Abstract
Chemokine receptors of both the CC and CXC families have been demonstrated to undergo a ligand-mediated homodimerization process required for Ca2+ flux and chemotaxis. We show that, in the chemokine response, heterodimerization is also permitted between given receptor pairs, specifically between CCR2 and CCR5. This has functional consequences, as the CCR2 and CCR5 ligands monocyte chemotactic protein-1 (MCP-1) and RANTES (regulated upon activation, normal T cell-expressed and secreted) cooperate to trigger calcium responses at concentrations 10- to 100-fold lower than the threshold for either chemokine alone. Heterodimerization results in recruitment of each receptor-associated signaling complex, but also recruits dissimilar signaling path ways such as Gq/11 association, and delays activation of phosphatidyl inositol 3-kinase. The consequences are a pertussis toxin-resistant Ca2+ flux and trig gering of cell adhesion rather than chemotaxis. These results show the effect of heterodimer formation on increasing the sensitivity and dynamic range of the chemokine response, and may aid in understanding the dynamics of leukocytes at limiting chemokine concentrations in vivo.
Keywords: chemokine receptor/dimerization/G proteins/phosphatidyl inositol 3-kinase
Introduction
The chemokines are a family of pro-inflammatory cyto kines that attract and activate specific leukocyte types (Baggiolini, 1998). Based on the position of the first two canonical cysteine residues and the chromosomal location of the corresponding genes, two main chemokine families have been identified: CC and CXC (Rollins, 1997; Baggiolini, 1998). They act on monocytes, lymphocytes, natural killer (NK) cells, basophils, eosinophils and neutrophils (Rossi and Zlotnick, 2000). Chemokines mediate their effects via interactions with seven-transmembrane-domain glycoprotein receptors coupled to a G protein signaling pathway (G-protein-coupled receptors or GPCRs). This type of receptor consists of a single polypeptide chain with an extracellular N-terminal domain and a cytoplasmic C-terminal domain. The N-terminus and the extracellular domains have been implicated in receptor–ligand interaction, whereas the C-terminus and the intracellular domains cooperate to bind and activate the G proteins (Bockaert and Pin, 1999).
After binding to their specific receptors, and as occurs for other GPCRs (Hebert et al., 1996; Romano et al., 1996; Cvejic et al., 1997; Bai et al., 1998; Zeng et al., 1999), chemokines induce receptor homodimerization and subsequently activate the receptor-associated JAK kinase, possibly by transphosphorylation on tyrosine residues (Mellado et al., 2001). This may create Src homology 2 (SH2) docking sites, leading to the recruitment of STAT (signal transducers and activators of transcription) transcription factors. The highly conserved Tyr present in the DRY motif is a primary target for chemokine receptor phosphorylation; a Tyr-to-Phe mutation impairs Gi-mediated Ca2+ flux triggered by chemokine binding, as well as Gi association with the chemokine receptor (Mellado et al., 1998). This mutant form of the receptor behaves as a dominant negative, since co-transfection with wild-type receptor impairs its function (Rodríguez-Frade et al., 1999b). In another member of the seven-transmembrane-domain receptor family, it has been demonstrated that the response to γ-aminobutyrate (GABA) requires heterodimerization of the GABA receptor type 1 (GBR1) and GBR2 receptors (Kaupmann et al., 1998; White et al., 1998; Kuner et al., 1999), as physical interaction between GBR1 and GBR2 appears to be essential for the activation of potassium channels. Another group of GPCRs, the opioid receptors, undergoes heterodimerization (Jordan and Devi, 1999). In this case, there is clear biochemical and pharmacological evidence for the heterodimeriza tion of two functional opioid receptors, κ and δ. Heterodimerization of these receptors causes synergistic agonist binding and potentiates the biological signal, yet there are no biochemical data to explain this phenomenon.
A polymorphism reported for the CCR2 receptor, in which Val64 is replaced by Ile (CCR2V64I) and which occurs at an allelic frequency of 10–25%, is associated with a 2–4 year delay in progression to acquired immunodeficiency sydrome (AIDS) (Lee et al., 1998). Relatively few viral strains are reported to use CCR2 in conjunction with CD4 to infect cells (Premack and Schall, 1996; Berger et al., 1999); we have shown that the mechanism underlying this protective effect may be the ability of the CCR2V64I mutant receptor to heterodimerize with CCR5 and/or CXCR4 (Mellado et al., 1999).
The majority of chemokine receptors bind more than one chemokine; in addition, most cell types express multiple chemokine receptors, so that if one ligand or receptor is defective, an alternative set of chemokines and their receptors can carry out the biological function (Wuyts et al., 1997; Wolf et al., 1998; Johnston et al., 1999). Although in vitro studies show overlapping functions for several chemokines, their in vivo expression and function appear to be finely controlled. Three mechanisms can be conceived to participate in this control: (i) chemo kine or chemokine receptor availability; (ii) ligand– receptor interaction; and (iii) the signal transduction mechanism activated by the chemokine receptor. Here we examine the dynamic interactions between chemokines and cell surface chemokine receptors, and analyze how the presence of several chemokine receptors regulates the response to a specific chemokine. Our results provide biochemical and functional evidence for CCR2 and CCR5 receptor heterodimerization. These heterodimers are more efficient at inducing biological responses, illustrated by the 10- to 100-fold lower chemokine concentration required to trigger these responses. This increase occurs via the synergistic interaction of several signaling complexes recruited by each individual receptor. Furthermore, receptor heterodimerization associates specific signaling pathways, such as recruitment of Gq/11, a G protein insensitive to pertussis toxin (PTx). Heterodimeric chemokine receptor interaction may have implications in understanding the in vivo processes that hinder leukocyte rolling on blood vessels and induce leukocyte parking in tissues during inflammatory responses.
Results
The simultaneous presence of chemokines triggers a synergistic response mediated by heterodimerization of their receptors
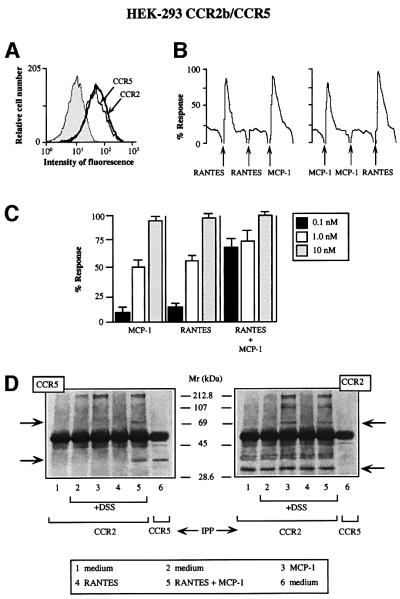
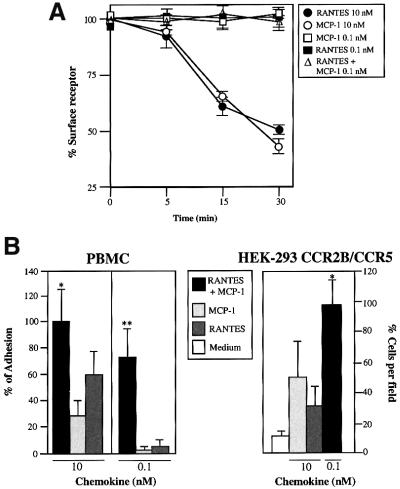
Using human embryonic kidney (HEK)-293 cells co-transfected with CCR2b and CCR5 receptors, we evaluated the potential of these chemokine receptors to induce functional responses following stimulation with a combination of chemokine ligands. The expression levels of the two receptors were quantified by flow cytometric analysis (Figure 1A) (Poncelet and Lavabre-Bertrand, 1993) and by their ability to respond in chemotaxis and in Ca2+ flux experiments to monocyte chemotactic protein-1 (MCP-1) or RANTES (regulated upon activation, normal T cell-expressed and secreted) (Figure 1B). In these cells, MCP-1 and RANTES sensitized responses to the homologous, but not to the heterologous chemokine. When MCP-1 and RANTES were added simultaneously to CCR2- and CCR5-co-transfected HEK-293 cells, Ca2+ flux was triggered at a concentration much lower than that required to induce a response by either chemokine alone (0.1 nM versus 1 nM; Figure 1C), indicating a cooperative effect when the two receptors bind their ligands simultaneously.
