Abstract
Background
The present study was conducted to evaluate and compare selective salivary properties and taste perception in subjects with and without oral submucous fibrosis (OSMF), since OSMF affects the properties of saliva and may lead to decline in the quality of life.
Materials and method
Eighty subjects were enrolled in the study and equally divided into two groups based on the presence or absence of OSMF, for estimating, analysing and comparing the salivary flow rate (SFR), pH and taste perception using Modified Schirmer strip, pH strip and taste solutions respectively, in both groups. Chi-square test and unpaired t-test were applied.
Results
Compared to the control group, a statistically significant decrease in SFR was noted among stage 4 OSMF subjects. Alkaline pH was present in OSMF group, regardless of the stage. Taste alterations were present in relation to sweet, salty and sour taste in subjects with OSMF.
Conclusion
There is a marked decrease in SFR, alteration in salivary pH and significant taste changes such as hypogeusia, dysgeusia and ageusia in sweet, salt and sour tastes in subjects with OSMF.
Keywords: Modified schirmer strips, Oral submucous fibrosis, pH strip, Salivary flow rate, Salivary pH, Taste perception, Taste solution
1. Introduction
Oral Submucous Fibrosis (OSMF) is a potentially malignant disorder associated with betel-nut/areca-nut chewing habit, characterized by fibro-elastic changes in oral mucosa.1 Areca-nut is the most common etiologic factor of OSMF and is made up of alkaloid and flavonoid components, of which arecoline is the most potent agent and plays a major role in the pathogenesis of OSMF by causing an abnormal increase in collagen production.2 Saliva is a critical fluid in the oral cavity whose main role includes transport of taste substances to the taste receptor.3 Eventually when areca-nut is leached in the oral cavity, it causes alteration in salivary properties and taste perception.4 Despite numerous studies on trismus and burning sensation in OSMF, limited data exists on functional changes in salivary flow and taste perception, which may contribute significantly to patient morbidity. Therefore, the objective of this study was to evaluate and compare salivary flow rate, salivary pH, and taste perception in individuals with and without oral submucous fibrosis (OSMF). Hence, we hypothesize that individuals with OSMF will exhibit reduced salivary flow, altered pH and impaired taste perception compared to healthy controls and reject null hypothesis.
2. Materials & methods
A comparative study was conducted to assess, evaluate and compare salivary flow rate, salivary pH and taste perception among 80 subject (40 OSMF + 40 Controls) (which was calculated keeping Level of significance (5 %), level of confidence (α = 0.05) and power (80 %)& two sided test was applied); between the age of 18–45 years, in the department of Oral Medicine and Radiology after getting approval from the institutional ethical committee (CDS/IEC/20210702/20). Written and informed consent was obtained from all participants, after explaining to them in detail about the present study in the regional language. The present study was conducted according to the guidelines of Helsinki Declaration.
40 subjects belonging to the age group of 18–45 years having habit of chewing areca-nut or betel-nut without tobacco for a minimum of six months, and having clinical signs of OSMF (diagnosed based on clinical criteria given by Kerr et al. & divided into groups based on Lai Dr et al.) were included in this study. For the control group, 40 age and gender matched healthy subjects, without any habit or medical history and without any sign of OSMF were included (based on clinical examination and history) on the basis of convenience sampling (i.e. hospital walk-ins). Only subjects who were not allergic to the contents of taste solution, i.e. Sucrose, NaCl, Citric acid and Bitter neem powder were included. However, subjects above the age of 45 years, with underlying systemic illness who have/are undergone/undergoing treatment of OSMF and/or who have/are undergone/undergoing radiation therapy were excluded based on history and clinical examination. The subjects were further divided in two groups, where Group 1 (n = 40) comprised of Areca-nut/betel-nut users, clinically diagnosed as OSMF divided equally into 4 groups of 10 each (Group A, B, C and D), based on stages of OSMF according to Lai DR et al.5 and Group 2 (n = 40) comprised of age and gender matched controls. The study was carried out in the morning hours, in order to prevent a bias due to circadian rhythm or any environmental controls (excessive heat/sun which could alter salivary properties). Demographic data along with a brief history was entered in a specially designed structured proforma for this study. The subjects were then made to sit comfortably in a dental chair and were asked to refrain from eating or drinking anything for 90 minutes, before beginning the analysis. All procedures were performed by a single examiner to ensure consistency.
