Abstract
ILKAP, a protein serine/threonine (S/T) phosphatase of the PP2C family, was isolated in a yeast two-hybrid screen baited with integrin-linked kinase, ILK1. Association of ILK1 and ILKAP was independent of the catalytic activity of either partner, as assayed in co-precipitation and two-hybrid experiments. Condi tional expression of ILKAP in HEK 293 cells resulted in selective inhibition of ECM- and growth factor-stimulated ILK1 activity, but did not inhibit Raf-1 kinase activity. A catalytic mutant of ILKAP, H154D, did not inhibit ILK1 kinase activity. Two cellular targets of ILK1, glycogen synthase kinase 3 β (GSK3β) and protein kinase B (PKB)/AKT, were differentially affected by ILKAP-mediated inhibition of ILK1. Catalytically active, but not mutant ILKAP, strongly inhibited insulin-like growth factor-1-stimulated GSK3β phosphorylation on Ser9, but did not affect phosphorylation of PKB on Ser473, suggesting that ILKAP selectively affects ILK-mediated GSK3β signalling. Consistent with this, active, but not H154D mutant or the related PP2Cα, selectively inhibited transactivation of a Tcf/Lef reporter gene, TOPFlash, in 293 cells. We propose that ILKAP regulates ILK1 activity, targeting ILK1 signalling of Wnt pathway components via modulation of GSK3β phosphorylation.
Keywords: β-catenin/GSK3β/integrin-linked kinase/protein phosphatase 2C/signal transduction
Introduction
Integrin-mediated cell adhesion to extracellular matrices (ECM) stimulates a number of protein kinase activities, such as phosphoinositide-3-OH kinase (PI3K), src family and FAK tyrosine kinases, as well as protein serine/threonine (S/T) kinases such as ILK1 and protein kinase B (PKB) (Dedhar and Hannigan, 1996; Hanks and Polte, 1997; Hannigan and Dedhar, 1997; Banfic et al., 1998; Dedhar, 1999). Integrin-linked kinase, ILK1, functions to mediate integrin signal transduction, and was originally identified in a yeast two-hybrid screen for protein partners of the β1 integrin subunit cytoplasmic domain (Hannigan et al., 1996). ECM and growth factors each stimulate rapid, transient activation of ILK1 activity and signalling. Failure to down-regulate ILK1-mediated signalling can lead to oncogenic transformation, since constitutive overexpression of p59ILK1 induces anchorage-independent growth of epithelial cells, reflecting induction of cyclin D1/cdk4 expression and activation (Hannigan et al., 1996; Radeva et al., 1997). More recently, it has been demonstrated that ILK1 stimulation by ECM or growth factors is dependent on the activity of PI3K, a widely acting mediator of cell growth and survival signals. Moreover, ILK1 is activated in vitro by phosphatidylinositol (3,4,5) trisphosphate (PIP3), suggesting physiological activation of ILK1 by lipid products of PI3K activity (Delcommenne et al., 1998; Morimoto et al., 2000).
A number of studies have shown that integrin-mediated cell adhesion stimulates PI3K-dependent activation of PKB (Khwaja et al., 1997; Banfic et al., 1998). A recent report on the inverse correlation between PIP3 lipid phosphatase (PTEN) and PKB expression in a series of primary acute leukaemias and non-Hodgkin’s lymphomas provides additional support for the role of PI3K signalling in regulating tumour progression and survival via PKB (Dahia et al., 1999). Phospholipid-dependent kinase-2 (PDK2) activity effects Ser473 phosphorylation and it has been shown that ILK1 stimulates Ser473 phosphoryl ation (Delcommenne et al., 1998; Lynch et al., 1999). Conversely, phosphorylation on Thr308 of PKB is effected by a distinct phospholipid-sensitive PDK1, both these phosphorylations being required for full activation of PKB. Although ILK1 is a candidate PDK2 regulating PKB-mediated cell survival, ILK1 has also been shown to target glycogen synthase kinase 3 β (GSK3β) (Delcommenne et al., 1998; D’Amico et al., 2000; Persad et al., 2000). GSK3β is an important mediator of developmental signalling of the Wnt/wingless pathway, affecting the transcriptional activity of Tcf/Lef factors through phosphorylation of the transcriptional co-factor β-catenin (Barker et al., 2000). GSK3β phosphorylation of β-catenin targets the latter for degradation, and overexpression of ILK1 in epithelial cells induces an inhibitory phosphorylation of GSK3β at Ser9 (Delcommenne et al., 1998; D’Amico et al., 2000; Tan et al., 2001), resulting in stabilization and nuclear translocation of β-catenin, with concomitant activation of Tcf/Lef factors (Novak et al., 1998; Dedhar et al., 1999). Thus, ILK1 is emerging as an important intracellular regulator of signalling via components of the Wnt/wingless pathway, specifically through modulation of GSK3β activity.
In this paper we describe the isolation of a novel member of the protein phosphatase 2C (PP2C) family in a yeast two-hybrid screen to identify proteins that interact with p59ILK1. We have named this PP2C family member ILKAP, for ILK1-associated phosphatase. ILK1 and ILKAP were co-precipitated from lysates of HEK 293 cells, independently of ILK1 or ILKAP catalytic activities. Induced expression of recombinant, catalytically active ILKAP in HEK 293 cells resulted in the inhibition of protein kinase activity in ILK1 immune complexes. Conversely, ILKAP did not affect Raf-1 immune complex kinase activity, and a catalytically inactive ILKAP mutant did not inhibit ILK1 activity. ILKAP expression strongly inhibited integrin-stimulated phosphorylation of GSK3β at Ser9; however, we did not observe an ILKAP-mediated block to integrin-induced phosphorylation of PKB. Finally, we show that ILKAP selectively inhibited activation of Tcf/Lef factors, consistent with the observed inhibition of GSK3β Ser9 phosphorylation. These results suggest that ILKAP complexes with ILK1 to selectively inhibit the GSK3β arm of ILK1 signalling. Taken together, our data describe a new cytoplasmic protein S/T phosphatase, ILKAP, that down-regulates integrin- and growth factor-stimulated ILK1 activity, and lead us to propose that ILKAP is a physiological regulator of ILK1 signalling.
Results
Identification and characterization of ILKAP
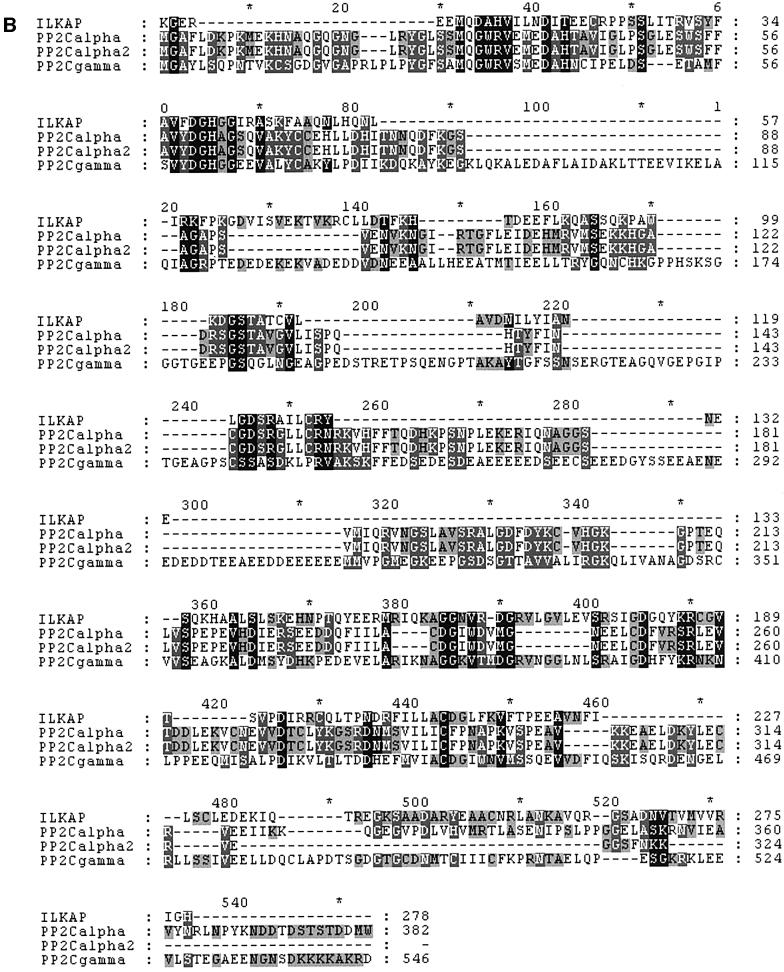
A yeast two-hybrid screen was performed, using a bait plasmid encoding a C-terminally truncated ILK1 protein comprising amino acid residues 1–276 (Hannigan et al., 1996). This construct contained the N-terminal ankyrin-like repeat domain of ILK1, the pleckstrin homology-like domain and the first few residues of the catalytic domain. One positive clone identified in a screen of HeLa cell cDNAs yielded a 700 bp cDNA (designated 5BT) possessing significant sequence similarity to S/T phosphatases of the PP2C family. Oligonucleotide primers derived from this cDNA sequence were synthesized and used to amplify clones from human placental and skeletal muscle cDNA libraries (OriGene Rapid Screen; see Materials and methods). A cDNA of 1.7 kb was isolated (Figure 1A), exhibiting a high degree of similarity to PP2C family phosphatases (Figure 1B). Northern blot analyses of human multi-tissue and muscle RNA blots indicated that this probably represented a full-length transcript (Figure 2). The 1.7 kb cDNA contains on open reading frame encoding a protein of 392 amino acid residues. The major features of this coding region are two PP2C boxes: one comprising residues 115–206 (PP2C box 1) and the second encompassing residues 215–392 (PP2C box 2). This architecture is typical of PP2C family members (Wera and Hemmings, 1995; Barford et al., 1998), and within these PP2C boxes highly conserved catalytic residues are present in the ILK1-trapped sequence. In particular, a DGH triplet within PP2C box 1 is absolutely conserved in all PP2Cs and represents the catalytic active site, as shown by mutagenesis and X-ray crystallography studies (Das et al., 1996; Sheen, 1998). Accordingly, we called the trapped PP2C, ILKAP. The deduced ILKAP amino acid sequence displays a high degree of similarity (95% identity) to rat PP2C, described by Tong et al. (1998), suggesting that ILKAP is the human homologue of rat PP2C.
