Abstract
Gas2 is a caspase-3 substrate that plays a role in regulating microfilament and cell shape changes during apoptosis. Here we provide evidence that overexpression of Gas2 efficiently increases cell susceptibility to apoptosis following UV irradiation, etoposide and methyl methanesulfonate treatments, and that these effects are dependent on increased p53 stability and transcription activity. To investigate possible pathways linking Gas2 to p53, a yeast two-hybrid screen swas performed, indicating m-calpain as a strong Gas2- interacting protein. Moreover, we demonstrate that Gas2 physically interacts with m-calpain in vivo and that recombinant Gas2 inhibits calpain-dependent processing of p53. Importantly, the Gas2 dominant-negative form (Gas2Δ171–314) that binds calpain but is unable to inhibit its activity abrogates Gas2’s ability to stabilize p53, to enhance p53 transcriptional activity and to induce p53-dependent apoptosis. Finally, we show that Gas2 is able to regulate the levels of p53 independently of Mdm2 status, suggesting that, like calpastatin, it may enhance p53 stability by inhibiting calpain activity.
Keywords: apoptosis/calpain/Gas2/p53/yeast two-hybrid
Introduction
Cell death by apoptosis is a fundamental process controlling normal development and homeostasis of multicellular organisms. Decreased apoptotic susceptibility contributes to the pathogenesis of several diseases including cancer. A central regulator of apoptotic susceptibility is the tumor suppressor protein p53, whose levels are regulated by several stress conditions including UV irradiation, DNA damage, hypoxia, changes in the redox potential, cell adhesion and expression of several oncogenes (Levine, 1997; Giaccia and Kastan, 1998). Increased susceptibility to p53-dependent apoptosis has been shown to be an important mechanism by which transformation and tumor growth are inhibited. The apoptotic mechanism of p53 has been dissected intensively and multiple pathways have been identified (Giaccia and Kastan, 1998).
An initial critical step in the p53 apoptotic response is the accumulation of the protein, where the Mdm2 oncoprotein plays a key role as regulator of p53 stability (Haupt et al., 1997). In addition to this well-characterized pathway, it has also been reported that p53 is proteolytically cleaved in vitro by calpains (Gonen et al., 1997; Pariat et al., 1997), a family of calcium-activated non-lysosomal neutral cysteine proteases. In vivo activation of calpain or expression of its inhibitor, calpastatin, can modulate p53 levels (Pariat et al., 1997) and, most importantly, recent reports suggest a link between the role of calpain in degradation of wild-type p53 and p53-dependent apoptosis (Kubbutat and Vousden, 1997; Pariat et al., 1997; Atencio et al., 2000).
The final outcome of the p53 apoptotic response is the activation of caspases, which play a critical role in the execution of the apoptotic program from nematodes to humans (Cohen, 1997; Yuan, 1997). Caspases govern the morphological changes by specifically cleaving selected cellular proteins involved in the apoptotic phenotype. Moreover, they can also regulate susceptibility to apoptosis by cleaving substrates controlling cell survival or cell death (Tan and Wang, 1998; Porter and Janicke, 1999).
Gas2 was identified initially as a growth arrest-specific gene (Schneider et al., 1988), since its expression is increased during G0 in cultured cell lines (Brancolini et al., 1992). Further studies showed that Gas2 is a death substrate cleaved by caspase-3 in vitro (Sgorbissa et al., 1999) and that the resulting processed form can regulate cell shape and morphological changes (Brancolini et al., 1995). In addition, Gas2 expression is regulated during mouse development and apoptosis (Brancolini and Schneider, 1997; Lee et al., 1999).
Here we provide evidence that Gas2 efficiently enhances cell susceptibility to apoptosis following treatments with DNA-damaging agents such as UV irradiation, etoposide or methyl methanesulfonate (MMS) and that these effects are dependent on a functional p53. In addition, we demonstrate that Gas2 binds m-calpain and modulates its proteolytic activity in vitro. Finally, we describe a dominant-negative form of Gas2 (Gas2Δ171– 314) that is able to prevent calpain inhibition and the downstream effects on p53 as mediated by Gas2, thus supporting the hypothesis that Gas2 could modulate susceptibility to p53-dependent apoptosis by inhibiting calpain activity.
Results
Gas2 enhances p53-induced apoptosis
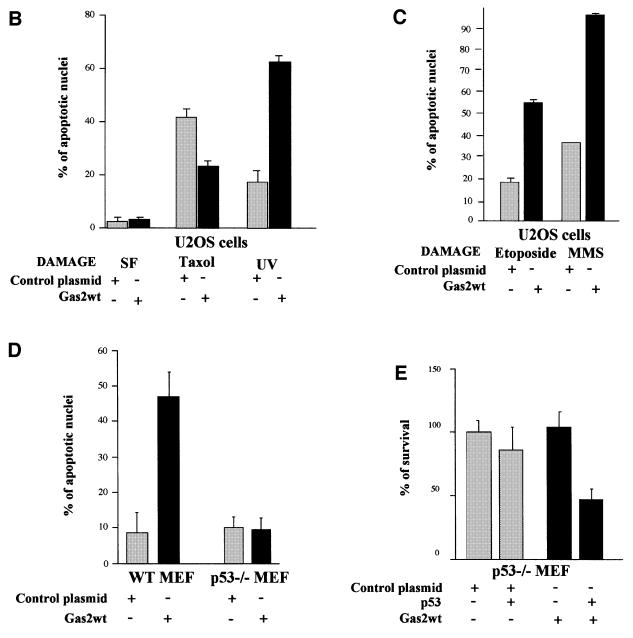
The ability of Gas2 to regulate susceptibility to apoptosis was analyzed by transfecting Gas2 in the U2OS human cell line. After transfection, cells were treated with different apoptotic stimuli such as UV irradiation, taxol and removal of survival signals (serum free). After 24 h, apoptosis was assessed by scoring nuclear alteration in cells overexpressing Gas2, as revealed by immunofluorescence analysis. Representative fields of the immunofluorescence analysis are shown in Figure 1A. Overexpression of Gas2 alone is unable to induce cell death (Brancolini et al., 1999); however, we observed that it significantly enhanced the UV-triggered apoptosis. The Gas2-dependent increased susceptibility to UV-induced apoptosis was highly reproducible over a total of five independent experiments, ranging from a 40 to 65% increase in apoptosis (58.4 ± 6.2) compared with a control plasmid (16.9 ± 5.9). Taxol treatment or growth in serum-free conditions for 24 h did not result in a significant increase in apoptosis in cells overexpressing Gas2. On the contrary, Gas2 appeared to provide protection from taxol-induced cell death (Figure 1B). Since UV irradiation induces apoptosis through a p53-dependent mechanism (Smith and Fornace, 1997), while both serum-free conditions and taxol act through a p53-independent mechanism (Woods et al., 1995; Sorger et al., 1997), we further analyzed the effect of other treatments known to stimulate p53-dependent apoptosis (Lakin and Jackson, 1999; Rodriguez-Lopez et al., 2001), such as MMS and etoposide on Gas2-overexpressing U2OS cells. At 12 h after transfection, cells were treated with 100 µM MMS for 4 h or with 100 µM etoposide for 24 h. Apoptosis was assessed 24 h after cell treatments by scoring nuclear alteration in cells overexpressing Gas2 or a control protein. Gas2-dependent increased susceptibility to apoptosis was observed in both cases. Gas2 overexpression caused an increase in apoptosis on etoposide-treated cells ranging from 40 to 45% and on MMS-treated cells ranging from 45 to 50% when compared with a control protein (Figure 1C). These data suggest that Gas2 could enhance susceptibility to apoptosis through a p53-dependent mechanism.
