Abstract
Background
Chitosan, a deacetylated chitin, is a dietary supplement reported to decrease body weight. It is widely available over the counter worldwide and although evaluated in a number of trials its efficacy remains in dispute.
Objectives
To assess the effects of chitosan as a treatment for overweight and obesity.
Search methods
We searched electronic databases (MEDLINE, EMBASE, BIOSIS, CINAHL, The Cochrane Library), specialised web sites (Controlled Trials, IBIDS, SIGLE, Reuter's Health Service, Natural Alternatives International, Pharmanutrients), bibliographies of relevant journal articles, and contacted relevant authors and manufacturers.
Selection criteria
Trials were included in the review if they were randomised controlled trials of chitosan for a minimum of four weeks duration in adults who were overweight or obese. Authors of included studies were contacted for additional information where appropriate.
Data collection and analysis
Details from eligible trials were extracted independently by two reviewers using a standardised data extraction form. Differences in data extraction were resolved by consensus. Continuous data were expressed as weighted mean differences and standard deviations. The pooled effect size was computed by using the inverse variance weighted method.
Main results
Fifteen trials including a total of 1219 participants met the inclusion criteria. No trial to date has measured the effect of chitosan on mortality or morbidity. Analyses indicated that chitosan preparations result in a significantly greater weight loss (weighted mean difference ‐1.7 kg; 95% confidence interval (CI) ‐2.1 to ‐1.3 kg, P < 0.00001), decrease in total cholesterol (‐0.2 mmol/L [95% CI ‐0.3 to ‐0.1], P < 0.00001), and a decrease in systolic and diastolic blood pressure compared with placebo. There were no clear differences between intervention and control groups in terms of frequency of adverse events or in faecal fat excretion. However, the quality of many studies was sub‐optimal and analyses restricted to studies that met allocation concealment criteria, were larger, or of longer duration showed that such trials produced substantially smaller decreases in weight and total cholesterol.
Authors' conclusions
There is some evidence that chitosan is more effective than placebo in the short‐term treatment of overweight and obesity. However, many trials to date have been of poor quality and results have been variable. Results obtained from high quality trials indicate that the effect of chitosan on body weight is minimal and unlikely to be of clinical significance.
Keywords: Adult, Female, Humans, Male, Dietary Supplements, Anti-Obesity Agents, Anti-Obesity Agents/therapeutic use, Chitosan, Chitosan/therapeutic use, Obesity, Obesity/drug therapy, Overweight, Overweight/drug therapy, Randomized Controlled Trials as Topic, Weight Loss
Plain language summary
Chitosan for overweight or obesity
Overweight and obesity are common health conditions worldwide but there are few effective treatments. Chitosan is a widely available dietary supplement that claims to aid weight loss and blood cholesterol levels. Fifteen studies which lasted between 4 to 24 weeks including a total of 1219 participants were analysed. Trials of chitosan to date have varied considerably in terms of quality. The review suggests that chitosan may have a small effect on body weight but results from high quality trials indicate that this effect is likely to be minimal.
Background
Description of the condition
Overweight and obesity are increasingly common health conditions globally. Obesity may be defined as the degree of fat storage associated with clearly elevated health risks. However, because fat mass is difficult to measure, the pragmatic definition of obesity is based upon body mass index (BMI). The World Health Organisation guidelines define a BMI of 18.5 to 24.9 kg/m2 as normal, 25 to 29.9 kg/m2 as grade 1 overweight and greater than 30 kg/m2 as grade 2 overweight (WHO, 1995).
Overweight is increasingly prevalent in developed and developing populations (Flegal 2002; NAO 2001; WHO 2000) and is an important contributor to cardiovascular disease (Whitlock 2002; Willett 1995; Rimm 1995) operating in part through effects of weight gain on blood pressure (Dyer 1994), blood lipids (Denke 1993; Denke 1994) and blood glucose (Grinker 2000). Excess body weight is also associated with increased incidence of type 2 diabetes mellitus (Carey 1997; Chan 1994; Colditz 1995), osteoarthritis (Must 1999) and several common cancers (Calle 2003; Bergstrom 2001), and in addition, it impairs health‐related quality of life (Fontaine 2001). The non‐fatal health problems associated with overweight include respiratory difficulties (Naimark 1960), chronic musculoskeletal problems (Felson 1992), skin problems (Dunn 1997) and infertility (Kirschner 1982). Estimations of the burden of disease attributable to excess weight indicate that high body mass index (BMI) is a leading cause of loss of healthy life leading to 33 million disability adjusted life years (DALY) worldwide (Ezzati 2002).
Description of the intervention
Intentional weight loss can reduce the mortality of overweight and obese people (Williamson 1995; Williamson 1999), reduce the incidence of diabetes, hypertension, hyperinsulinaemia and hypertriglyceridaemia (Sjostrom 1999), serum total cholesterol (Poobalan 2004), and lead to improvements in health‐related quality of life (Fontaine 1999). Traditional methods of weight loss focus on reducing energy intake through reduced fat or calorie‐controlled diets, increasing energy expenditure via an increase in physical activity, and behaviour modification. The effectiveness of these weight reduction methods is limited (Glenny 1997; Douketis 1999) with an overall pattern of moderate weight loss, followed by gradual weight regain. Numerous other weight management interventions are available, including surgery (for example liposuction, gastroplasty), vitamin and mineral supplements, meal replacements and pharmacological therapies. However, other than in the case of surgery (Sjostrom 1999) the long term effectiveness of many of these interventions is unproven.
Pharmacological therapy as an adjunct to diet and lifestyle changes may improve long term weight loss. Pharmacological therapies currently approved for weight loss fall into two broad categories: those that decrease food intake by reducing appetite or increasing satiety (appetite suppressants) and those that decrease nutrient absorption (Yanovski 2002). Examples of such treatments include sibutramine and orlistat. Sibutramine is a centrally acting re‐uptake inhibitor of both norepinephrine and serotonin and results in decreased appetite, earlier satiety and a slight increase in energy expenditure (Aronne 1998) while orlistat inhibits pancreatic and gastric lipases and thus decreases gastrointestinal uptake of dietary fat (Sjostrom 1998). However, both medications are expensive, have side effects (for example, dry mouth, constipation, insomnia, and headaches with sibutramine, and faecal urgency, flatulence and reduced absorption of fat‐soluble vitamins with orlistat) and are contraindicated in many people.
Unlike pharmacological therapies, dietary supplements are not required to provide proof of safety or efficacy. A US survey estimated that 7% of the population use non‐prescription weight loss supplements and such use is particularly common among young obese women (28%) (Blanck 2001). One such over‐the‐counter dietary supplement is chitosan, which is advertised and sold as a food supplement that lowers blood cholesterol concentration and aids weight loss. This supplement believed to reduce fat absorption is widely available and relatively inexpensive, and hence would offer many advantages if its effectiveness as a weight loss treatment were proven.
How the intervention might work
Chitosan, a partially deacetylated polymer of N‐acetyl glucosamine derived from the polysaccharide chitin (Shepherd 1997) appears to bind to negatively charged lipids in animal trials, hence reducing their gastrointestinal uptake (Deuchi 1995; Sugano 1980; Zacour 1992) and lowering serum cholesterol (Nagyvary 1979; Ormrod 1998). Some human trials have suggested that chitosan may decrease body weight (Schiller 2001) and a meta‐analysis (Ernst 1998) suggested a 3.3 kg greater weight loss with chitosan compared with placebo. However, other studies have found no effect of chitosan on body weight (Ho 2001; Pittler 1999). Several chitosan studies have been published since the 1998 meta‐analysis, the results of which have been variable with some indicating a positive effect on weight and some demonstrating no effect. In order resolve the uncertainty surrounding the effectiveness of this dietary supplement (Allison 2001; Egger 1999) this Cochrane review aims to summarise systematically all of the available evidence on the effectiveness of chitosan as a weight loss treatment.
Objectives
The primary objective was to assess the effect of the dietary supplement chitosan on mortality, morbidity, and quality of life of participants who were overweight or obese.
Methods
Criteria for considering studies for this review
Types of studies
The review was restricted to randomised controlled trials where chitosan was compared with placebo or standard care. Identified trials in all languages were included. There was no restriction in terms of sample size but trials had to have a minimum treatment duration of four weeks. Unpublished data were considered for inclusion on the same basis as published reports.
Types of participants
We included all free‐living adults (18 years and older), both male and female, defined as overweight or obese at baseline. Criteria for defining overweight or obesity included body mass index (BM)I cut‐points and percentage excess weight compared with ideal weight/height tables. Studies including children, pregnant women, or patients with serious medical conditions were excluded.
Types of interventions
Interventions eligible for inclusion in the review included:
chitosan versus placebo;
variable doses of chitosan versus placebo;
chitosan plus diet versus placebo plus diet;
chitosan versus any other pharmacological intervention;
chitosan versus a non‐pharmacological intervention (for example dietary or physical activity interventions).
Types of outcome measures
Primary outcomes
To be eligible for inclusion in the review trials had to report one or more of the following outcomes:
total mortality, obesity‐related mortality (death from coronary heart disease, atherosclerosis, sleep apnoea, pulmonary dysfunction, ischaemic stroke, gallbladder disease, musculoskeletal disease, cancer, sudden death);
morbidity (non‐fatal myocardial infarction, stroke, diabetes mellitus);
quality of life.
Secondary outcomes
indicators of body mass (body weight, BMI, hip‐to‐waist circumference, other anthropometric measurements);
blood pressure;
blood lipid levels (total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides);
adverse effects of the treatment;
faecal fat excretion;
glycosylated haemoglobin A1c (HbA1c);
costs.
Specific patient covariates, effect modifiers, confounders
The major covariate that may influence the effect of chitosan on mortality and morbidity is compliance with the treatment.
Timing of outcome assessment
short (four weeks);
medium (over four weeks but less than six months);
long term (six months or more).
Search methods for identification of studies
Electronic searches
We use the following sources for the identification of trials:
The Cochrane Library (issue 3, 2007);
MEDLINE (until September 2007);
EMBASE (until September 2007);
BIOSIS (until March 2004);
CINAHL (until March 2004).
We also searched databases of ongoing trials:
Controlled Trials (www.controlled‐trials.com)(searched 10 February 2004)
International Bibliographic Information on Dietary Supplements (IBIDS) Database from the National Institutes of Health Office of Dietary Supplements (http://dietary‐supplements.info.nih.gov/databases/ibids.html) (searched 10 February 2004)
System for Information on Grey Literature in Europe (SIGLE) (www.fiz‐informationsdienste.de/de/DB/sigle/) (searched 10 February 2004)
Reuter's Health Service (www.reutershealth.com) (searched 10 February 2004)
Natural Alternatives International (www.nai‐online.com) (searched 10 February 2004)
Pharmanutrients (www.pharmanutrients.com) (searched 10 February 2004)
The described search strategy (see for a detailed search strategy Appendix 1 was used for MEDLINE. For use with EMBASE, The Cochrane Library and the other databases this strategy was slightly adapted.
Searching other resources
Authors of all included studies were contacted to ask if they had knowledge of any additional references, unpublished trials, ongoing trials, and also to obtain unpublished data necessary for the review. Studies published in any language were included.
Efforts were made to identify additional studies by searching the reference lists of relevant trials and reviews identified.
Data collection and analysis
Selection of studies
For the original review, a single reviewer (CDM) reviewed the titles, abstracts and keywords of every record retrieved. Full articles were retrieved for further assessment if the information given suggested that the study: 1. included overweight or obese patients, 2. compared chitosan with placebo or any other active intervention, 3. assessed one or more relevant clinical outcome measure, 4. used random allocation to the comparison groups. If there was any doubt regarding these criteria from the information given the title and abstract, the full article was retrieved for clarification. Two reviewers (CDM, CNM) then independently selected the trials for inclusion in the review from the list of potentially eligible trials.
For the 2007 update of the review , a single reviewer (AJ) reviewed the titles, abstracts and keywords of every record retrieved. Full articles were retrieved for further assessment if the information given suggested that the study: 1. included overweight or obese patients, 2. compared chitosan with placebo or any other active intervention, 3. assessed one or more relevant clinical outcome measure, 4. used random allocation to the comparison groups. If there was any doubt regarding these criteria from the information given the title and abstract, the full article was retrieved for clarification. Two reviewers (AJ, CNM) then independently selected the trials for inclusion in the review from the list of potentially eligible trials.
Data extraction and management
Data concerning details of study population, intervention and outcomes were extracted independently by two reviewers (original review: CDM, CNM; 2007 update of the review: AJ, CNM) using a specially designed data extraction form. The standard data extraction form included the following items:
general information: published/unpublished, title, authors, source, contact address, country, setting, language of publication, year of publication, duplicate publications, sponsor;
trial characteristics: design, duration, methods of randomisation and allocation concealment, blinding (patients, people administering treatment, outcome assessors);
intervention(s): composition, dose, frequency of chitosan and placebo interventions, diet intervention(s), other intervention(s) (dose, timing);
participant characteristics: inclusion and exclusion criteria, number in each intervention group, sex, age, baseline characteristics, duration of overweight/obesity, similarity of groups at baseline (including any co‐morbidity), assessment of compliance, withdrawals/losses to follow‐up;
outcomes: outcomes specified above, any other outcomes assessed, length of follow‐up;
results: if necessary converted to measures of effect specified below, use of intention‐to‐treat analysis.
Differences in data extraction were resolved by consensus, referring back to the original article. When necessary, additional information was sought from the authors of the studies. Where differences in opinion still existed, a third party (AR) was consulted.
Assessment of risk of bias in included studies
Each of the studies included in the review was evaluated based on the following indicators of risk of bias:
Allocation concealment
The process of concealing randomised study assignments. Studies reporting appropriate methods of allocation concealment were considered "A grade" trials. Studies where the method of allocation concealment was unclear were considered "B grade" (Clarke 2003)
blinding of participants, investigators and outcome assessors;
completeness of follow‐up;
use of intention‐to‐treat (ITT) analyses;
similarity of the baseline characteristics of participants;
Quality of allocation concealment (A or B) was used in sensitivity analyses to determine the effect of including trials of lower quality.
Assessment of heterogeneity
Continuous data were expressed as weighted mean differences (WMD) and standard deviations (SD) where possible and summarised statistically. The pooled effect size was computed by using the inverse variance weighted method.
Where there was no evidence of statistical heterogeneity the results were pooled statistically using a fixed‐effects approach. Otherwise a random‐effects approach was also used as a sensitivity analysis. Statistical heterogeneity was tested for using the Q statistic devised by Cochran with significance being set at P < 0.10, because tests of statistical heterogeneity are known to have low power. Quantification of the effect of heterogeneity was assessed by means of I2, which ranges from 0% to 100%, and has associated 95% confidence intervals (Higgins 2002). I2 demonstrates the percentage of total variation across studies due to heterogeneity and was used to judge the consistency of evidence. Possible sources of heterogeneity were assessed by sensitivity analyses as described below. The existence of publication bias was checked using a funnel plot.
