Abstract
In sea urchin eggs, Ca2+ mobilization by nicotinic acid adenine dinucleotide phosphate (NAADP) potently self-inactivates but paradoxically induces long-term Ca2+ oscillations. We investigated whether NAADP-induced Ca2+ oscillations arise from the recruitment of other Ca2+ release pathways. NAADP, inositol trisphosphate (IP3) and cyclic ADP-ribose (cADPR) all mobilized Ca2+ from internal stores but only NAADP consistently induced Ca2+ oscillations. NAADP-induced Ca2+ oscillations were partially inhibited by heparin or 8-amino-cADPR alone, but eliminated by the presence of both, indicating a requirement for both IP3- and cADPR-dependent Ca2+ release. Thapsigargin completely blocked IP3 and cADPR responses as well as NAADP-induced Ca2+ oscillations, but only reduced the NAADP-mediated Ca2+ transient. Following NAADP-mediated release from this Ca2+ pool, the amount of Ca2+ in the Ca2+-induced Ca2+ release stores was increased. These results support a mechanism in which Ca2+ oscillations are initiated by Ca2+ release from NAADP-sensitive Ca2+ stores (pool 1) and perpetuated through cycles of Ca2+ uptake into and release from Ca2+-induced Ca2+ release stores (pool 2). These results provide the first direct evidence in support of a two-pool model for Ca2+ oscillations.
Keywords: cADPR/calcium/IP3/NAADP/oscillation
Introduction
Nicotinic acid adenine dinucleotide phosphate (NAADP) potently mobilizes Ca2+ in several cell types and species including sea urchin eggs (Lee and Aarhus, 1995), ascidian oocytes (Albrieux et al., 1998), starfish oocytes (Santella et al., 2000), mouse pancreatic acinar cells (Cancela et al., 1999), rat brain microsomes (Bak et al., 1999), plant microsomes (Navazio et al., 2000) and human T-lymphocytes (Berg et al., 2000). NAADP operates through a pathway that, although as yet undefined at the molecular level, is pharmacologically and physically distinct from the inositol 1,4,5-trisphosphate (IP3)- and cyclic ADP-ribose (cADPR)-sensitive Ca2+ release pathway (Lee, 1997). In the sea urchin egg, NAADP is unique among the Ca2+-releasing messengers in at least four respects (Genazzani and Galione, 1997; Lee, 1997; Galione et al., 2000). First, NAADP does not exhibit positive feedback by Ca2+, termed Ca2+-induced Ca2+ release (CICR) (Chini and Dousa, 1996). Secondly, NAADP potently self-inactivates even when present at concentrations less than those required to activate detectable Ca2+ release (Aarhus et al., 1996; Genazzani et al., 1996; Lee et al., 1997). Thirdly, once bound to its receptor, radiolabelled NAADP is occluded and cannot be displaced by non-labelled NAADP, which is thought to be related to its self-inactivation mechanism (Aarhus et al., 1996; Billington and Genazzani, 2000; Patel et al., 2000). Fourthly, NAADP mobilizes Ca2+ from a store that is distinct pharmacologically and physically from the IP3- and cADPR-sensitive Ca2+ stores (Lee and Aarhus, 1995, 2000; Genazzani and Galione, 1996).
NAADP stimulates Ca2+ oscillations in both pancreatic acinar cells (Cancela et al., 1999) and sea urchin eggs (Aarhus et al., 1996; Genazzani et al., 1996; Lee et al., 1997). In pancreatic acinar cells, NAADP releases Ca2+, which is then amplified by CICR through IP3 and ryanodine receptors (Cancela et al., 1999). Amplification of NAADP-induced Ca2+ release through CICR pathways also occurs in starfish eggs (Santella et al., 2000) and sea urchin eggs (Churchill and Galione, 2000). In sea urchin eggs, however, a ‘trigger’ mechanism is unlikely to underlie the long-term Ca2+ oscillations induced by NAADP because of self-inactivation (Aarhus et al., 1996; Billington and Genazzani, 2000; Patel et al., 2000). Based on this, Lee and coworkers (Aarhus et al., 1996; Lee, 1997; Lee et al., 1997) suggested that these Ca2+ oscillations are more likely to involve overloading and spontaneous release from the IP3- and/or cADPR-sensitive pathways. However, we demonstrated recently that NAADP self-inactivation is reversible in intact sea urchin eggs when NAADP release is submaximal and spatially localized (Churchill and Galione, 2001), raising the possibility that NAADP-induced Ca2+ oscillations are due to cycles of desensitization and re-sensitization.