Fig. 1. Gas2 overexpression increases susceptibility to apoptosis. (A) A confocal-generated overlay showing nuclear morphology in U2OS cells expressing Gas2 and treated further with the indicated apoptotic stimuli. U2OS cells transfected with Gas2wt were treated with the different apoptotic stimuli and 12 h later cells were processed for immunofluorescence to visualize Gas2 (green). Propidium iodide was used to visualize nuclei (red). (B) Diagram showing the percentage of apoptotic nuclei in U2OS cells (p53 wild-type) expressing Gas2 and treated with the indicated apoptotic stimuli. (C) Diagram showing the percentage of apoptotic nuclei in U2OS cells expressing Gas2 and a control protein, and treated with etoposide and MMS. (D) Diagram showing the percentage of apoptotic nuclei in wild-type and p53–/– MEFs, which differ only in their p53 status, expressing Gas2 and treated with UV. (E) Diagram showing the comparison of viability between cells microinjected with different combinations of vectors as indicated. Data represent the means of at least three independent experiments and error bars represent standard deviations.
To analyze specifically whether Gas2-enhanced apoptotic susceptibility required wild-type p53, we compared the behavior of mouse embryo fibroblasts (MEFs) containing wild-type p53 (wild-type MEFs) or deleted p53 alleles (p53–/– MEFs) by using the Gas2/UV irradiation model to induce cell death. The presence of Gas2, followed by UV treatment, increased cell susceptibility to apoptosis only in wild-type MEFs, while no significant effect was observed when Gas2 was overexpressed in p53–/– MEFs (Figure 1D). Moreover, p53–/– MEFs regained the Gas2 apoptotic effect when p53 was expressed ectopically by combined microinjection of Gas2 and wild-type p53, significantly decreasing cell recovery as compared with microinjection of the single plasmids, indicating that the apoptosis induced by ectopic expression of p53 was enhanced efficiently by Gas2 (Figure 1E).
In summary, while Gas2 overexpression by itself failed to induce apoptosis (Brancolini et al., 1999), further treatments with either UV, etoposide, MMS or ectopic expression of p53 significantly increased susceptibility to apoptosis. Furthermore, Gas2-induced sensitization to apoptotic stimuli required wild-type p53, thus linking Gas2-dependent apoptosis susceptibility to a p53- dependent mechanism.
Gas2 increases the steady-state levels of p53
The evidence that ectopic expression of Gas2 enhances p53-dependent apoptosis when cells are exposed to UV radiation led us to analyze whether overexpression of Gas2 might enhance the steady-state levels of p53, thus explaining the increased response to p53-dependent apoptosis.
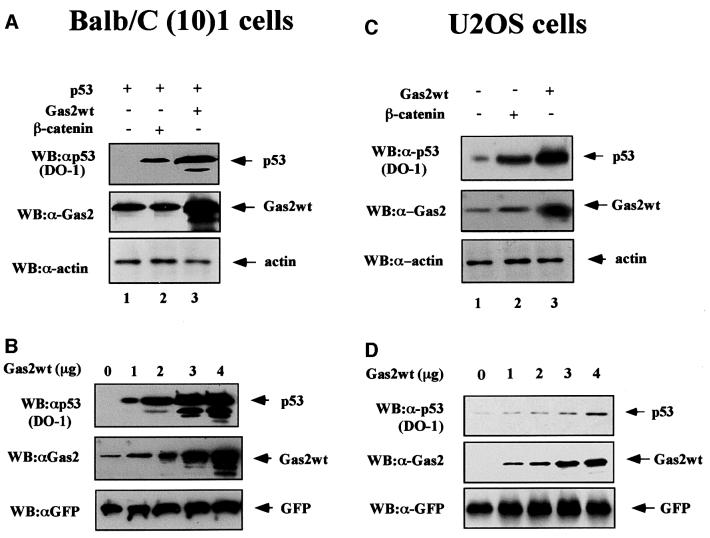
In order to analyze the effects of Gas2 on p53 stability, Balb/C (10)1 cells (null for p53) were transiently transfected with wild-type p53 either alone or in combination with Gas2. A significant enhancement of p53 levels was observed when Gas2 was overexpressed (Figure 2A, lane 3). As reported by others (Damalas et al., 1999), a similar effect on the steady-state level of p53 was observed when β-catenin was overexpressed under the same assay conditions as used for Gas2 (Figure 2A, lane 2). As shown in Figure 2B, the accumulation of p53 correlates with the amount of overexpressed Gas2 protein in a dose-dependent fashion. Similar results were obtained when p53–/– MEFs were used (data not shown).
Fig. 2. Gas2 overexpression regulates p53 levels. (A) Mouse Balb/C (10)1 fibroblasts were transfected with wild-type p53, either alone or together with Gas2wt or β-catenin, as indicated. Cells were lysed 24 h after transfection and subjected to western blot analysis with p53-specific DO-1 antibody (upper panel). The same membrane was probed subsequently with anti-Gas2 antibody (middle panel) and anti-actin antibody as a loading control (lower panel). (B) Balb/C (10)1 mouse fibroblasts were transfected with wild-type p53 together with empty vector or with increasing amounts of Gas2wt as indicated. A 100 ng aliquot of pEGFP was co-transfected to monitor transfection efficiency. Western blotting was performed with DO-1 antibody (upper panel), with anti-Gas2 antibody to confirm the expression of transfected Gas2 (middle panel) or with anti-GFP antibody to evaluate transfection efficiency (lower panel). (C) U2OS cells were transfected with empty vector, Gas2 and β-catenin as indicated. Cells were lysed 24 h after transfection and subjected to western blot analysis with DO-1 antibody. The same membrane subsequently was probed with anti-Gas2 antibody and anti-actin antibody to estimate the total amount of protein loaded in each lane. (D) U2OS cells were transfected with the indicated amounts of empty vector or Gas2. A 100 ng aliquot of pGFP was co-transfected to monitor the efficiency of transfection. Western blotting was performed with DO-1, with anti-Gas2 antibody to confirm the expression of transfected Gas2 or with anti-GFP antibody to evaluate transfection efficiency.
To understand whether ectopic expression of Gas2 could affect the levels of endogenous p53, U2OS cells were transfected with Gas2. As summarized in Figure 2C, introducing Gas2 elicited a significant increase in the levels of endogenous p53 (lane 3). Again, expression of β-catenin, used as a control, induced a stabilization of p53 protein (lane 2). In this case also, the levels of p53 accumulation were related to the amount of expressed Gas2 (Figure 2D). Taken together, these data indicate that ectopic expression of Gas2 is able to increase p53 levels.
The p53 protein, as stabilized by Gas2, is transcriptionally active
We next investigated whether the p53 protein induced by Gas2 was in an active form by evaluating the ability of Gas2 to stimulate p53-specific transcription in reporter gene assays. U2OS cells were co-transfected with a luciferase reporter construct (p21-LUC) containing the p21Waf1 promoter (El-Deiry et al., 1993): overexpression of Gas2 efficiently enhanced transcription from the p21 promoter when compared with the empty vector (Figure 3A). Similar results were obtained using the Bax and PIG3 promoters (data not shown). This topic was also approached by transfecting p53 and Gas2 in p53-null Balb/C (10)1 fibroblasts. Small amounts of ectopically expressed wild-type p53 caused a limited increase in p21Waf1 promoter activation, but such an induction became significantly more pronounced when Gas2 was co-transfected (Figure 3B). Increasing amounts of Gas2 elicited a dose-dependent accumulation of both the transfected and endogenous p53 transcriptionally active form (data not shown).
Fig. 3. Gas2 enhances the transcriptional activity of p53. (A) U2OS cells were transfected with the p21-Luc reporter together with empty vector or Gas2. Luciferase assays were performed 24 h later. (B) Balb/C (10)1 fibroblasts were transfected with p21-Luc reporter and wild-type p53, together with empty vector or Gas2. Luciferase assays were performed 24 h later. Data represent arithmetic means ± SD from three independent experiments. (C) MEFs were transfected with the indicated combinations of plasmids. Protein levels of p53, p21/Waf1, Mdm2 and Gas2 were determined by western blot analysis.
It was important to confirm that such enhanced transcriptional activity operates not only on reporter gene assays, but also on endogenous p53-responsive genes. Endogenous levels of the p53 target proteins p21Waf1 and Mdm2 were therefore monitored. Co-transfection of p53 with Gas2 alone or β-catenin used as positive control in p53–/– MEFs increased the expression of both p21Waf1 and Mdm2 proteins (Figure 3C).
