Abstract
The destruction box (D-box) consensus sequence has been defined as a motif mediating polyubiquitylation and proteolysis of B-type cyclins during mitosis. We show here that the regions with similarity to D-boxes are not required for mitotic degradation of Drosophila Cyclin A. Instead of a simple D-box, a complex N-terminal degradation signal is present in this cyclin. Mutations that impair or abolish mitotic Cyclin A destruction delay progression through metaphase, but only when overexpressed. Moreover, these mutations prevent epidermal cells from entering the first G1 phase of embryogenesis and lead to a complete extra division cycle instead of a timely cell proliferation arrest. Residual Cyclin A activity after mitosis, therefore, has S phase-promoting activity. In principle, an S phase defect could also explain why epidermal cells fail to enter mitosis 16 in mutants lacking zygotic Cyclin A function. However, we demonstrate that this failure of mitosis is not caused simply by DNA replication or damage checkpoints. Entry into mitosis requires a function of Cyclin A that does not depend on the presence of the N-terminal region.
Keywords: APC/cell proliferation/Cyclin A/degradation/mitosis
Introduction
Progression into and through mitosis is controlled by cyclin-dependent kinase 1 (cdk1). Metazoan species co-express A-, B- and B3-type cyclins, which all bind as regulatory subunits to cdk1 (Jacobs et al., 1998). The specific functions of these different heterodimeric cyclin– cdk1 complexes are poorly understood. Cyclin A–cdk1 complexes have been reported to be less prone to inhibition mediated by phosphorylation of cdk1 on conserved residues (Thr14, Tyr15) (Devault et al., 1992). Cyclin A–cdk1 might therefore be particularly effective in starting a positive feedback loop involving phosphorylation and consequential antagonistic regulation of kinases (wee1 and myt1) and phosphatases (cdc25B and C) targeting Thr14 and Tyr15. This feedback loop is thought to cause the abrupt maximal activation of cdk1, which enforces entry into mitosis. In addition, A- and B-type complexes appear to have different roles during spindle assembly. Cyclin A–cdk1 preferentially stimulates the microtubule-nucleating activity of centrosomes, and cyclin B–cdk1 increases microtubule turnover (Buendia et al., 1992).
While entry into mitosis requires cdk1 activity, exit from mitosis is dependent on inactivation of cdk1, which results from cyclin proteolysis (Murray et al., 1989). The degradation of A-, B- and B3-type cyclin proteins during mitosis involves ubiquitylation via the anaphase-promoting complex/cyclosome (APC/C) pathway (for a review see Zachariae and Nasmyth, 1999). This pathway is stimulated by cdk1 activity, which phosphorylates APC/C subunits during mitosis and allows binding of the temporary subunit Fizzy(FZY)/Cdc20 (Shteinberg et al., 1999; Kramer et al., 2000; Rudner and Murray, 2000). In contrast, another temporary subunit, Fizzy-related(FZR)/Hct1/Cdh1, is phosphorylated and inhibited from binding to the APC/C by cdk activity (Zachariae et al., 1998; Kramer et al., 2000). FZR therefore activates the APC/C only during late mitosis and G1 phase. FZY and FZR carry C-terminal WD40 repeat domains. Since substrate recruitment in the SCF ubiquitylation pathway can be mediated by WD40 repeat proteins, FZY and FZR might have an analogous role in the APC/C pathway.
The sequences required to recruit the mitotic cyclins to APC/C-dependent ubiquitylation have been analyzed most extensively in the case of B-type cyclins, which carry an RXXLXXXXN motif in the otherwise poorly conserved N-terminal regions. Mutations in the conserved positions of this destruction box (D-box) consensus interfere with ubiquitylation and mitotic degradation (Glotzer et al., 1991; King et al., 1996). The N-terminal region of A-type cyclins is clearly required for mitotic degradation (Luca et al., 1991; Sigrist et al., 1995), and D-box motifs are also present in the N-terminal region of most A-type cyclins. Mutations in these D-boxes were found to interfere with mitotic degradation in the case of a limpet and a Xenopus A-type cyclin (Kobayashi et al., 1992; Lorca et al., 1992).
Along with parallels, however, characteristic differences have also been described in the mitotic degradation of A- and B-type cyclins. Degradation of cyclin A is completed earlier in mitosis than cyclin B and B3 degradation (Lehner and O’Farrell, 1990; Minshull et al., 1990; Whitfield et al., 1990; Sigrist et al., 1995). In Drosophila embryos, overexpression of non-degradable Cyclins A, B and B3, lacking the N-terminal regions with the degradation signals, results in an enrichment of either metaphase figures, early anaphase figures or late anaphase figures, respectively (Rimmington et al., 1994; Sigrist et al., 1995). Thus, the sequential disappearance of Cyclins A, B and B3 might contribute to the temporal ordering of mitotic processes.
A striking difference is also observed in cells arrested in mitosis by microtubule poisons. While A-type cyclins are degraded in arrested cells, B-type cyclins are not (Whitfield et al., 1990). The inhibition of cyclin B degradation is mediated by a mitotic checkpoint pathway that detects the presence of kinetochores that are not correctly attached to a mitotic spindle (for a review see Shah and Cleveland, 2000). At unattached kinetochores, the Mad2 protein is thought to be converted into a form that binds and inhibits FZY/Cdc20. This suggestion can also explain why cell cycle progression beyond the metaphase–anaphase transition is dependent on the presence of a functional spindle with correctly attached chromosomes, since the FZY–APC/C complex is required not only for the degradation of cyclin B, but also for the degradation of securin proteins, which inhibit the separation of sister chromatids (Zachariae and Nasmyth, 1999). However, the fact that cyclin A degradation requires fzy function (Sigrist et al., 1995) and yet proceeds in checkpoint-arrested mitotic cells suggests that inhibition of FZY–APC/C activity by Mad2 is not complete, but specific for selected substrates like cyclin B and securin.
What is the mechanistic basis of this selectivity? What confers the temporally controlled disappearance of mitotic cyclins? This regulatory complexity rules out the simple assumption that degradation of both A- and B-type cyclins is controlled exclusively by functionally identical D-boxes. In fact, experiments in Xenopus extracts have demonstrated that degradation of cyclin A1 requires not only a D-box but also binding to cdk1, while the D-box of B1-type cyclins is also sufficient to confer mitotic degradation on some heterologous proteins (Stewart et al., 1994; King et al., 1996; Klotzbucher et al., 1996). To define the degradation signal of Drosophila Cyclin A in more detail, we have generated a series of mutants. Surprisingly, we find that the two putative D-boxes in the N-terminal region of Cyclin A are not required for mitotic degradation. Instead, mitotic Cyclin A degradation is dependent on the presence of a 53-amino-acid long N-terminal region. Stabilized Cyclin A versions prevent exit from the cell cycle and entry into G1 at the appropriate developmental stage. Moreover, they result in a metaphase delay, but only when overexpressed. Finally, we demonstrate that the C-terminal cyclin box region of Cyclin A is sufficient to provide a specific function required for entry into mitosis.
