Abstract
We have previously shown that the molecular chaperone heat shock protein 90 (Hsp90) is required to ensure proper centrosome function in Drosophila and vertebrate cells. This observation led to the hypothesis that this chaperone could be required for the stability of one or more centrosomal proteins. We have found that one of these is Polo, a protein kinase known to regulate several aspects of cell division including centrosome maturation and function. Inhibition of Hsp90 results in the inactivation of Polo kinase activity. It also leads to a loss in the ability of cytoplasmic extracts to complement the failure of salt-stripped preparations of centrosomes to nucleate microtubules. This effect can be rescued upon addition of active recombinant Polo. We also show that Polo and Hsp90 are part of a complex and conclude that stabilization of Polo is one of the mechanisms by which Hsp90 contributes to the maintenance of functional centrosomes.
Keywords: centrosome/Drosophila/Hsp90/Polo
Introduction
We have previously identified heat shock protein 83 (Hsp83; Cutforth and Rubin, 1994; van der Straten et al., 1997) as a centrosomal component in Drosophila and vertebrate cell lines (Lange et al., 2000). Hsp83 is the Drosophila member of the Hsp90 family, which includes highly conserved, abundant proteins that are expressed in all eukaryotic cells (reviewed in Parsell and Lindquist, 1993; Pratt, 1997; Pratt and Toft, 1997; Buchner, 1999). Hsp90 is a chaperone known to maintain the activity of a large number of proteins, including members of signal transduction pathways, and cell cycle regulatory proteins such as Raf, steroid hormone receptors and Wee1 (Aligue et al., 1994; Cutforth and Rubin, 1994; Nathan and Lindquist, 1995; Nathan et al., 1997; Pratt and Toft, 1997). A fraction of the total pool of Hsp90 is localized in the centrosome throughout the cell cycle at different stages of development in Drosophila. The centrosomal localization of Hsp90 does not depend on microtubules. Moreover, disruption of Hsp90 function, either by mutations or by treatment with the Hsp90 inhibitor geldanamycin, results in abnormal centrosome segregation and maturation, aberrant cell division spindles and impaired chromosome segregation. Therefore, Hsp90 behaves as a core centrosomal component and is required to ensure proper centrosome function (Lange et al., 2000).
Hsp90 is known to modulate protein structure, thus increasing the half-life of proteins and facilitating their interactions (Nathan and Lindquist, 1995; Pratt, 1997; Buchner, 1999). Inhibition of Hsp90 by geldanamycin has been shown to decrease the half-life of Hsp90-interacting proteins like Src, Raf and steroid receptor (Cake and Litwack, 1978; Moudgil and John, 1980; Barnett et al., 1983; Holbrook et al., 1983). Geldanamycin is a specific competitive inhibitor of Hsp90 that docks to its highly conserved ATP binding site, which is important in regulating Hsp90 function (Prodromou et al., 1997; Stebbins et al., 1997; Panaretou et al., 1998). Geldanamycin-bound Hsp90 cannot form heterocomplexes and this results in destabilization of the proteins that require Hsp90. Consequently, it has been proposed that this property of geldanamycin can be used to identify proteins that interact with Hsp90 and that might be inactivated or downregulated following treatment of live cells with this drug (Pratt and Toft, 1997). Such a method has been used successfully to demonstrate Hsp90 association with mutants of p53 (Puca et al., 1972) and the reverse transcriptase of hepatitis B virus (Baulieu and Jung, 1972).
We have followed this approach to test the hypothesis that the centrosomal defects produced by inhibition of Hsp90 may be a consequence of the inactivation of a centrosomal protein whose stability requires Hsp90. Among 15 centrosomal proteins studied, we have found that the protein kinase Polo is very sensitive to Hsp90 inhibition. Interestingly, the reported centrosomal phenotypes produced by inhibition of Polo in different systems bear striking similarity to the effects of geldanamycin treatment. We have also found that the ability of cytoplasmic extracts to complement salt-stripped centrosomes is lost following Hsp90 inactivation, but can be rescued by the addition of recombinant Polo. These observations strongly suggest that stabilization of Polo is one of the mechanisms by which Hsp90 contributes to maintaining functional centrosomes.
Results
Hsp90 is required to maintain the levels of Polo
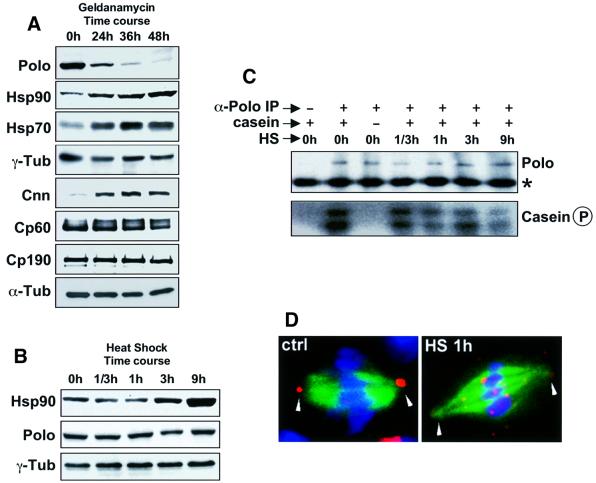
To identify the centrosomal proteins that may require Hsp90 for their function we followed the stability of a panel of proteins, which have been described as structural or regulatory components of centrosomes, in Drosophila SL2 cells treated with geldanamycin. Geldanamycin treatment of SL2 cells results in aberrant centrosome and mitotic spindle morphology (data not shown) comparable to the effects that it shows upon mammalian cells (Lange et al., 2000). Of 15 centrosomal proteins studied (see Materials and methods), only the Polo protein kinase followed a rapid degradation kinetics. This enzyme exhibited a reduction of its normal protein levels by 50% in 24 h and >90% after 48 h of geldanamycin treatment (Figure 1A). Geldanamycin treatment also results in an increase in the levels of Hsp70, Hsp90 and, to a lesser extent, centrosomin (Cnn). The moderate, but significant upregulation of Cnn is difficult to interpret since we are not aware of other examples of proteins upregulated by treatment with geldanamycin. It could be either a direct consequence of the destabilization of Polo or other proteins that require Hsp90, or an indirect consequence of the dispersion of centrosomal material and its failure to accumulate into organized centrosomes.