Isolation of m-calpain as a candidate Gas2-associated protein
The reported results suggest that ectopic expression of Gas2 could enhance the steady-state levels of endogenous p53, thus rendering cells more prone to die through a p53-dependent apoptosis when treated with DNA-damaging agents. To define better the mechanisms involved in the Gas2-mediated accumulation of p53, the yeast two-hybrid technique (Gyuris et al., 1993; Lamphere et al., 1997) was employed to identify Gas2-interacting proteins. Full-length wild-type Gas2 (Gaswt) fused to the LexA DNA-binding domain was used as bait to screen a cDNA library from quiescent human WI-38 fibroblast fused to the B42 transactivation domain. From the classification and sequence analysis, 94% of the primary clones were identified as cDNAs encoding the large subunit of human m-calpain (Sorimachi et al., 1997; Ono et al., 1998).
To identify the region of m-calpain (Figure 4A) involved in the interaction with Gas2wt, the N- (amino acids 1–355) and C-terminal (amino acids 355–700) halves of the large subunit were fused to the B42 transactivation domain and tested using the yeast two-hybrid system for their ability to interact with Gas2wt. The C-terminal region of m-calpain, which contains the calcium-binding region, binds to Gas2wt, while its N-terminal region, which contains the catalytic domain, was unable to interact (Figure 4B).
Fig. 4. Gas2 interacts with m-calpain. (A) Schematic representation of m-calpain. In the large subunit, the N-terminal region (domains I and II; amino acids 1–355) contains the catalytic domains, while the C-terminal region (domains III and IV; amino acids 355–700) represents the regulatory regions of the protease. EF-hand structures capable of binding calcium are indicated as white bars. (B) Interaction of calpain with Gas2wt in yeast. The LexA–Gas2 fusion employed is represented on the left and the various deletions of calpain fused to B42 are shown on the right. + indicates a positive interaction, as judged by β-galactosidase activity and ability to grow in the absence of leucine; – indicates no detectable interaction. (C) Balb/C (10)1 cells were transfected with Gas2 together with HA-m-calpain (lane 1) or HA-E4FΔ350 (lane 2). After 24 h, lysates were immunoprecipitated with an anti-Gas2 antibody. Total lysates: an aliquot of each lysate was checked for the expression of the transfected plasmid by staining with anti-HA. The same membrane was probed subsequently with an anti-Gas2 antibody. (D) Immunoprecipitation was performed on endogenous Gas2 and endogenous calpain. Balb/C 3T3 cell lysates were immunoprecipitated with an anti-Gas2 antibody (lane 1) or with a pre-immune serum (lane 2). Immuno complexes were resolved on a 10% SDS–polyacrylamide gel and subjected to western blot analysis with anti-calpain antibody. An aliquot of each lysate (total lysates) was checked for the expression of endogenous Gas2wt and calpain. Running positions of molecular weight markers and of the various proteins are indicated.
We then tested the ability of Gas2 to interact with m-calpain in mammalian cells by co-immunoprecipitation analysis. Expression vectors containing hemagglutinin (HA)-tagged m-calpain and Gas2wt were transiently expressed in Balb/C (10)1 fibroblasts. Co-transfection of Gas2wt and the HA-tagged protein E4FΔ350 (Sandy et al., 2000) was used as negative control. Cell lysates were immunoprecipitated with anti-Gas2 polyclonal antibody followed by western blotting with anti-HA antibody. As shown in Figure 4C, ectopically expressed Gas2wt can associate with HA-calpain (lane 1) but not with HA-E4FΔ350 (lane 2). Gas2 and HA-tagged protein levels on the respective total lysates are also shown.
Most importantly, we also observed the interaction between endogenous Gas2 and the large subunit of m-calpain in Balb/C 3T3 cells when cell lysates were immunoprecipitated with Gas2 antibody followed by western blotting with anti-calpain antibody (Figure 4D).
Gas2 is an inhibitor of m-calpain in vitro and, similarly to calpastatin, stabilizes p53 in an Mdm2-independent manner
The physical interaction between Gas2 and calpain mentioned above prompted us to examine whether Gas2 could be a substrate for calpain by using a previously described in vitro proteolytic assay (Pariat et al., 1997). Several reports have shown that calpain can cleave p53 in a cell-free system (Gonen et al., 1997; Pariat et al., 1997). In vitro translated (IVT) p53 was therefore used as a substrate to monitor calpain activity. p53 cleavage was inhibited by EGTA and was not due to other Ca2+-dependent proteases present in the reticulocyte lysate, since it did not occur in the absence of added m-calpain (data not shown).
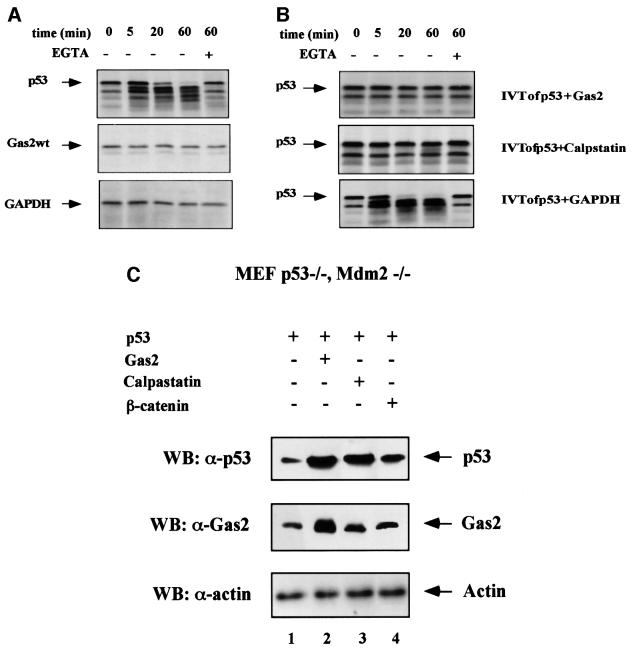
The same in vitro proteolytic assay was performed using IVT Gas2wt as substrate. Aliquots of the reaction were sampled at various time points, fractionated by SDS–PAGE and analyzed by autoradiography. As shown in Figure 5A, Gas2wt was not cleaved by calpain under these conditions, while p53 was cleaved within 20 min. Hamster glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which is not degraded by calpain, was used as negative control.
Fig. 5. Gas2 is not a substrate of calpain, inhibits calpain activity in vitro and stabilizes p53 in the absence of Mdm2. (A) Proteolysis experiments were carried out as described in the text. Aliquots were taken at the indicated time points, with time zero corresponding to the addition of calcium for activating calpain. EGTA was added at the concentration of 1 mM. Full-length proteins are indicated by arrows. (B) Equal amounts of in vitro translated Gas2, calpastatin and GAPDH proteins were added separately to in vitro translated p53 (indicated by an arrow). Reactions were incubated at room temperature with purified bovine m-calpain, and aliquots of samples were collected after 5, 20 and 60 min, as indicated. Time 0 corresponds to the addition of calcium. EGTA was added at the concentration of 1 mM. (C) p53–/– Mdm2–/– MEFs were transfected with p53 together with an equal amount of Gas2, calpastatin and β-catenin as indicated. Cells were harvested 24 h after transfection and subjected to western blot analysis using p53-specific antibody (DO-1) (upper panel), Gas2-specific antibody (middle panel) and anti-actin antibody (lower panel).
Next, we analyzed whether Gas2 might function as a regulator of m-calpain proteolytic activity, using p53 as a specific substrate. IVT p53 and Gas2wt were mixed with purified m-calpain in a standard cleavage buffer, and aliquots of the samples were taken at defined time points to follow the kinetics of degradation. As shown in Figure 5B, under conditions in which p53 is cleaved completely by calpain, the presence of IVT Gas2wt efficiently inhibited such processing. A similar degree of inhibition was observed when the assay was performed using IVT calpastatin, the well-defined calpain inhibitor, suggesting that Gas2 can modulate the proteolytic activity of calpain.