Fig. 1. ILKAP structure and alignment with PP2C. (A) Deduced protein sequence of ILKAP cDNA. Sequence was translated from the H4C11-30 cDNA sequence using Gene Inspector v. 1.5 software (Textco, Inc., New Hampshire). PP2C boxes 1 and 2 are underlined, and bold letters indicate the DGH active site triplet. (B) Amino acid residues 115–392 of ILKAP were aligned using the Clustal V algorithm (Gene Inspector 1.5) and the align ment edited to highlight identities (black) and conserved (shaded) amino acid residues, using Genedoc v. 2.6.01 (www.psc.edu/biomed/genedoc/).
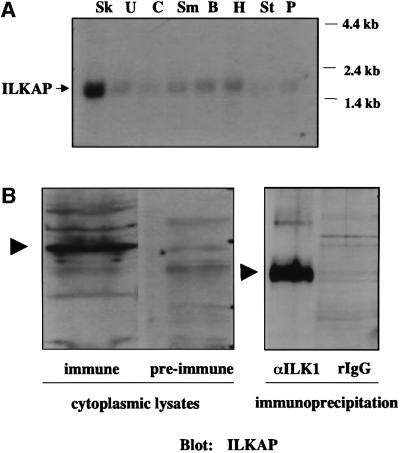
Fig. 2. ILKAP transcript is preferentially expressed in skeletal muscle. (A) A partial cDNA representing amino acid residues 269–392 of ILKAP was 32P labelled, and used to probe a northern blot of poly(A)+ RNAs from human striated and smooth muscle tissues. Sk, skeletal muscle; U, uterus; C, colon; Sm, small intestine; B, bladder; H, heart; St, stomach; P, prostate. (B) Left panel: a polyclonal antibody was raised to an ILKAP fusion protein (residues 269–392) and affinity purified over an immobilized ILKAP column. Pre-immune serum from the same rabbit was subjected to the same purification protocol, as a negative control. Cytoplasmic lysates of HEK 293 cells were analysed by western blotting using the ILKAP immune and pre-immune sera as indicated. Right panel: cytoplasmic 293 cell lysates were immunoprecipitated with rabbit IgG, or affinity-purified rabbit polyclonal ILK1 antibody, covalently coupled to CNBr-activated Sepharose beads. Unfractionated cytoplasmic lysates and ILK1 immunoprecipitates each indicated a single ILKAP band, migrating with an apparent mol. wt of 47 kDa. Immune complexes were analysed by western blotting, using affinity-purified ILKAP antibody. Arrowheads indicate p47ILKAP migration on 12% SDS–PAGE.
Northern blot analyses of human multi-tissue blots indicated that ILKAP is widely expressed, with the highest transcript levels expressed in striated muscle (data not shown). Since ILK is also important for muscle development in Caenorhabditis elegans (Dedhar et al., 1999), we examined ILKAP expression in human muscle tissue blots. ILKAP was preferentially expressed in striated muscle, with much lower levels evident in various smooth muscle tissues (Figure 2A). Dot-blot analyses of multi-tissue arrays containing 56 tissue RNAs indicated that ILKAP is ubiquitously expressed, and verified highest expression levels in cardiac and skeletal muscle, similar to ILK1 (not shown). In order to characterize an ILKAP-encoded protein, we generated a His6-tagged recombinant fusion protein by subcloning the 700 bp 5BT, C-terminal cDNA into pProExHT for expression and purification in Escherichia coli. Soluble recombinant protein was purified on Ni-NTA columns, and used to immunize rabbits. Affinity-purified ILKAP antibodies recognize a protein band migrating with an apparent mol. wt of 47 kDa, as estimated by SDS–PAGE, similar to the molecular weight of characterized PP2C family members (Figure 2B). These affinity-purified ILKAP antibodies recognized p47ILKAP in ILK1 immunoprecipitates of 293 cell lysates, indicating that p59ILK1 and p47ILKAP associate under physiological conditions (Figure 2B).
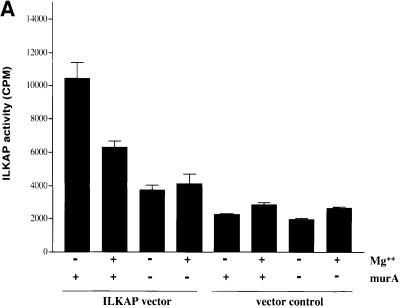
In order to investigate whether ILKAP is a functional PP2C, we expressed recombinant ILKAP proteins in eukaryotic cells, and characterized the catalytic activity of these proteins using in vitro phosphatase assays (Figure 3). For this purpose we cloned full-length ILKAP cDNAs, representing wild-type and mutant (see below) proteins, into the pIND/V5TOPO-His expression plasmid. When co-transfected with pVgRXR, encoding a modified ecdysone receptor, pIND/V5-ILKAP conferred ecdysone (muristerone A)-inducible expression of V5-tagged ILKAP in transiently transfected 293 cells (Figure 3). Control phosphatase assays indicated that 85–90% of the total protein S/T phosphatase activity in HEK 293 cells was sensitive to okadaic acid (OA) and EGTA, inhibitors of PP1, PP2A and PP2B phosphatases (data not shown). Muristerone-inducible PP2C (ILKAP) activity was thus assayed in vitro in the presence of OA and EGTA, using PKA-phosphorylated, 32P-labelled myelin basic protein (MBP) as substrate. Vector (pVgRXR) control HEK 293 transfectants showed no muristerone-inducible PP2C activity; however, the ILKAP transfectants showed ∼2.5-fold induction of PP2C activity (Figure 3A). Furthermore, ILKAP activity was inhibited by 50% in the presence of Mg2+ ions, as has been reported for rat PP2C (Tong et al., 1998). These assays produced only minimal background activity in the absence of Mn2+ ions (not shown), further indicating that the ILKAP cDNA encodes Mn2+-dependent PP2C activity.