Fig. 1. Simultaneous MCP-1 and RANTES co-activation of CCR2- and CCR5-expressing cells increases sensitivity of chemokine responses and promotes their heterodimerization. (A) CCR2b/CCR5 double-transfected HEK-293 cells were incubated with biotin-labeled mAbs against CCR2 and CCR5 or their respective isotype-matched control mAbs, followed by isothiocyanate-labeled streptavidin. (B) Ca2+ mobilization was induced by treatment with 10 nM MCP-1 or 10 nM RANTES in Fluo-3-loaded CCR2/CCR5-co-transfected HEK-293 cells. Results are expressed as a percentage of the chemokine-induced calcium response. Five experiments were performed; the figure depicts one representative experiment. Arrows indicate addition of stimulus. (C) Ca2+ mobilization was determined as in (B), following stimulation with different concentrations of MCP-1 or RANTES as indicated, added separately or simultaneously. Results are expressed as a percentage of the maximum chemokine-induced calcium response. The mean ± SD of four independent experiments is shown. (D) CCR2/CCR5-co-transfected HEK-293 cells were stimulated with chemokines (10 nM for 5 min at 37°C) and, where indicated, cross-linked with 1 mM DSS. Cell lysates were immunoprecipitated with anti-CCR2 antibody, electrophoresed and transferred to nitrocellulose membranes. The western blot was analyzed with anti-CCR5 antibody (left); as a positive control, unstimulated CCR2/CCR5-co-transfected HEK-293 cells were immunoprecipitated with anti-CCR5 antibody (lane 6). The membrane was stripped and reprobed with anti-CCR2 antibody as a control for protein loading (right). Arrows indicate the position to which monomers and dimers migrated.
We have shown that the initiation of chemokine signaling through the CCR2, CCR5 and CXCR4 chemokine receptors involves ligand-triggered receptor homodimerization (Rodríguez-Frade et al., 1999a,b; Vila-Coro et al., 1999). In an attempt to understand the mechanisms underlying the RANTES- and MCP-1-promoted synergistic response in Ca2+ mobilization, and based on the observed heterodimerization of other GPCRs, we tested whether chemokine binding also triggered receptor heterodimerization. CCR2b/CCR5-co-transfected HEK- 293 cells were stimulated with MCP-1, RANTES or equimolar concentrations of both. Cells were then cross-linked using disuccinimidyl suberate (DSS), lysed and immunoprecipitated with an anti-CCR2 antibody, and the western blot was developed with anti-CCR5 (Figure 1D, left) or anti-CCR2 antibodies (Figure 1D, right). In accordance with previous results (Rodríguez-Frade et al., 1999b), stimulation with MCP-1 alone induced dimerization of the CCR2 receptor (Figure 1D, right), but not of the CCR5 receptor (Figure 1D, left), as determined by the presence of higher molecular weight complexes. In the converse experiment, RANTES induced homodimerization of the CCR5 receptor, but not of the CCR2 receptor (not shown). The simultaneous presence of MCP-1 and RANTES promoted the formation of CCR2 homodimers (Figure 1D, right), CCR5 homodimers (not shown) and, interestingly, CCR2–CCR5 heterodimers (Figure 1D, left). The same results were obtained when double-transfectant HEK-293 cells were stimulated simultaneously with MCP-1 and RANTES, lysed and immunoprecipitated with anti-CCR5 antibody, and the western blot developed with anti-CCR2 antibody (not shown). We conclude, therefore, that the CCR2 and CCR5 receptors can form heterodimers following simultaneous stimulation with the ligands of both receptors. Neither synergistic chemokine responses nor heterodimerization were observed in cells expressing CCR2 and CXCR4 after stimulation with MCP-1 and SDF-1α (not shown).
Chemokine receptor heterodimerization regulates chemokine responses
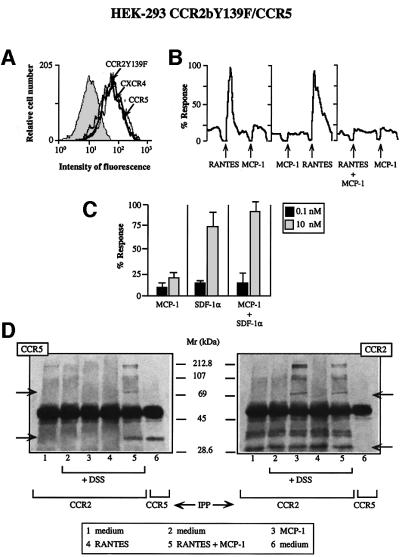
We showed previously that the CCR2bY139F mutant under goes receptor homodimerization in response to MCP-1 and heterodimerization with the CCR2b wild-type receptor, blocking the response to MCP-1 (Rodríguez-Frade et al., 1999b). To evaluate the functional consequences of CCR5 and CCR2 heterodimerization, CCR5 and the dominant-negative CCR2bY139F mutant receptor (Rodríguez-Frade et al., 1999b) were co-transfected into HEK-293 cells. Receptor expression in these cells was quantified by flow cytometry using specific antibodies (Figure 2A). CCR2bY139F expression in HEK-293 cells resulted in an impaired response to MCP-1 (Figure 2B), as would be predicted based on previous results. Co-expression of CCR5 and CCR2bY139F did not affect the RANTES Ca2+ mobilization response, but co-addition of MCP-1 and RANTES blocked this response (Figure 2B). In control experiments, CCR2bY139F did not block the response to SDF-1α, even with simultaneous addition of MCP-1 and SDF-1α (Figure 2C), whose receptor, CXCR4, is constitutively expressed in HEK-293 cells. Here we demonstrate that, in the presence of RANTES and MCP-1, the CCR2bY139F mutant also dimerizes with the CCR5 receptor. Indeed, when cells were cross-linked and immunoprecipitated with anti-CCR2b antibody and the western blot developed with anti-CCR5 antibody (Figure 2D, left), we observed heterodimer formation only in the presence of both MCP-1 and RANTES (Figure 2D, left, lane 5). In anti-CCR2b antibody immunoprecipitates developed in western blotting with the same antibody, CCR2 dimers were observed after stimulation with MCP-1 or MCP-1 plus RANTES (Figure 2D, right). We conclude that these two receptors, CCR5 and CCR2bY139F, undergo heterodimerization after stimulation with MCP-1 plus RANTES, impairing the downstream responses to RANTES.
Fig. 2. The mutant CCR2bY139F receptor impairs the response to RANTES but to SDF-1α. (A) CCR2bY139F/CCR5 double-transfected HEK-293 cells were incubated with biotin-labeled mAbs to CCR2, CCR5 or CXCR4 or their respective isotype-matched control mAbs, followed by isothiocyanate-labeled streptavidin. (B) Ca2+ flux was triggered by 10 nM RANTES, 10 nM MCP-1, or a combination of both chemokines (0.1 nM each) as indicated using CCR2bY139F/CCR5-co-transfected HEK-293 cells. Results are expressed as a percentage of the maximum chemokine-induced Ca2+ response. The figure depicts one representative experiment of four performed. (C) Ca2+ mobilization was determined as in Figure 1B, following stimulation of CCR2b Y139F/CCR5-co-transfected HEK-293 cells with different concen trations of MCP-1 or SDF-1α, separately or simultaneously as indicated. Results are expressed as a percentage of the maximum chemokine-induced response. The mean ± SD of three independent experiments is shown. (D) HEK-293 cells co-transfected with the CCR2bY139F and CCR5 receptors were processed as in Figure 1D. Arrows indicate the monomer and the dimer.
