Take Home Message
This network meta-analysis provides a comparative evaluation of four robotic surgical systems—Da Vinci, KangDuo, Hugo, and Hinotori—in radical prostatectomy for prostate cancer. Our findings demonstrate that while all platforms achieve comparable outcomes in terms of surgical safety, complication rates, hospital stay, blood loss, positive surgical margins, and urinary continence, the Da Vinci system consistently outperforms the others in operative and console times, indicating superior intraoperative efficiency. The KangDuo system, despite being more cost effective, showed longer operative durations, but may offer advantages in postoperative recovery and blood loss control. These results suggest that while all systems are clinically viable, the choice of platform should be guided by institutional resources, cost considerations, surgical expertise, and specific procedural priorities.
Keywords: Prostate cancer, Radical prostatectomy, Robot-assisted surgery, Network meta-analysis, Da Vinci, KangDuo, Hugo, Hinotori, Operative time, Postoperative recovery
Abstract
Background and objective
Prostate cancer (PCa) continues to pose significant public health challenges, with rising incidence and mortality rates. Robot-assisted surgery has become a preferred method for radical prostatectomy (RP) due to its precision and minimal invasiveness. This study aims to evaluate the clinical performance of four robotic surgical systems—Da Vinci (DV), KangDuo (KD), Hugo (HG), and Hinotori—in RP for PCa using a network meta-analysis (NMA).
Methods
We conducted a systematic review and NMA according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines, including studies published until January 2025. Studies comparing the four robotic systems in RP for PCa were included, with outcomes including operative time, blood loss, length of hospital stay, complications, 3-mo urinary incontinence rates, and positive surgical margin rates. Data were extracted from randomized controlled trials and observational studies, and statistical analyses were performed using Stata version 18.
Key findings and limitations
A total of 2064 patients from 15 studies were included. The results showed that the DV system had the shortest operative time (surface under the cumulative ranking curve [SUCRA] score: 90.7), while the KD system exhibited the longest operative time. There were no significant differences in estimated blood loss, hospital stay, or complication rates between the systems. The KD system showed a slight advantage in hospital stay (SUCRA score: 85.8), while the HG system had the lowest complication rate (SUCRA score: 84.3). No significant differences were found in positive surgical margin rates or 3-mo urinary incontinence rates among the systems.
Conclusions and clinical implications
The DV system demonstrated superior efficiency in terms of operative time, while the KD system showed potential advantages in postoperative recovery. However, all robotic systems provided similar safety profiles in terms of blood loss, complications, and long-term functional outcomes. These findings suggest that robotic systems can be selected based on clinical priorities, with further studies needed to optimize system performance and guide personalized treatment strategies.
Patient summary
This network meta-analysis compares four robotic surgical systems—Da Vinci, KangDuo, Hugo, and Hinotori—for prostate cancer patients. All platforms achieved comparable outcomes. However, the Da Vinci system demonstrated superior efficiency in terms of operative time, while the KangDuo system showed better postoperative recovery and blood loss control. These findings support safe integration of robotic surgical systems into clinical practice.
1. Introduction
Prostate cancer (PCa) has emerged as an increasingly prominent public health concern worldwide, with its incidence and mortality rates continuing to rise [1]. It remains the most frequently diagnosed cancer among men in Europe [2,3]. These trends are likely attributable to the widespread adoption of imaging modalities, the routine use of prostate-specific antigen (PSA) screening, and the growing prevalence of lifestyle-related risk factors, such as high-fat diets, obesity, and smoking [4]. The introduction of PSA screening has facilitated the early detection of localized PCa, thereby contributing to the rising rates of early diagnosis [5].
Radical prostatectomy (RP) is the standard surgical approach for managing localized PCa, particularly in patients with early-stage, confined disease. RP has been associated with a 48% reduction in PCa-specific mortality and an average survival benefit of 2.2 yr [6]. As minimally invasive surgical techniques have advanced, robot-assisted surgery (RAS) has gained widespread adoption in the management of PCa, especially in aging frail patients [7]. The precision and flexibility afforded by robotic platforms have significantly enhanced surgical accuracy and improved postoperative recovery, making RAS the preferred approach in many centers [8].