Results
The putative D-boxes are not required for mitotic degradation of Cyclin A
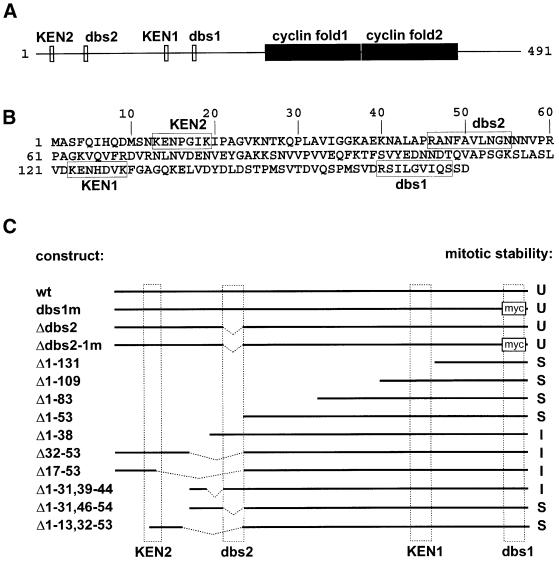
Our previous experiments with a heat-inducible transgene (Hs-ΔCycA) indicated that the first 170 amino acids of Drosophila Cyclin A are required for mitotic degradation (Sigrist et al., 1995). This region includes two putative D-boxes designated here as destruction box similarity regions 1 and 2 (dbs1 and dbs2) (Figure 1). To test whether these regions are required for mitotic Cyclin A degradation, we constructed mutant transgenes in which either one or the other, or both, were altered (Figure 1C). dbs1 was replaced by a myc epitope tag and dbs2 was deleted. The UAS/GAL4 system (Brand and Perrimon, 1993) was used to express these mutations.
Fig. 1. Identification of the degradation signal region in Drosophila Cyclin A. (A) The distribution of potential degradation signals with similarity to either the KEN-box (KEN1 and KEN2; Pfleger and Kirschner, 2000) or the D-box (dbs1 and dbs2; King et al., 1996) and the cyclin folds in Drosophila Cyclin A is illustrated. (B) The sequence of the first 170 amino acids of Drosophila Cyclin A is shown in one-letter code and potential degradation signals are boxed. Deletion of the first 170 amino acids results in a mutant protein that is stable during mitosis (Sigrist et al., 1995). (C) The N-terminal region in the different UAS transgenes allowing expression of either wild-type (wt) or mutant Cyclin A is illustrated. Dashed boxes indicate the position of KEN1, KEN2, dbs1 and dbs2. dbs1 is replaced with a myc epitope in the constructs dbs1m and Δdbs2-1m. During mitosis, the various transgene products were found to be unstable (U), of intermediate stability (I) or stable (S).
The prd-GAL4 driver results in UAS transgene expression in the epidermis of every other segment during the embryonic cell division cycles after cellularization. Since these embryonic division cycles occur in a stereotypic, segmentally repeated pattern, we were able to use those segments that do not express the UAS transgenes as internal controls, allowing for accurate staging and careful comparison of progression through the embryonic divisions in either the presence or absence of UAS transgene products.
Immunofluorescence experiments indicated that the different mutant Cyclin A proteins (Cyclin A-dbs1m, Cyclin A-Δdbs2, Cyclin A-Δdbs2-1m) were degraded during mitosis in a manner comparable to wild-type Cyclin A (Figures 1C and 2). The behavior of UAS transgene products was revealed by anti-Cyclin A labeling, because the UAS transgenes were expressed at higher levels than the endogenous CycA gene. The mutant Cyclin A proteins did not, or at most very subtly, interfere with progression through the cell division cycles (Figure 2 and data not shown, see below). These results indicate that dbs1 and dbs2 are not required for mitotic Cyclin A degradation.
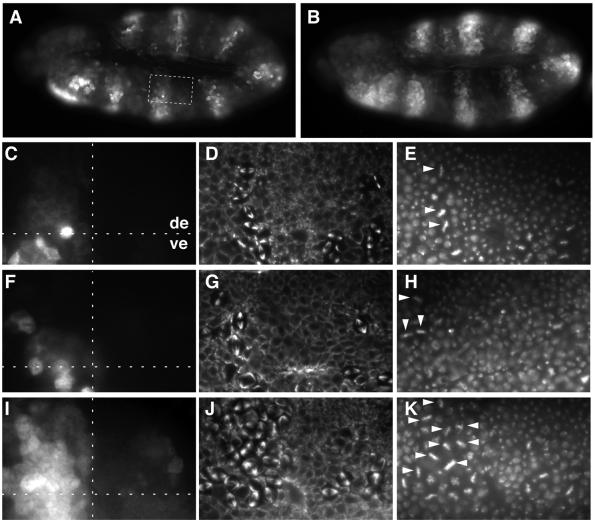
Fig. 2. Mitotic degradation of Cyclin A lacking D-box regions. UAS transgenes allowing expression of either wild-type Cyclin A (A and C–E; UAS-CycA), Cyclin A lacking regions with similarity to D-boxes (F–H; UAS-CycA-Δdbs2-1m) or the first 53 amino acids (B and I–K; UAS-CycA-Δ1–53) were overexpressed in alternating segments using prd-GAL4. Embryos at the stage when mitosis 16 is essentially completed in the dorsal epidermis (de) and just starting in the ventral epidermis (ve) were labeled with antibodies against Cyclin A (A–C, F and I), tubulin (D, G and J) and a DNA stain (E, H and K). Note that the expression from the endogenous CycA gene is barely detectable in (A–C), (F) and (I), because of the short exposure times chosen to visualize UAS transgene expression. High magnification views of the boxed region in (A) are shown in (C–E) and equivalent regions in (F–H) and (I–K). A horizontal dashed line marks the border between ventral and dorsal epidermis in (C), (F) and (I). A vertical dashed line in these panels indicates the border between regions that either express a UAS transgene (left side) or not (right side). Note that Cyclin A-Δdbs2-1m is degraded during mitosis 16 like wild-type Cyclin A. As a result, labeling with anti-Cyclin A is low in the upper left regions in (C) and (F). In contrast, Cyclin A-Δ1–53 is stable during mitosis. As a result, labeling with anti-Cyclin A persists (I) and metaphase figures are enriched in the upper left region (J and K). All metaphase figures within the upper left regions are indicated by arrowheads in (E), (H) and (K).
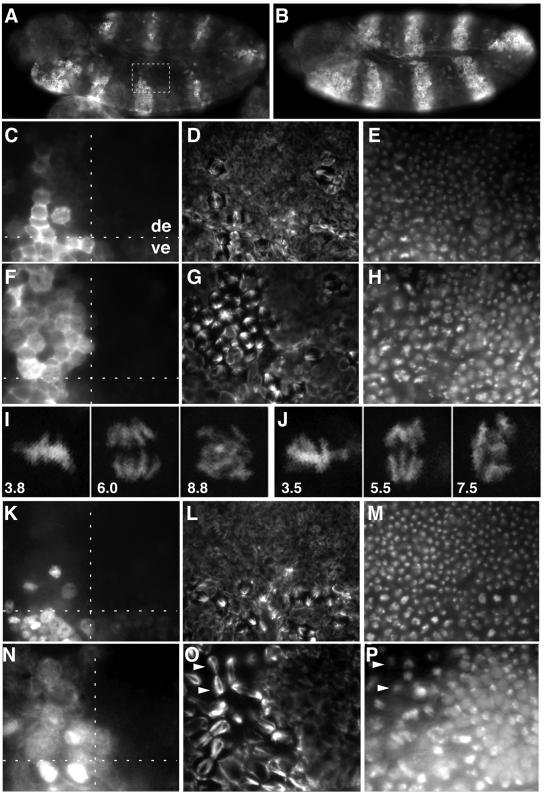
As the D-box-like regions in Drosophila Cyclin A were dispensable for mitotic degradation, we wondered whether the putative D-boxes in Drosophila Cyclin B and B3 are required for mitotic degradation. Therefore, we expressed transgenes encoding mutant Cyclin B or B3 protein with a myc epitope in place of the D-boxes (UAS-CycB-dbm, UAS-CycB3-dbm). These mutant cyclins were found to be stable during mitosis and to delay progression through mitosis (Figure 3). As previously observed with large N-terminal truncations (Sigrist et al., 1995), expression of the mitotically stable Cyclin B-dbm resulted in an enrichment of mitotic figures in which sister chromatids were separated but not segregated all the way to the spindle poles (Figure 3G–J), and expression of the mitotically stable Cyclin B3-dbm resulted in an enrichment of late mitotic figures in which sister chromatids were segregated to the spindle poles (Figure 3O and P). We conclude that the putative D-box regions in Cyclin B and B3 are required for mitotic degradation.