These results therefore suggest that the Gas2-mediated increase in p53 stability could be due to inhibition of calpain proteolytic activity towards p53. Similarly to Gas2, overexpression of the endogenous inhibitor of calpain, calpastatin, increases UV sensitivity (Hiwasa et al., 2000); this effect was not observed when calpastatin was overexpressed in p53 null cells (data not shown). We next tested whether Gas2 and calpastatin also stabilized p53 in the absence of Mdm2, a critical regulator of p53 stability (Prives, 1998; Prives and Hall, 1999). To perform this experiment, double knockout p53–/– Mdm2–/– MEFs were transfected with wild-type p53 alone or together with Gas2, calpastatin or β-catenin. It has been shown recently that β-catenin stabilizes p53 in the absence of Mdm2 (Damalas et al., 1999). As shown in Figure 5C, Gas2 and calpastatin were both also able to up-regulate p53 in the absence of Mdm2 (compare lanes 1, 2 and 3), thus suggesting that Gas2 does not interfere with Mdm2 functions to stabilize p53.
A deleted form of Gas2 acts as a dominant-negative, preventing Gas2-dependent calpain inhibition, p53 stabilization and susceptibility to apoptosis
Altogether, the previous results suggest that Gas2-mediated stabilization of p53 is independent of Mdm2 activity, strongly supporting a possible role for calpain in p53 degradation.
The ability of Gas2 to interfere with the stability and function of p53 in an Mdm2-independent manner prompted us to identify a deleted form of Gas2, which lacked the ability to inhibit calpain in vitro, and also lacked the reported cell shape modulation, thus differing from the apoptotic form.
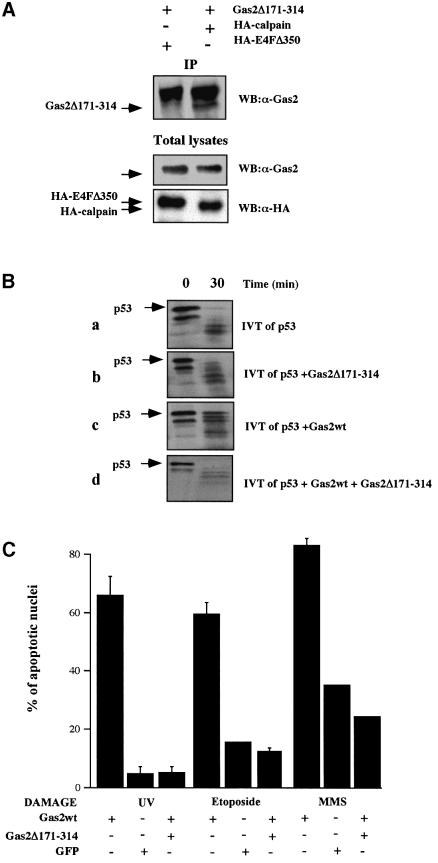
A C-deleted form of Gas2 (Gas2Δ171–314) previously shown to be impaired in regulating cell shape changes (Brancolini et al., 1995) was tested for its ability to bind m-calpain. Gas2Δ171–314 was shown to interact with m-calpain in yeast (data not shown). A similar interaction was also shown in mammalian cells (Figure 6A) when an expression vector containing Gas2Δ171–314 fused to green fluorescent protein (GFP) was transiently co-transfected with an HA-tagged m-calpain or an HA-tagged control protein (HA-E4FΔ350) into Balb/C (10)1 fibroblasts, as previously shown for the Gas2wt–calpain interaction. Cell lysates were immunoprecipitated with anti-HA antibody followed by western blot with anti-Gas2 antibody. As shown in Figure 6A, ectopically expressed Gas2Δ171–314 was able to interact specifically with HA-calpain (lane 2) but not with HA-E4FΔ350 (lane 1). Finally, we analyzed whether the deleted form of Gas2 was able to inhibit m-calpain in vitro: IVT Gas2Δ171–314 and p53 were mixed with purified m-calpain under the same conditions previously described and aliquots of the samples were taken at defined time points. As shown in Figure 6B (a and b), IVT Gas2Δ171–314 was unable to inhibit p53 processing. Most importantly, IVT Gas2Δ171– 314 blocked the inhibitory effect of Gas2wt on calpain as shown in Figure 6B (c and d), while the same amounts of IVT GAPDH failed to show an inhibitory effect, strongly suggesting a putative role for Gas2Δ171–314 as dominant-negative with respect to the wild-type form.
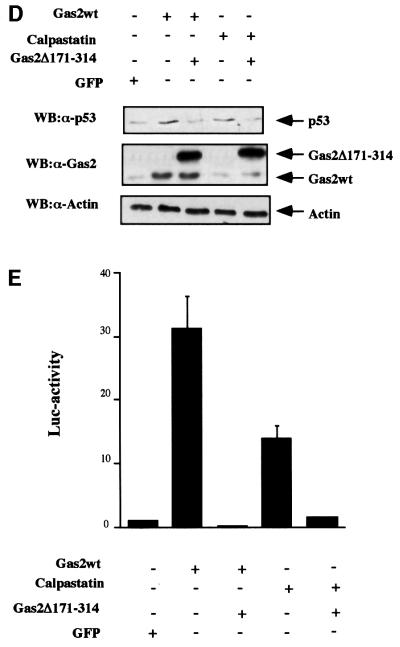
Fig. 6. Characterization of the dominant-negative form of Gas2. (A) Gas2Δ171–314 associates with the large subunit of m-calpain in mammalian cells. Balb/C (10)1 cells were transfected with GFP-tagged Gas2Δ171–314 together with HA-tagged E4FΔ350 (lane 1) and HA-tagged m-calpain (lane 2). Lysates were immunoprecipitated with anti-HA antibody. Immunocomplexes were resolved by SDS–PAGE and subjected to western blot analysis with anti-Gas2 antibody. (B) Gas2Δ171–314 is not able to inhibit calpain activity in vitro and to counteract the Gas2wt effect. (a and b) Equal amounts of in vitro translated Gas2Δ171–314 and GAPDH proteins were added separately to in vitro translated p53 (indicated by an arrow). Reactions were incubated at room temperature with purified bovine m-calpain (final concentration of 50 mg/ml) and aliquots of samples were collected at time 0, which corresponds to addition of calcium required for activating calpains, and after 30 min, as indicated. (c and d) Gas2wt and GAPDH or Gas2wt and Gas2Δ171–314 were added separately to in vitro translated p53 (indicated by an arrow). Reactions were incubated at room temperature with purified bovine m-calpain (50 mg/ml) and aliquots of samples were collected at time 0 and after 30 min, as indicated. (C) Diagram showing the percentage of apoptotic nuclei in U2OS cells expressing Gas2wt either alone or together with Gas2Δ171–314 in comparison with a control after UV irradiation, etoposide or MMS treatments. Data represent arithmetic means ± SD from three independent experiments. (D) Expression of Gas2Δ171–314 efficiently reduces the steady-state levels of p53 as stabilized by the wild-type form and by calpastatin. U2OS cells were co-transfected with Gas2wt or calpastatin alone or together with Gas2Δ171–314. Cells were subjected to western blot analysis using DO-1 antibody (upper panel), Gas2 antibody (middle panel) and anti-actin antibody (lower panel). (E) The presence of Gas2Δ171–314 efficiently reduces the Gas2wt-dependent increased transcription from the p21 promoter. U2OS cells were transfected with the p21-Luc reporter together with Gas2 or calpastatin either alone or together with Gas2Δ171–314. Luciferase assays were performed 24 h later. Data represent arithmetic means ± SD from five independent experiments.
We therefore tested the ability of the deleted form to counteract the observed effects of Gas2wt overexpression. Gas2-dependent increased susceptibility to apoptosis was assessed in U2OS cells treated with DNA-damaging agents such as UV irradiation, etoposide or MMS. As shown in Figure 6C, Gas2-dependent increased apoptosis was abrogated in all cases by overexpression of Gas2Δ171–314.