Currently, the Da Vinci Surgical System (Intuitive Surgical, Inc., Sunnyvale, CA, USA) is the most widely used robotic platform globally. Since its initial application in RP for PCa in 2000, the Da Vinci (DV) system has demonstrated substantial benefits, including improved three-dimensional (3D) visualization, magnified imaging, enhanced surgical precision, reduced intraoperative blood loss, and shorter hospital stays [9,10]. In recent years, emerging robotic platforms, including the KangDuo Surgical System (KangDuo Medical, Shanghai, China), Hinotori Surgical Robot System (Medicaroid, Kobe, Japan), and Hugo RAS System (Medtronic, Minneapolis, MN, USA), have gained traction worldwide.
Although these robotic systems offer similar advantages in clinical practice, their comparative efficacy regarding surgical outcomes, postoperative recovery, and functional preservation remains unclear. Therefore, evaluation of the clinical performance of the DV, KangDuo (KD), Hugo (HG), and Hinotori (HS) systems in RP is critical for informing clinical decision-making and optimizing treatment strategies.
This study aims to provide a comprehensive evaluation of the outcomes associated with these robotic systems in RP through a network meta-analysis (NMA). By integrating data on key outcomes, including operative time (OT) , blood loss, length of hospital stay, complications, 3-mo postoperative urinary incontinence, and oncological results (positive surgical margin rates), we seek to offer robust, evidence-based insights into PCa treatment. These findings are intended to guide personalized treatment strategies in future clinical practice.
2. Methods
This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines and has been registered (CRD42025634204; Supplementary Table 1) [11]. As this study is an NMA based on publicly available data and does not involve the collection of new patient information, institutional ethics approval was not required.
2.1. Literature search
A systematic review and NMA were conducted to compare the clinical outcomes of different RAS systems in RP for PCa. We searched the PubMed, Embase, Web of Science, and Cochrane Library databases using a search strategy designed by senior authors and executed by two independent reviewers. The search terms included “prostate cancer,” “robot-assisted surgery,” “radical prostatectomy,” “da Vinci,” “Hugo RAS,” “KangDuo,” and “Hinotori,” combined using the Boolean operator “AND” or “OR.” The search encompassed studies published up to December 27, 2024, and was limited to articles in English and Chinese. Retrieved records were deduplicated using software and manual review. Subsequently, two authors screened the titles and abstracts to identify relevant studies, followed by a comprehensive review of potentially eligible articles to ensure that each included study reported at least one outcome of interest Fig. 1.
Fig. 1.
PRISMA flowchart. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses.
2.2. Inclusion and exclusion criteria
This review adhered to the PICOS framework to include studies that met the following criteria: (1) published in English; (2) focused on patients with PCa undergoing RP performed using RAS systems, including the DV, HG RAS, KD, or HS platforms; (3) reported at least one of the following outcomes: OT, estimated blood loss, length of hospital stay, postoperative complications, 3-mo urinary incontinence rate, or positive surgical margin rate; and (4) designed as randomized controlled trials (RCTs) or observational studies. The exclusion criteria were the following: (1) studies with incomplete data or those unsuitable for a statistical analysis; (2) reviews, case reports, commentaries, or letters; and (3) duplicate publications or studies based on overlapping data, with only the most recent or comprehensive dataset included. These criteria ensured reliability and consistency in the NMA.
