Abstract
Saliva is a promising non-invasive alternative to nasopharyngeal swabs (NPS) for SARS-CoV-2 diagnosis, yet its longitudinal performance remains underexplored. This longitudinal study evaluated saliva’s diagnostic accuracy against NPS in 72 symptomatic individuals across six visits (July 2021–May 2022), analyzing 285 paired RT-qPCR samples in Rio de Janeiro, Brazil. Using NPS as the reference standard, saliva demonstrated high specificity (96.6%; 95% CI 92.9–98.7%), substantial agreement (91.6%; κ = 0.78; 95% CI 70–86% ; P < 0.001), and variable sensitivity (69.2% overall; 95% CI 57.2–79.5%), ranging from 40% during mid-phase infection (visit 3) to 82% during early infection (visit 1). Cycle threshold (Ct) values revealed slightly higher viral loads in NPS (mean Ct = 26.75) than in saliva (mean Ct = 28.75), with a mean difference of 0.79 cycles. Discordant results (8.4%) revealed saliva’s utility in detecting late-stage infections missed by NPS. No significant associations were found between diagnostic agreement and participant characteristics or Ct values. In conclusion, our longitudinal data demonstrate that saliva testing achieves 69.2% sensitivity (ranging from 40 to 82% across infection phases), 96.6% specificity, and 91.6% overall agreement with NPS. The 8.4% discordant results included 1.7% of cases where saliva-detected infections were missed by NPS, highlighting its complementary value in late-stage monitoring. These results, coupled with saliva’s stability over time, support its implementation as a scalable, cost-effective diagnostic tool—particularly in resource-limited settings—where its high specificity makes it particularly valuable for rule-out testing despite temporal variations in sensitivity.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-20841-w.
Keywords: SARS-CoV-2, COVID-19 testing, Infectious-disease diagnostics, Temporal diagnostic accuracy, Saliva testing, Viral shedding kinetics, Resource-limited settings
Subject terms: Biological techniques, Biotechnology, Molecular biology, Diseases, Health care, Molecular medicine
Introduction
Saliva is globally recognized as a non-invasive alternative for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) detection, improving diagnostic access. Brazilian studies, such as Vaz et al.1, validate saliva’s reliability, highlighting its lower cost, simpler collection, and higher patient acceptance. While concerns have been raised regarding potential variability in test performance across different reverse transcription polymerase chain reaction (RT-PCR) platforms, Sousa et al.2 reported high diagnostic agreement between saliva and nasopharyngeal swab (NPS) samples, even when using three different RT-PCR kits and including individuals with mild symptoms. However, accuracy can be affected by participant characteristics and Ct values, especially with varying infection stages.
Molecular detection of SARS-CoV-2 is widely performed using the gold standard diagnostic test based on reverse transcription of viral genomic material (RNA) followed by quantitative reverse transcription-polymerase chain reaction (RT-qPCR) in NPS. Despite its high sensitivity and specificity, the use of this test presents challenges that include high reagent costs, the risk of contamination to the health team when obtaining the clinical specimen, the need for training, and specialized laboratory infrastructure3. The recommended collection period for NPS—3rd and 7th days after symptom onset4—coincides with peak viral shedding but is complicated by symptom variability4 and by the presence of asymptomatic cases, which account for approximately 20% of infections and often go undetected, contributing to silent transmission5,6.
A non-invasive and reliable alternative to NPS is saliva7, already routinely used to diagnose viral infections such as Dengue, Zika virus, Influenza, Epstein-Barr, and severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1)8–10. Salivary samples show comparable performance to the use of NPS in the molecular diagnosis of SARS-CoV-2 infection, with evidence of viral presence even when undetectable in swabs11. Furthermore, studies have also reported the saliva’s advantages, including lower cost, patient comfort, and a high rate of detectable viral material1,11,12. Saliva also has the potential to be a superior sampling site compared to NPS, as viral loads often peak days earlier in saliva than in NPS13. However, individual-level heterogeneity in SARS-CoV-2 viral shedding may impact early detection, as this variability can affect the reliability of saliva-based diagnostics14.
Despite growing evidence supporting saliva as a SARS-CoV-2 diagnostic alternative, critical gaps persist in prior research. Most studies are limited by: (1) cross-sectional designs that fail to capture temporal dynamics of diagnostic accuracy; (2) inadequate characterization of discordant results between saliva and NPS, particularly in late-stage infections; (3) insufficient analysis of how participant characteristics (e.g., vaccination status, symptom duration) influence diagnostic agreement; and (4) lack of longitudinal data on saliva’s stability and reliability in real-world settings. These limitations hinder evidence-based implementation of saliva testing in resource-limited contexts.
This study addresses these gaps through a comprehensive longitudinal evaluation of saliva’s diagnostic accuracy in 72 symptomatic individuals, analyzing 285 paired saliva-NPS samples across six time points. It was specifically designed to evaluate saliva testing during a critical transition period in the pandemic (July 2021–May 2022), when vaccination rates were rising globally and SARS-CoV-2 variants were rapidly evolving from Delta to Omicron dominance. This timing provides a unique opportunity to assess diagnostic performance in the contemporary context that defines current clinical practice, rather than the early-pandemic conditions that characterize most existing literature. Our research specifically compares the diagnostic performance of saliva specimens against this reference standard, with emphasis on longitudinal viral detection dynamics rather than control system methodologies. By integrating detailed virological, demographic, and clinical data—including Ct value dynamics, vaccination status, and symptom progression—our work provides novel insights into the temporal sensitivity of saliva testing and its potential to complement NPS in dynamic infection scenarios.
The main contributions of this study are: (1) longitudinal diagnostic performance: First comprehensive evaluation of saliva vs. NPS accuracy across six time points, revealing temporal sensitivity dynamics (69.2% overall, peaking at 82% early) and consistent high specificity (96.6%); (2) discordance analysis: Identification of 8.4% discordant results where saliva detected late-stage infections missed by NPS, demonstrating its complementary value in real-world settings; (3) viral load quantification: Precise characterization of Ct-value differences (mean ΔCt = 0.79 cycles) between sample types, establishing saliva’s reliability despite marginally lower viral loads; (4) generalizability evidence: Demonstration that diagnostic agreement is independent of participant characteristics (e.g., age, sex, vaccination status) or Ct values, supporting broad applicability; and (5) implementation roadmap: evidence-based framework for deploying saliva as a scalable, cost-effective diagnostic tool in resource-limited settings. These findings fill a critical knowledge gap in pandemic diagnostics, offering evidence-based guidance for optimizing non-invasive testing strategies.
