Abstract
Pyrazines impart a pronounced burnt flavour and are essential components in many processed foods. While certain microorganisms, notably bacteria within the genera Bacillus and Corynebacterium, as well as some fungi, can synthesize pyrazines. Their biosynthetic pathways remain poorly elucidated, and synthesis efficiency is notoriously low. This review provides a comprehensive synthesis of the current knowledge on microbial pyrazines biosynthesis. We critically analyze the putative pathways and detail the functional roles of key enzymes, such as L-threonine dehydratases and aminotransferases. Furthermore, we evaluate scenario-based strategies for utilizing cheap carbon sources, to enhance the economic feasibility of bioproduction. The significant barriers of low product yield and inherent microbial toxicity are also systematically examined. Finally, we discuss the potential of emerging candidate strains and cell-free metabolic engineering systems for pyrazines synthesis, aiming to establish a foundational roadmap for future research and development in this field.
Keywords: Pyrazines, Biosynthesis, Genetic engineering, Cheap carbon source, Metabolic engineering
Graphical abstract

Highlights
-
•
The biosynthetic pathways and enzymatic diversity of pyrazines are discussed.
-
•
Inexpensive carbon sources hold great promise for the biosynthesis of pyrazines.
-
•
Biosynthetic barriers and product tolerance limit the synthesis of pyrazines.
-
•
The construction of engineering strains promoted the application of pyrazines.
1. Introduction
Pyrazines are a class of 1,4-dinitrogen-substituted benzene compounds abundant in nature. These compounds have an apparent burnt or roasted nut aroma. Moreover, the compounds have characteristics such as low vapor pressure, easy volatilization, and low aroma perception threshold, making them critically important flavour components in a wide range of foodstuffs. The flavour contribution of pyrazines is closely related to their molecular structure and exhibits strong olfactory characteristics (Mortzfeld et al., 2020a, Mortzfeld et al., 2020b). Its sensory threshold is often at the ppb or even ppt level. Different substitution positions of pyrazines impart distinct odours to food, and the perceivable odor depends on the substance's dilution and its combination with other flavour components. In foods, their concentrations typically range from 1 ppm to 10 ppm (Fayek et al., 2023a, Fayek et al., 2023b). Pyrazines can be classified into various groups according to the substituents at one or more of the four ring carbon atoms in pyrazines as follows: alkylpyrazines, hydroxypyrazines, methoxypyrazines and acylpyrazines (Wagner et al., 1999; Adams et al., 2002). The formation of food flavours is largely attributed to the diversity of pyrazines, as shown in Table 1, this paper provides a detailed overview of the specific types of pyrazines, their main sources and their unique flavour contributions.
Table 1.
Sources and flavour contributions of key pyrazines in food.
| Kind | Flavour contributions | Sources | Reference | |
|---|---|---|---|---|
| Alkyl pyrazines | TTMP | Cocoa, Smoky, Woody herbal notes | Maillard reaction (MR), Strecker degradation (SD), Fuel oil, White pine oil, Cocoa butter, Cocoa beans, Coffee beans, Beef, Fruno, Mung beans, Pepper, Potato, Peanuts, Tea, Soybeans, Ligusticum wallichii, Insects, Bacillus subtilis, Bacillus beleriensis, Bacillus amyloliquefaciens, Bacillus licheniformis, Bacillus coagulans, Corynebacterium glutamicum, Pseudomonas aeruginosa, Actinobacillus thermophilus, Saccharomyces cerevisiae, Lactobacillus, Serratia | (Maga et al., 1973; Mattey and Harle, 1976; GRIMONT2 and Grimont, 1985; Rizzi, 1988; Jung et al., 1997; Leejeerajumnean et al., 2001; Koehler et al., 2006; Bolhuis et al., 2013; Silva-Junior et al., 2018; Xu et al., 2018; Cui et al., 2020; Liu et al., 2020) |
| 2,3,5-trimethylpyrazine | Cocoa, Nutty, Potato baked | MR, SD, Chitin pyrolysis, Cocoa beans, Tea, Soybeans, B. subtilis, Bacillus sphaericus, B. licheniformis, B. natto, Lactococcus | (Knorr et al., 1985; Jung et al., 1997; Zhang et al., 2019a, Zhang et al., 2019b; Kłosowski et al., 2021; Wang et al., 2021; Fayek et al., 2023a, Fayek et al., 2023b; He et al., 2023; Liu et al., 2024; Noda et al., 2025) | |
| 2,5-dimethylpyrazine | Cocoa aroma, Nutty, Bacon, Caramelised roast, Slight soil notes | MR, SD, Chitin pyrolysis, B. subtilis, E. coli, B. natto, P. aeruginosa | (Knorr et al., 1985; Jung et al., 1997; Müller and Rappert, 2009; Guo et al., 2018; Zhang et al., 2019a, Zhang et al., 2019b; Xu et al., 2020; Kłosowski et al., 2021; Wang et al., 2021; Liu et al., 2023; Noda et al., 2025) | |
| 2,6-dimethylpyrazine | Chocolate, Nnutty | Commonly used for cigarettes | (Jung et al., 1997; Kłosowski et al., 2021; Wang et al., 2021; Fayek et al., 2023a, Fayek et al., 2023b) | |
| 2,3-dimethylpyrazine | Coffee, Nutty, Roasty | MR, SD, Chitin pyrolysis, Bacillus strains | (Knorr et al., 1985; Ohata et al., 2020; Kłosowski et al., 2021; Wang et al., 2021) | |
| 3,6-dimethylpyrazine | Toasted sesame Aroma, Smoky | MR,SD,Aspergillus, Bacillus thermophilus | (Rizz, 1972; Rajini et al., 2011; Wang et al., 2021) | |
| 2-methylpyrazine | Nutty, Meaty, Smoky, Caramelised | MR,SD,Chitin pyrolysis, Asparagus Barley, Beans, Beef, Beer, Buckwheat, Cabbage, Cashew nut, Cherimoya, Chicken, B. subtilis, P. aeruginosa | (Knorr et al., 1985; Jung et al., 1997, 1999; Kłosowski et al., 2021; Patel et al., 2021; Fayek et al., 2023a, Fayek et al., 2023b; Lee et al., 2023; Api et al., 2024a, Api et al., 2024b, Api et al., 2024c, Api et al., 2024d, Api et al., 2024e) | |
| 2,5-diisopropylpyrazine | Nutty, Roasted, and Earthy | Paenibacillus polymyxa, Paenibacillus peoriae | (Beck et al., 2003; Aspray et al., 2013; Orban et al., 2023) | |
| 2,5-diethylpyrazine | Nutty, Grilled, Earthy | MR, SD, Beets, Cocoa beans, Tea | (Yu et al., 2012; Cherniienko et al., 2022; Yin et al., 2023) | |
| 2-ethyl-3-methylpyrazine | Nutty, Musty, Corn, Raw, Earthy, Oily | MR, SD, Allium species, Beef, Maize, Cashew nut, Rye, Chicken, Soybean, Cocoa category, Tea Bacillus, S. cerevisiae | (Api et al., 2024; Fan et al., 2024) | |
| 2-ethyl-5-methylpyrazine | Green pepper, Earthy | MR, SD, Allium species, Popcorn, Cocoa category, Pork, Filbert, Hazelnut, Potato chips, Malt, Rice cake, Mentha oils, Rum, Streptomyces setonii | (Yang et al., 2023; Api et al., 2024a, Api et al., 2024b, Api et al., 2024c, Api et al., 2024d, Api et al., 2024e; Qin et al., 2024) | |
| 2-ethyl-6-methylpyrazine | Nutty aroma | MR, SD, Tea, Beef | (Yang et al., 2023; Wei et al., 2024; Gai et al., 2025) | |
| 2-ethyl-3,5-dimethylpyrazine | Caramelised, Smoky, Cocoa | MR, SD, Tea, S. cerevisiae | (Burdock and Carabin, 2008; Liu et al., 2024) | |
| 2-ethyl-3,6-dimethylpyrazine | Caramelised aroma, Smoky, Grassy | MR, SD, S. cerevisiae | Burdock and Carabin (2008) | |
| 3-ethyl-2,5-dimethylpyrazine | Roasted, Nutty | MR, SD, Coffee, Chocolate, Roasted meats, B. subtilis | (Motoyama et al., 2021; Gao et al., 2025) | |
| 3-ethyl-2,6-dimethylpyrazine | Roasted, Nutty | Beer, Malt, Cocoa category, Peanut, Coffee, Rapeseed, Licorice, Shrimps, Maize, Soybean | (Yin et al., 2023; Api et al., 2024a, Api et al., 2024b, Api et al., 2024c, Api et al., 2024d, Api et al., 2024e) | |
| 2,3-diethyl-5-methylpyrazine | Caramel | MR, SD, Beef, Pecan, Cocoa category, Pork, Coffee, Potato, Mustard, Pumpkin seed oil, Peanut, Rapeseed | (Guo et al., 2021; Yin et al., 2023; Api et al., 2024a, Api et al., 2024b, Api et al., 2024c, Api et al., 2024d, Api et al., 2024e) | |
| 3-isopropylpyrazine | Nutty, Roasted, Slightly herbal | Chondromyces crocatus, Alphaproteo bacteria | Dickschat et al. (2005) | |
| 3,5-diethyl-2-methylpyrazine | Parsley root | MR, SD, Oats and oat cereals | McGorrin (2019) | |
| Hydroxy pyrazines | Aspergillic acid | Commonly used in antimicrobials | Aspergillus, Penicillium | MacDonald (1970) |
| Pulcherriminic acid | Food preservative and antibacterial | Candida pulcherrima | MacDonald (1962) | |
| 2-hydroxyyrazine | Nutty, Burnt | MR, Grapes, Rhodocoocus erythropolis, Arthrobacter | (Müller and Rappert, 2009; Sun et al., 2024) | |
| 2-hydroxy-3,5-dimethyl-6-butylpyrazine | Caramelised, Pasty | MR | Zhao et al. (2020) | |
| 2-hydroxymethyl-3,6-diethyl-5-methylpyrazine | Caramelised, Pasty | MR | Zhao et al. (2018) | |
| 3-isobutyl-2-hydroxypyrazine | Fresh fragrance | Coffee beans, Vitis vinifera, Capsicum annuum, Potato, E. coli | (Ryona et al., 2010; Harris et al., 2012; Frato, 2019) | |
| 3-isopropyl-2-hydroxypyrazine | Plant fragrance | Coffee beans, Potato, E. coli | Frato (2019) | |
| Methoxy pyrazines | 2-methoxy-3-(1-methylpropyl) pyrazine | Fresh, Green, Woody, Ambery, Oriental, Musky, Minty, Herbaceous | Halomonas venusta, Myxobacterium Chondromyces crocatus, Marine Bacteria, Pea | (Bungert et al., 2001; Dickschat et al., 2005; Xu et al., 2020; Zhao et al., 2020) |
| 3-methoxy-2,5-dimethylpyrazine | Peppery, Earthy | Grapes, Bell peppers, Pea shoots, Tea, Chondromyces crocatus, Alphaproteo bacteria, Rhizobium excellensis | (Dickschat et al., 2005; Chatonnet et al., 2010) | |
| 2-isopropyl-3-methoxypyrazine | Raw green, Earthy | Lysobacter enzymogenes, Labidostomis lusitanica, Peppers, Grapes | (Emde et al., 1992; Cherniienko et al., 2022; Zhou et al., 2022; López et al., 2023) | |
| 3-isobutyl-2-methoxypyrazine | Herbaceous odours, Give wine a bad smell | Grape skins, Peas, Cucumbers, Wine | (Ling et al., 2021; Li et al., 2022; Zamolo and Wüst, 2022) | |
| 3-isopropyl-2-methoxypyrazine | Herbaceous odours, Give wine a bad smell | Grape skins, Peas, Cucumbers, Wine, Bell peppers | (Murray and Whitfield, 1975; Lei et al., 2023; Yuan et al., 2025) | |
| 2-isobutyl-3-methoxypyrazine | Herbaceous odours, Give wine a bad taste | Grape skins, Peas, Cucumbers, Wine, Labidostomis lusitanica | (Emde et al., 1992; López et al., 2023) | |
| 3-sec-butyl-2-methoxypyrazine | Cucumber | Cucumber, Beet, Carrot, Trehalose, Serratia, Cedecea Strains | (Gallois and Grimont Patrick, 1985; Ryona et al., 2010; Schneider et al., 2024) | |
| Acyl pyrazines | 2-acetylpyrazine | Popcorn, Nutty | MR, Lipid oxidation, Aspergillus oryzae | (Maga et al., 1973; Ali, 2010; Gao et al., 2024) |
| 2-acetyl-3-methylpyrazine | Toasted almonds | MR, Clam, Pork Cocoa category, Potato, Coffee, Scallop, Lobster, S. cerevisiae | (Api et al., 2023; Liu and Quan, 2024) | |
| 2,5-dimethyl-3-(methylsulfanyl) pyrazine | Nutty, Caramelised | Chondromyces crocatus, Alphaproteobacteria | Dickschat et al. (2005) | |
Researchers have widely explored alkylpyrazines due to their complex chemical structures and diverse biosynthetic pathways. Alkylpyrazines are used in several fields, such as pharmaceutical intermediates, intermediates of essence, fragrance and antimicrobial agents (Ho et al., 1989; Schmid et al., 2001; Masuda and Mihara, 2002; Schöck et al., 2018). The significance of alkylpyrazines is particularly evident in traditional fermented beverages. A prime example is Chinese Baijiu, where they are the primary flavour substances, significantly enhancing the aging flavour of high-quality liquor, especially the Daqu Maotai-flavour type. The content of 2,3,5,6-tetramethylpyrazine (TTMP) in Baijiu can range from a few micrograms per liter to dozens of milligrams per liter, providing a distinctive baking and burnt aroma. The TTMP contents in Maotai-flavour Baijiu, sesame-flavour Baijiu, and mixed-flavour Baijiu are significantly higher compared to other Baijiu flavours (Yan et al., 2020), demonstrating how structural diversity of pyrazines directly translates to specific sensory profiles.
In addition, the application of pyrazines in key fields such as medicine and agriculture has increased owing to scientific advances (MacDonald, 1973; Griffin et al., 1990; Dolezal et al., 2002; Moser, 2008). Quinoxaline is an important aromatic benzopyrazines heterocyclic compound widely used in pesticides (Sharma et al., 2017; Montero et al., 2022; Syam et al., 2022), medicine (Zayed et al., 2019; Ghorab et al., 2020; Zayed, 2022), dyes, and polymer synthesis (Sharma et al., 2017; Abu-Hashem and Al-Hussain, 2022; Peppas et al., 2022). Phenazine is a pyrazine with anti-tumor, antibacterial, and diuretic properties (Chaudhary and Khurana, 2017; Liu et al., 2020). TTMP have various medicinal activities, such as capturing superoxide anion, reducing the production of nitrogen oxides by human granulocytes, increasing coronary flow, reducing arterial pressure and coronary resistance, and inhibiting the effects of epinephrine and potassium chloride on the contraction of isolated rabbit arteries (Wu et al., 1999; Zhang et al., 2003). Consequently, several researchers have explored this compound. Hydroxypyrazines has antiviral activity, anthelmintic activity and special optical properties. Therefore, it has broad potential applications in the fields of medicine, agriculture, and material science. Pyrazines derivatives such as 6-fluoro-3-hydroxypyrazine-2-carboxamide and 3-hydroxypyrazine-2-carboxamide are effective antiviral drugs (Cai et al., 2017; Huchting et al., 2018; Nakayama and Honda, 2021). Hydroxypyrazines derivatives can be used to prepare optical nanomaterials, fluorescent probes, and optical sensors due to their unique optical properties. In addition, they have potential applications in the fields of biological imaging, cell labeling, and optical sensing (Zhang et al., 2014; Chen et al., 2015; Gawne et al., 2018). Acylpyrazines are oxygen-containing pyrazine derivatives formed by the substitution of the hydrogen atom on the pyrazine ring with an acyl group, and are used in drug synthesis (Opletalová et al., 2002; Siriwardana et al., 2004; Feng et al., 2007).
Population growth, enhanced lifespan, and other demands have significantly increased the need for biosynthesis of pyrazines and pyrazine derivatives. The demand for biosynthetic pyrazines food additives has increased due to enhanced public awareness of food safety (Román et al., 2017a, Román et al., 2017b). The increase in chronic diseases has significantly increased the demand for exploring the potential of pyrazines as a therapeutic drug. However, most of the pyrazines are commercially produced by chemical synthesis (Mortzfeld et al., 2020a, Mortzfeld et al., 2020b; Choudhary et al., 2022). Generally, chemical synthesis typically yields racemic mixtures, and the separation of racemic mixtures is often difficult to achieve chemically, resulting in the target product carrying unpleasant off-flavours. Due to the high stereospecificity of enzyme-catalysed biosynthesis, only a single enantiomer or specific isomer is typically produced. The aroma thresholds and characteristics of different enantiomers significantly influence the flavor profile. Flavor compounds derived from microbial fermentation constitute a complex mixture. This spectrum of secondary metabolites imparts a richer, more harmonious flavor profile that cannot be replicated by individual chemically synthesized molecules (Longo and Sanromán, 2005; Serra et al., 2005; Berger, 2009). Furthermore, consumers generally perceive “natural” foods as superior to synthetic alternatives in terms of taste, safety, and health benefits. This cognitive bias leads to significant preference differences in sensory evaluations (Román et al., 2017a, Román et al., 2017b). The price of pyrazines varies greatly, mainly depending on their production methods. For instance, the price of chemical synthesis or natural extraction of 2,5-dimethylpyrazine (2,5-DMP) ranges from 200 to 3500 $/kg (Mortzfeld et al., 2020a, Mortzfeld et al., 2020b). The use of pyrazines in various industries is expected to significantly increase due to global economic development and market progress. Currently, only a few studies have explored the pyrazines biosynthetic pathways. Some bacteria and fungi synthesize pyrazines through the MR and SD (Hwang et al., 2002). Pyrazines are typically produced from amino acids and sugars through various reactions, such as the MR. Low-molecular-weight alkylpyrazines are probably derived from dihydropyrazines, which are unstable and easily oxidized. Several high-molecular pyrazines are often synthesized from amino acids (Rajini et al., 2010).