Subgroup analysis and investigation of heterogeneity
It was agreed that subgroup analyses would only be done where there was a significant result for one of the main outcome measures and where numbers permitted as otherwise the risk of getting a spurious result is too great. Where data allowed we aimed to perform the following subgroup analyses:
weight level (BMI) at baseline;
age;
gender:
different comparison interventions;
dose:
co‐morbidity;
smoking;
medication;
duration of intervention.
Sensitivity analysis
Sensitivity analyses were undertaken to explore the influence of the following factors:
unpublished studies (if any);
non‐peer‐reviewed studies (if any);
studies not meeting the quality criteria (B grade or lower);
studies where the treatment used contained other weight loss agents in addition to chitosan;
study duration and study size;
studies where weight reduction was the secondary outcome (if any).
The sensitivity of the combined estimate to the influence of these factors was determined by calculating the combined effect for relevant studies, and also calculating the combined effect of the remaining studies, and then comparing both. Body weight and total cholesterol were chosen as outcomes to examine in the sensitivity analyses because they were deemed to be the most clinically relevant outcomes in terms of the proposed mechanism of action of chitosan and because most studies included in the review had measured these outcomes.
Results
Description of studies
Results of the search
Forty two potentially eligible studies were found. Twenty seven studies were excluded and 15 were included in the review.
Included studies
The 15 studies included in the review were all randomised controlled trials. Duplicate publications existed for two studies (Giustina 1995; Veneroni 1996) while others had more than one publication relating to the same study but a primary reference containing most or all of the relevant data could be identified (Ni Mhurchu 2004; Woodgate 2003; Wuolijoki 1999). One thousand two hundred and nineteen participants were included in total comprising 87,852 patient‐days of follow‐up, with 640 participants allocated to chitosan and 579 allocated to placebo. The mean trial duration was 8.3 weeks (range 4 to 24 weeks) and mean study size was 81 participants (range 24 to 250). Six of the 15 studies were conducted in Italy (Colombo 1996; Girola 1996; Giustina 1995; Macchi 1996; Sciutto 1995; Veneroni 1996) while the remainder were conduced in Singapore (Ho 2001), New Zealand (Ni Mhurchu 2004), the United Kingdom (Pittler 1999; Williams 1998), the United States (Kaats 2006; Schiller 2001), Canada (Woodgate 2003), Finland (Wuolijoki 1999) and Poland (Zahorska‐Markiewicz). Study participants ranged in age from 18 to 70 years (mean 44 years) and most studies included both men and women but three included only women (Schiller 2001; Wuolijoki 1999; Zahorska‐Markiewicz). All participants were overweight or obese at baseline although the criteria used to define overweight varied. Some studies specified that participants were 10% to 25% overweight based on ideal weight for height tables (Colombo 1996; Girola 1996; Giustina 1995; Macchi 1996; Sciutto 1995; Veneroni 1996) while others used varied BMI cut‐offs (Ni Mhurchu 2004; Pittler 1999; Schiller 2001; Woodgate 2003; Wuolijoki 1999). One study specified that eligible participants had greater than 20% to 30% body fat (Ho 2001) and two others stated that participants were overweight or obese but did not provide details of how this was measured (Williams 1998; Kaats 2006).
Interventions
All 15 studies compared chitosan with placebo. One study required participants to follow a self‐monitored behaviour modification programme without clarifying what behaviour was being modified (Kaats 2006). Eight studies advised participants to follow a low calorie or weight reducing diet in addition to taking chitosan or placebo treatment (Colombo 1996; Girola 1996; Giustina 1995; Macchi 1996; Ni Mhurchu 2004; Sciutto 1995; Veneroni 1996; Zahorska‐Markiewicz) while the remainder advised participants to maintain normal dietary and physical activity patterns (Ho 2001; Pittler 1999; Schiller 2001; Williams 1998; Woodgate 2003; Wuolijoki 1999). One study (Macchi 1996) compared chitosan alone versus chitosan plus diet versus placebo plus diet, while another study (Girola 1996) compared two different doses of chitosan with placebo.
The dose of chitosan used in studies ranged from 0.24 g/day to 15 g/day (mean 3.7 g/day) and five studies (Colombo 1996; Giustina 1995; Sciutto 1995; Veneroni 1996; Woodgate 2003) did not report any dose. Seven studies (Colombo 1996; Girola 1996; Giustina 1995; Kaats 2006; Sciutto 1995; Veneroni 1996; Woodgate 2003) used treatment preparations that contained other active ingredients in addition to chitosan while the remainder used chitosan alone. The treatment preparations used in four studies (Colombo 1996; Giustina 1995; Sciutto 1995; Veneroni 1996) all contained guar's meal, ascorbic acid, and other micronutrients in addition to chitosan, while the preparation used by Woodgate (Woodgate 2003) contained glucomannan, fenugreek, G. sylvestre, and vitamin C in addition to chitosan, and that used by Girola at al (Girola 1996) contained garcinia cambogic extract and chrome. The preparation used by Kaats et al (Kaats 2006) contained beta‐glucan, snow white oat fibre, betamine HCL and aloe saponins in addition to chitosan. One study used a powder preparation of chitosan that was made up into a drink (Williams 1998) while the others all used capsules or tablets.
Types of outcome measures
None of the 15 studies measured mortality or morbidity, probably because treatment durations were insufficient to detect adequate numbers of such events. Seven studies reported measuring quality of life (Colombo 1996; Girola 1996; Giustina 1995; Ni Mhurchu 2004; Pittler 1999; Sciutto 1995; Veneroni 1996) but only one study reported results (Ni Mhurchu 2004). All 15 studies included body weight as an outcome measure but one study (Wuolijoki 1999) did not report the results except in the form of a figure, which meant the mean weight change and the associated standard deviation over time could not be derived. Ten studies reported results for total blood cholesterol levels (Colombo 1996; Girola 1996; Ho 2001; Kaats 2006; Macchi 1996; Ni Mhurchu 2004; Pittler 1999; Veneroni 1996; Wuolijoki 1999; Zahorska‐Markiewicz) with a slightly smaller number reporting results for the lipid sub fractions LDL cholesterol (8), HDL cholesterol (8), and triglycerides (8). Seven studies reported results for systolic and diastolic blood pressure (Giustina 1995; Macchi 1996; Ni Mhurchu 2004; Pittler 1999; Sciutto 1995; Woodgate 2003; Zahorska‐Markiewicz). Fourteen trials measured and reported adverse events (side effects and/or fat‐soluble vitamin levels and/or blood chemistry) but one did not provide any quantitative data (Sciutto 1995). One trial did not report adverse events (Kaats 2006). Two studies examined treatment effects on faecal fat (Ni Mhurchu 2004; Schiller 2001) but both used different units of measurement (mmol versus grams). No studies measured effects on HbA1 or on costs. All studies measured outcomes post‐intervention only, i.e. without extended follow‐up beyond the end of the intervention periods.
For one study endpoint data were provided only in the form of mean difference between baseline and endpoints (Kaats 2006). Additional data for abstraction were provided by the author (personal communication, Gilbert Kaats). For several of the studies, additional calculations were necessary in order to transform the available data into a format suitable for inclusion in the review. Eight studies (Colombo 1996; Giustina 1995; Ho 2001; Pittler 1999; Schiller 2001; Veneroni 1996; Woodgate 2003; Zahorska‐Markiewicz) presented results at baseline and follow‐up but did not present change over time so we used the baseline mean and standard deviation (SD) and the follow‐up mean and SD to compute change over time. The mean change was computed as the (follow‐up mean ‐ baseline mean) within the study and the SD for change was found from sqrt (((nb‐1) sd^2(b) ‐ (nf‐1) sd^2(f))/(N‐2)) where nb is the number of participants at baseline, nf is the number of participants at follow‐up, sd(b) is the sd at baseline, sd(f) is the sd at follow‐up, and N= nf+nb. Unpublished data were provided by one author (Ho 2001) enabling the necessary calculations since the published results were only reported stratified by sex (seeTable 1 for details). Mean change over time and SD were calculated from published individual patient data in one study (Macchi 1996), and although data were available for three intervention groups (chitosan alone versus chitosan plus diet versus placebo plus diet) only data from two intervention groups (chitosan plus diet versus placebo plus diet) were included in the review. One study (Sciutto 1995) published cumulative changes over a period of four weeks so these were summed to estimate overall change over time and SD. One study did not provide data in a form suitable for inclusion in the review (Girola 1996) because results were reported as percentage change over time so a standard deviation could not be calculated. Repeated attempts were made to contact Girola et al but were unsuccessful.
1. Unpublished data provided by Ho et al.
| Outcome | Rx Baseline M | Rx Baseline SD | Rx Follow‐up Mean | Rx Follow‐up SD | Plac Baseline Mean | Plac Baseline SD | Plac Follow‐up Mean | Plac Follow‐up SD |
| Weight | 70.537 | 12.6423 | 69.600 | 11.7680 | 69.566 | 12.2740 | 69.256 | 12.9566 |
| BMI | 26.0378 | 3.90914 | 25.4143 | 3.18654 | 25.8704 | 3.16123 | 25.7903 | 3.48707 |
| Total cholesterol | 5.8947 | .74883 | 5.8512 | .66735 | 6.3471 | 1.28668 | 6.3556 | 1.49041 |
| LDL cholesterol | 3.9532 | .77550 | 3.7493 | .72309 | 3.9631 | .69987 | 3.9524 | .90638 |
| HDL cholesterol | 1.1728 | .31098 | 1.2203 | .27277 | 1.2424 | .43896 | 1.1955 | .39659 |
| Triglycerides | 1.8905 | 1.14381 | 1.9512 | 1.10312 | 2.2927 | 1.53025 | 2.4106 | 1.84905 |
Since none of the studies reported primary outcomes (mortality, morbidity, quality of life) it was decided it would be inappropriate to do sub‐group analyses since the chance of obtaining a spurious result was high. Sensitivity analyses to test the robustness of the overall findings were performed.
Excluded studies
Twenty seven studies were excluded for the following reasons:
ten were not randomised controlled trials (Gades 2002; Gades 2003; Gades 2005; Gallaher 2002; Maezaki 1993; Nopchinda 2001; Terada 1995; Thom 2000; Wadstein 2000; Yihua 1997);
seven were commentaries or reviews of previous studies (Ernst 2001; Muzzarelli 1997; Muzzarelli 1999; Muzzarelli 2000; Otto 2000; Pepping 2003; Pittler 2002);
four were of insufficient duration (Abelin 1994; Aranda 2002; Guerciolini 2001; Heldman 1997);
six involved participants that did not meet the inclusion criteria for the review because inclusion in the study was not based on being overweight at baseline (Ausar 2003; Bokura; Guha 2005; Kaats 1998; Tai 2000) or because they suffered from a serious medical condition (Jing 1997).
Risk of bias in included studies
Studies varied in quality and/or reporting of methods. The 15 studies included in the review were all described as randomised controlled trials.
Allocation
The process of concealing randomised study assignments is known as allocation concealment and when studies do not report any concealment approach, adequacy is considered unclear. Studies reporting appropriate methods of allocation concealment are considered "A grade" trials. Studies where the method of allocation concealment is unclear are considered "B grade". Using an appropriate method to prevent foreknowledge of treatment assignment is crucially important in preventing bias and distorting treatment comparisons and, as such, it is recommended that allocation concealment is used as a key method of measuring study quality (Clarke 2003). Although all studies included in the review were randomised the method of allocation concealment was unclear for all but three studies (Ni Mhurchu 2004; Pittler 1999; Schiller 2001), meaning that the quality of the remaining 12 studies could not be determined satisfactorily.
Blinding
All 15 studies reported that both participants and investigators were blinded to treatment allocation. However, only three studies (Ni Mhurchu 2004; Woodgate 2003; Zahorska‐Markiewicz) specifically reported that outcome assessors were also blinded.
Incomplete outcome data
One study had complete follow‐up of all participants (Macchi 1996) while the remainder all experienced some dropouts or loss to follow‐up of participants ranging from 2% (Sciutto 1995) to 36% (Zahorska‐Markiewicz). Only one study (Ni Mhurchu 2004) used intention‐to‐treat (ITT) analysis to account for loss to follow‐up, although the study by Macchi (Macchi 1996) did not have any loss to follow‐up. Pittler et al (Pittler 1999) stated that they used ITT analyses but in fact their analyses did not include four participants who dropped out or were lost to follow‐up. The remaining 11 studies either reported that dropouts and participants lost to follow‐up were not included in the analyses (Ho 2001; Kaats 2006; Schiller 2001; Williams 1998; Woodgate 2003; Zahorska‐Markiewicz) or it was unclear if their follow‐up analyses included such participants (Colombo 1996; Girola 1996; Giustina 1995; Sciutto 1995; Veneroni 1996; Wuolijoki 1999).
Other potential sources of bias
Similarity of baseline characteristics
Data provided by all 15 studies indicated that baseline characteristics were similar across intervention groups within studies.
Reporting of study findings
Wuolijoki et al (Wuolijoki 1999) reported that chitosan was effective in decreasing lipid levels but, in fact, statistically significant results were obtained only in sub‐group analyses and not in the main analyses. Zahorska‐Markiewicz et al reported a highly significant effect of chitosan on body weight in the abstract of their paper but it is important to note that this effect was only seen in the per‐protocol analyses limited to the 32 participants who completed the study. When data from the 50 randomised participants were included in analyses, the effect on body weight was reversed and the control group lost more weight than the intervention group.
Effects of interventions
Body weight
Combining the 13 trials that provided data on body weight (Colombo 1996; Giustina 1995; Ho 2001; Kaats 2006; Macchi 1996; Ni Mhurchu 2004; Pittler 1999; Schiller 2001; Sciutto 1995; Veneroni 1996; Williams 1998; Woodgate 2003; Zahorska‐Markiewicz) using a fixed‐effect model produced a weighted mean difference (WMD) in body weight of ‐1.7 kg (95% confidence interval (CI) ‐2.1 to ‐1.3) in favour of chitosan versus placebo (P < 0.00001, I2 = 73.6%). The use of a random‐effects model in a sensitivity analysis did not substantially alter this estimate (‐2.2 kg (95% CI ‐3.3 to ‐1.1), P = 0.0001).
Limiting analysis to trials that met the allocation concealment quality criteria (Ni Mhurchu 2004; Pittler 1999; Schiller 2001) reduced the estimated weight loss to ‐0.6 kg (95% CI ‐1.3 to 0.1, P = 0.09), versus an estimated WMD of ‐2.3 kg (95% CI ‐2.7 to ‐1.8, P < 0.00001) in the lower quality trials (Colombo 1996; Giustina 1995; Ho 2001; Kaats 2006; Macchi 1996; Sciutto 1995; Veneroni 1996; Williams 1998; Woodgate 2003; Zahorska‐Markiewicz). The I2‐statistic indicated no heterogeneity in the A grade trials (I2 = 0%) but there was substantial heterogeneity in the B grade trials (I2 = 69.5%).
Similarly, limiting analysis to trials that used chitosan alone as the intervention (without additional weight loss agents) (Ho 2001; Macchi 1996; Ni Mhurchu 2004; Pittler 1999; Schiller 2001; Wuolijoki 1999; Zahorska‐Markiewicz) reduced estimated weight loss to ‐0.9 kg (95% CI ‐1.4 to ‐0.4, P = 0.0009), compared with a WMD of ‐2.7 kg (95% CI ‐3.3 to ‐2.2, P = 0.0008) in trials where the chitosan intervention also included other agents (Colombo 1996; Giustina 1995; Kaats 2006; Sciutto 1995; Veneroni 1996; Williams 1998; Woodgate 2003). The I2‐statistic indicated no heterogeneity in the chitosan only trials (I2 = 0%) but there was substantial heterogeneity in the trials where other agents were used in the intervention (I2 = 71.7%).
Results were also sensitive to study duration. Limiting analysis to trials exceeding four weeks in duration (Ho 2001; Kaats 2006; Ni Mhurchu 2004; Schiller 2001; Williams 1998; Woodgate 2003; Zahorska‐Markiewicz) reduced the estimated weight loss to ‐0.8 kg (95% CI ‐1.4 to ‐0.3, P = 0.003), compared with a WMD of ‐2.7 kg (95% CI ‐3.3 to ‐2.1, P = 0.0008) in trials that only lasted four weeks (Colombo 1996; Giustina 1995; Macchi 1996; Pittler 1999; Sciutto 1995; Veneroni 1996). Once again, the I2‐statistic indicated no heterogeneity in trials exceeding four weeks in duration (I2 = 0%) but there was substantial heterogeneity in trials with a duration of four weeks or less (I2 = 76.2%). The two longest trials included in the review were both six‐month trials (Ni Mhurchu 2004; Zahorska‐Markiewicz) and together these trials produced a weighted mean weight loss of ‐0.6 kg (95% CI ‐1.2 to 0.14), P = 0.12, compared with the trials of shorter duration that produced a WMD of ‐2.3 kg (95% CI ‐2.7 to ‐1.8, P < 00001).
