Abstract
Yeast translation initiation factor 3 contains five core subunits (known as TIF32, PRT1, NIP1, TIF34 and TIF35) and a less tightly associated component known as HCR1. We found that a stable subcomplex of His8-PRT1, NIP1 and TIF32 (PN2 subcomplex) could be affinity purified from a strain overexpressing these eIF3 subunits. eIF5, eIF1 and HCR1 co-purified with this subcomplex, but not with distinct His8-PRT1– TIF34–TIF35 (P45) or His8-PRT1–TIF32 (P2) sub complexes. His8-PRT1 and NIP1 did not form a stable binary subcomplex. These results provide in vivo evidence that TIF32 bridges PRT1 and NIP1, and that eIFs 1 and 5 bind to NIP1, in native eIF3. Heat-treated prt1-1 extracts are defective for Met-tRNAiMet binding to 40S subunits, and we also observed defective 40S binding of mRNA, eIFs 1 and 5 and eIF3 itself in these extracts. We could rescue 40S binding of Met- tRNAiMet and mRNA, and translation of luciferase mRNA, in a prt1-1 extract almost as well with purified PN2 subcomplex as with five-subunit eIF3, whereas the P45 subcomplex was nearly inactive. Thus, several key functions of eIF3 can be carried out by the PRT1–TIF32–NIP1 subcomplex.
Keywords: eIF3/initiation/ribosome/translation/yeast
Introduction
The initiation of protein synthesis in eukaryotic cells is dependent on multiple eukaryotic initiation factors (eIFs) that stimulate binding of mRNA and methionyl-initiator tRNA (Met-tRNAiMet) to the 40S ribosome. In mammals, eIF3 is the most complex of these factors, containing 11 different subunits, and participates in multiple steps of the initiation pathway. Mammalian eIF3 forms a complex with the 40S subunit and stimulates binding of the eIF2–GTP–Met-tRNAiMet ternary complex (TC) to produce the 43S pre-initiation complex. Binding of mRNA to the 43S complex, producing the 48S complex, is stimulated by the m7G cap-binding initiation factor eIF4F and by eIF3. The 48S complex locates the start codon in a process known as scanning, and AUG recognition triggers hydrolysis of the GTP bound to eIF2 in a reaction stimulated by eIF5. This leads to dissociation of eIF2–GDP and other eIFs from the 40S subunit, and subsequent joining of the 60S subunit to form an 80S initiation complex (reviewed in Hershey and Merrick, 2000; Hinnebusch, 2000).
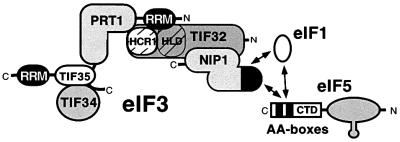
Yeast eIF3 contains five stoichiometric core subunits (known as TIF32, NIP1, PRT1, TIF34 and TIF35) that are orthologs of human eIF3 subunits p170, p116, p110, p36 and p44, respectively. The yeast factor was affinity purified and shown to restore high-level binding of Met-tRNAiMet to 40S ribosomes in a heat-treated prt1-1 mutant extract, showing that it possesses a key activity of eIF3 (Phan et al., 1998). Interactions among the five core subunits have been studied by yeast two-hybrid analysis and in vitro binding assays (Asano et al., 1998; Valášek et al., 2001). These studies suggest that PRT1 is central to the complex, with the two smallest core subunits (TIF34/TIF35) binding to its extreme C-terminus (and also to one another), and the largest subunit (TIF32) binding to the RNA recognition motif (RRM) at the N-terminus of PRT1. NIP1 binds to TIF32 but not to PRT1 (Figure 1). Mutational analysis has shown that PRT1 and NIP1 are both required for eIF3-dependent TC binding to 40S subunits in yeast extracts (Feinberg et al., 1982; Phan et al., 1998); however, their molecular functions in this activity are not understood.
Fig. 1. Predicted interactions among yeast eIF3 subunits, eIF5, eIF1 and HCR1. Yeast eIF3 contains five core subunits (TIF32, PRT1, NIP1, TIF34 and TIF35) and a less tightly associated component known as HCR1. PRT1 and TIF35 contain RRMs, and TIF32 contains an HCR1-like domain (HLD). Interactions among these proteins detected by yeast two-hybrid or in vitro binding assays are depicted schematically as points of contact between the representative shapes. The CTD of eIF5 contains a conserved bipartite motif (AA-boxes) required for its interaction with eIF3–NIP1. NIP1 additionally interacts with eIF1, and eIF1 interacts with the eIF5-CTD in these binding assays. The N-terminal portion of eIF5 contains a zinc-finger motif depicted as a prong. (See text for references.)
Yeast eIF3 also contains a protein related to human eIF3 subunit p35, known as HCR1. Although HCR1 was not detected in affinity-purified eIF3 preparations (Phan et al., 1998), it interacted genetically with TIF32 and PRT1, bound in vitro to both of these eIF3 subunits (Figure 1), and co-immunoprecipitated with eIF3 subunits from cell extracts. In vitro, HCR1 interacted with RRM in PRT1 and with TIF32 (Valášek et al., 2001). The absence of HCR1 from purified eIF3 preparations seems to result from its substoichiometric level and weak interaction with the core eIF3 complex (Valášek et al., 1999, 2001).
Interestingly, affinity-purified yeast eIF3 contained nearly stoichiometric amounts of eIF5 (Phan et al., 1998). eIF1 (SUI1) is also physically associated with yeast eIF3 (Naranda et al., 1996), although this interaction is salt labile (Phan et al., 1998). Both eIF1 and eIF5 interacted with recombinant forms of the NIP1 subunit of yeast eIF3 (Figure 1) (Asano et al., 1998, 1999; Phan et al., 1998). The association of eIF5 with eIF3 has also been observed in mammals (Bandyopadhyay and Maitra, 1999), and both eIF5 and eIF1 interacted with the mammalian counterpart of yeast NIP1 (eIF3-p110) (Fletcher et al., 1999; Das and Maitra, 2000). eIF5 and eIF1 have been implicated in recognition of initiation codons during the scanning process (Pestova et al., 1998; Donahue, 2000), and the rate of eIF5-dependent GTP hydrolysis in the TC is an important determinant of the stringent selection of AUG as start codon (Huang et al., 1997).
Although eIF3 stimulates binding of the TC to 40S ribosomes, no direct interaction has been observed between eIF3 and eIF2 that might underlie this function of eIF3. Interestingly, the C-terminal domain (CTD) of yeast eIF5 can interact simultaneously with the β-subunit of eIF2 and eIF3–NIP1, and thereby promotes formation of a multifactor complex (MFC) containing eIFs 1, 3, 5 and the TC. A mutation in the eIF5-CTD that destabilizes the MFC impaired translation initiation in yeast, suggesting that physical coupling between eIF3 and eIF2 bridged by the eIF5-CTD is required for efficient TC binding or AUG recognition (Asano et al., 2000).
The function of eIF3 in stimulating mRNA binding to the 40S ribosome is poorly understood at the molecular level. Interactions between eIF3 and the eIF4G subunit of eIF4F are thought to be instrumental in this activity; however, a strong eIF3–eIF4G interaction has not been reported in yeast. Because binding of the TC is a prerequisite for mRNA binding to 40S ribosomes in vitro (Hinnebusch, 2000), eIF3 may also stimulate mRNA binding indirectly by promoting recruitment of the TC.
In this study, we investigated the contributions of different yeast eIF3 subunits to its interactions with other initiation factors and its activities in recruiting TC and mRNA to 40S ribosomes. We overexpressed different combinations of eIF3 subunits, including a polyhistidine-tagged version of PRT1 (His8-PRT1), and affinity purified the subcomplexes by nickel chelation chromatography. Analysis of these subcomplexes confirmed our protein linkage map for eIF3 subunits (Asano et al., 1998) and demonstrated a requirement for NIP1 in the association of eIFs 1, 5 and HCR1 with native eIF3 in vivo. When the subcomplexes were tested for the ability to rescue TC and mRNA binding in a yeast prt1-1 mutant extract, the results showed that a PRT1–TIF32–NIP1 subcomplex restored both activities almost as well as the five-subunit eIF3 complex did, while a PRT1–TIF34–TIF35 subcomplex was nearly inactive. Thus, the key biochemical activities of eIF3, and its ability to interact with eIFs 1, 5 and HCR1, reside in the PRT1–TIF32–NIP1 subcomplex.