This review focuses on the biosynthetic route and key enzyme systems in pyrazines synthesis in the context of the food industry. In addition, approaches to achieve green, efficient and environmentally friendly biosynthesis of pyrazines using cheap carbon source substitution strategies are summarized. The limitations in biosynthesis and microbial tolerance caused by product accumulation, reducing the yield of pyrazines, are discussed in depth, and feasible solutions are proposed in this paper. Cutting-edge strategies for enhancing the biosynthetic potential of pyrazines in terms of screening and optimization of food-grade candidate microbial resources are outlined. Moreover, metabolic engineering studies under in vitro simulated food fermentation environments are proposed. These prospective discussions provide theoretical guidance for subsequent research on pyrazines synthesis to achieve higher yields and safer and better flavour.
2. Biosynthetic pathways of pyrazines
2.1. Amino acid metabolic pathways
Among the many biosynthetic pathways for pyrazines, the amino acid metabolic pathway is one of the more common. Alanine (Ala) and glycine (Gly) are the two main substrates for the formation of pyrazines in the MR, and previous studies have shown that Ala and Gly not only provide a nitrogen source but also contribute to the formation of the alkyl side chains of some alkylpyrazines (Amrani-Hemaimi et al., 2002; Van Lancker et al., 2011). In some microorganisms, lysine (Lys) is also involved in the biosynthesis of pyrazines (Rajini et al., 2011). Lys undergoes a series of enzymatic reactions to produce α-aminoadipic acid semialdehyde, which is condensed with another amino acid and undergoes cyclisation and dehydrogenation to form pyrazines with specific structures. This pathway has been demonstrated in some filamentous fungi, and the key enzymes and reaction conditions of this pathway vary among microorganisms (Ito et al., 1989; Hijarrubia et al., 2002).
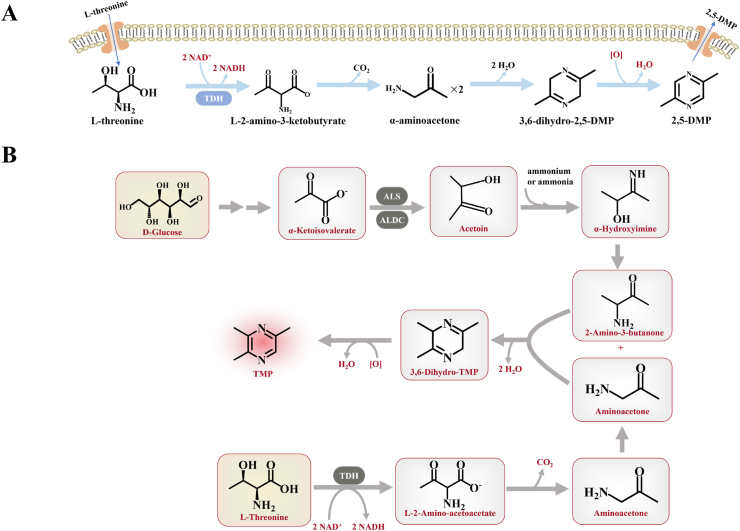
In addition, L-threonine (L-thr) is an important substrate for the synthesis of pyrazines. The synthesis of 2,5-DMP and 2,3,5-trimethylpyrazine (TMP), often derives its precursors from L-thr metabolites. Zhang et al. identified L-thr as the substrate for the synthesis of 2,5-DMP (Fig. 1 A), TMP was produced by Bacillus subtilis 168 using L-thr and D-glucose as the substrates. Both of these biosynthetic pathways involve enzyme-catalysed and non-enzyme-catalysed reactions (Fig. 1 B) (Zhang et al., 2019a, Zhang et al., 2019b). Researchers redesigned and optimized the biosynthesis pathway for 2,5-DMP and the mechanism of substrate transmembrane transport in an L-thr high-yield E. coli T6-47-7 strain, achieving a yield of 1.43 g/L of 2,5-DMP (Xu et al., 2020).
Fig. 1.
A) Proposed 2,5-DMP synthetic pathway in B. subtilis.B) Proposed TMP synthetic pathway in B. subtilis. L-threonine-3-dehydrogenase (TDH); α-acetolactate synthase (ALS) and α-acetolactate decarboxylase (ALDC).
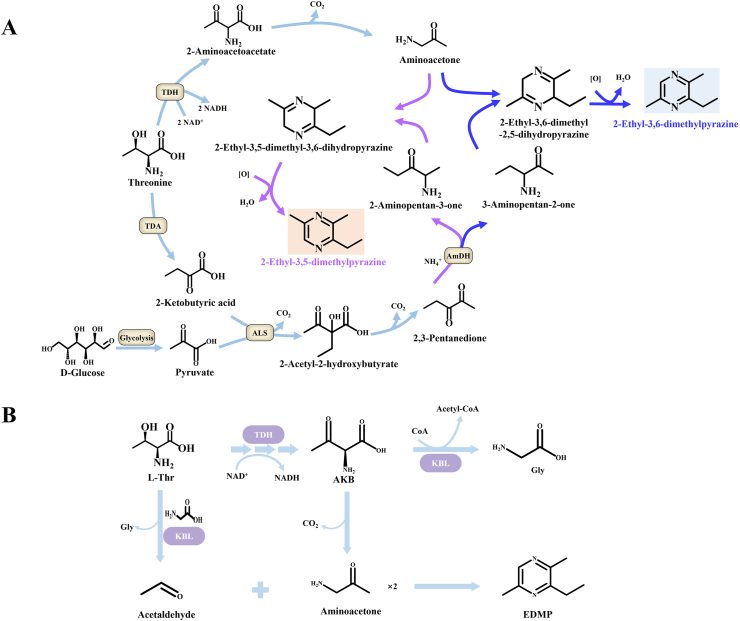
B. subtilis utilizes a biosynthetic pathway to produce 2-ethyl-3,5(3,6)-dimethylpyrazines (EDMPs) under mild conditions, using aminoacetone and 2,3-pentanedione as key intermediates (Fig. 2 A) (Zhang et al., 2020). A separate chemoenzymatic pathway enables the synthesis of 3-ethyl-2,5-dimethylpyrazine from L-thr. In this route, L-thr is first enzymatically converted to 2-amino-3-ketobutyrate (AKB), which subsequently provides acetaldehyde. The final step involves the condensation of two aminoacetone molecules with one acetaldehyde molecule (Fig. 2 B) (Motoyama et al., 2021a, Motoyama et al., 2021b). Notably, a key commonality is that both routes converge on aminoacetone as a crucial precursor for pyrazine ring formation. Therefore, enhancing the accumulation of aminoacetone could serve as a universal strategy for increasing the yield of EDMPs and their analogues across different production systems. Biosynthetic pathways for the production of methoxypyrazines, such as 2-methoxy-3-isopropylpyrazine (2M3IP) and 3-isobutyl-2-methoxypyrazine (IBMP), have been identified in living organisms. However, the enzymes involved in the synthetic process have not been identified (Cheng et al., 2002; Lei et al., 2018). A recent study showed that Pseudomonas fluorescens SBW25 can synthesize monocyclic pyrazines, and a pap genes cluster is involved in the formation of the pyrazine ring. This process can serve as a novel enzymatic tool for the synthesis of pyrazine derivatives from amino acids (Masuo et al., 2019).
Fig. 2.
A) Pathways for the biosynthesis of EDMPs in B. subtilis. Threonine deaminase (TDA) and amine dehydrogenase (AmDH). B) Proposed pathway for the synthesis of EDMP from L-thr using TDH and 2-Amino-3-ketobutyrate coenzyme A (CoA) ligase (KBL). TDH converts L-thr to AKB, which is metabolized to Gly through the acetyltransferase activity of KBL under normal conditions. However, under malnutrition and low CoA concentrations, the unstable AKB is gradually decarboxylated to aminopropanone, and the cleavage activity of the KBL enzyme breaks down L-thr into acetaldehyde and Gly. EDMP is chemically and enzymatically synthesized from L-thr through the condensation of two molecules of aminopropanone and one molecule of acetaldehyde.
Aspergillic acid is a hydroxamic acid-containing pyrazinone with antibiotic properties (White and Hill, 1943). Radiolabelled experiments demonstrate that aspergillic acid is produced from L-leucine (Leu) and L-isoleucine (Ile) residues (MacDonald, 1970). Lebar et al. reported gene cluster 11 responsible for aspergillic acid biosynthesis in Aspergillus Circumdati (Lebar et al., 2019).
Pulcherriminic acid is a cyclodipeptide derived from cyclo (L-Leu-L-Leu) in Bacillus and various yeast species (Uffen and Canale-Parola, 1972; Sipiczki, 2006; Cryle et al., 2010). Early studies achieved a high pulcherriminic acid yield of 556.1 mg/L through the Leu pathway. In addition, multistep metabolic engineering was exploited in B. licheniformis, involving overexpressing the Leu biosynthetic gene, deletion of Leu branched-chain pathway, upregulating leucyl-tRNA expression, replacing promoter, and enhancing pulcherriminic acid secretion (Fig. 3) (Wang et al., 2020). Some proteins involved in pucherriminic acid biosynthesis were identified in the past. For example, Snf2 regulates pucherriminic acid biosynthesis in yeast (Gore-Lloyd et al., 2019), and PchR is involved in pulcherriminic acid yield in B. subtilis (Randazzo et al., 2016). 3-hydroxypyra-zine-2-carboxylic acid, 3-hydroxy-5-methylpyrazine-2-carboxylic acid and 3-hydroxy-5-chloropyrazine-2-carboxylic acid were identified in Ralstonia/Burkholderia sp. strain DSM 6920. However, the biosynthetic pathways for these hydroxylated pyrazines are still unclear. Currently, the biosynthetic information for several hydroxylated pyrazines is lacking.
Fig. 3.
Metabolic engineering of B. licheniformis for enhanced production of pulcherriminic acid. Acetolactate synthase-like (IlvBH), ketol-acid reductoisomerase (IlvC), dihydroxy-acid dehydratase (IlvD), leucyl-tRNA synthetase (LeuS), 2-oxoisovalerate dehydrogenase E1 component subunit beta (BkdAB), cyclo (L-leucyl-L-leucyl) synthase (YvmC), pulcherriminic acid synthase (CypX), LeuABCD (2-isopropylmalate synthase, 3-isopropylmalate dehydrogenase).
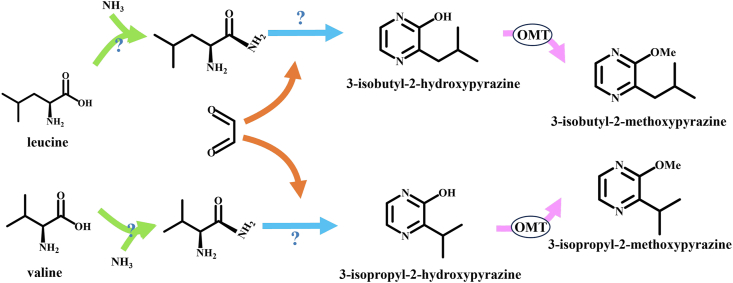
Oxygenate pyrazines, such as alkoxy-substituted pyrazines are present in several foodstuffs. In higher organisms, alkylpyrazines undergo several metabolic reactions and are excreted through the kidney after undergoing hydroxylation. Cytochrome P450 converts the alkylpyrazines to the corresponding alcohol substituents. Subsequently, they are oxidized to yield the corresponding carboxylic acids (Yamamoto et al., 1987; Kremer et al., 2019). However, the synthetic and degradation pathways of oxygenated pyrazines have not yet been confirmed. (Dihydro)pyrazine-N-oxides((d)PNOs) are synthesized in Pseudomonas entomophila L48. N-oxygenase enzyme PvfB catalyzes the N-oxygenation of the α-amine group in valine (Val). This oxygenation step is essential in the biosynthesis of (d) PNO (Morgan and Li, 2020). Currently, researchers have not elucidated the complete pathway of methoxypyrazines biosynthesis. However, various biosynthetic pathways have been proposed. The last step of the pathway involves condensation of amino acids (such as Leu and Val) and NH3 with dicarbonyl compounds to form 3-alkyl-2-hydroxypyrazine, which is further converted to methoxypyrazines under the catalytic activity of O-methyltransferase (OMT) (Fig. 4) (Murray and Whitfield, 2006; Dunlevy et al., 2010; Frato, 2018).
Fig. 4.
A proposed pathway for the biosynthesis of 3-isopropyl-2-methoxypyrazine (IPMP) and IBMP from Val and Leu amino acids, respectively. OMT catalyzes the last step, which involves methylation of hydroxypyrazine to methoxypyrazine.
Currently, there are two main hypotheses regarding the biosynthetic mechanism of 3-sec-butyl-2-methoxypyrazine (SBMP). Hypothesis one proposes that the precursors are amidated derivatives of branched-chain amino acids: IBMP, IPMP and SBMP are generated from Leu, Val, and Ile, respectively, by amidation, and then subsequently undergo condensation with α-/β-dicarbonyl compounds (e.g., glycolaldehyde, acetaldehyde) to form pyrazine cyclohexyls (Gallois et al., 1988). Hypothesis two suggests that the synthesis of SBMP begins with the formation of a cyclic dipeptide intermediate from two amino acid molecules via a condensation reaction, followed by oxidative modification and methoxylation to complete the structural assembly (Cheng et al., 1991). Although both pathways use amino acids as precursors and the end product synthesis steps involve condensation and cyclisation, it is unclear how the intermediate metabolites are synthesized, and most studies have focused on the last step.
2.2. Glucose metabolism pathway
The glucose metabolic pathway is also one of the important pathways for the biosynthesis of pyrazines. Glucose can be used as a key carbon skeleton precursor to activate the pyrazines synthesis pathway. Researchers isolated and screened two strains of P. aeruginosa from wine cork, and systematically regulated the ratio of carbon and nitrogen sources through in vitro fermentation experiments, and found that pyrazines synthesis was significantly and positively correlated with the increase in glucose concentration (Bañeras et al., 2013). Several microorganisms typically convert glucose to acetoin (3-hydroxy-2-butanone) through the glycolytic pathway, avoiding acidosis (Kosuge et al., 1962; Zhang et al., 2017). Acetoin is a precursor of methylpyrazines, such as TMP/TTMP (Karp et al., 2011; Xiao et al., 2014a, Xiao et al., 2014b). Acetoin is produced from acetolactate through glycolysis, and further TTMP is biosynthesized. However, the subsequent step in this process is unclear. The researchers developed an efficient TTMP synthesis strategy based on a temperature-controlled two-step process: firstly, glucose was bioconverted to 30.1 g/L acetoin by using B. subtilis at 37 °C, and then 8.34 g/L high-purity (99.9 %) TTMP crystals were generated by diammonium phosphate-mediated spontaneous conversion in a high-temperature reaction at 95 °C, whereby the by-products were self-decomposed by a high-temperature self-decomposition mechanism. The by-products were effectively eliminated by high-temperature self-decomposition mechanism. This strategy was validated in multiple strains by coupling biological and chemical processes in a staged temperature-controlled manner, which provides an innovative pathway for the green and efficient synthesis of TTMP (Xiao et al., 2014a, Xiao et al., 2014b).
In the biosynthesis of pyrazines, L-thr metabolism has been widely demonstrated as a central pathway for the construction of the pyrazines backbone (Zhang et al., 2020; Liu and Quan, 2024).The metabolic pathway of 2,5-DMP relies on efficient carbon flux supply of glucose substrates through the L-thr branch. To enhance this metabolic flux, Vytautas Petkevičius and other researchers used a metabolic engineering strategy to heterologously express the key L-thr biosynthesis genes, thrB and thrC, in Pseudomonas malodorata KT2440, ultimately achieving 2,5-DMP yields up to 168 ± 20 mg/L (Petkevičius et al., 2025). Previous studies have shown that the formation mechanism of pyrazines in vinegar production processes involves the dual contribution of non-enzymatic reactions (e.g., the MR) and microbially driven enzymatic degradation processes. Their production is characterised by a staged approach: glucose generates pyrazines precursors via glycolysis during the fermentation phase, and the increased efficiency of precursor conversion during the ageing phase contributes to a significant increase in pyrazines content. The key metabolic pathways and key genetic differences in the fermentation process lead to different kinetic responses of pyrazines at different phases (Zhang et al., 2022; Yang et al., 2024).