The effect of study size was examined by removing the largest trial (Ni Mhurchu 2004) from the analysis. The resulting WMD of ‐2.2 kg (95% CI ‐2.7 to ‐1.8, P < 0.00001), was larger than the combined estimate when the trial was included (‐1.7 kg (95% CI ‐2.1 to ‐1.3)), suggesting that the findings of the review were sensitive to study size.
Body mass index (BMI)
Combining the seven trials that provided data on BMI (Ho 2001; Macchi 1996; Ni Mhurchu 2004; Pittler 1999; Schiller 2001; Woodgate 2003; Zahorska‐Markiewicz) using a fixed‐effect model produced a WMD in BMI of ‐0.4 kg/m2 (95% CI ‐0.6 to ‐0.2) in favour of chitosan versus placebo (P = 0.0005, I2 = 37.6%).
Total cholesterol
Combining the nine trials that provided data on total cholesterol (Colombo 1996; Ho 2001; Kaats 2006; Macchi 1996; Ni Mhurchu 2004; Pittler 1999; Veneroni 1996; Wuolijoki 1999; Zahorska‐Markiewicz) using a fixed‐effect model produced a WMD in total cholesterol of ‐0.21 mmol/L (95% CI ‐0.28 to ‐0.13) in favour of chitosan versus placebo (P < 0.00001, I2 = 76.7%). The use of a random‐effects model in a sensitivity analysis increased this estimate (‐0.42 mmol/L (95% CI ‐0.69 to ‐0.15, P = 0.002)).
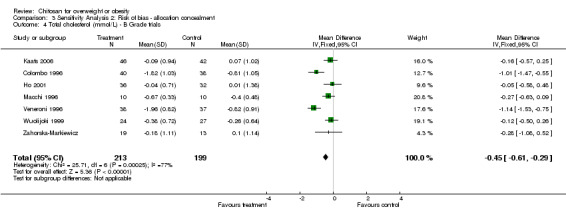
However, limiting included trials to those that met the allocation concealment quality criteria (Ni Mhurchu 2004; Pittler 1999) reduced the estimated reduction in cholesterol to ‐0.15 mmol/L (95% CI ‐0.23 to ‐0.07, P = 0.0004, versus an estimated WMD of ‐0.45 mmol/L (95% CI ‐0.61 to ‐0.29, P < 0.00001) in the lower quality trials (Colombo 1996; Ho 2001; Kaats 2006; Macchi 1996; Veneroni 1996; Wuolijoki 1999; Zahorska‐Markiewicz). The I2‐statistic indicated substantial heterogeneity in both the A grade trials (I2 = 59.5%) and the B grade trials (I2 = 76.7%).
Similarly, limiting included trials to those that used chitosan alone as the intervention (without additional weight loss agents) (Ho 2001; Macchi 1996; Ni Mhurchu 2004; Pittler 1999; Zahorska‐Markiewicz) reduced estimated cholesterol reduction to ‐0.15 mmol/L (95% CI ‐0.23 to ‐0.07, P = 0.0002), compared with a WMD of ‐0.58 mmol/L (95% CI ‐0.78 to ‐0.37, P < 0.00001) in trials where the chitosan intervention also included other agents (Colombo 1996; Kaats 2006; Veneroni 1996; Wuolijoki 1999). The I2‐statistic indicated no heterogeneity in the chitosan only trials (I2 = 0%) but there was substantial heterogeneity in the trials where other agents were used in the intervention (I2 = 85.7%).
Results were also sensitive to study duration. Limiting included trials to those exceeding four weeks in duration (Ho 2001; Kaats 2006; Ni Mhurchu 2004; Wuolijoki 1999; Zahorska‐Markiewicz) also reduced the estimated reduction in cholesterol to ‐0.14 mmol/L (95% CI ‐0.22 to ‐0.06, P = 0.0005), compared with a WMD of ‐0.75 mmol/L (95% CI ‐0.97 to ‐0.53, P < 0.00001) in trials that only lasted four weeks (Colombo 1996; Macchi 1996; Pittler 1999; Veneroni 1996). Once again, the I2‐statistic indicated no heterogeneity in trials exceeding four weeks in duration (I2 = 0%) but there was substantial heterogeneity in the trials where other agents were used in the intervention (I2 = 74.6%). The two six‐month trials (Ni Mhurchu 2004; Zahorska‐Markiewicz) produced a weighted mean weight loss of ‐0.14 mmol/L (95% CI ‐0.22 to ‐0.06, P = 0.0008), compared with the trials of shorter duration that produced a WMD of ‐0.47 mmol/L (95% CI ‐0.63 to ‐0.31, P < 0.00001).
In addition, results were sensitive to study size and removal of the largest trial (Ni Mhurchu 2004) from the analysis produced a WMD of ‐0.46 mmol/L (95% CI ‐0.62 to ‐0.30, P = 0.00001), which was larger than the combined estimate when the trial was included (‐0.21 mmol/L (95% CI ‐0.28 to ‐0.13)).
LDL cholesterol
Combining the seven trials that provided data on LDL cholesterol levels (Colombo 1996; Ho 2001; Kaats 2006; Ni Mhurchu 2004; Veneroni 1996; Wuolijoki 1999; Zahorska‐Markiewicz) using a fixed‐effect model produced a WMD in LDL cholesterol of ‐0.16 mmol/L (95% CI ‐0.23 to ‐0.10) in favour of chitosan versus placebo (P < 0.00001, I2 = 81.7%)).
HDL cholesterol
Combining the seven trials that provided data on HDL cholesterol levels (Colombo 1996; Ho 2001; Kaats 2006; Macchi 1996; Ni Mhurchu 2004; Veneroni 1996; Zahorska‐Markiewicz) using a fixed‐effect model produced a WMD in HDL cholesterol of 0.03 mmol/L (95% CI 0.01 to 0.05) in favour of chitosan versus placebo (P = 0.01, I2 = 90.8%).
Triglycerides
Combining the seven trials that provided data on triglyceride levels (Colombo 1996; Ho 2001; Macchi 1996; Ni Mhurchu 2004; Pittler 1999; Veneroni 1996; Zahorska‐Markiewicz) using a fixed‐effect model produced a WMD in triglycerides of ‐0.12 mmol/L (95% CI ‐0.19 to ‐0.06) in favour of chitosan versus placebo (P < 0.0001, I2 = 75.2%).
Systolic blood pressure (SBP)
Combining the seven trials that provided data on SBP (Giustina 1995; Macchi 1996; Ni Mhurchu 2004; Pittler 1999; Sciutto 1995; Woodgate 2003; Zahorska‐Markiewicz) using a fixed‐effect model produced a WMD in SBP of ‐6 mm Hg (95% CI ‐7 to ‐5) in favour of chitosan versus placebo (P < 0.00001, I2 = 78.5%).
Diastolic blood pressure (DBP)
Combining the seven trials that provided data on DBP (Giustina 1995; Macchi 1996; Ni Mhurchu 2004; Pittler 1999; Sciutto 1995; Woodgate 2003; Zahorska‐Markiewicz) using a fixed‐effect model produced a WMD in DBP of ‐3 mm Hg (95% CI ‐4 to ‐2) in favour of chitosan versus placebo (P < 0.00001, I2 = 86.3%).
Adverse effects
Combining data from the 13 trials that provided quantitative data on numbers of adverse events (Colombo 1996; Girola 1996; Giustina 1995; Ho 2001; Macchi 1996; Ni Mhurchu 2004; Pittler 1999; Sciutto 1995; Veneroni 1996; Williams 1998; Woodgate 2003; Wuolijoki 1999; Zahorska‐Markiewicz) showed that there were no clear differences between intervention and control groups in terms of frequency of adverse events: odds ratio of 1.09 (95% CI 0.72, 1.66, P = 0.67, I2 = 75.8%). Common side effects reported in most trials included constipation, nausea, bloating, indigestion and abdominal pain, but only two studies (Ni Mhurchu 2004; Williams 1998) found that these were significantly increased in participants taking chitosan. In addition to general side effects, some trials monitored changes in blood chemistry (Colombo 1996; Girola 1996; Giustina 1995; Macchi 1996; Veneroni 1996) or fat‐soluble vitamin levels (Colombo 1996; Ni Mhurchu 2004; Pittler 1999; Wuolijoki 1999) but none found any significant effect of chitosan on these parameters.
Faecal fat excretion
Only two trials included in the review measured the effect of chitosan on faecal fat excretion (Ni Mhurchu 2004; Schiller 2001). Ni Mhurchu et al measured faecal fat excretion in a sub‐sample of 51 study participants and found a non significant mean difference between intervention groups. Schiller et al measured faecal fat excretion in a sub‐sample of seven study participants and although there was an increase in faecal fat excreted by the treatment group versus the placebo group the sample size was too small for statistical significance.
Quality of life
Only one trial reported the effects of the study interventions on health‐related quality of life (Ni Mhurchu 2004). No significant differences were seen between intervention groups in the physical and mental component sub scales of the SF‐36 questionnaire throughout the duration of the trial.
Compliance as an effect modifier
Twelve of the 15 trials measured compliance using capsule counts (Colombo 1996; Girola 1996; Giustina 1995; Ho 2001; Ni Mhurchu 2004; Pittler 1999;Schiller 2001; Sciutto 1995; Veneroni 1996; Woodgate 2003; Wuolijoki 1999) or diaries to record doses taken (Williams 1998). However, only four trials reported results of capsule counts (Ho 2001; Ni Mhurchu 2004; Pittler 1999; Woodgate 2003), all of which ranged from approximately 78% (Ho 2001) to 95% (Woodgate 2003).
Discussion
This review examined the efficacy of chitosan as a weight loss treatment. Combining data from all trials that provided data on body weight suggests that chitosan may be an effective treatment for overweight and obesity. However, this finding should be interpreted with caution because the results were sensitive to several indicators of study quality including allocation concealment, study duration, study size, and the use of treatments that included active agents other than chitosan.
Limitation of analyses to those trials that met the allocation concealment criteria suggested an estimated mean weight difference of ‐0.6 kg (95% CI ‐1.3 to 0.10) between people taking chitosan verus placebo (P = 0.09). This mean weight difference of approximately half a kilogram achieved over trial periods ranging from four weeks to six months is minimal and therefore unlikely to be of clinical significance. The effect of chitosan on total cholesterol levels was similarly reduced when analyses were restricted to studies that met the allocation concealment criteria (‐0.15 mmol/L (95% CI ‐0.23 to ‐0.07)) but remained statistically significant (P = 0.0004) although its clinical significance is also questionable. Sensitivity analyses also demonstrated that trials of longer duration and larger size produced smaller effects, as did trials where the intervention treatment was limited to chitosan alone (i.e. unadulterated with other active weight loss agents).
Several small studies reported surprisingly small standard deviations associated with changes in outcomes. Three studies in particular had sample sizes of only 20 (Macchi 1996), 27 (Williams 1998), and 88 (Sciutto 1995) and yet reported standard deviations for weight change that were less than those reported in a trial involving 250 participants (Ni Mhurchu 2004). This suggests either remarkably homogeneous sample populations or a data error. As the weight allocated to each study is inversely proportional to their precision these small trials (with apparent high precision) were awarded more weight in the summary analyses and thus had a large influence on the combined estimate.
A previous meta‐analysis of chitosan as a treatment for body weight reduction (Ernst 1998) found a mean difference in terms of weight reduction between chitosan and placebo groups of 3.3 kg (95% CI 1.5 to 5.1), which is considerably greater than that found in this review. However, the meta‐analysis by Ernst and Pittler (Ernst 1998) was based on only five Italian studies published in 1995 and 1996 (Giustina 1995; Sciutto 1995; Colombo 1996; Macchi 1996; Veneroni 1996) and since then many additional studies of chitosan have been published, ten of which have been included in this review. Ernst and Pittler expressed concerns about the possibility of a systematic bias in the studies included in their meta‐analysis because they were all supplied by one manufacturer, were similar in design, and appeared in the same Italian journal. It is worth noting that these five studies consistently demonstrated the greatest effects in this review, did not meet the allocation concealment criteria, were all of short duration, and four of them also included other agents in their chitosan preparations. As a result, these studies were excluded from many of the sensitivity analyses, thus partly explaining the smaller effects seen in the restricted analyses. Correspondence with the authors of these studies was unsuccessful and therefore, these issues remain unresolved.
Limitations of the review include the exclusion of some potentially informative trials where trial participants were selected for criteria other than being overweight or obese (Ausar 2003; Bokura, Guha 2005; Kaats 1998; Tai 2000) and thus did not meet the criteria for this review. In addition, the small number of studies included in the review and the sensitivity of results to several indicators of study quality suggest that the results should be interpreted with caution.
In summary, this review has indicated that chitosan may be an effective aid to weight loss but many of the included trials have been limited by poor methodology and reporting. Therefore, caution should be exercised in interpreting the findings of the review and further high quality trials are required in order to evaluate appropriately its effectiveness as a weight loss agent.
Authors' conclusions
Implications for practice.
There is some evidence that chitosan is more effective than placebo in the short‐term treatment of overweight and obesity. However, many of the included trials to date have been of poor quality. Results obtained from high quality trials indicate that the effect of chitosan on body weight is minimal and unlikely to be of clinical significance.
Implications for research.
The quality of trials of chitosan and reporting of trial methodology has been generally poor. Recommendations for future studies of chitosan are:
To conduct longer term trials: 24 weeks should be the minimum duration for a weight loss trial.
To measure important outcomes including mortality, morbidity, and quality of life.
To describe in detail the composition and dose of chitosan used.
To report trial methodology more comprehensively, particularly in relation to methods of randomisation, blinding and allocation concealment.
To conduct intention‐to‐treat (ITT) analyses in order to account for any loss to follow‐up following randomisation.
To incorporate economic evaluations into future trials.
What's new
| Date | Event | Description |
|---|---|---|
| 8 May 2008 | New search has been performed | AJ has taken over as lead reviewer. Three new studies were identified and one new study has been included in the review |
| 8 May 2008 | New citation required but conclusions have not changed | Three new studies were identified and one new study has been included in the review |
Acknowledgements
We are grateful to Maree Hackett and Andrew Jull, Clinical Trials Research Unit, University of Auckland, for their advice and help with the original review.
Appendices
Appendix 1. Search strategy
Part I: Chitosan
1 exp Chitin/
2 (chitin$ or chitosan$ or poliglusam$ or RW94).ab,ti,ot.
3 1 or 2
Part II: Obesity and overweight
4 exp Obesity/
5 exp Weight Gain/
6 exp Weight Loss/
7 body mass index/
8 exp skinfold thickness/
9 exp waist‐hip ratio/
10 exp abdominal fat/
11 exp overweight/
12 exp Abdominal Fat/
13 (overweight or over weight).ab,ti,ot.
14 fat overload syndrom$.ab,ti,ot.
15 (overeat or over eat).ab,ti,ot.
16 (overfeed or over feed).ab,ti,ot.
17 (adipos$ or obes$).ab,ti,ot.
18 (weight adj6 (cyc$ or reduc$ or los$ or maint$ or decreas$ or watch$ or control$ or gain
or chang$)).ab,ti,ot.
19 body mass inde$.ab,ti,ot.
20 waist‐hip ratio.ab,ti,ot.
21 skinfold thickness.ab,ti,ot.
22 abdominal fat.ab,ti,ot.
23 or/4‐23
Part III: RCT/CCT
24 randomized controlled trial.pt.
25 controlled clinical trial.pt.
26 randomized controlled trials.sh.
27 random allocation.sh.
28 double‐blind method.sh.
29 single‐blind method.sh.
30 or/25‐30
31 clinical trial.pt.
32 exp clinical trials/
33 (clinic$ adj25 trial$).ab,ti,ot.
34 ((singl$ or doubl$ or trebl$ or tripl$) adj (mask$ or blind$)).ab,ti,ot.
35 placebos.sh.
36 placebo$.ab,ti,ot.
37 random$.ab,ti,ot.
38 research design.sh.
39 (latin adj square).ab,ti,ot.
40 or/32‐40
41 comparative study.pt.
42 exp evaluation studies/
43 follow‐up studies.sh.
44 prospective studies.sh.
45 (control$ or prospectiv$ or volunteer$).ab,ti,ot.
46 cross‐over studies.sh.
47 or/42‐47
48 30 or 40 or 47
Part IV: Meta‐analysis/ Reviews/ HTA
49 exp meta‐analysis/
50 exp Review Literature/
51 meta‐analysis.pt.
52 (meta‐analy$ or meta?analy$).ab,ti,ot.
53 ((review$ or search$) and (medical databas$ or medline or pubmed or embase or
cochrane or systematic$)).ab,ti,ot.
54 or/49‐53
55 letter.pt.
56 comment.pt.
57 editorial.pt.
58 historical‐article.pt.
59 or/55‐58
60 ((systematic$ or quantitativ$ or methodologic$) adj (review$ or overview$)).ab,ti,ot.
61 (integrativ$ research review$ or research integration$).ab,ti,ot.
62 quantitativ$ synthes$.ab,ti,ot.
63 (pooling$ or pooled analys$ or mantel$ haenszel$).ab,ti,ot.
64 (peto$ or der?simonian$ or fixed effect$ or random effect$).ab,ti,ot.
65 or/60‐64
66 54 not 59
67 65 or 66
68 exp Technology Assessment, Biomedical/
69 HTA.ab,ti,ot.
70 (health technology adj6 assessment$).ab,ti,ot.
71 (biomedical adj6 technology assessment$).ab,ti,ot.
72 or/68‐71
Part V: III + IV
73 or/ 67 or 72
Part VI: I and II and V
74 23 and 73
75 limit 74 to animals
76 limit 74 to humans
77 75 not 76
78 74 not 77
Data and analyses
Comparison 1. Primary Comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body weight (kg) | 13 | 932 | Mean Difference (IV, Fixed, 95% CI) | ‐1.71 [‐2.09, ‐1.32] |
| 2 Change in body mass index (kg/m2) | 7 | 493 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.55, ‐0.15] |
| 2.1 Sub‐category | 7 | 493 | Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.55, ‐0.15] |
| 3 Change in total cholesterol (mmol/L) | 9 | 686 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.28, ‐0.13] |
| 4 Change in HDL cholesterol (mmol/L) | 7 | 605 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [0.01, 0.05] |
| 5 Change in LDL cholesterol (mmol/L) | 7 | 635 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.23, ‐0.10] |
| 6 Change in triglycerides (mmol/L) | 7 | 547 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.19, ‐0.06] |
| 7 Change in systolic blood pressure (mm Hg) | 7 | 537 | Mean Difference (IV, Fixed, 95% CI) | ‐5.94 [‐7.25, ‐4.63] |
| 8 Change in diastolic blood pressure (mm Hg) | 7 | 537 | Mean Difference (IV, Fixed, 95% CI) | ‐3.38 [‐4.35, ‐2.42] |
| 9 Adverse events (counts) | 13 | 1006 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.72, 1.66] |
1.1. Analysis.

