Abstract
Plants contain a novel unique subfamily of Rho GTPases, vital components of cellular signalling networks. Here we report a general role for some members of this family in polarized plant growth processes. We show that Arabidopsis AtRop4 and AtRop6 encode functional GTPases with similar intrinsic GTP hydrolysis rates. We localized AtRop proteins in root meristem cells to the cross-wall and cell plate membranes. Polar localization of AtRops in trichoblasts specifies the growth sites for emerging root hairs. These sites were visible before budding and elongation of the Arabidopsis root hair when AtRops accumulated at their tips. Expression of constitutively active AtRop4 and AtRop6 mutant proteins in root hairs of transgenic Arabidopsis plants abolished polarized growth and delocalized the tip-focused Ca2+ gradient. Polar localization of AtRops was inhibited by brefeldin A, but not by other drugs such as latrunculin B, cytochalasin D or caffeine. Our results demonstrate a general function of AtRop GTPases in tip growth and in polar diffuse growth.
Keywords: Arabidopsis thaliana/polarity/Rho GTPase/root development/root hair
Introduction
Rho GTPases are a subgroup of the Ras superfamily of small GTPases and include RHO, Rac and Cdc42 sub families. They are key components in signalling pathways that link stimulation by extracellular ligands or intracellular signals with changes in the actin cytoskeleton (for reviews see Hall, 1998; Schmidt and Hall, 1998; Kjøller and Hall, 1999). Coordinately with regulation of the actin cytoskeleton, Rho GTPases regulate specific MAP kinase pathways and interact with a variety of other targets, resulting in a central role for Rho GTPases in diverse cellular processes such as cell motility, cell shape determination, membrane trafficking, transcriptional regulation and cell cycle progression (reviews by Van Aelst and D’Souza-Schorey, 1997; Hall, 1998). In Saccharomyces cerevisiae, RHO-like GTPases and Cdc42 have been implicated in the control of bud formation. Cdc42 functions in the establishment of cell polarity and localizes to the plasma membrane at the presumptive bud site and at the bud tip (review by Johnson, 1999). Rho1p also localizes to the bud site, where it effects actin assembly via Bni1p and promotes cell wall synthesis via activation of glucan synthase (Imamura et al., 1997; review by Schmidt and Hall, 1998).
Rho GTPases are molecular switches, cycling between GTP-bound (on) and GDP-bound (off) states. This cycle is controlled by GDP/GTP exchange factors (GEFs) and GTPase activating proteins. Specific mutations, such as those corresponding to 12V and 17N in Ras, result in constitutively active (GTP-locked form) and dominant-negative Rho (GDP-locked form), respectively. Rho GTPases also cycle between membrane-bound and cytosolic locations, and are only active when associated with membranes. Inactive GDP-bound Rho GTPases reside in the cytosol in a complex with guanine nucleotide dissociation inhibitors (RhoGDIs), and are released from the GDI protein and translocate to membranes upon activation (Takahashi et al., 1997). Since RhoGDI captures membrane-bound Rho, it has an important role in the localization cycle and thereby in the regulation of Rho activity (reviewed by Olofsson, 1999).
The Rho-related Rop (Rho of plant) GTPases have been implicated in diverse pathways including pathogen defence and CLAVATA signal transduction and are present as large multigene families in higher plants (Yang and Watson, 1993; Delmer et al., 1995; Winge et al., 1997; Li et al., 1998; Bischoff et al., 1999; Kawasaki et al., 1999; Trotochaud et al., 1999; Schiene et al., 2000; Valster et al., 2000). Rop mRNA is detected in all plant tissues, although certain Rop genes such as Arabidopsis AtRop1 and AtRac2 are specifically expressed in pollen (Delmer et al., 1995; Winge et al., 1997; Li et al., 1998; Kost et al., 1999). In elongating pea pollen tubes, Rop GTPase is concentrated in the cortical region of the tube apex (Lin et al., 1996). Morphological changes due to expression of constitutively active or dominant-negative forms of AtRac2 and AtRop1 have demonstrated a central role for these proteins in pollen tube tip growth (Kost et al., 1999; Li et al., 1999; Zheng and Yang, 2000). Whether Rop GTPases might have a more general role in the regulation of plant cell polarity remains to be elucidated (Yang, 1998). Due to the technical limitations of the ‘single cell’ pollen model, better and more easily accessible systems are needed to study the role of different members of this family in cell polarity control. Roots with their well defined architecture and emerging root hairs may represent such a model system (Scheres and Heidstra, 1999; Barlow and Baluška, 2000). We have therefore tested roots to study a more general role for Rop GTPases in polar plant cell growth. Here we demonstrate the usefulness of this model system for the functional analysis of Rho GTPases. We show the intracellular localization of Rop GTPases in Arabidopsis roots and in transgenic tobacco BY-2 cells. Rop GTPases were associated mainly with the plasma membrane and polarly localized during root hair formation. Using analysis of dominant mutants we demonstrate a role for Rop GTPases in root hair tip growth and that these proteins function in polarized cell growth in plants.
Results
Arabidopsis Rop homologues have similar intrinsic GTP hydrolysis rates
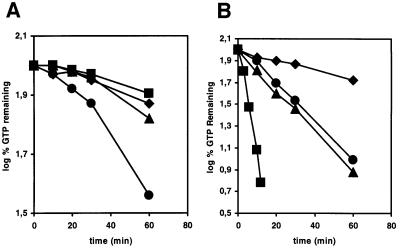
AtRop4 and AtRop6 GTPases belong to the plant-specific Rop group of Rho GTPases (Yang and Watson, 1993). AtRop4 and AtRop6 are 89% identical and, from the presence of C-terminal CAAX isoprenylation motifs, are substrates for geranylation (Moores et al., 1991) (Figure 1). To determine the intrinsic GTP hydrolysis rates, Arabidopsis cDNAs corresponding to GTPases AtRop4, AtRop6, AtRab11c and a Rac-like GTPase (Collins and Johnson, 1997), were cloned in a pGEX vector (Smith and Johnson, 1988), to obtain the corresponding glutathione S-transferase (GST) fusion proteins. The Rac-like sequence is derived from an Arabidopsis cDNA library (Newman et al., 1994; Collins and Johnson, 1997), but is absent from the completed Arabidopsis genome (Arabidopsis Genome Initiative, 2000). The Rac-like sequence was not detected in genomic Southern hybridization (data not shown), suggesting that it is not an Arabidopsis clone. Both [γ-32P]GTP and [α-32P]GTP were used in filter binding assays to allow for determination of the relative contribution of intrinsic GTP hydrolysis and GTP–GDP dissociation. The GTP–GDP off rates for the plant Rho GTPases were found to be low relative to GTP hydrolysis, as shown by the different kinetics of loss of label for [α-32P]- and [γ-32P]GTP, respectively (Figure 2). The GTP–GDP off rate for AtRop4 was higher than for AtRop6. Both Rop GTPases had similar intrinsic GTP hydrolysis rates of 0.040/min and 0.043/min, respectively. A constitutively active mutant AtRop4 G15V had no measurable GTP hydrolysis (result not shown). The different GTPase activities of AtRab11c and Rac-like protein (0.23/min) reflected the functional difference from the Rop GTPases, whereas the similar rates of GTP hydrolysis for AtRop4 and AtRop6 suggested a high degree of functional conservation between these Rop genes.