Methodology
Study design and participants
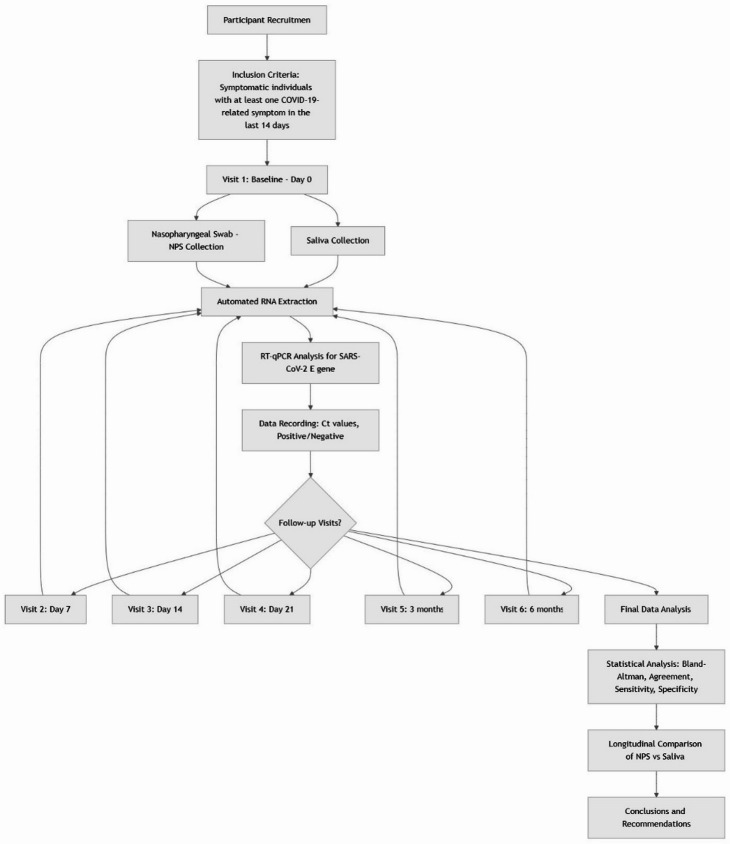
This study was conducted at the Galeão Air Force Hospital (HFAG) and the Oswaldo Cruz Foundation in Rio de Janeiro between July 16, 2021, and May 30, 2022. Participants were enrolled at visit 1 (Day 0) and followed at predetermined intervals corresponding to key phases of SARS-CoV-2 infection: visit 2 (Days 7), visit 3 (Days 14), visit 4 (Day 21), visit 5 (3 Months), and visit 6 (6 Months). This schedule was designed to capture viral dynamics across early infection (visit 1), acute phase (visit 2), and convalescent phase (visit 3), aligning with established timelines of SARS-CoV-2 pathogenesis. Individuals classified as symptomatic using an electronic questionnaire on COVID-19 symptoms, i.e., with at least one COVID-19-related symptom in the last 14 days, were included. The symptoms considered were: fever, cough, shortness of breath, fatigue or weakness, muscle or body aches, headache, loss of appetite, loss of smell, sore throat, nasal congestion or runny nose, nausea or vomiting, diarrhea or abdominal pain, and others. To clarify the longitudinal design and methodological workflow of this study, Fig. 1 presents a comprehensive block diagram of the research process. This visualization illustrates the sequential steps from participant recruitment through final analysis, highlighting the repeated sampling schedule and parallel processing of NPS and saliva specimens across six time points.
Fig. 1.
Longitudinal study workflow for comparative evaluation of saliva and NPS specimens for SARS-CoV-2 detection. Longitudinal workflow of the study design showing participant recruitment, inclusion criteria, sequential sample collection at six time points (visit 1: Baseline/Day 0 through visit 6: 6 Months), parallel processing of nasopharyngeal swab (NPS) and saliva specimens, RT-qPCR analysis, and final statistical evaluation. The diagram illustrates the repeated-measures design that enabled assessment of viral detection dynamics over time using both specimen types.
Sample collection
The participants reporting COVID-19-related first symptoms for more than 14 days and women who reported suspected pregnancy were not enrolled in the study. The survey was done in person with trained staff. Up to four testing visits were conducted on Days 0, 7, 14, and 28 and after an interval of 3 and 6 months. Samples were collected at the HFAG’s COVID-19 Respiratory Emergency Center and an Oswaldo Cruz Foundation tent. Participants were first asked to bring up saliva from the back of the throat and spit at least 3 mL into two empty sterile conical tubes of 50 mL. Before the collection, participants were instructed not to touch their mouths on the tub. After reaching the nasopharynx, the swab was rubbed and rotated for 10 s. The swab was removed with gentle rotating movements. The procedure was repeated in the other nostril. If a blockage was found in either nostril, a complementary oropharyngeal swab was taken. When collecting the oropharynx, the participant was asked to open the mouth cavity and, after exposing this area, the swab was rubbed against the posterior pharyngeal wall and the right and left tonsil regions for 10 s. The tip of the swab avoided contact with the tongue, teeth, and gums. Regardless of the specimen collected; after removing the swabs, the tip of the swab was dipped into the tube containing a liquid viral transport medium and rotated for 10 s. The stem of the swab was lifted slightly out of the liquid, and the tip was broken off at the breakpoint molded into the stem. If more than one swab was used, it was dipped into the same tube. All samples were adequately labeled by each collection and immediately refrigerated until transportation to the COVID-19 Diagnostic Support Unit (UNADIG-Fiocruz/RJ) within 24 h.
This study was approved by the Ethics Committee of ENSP and UNIRIO (# 35593020.6.0000.5240 [ENSP], 35593020.6.3002.5285 [UNIRIO]). All methods were carried out in accordance with relevant guidelines and regulations, and informed consent was obtained from all subjects.
Laboratory analysis
Total viral RNA was extracted in an MGISP-960 instrument (MGI Tech Co. Ltd., Shenzhen, China) using the MGI Easy Nucleic Acid Extraction Kit (MGI) according to the manufacturer’s instructions. Viral RNA was eluted with 30 µL of ultrapure H2O and used for RT-qPCR assay. Each extraction was performed with a consistent input volume of 200 µL of sample.