2.3. Newly discovered biosynthetic pathways
In recent years, some new biosynthetic pathways of pyrazines have been revealed with increasing research. One study found a pyrazines synthesis pathway dependent on special coenzymes in certain extremophile microorganisms. These microorganisms are able to convert simple nitrogenous and carbonaceous substrates into pyrazines under special environmental conditions using unique enzyme systems. In the production of soy sauce-flavoured Baijiu, chose to cultivate heat-resistant strains in a high-temperature environment, and these microorganisms are able to produce the unique pyrazines that are the chemical roots of its soy sauce flavour. Li et al. (2019) screened a heat-resistant actinomycete strain, Laceyella sacchari FBKL4.010, from Daqu, which was able to metabolise and produce large amounts of pyrazines under a solid-state fermentation system. Through genomic mining of the halophilic strain Halomonas elongata 153B, researchers discovered a gene encoding a L-thr dehydratase-like enzyme (euIB) in its genome, which is a key component of the pyrazines synthesis pathway. Additionally, this strain exhibits a robust aspartate metabolic flux, providing abundant precursor materials (Thr) for pyrazines synthesis. However, the complete pyrazines synthesis pathway still requires further bioinformatics analysis and experimental validation (Enuh and Aytar Çelik, 2022). Notably, another study on a salt field isolate of a halophilic strain showed that the strain was capable of synthesising 2,3-butanediol (2,3-BD) and pyrazines derivatives, but the specific biosynthetic mechanism of its pyrazines remains to be elucidated (Selvarajan et al., 2017).
Unique pyrazines biosynthetic pathways have also been identified in some marine microorganisms. The special environment of marine microorganisms, such as high salinity and low temperature, has led them to evolve different metabolic patterns from terrestrial microorganisms. It has been shown that more than twenty pyrazines are present in the volatiles released by two strains of the slime mold Chondromyces crocatus and seven strains of the marine Alphaproteobacteria from the ocean, which are significantly different in structure and function (Dickschat et al., 2005). By high performance liquid chromatography coupled with ultraviolet detection (HPLC-UV), Jin et al. (Kim et al., 2022) fractionated and purified the crude extracts of Streptomyces sp. CNP-944, a strain of Alphaproteobacteria isolated from marine sediments, and ultimately succeeded in isolating and identifying two structurally novel pyrazine alkaloid compounds.
3. Key enzymes in biosynthetic processes
3.1. Transfer enzyme
3.1.1. Transaminases
In the biosynthesis of pyrazines, transaminases (Tra) provide key precursors for the formation of the pyrazine ring by catalysing amino transfer reactions. Tra have a broad substrate specificity and are able to act on a wide range of amino acids and α-keto acids (Bezsudnova et al., 2020). Their catalytic activity is influenced by coenzymes, which bind to enzyme proteins to form active centres that participate in the amino transfer process of the substrate molecule. Before the MR, proteins or amino acids are first aminated by stereoselective Tra mediating the amination of specific amino donors, followed by oxidative dimerisation to form pyrazine ring structures (Rizzi, 2005). Peng et al. developed a biological-chemical approach to form TTMP from acetaldehyde. The process involves the optimization of formolase (FLS) expression and activity, leading to a 94 g/L yield of TTMP, which is the highest TTMP yield obtained through biosynthesis (Peng et al., 2020). Several enzymes, such as amino acid dehydrogenase (ADH) and other Tra, are involved in TTMP biosynthesis (Fig. 5 A). These enzymes provide ammonium ions in the last step of TTMP synthesis (Li et al., 2019).
Fig. 5.
A) TTMP biosynthetic pathway and other related metabolic pathways in B. licheniformis BL1. alsS, aldC, bdhA, and ldh genes encode ALS, ALDC, BDH and lactic dehydrogenase (LDH), respectively. The tricarboxylic acid cycle (TCA), nitric oxide dioxygenase (NOD). Oxidative decarboxylation occurs through non-enzymatic catalysis. Disrupted pathway steps are indicated by arrow breaks. B) Proteins are converted to free amino acids by proteases and Tra and further acted upon by ADH to provide ammonium ions for the final step in TTMP synthesis. Letter S indicates nonenzymatic spontaneous condensation reactions.
3.1.2. O-methyltransferase
OMT methylates a variety of secondary metabolites such as pyrazines, alkaloids and flavonoids (Lam et al., 2007; Byeon et al., 2014). To date, researchers have identified four OMT genes involved in methoxypyrazines biosynthesis (VvOMT1, VvOMT2, VvOMT3 and VvOMT4) (Vallarino et al., 2011; Dunlevy et al., 2013a, Dunlevy et al., 2013b; Lei et al., 2018). In recent years, the involvement of OMT as a key enzyme in the synthesis of alkylpyrazines has been reported. Previous findings indicate that OMT identified in from Vitis vinifera L. cv. Cabernet Sauvignon can methylate hydroxy-alkylpyrazine, which is the last step in the biosynthesis of methoxy-alkylpyrazine (Dunlevy et al., 2013a, Dunlevy et al., 2013b; Hashizume et al., 2014).
3.2. Dehydrogenases
3.2.1. Amino acid dehydrogenase
Dehydrogenases play a role in several aspects of pyrazines biosynthesis, and are mainly responsible for catalysing the dehydrogenation of substrate molecules, promoting pyrazines ring formation and structural stabilisation. TDH is a NAD-dependent enzyme that catalyzes the dehydrogenation of the hydroxyl groups of the side chain of L-thr, generating 2-amino-3-keto-butyrate (Boylan and Dekker, 1981; Marcus and Dekker, 1993; Shimizu et al., 2005), which can spontaneously under non-enzymatic conditions The latter can spontaneously decarboxylate under non-enzymatic conditions to form aminoacetone (Laver et al., 1959; Elliott, 1960). It was shown that aminoacetone can be converted to 3,6-dihydro-2,5-DMP by non-enzymatic dimerisation and dehydration reactions, and further converted to 2,5-DMP under oxidative conditions (Robacker et al., 2009). However, another report describes the presence of an aminopyrazine oxidase (AAO) in Streptococcus pyrazineus that catalyzes the condensation of two aminopyrazine molecules to form 3,6-dihydro-2,5-DMP, followed by oxidation to form 2,5-DMP (Molla et al., 2014). This finding indicates that there are two pathways for the synthesis of pyrazines, non-enzymatic and enzymatic, and its dominant mechanism is regulated by species specificity and environmental conditions. Several researchers believe that acetoin combines with the ammonia from amino acid residues through the action of amino dehydrogenase to form TTMP by thermodynamic action, indicating that the step is a non-enzymatic spontaneous reaction (Rizzi, 2002).
3.2.2. Butanediol dehydrogenase
2,3-Butanediol dehydrogenase (BDH) is a key enzyme in the synthesis of TTMP, catalysing the conversion between ethylene diphosgene and 2,3-BD, which affects the yield of TTMP. Meng et al. used Bacillus licheniformis BL1 as an experimental strain to overexpress ALDC. Acetaldehyde was added to the fermentation process, resulting in a TTMP yield of 43.75 g/L (Meng et al., 2020). Moreover, TTMP production was enhanced by disrupting bdhA in B. licheniformis BL1 and the addition of 2,3-BD. The engineered strain produced 46.98 g/L of TTMP through microaerobic fermentation (Fig. 5 B) (Meng et al., 2016). In general, metabolic engineering for TTMP biosynthesis comprises three strategies: (i) over-expression of ALDC and ALS; (ii) mutation of key enzymes in the branch pathway, such as BDH, an enzyme that catalyzes conversion of acetoin to 2,3- butanediol; (iii) addition of intermediate metabolites in the fermentation process, such as 2,3-BD or acetaldehyde. These strategies ensure a significant increase in the flux from pyruvate to acetoin, subsequently increasing TTMP yields.
4. Pathway scenarios for the synthesis of pyrazines using cheap carbon sources
Glucose is a major carbon source for pyrazines synthesis. Substrate costs typically account for 20 %–40 % of the total cost of microbial fermentation processes, it is an important factor affecting the cost of pyrazines synthesis (Jin et al., 2025). The available pathways for pyrazines biosynthesis are limited and technically demanding. These pathways require advanced knowledge and technology from various fields, such as genetic engineering, effective control of culture conditions and enzyme engineering (Xiao et al., 2006, 2007; Xu et al., 2018). In addition, the extraction of natural pyrazines is costly, inefficient and unsustainable. Conversely, pyrazines biosynthesis is expensive, significantly limiting the application of pyrazines (Zhu et al., 2009; Xiao et al., 2014a, Xiao et al., 2014b; R.-B. Jia et al., 2017; Peng et al., 2020). Several industrial by-products, agricultural or forestry wastes, as well as some fruits and vegetables with high sugar content, such as molasses, corn stover, wheat bran, lignocellulose, bagasse, and organic matter in wastewater, can be used as raw materials by micro-organisms. These materials are widely available, renewable, and cheap. Consequently, they are suitable for various biosynthesis processes and can be used to replace glucose as the carbon source for pyrazines synthesis (El-Kadi et al., 2021; Li et al., 2022; Bibi et al., 2023; Kumar et al., 2023). Table 2 provides a systematic comparison of studies on the synthesis of pyrazines and their precursor substances using various cheap carbon sources, revealing core characteristics and key driving factors in this field. Pretreatment is a critical first step in unlocking the value of waste materials. Whether through the optimized acid treatment of distillers' grains from white wine to enhance yield, or the necessary sulfuric acid treatment of sugarcane molasses to remove inhibitors and achieve significant productivity, pretreatment plays an indispensable role. Additionally, the characteristics of different cheap carbon sources significantly influence the selection of technological routes. Complex or inhibitor-rich carbon sources (such as molasses and lignocellulosic hydrolysate) require efficient pretreatment detoxification as well as potential strain tolerance modifications. Waste by-products containing mixed sugars necessitate metabolic engineering to enable co-utilization of sugars. Nutrient-rich carbon sources (such as soybean meal) can leverage their inherent carbon-nitrogen balance to simplify the process. Although current research focuses on TTMP and its precursors, this review demonstrates that by integrating the ‘pretreatment → engineered strains → fermentation optimization’ strategy, this approach has the potential to efficiently convert widely available, low-cost waste carbon sources into high-value pyrazines and their precursors, offering significant cost advantages.
Table 2.
A comparison of research cases on the synthesis of pyrazines using different cheap carbon sources.
| Carbon source type | Pretreatment/processing method | Key process | Target product | Yield | Costs | References |
|---|---|---|---|---|---|---|
| Distiller 's grains | Dilute acid pretreatment; Fermentated by B. subtilis TTMP20. | Optimization of detoxification conditions for distiller's grains hydrolysate. | TTMP | 705.27 mg/L | 34-66 $/t | (Gan et al., 2023; Zheng et al., 2024) |
| Corncob | Alkali-treated; Fermentated by B. subtilis IPE5-4-UD-4. | Composite mutation;Isothermal simultaneous saccharification and fermentation. | Acetoin | 0.46 g/g | 50-30 $/t | (Jia et al., 2017; Zhang, 2023) |
| Ignocellulosic hydrolysates | Fermentated by engineered B. subtilis ZB02. | The overexpression of transporter (AraE) and key enzymes (XylA and XylB) enables it to utilize glucose, xylose and arabinose simultaneously. | Acetoin | 11.20 g/L | / | Zhang et al. (2016) |
| Sugarcane molasses | Fermentation after treatment with sulfuric acid; Soybean meal as nitrogen sources. | Knockout the key genes bdhA and acoA in the acetoin catabolism pathway. | TTMP | 1328.95 mg/L | 120 $/t | (Zhou et al., 2019; Li et al., 2023) |
| Okara hydrolysate | Batch fermentation and fed-batch fermentation; Fermentated by B. subtilis BSO3. | Knockout the key genes bdhA and acoA; overexpression of the arabinose transporter-encoding gene (araE). | TTMP | 5.33 g/L (Batch fermentation);13.37 g/L (Fed-batch fermentation) | 32-83 $/t | Li et al. (2023) |
5. Limitations to the biosynthesis of pyrazines and product tolerance challenges
Pyrazines biosynthesis is a complex process involving multiple enzymes. Mutations in the genes that encode the enzymes involved in the synthetic pathway (such as α-acetyllactate decarboxylase and α-acetyllactate synthetase) can inhibit the pyrazines synthesis. In addition, the microbial strains and the activities of enzymes involved in pyrazines synthesis are affected by environmental factors such as temperature, pH, and light. Alkylpyrazines are mainly derived from the MR and microbial metabolism. The effectiveness of the MR is highly correlated with various factors, such as the reaction time, temperature, and pH. The optimum pH of the MR is 5.0-8.0. Consequently, the generation of pyrazines is more effective in weakly alkaline conditions. Under suitable conditions, more pyrazines are generated, and higher pyrazines yields are obtained under higher temperatures and longer reaction times (Sun et al., 2021). Microbial diversity decreases at high temperatures during the fermentation of soy sauce-flavoured Baijiu. However, thermophilic bacteria (such as B. subtilis) that produce pyrazines become the dominant flora (Wang et al., 2017).
The tolerance of microorganisms to substrates is also a major factor influencing the pyrazines synthesis rate. Acetoin is the precursor of TTMP. This compound can be synthesized by various microorganisms (Bae et al., 2016; Jang et al., 2017; Mao et al., 2017; Dai et al., 2018; Lu et al., 2020). However, it is toxic to cells, and an increase in acetoin concentration decreases the metabolic rate of the microorganisms, preventing further increase in pyrazines yield (Wang et al., 2019). Cui et al. achieved efficient production of acetoin by heterologous co-expression of fusion proteins (ALS and ALDC), lactate dehydrogenase and NADH oxidase, and optimization of the cell biotransformation conditions (Cui et al., 2023). Metabolic pathway competition or alterations in the metabolic pathway can also affect pyrazines synthesis. Zhang et al. observed that the presence of 2-amino-3-ketobutyric acid CoA ligase, which catalyzes the synthesis of Gly from L-thr in the 2,5-DMP synthetic pathway, affects the synthetic efficiency of 2,5-DMP (Zhang et al., 2019a, Zhang et al., 2019b). Genetic engineering techniques allow the modulation of microbial gene expression and metabolic pathways to improve pyrazines yields. However, heterologous expression of genes imposes a metabolic burden on the host cell. Studies report that dysregulated expression of NADH oxidase causes intracellular NAD+/NADH ratio imbalance, which significantly affects cell growth and metabolic activities and ultimately hinders pyrazines biosynthesis (Zhang et al., 2016). The use of microbial fermentation for pyrazines production has developed rapidly in recent years. However, strains with high pyrazines production potential have challenges in adapting to actual fermentation environments. In addition, it is challenging to determine the mode and number of additions, achieve nutrient regulation, and their tolerance to substrates.
6. Prospects for pyrazines synthesis
6.1. Mining and transformation of candidate strains
Pyrazines biosynthesis represents a promising green alternative to chemical synthesis. While enzyme catalysis, genetic engineering, and microbial modification offer viable routes, the selection of optimal microbial chassis is critical for scaling production. Current research primarily leverages two bacterial hosts: B. subtilis (native pathway specialist) and E. coli (engineered platform), each with distinct advantages and limitations as systematically compared in Table 3. B. subtilis is widely used in pyrazines synthesis. B. subtilis isolated from dacron, natto, vinegar spirits, and distillers' grains effectively biosynthesizes various alkylpyrazines (Zhang et al., 2017; Kłosowski et al., 2021; Jäger et al., 2022). Some scholars have genetically modified B. subtilis by knocking out the dehydrogenase gene (bdhA) to achieve different degrees of pyrazines content enhancement. E. coli is an important strain widely used in the production of commercial chemicals. E. coli has several advantages such as fast growth, easy cultivation and handling, high product expression, metabolic diversity, and higher safety (Zhang et al., 2016, Zhang et al., 2016; Yang et al., 2020), and can be genetically engineered, making it suitable for pyrazines biosynthesis.
Table 3.
Comparison of B. subtilis and E. coli as chassis for pyrazines biosynthesis.
| Attribute | B. subtilis | E. coli |
|---|---|---|
| Native pathway capacity | High (TTMP/alkylpyrazines via acetoin) | None (requires full heterologous pathway) |
| Max. reported titer | 13.37 g/L TTMP (Li et al., 2023) | 2.90 g/L 2,5-DMP (Liu et al., 2024) |
| Metabolic flexibility | Limited (alkylpyrazines dominant) | High (engineered for various pyrazines) |
| Key engineering targets | Precursor retention (bdhA, acoA) | Pathway assembly + Cofactor balancing (NADH/NADPH) |
| Process scalability | Robust in waste-based media (e.g., molasses) | Demands defined media; Sensitive to inhibitors |
B. subtilis excels in native alkylpyrazines synthesis owing to its endogenous acetoin pathway and spontaneous non-enzymatic cyclisation, whereas E. coli requires complete heterologous pathway engineering but offers superior metabolic flexibility for non-natural pyrazines. The rapid growth and well-characterized genetics of E. coli facilitate rapid prototyping, though its titers for classical pyrazines like TTMP consistently lag behind B. subtilis as quantified in Table 3. Metabolic engineering breakthroughs in both hosts demonstrate scalable production potential. Significant E. coli achievements include combinatorial expression of acetoin biosynthesis genes and NADH oxidase yielding 68.4 g/L acetoin (Xu et al., 2018). Xu et al. established a genetically engineered E. coli for efficient production of (3R)-Ediochrome. Optimization of fermentation variables and supplemented batch culture resulted in a maximum (3R)-Ediochrome yield of 60.3 g/L (Xu et al., 2015). In a previous study, a metabolic pathway for the fermentative production of 2,5-DMP was established using glucose as a raw material with a threonine-producing E. coli strain as a host. This study provided novel insights into the ab initio synthesis of 2,5-DMP in microorganisms (Xu et al., 2020). Yang constructed a recombinant E. coli strain by CRISPR-cas9 knockdown of the kbl gene to inhibit the strongly competing carbon flow branching metabolic pathway of the 2,5-DMP synthetic pathway. A 2,5-DMP yield of 1528.0 mg/L was achieved through a whole-cell catalytic synthesis using the recombinant strain (Yang, 2021), and integration of L-thr dehydrogenase with NADH oxidase achieving 2.9 g/L 2,5-DMP. This high yield demonstrates the great potential of metabolic pathway optimization in improving the efficiency of target product synthesis (Liu et al., 2024). In contrast, B. subtilis genetic modifications remain primarily focused on precursor retention strategies such as bdhA deletion, with fewer reports of complex pathway integrations. This dichotomy underscores a fundamental trade-off—B. subtilis provides 'plug-and-play' simplicity for natural pyrazines production, while E. coli enables bespoke design of novel structures at the cost of extensive genetic engineering, as systematically compared in Table 3.
