in each of the selected sites were either deleted or replaced by alanine and, in the case of S47, by aspartate. The resulting mutant constructs (Figure 1B) and wild-type sortilin-cd were then combined with the extracellular and transmembrane segments of interleukin-2 receptor-α (IL2Rα) (CD25, Tac) and stably expressed as IL2R–sort chimeric receptors in CHO-K1 cells. Alternatively, the tail constructs were combined with the luminal and the transmembrane domains of MPR300 (MPR300–sort chimeras) and expressed in mouse fibroblasts deficient for both mannose 6-phosphate receptors.
Fig. 1. (A) Primary sequence of the sortilin cytoplasmic domain. Motifs that are known to constitute active sorting signals in other transmembrane proteins are underlined, putative key residues are boxed. (B) Schematic presentation of the chimeric receptors (upper panel) and the cytoplasmic domain constructs (lower panel) used in this study. The luminal domains of IL2Rα and of MPR300 are indicated.
Endocytosis of chimeric receptors
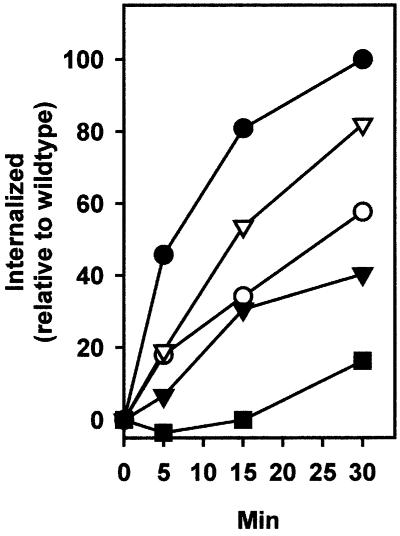
To examine the endocytic capability of the IL2R–sort chimeras, CHO transfectants were incubated with iodinated anti-Tac at 4°C. Following incubation, unbound antibody was removed and the cells were re-incubated in warm medium (37°C, zero time). At given times, reactions were stopped on ice and the degree of endocytosis was determined by incubation at pH 2.5, i.e. not internalized tracer was defined as the amount of cell-associated radioactivity that could be dissociated at low pH. Using this method, a maximum of ∼67% of the total amount of anti-Tac bound by IL2R–sort-wt was internalized within 2 h, which is similar to the amount (∼75%) of labelled anti-sortilin internalized by CHO cells expressing full-length sortilin (data not shown). Results obtained with the individual IL2R–sort chimeric constructs are summarized in Table I. The table shows the percentage of tracer not internalized (dissociable at pH 2.5) found on the respective transfectants at 30 min, i.e. at a time when >85% of maximum endocytosis had taken place. It appears that only alterations involving the tyrosine-based YXXΦ motif, near the membrane-spanning segment, and the C-terminal dileucine had any significant effect on internalization. The time course of [125I]anti-Tac internalization by IL2R–sort chimeras is delineated in Figure 2 and clearly designates Y14SVL17 as the main signal for endocytosis, being responsible for ∼60% of the activity, and the tyrosine as the single most important residue. Deletion of the dileucine L51L52 caused a relatively minor decrease in internalization, but in combination, disruption of the dileucine- and tyrosine-based signals accounted for an ∼80% reduction of the endocytosis conveyed by the sortilin wild-type tail.
Table I. Internalization of ILR–sort chimeras.
| Sortilin-tail constructs | Surface-associated liganda |
|---|---|
| Wild type | 41.5 ± 6.3 |
| F9A | 47.5 ± 2.6 |
| G42A/Y43A | 37.9 ± 1.2 |
| S47A | 42.8 ± 0.2 |
| Del H44DDSDED50 | 42.1 ± 4.7 |
| Del E49DLLE53 | 53.6 ± 8.0 |
| Del L51L52 | 52.3 ± 7.1 |
| Y14A | 66.4 ± 4.7 |
| Y14A/L17A | 76.5 ± 4.5 |
| Y14A/L17A and Del H44DDSDED50 | 77.9 ± 1.0 |
| Y14A/L17A and Del L51L52 | 90.5 ± 1.2 |
aPercentage ligand bound to CHO cells at 4°C (zero time) after 30 min at 37°C (mean ± SD, n = 3).
Fig. 2. Time course of [125I]anti-Tac internalization in transfected CHO cells expressing IL2R–sort chimeras. Following incubation at 4°C, unbound tracer was removed by washing and the cells were re-incubated in warm medium (zero time). At the given times, incubation was stopped on ice and internalization was determined as the amount of cell-associated radioactivity that was not released upon incubation at pH 2.5. Each point represents a mean of triplicates and all values are shown relative to the maximal internalization (100% at 30 min) obtained in transfectants expressing IL2R–sort-wt. Wild-type tail, closed circles; L51L52 deleted, open triangles; Y14A, open circles; Y14/L17A, closed triangles; Y14A/L17A and L51L52 deleted, closed squares.
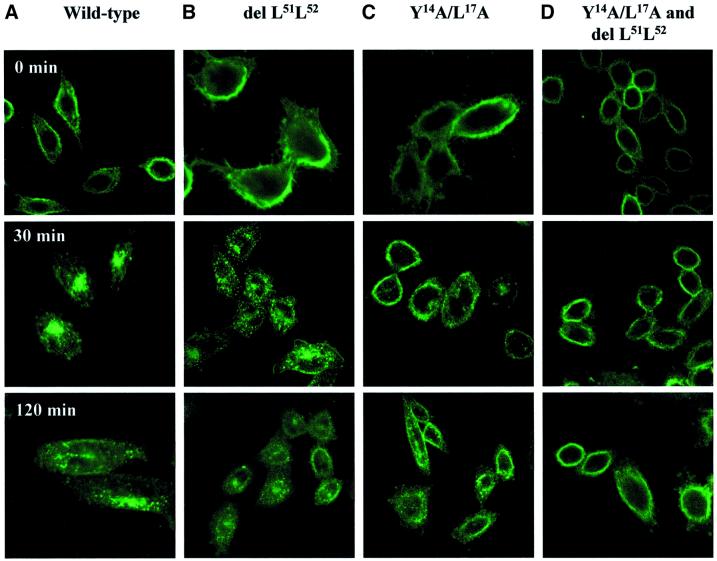
Matching results were obtained by confocal microscopy (Figure 3). Thus, anti-Tac, bound at 4°C by CHO cells expressing IL2R–sort-wt, was translocated from the cell surface to intracellular vesicles within minutes of incubation in warm medium (Figure 3A). After 30 min, practically all staining for antibody was concentrated in perinuclear compartments, leaving little or no staining on the surface membrane. By comparison, translocation of anti-Tac by chimeras with a deleted dileucine (Figure 3B) or a disrupted YXXΦ motif (Figure 3C) was slow and incomplete, and in cells transfected with constructs carrying the combined defects (Figure 3D) anti-Tac was found exclusively on the cell surface, even after 2 h at 37°C.
Fig. 3. Internalization of IL2R–sort chimeras in transfected CHO cells (confocal microscopy). After binding of anti-Tac at 4°C, transfected CHO cells expressing chimeric receptors comprising (A) wild-type sortilin-cd or (B) cd constructs containing a deletion of L51L52, (C) a Y14A/L17A mutation or (D) both were washed and re-incubated in warm medium. At the given times, the cells were fixed, stained by Alexa 488-conjugated goat anti-mouse Ig and analysed by confocal microscopy.