Fig. 1. Stability of centrosomal proteins following inhibition of Hsp90 and heat shock. Cell extracts obtained from Drosophila SL2 cells treated with the Hsp90 inhibitor geldanamycin (A) or heat shock (B) were prepared at different time points, run in SDS–PAGE gels and blotted with antibodies against a panel of centrosomal proteins. Of 15 centrosomal proteins examined, Polo was destabilized following geldanamycin treatment, Hsp90, Hsp70 and Cnn increased slightly and the rest of the proteins did not change. Heat shock, which also leads to an increase in Hsp90, does not have any noticeable effect on the levels of Polo (B). Immunoprecipitated Polo from heat shocked cells maintains kinase activity (C). Heat shock-induced centrosome dispersion can be observed as early as 1 h (D). The centrosomal marker CP190, microtubules and DNA are shown in red, green and blue. The spindle poles are labelled with arrowheads.
The upregulation of Hsp70 and Hsp90 following geldanamycin treatment has been reported previously in mammalian cells (Loo et al., 1998) and has been interpreted as a stress response to the presence of this drug (Zou et al., 1998). Indeed, heat shock also results in an increase in the levels of Hsp70 and Hsp90 and is known to induce the reversible dispersion of the pericentriolar material (Debec et al., 1990). Thus, to rule out a general stress response as the cause of the degradation of Polo in geldanamycin-treated cells, we followed the effect of heat shock on the levels of the enzyme in total cell extracts (Figure 1B). We found that heat shock does not have any noticeable effect on Polo levels, which remain fairly constant, while Hsp90 levels increase >5-fold. We then immunoprecipitated Polo from these extracts and assayed its kinase activity. We found that the Polo kinase activity is fairly stable even after 9 h of heat shock (Figure 1C), much longer than it takes for centrosomes to get disrupted under these conditions (Figure 1D). Thus, the degradation of Polo in geldanamycin-treated cells does not appear to be a consequence of a general stress response. Moreover, the effect upon centrosome function of heat shock seems not to be mediated by the inactivation of Polo. Interestingly, stress induced by cadmium chloride or hydrogen peroxide treatment, which upregulates most heat shock proteins, neither leads to dispersion of the centrosome nor affects its microtubule nucleation capacity (Debec et al., 1990). Therefore, dispersion of the centrosome is not an automatic consequence of stress.
Hsp90 and Polo are part of a complex
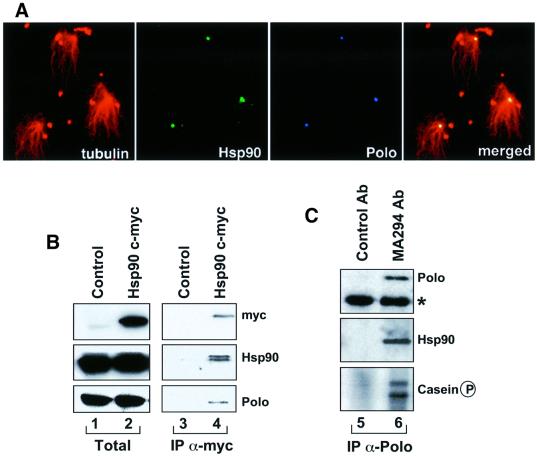
Having shown that maintenance of the levels of Polo requires functional Hsp90, we wished to determine whether these two proteins interact. We first observed that Hsp90 and Polo co-localize in purified Drosophila embryo centrosomes that are competent to polymerize microtubules (Figure 2A). We then studied whether these two proteins could be found in the same complex. To this end we overexpressed myc-tagged Drosophila Hsp90 in SL2 cells and determined whether Polo could co-immunoprecipitate with Hsp90 (Figure 2B). In total extracts, myc can be detected in cells transfected with the myc–Hsp90 (lane 2), but not in the control (lane 1). Hsp90 and Polo can also be observed in these extracts. In the anti-myc-derived immunoprecipitates, myc and Hsp90 can be observed in the transfected cells (lane 4), but not in the control (lane 3). The two bands revealed with the anti-Hsp90 antibody are due to dimerization of tagged (low mobility band) and endogenous non-tagged Hsp90 molecules. The presence of Polo in lane 4 suggests that Polo co-immunoprecipitated with Hsp90. We also studied the presence of Hsp90 in immunoprecipitates obtained with anti-Polo antibodies from SL2 cells that do not overexpress either of these two proteins (Figure 2C) and found that Hsp90 co-immunoprecipitates with Polo (lane 6). These observations suggest that Polo and Hsp90 form part of a complex. The co-immunoprecipitation of Polo and Hsp90 is not disrupted by heat shock (not shown) as expected from the stability of Polo under heat shock conditions.