Several theoretical models have been proposed to explain Ca2+ oscillations, and can be classified by the number of Ca2+ stores required (one or two) and whether the level of IP3 must also oscillate (Berridge and Galione, 1988; Tsien and Tsien, 1990; Berridge, 1993). Depending on the cell type and experimental conditions, evidence exists for mechanisms requiring oscillating levels of IP3 (Harootunian et al., 1991; Hirose et al., 1999) and control by positive and/or negative feedback of Ca2+ on Ca2+ release channels with a steady level of IP3 (Wakui et al., 1989; Hajnoczky and Thomas, 1997). Direct evidence does not exist for the two-pool mechanism proposed by Berridge and colleagues (Berridge, 1988, 1991; Berridge and Galione, 1988), which envisages transfer of Ca2+ from IP3-sensitive stores into CICR stores, which overload and spontaneously release Ca2+.
The sea urchin egg is highly responsive to IP3, cADPR and NAADP (Clapper and Lee, 1985; Clapper et al., 1987; Lee and Aarhus, 1995) and thus provides an excellent system in which to study the interaction between Ca2+ release pathways. Here we show that NAADP induces long-term Ca2+ oscillations by releasing Ca2+ from a separate store (pool 1) and is then taken up by CICR stores (pool 2). The shuttling of Ca2+ primes the CICR stores and results in cycles of Ca2+ overloading, release and re-uptake.
Results and discussion
NAADP but not IP3 or cADPR induces long-term Ca2+ oscillations
Lee and coworkers (Aarhus et al., 1996; Lee, 1997; Lee et al., 1997) reported that NAADP but not IP3 or cADPR resulted in Ca2+ oscillations in sea urchin eggs, and photorelease was much more effective than microinjection of NAADP (Aarhus et al., 1996). However, Swann and Whitaker (1986) reported that microinjection of IP3 also resulted in a second Ca2+ increase that occurred several minutes after the primary Ca2+ increase. Therefore, to investigate the mechanism by which NAADP gives rise to Ca2+ oscillations, we first compared the Ca2+ responses of sea urchin eggs to NAADP, IP3 and cADPR.
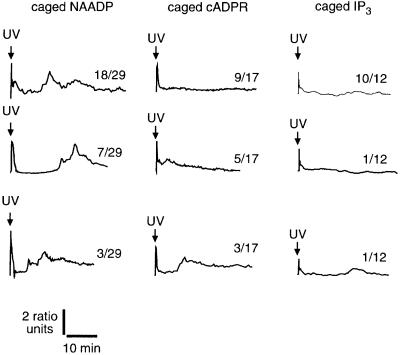
The photorelease of supramaximal amounts of messenger revealed that NAADP released more Ca2+ than cADPR, which in turn released more than IP3 (Figures 1 and 6A). That NAADP evokes a larger increase than either IP3 or cADPR supports the notion that NAADP is releasing Ca2+ from both a common store and a store responsive to only NAADP, as suggested by the data from sea urchin homogenates (Lee and Aarhus, 1995; Genazzani and Galione, 1996). NAADP induced long-lasting (at least 80 min) Ca2+ oscillations in 26 of 29 experiments (Figure 1), as demonstrated previously (Aarhus et al., 1996; Genazzani et al., 1996; Lee, 1997). NAADP-induced Ca2+ oscillations persisted in the absence of extracellular Ca2+ (data not shown), indicating that the oscillations involved mobilization from intracellular Ca2+ stores, as reported previously (Aarhus et al., 1996; Lee et al., 1997). In contrast to the high proportion of eggs exhibiting Ca2+ oscillations after photorelease of NAADP, Ca2+ oscillations were induced less frequently after photorelease of cADPR (eight of 17 experiments, p = 0.004) and IP3 (one of nine experiments, p <0.0001; Figure 1). Additionally, the magnitude of the Ca2+ oscillations was much larger after NAADP-mediated Ca2+ release than after either cADPR- or IP3-mediated Ca2+ release (Figure 1). Taken together, it is clear that Ca2+ oscillations are more robust and frequent after a Ca2+ increase mediated by NAADP than after either cADPR or IP3.
Fig. 1. Induction of Ca2+ oscillations by photorelease of NAADP, cADPR and IP3. The number of similar responses (numerator) relative to the total number of experiments (denominator) is shown next to each trace. The final intracellular concentrations were: Oregon Green 488 BAPTA Dextran, 10 µM; caged NAADP, 0.5 µM; caged cADPR, 5 µM and caged IP3, 5 µM.
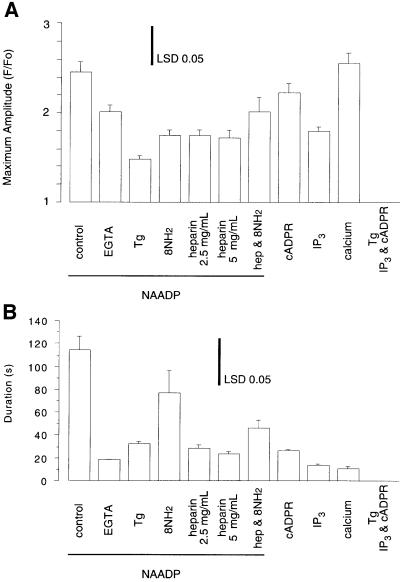
Fig. 6. Histograms of the maximal amplitude and duration of the Ca2+ transients elicited by the designated treatments. Maximal amplitude (A) was calculated as F/Fo and duration (B) was calculated as the time during which F/Fo remained above half the maximal amplitude. All data are from the Ca2+ transient elicited by photorelease of the messenger indicated. Histograms are the mean ± standard error of the mean for n = 3–29. The inset bar represents the least significant difference (LSD), which was calculated from the pooled error.