Fig. 3. D-boxes are required for mitotic degradation of Cyclin B and Cyclin B3. UAS transgenes allowing expression of either wild-type Cyclin B (A, C–E; UAS-CycB), Cyclin B without D-box (B and F–J; UAS-CycB-dbm), Cyclin B3 (K–M; UAS-CycB3) or Cyclin B3 without D-box (N–P; UAS-CycB3-dbm) were overexpressed in alternating segments using prd-GAL4 (A–H and K–P). Embryos at the stage when mitosis 16 is essentially completed in the dorsal epidermis (de) and just starting in the ventral epidermis (ve) were labeled with antibodies against Cyclin B (A–C and F), Cyclin B3 (K and N), tubulin (D, G, L and O) and a DNA stain (E, H, M and P). High magnification views of the boxed region in (A) are shown in (C–E) and equivalent regions in (F–H) and (K–P). The regions shown in (N–P) are at slightly higher magnification to illustrate the characteristic accumulation of late mitotic figures clearly. A typical example is indicated by white arrowheads in (O) and (P). A horizontal dashed line marks the border between ventral and dorsal epidermis in (C), (F), (K) and (N). A vertical dashed line in these panels indicates the border between regions that express a UAS transgene (left side) or not (right side). Note that Cyclin B-dbm and Cyclin B3-dbm are not degraded during mitosis 16. As a result, labeling with anti-Cyclin B (F) and anti-Cyclin B3 (N) persists, and mitotic figures are enriched in the upper left regions (G and H, and O and P, respectively). In vivo imaging of histone–GFP (I and J) demonstrates that UAS-CycB-dbm expression results in an arrest after sister chromatid separation. The cell in (I) is shown at 3.8, 6.0 and 8.8 min, and the cell in (J) at 3.5, 5.5 and 7.5 min after entry into mitosis 14.
To identify the elusive degradation signal of Cyclin A, we analyzed a series of N-terminal deletions (Figure 1C). Immunofluorescence experiments indicated that the first 53 N-terminal residues are required for mitotic degradation (Figures 1C and 2 and data not shown).
Cyclin A lacking the N-terminal residues 1–38 appeared to be partially stabilized during mitosis (Figure 1C). Therefore, we searched for a degradation signal within the surrounding regions by constructing several transgenes with internal deletions. The encoded mutant Cyclin A proteins still did not appear to be completely stable during mitosis. However, the combination of a small N-terminal deletion with an internal deletion (UAS-CycA-Δ1–13, 32–53) resulted in a protein that was essentially stable during mitosis (Figure 1C). Thus, the N-terminal residues 1–13 of Cyclin A appear to contribute to the mitotic degradation signal. Interestingly, this region includes a KEN-box, a motif which has recently been shown to mediate APC/C-dependent ubiquitylation of Xenopus FZY/Cdc20, as well as of other proteins (Petersen et al., 2000; Pfleger and Kirschner, 2000). However, we emphasize that this region (Figure 1, KEN 2) is clearly not sufficient to bring about normal mitotic degradation, as evidenced by the compromised degradation of Cyclin A-Δ31–53, which carries the N-terminal region including KEN 2. Moreover, a mutant Cyclin B protein having the first 28 amino acids of Cyclin A, which include KEN 2, instead of the first 30 amino acids, as well as a myc epitope instead of the D-box, was found to be stable during mitosis (data not shown). The second more C-terminal KEN-box (KEN 1) is also unable to bring about mitotic degradation of Cyclin A (Figure 1C).
In summary, our findings demonstrate that amino acids 1–53 are required for normal Cyclin A degradation. Moreover, our results suggest that cooperative effects of several elements within this region bring about the efficient mitotic degradation of Cyclin A. It is conceivable that Cyclin A degradation results from an additive effect of KEN-box 2 and dbs2, neither of which is sufficient to bring about normal mitotic degradation. We emphasize that we were unable to quantify the degree of mitotic stability of the various mutant Cyclin A proteins precisely with our immunofluorescence experiments. Nevertheless, our findings clearly demonstrate that mitotic degradation of Cyclin A is not regulated by a simple D-box, as has been widely assumed previously based on the analysis of the D-box of B-type cyclins and a few limited examples of A-type cyclins.
Metaphase delay caused by overexpression of non-degradable Cyclin A
Apart from analyzing the degradation of the various mutant Cyclin A proteins during mitosis, we also studied their effect on progression through the embryonic cell division cycles. prd-GAL4-driven overexpression of wild-type and all of the different mutant Cyclin A proteins did not advance the G2–M transition, since the pattern of entry into mitosis was found to be indistinguishable within expressing and non-expressing segments. Moreover, overexpression of wild-type Cyclin A also had no effect on progression through mitosis. The same frequencies and types of mitotic figures were observed in expressing and non-expressing segments (Figure 2C–E). In contrast, we observed a clear enrichment of metaphase plates in segments expressing mitotically stable mutant Cyclin A proteins (Figure 2J and K and data not shown), as previously observed with Cyclin A-Δ1–170 (Sigrist et al., 1995).
As in our previous experiments, the delay in metaphase was transient and the length of the delay correlated with the level of non-degradable Cyclin A. The extent of metaphase plate enrichment in the UAS transgene-expressing segments varied with the expression levels of independent transgene insertions. Moreover, the extent of metaphase plate enrichment was also correlated with the mitotic stability of the various Cyclin A mutant proteins. Expression of partially stabilized variants (Figure 1C) only resulted in a slight enrichment.
To define the effects of non-degradable Cyclin A on progression through mitosis more precisely, we performed time-lapse analyses of embryonic divisions in vivo. For this purpose, we expressed UAS transgenes in embryos carrying a histone–green fluorescent protein (GFP) transgene using nos-GAL4-GCN4-bcd3′UTR, which results in maximal expression before mitosis 14 when cells are relatively large, facilitating in vivo imaging. All our observations refer to cells that progress through mitosis 14 as domain 18 (Foe, 1989). At the onset of mitosis, the GFP signal readily revealed chromosome condensation. Moreover, while fluorescent signals were absent from the cytoplasm during interphase, we observed that some signal was rapidly redistributed from the nucleus to throughout the cell at the onset of mitosis, presumably as a consequence of nuclear envelope breakdown. Since the time of this redistribution could be defined more precisely than the onset of chromosome condensation, we defined this time as zero and determined the time until the onset of sister chromatid separation (ΔtNEB–OSS). Moreover, we also determined the time from onset till the end of sister chromatid separation (the time when the last pair of sister chromatids lost its last contacts; ΔtOSS–ESS), as well as the time from the end of sister chromatid separation until telophase (perfectly round daughter nuclei; ΔtESS–TP).