Fig. 2. Polo co-localizes with Hsp90 in purified centrosomes and co-immunoprecipitates with Hsp90. (A) Immunofluorescence showing co-localization of Hsp90 (green) and Polo (blue) in purified Drosophila centrosomes that are competent to organize microtubule asters (red). (B) Total homogenate and a fraction immunoprecipitated with an anti-myc antibody (IP α-myc) prepared from control cells (lanes 1 and 3) and cells carrying a myc-tagged version of Hsp90 (lanes 2 and 4). These samples were blotted with antibodies against myc, Hsp90 and Polo. Polo co-immunoprecipitates with overexpressed myc-Hsp90 (lane 4). (C) A fraction immunoprecipitated with an anti-Polo (IP α-Polo) prepared from SL2 cells, and blotted with anti-Hsp90 antibodies. The asterisk corresponds to the heavy chain of immunoglobulin. The IP α-Polo fraction was also assayed for kinase activity. Hsp90 co-immunoprecipitates with the endogenous Polo (lane 6).
Inhibition of Hsp90 results in inactivation and proteasome-mediated degradation of Polo
To determine whether the degradation of Polo that follows geldanamycin treatment in SL2 cells is dependent upon the proteasome, we studied the course of Polo degradation in geldanamycin-treated cells in the presence and absence of the proteasome inhibitor lactacystin. To minimize apoptosis, lactacystin was used at a concentration of 1 µM, that is one-tenth of its IC50. Even at these relatively low levels, lactacystin is able to significantly retard the rate of Polo degradation. This observation suggests that the degradation of Polo induced by geldanamycin is mediated by the proteasome (Figure 3A). We then decided to determine the kinase activity of the Polo protein that had been prevented from degradation following geldanamycin treatment by inhibiting the proteasome. With this aim we determined the casein kinase activity of the Polo protein immunoprecipitated from cells treated with geldanamycin and lactacystin (Figure 3B). Polo kinase activity is lost as early as 24 h from the start of geldanamycin treatment, even though the levels of Polo protein remain hardly affected. These observations strongly suggest that the eventual degradation of Polo in geldanamycin-treated cells is preceded by the inactivation of the Polo kinase activity.
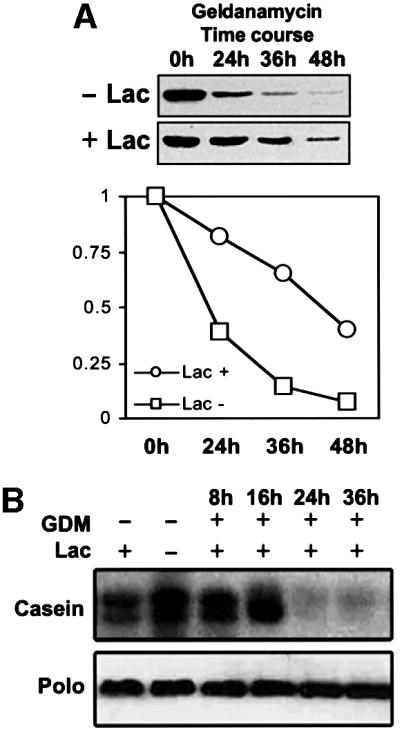
Fig. 3. Polo degradation is mediated by the proteasome. (A) Inhibition of the proteasome with lactacystin significantly slows down the rate of Polo degradation in geldanamycin-treated cells. (B) The kinase activity of Polo drops dramatically as early as 24 h after geldanamycin treatment, even when Polo degradation is retarded by lactacystin.
The ability of cytoplasmic extracts to complement salt-stripped centrosomes is lost following Hsp90 inactivation, but can be rescued by the addition of recombinant Polo
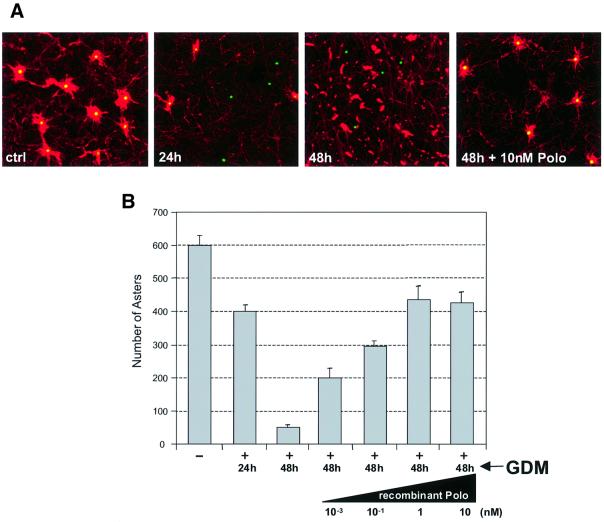
In vitro studies have demonstrated that preparations of centrosomes become unable to nucleate asters of microtubules following treatment with high salt concentrations. This can be restored by the provision of cytoplasmic fractions containing specifically the γ-tubulin ring complex and Asp protein (Moritz et al., 1998; Avides and Glover, 1999). Recent studies indicate that the Asp protein is a substrate of Polo kinase and suggest that Asp has to become phosphorylated to be able to nucleate microtubule organizing centre (MTOC) activity at the centrosome (do Carmo Avides et al., 2001). This would predict that cytoplasm from cells treated to inactivate Hsp90 would be unable to provide such activity. To test this hypothesis we examined the ability of cytoplasmic extracts of cells treated with geldanamycin to complement the loss of MTOC activity of salt-stripped centrosomes (Figure 4A and B). We found that microtubule nucleation is markedly less efficient in extracts derived from cells in which Hsp90 had been inhibited with geldanamycin for 24 h than in control untreated cells, and is essentially absent in extracts derived from cells treated for 48 h. This correlates with the activity and stability of Polo kinase in these extracts and so is consistent with a requirement for Polo to phosphorylate components that are removed from centrosomes by the salt-stripping process.