2.3. Data extraction
Two independent reviewers (J.W. and Y.L.) extracted data using standardized forms. Discrepancies were resolved through discussion or consultation with a third reviewer. Extracted data included study characteristics (eg, first author, publication year, country/region, and study design), patient demographics (eg, sample size, age range, body mass index, PSA levels, and treatment group details), interventions and comparators (eg, robotic platform used), and surgical outcomes (eg, OT, blood loss, and length of stay). The key outcomes included OT, blood loss, length of stay, 3-mo urinary incontinence rates, and positive surgical margin rates. Complications were extracted and classified based on the Clavien-Dindo grading system, as reported in the original studies. However, since no Clavien-Dindo grade III–V complications were observed in the vast majority of studies—except for a single study comparing the HS and DV systems, in which one grade III complication was reported in each group—we did not perform subgroup analyses of minor (grades I–II) versus major (grades III–V) complications. Instead, we used the overall complication rate (grades I–V combined) as the primary safety outcome to ensure consistency and comparability across studies. Relevant statistical measures (eg, mean, standard deviation, odds ratios [ORs], and weighted mean differences [WMDs]) were also extracted for a subsequent analysis.
2.4. Quality assessment
Study quality was independently assessed by two reviewers (J.W. and L.W.) using the Cochrane Risk of Bias Tool for RCTs (Fig. 2) [12] and the Newcastle-Ottawa Scale (NOS) for observational studies [13]. Studies scoring ≤5 on the NOS were classified as of low quality, scores of 6–7 as of moderate quality, and scores of 8–9 as of high quality (Table 1). Discrepancies were resolved through discussion or by consulting a third reviewer.
Fig. 2.
Quality evaluation chart utilizing the Cochrane Risk of Bias Assessment tool.
Table 1.
Study characteristics
| Reference | Country | Study type | Technique | Patients | Age (yr) | BMI (kg/m2) | Prostate volume (ml) | Preoperative PSA level (ng/ml) | Outcomea | NOS |
|---|---|---|---|---|---|---|---|---|---|---|
| Fan et al (2023) [27] | China | Prospective | KangDuo | 16 | 66.17 (4.81) | 23.70 (2.35) | 43.33 (19.22) | 7.59 (4.83) | ①②③⑤⑥⑦ | 7 |
| Da Vinci Si | 16 | 68.5 (5.94) | 25.12 (2.90) | 34.5 (15.55) | 8.56 (7.39) | |||||
| Shen et al (2024) [26] | China | RCT | KangDuo | 40 | 66.50 (4.96) | 25 (2.49) | 42.32 (19.25) | 9.81 (4.41) | ①②③④⑤⑥ | RCT |
| Da Vinci Si | 39 | 65.31 (6.08) | 24.70 (2.68) | 34.98 (15.14) | 40.59 (70.67) | |||||
| Dong et al (2024) [28] | China | RCT | KangDuo | 20 | 65.74 (4.55) | 24.42 (2.31) | NA | 8.25 (1.95) | ①②③④⑤⑥ | RCT |
| Da Vinci S | 19 | 65 (4.88) | 23.53 (2.72) | NA | 11.71 (2.92) | |||||