Fig. 1. Alignment of AtRop4 and AtRop6 sequences. Added mutations G15V, Q64L (both constitutively active) and T17N (dominant-negative) are marked by arrows.
Fig. 2. Off rates (A) and intrinsic hydrolysis rates (B) for AtRab11c (diamond), AtRop6 (triangle), AtRop4 (circle) and a Rac-like GTPase (square) in high magnesium. Proteins were preloaded with either [α-32P]GTP (A) or [γ-32P]GTP (B), and then an excess of Mg2+ and cold GTP were added. Reaction aliquots were removed and the radioactivity bound to protein was determined by a nitrocellulose filter binding assay. The remaining GTP is expressed as log of the percentage of bound label at time zero.
Cellular localization of AtRop4 GTPase
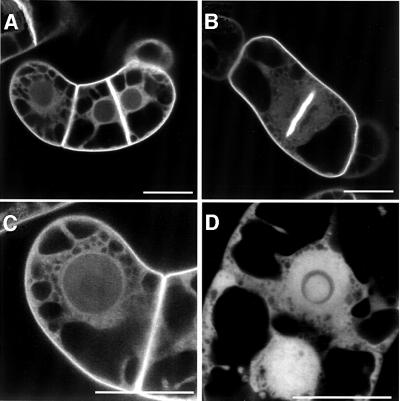
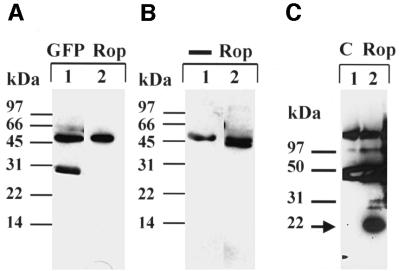
To study the localization of AtRop4 in the living cell, the N-terminally tagged green fluorescent protein (GFP)–Rho GTPase was expressed in stably transformed tobacco BY-2 cells using the dexamethasone-inducible GVG system (Aoyama and Chua, 1997). GFP–AtRop4 was predominantly localized to the plasma membrane, especially to the cross-wall and cell plate membranes, with occasional localization in vesicles. A fraction of GFP– AtRop4 was localized in the cytoplasm, as indicated by a diffuse staining of the cytoplasmic strands and the region surrounding the nucleus (Figure 3A–C). In contrast, control GFP accumulated in the nucleus and showed a diffuse labelling of the cytoplasm without plasma membrane staining (Figure 3D). Using anti-GFP monoclonal antibodies, GFP–AtRop4 fusion protein of the predicted size could be immunoprecipitated from a cell extract of the transgenic line, with some evidence of protein degradation (Figure 4A). To determine the localization of endogenous Rop GTPases, antiserum was raised against AtRop4 and tested using the transgenic BY-2 culture. GFP–AtRop4 fusion protein was detected using the affinity-purified anti serum (Figure 4A). In addition, the antibody was effective in specifically immunoprecipitating GFP–AtRop4 fusion protein from whole cell extract (Figure 4B). Longer exposures revealed one additional band (21 kDa) of the right size for endogenous tobacco Rop proteins (not shown). Endogenous Arabidopsis Rop proteins were also detected specifically in immunoprecipitates from whole seedling extracts (Figure 4C). Due to the high homology of the different Rop proteins, the AtRop4 antibody is likely to be reactive against all Rop isoforms.
Fig. 3. Localization of GFP–AtRop4 in transgenic tobacco BY-2 cells. GFP–AtRop4 (A–C) and GFP control (D). (B) Dividing GFP–AtRop4 cell with labelled cell plate membranes. Bar, 25 µm.
Fig. 4. Western analysis of GFP-tagged AtRop4 expressed in a transgenic BY-2 line. (A) Immunoprecipitation with anti-GFP monoclonal antibodies. Western analysis with anti-GFP polyclonals (lane 1) or anti-AtRop4 polyclonal antibody (lane 2). Molecular weight markers as indicated. (B) Immunoprecipitation with anti-AtRop4 antibody. Western with secondary reagent only (lane 1) or anti-AtRop4 polyclonal antibody (lane 2). A band corresponding to IgG heavy chain (50 kDa) is visible in both lanes. Molecular weight markers as indicated. (C) Immunoprecipitation of Rop proteins from whole Arabidopsis seedling extract with preimmune serum (lane 1) or anti-AtRop4 polyclonal antibody (lane 2). Western with anti-AtRop4 polyclonal antibody. Background Ig bands detected by secondary reagent are present in both lanes. The position of the Rop band is marked by an arrow.
Polar localization of Rop during root hair formation
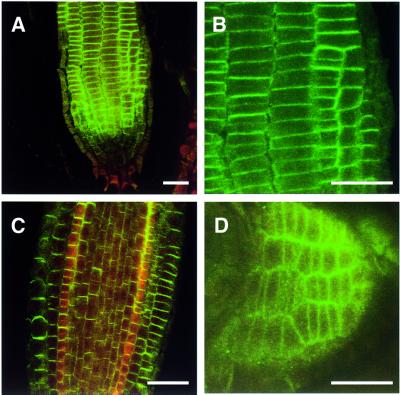
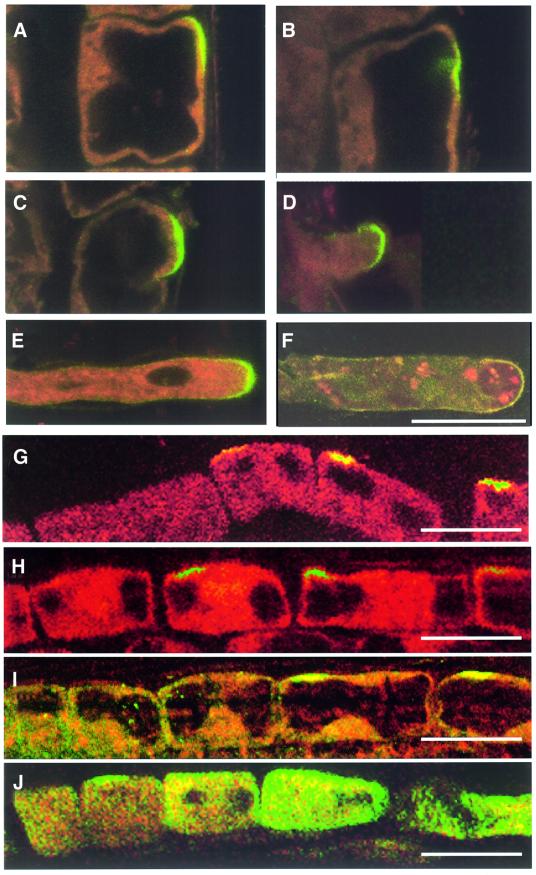
Root hair elongation is an example of tip growth in plants and shares many similarities with growing pollen tubes. To determine a possible role for Rop GTPases in root hair formation and probably of other root cells as well, the antiserum against AtRop4 was used for immunolocalization in Arabidopsis seedlings. Rop GTPases were highly enriched in the basal and apical plasma membranes in cells of the division zone of the primary root (Figure 5A–C), and in the cell membranes of lateral root primordia (Figure 5D). In the cell elongation zone, AtRop proteins were found to be polarly localized in the plasma membranes of the trichoblast cell files before the root hair bulges became visible (Figure 6A–F). In the differentiation zone of the root, AtRop proteins remained concentrated in the apical membrane of root hair bulges and at tips of growing root hairs, but were absent from the apical membrane of fully grown root hairs from the older zone of the root.