Results
The prt1-1 mutation impairs mRNA binding to 40S subunits in vitro
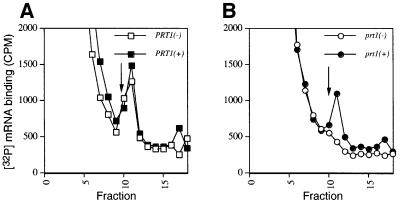
The prt1-1 mutation in eIF3 subunit PRT1 leads to temperature-sensitive growth and a severe reduction in translation initiation in vivo (Hartwell and McLaughlin, 1969). In vitro analysis of heat-inactivated prt1-1 extracts revealed a defect in binding of the TC to 40S ribosomal subunits that could be complemented with purified eIF3 complexes (Danaie et al., 1995; Phan et al., 1998). As mammalian eIF3 stimulates binding of mRNA and TC to 40S subunits (Hinnebusch, 2000), we investigated whether mRNA binding was temperature sensitive in the prt1-1 extract. Aliquots of PRT1 and prt1-1 extracts were heat treated at 37°C for 5 min and incubated with 32P-labeled MFA2 mRNA at 26°C in a reaction containing all components required for in vitro protein synthesis. The non-hydrolyzable GTP analogue guanylyl-(β,γ-imido)diphosphonate (GMPPNP) was included to promote accumulation of 48S complexes by preventing release of factors from 48S complexes and joining of 60S subunits. Reactions were resolved by sedimentation through sucrose gradients and the labeled mRNA in each fraction was determined. Whereas the wild-type extract showed substantial binding of [32P]MFA2 mRNA to 40S ribosomes (Figure 2A), the mutant extract was almost completely defective for binding (Figure 2B). Importantly, mRNA binding was rescued in the prt1-1 extract with purified eIF3 (Figure 2B), while addition of eIF3 did not stimulate mRNA binding in the wild-type extract (Figure 2A). Thus, yeast eIF3 is required for efficient mRNA binding to 40S ribosomes in cell extracts.
Fig. 2. Rescue of [32P]mRNA binding to the 40S ribosome in heat-inactivated prt1-1 extract by purified eIF3. Twenty microliters (∼300 µg) of extract prepared from PRT1 strain LPY200 (A) or prt1-1 strain H1676 (B) were heat treated at 37°C for 5 min and incubated in a 40 µl reaction containing ∼2 pmol of [32P]MFA2 mRNA (∼200 000 c.p.m.), 1× translation buffer, 1.2 mM GMPPNP, and either 1.5 pmol purified eIF3 (+) or buffer alone (–), at 26°C for 20 min. The reactions were stopped by adding formaldehyde to 0.3% and chilled on ice for 10 min before loading on a 7.5–30% sucrose gradient and centrifuging for 5 h at 41 000 r.p.m. in an SW41 rotor at 4°C. Fractions of 0.6 ml were collected using an ISCO gradient fraction collector and assayed for [32P]mRNA by mixing 0.2 ml of each fraction with 1 ml of water and 10 ml of scintillation fluid, and counting in a scintillation counter. The arrow in each panel marks the migration position of 40S ribosomes.
The prt1-1 mutation reduced binding of eIF3 and initiation factors 1, 2 and 5 to the 40S ribosome in cell extracts
It was possible that prt1-1 impaired binding of TC and mRNA to 40S ribosomes because the mutant eIF3 complexes dissociated following heat treatment. The levels of all five eIF3 subunits were indistinguishable between the prt1-1 and PRT1 extracts following heat treatment (see Figure 4, 5% input lane), providing no indication of accelerated degradation in the mutant extract. To examine directly the stability of the mutant complex, we inserted a polyhistidine tag at the C-terminus of the prt1-1 allele (producing prt1-1-His) to allow affinity purification of the mutant complexes. Extracts were prepared from isogenic strains LPY202 and LPY201 containing prt1-1-His or the corresponding tagged wild-type allele PRT1-His (Phan et al., 1998), respectively. Aliquots were incubated at 37°C for 5 min, or maintained at 25°C, and complexes containing His8-prt1-1 or His8- PRT1 were isolated on Ni2+-nitrilotriacetic acid (NTA)– silica resin. Western analysis revealed similar amounts of the mutant or wild-type PRT1 proteins and other eIF3 subunits (TIF32, NIP1, TIF34 and TIF35) in equivalent proportions of all four purified preparations. Additionally, similar amounts of eIF5 and eIF1 were associated with the mutant and wild-type eIF3 complexes with or without prior heat treatment of the extracts (Figure 3). We conclude that heat treatment of prt1-1 extracts did not lead to dissociation of eIF3 or disruption of its interactions with eIF5 and eIF1.
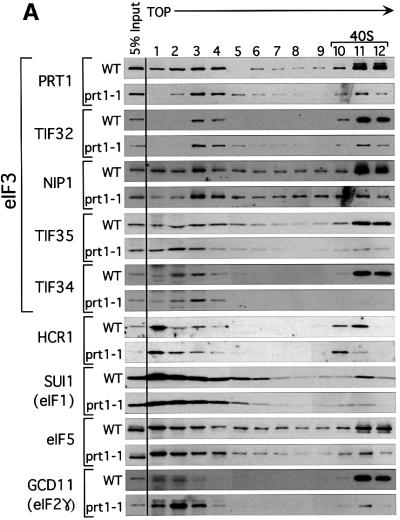
Fig. 4. Binding of eIF3, eIF2, eIF5 and eIF1 to 40S ribosomes is defective in the heat-treated prt1-1 extract. (A) Twenty microliters (∼300 µg) of translation extracts prepared from PRT1 strain LPY200 or prt1-1 strain H1676 were heat treated at 37°C for 5 min and incubated in a 40 µl reaction containing 1× translation buffer and 1.2 mM GMPPNP at 26°C for 20 min. The reactions were stopped by adding formaldehyde to 0.3% and incubating on ice for 10 min. A portion of each reaction (5%) was removed (input samples) and the remainder was separated on a 7.5–30% sucrose gradient as described in Figure 2. Fractions (0.6 ml) were collected and precipitated with 1.0 ml of ethanol at –20°C. The precipitates were washed once with ethanol, dried and resuspended in 50 µl of loading buffer, and separated by SDS–PAGE using 4–20% gradient gels. The separated proteins were subjected to immunoblot analysis using antibodies against the proteins indicated on the left. Antibodies were used at the same dilutions described in Figure 3, with the addition of antibodies against HCR1 (1:500) (Valášek et al., 2001) and GCD11 (1:10000). The position of 40S ribosomes in the gradients is indicated over fractions 10–12. (B) Heat-treated PRT1 and prt1-1 extracts were analyzed as in (A) except that 4.5 pmol of highly purified eIF3 (+) or buffer alone (–) were added to each reaction, as indicated on the right, prior to incubation at 26°C for 20 min. The reactions were stopped and analyzed as described in (A).
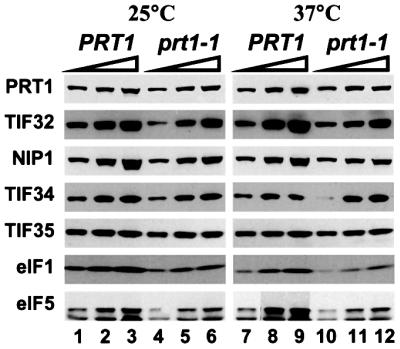
Fig. 3. Ni2+ affinity purification of intact eIF3 complexes containing eIF1 and eIF5 from heat-treated extract containing His8-prt1-1. Translation extracts were prepared from strains LPY201 (PRT1-His) and LPY202 (prt1-1-His) grown in YPD medium (Sherman et al., 1974) at 26°C to an OD600 of 1.0. The extracts were supplemented with imidazole to a final concentration of 20 mM, incubated at 25 or 37°C for 5 min, as indicated, and subjected to Ni2+ chelation chromatography using buffer A (see Materials and methods). Equivalent aliquots (2.5, 5 and 10 µl) of the Ni2+-NTA–silica eluates were separated by SDS–PAGE using 4–20% gels and subjected to immunoblot analysis using rabbit polyclonal antibodies against the proteins shown on the left, at the following dilutions: PRT1, 1:3000; TIF32, 1:3000; NIP1, 1:1000; TIF34, 1:500; TIF35, 1:5000; eIF5, 1:10000; SUI1/eIF1, 1:1000. Immune complexes were detected by chemiluminescence (ECL™, Amersham Pharmacia Biotech) using horseradish peroxidase-conjugated secondary antibodies (Amersham Pharmacia Biotech).