Comparison 1 Primary Comparisons, Outcome 1 Change in body weight (kg).
1.2. Analysis.

Comparison 1 Primary Comparisons, Outcome 2 Change in body mass index (kg/m2).
1.3. Analysis.

Comparison 1 Primary Comparisons, Outcome 3 Change in total cholesterol (mmol/L).
1.4. Analysis.

Comparison 1 Primary Comparisons, Outcome 4 Change in HDL cholesterol (mmol/L).
1.5. Analysis.

Comparison 1 Primary Comparisons, Outcome 5 Change in LDL cholesterol (mmol/L).
1.6. Analysis.

Comparison 1 Primary Comparisons, Outcome 6 Change in triglycerides (mmol/L).
1.7. Analysis.

Comparison 1 Primary Comparisons, Outcome 7 Change in systolic blood pressure (mm Hg).
1.8. Analysis.

Comparison 1 Primary Comparisons, Outcome 8 Change in diastolic blood pressure (mm Hg).
1.9. Analysis.

Comparison 1 Primary Comparisons, Outcome 9 Adverse events (counts).
Comparison 2. Sensitivity Analysis 1: Random‐effects model.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body weight (kg) | 13 | 932 | Mean Difference (IV, Random, 95% CI) | ‐2.22 [‐3.33, ‐1.10] |
| 2 Total cholesterol (mmol/L) | 9 | 686 | Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.69, ‐0.15] |
2.1. Analysis.

Comparison 2 Sensitivity Analysis 1: Random‐effects model, Outcome 1 Body weight (kg).
2.2. Analysis.

Comparison 2 Sensitivity Analysis 1: Random‐effects model, Outcome 2 Total cholesterol (mmol/L).
Comparison 3. Sensitivity Analysis 2: Risk of bias ‐ allocation concealment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body weight (kg) ‐ A Grade trials | 3 | 339 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.26, 0.10] |
| 2 Body weight (kg) ‐ B Grade trials | 10 | 593 | Mean Difference (IV, Fixed, 95% CI) | ‐2.25 [‐2.72, ‐1.77] |
| 3 Total cholesterol (mmol/L) ‐ A Grade trials | 2 | 274 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.23, ‐0.07] |
| 4 Total cholesterol (mmol/L) ‐ B Grade trials | 7 | 412 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.61, ‐0.29] |
3.1. Analysis.

Comparison 3 Sensitivity Analysis 2: Risk of bias ‐ allocation concealment, Outcome 1 Body weight (kg) ‐ A Grade trials.
3.2. Analysis.

Comparison 3 Sensitivity Analysis 2: Risk of bias ‐ allocation concealment, Outcome 2 Body weight (kg) ‐ B Grade trials.
3.3. Analysis.

Comparison 3 Sensitivity Analysis 2: Risk of bias ‐ allocation concealment, Outcome 3 Total cholesterol (mmol/L) ‐ A Grade trials.
3.4. Analysis.
Comparison 3 Sensitivity Analysis 2: Risk of bias ‐ allocation concealment, Outcome 4 Total cholesterol (mmol/L) ‐ B Grade trials.
Comparison 4. Sensitivity Analysis 3: Effect of additional weight loss agents.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body weight (kg) ‐ Chitosan alone | 6 | 459 | Mean Difference (IV, Fixed, 95% CI) | ‐0.88 [‐1.40, ‐0.36] |
| 2 Body weight (kg) ‐ Chitosan + other weight loss agents | 7 | 473 | Mean Difference (IV, Fixed, 95% CI) | ‐2.73 [‐3.31, ‐2.15] |
| 3 Total cholesterol (mmol/L) ‐ Chitosan alone | 5 | 394 | Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.23, ‐0.07] |
| 4 Total cholesterol (mmol/L) ‐ Chitosan + other weight loss agents | 4 | 292 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐0.78, ‐0.37] |
4.1. Analysis.

Comparison 4 Sensitivity Analysis 3: Effect of additional weight loss agents, Outcome 1 Body weight (kg) ‐ Chitosan alone.
4.2. Analysis.

Comparison 4 Sensitivity Analysis 3: Effect of additional weight loss agents, Outcome 2 Body weight (kg) ‐ Chitosan + other weight loss agents.
4.3. Analysis.

Comparison 4 Sensitivity Analysis 3: Effect of additional weight loss agents, Outcome 3 Total cholesterol (mmol/L) ‐ Chitosan alone.
4.4. Analysis.

Comparison 4 Sensitivity Analysis 3: Effect of additional weight loss agents, Outcome 4 Total cholesterol (mmol/L) ‐ Chitosan + other weight loss agents.
Comparison 5. Sensitivity Analysis 4: Study duration ‐ 4 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body weight (kg) ‐ > 4 weeks in duration | 7 | 546 | Mean Difference (IV, Fixed, 95% CI) | ‐0.81 [‐1.35, ‐0.28] |
| 2 Body weight (kg) ‐ 4 weeks in duration | 6 | 386 | Mean Difference (IV, Fixed, 95% CI) | ‐2.69 [‐3.25, ‐2.13] |
| 3 Total cholesterol (mmol/L) ‐ > 4 weeks in duration | 5 | 483 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.22, ‐0.06] |
| 4 Total cholesterol (mmol/L) ‐ 4 weeks in duration | 4 | 203 | Mean Difference (IV, Fixed, 95% CI) | ‐0.75 [‐0.97, ‐0.53] |
5.1. Analysis.

Comparison 5 Sensitivity Analysis 4: Study duration ‐ 4 weeks, Outcome 1 Body weight (kg) ‐ > 4 weeks in duration.
5.2. Analysis.

Comparison 5 Sensitivity Analysis 4: Study duration ‐ 4 weeks, Outcome 2 Body weight (kg) ‐ 4 weeks in duration.
5.3. Analysis.

Comparison 5 Sensitivity Analysis 4: Study duration ‐ 4 weeks, Outcome 3 Total cholesterol (mmol/L) ‐ > 4 weeks in duration.
5.4. Analysis.

Comparison 5 Sensitivity Analysis 4: Study duration ‐ 4 weeks, Outcome 4 Total cholesterol (mmol/L) ‐ 4 weeks in duration.
Comparison 6. Sensitvity Analysis 5: Study duration ‐ 6 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body weight (kg) ‐ 6 months in duration | 2 | 282 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐1.23, 0.14] |
| 2 Body weight (kg) ‐ < 6 months in duration | 11 | 650 | Mean Difference (IV, Fixed, 95% CI) | ‐2.24 [‐2.71, ‐1.78] |
| 3 Total cholesterol (mmol/L) ‐ 6 months in duration | 2 | 276 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.22, ‐0.06] |
| 4 Total cholesterol (mmol/L) ‐ < 6 months in duration | 7 | 410 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.63, ‐0.31] |
6.1. Analysis.

Comparison 6 Sensitvity Analysis 5: Study duration ‐ 6 months, Outcome 1 Body weight (kg) ‐ 6 months in duration.
6.2. Analysis.

Comparison 6 Sensitvity Analysis 5: Study duration ‐ 6 months, Outcome 2 Body weight (kg) ‐ < 6 months in duration.
6.3. Analysis.

Comparison 6 Sensitvity Analysis 5: Study duration ‐ 6 months, Outcome 3 Total cholesterol (mmol/L) ‐ 6 months in duration.
6.4. Analysis.