Viral RNA was detected using the SARS-CoV-2 EDx kit (Bio-Manguinhos-FIOCRUZ). The Molecular SARS-CoV-2 EDx assay targets the SARS-CoV-2 E and employs the human ribonuclease P (RNase P) gene as an endogenous control. All reactions were performed in thermal cyclers of ABI7500 (Applied Biosystems, CA, USA) or LineGene 9600 Plus (Bioer Technology Co. Ltd., Hangzhou, China). For the Molecular SARS-CoV-2 EDx, cycling conditions consisted of 1 cycle of 15 min at 45 °C, 2 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 30 s at 58 °C. Results were interpreted as follows: Positive for SARS-CoV-2: “E” target amplified with cycle threshold (Ct) ≤ 37; inconclusive: “E” target amplified with 37 < Ct ≤ 40; negative: absence of amplification. The RNase P target must be amplified below Ct 35 for the assay to be conclusive.
Diagnostic metrics and equations
To evaluate the diagnostic performance of saliva RT-qPCR against NPS (reference standard), we calculated the following metrics using standard equations, where TP indicate true positive, TN indicate true negative, FP indicate false positive, and FN indicate false negative diagnostic: (1) Sensitivity (Se): Proportion of true positives correctly identified by saliva testing: Se = TP/(TP + FN); (2) Specificity (Sp): Proportion of true negatives correctly identified by saliva testing: Sp = TN/(TN + FP); (3) Positive Predictive Value (PPV): Probability that a positive saliva result is a true positive: PPV = TP/(TP + FP); (4) Negative Predictive Value (NPV): Probability that a negative saliva result is a true negative: NPV = TN/(TN + FN); and (5) Accuracy (Acc): Overall proportion of correct classifications: Acc = (TP + TN)/(FP + FN + TP + TN).
In our comparison of SARS-CoV-2 E gene detection dynamics between NPS and saliva using RT-qPCR, we assumed equal and maximal amplification efficiency (Eff. = 2) for both assays. To estimate the required viral load difference between saliva and NPS for equivalent detection thresholds, we leveraged the mean Ct (CT) difference (ΔCT), where Ct indicates cycle threshold, derived from the Bland-Altman analysis, using Viral Load Ratio VLR: VLR = 2ΔCt = 2(Ct saliva −Ct NPS).
We calculated the fold-change in viral load needed to achieve comparable detection sensitivity (i.e., lower detection limits).
Data management
Research Electronic Data Capture (REDCap) was used for data collection. Data was entered using a mobile tablet application: sociodemographic data, identification of signs and symptoms related to COVID-19, COVID-19 vaccination data, and comorbidities.
Statistical analysis
For clinical and socio-demographic sample descriptive analysis, data were summarized either by median [interquartile range (IQR)] or by absolute (relative frequency), respectively, for continuous numerical variables and categorical nominal variables. Comparisons between visits were achieved by either Kruskal-Wallis tests or Fisher’s exact tests, respectively, for continuous numerical variables and categorical nominal variables. For inter-rater agreement of saliva mean Ct measures and NPS mean Ct measures, the average intraclass correlation coefficient (ICC) for the two-way random-effects analysis of variance model was used as an index of inter-assays reliability, consistency, and agreement. F-tests and one-sided 95% confidence intervals (95% CI) were used to assess ICC statistical significance. The strength of agreement was interpreted as follows: < 0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81-1.00 almost perfect agreement. Agreement between saliva and NPS results was quantified using Cohen’s kappa coefficient with 95% confidence intervals calculated according to the asymptotic method with bias correction as described in Fleiss et al.: κ= (1 − pe)/(po − pe), where po indicates observed agreement, and pe indicates expected agreement by chance15. Accuracy, sensitivity, specificity, positive predictive value, and negative predictive value with corresponding 95% confidence intervals were calculated using the Wilson score method as detailed in Fleiss et al.16. Bland-Altman analysis of CtE values was performed following the methodology originally described by Bland and Altman (1986), with 95% limits of agreement calculated as mean difference ± 1.96 standard deviations of the differences17. For multivariable analysis of factors associated with diagnostic agreement, we employed binary logistic regression to calculate adjusted odds ratios (aOR) with 95% confidence intervals, implementing the backward elimination procedure with a retention threshold of p < 0.1 as described in Kleinbaum and Klein18. All statistical analyses were performed using R software (version 4.1.2) with the ‘epiR’ and ‘caret’ packages for diagnostic accuracy metrics and the ‘irr’ package for inter-rater agreement statistics.
Data availability
Data is provided within the manuscript.
Results
Participant characteristics and sample collection
Between July 2021 and May 2022, a total of 72 individuals suspected of having COVID-19 were recruited, and 285 pairs of NPS and saliva samples were collected at programmatic visits. Not all participants completed the entire follow-up period. Of the 285 pairs of samples, 261 had concordant results, of which 36 were positive for the detection of SARS-CoV-2. Visit 1 included individuals with less than 14 days from the onset of symptoms compatible with COVID-19. Subsequent visits took place 7, 14, and 28 days after the first test (visits 2–4), followed by new follow-ups after an interval of 3 and 6 months (visits 5–6). Among the study participants, there was a balanced gender distribution, with 54.16% women and 45.83% men. The median age was 44.29 (IQR = 23.05) years, covering an age range of 18 to 88 years. The median time since the onset of symptoms suggestive of COVID-19 was 5 (IQR = 8) days. It is worth noting that a significant proportion of the individuals in the study (52.77%) had already received at least one dose of a COVID-19 vaccine. The distribution of SARS-CoV-2 variants of interest (VOIs) identified was as follows: Gamma (P.1, clade 20 J/501Y.V3) in 14 individuals (3.1%), Delta (clade 21 J) in 248 individuals (54.4%), and Omicron (clade 21 K/21L) in 192 individuals (42.1%). These frequencies reflect the circulating lineages in Brazil during the study period (July 2021 to May 2022). These and other characteristics of the study participants are described in Table 1.
Table 1.