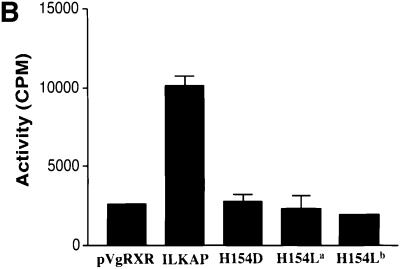
Fig. 3. PP2C activity of recombinant ILKAP expressed in mammalian cells. (A) HEK 293 cells stably expressing the synthetic ecdysone receptor (pVgRXR) were transiently transfected with pIND/V5-ILKAP, in order to characterize muristerone A-induced phosphatase activity. Vector control cells were the stable pVgRXR 293 transfectants. Muristerone A treatment of the pIND/V5-ILKAP cells induced OA- and EGTA-resistant PP2C ∼3-fold relative to uninduced pIND/V5-ILKAP transfected cells; however, it did not raise PP2C activity of vector control cells above background. Muristerone-induced S/T phosphatase activity was partially sensitive to inhibition by Mg2+, as reported for rat PP2C (Tong et al., 1998). (B) pVgRXR-expressing 293 cells were transiently transfected with pIND/V5-ILKAP plasmids encoding wild-type ILKAP, or the indicated ILKAP catalytic mutants. Muristerone-induced PP2C activity from the wild-type ILKAP transfectants was ∼5-fold above background levels seen with the mutant ILKAP transfectants. All assays were performed in triplicate, with error bars indicating 1 SEM. Control experiments confirmed that this phosphatase activity was dependent on Mn2+, and not inhibited by OA or EGTA, as expected of PP2C enzymes. Thus, all assays in (A) and (B) were carried out in the presence of Mn2+, OA and EGTA.
To characterize ILKAP catalytic activity further, mutant H154L and H154D proteins were generated by site-directed mutagenesis of the putative active site, 152DGH154 (see Materials and methods). To assay for catalytic inactivation of these ILKAP mutants, HEK 293 cells were transiently transfected with pVgRXR and pIND/V5-ILKAP, or pIND/V5-ILKAP mutant cDNAs. Three independently constructed mutant ILKAP plasmids, two encoding H154L and one encoding H154D, were transfected in parallel and assayed for phosphatase activity, as above. Wild-type ILKAP transfectant cells demonstrated muristerone-inducible PP2C activity; however, despite robust induction of recombinant mutant proteins, PP2C activity in the mutant transfectants was equal to the background activity assayed in the pVgRXR/293 control transfectants (Figure 3B). Taken together, these structural and functional data strongly support the identity of ILKAP as a PP2C.
Specific association of ILK1 and ILKAP is independent of ILKAP catalytic activity
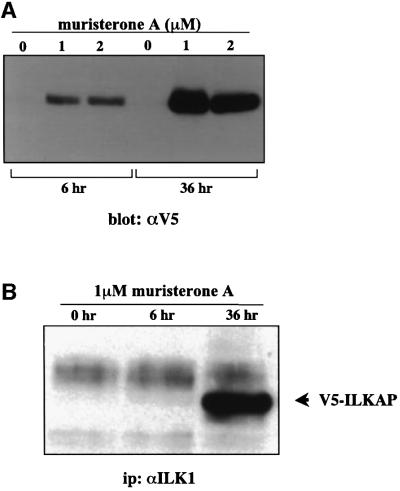
Having demonstrated that the ILKAP cDNA encoded a functional PP2C, it was important to confirm and further characterize the association of ILK1 and ILKAP proteins in mammalian cells. In the transient pIND/V5-ILKAP transfectants, maximal induction of V5-ILKAP was seen at 1 µM muristerone A (Figure 4A), and expression was clearly seen at 6 h post-induction. After 36 h, higher levels of V5-ILKAP accumulation were evident. Therefore, we examined the association of V5-ILKAP with endogenous p59ILK1 at these times (Figure 4B). Affinity-purified ILK1 antibodies were used to immunoprecipitate cytoplasmic lysates of uninduced, 6 h and 36 h muristerone-induced transfectants. At 6 h induction we could not detect co-precipitating V5-ILKAP; however, after 36 h, significant levels were detected in the immune complexes (Figure 4B). V5-tagged ILKAP was not detected in Raf-1 immunoprecipitates of these same lysates (Figure 4C). These results indicate that the observed association of ILKAP and ILK in intact cells is specific, and is not due to a post-lysis artefact. Equally, as we also detect p47ILKAP in ILK1 immune complexes from lysates of untransfected, parental 293 cells (Figure 2B), it is evident that ILK1 association of V5-tagged ILKAP is not a consequence of overexpression and occurs under physiological conditions.
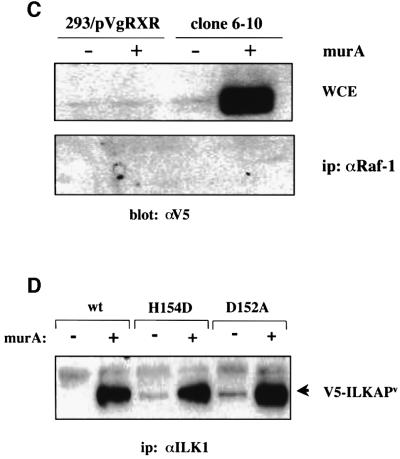
Fig. 4. ILKAP and ILK1 associate in mammalian cells. (A) HEK 293 were transiently co-transfected with pIND/V5-ILKAP and pVgRXR plasmids. After 24 h of culture, transfected cells were then induced with 1 or 2 µM muristerone A, or left untreated, for the indicated periods. Cytoplasmic lysates were analysed by western blotting for expression of the V5 epitope, migrating at the appropriate size of ∼50 kDa. (B) 293 cells were transiently co-transfected, as above. After 24 h, transfectants were induced with muristerone A for the indicated times. Cytoplasmic lysates of these cells were immunoprecipitated with affinity-purified ILK1 antibodies, and the resulting immune complexes analysed by SDS–PAGE and western blotting for the presence of the V5-tagged ILKAP protein. (C) 293/pVgRXR and wt/6-10 clones were cultured under normal conditions, prior to induction with 1 μM muristerone A for 36 h. Cytoplasmic lysates (WCE; top panel) were immunoprecipitated (bottom panel) with anti-Raf-1 antibody (C-20; Santa Cruz). Immune complexes were run on 10% SDS–PAGE and transferred to PVDF membranes. Membranes were blotted with anti-V5 monoclonal antibody, and visualization was by ECL. (D) 293/pVgRXR cells were transiently transfected as in (A), with the indicated ILKAP mutants. Transfectant cultures were lysed and subjected to immunoprecipitation with affinity-purified ILK1 antibodies. Complexes were analysed for co-precipitating V5-tagged ILKAP and mutants, by SDS–PAGE and western blotting with anti-V5 monoclonal antibody. ILKAPV indicates variant ILKAP recombinant proteins.
In order to gain additional insight into the regulation of ILK1 by ILKAP, it was important to determine the requirement for ILKAP catalytic activity in the association of p47ILKAP with p59ILK1. Wild-type, H154D and D152A ILKAP plasmids were transiently transfected into pVgRXR-293 cells, which were subsequently induced with muristerone A, and cell lysates subjected to co-immunoprecipitation analyses using affinity-purified ILK1 antibodies. The ILKAP point mutations affect PP2C box 1 and do not directly involve PP2C box 2, which encompasses the ILK1-binding region. The H154D and D152A mutant proteins co-precipitated with p59ILK1 as efficiently as the wild-type protein (Figure 4D), demonstrating that phosphatase activity is not required for the physical interaction of p47ILKAP and p59ILK1. These data suggest that integrin-induced signals mediate inactivation of p47ILKAP complexed with p59ILK1 to regulate transient ILK1 activation in response to ECM.
ILKAP selectively inhibits ILK1 activity and signal transduction
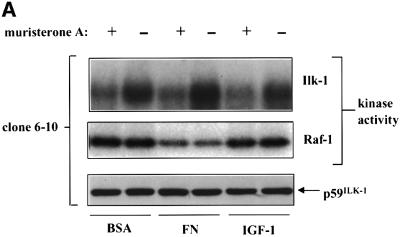
We next examined the ability of ILKAP to inhibit ILK1 kinase activity and signal transduction. Stable HEK 293 cell lines expressing muristerone A-inducible, V5 epitope-tagged ILKAP were derived for this purpose. Two such independently isolated clones, wt/6-4 and wt/6-10, were chosen for detailed study. In the absence of treatment with muristerone, we could not detect any V5-tagged ILKAP in either wt/6-4 or wt/6-10 clones, indicating that expression was tightly regulated. Cells were untreated or induced with muristerone A for 24 h. These were then re-plated on fibronectin (FN), control bovine serum albumin (BSA), or treated with 100 nM insulin for 20 min to activate endogenous ILK1. Cells were then lysed and subjected to ILK1 immune complex kinase assays. We observed muristerone A-dependent inhibition of ILK1 kinase activity in both wt/6-4 and wt/6-10 lines (Figure 5A). Basal ILK1 activity in these cells is relatively high due to the presence of serum in the cultures, making ECM and insulin stimulation of ILK1 apparently less robust than in serum-starved cultures. ILKAP induction significantly inhibited the high levels of ILK1 activity observed under these conditions. We wished to confirm that this inhibition was not generalized to other, S/T protein kinases. Raf-1 is a cytoplasmic S/T kinase whose activity is modulated by extracellular signals. Therefore, as a specificity control, we assayed Raf-1 kinase activity in the same lysates used for ILK1 activity. Raf-1 activity was decreased by plating on FN, as expected under conditions where PKB is activated (Zimmermann and Moelling, 1999); however, there was no muristerone A effect on Raf-1 kinase activity, demonstrating that induction of ILKAP selectively inhibited ILK1 immune complex kinase activity.