Fig. 4. The complementing activity of cytoplasmic extracts required to restore microtubule polymerization in salt-stripped centrosomes is inhibited by geldanamycin and rescued by Polo. (A) Immunofluorescence showing the ability of SL2 cell extracts to complement KI-extracted centrosomes (ctrl). Treatment with geldanamycin progressively inhibits the complementation activity (24 h, 48 h), but the effect can be reversed by the addition of 10 nM recombinant Polo (48 h +10 nM Polo). Centrosomes and microtubules were stained with anti-γ-tubulin (green) and anti-α-tubulin (red), respectively. (B) Quantification of the inhibition produced by geldanamycin and the effect of recombinant Polo added to the extracts. Geldanamycin treatment reduces the complementing activity of cell extracts by 30% after 24 h and >90% after 48 h. Increasing concentrations of Polo added to these extracts can rescue up to 70% of the control complementing activity.
To establish whether the inactivation of Polo alone could account for the loss of complementing activity in geldanamycin-treated cytoplasmic extracts, we quantified the ability of pure recombinant Polo to rescue this effect. We found that the addition of Polo in the range 1–10 nM, to extracts that had been treated with geldanamycin for 48 h, rescues up to 70% of the control activity (Figure 4B). Therefore, Polo degradation seems to be one of the major causes of the abnormal centrosomes induced by geldanamycin treatment. The failure of recombinant Polo to rescue 100% of the control aster formation activity suggests, but does not prove, that other activities may also be involved.
Discussion
We have shown previously that Hsp90 is required for proper centrosome function (Lange et al., 2000). The centrosomal defects observed in loss-of-function conditions for Hsp90 include dispersion of the pericentriolar material (PCM), failure of some mitotic PCM markers to be recruited to mitotic centrosomes, and the presence of only a single MTOC in mitotic cells. Concomitantly, in agreement with previous observations (Yue et al., 1999), we have observed that the loss of Hsp90 also results in defects in microtubule organization and chromosome segregation (Lange et al., 2000). The fact that mitotic centrosomes, which grow significantly at the onset of mitosis, are much more sensitive to the loss of Hsp90 function than interphase centrosomes strongly suggests that the process of centrosome maturation (Lane and Nigg, 1996) may be particularly dependent upon Hsp90. The presence of single MTOCs in mitotic cells with reduced Hsp90 function suggests that this chaperone may also be required for centrosome duplication and/or segregation.
Hsp90 is a molecular chaperone known to interact with proteins to modulate their structure, increase their half-life and facilitate protein–protein interactions. Thus, a plausible hypothesis to interpret the centrosomal defects brought about by Hsp90 inhibition is that Hsp90 may interact with one or more centrosomal proteins that require this interaction for their function. Our current observations indicate that one such protein is Polo kinase. This conclusion is fully consistent with the striking similarity between the phenotypes induced by inhibition of Hsp90 and those that have been reported in cells with reduced Polo function. Drosophila Polo (Llamazares et al., 1991) is the founder member of the polo-like kinase (Plk) family, which has homologues in a wide range of organisms (for reviews see Glover et al., 1998; Nigg, 1998). The Plks are found at a number of cellular locations during mitosis, including the spindle pole bodies of yeasts and the centrosomes of metazoans. Females homozygous for polo1, the first mutant allele of this gene isolated in Drosophila, give rise to embryos that show abnormal mitotic spindles and in which centrosomal components fail to organize into discrete structures (Sunkel and Glover, 1988; Riparbelli et al., 2000). In both Drosophila and human cells, reduction of Plk kinase function results in a failure of centrosome segregation that leads to the formation of monopolar spindles (Llamazares et al., 1991; Lane and Nigg, 1996). A similar phenotype is observed by mutation in plo1, the fission yeast homologue of polo (Ohkura et al., 1995). Moreover, inhibition of human Plk-1 by microinjection of blocking antibodies arrests cells in mitosis and results in small centrosomes unable to undergo the increase in size that precedes the onset of mitosis (Lane and Nigg, 1996). Identical results can be observed in human Hs68 cells treated with geldanamycin (G.de Cárcer, unpublished).
The similarity between the phenotypes produced by the loss of Polo or Hsp90, together with the remarkable destabilization of Polo following the loss of Hsp90 function, strongly suggests that the centrosome alterations produced by inhibition of Hsp90 are due, at least partially, to the inactivation of Polo. This conclusion is strengthened by the ability of Polo to rescue the inhibitory effect of geldanamycin upon the complementation activity of cytoplasmic extracts to restore microtubule nucleation from salt-stripped centrosome cores. Therefore, notwithstanding the possible involvement of other Hsp90 partners that we have not yet identified, we conclude that stabilization of Polo is one of the mechanisms by which Hsp90 contributes to the maintenance of functional centrosomes.