This suggested that chimeras with an impaired endocytic function accumulated on the cell surface. We therefore assessed whether the IL2R–sort constructs in question were relatively overexpressed on the plasma membrane. Surface-associated and intracellular receptors were separated from biolabelled CHO transfectants by stepwise immunoprecipitation and quantitated, after SDS–PAGE, by autoradiography and densitometry. Using this approach, we found that the fraction of mutant receptors exposed on the plasma membrane was 1.3- (L51L52 deleted), 2.8- (Y14A/L17A) and 3.8- (the two combined) fold higher than that of IL2R–sort-wt. Another set-up, based on biotinylation of surface proteins on whole cells and subsequent separation of the two receptor pools by streptavidin beads, gave similar results, confirming that a decrease in endocytic capacity was accompanied by an increased expression of receptors on the cell membrane (data not shown).
Internalized IL2R–sort-wt chimeras are directed to the TGN without recycling
As depicted in Figure 3A, internalized IL2R–sort-wt in complex with anti-Tac rapidly accumulated in perinuclear compartments. Even after prolonged incubation, staining seemed restricted to intracellular vesicles, suggesting insignificant recycling of the antibody–receptor complexes. Accordingly, during a 4 h chase at 37°C, little or no internalized 125I-labelled anti-Tac returned to the surface in a pH 2.5-releasable form.
The routing of chimeric receptors in complex with anti-Tac was examined further by electron microscopy. The results are shown in Figure 4. It appears that within 15–20 min, the anti-Tac–receptor complexes, initially scattered on the plasma membrane or assembled in coated pits (Figure 4, panel 1), became localized in coated vesicles (Figure 4, panel 2, inserts) and in Lamp-1-negative early endosomes (Figure 4, panel 2). At 60 min, staining predominated in vesiculotubular structures in close proximity to the nucleus, and double staining demonstrated that the antibody–receptor complexes to a large extent co-localized with TGN38 (Figure 4, panel 3). At this point (60 min), minor staining was also seen in uncoated vesicles, some resembling multivesicular bodies, and in a few Lamp-1-positive structures (Figure 4, panel 4). It is remarkable that co-localization with Lamp-1 was scarce and only seen upon prolonged incubation (>45 min).
Fig. 4. Endocytosis of IL2R–sort-wt and co-localization with TGN38 and Lamp-1. After binding of anti-Tac at 4°C and removal of unbound antibody, CHO transfectants expressing IL2R–sort-wt were re-incubated at 37°C. At the given times, the incubation was stopped on ice and the cells were fixed. Staining was performed using gold beads coupled to goat anti-mouse antibody. For double staining, fixed cells were incubated with rabbit anti-Lamp-1 or rabbit anti-TGN38 prior to staining with gold beads coupled to goat anti-rabbit antibody. Staining: panel 1 (zero time), IL2R–sort-wt; panel 2 (20 min), IL2R–sort-wt (black arrowheads) and Lamp-1 (black arrows); panel 2 insets, IL2R–sort-wt; panel 3 (60 min), IL2R–sort-wt (black arrowheads) and TGN38 (black arrows); panel 4 (60 min), IL2R–sort-wt (black arrowheads) and Lamp-1 (black arrows). Magnification in all panels ×114 000.
It can be concluded from the above that sortilin-cd mediates endocytosis of IL2R–sort-wt in clathrin-coated vesicles, avoids recycling and directs the receptors to the TGN via Lamp-1-negative endosomes.
Sortilin-cd contains an acidic cluster (H44DDSDED50), which shows great similarity to clusters that are known to play an important part in the surface-to-Golgi sorting of other transmembrane proteins, e.g. furin. Separate experiments were therefore performed to determine the involvement of the sortilin acidic cluster. It was established that S47 is readily phosphorylated by casein kinase II in vitro (Figure 5) and small amounts of phosphorylated full-length sortilin could be immunoprecipitated from CHO transfectants treated with the phosphatase inhibitor calyculin, but not from untreated cells (data not shown). This suggested that sortilin may be a substrate for CKII in vivo, but as described above (Table I), the acidic cluster does not influence endocytosis, and various measures, including deletion of the entire segment and substituting aspartate for S47 (to mimic permanent phosphorylation), failed to induce any change in the apparent lack of IL2R–sort-wt recycling. In contrast, confocal microscopy did leave the overall impression that conditions mimicking or favouring a state of phosphorylation (S47D, or the presence of calyculin) promoted the TGN localization of internalized antibody–receptor complexes. However, the cell-to-cell variation was not insignificant and further evidence is needed before final conclusions can be drawn.
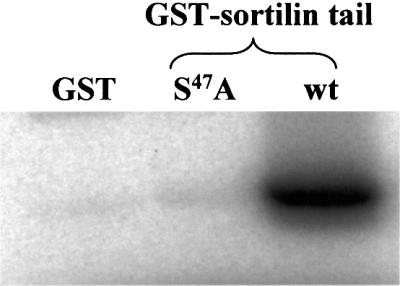
Fig. 5. In vitro phosphorylation of the sortilin cytoplasmic domain. Constructs of the sortilin-cd were expressed with a GST tag and purified. The purified fusion proteins and GST were incubated at 2 µM in MOPS buffer pH 7.0 containing 0.5 nM casein kinase II. Phosphorylation was started by addition of 20 µM [γ-32P]ATP, stopped after 45 min in 5% sample buffer, and analysed by SDS–PAGE and autoradiography. Lane 1, GST; lane 2, mutant construct S47A; lane 3, wild-type sortilin tail.
Sortilin‘s cytoplasmic tail conveys Golgi–endosome transport
Information on direct sorting from the synthetic pathway to endo- and lysosomal compartments was obtained in a previously described model, using mouse embryonic fibroblasts deficient in both mannose 6-phosphate receptors (Pohlmann et al., 1995). Owing to their receptor deficiency, these (mpr–) cells are unable to transfer newly synthesized lysosomal enzymes, normally targeted by the mannose 6-phosphate receptors, from the secretory pathway to the late endosomes. The cells consequently release the ligands into the medium and are left with abnormal lysosomes. We transfected mpr– cells with chimeric receptor constructs containing the sortilin tail, mutant or wild type, combined with the luminal (ligand binding) and transmembrane parts of MPR300. Since these chimeras (MPR300–sort) bind the mannose 6-phosphate moieties of proenzymes via the MPR300 luminal domain, we were then able to determine their sorting by monitoring the fate and cellular release of ligands, i.e. β-hexosaminidase. The results shown in Figure 6 (left panel) demonstrate that the levels of β-hexosaminidase were found to be very similar in the culture medium of transfected mpr– cells expressing comparable amounts of either MPR300–sort-wt or wild-type MPR300. At the optimal level of receptor expression (≥1.0), the medium from transfected cultures contained ∼80% less β-hexosaminidase than that of untransfected cells and in cultures supplemented with mannose 6-phosphate, <40% of the enzyme was found in the medium. Since mannose 6-phosphate inhibits ligand binding at the cell surface and thereby prevents uptake from the medium, it follows that the chimeric receptor, similarly to MPR300 itself, downregulates the cellular secretion of β-hexosaminidase.