Initial experiments involving expression of a single UAS-CycA-Δ1–53 transgene copy did not appear to result in a significant prolongation of mitosis, even though transgene expression was clearly detectable by immunolabeling with anti-Cyclin A (data not shown). However, expression of two UAS-CycA-Δ1–53 transgene copies resulted in a significant mitotic delay (Table I). A delay was already apparent during the first mitotic interval (ΔtNEB–OSS) as well as during the subsequent mitotic stages (ΔtOSS–ESS and ΔtESS–TP). The onset of the mitotic delay appeared to be within metaphase. However, we can not rule out an earlier onset already during prometaphase because the spatial resolution of our imaging experiments was not sufficiently high to allow a precise definition of the onset of metaphase (i.e. the time when the last chromosome had reached bi-orientation).
Table I. Delay of mitosis by non-degradable cyclins.
| UAS transgene | Duration of mitotic phases (seconds ± SD)a |
||
|---|---|---|---|
| NEB–OSSb | OSS–ESSc | ESS–TPd | |
| None | 216 ± 18 | 41 ± 5 | 84 ± 17 |
| CycA-Δ1–53 | 250 ± 20 | 69 ± 21 | 165 ± 50 |
| CycB-dbme | 240 ± 28 | n.a.f | n.a.f |
| CycB-dbme | 233 ± 18 | 91 ± 20 | 128 ± 27 |
| CycB3-dbm | 247 ± 35 | 58 ± 9 | >403g |
aThe duration of different phases of mitosis was determined by time-lapse in vivo imaging of embryos expressing His2avD-GFP and UAS transgenes during mitosis 14 (see Materials and methods). The average and standard deviation (SD) of the values obtained from at least 15 different mitotic cells from at least five different embryos are indicated.
bNuclear envelope breakdown (NEB) until onset of sister chromatid separation (OSS).
cOnset until end of sister chromatid separation (ESS).
dEnd of sister chromatid separation until telophase (TP).
eWhile a mitotic arrest after ESS until the end of our imaging experiments was observed in 20% of the UAS-CycB-dbm-expressing embryos, cells in the other embryos eventually completed mitosis 14 (see text). Values from cells that became arrested are given in the upper line; values from cells that progressed through mitosis are given in the lower line.
fn.a., not available. Because sister chromatids were not effectively pulled apart, we could not determine the precise time when all the contacts between sister chromatids were resolved.
gBecause many cells failed to reach telophase within the time of our experiments, we can only give a lower limit of the average delay.
To compare the effects of non-degradable Cyclin A with those caused by non-degradable Cyclin B and B3, we performed analogous experiments with UAS-CycB-dbm and UAS-CycB3-dbm transgenes. In 20% of the UAS-CycB-dbm-expressing embryos, all cells became arrested in mitosis until the end of our imaging experiments. This mitotic arrest occurred after sister chromatid separation and an initial segregation phase. However, sister chromatids did not reach the poles completely and continued to move around individually and often backwards to the central region of the arrested cells (Figure 3I and J). In the remainder of the UAS-CycB-dbm-expressing embryos, the effects on mitosis were less severe and similar, but not completely identical, to the effects of non-degradable Cyclin A (Table I).
UAS-CycB3-dbm expression also had some effect on the initial mitotic phases. However, as suggested by the analysis of fixed embryos (Figure 3), the strongest delay was observed during the late mitotic phase (ΔtESS–TP; Table I). This preferential effect of non-degradable Cyclin B3 on late mitosis was clearly distinct from the effects of the other non-degradable cyclins.
The mitotic delay caused by non-degradable cyclins was clearly dependent on the level of expression. To evaluate the effects of physiological levels, we expressed non-degradable Cyclin A or B using prd-GAL4 in mutant embryos (Jacobs et al., 1998) that do not express protein products from the endogenous CycA or CycB genes, respectively. After immunolabeling with antibodies reacting equally well with wild-type and non-degradable cyclins, the frequency and types of mitotic figures could be compared in regions of sibling embryos with comparable levels of only non-degradable or wild-type cyclin. These observations indicated that physiological levels of non-degradable Cyclin B caused a detectable enrichment of anaphase figures (∼2-fold). In contrast, physiological levels of non-degradable Cyclin A did not appear to cause a delay (data not shown).
Non-degradable Cyclin A causes an extra cell cycle
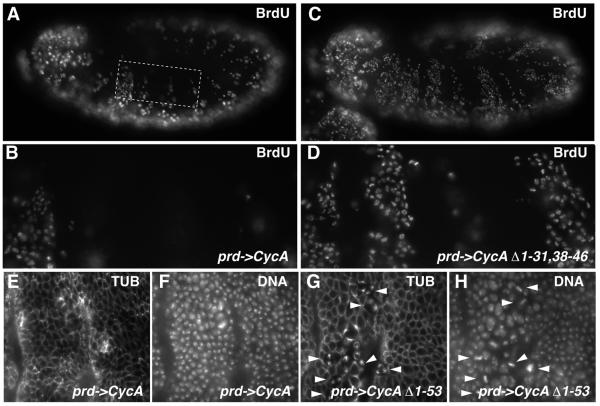
Non-degradable Cyclin A was found to interfere with a timely arrest of the epidermal cell proliferation. In wild-type embryos, the great majority of epidermal cells exit from the cell cycle after mitosis 16, except for a patch of cells in the thoracic region. After mitosis 16, therefore, 5′-bromo-deoxyuridine (BrdU) is no longer incorporated into the postmitotic epidermal cells, while BrdU incorporation still occurs in the thoracic epidermal patch and in the peripheral and central nervous system where some cells continue to proliferate (Knoblich et al., 1994). A normal pattern of BrdU incorporation was also observed in prd-GAL4 UAS-CycA embryos (Figure 4A and B). In contrast, the pattern of BrdU incorporation was clearly abnormal in prd-GAL4 UAS-CycA-Δ1–31,38–46 embryos (Figure 4C and D). In the segments expressing UAS-CycA-Δ1–31,38–46, BrdU was incorporated in the majority of the epidermal cells. UAS-CycA-Δ1–31,38–46 expression caused only a slight delay during mitosis 16 (data not shown) and the expressing segments started to incorporate BrdU immediately after exit from mitosis 16. A comparable BrdU incorporation following exit from mitosis 16 was observed with all of the Cyclin A mutants that were either partially or fully stable during mitosis (data not shown). In the case of mitotically stable mutants, expressing segments started to incorporate BrdU after exit from mitosis even though the metaphase delay was substantial, exit from mitosis was aberrant and cytokinesis often not successful.
Fig. 4. Mitotic degradation of Cyclin A is required for cell cycle arrest in G1. UAS-CycA (A, B, E and F), UAS-CycA-Δ1–31,38–46 (C and D) or UAS-CycA-Δ1–53 (G and H) were overexpressed in alternating segments using prd-GAL4. Embryos were labeled after mitosis 16 in early stage 12 with BrdU and anti-BrdU (A–D), and in stage 13 with antibodies against tubulin (E and G) and a DNA stain (F and H). The boxed region in (A) is shown at a higher magnification in (B), and an equivalent region in (D). High magnification views of epidermal regions are shown in (E–H) with segments expressing a UAS transgene (left side) or not (right side). Note that epidermal regions expressing UAS-CycA-Δ1–31,38–46 fail to become postmitotic and incorporate BrdU after mitosis 16. Moreover, after completion of this extra S phase, entry into mitosis is observed as illustrated with UAS-CycA-Δ1–53-expressing embryos (G and H), where metaphase figures (arrowheads) accumulate transiently in the UAS transgene-expressing region during this extra mitosis.