The mitotic centrosomes of polo1 have been shown to fail to accumulate the mitosis-specific phospho-epitopes identified in wild-type centrosomes by the MPM2 antibody (Logarinho and Sunkel, 1998). Inhibition of Hsp90 also prevents the accumulation of these mitosis-specific phospho-epitopes (G.de Cárcer, unpublished). One of the MPM2 phospho-epitopes that requires functional Polo for its accumulation is the phosphorylated form of the Asp protein (do Carmo Avides et al., 2001). Asp is a 220 kDa microtubule-associated protein found at the spindle poles from prophase to early telophase (Saunders et al., 1997; Avides and Glover, 1999). Asp is required to restore the microtubule nucleating activity of isolated preparations of centrosomes. This activity is not provided by the Asp protein supplied by extracts derived from polo mutant embryos. Our present finding that the complementing activity of cell extracts is also lost following the destabilization of Polo kinase induced by geldanamycin treatment strongly suggests that phosphorylation of Asp by Polo is required for centrosome function. This hypothesis is consistent with the similarities between the phenotypes of asp and polo mutants and their synergic interaction (Gonzalez et al., 1998).
The number of proteins that are known to require Hsp90 for their function is growing rapidly. Recent studies have expanded the original family of Hsp90 substrates—steroid hormone receptors and protein kinases—to include other classes of proteins such as nitric oxide synthase (Garcia-Cardena et al., 1998) and telomerase (Holt et al., 1999). Hsp90 has also been shown to form complexes with and be required for the function of wee1 (Aligue et al., 1994) and cdc2 (Muñoz and Jimenez, 1999), two kinases that play a major role in the control of the cell cycle. In this work we have shown that an additional mechanism by which Hsp90 can facilitate cell cycle regulation is by maintaining the level of Plks. Our work is consistent with that of Simizu and Osada (2000) who showed that mammalian Plk1 interacts with Hsp90, and that the instability of Plk1 in some human tumours and its insensitivity to geldanamycin may be due to mutations in the C-terminal non-catalytic domain.
Although our work serves to emphasize the role of Plks in regulating the centrosome cycle, we should not overlook the functions of the enzyme at other mitotic stages. We have also found that geldanamycin-treated HeLa cells tend to arrest at metaphase (Lange et al., 2000). In such cells Cdc27, a component of the anaphase-promoting complex suggested by Kotani et al. (1998) to be phosphorylated and activated by Plk1, is found in its unphosphorylated form and levels of Plk1 are reduced (G.de Cárcer, unpublished data). This points to a requirement for Hsp90 to stabilize polo kinase to regulate the metaphase–anaphase transition. Hence, Hsp90 seems to serve as a chaperone of this essential mitotic kinase, and perhaps other centrosomal and cell cycle regulatory proteins yet to be identified, throughout mitotic progression.
Materials and methods
Antibodies
The following antibodies were used: mouse monoclonal MA294 anti-Polo (Llamazares et al., 1991); rabbit Rb1011 anti-γ-tubulin (Tavosanis et al., 1997); mouse monoclonal GTU-88 anti-γ-tubulin (Sigma); rabbit anti-Cp60 (Kellogg et al., 1995); rabbit Rb188 anti-Cp190 (Whitfield et al., 1988); rabbit anti-Cnn (Li and Kaufman, 1996); mouse monoclonal N356 anti-α-tubulin (Amersham); rat monoclonal 16F1 anti-Hsp90α (Stressgen); mouse monoclonal anti-Hsp70 (Stressgen); rabbit anti-LK6 (Kidd and Raff, 1997), anti-centrin (Salisbury et al., 1988); rabbit anti-TCP-1α (Stressgen); rabbit anti-14-3-3β (Santa Cruz Technologies); rabbit MA8 anti-D-TACC (Gergely et al., 2000); sheep anti-PP4 (Helps et al., 1998) and rabbit ANGT anti-Drosophila Nek2 (C.Gonzalez and C.E.Sunkel, unpublished).
Cell culture and drug treatments
Drosophila Schneider cells (SL2) were maintained at 25°C in Schneider medium (Gibco-BRL) supplemented with 10% fetal bovine serum. Where indicated, 1.78 µM geldanamycin (Sigma) and 1 or 10 µM lactacystin (Calbiochem) were added to the culture medium. For heat shock treatment, SL2 cells were shifted to 37°C for the times indicated.
Cell extracts, western blots and immunoprecipitation assays
Cells were harvested from Petri dishes, washed in phosphate-buffered saline (PBS) and resuspended in lysis buffer (50 mM Tris–HCl pH 7.5, 0.5 M NaCl, 6 mM EGTA, 6 mM EDTA, 0.1% NP-40) supplemented with 1 µg/ml of aprotinin, 1 µg/ml of leupeptin, 1 µg/ml of pepstatin, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 25 mM sodium β-glycerophosphate, 1 mM sodium fluoride and 1 mM sodium orthovanadate. Cell lysates were spun down and the proteins in the supernatant were resolved in 10% gradient SDS–polyacrylamide gels and transferred on to nitrocellulose membranes. Blots were incubated in blocking buffer [10% milk in PBS with 0.05% Tween-20 (PBST)] for 1 h and then incubated in PBST containing the primary antibody dilution for 1 h. They were then washed in PBST and incubated with horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) for 45 min. After several washes, blots were developed with ECL chemiluminescence reagent (Amersham).