6.2. In vitro metabolic pathway studies
In vitro metabolism can overcome the influence of other metabolic pathways in the cell. Precise control of the synthetic process is achieved by precisely modulating the reaction conditions and substrate supply, resulting in high purity and yield of the synthesized product (Zhu and Percival Zhang, 2015). In vitro metabolism allows access to enzymatic systems or cellular extracts for pyrazines synthesis from multiple sources, increasing the diversity of the synthesized pyrazines. Furthermore, in vitro metabolism does not require the entire microbial cell growth and reproduction process, minimizing environmental pollution and the waste of resources (Ricca et al., 2011). In vitro metabolism has several potential applications in drug synthesis, chemical synthesis, biosensors, and bioenergy (Zhang, 2010, 2011; Zhang et al., 2020; Chiba et al., 2021; Kang et al., 2023). Recently, an innovative in-situ cofactor regeneration strategy was designed and verified. This method achieves the direct conversion of D-xylose to high optical purity (R) -acetoin and ethylene glycol in a single reaction system without additional ATP input, showing the advances in the field of in vitro synthesis (Jia et al., 2018). The biological systems that produce acetoin through in vitro metabolic engineering include three types: single enzyme system, double enzyme system and multi-enzyme system. The single-enzyme, double-enzyme and multi-enzyme systems have high feasibility for cell-free biocatalytic production of acetoin (Cui et al., 2021). Using in vitro metabolic engineering for pyrazines biosynthesis and optimizing the pyrazines synthetic pathway can provide novel approaches for the synthesis and further applications of pyrazines.
6.3. Multi-omics techniques to resolve synthesis mechanisms
Research on the specific mechanism of pyrazines biosynthesis remains limited, with most studies presenting contradictory findings. Multi-omics techniques, by integrating genomics, isotope tracing, transcriptomics and metabolomics, provide a systematic solution to resolve these ambiguities.
6.3.1. Genomics: Decoding genetic blueprints for pyrazines biosynthesis
Genomics serves as the foundational framework for understanding pyrazines biosynthesis, enabling researchers to identify core gene clusters, evolutionary conservation, and potential functional genes that underpin metabolic capabilities. By sequencing and annotating the entire genome of pyrazines-producing microorganisms, this approach reveals the genetic potential for pyrazines synthesis, which can then be validated through transcriptomics or isotope tracing. Whole-genome sequencing of L. FBKL4.010 has identified key enzymes (e.g., proteases, peptidases, and acetolactate synthase) in the TTMP metabolic pathway, enabling reconstruction of its biosynthetic network. Annotation analysis reveals two novel gene clusters distinct from known TTMP synthases: one encoding an ADH and another encoding a TTMP-essential transaminase (Li et al., 2019). Through whole-genome sequencing analysis, Gan et al. (2024) confirmed the presence of complete acetolactate and ammonia synthesis pathways in Bacillus sp. TTMP2, thereby elucidating the biosynthetic route of TTMP production. However, the underlying regulatory mechanisms remain unresolved. Li et al. (2017) isolated a novel strain Paenibacillus aceti L14T from vinegar mash. Genome sequencing analysis identified 50 peptidase-related and 4 amino acid dehydrogenase-related coding sequences, and the strain harbors complete biosynthetic pathways for Val, Leu and Ile, as well as genes encoding L-thr dehydratase and ketone acid reductase isomerase, both crucial for pyrazines biosynthesis.
6.3.2. Isotope tracing: resolving precursor flux and catalytic nodes
Intramolecular isotope labeling provides indispensable validation of metabolic fluxes in pyrazines biosynthesis by enabling atom-specific tracking. Cheng et al. targeted the ambiguous precursor of 2M3IP in Pseudomonas. They used position-specific 13C-labeled pyruvate ([2-13C] pyruvate and [3-13C] pyruvate). A choice based on pyruvate’s role as a central metabolite for amino acid synthesis to track isotope incorporation into 2M3IP. Mass spectrometry revealed 13C enrichment in the isopropyl and methoxy moieties, directly confirming Val (isopropyl donor), Gly (pyrazine ring carbon), and methionine (methoxy source) as precursors. (Cheng et al., 2002). B. subtilis 168 was previously used as the experimental strain and the microbial synthesis pathways of 2,5-DMP and TMP were successfully evaluated by isotope tracing and genetic engineering techniques. Combined with the results of whole-cell catalysis, it was confirmed that L-thr can be the sole substrate for the production of 2,5-DMP by B. subtilis. (Zhang et al., 2019a, Zhang et al., 2019b). Nawrath et al. focused on Myxobacteria’s synthesis of 2,5-isopropylpyrazine and 2,6-isopropylpyrazine, which were hypothesized to derive from Val. The microbial synthesis pathway of 2,5-isopropylpyrazine and 2,6-isopropylpyrazine in Myxobacteria was explored using differently labeled Val anhydride and Val as exogenous substrates via isotopic tracer techniques (Nawrath et al., 2010).
6.3.3. Transcriptomics and beyond: Elucidating gene regulation and metabolic networks
Transcriptome sequencing has emerged as a pivotal tool for elucidating key genes and regulatory factors in pyrazines biosynthesis, particularly in non-model strains. In their seminal work, Wu Huang (Huang, 2022; Wang et al., 2024) leveraged this technology to map the TTMP synthetic pathway by profiling gene expression dynamics, revealing critical enzymatic bottlenecks. To dissect metabolic regulation, the team further introduced exogenous sodium acetate at graded concentrations, implementing an integrated monitoring framework that tracked real-time biomass kinetics, quantified ackA (acetate kinase gene) expression via qPCR, and correlated these shifts with acetic acid, acetoin, and TTMP accumulation. This multi-omics approach uncovered how sodium acetate reprograms central metabolism, redirecting carbon flux through acetate kinase, modulating NADH/NAD+ redox balance. Parallel efforts in strong-aroma Baijiu microbiota identified a high-yield TTMP strain through comparative transcriptomics, which implicated three core metabolic modules, including carbohydrate metabolism, cell motility, and amino acid metabolism. Crucially, novel regulators were discovered, including uracil phosphoribosyltransferase and glycosyltransferases, providing not only mechanistic insights but actionable targets to amplify TTMP production in industrial fermentation (Liu et al., 2023). Collectively, these advances demonstrate how transcriptomics transcends gene cataloging to resolve metabolic checkpoints and adaptive responses, establishing a blueprint for precision engineering of microbial pyrazines factories.
Current studies have validated the value of single omics, but integrated multi-omics will be more powerful for resolving complex pyrazines synthesis mechanisms. These findings indicate that genome sequencing, transcriptome analysis, metabolome and other multi-omics techniques can be used to evaluate the expression differences of various genes, key enzymes and metabolic pathways associated with the biosynthesis of different pyrazines types in the future. In addition, these findings provide a basis to evaluate the correlation between substrates, microorganisms and metabolites, explore the biosynthetic mechanisms of pyrazines, providing new research ideas.
6.4. Current status of pyrazines metabolic pathway exploration
To the best of our knowledge, there are no studies describing the natural degradation or metabolism of pyrazines in the public literature, and there is no information on the degradation of alkylpyrazines by fungi. Few studies report the degradation of alkylpyrazines using bacterial isolates. Rappert isolated and described bacterial strains that can use various alkylpyrazines as the sole carbon and energy source (Rappert et al., 2006; Müller and Rappert, 2009; Kutanovas et al., 2013a, Kutanovas et al., 2013b). However, there are no reports describing the pathways involved in the degradation of alkylpyrazines in these bacteria. Kutanovas et al. identified the genes involved in the degradation of pyrazines by bacteria and isolated the intermediate metabolites in the TTMP degradation pathway (Kutanovas et al., 2013a, Kutanovas et al., 2013b). The study provided key information on the biodegradation of these N-heterocyclic compounds and revealed that various enzymes can be used for selective and specific biotransformation reactions. These few reports indicate that the metabolic pathways involved in pyrazines degradation have not been widely explored.
7. Conclusion and future prospective
This review systematically summarizes the current knowledge base on microbial pyrazines synthesis, identifies multiple biosynthetic pathways centered on amino acid and glucose metabolism, and elaborates on the functional roles of key enzymes including TDH, ALS, ALDC, and OMT. By summarising the reported synthetic pathways for alkylpyrazines and alkoxypyrazines (Fig. 6), a clear framework is provided for a deeper understanding of their biosynthetic mechanisms. By analyzing strategies for utilizing cheap carbon sources, it demonstrates the feasibility of replacing traditional raw materials with industrial and agricultural waste for green manufacturing. Simultaneously, it reveals two core challenges facing the field. On the one hand, low product synthesis efficiency stems from incomplete pathway elucidation, insufficient catalytic capacity of key enzymes, and complex intracellular metabolic competition. On the other hand, poor tolerance of microbial hosts to pyrazine and its precursors severely limits the construction of high-yield strains and fermentation process optimization.
Fig. 6.
Summary of the pathways for biosynthesis of alkylpyrazines and alkoxypyrazines.
To advance current laboratory findings toward industrial application, future research may focus on the following actionable technical pathways. At the mechanism elucidation level, efforts must go beyond simple gene cluster annotation. Isotope labeling combined with high-resolution mass spectrometry should be employed to track intermediates and validate the primary synthetic pathway for target products. Concurrently, adaptive laboratory evolution techniques should be used to screen for tolerant mutant strains. Whole-genome sequencing comparisons can then precisely identify key genes responsible for toxicity tolerance to acetoin and pyrazines. Regarding synthetic system construction, strategies should be refined. Within the cell, the core metabolic network of engineered strains can be reprogrammed. For instance, the pyruvate node can be dynamically regulated using CRISPRi technology to precisely redirect carbon flux toward the L-thr branch, while heterologously assembling highly active dehydrogenases, transaminases, and other enzymes. At the extracellular level, a cell-free system integrating dehydrogenase, transaminase, and coenzyme regeneration modules must be developed. This system will focus on achieving one-pot synthesis of pyrazine compounds using inexpensive carbon and nitrogen substrates, thereby circumventing limitations imposed by cell membrane transport and metabolic homeostasis. Finally, in process integration, targeted enzymatic pretreatment will be developed for different complex feedstocks to eliminate inhibitors. Intelligent fermentation strategies will be designed to promote cell proliferation during the growth phase and accelerate pyrazines synthesis during the stationary phase, maximizing the spatiotemporal yield of the final product. Beyond economic and environmental advantages, a critical task in biosynthesis involves precisely comparing sensory quality differences between biosynthesized and chemically synthesized pyrazines, including aroma intensity, threshold levels, and flavor harmony. This not only constitutes the core value for high-end applications of bio-manufactured products but also provides essential data support and rational justification for market access.
In summary, despite the tremendous potential demonstrated by microbial synthesis of pyrazines, the path from fundamental research to industrialization remains fraught with challenges. By implementing the aforementioned specific research strategies, we aim to systematically overcome existing bottlenecks. The research team will leverage the high-yield pyrazine strains already screened to prioritize breakthroughs in the efficient in vitro and in vivo synthesis of alkylpyrazines. We are committed to establishing cost-competitive green manufacturing pathways to drive large-scale application.
CRediT authorship contribution statement
Wenhua Tong: Methodology, Investigation, Writing-original draft, Project administration, Formal analysis. Yan Wang: Software, Visualization, Formal analysis, Writing-original draft. Ying Yang: Conceptualization, Supervision, Project administration. Yongfang Zou: Supervision, Resources. Huibo Luo: Supervision. Jizhou Pu: Investigation, Resources. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Innovation Fund of Postgraduate, Sichuan University of Science & Engineering (NO. Y2024199).
Handling Editor: Professor Aiqian Ye
Contributor Information
Wenhua Tong, Email: tongwh@suse.edu.cn.
Jizhou Pu, Email: power@tuopai.biz.