The extra S phase resulting from expression of the mutant Cyclin A proteins was later also followed by an extra M phase. In embryos expressing partially stabilized versions, this extra division was ultimately successful, resulting in an abnormally high epidermal cell density (data not shown). In embryos expressing fully stabilized mutants, the extra division was aberrant, just like the previous divisions, explaining the frequent presence of metaphase figures and the decreased epidermal cell density in the expressing segments (Figure 4G and H).
Our observations indicate that a complete degradation of Cyclin A during the final mitosis is essential for a timely exit from the cell division cycle. Residual Cyclin A present after the terminal division is extremely efficient in triggering progression through an extra division cycle.
Cyclin A is required for entry into mitosis
We have described previously that zygotic CycA function is required for mitosis 16 in the embryonic epidermis (Lehner and O’Farrell, 1989). The maternal CycA contribution present in embryos homozygous for null mutations in CycA allows normal development up to mitosis 16. Moreover, Cyclin B and B3, as well as Cdk1, are also normally expressed in CycA mutant embryos during cycle 16, and yet this cycle is not completed with a division. In principle, mitosis 16 might be inhibited in CycA mutants by a checkpoint mechanism recognizing a DNA replication defect during S phase 16. The observation that BrdU is incorporated during S phase 16 in CycA mutant embryos (Lehner et al., 1991) does not exclude the possibility of a subtle DNA replication defect. The demonstrated role of Cyclin A in the regulation of S phase in vertebrates, in combination with the observations that mitotically stabilized CycA mutant proteins are extremely potent in triggering an extra S phase (Figure 4; Sprenger et al., 1997), supports the idea that zygotic CycA function is primarily required for S phase 16 and only indirectly for mitosis 16.
The recent characterization of mutations in the Drosophila gene double-parked (dup) allowed us to test whether CycA is directly required for mitosis 16. dup encodes a conserved protein (a homolog of Schizosaccharomyces pombe cdt1+) that is required for the assembly of DNA replication complexes in eukaryotic cells (Whittaker et al., 2000). In the absence of zygotic dup expression, Drosophila embryos initially develop normally because of a maternal dup contribution. However, after completion of mitosis 15, cells fail to enter S phase 16 in dup mutants. This failure of S phase 16 is not detected by a checkpoint mechanism and cells enter mitosis 16 at the appropriate stage. However, the mitotic chromosome attachment and spindle assembly checkpoint apparently senses the absence of paired sister chromatids and arrests cells during mitosis 16. Eventually, cells adapt in dup mutants and exit from the mitotic arrest.
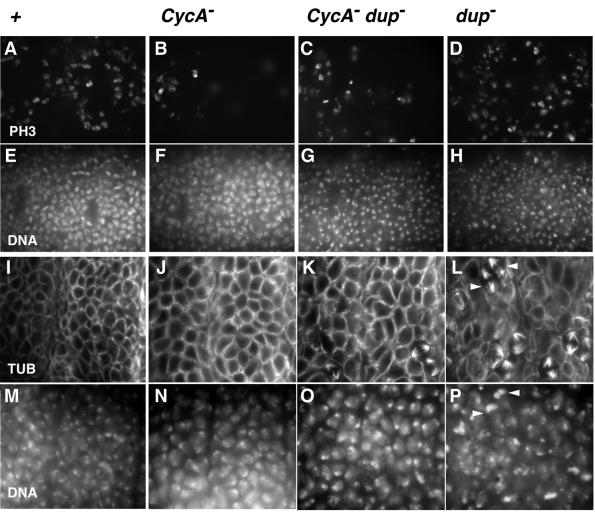
Since mutations in dup uncouple entry into mitosis 16 from progression through the preceding S phase (Whittaker et al., 2000), we analyzed whether zygotic CycA function is still required for mitosis 16 in dup mutants. If CycA was required for S phase, and thus only indirectly for mitosis 16, dup CycA double mutants would be expected to display the dup mutant phenotype and enter mitosis 16. Conversely, if CycA is also required directly for entry into mitosis, dup CycA double mutants would be expected to arrest before mitosis 16, just like CycA mutants. Our immunolabeling experiments clearly indicated that the great majority of the epidermal cells in dup CycA double mutants failed to enter mitosis 16 (Figure 5). BrdU pulse-labeling experiments indicated that S phase 16 is not restored in dup CycA double mutant embryos (data not shown). The phenotype of dup CycA double mutants, therefore, strongly indicates that CycA is directly required for entry into mitosis.
Fig. 5. Cyclin A is required for entry to mitosis in CycA dup double mutants. Embryos at the stage of mitosis 16 (A–H) were double-labeled with a DNA stain (E–H) and an antibody against a phosphorylated form of histone H3 (A–D), which is only present during mitosis. Embryos after completion of mitosis during stage 12 (I–P) were double-labeled with a DNA stain (M–P) and antibodies against tubulin (I–L). As all these embryos were the progeny of parents doubly heterozygous for mutations in CycA and dup, they were lacking zygotic function of either only CycA (B and F; J and N; CycA–), only dup (D and H; L and P; dup–), both CycA and dup (C and G; K and O; CycA– dup–) or neither CycA nor dup (A and E; I and M; +). Their identification was achieved with blue balancer chromosomes and anti-β-galactosidase labeling (not shown). High magnification views of thoracic epidermal regions are shown. While the great majority of the epidermal cells in CycA mutants fail to enter mitosis 16, a few exceptional mitoses are always observed in the thoracic region. These are detected by the anti-phospho-histone H3 labeling (B). In contrast, far more mitotic cells are detected in control embryos (A), where all epidermal cells eventually complete mitosis 16. Even higher numbers of mitotic cells were detected in dup embryos (D) because of the transient checkpoint arrest during mitosis 16. The number of mitotic cells in CycA dup double mutants (C) was slightly higher than in CycA mutants (B), but significantly lower than in dup mutants (D) and control embryos (A). At stage 12, when control embryos (I and M) have completed mitosis 16, dup mutant embryos (L and P) are characterized by the high frequency of cells still arrested in mitosis 16 and the irregular organization of the other interphase cells. Examples of mitotic cells are indicated by arrowheads in (L) and (P). In contrast, the epidermal organization is very regular in CycA dup double mutants (K and O) and very similar to CycA mutants (J and N).
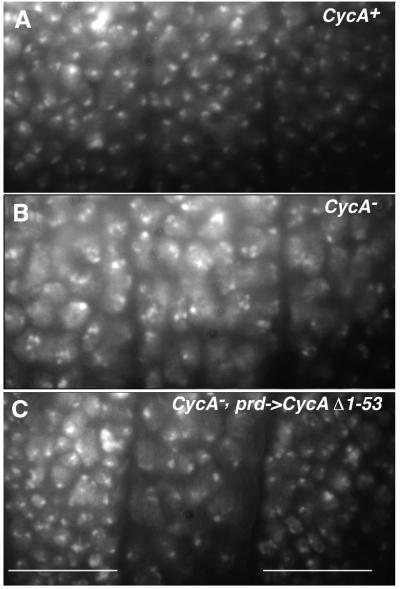
The fact that mitosis 16 does not occur in CycA mutants, despite the presence of Cyclin B and B3, indicates that Cyclin A provides a specific mitosis-promoting function. This specific function might be expected to require the presence of the N-terminal region of Cyclin A, as the different mitotic cyclin types are most divergent in the N-terminal regions. Thus, we analyzed whether the various N-terminal truncations of Cyclin A are still capable of restoring mitosis 16 in CycA mutants by expressing the corresponding UAS transgenes with prd-GAL4 in CycA mutant embryos. As illustrated in Figure 6, we found that all of the truncated Cyclin A proteins are capable of triggering mitosis 16 in CycA mutants. In CycA mutants, as in wild-type embryos, mitotically stable Cyclin A truncations also resulted in an enrichment of metaphase figures and frequently interfered with successful completion of cell division (data not shown). While deletion of the N-terminal degradation signals, therefore, clearly affects progression through mitosis, it appears that the N-terminal region is not required to provide the specific mitosis-promoting function required for entry into mitosis 16.