To determine whether prt1-1 impaired binding of eIF3 to 40S subunits, aliquots of PRT1 and prt1-1 extracts were heated at 37°C for 5 min and added to translation reaction mixtures containing GMPPNP, as described above. After incubating at 26°C, the reactions were separated on sucrose gradients and the fractions were analyzed by western blotting. As expected, large proportions of all five core eIF3 subunits in the wild-type extract co-sedimented with small ribosomal subunits in the 40–48S region of the gradient (Figure 4A, WT, fractions 10–12 versus 1–4). In the prt1-1 extract, co-sedimention of eIF3 core subunits with 40S subunits was greatly reduced. As we did not see a commensurate increase in the amounts of unbound eIF3 subunits in fractions 1–4 for the mutant extract, it appears that a large proportion of the mutant complexes were degraded during centrifugation. Nevertheless, prt1-1 clearly reduced the absolute amounts and proportion of eIF3 subunits that co-sedimented with 40S ribosomes following heat treatment of the extract.
Substantial fractions of eIF1, eIF5 and eIF2γ also co-sedimented with 40S ribosomes in the PRT1 extract, and this behavior was impaired by heat treatment of the prt1-1 extract (Figure 4A). These data suggest that eIF3 promotes binding of all these factors to 40S subunits, dependent on PRT1. The fact that eIF2γ binding to 40S subunits was reduced by prt1-1 is in keeping with previous observations that binding of initiator tRNAMet is impaired in heat-treated prt1-1 extracts (Danaie et al., 1995; Phan et al., 1998). As noted above, eIF1 and eIF5 co-purified with eIF3, and both factors interacted with the NIP1 subunit of eIF3. The results in Figure 4A confirm that physical association of eIF1 and eIF5 with eIF3 stimulates incorporation of these factors into 43–48S initiation complexes. Thus, the prt1-1 mutation impairs 40S binding by all components of the MFC (Asano et al., 2000) in heat-treated extracts.
The effect of prt1-1 on 40S binding by HCR1 was distinctive. In the wild-type extract, HCR1 consistently peaked in the 40S region one fraction earlier than the other factors (Figure 4A). In the mutant extract, the most rapidly sedimenting form of HCR1 in fraction 11 was diminished, but a substantial proportion of HCR1 was retained in fraction 10. Recently, we found that HCR1 has a dual function in translation initiation, being required for normal levels of 40S ribosomes and for high levels of the MFC (Valášek et al., 2001). We suggest that HCR1 binds to 40S subunits with the MFC, but remains bound to the ribosome following dissociation of other factors on heat treatment of the prt1-1 extract. This eIF3-independent binding of HCR1 to 40S subunits may be related to its function in ribosome biogenesis.
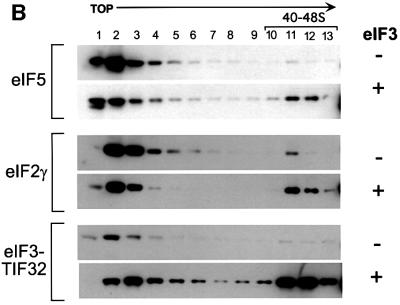
To confirm the involvement of eIF3 in recruitment of eIF5 to the 40S ribosome, we attempted to rescue 40S binding of eIF5 in the heat-treated prt1-1 mutant extract with purified eIF3. As shown in Figure 4B, addition of eIF3 to the mutant extract significantly increased the proportions of eIF5, eIF2γ and eIF3–TIF32 sedimenting in the 40–48S region (fractions 10–12) at the expense of the unbound forms of these factors (fractions 1–4). These results provide strong evidence that eIF3 promotes stable incorporation of eIF5 into 43–48S initiation complexes.
Physical association between overexpressed eIF3 subcomplexes and other initiation factors
In an effort to determine which subunits of eIF3 are required for its ability to stimulate binding of TC, mRNA and other initiation factors to 40S ribosomes, we overexpressed and purified various eIF3 subcomplexes containing His8-PRT1, and tested them for the presence of co-purifying initiation factors and the ability to rescue Met-tRNAiMet and mRNA binding in heat-treated prt1-1 extracts. Our previous analysis of protein linkages among eIF3 subunits revealed that PRT1, TIF34 and TIF35 interacted with one another, that PRT1 interacted with TIF32, but that NIP1 interacted only with TIF32 (Figure 1) (Asano et al., 1998). Based on these findings, we predicted that two different trimeric subcomplexes could be overproduced, one containing His8-PRT1, TIF34 and TIF35, and the other containing His8-PRT1, TIF32 and NIP1. We also expected to observe a stable His8-PRT1–TIF32 binary complex, but since PRT1 did not interact directly with NIP1 (Asano et al., 1998), a His8-PRT1–NIP1 binary complex should not exist. Similarly, a four-subunit complex containing His8-PRT1, TIF34, TIF35 and TIF32 was predicted, whereas one containing NIP1 in place of TIF32 should not occur.
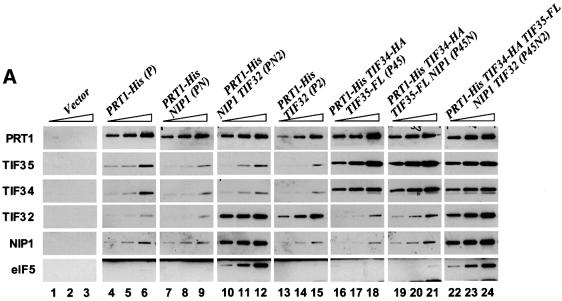
To test these predictions, we constructed yeast strains containing different subsets of eIF3 subunit genes under their native promoters on high-copy plasmids. All of the strains overexpressed His8-PRT1, and the overexpressed TIF34 and TIF35 subunits contained hemagglutinin (HA)- and FLAG-epitope tags, respectively. Western analysis of whole-cell extracts (WCEs) confirmed that eIF3 subunits were overexpressed in the appropriate strains between 10- and 20-fold above the endogenous levels, except for NIP1, which was only ∼5-fold overexpressed (data not shown). We affinity purified His8-PRT1 and associated proteins on Ni2+-NTA–silica from ribosomal high-salt washes (RSWs) and the eluates were subjected to western analysis using PRT1 antibodies. Aliquots containing equivalent amounts of His8-PRT1 were then examined for levels of other eIF3 subunits (Figure 5A). A mock purification using RSW from a strain containing untagged chromosomal PRT1 was conducted to ensure that purification of eIF3 subunits was dependent on binding of His-tagged PRT1 to the Ni2+-NTA resin. Indeed, none of the eIF3 subunits was detectable in this control preparation, henceforth designated ‘Vector’ (Figure 5A, lanes 1–3). As expected, the sample purified from the strain overexpressing only His8-PRT1 (designated sample P) contained detectable amounts of the other four eIF3 subunits (Figure 5A, lanes 4–6), which we attribute to incorporation of His8-PRT1 into endogenous eIF3 in place of untagged PRT1 expressed from the chromosome. This background level of five-subunit eIF3 should also occur in the preparations containing His8-PRT1 and other overexpressed eIF3 subunits.