Fig. 5. Immunolocalization of Rop proteins in Arabidopsis root tips using confocal microscopy. Epidermal meristem (A), close-up of epidermal meristem with newly completed cell plates (B), longitudinal section with central cylinder (C) and lateral root primordium (D). Images (A–C) are oriented with the root tip down. Bar, 25 µm.
Fig. 6. Immunolocalization of Rop proteins in the root hair-forming zone of Arabidopsis roots using confocal microscopy. (A–F) Sequence of root hair formation showing early establishment of a Rop-positive bud site (A and B), bulging (C), tip-growth (D and E) and Rop-negative finished root hair (F). Images are oriented with the root tip up. Bar, 20 µm. (G–J) Rop localization in trichoblast cell files of inhibitor-treated seedlings. Control (G), cytochalasin D (H), caffeine (I) and BFA (J). Images are oriented with the root tip left. Bar, 25 µm.
Polar localization of Arabidopsis Rop proteins is sensitive to brefeldin A
To find the requirements for polar localization of AtRop proteins on the trichoblast plasma membrane, we tested several inhibitors of root hair tip growth. Actin-disrupting drugs latrunculin B (1 µM, 2 h) and cytochalasin D (10 µM, 2 h) did not inhibit the early polar localization of AtRop proteins in trichoblasts (as shown for cytochalasin D; Figure 6H), in agreement with the reported lack of effect of these drugs on root hair initiation (Miller et al., 1999; Ovecka et al., 2000). Likewise, tubulin-depolymerizing drugs propyzamide (10 µg/ml, 2 h) and colchicine (10 mM, 2 h) did not affect the polar localization of AtRop proteins at the early stage (not shown). Also, caffeine (10 mM, 2 h, used as a putative Ca2+ flux antagonist) had no effect on early AtRop protein localization (Figure 6I), in agreement with the reported lack of effect of Ca2+ channel blocker verapamil on root hair budding (Wymer et al., 1997). However, brefeldin A (BFA, 5–50 µg/ml, 0.5–2 h), a pharmacological inhibitor of ARF1 guanine nucleotide exchange activity, was effective in inhibiting polar localization of Rop in trichoblast cell files (total of 20 independent treatments). Only four of 20 samples contained roots with occasional Rop spots left at wrong positions in the trichoblast plasma membrane, or showed incomplete inhibition of localization as shown in Figure 6J. A difference in AtRop protein contents of trichoblasts and atrichoblasts was visible, which might be caused by trichoblast-specific AtRop gene expression. BFA also inhibited localization of AtRop proteins in the transverse plasma membranes in the cell division zone of the root (not shown). Washing away BFA reversibly allowed the relocalization of AtRop proteins to the bud sites (nine treatments) sometimes with trichoblasts having spots at wrong positions (in three of nine treatments). This recovery could be observed after 30 min and was insensitive to 1 µM latrunculin B (not shown), confirming that early polar localization of Rop GTPase is independent of the actin cytoskeleton.
Constitutively active AtRop4 and AtRop6 abolish polar cell growth
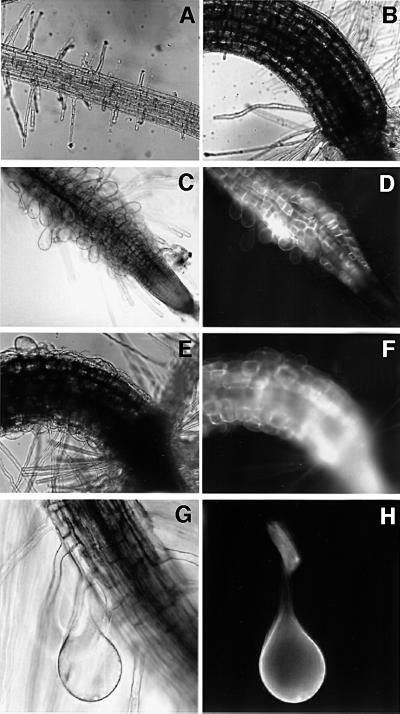
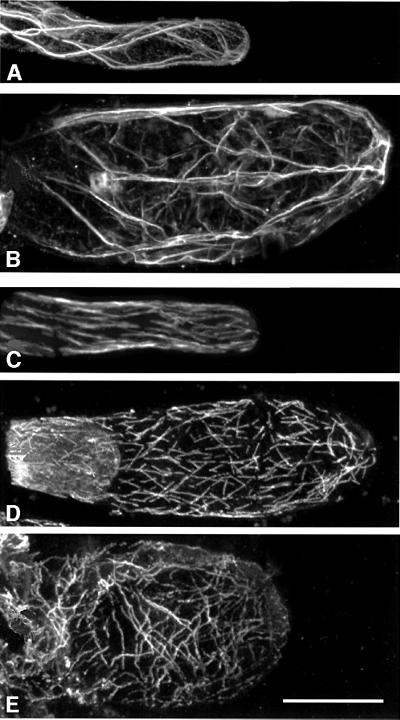
To determine a role for Arabidopsis Rop GTPases in root hair formation, transgenic Arabidopsis lines were generated that inducibly expressed wild-type, constitutively active and dominant-negative forms of GFP-tagged Rop GTPases. In addition, transgenic lines were made that expressed GFP fused to the C-terminal 20 amino acids of AtRop4 (GFP–AtRop4 tail). The GVG system was used to avoid gene silencing or toxic effects associated with constitutive AtRop GTPase expression (Aoyama and Chua, 1997). Different transgenic lines expressed variable quantities of inducible GFP fusion protein, mostly with low levels in the stem and leaves, relatively strong labelling of guard cells and trichomes, high levels in roots, but not in root meristem. Expression in pollen tubes was absent, precluding determination of a role for these Rop GTPases in pollen tube tip growth. To circumvent possible phenotypes caused by expression of transcriptional activator GVG alone (Kang et al., 1999), multiple lines were screened for each construct allowing the identification of specific phenotypes. Seedlings expressing the constitutively active GFP–AtRop4 G15V mutant showed strong swelling of the epidermal cells of the hypocotyl and cotyledons (12 of 16 lines) concomitant with loss of tip growth in three lines (Figure 7E). Swollen epidermal cells were always green fluorescent, indicating that the swelling was caused by expression of the GFP fusion protein in individual cells. In five transgenic lines showing green fluorescent tip-growing root hairs, both constitutively active GFP–AtRop4 G15V (three of 16 lines) and GFP–AtRop6 Q64L (two of three lines) caused loss of tip growth