Drosophila SL2 cells were transfected using the Effectene kit (Qiagen) with the pACT5 expression vector carrying Hsp83–myc under the control of the actin promoter (van der Straten et al., 1997). Cells were lysed 48 h after transfection in lysis buffer. Two hundred micrograms of each extract were incubated with 0.2 µg of rabbit polyclonal anti-myc (Signal Transduction) for 2 h at 4°C. Protein A–Sepharose beads (Amersham) were then added to the extracts, after which the beads were washed three times with 50 mM Tris–HCl pH 7.5, 150 mM NaCl, 2 mM EDTA, 1 mM EGTA, 1% TX-100 and boiled in sample buffer. Finally, samples were run in SDS–PAGE gels.
Purification of centrosomes and complementation assays
Purification of centrosomes from Drosophila embryos, immunofluorescence and complementation assays on KI-treated centrosomes were carried out as described by Moritz et al. (1995) and Moritz and Alberts (1999). Complementation extracts were prepared from Drosophila SL2 cells treated with geldanamycin for 24 and 48 h. Cells were centrifuged at low speed on a bench-top centrifuge and washed twice in buffer A (50 mM K-HEPES pH 7.6, 100 mM KCl, 1 mM MgCl2, 1 mM Na3EGTA, 10% glycerol, 100 µM nocodazole, 1 mM PMSF; 1:100 protease inhibitor stock). Protease inhibitor stock is 10 mM benzamidine–HCl, 0.1 mg/ml phenanthroline, 1 mg/ml aprotinin, 1 mg/ml leupeptin, 1 mg/ml pepstatin. The pellet was resuspended in cold buffer A (typically 200 µl of buffer for each ml of culture) and homogenized using a Dounce homogenizer at 4°C. The extract was cleared by centrifugation (twice) at top speed for 30 min in a refrigerated Eppendorf centrifuge. Polo rescue experiments were made, supplementing cell extracts that had been treated with geldanamycin for 48 h with increasing concentrations of His-tagged recombinant Polo obtained from a baculovirus expression system (Qian et al., 1998).
Polo kinase assays
Drosophila SL2 extracts, either Hsp90 inhibited or heat shocked, were prepared as described for centrosome complementation assay. Extracts were immunoprecipitated with Dynabeads (Dynal) coated with the anti-Polo monoclonal antibody MA294 (typically using 200 µl of extract and 20 µl of antibody). The beads were extensively washed in buffer A and then resuspended in 50 µl of kinase buffer (10 mM K-HEPES pH 7.5, 75 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol, 0.5 mM EGTA). Dephosphorylated casein (1 µl of a 20 mg/ml stock) was mixed with 10 µl of the immunoprecipitate, 1 µl 2 mM ATP and 0.5 µl [γ-32P]ATP. Reactions were incubated for 20 min at room temperature and analysed by SDS–PAGE and autoradiography.
Immunofluorescence microscopy
Drosophila SL2 were spun down on to coverslips, fixed in 4% formaldehyde in PBS for 10 min, and permeabilized in cold (–20°C) methanol. They were then incubated with glycine 20 mM/PBS for 10 min, followed by a further incubation with bovine serum albumin 3% in PBS for 20 min. Microtubules and centrosomes were stained with antibodies against α-tubulin (Amersham N356) and Cp190, respectively, and the corresponding fluorescein and Texas-Red-tagged secondary antibodies. Chromosomes were counter-stained with 4′-6′-diamidine-2-phenylindole (2 µg/ml). The cells were mounted with Mowiol and analysed with a Leica epifluorescence microscope.
Acknowledgments
Acknowledgements
We are very grateful to T.C.Kaufman, W.Whitfield, P.T.Cohen and J.Raff for valuable antibody probes and E.Hafen for the Hsp90–myc constructs. G.d.C. is supported by a postdoctoral fellowship from the Spanish ‘Ministerio de Educacion’. Work in our two laboratories is supported by the Human Capital and Mobility Programme of the EU, and by a grant from the Cancer Research Campaign of Great Britain to D.M.G.