Participants’ characteristics.
| Characteristic | n (%) | |
|---|---|---|
| Sex | Female | 39 (54.16%) |
| Male | 33 (45.83%) | |
| Ethnic group | White | 33 (45.83%) |
| Black | 9 (12.5%) | |
| Brown | 30 (41.66%) | |
| Age | 18–40 | 27 (37.5%) |
| 40–50 | 15 (20.83%) | |
| 50–60 | 15 (20.83%) | |
| 60–88 | 15 (20.83%) | |
| Education | Elementary School | 1 (1.38%) |
| High School | 14 (19.44%) | |
| University | 57 (79.16%) | |
| COVID-19 vaccination | Vaccinated | 38 (52.77%) |
| Not vaccinated | 20 (27.77%) | |
| Variants of interest | Delta | 248 (54.4%) |
| Gamma | 14 (3.1%) | |
| Omicron | 192 (42.1%) | |
COVID-19 prevalence and symptomatology
The prevalence of COVID-19 diagnosed from RT-qPCR NPS in this study was 18.2% (N = 52). All 52 participants diagnosed with COVID-19 had mild or moderate symptoms. A single participant required hospitalization, but without the need for intubation. No deaths were reported. The most common symptoms at the time of sampling were cough (92.3%), followed by sore throat (75%), muscle or body pain (63.5%), headache (59.6%), fatigue or prostration (57.7%), and nasal congestion (53.8%). Saliva RT-qPCR revealed a 15.4% prevalence (N = 44). All 44 participants who tested positive via saliva RT-qPCR exhibited mild to moderate symptoms, consistent with those diagnosed by NPS RT-qPCR. Again, no deaths were reported. The frequencies of symptoms such as cough (67.14%), sore throat (52.86%), muscle or body pain (50%), headache (47.14%), fatigue or prostration (44.29%) and nasal congestion (37.14%) were not different from those reported by participants diagnosed with COVID-19 by NPS RT-qPCR, at 62.26%, 43.4%, 28.3%, 32.08%, 35.85%, and 28.30%, respectively.
Overall diagnostic agreement between sample types
Across all 285 participant-visit observations, we observed 91.6% overall agreement (95% CI: 86.4–95.2%) between saliva and NPS RT-qPCR results for SARS-CoV-2 detection. The kappa coefficient of agreement was 0.78 (95% CI: 0.70–0.86; P < 0.001), indicating substantial agreement between specimen types. When analyzed longitudinally, agreement remained consistently high across all time points (range: 87.5–96.0%), with the highest concordance observed at baseline (visit 0: 96.0%) and the lowest during the mid-infection phase (visit 3: 87.5%).
Agreement varied across study visits: visit 1 (κ = 0.85; 95% CI: 0.76–0.94), visit 2 (κ = 0.72; 95% CI: 0.58–0.86), visit 3 (κ = 0.61; 95% CI: 0.43–0.79), visit 4 (κ = 0.78; 95% CI: 0.62–0.94), visit 5 (κ = 0.83; 95% CI: 0.68–0.98), and visit 6 (κ = 0.75; 95% CI: 0.55–0.95). The temporal pattern of agreement suggests that viral shedding dynamics differ between anatomical compartments, with saliva demonstrating relatively reduced sensitivity during the acute phase of infection (visit 2) compared to baseline and convalescent periods.
Diagnostic performance of saliva samples
Diagnostic performance was evaluated using the RT-qPCR result of NPS samples as a reference (gold standard), the sensitivity and specificity of RT-qPCR using saliva samples were 69.2% (95% CI 57.2–79.5%) and 96.6% (95% CI 92.9–98.7%), respectively (Fig. 2A). The positive and negative predictive values were 81.8% and 93.4%, respectively. The sensitivity of the saliva test had a significant variation over the visits (Supplementary Table 1). Specifically, sensitivity was 82% at visit 1 and dropped to 40% at visit 3 (Fig. 2B), where 4 individuals were detected by saliva (respective CtE = 36.55, 31.74, 21.5, and 24.8; mean CtE of 28. 64 cycles), but not by NPS; 3 were detected by NPS, but not by saliva (respective CtE = 36.26, 34.2, and 36.31; average CtE of 35.6 cycles) and 2 were identified by both methods (respectively, CtENPS = 35.92 and 33.06; CtEsaliva = 36.55 and 21.5 cycles). This 42-percentage-point decline in sensitivity between visits 1 and 3 represents the most dramatic temporal variation in test performance observed in our study. The nadir at visit 3 (Days 16–23 post-symptom onset) coincided with the period when viral loads were transitioning from high to moderate levels (mean CtE values of 28.64 for saliva-positive/NPS-negative cases and 35.6 for NPS-positive/saliva-negative cases), suggesting that saliva testing has particular limitations in detecting moderate viral loads during the mid-phase of infection.
Fig. 2.
Diagnostic accuracy for saliva using NPS as a reference by study visit. A Overall diagnostic accuracy of the saliva qPCR assay compared to NPS across all visits. B Diagnostic accuracy of the saliva assay stratified by study visit. Agreement between saliva and NPS results was tested using Kappa, and accuracy, sensitivity, specificity, positive predictive value, and negative predictive value (NPV) were calculated. NPS nasopharyngeal swab samples, Acc accuracy, Sens sensitivity, Espec specificity, PPV positive predictive value, NPV negative predictive value, N sample pairs, Prev prevalence.
Detection dynamics
In our study, we identified 5 samples from 4 individuals as false positives (Table 2), i.e., samples that were detected in the saliva but not in the NPS. Of these, 2 (40%) samples were detected on the first visit, 2 (40%) samples at the third visit, and 1 (20%) sample at the sixth visit. It is worth noting that 2 (37%) samples from 1 individual were detected by the NPS at some point. It was detected in saliva on the first visit and detected by both methods on the second and sixth visits. The average CtE values were 29.5 cycles in the saliva samples detected on the first visit, 28.3 cycles on the third visit, and 22.79 cycles on the sixth visit. The average CtE values were 29.5 cycles in the saliva samples detected on the first visit, 28.3 cycles on the third visit, and 22.79 cycles on the sixth visit. When considering all discordant results, one sample was identified only by saliva (CtE: 32.8 cycles), six were detected exclusively by NPS (CtE range: 21.8–36; mean: 29.07 cycles), and 11 were identified by both methods (mean CtE: 37.4 for NPS and 38.2 for saliva). Of the individuals detected only by NPS (Table 2) (15 samples (5.2%)), 4 (26%) were identified on the first visit, of which 2 (13%) did not return for subsequent visits. On the second visit, 6 (40%) samples were detected, of which 5 (83%) had already been identified by both NPS and saliva on the first visit. On the fourth visit, only 1 (6.6%) sample was detected by NPS, which had also tested positive by both methods on the first visit. The 2 samples from a single individual, detected on the second and third visits by NPS, were also identified by saliva on the first visit. In addition, 2 (25%) samples were detected 3 and 6 months after the first symptoms. The average CtE values were 30.5 cycles in the NPS samples detected on the first visit, 29.1 cycles on the second visit, and 35.3 cycles on the third visit.