resulting in root hair swelling (Figure 7C). The expression of GFP–Rop in most lines was patchy, and in such lines expressing constitutively active Rop only fluorescent root hair cells were swollen, while non-expressing root hairs developed normally (Figure 7G and H). The occurrence of the root hair phenotype was associated with an increase in diameter of the root elongation zone. Mature epidermal root cells were not affected, suggesting that the phenotype was specific for elongating cells. The finished bulges were found to contain extensive actin and tubulin cytoskeletons (Figure 8). Long actin bundles were present, with a net-like arrangement of finer filaments. Microtubules were more randomly oriented and shorter than microtubules in mature root hairs or root epidermal cells, possibly as a consequence of isotropic growth. Swollen epidermal cells of the hypocotyl and swollen root hairs were not observed in dexamethasone-treated Arabidopsis wild-type seedlings nor in induced transgenic lines expressing either wild-type GFP–AtRop4 (nine lines), dominant-negative GFP– AtRop4 T20N (25 lines) or GFP–AtRop4 tail (20 lines). The phenotype was reproducible in T2 and T3 generations. The experiment indicated that cell swelling was specific for constitutively activated Rop, and thus pointed to an important role for Rop GTPases in root hair tip growth and also in elongation of epidermal cells of the hypocotyl.
Fig. 7. Constitutively active GFP–AtRop causes root hair and epidermal cell swelling in transgenic Arabidopsis. (A and B) Root tip (A) and hypocotyl (B) of uninduced GFP–AtRop6 Q64L line. (C and D) Root tip of induced GFP–AtRop6 Q64L line (D, fluorescence image). (E and F) Hypocotyl of induced GFP–AtRop4 G15V line (F, fluorescence image). (G and H) Swollen root hair cell of induced GFP–Rop6 Q64L line (H, fluorescence image).
Fig. 8. Immunolocalization of the actin and microtubule cytoskeletons in swollen root hairs. (A) Actin, mature wild-type root hair, projection of 12 confocal planes (depth, 10 µm). (B) Actin, GFP–AtRop6 Q64L finished swelling, projection of 27 planes (depth, 50 µm). (C) Tubulin, mature wild-type root hair, projection of eight planes (depth, 8 µm). (D and E) Tubulin, GFP–AtRop6 Q64L finished swellings, projections of nine planes each (depth, 11 µm). Bar, 25 µm.
Root hair swelling is characterized by delocalized Ca2+ gradients
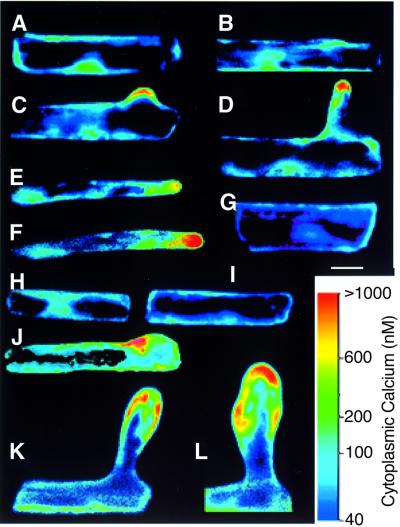
To study Ca2+ gradients in wild-type and mutant root hairs, we microinjected root hairs with Indo-dextran for confocal ratio imaging (Wymer et al., 1997). In wild-type root hairs (e.g. Wymer et al., 1997; Bibikova et al., 1999) and in uninduced GFP–Rop6 Q64L roots (Figure 9A–F), a tip-localized Ca2+ gradient was first visible after early bulge formation and disappeared at the end of tip growth. Early trichoblasts from induced seedlings had low Ca2+ levels similar to the uninduced control (Figure 9H and I). Swelling root hairs from induced GFP–Rop6 Q64L roots had delocalized Ca2+ gradients focused on where growth was occurring (Figure 9K and L). Delocalization of the Ca2+ gradient could already be observed at the early stage of tip growth (Figure 9J). GFP fusion protein was evenly distributed over the trichoblast plasma membrane (e.g. Figure 7H), and did not colocalize with the tip-high Ca2+ gradient. Also, expression of GFP–Rop6 Q64L in root cortical cells did not result in higher Ca2+ levels (Figure 9G), indicating that GTP-bound Rop is not sufficient to generate high Ca2+.
Fig. 9. Swelling root hairs contain multiple Ca2+ gradients. (A–F) Sequence of root hair formation in uninduced GFP–AtRop6 Q64L line. Early trichoblast (A) and early bulge formation (B) without high Ca2+ levels. (C) Start of tip growth with Ca2+ gradient in bulge. (D–F) Tip growth with tip-focused Ca2+ gradient. (G) Cortical cell (induced) with low Ca2+. (H–L) Sequence of root hair swelling in induced GFP–AtRop6 Q64L line. (H and I) Early trichoblasts with low Ca2+ levels. (J) Start of tip growth with slightly mislocalized Ca2+ gradient. (K and L) Isotropic growth with multiple Ca2+ foci. Ca2+ concentrations have been pseudocolour coded according to the inset scale. Bar, 25 µm.
Discussion
Striking differences exist in the conservation of several members of the small GTPase family in plants, indicating that some of the basic signalling pathways are fundamentally differently organized. This may be particularly relevant for the pathways controlling cellular polarity. The absence of homologues of Cdc42 in plants and the relative abundance of a Rho-related plant-specific family, named Rop, raises questions as to their precise functions. In this paper we show for the first time that two members of this family, AtRop4 and AtRop6, are involved in cell polarity control in Arabidopsis roots.