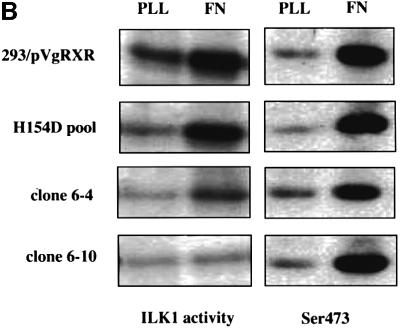
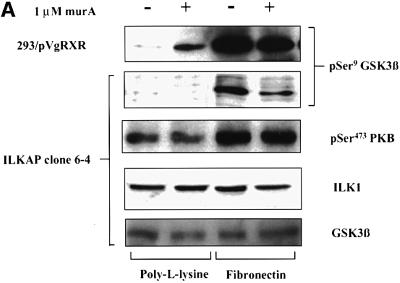
Fig. 5. ILKAP selectively inhibits ILK1 kinase activity. (A) Cytoplasmic lysates from ILKAP clone wt/6-10 were analysed in ILK1 (top panel) or Raf-1 (middle panel) immune complex kinase assays using MBP as exogenous substrate. Cells were pre-treated for 36 h with or without muristerone A, detached, and replated for 20 min on BSA or FN in 10% serum, or treated for 10 min with 100 ng/ml IGF-1, to induce high levels of ILK1 activity. Lysates were controlled for equal levels of p59ILK1 protein (bottom panel). (B) The stable ILKAP clones wt/6-4 and wt/6-10, and a non-clonal H154D pooled transfectant cell line, were treated with muristerone A for 36 h to induce recombinant protein expression. Subsequently, these cells were plated on FN or poly-l-lysine (PLL) for 10 min, after which cell lysates were assayed for ILK1 activity by immune complex kinase assays (MBP substrate), and also for PKB Ser473 phoshorylation by western blotting with a pSer473-specific antibody. Induction of ILKAP, but not the H154D mutant, inhibited ILK1 immune complex kinase activity. Ser473 phosphorylation was not inhibited in parallel with ILK1 inhibition. Plating on PLL did not induce ILK1 activity or Ser473 phosphorylation.
We next examined the requirement for ILKAP catalytic activity in the observed inhibition of ILK1 activity. A stable, non-clonal cell line was derived from HEK 293 by transfection of the H154D catalytic mutant. Induction of H154D expression did not inhibit ILK1 kinase activity, as seen with ILKAP-expressing wt/6-4 and wt/6-10 cells (Figure 5B). A number of groups have shown that ILK1 activates the PKB survival pathway, by either directly or indirectly effecting phosphorylation of PKB on Ser473 (Delcommenne et al., 1998; Lynch et al., 1999; Persad et al., 2000). Surprisingly, ILKAP inhibition of FN-induced ILK1 activity in the wt/6-4 and wt/6-10 clones did not affect FN-induced phosphorylation of PKB Ser473. Induction of the H154D mutant also had no inhibitory effect on Ser473 phosphorylation (Figure 5B). Inhibition of ILK1 kinase activity in these experiments requires catalytically active ILKAP.
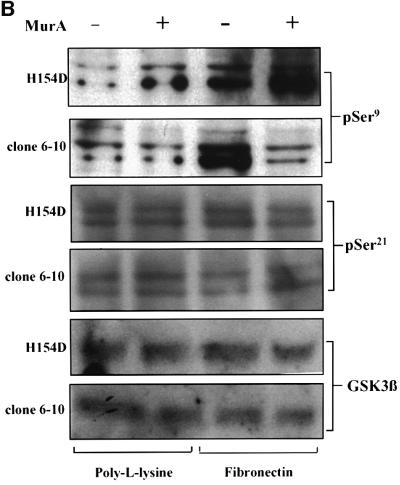
In light of the unexpected Ser473 results in ILK1-inhibited cultures, it was important to determine the signalling outcome of ILKAP inhibition. ILK1-mediated phosphorylation of GSK3β has suggested a mechanism for the regulation of β-catenin nuclear translocation and activation of β-catenin–Lef transcription complexes, observed in ILK1-overexpressing epithelial cells (Novak et al., 1998). We therefore examined whether ILKAP affected ILK1-stimulated phosphorylation of GSK3β, using phosphospecific antibodies to GSK3 Ser9 and Ser21. Transfectant clones were serum starved prior to stimulation with FN or insulin-like growth factor (IGF-1). As expected, these treatments induced robust phosphorylation on Ser9. Muristerone-induced ILKAP inhibited both FN- and IGF-1-induced Ser9 phosphorylation in the wt/6-4 and wt/6-10 clones (Figure 6), consistent with the reported effect of ILK1-stimulated Ser9 phosphorylation of GSK3β. We verified that phosphorylation of PKB on Ser473 was normal in the same wt/6-4 lysates (Figure 6A). To examine the requirement for ILKAP catalytic activity in the observed inhibition, we examined Ser9 phosphorylation in a stable cell line conditionally expressing the H154D catalytic mutant. Muristerone induction of the H154D line mutant did not inhibit FN-induced Ser9 phosphorylation (Figure 6B), indicating that ILKAP catalytic activity is required for inhibition of GSK3β Ser9 phosphorylation.
Fig. 6. ILKAP selectively inhibits phosphorylation of GSK3β at Ser9. (A) ILKAP clone wt/6-4 and vector control transfectant cells were serum starved and pre-treated or left untreated, prior to plating on FN. Control cells showed FN-dependent induction of Ser9 phosphorylation, which was inhibited in the wt/6-4 cells induced with muristerone A. Parallel blots were probed with phosphospecific antibody for PKB Ser473. Induction of ILKAP had no effect on Ser473 phosphorylation. Blots were stripped and reprobed with appropriate antibodies, for total ILK1 and GSK3 protein levels. (B) ILKAP clone wt/6-10 was treated as in (A), in parallel with the non-clonal transfectant cell line expressing a catalytically inactive H154D ILKAP mutant. Muristerone induction of ILKAP suppressed FN-induced Ser9 phosphorylation; however, the catalytic mutant did not inhibit this phosphorylation. Parallel blots were probed for changes in Ser 21 of GSK3α/β, which was not detectably affected by ILKAP activity. Blots were stripped and reprobed for total GSKβ protein levels.
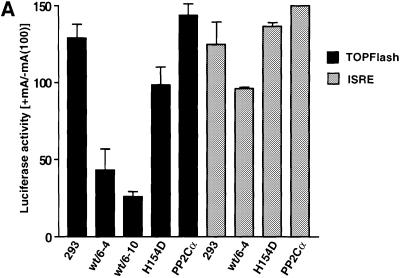
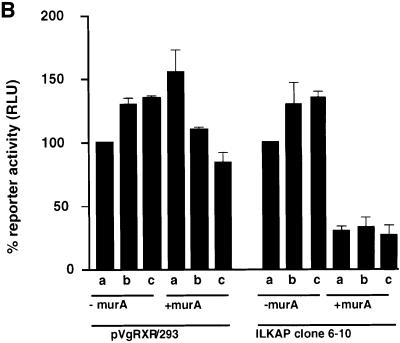
Overexpression of p59ILK1 and ILK1 activity has been shown to induce nuclear translocation of β-catenin–Lef complexes and activation of a Tcf-responsive promoter. We reasoned that inhibition or blocking of Ser9 phosphorylation by overexpression of p47ILKAP activity would result in inhibition of growth factor-stimulated β-catenin–Lef activity. We chose to assay inhibition of β-catenin–Lef transactivation using the TOPFlash/FOPFlash reporter genes, which are widely used for this purpose. To test the requirement for ILKAP catalytic activity, we also assayed the H154D mutant for effects on TOPFlash reporter gene activation. The ILKAP-related PP2Cα has been reported to activate this pathway (Strovel et al., 2000), thus providing a stringent specificity control for regulation of β-catenin–Lef transactivation. Three independently derived stable cell lines, expressing muristerone-inducible V5-PP2Cα, were used as controls for ILKAP activity. We confirmed muristerone-dependent expression of V5-tagged PP2Cα recombinant protein and PP2C activity in these PP2Cα transfectant lines (not shown). Serum stimulated high levels of luciferase activity from the TOPFlash reporter, as expected. This β-catenin-dependent Tcf transcription factor activity was dramatically (50–70%) inhibited by muristerone induction of ILKAP, whereas induction of H154D mutant or PP2Cα did not inhibit β-catenin–Lef activity. Next we tested the ILKAP-mediated inhibition of β-catenin–Lef transactivation in response to insulin and IGF-1, conditions shown above to strongly induce GSK3β Ser9 phosphorylation in the 293 cells. Insulin- or IGF1-stimulated transactivation was inhibited by 60–75% in muristerone-treated wt/6-10 cells compared with levels in non-muristerone treated wt/6-10 and vector control cells, consistent with the inhibition of GSK3β Ser9 phosphorylation under these conditions (Figure 7B). These results indicate that ILKAP catalytic activity selectively inhibits β-catenin–Lef transactivation.