Receptors likewise regulate the secretion of β-glucuronidase (data not shown), a lysosomal enzyme that is converted upon arrival in the late endosomes/lysosomes from its 72 kDa proform to its mature cleaved 69 kDa form. SDS–PAGE analysis (Figure 6, left panel inset) demonstrates that while β-glucuronidase was released into the medium in its proform, the cells contained only the converted mature enzyme, providing evidence that retained (not secreted) enzyme had been directed to the lysosomes. Moreover, as transfection with MPR300–sort-wt chimeras, as well as with wild-type MPR300, normalized the appearance of lysosomes in the mpr– mouse fibroblasts (Figure 7), it can be concluded that the sortilin cytoplasmic domain conveys transport between the Golgi and lysosomes.
Fig. 6. Sorting of lysosomal enzymes by MPR300–sortilin chimeras. The left panel shows the percentage of newly synthesized β-hexosaminidase that could be detected in the culture medium of untransfected mpr– cells (black circles, at expression level zero) and of mpr– cells transfected with wild-type MPR300 (black circles, full line) or with the MPR300–sort-wt chimera (circle with cross). Corresponding data obtained in cultures supplemented with mannose 6-phosphate (5 mM), which inhibits uptake of ligand from the medium, are indicated by a stippled line and arrowheads. The points represent results with individual clones exhibiting different levels of receptor expression. The inset shows an SDS–PAGE analysis (autoradiography) of samples of biolabelled β-glucuronidase immunoprecipitated from the medium (right lane) and lysate (left lane) of mpr– cells expressing MPR300–sort-wt and cultured in the presence of mannose 6-phosphate. The positions of the mature converted enzyme (69 kDa) and that of its proform (72 kDa) are indicated. The right panel shows the amount of β-hexosaminidase found in the medium of mpr–-cells transfected with MPR300–sort chimeras containing the wild-type or various mutated constructs of sortilin-cd. Arrowheads indicate values obtained in cultures containing mannose 6-phosphate.
Fig. 7. Morphology of late endosomes/lysosomes in normal mouse embryonic fibroblasts (MEF), in mpr– (i.e. MEF cells deficient in mannose 6-phosphate receptors) and in mpr– transfected with MPR300–sortilin-cd chimeras or the wild-type MPR300. Following fixation, the cells were permeabilized with 0.5% saponin. The distribution of receptors carrying the MPR300 luminal domain and of Lamp-1, a marker of late endosomes and lysosomes, was detected by immunofluorescence using conjugated secondary antibodies.
The distribution of β-hexosaminidase was also determined in mpr– cultures expressing MPR300–sort chimeras containing mutations or deletions at selected sites in sortilin-cd. Figure 6 (right panel) shows representative experiments with the different constructs. It appears that only alterations addressing the YXXΦ motif (Y14A/L17A) and the C-terminal dileucine (L51A/L52A) had any significant effect on enzyme sorting. The importance of these sites was confirmed by repetitive experiments using different clones expressing the same constructs at comparative and near optimal levels (expression level 1.0–2.0, Table II). From findings obtained in the absence and presence of mannose 6-phosphate, it can further be deduced that the dileucine almost only contributes to the direct intracellular transport, whereas residues of the YXXΦ motif promote transport by endocytosis as well as by sorting from the Golgi (Figure 6, right panel; Table II). This is in good agreement with the data on IL2R–sort internalization in CHO cells (Table I) and therefore not surprising. However, it should be noted that chimeras carrying Y14A as a single mutation showed no signs of missorting. In contrast, missorting by double mutants (Y14A/L17A) was profound even at high receptor expression levels. It follows that in terms of sorting from the synthetic pathway, L17 may be a key residue, but tyrosine and the YXXΦ motif as such are not functionally significant.
Table II. Sorting by MPR–sort chimeras in mpr– cells.
| Sortilin-tail constructsa | Level of expression | Extracellular β-hexosaminidaseb (mean % ± SD of total) |
|
|---|---|---|---|
| – M6P | + M6P | ||
| Non-transfected (n = 2) | – | 89.5 | 88.5 |
| Wt-tail (n = 9, cl = 6) | 0.98 ± 0.2 | 25.34 ± 7.32 | 36.13 ± 7.75 |
| Y14A/L17A (n = 3, cl = 2) | 1.61 ± 0.38 | 58.2 ± 3.38 | 63.0 ± 1.11 |
| L51A/L52A (n = 4, cl = 3) | 1.07 ± 0.12 | 32.6 ± 6.15 | 57.18 ± 5.72 |
aIndicating the numbers of experiments and numbers of different clones used (cl).
bIn culture medium without (–) and with (+) 5 mM mannose 6-phosphate (M6P).
The findings establish that sortilin-cd has the capacity for Golgi–endosome sorting and the evidence is that this function relies on sorting motifs other than those governing endocytosis.
Sortilin binds the cytosolic sorting protein GGA2
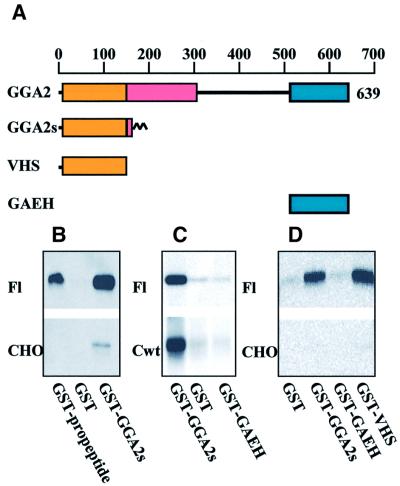
For the purpose of finding cytosolic proteins partaking in sortilin trafficking, a two-hybrid screen was set up using sortilin-cd as bait. Among the resulting confirmed positive clones, which did not include any adaptor protein (µ) subunits, one was found to be a differentially spliced version of human GGA2 cDNA (DDBJ/EMBL/GenBank accession No. AF323754). This was determined by alignment with the human GGA2 genomic sequence (DDBJ/EMBL/GenBank accession No. AC002400). The GGA2 two-hybrid clone contains an additional exon positioned between exons 6 and 7 and spanning the genomic positions 117 165–117 304. This exon encodes 29 amino acids followed by an in-frame stop codon at position 117 252. The translated product of the isolated clone (GGA2s) therefore represents a truncated form of GGA2, which contains the first 193 N-terminal residues of the published GGA2 sequence, including the entire VHS domain, followed by the amino acid sequence LFLSASEPGPIHFPSTMNSPNRYSLDISI. In control experiments, both full-length GGA2 and GGA2s induced a positive response with sortilin-cd as bait (data not shown). To elaborate on this finding, various GGA2 constructs (Figure 8A) were expressed as glutathione S-transferase (GST) fusion proteins in Escherichia coli. Full-length GGA2 could not be purified as a uniform protein, but truncated constructs covering the 183 N-terminal residues (VHS) and the 100 C-terminal residues [(containing the γ-adaptin ear homology domain (GAEH)] were obtained as uniform soluble proteins. The purified constructs were then tested in pull-down experiments (Figure 8B–D) performed on lysates of untransfected CHO cells or transfectants expressing full-length sortilin (Fl), IL2R–sort-wt chimeras (Cwt) or a soluble sortilin minireceptor without the cytoplasmic domain (data not shown). The results demonstrate that neither full-length sortilin nor the chimeric receptor was precipitated upon incubation with the C-terminal GAEH construct (Figure 8C and D). In contrast, the splice variant GGA2s construct mediated pull-down, in intra- (data not shown) as well as extracellular buffer, of both sortilin and IL2R–sort-wt (e.g. Figure 8C), and separate experiments, using biolabelled CHO transfectants, demonstrated that >10% of the labelled sortilin pool was precipitated by GST–GGA2s. Control experiments performed with GST alone yielded no precipitation (e.g. Figure 8B and C) and only receptors containing the luminal part of sortilin were precipitated by sortilin (GST–) propeptide (e.g. Figure 8B, upper panel). Figure 8 further shows that pull-down in non-transfected CHO was unproductive apart from occasional weak bands (best seen in Figure 8B, lower panel) reflecting a minor pool of endogenous receptor. Finally, a GST fusion protein comprising the N-terminal VHS domain of GGA2, but not the extra 29 residues found in GGA2s (encoded by the differentially spliced clone), was found to be just as effective for sortilin pull-down as GGA2s (Figure 8C).