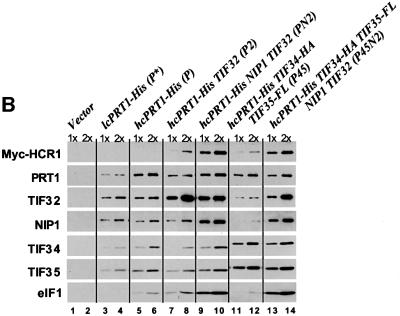
Fig. 5. eIF5 and eIF1 co-purify with eIF3 subcomplexes containing His8-PRT1, NIP1 and TIF32. (A) His8-PRT1 and associated proteins were purified from the PRS by nickel chelation chromatography in buffer containing 350 mM KCl (buffer B; see Materials and methods) from the following strains overexpressing different combinations of eIF3 subunits: LPY60 (empty vectors), LPY65 (P), LPY66 (PN), LPY67 (PN2), LPY68 (P2), LPY85 (P45), LPY86 (P45N) and LPY87(P45N2). The letters and numbers in parentheses designate overexpression of His8-PRT1 (P), HA-TIF34 (4), FLAG-TIF35 (5), NIP1(N) and TIF32 (2). Three serial dilutions of the Ni2+-NTA–silica eluates for each preparation were resolved by 4–20% SDS–PAGE and subjected to immunoblot analysis as described in Figures 3 and 4. Samples of the control extract loaded in lanes 1–3 (Vector) contained 0.5, 1 and 2 µg of total protein, respectively. Samples of the P preparation containing overexpressed His8-PRT1 alone in lanes 4–6 contained 0.38, 0.75 and 1.5 µg total protein, respectively. For the remaining preparations, the amounts loaded were predetermined to contain the same quantities of His8-PRT1 as in lanes 4–6. (B) Yeast strains LPY60, LPY65, LPY67, LPY68, LPY85 and LPY87, described in (A), were transformed with low-copy-number plasmid YCpLVHM-T encoding c-myc-tagged HCR1 to create strains LPY142 (Vector), LPY134 (P), LPY136 (PN2), LPY137 (P2), LPY138 (P45) and LPY140 (P45N2), respectively. In addition, strain LPY191 (P*) was constructed containing YCpLVHM-T and a low-copy plasmid bearing PRT1-His. His8-PRT1 and associated proteins were purified from PRS by nickel chelation chromatography in a buffer containing 100 mM KCl (buffer A; see Materials and methods). Two dilutions of the Ni2+-NTA–silica eluates for each preparation were resolved by 4–20% SDS–PAGE and subjected to immunoblot analysis as described in Figures 3 and 4, except that anti-c-Myc monoclonal antibodies (Boehringer-Mannheim; 1:2000) were used to probe for c-Myc-tagged HCR1. Samples of the control preparation in lanes 1 and 2 (Vector) contained 0.25 (1×) and 0.5 µg (2×) of total protein, respectively, as did the P* preparation in lanes 3 and 4. Samples of the P preparation in lanes 5 and 6 contained 0.125 (1×) and 0.25 µg (2×) of total protein. For the remaining preparations, the samples were predetermined to contain approximately the same amounts of His8-PRT1 as in lanes 5 and 6.
As expected, higher levels of the other four eIF3 subunits co-purified with His8-PRT1 from the strain overexpressing all five subunits (sample P45N2) (Figure 5A, lanes 22–24 versus 4–6). Interestingly, the sample purified from the extract containing overexpressed HA-TIF34, FLAG-TIF35 and His8-PRT1 (P45) contained high levels of HA-TIF34 and FLAG-TIF35 compared with their levels in the P sample (Figure 5A, lanes 16–18 versus 4–6). These data support our prediction that HA-TIF34 and FLAG-TIF35 can form a stable subcomplex with His8-PRT1 in the absence of TIF32 and NIP1. Analogous results were obtained for the PN2 sample purified from the extract containing overexpressed His8-PRT1, NIP1 and TIF32, showing that TIF32 and NIP1 can form a stable subcomplex with PRT1 in the absence of TIF34 and TIF35 (Figure 5A, lanes 10–12). As predicted, TIF32 and PRT1 formed a stable binary complex, whereas NIP1 and PRT1 did not (Figure 5A, lanes 13–15 and 7–9, respectively). Similarly, the overexpressed NIP1 did not co-purify with the His8-PRT1–HA-TIF34–FLAG-TIF35 subcomplex in the P45N preparation, whereas a fraction of the overexpressed TIF32 was recovered with His8-PRT1– HA-TIF34–FLAG-TIF35 from the P452 preparation (Figure 5A, lanes 19–21 and data not shown). Thus, in accordance with our subunit interaction map for eIF3, TIF32 is required to bridge interaction between NIP1 and His8-PRT1 in vivo.
The P45N2 sample contained a much higher amount of eIF5 compared with that present in the P sample, consistent with physical association of eIF5 with the eIF3 complex (Phan et al., 1998). (A low level of eIF5 was present in the P sample, but was not visible at the exposure chosen for Figure 5A.) Additionally, the increased yield of eIF5 co-purifying with His8-PRT1 was dependent on the presence of excess NIP1, occurring only for the PN2 and P45N2 complexes (Figure 5A, lanes 10–12 and 22–24). In the experiments of Figure 5A, we detected only a small amount of eIF1 associated with the P45N2 complex (data not shown). This can be attributed to the fact that association of eIF1 with eIF3 is salt labile (Phan et al., 1998) and the complex was purified from high-salt extracts of ribosomes. In contrast, high levels of eIF1 were recovered with the P45N2 and PN2 complexes when they were purified from WCEs at a lower salt concentration (Figure 5B). Under these conditions, increased amounts of eIF1 co-purified with His8-PRT1 only for the PN2 and P45N2 complexes, which contained NIP1 (Figure 5B, lanes 9–10 and 13–14 versus 5–6). These results provide in vivo evidence that NIP1 is the principal binding partner for eIFs 1 and 5 in the eIF3 complex (Figure 1).
For the experiment in Figure 5B, we employed a strain overexpressing Myc-tagged HCR1 (Myc-HCR1) in addition to untagged HCR1 produced from the chromosomal allele. Using Myc antibodies to probe the eIF3 subcomplexes, we found that Myc-HCR1 specifically co-purified with the PN2 trimeric complex and with five-subunit eIF3 (Figure 5B, lanes 9–10 and 13–14 versus 5–6). Thus, HCR1 interacts with the same trimeric subcomplex that binds eIFs 1 and 5. Recently, we found that recombinant TIF32 and PRT1 contain independent binding domains for HCR1 (Valášek et al., 2001); however, here we observed only weak association of Myc-HCR1 with the P2 binary complex (Figure 5B, lanes 7–8). Thus, it appears that NIP1 cooperates with PRT1 and TIF32 to promote tight binding of HCR1 to native eIF3 in vivo.
The PN2 eIF3 subcomplex restored 40S binding of Met-tRNAiMet and MFA2 mRNA, and the translation of LUC mRNA, in heat-treated prt1-1 extracts
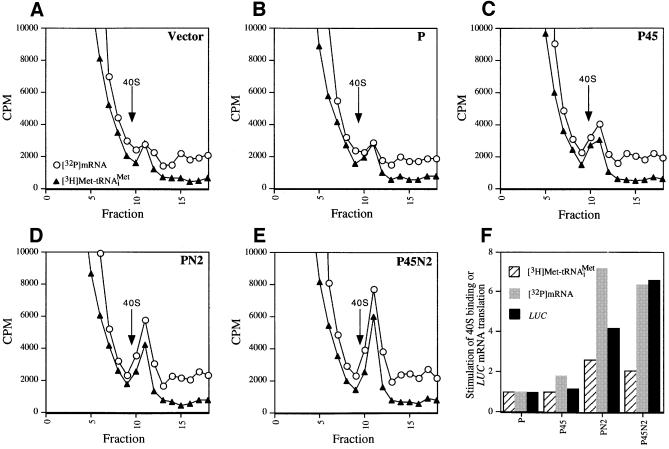
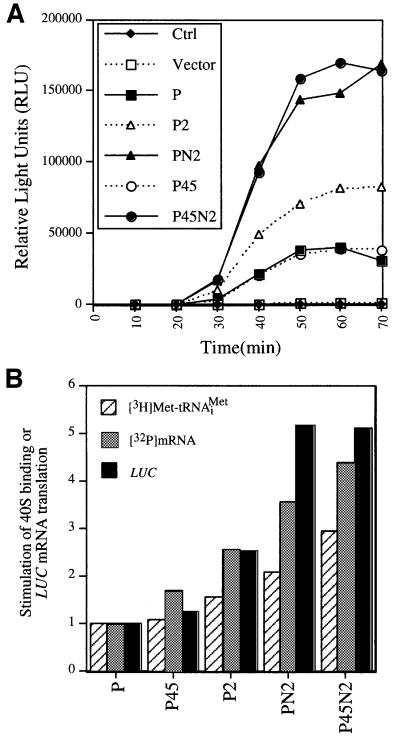
We have shown that binding of eIF3, eIF5, TC and mRNA to the 40S ribosome is defective in the heat-inactivated prt1-1 extract, and that all of these activities can be rescued by five-subunit eIF3 containing His8-PRT1. It was important to determine whether His8-PRT1 alone could rescue these activities, and if not, whether any of the eIF3 subcomplexes described above could do so. In a first approach, we prepared post-ribosomal supernatants (PRS) from cell extracts containing the combinations of overexpressed eIF3 subunits described above and tested them for restoration of 40S binding by [3H]Met-tRNAiMet and [32P]MFA2 mRNA in a heat-treated prt1-1 extract. In agreement with previous observations, binding of exogenous [3H]Met-tRNAiMet to 40S subunits occurred at low levels in the heat-treated mutant extract supplemented with PRS from the Vector (V) extract used as negative control (Figure 6A). PRS from the P45N2 extract stimulated [32P]MFA2 mRNA and [3H]Met-tRNAiMet binding to 40S ribosomes at levels higher than did PRS from the P extract containing overexpressed PRT1 alone (Figure 6B and E). After normalizing for amounts of His8-PRT1 in the PRS fractions, we calculated that the P45N2 PRS gave 2- and ∼6-fold greater stimulation of [3H]Met-tRNAiMet and [32P]MFA2 mRNA binding to 40S subunits, respectively, than an equivalent amount of P extract (Figure 6F). The P45 PRS gave little or no stimulation above that given by the P extract, whereas the PN2 preparation was nearly indistinguishable from the P45N2 sample in stimulating [32P]MFA2 mRNA and [3H]Met-tRNAiMet binding to 40S ribosomes (Figure 6D and F).