Fig. 7. Tcf/Lef factor activation is selectively inhibited by ILKAP. (A) TOPFlash and FOPFlash (mutant Tcf site) reporter plasmids were co-transfected with pRSV/βgal into 293/pVgRXR vector control, ILKAP wt/6-4 and wt/6-10, H154D and PP2Cα expressing lines (dark bars). Cells were untreated or muristerone A was added for 36 h to induce recombinant protein expression, and resulting Tcf-driven or ISRE-luc-driven luciferase reporter gene activity assayed. In the case of the ISRE-luc transfectants, recombinant human IFN-α was added (500 IU/ml) for the last 18 h of the induction period. Control experiments confirmed ∼3-fold induction of ISRE-luc activity by this IFN treatment (data not shown). Luciferase activity is presented as the muristerone-induced/uninduced ratio, also corrected for luciferase activity from duplicate FOPFlash mutant reporter transfections in the case of FOPFlash results. (B) The Tcf-luciferase reporter construct TCF-pGL2 (see Materials and methods) was co-transfected with pRSV/βgal into the pVgRXR/293 vector control and ILKAP clone wt/6-10 cell lines. Transfectants cultured in 10% FCS (a), 10% FCS + 50 ng/ml IGF-1 (b) or 10% FCS + 100 nM insulin (c) were not induced or induced with muristerone A, and subsequently assayed for TCF-driven luciferase activity. Each transfection in (a), (b) and (c) was controlled internally and normalized to β-galactosidase activity, to account for transfection efficiencies.
It was important to confirm that ILKAP inhibition of β-catenin–Lef transactivation is not generalized for other cytoplasmically activated transcription pathways. Type I interferon (IFN) activation of ISRE-dependent transcription requires cytoplasmic S/T (p38 MAP kinase) and tyrosine kinase (Tyk2 and JAK) activities for phosphorylation of STAT factors, and PI3K activity is also stimulated by IFN treatment of cells. We transfected ISRE-luciferase and TOPFlash reporter plasmids into the ILKAP clones, wt/6-4 and wt/6-10, as well as the H154D mutant and PP2Cα cell lines. Induction of ILKAP, H154D or PP2Cα did not inhibit IFN-stimulated ISRE reporter gene activity (Figure 7A). Taken together, the reporter assays are consistent with the data on inhibition of GSK3β phosphorylation, and indicate that ILKAP selectively targets GSK3β-regulated β-catenin–Lef transactivation.
Discussion
As demonstrated by sequence similarity and biochemical characterization of its catalytic function, ILKAP is clearly a PP2C. The PP2C family is a structurally diverse group of Mg2+/Mn2+-dependent S/T phosphatases, members of which have been implicated in environmental stress responses in yeasts, plants and mammals, as well as regulating abscisic acid signal transduction in Arabidopsis thaliana (Rodriguez, 1998). Rat PP2C, which shares 95% amino acid identity with ILKAP, was cloned using an amplification strategy to isolate conserved PP2C isoforms (Tong et al., 1998). This orthologue is the most closely related protein to ILKAP that we have identified through searches of SwissProt and GenPept databases. The biochemical distinction of ILKAP and rat PP2C from other PP2C enzymes, i.e. being inhibited rather than stimulated by Mg2+, further suggests that they are homologues and, thus, PP2C/ILKAP regulation of ILK signal transduction is likely to be conserved among mammals.
The crystal structure of PP2C has been resolved to 2 Å (Das et al., 1996). This structure, along with extensive mutational analyses of mouse PP2Cβ (Kusuda et al., 1998) and the related AB1l and AtPP2C phosphatases of Arabidopsis sp. (Sheen, 1998), have identified highly conserved PP2C residues that are critical for catalysis. Our site-directed mutations of ILKAP targeted the putative 152DGH154 catalytic site, as analogous mutations in the Arabidopsis and mouse PP2Cβ enzymes have been shown to abrogate catalytic activity. Three ILKAP mutations, D152A, H154L and H154D, were markedly catalytically deficient, losing >90% of activity in in vitro phosphatase assays (Figure 3B). These data indicate that the 152DGH154 amino acid triplet does constitute the ILKAP active site, typical of PP2C. Each of the ILKAP catalytic mutants formed stable complexes with p59ILK1; however, they did not inhibit FN-induced ILK1 activity, GSK3β Ser9 phosphorylation or β-catenin–Lef transactivation, indicating that they are not dominant-negative mutations. Our initial studies on ILK1 indicated that its constitutive overexpression in epithelial cells leads to oncogenic transformation, as assayed in soft agar colony and nude mouse tumorigenesis assays (Hannigan et al., 1996; Radeva et al., 1997). Constitutive expression from either antisense or dominant-negative ILK1 constructs has demonstrated that increased ILK1 activity is sufficient to effect transformation (Dedhar and Hannigan, 1996; Novak et al., 1998; Wu et al., 1998). We are currently testing the ILKAP catalytic mutants to determine whether they effect persistent, rather than transient ILK1 activation.
Our data support the view that p59ILK1 is a bona fide protein kinase, as we have previously demonstrated (Hannigan et al., 1996), and suggest that ILKAP selectively inhibits ILK1 protein kinase activity and signalling. Recently, the catalytic subunit of PP2A has been reported to co-precipitate and co-localize with ILK1 and β1 integrin, in focal adhesions of F9 stem cells. The ILK1–integrin association in these cells is apparently regulated by PP2A-sensitive phosphorylation of β1 integrin, since ILK1 dissociates from phosphorylated β1 in OA-treated F9 cells (Mulrooney et al., 2000). ILKAP activity is not OA sensitive, clearly distinguishing it from integrin-associated PP2A activity. The demonstrated selectivity of ILKAP in modulating ECM- or growth factor-induced phosphorylation of GSK3β Ser9, while having little or no effect on PKB Ser473 phosphorylation, suggests that ILKAP might directly dephosphorylate Ser9. However, our data on inhibition of ILK1 activity lead us to favour a mechanism whereby ILKAP inhibits ILK1-mediated Ser9 phosphorylation. We cannot rule out the possibility that GSK3β complexes with and inhibits ILK1 activity. In vitro studies using recombinant proteins should clarify these issues.
It is not known whether ILK1 activity is developmentally regulated by Wnt factors; however, recent evidence suggests differential regulation of β-catenin signalling by insulin and Wnt (Ding et al., 2000). These authors show that Wnt, in contrast to insulin, does not induce phosphorylation of GSK3β or PKB, suggesting that the ILK1–GSK3β axis is not sensitive to Wnt. GSK3β activity phosphorylates β-catenin and targets it for destruction via the ubiquitin–proteasome pathway (Aberle et al., 1997; Easwaran et al., 1999; Barker et al., 2000). ILK1-mediated inhibition of GSK3β induces nuclear translocation of stabilized β-catenin–Lef-1 complexes and activation of Tcf transcription factors (Novak et al., 1998). In contrast to the effects of ILKAP on Tcf activity, we show that PP2Cα does not inhibit transactivation (Figure 7), and indeed PP2Cα has recently been shown to stimulate β-catenin–Lef signalling (Strovel et al., 2000). Pharmacological inhibition of ILK1 activity results in reduced phosphorylation of GSK3β on Ser9 in SW480 colon carcinoma cells (Tan et al., 2001), consistent with results presented here indicating the modulation of ECM- and insulin/IGF-induced GSK3β phosphorylation by the activities of ILK1 and ILKAP. Our data suggest further that ILK1/ILKAP function coordinately to regulate PKB-independent signalling of GSK3β, and clearly point to distinct mechanisms regulating ILK1 signalling of these protein kinases. Finally, the question of ILKAP regulation by extracellular signals provides a focus for studies on the mechanisms of activation of ILK1 signalling.