References
- Abu-Hashem A.A., Al-Hussain S.A. Design, synthesis of new 1,2,4-Triazole/1,3,4-Thiadiazole with spiroindoline, Imidazo[4,5-b]quinoxaline and Thieno[2,3-d]pyrimidine from Isatin derivatives as anticancer agents. Molecules. 2022;(3):27. doi: 10.3390/molecules27030835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams T.B., Doull J., Feron V.J., Goodman J.I., Marnett L.J., Munro I.C., Newberne P.M., Portoghese P.S., Smith R.L., Waddell W.J., Wagner B.M. The FEMA GRAS assessment of pyrazine derivatives used as flavor ingredients. Food Chem. Toxicol. 2002;40(4):429–451. doi: 10.1016/s0278-6915(01)00123-5. [DOI] [PubMed] [Google Scholar]
- Ali M. Production of pyrazine flavours by mycelial fungi. https://api.semanticscholar.org/CorpusID:82822686
- Amrani-Hemaimi M., Cerny C., Fay L.B. Mechanisms of formation of alkylpyrazines in the maillard reaction. J. Agric. Food Chem. 2002;43(11):2818–2822. doi: 10.1021/jf00059a009. [DOI] [Google Scholar]
- Api A.M., Belsito D., Botelho D., Bruze M., Burton G.A., Cancellieri M.A., Chon H., Dagli M.L., Dekant W., Deodhar C., Fryer A.D., Jones L., Joshi K., Kumar M., Lapczynski A., Lavelle M., Lee I., Liebler D.C., Moustakas H., Muldoon J., Penning T.M., Ritacco G., Romine J., Sadekar N., Schultz T.W., Selechnik D., Siddiqi F., Sipes I.G., Sullivan G., Thakkar Y., Tokura Y. RIFM fragrance ingredient safety assessment, 3-ethyl-2,6-dimethylpyrazine, CAS Registry Number 13925-07-0. Food Chem. Toxicol. 2024;183 doi: 10.1016/j.fct.2023.114266. [DOI] [PubMed] [Google Scholar]
- Api A.M., Belsito D., Botelho D., Bruze M., Burton G.A., Cancellieri M.A., Chon H., Dagli M.L., Dekant W., Deodhar C., Fryer A.D., Jones L., Joshi K., Kumar M., Lapczynski A., Lavelle M., Lee I., Liebler D.C., Moustakas H., Na M., Penning T.M., Ritacco G., Romine J., Sadekar N., Schultz T.W., Selechnik D., Siddiqi F., Sipes I.G., Sullivan G., Thakkar Y., Tokura Y. RIFM fragrance ingredient safety assessment, 2-ethyl-3-methylpyrazine, CAS Registry Number 15707-23-0. Food Chem. Toxicol. 2024;183 doi: 10.1016/j.fct.2023.114360. [DOI] [PubMed] [Google Scholar]
- Api A.M., Belsito D., Botelho D., Bruze M., Burton G.A., Cancellieri M.A., Chon H., Dagli M.L., Dekant W., Deodhar C., Fryer A.D., Jones L., Joshi K., Kumar M., Lapczynski A., Lavelle M., Lee I., Liebler D.C., Moustakas H., Na M., Penning T.M., Ritacco G., Romine J., Sadekar N., Schultz T.W., Selechnik D., Siddiqi F., Sipes I.G., Sullivan G., Thakkar Y., Tokura Y. RIFM fragrance ingredient safety assessment, 2-ethyl-5-methylpyrazine, CAS registry number 13360-64-0. Food Chem. Toxicol. 2024;183 doi: 10.1016/j.fct.2023.114367. [DOI] [PubMed] [Google Scholar]
- Api A.M., Belsito D., Botelho D., Bruze M., Burton G.A., Cancellieri M.A., Chon H., Dagli M.L., Dekant W., Deodhar C., Fryer A.D., Jones L., Joshi K., Kumar M., Lapczynski A., Lavelle M., Lee I., Liebler D.C., Moustakas H., Na M., Penning T.M., Ritacco G., Romine J., Sadekar N., Schultz T.W., Selechnik D., Siddiqi F., Sipes I.G., Sullivan G., Thakkar Y., Tokura Y. RIFM fragrance ingredient safety assessment, 2-methylpyrazine, CAS Registry Number 109-08-0. Food Chem. Toxicol. 2024;183 doi: 10.1016/j.fct.2023.114377. [DOI] [PubMed] [Google Scholar]
- Api A.M., Belsito D., Botelho D., Bruze M., Burton G.A., Cancellieri M.A., Chon H., Dagli M.L., Dekant W., Deodhar C., Fryer A.D., Jones L., Joshi K., Kumar M., Lapczynski A., Lavelle M., Lee I., Liebler D.C., Moustakas H., Na M., Penning T.M., Ritacco G., Romine J., Sadekar N., Schultz T.W., Selechnik D., Siddiqi F., Sipes I.G., Sullivan G., Thakkar Y., Tokura Y. RIFM fragrance ingredient safety assessment, 2,3-diethyl-5-methylpyrazine, CAS Registry Number 18138-04-0. Food Chem. Toxicol. 2024;183 doi: 10.1016/j.fct.2023.114343. [DOI] [PubMed] [Google Scholar]
- Api A.M., Belsito D., Botelho D., Bruze M., Burton G.A., Jr., Cancellieri M.A., Chon H., Dagli M.L., Dekant W., Deodhar C., Fryer A.D., Jones L., Joshi K., Kumar M., Lapczynski A., Lavelle M., Lee I., Liebler D.C., Moustakas H., Na M., Penning T.M., Ritacco G., Romine J., Sadekar N., Schultz T.W., Selechnik D., Siddiqi F., Sipes I.G., Sullivan G., Thakkar Y., Tokura Y. RIFM fragrance ingredient safety assessment, 2-acetyl-3-methylpyrazine, CAS Registry number 23787-80-6. Food Chem. Toxicol. 2023;179(Suppl. 1) doi: 10.1016/j.fct.2023.113853. [DOI] [PubMed] [Google Scholar]
- Aspray T.J., Jones E.E., Davies M.W., Shipman M., Bending G.D. Increased hyphal branching and growth of ectomycorrhizal fungus Lactarius rufus by the helper bacterium Paenibacillus sp. Mycorrhiza. 2013;23(5):403–410. doi: 10.1007/s00572-013-0483-1. [DOI] [PubMed] [Google Scholar]
- Bae S.-J., Kim S., Hahn J.-S. Efficient production of acetoin in Saccharomyces cerevisiae by disruption of 2,3-butanediol dehydrogenase and expression of NADH oxidase. Sci. Rep. 2016;6(1) doi: 10.1038/srep27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bañeras L., Trias R., Godayol A., Cerdán L., Nawrath T., Schulz S., Anticó E. Mass spectrometry identification of alkyl-substituted pyrazines produced by Pseudomonas spp. isolates obtained from wine corks. Food Chem. 2013;138(4):2382–2389. doi: 10.1016/j.foodchem.2012.12.030. [DOI] [PubMed] [Google Scholar]
- Beck H.C., Hansen A.M., Lauritsen F.R. Novel pyrazine metabolites found in polymyxin biosynthesis by Paenibacillus polymyxa. FEMS Microbiol. Lett. 2003;220(1):67–73. doi: 10.1016/s0378-1097(03)00054-5. [DOI] [PubMed] [Google Scholar]
- Berger R.G. Biotechnology of flavours—the next generation. Biotechnol. Lett. 2009;31(11):1651–1659. doi: 10.1007/s10529-009-0083-5. [DOI] [PubMed] [Google Scholar]
- Bezsudnova E.Y., Popov V.O., Boyko K.M. Structural insight into the substrate specificity of PLP fold type IV transaminases. Appl. Microbiol. Biotechnol. 2020;104(6):2343–2357. doi: 10.1007/s00253-020-10369-6. [DOI] [PubMed] [Google Scholar]
- Bibi F., Ilyas N., Saeed M., Shabir S., Shati A.A., Alfaifi M.Y., Amesho K.T.T., Chowdhury S., Sayyed R.Z. Innovative production of value-added products using agro-industrial wastes via solid-state fermentation. Environ. Sci. Pollut. Res. 2023 doi: 10.1007/s11356-023-28765-6. [DOI] [PubMed] [Google Scholar]
- Bolhuis J.J., Osada K., Kurihara K., Izumi H., Kashiwayanagi M. Pyrazine analogues are active components of Wolf Urine that induce avoidance and freezing behaviours in mice. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0061753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan S.A., Dekker E.E. L-threonine dehydrogenase. Purification and properties of the homogeneous enzyme from Escherichia coli K-12. J. Biol. Chem. 1981;256(4):1809–1815. doi: 10.1016/S0021-9258(19)69880-7. [DOI] [PubMed] [Google Scholar]
- Bungert M., Jahns T., Becker H. 2-Methoxy-3-(1′-methylpropyl)pyrazine, pea odour, from the marine bacterium Halomonas venusta. Flavour Fragrance J. 2001;16(5):329–333. doi: 10.1002/ffj.1004. [DOI] [Google Scholar]
- Burdock G.A., Carabin I.G. Safety assessment of 2-ethyl-3,(5 or 6) dimethylpyrazine as a food ingredient. Regul. Toxicol. Pharmacol. 2008;50(3):303–312. doi: 10.1016/j.yrtph.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Byeon Y., Lee H.Y., Lee K., Back K. Caffeic acid O-methyltransferase is involved in the synthesis of melatonin by methylating N-acetylserotonin in Arabidopsis. J. Pineal Res. 2014;57(2):219–227. doi: 10.1111/jpi.12160. [DOI] [PubMed] [Google Scholar]
- Cai L., Sun Y., Song Y., Xu L., Bei Z., Zhang D., Dou Y., Wang H. Viral polymerase inhibitors T-705 and T-1105 are potential inhibitors of Zika virus replication. Arch. Virol. 2017;162(9):2847–2853. doi: 10.1007/s00705-017-3436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatonnet P., Fleury A., Boutou S. Origin and incidence of 2-Methoxy-3,5-dimethylpyrazine, a compound with a “Fungal” and “Corky” aroma found in Cork stoppers and oak chips in contact with wines. J. Agric. Food Chem. 2010;58(23):12481–12490. doi: 10.1021/jf102874f. [DOI] [PubMed] [Google Scholar]
- Chaudhary A., Khurana J.M. Synthetic routes for phenazines: an overview. Res. Chem. Intermed. 2017;44(2):1045–1083. doi: 10.1007/s11164-017-3152-8. [DOI] [Google Scholar]
- Chen X.-L., Lin C.-S., Wu X.-Y., Yu R., Teng T., Zhang Q.-K., Zhang Q., Yang W.-B., Lu C.-Z. Correction: highly efficient cuprous complexes with thermally activated delayed fluorescence and simplified solution process OLEDs using the ligand as host. J. Mater. Chem. C. 2015;3(6):1408. doi: 10.1039/c5tc90020d. 1408. [DOI] [Google Scholar]
- Cheng T.B., Reineccius G.A., Bjorklund J.A., Leete E. Biosynthesis of 2-methoxy-3-isopropylpyrazine in Pseudomonas perolens. J. Agric. Food Chem. 1991;39(5):1009–1012. doi: 10.1021/jf00005a042. [DOI] [Google Scholar]
- Cheng T.B., Reineccius G.A., Bjorklund J.A., Leete E. Biosynthesis of 2-methoxy-3-isopropylpyrazine in Pseudomonas perolens. J. Agric. Food Chem. 2002;39(5):1009–1012. doi: 10.1021/jf00005a042. [DOI] [Google Scholar]
- Cherniienko A., Pawełczyk A., Zaprutko L. Antimicrobial and odour qualities of alkylpyrazines occurring in chocolate and cocoa products. Appl. Sci. 2022;12 doi: 10.3390/app122211361. [DOI] [Google Scholar]
- Chiba C.H., Knirsch M.C., Azzoni A.R., Moreira A.R., Stephano M.A. Cell-free protein synthesis: advances on production process for biopharmaceuticals and immunobiological products. Biotechniques. 2021;70(2):126–133. doi: 10.2144/btn-2020-0155. [DOI] [PubMed] [Google Scholar]
- Choudhary D., Garg S., Kaur M., Sohal H.S., Malhi D.S., Kaur L., Verma M., Sharma A., Mutreja V. Advances in the synthesis and bio-applications of pyrazine derivatives: a review. Polycycl. Aromat. Compd. 2022;43(5):4512–4578. doi: 10.1080/10406638.2022.2092873. [DOI] [Google Scholar]
- Cryle M.J., Bell S.G., Schlichting I. Structural and biochemical characterization of the cytochrome P450 CypX (CYP134A1) from Bacillus subtilis: a Cyclo-l-leucyl-l-leucyl dipeptide oxidase. Biochemistry. 2010;49(34):7282–7296. doi: 10.1021/bi100910y. [DOI] [PubMed] [Google Scholar]
- Cui D.-Y., Wei Y.-N., Lin L.-C., Chen S.-J., Feng P.-P., Xiao D.-G., Lin X., Zhang C.-Y. Increasing yield of 2,3,5,6-Tetramethylpyrazine in baijiu through Saccharomyces cerevisiae metabolic engineering. Front. Microbiol. 2020;11 doi: 10.3389/fmicb.2020.596306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Z., Wang Z., Zheng M., Chen T. Advances in biological production of acetoin: a comprehensive overview. Crit. Rev. Biotechnol. 2021;42(8):1135–1156. doi: 10.1080/07388551.2021.1995319. [DOI] [PubMed] [Google Scholar]
- Cui Z., Zheng M., Ding M., Dai W., Wang Z., Chen T. Efficient production of acetoin from lactate by engineered Escherichia coli whole-cell biocatalyst. Appl. Microbiol. Biotechnol. 2023;107(12):3911–3924. doi: 10.1007/s00253-023-12560-x. [DOI] [PubMed] [Google Scholar]
- Dai J., Wang Z., Xiu Z.-L. High production of optically pure (3R)-acetoin by a newly isolated marine strain of Bacillus subtilis CGMCC 13141. Bioproc. Biosyst. Eng. 2018;42(3):475–483. doi: 10.1007/s00449-018-2051-8. [DOI] [PubMed] [Google Scholar]
- Dickschat J.S., Reichenbach H., Wagner-Döbler I., Schulz S. Novel pyrazines from the Myxobacterium Chondromyces crocatus and marine bacteria. Eur. J. Org. Chem. 2005;2005(19):4141–4153. doi: 10.1002/ejoc.200500280. [DOI] [Google Scholar]
- Dolezal M., Miletin M., Kunes J., Kralova K. Substituted Amides of Pyrazine-2-carboxylic acids: synthesis and Biological Activity. Molecules. 2002;7(3):363–373. doi: 10.3390/70300363. [DOI] [Google Scholar]
- Dunlevy J.D., Dennis E.G., Soole K.L., Perkins M.V., Davies C., Boss P.K. A methyltransferase essential for the methoxypyrazine-derived flavour of wine. Plant J. 2013;75(4):606–617. doi: 10.1111/tpj.12224. [DOI] [PubMed] [Google Scholar]
- Dunlevy J.D., Soole K.L., Perkins M.V., Dennis E.G., Keyzers R.A., Kalua C.M., Boss P.K. Two O-methyltransferases involved in the biosynthesis of methoxypyrazines: grape-derived aroma compounds important to wine flavour. Plant Mol. Biol. 2010;74(1–2):77–89. doi: 10.1007/s11103-010-9655-y. [DOI] [PubMed] [Google Scholar]
- Dunlevy J.D., Soole K.L., Perkins M.V., Dennis E.G., Keyzers R.A., Kalua C.M., Boss P.K. Erratum to: two O-methyltransferases involved in the biosynthesis of methoxypyrazines: grape-derived aroma compounds important to wine flavour. Plant Mol. Biol. 2013;81(4–5):523. doi: 10.1007/s11103-013-0012-9. 523. [DOI] [PubMed] [Google Scholar]
- El-Kadi S.M., Elbagory M., El-Zawawy H.A.H., El-Shaer H.F.A., Shoukry A.A., El-Nahrawy S., Omara A.E.-D., Ali D.F.I. Biosynthesis of Poly-ß-Hydroxybutyrate (PHB) from different bacterial strains grown on alternative cheap carbon sources. Polymers. 2021;13(21) doi: 10.3390/polym13213801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott W.H. Aminoacetone formation by Staphylococcus aureus. Biochem. J. 1960;74(3):478–485. doi: 10.1042/bj0740478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emde K.M.E., Norine B., E S., Hrudey Production of the potent odour agent, isopropyl methoxypyrazine, by Lysobacter enzymogenes. Environ. Technol. 1992;13(3):201–206. doi: 10.1080/09593339209385146. [DOI] [Google Scholar]
- Enuh B.M., Aytar Çelik P. Genome analysis of Halomonas elongata strain 153B and insights into polyhydroxyalkanoate synthesis and adaptive mechanisms to high saline environments. Curr. Microbiol. 2022;80(1):18. doi: 10.1007/s00284-022-03115-w. [DOI] [PubMed] [Google Scholar]
- Fan X., Zhong M., Feng L., Huo Y., Pan L. Evaluation of flavor characteristics in tartary buckwheat (Fagopyrum tataricum) by E-nose, GC-IMS, and HS-SPME-GC-MS: influence of different roasting temperatures. Lebensm. Wiss. Technol. 2024;191 doi: 10.1016/j.lwt.2023.115672. [DOI] [Google Scholar]
- Fayek N.M., Jianbo X., A M., Farag A multifunctional study of naturally occurring pyrazines in biological systems; formation mechanisms, metabolism, food applications and functional properties. Crit. Rev. Food Sci. Nutr. 2023;63(21):5322–5338. doi: 10.1080/10408398.2021.2017260. [DOI] [PubMed] [Google Scholar]
- Fayek N.M., Xiao J., Farag M.A. A multifunctional study of naturally occurring pyrazines in biological systems; formation mechanisms, metabolism, food applications and functional properties. Crit. Rev. Food Sci. Nutr. 2023;63(21):5322–5338. doi: 10.1080/10408398.2021.2017260. [DOI] [PubMed] [Google Scholar]
- Feng D., Chen R., Huang Y., Song H. A new convenient way to synthesize 1‐hydroxyphosphonates from heterocyclic aldehydes and ketones under microwave irradiation. Heteroat. Chem. 2007;18(4):347–353. doi: 10.1002/hc.20304. [DOI] [Google Scholar]
- Frato K.E. Identification of hydroxypyrazine O-Methyltransferase genes in coffea arabica: a potential source of methoxypyrazines that cause potato taste defect. J. Agric. Food Chem. 2018;67(1):341–351. doi: 10.1021/acs.jafc.8b04541. [DOI] [PubMed] [Google Scholar]
- Frato K.E. Identification of hydroxypyrazine O-Methyltransferase genes in coffea arabica: a potential source of methoxypyrazines that cause potato taste defect. J. Agric. Food Chem. 2019;67(1):341–351. doi: 10.1021/acs.jafc.8b04541. [DOI] [PubMed] [Google Scholar]
- Gai L., Li K., Niu D. The scent of time: analyzing the differences in volatile organic compounds of camellia oleifera oil with different oil-tea tree ages using GC–IMS and GC–MS. Food Chem. 2025;482 doi: 10.1016/j.foodchem.2025.144016. [DOI] [PubMed] [Google Scholar]