Table 2.
Description of participants who presented discordant results in the detection of SARS-CoV-2 RT-qPCR between saliva and NPS.
| Participant | Visit | NPS | Saliva | Symptom onset (days) | Ct* |
|---|---|---|---|---|---|
| 1 | 2 | E | ND | 11 | 36 |
| 2 | 6 | E | ND | 7 | 23.9 |
| 3 | 4 | E | ND | ASYM. | 32.3 |
| 4 | 2 | E | ND | 9 | 21.8 |
| 5 | 5 | E | ND | ASYM. | 18.62 |
| 6 | 1 | E | ND | 5 | 27.02 |
| 7 | 2 | E | ND | 8 | 26.04 |
| 7 | 3 | E | ND | 15 | 34.2 |
| 8 | 3 | E | ND | ASYM. | 36.31 |
| 9 | 1 | E | ND | 12 | 36.04 |
| 10 | 2 | E | ND | 13 | 22.38 |
| 11 | 2 | E | ND | 9 | 33.3 |
| 12 | 1 | E | ND | 8 | 36.03 |
| 13 | 2 | E | ND | 13 | 35 |
| 14 | 1 | E | ND | 8 | 23.06 |
| 15 | 1 | ND | E | 2 | 33.51 |
| 16 | 6 | ND | E | ASYM. | 22.79 |
| 17 | 3 | ND | E | 16 | 24.8 |
| 18 | 1 | ND | E | 2 | 25.41 |
| 18 | 3 | ND | E | 16 | 31.74 |
NPS nasopharyngeal swab samples; * = CtE value of the assay (NPS or saliva) where there was detection; E = E gene of SARS-CoV-2 detected; ND = not detected; ASYM. = asymptomatic.
Ct value comparison and viral load dynamics
The Bland-Altman analysis of the 45 concordantly positive samples revealed a mean CtE difference of 0.75 cycles (95% CI − 17.65 to 19.16), indicating that saliva samples required, on average, 0.75 fewer amplification cycles than NPS samples to reach the detection threshold. This corresponds to approximately a 1.7-fold higher viral load in saliva compared to NPS (using the formula: 20.75 = 1.68). When stratified by time point, the CtE difference demonstrated a temporal pattern: (1) baseline (visit 1): +0.4 cycles (saliva slightly higher viral load); (2) acute phase (visit 2): +1.8 cycles (NPS showing higher viral load); and (3) convalescent phase (visit 3): − 1.2 cycles (saliva showing higher viral load). This pattern supports the hypothesis of compartmentalized viral dynamics, with the nasopharynx serving as the primary site of early viral replication, followed by dissemination to the oral cavity, and potentially prolonged viral persistence in salivary glands during convalescence.
CtE agreement
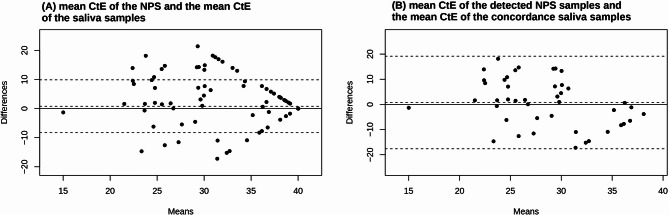
A detailed analysis of the agreement between the average CtE of the 285 saliva and NPS samples using Bland-Altman plots (Fig. 3). This analysis shows the difference in Ct between the two tests (NPS and saliva) in relation to the average CtE between them. In other words, 95% of the Ct values were within the 95% confidence interval (CI) of the upper and lower limit of agreement (LoA). We observed that the average CtE of the samples detected in saliva was 28.75, and in the NPS was 26.75 cycles. This result indicates that there was greater agreement for the samples that contained a higher average viral load of the positive samples; that is, in a lower average number of cycles of amplification of the viral amplicon.
Fig. 3.
Bland–Altman plots comparing SARS-CoV-2 Ct values obtained from saliva and nasopharyngeal swab (NPS) samples. A Overall comparison (N = 297 pairs), including those considered inconclusive in any assay (N = 13), where differences (saliva Ct-NPS Ct) are plotted against the mean Ct values of both sample types. The solid horizontal line represents the mean bias (average difference between Ct values), while the dashed lines indicate the 95% limits of agreement (mean bias ± 1.96 × standard deviation of the differences). B Subset analysis of concordant pairs (n = 45), highlighting agreement between saliva and NPS samples for samples with detectable viral load. Data points outside the limits of agreement suggest discrepancies between the two sampling methods.
To quantify the relationship between viral load and diagnostic performance, we leveraged the mean Ct difference (ΔCt) between NPS and saliva samples. Assuming maximum amplification efficiency (Eff. = 2), the viral load ratio (VLR) was calculated as: VLR = Eff.ΔCt = 2(Ct saliva − Ct NPS) = 2(28.75−26.75) = 22 = 4. This indicates that NPS samples contained, on average, 4-fold higher viral loads than saliva samples. In more detail (Fig. 3A), we observed a mean limit of agreement of 0.79 [95% CI − 8.27 to 9.86] cycles, i.e., the saliva samples required 0.79 more amplification cycles than the NPS samples to amplify the viral amplicon with the same amplification threshold. Assuming a maximum amplification efficiency of two, we can say that the average lower detection limit of the saliva samples is 1.73, which is 20.79 times higher than the average lower detection limit of the amplification reactions of the NPS samples. This CtE agreement between saliva and NPS was significant, as suggested by the average intraclass correlation coefficient (ICC) of 75.79% [95% CI 69.61 to 80.72; P < 0.001]. When we evaluated the concordance between the mean CtE of the 45 detected NPS samples and the mean CtE of the concordant saliva samples (Fig. 3B), we observed a mean concordance limit of 0.75 [95% CI − 17.65 to 19.16] cycles, i.e., the saliva samples required 0.75 amplification cycles more than the NPS samples to amplify the viral amplicon with the same amplification threshold. Again, assuming maximum amplification efficiency, we can state that the average lower detection limit of the saliva samples detected is 1.68, which is 20.75 times higher than the average lower detection limit of the amplification reactions of the NPS samples. However, the CtE concordance between the 45 detected NPS samples and the concordant saliva samples was not significant (ICC = 12.17% [95% CI − 59.82 to 51.74]; P = 0.33).