Fig. 8. Pull-down of full-length sortilin and IL2R–sort-wt chimeric receptors by GGA2s and GGA2 domain constructs. Untransfected CHO cells and CHO cells transfected with full-length sortilin (Fl) or the IL2R–sort-wt chimera (Cwt) were lysed at 4°C in 1% Triton X-100 incubation buffer supplemented with proteinase inhibitors. After removal of debris, 100 µl of lysate were supplemented with 900 µl of incubation buffer (150 mM NaCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES pH 7.4) and 10 µg of the indicated GST fusion proteins, and incubated overnight at 4°C. Following addition of 50 µl of glutathione–Sepharose and a 4 h incubation (4°C), the beads were washed and precipitated protein was analysed by western blotting using anti-sortilin or anti-Tac (lower centre panel).
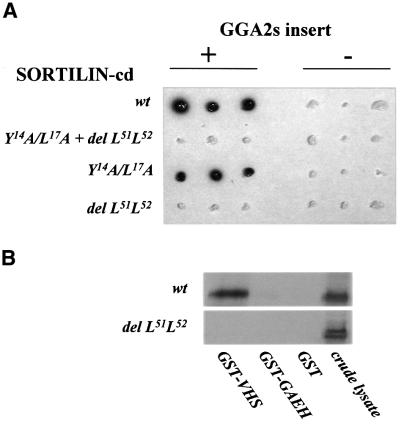
The combined results give strong evidence of a direct interaction between sortilin-cd and the VHS domain of GGA2. To determine whether GGA2 might be involved in trafficking conditioned by the YXXΦ or the dileucine motif, sortilin tail constructs (wild type, Y14A/L17A, del L51L52 or Y14A/L17A + del L51L52) were finally tested for interaction with GGA2s in a yeast two-hybrid screen. The results (Figure 9A) demonstrate that constructs not containing the C-terminal dileucine failed to induce a response in yeast cells (blue staining), suggesting that disruption of this motif abolished proper binding of GGA2. In accordance, GST–VHS mediated precipitation of a considerable fraction of the IL2R–sort-wt contained in CHO lysates but produced little or no pull-down of the IL2R–sort-del L51L52 (Figure 9B).
Fig. 9. Two-hybrid and pull-down analysis of the interaction between constructs of the sortilin-cd and GGA2. (A) cDNA encoding the sortilin-cd (wild type or the indicated mutants) was inserted into the pLexA vector and tested against the pBAD42 plasmid with or without the GGA2s insert. Stained colonies, indicating interaction between the expressed proteins, were detected on gal/raf induction plates containing X-gal. (B) CHO cells transfected with IL2R–sort chimeric receptors carrying the sortilin-cd with (del L51L52) or without (wt) a deletion of the C-terminal dileucine were lysed and receptor pull-down was performed as described (legend Figure 8) using the indicated GST fusion proteins and glutathione–Sepharose. Precipitates were analysed by western blotting and anti-Tac. The far right lane demonstrates the presence of receptors in samples of crude cell lysate (10 µl) prior to incubation with the GST fusion proteins.
Discussion
Sortilin was the first member of the new and growing family of Vps10p-domain receptors to be identified. The physiological significance of these receptors is still unknown, but current information based on sortilin and sorLA suggests that they are multifunctional (Jacobsen et al., 1996; Yamazaki et al., 1996; Hampe et al., 1999; Nielsen et al., 1999; Petersen et al., 1999).
Sortilin binds a number of unrelated ligands known to participate in a diverse range of cellular processes, including lipid metabolism and intracellular signalling. It is remarkable that all of the ligands identified so far, which include LpL, NT (Mazella et al., 1998; Nielsen et al., 1999) and at least two more proteins (M.S.Nielsen and C.M.Petersen, unpublished observations), are known to target other established receptors for endocytosis and signalling, e.g. LpL binds members of the LDLR family and NT binds NTR-1 and -2 (Vincent, 1995; Gliemann, 1998). Sortilin lacks the typical features of a signalling receptor, and although it promotes uptake and internalization of exogenous ligands like LpL, the presence of ubiquitous competing endocytic receptors, such as members of the LDL receptor family, indicates that sortilin may have other more important functions not covered by the alternative receptors. A bulk of circumstantial evidence, including structural features and cellular localization, indicates that such a function could be sorting and transporting inside the cell (Petersen et al., 1997; Morris et al., 1998).
The present study provides the first direct evidence suggesting that sortilin functions as a sorting receptor and demonstrates that in addition to endocytosis the sortilin cytoplasmic domain conveys Golgi localization and Golgi–endosome transport.
Motifs for sortilin internalization
We have previously shown that LpL bound to full-length sortilin on transfected cells is subject to internalization and degradation (Nielsen et al., 1999). In good agreement, the present findings demonstrate that the sortilin cytoplasmic domain mediates rapid endocytosis of chimeric receptors (IL2R–sort) in complex with anti-Tac antibodies. The comparative analysis of chimeras containing various tail constructs further establishes that four residues, constituting two separate signals, condition endocytosis. The single most important residue is clearly Y14, but as the nearby L17 also contributes, the tyrosine should probably be seen in the context of Y14SVL17 and not of the six-amino-acid peptide F9–Y14. Y14SVL17 conforms to the consensus motif YXXΦ (where X is any amino acid and Φ is a bulky hydrophobic residue), which is known to signal rapid endocytosis through coated pits of several other transmembrane proteins like TGN38 and the MPR300 (Canfield et al., 1991; Bos et al., 1993; Humphrey et al., 1993). The two remaining residues (L51L52) affecting internalization, although to a minor degree, form a dileucine motif situated in the C-terminus of the sortilin tail. It can be concluded that the endocytic capacity of sortilin depends on two signals conforming to classical motifs known to facilitate binding to µ and β subunits of AP complexes and to destine several other receptors for internalization (for review see Ktistakis and Roth, 1996; Hirst and Robinson, 1998; Le Borgne and Hoflack, 1998; Bonifacino and Dell’Angelica, 1999; Kirchhausen, 1999).