Next, the effects of ectopic expression of Gas2 and calpastatin on endogenous p53 levels were analyzed in U2OS cells, either alone or together with Gas2Δ171–314. As can be observed in Figure 6D, Gas2Δ171–314 abrogated the ability of both Gas2wt and calpastatin to increase the levels of endogenous p53 (Figure 6D, lanes 3 and 5 as compared with lanes 2 and 4). Finally, we analyzed whether Gas2Δ171–314 was also able to decrease the ability of Gas2wt and calpastatin to enhance transcription of a p53-dependent reporter gene. As shown in Figure 6E, Gas2Δ171–314 reduced transcription by an average of 60 times the induction of the p21 promoter by Gas2wt. When the p21 promoter was induced by calpastatin, Gas2Δ171– 314 reduced transcription by an average of 14 times. The same results were obtained when a p53-specific responsive promoter (pG13Luc) was used (data not shown), thus supporting a link between Gas2, calpain and p53 in this pathway.
In summary, Gas2Δ171–314 behaves as a dominant-negative form of Gas2wt, which is unable to inhibit calpain activity and can counteract efficiently the effects of Gas2wt on p53 stability.
The dominant-negative form of Gas2 decreases the endogenous steady-state levels of p53 and its transcriptional activity
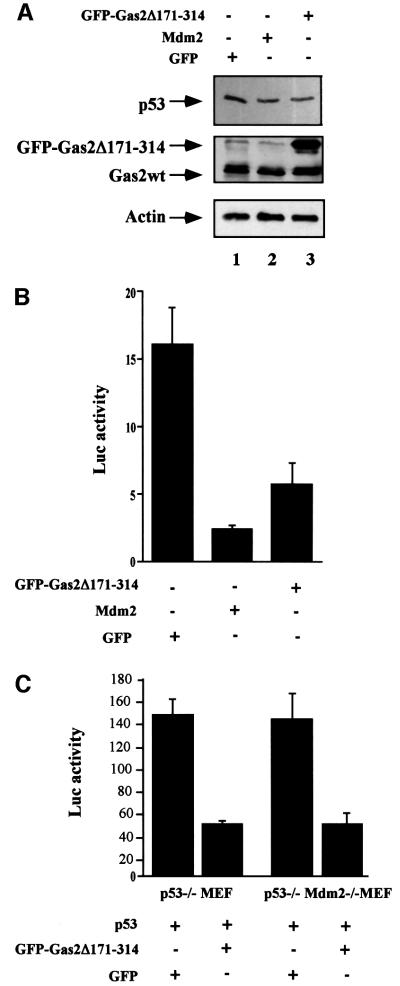
To characterize further the effect of the Gas2 dominant-negative, we separately transfected U2OS cells with GFP, Gas2Δ171–314 and Mdm2, as indicated in Figure 7A. A decrease in the endogenous levels of p53 was observed when Gas2Δ171–314 was present, compared with cells transfected with GFP alone (Figure 7A, lane 1 as compared with lane 3). A similar effect on the steady-state level of p53 was observed when Mdm2 was overexpressed (Figure 7A, lane 2). Moreover, the presence of Gas2Δ171–314 clearly reduced endogenous p53 transcriptional activity on the p21-Luc reporter, as can be observed in Figure 7B. The same results were obtained when the p53-specific responsive promoter pG13Luc was used (data not shown). The ability of Gas2 to also stabilize p53 in the absence of Mdm2 (p53–/– Mdm2–/– MEFs, Figure 5C) suggested that Gas2 works in an Mdm2-independent manner. Accordingly, Gas2Δ171–314 should be able to affect p53 function independently of Mdm2. To demonstrate this, we co-transfected isogenic cell lines that differ only in their Mdm2 status (p53–/– MEFs and p53–/– Mdm2–/– MEFs) with wild-type p53 and Gas2Δ171–314. We observed that Gas2Δ171–314 is able to down-regulate p53 activity in both the presence and absence of Mdm2 (Figure 7C). The same results were obtained when a different experimental model was used to analyze whether the effect of Gas2Δ171–314 was synergistic with that of Mdm2 in reducing endogenous p53 activity. U2OS cells were transfected with increasing amounts of Gas2Δ171– 314 to set conditions showing maximum repression of p53-dependent transcription of the pG13LUC construct. Under such experimental conditions, we observed that expression of Mdm2 could still down-regulate p53 activity (data not shown), suggesting independent pathways to regulate p53.
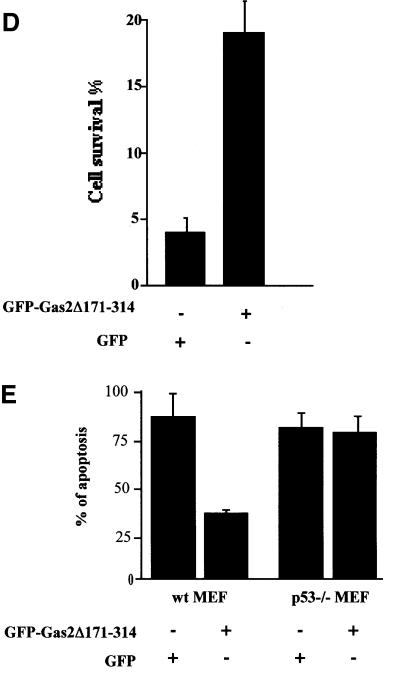
Fig. 7. Gas2Δ171–314 decreases endogenous p53 levels and its transcriptional activity. (A) U2OS cells were transfected with GFP, Mdm2 or Gas2Δ171–314 as indicated. Western blotting of total lysates was performed with DO-1 to monitor the p53 levels, with anti-Gas2 antibody to confirm the expression of transfected Gas2 and polyclonal anti-actin antibody as loading control. (B) U2OS cells were transfected with the p21-Luc reporter together with a control vector, GasΔ171–314 or Mdm2. Luciferase assays were performed 24 h later. Data represent arithmetic means ± SD from three independent experiments. (C) p53–/– or p53–/– Mdm2–/– MEFs were transfected with the p21-Luc reporter together with GFP or GFP-tagged Gas2Δ171–314. Luciferase assays were performed 24 h later. Data represent arithmetic means ± SD from five independent experiments. (D) Diagram showing the viability of Balb/C 3T3 cells microinjected with GFP or GFP-tagged Gas2Δ171–314 after UV damage and in conditions where Gas2wt is physiologic ally induced. After microinjection and Gas2 induction, cells were treated with UV and 12 h later GFP staining was visualized to calculate cell recovery. (E) Diagram showing the apoptotic susceptibility of wild-type or p53–/– MEFs transfected with GFP or GFP-tagged Gas2Δ171–314 after UV damage and in conditions where Gas2wt is physiologically induced. After transfection and Gas2 induction, cells were treated with UV and 12 h later GFP staining was visualized to calculate cell recovery.
Finally, we investigated whether such a dominant-negative form of Gas2 could prevent UV-dependent apoptosis, using a model in which Gas2 is known to be physiologically up-regulated. In Balb/C 3T3 cells, Gas2 increases dramatically after 48 h of serum starvation (Brancolini et al., 1992). GFP-tagged Gas2Δ171–314 or GFP alone was microinjected into Balb/C 3T3 cells and, 6 h later, cells were serum starved for 48 h in order to increase expression of Gas2. After this time, cells were treated with UV and 24 h later cell recovery was assessed by scoring GFP staining in microinjected cells. In the absence of UV treatment, recovery of cells was not affected by overexpression of either GFP or Gas2Δ171– 314 (not shown). On the contrary, as shown in Figure 7D, a significant increase in cell recovery was observed in cells microinjected with Gas2Δ171–314 as compared with cells microinjected with GFP alone, thus indicating that physiologically induced Gas2 could play a role in apoptosis and that the dominant-negative form could specifically abrogate such an effect.