- Gallois A., Grimont Patrick A.D. "Pyrazines Responsible for the Potatolike Odor Produced by Some Serratia and Cedecea Strains.". Appl. Environ. Microbiol. 1985;50(4):1048–1051. doi: 10.1128/aem.50.4.1048-1051.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallois A., Kergomard A., Adda J. Study of the biosynthesis of 3-isopropyl-2-methoxypyrazine produced by Pseudomonas taetrolens. Food Chem. 1988;28(4):299–309. doi: 10.1016/0308-8146(88)90105-7. [DOI] [Google Scholar]
- Gan S., Li Y., Zhang X., Luo L., Xu X., Jiang J., Huo Y., Shang C. Optimizing the detoxification conditions of distiller's grains hydrolysate for tetramethylpyrazine fermentation by Bacillus sp. TTMP20. Ind. Crops Prod. 2023;206 doi: 10.1016/j.indcrop.2023.117608. [DOI] [Google Scholar]
- Gan S., Ruan L., Xu X., Luo L., Huo Y., Jiang J., Zhang X., Shang C. Whole genome sequencing and analysis of Bacillus sp. TTMP2, a tetramethylpyrazine-producing bacterium. Mol. Biol. Rep. 2024;51(1):863. doi: 10.1007/s11033-024-09749-2. [DOI] [PubMed] [Google Scholar]
- Gao P., Wang S., Feng B., Liu C., Wang Y., Dou S., Dong L. Volatile profiling from thermal decomposition of Amadori compounds in the alanine-glucose Maillard reaction: an DFT insight of 3-ethyl-2,5-dimethylpyrazine forming mechanism. Food Chem. X. 2025;27 doi: 10.1016/j.fochx.2025.102446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Yu M., Han X., Song H., Pan W., Chen W., Xiong W. Characterization of odor-active compounds in Liuzhou River snail rice noodles soup by sensory-directed flavor analysis. J. Food Compos. Anal. 2024;131 doi: 10.1016/j.jfca.2024.106276. [DOI] [Google Scholar]
- Gawne P., Man F., Fonslet J., Radia R., Bordoloi J., Cleveland M., Jimenez-Royo P., Gabizon A., Blower P.J., Long N., de Rosales R.T.M. Manganese-52: applications in cell radiolabelling and liposomal nanomedicine PET imaging using oxine (8-hydroxyquinoline) as an ionophore. Dalton Trans. 2018;47(28):9283–9293. doi: 10.1039/c8dt00100f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorab M.M., Abdel-Kader M.S., Alqahtani A.S., Soliman A.M. Synthesis of some quinazolinones inspired from the natural alkaloid L-norephedrine as EGFR inhibitors and radiosensitizers. J. Enzyme Inhib. Med. Chem. 2020;36(1):218–238. doi: 10.1080/14756366.2020.1854243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore‐Lloyd D., Sumann I., Brachmann A.O., Schneeberger K., Ortiz‐Merino R.A., Moreno‐Beltrán M., Schläfli M., Kirner P., Santos Kron A., Rueda‐Mejia M.P., Somerville V., Wolfe K.H., Piel J., Ahrens C.H., Henk D., Freimoser F.M. Snf2 controls pulcherriminic acid biosynthesis and antifungal activity of the biocontrol yeast Metschnikowia pulcherrima. Mol. Microbiol. 2019;112(1):317–332. doi: 10.1111/mmi.14272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D.A., Rice M.J., Elliott R. Pyrazine compound useful as plant growth regulators. 1990. https://pubchem.ncbi.nlm.nih.gov/patent/US-4975112-A
- Guo X., Ho C.-T., Schwab W., Wan X. Effect of the roasting degree on flavor quality of large-leaf yellow tea. Food Chem. 2021;347 doi: 10.1016/j.foodchem.2021.129016. [DOI] [PubMed] [Google Scholar]
- Guo X., Song C., Ho C.-T., Wan X. Contribution of l-theanine to the formation of 2,5-dimethylpyrazine, a key roasted peanutty flavor in Oolong tea during manufacturing processes. Food Chem. 2018;263:18–28. doi: 10.1016/j.foodchem.2018.04.117. [DOI] [PubMed] [Google Scholar]
- Harris S.A., Ryona I., Sacks G.L. Behavior of 3-Isobutyl-2-hydroxypyrazine (IBHP), a key intermediate in 3-Isobutyl-2-methoxypyrazine (IBMP) metabolism, in ripening wine grapes. J. Agric. Food Chem. 2012;60(48):11901–11908. doi: 10.1021/jf302990m. [DOI] [PubMed] [Google Scholar]
- Hashizume K., Tozawa K., Hiraga Y., Aramaki I. Purification and characterization of a O-Methyltransferase capable of methylating 2-Hydroxy-3-alkylpyrazine from Vitis vinifera L. (cv. Cabernet sauvignon) Biosci. Biotechnol. Biochem. 2014;65(10):2213–2219. doi: 10.1271/bbb.65.2213. [DOI] [PubMed] [Google Scholar]
- He Y., Chen Y., Li J., Wang D., Song S., Dong F., He Z. Efficient degradation of 2,3,5-trimethylpyrazine by catalytic ozonation over MnOx supported on biochar derived from waste tea leaves. Chem. Eng. J. 2023;464 doi: 10.1016/j.cej.2023.142525. [DOI] [Google Scholar]
- Hijarrubia M.J., Aparicio Jf Fau - Martín J.F., Martín J.F. Nitrate regulation of alpha-aminoadipate reductase formation and lysine inhibition of its activity in Penicillium chrysogenum and Acremonium chrysogenum. 2002. 0175-7598. [DOI] [PubMed]
- Ho W.K., Wen H.L., Lee C.M. Tetramethylpyrazine for treatment of experimentally induced stroke in Mongolian gerbils. Stroke. 1989;20(1):96–99. doi: 10.1161/01.Str.20.1.96. [DOI] [PubMed] [Google Scholar]
- Huang W. Guizhou University; 2022. Exploring Laceyella sacchari FBKL4.010 Based on Transcriptomics Study on the Production of Tetramethylpyrazine. Master. [DOI] [Google Scholar]
- Huchting J., Vanderlinden E., Winkler M., Nasser H., Naesens L., Meier C. Prodrugs of the phosphoribosylated forms of hydroxypyrazinecarboxamide pseudobase T-705 and its De-Fluoro analogue T-1105 as potent influenza virus inhibitors. J. Med. Chem. 2018;61(14):6193–6210. doi: 10.1021/acs.jmedchem.8b00617. [DOI] [PubMed] [Google Scholar]
- Hwang H.-I., Hartman T.G., Rosen R.T., Lech J., Ho C.-T. Formation of pyrazines from the maillard reaction of glucose and Lysine-.alpha.-amine-15N. J. Agric. Food Chem. 2002;42(4):1000–1004. doi: 10.1021/jf00040a031. [DOI] [Google Scholar]
- Ito T., Sugawara E., Miyanohara J.-i., Sakurai Y., Odagiri S. Effect of amino acids as nitrogen sources on microbiological formation of pyrazines. J. Jpn. Soc. Food Sci. Technol. 1989;36(9):762–764. doi: 10.3136/nskkk1962.36.9_762. [DOI] [Google Scholar]
- Jäger T., Mokos A., Prasianakis N.I., Leyer S. Pore-level multiphase simulations of realistic distillation membranes for water desalination. Membranes. 2022;12(11) doi: 10.3390/membranes12111112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J.-W., Jung H.-M., Im D.-K., Jung M.-Y., Oh M.-K. Pathway engineering of Enterobacter aerogenes to improve acetoin production by reducing by-products formation. Enzym. Microb. Technol. 2017;106:114–118. doi: 10.1016/j.enzmictec.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Jia R.-B., Guo W.-L., Zhou W.-B., Jiang Y.-J., Zhu F.-F., Chen J.-H., Li Y., Liu B., Chen S.-J., Chen J.-C., Ni L., Rao P.-F., Lv X.-C. Screening and identification of Monacusstrain with high TMP production and statistical optimization of its culture medium composition and liquid state fermentation conditions using response surface methodology (RSM) Biotechnol. Biotechnol. Equip. 2017:1–11. doi: 10.1080/13102818.2017.1335176. [DOI] [Google Scholar]
- Jia X., Kelly R.M., Han Y. Simultaneous biosynthesis of (R)-acetoin and ethylene glycol from D-xylose through in vitro metabolic engineering. Metab. Eng. Commun. 2018;7 doi: 10.1016/j.mec.2018.e00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X., Peng X., Liu Y., Han Y. Conversion of cellulose and hemicellulose of biomass simultaneously to acetoin by thermophilic simultaneous saccharification and fermentation. Biotechnol. Biofuels. 2017;10(1):232. doi: 10.1186/s13068-017-0924-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X., Wang S., Gao Y., Qi Q., Liang Q. Combinatorial metabolic engineering of Escherichia coli to efficiently produce L-threonine from untreated cane molasses. Bioresour. Technol. 2025;419 doi: 10.1016/j.biortech.2025.132058. [DOI] [PubMed] [Google Scholar]
- Jung M.Y., Bock J.Y., Back S.O., Lee T.K., Kim J.H. Pyrazine contents and oxidative stabilities of roasted soybean oils. Food Chem. 1997;60(1):95–102. doi: 10.1016/S0308-8146(96)00316-0. [DOI] [Google Scholar]
- Jung M.Y., Bock J.Y., Baik S.O., Lee J.H., Lee T.K. Effects of roasting on pyrazine contents and oxidative stability of red pepper seed oil prior to its extraction. J. Agric. Food Chem. 1999;47(4):1700–1704. doi: 10.1021/jf981028l. [DOI] [PubMed] [Google Scholar]
- Kang Q., Fang H., Xiang M., Xiao K., Jiang P., You C., Lee S.Y., Zhang D. A synthetic cell-free 36-enzyme reaction system for vitamin B12 production. Nat. Commun. 2023;14(1) doi: 10.1038/s41467-023-40932-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp P.D., Latendresse M., Caspi R. The pathway tools pathway prediction Algorithm. Stand. Genomic Sci. 2011;5(3):424–429. doi: 10.4056/sigs.1794338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Hillman P.F., Lee J.Y., Lee J., Lee J., Cha S.-S., Oh D.-C., Nam S.-J., Fenical W. Actinopolymorphols E and F, pyrazine alkaloids from a marine sediment-derived bacterium streptomyces sp. J. Antibiot. 2022;75(11):619–625. doi: 10.1038/s41429-022-00562-2. [DOI] [PubMed] [Google Scholar]
- Kłosowski G., Mikulski D., Pielech-Przybylska K. Pyrazines biosynthesis by Bacillus Strains isolated from Natto fermented soybean. Biomolecules. 2021;11(11) doi: 10.3390/biom11111736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorr D., Wampler T.P., Teutonico R.A. Formation of pyrazines by chitin pyrolysis. J. Food Sci. 1985;50(6):1762–1763. doi: 10.1111/j.1365-2621.1985.tb10589.x. [DOI] [Google Scholar]
- Koehler P.E., Mason M.E., Odell G.V. Odor threshold levels of pyrazine compounds and assessment of their role in the flavor of roasted foods. J. Food Sci. 2006;36(5):816–818. doi: 10.1111/j.1365-2621.1971.tb03314.x. [DOI] [Google Scholar]
- Kosuge T., Adachi T., Kamiya H. Isolation of tetramethylpyrazine from culture of Bacillus natto, and biosynthetic pathways of tetramethylpyrazine. Nature. 1962;195(4846):1103. doi: 10.1038/1951103a0. 1103. [DOI] [PubMed] [Google Scholar]
- Kremer J.I., Pickard S., Stadlmair L.F., Glaß‐Theis A., Buckel L., Bakuradze T., Eisenbrand G., Richling E. Alkylpyrazines from coffee are extensively metabolized to pyrazine carboxylic acids in the human body. Mol. Nutr. Food Res. 2019;63(14) doi: 10.1002/mnfr.201801341. [DOI] [PubMed] [Google Scholar]
- Kumar J.A., Sathish S., Prabu D., Renita A.A., Saravanan A., Deivayanai V.C., Anish M., Jayaprabakar J., Baigenzhenov O., Hosseini-Bandegharaei A. Agricultural waste biomass for sustainable bioenergy production: feedstock, characterization and pre-treatment methodologies. Chemosphere. 2023:331. doi: 10.1016/j.chemosphere.2023.138680. [DOI] [PubMed] [Google Scholar]
- Kutanovas S., Rutkiene R., Urbelis G., Tauraite D., Stankeviciute J., Meskys R. Bioconversion of methylpyrazines and pyridines using novel pyrazines-degrading microorganisms. Chemija. 2013;24(1):67–73. https://api.semanticscholar.org/CorpusID:96771168 [Google Scholar]
- Kutanovas S., Stankeviciute J., Urbelis G., Tauraite D., Rutkiene R., Meskys R. Identification and characterization of a tetramethylpyrazine catabolic pathway in Rhodococcus jostii TMP1. Appl. Environ. Microbiol. 2013;79(12):3649–3657. doi: 10.1128/aem.00011-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam K.C., Ibrahim R.K., Behdad B., Dayanandan S. Structure, function, and evolution of plant O-methyltransferases. Genome. 2007;50(11):1001–1013. doi: 10.1139/g07-077. [DOI] [PubMed] [Google Scholar]
- Laver W.G., Neuberger A., Scott J.J. 287. α-Amino-β-keto-acids. Part II. Rates of decarboxylation of the free acids and the behaviour of derivatives on titration. J. Am. Chem. Soc. 1959;(0):1483–1491. doi: 10.1039/JR9590001483. [DOI] [Google Scholar]
- Lebar M.D., Mack B.M., Carter-Wientjes C.H., Gilbert M.K. The aspergillic acid biosynthetic gene cluster predicts neoaspergillic acid production in Aspergillus section Circumdati. World Mycotoxin J. 2019;12(3):213–222. doi: 10.3920/wmj2018.2397. [DOI] [Google Scholar]
- Lee K., Kwon S.-H., Song S., Lee D.-Y., Park M.K., Kim Y.-S. Comparative analysis of volatile and non-volatile metabolites derived from Bacillus subtilis strains producing different levels of biogenic amines. Metabolites. 2023:13. doi: 10.3390/metabo13020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leejeerajumnean A., Duckham S.C., Owens J.D., Ames J.M. Volatile compounds in Bacillus‐fermented soybeans. J. Sci. Food Agric. 2001;81(5):525–529. doi: 10.1002/jsfa.843. [DOI] [Google Scholar]
- Lei Y., Ma Z., Wang P., Qin X., Guan X., Zhang Z. Effect of 2,5-Dicarbonyl-3-Isobutyl-Piperazine on 3-Isobutyl-2-Methoxypyrazine biosynthesis in wine grape. Foods. 2023:12. doi: 10.3390/foods12173258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei Y., Xie S., Guan X., Song C., Zhang Z., Meng J. Methoxypyrazines biosynthesis and metabolism in grape: a review. Food Chem. 2018;245:1141–1147. doi: 10.1016/j.foodchem.2017.11.056. [DOI] [PubMed] [Google Scholar]
- Li D., Huang W., Wang C., Qiu S. The complete genome sequence of the thermophilic bacterium Laceyella sacchari FBKL4.010 reveals the basis for tetramethylpyrazine biosynthesis in Moutai-flavor Daqu. Microbiol. Open. 2019;8(12) doi: 10.1002/mbo3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Lu J., Ma Z., Li J., Chen X., Diao M., Xie N. A green route for high-yield production of tetramethylpyrazine from non-food raw materials. Front. Bioeng. Biotechnol. 2022;9 doi: 10.3389/fbioe.2021.792023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.C., Wilkinson K.L., Ford C.M., Jiranek V. Effect of non-Saccharomyces yeast strains on 3-isobutyl-2-methoxypyrazine concentration and aroma properties in Sauvignon Blanc wines during fermentation. Aust. J. Grape Wine Res. 2022;28(4):607–620. doi: 10.1111/ajgw.12563. [DOI] [Google Scholar]
- Li P., Gan X., Luo L., Du B. Genomic analysis of type strain Paenibacillus aceti L14T, a highly efficient producer of pyrazines. Ann. Microbiol. 2017;67(5):391–393. doi: 10.1007/s13213-017-1267-1. [DOI] [Google Scholar]
- Li T., Liu P., Guo G., Liu Z., Zhong L., Guo L., Chen C., Hao N., Ouyang P. Production of acetoin and its derivative tetramethylpyrazine from okara hydrolysate with Bacillus subtilis. AMB Express. 2023;13(1):25. doi: 10.1186/s13568-023-01532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Gan S., Luo L., Yang W., Mo L., Shang C. Optimization of molasses and soybean meal content to enhance tetramethylpyrazine yield by Bacillus sp. TTMP20. Molecules. 2023:28. doi: 10.3390/molecules28186515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling M., Zhou Y., Lan Y., Cheng C., Wu G., Duan C., Shi Y. Modification of sensory expression of 3-Isobutyl-2-methoxypyrazine in wines through blending technique. Molecules. 2021:26. doi: 10.1186/s13568-023-01532-z10.3390/molecules26113172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.-M., Chen L., Dorau R., Lillevang S.K., Jensen P.R., Solem C. From waste to Taste—Efficient production of the butter aroma compound acetoin from low-value dairy side streams using a natural (Nonengineered) Lactococcus lactis dairy isolate. J. Agric. Food Chem. 2020;68(21):5891–5899. doi: 10.1021/acs.jafc.0c00882. [DOI] [PubMed] [Google Scholar]
- Liu Q., Huang W., Sheng C., Wu Y., Lu M., Li T., Zhang J., Wei Y., Wang Y., Ning J. Contribution of tea stems to large-leaf yellow tea aroma. Food Chem. 2024;460 doi: 10.1016/j.foodchem.2024.140472. [DOI] [PubMed] [Google Scholar]
- Liu X.-X., Wang Y., Zhang J.-H., Lu Y.-F., Dong Z.-X., Yue C., Huang X.-Q., Zhang S.-P., Li D.-D., Yao L.-G., Tang C.-D. Engineering Escherichia coli for high-yielding 2,5-Dimethylpyrazine synthesis from L-Threonine by reconstructing metabolic pathways and enhancing cofactors regeneration. Biotechnol. Biofuels Bioprod. 2024;17(1) doi: 10.1186/s13068-024-02487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.-X., Xi X.-Q., Shen T.-S., Shi H.-L., Zhang Y.-J., Kan Y.-C., Yao L.-G., Tang C.-D. Enhancing biosynthesis efficiency of 2,5-dimethylpyrazine by overexpressing l-threonine dehydrogenase and NADH oxidase in Escherichia coli. Mol. Catal. 2023;550 doi: 10.1016/j.mcat.2023.113550. [DOI] [Google Scholar]