The Ca2+ increase that occurs 1–3 min after the NAADP-mediated Ca2+ increase occurs before Ca2+ recovers to baseline and appears as either a spike clearly separated from the primary increase or as only a shoulder during recovery from the primary increase (Figure 1) (Aarhus et al., 1996; Genazzani et al., 1996; Lee, 1997). To enable quantitative comparisons of data with and without a discernible Ca2+ spike, we determined the duration that Ca2+ was elevated above its half-maximal concentration (Figure 6B). This measure validly described all treatments except for the effect of removing extracellular Ca2+ (Figure 6B), in which case it is an underestimate because the first oscillation is separated from the NAADP-induced Ca2+ increase to the extent that Ca2+ decreases below its half-maximal concentration before the first oscillation occurs (Aarhus et al., 1996; Lee et al., 1997). Duration data show that the initial Ca2+ increase was more prolonged when initiated by NAADP than when initiated by either IP3 or cADPR (Figures 1 and 6B).
The later Ca2+ increases that occur 5–15 min after the NAADP-induced Ca2+ increase exhibit irregular patterns (Figure 1) (Aarhus et al., 1996; Genazzani et al., 1996; Lee, 1997). The time of onset, duration, amplitude and frequency of the Ca2+ increases is decidedly variable (Figure 1, NAADP). Often there are small Ca2+ transients of short duration (∼1 min) superimposed on larger prolonged Ca2+ transients (∼5–15 min). This suggests that there are at least two independent oscillatory mechanisms operating simultaneously. Ca2+ oscillations with a similar ‘signature’ occur after fertilization of sea urchin eggs with increases occurring before nuclear envelope breakdown, metaphase–anaphase transition and cytokinesis (Poenie et al., 1985; Whitaker and Larman, 2001).
In view of our recent data showing that NAADP desensitization is reversible in intact eggs (Churchill and Galione, 2001), we determined whether re-sensitization could explain NAADP-induced Ca2+ oscillations. Under the conditions used in the current study the eggs remained insensitive to NAADP for at least 2 h (data not shown), probably due to the use of supramaximal concentrations of NAADP. Thus, Lee and colleagues’ original argument that the long-term Ca2+ oscillations cannot be due to NAADP because of desensitization (Aarhus et al., 1996; Lee, 1997; Lee et al., 1997) remains valid.
NAADP-induced Ca2+ oscillations require both IP3- and cADPR-dependent Ca2+ release pathways
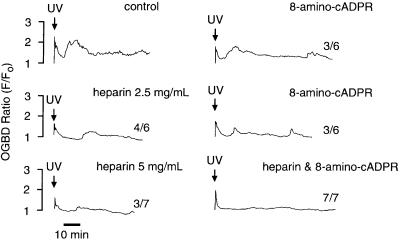
To determine the potential roles of IP3- and cADPR-dependent Ca2+ release pathways in the later oscillations (≥5 min after NAADP photorelease), the effects of IP3 and cADPR antagonists on NAADP-induced Ca2+ oscillations were investigated. The involvement of IP3 was probed with heparin, which inhibits IP3 binding to its receptor (Taylor and Broad, 1998). NAADP induced oscillations in four of six experiments (p = 0.20) in the presence of 2.5 mg/ml heparin and in three of seven experiments (p = 0.07) in the presence of 5 mg/ml heparin (Figure 2). Heparin, however, inhibited the first Ca2+ oscillation after the NAADP-induced Ca2+ increase and shortened the duration of this Ca2+ transient (Figures 2 and 6B). The involvement of cADPR was probed with 8-amino-cADPR (8NH2, Figure 6), which inhibits cADPR binding to its receptor (Walseth and Lee, 1993). The presence of 8-amino-cADPR (5 µM) did not block Ca2+ oscillations in six of six experiments (Figure 2, p >0.99). Nevertheless, 8-amino-cADPR, as did heparin, decreased the amplitude and frequency of the Ca2+ oscillations (Figure 2), indicating a partially inhibitory effect. In contrast to the variable and partially inhibitory effects of either heparin or 8-amino-cADPR alone, the combination of both compounds eliminated NAADP-induced later Ca2+ oscillations in seven of seven experiments (Figure 2, p <0.0001). In the presence of both inhibitors, NAADP released more Ca2+ than it did in the presence of either inhibitor alone (Figures 2 and 6A), possibly due to prevention of a basal leak of Ca2+ from a common store, which would lead to greater loading of this common store. These data demonstrate that both IP3- and cADPR-mediated pathways for Ca2+ release are required for NAADP-induced Ca2+ oscillations.