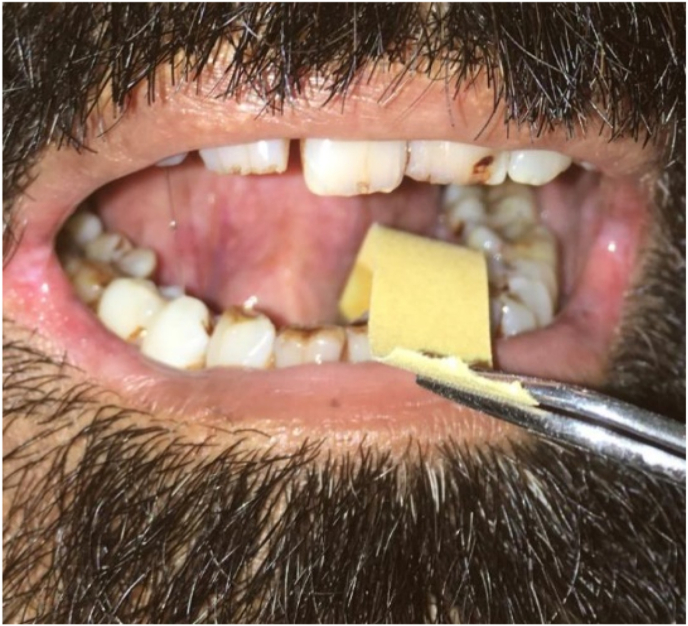
Before beginning the measurement for salivary flow rate, the patient was asked to relax for 3–5 minutes and then swallow the whole saliva present in the mouth. Then, the patient was asked to rest tongue on hard palate, and instructed not to swallow any saliva from this point forward. MST strip (Tear Touch from Madu Instruments Pvt. Ltd. MIPL/A1/84)) was held vertically with a cotton plier and rounded end of the strip was positioned on the floor of mouth (Fig. 1) and as saliva travelled up the strip, its distance was read immediately and after 1st, 2nd & 3rd minute. Based on the distance travelled on the SFR strip, the subjects were categorized into 5 categories: 0–1 mm = Severely dry mouth; 2–5 mm = Moderately dry mouth; 6–10 mm = Mild dry mouth; 10–15 mm = Dry mouth; 15–25 mm = Questionable and 25–30 mm = Normal according to Dyasanoor et al.6
Fig. 1.
To measure pH, a pH strip (Indikrom pH Strip Range of pH 1.0–14.0) was held vertically with a pair of tweezers in floor of mouth (Fig. 2) for 10 seconds. The change in colour of the strip was noted and matched with colour coding given on the packet and accordingly the pH value was recorded. pH below 6.5 was considered acidic and pH above 7.5 was considered alkaline, according to Tamgadge P et al. (2020).7
Fig. 2.