| Antonelli et al (2024) [18] | Italy | Prospective | Hugo | 50 | 65.9 (5.9) | 25.93 (2.51) | 39.64 (16.1) | 8.23 (3.89) | ② | 6 |
| Da Vinci Xi | 50 | 66.4 (5.5) | 27.07 (3.96) | 45.12 (16.86) | 6.50 (2.97) | |||||
| Bravi et al (2024) [19] | Belgium | Retrospective | Hugo | 164 | 65 (7.48) | 26.35 (3.74) | 44.2 (20.83) | 8.28 (4.03) | ①②③⑥⑦ | 7 |
| Da Vinci X or Xi | 378 | 66 (7.44) | 27.35 (3.72) | 44.45 (18.69) | 8.02 (4.61) | |||||
| Gandi et al (2024) [21] | Italy | Retrospective | Hugo | 99 | 67.47 (7.14) | 26.08 (2.82) | 49.47 (22.94) | 7.73 (3.46) | ①③⑥ | 6 |
| Da Vinci Xi | 99 | 66.65 (6.77) | 26.79 (2.54) | 46.05 (21.81) | 8.54 (4.32) | |||||
| Brime Menendez et al (2024) [20] | Spain | Prospective | Hugo | 75 | 65.81 (8.10) | 26.20 (3,98) | 41.68 (16.32) | 6.41 (1.9) | ①②③⑥ | 6 |
| Da Vinci Xi | 75 | 66.33 (7.06) | 25.40 (4.15) | 46.50 (15.45) | 6.88 (2.76) | |||||
| Olsen et al (2024) [25] | Denmark | Retrospective | Hugo | 19 | 67.44 (8.01) | 25.57 (3.04) | 50.96 (36.04) | ≤10:12;≥10:7 | ②③④⑤⑥⑦ | 7 |
| Da Vinci | 11 | 59.73 (3.39) | 25.81 (1.69) | 44.83 (26.29) | ≤10:7;≥10:4 | |||||
| Ou et al (2024) [22] | Taiwan | Retrospective | Hugo | 30 | 66.5 (7.41) | 25.79 (3.16) | 40 (12.22) | 8.81 (13.33) | ②④⑤⑥⑦ | 6 |
| Da Vinci | 30 | 67.5 (8.52) | 26 (3.21) | 41 (14.07) | 9.46 (7.06) | |||||
| Ragavan et al (2023) [24] | India | Prospective | Hugo | 17 | 68.72 (4.85) | 24.62 (3.21) | NA | 16.37 (14.71) | ①③④⑥⑦ | 7 |
| Da Vinci Xi | 17 | 68.72 (6.46) | 25.09 (3.18) | NA | 23.94 (27.98) | |||||
| Sighinolfi et al (2024) [23] | Italy | Retrospective | Hugo | 14 | 63.91 (5.76) | NA | 48 (21.41) | 7.08 (3.54) | ①③ | 6 |
| Da Vinci | 27 | 64.64 (8.61) | NA | 41.92 (22.69) | 7.81 (4.3) | |||||
| Nakayama et al (2024) [15] | Japan | Retrospective | Hinotori | 97 | 68.47 (7.15) | 24.49 (3.54) | 32.97 (10.54) | 7.83 (4.06) | ①②⑥⑦ | 6 |
| Da Vinci Xi | 246 | 68.65 (5.22) | 23.88 (3.06) | 32.25 (11.33) | 8.07 (4.40) | |||||
| Kohjimoto et al (2024) [16] | Japan | Retrospective | Hinotori | 43 | 69.65 (6.90) | 24.71 (3.07) | NA | 8.68 (3.60) | ①②③④⑥⑦ | 7 |
| Da Vinci Si or Xi | 43 | 70.23 (6.90) | 24.29 (3.07) | NA | 8.31 (3.60) | |||||
| Sasaki et al (2024) [14] | Japan | Retrospective | Hinotori | 48 | 71.65 (5.35) | 23.61 (3.13) | NA | 9.6 (6.11) | ①③④⑤⑥⑦ | 7 |
| Da Vinci Xi | 46 | 72.35 (5.36) | 23.80 (3.29) | NA | 9.02 (3.44) | |||||
| Tsujioka et al (2024) [17] | Japan | Retrospective | Hinotori | 118 | 69.65 (6.75) | 24.25 (3.23) | 34.09 (12.38) | 8.30 (4.73) | ①②④⑤⑥ | 6 |
| Da Vinci Xi | 118 | 69.65 (5.25) | 24.11 (3.15) | 11.86 (33.72) | 7.79 (3.38) |
BMI = body mass index; NA = not available; NOS = the Newcastle-Ottawa Scale; PSA = prostate-specific antigen; RCT = randomized controlled trial.
Outcome: ① operative time, ② estimated blood loss, ③ length of stay, ④ console time, ⑤ complications, ⑥ positive surgical margin, and ⑦ urinary incontinence.