Fig. 2. Requirement of NAADP-induced Ca2+ oscillations on Ca2+ release pathways mediated by IP3 and cADPR. The final intracellular concentrations were: Oregon Green 488 BAPTA Dextran, 10 µM; caged NAADP, 0.5 µM; heparin, 2.5 or 5 mg/ml; 8-amino-cADPR, 5 µM; and heparin and 8-amino-cADPR combined, 2.5 mg/ml and 5 µM, respectively. The number of similar responses relative to the total number of experiments is shown next to each trace. The positive control for NAADP-induced Ca2+ oscillations was from the same batch of eggs.
That NAADP-induced Ca2+ oscillations could only be blocked completely by inhibition of both CICR pathways is similar to the findings reported for inhibition of fertilization-induced Ca2+ waves in sea urchin eggs (Galione et al., 1993; Lee et al., 1993), NAADP-induced Ca2+ release in starfish eggs (Santella et al., 2000) and for amplification of NAADP-mediated Ca2+ waves in sea urchin eggs (Churchill and Galione, 2000). The situation is different from that in pancreatic acinar cells, where NAADP-induced Ca2+ oscillations can be blocked by inhibiting either the IP3 or cADPR pathway (Cancela et al., 1999). In the case of pancreatic acinar cells, the interaction was suggested to occur at the level of CICR, with Ca2+ released via the NAADP-mediated pathway serving as ‘trigger Ca2+’, which feeds forward on to either IP3 or ryanodine receptors thereby amplifying the response (Cancela et al., 1999). Such a mechanism cannot explain the Ca2+ oscillations that occur after NAADP-induced Ca2+ mobilization because the NAADP receptors are completely desensitized to NAADP for at least 2 h after the initial release using this particular protocol (data not shown). Indeed, the first Ca2+ oscillation, which is sometimes distinct and at other times appears as a shoulder after the initial NAADP-mediated Ca2+ increase (Figure 1) (Aarhus et al., 1996; Genazzani et al., 1996; Lee, 1997), occurs during recovery from the NAADP-induced Ca2+ transient when Ca2+ is decreasing. This is at odds with a cytosol-based CICR mechanism, which would provide amplification during the increase in Ca2+.
A Ca2+ store that is thapsigargin insensitive and NAADP sensitive is present in intact eggs
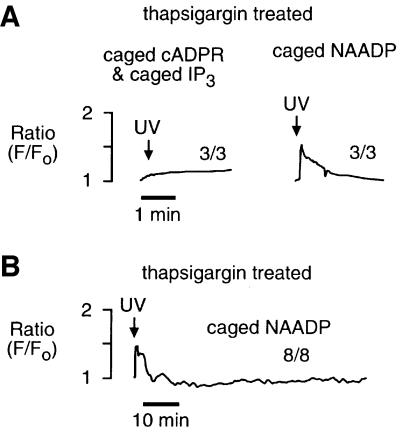
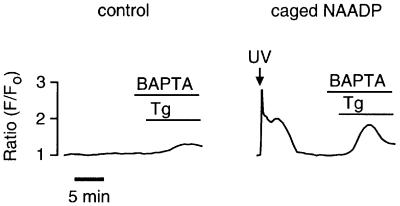
If NAADP-induced Ca2+ oscillations are due to overloading and spontaneous Ca2+ release from CICR stores, then Ca2+ would have to be moved into the CICR stores from an NAADP-sensitive Ca2+ store that is functionally and physically separate. The NAADP-sensitive Ca2+ store is pharmacologically and physically distinct in sea urchin egg homogenates (Lee and Aarhus, 1995; Genazzani and Galione, 1996). The presence of such a store in intact sea urchin eggs, however, was unknown until a recent report that demonstrated spatial separation of the NAADP-sensitive Ca2+ stores from the IP3- and cADPR-sensitive Ca2+ stores in intact sea urchin eggs whose organelles were stratified by centrifugation (Lee and Aarhus, 2000). We used a pharmacological approach to determine whether NAADP-sensitive Ca2+ stores in intact sea urchin eggs were functionally distinct from IP3- and cADPR-sensitive Ca2+ stores. Treatment of intact eggs with the Ca2+ pump inhibitor thapsigargin (2 µM) for 30 min or more completely eliminated the response to both IP3 and cADPR (Figures 3A and 6A), as reported previously for homogenates (Genazzani and Galione, 1996). In contrast, thapsigargin reduced but did not eliminate the initial response to NAADP (Figures 3A and 6A). This result demonstrates that in the intact egg NAADP targets Ca2+ stores that are functionally separate from the CICR stores. This conclusion is consistent with that of Lee and Aarhus (2000), who demonstrated recently that the NAADP-sensitive store was separable from the endoplasmic reticulum and segregated with mitochondria. However, it is unlikely that mitochondria are the target for NAADP because NAADP-sensitive stores are distinct from cytochrome C oxidase, a mitochondrial marker, in fractionated sea urchin egg homogenates (Lee and Aarhus, 1995), and mitochondria are sinks not sources for Ca2+ in non-fertilized eggs (Eisen and Reynolds, 1985). Therefore, NAADP probably targets an organelle that remains to be characterized.