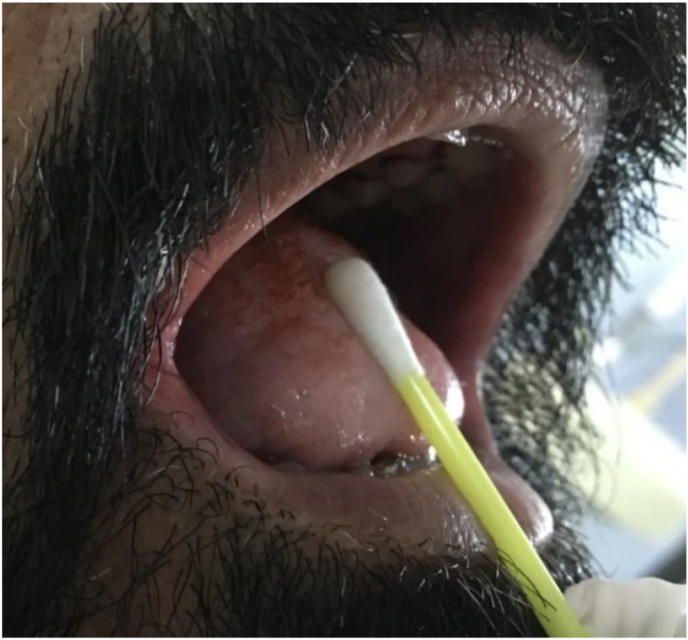
For taste determination, tastants of four different tastes i.e. Sweet, Salty, Sour and Bitter were freshly prepared, by adding Sucrose, NaCl, Citric acid and bitter neem powder obtained from pharmacology lab at three different concentrations (½ tsp, 1tsp & ½ tbsp) to the same proportion of water to prepare low, medium and high concentrations of each tastant respectively. A Spatial test (Fig. 3) was done to assess taste perception, by applying the tastant solution all over the dorsum and lateral part of tongue using a cotton swab, in progressively increased concentrations for approximately 10 s for all four tastes. In between drinking water was given to rinse mouth to avoid overlapping of different tastes, leading to discrepancies in reporting tastes by the subjects. The response of identification of taste was then recorded by verbally asking subject to guess the taste. If patient identified taste at a lower concentration, then no further test was done with a higher concentration.
Fig. 3.
Taste determination was analysed using method given by Bangi BB et al. (2019)8 and categorized as having: Normal Taste; Hypogeusia: Decreased sensitivity to taste; Dysgeusia: Taste confusion and Ageusia: Complete loss of taste.
These methods were used to measure salivary properties and taste analysis, as they are non-invasive and less time-consuming chairside investigative; it has the advantages of being simple, inexpensive, and readily available in sterilized packs for routine use in clinical settings to assess subjective and objective salivary discrepancies.
The data was entered in MS Excel and analysed using IBM SPSS statistical software (SPSS version 25; IBM, Armonk, NY, USA) and was statistically analysed for both groups using Chi-square test and Unpaired t Test. “p” is level of significance, p > 0.05 not significant, p < 0.05 significant.
3. Results
Since the subjects were age and gender matched, group 1 and group 2 both consisted of 40 subjects each, of which 31 (77.5 %) were males and 9 (22.5 %) were females and 9 (22.5 %) belonged to age group 18–25 years, 12 (30 %) belonged to age group 26–35 years and 19 (47.5 %) belonged to age group 36–45 years. Table 1 shows the comparison of salivary flow rate amongst both groups. Table 2 shows the salivary pH of both groups. Table 3 depicts the response of both groups to various tastants.
Table 1.
Comparison of salivary flow rate among study groups at the end of 3 minutes.
| GROUPS | SFR at 3 min (mm) |
TOTAL | p Value | Mean SFR at 3 min | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–1 | 2–5 | 6–10 | 10–15 | 15–25 | 25–30 | 31–35 | |||||
| Group 1 | 0 (0 %) | 0 (0 %) | 2 (5 %) | 2 (5 %) | 17 (42.5 %) | 9 (22.5 %) | 10 (25 %) | 40 (100 %) | 0.011∗ | 18.89 ± 7.83 | 0.146 |
| Group 2 | 0 (0 %) | 0 (0 %) | 1 (2.5 %) | 2 (5 %) | 13 (32.5 %) | 14 (35 %) | 10 (25 %) | 40 (100 %) | 19.75 ± 5.20 | ||
Table 2.