Cellular localization of Rop GTPases
In root meristem, endogenous Arabidopsis Rop GTPases localized predominantly to the plasma membranes, as was also true for GFP-tagged AtRop4 in BY-2 cells. N-terminal GFP tagging preserves the function and accurately reflects the intracellular localization of Rho GTPases (e.g. Larochelle et al., 1997; Kost et al., 1999; Bischoff et al., 2000; Michaelson et al., 2001), although overexpression of GFP–Rop may result in apolar localization in growing pollen tubes (Li et al., 1999). We also noticed that tip localization in growing root hairs detected with immunolocalization was much more pronounced than with GFP-tagged Rop, probably as a result of overexpression of the GFP fusion protein using the GVG system. The apolar plasma membrane localization of overexpressed Rop GTPases might be caused by insufficient levels of RhoGDI, needed for recycling of Rop to defined plasma membrane locations. In dividing tobacco BY-2 cells, GFP–AtRop4 strongly labelled the cell plate membrane. This could also be observed in cells induced for shorter periods, containing lower levels of GFP fusion protein just visible by epifluorescence microscopy, suggesting that cell plate localization was not a result of overexpression (data not shown). The cell plate is a site of actin assembly (Endlé et al., 1998), which might suggest a possible regulatory role for Rop GTPases in actin dynamics at this location. However, expression of wild-type or constitutively active GFP–AtRop4 in tobacco BY-2 cells did not visibly affect cell plate formation (data not shown). Our localization data raise the question of whether AtRop proteins would function similarly in cell plate formation and in tip growth, acting on the same effectors.
Involvement in root hair development
Genetic analysis indicates the existence of discrete steps in root hair development in Arabidopsis, namely bud site selection, budding and tip growth (as reviewed by Hülskamp et al., 1998; Schiefelbein, 2000). Pollen tube and root hair tip growth are highly similar with respect to the presence of a vesicle-rich apical region, a reverse-fountain type of cytoplasmic streaming and a tip-high Ca2+ gradient (Pierson et al., 1996; Wymer et al., 1997; de Ruijter et al., 1998; Yang, 1998). In elongating pollen tubes Rop GTPase is associated with the apical plasma membrane and has an important regulatory role in polar pollen tube growth (Lin et al., 1996; Lin and Yang, 1997; Kost et al., 1999; Li et al., 1999). The apical localization of Rop in tip-growing root hairs indicated a more general role for Rop GTPases in plant tip growth. This was verified here by the expression of constitutively active AtRop4 and AtRop6 in growing root hairs leading to loss of polarity. Our data correlate well with previous observations when constitutively active pollen Rop genes AtRac2 and AtRop1 were expressed in pollen tubes (Kost et al., 1999; Li et al., 1999), thus demonstrating a close conservation of Rop function in tip growth in sporophyte and gametophyte alike.
Pollen Rop proteins have been implicated in mediating a Ca2+-dependent pathway leading to exocytosis (Lin and Yang, 1997; Li et al., 1999). Whether pollen Rop proteins directly regulate Ca2+ influx or whether the effect is mediated by reorganization of cortical actin at the tube apex remains an important open question (Li et al., 1999; Zheng and Yang, 2000). The recently reported association of pollen Rop with PtdIns kinase activity, and the localization of PtdIns (4,5)P2 at the apical plasma membrane of pollen tubes, have provided evidence for an essential function of apical PtdIns (4,5)P2 in pollen tube elongation (Kost et al., 1999). In addition to its effect on actin organization, PtdIns (4,5)P2 was also suggested to be important as a substrate in inositol trisphosphate production and, consequently, in the generation of the tip-high Ca2+ gradient (Kost et al., 1999; Zheng and Yang, 2000). Our observation that polar localization of Arabidopsis Rop proteins precedes visible bulging on the trichoblast indicates a role for Rop proteins in root hair initiation as well as in tip growth. The early bulging of the trichoblast, unlike tip growth, is not accompanied by a tip-high Ca2+ gradient and is insensitive to Ca2+ channel blocker verapamil, suggesting that Ca2+ does not trigger the initiation of root hairs (Wymer et al., 1997). This indicates that polar localization of Rop proteins is independent of a localized Ca2+ influx, and might suggest that Rop proteins are not sufficient to create the tip-high Ca2+ gradient observed during the later stage of tip growth.
Root hair formation requires a local reorganization of the actin cytoskeleton. The axial actin bundles in growing root hairs are perpendicular to their orientation in the trichoblast (Baluška et al., 2000b). In the subapical region of growing root hairs these thick bundles branch off into ever thinner bundles. The apical vesicle-rich region is enriched with dense F-actin meshwork (Baluška et al., 2000b). This dynamic configuration of the actin cytoskeleton is thought to be required for delivery of secretory vesicles to the growth site. Bulging of the trichoblast, unlike tip growth, is insensitive to actin drugs (Miller et al., 1999; Ovecka et al., 2000). However, untreated bulges contain stabilizing actin filaments and F-actin-devoid bulges are mechanically unstable (Baluška et al., 2000b). Although it is not clear whether Rop GTPase regulates actin during trichoblast bulging, it might regulate the actin cytoskeleton during and after the transition from bulging to tip growth, which is F-actin dependent and coincides with a severing and rearrangement of actin filaments (Braun et al., 1999; Miller et al., 1999; Baluška et al., 2000b). The localization of Rop GTPase at the bud site was reminiscent of the polar localization of Cdc42 or Rho1p in yeast bud formation (Ziman et al., 1993; Yamochi et al., 1994).
Rop proteins in root meristem were present in transverse plasma membranes. Since the corresponding cross-walls are non-growing (Baluška et al., 2000a), this indicated that Rop GTPases are not exclusively associated with sites of high secretion. The cross-wall domains in root meristem are enriched in myosin VIII and actin, and act as putative F-actin organizing centres during mitosis and elongation (Reichelt et al., 1999; Baluška et al., 2000a). The corresponding localization of putative F-actin organizing centres and Rop GTPases in root meristem further suggested a regulatory role for Rop in actin dynamics.
Actin-disrupting drugs did not interfere with Rop localization to the root hair bud site. In contrast, BFA inhibited the early localization of Rop at the bud site, implying an essential role for Arf GTPase in the establishment of polarity in plant cells. BFA inhibits the enzyme activity of Arabidopsis GNOM Arf GEF, and causes internalization of the auxin efflux carrier PIN1 from its usual polar localization on the plasma membrane (Gälweiler et al., 1998; Müller et al., 1998; Steinmann et al., 1999). However, inhibition of polar auxin transport by NPA does not affect root hair initiation (Masucci and Schiefelbein, 1994), indicating that BFA does not inhibit polar localization of Rop by blocking polar auxin transport. An attractive possibility is that root hair initiation begins with an Arf-dependent, actin-independent process, such as secretion at an internal cue, and is then followed by polar localization of Rop.