Comparison 6 Sensitvity Analysis 5: Study duration ‐ 6 months, Outcome 4 Total cholesterol (mmol/L) ‐ < 6 months in duration.
Comparison 7. Sensitvity Analysis 6: Study size.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Body weight (kg) ‐ large studies (> 150 participants) | 1 | 250 | Mean Difference (IV, Fixed, 95% CI) | ‐0.56 [‐1.25, 0.13] |
| 2 Body weight (kg) ‐ smaller studies (< 150 participants) | 12 | 682 | Mean Difference (IV, Fixed, 95% CI) | ‐2.24 [‐2.71, ‐1.77] |
| 3 Total cholesterol (mmol/L) ‐ large studies (> 150 participants) | 1 | 244 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.22, ‐0.06] |
| 4 Total cholesterol (mmol/L) ‐ smaller studies (< 150 participants) | 8 | 442 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.62, ‐0.30] |
7.1. Analysis.

Comparison 7 Sensitvity Analysis 6: Study size, Outcome 1 Body weight (kg) ‐ large studies (> 150 participants).
7.2. Analysis.

Comparison 7 Sensitvity Analysis 6: Study size, Outcome 2 Body weight (kg) ‐ smaller studies (< 150 participants).
7.3. Analysis.

Comparison 7 Sensitvity Analysis 6: Study size, Outcome 3 Total cholesterol (mmol/L) ‐ large studies (> 150 participants).
7.4. Analysis.