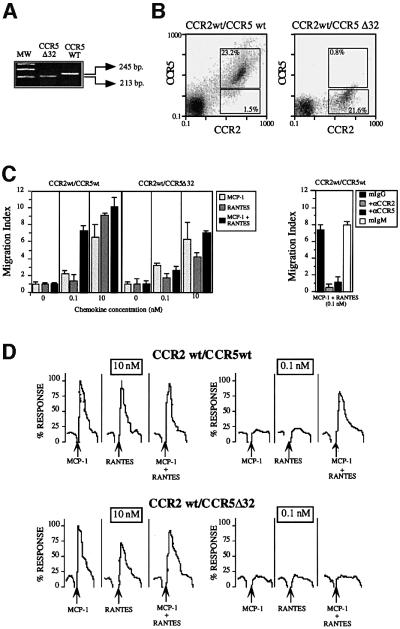
To exclude the possibility that the synergistic response observed with the combination of RANTES and MCP-1 was due to CCR5 and CCR2 receptor overexpression, we tested the effects of these two chemokines on peripheral blood mononuclear cells (PBMC) derived from a CCR5 homozygotic donor and on PBMC derived from a donor bearing the CCR5Δ32 mutation and therefore not expressing the CCR5 receptor (Benkirane, 1997) (Figure 3A). Expression of CCR2 and CCR5 was assessed by flow cytometry using specific antibodies. In CCR5 wild-type donors, 15–20% of CD3+ and 30–35% of CD14+ cells expressed both types of receptor, and very few cells were detected that expressed CCR2 or CCR5 alone. PBMC from the CCR5Δ32 donor showed similar CCR2 levels and cell distribution, and no significant expression of CCR5 (Figure 3B). When combined, RANTES and MCP-1 triggered both a chemotactic response (Figure 3C, left) and Ca2+ flux (Figure 3D, upper panel) at a concentration 10–100 times lower than that of either one alone in CCR5 homozygotic donors. This synergistic effect was not seen for the CCR5Δ32 (Figure 3D, lower panel), although we observed a RANTES-mediated response when PBMC from this donor were stimulated with the chemokine. This synergistic effect may be due to a RANTES-mediated response using a receptor other than CCR5; in fact, MIP-1β, a specific ligand for CCR5, promoted no response (not shown). To confirm that heterodimerization requires specific, simultaneous activation of both receptors, we used antagonistic anti-CCR2 and -CCR5 monoclonal antibodies (mAbs) (Mellado et al., 1998; Rodríguez-Frade et al., 1999a). Both of these mAbs completely blocked the synergistic response in PBMC from CCR5 homozygous donors (Figure 3C, right), suggesting that heterodimerization is involved in this response.
Fig. 3. Simultaneous MCP-1 and RANTES co-activation of PBMC increases sensitivity of chemokine responses. (A) Amplification of gene fragments corresponding to the CCR5 (245 bp) and CCR5Δ32 (213 bp) with specific primers as described in Materials and methods using genomic DNA from PBMC isolated from CCR5-homozygous and CCR5Δ32-homozygous donors. (B) PBMC from CCR5 wild-type and CCR5Δ32 donors were incubated with anti-CCR2, anti-CCR5 mAbs or their respective isotype-matched control mAbs in the presence of an excess of human immunoglobulins, followed by fluorescein isothiocyanate-labeled anti-mouse IgG and phycoerythrin (PE)-labeled anti-mouse IgM antibodies. The figure also shows the percentage of double-staining cells and single positives. (C) PBMC from CCR5- and CCR5Δ32-homozygous donors were allowed to migrate following stimulation with MCP-1 or RANTES, added separately or simul taneously as indicated. The migration index was calculated as described in Materials and methods. Data represent the mean of quadruplicate determinations, with the SD indicated. Migration of PBMC from CCR5-homozygous donors in response to 0.1 nM MCP-1 plus 0.1 nM RANTES was blocked by pre-treatment of the cells with antibodies against CCR2 and CCR5 (50 µg/ml for 30 min at 37°C). As a control, pre-treatment with isotype-matched antibodies is also shown. (D) Ca2+ flux was triggered by 10 nM or 0.1 nM RANTES, MCP-1, or a combination, using PBMC from CCR5- and CCR5Δ32-homozygous donors. Results are expressed as a percentage of the maximum chemokine-induced calcium response. The figure depicts one representative experiment of four performed.
Receptor heterodimerization results in recruitment of both receptor-associated signaling complexes
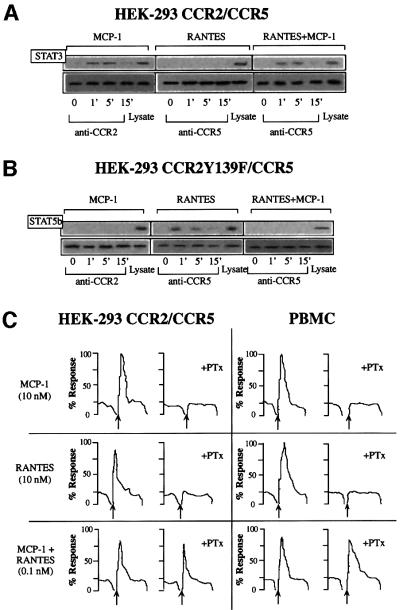
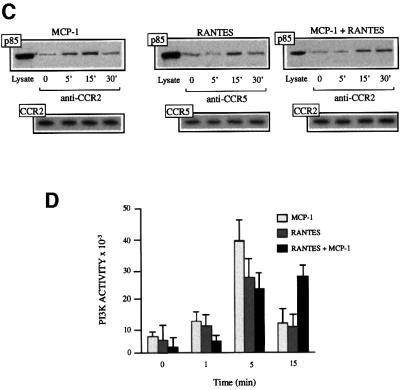
We next analyzed whether heterodimers could recruit the signaling complex associated with either receptor. In RANTES-stimulated, CCR5-transfected HEK-293 cells, we identified JAK1, but not JAK2 or JAK3, associated with the CCR5 receptor, whereas in CCR2-transfected HEK-293 cells, MCP-1 stimulation promoted JAK2 association with the receptor (Mellado et al., 1998; Rodríguez-Frade et al., 1999a). The identification of the downstream signaling pathway activated by JAK1/JAK2 kinases has also revealed phosphorylated STAT5 transcription factors in anti-CCR5 immunoprecipitates (Rodríguez-Frade et al., 1999a) and STAT3 in anti-CCR2 immunoprecipitates (Mellado et al., 1998). As predicted, when CCR2- and CCR5-co-transfected HEK- 293 cells were stimulated with MCP-1, STAT3 was observed in the immunoprecipitates obtained with the anti-CCR2 antibody (Figure 4A, left). When these cells were stimulated with RANTES, however, anti-CCR5 antibody did not precipitate STAT3, as seen in the western blot (Figure 4A, center). When the cells were stimulated with both MCP-1 and RANTES, STAT3 was found in the CCR5 receptor immunoprecipitates (Figure 4A, right). To check that equivalent amounts of protein had been loaded, membranes were stripped and probed with the immunoprecipitating antibodies (Figure 4A, lower panels). Since STAT3 does not associate with RANTES-activated CCR5, it can be inferred that the simultaneous presence of MCP-1 and RANTES triggers the formation of a CCR2–CCR5 complex, which recruits the signaling machinery associated with each of the receptors.
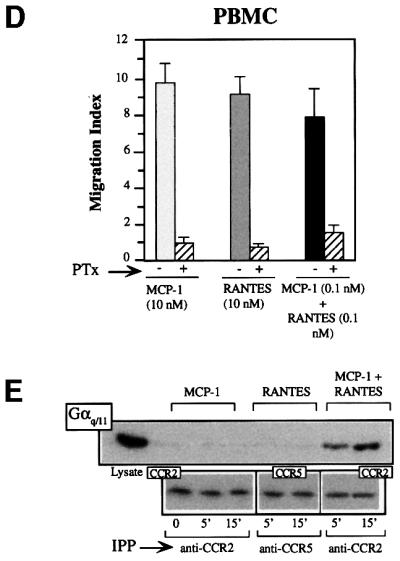
Fig. 4. Heterodimerization of chemokine receptors results in recruitment of specific signaling events. (A) Serum-starved CCR2/CCR5-transfected HEK-293 cells (10 × 106) were used alone or treated with 10 nM MCP-1, RANTES, or a combination of both for the times indicated. Cell lysates were immunoprecipitated with anti-CCR2 (left) or anti-CCR5 antibody (center and right), and western blots were developed with anti-STAT3 antibody. As a control, an unstimulated, unprecipitated transfected HEK-293 cell lysate was analyzed in a western blot with an anti-STAT3 antibody. In each case, CCR2 or CCR5 protein loading was assessed by reprobing membranes with anti-CCR2 or -CCR5 mAb. (B) Serum-starved, CCR2bY139F/CCR5-transfected HEK-293 cells were treated with 10 nM MCP-1, 10 nM RANTES, or a combination of MCP-1 and RANTES (10 nM of each). Cell lysates were immunoprecipitated with anti-CCR2 (left) or anti-CCR5 antibody (center and right) and western blots were developed with anti-STAT5b antibody. Protein loading was controlled for as in (A). (C) Ca2+ mobilization induced by MCP-1 (10 nM), RANTES (10 nM) or MCP-1 plus RANTES (0.1 nM of each) was determined in CCR2- and CCR5-co-transfected HEK-293 cells, and in PBMC from CCR5 wild-type donors untreated or pre-incubated with PTx. The figure depicts one representative experiment of three performed. (D) PBMC from CCR5 wild-type donors were used alone or pre-incubated with PTx, as indicated, and allowed to migrate following stimulation with MCP-1 or RANTES, added separately (10 nM each) or simultaneously (0.1 nM of each) as indicated. The migration index was calculated as described in Materials and methods. Data represent the mean of quadruplicate determinations, with the SD indicated. (E) Serum-starved CCR2- and CCR5-transfected HEK-293 cells (10 × 106) were used alone or treated with 10 nM MCP-1, RANTES, or a combination of both ligands for the times indicated. Cell lysates were immunoprecipitated with anti-CCR2 or anti-CCR5 antibody, and western blots developed with anti-Gq/11 antibody. As a control, an unstimulated, unprecipitated transfected HEK-293 cell lysate was analyzed in a western blot with anti-Gq/11 antibody. As a protein loading control, membranes were reprobed with the immunoprecipitating antibody.