Fig. 6. The Cyclin A function required for entry into mitosis does not depend on the presence of the N-terminal region. The cell density present after completion of the epidermal cell proliferation during stage 12 in control embryos (A; CycA+), CycA mutant embryos (B; CycA–) and CycA mutant embryos with prd-GAL4 and UAS-CycA-Δ1–53 (C; CycA–, prd->CycAΔ1–53) is revealed by a DNA stain. White bars in (C) indicate segments that express UAS-CycA-Δ1–53. These segments have a cell density comparable to control embryos (A) and clearly higher than the intervening non-expressing segment. This non-expressing segment has the same reduced cell density as in CycA mutants (B), reflecting the failure of mitosis 16.
Discussion
The different mitotic cyclins A, B and B3 are all degraded during mitosis. Experiments with various B-type cyclins have led to the identification of the D-box motif, which mediates mitotic degradation. As expected, we find that the D-box motifs present in Drosophila Cyclin B and B3 are indeed required for mitotic degradation. In contrast, however, we find that the mitotic degradation of Drosophila Cyclin A does not depend on regions that fit the D-box consensus. Instead of a simple brief motif as in B-type cyclins, degradation of Drosophila Cyclin A is mediated by a complex degradation signal. Mitotic degradation of human cyclin A also involves a complex degradation signal (Geley et al., 2001). Complex degradation signals might thus be more common among A-type cyclins than initially suggested (Kobayashi et al., 1992; Lorca et al., 1992). We point out that a number of cyclin A sequences from different species (i.e. mouse and hamster cyclin A2) do not contain regions matching even a very relaxed D-box consensus (RXXL) within the first hundred N-terminal amino acids.
In addition to the D-box, the KEN box has recently been shown to mediate APC/C-dependent degradation of proteins like human p55CDC, Nek2, Cdc6 and mouse B99 (Petersen et al., 2000; Pfleger and Kirschner, 2000). The N-terminus of Drosophila Cyclin A also includes KEN box-like sequences, but these sequences are clearly not sufficient for normal mitotic Cyclin A degradation. It is not excluded that KEN-box 2 contributes to the degradation signal, perhaps by cooperating with one of the D-box-like motifs, dbs2, which alone is also not sufficient for normal degradation. The combined function of a KEN and a D-box recently has been shown to mediate degradation of human Cdc6 during G1 (Petersen et al., 2000). However, the same signal combination is unlikely to operate in the case of Drosophila Cyclin A because KEN-boxes are thought to mediate ubiquitylation exclusively via FZR/Cdh1, which does not appear to be expressed during the early embryonic mitoses, during which Cyclin A, nevertheless, is efficiently degraded (Sigrist and Lehner, 1997).
Our finding that the degradation of A- and B-type cyclins does not rely on identical signals might be a first step towards understanding why Cyclin A but not Cyclin B is degraded in cells arrested by the spindle assembly and chromosome attachment checkpoint. It is conceivable, for instance, that FZY/Cdc20 might have different binding sites for A- and B-type degradation signals. If Mad2 was to block only the binding site for B-type signals, Cyclin A could still be degraded in arrested cells in a FZY/Cdc20-dependent manner.
Our results clarify further the physiological significance of mitotic cyclin degradation. The sequential disappearance of A-, B- and B3-type cyclins during mitosis appears to have been conserved in evolution, and our previous experiments with non-degradable mutant cyclins have suggested that this sequential disappearance contributes to the temporal ordering of mitotic processes (Sigrist et al., 1995). Our present work, which includes a number of technical improvements, explores this suggestion further. Instead of large N-terminal deletions, we have now analyzed mutations targeted specifically to the degradation signals. Instead of heat-inducible expression, we have now used the UAS/GAL4 system, which eliminates possible heat shock artefacts. Moreover, in addition to analysis of fixed samples, we have now also used in vivo imaging for phenotypic characterizations.
By immunofluorescence, Cyclin B3 is the last of the mitotic cyclins to become undetectable during mitosis (Sigrist et al., 1995). Non-degradable Cyclin B3 delays mitotic progression primarily in late anaphase. It appears, therefore, that substrates of Cyclin B3–Cdk1 complexes need to be dephosphorylated to allow the telophase processes (chromosome decondensation, nuclear envelope reformation, spindle disassembly, cytokinesis). The phenotype caused by non-degradable Cyclin B3 is clearly characteristic. Changing the expression level affects the length but not the mitotic stage of the delay. The phenotype can not be mimicked by expression of non-degradable Cyclin A or B over a range of concentrations.
Expression of non-degradable Cyclin B results in a delay primarily after sister chromatid separation. We have shown previously that non-degradable Cyclin B does not interfere with Cyclin A and B3 degradation. Thus, it appears that the complete segregation of separated sister chromatids to the spindle poles, as well as the following telophase processes, are dependent on dephosphorylation of substrates of Cyclin B–Cdk1 complexes.
Non-degradable Cyclin B appears to maintain the polar ejection forces at a much higher level than non-degradable Cyclin B3. In the presence of stabilized Cyclin B, the sister chromatids remain dynamic in the arrested cells and some move back after an initial phase of separation and poleward migration that is almost normal.
Strong mitotic delays require overexpression of non-degradable cyclins. This is particularly true in the case of Cyclin A. At physiological levels, the effects of non-degradable cyclins are surprisingly subtle. While presumably contributing, sequential degradation of Cyclins A, B and B3 is therefore unlikely to be the only regulatory mechanism ordering the various cellular processes during exit from mitosis. We point out that sister chromatid separation in Drosophila appears to be regulated by degradation of Pimples, a securin protein that in contrast to Cyclin A is stabilized in cells arrested by the chromosome attachment and spindle assembly checkpoint (Leismann et al., 2000).
While mitotic degradation of Cyclin A does not appear to be absolutely required for progression through mitosis, it is very crucial for entry into G1. The first G1 phase during the development of the embryonic epidermis is observed after mitosis 16, which is also the last mitotic division in this tissue. It has previously been shown that strong overexpression of Cyclin A and in particular of Cyclin A-Δ1–170 from heat-inducible transgenes during this G1 can trigger entry into S phase (Sprenger et al., 1997). Our experiments emphasize further the crucial role of mitotic Cyclin A degradation for a timely cell proliferation arrest. Mutations that impair mitotic degradation of Cyclin A and progression through mitosis only very slightly triggered a complete extra division cycle in the embryonic epidermis with very high efficiency. This extra division cycle started immediately after mitosis 16 and before Cyclin A had re-accumulated to detectable levels. Therefore, Cyclin A complexes surviving the terminal mitosis, at concentrations well below the maximal physiological levels, generate the same phenotype as observed previously after overexpression of Cyclin E or in dacapo (which encodes a CIP/KIP-type Cyclin E–Cdk2 inhibitor) and fizzy-related (fzr) mutant embryos (Knoblich et al., 1994; DeNooij et al., 1996; Lane et al., 1996; Sigrist and Lehner, 1997). It is likely, therefore, that the extra division cycle that occurs in fzr mutants results from an inability to degrade Cyclin A, and the resulting Cyclin A complexes present after mitosis might either mimic or activate Cyclin E–Cdk2 activity. A similar inappropriate and Cyclin A-dependent progression into the cell cycle is also observed in roughex mutant eye imaginal discs (Thomas et al., 1994). roughex encodes an inhibitor of Cyclin A–Cdk1 complexes (Foley et al., 1999). Moreover, the Drosophila lats/warts tumor suppressor gene, which encodes a kinase of the dbf2/20 family implicated in the mitotic exit network in budding yeast, might also limit cell proliferation by controlling postmitotic Cyclin A levels (Tao et al., 1999). All these findings emphasize that the complete elimination of Cyclin A during mitosis is extremely important for a cell cycle arrest during G1.