Comparison 7 Sensitvity Analysis 6: Study size, Outcome 4 Total cholesterol (mmol/L) ‐ smaller studies (< 150 participants).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Colombo 1996.
| Methods | Trial design: randomised placebo‐controlled double‐blind trial comparing chitosan v. placebo Randomisation and allocation concealment: participants were randomised using random numbers. No details given of allocation concealment. Baseline characteristics: similar | |
| Participants | Country: Italy Participants: 86 ambulatory males and females aged 20‐70 years with mild obesity (overweight by 10% to 25% as compared with ideal weight‐height tables) and hyperlipoproteinemia Exclusion criteria: known or presumed hypersensitivity to any component of the drug, non‐adherence to trial protocol, severe hepatic or gastrointestinal disease, renal insufficiency, severe chronic disease, pregnancy, concomitant administration of drugs that could interfere with results of the study. Total randomised: 86 (43 placebo, 43 chitosan). Loss to follow‐up: 8 participants (3 chitosan, 5 placebo) withdrew from the study. Final number analysed: Unclear if drop‐outs were included in analysis. | |
| Interventions | Chitosan: 2 capsules twice daily (dose not stated) of "Somagril" (Lifepharma). Preparation was a mixture of chitosan, guar's meal, ascorbic acid and other micronutrients. Placebo: 2 capsules twice daily (dose and composition not stated). Timing: capsules advised to be taken with main meals (lunch/dinner). Co‐interventions: low calorie diet (1000 kcal based on 34% fat, 41% carbohydrate and 25% protein). | |
| Outcomes | Body weight, percentage overweight, serum total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides, incidence of side effects, quality of life, diet and drug compliance, appearance of faeces, haematological and blood chemistry analysis (hemoglobin, hematocrit, leukocytes, erythrocytes, glucose, urea nitrogen, creatinine, bilirubin, AST and ALT transaminases, gamma‐GT, sodium, potassium, calcium, magnesium, ferritin and iron). Blood concentrations of sodium, potassoim, calcium, magnesium, zinc, iron, copper, vitamins A, D, and E. Outcomes asessed following 4 weeks of treatment. | |
| Notes | Unclear if intention‐to‐treat analyses were used. Funding source not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Girola 1996.
| Methods | Trial design: randomised placebo‐controlled double‐blind trial comparing chitosan v. placebo Randomisation and allocation concealment: participants were randomised using random numbers. No details given of allocation concealment Baseline characteristics: similar | |
| Participants | Country: Italy Participants: 150 ambulatory males and females aged 20‐70 years with mild obesity (overweight by 10% to 25% as compared with ideal weight‐height tables) and hyperlipoproteinemia Exclusion criteria: known or presumed hypersensitivity to any component of the drugs, non‐adherence to the trial protocol, severe hepatic or gasrointestinal diseases, renal insufficiency, severe chronic disease, pregnancy, concomitant administration of drugs that could interfere with the results of the study. Total randomised: 150 (50 = placebo, 50 = 1 capsule chitosan/d, 50 = 2 capsules chitosan/d) Loss to follow‐up: 6 participants (3 chitosan, 3 placebo) withdrew from the study Final number analysed: unclear if drop‐outs were included in analysis | |
| Interventions | Chitosan: One group (n = 50) took 2 capsules daily of "Colenon" (480 mg chitosan) and another (n = 50) took 1 capsule daily (240 mg). Preparation was a mixture of chitosan, garcinia cambogia extract and chrome. Placebo: 2 capsules daily (dose and composition not stated) Timing: capsules advised to be taken with main meals (lunch/dinner) Co‐interventions: low calorie diet (1000 kcal based on 34% fat, 41% carbohydrate and 25% protein) | |
| Outcomes | Body weight, percentage overweight, serum total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides, incidence of side effects, quality of life, diet and drug compliance, appearance of faeces, haematological and blood chemistry analysis (hemoglobin, hematocrit, leukocytes, erythrocytes, glucose, urea nitrogen, creatinine, bilirubin, AST and ALT transaminases, gamma‐GT, sodium, potassium, calcium, magnesium, ferritin and iron), treatment compliance, adverse events. Outcomes asessed following 4 weeks of treatment. | |
| Notes | Unclear if intention‐to‐treat analyses were used. Funding source not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Giustina 1995.
| Methods | Trial design:randomised placebo‐controlled double‐blind trial comparing chitosan v. placebo Randomisation and allocation concealment: participants were randomised using random numbers. No details given of allocation concealment. Baseline characteristics: similar | |
| Participants | Country: Italy Participants: 100 ambulatory males and females aged 20‐70 years with mild obesity (overweight by 10% to 25% as compared with ideal weight‐height tables) and mild hypertension Exclusion criteria: known or presumed hypersensitivity to any component of the drugs, non‐adherence to the trial protocol, severe hepatic or gasrointestinal diseases, renal insufficiency, severe chronic disease, pregnancy, concomitant administration of drugs that could interfere with the results of the study. Total randomised: 100 (50 placebo, 50 chitosan). Loss to follow‐up: 5 participants (2 chitosan, 3 placebo) withdrew from the study. Final number analysed: unclear if drop‐outs were included in analysis. | |
| Interventions | Chitosan: 2 tablets twice daily (dose not stated) of "Nofat". Preparation was a mixture of chitosan, guar's meals, ascorbic acid and other micronutrients. Placebo: 2 tablets twice daily (dose and composition not stated). Timing: capsules advised to be taken with main meals (lunch/dinner). Co‐intervetnions: low calorie diet (1000‐1100 kcal based on 34% fat, 41% carbohydrate and 25% protein). | |
| Outcomes | Body weight, percentage overweight, arterial pressure (systolic and diastolic), heart, respiratory rate, incidence of side effects, quality of life, diet. and drug compliance, appearance of faeces were assessed at 7, 14, 21, and 28 days of treatment. Hematological and blood chemistry analysis (hemoglobin, hematocrit, leukocytes, erythrocytes, glucose, urea nitrogen, creatinine, bilirubin, AST and ALT transaminases, gamma‐GT, sodium, potassium, calcium, magnesium, ferritin and iron) were assessed at 28 days only. | |
| Notes | Unclear if intention‐to‐treat analyses were used. Funding source not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ho 2001.
| Methods | Trial design: randomised placebo‐controlled double‐blind trial comparing chitosan v. placebo. Randomisation and allocation concealment: no details given Baseline characteristics: broadly similar but females in the chitosan group had significantly higher waist‐hip ratios than the placebo treated group (p=0.046) whereas males in the chitosan group had lower % body fat compared to placebo group (p=0.044). | |
| Participants | Country: Singapore Participants: 88 normoglycaemic obese males and females (body fat % > 20% in males and > 30% in females) who were hypercholesterolaemic (total cholesterol > 5.20 mmol/L) and had no history of chronic illnesses. Exclusion criteria: seafood allergy, history of alcohol and drug usage, chronic illnesses such as diabetes mellitus, hypertension, ischemic heart disease, stroke, and chronic liver or renal dysfunction. Total enrolled: 88 Total randomised: 85 Total included in analyses: 68 (32 placebo, 36 chitosan). | |
| Interventions | Chitosan: 4 capsules of chitosan (257 mg) 3 times daily for 12 weeks i.e. 3.1g/day chitosan. Preparation was "Absorbitol", a salt of chitosan. Placebo: No details given. Co‐interventions: no dietary restriction | |
| Outcomes | Body weight, BMI, waist and hip circumferences, blood pressure (systolic and diastolic), fat free mass, % body fat, serum total cholesterol, triglyceride, HDL cholesterol, and fasting insulin, adverse events Outcomes assessed at enrolment, baseline (following 4 weeks placebo run‐in phase) and after 12 weeks of treatment. | |
| Notes | Paper states analyses were intention‐to‐treat but data are only presented on 68 who completed treatment. Funding source not stated. Results in paper are stratified by sex. Investigator provided data by intervention group, which have been used for the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kaats 2006.
| Methods | Trial design: randomised placebo controlled double‐blind three‐arm parallel group trial comparing chitosan v. placebo v. minimal intervention control (Only chitosan and placebo arms included in this review and described here) Randomisation and allocation concealment: no details given Baseline characteristics: similar, although the placebo group had higher levels of HDL cholesterol than the chitosan group (61.2 mg/dl v. 53.3 mg/dl, p=0.01). | |
| Participants | Country: United States of America Participants: 100 adults described as overweight. Exclusion criteria: Chronically ill, pregnant or lactating women. Total randomised: 50 participants were randomised to each group. Total included in analyses: 88 (42 placebo, 46 chitosan) | |
| Interventions | Chitosan: 6 tablets chitosan (500 mg) daily i.e. 3 g/day. Preparation was a mixture of chitosan, and 1mg per tablet of beta‐glucan, snow white oat fibre, betamine HCL and aloe saponins. Placebo: 6 tablets daily (composition not stated). Co‐interventions: Self‐monitored behavioural modification programme consisting of a work book for estimating caloric intake, calculator with nutritional information on 5000 foods, log book for calculating and estimating daily calorie balances and dietary fat intake, pedometer and recording of daily steps. | |
| Outcomes | Body weight (lbs), fat mass, fat free mass, % body fat, serum total cholesterol, HDL cholesterol, LDL cholesterol. Outcomes assessed at baseline and after 60 days of treatment. | |
| Notes | Baseline characteristics only provided on those who completed the trial, not the total number of randomised participants. Participants recruited from those who participated in previous studies by the investigators and from other people referred by participants. Funding source not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Macchi 1996.
| Methods | Trial design: randomised, placebo‐controlled double‐blind 3‐arm trial comparing chitosan alone v. chitosan plus diet v. placebo plus diet. Randomisation and allocation concealment.: no details given Baseline characteristics: similar | |
| Participants | Country: Italy Participants: 30 obese males and females aged 30 and 80 years old, with 25% excess body weight. Exclusion criteria: concomitant diseases (kidney or hepatic disease, intestinal disturbances, cardiac failure), excessive use of alcohol, pregnancy or the necessity of drugs administration. Total randomised: 10 participants were randomised to each treatment group. Loss to follow‐up: there were no drop‐outs. | |
| Interventions | Chitosan: 4 tablets chitosan (250 mg) daily i.e. 1 g/day. Preparation was purified electrostatically charged chitosan (PAT RM q5A 000 772) Placebo: 4 tablets daily (composition not stated). Timing: capsules advised to be taken just before main meals. Co‐interventions: low calorie diet (1200 kcal based on 30% fat, 45% carbohydrate and 25% protein). | |
| Outcomes | Body weight, BMI, % body fat, emochrome, iron, electrolytes, transaminase, total cholesterol, HDL cholesterol, triglycerides, glucose, urea nitrogen and creatinine, side effects, well‐being and appetite. Outcomes assessed following 4 weeks of treatment. | |
| Notes | Individual participant data provided in paper. Funding source not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ni Mhurchu 2004.
| Methods | Trial design: randomised, placebo‐controlled double‐blind trial comparing chitosan v. placebo. Randomisation and allocation concealment: participants were randomised using computer‐generated random numbers with mixed block sizes. Treatment assignment codes were held centrally and were not available to investigators, research staff or data entry staff. Baseline characteristics: similar Analysis: Intention‐to‐treat analysis with last value carried forward. | |
| Participants | Country: New Zealand Participants: 250 overweight or obese (BMI 28‐50kg/m2) males and females aged at least 18 years who wished to lose weight. Exclusion criteria: current treatment with chitosan, current or recent treatment with weight loss medications, current or recent attendance at a commercial weight loss clinic/programme, allergy to seafood, pregnancy or lactation, active gastrointestinal disease or obesity surgery, involvement in another clinical trial, unlikely to comply with study treatment / follow‐up procedures. Total randomised: 250 (125 placebo, 125 chitosan). Total included in analyses: 250 (125 placebo, 125 chitosan). Loss to follow‐up: 86 participants dropped out (44 placebo, 42 chitosan). | |
| Interventions | Chitosan: 4 capsules of chitosan (250 mg) three times daily for 24 weeks i.e. 3 g/day chitosan. Chitosan was beta‐chitosan derived from NZ squid pens, had a molecular weight of 130,000, and deacetylation was 75.5%. Placebo: 4 capsules of maize cornflour (250 mg) three times daily for 24 weeks Timing: capsules advised to be taken 30 minutes before meals. Co‐interventions: standarised dietary and lifestyle advice | |
| Outcomes | Body weight, BMI, waist circumference, % fat, blood pressure (systolic and diastolic), glucose, total cholesterol, LDL and HDL cholesterol, triglycerides, vitamin A, beta‐carotene, vitamin D, prothrombin time, health‐related quality of life, adverse events, adherence to treatment, faecal fat (subgroup of 50 volunteers) Outcomes assessed at 4‐weekly visits and overall repsonse was measured over entire 24‐week period. | |
| Notes | Funded by the Health Research Council of New Zealand and Healtheries of New Zealand | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pittler 1999.
| Methods | Trial design: randomised placebo‐controlled double‐blind trial comparing chitosan v. placebo Radomisation and allocation concealment: block randomisation using random number tables performed by an individual not involved in the study. The randomisation code was kept in a sealed, opaque envelope and broken only after conclusion of the experimental phase of the trial. Baseline characteristics: similar Analysis: methods state that intention to‐treat analysis was used but analyses were only carried out on those who completed study. | |
| Participants | Country: United Kingdom Participants: 34 overweight male and female volunteers (BMI 23.9‐28.5 kg/m2 for women, 25.0‐29.9 kg/m2 for men), aged 18 to 60 years. Exclusion criteria: intestinal disorders, diabetes mellitus, concomitant medication, pregnancy, known/suspected hypersensitivity to drug Total randomised: 34 (17 placebo, 17 chitosan) Total analysed: 30 (15 placebo, 15 chitosan) Loss to follow‐up: 4 participants (2 placebo, 2 chitosan) withdrew from the study | |
| Interventions | Chitosan: 4 capsules of chitosan (250 mg) daily for 28 days i.e. 1 g/day chitosan. Chitosan was deacetylated chitin biopolymer. Placebo: 4 capsules daily for 28 days (no details of composition). Co‐interventions: participants were advised to maintain their normal diet. | |
| Outcomes | Body weight, blood pressure, quality of life (SF‐36), total cholesterol, triglycerides, vitamins A, D, E, K, beta‐carotene, adverse effects, compliance. Outcomes assessed at baseline, 2 weeks, and 4 weeks of treatment. | |
| Notes | Capsules only contained 42% chitosan i.e. less than 71% (250 mg) claimed by distributor. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Schiller 2001.
| Methods | Trial design: randomised, placebo‐ controlled double‐blind trial comparing chitosan v. placebo. Randomisation and allocation concealment: participants were randomly assigned to either the treatment or placebo group using random numbers table. Both subjects and investigators were blinded to group assignment. Baseline characteristics: similar Analysis: not an intention‐to‐treat analysis (completers only) | |
| Participants | Country: USA Participants: 69 overweight and mildly obese (BMI 27‐40) but otherwise healthy females aged 21‐55 years, with a stable weight history of at least 6 months and a history of daily fat consumption greater than or equal to 30% of calories. Exclusion criteria: history of metabolic, hepatic, renal, autoimmune, gastrointestinal or neurological disease, consumption of supplements, OTC products and/or pharmaceutical agents that might influence the outcome of the study, history of eating disorders, bipolar disorder or severe depression, perimenopausal or menopausal symptoms, pregnancy or breastfeeding Total randomised: 69 (35 to placebo, 34 to chitosan). Total analysed: 59 (30 placebo, 29 chitosan) Loss to follow‐up: 10 participants (placebo = 5, chitosan = 5) excluded/withdrew from the study. Of the 10 subjects who did not complete the study, 7 were dropped due to non‐compliance with the study protocol, 1 was dropped due to non‐study related illness, and 2 were lost during follow‐up. | |
| Interventions | Chitosan: 3 capsules of chitosan (500 mg) twice daily for 8 weeks i.e. 3 g/day chitosan. Preparation was LipoSan Ultra consisting of >90% chitosan and <10% succinic acid, deacetylation value of >78%, viscosity of approx. 155 mPas, molecular weight >100,000 daltons. Placebo: 3 capsules of a maltodextrin‐semolina flour blend (500 mg) twice daily for 8 weeks Timing: Capsules advised to be taken immediately prior to 2 largest meals of the day. Co‐interventions: participants instructed not to deviate from normal diet or exercise routines. | |
| Outcomes | Body weight, BMI, waist‐to‐hip ratio, % body fat, % lean body mass, fasting serum lipid levels, fecal fat (in a subset of 7 participants). Beck depression inventory, medical outcome survey (short form 36), diet (food frequency questionnaire and diet diary), functional gastrointestinal and elimination symptoms. Outcomes assessed at baseline and 8 weeks. | |
| Notes | Funded by Vanson Inc., the manufacturer of Liposan Ultra (used in study) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Sciutto 1995.
| Methods | Randomised, placebo‐controlled double‐blind trial comparing chitosan v. placebo Randomisation and allocation concealment: participants were randomised using random numbers. No details given of allocation concealment. Baseline characteristics: similar | |