Following internalization in coated pits, chimeric receptors (and full-length sortilin) travel to the TGN seemingly by a route bypassing Lamp-1-positive (late) endosomes. Internalized receptors, at least when complexed with antibodies, show little or no recycling. Interestingly, the proprotein convertase furin undergoes a similar Golgi-directed endocytosis, which involves a recently described cytosolic protein called PACS-1 and an acidic cluster incorporating a casein kinase II phosphorylation site in the furin cytoplasmic domain. It appears that PACS-1 mediates translocation of dephosphorylated-cluster proteins from one (surface associated) recycling compartment to another (Golgi associated), whereas phosphorylated proteins are returned to their starting point, i.e. the surface membrane or TGN (for review see Molloy et al., 1999). Since the sortilin tail binds PACS-1 in vitro (G.Thomas, personal communication) and contains an acidic cluster (H44DDSDED50), which is a substrate for regulated in vivo phosphorylation (Figure 5), it could be speculated that PACS-1 is similarly involved in the routing of sortilin. However, although confocal analysis of chimeras mimicking phosphorylation, i.e. S47D, did leave the impression of an accentuated TGN localization, the findings were not conclusive, and neither inhibition of cellular phosphatase activity nor point mutations and deletions in the acidic cluster allowed us to verify any increase in receptor recycling. Thus, further evidence is needed to establish whether PACS-1 partakes in sortilin trafficking.
The sortilin tail conveys Golgi–endosome transport and binds GGA2
Our data on the transport of β-hexosaminidase in cultures of mpr– cells demonstrate that the MPR300–sort-wt chimera is almost as efficient as MPR300 in correcting the secretion of newly synthesized β-hexosaminidase and β-glucuronidase. Furthermore, transfection of mpr– cells with the chimeric receptor normalizes lysosome morphology and promotes the conversion of the immature precursor enzymes (released by untransfected cells) to their mature form—even when re-uptake from the medium is prevented (by addition of mannose 6-phosphate; Figure 6). Thus, unlike the cytoplasmic domains of other transmembrane proteins residing in the TGN, e.g. TGN38 and furin (D.Kasper and R.Pohlmann, unpublished results), the sortilin-cd can substitute for the MPR300-cd and provide direct transport to lysosomes for ligands bound in the synthetic pathway as well as on the plasma membrane. This is in good agreement with previous evidence on sortilin and MPR300 co-localization, and in strong support of full-length sortilin as a functional sorting receptor (Petersen et al., 1997; Morris et al., 1998).
These findings show that the sortilin-cd contains both N- and C-terminal residues that are functional determinants in Golgi–endosome trafficking. Neither the acidic cluster (H44–D50) nor S41GY43 (a signal in Lamp-1 and -2) appears to constitute active signals in sortilin. In contrast, disruption af the L51L52 dileucine significantly affected the distribution of β-hexosaminidase in transfected mpr– cultures, and since L51A/L52A had little impact on the cellular uptake, in agreement with findings in CHO cells, the marked accumulation of lysosomal β-hexosaminidase in the medium must relate to a failure in direct Golgi–endosome transport. As expected, profound missorting also resulted from alterations in the YXXΦ motif, but, surprisingly, only L17A and not Y14A had a clear impact on intracellular transport. The latter finding indicates that in terms of transport originating in the TGN, the functional sorting signal may be the dileucine-like V16L17 rather than the YXXΦ motif. In any case, both types of motif mediate binding to AP complexes involved in budding of TGN transport vesicles (AP-1 and -3) as well as endocytosis (AP-2) (reviewed in Bonifacino and Dell’Angelica, 1999; Kirchhausen, 1999).
However, as demonstrated by the two-hybrid screen and pull-down experiments involving sortilin and GGA2, alternative transporter molecules may target the sortilin tail. GGA2 belongs to a recently described family of monomeric cytosolic proteins that includes three mammalian and two (gga) yeast members (Boman et al., 2000; Dell’Angelica et al., 2000; Hirst et al., 2000; Poussu et al., 2000; Takatsu et al., 2000). GGAs are ARF dependent and Golgi localizing, and comprise, from the N-terminus, a VHS domain, a so-called GAT domain, a linker region and a C-terminal GAEH domain (Dell’Angelica et al., 2000). Information concerning their physiological function is still limited, but important findings in yeast convincingly implicate the family in Golgi–endosome/lysosome transport. Thus, Vps10p-mediated sorting of CPY is impaired in gga null cells (Hirst et al., 2000) and the incorporation of the syntaxin Pep12p in late endosomes is likewise dependent on gga proteins (Black and Pelham, 2000). Since sorting of CPY is clathrin independent, i.e. restores in the absence of functional clathrin heavy chains (Seeger and Payne, 1992), whereas that of Pep12p is not, it is conceivable that the GGAs are involved in more than one type or aspect of protein transport (Payne et al., 1988; Black and Pelham, 2000). The routing of sortilin may rely on interaction with several cytosolic partners, but in view of the similarity between sortilin and the CPY sorting receptor Vps10p and the present evidence of GGA2 binding to sortilin, it is an attractive possibility that the GGAs are likewise involved in the trafficking of mammalian sortilin. In fact, the finding that chimeric receptors lacking the C-terminal dileucine fail to interact with GGA2 provides strong evidence of a direct link between GGA binding and sortilin sorting.
In conclusion, we have demonstrated that the sortilin cytoplasmic domain conveys endocytosis and TGN localization as well as transport from the synthetic pathway to lysosomes, and we have identified specific residues in the C-terminal sequence that condition this traffic. Our results, for the first time, demonstrate a direct binding between a luminal receptor and a member of the GGA family of cytosolic proteins, and suggest that GGA2 is a participant in sortilin sorting. Finally, the finding that GGA2 binds to the sortilin-cd via its VHS domain provides new information concerning VHS domains in general and on the functional organization of GGAs in particular.
Materials and methods
DNA constructs
Plasmids containing the coding sequences for the sortilin propeptide, the luminal domain of sortilin (s-sortilin), full-length sortilin, and for the luminal and transmembrane part of the MPR300 have been described elsewhere (Petersen et al., 1999). For the construction of chimeric receptors, cDNA encoding the wild-type cytoplasmic domain of sortilin was amplified by standard PCR technique using primers generating a HindIII site at the 5′-end and a XhoI site at the 3′-end. Modified tail constructs containing deletions and/or amino acid substitutions at selected sites were made using appropriate oligonucleotides. Each of the resulting products was inserted into a pCMV-IL2R/CD25/Tac vector (LaFlamme et al., 1994) to form the IL2R–sort constructs encoding receptors containing the luminal and transmembrane segments of IL2Rα and the cytoplasmic tail, wild type or mutant, of sortilin. All IL2R–sort constructs were finally cut free via a NheI and a XhoI site, and transferred to pcDNA3.1/Zeo(–) (Invitrogen, Groningen, The Netherlands). A similar procedure was used to generate chimeric receptor constructs containing the MPR300 luminal and transmembrane parts in combination with the sortilin tails. The latter constructs were subcloned into an internal NdeI site and a recombinant MluI site of the pMPSVHE(-MPR300) vector as described in Sandholzer et al. (2000). For prokaryote expression, sortilin tail constructs were amplified and transferred to the pGEX4T-1-plasmid (Amersham Pharmacia, Buckinghamshire, UK) using primer-generated cut sites (5′-end BamHI and 3′-end EcoRI). The correct composition of the above constructs was ensured by sequencing in a Licore 4200 or the ABI 373A system (MWG, Germany).