In mammalian cells, Cyclin A associates not only with cdk1, but also with cdk2, and a number of observations have suggested that Cyclin A is important not only for M phase, but also for S phase. In Drosophila embryos, we have failed to detect complexes with Cdk2, while an association with Cdk1 is readily observed. Our characterization of CycA mutants has suggested that Cyclin A is required for entry into mitosis (Knoblich and Lehner, 1993). However, our previous analyses had not excluded the possibility that the failure to enter mitosis 16 in the CycA mutant embryonic epidermis is caused by a checkpoint detecting subtle S-phase defects. With dup mutants (Whittaker et al., 2000) we were able to address this issue. dup encodes a conserved assembly factor for DNA replication initation complexes. In dup mutants, mitosis 16 is uncoupled from progression through the preceding S phase. Our double mutant analysis demonstrated that dup mutants require Cyclin A function for entry into this uncoupled mitosis. Therefore, Cyclin A is required for entry into mitosis, at least in the embryonic epidermis.
We emphasize that the failure of mitosis 16 occurs in CycA mutants despite the presence of Cyclin B and B3. Moreover, mitosis 16 can not be rescued in CycA mutants by overexpression of either wild-type or non-degradable Cyclin B (Sigrist et al., 1995 and data not shown). However, the Cyclin A-specific function required for mitosis 16 can be fulfilled with the C-terminal cyclin box region and thus does not require the N-terminal region of Cyclin A.
While mitosis 16 clearly requires CycA function, we have shown that null mutations in Cyclin B and B3 do not prevent mitoses (Jacobs et al., 1998). Therefore, at least in Drosophila, Cyclin A–Cdk1 qualifies more readily as MPF in the sense of ‘mitosis-promoting factor’ than the B-type cyclin complexes. In HeLa cells, cyclin A also provides a mitosis-promoting function upstream of B-type cyclin– cdk1 complexes, although in a cdk2 complex (Furuno et al., 1999).
In summary, all of our genetic analyses of the different mitotic cyclins in Drosophila indicate overlapping as well as specific functions. Understanding these specific functions will be dependent on the identification of specific substrates, a difficult task that might, however, be aided in the future by the rapid development of powerful proteomics methods.
Materials and methods
Fly stocks
dupa1 and dupa3 (Whittaker et al., 2000), His2avD-GFP (Clarkson and Saint, 1999), prd-GAL4 (Brand and Perrimon, 1993), nos-GAL4-GCN4-bcd3′UTR (Janody et al., 2000) CycAC8LR1, CycA5, UAS-CycA, UAS-CycB and UAS-CycB3 (Lehner and O’Farrell, 1989; Sigrist and Lehner, 1997) have been described previously. Blue balancer chromosomes were used for the identification of mutant embryos.
Transgenic lines were generated by germ line transformation with pUAST (Brand and Perrimon, 1993) constructs. These contained cDNA-derived fragments of Drosophila CycA, CycB and CycB3. D-boxes (db) and regions with similarity to D-boxes (dbs) were deleted and replaced by a KpnI site using inverse PCR, followed by the introduction of a double-stranded oligonucleotide including a single myc epitope sequence into this KpnI site in the case of the dbm and dbs1m constructs (m, myc). N-terminally deleted CycA fragments were amplified with a series of 5′ primers introducing a NcoI site with a start codon, and modified further in the case of the constructs with internal deletions. All of the altered cDNA fragments were sequenced to exclude construction artefacts. Several independent insertions of each construct were established and analyzed.
BrdU- and immunolabeling
Pulse labeling of permeabilized embryos with BrdU and immunolabeling with antibodies against BrdU (Becton-Dickinson), β-galactosidase (ICN, Cappel and Promega), α-tubulin (Sigma), phospho-histone H3 (Upstate Biotechnology), Drosophila Cyclin A, Cyclin B, Cyclin B3 and Hoechst 33258 were carried out essentially as described previously (Knoblich et al., 1994; Jacobs et al., 1998). A cooled CCD camera (Photometrics) and IPLab software (Signal Analytics) in combination with a Zeiss Axiophot fluorescence microscope were used for the capture and quantification of immunofluorescence signal intensities. Relative expression levels of different UAS transgenes in combination with prd-GAL4 were estimated by comparing the ratios between signal intensities within regions expressing or not expressing the UAS transgene before mitosis 16.
In vivo imaging
Females homozygous for His2avD-GFP on the second and nos-GAL4-GCN4-bcd3′UTR on the third chromosome were crossed with either w1 males for controls or males carrying a chromosome with two UAS transgene insertions (UAS-CycAΔ1–53 2.41 and 2.77, or UAS-CycB-dbm II.1 and II.4, or UAS-CycB3-dbm III.1 and III.2). Eggs were collected during 30 min and aged for 2.5 h. After dechorionization, embryos were mounted on a glass slide and covered with halo carbon oil. Time-lapse analyses were carried out by scanning frames with an inverted Leica TCS-SP confocal microscope at 10 s intervals using a 16× oil immersion lens and a minimal laser intensity. All analyses were completed within 20 min of mounting the slides on the microscope.
Acknowledgments
Acknowledgements
We thank A.Weiss, A.Prell, D.Richter and J.Günther for help during the construction and analysis of various transgenes. We thank T.Orr-Weaver and R.Saint for fly stocks, and S.Geley, T.Hunt, P.O’Farrell and F.Sprenger for communication of results prior to publication. This work was supported by grants from the DFG (Le 987/1-2 and Le 987/2-1).