Fig. 3. NAADP-induced Ca2+ oscillations require thapsigargin-sensitive Ca2+ stores. (A) Effect of thapsigargin on the initial response to photorelease of an IP3–cADPR mixture and NAADP. (B) Effect of thapsigargin on long-term NAADP-induced Ca2+ oscillations shown on a different time scale. Eggs were treated with thapsigargin (2 µM) for ≥30 min and then exposed to UV. The final intracellular concentrations were: Oregon Green 488 BAPTA Dextran, 10 µM; caged NAADP, 0.5 µM, and both caged cADPR, 5 µM and caged IP3, 5 µM.
NAADP-induced Ca2+ oscillations require thapsigargin-sensitive Ca2+ stores
Pretreatment with thapsigargin eliminated NAADP- induced Ca2+ oscillations in eight of eight experiments (Figure 3B; p <0.001 compared with the NAADP control), demonstrating a requirement for IP3- and cADPR-sensitive Ca2+ stores. These data are consistent with the elimination of NAADP-induced Ca2+ oscillations obtained with heparin and 8-amino-cADPR (Figure 2), and consistent with a mechanism in which the CICR stores (IP3- and cADPR-sensitive) play a role in the NAADP-induced Ca2+ oscillations.
CICR stores contain more Ca2+ after an NAADP-mediated Ca2+ transient
Next we directly assessed the effect of an NAADP-mediated Ca2+ release on the amount of Ca2+ in the CICR stores. As only the CICR stores are sensitive to thapsigargin (Genazzani and Galione, 1996 and Figure 3), the size of the Ca2+ transient induced by thapsigargin was used as a measure of CICR store loading. Figure 4 shows that thapsigargin (5 µM) induced a significantly (p = 0.01) larger Ca2+ transient after an NAADP-induced Ca2+ transient (maximum amplitude of 2.0 ± 0.27 ratio units, n = 10) than in control eggs (maximum amplitude of 1.3 ± 0.07 ratio units, n = 6). These data indicate that a substantial amount of the Ca2+ released by NAADP is taken up by the CICR stores. In contrast, the amplitude of Ca2+ release upon photorelease of NAADP was not increased after Ca2+ mobilization by either IP3 (maximum amplitude of 2.2 ± 0.14 ratio units, t-test, p = 0.53) or both IP3 and cADPR (maximum amplitude of 2.2 ± 0.13 ratio units, t-test, p = 0.62). The mechanism for the preferential accumulation of NAADP-released Ca2+ into the CICR stores is unknown. A possible mechanism could be faster removal of Ca2+ by thapsigargin-sensitive Ca2+ pumps than by thapsigargin-insensitive Ca2+ pumps.
Fig. 4. Overloading of CICR stores in response to an NAADP-mediated Ca2+ release. The extracellular solution was changed from artificial sea water containing 10 mM Ca2+ to one containing no added Ca2+ and 2 mM BAPTA. Thapsigargin (5 µM) was added 2 min later. The final intracellular concentrations were: Oregon Green 488 BAPTA Dextran, 10 µM and caged NAADP, 0.5 µM.
Ca2+ oscillations can be induced by photorelease of Ca2+
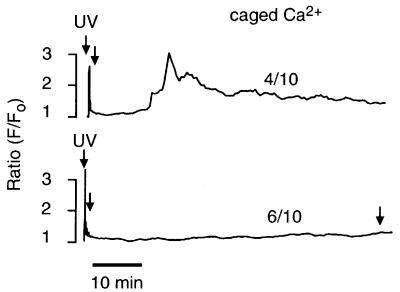
With a Ca2+-overloading mechanism, any means of overloading Ca2+ stores should induce long-term Ca2+ oscillations (Berridge and Galione, 1988; Berridge, 1991). Photoreleasing Ca2+ (NP-EGTA) (Ellis-Davies and Kaplan, 1994) resulted in an increase equivalent to that induced by NAADP (Figures 5 and 6A), and induced long-term Ca2+ oscillations in four of 10 experiments (Figure 5). The pattern of the oscillations generated by photoreleasing Ca2+ (Figure 5) was similar but not identical to that generated by NAADP (Figure 1). The amount of caged Ca2+ remaining after the first period of photorelease was determined by two additional periods of photorelease, one immediately after recovery to basal levels and one 70 min after the first photorelease (Figure 5). These additional UV illuminations failed to increase Ca2+, demonstrating that the first period of photorelease destroyed most of the NP-EGTA. The amount of Ca2+ can be approximated from this information. Given that NP-EGTA was at a concentration of 500 µM and that the resting Ca2+ (∼100 nM) is approximately at the Kd (80 nM) for NP-EGTA (Ellis-Davies and Kaplan, 1994), there would be ∼250 µM caged Ca2+ (and 250 µM free NP-EGTA). Complete photorelease of the caged Ca2+ would liberate ∼250 µM Ca2+. Although an increase in Ca2+ is sufficient for inducing Ca2+ oscillations in sea urchin eggs about half the time, it does not induce Ca2+ oscillations as often as NAADP, and fails to induce the higher frequency component. Therefore, Ca2+ alone can partially, but not completely, mimic NAADP-induced Ca2+ oscillations.