The isotropic growth phenotype of root hairs was characterized by the presence of multiple Ca2+ foci that marked the sites where growth was occurring. In this respect, the swellings resembled root hairs treated with tubulin drugs (Bibikova et al., 1999). Since tubulin and actin drugs, exocytosis inhibitors and phytohormones may all cause root hair branching and swelling (Ovecka et al., 2000), this indicates that tip growth can be inhibited in various ways resulting in isotropic growth. In growing root hairs, new membrane is continuously added at the tip by secretory vesicle fusion, and the polar localization of Rop GTPase probably relies on inactivation and recycling from the flank to the tip. Overexpression of constitutively active Rop GTPase caused isotropic growth, indicating that inactivation or retrieval of Rop GTPase from the root hair flanks is required to restrict secretory growth at the tip. Since tip growth relies on the integrity of fine actin filament bundles and a dense F-actin meshwork at the root hair tips (Baluška et al., 2000b; Esseling et al., 2000), it is possible that the phenotype results primarily from an effect of constitutively active Rop GTPase on actin dynamics. The large increase in cell volume and surface compared with wild-type root hairs might result from persistent oversecretion of expansins (Baluška et al., 2000b), or else, be driven primarily by excessive vacuolar expansion. Cell elongation in the hypocotyl is an example of polar diffuse growth in plants and is dependent on the integrity of both the microtubule and actin cytoskeletons (Kropf et al., 1998; Baluška et al., 2000b). Swelling in elongating epidermal cells of the hypocotyl might thus result from a disorientation of the cytoskeleton in cells containing constitutively active Rop GTPase, but the precise steps of this process remain to be determined. The root hair and hypocotyl phenotypes indicated a central role for Rop proteins in both ways of polar growth.
Concluding remarks
In Arabidopsis root meristem, Rop GTPase was predominantly localized to transverse plasma membranes, and in trichoblasts was polarly localized during root hair formation, implying a role for Rop proteins in root hair budding as well as in tip growth. The inhibition of polar localization of Arabidopsis Rop proteins by BFA indicated an early role for Arf GTPase in root hair formation and also implied that AtRop function is closely connected to Arf-dependent vesicle trafficking. The cell swelling in the hypocotyl of transgenic seedlings expressing constitutively active AtRop4 implied a role for this particular Rop protein in elongation growth. The isotropic growth phenotypes of constitutively active AtRop4 and AtRop6 in root hairs demonstrated a similar role for different Rop proteins in both pollen and root hair tip growth. Moreover, our results show a general function of AtRop GTPases in polarized cell growth in plants.
Materials and methods
PCR and mutagenesis
AtRop4 (U52350), AtRop6 (U43501) and Rac-like (U88402) ESTs were obtained from the Arabidopsis Biological Resource Center. AtRab11c cDNA (U74669) was isolated from an Arabidopsis cDNA library (Bischoff et al., 2000). Mutations were made in AtRop4 (G15V and T20N) and in AtRop6 (Q64L). Wild-type inserts, mutant inserts and an AtRop4 fragment corresponding to the C-terminal 20 amino acids, were obtained by PCR using Pfu polymerase. PCR products were cloned into the EcoRI and SalI sites of pBluescript II SK, and sequenced to confirm the presence of desired mutations and the absence of PCR errors.
GFP DNA constructs
Rho GTPase cDNA inserts were directionally cloned behind the GFP open reading frame (ORF) of a modified version of pCKGFP (Reichel et al., 1996). pCATGFPnostop has a plant expression cassette containing the CaMV 35S promoter with a duplicated transcriptional enhancer, a tobacco etch virus translational enhancer (TL), a GFP S65C ORF with additional M153T and V163A mutations and no stop codon, and at the end the CaMV 35S polyadenylation signal. pBluescript Rho PCR clones were cut with BamHI–SalI and the inserts were cloned into the BamHI–XbaI sites of pCATGFPnostop. XhoI–HindIII blunt fragments lacking the enhanced CaMV 35S promoter were directionally cloned into the XhoI–SpeI blunt sites of pTA7002, which allows dexamethasone-inducible expression (Aoyama and Chua, 1997).
GTPase assays
Small GTPase cDNAs were cloned in-frame into pGEX-5x-1 (Smith and Johnson, 1988). GST–GTPase fusion proteins were purified from Escherichia coli strain DH5α, and GTPase assays were carried out using a preloading step with [γ-32P]GTP (Self and Hall, 1995).
Cultivation and transformation of tobacco BY-2 cells
Growth of tobacco BY-2 cells was according to Nagata et al. (1992), and transformation was performed using Agrobacterium strain GV3101 (pMP90). pTA7002 transformants were replica-plated on BY-2/agar medium containing 60 µM dexamethasone and allowed to grow for 48 h. Calli expressing inducible GFP fusion proteins were identified using fluorescence microscopy. GFP-positive calli were transferred to liquid BY-2 medium with selection and grown as cell suspensions.
Arabidopsis transformation and induction
Arabidopsis transgenic plants were obtained using Agrobacterium-mediated transformation. To observe root hair phenotypes, GFP–Rop fusion proteins were induced by growing seedlings in medium containing 0.5× MS salts, 1% sucrose and B5 vitamins, and 10 µM dexamethasone for 16–48 h.
Antibodies
Rabbit antisera were raised against gel-purified bacterial GST–AtRop4 antigen and affinity purified using His6-tagged AtRop4 protein.
Immunoprecipitation
Six-day-old suspension cultures of BY-2 transgenic lines were induced with 30 µM dexamethasone for 20 h. Cells were pelleted, resuspended in buffer A (50 mM Tris–HCl pH 7.6, 50 mM NaCl, 5 mM MgCl2, 20 µM dithiothreitol, 1% NP-40, 0.05% SDS and protease inhibitors), sonicated on ice and then centrifuged at 10 000 g for 15 min at 4°C. Anti-GFP monoclonal antibodies (Roche) were added to the supernatant, immune complexes were bound to protein G–CL-4B agarose beads and analysed by SDS–PAGE and western blotting according to standard procedures. Blots were incubated with affinity-purified anti-AtRop4 antibody, or with a polyclonal anti-GFP antibody (Molecular Probes), peroxidase-conjugated protein A (Pierce), followed by antigen detection using a chemiluminescent substrate (Pierce). AtRop4 antiserum was used in a similar way but using protein A–CL-4B agarose beads.
Immunolocalization and confocal microscopy
Whole mount immunofluorescence staining of Arabidopsis seedlings was performed according to Müller et al. (1998). Immunofluorescence detection of actin and tubulin in Arabidopsis seedlings was performed using the freeze-shattering procedure according to Braun et al. (1999).
Cytoplasmic [Ca2+] measurements
Root cells were microinjected with the fluorescent Ca2+-indicating dye Indo-1 conjugated to a 10 kDa dextran (Molecular Probes, Eugene, OR), and [Ca2+] was monitored by confocal ratio imaging, according to Wymer et al. (1997). When roots were induced with 10 µM dexamethasone, only cells showing GFP expression were used in subsequent microinjection and [Ca2+] measurements. In situ calibration using 10 µM Ca2+ ionophore (Br A23187) and a range of BAPTA Ca2+ buffers (Wymer et al., 1997) indicated that the responsiveness of Indo-1 to changes in [Ca2+] was unchanged in the GFP-expressing cells.