- Liu X., Quan W. Progress on the synthesis pathways and pharmacological effects of naturally occurring pyrazines. Molecules. 2024:29. doi: 10.3390/molecules29153597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Yang W., Gu H., Bughio A.A., Liu J. Optimization of fermentation conditions for 2,3,5-Trimethylpyrazine produced by Bacillus amyloliquefaciens from Daqu. Fermentation. 2024:10. doi: 10.3390/fermentation10020112. [DOI] [Google Scholar]
- Liu Y., Li M., Hong X., Li H., Huang R., Han S., Hou J., Pan C. Screening and identification of high yield tetramethylpyrazine strains in Nongxiangxing liquor Daqu and study on the mechanism of tetramethylpyrazine production. J. Sci. Food Agric. 2023;103(14):6849–6860. doi: 10.1002/jsfa.12773. [DOI] [PubMed] [Google Scholar]
- Longo M., Sanromán M. Production of food aroma compounds: microbial and enzymatic methodologies. Food Technol. Biotechnol. 2005:44. https://www.researchgate.net/publication/228621109 [Google Scholar]
- López S., Rodrigo-Gómez S., Fernández-Carrillo E., Corbella-Martorell C., Quero C. Laboratory evidence of 2-Isobutyl-3-methoxypyrazine as a male-released aggregative cue in Labidostomis lusitanica (Germar) (Coleoptera: Chrysomelidae) Insects. 2023:14. doi: 10.3390/insects14020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Mao Y., Kou M., Cui Z., Jin B., Chang Z., Wang Z., Ma H., Chen T. Engineering central pathways for industrial-level (3R)-acetoin biosynthesis in Corynebacterium glutamicum. Microb. Cell Fact. 2020;19(1) doi: 10.1186/s12934-020-01363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J.C. Biosynthesis of hydroxyaspergillic acid. J. Biol. Chem. 1962;237(6):1977–1981. doi: 10.1016/S0021-9258(19)73969-6. [DOI] [PubMed] [Google Scholar]
- MacDonald J.C. Biosynthesis of compounds similar to aspergillic acid. Can. J. Biochem. 1970;48(10):1165–1174. doi: 10.1139/o70-181. [DOI] [PubMed] [Google Scholar]
- MacDonald J.C. Toxicity, analysis, and production of aspergillic acid and its analogues. Can. J. Biochem. 1973;51(9):1311–1315. doi: 10.1139/o73-172. [DOI] [PubMed] [Google Scholar]
- Maga J.A., Sizer C.E., Myhre D.V. Pyrazines in foods. CRC Crit. Rev. Food Technol. 1973;4(1):39–115. doi: 10.1080/10408397309527153. [DOI] [Google Scholar]
- Mao Y., Fu J., Tao R., Huang C., Wang Z., Tang Y.-J., Chen T., Zhao X. Systematic metabolic engineering of Corynebacterium glutamicum for the industrial-level production of optically pure d-(−)-acetoin. Green Chem. 2017;19(23):5691–5702. doi: 10.1039/c7gc02753b. [DOI] [Google Scholar]
- Marcus J.P., Dekker E.E. pH-dependent decarboxylation of 2-amino-3-ketobutyrate, the unstable intermediate in the threonine dehydrogenase-initiated pathway for threonine utilization. Biochem. Biophys. Res. Commun. 1993;190(3):1066–1072. doi: 10.1006/bbrc.1993.1157. [DOI] [PubMed] [Google Scholar]
- Masuda H., Mihara S. Olfactive properties of alkylpyrazines and 3-substituted 2-alkylpyrazines. J. Agric. Food Chem. 2002;36(3):584–587. doi: 10.1021/jf00081a044. [DOI] [Google Scholar]
- Masuo S., Tsuda Y., Namai T., Minakawa H., Shigemoto R., Takaya N. Enzymatic Cascade in pseudomonas that produces pyrazine from α‐Amino acids. Chembiochem. 2019;21(3):353–359. doi: 10.1002/cbic.201900448. [DOI] [PubMed] [Google Scholar]
- Mattey M., Harle E.M. Aerobic metabolism of pyrazine compounds by a Pseudomonas Species. Biochem. Soc. Trans. 1976;4(3):492–494. doi: 10.1042/bst0040492. [DOI] [PubMed] [Google Scholar]
- McGorrin R.J. Key Aroma compounds in oats and oat cereals. J. Agric. Food Chem. 2019;67(50):13778–13789. doi: 10.1021/acs.jafc.9b00994. [DOI] [PubMed] [Google Scholar]
- Meng W., Ding F., Wang R.-M., Wang T.-F. Enhanced production of tetramethylpyrazine in Bacillus licheniformis BL1 through aldC over-expression and acetaldehyde supplementation. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-60345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng W., Xiao D., Wang R. Enhanced production of tetramethylpyrazine in Bacillus licheniformis BL1 by bdhA disruption and 2,3-butanediol supplementation. World J. Microbiol. Biotechnol. 2016;32(3) doi: 10.1007/s11274-015-1992-1. [DOI] [PubMed] [Google Scholar]
- Molla G., Nardini M., Motta P., D'Arrigo P., Panzeri W., Pollegioni L. Aminoacetone oxidase from Streptococcus oligofermentans belongs to a new three-domain family of bacterial flavoproteins. Biochem. J. 2014;464(3):387–399. doi: 10.1042/bj20140972. [DOI] [PubMed] [Google Scholar]
- Montero V., Montana M., Khoumeri O., Correard F., Estève M.-A., Vanelle P. Synthesis, in vitro antiproliferative activity, and in Silico evaluation of novel Oxiranyl-Quinoxaline derivatives. Pharmaceuticals. 2022;15(7) doi: 10.3390/ph15070781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G.L., Li B. In vitro reconstitution reveals a central role for the n‐oxygenase PvfB in (Dihydro)pyrazine‐N‐oxide and valdiazen biosynthesis. Angew. Chem. Int. Ed. 2020;59(48):21387–21391. doi: 10.1002/anie.202005554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortzfeld F.B., Hashem C., Vranková K., Winkler M., Rudroff F. Pyrazines: synthesis and industrial application of these valuable flavor and fragrance compounds. Biotechnol. J. 2020;15(11) doi: 10.1002/biot.202000064. [DOI] [Google Scholar]
- Mortzfeld F.B., Hashem C., Vranková K., Winkler M., Rudroff F. Pyrazines: synthesis and industrial application of these valuable flavor and fragrance compounds. Biotechnol. J. 2020;15(11) doi: 10.1002/biot.202000064. [DOI] [Google Scholar]
- Moser B.R. Review of cytotoxic cephalostatins and ritterazines: isolation and synthesis. J. Nat. Prod. 2008;71(3):487–491. doi: 10.1021/np070536z. [DOI] [PubMed] [Google Scholar]
- Motoyama T., Nakano S., Hasebe F., Miyata R., Kumazawa S., Miyoshi N., Ito S. Chemoenzymatic synthesis of 3-ethyl-2,5-dimethylpyrazine by L-threonine 3-dehydrogenase and 2-amino-3-ketobutyrate CoA ligase/L-threonine aldolase. Commun. Chem. 2021;4(1) doi: 10.1038/s42004-021-00545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoyama T., Nakano S., Hasebe F., Miyata R., Kumazawa S., Miyoshi N., Ito S. Chemoenzymatic synthesis of 3-ethyl-2,5-dimethylpyrazine by L-threonine 3-dehydrogenase and 2-amino-3-ketobutyrate CoA ligase/L-threonine aldolase. Commun. Chem. 2021;4(1):108. doi: 10.1038/s42004-021-00545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Rappert S. Pyrazines: occurrence, formation and biodegradation. Appl. Microbiol. Biotechnol. 2009;85(5):1315–1320. doi: 10.1007/s00253-009-2362-4. [DOI] [PubMed] [Google Scholar]
- Murray K.E., Whitfield F.B. The occurrence of 3-alkyl-2-methoxypyrazines in raw vegetables. J. Sci. Food Agric. 1975;26(7):973–986. doi: 10.1002/jsfa.2740260714. [DOI] [Google Scholar]
- Murray K.E., Whitfield F.B. The occurrence of 3‐alkyl‐2‐methoxypyrazines in raw vegetables. J. Sci. Food Agric. 2006;26(7):973–986. doi: 10.1002/jsfa.2740260714. [DOI] [Google Scholar]
- Nakayama T., Honda R. Electrochemical and mechanistic study of oxidative degradation of favipiravir by electrogenerated superoxide through proton-coupled electron transfer. ACS Omega. 2021;6(33):21730–21740. doi: 10.1021/acsomega.1c03230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath T., Dickschat J.S., Kunze B., Schulz S. The biosynthesis of branched dialkylpyrazines in Myxobacteria. Chem. Biodivers. 2010;7(9):2129–2144. doi: 10.1002/cbdv.201000158. [DOI] [PubMed] [Google Scholar]
- Noda K., Igarashi M., Morimitsu Y., Tsutsuura S., Murata M. 2,5-Dimethylpyrazine and 2,3,5-trimethylpyrazine in natto are formed metabolically from threonine through aminoacetone by Bacillus natto during the fermentation. Food Sci. Technol. Res. 2025 doi: 10.3136/fstr.FSTR-D-25-00040. advpub. [DOI] [Google Scholar]
- Ohata M., Zhou L., Yada Y., Yokoyama I., Arihara K. 2,3-Dimethylpyrazine (3DP) and 2,5-dimethyl-4-hydroxy-3(2H)-furanone (DMHF) generated by the Maillard reaction in foods affect autonomic nervous activity and central nervous activity in human. Biosci. Biotechnol. Biochem. 2020;84(9):1894–1902. doi: 10.1080/09168451.2020.1775066. [DOI] [PubMed] [Google Scholar]
- Opletalová V., Hartl J., Patel A., Palát K., Buchta V.r. Ring substituted 3-phenyl-1-(2-pyrazinyl)-2-propen-1-ones as potential photosynthesis-inhibiting, antifungal and antimycobacterial agents. Il Farmaco. 2002;57(2):135–144. doi: 10.1016/s0014-827x(01)01187-9. [DOI] [PubMed] [Google Scholar]
- Orban A., Jerschow J.J., Birk F., Suarez C., Schnell S., Rühl M. Effect of bacterial volatiles on the mycelial growth of mushrooms. Microbiol. Res. 2023;266 doi: 10.1016/j.micres.2022.127250. [DOI] [PubMed] [Google Scholar]
- Patel A., Kumar A., Sheoran N., Kumar M., Sahu K.P., Ganeshan P., Ashajyothi M., Gopalakrishnan S., Gogoi R. Antifungal and defense elicitor activities of pyrazines identified in endophytic Pseudomonas putida BP25 against fungal blast incited by Magnaporthe oryzae in rice. J. Plant Dis. Prot. 2021;128(1):261–272. doi: 10.1007/s41348-020-00373-3. [DOI] [Google Scholar]
- Peng K., Guo D., Lou Q., Lu X., Cheng J., Qiao J., Lu L., Cai T., Liu Y., Jiang H. Synthesis of ligustrazine from acetaldehyde by a combined biological–chemical approach. ACS Synth. Biol. 2020;9(11):2902–2908. doi: 10.1021/acssynbio.0c00113. [DOI] [PubMed] [Google Scholar]
- Peppas A., Sokalis D., Perganti D., Schnakenburg G., Falaras P., Philippopoulos A.I. Sterically demanding pyridine-quinoline anchoring ligands as building blocks for copper(i)-based dye-sensitized solar cell (DSSC) complexes. Dalton Trans. 2022;51(39):15049–15066. doi: 10.1039/d2dt02382b. [DOI] [PubMed] [Google Scholar]
- Petkevičius V., Juknevičiūtė J., Mašonis D., Meškys R. Synthetic pathways for microbial biosynthesis of valuable pyrazine derivatives using genetically modified Pseudomonas putida KT2440. Metab. Eng. Commun. 2025;20 doi: 10.1016/j.mec.2025.e00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y.-Y., Gong Y., Kong S.-Y., Wan Z.-Y., Liu J.-Q., Xing K., Qin S. Aerial signaling by plant-associated Streptomyces setonii WY228 regulates plant growth and enhances salt stress tolerance. Microbiol. Res. 2024;286 doi: 10.1016/j.micres.2024.127823. [DOI] [PubMed] [Google Scholar]
- Rajini K.S., A P., Ch S., V C., Ramana Microbial metabolism of pyrazines. Crit. Rev. Microbiol. 2011;37(2):99–112. doi: 10.3109/1040841X.2010.512267. [DOI] [PubMed] [Google Scholar]
- Rajini K.S., Sasikala C., Ramana C.V. Reductive degradation of pyrazine-2-carboxylate by a newly isolated Stenotrophomonas sp. HCU1. Biodegradation. 2010;21(5):801–813. doi: 10.1007/s10532-010-9345-0. [DOI] [PubMed] [Google Scholar]
- Randazzo P., Aubert-Frambourg A., Guillot A., Auger S. The MarR-like protein PchR (YvmB) regulates expression of genes involved in pulcherriminic acid biosynthesis and in the initiation of sporulation in Bacillus subtilis. BMC Microbiol. 2016;16(1) doi: 10.1186/s12866-016-0807-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappert S., Li R., Kokova M., Antholz M., Nagorny S., Francke W., Müller R. Degradation of 2,5-dimethylpyrazine by Rhodococcus erythropolis strain DP-45 isolated from a waste gas treatment plant of a fishmeal processing company. Biodegradation. 2006;18(5):585–596. doi: 10.1007/s10532-006-9091-5. [DOI] [PubMed] [Google Scholar]
- Ricca E., Brucher B., Schrittwieser J.H. Multi‐Enzymatic Cascade reactions: overview and perspectives. Adv. Synth. Catal. 2011;353(13):2239–2262. doi: 10.1002/adsc.201100256. [DOI] [Google Scholar]
- Rizz G.P. Mechanistic study of alkylpyrazine formation in model systems. J. Agric. Food Chem. 1972;20(5):1081–1085. doi: 10.1021/jf60183a022. [DOI] [Google Scholar]
- Rizzi G.P. The biogenesis of food‐related Pyrazines. Food Rev. Int. 1988;4(3):375–400. doi: 10.1080/87559128809540839. [DOI] [Google Scholar]
- Rizzi G.P. Formation of pyrazines from acyloin precursors under mild conditions. J. Agric. Food Chem. 2002;36(2):349–352. doi: 10.1021/jf00080a026. [DOI] [Google Scholar]
- Rizzi G.P. In: Chemistry, Food and Health. Labuza T.P., et al., editors. Woodhead Publishing; 2005. The maillard reaction in foods. Maillard reactions; pp. 11–19. [DOI] [Google Scholar]
- Robacker D.C., Aluja M., Bartelt R.J., Patt J. Identification of chemicals emitted by calling males of the Sapote fruit fly, Anastrepha serpentina. J. Chem. Ecol. 2009;35(5):601–609. doi: 10.1007/s10886-009-9631-7. [DOI] [PubMed] [Google Scholar]
- Román S., Sánchez-Siles L.M., Siegrist M. The importance of food naturalness for consumers: results of a systematic review. Trends Food Sci. Technol. 2017;67:44–57. doi: 10.1016/j.tifs.2017.06.010. [DOI] [Google Scholar]
- Román S., Sánchez-Siles L.M., Siegrist M. The importance of food naturalness for consumers: results of a systematic review. Trends Food Sci. Technol. 2017;67:44–57. doi: 10.1016/j.tifs.2017.06.010. [DOI] [Google Scholar]
- Ryona I., Leclerc S., Sacks G.L. Correlation of 3-Isobutyl-2-methoxypyrazine to 3-Isobutyl-2-hydroxypyrazine during maturation of bell pepper (Capsicum annuum) and wine grapes (Vitis vinifera) J. Agric. Food Chem. 2010;58(17):9723–9730. doi: 10.1021/jf102072w. [DOI] [PubMed] [Google Scholar]
- Schmid A., Dordick J.S., Hauer B., Kiener A., Wubbolts M., Witholt B. Industrial biocatalysis today and tomorrow. Nature. 2001;409(6817):258–268. doi: 10.1016/j.tibtech.2019.01.011. [DOI] [PubMed] [Google Scholar]
- Schneider K., Assumpção W.T., Soldi C., Guerra M.P. Evidence of 2-methoxypyrazine reduction in cabernet sauvignon wines via spontaneous fermentation. Eur. Food Res. Technol. 2024;250(6):1815–1821. doi: 10.1007/s00217-024-04518-8. [DOI] [Google Scholar]
- Schöck M., Liebminger S., Berg G., Cernava T. First evaluation of alkylpyrazine application as a novel method to decrease microbial contaminations in processed meat products. AMB Express. 2018;8(1) doi: 10.1186/s13568-018-0583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajan R., Sibanda T., Tekere M., Nyoni H., Meddows-Taylor S. Diversity analysis and bioresource characterization of halophilic bacteria isolated from a South African saltpan. Molecules. 2017:22. doi: 10.3390/molecules22040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra S., Fuganti C., Brenna E. Biocatalytic preparation of natural flavours and fragrances. Trends Biotechnol. 2005;23(4):193–198. doi: 10.1016/j.tibtech.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Sharma B.K., Shaikh A.M., Chacko S., Kamble R.M. Synthesis, spectral, electrochemical and theoretical investigation of indolo[2,3-b]quinoxaline dyes derived from anthraquinone for n–type materials. J. Chem. Sci. 2017;129(4):483–494. doi: 10.1007/s12039-017-1252-z. [DOI] [Google Scholar]
- Shimizu Y., Sakuraba H., Kawakami R., Goda S., Kawarabayasi Y., Ohshima T. L-Threonine dehydrogenase from the hyperthermophilic archaeon Pyrococcus horikoshii OT3: gene cloning and enzymatic characterization. Extremophiles. 2005;9(4):317–324. doi: 10.1007/s00792-005-0447-2. [DOI] [PubMed] [Google Scholar]