References
- Aligue R., Akhavan-Niak,H. and Russell,P. (1994) A role for Hsp90 in cell cycle control: Wee1 tyrosine kinase activity requires interaction with Hsp90. EMBO J., 13, 6099–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avides M.C. and Glover,D.M. (1999) Abnormal spindle protein, Asp and the integrity of mitotic centrosomal microtubule organizing centres. Science, 283, 1733–1735. [DOI] [PubMed] [Google Scholar]
- Barnett C.A., Palmour,R.M., Litwack,G. and Seegmiller,J.E. (1983) In vitro stabilization of the unoccupied glucocorticoid receptor by adenosine 5′-diphosphate. Endocrinology, 112, 2059–2068. [DOI] [PubMed] [Google Scholar]
- Baulieu E.E. and Jung,I. (1972) Aprostatic cytosol ‘receptor’ in the rat levator ani muscle. Nature New Biol., 237, 24–26. [DOI] [PubMed] [Google Scholar]
- Buchner J. (1999) Hsp90 & Co. a holding for folding. Trends Biochem. Sci., 24, 136–141. [DOI] [PubMed] [Google Scholar]
- Cake M.H. and Litwack,G. (1978) Effect of calf intestinal alkaline phosphatase, phosphatase inhibitors and phosphorylated compounds on the rat of activation of glucocorticoid–receptor complexes. Biochemistry, 19, 5446–5455. [DOI] [PubMed] [Google Scholar]
- Cutforth T. and Rubin,G.M. (1994) Mutations in Hsp83 and cdc37 impair signalling by the sevenless receptor tyrosine kinase in Drosophila. Cell, 77, 1027–1036. [DOI] [PubMed] [Google Scholar]
- Debec A., Courgeon,A.M., Maingourd,M. and Maisonhaute,C. (1990) The response of the centrosome to heat shock and related stresses in a Drosophila cell line. J. Cell Sci., 96, 403–412. [DOI] [PubMed] [Google Scholar]
- do Carmo Avides M., Tavares,A. and Glover,D.M. (2001) Polo kinase and Asp are needed to promote the mitotic organizing activity of centrosomes. Nature Cell Biol., 4, 421–424. [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G., Fan,R., Shah,V., Sorrentino,R., Cirino,G., Papapetropoulos,A. and Sessa,W.C. (1998) Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature, 392, 821–824. [DOI] [PubMed] [Google Scholar]
- Gergely F., Kidd,D., Jeffers,K., Wakefield,J.G. and Raff,J.W. (2000) D-TACC: a novel centrosomal protein required for normal spindle function in the early Drosophila embryo. EMBO J., 19, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D.M., Hagan,I.M. and Tavares,A.A. (1998) Polo-like kinases: a team that plays throughout mitosis. Genes Dev., 12, 3777–3787. [DOI] [PubMed] [Google Scholar]
- Gonzalez C., Sunkel,C.E. and Glover,D.M. (1998) Interactions between mgr, asp and polo: asp function modulated by polo and needed to maintain the poles of monopolar and bipolar spindles. Chromosoma, 107, 452–460. [DOI] [PubMed] [Google Scholar]
- Helps N.R., Brewis,N.D., Lineruth,K., Davis,T., Kaiser,K. and Cohen,P.T. (1998) Protein phosphatase 4 is an essential enzyme required for organisation of microtubules at centrosomes in Drosophila embryos. J. Cell Sci., 111, 1331–1340. [DOI] [PubMed] [Google Scholar]
- Holbrook N.J., Bodwell,J.E. and Munck,A. (1983) Effect of ATP and pyrophosphate on properties of glycocorticoid–receptor complexes from rat thymus cells. J. Biol. Chem., 258, 14885–14894. [PubMed] [Google Scholar]
- Holt S.E. et al. (1999) Functional requirement of p23 and Hsp90 in telomerase complexes. Genes Dev., 13, 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D.R., Oegema,K., Raff,J., Schneider,K. and Alberts,B.M. (1995) CP60: a microtubule-associated protein that is localized to the centrosome in a cell cycle-specific manner. Mol. Biol. Cell, 6, 1673–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd D. and Raff,J.W. (1997) LK6, a short lived protein kinase in Drosophila that can associate with microtubules and centrosomes. J. Cell Sci., 110, 209–219. [DOI] [PubMed] [Google Scholar]
- Kotani S., Tugendreich,S., Fujii,M., Jorgensen,P.M., Watanabe,N., Hoog,C., Hieter,P. and Todokoro,K. (1998) PKA and MPF-activated Polo-like kinase regulate anaphase-promoting complex activity and mitosis progression. Mol. Cell, 1, 371–380. [DOI] [PubMed] [Google Scholar]
- Lane H.A. and Nigg,E.A. (1996) Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol., 135, 1701–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange B.M., Bachi,A., Wilm,M. and Gonzalez,C. (2000) Hsp90 is a core centrosomal component and is required at different stages of the centrosome cycle in Drosophila and vertebrates. EMBO J., 19, 1252–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K. and Kaufman,T.C. (1996) The homeotic target gene centrosomin encodes an essential centrosomal component. Cell, 85, 585–596. [DOI] [PubMed] [Google Scholar]
- Llamazares S., Moreira,A., Tavares,A., Girdham,C., Spruce,B.A., Gonzalez,C., Karess,R.E., Glover,D.M. and Sunkel,C.E. (1991) Polo encodes a protein kinase homologue required for mitosis in Drosophila. Genes Dev., 5, 2153–2165. [DOI] [PubMed] [Google Scholar]
- Logarinho E. and Sunkel,C.E. (1998) The Drosophila POLO kinase localises to multiple compartments of the mitotic apparatus and is required for the phosphorylation of MPM2 reactive epitopes. J. Cell Sci., 111, 2897–2909. [DOI] [PubMed] [Google Scholar]
- Loo M.A., Jensen,T.J., Cui,L., Hou,Y., Chang,X.B. and Riordan,J.R. (1998) Perturbation of Hsp90 interaction with nascent CFTR prevents its maturation and accelerates its degradation by the proteasome. EMBO J., 17, 6879–6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M. and Alberts,B.M. (1999) Isolation of centrosomes from Drosophila embryos. Methods Cell Biol., 61, 1–12. [DOI] [PubMed] [Google Scholar]