Acknowledgments
Acknowledgements
We thank Drs Frantisek Baluška, Matthias Godde, Peter Huijser, Lars Vahlkamp and Dieter Volkmann for help, criticism and constructive reading of the manuscript. We are grateful to Dr N.-H.Chua for providing us with pTA7002. The work was funded by the DFG, the European Communities Biotechnology Programme (Bio4-CT98 0239) and the INCO Copernicus Programme (IC15-CT96-0920). C.S.V.R. is the recipient of an Alexander von Humboldt fellowship and J.F. of a DAAD fellowship.
References
- Aoyama T. and Chua,N.-H. (1997) A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J., 11, 605–612. [DOI] [PubMed] [Google Scholar]
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature, 408, 796–815. [DOI] [PubMed] [Google Scholar]
- Baluška F., Barlow,P.W. and Volkmann,D. (2000a) Actin and myosin VII in developing root cells. In Staiger,C.J., Baluška,F., Volkmann,D. and Barlow,P.W. (eds), Actin: A Dynamic Framework for Multiple Plant Cell Function. Kluwer Academic, Dordrecht, The Netherlands, pp. 457–476.
- Baluška F., Salaj,J., Mathur,J., Braun,M., Jasper,F., Šamaj,J., Chua, N.-H., Barlow,P.W. and Volkmann,D. (2000b) Root hair formation: F-actin dependent tip growth is initiated by a local assembly of profilin-supported F-actin meshworks accumulated within expansin-enriched bulges. Dev. Biol., 227, 618–632. [DOI] [PubMed] [Google Scholar]
- Barlow P.W. and Baluška,F. (2000) Cytoskeletal perspectives on root growth and morphogenesis. Annu. Rev. Plant Physiol. Plant Mol. Biol., 51, 289–322. [DOI] [PubMed] [Google Scholar]
- Bibikova T.N., Blancaflor,E.B. and Gilroy,S. (1999) Microtubules regulate tip growth in Arabidopsis root hairs. Plant J., 17, 657–665. [DOI] [PubMed] [Google Scholar]
- Bischoff F., Molendijk,A., Rajendrakumar,C.S.V. and Palme,K. (1999) GTP-binding proteins in plants. Cell. Mol. Life Sci., 55, 233–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff F., Vahlkamp,L., Molendijk,A. and Palme,K. (2000) Localiz ation of AtRop4 and AtRop6 and interaction with the guanine nucleotide dissociation inhibitor AtRhoGDI1 from Arabidopsis. Plant Mol. Biol., 42, 515–530. [DOI] [PubMed] [Google Scholar]
- Braun M., Baluska,F., von Witsch,M. and Menzel,D. (1999) Redistribution of actin, profilin and phosphatidylinositol-4,5-bisphosphate in growing and maturing root hairs. Planta, 209, 435–443. [DOI] [PubMed] [Google Scholar]
- Collins C.C. and Johnson,D.I. (1997) An Arabidopsis thaliana expressed sequence tag cDNA that encodes a Rac-like protein. Plant Physiol., 113, 1463.9112785 [Google Scholar]
- Delmer D.P., Pear,J.R., Andrawis,A. and Stalker,D.M. (1995) Genes encoding small GTP-binding proteins analogous to mammalian rac are preferentially expressed in developing cotton fibers. Mol. Gen. Genet., 248, 43–51. [DOI] [PubMed] [Google Scholar]
- De Ruijter N.C.A., Rook,M.B., Bisseling,T. and Emons,A.M.C. (1998) Lipochito-oligosaccharides re-initiate root hair tip growth in Vicia sativa with high calcium and spectrin-like antigen at the tip. Plant J., 13, 341–350. [Google Scholar]
- Endlé M.-C., Stoppin,V., Lambert,A.-M. and Schmit,A.-C. (1998) The growing cell plate of higher plants is a site of both actin assembly and vinculin-like antigen recruitment. Eur. J. Cell Biol., 77, 10–18. [DOI] [PubMed] [Google Scholar]
- Esseling J., de Ruijter,N. and Emons,A.M.C. (2000) The root hair actin cytoskeleton as backbone, highway, morphogenetic instrument and target for signalling. In Ridge,R.W. and Emons,A.M.C. (eds), Root Hairs: Cell and Molecular Biology. Springer, Germany, pp. 29–52.
- Gälweiler L., Guan,C., Müller,A., Wisman,E., Mendgen,K., Yephremov,A. and Palme,K. (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science, 282, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Hülskamp M., Folkerts,U. and Grini,P.E. (1998) Cell morphogenesis in Arabidopsis. BioEssays, 20, 20–29. [DOI] [PubMed] [Google Scholar]
- Imamura H., Tanaka,K., Hihara,T., Umikawa,M., Kamei,T., Takahashi,K., Sasaki,T. and Takai,Y. (1997) Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J., 16, 2745–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D.I. (1999) Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev., 63, 54–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.-G., Fang,Y. and Singh,K.B. (1999) A glucocorticoid-inducible transcription system causes severe growth defects in Arabidopsis and induces defence-related genes. Plant J., 20, 127–133. [DOI] [PubMed] [Google Scholar]
- Kawasaki T., Henmi,K., Ono,E., Hatakeyama,S., Iwano M., Satoh,H. and Shimamoto,K. (1999) The small GTP-binding protein Rac is a regulator of cell death in plants. Proc. Natl Acad. Sci. USA, 96, 10922–10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjøller L. and Hall,A. (1999) Signaling to Rho GTPases. Exp. Cell Res., 253, 166–179. [DOI] [PubMed] [Google Scholar]
- Kost B., Lemichez,E., Spielhofer,P., Hong,Y., Tolias,K., Carpenter,C. and Chua,N.-H. (1999) Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J. Cell Biol., 145, 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf D.L., Bisgrove,S.R. and Hable,W.E. (1998) Cytoskeletal control of polar growth in plant cells. Curr. Opin. Cell Biol., 10, 117–122. [DOI] [PubMed] [Google Scholar]
- Larochelle D.A., Vithalani,K.K. and De Lozanne,A. (1997) Role of Dictyostelium racE in cytokinesis: mutational analysis and localization studies by use of green fluorescent protein. Mol. Biol. Cell, 8, 935–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Wu,G., Ware,D., Davis,K.R. and Yang,Z. (1998) Arabidopsis Rho-related GTPases: differential gene expression in pollen and polar localization in fission yeast. Plant Physiol., 118, 407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Lin,Y.K., Heath,R.M., Zhu,M.X. and Yang,Z.B. (1999) Control of pollen tube tip growth by a Rop GTPase dependent pathway that leads to tip-localized calcium influx. Plant Cell, 11, 1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y. and Yang,Z. (1997) Inhibition of pollen tube elongation by microinjected anti-Rop1Ps antibodies suggests a crucial role for Rho-type GTPases in the control of tip growth. Plant Cell, 9, 1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Wang,Y., Zhu,J. and Yang,Z. (1996) Localization of Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell, 8, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci J.D. and Schiefelbein,J.W. (1994) The RHD6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene-associated process. Plant Physiol., 106, 1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelson D., Siletti,J., Murphy,G., D’Eustachio,P., Rush,M. and Philips,M.R. (2001) Differential localization of Rho GTPases in live cells: regulation by hypervariable regions and RhoGDI binding. J. Cell Biol., 152, 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D.D., de Ruijter,N.C.A., Bisseling,T. and Emons,A.M.C. (1999) The role of actin in root hair morphogenesis: studies with lipochito-oligosaccharide as a growth stimulator and cytochalasin as an actin perturbing drug. Plant J., 17, 141–154. [Google Scholar]