Materials and methods
cDNA library screens and construction of inducible expression vectors
Two libraries (human placenta and human muscle) from OriGene (OriGene, Rockville, MD) were screened using an external vector-specific 5′ primer (Ori-3, 5′-GCAGAGCTCGTTTAGTGAACC-3′) and an internal ILKAP primer spanning the 3′ stop codon (CJLH-3, 5′-TCA GTGCCCTAT-3′). Nested PCR was performed with Ori-2 (5′-TGG GCGGTAGGCGTGTACGG-3′) and 3′ gene-specific primers (CJLH-1, 5′-ATAACGACACAAGAT-3′; or CJLH-4, 5′-CCCGAGTAATGA GGG-3′). Two full-length ILKAP clones were obtained from the human muscle library. A partial ILKAP clone, missing the 5′ end of the gene (encoding amino acid residues 269–392), was obtained from the human placental cDNA library.
OriGene library screening was as recommended by the manufacturer. Four microlitres of each well from the master plate were screened using the Qiagen Taq DNA polymerase kit. Reactions contained 10 ng of vector-specific primer Ori1, 10 ng of ILKAP-specific primers, 2 µl of 10× reaction buffer, 0.2 µl of Taq, 0.4 µl of 2 mM dNTP, 0.4 µl of 25 mM MgCl2, 4 µl of ‘Q’ buffer and 4 µl of library DNA. Thermocycles were as follows: 94°C for 10 min; (94°C for 1.15 min denaturation, 65°C for 1.0 min annealing with 0.5°C touch down per cycle, 72°C for 2 min extension) × 20 cycles; (94°C for 1.5 min, 55°C for 1.0 min, 72°C for 2 min) × 10 cycles; and a final 72°C for 7 min final extension (PE Biosystems GeneAmp 9700). Screening of the OriGene subplates was as recommended by OriGene, in which 5 µl of bacterial cells were used as in the above PCR reactions. Positive wells from the subplates were plated out onto LB/ampicillin agar plates and colony hybridizations to an ILKAP-specific probe were used to identify positives. The complete ILKAP clones were sequenced using either Amersham’s Thermo sequenase kit or by automated sequencing.
A full-length ILKAP cDNA (H4C11) was used as a template for PCR cloning into the pIND/V5TOPO-His expression vector (Invitrogen) allowing for ecdysone-inducible expression of V5 epitope-tagged recombinant ILKAP. A primer at the 5′ UTR of ILKAP (5′-GGC ACCAGGCCCGC-3′) and a 3′ primer in which the TGA stop codon was removed (5′-GTGCCCTATCCGCAC-3′) were used to amplify the complete ILKAP using the OriGene clones as templates and Stratagene’s high-fidelity Pfu DNA polymerase. Two microlitres of PCR product were immediately ligated into the pIND/TOPOV5-His vector. Colonies recovered following transformation were screened for the presence of inserts using BamHI and XbaI restriction digests. Positive clones were sequenced to verify the ILKAP insert and to check for in-frame expression between ILKAP and V5/His coding regions. A clone designated H4C11-30 was used for subsequent transfections. Control plasmids expressing human PP2Cα were generated via the same strategy, using gene-specific primers (forward: 5′-ATAATGGGAGCATTTTTA GACAAG-3′; reverse: 5′-CCACATATCATCTGTTGATGTAGA-3′) to generate an amplification product from a pcDNA3.1-PP2Cα plasmid template (Invitrogen). Independent pIND/V5TOPO-His/PP2Cα plasmid isolates were used to derive three stable pooled transfectant lines from the 293 pVgRXR clone described above.
Cell culture, transfections and establishment of stable transfectants
HEK 293 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), high glucose (Ontario Cancer Institute, Toronto, Ontario, Canada) supplemented with 10% fetal calf serum, 2 mM l-glutamine and 0.1 mM non-essential amino acids (Life Technologies) at 5% CO2 and 37°C. Transfected cells were selected in 125 µg/ml zeocin (Invitrogen, Carlsbad, CA) and/or 400 µg/ml Geneticin G418 (Life Technologies, Burlington, Canada). Cells in 35 mm dishes were transfected with 1 µg of plasmid DNA in the presence of 4 µl of Lipofectamine Plus reagent (Life Technologies) under serum-free conditions (Optimem; Life Technologies). Cells were cultured for 16 h at 37°C; Lipofectamine/Optimem was removed and replaced with regular 10% fetal calf serum (FCS)/DMEM. Muristerone A (Invitrogen) was added to transiently transfected cultures at a final concentration of 1 µM for 24–36 h for induction of transgene expression. A stable HEK 293 derivative was generated by transfection of the pVgRXR (Invitrogen) expression plasmid, encoding a modified ecdysone receptor. This line was cloned out by limiting dilution and used as a recipient line for transfection of pIND expression plasmids. Double transfectants were maintained under G418 and zeocin selections. For stable lines, transfectant cells were allowed to recover for 48 h, following which G418 and/or zeocin was added for selection. Killing curves on the parental 293 cells established the concentration of each drug used for selections. Twelve independent isolates of HEK 293 transfectant clones were analysed for muristerone A-inducible V5-ILKAP expression levels. Lines were chosen for further study based on intermediate levels, relative to constitutively expressing clones, of recombinant ILKAP protein expression. For study of mutant ILKAP expression, a stable, pooled transfectant line was established by transfecting a mutated (see below) pIND/V5-ILKAP(H154D) plasmid into 293/pVgRXR cells and selection in G418 and zeocin. Stable clonal and pooled, non-clonal lines of HEK 293 transfectants were analysed for expression levels of recombinant V5-tagged ILKAP by western blotting, using V5 monoclonal antibody. Independent clones were selected by two rounds of limiting dilutions in 96-well tissue culture plates.
Site-directed mutagenesis
Three mutants were created using the full-length pIND/V5-ILKAP cDNA (H4C11-30, above) as template. These mutations were created by converting Asp152 to alanine (D152A), His154 to leucine or to aspartic acid (H154L or H154D). The following primers were used. The 5′ primer for all reactions was C26: 5′-GGCCGGATCCGGCACCAGGCC CGCTGCTGC. Reactions were 3′ reverse-primed by one of the following internal primers, incorporating the underlined point mutations: C27 (5′-CTCGAATTCCTCCATCTCCATC), C28 (5′-CTCGAATTCCTC CTGTCCAGCAA) and C29 (5′-ACTCGAATTCCTCCAAGTCCATC) using the parental H4C11-30 clone as template. PCR was performed using 10 ng of 5′ primer C26 and either of the 3′ primers C27, C28 and C29, 2 µl of 10× reaction buffer, 0.2 µl of Pfu thermostable polymerase (high-fidelity Taq), 0.4 µl of 25 mM dNTP, 0.4 µl of 25 mM MgCl2 and 4 µl of template. The amplification cycles were as follows: 94°C for 10 min; (94°C for 1.25 min denaturation, 65°C for 1.0 min annealing with 0.5°C touch down per cycle, 72°C for 2 min extension) × 20 cycles; (94°C for 1.5 min, 55°C for 1 min, 72°C for 2 min) × 10 cycles; and a final 72°C for 10 min extension. A 500 bp BamHI–EcoRI fragment was cut out of the cDNA clone H4C11-30, and the BamHI–EcoRI linkered mutant PCR product was directionally cloned in, to replace the wild-type fragment in the template vector. Positive clones were sequenced to verify the presence of the mutation and to check for in-frame fusion of ILKAP with the V5 epitope.
Fibronectin, IGF-1 stimulation and immune complex kinase assays
Sterile non-tissue culture dishes (100 mm) were coated overnight with 10 µg/ml FN (Life Technologies) or poly-l-lysine (Sigma, St Louis, MO) in phosphate-buffered saline (PBS) pH 7.4 at 4°C with gentle rotation. Prior to use, these plates were blocked for 2 h at 37°C with 2.5 mg/ml BSA (Sigma; Fraction V) in DMEM. Cells were harvested and washed twice in 2.5 mg/ml BSA/DMEM. A total of 2 × 106 cells were added to each plate in 5 ml volume and incubated for 20 min at 37°C. Non-adherent cells were removed and the plates were washed twice with ice-cold PBS. Cell lysis was performed directly on the plates in 200 µl of NP-40 lysis buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 50 mM HEPES pH 7.5, 1 µg/ml leupeptin, 1 µg/ml aprotinin, 3 mM phenylmethylsulfonyl fluoride). Following 20 min incubation on ice, the soluble lysate was collected by centrifugation at 15 000 g for 20 min at 4°C. A non-adherent control was performed on 100 mm non-tissue culture plates pre-coated with BSA/DMEM. For IGF-1 (Sigma) and insulin stimulations, cells were pre-starved in serum-free medium overnight. Cells were treated with 50 ng/ml IGF-1 or 100 nM insulin for the specified times at 37°C, and washed twice with cold PBS followed by lysis at 4°C.