| Participants | Country: Italy Participants: 90 ambulatory males and females aged 20‐70 years with mild obesity (overweight by 10% to 25% as compared with ideal weight‐height tables), mild hypertension, and hyperlipoproteinemia. Exclusion criteria: known or presumed hypersensitivity to any component of the drugs, no guarantee of total adherence to the trial protocol, severe hepatic or gastrointestinal diseases, renal insufficiency, severe chronic disease, pregnancy, concomitant administration of the drug that could interfere with the results of the study. Total randomised: 90 (45 placebo, 45 chitosan). Loss to follow‐up: 2 participants (1 placebo, 1 chitosan) withdrew from the study. Unclear if drop‐outs were included in analysis. | |
| Interventions | Chitosan: 2 tablets twice daily of "Somagril" (dose not stated) for 4 weeks. Preparation was a mixture of chitosan, guar's meals, ascorbic acid and other micronutrients. Placebo: 2 tablets twice daily (dose and composition not stated) for 4 weeks. Timing: capsules advised to be taken with main meals (lunch/dinner). Co‐interventions: low calorie diet (1000 kcal based on 34% fat, 41% carbohydrate and 25% protein). | |
| Outcomes | Body weight, percentage overweight, arterial pressure (systolic and diastolic), incidence of side effects, quality of life, diet. and drug compliance, appearance of faeces were assessed at 7, 14, 21, and 28 days of treatment. Serum concentration of total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides were assessed at 0, 7, and 28 days. Hematological and blood chemistry analysis (hemoglobin, hematocrit, leukocytes, eryhrocytes, glucose, urea nitrogen, creatinine, bilirubin, AST and ALT transaminases, gamma‐GT, sodium, potassium, calcium, magnesium, ferritin and iron) were assessed at 0 and 28 days. | |
| Notes | Unclear if intention‐to‐treat analyses were used. Funding source not stated. Lipid results are percentage reductions rather than actual values so SD could not be calculated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Veneroni 1996.
| Methods | Randomised, placebo‐controlled double‐blind trial comparing chitosan v. placebo Randomisation and allocation concealment: participants were randomised using random numbers. No details given of allocation concealment. Baseline characteristics: similar | |
| Participants | Country: Italy Participants: 80 ambulatory males and females aged 20‐70 years with mild obesity (overweight by 10% to 25% as compared with ideal weight‐height tables), and hyperlipidemia. Exclusion criteria: known or presumed hypersensitivity to any component of the drugs, no guarantee of total adherence to the trial protocol, severe hepatic or gastrointestinal diseases, renal insufficiency, severe chronic disease, pregnancy, concomitant administration of the drug that could interfere with the results of the study. Total randomised: 80 (40 placebo, 40 chitosan). Loss to follow‐up: 5 participants (3 placebo, 2 chitosan) withdrew from the study. Unclear if drop‐outs were included in analysis. | |
| Interventions | Chitosan: 2 tablets twice daily of "Nofat" (dose not stated) for 4 weeks. Preparation was a mixture of chitosan, guar's meals, ascorbic acid and other micronutrients. Placebo: 2 tablets twice daily (dose and composition not stated) for 4 weeks. Timing: capsules advised to be taken with main meals (lunch/dinner). Co‐interventions: low calorie diet (1000 kcal based on 34% fat, 41% carbohydrate and 25% protein). | |
| Outcomes | Body weight, percentage overweight, diet, physical fitness program and drug compliance, appearance of faeces, incidence of adverse events, quality of life were assessed at 0, 7, 14, 21, and 28 days of treatment. Serum concentration of total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, hematological and blood chemistry analysis (hemoglobin, hematocrit, leukocytes, eryhrocytes, glucose, urea nitrogen, creatinine, bilirubin, AST and ALT transaminases, gamma‐GT, sodium, potassium, calcium, magnesium, ferritin and iron) were assessed at 0 and 28 days. | |
| Notes | Unclear if intention‐to‐treat analyses were used. Funding source not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Williams 1998.
| Methods | Randomised, placebo‐controlled double‐blind trial comparing RW94 v. placebo. Randomisation and allocation concealment: participants were randomised using a pre‐determined schedule. Allocation concealment unclear. Baseline characteristics: similar Analysis: not an intention‐to‐treat analysis. | |
| Participants | Country: United Kingdom Participants: 32 adult overweight but otherwise healthy male and female volunteers aged 23‐57 years, meetng standard inclusion criteria for Phase I clinical trials. Exclusion criteria: standard exclusion criteria for Phase I clinical trials. Total randomised: 32 participants (16 placebo, 16 RW94). Total analysed :27 participants (14 placebo, 13 RW94). Loss to follow‐up: 4 participants (2 placebo, 2 RW94) withdrew from the study, and 1 participant (RW94) was excluded for non‐compliance. | |
| Interventions | RW94: 5g RW94 powder stirred into a glass of fruit squash 3 times daily for 6 weeks. Placebo: 5g maize starch stirred into a glass of fruit squash 3 times daily for 6 weeks. Timing: powders advised to be taken half an hour after food. Co‐interventions: participants instructed not to vary their diet, smoking or drinking habits for the duration of the study. | |
| Outcomes | Body weight, state of health, adverse events. Empty medication packs and completed food diaried were collected. Outcomes assessed at 0, 1, 2, 3, 4, 5, and 6 weeks. | |
| Notes | 15g/day = much higher than other studies. Taken as a powder rather than capsules/tablets. Taken after meals rather than before meals. Not explicitly stated anywhere that RW94 is chitosan. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Woodgate 2003.
| Methods | Randomised, placebo‐controlled double‐blind trial comparing chitosan v. placebo. Randomisation and allocation concealment: participants were randomised by drawing cards containing randomisation codes. Allocation concealment unclear. Baseline characteristics: similar Analysis: not an intention to treat analysis. | |
| Participants | Country: Canada Participants: 24 obese (BMI >/= 30) male and female volunteers aged 20‐ 50 years. Exclusion criteria: history of cardiac disease, diabetes mellitus, hypertension, psychiatric disorders, use of monoamine oxidase inhibitors, pregnancy or lactation, thyroid disease, use of other dietary aids or enrolment in a weight loss program, allergy to any of the ingredients used in the treatment. Total randomised: 24 (12 placebo, 12 chitosan) Total analysed: 22 (11 placebo, 11 chitosan) Loss to follow‐up: 2 participants (1 placebo, 1 chitosan) dropped out for personal reasons unrelated to study. Not an intention to treat analysis. | |
| Interventions | Chitosan: 2 capsules of chitosan (dose unknown) three times daily for 6 weeks. Preparation was a proprietary blend of glucomannan, chitosan, fenugreek, G sylvestre, and vitamin C (1 capsule = 1395 mg). Placebo: 2 capsules of rice flour three times daily for 6 weeks. Timing: capsules advised to be taken before 1 hour meals. Co‐interventions: participants instructed to continue their regular diet and exercise patterns. | |
| Outcomes | Body weight, % body fat, fat mass, lean body mass, BMI, blood pressure, resting heart rate, upper abdominal circumference, waist and hip circumferences. Measured at baseline and 6 weeks. | |
| Notes | First author is president and owner of NxCare Inc, which produces the dietary supplement used in the study (Calorie Care). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wuolijoki 1999.
| Methods | Randomised, placebo‐controlled double‐blind trial comparing chitosan v. placebo. Randomisation and allocation concealment: no details given Baseline characteristics: similar Analysis: sub‐group analyses conducted. | |
| Participants | Country: Finland Participants: 53 healthy women with a BMI of 28‐34.99 and employed at Tampere University Hospital. Exclusion criteria: regular medication, gravidity, vegetarianism, allergy to crustaceans, age < 18 or > 60, lack of reliable contraception. Total randomised: 53 (27 placebo, 24 chitosan). Total analysed: unclear because depends on subgroup. Loss to follow‐up: 2 participants (1 placebo, 1 chitosan) withdrew from the study. 4 participants (3 placebo, 1 chitosan) changed their eating habits during trial. | |
| Interventions | Chitosan: 3 capsules of chitosan (400 mg) twice daily for 8 weeks i.e. 2.4 g/day chitosan. Chitosan was microcrystallised chitosan (Novamic). Placebo: 3 capsules of starch twice daily for 8 weeks. Timing: capsules advised to be taken before lunch and dinner. Co‐interventions: no additional weight reduction procedures allowed. | |
| Outcomes | Body weight, adverse events, and volunteer and nurse opinions on living habits, compliance, and treatment efficacy at baseline, 4, 6, and 8 weeks of treatment. Serum lipids (total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides at baseline, 4, and 8 weeks. Fe++, transferrin, total A and E vitamins, ALAT, ASAT, alkaline phosphatase, creatinine, Na+, K+, blood cell (erythrocyte, white cell and platelet) counts and urine sediment at baseline and 8 weeks. | |
| Notes | Actual body weight data not reported (only available as figure). Significant lipid results only obtained in sub‐group analyses (BMI >/= 30 who had not changed their eating habits). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zahorska‐Markiewicz.
| Methods | Randomised, placebo‐controlled double‐blind trial comparing chitosan v. placebo. Randomisation and allocation concealment: methods unknown Baseline characteristics: similar. Analysis: per‐protocol analysis. | |
| Participants | Country: Poland Participants: 50 obese (BMI > 30) but otherwise healthy females aged between 22 and 59 years. Exclusion criteria: unknown. Total randomised: 50 (25 placebo, 25 chitosan) Total analysed: 32 (13 placebo, 19 chitosan) Loss to follow‐up: 18 participants (12 placebo, 6 chitosan) were lost to follow up. | |
| Interventions | Chitosan: 2 tablets of chitosan (750 mg) three times daily for 6 months i.e. 4.5g/day chitosan. Preparation was ChitininN. Placebo: Dose and conposition unknown. Timing: capsules advised to be taken before main meals. Co‐interventions: 6 month programme that consisted of 2‐hour group meetings with a physician, psychologist and dietetian every 2 weeks. A low calorie diet (1000 kCal/d), physical activity and behaviour modification were recommended. | |
| Outcomes | Body weight, BMI, body fat, fat free mass, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, glucose, systolic blood pressure, diastolic blood pressure, mean blood pressure. Measured at baseline, 3 months and 6 months. | |
| Notes | Published in the Polish Medical Journal and translated by third party. Results quoted in (English) abstract are per‐protocol results but if analysis includes all participants at baseline different results are obtained (different direction of effect). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
HDL: High density lipoprotein LDL: Low density lipoprotein AST: Aspartate aminotransferase ALT: Alanine aminotransferase Gamma‐GT: gamma‐glutamyl‐transpeptidase BMI: Body mass index SF‐36: Short Form 36‐question Health Survey
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abelin 1994 | RCT of chitosan v placebo (n = 20). Duration of main trial was only 2 weeks although some volunteers continued to take treatment beyond this time. |
| Aranda 2002 | A crossover trial comparing chitosan formulation (chitosan plus psyllium husk seeds) v placebo on faecal fat excretion. Trial duration was only 24 days. |
| Ausar 2003 | RCT of bread containing chitosan v standard bread (n = 18). Main inclusion criteria were diabetes mellitus and hyperlipidaemia i.e. participants were not necessarily overweight and therefore did not meet inclusion criteria for this review. |
| Bokura | RCT of chitosan v placebo (n = 90). Main inclusion criteria were mild to moderate hyperlipidaemia i.e. participants were not necessarily overweight and therefore did not meet inclusion criteria for this review. |
| Ernst 2001 | Commentary on RCT conducted by Ho 2001. |
| Gades 2002 | A before and after study evaluating the effect of chitosan supplementation on fat absorption (n = 7). Not a randomised controlled trial. Study duration was only 12 days. |
| Gades 2003 | A before and after study evaluating the effect of chitosan supplementation on fat absorption (n = 15). Not a randomised controlled trial. Study duration was only 12 days. |
| Gades 2005 | A before and after study evaluating the effect of chitosan supplementation on fat absorption (n=24). Not a randomised controlled trial. Study duration only 12 days. |
| Gallaher 2002 | A before and after study evaluating the effect of a glucomannan and chitosan supplement on blood lipids and faecal fat excretion (n = 21). Not a randomised controlled trial. |
| Guerciolini 2001 | A crossover trial comparing effects of chitosan and orlistat on faecal fat excretion. Trial duration was only 21 days. |
| Guha 2005 | A 2‐arm parallel group trial in patients with congestive heart failure comparing effects of chitosan and atorvastation compared to placebo and atorvastatin. Unable to determine whether participants were overweight and therefore did not meet inclusion criteria for review. |
| Heldman 1997 | A crossover trial comparing effects of chitosan v placebo on fat absorption (n = 12). Trial duration was only 24 hours |
| Jing 1997 | A study of the effects of chitosan in patients with renal failure (n = 80). Participants had renal failure (serious medical condition) and therefore did not meet inclusion criteria. |
| Kaats 1998 | A 4‐arm RCT comparing biotrol (trace elements) and biozan (chitosan) with placebo and control groups (n = 200). Study included participants with BMI < 25 (normal weight) and therefore did not meet the inclusion criteria for this review. |
| Maezaki 1993 | A before and after study evaluating the effects of chitosan in the form of biscuits (n = 8). Not a randomised controlled trial. |
| Muzzarelli 1997 | A review. |
| Muzzarelli 1999 | A review. |
| Muzzarelli 2000 | A review. |
| Nopchinda 2001 | A before and after study evaluating effect of chitosan on weight, blood pressure, lipids and fat‐soluble vitamins (n = 23). Not a randomised controlled trial. |
| Otto 2000 | Commentary on RCT conducted by Pittler 1999. |
| Pepping 2003 | A review |
| Pittler 2002 | Commentary on RCT conducted by Schiller 2001. |
| Tai 2000 | RCT of chitosan v placebo (n = 40). Main inclusion criteria were type 2 diabetes mellitus and hyperlipidaemia i.e. participants were not necessarily overweight and therefore did not meet inclusion criteria for this review. |
| Terada 1995 | A before and after study of effects of chitosan on faecal microbiota and metabolites (n = 8). Not a randomised controlled trial. |
| Thom 2000 | Open consumer study of effect of chitosan on self‐reported body weight and tolerability (n = 332). Not a randomised controlled trial. |
| Wadstein 2000 | Same consumer study as that reported by Thom 2000 |
| Yihua 1997 | In vitro study. Not a randomised controlled trial. |
Contributions of authors
CLIONA NI MHURCHU: Design and coordination of first review, study selection, data extraction, data analysis, drafting of review, interpretation of data, data presentation.
CHRISTEL DUNSHEA‐MOOIJ: Protocol development, literature searching, study selection, data collection, data extraction, co‐drafting of review.
DERRICK BENNETT: Statistics, data analysis, data presentation, co‐drafting of review.
ANTHONY RODGERS: Co‐drafting of protocol/review, interpretation of data.
ANDREW JULL: Coordination of updated review, study selection, data extraction, data analysis, redrafting review.
Sources of support
Internal sources
Clinical Trials Research Unit (CTRU), New Zealand.
External sources
No sources of support supplied
Declarations of interest
CNM, DB and AR were investigators on one trial included in the review. The majority of the funding for this trial was obtained from the Health Research Council of New Zealand. Study treatments and some additional funding were obtained from Healtheries of New Zealand Ltd (manufacturers of chitosan). No external funding was received for undertaking this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Colombo 1996 {published data only}
- Colombo P, Sciutto AM. Nutritional aspects of chitosan employment in hypocaloric diet [Aspetti nutrizionali dell'impiego dell'integratore dietetico chitosano nelle diete ipocaloriche]. Acta Toxicologica et Therapeutica 1996;17(4):287‐302. [Google Scholar]
Girola 1996 {published data only}
- Girola M, Bernardi M, Contos S, Tripodi S, Ventura P, Guarino C, Marletta M. Dose effect in lipid‐lowering activity of a new dietary integrator (chitosan, Garcinia cambogia extract and chrome) [Effetto della dose sull'attivita antilipemica di un nuovo integratore dietetico (chitosano, estratto di Garcinia cambogia e cromo)]. Acta Toxicologica et Therapeutica 1996;17(1):25‐40. [Google Scholar]
Giustina 1995 {published data only}
- Giustina A, Ventura P. Weight‐reducing regimes in obese subjects: effects of a new dietary fiber integrator [Programmi per la riduzione del peso in pazienti obesi: effetti di un nuovo integratore di fibra dietetica]. Acta Toxicologica et Therapeutica 1995;16(4):199‐214. [Google Scholar]
- Ventura P. Lipid lowering activity of chitosan a new dietary integrator. Chitin Enzymology 1996;2:55‐62. [Google Scholar]
Ho 2001 {published and unpublished data}
- Ho SC, Tai ES, Eng PHK, Tan CE, Fok ACK. In the absence of dietary surviellance, chitosan does not reduce plasma lipids or obesity in hypercholesterolaemic obese asian subject. Singapore Medical Journal 2001;42(1):6‐10. [PubMed] [Google Scholar]
Kaats 2006 {published and unpublished data}
- Kaats G, Michalek JE, Preuss HG. Evaluating efficacy of a chitosan product using a double‐blinded, placebo‐controlled protocol. Journal of the American College of Nutrition 2006;25(5):389‐394. [DOI] [PubMed] [Google Scholar]
Macchi 1996 {published data only}
- Macchi G. A new approach to the treatment of obesity: chitosan's effects on body weight reduction and plasma cholesterol's levels [Un nuovo approccio al trattamento dell'obesita: effetti del chitosano sulla riduzione del peso corporeo e sulla colesterolemia]. Acta Toxicologica et Therapeutica 1996;17(4):301‐20. [Google Scholar]
Ni Mhurchu 2004 {published data only}
- Ni Mhurchu C, Poppitt SD, McGill A‐T, Leahy FE, Bennett DA, Lin RB, Ormrod D, Ward L, Strik C, Rodgers A, on behalf of the ECHO Collaboration. The effect of the dietary supplement, chitosan, on body weight: a randomised controlled trial in 250 overweight and obese adults. International Journal of Obesity 2004;28(9):1149‐1156. [DOI] [PubMed] [Google Scholar]
- Ni Mhurchu C, on behalf of the ECHO Collaboration. The effect of the dietary supplement, chitosan, on body weight: a randomised controlled trial in 250 overweight and obese adults. Proceedings of the Nutrition Society of New Zealand. 2003; Vol. 28:143. [DOI] [PubMed]