- Moores S.L., Schaber,M.D., Mosser,S.D., Rands,E., O’Hara,M.B., Garsky,V.M., Marshall,M.S., Pompliano,D.L. and Gibbs,J.B. (1991) Sequence dependence of protein isoprenylation. J. Biol. Chem., 266, 14603–14610. [PubMed] [Google Scholar]
- Müller A. et al. (1998) AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J., 17, 6903–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T., Nemoto,Y. and Hasezawa,S. (1992) Tobacco BY2 cell line as the ‘HeLa’ cell in the cell biology of higher plants. Int. Rev. Cytol., 132, 1–30. [Google Scholar]
- Newman T. et al. (1994) Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol., 106, 1241–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson B. (1999) Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell. Signal., 11, 545–554. [DOI] [PubMed] [Google Scholar]
- Ovecka M., Nadubinska,M., Volkmann,D. and Baluška,F. (2000) Actomyosin and exocytosis inhibitors alter root hair morphology in Poa annua. Biologia, 55, 105–114. [Google Scholar]
- Pierson E.S., Miller,D.D., Callaham,D.A., Van Aken,J., Hackett,G. and Hepler,P.K. (1996) Tip-localized calcium entry fluctuates during pollen tube growth. Dev. Biol., 174, 160–173. [DOI] [PubMed] [Google Scholar]
- Reichel C., Mathur,J., Eckes,P., Langenkemper,K., Koncz,C., Schell,J., Reiss,B. and Maas,C. (1996) Enhanced green fluorescence by expression of an Aequoria victoria green fluorescent protein mutant in mono- and dicotyledonous plant cells. Proc. Natl Acad. Sci. USA, 93, 5888–5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt S., Knight,A.E., Hodge,T.P., Baluška,F., Šamaj,J., Volkmann,D. and Kendrick-Jones,J. (1999) Characterization of the unconventional myosin VIII in plant cells and its localization at the post-cytokinetic wall. Plant J., 19, 555–569. [DOI] [PubMed] [Google Scholar]
- Scheres B. and Heidstra,R. (1999) Digging out roots: pattern formation, cell division and morphogenesis in plants. Curr. Top. Dev. Biol., 45, 207–247. [DOI] [PubMed] [Google Scholar]
- Schiefelbein J.W. (2000) Constructing a plant cell. The genetic control of root hair development. Plant Physiol., 124, 1525–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiene K., Pühler,A. and Niehaus,K. (2000) Transgenic tobacco plants that express an antisense construct derived from a Medicago sativa cDNA encoding a Rac-related small GTP-binding protein fail to develop necrotic lesions upon elicitor infiltration. Mol. Gen. Genet., 263, 761–770. [DOI] [PubMed] [Google Scholar]
- Schmidt A. and Hall,M.N. (1998) Signaling to the actin cytoskeleton. Annu. Rev. Cell Dev. Biol., 14, 305–338. [DOI] [PubMed] [Google Scholar]
- Self A.J. and Hall,A. (1995) Measurement of intrinsic nucleotide exchange and GTP hydrolysis rates. Methods Enzymol., 256, 67–76. [DOI] [PubMed] [Google Scholar]
- Smith D.B. and Johnson,K.S. (1988) Single-step purification of polypeptides in Escherichia coli as fusions with glutathione S-transferase. Gene, 67, 31–40. [DOI] [PubMed] [Google Scholar]
- Steinmann T., Geldner,N., Grebe,M., Mangold,S., Jackson,C.L., Paris,S., Gälweiler,L., Palme,K. and Jurgens,G. (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science, 286, 316–318. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Sasaki,T., Mammoto,A., Takaishi,K., Kameyama,T., Tsukita,S. and Takai,Y. (1997) Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J. Biol. Chem., 272, 23371–23375. [DOI] [PubMed] [Google Scholar]
- Trotochaud A.E., Hao,T., Wu,G., Yang,Z. and Clark,S.E. (1999) The CLAVATA1 receptor-like kinase requires CLAVATA3 for the assembly into a signalling complex that includes KAPP and a Rho-related protein. Plant Cell, 11, 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valster A.H., Hepler,P.K. and Chernoff,J. (2000) Plant GTPases: the Rhos in bloom. Trends Cell Biol., 10, 141–146. [DOI] [PubMed] [Google Scholar]
- Van Aelst L. and D’Souza-Schorey,C. (1997) Rho GTPases and signaling networks. Genes Dev., 11, 2295–2322. [DOI] [PubMed] [Google Scholar]
- Winge P., Brembu,T. and Bones,A.M. (1997) Cloning and characterization of rac-like cDNAs from Arabidopsis thaliana. Plant Mol. Biol., 35, 483–495. [DOI] [PubMed] [Google Scholar]
- Wymer C.L., Bibikova,T.N. and Gilroy,S. (1997) Cytoplasmic free calcium distributions during the development of root hairs of Arabidopsis thaliana. Plant J., 12, 427–439. [DOI] [PubMed] [Google Scholar]
- Yamochi W., Tanaka,K., Nonaka,H., Maeda,A., Musha,T. and Takai,Y. (1994) Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J. Cell Biol., 125, 1077–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. (1998) Signaling tip growth in plants. Curr. Opin. Plant Biol., 1, 525–530. [DOI] [PubMed] [Google Scholar]
- Yang Z. and Watson,J.C. (1993) Molecular cloning and characterization of rho, a ras-related small GTP-binding protein from the garden pea. Proc. Natl Acad. Sci. USA, 90, 8732–8736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z.-L. and Yang,Z. (2000) The Rop GTPase switch turns on polar growth in pollen. Trends Plant Sci., 5, 298–303. [DOI] [PubMed] [Google Scholar]
- Ziman M., Preuss,D., Mulholland,J., O’Brien,J.M., Botstein,D. and Johnson,D.I. (1993) Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol. Biol. Cell, 4, 1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]