Kinase assays were carried out as described in Hannigan et al. (1996). Protein concentration was determined by Bradford assays (Bio-Rad, Richmond, CA). Cytoplasmic lysates (0.2–0.5 mg) were immunoprecipitated with 1.5 µg of affinity-purified rabbit anti-ILK [cat. #06-592; Upstate Biotechnology Inc. (UBI), Lake Placid, NY] overnight at 4°C, with rotation. Protein A–Sepharose (Sigma), pre-swollen in NP-40 lysis buffer, was added for 2 h at 4°C to capture the antibodies. Following two washes with NP-40 lysis buffer and two washes with kinase wash buffer [10 mM MgCl2, 10 mM MnCl2, 50 mM HEPES pH 7.5, 0.1 mM sodium orthovanadate, 1 mM dithiothreitol (DTT)], kinase assays were performed directly on the protein A beads. Kinase assay reaction was performed in 25 µl volume containing 10 mM MgCl2, 10 mM MnCl2, 50 mM HEPES pH 7.5, 1 mM sodium orthovanadate, 2 mM sodium fluoride, 5 mCi [γ-32P]ATP (Amersham, Piscataway, NJ) and 12.5 µg of MBP (UBI). Incubation was for 30 min at 30°C. The reaction was stopped with 10 µl of SDS–PAGE non-reducing stop buffer and heated for 5 min at 95°C. Phosphorylated MBP bands were visualized by 10% SDS–PAGE and autoradiography on Kodak X-OMAT film (Eastman Kodak, Rochester, NY) or by PhosphorImager analysis (Storm; Molecular Dynamics). Kinase assays to assess the activity of ILKAP on ILK1-mediated phosphorylation were carried out in the absence of sodium orthovanadate or sodium fluoride phosphatase inhibitors.
In vitro assay for S/T phosphatase activity
Phosphatase assay was carried out as outlined in the New England Biolabs instruction manual. The protein concentration of all samples was determined by Bradford assays (Bio-Rad). Protein (20–50 µg) was pre-incubated at 30°C for 2–5 min with assay buffer (50 mM Tris–HCl pH 7.0, 0.1 mM Na2EDTA, 5 mM DTT, 0.01% v/v Brij 35) plus 5 µM OA (PP1, PP2A inhibitor) and 0.1 mM EGTA (PP2B inhibitor), 4 mM Mn2+ and 4 mM Mg2+ (when indicated). Reactions were started by adding 10 µl of [γ-32P]ATP-labelled MBP and incubating for 10 min at 30°C. The reaction was then terminated by adding 200 µl of ice-cold 20% trichloroacetic acid (Sigma) and placing on ice for 5–10 min. Tubes were spun at 12 000 g for 5 min at 4°C. Finally, 200 µl of supernatant were added to 2 ml of aqueous scintillation fluid and counted.
Western blot analyses of protein expression
SDS–PAGE gels were transferred to polyvinyldifluoride membrane (Immobilon-P; Millipore, Bedford, MD) by electrophoresis in 25 mM Tris, 192 mM glycine and 20% methanol. For anti-ILK, anti-ILKAP, anti-V5 westerns, membranes were blocked in 5% milk in TBST (20 mM Tris pH 7.5, 500 mM NaCl, 0.1% Tween-20). Primary antibodies against ILK1 (UBI), V5 epitope (Invitrogen) and anti-ILKAP (affinity-purified rabbit polyclonal) were diluted to 1 µg/ml in TBS with 1% (w/v) BSA (Fraction V). Incubation was for 2 h at room temperature with gentle shaking. Secondary antibodies of either horseradish peroxidase (HRP)-conjugated goat anti-rabbit or rabbit anti-mouse (Jackson ImmunoResearch) were incubated at 1/20 000 dilutions in TBST, for 45 min at room temperature. Bands were visualized with chemiluminescent substrate (ECL; Amersham).
Phosphospecific antibodies, recognizing PKB Ser473 and Thr308 (New England Biolabs), as well as GSK-3 Ser9 (courtesy of Dr J.Woodgett, Ontario Cancer Institute, and BioSource, Camarillo, CA) and Ser21 (UBI), were used for analyses of the phosphorylation status of PKB and GSK3. A GSK3 antibody (clone 4G-1E; UBI) was used for determination of total GSK3α/β protein levels. Western blot protocols supplied by the manufacturer (NEB, Beverly, MA) were strictly adhered to. Primary antibodies were diluted in 0.1% Tween-20, 20 mM Tris pH 7.6 and 136 mM NaCl, and incubated overnight at 4°C with rotation. Secondary antibodies were used at 1/20 000 for 45 min at room temperature.
Immunoprecipitation
Cells transiently transfected with the H4C11-30 ILKAP (full-length) constructs were induced for 18 or 36 h with 1 µM muristerone A. Cells were harvested in NP-40 lysis buffer and protein concentrations determined by Bradford assay. Lysate (1 mg) was first pre-cleared with 100 µl of protein A (50% slurry equilibrated in NP-40 buffer) for 2 h at 4°C, then incubated with 1–2 µg of affinity-purified rabbit anti-ILK, or rabbit anti-ILKAP antibodies, overnight at 4°C. Protein A–Sepharose was added for an additional 2 h at 4°C, to capture primary immune complexes. Complexes were washed 8–10 times in NP-40 lysis buffer, samples run on 12% SDS–PAGE gels and transferred to Immobilon-P membranes. Westerns were performed with anti-V5HRP (Invitrogen), affinity-purified anti-ILK (UBI) or affinity-purified anti-ILKAP antibodies.
Alternatively, immunoaffinity columns were prepared in which rabbit anti-ILK was coupled to cyanogen bromide-activated Sepharose 4B (Pharmacia, Montreal, Quebec, Canada). Lysates were applied to the immobilized protein beads and rotated overnight at 4°C. Following extensive washes with 10× volume lysis buffers, the bound material was eluted with 100 mM glycine pH 3.0 and/or 100 mM triethanolamine pH 11.5, and immediately neutralized with 1 M Tris pH 8.0. The eluted material was run on SDS–PAGE, transferred to PVDF membranes and blotted with appropriate antibodies.
Luciferase reporter gene assays
TOPFlash and FOPFlash reporter plasmids (UBI) (1 µg/35 mm dish) were individually co-transfected with 1 µg of pRSV β-gal into stable ILKAP transfectant and vector control lines, to assay Tcf/Lef factor activation. As a second test of Tcf activation, cells were transiently transfected with 1 µg of TCF-pGL2, containing five tandem repeats of the TCF response element (Kinetek, Vancouver, BC, Canada) and 1 µg of pRSV-β-gal with Lipofectamine Plus reagent. For induction of recombinant ILKAP and mutant proteins, muristerone A was added for 36–48 h post-transfection. After the induction period, cells were washed twice with PBS and harvested in 100 µl of Promega reporter lysis buffer (Promega, Madison, WI). Twenty microlitres of lysates were mixed with 100 µl of Luciferase Assay Reagents (Promega) and read immediately in a Berthold Lumat LB9501 luminometer. β-gal assays were performed according to Sambrook et al. (1989). Briefly, 20 µl of lysates were mixed with 3 µl of 0.1 M MgCl2, 4.5 M β-mercaptoethanol, 66 µl of o-nitrophenyl-β-d-galactopyranoside and 0.1 M sodium phosphate pH 7.5. Incubation was for 30 min at 37°C. Colorimetric optical density was read at 420 nm in an ELISA reader (Molecular Devices, SpectroMax 250). Results are expressed as luciferase units (× 106)/β-gal values.
DDBJ/EMBL/GenBank accession No.
The DDBJ/EMBL/GenBank accession No. for ILKAP cDNA is AY024365.