Finally, we performed similar experiments in which HEK-293 cells co-expressing CCR5 and the CCR2b Y139F mutant were stimulated with MCP-1 and/or RANTES. STAT5b was detected in anti-CCR5 immunoprecipitates after stimulation with RANTES, but not after stimulation with MCP-1 and RANTES together (Figure 4B). These results and the impaired response to the combined stimulus in these co-transfected cells (Figure 2A) lead us to conclude that CCR2bY139F acts as a trans dominant-negative mutant, blocking RANTES responses by its ability to form non-productive complexes with partners containing the functional domain; this demonstrates the biological relevance of dimerization in chemokine responses.
Chemokine receptor heterodimers recruit unique signaling pathways
We have attempted to establish the molecular basis of this reduction in the threshold required to induce a biological response. Treatment with PTx abrogated both calcium release and migration in response to MCP-1 or RANTES (Figure 4C). However, when HEK-293 cells transfected with both CCR2b and CCR5 were stimulated simultaneously with 0.1 nM MCP-1 and 0.1 nM RANTES, PTx did not block the response (Figure 4C, left), illustrating the presence of a unique signaling pathway activated through receptor heterodimerization. Similar results were obtained when this assay was performed using PBMC derived from a normal donor, ruling out the possibility that this effect is an artifact due to the use of transfected cells (Figure 4C, right). In contrast, the synergistic migration induced by heterodimerization was sensitive to PTx (Figure 4D), suggesting that although Gαi is needed for chemotaxis, other factors are probably also required.
Some studies report that the calcium response to chemokines is not completely blocked by PTx (Al-Aoukaty et al., 1996; Arai and Charo, 1996; Kuang et al., 1996), suggesting that chemokine receptors may couple to multiple G proteins, such as Gi, Gq or Gs, depending on the chemokine receptor and/or the chemokine used. A CCR2–CCR5 receptor-associated protein distinct from Gi was immunoprecipitated and detected in western blots using an anti-Gq/11-specific antibody when cells were stimulated with the MCP-1–RANTES mixture, but not when the chemokines were used individually (Figure 4E). This association was also observed after immunoprecipitation with the CCR5-03 antibody following heterodimer formation, but not in homodimers. Finally, the failure of a specific antibody to detect Gq (not shown) prompted the conclusion that heterodimeric CCR2–CCR5 receptors associate specifically with G11. We conclude, therefore, that, in chemokine responses, distinct pathways can be activated in the simultaneous presence of chemokines able to bind receptors susceptible to heterodimerization. The implication of G11 in heterodimer activation would explain the resistance of Ca2+ flux to PTx treatment and the reduction in the chemokine response threshold.
Signaling through heterodimeric chemokine receptors fails to induce receptor down-regulation and triggers cell adhesion
To characterize the biological consequences of the specific signaling pathways activated by the heterodimer in greater detail, we analyzed whether the simultaneous presence of MCP-1 and RANTES modified the internalization process mediated by the individual activation of CCR2 and CCR5 by their respective ligands. Surprisingly, the simultaneous presence of these chemokines at concentrations that trigger receptor heterodimerization and Ca2+ mobilization did not promote receptor down-regulation, as measured by flow cytometry (Figure 5A).
Fig. 5. Chemokine receptor heterodimerization promotes specific signaling events but not receptor down-regulation. (A) Serum-starved CCR2/CCR5-transfected HEK-293 cells (10 × 106) were incubated for the times indicated with 0.1 nM and 10 nM RANTES, 0.1 nM and 10 nM MCP-1, or 0.1 nM of both chemokines at 37°C. Surface CCR2 or CCR5 was detected by fluorescence-activated cell sorting (FACS) analysis using biotin-labeled CCR2-03 mAb or CCR5-03 mAb, followed by streptavidin–PE; an isotype-matched mAb was used as control. Results are expressed as the percentage of maximum binding obtained in the absence of chemokines, with the SD indicated. (B) A static adhesion assay was performed using CCR2b- and CCR5-transfected HEK-293 cells (right) or PBMC from CCR5 wild-type donors (left), as described in Materials and methods. Stimuli include MCP-1 (10 nM), RANTES (10 nM), or MCP-1 plus RANTES (0.1 nM of each) for transfected cells, and 10 nM or 0.1 nM of MCP-1, RANTES, or both chemokines together for PBMC, as indicated. Results are expressed as a percentage of the maximum adhesion observed after stimulation with a mixture of RANTES plus MCP-1. The figure shows the mean ± SD of seven independent experiments. *Significantly different (p <0.05); **significantly different (p <0.01) (Student’s t-test). (C) Serum-starved CCR2- and CCR5-transfected HEK-293 cells (10 × 106) were incubated for the times indicated with 10 nM RANTES, 10 nM MCP-1, or 0.1 nM of each of these chemokines at 37°C. Cell lysates were immunoprecipitated with anti-CCR2 or anti-CCR5 antibody as indicated, and western blots were developed with anti-p85 antibodies (upper panel). CCR2 or CCR5 protein loading was controlled for as in Figure 4A (lower panel). (D) Serum-starved CCR2- and CCR5-transfected HEK-293 cells (10 × 106) were incubated for the indicated times with 10 nM RANTES, 10 nM MCP-1, or 0.1 nM of each of these chemokines combined at 37°C. Cell lysates were immunoprecipitated with anti-CCR2 or anti -CCR5 antibody, and an in vitro kinase assay was performed as indicated (Materials and methods). The mean ± SD of three independent experiments is shown.
Leukocyte recruitment is a multistep process that involves cell rolling, adhesion and migration (Springer, 1994); intracellular Ca2+ levels play an important role in all of these steps. We therefore analyzed adhesion of PBMC and CCR2–CCR5-co-transfected HEK-293 cells using collagen type VI as substrate. Simultaneous addition of these chemokines promoted more efficient cell adhesion than did the individual chemokines alone (Figure 5B). Other signals in addition to Ca2+ are proposed to regulate lymphocyte chemotaxis. Using phosphatidyl inositol 3-kinase (PI3K)γ–/– mice, the crucial role of this kinase has been demonstrated in neutrophil migration (Li et al., 2000) and in macrophage accumulation in inflammatory processes (Sasaki et al., 2000). Other PI3K family members have active roles in chemokine-mediated adhesion, polarization and migration (Turner et al., 1995; Vicente-Manzanares et al., 1999). Stimulation with MCP-1 or RANTES alone promoted rapid association of the p85 regulatory subunit of PI3K with CCR2 and CCR5, respectively, reaching a maximum 5 min after activation (Figure 5C). This association was followed by rapid dissociation, which was almost complete 15–30 min after stimulation. When PI3K activity was evaluated in anti-CCR2 and anti-CCR5 immunoprecipitates, results agreed with those of p85 association (Figure 5D), concurring with earlier reports (Turner et al., 1995, 1998). In contrast, simultaneous receptor activation with both ligands did not promote early PI3K activation or p85 association with the chemokine receptor, but rather led to a delayed and sustained activation, with a response peak at 15–30 min (Figure 5C and D). We conclude that there is a clear difference in PI3K activation by homo- or heterodimers: the former is early and transient, whereas the latter is slow but sustained. The reduction in the chemokine response threshold, the inability to trigger receptor down-regulation, the resistance to PTx and the sustained PI3K activation promoted by the heterodimers indicate activation of a different pathway as a consequence of heterodimer formation.