- Silva-Junior E.A., Ruzzini A.C., Paludo C.R., Nascimento F.S., Currie C.R., Clardy J., Pupo M.T. Pyrazines from bacteria and ants: convergent chemistry within an ecological niche. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-20953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipiczki M. Metschnikowia strains isolated from botrytized grapes antagonize fungal and bacterial growth by iron depletion. Appl. Environ. Microbiol. 2006;72(10):6716–6724. doi: 10.1128/aem.01275-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriwardana A.I., Kathriarachchi K.K.A.D.S., Nakamura I., Gridnev I.D., Yamamoto Y. Synthesis of pyridinylpyrrole derivatives via the palladium-catalyzed reaction of acetylpyridines with methyleneaziridines. J. Am. Chem. Soc. 2004;126(43):13898–13899. doi: 10.1021/ja0455401. [DOI] [PubMed] [Google Scholar]
- Sun C., Zeng R., Chen T., Yang Y., Song Y., Li Q., Cheng J., Liu B. Recent advances and challenges in the production of hydroxylated natural products using microorganisms. Fermentation. 2024:10. doi: 10.3390/fermentation10120604. [DOI] [Google Scholar]
- Sun Y., Lin L., Zhang P. Color development kinetics of maillard reactions. Ind. Eng. Chem. Res. 2021;60(9):3495–3501. doi: 10.1021/acs.iecr.1c00026. [DOI] [Google Scholar]
- Syam Y.M., Anwar M.M., Abd El-Karim S.S., Elokely K.M., Abdelwahed S.H. New quinoxaline-based derivatives as PARP-1 inhibitors: design, synthesis, antiproliferative, and computational studies. Molecules. 2022;27(15) doi: 10.3390/molecules27154924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R.L., Canale-Parola E. Synthesis of pulcherriminic acid by Bacillus subtilis. J. Bacteriol. 1972;111(1):86–93. doi: 10.1128/jb.111.1.86-93.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallarino J.G., López-Cortés X.A., Dunlevy J.D., Boss P.K., González-Nilo F.D., Moreno Y.M. Biosynthesis of methoxypyrazines: elucidating the structural/functional relationship of two vitis viniferaO-Methyltransferases capable of catalyzing the putative final step of the biosynthesis of 3-Alkyl-2-Methoxypyrazine. J. Agric. Food Chem. 2011;59(13):7310–7316. doi: 10.1021/jf200542w. [DOI] [PubMed] [Google Scholar]
- Van Lancker F., Adams A., De Kimpe N. Chemical modifications of peptides and their impact on food properties. Chem. Rev. 2011;111(12):7876–7903. doi: 10.1021/cr200032j. [DOI] [PubMed] [Google Scholar]
- Wagner R., Czerny M., Bielohradsky J., Grosch W. Structure-odour-activity relationships of alkylpyrazines. Zeitschrift fr Lebensmitteluntersuchung und -Forschung A. 1999;208(5–6):308–316. doi: 10.1007/s002170050422. [DOI] [Google Scholar]
- Wang F., Shen H., Liu T., Yang X., Yang Y., Guo Y. Formation of pyrazines in maillard model systems: effects of structures of lysine-containing dipeptides/tripeptides. Foods. 2021:10. doi: 10.3390/foods10020273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Wu Q., Jiang X., Wang Z., Tang J., Xu Y. Bacillus licheniformis affects the microbial community and metabolic profile in the spontaneous fermentation of Daqu starter for Chinese liquor making. Int. J. Food Microbiol. 2017;250:59–67. doi: 10.1016/j.ijfoodmicro.2017.03.010. [DOI] [PubMed] [Google Scholar]
- Wang S., Hou Y., Chen X., Liu L. Kick-starting evolution efficiency with an autonomous evolution mutation system. Metab. Eng. 2019;54:127–136. doi: 10.1016/j.ymben.2019.03.010. [DOI] [PubMed] [Google Scholar]
- Wang S., Wang H., Zhang D., Li X., Zhu J., Zhan Y., Cai D., Wang Q., Ma X., Wang D., Chen S., Kelly R.M. Multistep metabolic engineering of Bacillus licheniformis to improve pulcherriminic acid production. Appl. Environ. Microbiol. 2020;86(9) doi: 10.1128/aem.03041-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Huang W., Huang J., Luo X., Nie M., Jiang T., Ban S., Li P. The mechanism of Laceyella sacchari FBKL4.010 produced tetramethylpyrazine in the liquid fermentation by comparative transcriptomic techniques. Front. Microbiol. 2024:15. doi: 10.3389/fmicb.2024.1414203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei M., Liu X., Xie P., Han A., Lei Y., Yang X., Liu Y., Zhang S., Sun B. Investigating the interactions between selected heterocyclic flavor compounds and beef myofibrillar proteins using SPME-GC–MS, spectroscopic, and molecular docking approaches. J. Mol. Liq. 2024;403 doi: 10.1016/j.molliq.2024.124878. [DOI] [Google Scholar]
- White E.C., Hill J.H. Studies on antibacterial products formed by molds: I. Aspergillic acid, a product of a strain of Aspergillus flavus. J. Bacteriol. 1943;45(5):433–443. doi: 10.1128/jb.45.5.433-443.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C.C., Liao M.H., Chen S.J., Yen M.H. Tetramethylpyrazine prevents inducible NO synthase expression and improves survival in rodent models of endotoxic shock. Naunyn-Schmiedebergs Arch Pharmacol. 1999;360(4):435–444. doi: 10.1007/s002109900046. [DOI] [PubMed] [Google Scholar]
- Xiao Z., Hou X., Lyu X., Xi L., Zhao J.-y. Accelerated green process of tetramethylpyrazine production from glucose and diammonium phosphate. Biotechnol. Biofuels. 2014;7(1) doi: 10.1186/1754-6834-7-106. [DOI] [Google Scholar]
- Xiao Z., Hou X., Lyu X., Xi L., Zhao J.-y. Accelerated green process of tetramethylpyrazine production from glucose and diammonium phosphate. Biotechnol. Biofuels. 2014;7(1):106. doi: 10.1186/1754-6834-7-106. [DOI] [Google Scholar]
- Xiao Z.J., Liu P.H., Qin J.Y., Xu P. Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl. Microbiol. Biotechnol. 2007;74(1):61–68. doi: 10.1007/s00253-006-0646-5. [DOI] [PubMed] [Google Scholar]
- Xiao Z.J., Xie N.Z., Liu P.H., Hua D.L., Xu P. Tetramethylpyrazine production from glucose by a newly isolated Bacillus mutant. Appl. Microbiol. Biotechnol. 2006;73(3):512–518. doi: 10.1007/s00253-006-0491-6. [DOI] [PubMed] [Google Scholar]
- Xu J., Green A.P., Turner N.J. Chemo‐Enzymatic synthesis of pyrazines and pyrroles. Angew. Chem. Int. Ed. 2018;57(51):16760–16763. doi: 10.1002/anie.201810555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Yu H., Chen X., Liu L., Zhang W. Accelerated green process of 2,5-Dimethylpyrazine production from glucose by genetically modified Escherichia coli. ACS Synth. Biol. 2020;9(9):2576–2587. doi: 10.1021/acssynbio.0c00329. [DOI] [PubMed] [Google Scholar]
- Xu M., Jin Z., Gu Z., Rao J., Chen B. Changes in odor characteristics of pulse protein isolates from germinated chickpea, lentil, and yellow pea: role of lipoxygenase and free radicals. Food Chem. 2020;314 doi: 10.1016/j.foodchem.2020.126184. [DOI] [PubMed] [Google Scholar]
- Xu Q., Xie L., Li Y., Lin H., Sun S., Guan X., Hu K., Shen Y., Zhang L. Metabolic engineering of Escherichia coli for efficient production of (3R)-acetoin. J. Chem. Technol. Biotechnol. 2015;90(1):93–100. doi: 10.1002/jctb.4293. [DOI] [Google Scholar]
- Xu Y., Xu C., Li X., Sun B., Eldin A.A., Jia Y. A combinational optimization method for efficient synthesis of tetramethylpyrazine by the recombinant Escherichia coli. Biochem. Eng. J. 2018;129:33–43. doi: 10.1016/j.bej.2017.10.010. [DOI] [Google Scholar]
- Yamamoto T., Moriwaki Y., Takahashi S., Hada T., Higashino K. In vitro conversion of pyrazinamide into 5-hydroxypyrazinamide and that of pyrazinoic acid into 5-hydroxypyrazinoic acid by xanthine oxidase from human liver. Biochem. Pharmacol. 1987;36(19):3317–3318. doi: 10.1016/0006-2952(87)90654-x. [DOI] [PubMed] [Google Scholar]
- Yan Y., Chen S., He Y., Nie Y., Xu Y. Quantitation of pyrazines in Baijiu and during production process by a rapid and sensitive direct injection UPLC-MS/MS approach. Lwt. 2020:128. doi: 10.1016/j.lwt.2020.109371. [DOI] [Google Scholar]
- Yang C. Efficient synthesis of 2,5-dimethylpyrazine by constructing recombinant Escherichia coli. Master. 2021. 2021. [DOI]
- Yang D., Park S.Y., Park Y.S., Eun H., Lee S.Y. Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends Biotechnol. 2020;38(7):745–765. doi: 10.1016/j.tibtech.2019.11.007. [DOI] [PubMed] [Google Scholar]
- Yang P., Wang H., Cao Q., Song H., Xu Y., Lin Y. Aroma-active compounds related to Maillard reaction during roasting in Wuyi Rock tea. J. Food Compos. Anal. 2023;115 doi: 10.1016/j.jfca.2022.104954. [DOI] [Google Scholar]
- Yang Z., Xu H., Chen X., Tian J., Chen J. Development of a method for the determination of alkylpyrazine in traditional vinegar and its formation kinetics. J. Food Compos. Anal. 2024;131 doi: 10.1016/j.jfca.2024.106272. [DOI] [Google Scholar]
- Yin X., Wei Y., Li T., Zhang J., Zou L., Cui Q., Lu C., Ning J. Heterocyclic compounds formation in large-leaf yellow tea induced by the Maillard reaction at different roasting temperatures. Lebensm. Wiss. Technol. 2023;182 doi: 10.1016/j.lwt.2023.114856. [DOI] [Google Scholar]
- Yu A.-N., Tan Z.-W., Shi B.-A. Influence of the pH on the formation of pyrazine compounds by the Maillard reaction of L-ascorbic acid with acidic, basic and neutral amino acids. Asia Pac. J. Chem. Eng. 2012;7(3):455–462. doi: 10.1002/apj.594. [DOI] [Google Scholar]
- Yuan M., Zhang Q., Li Y., Li J., Meng N., Sun J., Wang B., Sun B. Mixed fermentation innovation: manipulating nitrogen and carbon levels for organoleptic masking of undesirable green character attributed to 3-isobutyl-2-Methoxypyrazine in wines. Food Biosci. 2025;64 doi: 10.1016/j.fbio.2025.105914. [DOI] [Google Scholar]
- Zamolo F., Wüst M. Investigation of biosynthetic precursors of 3-Isobutyl-2-Methoxypyrazine using stable isotope labeling studies in Bell pepper fruits (Capsicum annuum L.) J. Agric. Food Chem. 2022;70(22):6719–6725. doi: 10.1021/acs.jafc.2c01747. [DOI] [PubMed] [Google Scholar]
- Zayed M.F. Medicinal chemistry of quinazolines as analgesic and anti-inflammatory agents. ChemEng. 2022;6(6) doi: 10.3390/chemengineering6060094. [DOI] [Google Scholar]
- Zayed M.F., Ibrahim S.R.M., Habib E.L.S.E., Hassan M.H., Ahmed S., Rateb H.S. Design, synthesis, antimicrobial and anti-biofilm evaluation, and molecular docking of newly substituted fluoroquinazolinones. Med. Chem. 2019;15(6):659–675. doi: 10.2174/1573406414666181109092944. [DOI] [PubMed] [Google Scholar]
- Zhang B., Li X.-l., Fu J., Li N., Wang Z., Tang Y.-j., Chen T. Production of acetoin through simultaneous utilization of glucose, Xylose, and arabinose by engineered Bacillus subtilis. PLoS One. 2016;11(7) doi: 10.1371/journal.pone.0159298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Li L., Liu J., Cai J., Fu J., Li N., Cao H., Xu H., Zhang Y., Cao R. Comparing the metabolite components of Sichuan Sun vinegar and other kinds of vinegar based on non-targeted metabolomic. Lebensm. Wiss. Technol. 2022;164 doi: 10.1016/j.lwt.2022.113640. [DOI] [Google Scholar]
- Zhang H., Zhang L., Yu X., Xu Y. The biosynthesis mechanism involving 2,3-Pentanedione and aminoacetone describes the production of 2-Ethyl-3,5-dimethylpyrazine and 2-Ethyl-3,6-dimethylpyrazine by Bacillus subtilis. J. Agric. Food Chem. 2020;68(11):3558–3567. doi: 10.1021/acs.jafc.9b07809. [DOI] [PubMed] [Google Scholar]
- Zhang L. Marketable biorefinery strategy research: Split sugar refining process from corn cob. World Sci. Res. J. 2023;9(3) doi: 10.6911/WSRJ.202303_9(3).0004. [DOI] [Google Scholar]
- Zhang L., Cao Y., Tong J., Xu Y. An alkylpyrazine synthesis mechanism involving l-Threonine-3-Dehydrogenase describes the production of 2,5-Dimethylpyrazine and 2,3,5-Trimethylpyrazine by Bacillus subtilis. Appl. Environ. Microbiol. 2019;85(24) doi: 10.1128/AEM.01807-19. 01819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Cao Y., Tong J., Xu Y., Björkroth J. An alkylpyrazine synthesis mechanism involving l-Threonine-3-Dehydrogenase describes the production of 2,5-Dimethylpyrazine and 2,3,5-Trimethylpyrazine by Bacillus subtilis. Appl. Environ. Microbiol. 2019;85(24) doi: 10.1128/aem.01807-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Guo W., Lu Y. Advances in cell‐free biosensors: principle, mechanism, and applications. Biotechnol. J. 2020;15(9) doi: 10.1002/biot.202000187. [DOI] [PubMed] [Google Scholar]
- Zhang L., Huang J., Zhou R., Wu C. Evaluating the feasibility of fermentation starter inoculated with Bacillus amyloliquefaciens for improving acetoin and tetramethylpyrazine in Baoning bran vinegar. Int. J. Food Microbiol. 2017;255:42–50. doi: 10.1016/j.ijfoodmicro.2017.05.021. [DOI] [PubMed] [Google Scholar]
- Zhang L., Liu Q., Ge Y., Li L., Gao C., Xu P., Ma C. Biotechnological production of acetoin, a bio-based platform chemical, from a lignocellulosic resource by metabolically engineered Enterobacter cloacae. Green Chem. 2016;18(6):1560–1570. doi: 10.1039/c5gc01638j. [DOI] [Google Scholar]
- Zhang X., Tervo C.J., Reed J.L. Metabolic assessment of E. coli as a biofactory for commercial products. Metab. Eng. 2016;35:64–74. doi: 10.1016/j.ymben.2016.01.007. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zeng X., Mu L., Chen Y., Zhang J.-X., Redshaw C., Wei G. Rhodamine-triazine based probes for Cu2+ in aqueous media and living cells. Sens. Actuators, B. 2014;204:24–30. doi: 10.1016/j.snb.2014.06.125. [DOI] [Google Scholar]
- Zhang Y.H.P. Production of biocommodities and bioelectricity by cell‐free synthetic enzymatic pathway biotransformations: challenges and opportunities. Biotechnol. Bioeng. 2010;105(4):663–677. doi: 10.1002/bit.22630. [DOI] [PubMed] [Google Scholar]
- Zhang Y.H.P. Simpler is better: high-yield and potential low-cost biofuels production through cell-free synthetic pathway biotransformation (SyPaB) ACS Catal. 2011;1(9):998–1009. doi: 10.1021/cs200218f. [DOI] [Google Scholar]
- Zhang Z., Wei T., Hou J., Li G., Yu S., Xin W. Tetramethylpyrazine scavenges superoxide anion and decreases nitric oxide production in human polymorphonuclear leukocytes. Life Sci. 2003;72(22):2465–2472. doi: 10.1016/s0024-3205(03)00139-5. [DOI] [PubMed] [Google Scholar]
- Zhao C., Cao H., Xiao J. Handbook of Dietary Phytochemicals. J. Xiao et al. Singapore. Springer Singapore; 2020. Pyrazines in food; pp. 1–25. [DOI] [Google Scholar]
- Zhao T., Chen S., Li H., Xu Y. Identification of 2-Hydroxymethyl-3,6-diethyl-5-methylpyrazine as a key retronasal burnt flavor compound in soy sauce aroma type baijiu using sensory-guided isolation assisted by multivariate data analysis. J. Agric. Food Chem. 2018;66(40):10496–10505. doi: 10.1021/acs.jafc.8b03980. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Ngo H.H., Luo H., Wang R., Li C., Zhang C., Wang X. Production of cost-competitive bioethanol and value-added co-products from distillers' grains: Techno-economic evaluation and environmental impact analysis. Bioresour. Technol. 2024;397 doi: 10.1016/j.biortech.2024.130470. [DOI] [PubMed] [Google Scholar]
- Zhou C., Xu Z., Shi H., Zhang G., Cao W., Xu X. Simple and rapid method for the quantitation of trace-level 3-Alkyl-2-methoxypyrazines in fragrant vegetable oils by gas chromatography–tandem mass spectrometry. J. Agric. Food Chem. 2022;70(20):6247–6252. doi: 10.1021/acs.jafc.1c07380. [DOI] [PubMed] [Google Scholar]
- Zhou X., Zhang Y., Shen Y., Zhang X., Zhang Z., Xu S., Luo J., Xia M., Wang M. Economical production of androstenedione and 9α-hydroxyandrostenedione using untreated cane molasses by recombinant mycobacteria. Bioresour. Technol. 2019;290 doi: 10.1016/j.biortech.2019.121750. [DOI] [PubMed] [Google Scholar]
- Zhu B.-F., Xu Y., Fan W.-L. High-yield fermentative preparation of tetramethylpyrazine by Bacillus sp. using an endogenous precursor approach. J. Ind. Microbiol. Biotechnol. 2009;37(2):179–186. doi: 10.1007/s10295-009-0661-5. [DOI] [PubMed] [Google Scholar]
- Zhu Z., Percival Zhang Y.-H. Chemical biotechnology of in vitro synthetic biosystems for biomanufacturing. Chem Bio Eng. 2015:98–121. doi: 10.1039/9781782620129. [DOI] [Google Scholar]