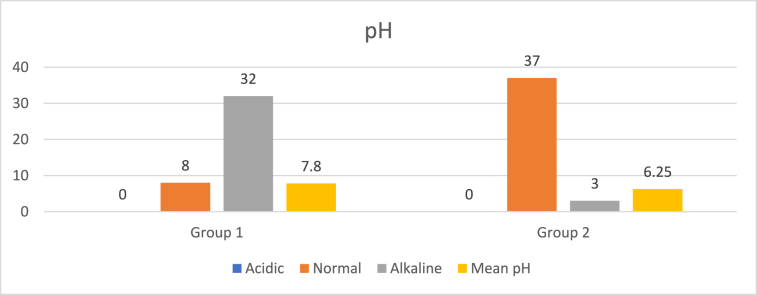
Comparison of salivary ph between study groups.
| GROUPS | PH |
TOTAL | p Value | MEAN pH | p Value | ||
|---|---|---|---|---|---|---|---|
| ACIDIC [<6.5] | NORMAL [6.5–7.5] | ALKALINE [>7.5] | |||||
| Group 1 | 0 (0 %) | 8 (20 %) | 32 (80 %) | 40 (100 %) | 0.001∗∗ | 7.8 ± 1.04 | 0.021∗ |
| Group 2 | 0 (0 %) | 37 (92.5 %) | 3 (7.5 %) | 40 (100 %) | 6.25 ± 0.66 | ||
Table 3.
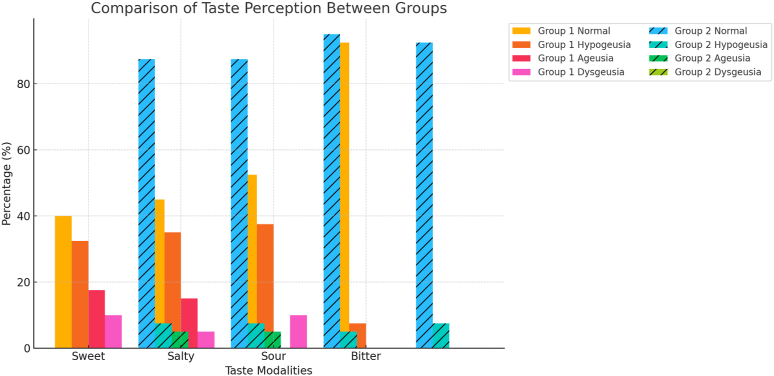
Comparison of response to sweet, salt, sour and bitter tastes among study groups.
| TASTE | STUDY GROUPS | NORMAL | HYPOGEUSIA | AGEUSIA | DYSGEUSIA | TOTAL | p-Value |
|---|---|---|---|---|---|---|---|
| SWEET | Group 1 | 16 (40 %) | 13 (32.5 %) | 7 (17.5 %) | 4 (10 %) | 40 (100 %) | 0.000∗ |
| Group 2 | 35 (87.5 %) | 3 (7.5 %) | 2 (5 %) | 0 (0 %) | 40 (100 %) | ||
| SALTY | Group 1 | 18 (45 %) | 14 (35 %) | 6 (15 %) | 2 (5 %) | 40 (100 %) | 0.001∗ |
| Group 2 | 35 (87.5 %) | 3 (7.5 %) | 2 (5 %) | 0 (0 %) | 40 (100 %) | ||
| SOUR | Group 1 | 21 (52.5 %) | 15 (37.5 %) | 0 (0 %) | 4 (10 %) | 40 (100 %) | 0.001∗ |
| Group 2 | 38 (95 %) | 2 (5 %) | 0 (0 %) | 0 (0 %) | 40 (100 %) | ||
| BITTER | Group 1 | 37 (92.5 %) | 3 (7.5 %) | 0 (0 %) | 0 (0 %) | 40 (100 %) | 1 |
| Group 2 | 37 (92.5 %) | 3 (7.5 %) | 0 (0 %) | 0 (0 %) | 40 (100 %) |
4. Discussion
The signs and symptoms of OSMF are due to fibrosis and hyalinization of sub epithelial tissues. The most frequently affected locations are the buccal mucosa and the retromolar areas.9 It manifests as a burning sensation in the mouth, intolerance to eating hot and spicy foods, blanching and stiffness of the oral mucosa, trismus, vesiculation, excessive salivation, ulceration, pigmentation change, recurrent stomatitis, defective gustatory sensation, dryness of the mouth, gradual stiffening and reduced mobility of the soft palate and the tongue leading to difficulty in swallowing and hyper nasality of voice, hoarseness of voice (with laryngeal involvement) and occasionally, mild hearing loss due to blockage of Eustachian tube.9
In literature, numerous reasons for alteration in gustatory perception is documented. The most acceptable and common causes for the same include inflammatory reactions with or without infection in oral cavity, which reduces the blood flow causing alteration in normal physiology and function of taste buds.