Fig. 6. Rescue of 40S binding of [32P]mRNA and [3H]Met-tRNAiMet and LUC mRNA translation in the prt1-1 extract by the PN2 subcomplex in a PRS. (A–E) For each panel, 20 µl (∼300 µg) of heat-treated translation extract from the prt1-1 strain were incubated in a 40 µl reaction containing 1× translation buffer, 1.2 mM GMPPNP, 1.2 pmol of [3H]Met-tRNAiMet (∼90 000 c.p.m.), 2 pmol of [32P]MFA2 mRNA (∼200 000 c.p.m.) and 15 µg of PRS (prepared as described in Materials and methods using buffer A) from the strains described in Figure 5A containing different combinations of overexpressed eIF3 subunits. The identity of the PRS added to the reaction is indicated in the upper right corner of the panel by the designations defined in Figure 5A. The reactions were carried out at 26°C for 20 min, stopped by addition of formaldehyde to 0.3% on ice for 10 min, and then separated on sucrose gradients. An aliquot (0.2 ml) of each fraction was assayed for [3H]Met-tRNAiMet and [32P]MFA2 mRNA by liquid scintillation counting, as described in Figure 2. The arrow in each panel marks the position of the 40S ribosomes in the gradient. (F) In vitro translation reactions were carried out using 35 µl of heat-treated prt1-1 extract in a 70 µl reaction containing 1× translation buffer, 1.2 mM GTP, 4 µg of capped LUC mRNA, 10 U of RNAsin (Promega), all 20 amino acids at 0.1 mM and 15 µg of the PRS fractions. The reactions were incubated at 26°C, and 10 µl aliquots were removed every 10 min and assayed for luciferase production by adding 100 µl of pre-mixed luciferase assay reagents (Promega) in an automated injection luminometer (Analytical Luminescence Laboratory) and measuring the emitted light. The relative stimulation of LUC mRNA translation by the P45, PN2 and P45N2 PRS fractions was calculated by normalizing the relative light units (RLU) measured after a 70 min incubation for the amount of His8-PRT1 present in the PRS fraction as determined by immunoblot analysis (data not shown). The normalized RLU values for the P45, PN2 and P45N2 reactions were divided by the normalized RLU value for the P reaction and plotted graphically (LUC, black bars). The stimulation of [3H]Met-tRNAiMet and [32P]MFA2 mRNA binding to 40S subunits in reactions (C–E) relative to that observed in (B) was also depicted graphically as hatched and gray bars, respectively. The relative amount of [3H]Met-tRNAiMet or [32P]MFA2 mRNA bound to 40S ribosomes in each experiment was determined by measuring the total area under the c.p.m. peak in the 40S region of the gradient using NIH Image software 1.61. The peak area measured for the Vector sample (A) was subtracted from the peak areas measured in (B–E), and the remainders were normalized for the quantity of Prt1-His8 in the PRS fraction added to the reaction. The resulting values for (C–E) were depicted graphically relative to that obtained for the P complex (B), which was set to 1.0.
The PRS fractions were also compared for the ability to rescue translation of a luciferase (LUC) reporter mRNA added to the prt1-1 extract. The heat-treated mutant extract had no detectable translation activity and addition of the control (V) PRS did not stimulate luciferase synthesis (data not shown). The P and P45 preparations each gave low-level stimulation of LUC mRNA translation, which we attribute to endogenous five-subunit eIF3 complexes in these samples. As expected, P45N2 PRS conferred high-level translation and, interestingly, the PN2 preparation had 60% of the stimulatory activity given by the P45N2 sample (Figure 6F). Together, the results in Figure 6 suggest that the PN2 trimeric complex can stimulate 40S binding of [3H]Met-tRNAiMet and [32P]MFA2 mRNA, and the translation of LUC mRNA, in prt1-1 extracts nearly to the same extent as five-subunit eIF3, whereas the P45 complex has little activity.
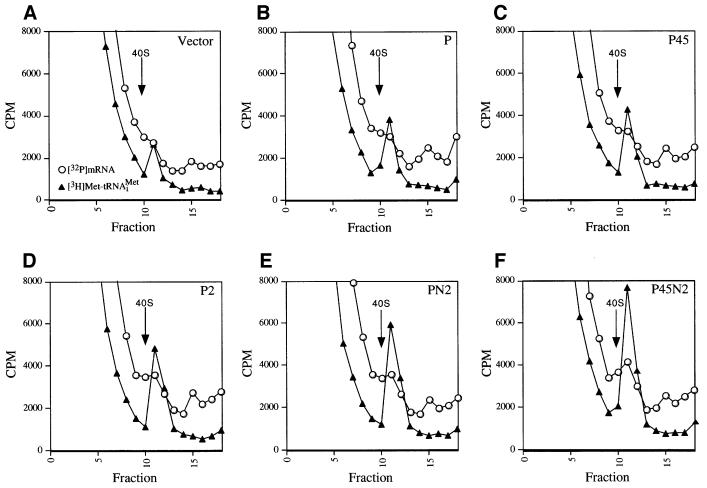
To confirm these conclusions, the same assays were repeated using the affinity-purified complexes described in Figure 5A. Binding of [3H]Met-tRNAiMet to 40S subunits was stimulated by the purified P45N2 complex to a much greater extent than by the P preparation, whereas the P45 subcomplex gave no greater stimulation of [3H]Met- tRNAiMet binding than did the P sample (Figures 7B, F and 8B). The P2 and PN2 subcomplexes had one-half and two-thirds of the stimulatory activity of the P45N2 complex, respectively (Figures 7C–E and 8B). Analysis of [32P]MFA2 mRNA binding to 40S subunits showed that the purified P2 and PN2 subcomplexes had more activity than did the P45 subcomplex, which was only slightly more active than the P preparation. The purified PN2 subcomplex had ∼80% of the activity given by P45N2 (Figures 7A–F and 8B). Finally, the P preparation and purified P45 subcomplex each gave low-level stimulation of LUC mRNA translation, whereas the PN2 subcomplex showed activity comparable to that of P45N2 (Figure 8A and B). The data in Figures 7 and 8 support our conclusion that the PN2 trimeric subcomplex is comparable to five-subunit eIF3 in restoring 40S binding of [3H]Met- tRNAiMet and [32P]MFA2 mRNA, and in stimulating LUC mRNA translation, in prt1-1 extracts.
Fig. 7. Rescue of [32P]mRNA and [3H]Met-tRNAiMet binding to 40S ribosomes in prt1-1 extract by affinity-purified PN2 subcomplex. (A–F) For each panel, 20 µl (∼300 µg) of heat-treated translation extract from prt1-1 strain H1676 were incubated in a 40 µl reaction containing 1× translation buffer, 1.2 mM GMPPNP, 1.2 pmol of [3H]Met-tRNAiMet (∼90 000 c.p.m.), 2 pmol of [32P]MFA2 mRNA (∼200 000 c.p.m.) and an aliquot of the Ni2+-affinity-purified preparations containing His8-PRT1 described in Figure 5A. Two to three picomoles of the P45N2 preparation were added to the reaction shown in (F). The amounts of the other His8-PRT1-containing complexes added in (B–E) contained the same amount of His8-PRT1 used in (F), as judged by immunoblot analysis (Figure 5A) and Coomassie Blue staining (data not shown) of the different preparations. The aliquot of Vector preparation used in (A) was identical in volume to that of the P preparation used in (B). The reactions were incubated at 26°C for 20 min, and analyzed for the amounts of [32P]mRNA and [3H]Met-tRNAiMet bound to 40S subunits as described in Figures 2 and 6.