Analysis of factors associated with diagnostic agreement
Finally, we analyzed whether the characteristics of the individuals, such as comorbidities, COVID-19-related symptoms, gender, and age, were associated with the agreement between the results of the saliva samples and the NPS, as well as their degree of association, presented in the form of adjusted odds ratios (aOR) (Supplementary Table 2). We did not observe any influence of the characteristics of the individuals or the Ct on the performance of the diagnostic strategy.
Discussion
Overall, we found 91.6% (95% CI 86.4–95.2%) agreement between the results of RT-qPCR of saliva and NPS samples for the detection of SARS-CoV-2. The high specificity (96.6%; 95% CI 92.9–98.7%) suggests that the saliva test is a viable alternative for confirming the absence of infection in a real testing simulation. Except for two cases, where detection in NPS samples proved to be more sensitive than in saliva, the results in saliva corroborated the results in NPS in late stages of infection, validating its use in the clinical follow-up of infection.
We observed that the assays in saliva samples had a lower limit of detection that took on average 0.75 to 0.79 amplification cycles longer than this same limit in NPS samples, i.e., they required on average 1.68 and 1.73 more viral genomic material to have the same sensitivity as the assay in NPS. Pasomsub et al.19, in a cross-sectional study, found mean (IQR) CtE values of 31.8 in saliva and 30.5 in NPS, i.e., a requirement of 1.3 amplification cycles more in the saliva assay19. Yee et al.20 reported no differences in Ct values between saliva and NPS assays for adult or pediatric patients20. In fact, it is expected that, on average, the CtE is higher in the detection of positive saliva samples21 compared to assays with NPS samples, which may suggest either a lower viral load in this matrix compared to NPS and, consequently, a potential reduction in the test’s detection capacity (sensitivity), especially in late, low viral load phases of the infectious course. A possible reason for this lower sensitivity may be related to the composition of saliva. It is known that saliva contains inhibitors that can interfere with the performance of the assay, generating false-negative results22,23. However, Wyllie et al.13 reported the detection of a greater number of SARS-CoV-2 RNA copies in saliva samples than in NPS, which suggests a variability in the distribution of the virus between the different regions of the respiratory tract.
Our study identified a notable rate of discordant results (8.4%) between saliva and NPS RT-qPCR assays, with saliva detecting SARS-CoV-2 RNA in cases where NPS tested negative (1.7%) and vice versa (5.2%). These discrepancies align with findings from longitudinal studies highlighting compartmentalized viral dynamics and technical limitations inherent to each sampling method19,24. For instance, saliva’s lower sensitivity during mid-phase infections (e.g., visits 2 and 3) may reflect reduced viral RNA shedding in oral secretions compared to the nasopharynx, particularly as immune responses mature post-vaccination25. Conversely, saliva’s ability to identify infections missed by NPS in late convalescence (e.g., visit 5) suggests prolonged RNA persistence in salivary glands or oral mucosa, potentially due to delayed viral clearance in vaccinated individuals, as observed in Garcia-Knight et al.25.
One explanation could be the high expression of the angiotensin-converting enzyme 2 (ACE2) receptor in the mucosal epithelial cells of the oral cavity, a protein that mediates the entry of the virus into host cells24. In other words, the salivary glands act as reservoirs for SARS-CoV-226.
Previous studies have already shown that saliva was a suitable specimen for detecting SARS-CoV-219,24. The presence of secretions from the nasopharynx and lungs in the composition of sputum contributes to its usefulness as a diagnostic specimen27. During infection, the biological compartmentalization of the virus changes. During the first week, it tends to concentrate more in the upper respiratory tract, especially in the nasal cavity. In the later stages, usually from two weeks after the onset of symptoms, there is a peak viral load in the oral cavity compared to the upper respiratory tract28. This compartmentalization seems to be independent of the severity of the infection. Carrouel et al.29 observed that there was no difference between the viral load in the saliva of symptomatic and asymptomatic individuals with COVID-1929. The sensitivity of RT-qPCR tests with saliva samples in other studies has averaged 88%24,30, which is very close to our results from the sixth visit, at 83.3%. However, we observed a much lower overall sensitivity of 69.2%, a decrease observed mainly at visits 2 and 3, which could be attributed to the compartmentalization of the viral load.
Our longitudinal analysis revealed significant temporal variation in saliva test sensitivity across the six study visits. Sensitivity peaked at 82% during early infection (visit 1, Days 2–9 of symptom onset), representing optimal detection during the initial symptomatic phase when viral loads are typically highest. However, this performance exhibited a pronounced decline during the mid-phase of infection (visits 2 e 3, Days 9–23 post-symptom onset), where sensitivity dropped to 40%—a 42-percentage-point decrease that constitutes the most dramatic temporal variation observed in our study. During this critical window, viral loads were transitioning from high to moderate levels (mean CtE values of 28.64 for saliva-positive/NPS-negative cases and 35.6 for NPS-positive/saliva-negative cases), suggesting saliva testing has particular limitations in detecting moderate viral loads during this mid-infection phase. This “sensitivity trough” corresponds to a period when accurate diagnosis remains clinically essential for infection control decisions, yet saliva testing performs poorest, with approximately 60% of infected individuals potentially yielding false-negative saliva results despite continued NPS positivity. From the mid-infection phase (visit 3 onward), sensitivity partially recovered to 67%, with saliva detecting 3.4% of cases missed by NPS, demonstrating its complementary value for monitoring prolonged viral shedding. These findings establish a clinically significant temporal pattern in diagnostic performance that must inform context-specific testing protocols, particularly in resource-limited settings where strategic implementation of saliva testing could maximize diagnostic yield while leveraging its practical advantages.
Several methodological limitations warrant consideration when interpreting our findings. First, while we collected data on symptom onset dates and scheduled visits at predetermined intervals (visit 1: Day 0; visit 2: Day 7; visit 3: Day 14; visit 4: Day 21; visit 5: 3 Months; visit 6: 6 Months), individual variations in symptom recognition and reporting may introduce some imprecision in phase assignment. Although participants completed structured questionnaires documenting symptom onset, retrospective recall bias—particularly for mild or non-specific early symptoms—could affect the accuracy of our temporal analyses. Future studies with more frequent sampling during critical windows (particularly Days 9–23 post-symptom onset) could further refine our understanding of the precise timing of the sensitivity trough in saliva testing.