Arecoline present in areca-nut has para-sympathomimetic activity. So, when it is leached into the oral cavity, it causes alteration in salivary properties; as it is the first biological fluid exposed to such products.6,7,10 Studies have found that there is an alteration of taste perception in OSMF patients too due to xerostomia and atrophy of papillae seen in OSMF, thus hampering nutritional status of the affected individual and oral mucosa, which subsequently becomes even more vulnerable for further initiation of pathologic changes. Many studies have been done for other features of OSMF; but correlating intensity of changes in saliva and taste impairment has not received much consideration in past so, present study was undertaken.11,12
At the end of 3rd minute significant difference was noted amongst study groups, where maximum participants (42.5 %) in OSMF group had questionable SFR whereas maximum participants (35 %) in control group had normal SFR. The cumulative mean salivary flow rate at the end of 3 was 18.89 ± 7.83 mm in group 1 while in group 2 it was 19.75 ± 5.20 mm, which shows that subjects with OSMF showed lower salivary flow compared to controls. Saraswathi G K et al. (2019)13 & Dyasanoor S et al. (2016)6 also showed decrease in mean salivary flow rate in OSMF group i.e. 16.4 mm and 23.4 mm respectively. Decrease in SFR among OSMF participants could be due to conversion by lime from arecoline to arecadine. Moreover, Nyachhyon et al. (2011)14 showed acinar degeneration that manifested as small clear cytoplasmic vacuoles with pyknotic nuclei which causes decrease in SFR among OSMF individuals. However, Siddabasappa S et al. (2014)15 showed increase in SFR among OSMF group (0.60 ± 0.09 ml/min) compared to normal individuals (0.39 ± 0.08 ml/min) because spitting method was used for salivary flow assessment for 10 minutes and in this method, the chances of spitting frothy saliva increases which may result in incorrect measurement.
In the present study significant results were found among study groups, in which maximum participants (80 %) in the OSMF group had an alkaline salivary pH (>7.5), whereas maximum participants (92.5 %) in the control group had a normal salivary pH (6.5–7.5). Mean salivary pH among OSMF group was 7.8 ± 1.04 while in control group it was 6.25 ± 0.66. None of the participants in both the groups showed an acidic pH (<6.5). This was in accordance to studies done by Sahu R et al. (2021)16 who showed 7.27 ± 0.52 in betel betelnut chewers compared to non-chewers, Donoghue M et al. (2015).17 C. Mackenzie et al. (1961)18 in his study found that sustained alkaline salivary pH can lead to fibroblast toxicity and death. These have a lower amount of MMP's & TIMP, due to surrounding alkaline pH.
In the present study, OSMF group showed hypogeusia to sweet taste 13(32.5 %) which was similar to study done by Ila A et al. (2022)19 & Dyasanoor S et al. (2016).6 These, result could be due to type 2 receptors (fungiform & circumvallate papillae) responsible for sweet taste perception i.e. their depapillation and atrophy of papillae. Saliva also plays an important role for taste maintenance which was in accordance to Matsuo R. (2000),20 whose flow has been found to be less in OSMF participants.