Fig. 8. Rescue of LUC mRNA translation in prt1-1 extract by affinity-purified PN2 subcomplex. (A) In vitro translation reactions were carried out using 35 µl of heat-treated translation extract from prt1-1 strain in a reaction volume of 70 µl containing 1× translation buffer, 1.2 mM GTP, 4 µg of capped LUC mRNA, 10 U of RNAsin (Promega), each of the 20 amino acids at 0.1 mM, and an aliquot of one of the Ni2+-affinity-purified preparations containing His8-PRT1 described in Figure 5A. The latter were added in the same relative proportions described in Figure 7A–F, using 1.5 pmol of the P45N2 preparation. The reactions were incubated at 26°C and assayed for luciferase production as described in Figure 6. (B) Quantification of the relative activities of eIF3 subcomplexes in stimulating 40S binding of [3H]Met-tRNAiMet and [32P]MFA2 mRNA and of LUC mRNA translation in the prt1-1 extract. The stimulation of LUC mRNA translation by the P2, P45, PN2 and P45N2 complexes observed in (A) was quantified by dividing each RLU value obtained after a 70 min incubation by the corresponding RLU value for the P reaction and plotted graphically (LUC, black bars). The relative amount of [3H]Met-tRNAiMet or [32P]MFA2 mRNA bound to 40S ribosomes in each experiment shown in Figure 7 was determined as described in Figure 6. The peak area measured for the Vector sample (Figure 7A) was subtracted from the peak areas measured in Figure 7B–F. The remainders for the P45N2, PN2, P2 and P45 complexes were depicted graphically relative to the value obtained for the P complex, which was set to 1.0.
Discussion
eIF5, eIF1 and HCR1 are associated with the PRT1–TIF32–NIP1 eIF3 subcomplex in vivo
Our results show that PRT1 can form two trimeric subcomplexes containing either TIF32 and NIP1 or TIF34 and TIF35, designated PN2 and P45 subcomplexes, respectively. Both subcomplexes were purified from yeast, and characterized for interactions with other factors and the ability to carry out biochemical functions of eIF3. eIF5, eIF1 and HCR1 specifically co-purified with the PN2 subcomplex. A stable PRT1–TIF32 binary complex was also detected, but it did not form a strong association with eIFs 1 and 5 or HCR1. These findings are consistent with our previous conclusion, based on interactions between recombinant proteins, that NIP1 is the principal binding partner in eIF3 for eIFs 1 and 5 (Asano et al., 1998; Phan et al., 1998). A stable PRT1–NIP1 binary complex was not detected, nor did we observe co-purification of NIP1 with the P45 subcomplex, in keeping with our conclusion that PRT1 and NIP1 do not interact directly and their association in eIF3 is bridged by TIF32 (Asano et al., 1998).
We found recently that eIF1 and eIF5 both interact with the N-terminal 20% of NIP1, and that these interactions can occur simultaneously in vitro (Asano et al., 2000). Both eIFs 1 and 5 have been implicated in accurate start codon selection in yeast (Donahue, 2000). Our findings suggest that these factors are held in proximity in the pre-initiation complex by their mutual association with the N-terminal domain of NIP1. This may enable eIF1 to influence the function of eIF5 in stimulating GTP hydrolysis by the TC on base pairing between initiator tRNAMet and the start codon.
The CTD of eIF5 can bind simultaneously to eIF2β and eIF3–NIP1, bridging these factors in the MFC containing eIFs 1, 3, 5 and the TC (Asano et al., 2000). We proposed that the constituents of the MFC bind to the 40S ribosome as a preformed unit and that eIF5 is present continuously in 43–48S initiation complexes, tethered to eIF3–NIP1 and the β subunit of eIF2. This view departs from conventional depictions of the initiation pathway, where eIF5 interacts transiently with the 48S complex following recognition of the start codon (Hershey and Merrick, 2000). Supporting this hypothesis, we have shown here that binding of eIF5 to 40S ribosomes was defective in a heat-treated prt1-1 extract and was stimulated by addition of purified eIF3 to the mutant extract.
The PN2 eIF3 subcomplex can restore initiatior tRNAMet and mRNA binding in prt1-1 extracts
The defect in 40S binding of Met-tRNAiMet in prt1-1 extracts could be rescued by purified PN2 subcomplex to nearly the same extent observed for five-subunit eIF3. In contrast, the purified P45 subcomplex had little activity in this assay. Even the P2 binary complex had more activity than the P45 subcomplex. Thus, PRT1 can not stimulate TC binding on its own, or in combination with TIF34 and TIF34, whereas the PRT1–TIF32 binary complex is capable of this important activity, in a manner stimulated by NIP1.
Recently, we found that association between eIF3 and the TC bridged by eIF5 in the MFC enhances the rate of TC binding to 40S subunits in vitro (Asano et al., 2001). Hence, the high activity of the PN2 subcomplex in stimulating TC binding may derive partly from its ability to interact with eIF5 as a bridge to the TC. The P2 complex also stimulated TC binding, albeit to a lesser extent than PN2, even though P2 did not interact with eIF5. Perhaps the P2 complex can interact directly with eIF2 on the surface of the ribosome, or produce an allosteric alteration of the ribosome that stimulates TC binding indirectly.
We have provided the first evidence that yeast eIF3 promotes efficient binding of mRNA to 40S subunits by rescuing this activity in a heat-treated prt1-1 extract with purified eIF3. Again, the PN2 subcomplex was very active, while the P45 subcomplex had little or no activity in promoting mRNA binding. The ability of mammalian eIF3 to stimulate mRNA binding to 40S subunits is frequently attributed to its interaction with the eIF4G subunit of eIF4F. It is unclear whether yeast eIF3 can interact directly with eIF4G. Previously, we detected a small proportion of cellular eIF4G in Ni2+ affinity-purified preparations of eIF3 (Phan et al., 1998); however, we were unsuccessful in assigning this interaction to one of the eIF3 subcomplexes studied here (data not shown). Interestingly, the affinity-purified P45N2 and PN2 complexes were less active than the corresponding PRS fractions in stimulating [32P]MFA2 mRNA binding to 40S subunits (compare results in Figures 6 and 7). Perhaps this function was enhanced by physical association of the P45N2 and PN2 complexes with eIF4G in the PRS, which was disrupted during affinity purification.
It was surprising that the PN2 complex could rescue translation of LUC mRNA in the heat-treated prt1-1 extract to nearly the same extent observed for the five-subunit complex (Figures 6 and 8). There are several ways to explain this finding. One possibility is that the PN2 subcomplex would titrate TIF34 and TIF35 from the mutant complexes in the prt1-1 extract to regenerate wild-type five-subunit eIF3. Given the relative amounts of the PN2 subcomplex and endogenous eIF3 in the experiments of Figure 8, most of the TIF34 and TIF35 would have to be titrated from endogenous eIF3 to account for these findings. At odds with this requirement, the prt1-1 mutant eIF3 complexes were highly stable and could be purified following heat treatment without loss of subunits (Figure 3), and the P45 subcomplex was extremely stable during purification (data not shown). Additionally, the hypothetical titration of subunits from endogenous eIF3 by overexpressed subcomplexes should occur for the P45 and P preparations as well, whereas these samples had little activity in the translation assay (Figures 6 and 8). Thus, it seems very unlikely that TIF34 and TIF35 were titrated from endogenous eIF3 by the exogenous PN2 at levels sufficient to explain the high activity of this subcomplex.
An alternative hypothesis would be that TIF34 and TIF35 function at a step following the binding of TC and mRNA to the 40S ribosome (e.g. subunit joining), and their functions in LUC mRNA translation were performed by the endogenous mutant eIF3 or by the small amount of wild-type five-subunit complex present in the PN2 preparation. This explanation would require replacement of PN2 with intact five-subunit eIF3 in the 48S initiation complex midway through the initiation pathway. While this seems at odds with the proposed function of eIF3 as a scaffold for other initiation factors on the ribosome, PN2 may have a weaker, and hence more dynamic, interaction with the ribosome compared with five-subunit eIF3. Finally, it is possible that TIF34 and TIF35 are dispensable for translation of LUC mRNA in vitro. Even though both proteins are essential (Naranda et al., 1997; Hanachi et al., 1999), they may be required in vivo primarily to augment the activities of other eIF3 subunits and ensure maximal translation of a subset of critical mRNAs.