Cell lines and protein expression
CHO-K1 cells were cultured in serum-free HyQ-CCM5 CHO medium (HyClone, Logan, UT) and the previously described immortalized mouse embryonic fibroblasts deficient in both mannose 6-phosphate receptors (Kasper et al., 1996) were grown in 10% fetal calf serim (FCS) Dulbecco’s modified Eagle’s medium (Bio-Whittaker, Verviers, Belgium). All culture medium was supplemented with antibiotics and Glutamax-I (Gibco-BRL, UK). The cells were transfected with pcDNA3.1/Zeo(–) (CHO) or pMPSVHE (mouse fibroblasts) constructs by use of DC-Chol or calcium phosphate. Stably transfected clones were selected in medium containing 500 µg/ml zeocin and 500 µg/ml hygromycin (Gibco-BRL), respectively. Recombinant H6FXRAP was expressed and purified as previously described (Petersen et al., 1997). The GST fusion constructs encoding the sortilin cytoplasmic domain, wild type or mutant, were expressed in E.coli BL21 DE3 and purified on a glutathione–Sepharose 4B column (Amersham Pharmacia).
Metabolic labelling and receptor expression levels
To determine the relative amount of receptors presented on the surface cell membrane CHO transfectants were biolabelled overnight using ∼200 μCi/ml [35S]l-cysteine and [35S]l-methionine (Pro-mix; Amersham Pharmacia) in cysteine- and methionine-free medium supplemented with 5% CHO medium (modified Eagle’s medium; Sigma, St Louis, MO). The cells were subsequently washed and incubated for 3 h in CHO medium (37°C) followed by incubation for 3 h at 4°C with anti-sortilin or anti-CD25 antibodies (Roche Diagnostics GmbH, Germany). After removal of unbound antibody by washing (4°C), the cells were lysed in Triton X-100 and surface membrane receptor–antibody complexes were precipitated by GammaBind G–Sepharose beads (Amersham Pharmacia). The remaining receptors, representing the intracellular pool, were finally precipitated from the supernatant by beads coated with anti-receptor antibodies. The precipitated samples were subjected to SDS–PAGE and the relative distribution of receptors between the surface membrane and the intracellular compartment was determined using a FLA-3000 (Fuji, Japan) by comparing the activity of specific bands obtained from the first and second precipitation in each lysate. Additional experiments, carried out to distinguish between the two receptor pools in unlabelled cells, were performed using surface biotinylation as previously described (Nielsen et al., 1999).
The expression level of chimeric receptors in transfected mannose 6-phosphate receptor-deficient mouse fibroblasts (mpr–) was determined as described elsewhere (Chao et al., 1990; Kasper et al., 1996). In brief, mpr– cells transfected with MPR300–sort-cd chimeras and cultured in 35 mm dishes were permeabilized and incubated with ∼4 × 105 c.p.m. of a 125I-labelled monoclonal antibody (2C2) recognizing a luminal epitope of MPR300. Following removal of unbound antibody, cell-bound radioactivity was determined and the corresponding amount of total cellular protein was determined by the Lowry method.
Assays for receptor internalization and sorting
Transfected cells were incubated in CHO medium at 4°C in four-well plates with 125I-labelled antibody (30 × 103 c.p.m./ml) directed against the luminal domain of IL2R–sort chimeras (anti-CD25/Tac; Roche) or sortilin. Antibodies were iodinated using chloramine T or iodobeads. After 2 h, unbound tracer was removed by washing and the cells were re-incubated at 37°C in fresh medium for 0 or up to 120 min. At given times, incubation was stopped and the cells were treated on ice in a Tris–HCl buffer pH 2.5. After 5 min, the supernatant was recovered and the cells were lysed in 1 M NaOH. The radioactivity contained in both fractions was finally counted and defined as surface-associated and internalized ligand, respectively.
Lysosomal β-hexosaminidase activity was detected using fluorometric assays as described (Köster et al., 1994).
Immunocytochemistry
Fluorescence microscopy was performed using a laser scanning confocal unit (LSM510; Carl Zeiss, Germany) attached to an Axiovert microscope (Carl Zeiss) with a 63 × 1.2 NA plan C-Apochromat objective. For analysis, CHO cells were washed in 10 mM phosphate, 150 mM NaCl pH 7.3, fixed for 10 min in the same buffer containing 3.7% formaldehyde, and finally washed and permeabilized in buffer containing 0.5% Triton X-100. To visualize internalization, the cells were labelled with primary antibodies at 4°C prior to incubation in warm medium (0–120 min) and fixation. Alexa 488-conjugated goat anti-mouse antibodies were from Molecular Probes (Eugene, OR), anti-TGN38 was kindly provided by M.A.McNiven and anti-Lamp-1 by S.Carlsson. To describe the lysosomal morphology in mouse embryonic fibroblasts and mpr– cells, the cells were permeabilized using 0.5% saponin and stained using anti-mouse Lamp-1 hybridoma supernatant 1D4B (Hybridoma Bank, University of Iowa).
For electron microscopy, CHO cells were fixed with 2% formaldehyde in 0.1 M sodium cacodylate buffer pH 7.4 for 1 h and subsequently post-fixed for up to 18 h in 2% formaldehyde in the same buffer. The cells were removed from the support with a rubber policeman in the same buffer, containing 1% gelatine (Merck), pelleted and embedded in 12% gelatine, and finally infiltrated with 2.3 M sucrose in phosphate-buffered saline for 30 min, and frozen in liquid nitrogen. Ultrathin cryosections (70–90 nm) were obtained with a FCS Reichert Ultracut S cryoultramicrotome at about –100°C and collected on 200 mesh Ni grids. The sections were incubated overnight with a polyclonal affinity-purified rabbit anti-TGN38 (3 mg/ml; 1:100) or a rabbit anti-LAMP-1 serum (1:100–500) prior to 2 h of incubation with goat anti-rabbit–gold (10–15 nm) and goat anti-mouse–gold (5 nm) (BioCell, Cardiff, UK). All incubations were performed at 4°C. The sections were finally contrasted with methyl cellulose containing 0.3% uranylacetate and studied in a Philips CM100 electron microscope.
Phosphorylation assays
CHO cells were cultured in monolayers as described above. At ∼80% confluency (3–4 × 106 cells per well), the cells were washed three times and incubated for 3 h in Dulbecco’s pyrophosphate-free MEM medium (Sigma) supplemented with carrier-free 32P-labelled o-phosphate (0.5 mCi/ml) prior to an additional hour of incubation in the presence or absence of 80 nM calyculin (Alexis Biochemicals, San Diego, CA). The cells were then washed three times at 4°C in a 20 mM Tris–HCl buffer (140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, 5.5 mM glucose pH 7.35) containing phosphatase inhibitors (10 mM EDTA, 5 mM EGTA, 10 mM NaF and 1 mM Na3VO4). Finally, the cells were lysed in 1% Triton X-100 containing phosphatase inhibitors and sortilin was immunoprecipitated using polyclonal rabbit anti-sortilin Ig and GammaBind G beads. In vitro phosphorylation of recombinant sortilin-tail fusion proteins (∼2 μM) was performed in MOPS buffer (20 mM MOPS, 130 mM KCl, 10 mM MgCl2, 5 mM dithiothreitol pH 7.0), in the absence or presence of 0.5 nM casein kinase II (Roche). Reactions were started by addition of 20 µM [γ-32P]ATP (activity ∼6 μCi/ml) and stopped after 45 min in SDS sample buffer (5% SDS, 20 mM Tris, 18% glycerol, pyronin). Western blotting was performed as previously described (Petersen et al., 1996).