Discussion
The classical view of chemoattractant receptor signaling requires activation of the G protein pathway after chemokine binding. Signaling studies have revealed potent, chemokine-dependent inhibition of adenylyl cyclase and mobilization of intracellular calcium, consistent with receptor coupling to Gαi. We have described the physical association of Gαi with CCR2, CCR5 and CXCR4 in response to MCP-1, RANTES and SDF-1α, respectively (Mellado et al., 1998; Rodríguez-Frade et al., 1999a; Vila-Coro et al., 1999). Consistent with Gi association, the majority of these responses are inhibited by treatment with PTx. Prior to Gi activation, initiation of chemokine signaling through the CCR2, CCR5 and CXCR4 receptors involves ligand-triggered receptor dimerization that enables activation of the JAK signaling pathway and chemokine-mediated STAT activation (Wong and Fish, 1998; Mellado et al., 2001).
Here we show that simultaneous stimulation with MCP-1 and RANTES induces the formation of CCR2– CCR5 heterodimers. Compatible with this, the CCR2b Y139F mutant, which acts as dominant negative, blocking wild-type CCR2 (Rodríguez-Frade et al., 1999b), also impairs CCR5 signaling through the formation of non-functional heterodimers. To our knowledge, this is the first case in which mutations in one chemokine receptor affect, in trans, the response to a ligand acting on a distinct, non-cross-reactive receptor. These results increase the complexity of chemokine response biology, as the simultaneous presence of more than one chemokine may cause synergistic responses or, quite the opposite, suppress physiological effects in the response to unrelated chemokines. This is in accordance with the fact that heterodimerization probably occurs efficiently only between certain pairs of chemokine receptors. The implications of this finding may be important in understanding the role of chemokines in inflammation or as suppressors of human immunodeficiency virus (HIV)-1 infection, to mention just two cases in which these molecules arouse interest (Cairns and D’Souza, 1998; Lee et al., 1998; Littman, 1998; Berger et al., 1999).
The response to GABA requires heterodimerization of its two receptors (Kaupmann et al., 1998; White et al., 1998; Kuner et al., 1999). Physical interaction between GBR1 and GBR2 seems to be essential for the coupling of GABA receptors to G protein-coupled inwardly rectifying potassium channels, and for subsequent modulation of neurotransmission. GABA receptor dimerization appears to take place between the intracellular C-termini, probably through conserved coiled-coil domains, although other receptor regions may also intervene. The results presented here, together with those showing the ability of CXCR4 to dimerize with the CCR2V64I mutant but not with wild-type CCR2 (Mellado et al., 1999), suggest that V64, a residue located in the transmembrane I domain, is critical for dimer stabilization. It is also known that some GPCRs may dimerize using transmembrane domains; for example, dimerization of the β-adrenergic receptor involves transmembrane domain VI (Hebert et al, 1996).
The κ and δ opioid receptors also heterodimerize (Jordan and Devi, 1999), forming a new receptor, with ligand-binding and functional properties distinct from those of either receptor alone; this new receptor can be activated synergistically by selective ligands (Jordan and Devi, 1999). The heterodimeric chemokine receptor also exhibits unique features, as PTx-independent Ca2+ flux indicates distinct properties. The heterodimer promotes specific Gq/11 recruitment, explaining the PTx-resistant calcium flux, and also shows differences in PI3K activation. The fact that PTx blocks chemotaxis triggered by the combination of chemokines may be surprising. Nonetheless, when this fact is considered together with Gq/11 activation, it concurs with previous reports showing that Gq/11 is not required for migration (Bowman et al., 1998; Soede et al., 2000). Gq/11 is, however, probably implicated in other effects necessary for chemotaxis, such as integrin activation, which may be mediated via RhoA (Katoh et al., 1998). It has also been shown that chemokines can couple to more than one G protein (Al-Aoukaty et al., 1996; Maghazachi, 1999), as has been reported for other GPCRs (Daaka et al., 1997; Luo et al., 1999). Regulator of G-protein signaling (RGS) family members may also be involved in this process, as RGS proteins regulate cellular migratory and pro-adhesive responses to chemoattractants (Bowman et al., 1998), a fact related to the selectivity of RGS for Gi or Gq (Carman et al., 1999). Both signals have an important role in the sequential steps leading to leukocyte recruitment, including cell rolling, adhesion and migration (Springer, 1994). The crucial role of PI3Kγ in neutrophil migration (Li et al, 2000) and in the accumulation of macrophages in inflammatory processes has recently been shown (Sasaki et al, 2000). Although it appears that neutrophils from PI3Kγ–/– mice adhere to fibronectin-coated surfaces (Sasaki et al, 2000) as tightly as do PI3Kγ+/– cells, suggesting that decreased chemotaxis is due to impaired motility and not to altered adhesion, it is known that other PI3K family members regulate cell adhesion and polarization (Sánchez-Madrid et al., 1999). PI3Kγ activation after chemokine stimulation nonetheless occurs very rapidly, and is activated in vitro directly by G protein βγ subunits (Toker and Cantley, 1997). It is plausible, therefore, that under our experimental conditions we were detecting activation not of this particular PI3K isoform, but rather of other classical PI3K family members. We observed association with the receptor after both homo- and heterodimer activation of the p85 regulatory subunit of PI3K class Ia (not shown). In any case, the differences between homo- and heterodimers affect mainly a second, delayed wave of PI3K activity, and may be related to differences in cell adhesion. The heterodimer receptor also promotes a reduction in the chemokine response threshold. Increased sensitivity of hetero- compared with homodimers is also reported in the opioid response, in which the κ–δ heterodimer synergistically binds highly selective agonists and potentiates biological responses (Jordan and Devi, 1999).
In chemotaxis in bacteria, the chemotactic receptors form higher-order complexes, thought to be important for diversification of biological responses (Bray et al., 1998; Alon et al., 1999). We have shown that chemotactic responses in leukocytes are also achieved by adaptive receptor clustering (Rodríguez-Frade et al., 1999a,b; Vila-Coro et al., 1999). One implication of receptor clustering as a consequence of chemotactic responses is that the activity of one receptor would influence that of its neighbors, such that a ligand could act in trans on a receptor for which it is not specific. In this manner, the bound ligand induces changes in receptor signaling activity, which are propagated to a large number of neighboring receptors, amplifying the effect of a binding event. In other words, chemokine binding triggers receptor clustering that adapts to external stimuli. As shown here and previously (Rodríguez-Frade et al., 1999a,b; Vila-Coro et al., 1999), ligand binding causes aggregation of chemokine receptors, allowing tyrosine phosphorylation of cytoplasmic domains, recruitment of JAK tyrosine kinases and STAT transcription factors, and downstream signal transmission. Here we broaden this to include receptor heterodimers as complexes able to mediate activities different from those governed by homodimers.
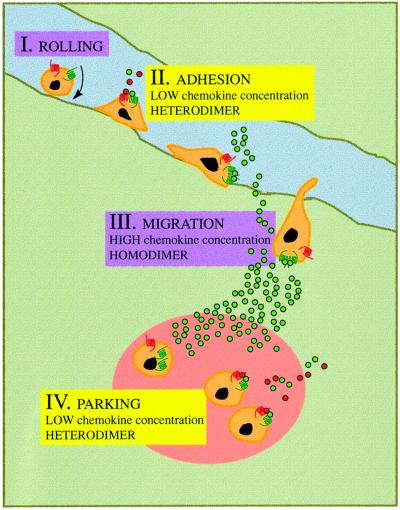
Receptor sensitivity can be regulated by the formation of signaling domain complexes dictated qualitatively by chemokine availability. Chemokines are produced within specific tissues and immobilized by low-affinity binding to heparin-bearing proteoglycans on the vascular endothelial barrier (Rollins, 1997); this would permit effective chemokine presentation to the rolling leukocytes. Variations in the availability of these presentation molecules or the chemokines produced would thus dramatically affect the ability of a chemokine to interact with a receptor. At low chemokine concentrations, receptor heterodimerization would be favored, and so cell adhesion would be triggered and leukocyte rolling stopped. Under these conditions, following leukocyte migration into tissues, heterodimers would preferentially favor ‘parking’ of leukocytes (Figure 6). The sum of these findings may help understand the molecular mechanisms involved in chemokine receptor signaling desensitization through heterologous chemokines, as well as the mechanisms that regulate cell migration during immune and inflammatory responses. One selective advantage of receptor homo- or heterodimerization lies in the augmented sensitivity of the system, such that chemokine responses increase with the spread of activity through a receptor array, illustrated by the abundance of chemokine receptors expressed on cell surfaces.