Pittler 1999 {published data only}
- Pittler MH, Abbot NC, Harkness EF, Ernst E. Randomized, double‐blind trial of chitosan for body weight reduction. European Journal of Clinical Nutrition 1999;53:379‐81. [DOI] [PubMed] [Google Scholar]
Schiller 2001 {published data only}
- Schiller RN, Barrager E, Schauss AG, Nichols EJ. A randomized, double‐blind, placebo‐controlled study examining the effects of a rapidly soluble chitosan dietary supplement on weight loss and body composition in overweight and mildly obese individuals. Journal of the American Nutraceutical Association 2001;4:42‐9. [Google Scholar]
Sciutto 1995 {published data only}
- Sciutto AM, Colombo P. Lipid‐lowering effect of chitosan dietary integrator and hypocaloric diet in obese subjects [Effetto antilipemico dell'integratore dietetico Chitosano e della dieta ipocalorica in soggetti obesi]. Acta Toxicologica et Therapeutica 1995;16(4):215‐30. [Google Scholar]
Veneroni 1996 {published data only}
- Veneroni G, Veneroni F, Contos S, Tripodi S, Bernardi M, Guarino C, Marletta M. Effect of a new chitosan dietary integrator and hypocaloric diet on hyperlipidemia and overweight in obese patients [Effetto di un nuovo integratore dietetico a base di chitosano e della dieta ipocalorica sulla dislipidemia ed il sovrappeso in pazienti obesi]. Acta Toxicologica et Therapeutica 1996;17(1):53‐70. [Google Scholar]
- Veneroni G, Veneroni F, Contos S, Tripodi S, Bernardi M, Guarino C, Marletta M. Effect of a new chitosan on hyperlipidemia and overweight in obese patients. Chitin Enzymology 1996;2:63‐7. [Google Scholar]
Williams 1998 {published data only}
- Williams AR. A double‐blind, placebo‐controlled evaluation of the effects of RW94 on the body weight of both overweight and obese healthy volunteers. Current Medical Research and Opinion 1998;14(4):243. [DOI] [PubMed] [Google Scholar]
Woodgate 2003 {published data only}
- Woodgate D, Conquer J. Double‐blind study evaluating the effects of a proprietary blend of glucomannan and chitosan on weight loss in overweight adults. Journal of Parenteral and Enteral Nutrition. 2001; Vol. 25, issue 1:S10.
- Woodgate DE, Conquer JA. Effects of a stimulant‐free dietary supplement on body weight and fat loss in obese adults: a six‐week exploratory study. Current Therapeutic Research 2003;64(4):248‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wuolijoki 1999 {published data only}
- Lehtimaki T, Wuolijoki E, Hirvela T, Lehtinen S, Ylitalo R. Decrease in serum LDL cholesterol with microcrystalline chitosan in healthy subjects. Atherosclerosis. 1999; Vol. 144, issue 1:189.
- Wuolijoki E, Hirvela T, Ylitalo P. Decrease in serum LDL cholesterol with microcrystalline chitosan. Methods and Findings in Experimental Clinical Pharmacology 1999;21(5):357‐61. [DOI] [PubMed] [Google Scholar]
Zahorska‐Markiewicz {published data only}
- Zahorska‐Markiewicz B, Krotkiewski M, Olszanecka‐Glinianowicz M, Zurakowski A. Effect of chitosan in complex management of obesity [Ocena Zastosowania chitosanu w kompleksowym leczeniu otylosci]. Polski Merkuriusz Lekarski 2002;13(74):129‐32. [PubMed] [Google Scholar]
References to studies excluded from this review
Abelin 1994 {published and unpublished data}
- Abelin J, Lassus A. L‐112 Biopolymer‐fat binder as a weight reducer in patients with moderate obesity. Medical Research Report 1994.
Aranda 2002 {published data only}
- Aranda JB, Contreras F, Bagchi D, Preuss HG. Efficacy of a novel chitosan formulation on fecal fat excretion: a double‐blind, crossover, placebo‐controlled study. Journal of Medicine 2002;33(1‐4):209‐25. [PubMed] [Google Scholar]
Ausar 2003 {published data only}
- Ausar SF, Morcillo M, Leon AE, Ribotta PD, Masih R, Vilaro Mainero M, Amigone JL, Rubin G, Lescano C, Castagna LF, Beltramo DM, Diaz G, Bianco ID. Improvement of HDL‐ and LDL‐cholesterol levels in diabetic subjects by feeding bread containing chitosan. Journal of Medicinal Food 2003;6(4):397‐9. [DOI] [PubMed] [Google Scholar]
Bokura {published data only}
- Bokura H, Kobayashi S. Chitosan decreases total cholesterol in women: a randomized, double‐blind, placebo‐controlled trial. European Journal of Clinical Nutrition 2003;57:721‐5. [DOI] [PubMed] [Google Scholar]
Ernst 2001 {published data only}
- Ernst E. Another negative chitosan trial. Focus on Alternative & Complementary therapies 2001;6(3):203‐4. [Google Scholar]
Gades 2002 {published data only}
- Gades MD, Stern JS. Chitosan supplementation does not affect fat absorption in healthy males fed a high‐fat diet, a pilot study. International Journal of Obesity 2002;26:119‐22. [DOI] [PubMed] [Google Scholar]
Gades 2003 {published data only}
- Gades MD, Stern JS. Chitosan supplementation and fecal fat excretion in men. Obesity Research 2003;11:683‐688. [DOI] [PubMed] [Google Scholar]
Gades 2005 {published data only}
- Gades MD, Stern JS. Chitosan supplementation and fat absorption in men and women. Journal of the American Dietetic Association 2005;105:72‐77. [DOI] [PubMed] [Google Scholar]
Gallaher 2002 {published data only}
- Gallaher DD, Gallaher CM, Mahrt GJ, Carr TP, Hollingshead, CH Hesslink R Jr, Wise J. A Glucomannan and Chitosan Fiber Supplement Decreases Plasma Cholesterol and Increses Cholesterol Excretion in Overweight Normocholesterolemic Humans. Journal of the American College of Nutrition 2002;21(5):428‐33. [DOI] [PubMed] [Google Scholar]
Guerciolini 2001 {published data only}
- Guerciolini R, Radu‐Radulescu L, Boldrin M, Dallas J, Moore R. Comparative Evaluation of Fecal Fat Excretion Induced by Orlistat and Chitosan. Obesity Research 2001;9(6):364‐7. [DOI] [PubMed] [Google Scholar]
Guha 2005 {published data only}
- Guha S, Pal SK, Chatterjee N, Sarkar G, Pal S, Guha S, Basu AK, Banerjee R. Effect of chitosan in lipid levels when administered concurrently with atorvastatin ‐ a placebo controlled study. Journal of the Indian Medical Association 2005;103(8):419‐420. [PubMed] [Google Scholar]
Heldman 1997 {unpublished data only}
- Heldman E, Gudmundsson S, Lilja B, Wadstein J. Effect of absorbitol L112 on animal fat resorption in humans. Report: Malmo University Hospital 7:8‐23 1997.
Jing 1997 {published data only}
- Jing SB, Li L, Daxi JI, Takiguchi Y, Yamaguchi T. Effect of Chitosan on Renal Function in Patients with Chronic Renal Failure. Journal of Pharmacy and Pharmacology 1997;49:721‐3. [DOI] [PubMed] [Google Scholar]
Kaats 1998 {published data only}
- Kaats GR, Keith SA, Pullin D, Squires W, Murrieta TG. An Evaluation of the Safety and Efficacy of Biotrol, a Propricary Blend of Trace Minerals, and Biozan, a Chitosan Fiber Product. Health and Medical Research Council 1998.
Maezaki 1993 {published data only}
- Maezaki Y, Tsuji K, Nakagawa Y, Kawai Y, Akimoto M, Tsugita T, Takekawa W, Terada A, Terada A, Hara H, Mitsuoka T. Hypocholesterolemic Effect of Chitosan in Adult Males. Biosci Biotech Biochemstry 1993;57(9):1439‐44. [Google Scholar]
Muzzarelli 1997 {published data only}
- Muzzarelli RAA. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cellular Molucular Life Science 1997;53:131‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muzzarelli 1999 {published data only}
- Muzzarelli RAA. Clinical and biochemical evaluation of chitosan for hypercholesterolemia and overweight control. Chitin and Chitinases 1999;87:293‐304. [DOI] [PubMed] [Google Scholar]
Muzzarelli 2000 {published data only}
- Muzzarelli RAA. Recent results in the oral administration of chitosan. Advances in Chitin Science 2000;4:212‐6. [Google Scholar]
Nopchinda 2001 {published data only}
- Nopchinda S, Kongsomboonvedth S, Komindr S, Puchaiwatanonon O, Taechagham S. Effect of Chitosan and Vitamin C on Weight, Lipid profile and Fat Soluble Vitamin. Annals of Nutrition & Metabolism 2001;45(suppl 1):136. [Google Scholar]
Otto 2000 {published data only}
- Otto C. Aloe ferox and chitosan in diabetes mellitus [Aloe ferox und chitosan bei diabetes mellitus]. Tagliche Praxis 2000;41(3):661‐2. [Google Scholar]
Pepping 2003 {published data only}
- Pepping J. Chitosan for weight loss and cholesterol management. American Journal of Health‐System Pharmacy 2003;60:1310‐1316. [DOI] [PubMed] [Google Scholar]
Pittler 2002 {published data only}
- Pittler MH. Chitosan, an effective means to reduce body‐weight?. Focus on Alternative & Complementary Therapies 2002;7(1):25‐6. [Google Scholar]
Tai 2000 {published data only}
Terada 1995 {published data only}
- Terada A, Hara H, Sato D, Higashi T, Nakayama S, Tsuji K, Sakamoto K, Ishioka E, Maezaki Y, Tsugita T, Takekawa T, Mitsuoka T. Effect of dietary chitosan on faecal microbiota and faecal metabolites of humans. Microbial Ecology in Health & Disease 1995;8(1):15‐21. [Google Scholar]
Thom 2000 {published data only}
- Thom E, Wadstein J. Chitosan in weight reduction. Advances in Chitin Science 2000;4:229‐32. [Google Scholar]
Wadstein 2000 {published data only}
- Wadstein J, Thom E, Heldman E, Gudmunsson S, Lilja B. Biopolymer L112, a chitosan with fat binding properties and potential as a weight reducing agent: a review of in vitro and in vivo experiments. Chitosan per os ‐ from dietary supplement to drug carrier. In: Ricardo A.A.Muzzarelli (ed.) Atec Edizioni, Grottammare, 2000:65‐76. [Google Scholar]
Yihua 1997 {published data only}
- Yihua Y, Binglin HE. A new low density lipoprotein (LDL) absorbent. Artificial Cells, Blood Substitutes and Immobilization Biotechnology 25;5:445‐50. [DOI] [PubMed] [Google Scholar]
Additional references
Allison 2001
- Allison DB, Fontaine KR, Heshka S, Mentore JL, Heymsfield SB. Alternative treatments for weight loss: a critical review. Critical Reviews in Food Science and Nutrition 2001;41(1):1‐28. [DOI] [PubMed] [Google Scholar]
Aronne 1998
- Aronne LJ. Modern medical management of obesity: the role of pharmaceutical intervention. Journal of the American Dietetic Association 1998;98:S23‐6. [DOI] [PubMed] [Google Scholar]
Bergstrom 2001
- Bergstrom A, Pisani P, Tenet V, et al. Overweight as an avoidable cause of cancer in Europe. International Journal of Cancer. 2001;91:421‐30. [DOI] [PubMed] [Google Scholar]
Blanck 2001
- Blanck HM, Khan LK, Serdula MK. Use of nonprescription weight loss products. Results from a multistate survey. Journal of the American Medical Association 2001;286:930‐935. [DOI] [PubMed] [Google Scholar]
Calle 2003
- Calle EE, Rodriguez C, Walker‐Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. New England Journal of Medicine 2003;348(17):1625‐1638. [DOI] [PubMed] [Google Scholar]
Carey 1997
- Carey VJ, Walters EE, Colditz GA, et al. Body fat distribution and risk of non‐insulin‐dependent diabetes mellitus in women. The Nurses' Health Study. American Journal of Epidemiology 1997;145:614‐9. [DOI] [PubMed] [Google Scholar]
Chan 1994
- Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care 1994;17(9):961‐9. [DOI] [PubMed] [Google Scholar]
Clarke 2003
- Clarke M, Oxman AD, eds. Cochrane Reviewers' Handbook 4.2.0 [updated March 2003]. Oxford: Update Software, In: The Cochrane Library, Issue 2, 2003. [Google Scholar]
Colditz 1995
- Colditz GA, Willett WC, Rotnitzky A, Manson JE. Weight gain as a risk factor for clinical diabetes mellitus in women. Annals of Internal Medicine 1995;122:481‐6. [DOI] [PubMed] [Google Scholar]
Denke 1993
- Denke MA, Sempos CT, Grundy SM. Excess body weight. An underrecognized contributor to high blood cholesterol levels in white American men. Archives of Internal Medicine 1993;153:1093‐1103. [DOI] [PubMed] [Google Scholar]
Denke 1994
- Denke MA, Sempos CT, Grundy SM. Excess body weight. An underrecognized contributor to dyslipidemia in white American women. Archives of Internal Medicine 1994;154:401‐10. [DOI] [PubMed] [Google Scholar]
Deuchi 1995
- Deuchi K, Kanauchi O, Imasato Y, Kobayashi E. Effect of the viscosity or deacetylation degree of chitosan on fecal fat excreted from rats fed on a high fat diet. Bioscience Biotechnology and Biochemistry 1995;59:781‐5. [DOI] [PubMed] [Google Scholar]
Douketis 1999
- Douketis JD, Feightner JW, Attia J, Feldman WF, with the Canadian Task Force on Preventive Health Care. Periodic health examination, 1999 update: 1. Detection, prevention and treatment of obesity. Canadian Medical Association Journal. 1999;160(4):513‐25. [PMC free article] [PubMed] [Google Scholar]
Dunn 1997
- Dunn LB, Damesyn M, Moore AA, Reuben DB, Greendale GA. Does Estrogen Prevent Skin Aging?: Results From the First National Health and Nutrition Examination Survey (NHANES I). Archives of Dermatology 1997;133(3):339‐42. [DOI] [PubMed] [Google Scholar]
Dyer 1994
- Dyer AR, Elliott P, Shipley M, Stamler R, Stamler J, on behalf of the INTERSALT Cooperative Research Group. Body mass index and associations of sodium and potassium with blood pressure in INTERSALT. Hypertension 1994;23(1):729‐36. [DOI] [PubMed] [Google Scholar]
Egger 1999
- Egger G, Cameron‐Smith D, Stanton R. The effectiveness of popular, non‐prescription weight loss supplements. Medical Journal of Australia 1999;171:604‐8. [DOI] [PubMed] [Google Scholar]
Ernst 1998
- Ernst E, Pittler MH. Chitosan as a treatment for body weight reduction? A meta‐analysis. Perfusion 1998;11:461‐5. [Google Scholar]
Ezzati 2002
- Ezzati MLA, Rodgers A, Vander Hoorn S, Murray CJL, and the Comparative Risk Assessment Collaborative Group. Selected major risk factors and global and regional burden of disease. The Lancet 2002;360:1347‐60. [DOI] [PubMed] [Google Scholar]
Felson 1992
- Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Annals of Internal Medicine 1992;116:535‐9. [DOI] [PubMed] [Google Scholar]
Flegal 2002
- Flegal KM, Carroll MD, Ogden LG, et al. Prevalence and trends in obesity among US adults. Journal of the American Medical Association 2002;288:1723‐7. [DOI] [PubMed] [Google Scholar]
Fontaine 1999
- Fontaine KR, Barofsky I, Andersen RE, et al. Impact of weight loss on health‐related quality of life. Quality of Life Research 1999;8:275‐7. [DOI] [PubMed] [Google Scholar]
Fontaine 2001
- Fontaine KR, Barofsky I. Obesity and health‐related quality of life.. Obesity Reviews. 2001;2:173‐82. [DOI] [PubMed] [Google Scholar]
Glenny 1997
- Glenny AM, O'Meara S, Melville A, Sheldon TA, Wilson C. The treatment and prevention of obesity: a systematic review of the literature. International Journal of Obesity 1997;21:715‐37. [DOI] [PubMed] [Google Scholar]
Grinker 2000
- Grinker JA, Tucker KL, Vokonas PS, Rush D. Changes in patterns of fatness in adult men in relation to serum indices of cardiovascular risk: the normative aging study. International Journal of Obesity 2000;24:1369‐1378. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Kirschner 1982
- Kirschner MA, Schneider G, Erter NH, Worton E. Obesity, androgens, oestrogens, and cancer risk. Cancer Research 1982;42(Suppl 8):3281S‐5S. [PubMed] [Google Scholar]
Must 1999
- Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. Journal of the American Medical Association 1999;282(16):1523‐9. [DOI] [PubMed] [Google Scholar]
Nagyvary 1979
- Nagyvary JJ, Falk JD, Hill ML, Schmidt ML, Wilkins AK, Brdbury EL. The hypolipidemic activity of chitosan and other polysaccharides in rats. Nutrition Reports International 1979;30(5):677‐84. [Google Scholar]
Naimark 1960
- Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. Journal of Applied Physiology 1960;15:377‐82. [DOI] [PubMed] [Google Scholar]
NAO 2001
- National Audit Office. Tackling obesity in England. London: The Stationery Office, 2001. [Google Scholar]
Ormrod 1998
- Ormrod DJ, Holmes CC, Miller TE. Dietary chitosan inhibits hypercholesterolaemia and atherogenesis in the apolipoprotein E‐deficient mouse model of atherosclerosis.. Atherosclerosis 1998;138:329‐34. [DOI] [PubMed] [Google Scholar]
Poobalan 2004
- Poobalan A, Aucott L, Smith WCS, Avenell A, Jung R, Broom J, Grant AM. Effects of weight loss in overweight/obese individuals and long‐term lipid outcomes ‐ a systematic review. Obesity Reviews 2004;5(1):43. [DOI] [PubMed] [Google Scholar]
Rimm 1995
- Rimm EB, Stampfer MJ, Giovanucci E, et al. Body size and fat distribution as predictors of coronary heart disease among middle‐aged and older US men. American Journal of Epidemiology 1995;141(12):1117‐27. [DOI] [PubMed] [Google Scholar]
Shepherd 1997
- Shepherd R, Reader S, Falshaw A. Chitosan functional properties. Glycoconjugate Journal 1997;14:535‐542. [DOI] [PubMed] [Google Scholar]
Sjostrom 1998
- Sjostrom L, Rissanen A, Andersen T. Randomised placebo‐controlled trials of Orlistat for weight loss and prevention of weight regain in obese patients. The Lancet 1998;352:167‐73. [DOI] [PubMed] [Google Scholar]
Sjostrom 1999
- Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS intervention study. Obesity Research 1999;7(5):477‐84. [DOI] [PubMed] [Google Scholar]
Sugano 1980
- Sugano M, Fujikawa T, Hiratsuji Y, Nakashima K, Fukuda N, Hasegawa Y. A novel use of chitosan as a hypocholesterolemic agent in rats. American Journal of Clinical Nutrition 1980;33:787‐93. [DOI] [PubMed] [Google Scholar]
Whitlock 2002
- Whitlock G, Lewington S, Ni Mhurchu C. Coronary heart disease and body mass index: a systematic review of the evidence from large prospective cohort studies. Seminars in Vascular Medicine 2002;2(4):369‐81. [DOI] [PubMed] [Google Scholar]
WHO 2000
- World Health Organization. Obesity: preventing and managing the global epidemic. Vol. 894, Geneva: World Health Organisation, 2000. [PubMed] [Google Scholar]
WHO, 1995
- World Health Organization. Physical status: the use and interpetation of anthropometry. Vol. 854, Geneva: World Health Organization, 1995. [Google Scholar]
Willett 1995
- Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the 'normal' weight range. Journal of the American Medical Association 1995;273(6):461‐5. [DOI] [PubMed] [Google Scholar]
Williamson 1995
- Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in never‐smoking overweight US white women aged 40‐64 years. American Journal of Epidemiology. 1995;141:1128‐41. [DOI] [PubMed] [Google Scholar]
Williamson 1999
- Williamson DF, Pamuk E, Thun M, Flanders D, Byers T, Heath C. Prospective study of intentional weight loss and mortality in overweight white men aged 40‐64 years. American Journal of Epidemiology 1999;149:491‐503. [DOI] [PubMed] [Google Scholar]
Yanovski 2002
- Yanovski SZ, Yanovski JA. Obesity. New England Journal of Medicine 2002;346(8):591‐602. [DOI] [PubMed] [Google Scholar]
Zacour 1992
- Zacour AC, Silva ME, Cecon PR, Bambirra EA, Vieira EC. Effect of dietary chiton on cholesterol absorption and metabolism in rats. Journal of Nutritional Science and Vitaminology 1992;38:609‐13. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ni Mhurchu 2005
- Ni Mhurchu C, Dunshea‐Mooij C, Bennett D, Rodgers A. Effect of chitosan on weight loss in overweight and obese individuals: a systematic review of randomized controlled trials. Obesity Reviews 2005;6(1):35‐42. [DOI] [PubMed] [Google Scholar]