For salty taste in OSMF group 14(35 %) participants showed hypogeusia which was in accordance to study done by Ila A et al. (2022)19, Bangi BB et al. (2019)8 & Dyasanoor S et al. (2016).6 In the present study, salty taste was affected as depapillation starts from anterior region of tongue and habit of chewing/placement of areca-nut is on lateral border of tongue which causes atrophy of taste papillae according to Zhang G H et al. (2009)21 fungiform papillae are most affected compared to foliate and circumvallate.
In present study 15(37.5 %) participants in OSMF group had hypogeusia to sour taste while only 3(7.5 %) participants had hypogeusia to bitter taste which was in accordance to study done by Ila A et al. (2022)19. According to Wang R et al. (2014)22 because OSMF causes inflammation throughout the oral cavity, there is also a high level of inflammatory mediators, which in turn regulates response to bitter taste (i.e., TNF α, igf-1, and leptin levels). As a result, bitter taste is unaffected by the severity of the disease, but sweet and salt tastes are affected by both the depapillation, which is primarily in the anterior region of the tongue.
4.1. Limitations
-
1)
Small and single-centred sample size (n = 80); convenience sampling was done which have limit the generalizability of the results.
-
2)
No scientific tests were performed for hyposalivation and taste sensation (i.e. electro-gustatory) to rule out problem gustatory impulses or any histological examination of taste receptors to know about effect of contents of areca-nut on structure of taste cells.
-
3)
Only two salivary parameters were assessed; while the biochemical parameters (buffering capacity, oxidative stress markers, protein/enzyme content) were not assessed
-
4)
It is a cross-sectional study it captures at single point of time; duration and frequency of areca-nut chewing in any of the forms were not considered in the study.
5. Conclusion
Thus, from the present study it can be concluded that there is a definite alteration in salivary flow, salivary pH and taste sensation in OSMF. These alterations may represent early functional changes that in, conjunction with other risk factors, could contribute to oral mucosal deterioration. Altered taste sensation is also common clinical symptom among OSMF cases, which is not routinely encountered. Besides these alterations, there is an increased risk of malignant transformation due to hampering of nutritional status in affected individuals, which further leads to anorexia, depression and weight loss.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
References
- 1.Gururaj Arakeri, Shekar Gowda Patil, Abdulsalam S. Aljabab, Kuan-Chou Lin, M. A. W. Merkx, Shan Gao, Peter A. Brennan Oral submucous fibrosis: an update on pathophysiology of malignant transformation, Oral Pathol Med. [DOI] [PubMed]
- 2.Xu H., Lyu F.Y., Song J.Y., et al. Research achievements of oral submucous fibrosis: progress and prospect. BioMed Res Int. 2021 March doi: 10.1155/2021/6631856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuo R. Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med. 2000;11(2):216–229. doi: 10.1177/10454411000110020501. [DOI] [PubMed] [Google Scholar]
- 4.Mathew Philips. Role of areca nut and its commercial products in oral submucous fibrosis-a review. J Adv Med Dent Sci Res. 2014 [Google Scholar]
- 5.Lai D.R., Chen H.R., Lin L.M., Huang Y.L., Tsai C.C. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10-year experience with 150 cases. J Oral Pathol Med. 1995;24(9):402–406. doi: 10.1111/j.1600-0714.1995.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 6.Dyasanoor S., Abdul Khader N.F. Alteration in salivary properties and taste perception in OSMF. Contemp Clin Dent. 2016 Apr-Jun;7(2):146–152. doi: 10.4103/0976-237X.183042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tamgadge P. Comparative evaluation of alteration in salivary pH among gutkha chewers with and without oral submucous fibrosis and healthy subjects: a prospective casecontrol study. J Curr Oncol. 2020;3:8–16. [Google Scholar]
- 8.Bangi B.B., Ginjupally U., Nadendla L.K., Mekala M.R., Lakshmi J., Kakumani A. Evaluation of gustatory function in oral submucous fibrosis patients and gutka chewers. Asian Pac J Cancer Prev APJCP. 2019;20(2):569–573. doi: 10.31557/APJCP.2019.20.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vikas Berwal1, Monika Khangwal1 , Ravinder Solanki1 , Rakshit Khandeparker2. Kiran Savant2 , Omkar Shetye;Systems for Oral C. A REVIEW.