Fig. 6. The physiological role of chemokine receptor dimerization. Leukocytes roll along the blood vessel endothelium (I); exposure to low chemokine concentrations causes the formation of chemokine receptor heterodimers and the cell adheres to the endothelium (II). The inflammatory response produces higher chemokine concentrations, triggering receptor homodimerization; this induces cell migration through the endothelium to inflammation sites (III), where low chemokine concentrations favor heterodimerization, leading the cell to adhere (‘park’) in the tissues (IV).
Finally, chemokine receptors are critical in the control of inflammatory responses, and so are potential targets for the treatment of chronic disease (Broxmeyer et al., 1993). Through these receptors, cells sense attractants over a concentration range of several orders of magnitude, raising anew the unsolved question as to how chemotactic responses achieve their combination of sensitivity and dynamic range. The results provided here may help to resolve this conundrum.
Materials and methods
Biological materials, proteins and antibodies
HEK-293 cells (ATCC TIB202) were from the American Type Culture Collection (Manassas, VA). mAbs against CCR2, CCR5 and CXCR4 were generated in our laboratory (Mellado et al., 1998; Rodríguez-Frade et al., 1999a; Vila-Coro et al., 1999). MCP-1, RANTES and SDF-1α were from Peprotech (London, UK). Antibodies against STAT3 and Gαq/11 were from Santa Cruz Biotech (Santa Cruz, CA), while that against PI3-kinase p85 was from Upstate Biotech (Lake Placid, NY). mAbs against human CD3, CD14 and CD19 were from Immunotech (Marseille, France).
PBMC
Freshly isolated whole blood from healthy donors, CCR5 homozygotes and the CCR5Δ32 homozygote was obtained in citrate buffer. Blood at room temperature (RT) was added to Accuspin tubes (Sigma) and centrifuged (700 g for 15 min). The PBMC band was collected and washed twice (220 g for 10 min at RT) and three times (100 g for 10 min at RT) with Dulbecco’s phosphate-buffered saline (PBS), resulting in a population containing 65–75% CD3+, 15–20% CD14+ and 5–15% CD19+ cells.
Flow cytometric analysis
Cells were centrifuged (250 g for 10 min at RT), plated in V-bottomed 96-well plates (2.5 × 105 cells/well) and incubated with 50 µl/well biotin-labeled mAb (5 µg/ml for 30 min at 4°C). Cells were washed twice in PBS with 2% bovine serum albumin (BSA) and 2% fetal calf serum (FCS) and centrifuged (250 g for 5 min at 4°C). Fluorescein isothiocyanate-labeled streptavidin (Southern Biotechnologies, Birmingham, AL) was added, the mixture was incubated (30 min at 4°C) and plates were washed twice. Cell-bound fluorescence was measured at 525 nm in a Profile XL flow cytometer (Coulter, Miami, FL). Chemokine receptor expression was assessed by flow cytometry and quantified using a modification of the Dako Qifikit (Dako, Glostrup, Denmark), as described (Poncelet and Lavabre-Bertrand, 1993).
Calcium determination
Cells (2.5 × 106 cells/ml) were resuspended in RPMI containing 10% FCS and 10 mM HEPES, and incubated with Fluo-3 (Calbiochem; 300 µM in dimethylsulfoxide, 10 µl/106 cells, 30 min, 37°C). Cells were then washed, resuspended in RPMI containing 2 mM CaCl2 and maintained at 4°C before adding MCP-1, RANTES or SDF-1α. Calcium flux was measured at 525 nm in an EPICS XL flow cytometer (Coulter). When PTx pre-treatment was required, cells were incubated in culture medium with 0.1 µg/ml PTx (Sigma, St Louis, MO) overnight at 37°C. After washing, cells were resuspended and Ca2+ flux determined as above. For PBMC analysis, cells were loaded as before and calcium response evaluated separately in monocytes and lymphocytes. In this case, when PTx pre-treatment was required, cells were incubated (2 h at 37°C) in culture medium with 0.1 µg/ml PTx.
Transfection
HEK-293 cells, which constitutively express the CXCR4 receptor, were co-transfected with CCR2, CCR2bY139F or CCR5 constructs by calcium phosphate precipitation (Mellado et al., 1998; Rodríguez-Frade et al., 1999b). Transfected cells were selected in G-418 (Calbiochem) and analyzed by flow cytometry for receptor expression using antibodies against CCR2, -CCR5 and -CXCR4.
Cell migration
Migration of HEK-293 cells stably transfected with CCR2/CCR5 or CCR2bY139F/CCR5 was studied in a 96-well microchamber (NeuroProbe Inc., Gaithersburg, MD). Chemokines at several concentrations were loaded in lower wells (30 µl/well), and cells (200 µl/well, 3 × 106 cells/ml) were loaded in upper wells. Polyvinylpyrrolidone-free filters with 10 µm pores (NeuroProbe) were pre-coated for 2 h at 37°C with 20 µg/ml type VI collagen (Sigma). The chamber was incubated (37°C, 5% CO2) for 5 h, after which filters were removed and the cells in the upper part wiped off. The cells present in the filters were fixed and stained with crystal violet (0.5% crystal violet, 20% methanol). Blue spots developed at positions at which cell migration had occurred, allowing densitometric quantification of migration (National Institutes of Health Image software). The migration index was calculated by mean spot intensity.
For analysis of PBMC migration, cells (0.25 × 106 cells in 0.1 ml) were placed in the upper well of 24-well transmigration chambers (5 µm pore size; Transwell; Costar Corp., Cambridge, MA) pre-coated for 2 h at 37°C with 20 µg/ml type VI collagen (Sigma), and 0.1–10 nM MCP-1 (in 0.6 ml RPMI containing 0.25% BSA) was added to the lower well. Plates were incubated at 37°C for 120 min and cells that had migrated to the lower chamber were counted. Cell migration was calculated as the x-fold increase in migration observed over the medium control. To block ligand-induced chemotaxis, cells (2.5 × 106 cells/ml) were first incubated for 30 min at 37°C with anti-CCR2, anti-CCR5 or isotype-matched control mAbs (50 µg/ml).
Immunoprecipitation, SDS–PAGE and western blotting
Serum-starved cells (10 × 106) were lysed in a detergent buffer (20 mM triethanolamine pH 8.0, 300 mM NaCl, 2 mM EDTA, 20% glycerol, 1% digitonin, with 10 µM sodium orthovanadate, 10 µg/ml leupeptin and 10 µg/ml aprotinin) for 30 min at 4°C with continuous rocking, then centrifuged (15 000 g for 15 min at 4°C) (Mellado et al., 1998). For immunoprecipitation, protein extracts that had been cleared by incubation with anti-mouse IgG– or anti-mouse IgM–agarose (Sigma; 10 µg for 60 min at 4°C) were centrifuged (15 000 g for 1 min at 4°C) and immunoprecipitated with the appropriate antibody (5 µg/sample for 120 min at 4°C), followed by anti-mouse IgG– or IgM–agarose (20 µg for 60 min at 4°C). Immunoprecipitates or protein extracts were separated by SDS–PAGE and transferred to nitrocellulose membranes. Western blot analysis was as described, using 5% non-fat dry milk in Tris-buffered saline (TBS) as a blocking agent (Mellado et al., 1998). Membranes were stripped using 62.5 mM Tris–HCl pH 7.8, containing 2% SDS and 0.5% β-mercaptoethanol (60 min, 60°C), washed with 0.1% Tween-20 in TBS for 2 h, reblocked, reprobed and developed as above. In all cases, protein loading was controlled using a protein detection kit (Pierce, Rockford, IL).
Cross-linking
Serum-starved HEK-293 cells co-transfected with chemokine receptors (10 × 106) were stimulated with the indicated chemokines. The reaction was terminated by adding 1 ml of cold PBS and centrifuged (250 g for 10 min ). After washing twice with cold PBS, 10 µl of 100 mM DSS (Pierce) were added for 10 min at 4°C with continuous rocking (Rodríguez-Frade et al., 1999b). The reaction was terminated by adding 1 ml of cold PBS, centrifuged (15 000 g for 60 s) and then washed three times. The pellet was lysed and immunoprecipitated as above.