Yeast two-hybrid screen, GGA2 constructs and co-precipitation experiments
Transformation of the Saccharomyces cerevisiae strain EGY48 and library screening were performed using a Matchmaker LexA Two-hybrid system (Clontech, Palo Alto, CA) in accordance with the manufacturer’s recommendations. In brief, a PCR fragment encoding the sortilin tail (748–800) was inserted into the pLexA bait vector and transformed into the EGY48 strain previously transformed with the reporter plasmid p8op-lacZ. The bait strain [EGY48(p8op-lacZ-pLexASort)] was next transformed with a human LexA mammary cDNA library and amplified on plates selecting for bait, library and reporter plasmids. The selected cells were then plated on X-gal/induction plates to identify two-hybrid interactions. Blue colonies were subcloned three times to eliminate non-interacting plasmids. Library plasmids (pBAD42) from positive colonies were rescued in E.coli KC8. False positives were identified and eliminated by co-transforming EGY48[p8op-lacZ] cells with the rescued library plasmids as well as bait plasmids without insert or with a non-interacting insert.
Three GST fusion proteins were constructed. The EcoRI fragment containing the GGA2s coding region was released from the pBAD42 vector and ligated into the pGEX4T-1 vector (Pharmacia). The cDNA encoding GGA2s has a deletion (224 bp), involving exons 11 and 12, which eliminates an EcoRI site present in full-length GGA2 (the data have been submitted to the GenBank database under accession No. AF323754). A truncated form of GGA2s, without the C-terminal 39 amino acids, was generated by digesting the pGEX-GGA2s construct with SacI (position 572) and XhoI, followed by treatment with T4 polymerase and self-ligation. Both fusion proteins (GGA2s and its truncated version) contain eight residues encoded by the 5′ UTR of the GGA2 cDNA. A fragment encoding the GAEH of GGA2 was amplified by PCR using the KIAA1080 clone (DDBJ/EMBL/GenBank accession No. AB029003) (Kikuno et al., 1999), the amplified fragment was digested with BamHI–XhoI and ligated into pGEX4T-1. The constructs were soluble and prepared by batch purification in the presence of proteinase inhibitors. A pBAD42 two-hybrid construct encoding the GGA2 protein was generated by exchanging a BspEI–XhoI fragment from the KIAA1080 clone into the pBAD42-GGA2s plasmid.
For pull-down experiments, CHO cells (80% confluent), wild type or transfected with full-length sortilin or IL2R–sort-tail chimeric constructs, were lysed on ice in a 1% Triton X-100 buffer containing either 150 mM KCl, 2 mM MgCl2, 0.1 mM EGTA, 1 mM DTE, 10 mM HEPES pH 7.4 (intracellular buffer) or 150 mM NaCl, 2 mM MgCl2, 2 mM CaCl2, 10 mM HEPES pH 7.4 (extracellular buffer) and supplemented with proteinase inhibitors (CompleteMini, Roche). Debris was removed by pelleting and 100 µl of lysate supernatant were supplemented with 900 µl of buffer (with proteinase inhibitors) prior to addition of 10 µg of GST fusion protein (GST–propeptide, –GGA2s, –VHS and –GAEH) or GST alone. Following overnight incubation (4°C), the samples were supplemented with 50 µl of glutathione–Sepharose (Amersham Pharmacia) and incubated for an additional 4 h at 4°C. The glutathione–Sepharose was pelleted and the beads were washed (3 × 5 min) at 4°C in buffer A and buffer B containing 0.1% Triton X-100 before resuspension in sample buffer and SDS–PAGE. For quantitation, proteins precipitated from biolabelled cells were dissociated from the Sepharose beads by addition of surplus glutathione (Sigma) and sortilin was subsequently immunoprecipitated. The amounts of sortilin found in lysates prior to precipitation and the quantity isolated by affinity beads were finally compared by SDS–PAGE and autoradiography.
Acknowledgments
Acknowledgements
Nina Jørgensen, Else Walbūm and Mitra Shamsali are thanked for technical assistance. The study was supported by The Danish Medical Research foundation, The Novo Nordic Foundation, Aarhus University Research Foundation and The Plasmid Foundation.
References
- Barbero P., Rovere,C., De Bie,I., Seidah,N., Beaudet,A. and Kitabgi,P. (1998) PC5-A mediated processing of pro-neurotensin in early compartments of the regulated secretory pathway of PC5-transfected PC12 cells. J. Biol. Chem., 273, 25339–25346. [DOI] [PubMed] [Google Scholar]
- Black M.W. and Pelham,H.R.B. (2000) A selective transport route from Golgi to late endosomes that requires yeast GGA proteins. J. Cell Biol., 151, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman A.L., Zhang,C.-j., Zhu,X. and Kahn,R.A. (2000) A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol. Biol. Cell, 11, 1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J.S. and Dell’Angelica,E.C. (1999) Molecular basis for the recognition of tyrosine-based sorting signals. J. Cell Biol., 145, 923–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos K., Wraight,C. and Stanley,K.K. (1993) TGN38 is maintained in the trans-Golgi network by a tyrosine-containing motif in the cytoplasmic domain. EMBO J., 12, 2219–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J.E. and Severson,D.L. (1992) Regulation of the synthesis, processing and translocation of lipoprotein lipase. Biochem. J., 287, 337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G. and Schwartz,A.L. (1998) RAP, a novel type of ER chaperone. Trends Cell Biol., 8, 272–276. [DOI] [PubMed] [Google Scholar]
- Canfield W.M., Johnson,K.F., Ye,R.D., Gregory,W. and Kornfeld,S. (1991) Localization of the signal for rapid internalization of the bovine cation-independent mannose 6-phosphate/insulin-like growth factor-II receptor to amino acids 24–29 of the cytoplasmic tail. J. Biol. Chem., 266, 5682–5688. [PubMed] [Google Scholar]
- Chao H.H.-J., Waheed,A., Pohlmann,R., Hille,A. and von Figura,K. (1990) Mannose 6-phosphate receptor dependent secretion of lysosomal enzymes. EMBO J., 9, 3507–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. and Howell,B.W. (1999) Lipoprotein receptors: signaling functions in the brain? Cell, 97, 671–674. [DOI] [PubMed] [Google Scholar]
- Dell’Angelica E.C., Puertollano,R., Mullins,C., Aguilar,R.C., Vargas, J.D., Hartnell,L.M. and Bonifacino,J.S. (2000) GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol., 149, 81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliemann J. (1998) Receptors of the low density lipoprotein (LDL) receptor family in man. Multiple functions of the large members via interaction with complex ligands. Biol. Chem., 379, 951–964. [PubMed] [Google Scholar]
- Hampe W., Urny,J., Franke,I., Hoffmeister-Ullerich,S.A.H., Herrmann, D., Petersen,C.M., Lohmann,J. and Schaller,H.C. (1999) A head-activator binding protein is present in hydra in a soluble and a membrane-anchored form. Development, 126, 4077–4086. [DOI] [PubMed] [Google Scholar]
- Hermans-Borgmeyer I., Hermey,G., Nykjær,A. and Schaller,H.C. (1999) Expression of the 100 kDa neurotensin receptor sortilin during mouse embryonal development. Mol. Brain Res., 65, 216–219. [DOI] [PubMed] [Google Scholar]