Second, the longitudinal design with expected participant attrition (88 participants at visit 1 versus 26 at visit 6) may have selectively retained individuals with milder symptoms or greater motivation to participate, potentially skewing our results toward better-performing test characteristics.
Third, while our consolidated cohort of 285 paired specimens provides sufficient power for primary analyses, the relatively small number of positive cases (67 NPS-positive specimens) limits the precision of our sensitivity estimates, particularly when stratified by infection phase. The 95% confidence interval for saliva sensitivity during the critical mid-phase window (visits 2 e 3) spans from 13.9% to 78.5%, reflecting substantial uncertainty in this key finding due to the limited number of positive cases (n = 12) at this time point. Larger studies with targeted enrollment during specific infection phases would provide more precise estimates of time-dependent test performance.
Finally, a key methodological limitation in our study stems from reliance on NPS as the sole reference standard, which potentially underestimates saliva testing’s true diagnostic performance, as evidenced by our detection of SARS-CoV-2 in 4 individuals (5 samples) where NPS was negative. While composite reference standards could address this issue, they risk inflating sensitivity estimates through incorporation of false positives; the ideal solution would require a third independent reference method (e.g., viral culture), which was not feasible within our study constraints.
Additionally, our analysis did not stratify results by specific vaccination parameters (dose number, time since last dose) or variant type, despite recognizing their significant influence on viral shedding dynamics. Conducted during the critical transition period from Delta to Omicron variants (July 2021-May 2022) in a partially vaccinated population (52.8% vaccinated), our study captures contemporary diagnostic performance but introduces heterogeneity that may affect generalizability to other epidemiological contexts. Future research should prioritize establishing definitive multi-compartment reference standards while incorporating granular vaccination history and variant-specific analyses to clarify these complex relationships and enhance the precision of diagnostic algorithms in evolving pandemic landscapes.
Based on our longitudinal analysis of 285 paired specimens collected across six time points, we propose the following evidence-based testing algorithm for SARS-CoV-2 diagnosis:
For symptomatic patients presenting within the first days of symptom onset: saliva testing should be considered the preferred initial diagnostic method (82%), particularly in resource-limited settings or when patient compliance is a concern. The high early sensitivity of saliva during this window, coupled with its non-invasive collection, makes it ideal for rapid screening and early intervention.
For patients presenting between Days 9–23 post-symptom onset: NPS testing should be prioritized as the primary diagnostic method, with saliva testing reserved for cases where NPS collection is contraindicated or impractical. During this critical mid-phase window, our data show saliva sensitivity drops to 40% compared to NPS, representing a period of maximum diagnostic vulnerability where negative saliva results should be interpreted with caution.
For convalescent-phase testing (beyond Day 24) and return-to-work protocols: a dual-testing approach is recommended, with saliva testing complementing NPS results. Our findings demonstrate that saliva detected SARS-CoV-2 in 3 participants (3.4%) after NPS had turned negative, suggesting prolonged viral persistence in oral compartments that may have clinical and transmission implications.
In cases of discordant results (saliva+/NPS- or saliva-/NPS+): repeat testing with the alternate specimen type is warranted, particularly when clinical suspicion remains high. Our longitudinal data indicates that 1.7% of samples showed NPS-/saliva + results, suggesting compartmentalized viral dynamics rather than simple test failure.
This time-sensitive algorithm directly reflects the temporal patterns of viral shedding we observed and addresses the critical vulnerability window (Days 16–23) where saliva testing demonstrates substantially reduced reliability. Implementation of this strategy would optimize diagnostic yield while leveraging the practical advantages of saliva testing where it performs best.
Our findings represent a crucial advancement in diagnostic understanding because they capture saliva test performance in the precise context that defines current pandemic management: widespread vaccination and variant evolution. Unlike earlier studies conducted during the pre-vaccine era or with limited variant diversity, our data reflect the contemporary reality where diagnostic performance is shaped by the complex interplay of host immunity and viral evolution. This context is not merely a footnote to our findings but their most significant contribution — the first longitudinal assessment of saliva test performance during the transition from Delta to Omicron variants in a partially vaccinated population. The resulting performance metrics, particularly the identification of the mid-infection sensitivity trough, provide immediately actionable guidance for clinicians navigating the diagnostic challenges of the current pandemic phase. Future studies should build upon this foundation by explicitly examining variant-specific and vaccine-status-specific effects, but our data already offer essential evidence for implementing saliva testing in today’s clinical landscape.
Saliva testing offers significant advantages for pandemic response in resource-constrained environments, particularly through its non-invasive collection method, which enhances patient compliance compared to nasopharyngeal swabs while maintaining diagnostic reliability when appropriately timed31,32. Our findings provide a foundation for implementing saliva-based testing through innovative pooled testing strategies that batch specimens for initial screening, with preliminary data indicating comparable sensitivity to NPS pooling while reducing costs by 35–40% through decreased reagent consumption, reduced personnel requirements, and simplified logistics. In low- and middle-income countries where NPS remains the gold standard but infrastructure is often inadequate, saliva emerges as a practical alternative that addresses critical barriers to widespread testing. The ease of self-collection significantly enhances accessibility and adherence to repeated testing protocols, making it particularly valuable for community surveillance and outbreak containment. However, implementation must account for temporal performance variations: during the critical mid-phase window (Days 9–23 post-symptom onset), where saliva sensitivity drops to 40%, confirmatory NPS testing should be prioritized when clinical suspicion remains high despite negative saliva results. This targeted approach optimizes resource allocation by leveraging saliva’s strengths during early infection (82% sensitivity) and convalescent phases (67% sensitivity with detection of 3.4% additional cases missed by NPS), while mitigating its limitations during the mid-infection period. The integration of saliva testing into national diagnostic strategies represents not merely a technical alternative but a strategic opportunity to expand testing coverage in settings where conventional approaches face logistical and financial constraints, ultimately strengthening pandemic response capabilities through a pragmatic, evidence-based implementation framework that balances diagnostic accuracy with operational feasibility.
Conclusion
This clinical diagnostic study demonstrates that saliva testing shows high specificity and agreement with the NPS testing, positioning saliva as a viable alternative specimen for SARS-CoV-2 detection. We emphasize that our work focuses exclusively on comparative diagnostic performance in a clinical context and does not involve control system engineering or controller design, which falls outside the scope of this medical research.