- Moritz M., Braunfeld,M.B., Fung,J.C., Sedat,J.W., Alberts,B.M. and Agard,D.A. (1995) Three-dimensional structural characterization of centrosomes from early Drosophila embryos. J. Cell Biol., 130, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz M., Zheng,Y., Alberts,B.M. and Oegema,K. (1998) Recruitment of the γ-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J. Cell Biol., 142, 775–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudgil V.K. and John,J.K. (1980) ATP-dependent activation of glucocorticoid receptor from rat-liver cytosol. Biochem. J., 190, 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz M.J. and Jimenez,J. (1999) Genetic interactions between Hsp90 and the Cdc2 mitotic machinery in the fission yeast Schizosaccharomyces pombe. Mol. Gen. Genet., 261, 242–250. [DOI] [PubMed] [Google Scholar]
- Nathan D.F. and Lindquist,S. (1995) Mutational analysis of Hsp90 function: interactions with a steroid receptor and a protein kinase. Mol. Cell. Biol., 15, 3917–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D.F., Vos,M.H. and Lindquist,S. (1997) In vivo functions of the Saccharomyces cerevisiae Hsp90 chaperone. Proc. Natl Acad. Sci. USA, 94, 12949–12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E.A. (1998) Polo-like kinases: positive regulators of cell division from start to finish. Curr. Opin. Cell Biol., 10, 776–783. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Hagan,I.M. and Glover,D.M. (1995) The conserved Schizosaccharomyces pombe kinase plo1, required to form a bipolar spindle, the actin ring and septum, can drive septum formation in G1 and G2 cells. Genes Dev., 9, 1059–1073. [DOI] [PubMed] [Google Scholar]
- Panaretou B., Prodromou,C., Roe,S.M., O’Brien,R., Ladbury,J.E., Piper,P.W. and Pearl,L.H. (1998) ATP binding and hydrolysis are essential to the function of the Hsp90 molecular chaperone in vivo. EMBO J., 17, 4829–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsell D.A. and Lindquist,S. (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu. Rev. Genet., 27, 437–496. [DOI] [PubMed] [Google Scholar]
- Pratt W.B. (1997) The role of the hsp90-based chaperone system in signal transduction by nuclear receptors and receptors signaling via MAP kinase. Annu. Rev. Pharmacol. Toxicol., 37, 297–326. [DOI] [PubMed] [Google Scholar]
- Pratt W.B. and Toft,D.O. (1997) Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev., 18, 306–360. [DOI] [PubMed] [Google Scholar]
- Prodromou C., Roe,S.M., O’Brien,R., Ladbury,J.E., Piper,P.W. and Pearl,L.H. (1997) Identification and structural characterization of the ATP/ADP-binding site in the Hsp90 molecular chaperone. Cell, 90, 65–75. [DOI] [PubMed] [Google Scholar]
- Puca G.A., Nola,E., Sica,V. and Bresciani,F. (1972) Estrogen-binding proteins of calf uterus. Interrelationship between various forms and identification of a receptor transforming factor. Biochemistry, 11, 4157–4165. [DOI] [PubMed] [Google Scholar]
- Qian Y.W., Erikson,E., Li,C. and Maller,J.L. (1998) Activated polo-like kinase is required at multiple points during mitosis in Xenopus laevis. Mol. Cell. Biol., 18, 4262–4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riparbelli M.G., Callaini,G. and Glover,D.M. (2000) Failure of pronuclear migration and repeated divisions of polar body nuclei associated with MTOC defects in polo eggs of Drosophila. J. Cell Sci., 113, 3341–3350. [DOI] [PubMed] [Google Scholar]
- Salisbury J.L., Baron,A.T. and Sanders,M.A. (1988) The centrin-based cytoskeleton of Chlamydomonas reinhardtii: distribution in interphase and mitotic cells. J. Cell Biol., 107, 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders R.D., Avides,M.C., Howard,T., Gonzalez,C. and Glover,D.M. (1997) The Drosophila gene abnormal spindle encodes a novel microtubule-associated protein that associates with the polar regions of the mitotic spindle. J. Cell Biol., 137, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simizu S. and Osada,H. (2000) Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nature Cell Biol., 2, 852–854. [DOI] [PubMed] [Google Scholar]
- Stebbins C.E., Russo,A.A., Schneider,C., Rosen,N., Hartl,F.U. and Pavletich,N.P. (1997) Crystal structure of an Hsp90–geldanamycin complex: targeting of a protein chaperone by an antitumor agent. Cell, 89, 239–250. [DOI] [PubMed] [Google Scholar]
- Sunkel C.E. and Glover,D.M. (1988) Polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J. Cell Sci., 89, 25–38. [DOI] [PubMed] [Google Scholar]
- Tavosanis G., Llamazares,S., Goulielmos,G. and Gonzalez,C. (1997) Essential role for γ-tubulin in the acentriolar female meiotic spindle of Drosophila. EMBO J., 16, 1809–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Straten A., Rommel,C., Dickson,B. and Hafen,E. (1997) The heat shock protein 83 (Hsp83) is required for Raf-mediated signalling in Drosophila. EMBO J., 16, 1961–1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield W.G., Millar,S.E., Saumweber,H., Frasch,M. and Glover,D.M. (1988) Cloning of a gene encoding an antigen associated with the centrosome in Drosophila. J. Cell Sci., 89, 467–480. [DOI] [PubMed] [Google Scholar]
- Yue L., Karr,T.L., Nathan,D.F., Swift,H., Srinivasan,S. and Lindquist,S. (1999) Genetic analysis of viable hsp90 alleles reveals a critical role in Drosophila spermatogenesis. Genetics, 151, 1065–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Guo,Y., Guettouche,T., Smith,D.F. and Voellmy,R. (1998) Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell, 94, 471–480. [DOI] [PubMed] [Google Scholar]