Fig. 5. Ca2+ oscillations induced by photorelease of Ca2+. The final intracellular concentrations were: Oregon Green 488 BAPTA Dextran, 10 µM and NP-EGTA, 500 µM. The number of similar responses relative to the total number of experiments is shown next to each trace.
Mechanism for NAADP-induced Ca2+ oscillations
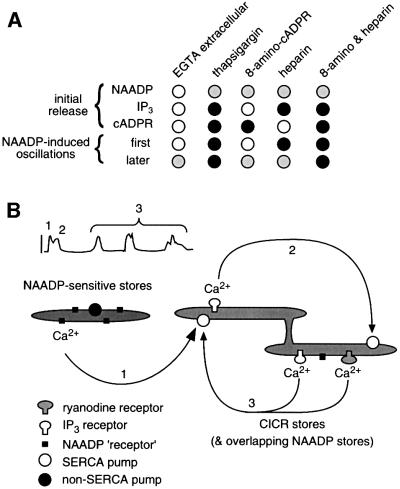
The mechanism by which NAADP induces Ca2+ oscillations and a summary of the evidence for this mechanism are presented in Figure 7. The initial Ca2+ release mediated by NAADP is largely independent of any additional processes (pathway 1, Figure 7), except for an amplification by CICR pathways, as described previously (Churchill and Galione, 2000). A portion (approximately one-third) of the Ca2+ mobilized by NAADP comes from a thapsigargin-resistant store that is distinct from the CICR stores. This released Ca2+ is then taken up into CICR stores. Ca2+ uptake continues until the stores overload and spontaneously release Ca2+ as demonstrated previously (Galione et al., 1991; Missiaen et al., 1991). A necessary feature of this mechanism is that the released Ca2+ is taken up into thapsigargin-sensitive stores more quickly than into thapsigargin-insensitive (NAADP-sensitive) stores, but how this is accomplished is unknown. The first Ca2+ oscillation after the NAADP-induced transient requires IP3-dependent Ca2+ release (pathway 2, Figure 7). Both IP3- and cADPR-sensitive Ca2+ stores participate in the later cycles of Ca2+ uptake, overload and release (pathway 3, Figure 7). This Ca2+-overloading mechanism can also explain the occasional induction of long-term Ca2+ oscillations by IP3 (Figure 1 and Swann and Whitaker, 1986) and cADPR (Figure 1), in which overloading would occur via Ca2+ influx. This mechanism meets the criteria of a two-pool model for Ca2+ oscillations (Berridge, 1988; Berridge and Galione, 1988; Tsien and Tsien, 1990) because one Ca2+ store is the source of priming Ca2+ (NAADP sensitive) and a separate Ca2+ store mediates the oscillations (CICR), and oscillations can be triggered by any increase in Ca2+ that primes the CICR stores. Therefore, this mechanism represents the first direct demonstration of the two-pool model for Ca2+ oscillations.
Fig. 7. NAADP induces Ca2+ oscillations by priming CICR stores. (A) The effect of each treatment is designated as having no effect (open circle), partial inhibition (grey circle) and complete inhibition (black circle). (B) Cartoon of the proposed mechanism. NAADP releases Ca2+ from a functionally separate Ca2+ store (designated 1). This Ca2+ is taken up into the CICR stores, thereby priming them. This results in overloading and spontaneous Ca2+ release initially from IP3-sensitive Ca2+ stores (designated 2). Subsequently, Ca2+ is taken up into and released from both IP3- and cADPR-sensitive Ca2+ stores (designated 3).
Materials and methods
Microinjection and Ca2+ imaging were performed as described previously (Churchill and Galione, 2000). Briefly, sea urchin (Lytechinus pictus) eggs (Marinus, Long Beach, CA) were obtained by intracoelomic injection of 0.5 M KCl, shed into artificial sea water (in mM, NaCl 435, MgCl2 40, MgSO4 15, CaCl2 11, KCl 10, NaHCO3 2.5, EDTA 1), dejellied by passing through 90 µm nylon mesh, and then washed twice by centrifugation. Eggs were transferred to polylysine-coated glass coverslips, pressure microinjected (Picospritzer, World Precision Instruments) with Oregon Green 488 BAPTA Dextran (Molecular Probes) and caged compounds and/or inhibitors. The Ca2+-sensitive dye was imaged by laser-scanning confocal microscopy (TCS NT, Leica) and caged compounds were photolysed with an ultraviolet laser. Images were processed with the software NIH Image to create a self-ratio by dividing each image by an image acquired before stimulation. Where appropriate, parametric data were subjected to analysis of variance with means separated by Fisher’s least significant difference test with significance taken as p ≤0.05. Proportions (e.g. number of eggs exhibiting oscillations versus number not) were analysed by creating a 2 × 2 contingency table, with NAADP compared with another treatment and then subjected to Fisher’s exact probability test.