References
- Brand A.H. and Perrimon,N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. [DOI] [PubMed] [Google Scholar]
- Buendia B., Draetta,G. and Karsenti,E. (1992) Regulation of the microtubule nucleating activity of centrosomes in Xenopus egg extracts: role of cyclin A-associated protein kinase. J. Cell Biol., 116, 1431–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson M. and Saint,R. (1999) A His2AvDGFP fusion gene complements a lethal His2AvD mutant allele and provides an in vivo marker for Drosophila chromosome behavior. DNA Cell Biol., 18, 457–462. [DOI] [PubMed] [Google Scholar]
- DeNooij J.C., Letendre,M.A. and Hariharan,I.K. (1996) A cyclin-dependent kinase inhibitor, dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell, 87, 1237–1247. [DOI] [PubMed] [Google Scholar]
- Devault A., Fesquet,D., Cavadore,J.C., Garrigues,A.M., Labbé,J.C., Lorca,T., Picard,A., Philippe,M. and Dorée,M. (1992) Cyclin A potentiates maturation-promoting factor activation in the early Xenopus embryo via inhibition of the tyrosine kinase that phosphorylates cdc2. J. Cell Biol., 118, 1109–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe V.E. (1989) Mitotic domains reveal early commitment of cells in Drosophila embryos. Development, 107, 1–22. [PubMed] [Google Scholar]
- Foley E., O’Farrell,P.H. and Sprenger,F. (1999) Rux is a cyclin-dependent kinase inhibitor (CKI) specific for mitotic cyclin–Cdk complexes. Curr. Biol., 9, 1392–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuno N., den Elzen,N. and Pines,J. (1999) Human cyclin A is required for mitosis until mid prophase. J. Cell Biol., 147, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geley S., Kramer,E., Gieffers,C., Gannon,J., Peters,J.-M. and Hunt,T. (2001) APC/C-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol., 153, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M., Murray,A.W. and Kirschner,M.W. (1991) Cyclin is degraded by the ubiquitin pathway. Nature, 349, 132–138. [DOI] [PubMed] [Google Scholar]
- Jacobs H.W., Knoblich,J.A. and Lehner,C.F. (1998) Drosophila Cyclin B3 is required for female fertility and is dispensable for mitosis like Cyclin B. Genes Dev., 12, 3741–3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janody F., Reischl,J. and Dostatni,N. (2000) Persistence of Hunchback in the terminal region of the Drosophila blastoderm embryo impairs anterior development. Development, 127, 1573–1582. [DOI] [PubMed] [Google Scholar]
- King R.W., Glotzer,M. and Kirschner,M.W. (1996) Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell, 7, 1343–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotzbucher A., Stewart,E., Harrison,D. and Hunt,T. (1996) The ‘destruction box’ of cyclin A allows B-type cyclins to be ubiquitinated, but not efficiently destroyed. EMBO J., 15, 3053–3064. [PMC free article] [PubMed] [Google Scholar]
- Knoblich J.A. and Lehner,C.F. (1993) Synergistic action of Drosophila cyclin A and cyclin B during the G2–M transition. EMBO J., 12, 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich J.A., Sauer,K., Jones,L., Richardson,H., Saint,R. and Lehner,C.F. (1994) Cyclin E controls S phase progression and its downregulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell, 77, 107–120. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Stewart,E., Poon,R., Adamczewski,J.P., Gannon,J. and Hunt,T. (1992) Identification of the domains in cyclin A required for binding to and activation of p34cdc2 and p32cdk2 protein kinase subunits. Mol. Biol. Cell, 3, 1279–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer E.R., Scheuringer,N., Podtelejnikov,A.V., Mann,M. and Peters,J.M. (2000) Mitotic regulation of the APC activator proteins CDC20 and CDH1. Mol. Biol. Cell, 11, 1555–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane M.E., Sauer,K., Wallace,K., Jan,Y.N., Lehner,C.F. and Vaessin,H. (1996) Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell, 87, 1225–1236. [DOI] [PubMed] [Google Scholar]
- Lehner C.F. and O’Farrell,P.H. (1989) Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell, 56, 957–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C.F. and O’Farrell,P.H. (1990) The roles of Drosophila cyclin A and cyclin B in mitotic control. Cell, 61, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C.F., Yakubovich,N. and O’Farrell,P.H. (1991) Exploring the role of Drosophila cyclin A in the regulation of S-phase. Cold Spring Harb. Symp. Quant. Biol., 56, 465–475. [DOI] [PubMed] [Google Scholar]
- Leismann O., Herzig,A., Heidmann,S. and Lehner,C.F. (2000) Degradation of Drosophila PIM regulates sister chromatid separation during mitosis. Genes Dev., 14, 2192–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T., Devault,A., Colas,P., Van Loon,A., Fesquet,D., Lazaro,J.B. and Doree,M. (1992) Cyclin A-Cys41 does not undergo cell cycle-dependent degradation in Xenopus extracts. FEBS Lett., 306, 90–93. [DOI] [PubMed] [Google Scholar]
- Luca F.C., Shibuya,E.K., Dohrmann,C.E. and Ruderman,J.V. (1991) Both cyclin A-Δ60 and B-Δ97 are stable and arrest cells in M-phase, but only cyclin B-Δ97 turns on cyclin destruction. EMBO J., 10, 4311–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshull J., Golsteyn,R., Hill,C.S. and Hunt,T. (1990) The A-type and B-type cyclin-associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J., 9, 2865–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A.W., Solomon,M.J. and Kirschner,M. (1989) The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature, 339, 280–285. [DOI] [PubMed] [Google Scholar]
- Petersen B.O. et al. (2000) Cell cycle- and cell growth-regulated proteolysis of mammalian CDC6 is dependent on APC-CDH1. Genes Dev., 14, 2330–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfleger C.M. and Kirschner,M.W. (2000) The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev., 14, 655–665. [PMC free article] [PubMed] [Google Scholar]
- Rimmington G., Dalby,B. and Glover,D.M. (1994) Expression of N-terminally truncated cyclin-B in the Drosophila larval brain leads to mitotic delay at late anaphase. J. Cell Sci., 107, 2729–2738. [DOI] [PubMed] [Google Scholar]
- Rudner A.D. and Murray,A.W. (2000) Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol., 149, 1377–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah J.V. and Cleveland,D.W. (2000) Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell, 103, 997–1000. [DOI] [PubMed] [Google Scholar]
- Shteinberg M., Protopopov,Y., Listovsky,T., Brandeis,M. and Hershko,A. (1999) Phosphorylation of the cyclosome is required for its stimulation by Fizzy/cdc20. Biochem. Biophys. Res. Commun., 260, 193–198. [DOI] [PubMed] [Google Scholar]
- Sigrist S.J. and Lehner,C.F. (1997) Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell, 90, 671–681. [DOI] [PubMed] [Google Scholar]
- Sigrist S., Jacobs,H., Stratmann,R. and Lehner,C.F. (1995) Exit from mitosis is regulated by Drosophila fizzy and the sequential destruction of cyclins A, B and B3. EMBO J., 14, 4827–4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger F., Yakubovich,N. and O’Farrell,P.H. (1997) S phase function of Drosophila cyclin A and its downregulation in G1 phase. Curr. Biol., 7, 488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart E., Kobayashi,H., Harrison,D. and Hunt,T. (1994) Destruction of Xenopus cyclin A and cyclin B2, but not cyclin B1, requires binding to p34cdc2. EMBO J., 13, 584–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W., Zhang,S., Turenchalk,G.S., Stewart,R.A., St John,M.A., Chen,W. and Xu,T. (1999) Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nature Genet., 21, 177–181. [DOI] [PubMed] [Google Scholar]
- Thomas B.J., Gunning,D.A., Cho,J. and Zipursky,S.L. (1994) Cell cycle progression in the developing Drosophila eye—roughex encodes a novel protein required for the establishment of G1. Cell, 77, 1003–1014. [DOI] [PubMed] [Google Scholar]
- Whitfield W.G.F., Gonzalez,C., Maldonado-Codina,G. and Glover,D.M. (1990) The A-type and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2–M transition. EMBO J., 9, 2563–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker A.J., Royzman,I. and Orr-Weaver,T.L. (2000) Drosophila double parked: a conserved, essential replication protein that colocalizes with the origin recognition complex and links DNA replication with mitosis and the down-regulation of S phase transcripts. Genes Dev., 14, 1765–1776. [PMC free article] [PubMed] [Google Scholar]
- Zachariae W. and Nasmyth,K. (1999) Whose end is destruction: cell division and the anaphase-promoting complex. Genes Dev., 13, 2039–2058. [DOI] [PubMed] [Google Scholar]
- Zachariae W., Schwab,M., Nasmyth,K. and Seufert,W. (1998) Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science, 282, 1721–1724. [DOI] [PubMed] [Google Scholar]