- Hermey G., Riedel,I.B., Hampe,W., Schaller,H.C. and Hermans-Borgmeyer,I. (1999) Identification and characterization of SorCS, a third member of a novel receptor family. Biochem. Biophys. Res. Commun., 266, 347–351. [DOI] [PubMed] [Google Scholar]
- Hirst J. and Robinson,M.S. (1998) Clathrin and adaptors. Biochim. Biophys. Acta, 1404, 173–193. [DOI] [PubMed] [Google Scholar]
- Hirst J., Lui,W.W.Y., Bright,N.A., Totty,N., Seaman,M.N.J. and Robinson,M.S. (2000) A family of proteins with γ-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol., 149, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey J.S., Peters,P.J., Yuan,L.C. and Bonifacino,J.S. (1993) Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J. Cell Biol., 120, 1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen L., Madsen,P., Moestrup,S.K., Lund,A.H., Tommerup,N., Nykjær,A., Sottrup-Jensen,L., Gliemann,J. and Petersen,C.M. (1996) Molecular characterization of a novel hybrid receptor that binds the α2-macroglobulin receptor-associated protein. J. Biol. Chem., 271, 31379–31383. [DOI] [PubMed] [Google Scholar]
- Johnson K.F. and Kornfeld,S. (1992a) A His-Leu-Leu sequence near the carboxyl terminus of the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor is necessary for the lysosomal enzyme sorting function J. Biol. Chem., 267, 17110–17115. [PubMed] [Google Scholar]
- Johnson K.F. and Kornfeld,S. (1992b) The cytoplasmic tail of the mannose 6-phosphate/insulin-like factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J. Cell Biol., 119, 249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper D., Dittmer,F., von Figura,K. and Pohlmann,R. (1996) Neither type of mannose 6-phosphate receptor is sufficient for targeting of lysosomal enzymes along intracellular routes. J. Cell Biol., 134, 615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuno R., Nagase,T., Ishikawa,K., Hirosawa,M., Miyajima,N., Tanaka,A., Kotani,H., Nomura,N. and Ohara,O. (1999) Prediction of the coding sequences of unidentified human genes. XIV. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res., 6, 197–205. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T. (1999) Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol., 15, 705–732. [DOI] [PubMed] [Google Scholar]
- Köster A., von Figura,K. and Pohlmann,R. (1994) Mistargeting of lysosomal enzymes in M(r) 46000 mannose 6-phosphate receptor-deficient mice is compensated by carbohydrate specific endocytotic receptors. Eur. J. Biochem., 224, 685–689. [DOI] [PubMed] [Google Scholar]
- Ktistakis N.T. and Roth,M.G. (1996) Protein sorting during endocytosis. In Hurtley,S.M. (ed.), Protein Targeting. Oxford University Press, Oxford, UK, pp. 177–212. [Google Scholar]
- LaFlamme S.E., Thomas,L.A., Yamada,S.S. and Yamada,K.M. (1994) Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J. Cell Biol., 126, 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R. and Hoflack,B. (1998) Mechanisms of protein sorting and coat assembly: insights from the clathrin-coated vesicle pathway. Curr. Opin. Cell Biol., 10, 499–503. [DOI] [PubMed] [Google Scholar]
- Marcusson E.G., Horazdovsky,B.F., Cereghino,J.L., Gharakhanian,E. and Emr,S.D. (1994) The sorting receptor for yeast vacuolar carboxypeptidase Y is encoded by the VPS10 gene. Cell, 77, 579–586. [DOI] [PubMed] [Google Scholar]
- Mazella J. et al. (1998) The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J. Biol. Chem., 273, 26273–26276. [DOI] [PubMed] [Google Scholar]
- Molloy S.S., Anderson,E.D., Jean,F. and Thomas,G. (1999) Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol., 9, 28–35. [DOI] [PubMed] [Google Scholar]
- Morris N.J., Ross,S.A., Lane,W.S., Moestrup,S.K., Petersen,C.M., Keller,S.R. and Lienhard,G.E. (1998) Sortilin is the major 110-kDa protein in Glut4 vesicles from adipocytes. J. Biol. Chem., 273, 3582–3587. [DOI] [PubMed] [Google Scholar]
- Nagase T., Kikuno,R., Ishikawa,K.I., Hirosawa,M. and Ohara,O. (2000) Prediction of the coding sequences of unidentified human genes. XVI. The complete sequences of 150 new cDNA clones from brain which code for large proteins in vitro. DNA Res., 7, 65–73. [DOI] [PubMed] [Google Scholar]
- Nielsen M.S., Jacobsen,C., Olivecrona,G., Gliemann,J. and Petersen, C.M. (1999) Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J. Biol. Chem., 274, 8832–8836. [DOI] [PubMed] [Google Scholar]
- Payne G.S., Baker,D., van Tuinen,E. and Schekman,R. (1988) Protein transport to the vacuole and receptor-mediated endocytosis in clathrin heavy chain-deficient yeast. J. Cell Biol., 106, 1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen C.M. et al. (1996) The receptor-associated protein (RAP) binds calmodulin and is phosphorylated by calmodulin-dependent kinase II. EMBO J., 15, 4165–4173. [PMC free article] [PubMed] [Google Scholar]
- Petersen C.M. et al. (1997) Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J. Biol. Chem., 272, 3599–3605. [DOI] [PubMed] [Google Scholar]
- Petersen C.M., Nielsen,M.S., Jacobsen,C., Tauris,J., Jacobsen,L., Gliemann,J., Moestrup,S.K. and Madsen,P. (1999) Propeptide cleavage conditions sortilin/neurotensin receptor-3 for ligand binding. EMBO J., 18, 595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlmann R., Wendland,M., Böker,C. and von Figura,K. (1995) The two mannose 6-phosphate receptors transport distinct complements of lysosomal enzymes. J. Biol. Chem., 270, 27311–27318. [DOI] [PubMed] [Google Scholar]
- Poussu A., Lohi,O. and Lehto,V.-P. (2000) Vear, a novel Golgi-associated protein with VHS and γ-adaptin ‘ear’ domains. J. Biol. Chem., 275, 7176–7183. [DOI] [PubMed] [Google Scholar]
- Sandholzer U., von Figura,K. and Pohlmann,R. (2000) Function and properties of chimeric MPR46–MPR300 mannose 6-phosphate receptors. J. Biol. Chem., 275, 14132–14138. [DOI] [PubMed] [Google Scholar]
- Seeger M. and Payne,G.S. (1992) A role for clathrin in the sorting of vacuolar proteins in the Golgi complex of yeast. EMBO J., 11, 2811–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takatsu H., Yoshino,K. and Nakayama,K. (2000) Adaptor γ ear homology domain conserved in γ-adaptin and GGA proteins that interact with γ-synergin. Biochem. Biophys. Res. Commun., 271, 719–725. [DOI] [PubMed] [Google Scholar]
- Vincent J.-P. (1995) Neurotensin receptors: binding properties, transduction pathways, and structure. Cell. Mol. Neurobiol., 15, 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow T.E. (1998) Receptor-associated protein (RAP): a specialized chaperone for endocytic receptors. Biol. Chem., 379, 1025–1031. [PubMed] [Google Scholar]
- Yamazaki H., Bujo,H., Kusunoki,J., Seimiya,K., Kanaki,T., Morisaki,N., Schneider,W.J. and Saito,Y. (1996) Elements of neural adhesion molecules and yeast vacuolar protein sorting receptor are present in a novel mammalian low density lipoprotein receptor family member. J. Biol. Chem., 271, 24761–24768. [DOI] [PubMed] [Google Scholar]