We added weight to this by showing that the documented effect is p53 dependent. A similar experiment was performed using wild-type and p53–/– MEFs. We previously observed that both wild-type and p53–/– MEFs show higher levels of endogenous Gas2 when synchronized in G0 (data not shown). We established that 108 J/m2 of UV were required to damage p53–/– MEFs, and 60 J/m2 to damage wild-type MEFs. We transfected wild-type and p53–/– MEFs with GFP-tagged Gas2Δ171–314 or GFP alone and 12 h later cells were starved for 48 h in order to induce expression of the endogenous Gas2. Cells were then treated with UV and 24 h later apoptosis was assessed by scoring apoptotic nuclei in transfected cells. A significant reduction in apoptotic nuclei was observed in cells transfected with Gas2Δ171–314 as compared with cells transfected with GFP alone (Figure 7E). Remarkably, this effect was observed only in wild-type MEFs, while no differences were observed in p53 knockout MEFs, indicating that physiologically induced Gas2 could play a role in p53-dependent apoptosis.
Discussion
Gas2-dependent susceptibility to cell death depends on functional p53
Here we have demonstrated that ectopic expression of Gas2 enhances cell susceptibility to apoptosis following exposure to UV irradiation or treatments with etoposide or MMS, and that these effects are dependent on p53 status. In line with the noticed effect, we observed that Gas2 is able to up-regulate p53 levels and transcriptional activity.
Although in the absence of apoptotic stimuli Gas2 overexpression was unable to induce cell death, we consistently observed that Gas2 enhanced apoptosis only when cells were treated with DNA-damaging agents capable of stimulating p53 (Figure 1B and C). Using wild-type and p53–/– MEFs, we demonstrated that Gas2 required wild-type p53 to exert sensitization to apoptosis (Figure 1D). Moreover, p53-deficient cells, resistant to Gas2-dependent increased susceptibility to apoptosis, became sensitive upon reintroduction of wild-type p53 (Figure 1E), strongly linking the Gas2-dependent susceptibility to apoptosis to a p53-dependent mechanism.
In order to analyze how Gas2 overexpression could enhance cell susceptibility to p53-dependent apoptosis, we measured p53 levels and transcriptional activity after Gas2 transient transfection. Consistent with our findings, we observed that increasing amounts of Gas2 stabilized both endogenous and ectopically expressed wild-type p53 in a dose-dependent manner (Figure 2B and D). Furthermore, we also demonstrated that the p53 accumulated by Gas2-forced expression is transcriptionally active. When a fragment of the p21Waf1 promoter containing the p53-binding site was used in reporter gene assays, Gas2 expression strongly enhanced its transcription in U2OS cells (Figure 3A). This effect was also observed using the PIG and Bax promoters or the p53-responsive construct pG13 (data not shown). In addition, Gas2 also enhanced the expression of endogenous p21Waf1 and Mdm2 proteins (Figure 3C).
Despite the heterogeneity of the stimuli acting on p53 and of the biological responses that can be induced, two common events accompany p53 activation: (i) stabilization and accumulation of the protein; and (ii) biochemical modifications and conformational shift that allow the protein to bind to DNA and transcriptionally regulate a number of target genes (Gostissa et al., 1999; Jimenez et al., 1999; Lakin and Jackson, 1999). A rapid increase in p53 concentration with no de novo transcription is particularly advantageous in cells that are more prone to respond to different stimuli. It is therefore possible that the noticed effect of Gas2 in maintaining higher steady-state levels of p53 reflects an accumulation of a p53 form not competent to induce apoptosis. Further modifications or different cellular environments could be required to trigger the apoptotic program, as exemplified by the increased susceptibility to apoptosis following UV irradiation, etoposide or MMS treatment.
In this context, we have observed that overexpression of Gas2 in cells lacking p21 is sufficient to induce apoptosis even in the absence of any stress (R.Benetti and C.Schneider, unpublished results), suggesting that p21 induction after p53 stabilization could be instrumental in increasing the survival potential, in the absence of other stimuli enhancing p53 apoptotic function.
Looking for possible pathways linking Gas2 to p53, a yeast two-hybrid screen was performed to identify Gas2-interacting proteins.
Gas2 binds m-calpain and regulates its activity
We have demonstrated that Gas2 physically interacts with m-calpain. Gas2 is able to bind m-calpain (Figure 4) and to inhibit its proteolytic activity on the p53 protein (Figure 5B). Calpains are a family of Ca2+-dependent cysteine proteases involved in many physiological and pathological processes including apoptosis (Sorimachi et al., 1997; Carafoli and Molinari, 1998; Ono et al., 1998). Different mechanisms responsible for m-calpain regulation have been reported and an important role has been ascribed to the specific inhibitor calpastatin (Maki et al., 1991; Carafoli and Molinari, 1998; Suzuki and Sorimachi, 1998). Different substrates for calpains have been determined; among them, p53 has been shown to be processed both in vitro (Gonen et al., 1997; Pariat et al., 1997) and in vivo (Pariat et al., 1997). As a consequence, overexpression of calpain’s specific inhibitor leads to consistent accumulation of transcriptionally active p53 (Pariat et al., 1997) and increased susceptibility to apoptosis (Atencio et al., 2000; Hiwasa et al., 2000).
To characterize further the relationship between Gas2 expression and p53 stabilization, we studied whether Mdm2 was involved in this event. As expected, both Gas2 and calpastatin up-regulate p53 levels and activity without interfering with Mdm2 activity, as demonstrated with Mdm2 knockout MEFs (Figure 5C), suggesting that their activity on p53 is through calpain inhibition.
We present evidence that a deleted form of Gas2 (Gas2Δ171–314), which binds to calpain but does not inhibit its activity, strongly abrogates Gas2wt functions in terms of inhibition of calpain activity (Figure 6B), susceptibility to apoptosis (Figure 6C) and p53 stabilization (Figure 6D and E). We observed that the deleted form of Gas2 partially interfered with calpastatin’s ability to modulate p53 activity, as shown in Figure 6E, possibly because both Gas2 and calpastatin share the binding region on calpain.
It is relevant to note that Gas2 expression levels are regulated under different growth conditions (Schneider et al., 1988; Brancolini and Schneider, 1997) and during differentiation (Lee et al., 1999), thus Gas2 should be involved in the regulation of p53-dependent cell death caused by genotoxic stresses during physiological processes. Moreover, we observed that Gas2 plays an important role in the apoptotic process caused by UV radiation in serum-starved cells, as tested with the Gas2 dominant-negative form. Remarkably, this effect was observed only in wild-type MEFs, while no differences were observed in p53-deficient MEFs, indicating that the physiological regulation of Gas2 could play a role in p53-dependent apoptosis (Figure 7D and E).
We provide evidence that Gas2 may regulate the levels of p53 via inhibition of calpain activity, as inferred by the following results: (i) Gas2 interacts with the regulatory domains of m-calpain; (ii) Gas2 is not cleaved by m-calpain in vitro; (iii) in an in vitro assay Gas2 can inhibit m-calpain proteolytic activity when p53 is used as substrate; and (iv) Gas2, like calpastatin, is able to stabilize p53 even in the absence of Mdm2. Gas2 appears to act like calpastatin, leading to a rapid accumulation of transcriptionally active p53 as strengthened by the fact that both Gas2 and calpastatin bind calpain in its regulatory domain and both are cleaved by caspase-3 (Brancolini et al., 1992; Porn-Ares et al., 1998), the respective processed forms showing reduced ability to inhibit m-calpain activity efficiently.
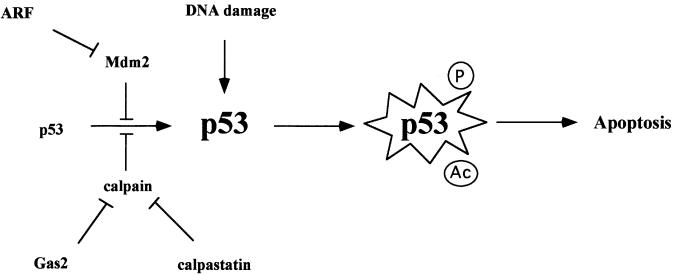
It can therefore be hypothesized that, during apoptosis, cleavage of Gas2 could represent an initial step for full activation of calpain, pointing to a complex cross-talk between members of these two families of cysteine proteases during cell death (Meredith et al., 1998; Porn-Ares et al., 1998): overexpression of either Gas2 or calpastatin can lead to p53 accumulation by inhibiting calpain. In the presence of stress-induced modification, p53 becomes competent to induce apoptosis (see Figure 8). Once the apoptotic process is activated, the caspase-processed forms of Gas2 or calpastatin lose the ability to inhibit calpain, which becomes fully competent to contribute to the apoptotic process (Porn-Ares et al., 1998).