Acknowledgments
Acknowledgements
We thank F.Boittin, R.Masgrau, Y.Okada, S.Patel and J.Thomas for helpful discussion. This work was funded by the Wellcome Trust.
References
- Aarhus R., Dickey,D.M., Graeff,R.M., Gee,K.R., Walseth,T.F. and Lee,H.C. (1996) Activation and inactivation of Ca2+ release by NADP+. J. Biol. Chem., 271, 8513–8516. [DOI] [PubMed] [Google Scholar]
- Albrieux M., Lee,H.C. and Villaz,M. (1998) Calcium signaling by cyclic ADP-ribose, NAADP, and inositol trisphosphate are involved in distinct functions in ascidian oocytes. J. Biol. Chem., 273, 14566–14574. [DOI] [PubMed] [Google Scholar]
- Bak J., White,P., Timar,G., Missiaen,L., Genazzani,A.A. and Galione,A. (1999) Nicotinic acid adenine dinucleotide phosphate triggers Ca2+ release from brain microsomes. Curr. Biol., 9, 751–754. [DOI] [PubMed] [Google Scholar]
- Berg I., Potter,B.V.L., Mayr,G.W. and Guse,A.H. (2000) Nicotinic acid adenine dinucleotide phosphate (NAADP+) is an essential regulator of T-lymphocyte Ca2+-signaling. J. Cell Biol., 150, 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. (1988) Inositol trisphosphate-induced membrane potential oscillations in Xenopus oocytes. J. Physiol. (Lond), 403, 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M.J. (1991) Cytoplasmic calcium oscillations: a two pool model. Cell Calcium, 12, 63–72. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. (1993) Inositol trisphosphate and calcium signalling. Nature, 361, 315–325. [DOI] [PubMed] [Google Scholar]
- Berridge M.J. and Galione,A. (1988) Cytosolic calcium oscillators. FASEB J., 2, 3074–3082. [DOI] [PubMed] [Google Scholar]
- Billington R.A. and Genazzani,A.A. (2000) Characterization of NAADP+ binding in sea urchin eggs. Biochem. Biophys. Res. Commun., 276, 112–116. [DOI] [PubMed] [Google Scholar]
- Cancela J.M., Churchill,G.C. and Galione,A. (1999) Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature, 398, 74–76. [DOI] [PubMed] [Google Scholar]
- Chini E.N. and Dousa,T.P. (1996) Nicotinate-adenine dinucleotide phosphate-induced Ca2+ release does not behave as a Ca2+-induced Ca2+-release system. Biochem. J., 316, 709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill G.C. and Galione,A. (2000) Spatial control of Ca2+ signaling by NAADP diffusion and gradients. J. Biol. Chem., 275, 38687–38692. [DOI] [PubMed] [Google Scholar]
- Churchill G.C. and Galione,A. (2001) Prolonged inactivation of NAADP-induced Ca2+ release mediates a spatiotemporal Ca2+ memory. J. Biol. Chem., 276, 11223–11225. [DOI] [PubMed] [Google Scholar]
- Clapper D.L. and Lee,H.C. (1985) Inositol trisphosphate induces calcium release from nonmitochondrial stores in sea urchin egg homogenates. J. Biol. Chem., 260, 13947–13954. [PubMed] [Google Scholar]
- Clapper D.L., Walseth,T.F., Dargie,P.J. and Lee,H.C. (1987) Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem., 262, 9561–9568. [PubMed] [Google Scholar]
- Eisen A. and Reynolds,G.T. (1985) Source and sinks for the calcium released during fertilization of single sea urchin eggs. J. Cell Biol., 100, 1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Davies G.C.R. and Kaplan,J.H. (1994) Nitrophenyl-EGTA, a photolabile chemator that selectively binds Ca2+ with high affinity and releases it upon photolysis. Proc. Natl Acad. Sci. USA, 91, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galione A., Lee,H.C. and Busa,W.B. (1991) Ca2+-induced Ca2+ release in sea-urchin egg homogenates—modulation by cyclic ADP-ribose. Science, 253, 1143–1146. [DOI] [PubMed] [Google Scholar]
- Galione A., McDougall,A., Busa,W.B., Willmott,N., Gillot,I. and Whitaker,M. (1993) Redundant mechanisms of calcium-induced calcium release underlying calcium waves during fertilization of sea-urchin eggs. Science, 261, 348–352. [DOI] [PubMed] [Google Scholar]
- Galione A., Patel,S. and Churchill,G.C. (2000) NAADP-induced calcium release in sea urchin eggs. Biol. Cell, 92, 197–204. [DOI] [PubMed] [Google Scholar]