Materials and methods
Strains, plasmids and antibodies
All yeast strains and plasmids employed are listed in Tables I and II, respectively. Details of their construction are provided in Supplementary data available at The EMBO Journal Online. Antibodies were raised against glutathione S-transferase fusions to TIF32 and TIF35 as described in the Supplementary data. Antibodies against SUI1 (eIF1) and eIF5 were provided by T.Donahue, and antibodies against GCD11 were provided by E.Hannig. Antibodies against PRT1 (Cigan et al., 1991), NIP1 (Greenberg et al., 1998), TIF34 (Asano et al., 1998) and HCR1 (Valášek et al., 2001) were as described previously.
Table I. Saccharomyces cerevisiae strains used in this study.
| Strain | Genotype | Plasmid | Source or reference |
|---|---|---|---|
| F353 | MATα ura3-52 trp1 leu2-Δ1 his3-Δ200 pep::HIS4 prb1-Δ1.6 can1 GAL+ | E.Jones | |
| F354 | MATa ura3-52 trp1 leu2-Δ1 his3-Δ200 pep::HIS4 prb1-Δ1.6 can1 GAL+ | E.Jones | |
| LPY29 | MATa ura3-52 trp1 leu2-Δ1 his3-Δ200 pep::HIS4 prb1-Δ1.6 can1 GAL+ | YEplac181 | this study |
| LPY30 | MATa ura3-52 trp1 leu2-Δ1 his3-Δ200 pep::HIS4 prb1-Δ1.6 can1 GAL+ | pLPY-PRT1His | this study |
| LPY34 | MATa ura3-52 trp1 leu2-Δ1 his3-Δ200 pep::HIS4 prb1-Δ1.6 can1 GAL+ | pLPY-PRT1His-TIF34HA-TIF35Flag | this study |
| LPY36 | MATα ura3-52 trp1 leu2-Δ1 his3-Δ200 pep::HIS4 prb1-Δ1.6 can1 GAL+ | YEplac195 | this study |
| LPY37 | MATa ura3-52 trp1 leu2-Δ1 his3-Δ200 pep::HIS4 prb1-Δ1.6 can1 GAL+ | pLPY-NIP1 | this study |
| LPY38 | MATα ura3-52 trp1 leu2-Δ1 his3-Δ200 pep::HIS4 prb1-Δ1.6 can1 GAL+ | pLPY-NIP1-TIF32 | this study |
| LPY39 | MATα ura3-52 trp1 leu2-Δ1 his3-Δ200 pep::HIS4 prb1-Δ1.6 can1 GAL+ | pLPY-TIF32 | this study |
| LPY60 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pYEplac181/YEplac195 | this study |
| LPY65 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His/YEplac195 | this study |
| LPY66 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His/pLPY-NIP1 | this study |
| LPY67 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His/pLPY-TIF32-NIP1 | this study |
| LPY68 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His/pLPY-TIF32 | this study |
| LPY85 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His-TIF34HA-TIF35Flag/YEplac195 | this study |
| LPY86 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His-TIF34HA-TIF35Flag/pLPY-NIP1 | this study |
| LPY87 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His-TIF34HA-TIF35Flag/pLPY-TIF32-NIP1 | this study |
| LPY134 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His/YEplac195/YCpLVHM-T | this study |
| LPY136 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His/pLPY-TIF32-NIP1/YCpLVHM-T | this study |
| LPY137 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His/pLPY-TIF32/YCpLVHM-T | this study |
| LPY138 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His-TIF34HA-TIF35Flag/YEplac195/YCpLVHM-T | this study |
| LPY140 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY-PRT1His-TIF34HA-TIF35Flag/pLPY-TIF32-NIP1/YCpLVHM-T | this study |
| LPY142 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pYEplac181/YEplac195//YCpLVHM-T | this study |
| LPY143 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | YEplac181/YEplac195/YCpLVHM-T | this study |
| LPY190 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY201 | |
| LPY191 | MATa/α ura3-52/ura3-52 trp1/trp1 leu2-Δ1/leu2-Δ1 his3-Δ200/his3-Δ200 pep::HIS4/pep::HIS4 prb1-Δ1.6/prb1-Δ1.6 can1/can1 GAL+ | pLPY201/YEplac195/YCpLVHM-T | this study |
| LPY199 | Mata leu2-3 leu2-112 prt1-KanMX | pLPY102 [PRT1 LEU2] | Greenberg et al. (1998) |
| LPY201 | Mata leu2-3 leu2-112 prt1-KanMX | pLPY201[PRT1-His URA3] | Greenberg et al. (1998) |
| LPY202 | Mata leu2-3 leu2-112 prt1-KanMX | pLPY202[prt1-1-His URA3] | this study |
| H1676 | Mata leu2-3 leu2-112 prt1-1 |
Table II. Plasmids used in this study.
| Plasmid | Description | Source or reference |
|---|---|---|
| pRS316 | low-copy URA3 vector | Sikorski and Hieter (1989) |
| YEplac181 | high-copy LEU2 vector | this study |
| pLPY-PRT1His | PRT1-His in YEplac181 | this study |
| pLPY-PRT1His-TIF34HA-TIF35Flag | PRT1-His, TIF34-HA, and TIF35-FLAG in YEplac181 | this study |
| YEplac195 | high-copy URA3 vector | this study |
| pLPY-NIP1 | NIP1 in Yeplac195 | this study |
| pLPY-NIP1-TIF32 | NIP1 and TIF32 in YEplac195 | this study |
| pLPY-TIF32 | TIF32 in Yeplac195 | this study |
| YCpLVHM-T | HCR1-Myc in TRP1 single-copy vector YCplac22 | Valášek et al. (2001) |
| pLPY201 | PRT1-His in pRS316 | this study |
| pLPY102 | PRT1 in low-copy LEU2 vector pRS315 | Greenberg et al. (1998) |
| pLPY202 | prt1-1-His in pRS316 | this study |
Purification of eIF3 holocomplex and subcomplexes containing His8-PRT1
His8-PRT1 and associated proteins were purified essentially as described previously (Phan et al., 1998). For the results in Figure 5B, cells were grown in 2 l of SD medium (Sherman et al., 1974) to an OD600 of 0.6–1.0 and harvested by centrifugation at 7000 g for 20 min, then washed with ice-cold water. All subsequent steps were performed at 4°C. The cell pellet was resuspended at 1 ml per 1 g of buffer A [20 mM Tris–HCl pH 7.5, 100 mM KCl, 5 mM MgCl2, 0.5 mM β-mercaptoethanol, 1 mM phenylmethylsulfonyl fluoride (PMSF), 20 mM imidazole, 10% glycerol, 1× complete protease inhibitor cocktail minus EDTA (Boehringer Mannheim)]. Cells were broken in a French press twice at 19 000 p.s.i., debris was removed by centrifugation at 7000 g, and the supernatant was centrifuged at 200 000 g for 1 h to pellet the ribosomes. The resulting supernatant was combined with Ni2+-NTA–silica resin (Qiagen) at 0.075 ml bed volume of resin per liter of starting culture, and mixed at 4°C for 1 h. The resin was pelleted by brief centrifugation and washed three times with buffer A. Bound proteins were eluted with buffer A containing 250 mM imidazole and stored in buffer A at –70°C.
For the results shown in Figure 5A, the above procedure was followed except that buffer B (buffer A + 350 mM KCl) was used during extract preparation and for the binding and initial washing steps during Ni2+ chelation chromatography. After the last wash, the resin was washed once with buffer A and bound proteins were eluted with buffer A containing 250 mM imidazole. The highly purified eIF3 employed in Figures 2 and 4B was purified from strain LPY87 essentially as described above, except that buffer C (buffer A + 750 mM KCl) was used for preparing the PRS. Following batch binding with Ni2+-NTA–silica resin (0.75 ml) for 1 h at 4°C, the resin was washed three times with buffer C, once with buffer A, and bound proteins were eluted with buffer A containing 250 mM imidazole. The eluate was diluted with 10 vols of 1× TBS (20 mM Tris–HCl pH 7.4, 150 mM NaCl) and the complex containing TIF35-FLAG was purified by incubating the eluate with 0.5 ml of anti-FLAG resin (Sigma) overnight at 4°C. The resin was washed three times with 5 ml of 1× TBS, and bound proteins were eluated with 0.5 ml of 1× TBS containing 25 pmol of FLAG peptide (Sigma). For the final step, the eluted proteins were purified by gel filtration chromatography as described (Phan et al., 1998).