Fig. 8. A working model for the regulatory network involving Gas2, calpain and p53. Mdm2 and calpain can negatively regulate p53 levels. Overexpression of either Gas2 or calpastatin can lead to p53 accumulation by inhibiting calpain. A rapid increase in p53 concentration with no de novo transcription is particularly advantageous in cells that are more prone to respond to different stimuli. Further modifications or different cellular environments are required to convert p53 into a fully active protein and to trigger the apoptotic program.
The existence of different calpain inhibitors could be instrumental in a differential calpain regulation in relation to specific extracellular stimuli and/or different subcellular compartments. Calpain inhibition may therefore render cells more sensitive to p53-dependent apoptosis. We report evidence suggesting that calpain activity is also critical for regulating p53 steady-state levels under normal conditions: overexpression of the dominant-negative form of Gas2 in U2OS cells significantly decreased the levels of p53 and its transcriptional activity. These results suggest that calpastatin and Gas2 should be regarded as relevant players that control p53 levels. In this context, we provide important evidence that the effect of p53 stabilization as mediated by Gas2 is independent of Mdm2 activity, thus elaborating a relevant contribution of calpains to the regulation of p53 stability.
Materials and methods
Cell lines and transfections
Cell lines were cultured at 37°C in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf seum (FCS), 2 mM l-glutamine, penicillin (100 U/ml) and streptomycin (100 µg/ml). UV treatment consisted of a 15 J/m2 irradiation as described (Del Sal et al., 1996). Taxol was added at the final concentration of 2.5 µM for 12 h. U2OS and MG-63 cells are human osteosarcoma cell lines, wild-type and null for p53, respectively. The Balb/C (10)1 fibroblast cell line is a murine cell line, null for p53.
Transfections were performed by the standard calcium phosphate method. Cells were seeded for 8 h before transfection and processed further for 24 h after removal of the precipitate.
For the luciferase assay, 3 cm Petri dishes were transfected with 500 ng of the reporter construct and 2 µg of the other plasmids. The assay was performed with the luciferase kit from Promega. The luciferase activity was determined in a Turner Design luminometer (Promega). The values obtained were normalized for protein concentration in each sample, as determined by a colorimetric assay (Bio-Rad Protein Assay).
Plasmids
To generate the LexA fusion protein, full-length Gas2wt was amplified by PCR and cloned into pLexA202 (Gyuris et al., 1993). To construct pcDNAHAcalpain, the calpain cDNA isolated from the two-hybrid screening was inserted as a HindIII–XhoI fragment into pcDNA3 (Invitrogen) downstream of a START codon and contiguous HA epitope. The C-terminal truncations of Gas2 described in the text were obtained by PCR amplification, and cloned into pEGFP-C1 vector (Clontech). pcDNA3p53wt contains the full-length human wild-type p53 cDNA cloned into the EcoRI site of the pcDNA3 expression vector (Invitrogen). The p53 reporter plasmid employed for luciferase assays (p21-Luc) and the calpastatin plasmid (pM194) have been described previously (El-Deiry et al., 1993; Pariat et al., 1997).
Yeast two-hybrid screening
Yeast two-hybrid screening was performed as described (Gostissa et al., 1999).
Cleavage assay in vitro
In vitro translated 35S-labeled products (4 µl) were mixed on ice with cleavage buffer [40 mM Tris–HCl pH 7.5, 50 mM NaCl, 1 mM dithiothreitol (DTT)]. CaCl2 (1 mM) and, when necessary, protease inhibitors were added in a final volume of 2 µl at time zero (t0). Kinetic analysis was carried out in the presence of pure bovine m-calpain (Sigma), added at a final concentration of 50 mg/ml by sampling aliquots of the reaction mix at various time points. Proteins were resolved by SDS–PAGE and 35S-labeled proteins were visualized by autoradiography.
Immunoprecipitation and western blot analysis
Petri dishes of 10 cm in diameter were transfected as indicated, and 36 h later cells were harvested in ice-cold lysis buffer containing 50 mM KPi pH 7.5, 100 mM KCl, 1 mM MgCl2, 10% glycerol, 1 mM DTT, 0.2% NP-40, 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM EGTA and 10 µg/ml each of chymostatin, leupeptin, antipain and pepstatin. Lysis was performed at 4°C for 10 min. The lysates were then clarified by centrifugation and pre-cleared with immunoprecipitin (Gibco). A 2.5 µg aliquot of the Gas2 antibody, pre-bound to 20 µl of protein A–Sepharose CL-4B (Amersham Pharmacia Biotech), was added and incubated at 4°C for 4 h. The beads were washed and bound proteins were solubilized in SDS–PAGE sample buffer. Western blot analysis was performed according to standard procedures using the following primary antibodies: polyclonal anti-Gas2 (Brancolini et al., 1992), monoclonal 12CA5 anti-HA (Boehringer Mannheim), monoclonal DO-1 anti-p53 (SantaCruz), polyclonal anti-GFP (Invitrogen), polyclonal anti-actin (Sigma), polyclonal anti-p21 C19 (SantaCruz), polyclonal anti-calpain II large subunit (Chemicon) and monoclonal 2A-10 anti-Mdm2 (Chen et al., 1993). Bound primary antibodies were visualized by enhanced chemiluminescence (Amersham).
Immunofluorescence, apoptosis and cell survival
Cells grown on coverslips in 35 mm Petri dishes were fixed in 3% paraformaldehyde (PFA) and Gas2 protein was stained using the anti-Gas2 antibody (Brancolini et al., 1992) followed by a fluorescein isothiocyanate (FITC)-conjugated secondary antibody (Sigma). Images were analyzed with a laser scan confocal microscope (Zeiss). Apoptosis was assessed by scoring nuclei with apoptotic morphology after DNA staining. Cell survival was determined by counting cell recovery after plasmid microinjection, performed with the Automated Injection System (Zeiss, Germany) as described (Brancolini et al., 1999).
Acknowledgments
Acknowledgements
We thank Stefania Marzinotto for help with microinjection experiments, Drs Collavin and Gostissa for helpful suggestions, Dr James Reid for critical reading of the manuscript, and G.Lozano (Anderson Cancer Center, Houston) for p53–/– Mdm2–/– MEFs. This work was supported by MURST COFIN 98 and AIRC.