Acknowledgments
Acknowledgements
We thank Dr M.Moran (University of Toronto) for the HEK 293 cells. The RSV-β-gal reporter plasmid was a gift of Drs J.Danska and C.Guidos (Hospital for Sick Children). Dr J.Woodgett (Ontario Cancer Institute, Toronto) kindly provided a Ser9 phosphospecific GSK3 antibody. Drs P.Costello and N.Yoganathan (Kinetek Pharmaceuticals, Vancouver) supplied the pGL2 luciferase vector, and Dr E.Fish (University of Toronto) generously provided the ISRE-luciferase construct. Thanks to Brenda Muskat (Bioinformatics Centre, HSC) for generating Figure 1B, and to Qiong Yi for technical assistance in the early stages of this work. This work was supported by grants to G.E.H. from the National Cancer Institute of Canada (with funds from the Terry Fox Run), and from the U.S. Department of Defence Breast Cancer Research Program. G.E.H. is a Scholar of the Canadian Institutes of Health Research.
References
- Aberle H., Bauer,A., Stappert,J., Kispert,A. and Kemler,R. (1997) β-catenin is a target for the ubiquitin–proteasome pathway. EMBO J., 16, 3797–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfic H., Tang,X., Batty,I.H., Downes,C.P., Chen,C. and Rittenhouse, S.E. (1998) A novel integrin-activated pathway forms PKB-stimulatory phosphatidylinositol 3,4-bisphosphate via phosphatidylinositol 3-phosphate in platelets. J. Biol. Chem., 273, 13–16. [DOI] [PubMed] [Google Scholar]
- Barford D., Das,A.K. and Egloff,M.P. (1998) The structure and mechanism of protein phosphatases: insights into catalysis and regulation. Annu. Rev. Biophys. Biomol. Struct., 27, 133–164. [DOI] [PubMed] [Google Scholar]
- Barker N., Morin,P.J. and Clevers,H. (2000) The Yin-Yang of TCF/β-catenin signaling. Adv. Cancer Res., 77, 1–24. [DOI] [PubMed] [Google Scholar]
- Dahia P.L. et al. (1999) PTEN is inversely correlated with the cell survival factor Akt/PKB and is inactivated via multiple mechanisms in haematological malignancies. Hum. Mol. Genet., 8, 185–193. [DOI] [PubMed] [Google Scholar]
- D’Amico M. et al. (2000) The integrin-linked kinase regulates the cyclin D1 gene through glycogen synthase kinase 3β and CREB-dependent pathways. J. Biol. Chem., 275, 32649–32657. [DOI] [PubMed] [Google Scholar]
- Das A.K., Helps,N.R., Cohen,P.T. and Barford,D. (1996) Crystal structure of the protein serine/threonine phosphatase 2C at 2.0 Å resolution. EMBO J., 15, 6798–6809. [PMC free article] [PubMed] [Google Scholar]
- Ding V., Chen,R. and McCormick,F. (2000) Differential regulation of glycogen synthase kinase 3β by insulin and wnt signalling. J. Biol. Chem., 275, 32475–32481. [DOI] [PubMed] [Google Scholar]
- Dedhar S. (1999) Integrins and signal transduction. Curr. Opin. Hematol., 6, 37–43. [DOI] [PubMed] [Google Scholar]
- Dedhar S. and Hannigan,G.E. (1996) Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Curr. Opin. Cell Biol., 8, 657–669. [DOI] [PubMed] [Google Scholar]
- Dedhar S., Williams,B. and Hannigan,G. (1999) Integrin-linked kinase (ILK): a regulator of integrin and growth factor signaling. Trends Cell Biol., 9, 319–323. [DOI] [PubMed] [Google Scholar]
- Delcommenne M., Tan,C., Gray,V., Rue,L., Woodgett,J. and Dedhar,S. (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl Acad. Sci. USA, 95, 11211–11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easwaran V., Song,V., Polakis,P. and Byers,S. (1999) The ubiquitin– proteasome pathway and serine kinase activity modulate adenomatous polyposis coli protein-mediated regulation of β-catenin-lymphocyte enhancer-binding factor signaling. J. Biol. Chem., 274, 16641–16645. [DOI] [PubMed] [Google Scholar]
- Hanks S.K. and Polte,T.R. (1997) Signaling through focal adhesion kinase. BioEssays, 19, 137–145. [DOI] [PubMed] [Google Scholar]
- Hannigan G.E. and Dedhar,S. (1997) Protein kinase mediators of integrin signal transduction. J. Mol. Med., 75, 35–44. [DOI] [PubMed] [Google Scholar]
- Hannigan G.E., Leung-Hagesteijn,C., Fitz-Gibbon,L., Coppolino,M.G., Radeva,G., Filmus,J., Bell,J.C. and Dedhar,S. (1996) Regulation of cell adhesion and anchorage-dependent growth by a new β 1-integrin-linked protein kinase. Nature, 379, 91–96. [DOI] [PubMed] [Google Scholar]
- Khwaja A., Rodriguez-Viciana,P., Wennstrom,S., Warne,P.H. and Downward,J. (1997) Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J., 16, 2783–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusuda K. et al. (1998) Mutational analysis of the domain structure of mouse protein phosphatase 2Cβ. Biochem. J., 332, 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D.K., Ellis,C.A., Edwards,P.A. and Hiles,I.D. (1999) Integrin-linked kinase regulates phosphorylation of serine 473 of protein kinase B by an indirect mechanism. Oncogene, 18, 8024–8032. [DOI] [PubMed] [Google Scholar]
- Morimoto A.M., Tomlinson,M.G., Nakatani,K., Bolen,J.B., Roth,R.A. and Herbst,R. (2000) The MMAC1 tumor suppressor phosphatase inhibits phospholipase C and integrin-linked kinase activity. Oncogene, 19, 200–209. [DOI] [PubMed] [Google Scholar]
- Mulrooney J., Foley,K., Vineberg,S., Barreuther,M. and Grabel,L. (2000) Phosphorylation of the β1 integrin cytoplasmic domain: toward an understanding of function and mechanism. Exp. Cell Res., 258, 332–341. [DOI] [PubMed] [Google Scholar]
- Novak A., Hsu,S.C., Leung-Hagesteijn,C., Radeva,G., Papkoff,J., Montesano,R., Roskelley,C., Grosschedl,R. and Dedhar,S. (1998) Cell adhesion and the integrin-linked kinase regulate the LEF-1 and β-catenin signaling pathways. Proc. Natl Acad. Sci. USA, 95, 4374–4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persad S., Attwell,S., Gray,V., Delcommenne,M., Troussard,A., Sanghera,J. and Dedhar,S. (2000) Inhibition of integrin-linked kinase (ILK) suppresses activation of protein kinase B/Akt and induces cell cycle arrest and apoptosis of PTEN-mutant prostate cancer cells. Proc. Natl Acad. Sci. USA, 97, 3207–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeva G., Petrocelli,T., Behrend,E., Leung-Hagesteijn,C., Filmus,J., Slingerland,J. and Dedhar,S. (1997) Overexpression of the integrin-linked kinase promotes anchorage-independent cell cycle progression. J. Biol. Chem., 272, 13937–13944. [DOI] [PubMed] [Google Scholar]
- Rodriguez P.L. (1998) Protein phosphatase 2C (PP2C) function in higher plants. Plant Mol. Biol., 38, 919–927. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sheen J. (1998) Mutational analysis of protein phosphatase 2C involved in abscisic acid signal transduction in higher plants. Proc. Natl Acad. Sci. USA, 95, 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strovel E.T., Wu,D. and Sussman,D.J. (2000) Protein phosphatase 2C α dephosphorylates axin and activates LEF-1-dependent transcription. J. Biol. Chem., 275, 2399–2403. [DOI] [PubMed] [Google Scholar]
- Tan C., Costello,P., Sanghera,J., Dominguez,D., Baulida,J., Garcia de Herreros,A. and Dedhar,S. (2001) Inhibition of integrin linked kinase (ILK) suppressed β-catenin-Lef/Tcf-dependent transcription and expression of the E-cadherin represor, snail, in APC–/– human colon carcinoma cells. Oncogene, 20, 133–140. [DOI] [PubMed] [Google Scholar]
- Tong Y., Quirion,R. and Shen,S.H. (1998) Cloning and characterization of a novel mammalian PP2C isozyme. J. Biol. Chem., 273, 35282–35290. [DOI] [PubMed] [Google Scholar]
- Wera S. and Hemmings,B.A. (1995) Serine/threonine protein phosphatases. Biochem. J., 311, 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Keightley,S.Y., Leung-Hagesteijn,C., Radeva,G., Coppolino,M., Goicoechea,S., McDonald,J.A. and Dedhar,S. (1998) Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression and tumorigenicity. J. Biol. Chem., 273, 528–536. [DOI] [PubMed] [Google Scholar]
- Zimmermann S. and Moelling,K. (1999) Phosphorylation and regulation of Raf by Akt (protein kinase B). Science, 286, 1741–1744. [DOI] [PubMed] [Google Scholar]