PI3 kinase assay
CCR2 or CCR5 immunoprecipitates were washed twice with lysis buffer, and twice with 50 mM Tris–HCl pH 7.4/100 µM EDTA before incubating with phosphatidyl inositol (PI) in reaction buffer (25 mM MgCl2, 20 µM ATP, 50 mM Tris–HCl pH 7.4, 10 µCi [γ-32P]ATP) (Amersham Pharmacia) for 10 min at room temperature. The phosphorylation reaction was terminated by adding 1 M HCl, and lipids were extracted with CHCl3/methanol. Radiolabeled lipids were resolved by thin-layer chromatography and radioactivity was analyzed with a GS-525 Molecular Imager System (Bio-Rad, Hercules, CA) with Molecular Analyst v. 2.2 software.
Static adhesion assays
Circles (0.5 cm diameter) were drawn on a glass slide using a wax pen (Dako) and coated with human collagen type VI (Sigma) at 20 µg/ml in 50 µl of endotoxin-free PBS (Gibco–BRL). As a negative control, 100% FCS was employed. After overnight incubation at 4°C, slides were washed three times in Dulbecco’s PBS and blocked for 1 h at 37°C with 100% FCS. HEK-293 CCR2/CCR5 transfectants were added (0.4 × 106 cells/ml in 50 µl), together with the indicated chemokines at a final concentration of 1 nM. After incubation (3 min at 37°C), slides were washed by dipping once in PBS to remove non-adherent cells, and bound cells were fixed in 1.5% glutaraldehyde/PBS. Data are expressed as the percentage of the maximum number of adherent cells counted in three random microscope fields/circle.
Cell adhesion assays
Flat-bottomed 96-well assay plates (Maxisorb, Nunc) were coated with 20 µg/ml collagen VI (Sigma) and blocked with 2% BSA. Freshly isolated PBMC were labeled with BCECF-AM [2′,7′-bis-(2-carboxyethyl)-5- (and -6)-carbofluorescein, acetoxymethyl ester; Molecular Probes, Eugene, OR] as described (Sanz-Rodríguez et al., 2001), and added in triplicate to the plate (105 cells/well). Plates were centrifuged at 30 g for 15 s and incubated at 37°C for 6 min, and unbound cells were removed by three washes with Dulbecco’s modified Eagle’s medium. Bound cells were quantified using a fluorescent analyzer (Cytofluor 2300; Millipore, Bedford, MA).
PCR reaction analysis of genomic DNA
Genomic DNA was isolated from PBMC of selected donors using an Easy DNA kit (Invitrogen). Upstream and downstream oligonucleotide primers used to amplify the CCR5 gene corresponded to the second extracellular region; the sequences of the 5′- and 3′ primers were CCTGGCTGTCGTCCATGCTG and CAAGCAGCGGCAGGACCAGC, respectively. Using this primer set, the wild-type CCR5 allele gives rise to a 245 bp PCR fragment, whereas the deleted allele gives a 213 bp fragment. For each PCR (100 µl), 1 µg of genomic of DNA was denatured at 95°C for 5 min, and amplified by five PCR cycles (94°C for 45 s; 55°C for 45 s; 72°C for 45 s), followed by 35 additional cycles (94°C for 45 s; 63°C for 45 s; 72°C for 30 s). The reaction products (25 µl) were run on 3% Nusieve GTG agarose gel and DNA bands were stained by ethidium bromide. In addition, CCR5 PCR fragments were subcloned and sequenced automatically.
Acknowledgments
Acknowledgements
We would like to thank Drs J.B.Stock, S.O’Brien and L.Birnbaumer for reading the manuscript. We also thank Drs J.Stein and J.Teixidó for help with adhesion experiments, M.C.Moreno and Dr I.López for help with flow cytometry, and C.Bastos and C.Mark for secretarial and editorial assistance, respectively. This work was partially supported by grants from Spanish National Plan for Scientific Development/FEDER EU and the Comunidad de Madrid. The Department of Immunology and Oncology was founded and is supported by the Spanish National Research Council (CSIC) and the Pharmacia Corporation.
References
- Al-Aoukaty A., Schall,T.J. and Maghazachi,A.A. (1996) Differential coupling of CC chemokine receptors to multiple heterotrimeric G proteins in human interleukin 2-activated natural killer cells. Blood, 87, 4255–4260. [PubMed] [Google Scholar]
- Alon U., Surette,M.G., Barkai,N. and Leibler,S. (1999) Robustness in bacterial chemotaxis. Nature, 397, 168–171. [DOI] [PubMed] [Google Scholar]
- Aragay A., Frade,J.M.R., Mellado,M., Serrano,A., Martínez-A.,C. and Mayor,F.,Jr (1998) MCP-1-induced CCR2B receptor desensitization by the G protein-coupled receptor kinase-2. Proc. Natl Acad. Sci. USA, 95, 2985–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai H. and Charo,I.F. (1996) Differential regulation of G-protein mediated signaling by chemokine receptors. J. Biol. Chem., 271, 21814–21819. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. (1998) Chemokines and leukocyte traffic. Nature, 392, 565–568. [DOI] [PubMed] [Google Scholar]
- Bai M., Trivedi,S. and Brown,E.M. (1998) Dimerization of the extracellular calcium-sensing receptor (CaR) on the cell surface of CaR-transfected HEK293 cells. J. Biol. Chem., 273, 23605–23610. [DOI] [PubMed] [Google Scholar]
- Benkirane M., Jin,D-Y., Chun,R.F., Koup,R.A. and Jeang,K-T. (1997) Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5Δ32. J. Biol. Chem., 272, 30603–30606. [DOI] [PubMed] [Google Scholar]
- Berger E.A., Murphy,P.M. and Farber,J.M. (1999) Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol., 17, 657–700. [DOI] [PubMed] [Google Scholar]
- Bockaert J. and Pin,J.P. (1999) Molecular tinkering of G protein-coupled receptors: an evolutionary success. EMBO J., 18, 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman E.P., Campbell,J.J., Druey,K.M., Scheschonka,A., Kehrl,J.H. and Butcher,E.C. (1998) Regulation of chemotactic and proadhesive responses to chemoattractant receptors by RGS (regulator of G-protein signaling) family members. J. Biol. Chem., 273, 28040–28048. [DOI] [PubMed] [Google Scholar]
- Bray D., Levin,M.D. and Morton-Firth,C.J. (1998) Receptor clustering as a cellular mechanism to control sensitivity. Nature, 393, 85–88. [DOI] [PubMed] [Google Scholar]
- Broxmeyer H.E., Sherry,B., Cooper,S., Lu,L., Maze,R.R., Beckmann,M.P., Cerami,A. and Ralph,P. (1993) Comparative analysis of the human macrophage inflammatory protein family of cytokines (chemokines) on proliferation of human myeloid progenitor cells. J. Immunol., 150, 3448–3458. [PubMed] [Google Scholar]
- Cairns J.S. and D’Souza,M.P. (1998) Chemokines and HIV-1 second receptors: the therapeutic connection. Nature Med., 4, 563–568. [DOI] [PubMed] [Google Scholar]
- Carman C.V., Parent,J.-L., Day,P.W., Pronin,A.N., Sternweis,P.M., Wedegaertner,P.B., Gilman,A.G., Benovic,J.L. and Kozasa,T. (1999) Selective regulation of Gαq/11 by an RGS domain in the G protein-coupled receptor kinase, GRK2. J. Biol. Chem., 274, 34483–34492. [DOI] [PubMed] [Google Scholar]
- Cvejic S. and Devi,L.A. (1997) Dimerization of the δ opioid receptor: implication for a role in receptor internalization. J. Biol. Chem., 272, 26959–26964. [DOI] [PubMed] [Google Scholar]
- Daaka Y., Luttrell,L.M. and Lefkowitz,R.J. (1997) Switching of the coupling of the β2-adrenergic receptor to different G proteins by protein kinase A. Nature, 390, 88–91. [DOI] [PubMed] [Google Scholar]
- Hebert T.E., Moffett,S., Morello,J.-P., Loisel,T.P., Bichet,D.G., Barret,C. and Bouvier,M. (1996) A peptide derived from a β2-adrenergic receptor transmembrane inhibits both receptor dimerization and activation. J. Biol. Chem., 271, 16384–16392. [DOI] [PubMed] [Google Scholar]