- Genazzani A.A. and Galione,A. (1996) Nicotinic acid-adenine dinucleotide phosphate mobilizes Ca2+ from a thapsigargin-insensitive pool. Biochem. J., 315, 721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani A.A. and Galione,A. (1997) A Ca2+ release mechanism gated by the novel pyridine nucleotide, NAADP. Trends Pharmacol. Sci., 18, 108–110. [DOI] [PubMed] [Google Scholar]
- Genazzani A.A., Empson,R.M. and Galione,A. (1996) Unique inactivation properties of NAADP-sensitive Ca2+ release. J. Biol. Chem., 271, 11599–11602. [DOI] [PubMed] [Google Scholar]
- Hajnoczky G. and Thomas,A.P. (1997) Minimal requirements for calcium oscillations driven by the IP3 receptor. EMBO J., 16, 3533–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harootunian A.T., Kao,J.P., Paranjape,S. and Tsien,R.Y. (1991) Generation of calcium oscillations in fibroblasts by positive feedback between calcium and IP3. Science, 251, 75–78. [DOI] [PubMed] [Google Scholar]
- Hirose K., Kadowaki,S., Tanabe,M., Takeshima,H. and Iino,M. (1999) Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science, 284, 1527–1530. [DOI] [PubMed] [Google Scholar]
- Lee H.C. (1997) Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol. Rev., 77, 1133–1164. [DOI] [PubMed] [Google Scholar]
- Lee H.C. and Aarhus,R. (1995) A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem., 270, 2152–2157. [DOI] [PubMed] [Google Scholar]
- Lee H.C. and Aarhus,R. (2000) Functional visualization of the separate but interacting calcium stores sensitive to NAADP and cyclic ADP-ribose. J. Cell Sci., 113, 4413–4420. [DOI] [PubMed] [Google Scholar]
- Lee H.C., Aarhus,R. and Walseth,T.F. (1993) Calcium mobilization by dual receptors during fertilization of sea urchin eggs. Science, 261, 352–355. [DOI] [PubMed] [Google Scholar]
- Lee H.C., Aarhus,R., Gee,K.R. and Kestner,T. (1997) Caged nicotinic acid adenine dinucleotide phosphate. J. Biol. Chem., 272, 4172–4178. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Taylor,C.W. and Berridge,M.J. (1991) Spontaneous calcium release from inositol trisphosphate-sensitive calcium stores. Nature, 352, 241–244. [DOI] [PubMed] [Google Scholar]
- Navazio L., Bewell,M.A., Siddiqua,A., Dickinson,G.D., Galione,A. and Sanders,D. (2000) Calcium release from the endoplasmic reticulum of higher plants elicited by the NADP metabolite nicotinic acid adenine dinucleotide phosphate. Proc. Natl Acad. Sci. USA, 97, 8693–8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Churchill,G.C. and Galione,A. (2000) Unique kinetics of NAADP binding enhance the sensitivity of NAADP receptors for their ligand. Biochem. J., 352, 725–729. [PMC free article] [PubMed] [Google Scholar]
- Poenie M., Alderton,J., Tsien,R.Y. and Steinhardt,R.A. (1985) Changes of free calcium levels with stages of the cell division cycle. Nature, 315, 147–149. [DOI] [PubMed] [Google Scholar]
- Santella L., Kyozuka,K., Genazzani,A.A., De Riso,L. and Carafoli,E. (2000) Nicotinic acid adenine dinucleotide phosphate-induced Ca2+ release. Interactions among distinct Ca2+ mobilizing mechanisms in starfish oocytes. J. Biol. Chem., 275, 8301–8306. [DOI] [PubMed] [Google Scholar]
- Swann K. and Whitaker,M. (1986) The part played by inositol trisphosphate and calcium in the propagation of the fertilization wave in sea urchin eggs. J. Cell Biol., 103, 2333–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C.W. and Broad,L.M. (1998) Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends Pharmacol. Sci., 19, 370–375. [DOI] [PubMed] [Google Scholar]
- Tsien R.W. and Tsien,R.Y. (1990) Calcium channels, stores, and oscillations. Annu. Rev. Cell Biol., 6, 715–760. [DOI] [PubMed] [Google Scholar]
- Wakui M., Potter,B.V. and Petersen,O.H. (1989) Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature, 339, 317–320. [DOI] [PubMed] [Google Scholar]
- Walseth T.F. and Lee,H.C. (1993) Synthesis and characterization of antagonists of cyclic-ADP-ribose-induced calcium release. Biochim. Biophys. Acta, 1178, 235–242. [DOI] [PubMed] [Google Scholar]
- Whitaker M. and Larman,M.G. (2001) Calcium and mitosis. Semin. Cell Dev. Biol., 12, 53–58. [DOI] [PubMed] [Google Scholar]