References
- Atencio I., Ramachandra,M., Shabram,P. and Demers,G. (2000) Calpain inhibitor 1 activates p53-dependent apoptosis in tumor cell lines. Cell Growth Differ., 11, 247–253. [PubMed] [Google Scholar]
- Brancolini C. and Schneider,C. (1997) Cut and die: proteolytic cascades regulating apoptosis. Adv. Clin. Pathol., 3, 177–189. [PubMed] [Google Scholar]
- Brancolini C., Bottega,S. and Schneider,C. (1992) Gas2, a growth arrest-specific protein, is a component of the microfilament network system. J. Cell Biol., 117, 1251–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C., Benedetti,M. and Schneider,C. (1995) Microfilament reorganization during apoptosis: the role of Gas2, a possible substrate for ICE-like proteases. EMBO J., 14, 5179–5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C., Marzinotto,S., Edomi,P., Agostoni,E., Fiorentini,C., Muller,H. and Schneider,C. (1999) Rho-dependent regulation of cell spreading by the tetraspan membrane protein Gas3/PMP22. Mol. Biol. Cell, 10, 2441–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. and Molinari,M. (1998) Calpain: a protease in search of a function? Biochem. Biophys. Res. Commun., 247, 193–203. [DOI] [PubMed] [Google Scholar]
- Chen J., Marechal,V. and Levine,A.J. (1993) Mapping of the p53 and mdm-2 interaction domains. Mol. Cell. Biol., 13, 4107–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G.M. (1997) Caspases: the executioners of apoptosis. Biochem. J., 326, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damalas A., Ben-Ze’ev,A., Simcha,I., Shtutman,M., Leal,J., Zhurinsky,J., Geiger,B. and Oren,M. (1999) Excess β-catenin promotes accumulation of transcriptionally active p53. EMBO J., 18, 3054–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Sal G., Murphy,M., Ruaro,E., Lazarevic,D., Levine,A.J. and Schneider,C. (1996) Cyclin D1 and p21/waf1 are both involved in p53 growth suppression. Oncogene, 12, 177–185. [PubMed] [Google Scholar]
- El-Deiry W.S. et al. (1993) WAF-1, a potential mediator of p53 tumor suppression. Cell, 75, 817–825. [DOI] [PubMed] [Google Scholar]
- Giaccia A.J. and Kastan,M.B. (1998) The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev., 12, 2973–2983. [DOI] [PubMed] [Google Scholar]
- Gonen H., Shkedy,D., Barnoy,S., Kosower,N.S. and Ciechanover,A. (1997) On the involvement of calpains in the degradation of the tumor suppressor protein p53. FEBS Lett., 406, 17–22. [DOI] [PubMed] [Google Scholar]
- Gostissa M., Hengstermann,A., Fogal,V., Sandy,P., Schwarz,E., Scheffner,M. and Del Sal,G. (1999) Activation of p53 by conjugation to the ubiquitin-like protein SUMO-1. EMBO J., 18, 6462–6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyuris J., Golemis,E., Chertkov,H. and Brent,R. (1993) Cdi1, a human G1 and S phase protein that associates with Cdk2. Cell, 75, 791–803. [DOI] [PubMed] [Google Scholar]
- Haupt Y., Maya,R., Kazaz,A. and Oren,M. (1997) Mdm2 promotes the rapid degradation of p53. Nature, 387, 296–299. [DOI] [PubMed] [Google Scholar]
- Hiwasa T. et al. (2000) Increase in ultraviolet sensitivity by overexpression of calpastatin in ultraviolet-resistant UVr-1 cells derived from ultraviolet-sensitive human RSa cells. Cell Death Differ., 7, 531–537. [DOI] [PubMed] [Google Scholar]
- Jimenez G.S., Khan,S.H., Stommel,J.M. and Wahl,G.M. (1999) p53 regulation by post-translational modification and nuclear retention in response to diverse stresses. Oncogene, 18, 7656–7665. [DOI] [PubMed] [Google Scholar]
- Kubbutat M.H. and Vousden,K.H. (1997) Proteolytic cleavage of human p53 by calpain: a potential regulator of protein stability. Mol. Cell. Biol., 17, 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakin N. and Jackson,S. (1999) Regulation of p53 in response to DNA damage. Oncogene, 18, 7644–7655. [DOI] [PubMed] [Google Scholar]
- Lamphere L., Fiore,F., Xu,X., Brizuela,L., Keezer,S., Sardet,C., Draetta,G.F. and Gyuris,J. (1997) Interaction between Cdc37 and Cdk4 in human cells. Oncogene, 14, 1999–2004. [DOI] [PubMed] [Google Scholar]
- Lee K.K., Tang,M.K., Yew,D.T., Chow,P.H., Yee,S.P., Schneider,C. and Brancolini,C. (1999) gas2 is a multifunctional gene involved in the regulation of apoptosis and chondrogenesis in the developing mouse limb. Dev. Biol., 207, 14–25. [DOI] [PubMed] [Google Scholar]
- Levine A.J. (1997) p53, the cellular gatekeeper for growth and division. Cell, 88, 323–331. [DOI] [PubMed] [Google Scholar]
- Maki M., Ma,H., Takano,E., Adachi,Y., Lee,W., Hatanaka,M. and Murachi,T. (1991) Calpastatins: biochemical and molecular biological studies. Biomed. Biochim. Acta, 50, 509–516. [PubMed] [Google Scholar]
- Meredith J.J., Mu,Z., Saido,T. and Du,X. (1998) Cleavage of the cytoplasmic domain of the integrin β3 subunit during endothelial cell apoptosis. J. Biol. Chem., 273, 19525–19531. [DOI] [PubMed] [Google Scholar]
- Ono Y., Sorimachi,H. and Suzuki,K. (1998) Structure and physiology of calpain, an enigmatic protease. Biochem. Biophys. Res. Commun., 245, 289–294. [DOI] [PubMed] [Google Scholar]
- Pariat M., Carillo,S., Molinari,M., Salvat,C., Debussche,L., Bracco,L., Milner,J. and Piechaczyk,M. (1997) Proteolysis by calpains: a possible contribution to degradation of p53. Mol. Cell. Biol., 17, 2806–2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porn-Ares M., Samali,A. and Orrenius,S. (1998) Cleavage of the calpain inhibitor, calpastatin, during apoptosis. Cell Death Differ., 5, 1028–1033. [DOI] [PubMed] [Google Scholar]
- Porter A.G. and Janicke,R.U. (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ., 6, 99–104. [DOI] [PubMed] [Google Scholar]
- Prives C. (1998) Signaling to p53: breaking the MDM2–p53 circuit. Cell, 95, 5–8. [DOI] [PubMed] [Google Scholar]
- Prives C. and Hall,P. (1999) The p53 pathway. EMBO J., 18, 112–126. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lopez A., Xenaki,D., Eden,T., Hickman,J. and Chresta,C. (2001) MDM2 mediated nuclear exclusion of p53 attenuates etoposide-induced apoptosis in neuroblastoma cells. Mol. Pharmacol., 59, 135–143. [DOI] [PubMed] [Google Scholar]
- Sandy P., Gostissa,M., Fogal,V., De Cecco,L., Szalay,K., Rooney,R., Schneider,C. and Del Sal,G. (2000) p53 is involved in the p120E4F-mediated growth arrest. Oncogene, 19, 188–199. [DOI] [PubMed] [Google Scholar]
- Schneider C., King,R. and Philipson,L. (1988) Genes specifically expressed at growth arrest of mammalian cells. Cell, 54, 787–793. [DOI] [PubMed] [Google Scholar]
- Sgorbissa A., Benetti,R., Marzinotto,S., Schneider,C. and Brancolini,C. (1999) Caspase-3 and caspase-7 but not caspase-6 cleave Gas2 in vitro: implications for microfilament reorganization during apoptosis. J. Cell Sci., 112, 4475–4482. [DOI] [PubMed] [Google Scholar]
- Smith M. and Fornace,A.J. (1997) p53-mediated protective responses to UV irradiation. Proc. Natl Acad. Sci. USA, 94, 12255–12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger P., Dobles,M., Tournebize,R. and Hyman,A. (1997) Coupling cell division and cell death to microtubule dynamics. Curr. Opin. Cell Biol., 9, 807–814. [DOI] [PubMed] [Google Scholar]
- Sorimachi H., Ishiura,S. and Suzuki,K. (1997) Structure and physio logical function of calpains. Biochem. J., 328, 721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K. and Sorimachi,H. (1998) A novel aspect of calpain activation. FEBS Lett., 433, 1–4. [DOI] [PubMed] [Google Scholar]
- Tan X. and Wang,J.Y. (1998) The caspase–RB connection in cell death. Trends Cell Biol., 8, 116–120. [DOI] [PubMed] [Google Scholar]
- Woods C.M., Zhu,J., McQueney,P.A., Bollag,D. and Lazarides,E. (1995) Taxol-induced mitotic block triggers rapid onset of a p53-independent apoptotic pathway. Mol. Med., 1, 506–526. [PMC free article] [PubMed] [Google Scholar]
- Yuan J. (1997) Transducing signals of life and death. Curr. Opin. Cell Biol., 9, 247–251. [DOI] [PubMed] [Google Scholar]