- Hebert T.E., Loisel,T.P., Adam,L., Ethier,N., Onge,S.S. and Bouvier,M. (1998) Functional rescue of a constitutively desensitized β2AR through receptor dimerization. Biochem. J., 330, 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston B., Burns,A.R., Suematsu,M., Issekutz,T.B., Woodman,R.C. and Kubes,P. (1999) Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J. Clin. Invest., 103, 1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan B.A. and Devi,L.A. (1999) G-protein-coupled receptor heterodimerization modulates receptor function. Nature, 399, 697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H., Aoki,J., Yamaguchi,Y., Kitano,Y., Ichikawa,A. and Negishi,M. (1998) Constitutively active Gα12, Gα13, and Gαq induce Rho-dependent neurite retraction through different signaling pathways. J. Biol. Chem., 273, 28700–28707. [DOI] [PubMed] [Google Scholar]
- Kaupmann K. et al. (1998) GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature, 396, 683–687. [DOI] [PubMed] [Google Scholar]
- Kuang Y., Wu,Y., Jiang,H. and Wu,D. (1996) Selective G protein coupling by C-C chemokine receptors. J. Biol. Chem., 271, 3975–3978. [DOI] [PubMed] [Google Scholar]
- Kuner R., Köhr,G., Grünewald,S., Eisenhardt,G., Bah,A. and Kornau, H.-C. (1999) Role of heteromer formation in GABAB receptor function. Science, 283, 74–77. [DOI] [PubMed] [Google Scholar]
- Lee B. et al. (1998) Influence of the CCR2-V64I polymorphism on HIV-1 coreceptor activity and chemokine receptor function of CCR2b, CCR3, CCR5 and CXCR4. J. Virol., 72, 7450–7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Jiang,H., Xie,W., Zhang,Z., Smrcka,A.V. and Wu,D. (2000) Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science, 287, 1046–1049. [DOI] [PubMed] [Google Scholar]
- Littman D.R. (1998) Chemokine receptors: keys to AIDS pathogenesis. Cell, 93, 677–680. [DOI] [PubMed] [Google Scholar]
- Luo X., Zeng,W., Xu,X., Popov,S., Davignon,I., Wilkie,T.M., Mumby,S.M. and Muallem,S. (1999) Alternate coupling of receptors to Gs and Gi in pancreatic and submandibular gland cells. J. Biol. Chem., 274, 17684–17690. [DOI] [PubMed] [Google Scholar]
- Maghazachi A.A. (1999) Intracellular signaling pathways induced by chemokines in natural killer cells. Cell. Signal., 11, 385–390. [DOI] [PubMed] [Google Scholar]
- Mellado M., Rodríguez-Frade,J.M., Aragay,A.M., del Real,G., Vila-Coro,A.J., Martín de Ana,A.M., Serrano,A., Mayor,F.,Jr and Martínez-A.,C. (1998) The chemokine MCP-1 triggers tyrosine phosphorylation of the CCR2B receptor and the JAK2/STAT3 pathway. J. Immunol., 161, 805–813. [PubMed] [Google Scholar]
- Mellado M., Rodríguez-Frade,J.M., Vila-Coro,A.J., Martín de Ana,A.M. and Martínez-A.,C. (1999) Chemokine control of HIV-1 infection. Nature, 400, 273–274. [DOI] [PubMed] [Google Scholar]
- Mellado M. Rodríguez-Frade,J.M., Mañes,S. and Martínez-A.,C. (2001) Chemokine signaling and functional responses: the role of receptor dimerization and TK pathway activation. Annu. Rev. Immunol., 19, 397–421. [DOI] [PubMed] [Google Scholar]
- Poncelet P.G. and Lavabre-Bertrand,T. (1993) Immunological detection of membrane-bound antigens and receptors. In Masseyeff,R.F., Albert,W.H. and Staines,N.A. (eds), Methods of Immunological Analysis. VCH, Weinheim, Germany, pp. 388–418.
- Premack B.A. and Schall,T.J. (1996) Chemokine receptors: gateways to inflammation and infection. Nature Med., 2, 1174–1178. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Frade J.M., Vila-Coro,A.J., Martín de Ana,A.M., Nieto,M., Sánchez-Madrid,F., Proudfoot,A.E.I., Wells,T.N.C., Martínez-A.,C. and Mellado,M. (1999a) Similarities and differences in RANTES- and (AOP)-RANTES-triggered signals: implications for chemotaxis. J. Cell Biol., 144, 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Frade J.M., Vila-Coro,A., Martín de Ana,A.M., Albar,J.P., Martínez-A.,C. and Mellado,M. (1999b) The chemokine monocyte chemoattractant protein-1 induces functional responses through dimerization of its receptor CCR2. Proc. Natl Acad. Sci. USA, 96, 3628–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B.J. (1997) Chemokines. Blood, 90, 909–928. [PubMed] [Google Scholar]
- Romano C., Yang,W.L. and O’Malley,K.L. (1996) Metabotropic glutamate receptor 5 is a disulfide-linked dimer. J. Biol. Chem., 271, 28612–28616. [DOI] [PubMed] [Google Scholar]
- Rossi D. and Zlotnik,A. (2000) The biology of chemokines and their receptors. Annu. Rev. Immunol., 18, 217–242. [DOI] [PubMed] [Google Scholar]
- Sánchez-Madrid F. and del Pozo,M.A. (1999) Leukocyte polarization in cell migration and immune interactions. EMBO J., 18, 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Rodríguez F., Hidalgo,A. and Teixidó,J. (2001) Chemokine stromal cell-derived factor-1a modulates VLA-4 integrin-mediated multiple myeloma cell adhesion to CS-1/fibronectin and VCAM-1. Blood, 97, 346–351. [DOI] [PubMed] [Google Scholar]
- Sasaki T. et al. (2000) Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science, 287, 1040–1046. [DOI] [PubMed] [Google Scholar]
- Soede R.D.M., Wijnands,Y.M., Kamp,M., van der Valk,M.A. and Roos,E. (2000) Gi and Gq/11 proteins are involved in dissemination of myeloid leukemia cells to the liver and spleen, whereas bone marrow colonization involves Gq/11 but not Gi. Blood, 96, 691–698. [PubMed] [Google Scholar]
- Springer T.A. (1994) Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell, 76, 301–314. [DOI] [PubMed] [Google Scholar]
- Toker A. and Cantley,L.C. (1997) Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature, 387, 673–676. [DOI] [PubMed] [Google Scholar]
- Turner L., Ward,S.G. and Westwick,J. (1995) RANTES-activated human T lymphocytes: a role for phosphoinositide 3-kinase. J. Immunol., 155, 2437–2444. [PubMed] [Google Scholar]
- Turner S.J., Domin,J., Waterfield,M.D., Ward,S.G. and Westwick,J. (1998) The CC chemokine monocyte chemotactic peptide-1 activates both the class Y p85/p110 phosphatidylinositol 3-kinase and the class II PI3K-C2α. J. Biol. Chem., 273, 25987–25995. [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M. et al. (1999) Involvement of phosphatidyl inositol 3-kinase in stromal cell-derived factor-1α-induced lymphocyte polarization and chemotaxis. J. Immunol., 163, 4001–4012. [PubMed] [Google Scholar]
- Vila-Coro A.J., Rodríguez-Frade,J.M., Martín de Ana,A.M., Moreno-Ortíz,M.C., Martínez-A.,C. and Mellado,M. (1999) The chemokine SDF-1α triggers CXCR4 receptor dimerization and activates the JAK/STAT pathway. FASEB J., 13, 1699–1710. [PubMed] [Google Scholar]
- White J.H. et al. (1998) Heterodimerization is required for the formation of a functional GABAB receptor. Nature, 396, 679–682. [DOI] [PubMed] [Google Scholar]
- Wolf M., Delgado,M.B., Jones,S.A., Dewald,B., Clark-Lewis,I. and Baggiolini,M. (1998) Granulocyte chemotactic protein 2 acts via both IL-8 receptors CXCR1 and CXCR2. Eur. J. Immunol., 28, 164–170. [DOI] [PubMed] [Google Scholar]
- Wong M. and Fish,E.N. (1998) RANTES and MIP-1α activate stats in T cells. J. Biol. Chem., 273, 309–314. [DOI] [PubMed] [Google Scholar]
- Wuyts A., Van Osselaer,N., Haelens,A., Samson,Y., Herdewijn,P., Ben-Baruch,A., Oppenheim,J.J., Proost,P. and Van Damme,J. (1997) Characterization of synthetic human granulocyte chemotactic protein 2: usage of chemokine receptors CXCR1 and CXCR2 and in vivo inflammatory properties. Biochemistry, 36, 2716–2723. [DOI] [PubMed] [Google Scholar]
- Zeng F.Y. and Wess,J. (1999) Identification and molecular characterization of m3 muscarinic receptor dimers. J. Biol. Chem., 274, 19487–19497. [DOI] [PubMed] [Google Scholar]