In vitro assays of eIF3 function
For the experiments shown in Figures 2, 4, 6, 7 and 8, translation extracts were prepared as described previously (Phan et al., 1998) and 0.25 ml aliquots were stored in liquid nitrogen. The extracts were thawed on ice, heated at 37°C for 5 min, and mixed with 2× translation buffer (40 mM HEPES pH 7.4, 60 mM potassium acetate, 4 mM magnesium acetate, 1.5 mM ATP, 3 mM dithiothreitol, 50 mg/ml creatine phosphate, 0.3 mg/ml creatine phosphate kinase), supplemented with 1.2 mM non-hydrolyzable GTP analog GMPPNP (Boehringer Mannheim) where indicated, for a final concentration of 1× translation buffer components and 1.2 mM GMPNP. Depending on the assay, [32P]MFA2 mRNA, [3H]Met-tRNAiMet or LUC mRNA was also added in the amounts indicated in the figure legends.
[32P]MFA2 mRNA was prepared from plasmid pAS225 linearized by digestion with PstI (Tarun et al., 1997) and used as template for in vitro transcription with the SP6 mMessage mMachine kit (Ambion) in the presence of [32P]UTP (10 µCi, 10 Ci/ml; Amersham). The labeled mRNA was purified using the RNAeasy kit (Qiagen) according to the manufacturer’s instructions and stored at –70°C in water pre-treated with diethyl pyrocarbonate. [3H]Met-tRNAiMet and capped luciferase (LUC) mRNA were prepared as described previously (Phan et al., 1998).
Supplementary data
Supplementary data for this paper are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
We thank Tom Donahue, Ernie Hannig and David Goldfarb for gifts of antibodies against eIFs 1 and 5, eIF2γ and NIP1, respectively. We thank members of the Hinnebusch and Dever laboratories for helpful suggestions during the course of this work.
References
- Asano K., Phan,L., Anderson,J. and Hinnebusch,A.G. (1998) Complex formation by all five homologues of mammalian translation initiation factor 3 subunits from yeast Saccharomyces cerevisiae. J. Biol. Chem., 273, 18573–18585. [DOI] [PubMed] [Google Scholar]
- Asano K., Krishnamoorthy,T., Phan,L., Pavitt,G.D. and Hinnebusch,A.G. (1999) Conserved bipartite motifs in yeast eIF5 and eIF2Bε, GTPase-activating and GDP–GTP exchange factors in translation initiation, mediate binding to their common substrate eIF2. EMBO J., 18, 1673–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano K., Clayton,J., Shalev,A. and Hinnebusch,A.G. (2000) A multifactor complex of eukaryotic initiation factors eIF1, eIF2, eIF3, eIF5 and initiator tRNAMet is an important translation initiation intermediate in vivo. Genes Dev., 14, 2534–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano K., Shalev,A., Phan,L., Nielsen,K., Clayton,J., Valášek,L., Donahue,T.F. and Hinnebusch,A.G. (2001) Multiple roles for the C-terminal domain of eIF5 in initiation complex assembly and GTPase activation. EMBO J., 20, 2326–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay A. and Maitra,U. (1999) Cloning and characterization of the p42 subunit of mammalian translation initiation factor 3 (eIF3): demonstration that eIF3 interacts with eIF5 in mammalian cells. Nucleic Acids Res., 27, 1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigan A.M., Foiani,M., Hannig,E.M. and Hinnebusch,A.G. (1991) Complex formation by positive and negative translational regulators of GCN4. Mol. Cell. Biol., 11, 3217–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaie P., Wittmer,B., Altmann,M. and Trachsel,H. (1995) Isolation of a protein complex containing translation initiation factor Prt1 from Saccharomyces cerevisiae. J. Biol. Chem., 270, 4288–4292. [DOI] [PubMed] [Google Scholar]
- Das S. and Maitra,U. (2000) Mutational analysis of mammalian translation initiation factor 5 (eIF5): role of interaction between the β subunit of eIF2 and eIF5 in eIF5 function in vitro and in vivo. Mol. Cell. Biol., 20, 3942–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue T. (2000) Genetic approaches to translation initiation in Saccharomyces cerevisiae. In Sonenberg,N., Hershey,J.W.B. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 487–502.
- Feinberg B., McLaughlin,C.S. and Moldave,K. (1982) Analysis of temperature-sensitive mutant ts187 of Saccharomyces cerevisiae altered in a component required for the initiation of protein synthesis. J. Biol. Chem., 257, 10846–10851. [PubMed] [Google Scholar]
- Fletcher C.M., Pestova,T.V., Hellen,C.U.T. and Wagner,G. (1999) Structure and interactions of the translation initiation factor eIF1. EMBO J., 18, 2631–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg J.R., Phan,L., Gu,Z., deSilva,A., Apolito,C., Sherman,F., Hinnebusch,A.G. and Goldfarb,D.S. (1998) Nip1p associates with 40S ribosomes and the Prt1p subunit of eIF3 and is required for efficient translation initiation. J. Biol. Chem., 273, 23485–23494. [DOI] [PubMed] [Google Scholar]
- Hanachi P., Hershey,J.W.B. and Vornlocher,H.P. (1999) Characterization of the p33 subunit of eukaryotic translation initiation factor-3 from Saccharomyces cerevisiae. J. Biol. Chem., 274, 8546–8553. [DOI] [PubMed] [Google Scholar]
- Hartwell L.H. and McLaughlin,C.S. (1969) A mutant of yeast apparently defective in the initiation of protein synthesis. Proc. Natl Acad. Sci. USA, 62, 468–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey J.W.B. and Merrick,W.C. (2000) Pathway and mechanism of initiation of protein synthesis. In Sonenberg,N., Hershey,J.W.B. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 33–88.
- Hinnebusch A.G. (2000) Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. In Sonenberg,N., Hershey,J.W.B. and Mathews,M.B. (eds), Translational Control of Gene Expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 185–243.
- Huang H., Yoon,H., Hannig,E.M. and Donahue,T.F. (1997) GTP hydrolysis controls stringent selection of the AUG start codon during translation initiation in Saccharomyces cerevisiae. Genes Dev., 11, 2396–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranda T., MacMillan,S.E., Donahue,T.F. and Hershey,J.W. (1996) SUI1/p16 is required for the activity of eukaryotic translation initiation factor 3 in Saccharomyces cerevisiae. Mol. Cell. Biol., 16, 2307–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranda T., Kainuma,M., McMillan,S.E. and Hershey,J.W.B. (1997) The 39-kilodalton subunit of eukaryotic translation initiation factor 3 is essential for the complex’s integrity and for cell viability in Saccharomyces cerevisiae. Mol. Cell. Biol., 17, 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova T.V., Borukhov,S.I. and Hellen,C.U.T. (1998) Eukaryotic ribosomes require initiation factors 1 and 1A to locate initiation codons. Nature, 394, 854–859. [DOI] [PubMed] [Google Scholar]
- Phan L., Zhang,X., Asano,K., Anderson,J., Vornlocher,H.P., Greenberg,J.R., Qin,J. and Hinnebusch,A.G. (1998) Identification of a translation initiation factor 3 (eIF3) core complex, conserved in yeast and mammals, that interacts with eIF5. Mol. Cell. Biol., 18, 4935–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman F., Fink,G.R. and Lawrence,C. (1974) Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 61–64.
- Sikorski R.S. and Hieter,P. (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics, 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarun S.Z., Wells,S.E., Deardorff,J.A. and Sachs,A.B. (1997) Translation initiation factor eIF4G mediates in vitro poly (A) tail-dependent translation. Proc. Natl Acad. Sci. USA, 94, 9046–9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valášek L., Hasek,J., Trachsel,H., Imre,E.M. and Ruis,H. (1999) The Saccharomyces cerevisiae HCRI gene encoding a homologue of the p35 subunit of human translation eukaryotic initiation factor 3 (eIF3) is a high copy suppressor of a temperature-sensitive mutation in the Rpg1p subunit of yeast eIF3. J. Biol. Chem., 274, 27567–27572. [DOI] [PubMed] [Google Scholar]
- Valášek L., Phan,L., Schoenfeld,L.W., Valášková,V. and Hinnebusch,A.G. (2001) Related eIF3 subunits TIF32 and HCR1 interact with an RNA recognition motif in PRT1 required for eIF3 integrity and ribosome binding. EMBO J., 20, 891–904. [DOI] [PMC free article] [PubMed] [Google Scholar]