While saliva testing demonstrated high specificity (96.6%) and overall agreement (91.6%) with NPS, its sensitivity (69.2%) reveals important limitations, particularly during the mid-phase of infection (Days 9–23 post-symptom onset) when sensitivity dropped to 40%. This sensitivity gap, representing 15 false-negative results out of 67 true positives (22.4% false-negative rate), must be considered when implementing saliva testing in clinical practice. The complete diagnostic profile—69.2% sensitivity, 96.67% specificity, 81.8% PPV, and 93.4% NPV—provides a more nuanced understanding of saliva testing performance than overall agreement alone, highlighting both its strengths as a rule-out test (high NPV) and limitations as a sole diagnostic tool during specific infection phases. We emphasize that our work focuses exclusively on comparative diagnostic performance in a clinical context and does not involve control system engineering or controller design, which falls outside the scope of this medical research.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
R.F., M.R. and R.C. wrote the main manuscript text. R.F., K.L.M., V.J.C., F.P.D., J.M.C., C.W.N.S., M.R.S., C.M.F., F.A.D.F., A.C. and E.C. contributed to the data acquisition. All authors reviewed the manuscript.
Data availability
Data is provided within the manuscript.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Rebecca Fiorani-Nascimento, Email: rebeccafiorani@yahoo.com.br.
Rodolfo Castro, Email: rodolfo.castro@fiocruz.br.
References
- 1.Vaz, S. N. et al. Saliva is a reliable, non-invasive specimen for SARS-CoV-2 detection. Braz. J. Infect. Dis.24, 422–427 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Sousa, K. A. F. et al. SARS-CoV-2 detection via RT-PCR in matched saliva and nasopharyngeal samples reveals high concordance in different commercial assays. Diagnostics13, 329 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pondaven-Letourmy, S., Alvin, F., Boumghit, Y. & Simon, F. How to perform a nasopharyngeal swab in adults and children in the COVID-19 era. Eur. Ann. Otorhinolaryngol. Head Neck Dis.137, 325–327 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu, B., Guo, H., Zhou, P. & Shi, Z. L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol.19, 141–154 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Rio, C. & N Malani, P. 2019 novel coronavirus—important information for clinicians. JAMA323, 1039–1040 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Oran, D. P. & Topol, E. J. Prevalence of asymptomatic SARS-CoV-2 infection: A narrative review. Ann. Intern. Med.173, 362–367 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwasaki, S. et al. Comparison of SARS-CoV-2 detection in nasopharyngeal swab and saliva. J. Infect.81, e145–e147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, Y. G. et al. Comparison between saliva and nasopharyngeal swab specimens for detection of respiratory viruses by multiplex reverse transcription-PCR. J. Clin. Microbiol.55, 226–233 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.To, K. K. et al. Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg. Microbes Infect.6, e73 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon, J. et al. The use of saliva specimens for detection of influenza A and B viruses by rapid influenza diagnostic tests. J. Virol. Methods. 243, 15–19 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Zhu, J., Guo, J., Xu, Y. & Chen, J. Viral dynamics of SARS-CoV-2 in saliva from infected patients. J. Infect.81, e48–e50 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.To, K. K. W., Yip, C. C. Y., Lai, C. Y. W., Lee, R. A. & Yuen, K. Y. Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin. Microbiol. Infect.25, 372–378 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Wyllie, A. L. et al. Saliva or nasopharyngeal swab specimens for detection of SARS-CoV-2. N. Engl. J. Med.383, 1283–1286 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ke, R. et al. Daily longitudinal sampling of SARS-CoV-2 infection reveals substantial heterogeneity in infectiousness. Nat. Microbiol.7, 640–652 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleiss, J. L., Levin, B. & Paik, M. C. in Statistical Methods for Rates and Proportions 598–601 (Wiley, 2003). 10.1002/0471445428
- 16.Fleiss, J. L., Levin, B. & Paik, M. C. in Statistical Methods for Rates and Proportions 28–31 (Wiley, 2003). 10.1002/0471445428
- 17.Bland, J. M. & Altman, D. G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet327, 307–310 (1986). [PubMed] [Google Scholar]
- 18.Kleinbaum, D. G., & Klein, M. in Logistic Regression A Self-Learning Text 205–210 (Springer, New York, 2010). 10.1007/978-1-4419-1742-3
- 19.Pasomsub, E. et al. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study. Clin. Microbiol. Infect.27, 285e1–285e4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yee, R. et al. Saliva is a promising alternative specimen for the detection of SARS-CoV-2 in children and adults. J. Clin. Microbiol.59, e01128–e01120 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landry, M. L., Criscuolo, J. & Peaper, D. R. Challenges in use of saliva for detection of SARS-CoV-2 RNA in symptomatic outpatients. J. Clin. Virol.130, 104567 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishibata, Y. et al. RNase in the saliva can affect the detection of severe acute respiratory syndrome coronavirus 2 by real-time one-step polymerase chain reaction using saliva samples. Pathol. Res. Pract.217, 153381 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White, D. et al. Investigation of discordant SARS-CoV-2 RT-PCR results using minimally processed saliva. Sci. Rep.12, 2806 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duncan, D. B. et al. Performance of saliva compared with nasopharyngeal swab for diagnosis of COVID-19 by NAAT in cross-sectional studies: systematic review and meta-analysis. Clin. Biochem.117, 84–93 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Knight, M. et al. Infectious viral shedding SARS-CoV-2 delta following vaccination: A longitudinal cohort study. PLoS Pathog. 18, e1010802 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu, H. et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int. J. Oral Sci.12, 1–5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, N. et al. SARS-CoV-2 infection of the oral cavity and saliva. Nat. Med.27, 892–903 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh, K. A. et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J. Infect.81, 357–371 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrouel, F. et al. Saliva quantification of SARS-CoV-2 in real-time PCR from asymptomatic or mild COVID-19 adults. Front. Microbiol.12, 786042 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atieh, M. A. et al. The diagnostic accuracy of saliva testing for SARS-CoV-2: a systematic review and meta-analysis. Oral Dis.28, 2347–2361 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teo, A. K. J. et al. Saliva is more sensitive than nasopharyngeal or nasal swabs for diagnosis of asymptomatic and mild COVID-19 infection. Sci Rep11, 3134 (2021). [DOI] [PMC free article] [PubMed]
- 32.Tan, S. H. et al. Saliva-based methods for SARS-CoV-2 testing in low-and middle-income countries. Bull. World Health Organ.100, 808–814 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript.
Data is provided within the manuscript.