- 10.Nayyar A., Lalfamkima F., Bommaji S., et al. Comparative evaluation of alteration in salivary flow rate between betal Nut/Gutkha chewers with and without OSMF, and healthy subjects: a prospective case-control study. Oncol J India. 2021;5(1):1. [Google Scholar]
- 11.Deeplaxmi R, B Sakarde S, Sur J, Pratap Singh A, Jain S, Mujoo S. Altered taste perception in oral submucous fibrosis: a research. J Indian Acad Oral.
- 12.Soni NK, Chatterji P, Tyagi UN, Nahata SK, Bansal M. Gustation in Oral Submucous.
- 13.Saraswathi G.K., Kumar H.D.M., Professor A. 49) alteration in taste perception salivary Ph & flow rate among OSMF & Leukoplakia - a case control study Dr. Sushmitha S, post graduate student Dr fibrosis. Indian J Otolaryngol. 1981;33(2):69–70. [Google Scholar]
- 14.Nyachhyon R., Boaz K., Sumanth K.N. Minor salivary gland changes in OSMF: retrospective pilot study. J Nepal Dental Assoc. 2011;12(1):26–28. Jan.-Jun. [Google Scholar]
- 15.Siddabasappa S., Ashok L., Sujatha G.P. Estimation of unstimulated salivary flow rate, pH, copper and iron in ghutka chewers with and without oral submucous fibrosis: a preliminary study. Res J Pharmaceut Biol Chem Sci. 2014;5:300–306. [Google Scholar]
- 16.Sahu R., Patro S., Nayak B., Bardhan D., Panda S., Rajguru J. Habit-associated salivary Ph changes in oral submucous fibrosis: a cross-sectional study. Natl J Maxillofac Surg. 2021;12(1):78. doi: 10.4103/njms.NJMS_39_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donoghue M., Basandi P., Adarsh H., Madhushankari G.S., Selvamani M., Nayak P. Habit associated salivary pH changes in oral submucous fibrosis-A controlled cross-sectional study. J Oral Maxillofac Pathol. 2015;19(2):175. doi: 10.4103/0973-029X.164529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackenzie C.G., Mackenzie J.B., Beck P. The effect of ph on growth, protein synthesis, and lipid-rich particles of cultured mammalian cells. J Cell Biol. 1961;9(1):141–156. doi: 10.1083/jcb.9.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ila A., Harini T.C., Nagarajappa A., Chandran A., Kolte D., Gunturu S. Comparative evaluation of alteration in taste perception among Gutkha chewers with and without OSMF and healthy subjects: a prospective case-control study. J Oral Maxillofac Pathol. 2022;26(2):208. doi: 10.4103/jomfp.jomfp_38_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuo R. Role of saliva in the maintenance of taste sensitivity. Crit Rev Oral Biol Med. 2000;11(2):216–229. doi: 10.1177/10454411000110020501. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G.-H., Zhang H.-Y., Wang X.-F., Zhan Y.-H., Deng S.-P., Qin Y.-M. The relationship between fungiform papillae density and detection threshold for sucrose in the young males. Chem Senses. 2009;34(1):93–99. doi: 10.1093/chemse/bjn059. [DOI] [PubMed] [Google Scholar]
- 22.Wang R., van Keeken N.M.A., Siddiqui S., et al. Higher TNF-α, IGF-1, and Leptin levels are found in tasters than non-tasters. Front Endocrinol (Lausanne) 2014;5:125. doi: 10.3389/fendo.2014.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]