2.5. Statistical analysis
Continuous variables were analyzed using WMDs, and dichotomous variables were assessed using ORs with corresponding 95% confidence intervals (CIs). For conventional pairwise meta-analyses, statistical analyses were conducted using Review Manager version 5.3. Heterogeneity was assessed using Cochran’s Q test, with p < 0.05 indicating significant heterogeneity. When significant between-study heterogeneity was detected, a random-effect model was applied; otherwise, a fixed-effect model was used. In addition, we generally preferred a random-effect model to account for potential heterogeneity among studies, while acknowledging that this approach cannot eliminate heterogeneity and that pooled estimates should therefore be interpreted with caution.
For the NMA, statistical analyses were performed in Stata version 18 (Stata Corp., College Station, TX, USA) using the “network” command. Evidence maps were generated, in which node size reflected sample size and edge thickness indicated the amount of direct evidence. Relative effect estimates were summarized in league tables. The surface under the cumulative ranking curve (SUCRA) scores were also calculated to provide a supplementary, probabilistic ranking of interventions in terms of their relative effectiveness. We interpreted these rankings with caution, acknowledging that SUCRA does not account for study quality or the statistical and clinical significance of differences between interventions, and therefore should be considered alongside effect estimates, CIs, and risk of bias assessments. Sensitivity analyses were conducted using a leave-one-out approach to evaluate the robustness of the findings, and the potential publication bias was assessed using funnel plots.
3. Results
3.1. Literature search results
Based on the literature search and inclusion criteria, we included 2064 patients from 15 studies for the meta-analysis [[14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]]. These studies, published between 2023 and 2024, assessed the clinical outcomes of four different robotic surgery systems: KD, DV, HG, and HS. All patients were diagnosed with PCa. Each study employed a parallel-group design, with detailed information provided in Table 1.
3.2. Pairwise meta-analysis
The results of the pairwise meta-analysis are shown in Table 2 (Supplementary Fig. 1 and 2), where we compared the performance of four robotic systems across several key intra- and postoperative outcomes. Regarding OT, the KD system prolonged the duration significantly compared with the DV system (WMD: 41.83 min [95% CI: 16.60–67.07]), while the HS system also exhibited a similar trend (WMD: 23.58 min [95% CI: 15.20–31.96]). However, the HG system did not show a statistically significant difference compared with the DV system (WMD: 4.97 min [95% CI: –6.62 to 16.55]). In terms of estimated blood loss, no significant differences were observed between the KD, HG, or HS system and the DV system (WMD: –7.34 ml [95% CI: –34.76 to 20.07]; WMD: 7.29 ml [95% CI: –63.26 to 77.85]; and WMD: –13.06 ml [95% CI: –32.17 to 6.06], respectively). Regarding hospital stay, no significant differences were found between the robotic systems and the DV system. The KD system showed a slightly shorter hospital stay than the DV system (WMD: –0.32 d [95% CI: –1.30 to 0.67]), while both the HG system (WMD: 0.10 d [95% CI: –0.07 to 0.26]) and the HS system (WMD: 0.20 d [95% CI: –0.25 to 0.28]) were comparable with the DV system. For console time, the HS system required significantly more time than the DV system (WMD: 17.78 min [95% CI: 3.83–31.72]). The differences between the KD system (WMD: 12.10 min [95% CI: –9.62 to 33.82]) and the HG system (WMD: 6.43 min [95% CI: –9.71 to 22.57]) compared with the DV system were not statistically significant. Regarding the complication rate, no significant differences were found between the alternative robotic systems and the DV system. Specifically, the OR was 1.15 (95% CI: 0.50–2.63) for the KD system, 0.34 (95% CI: 0.07–1.52) for the HG system, and 0.58 (95% CI: 0.34–0.98) for the HS system. For positive surgical margin rates, no significant differences were observed between the KD, HG, or HS system and the DV system, with ORs of 0.90 (95% CI: 0.45–1.81), 0.95 (95% CI: 0.68–1.34), and 0.90 (95% CI: 0.65–1.24), respectively. Similarly, in terms of postoperative incontinence, no significant differences were noted between the alternative robotic systems and the DV system, with ORs of 0.47 (95% CI: 0.04–5.73) for KD, 0.84 (95% CI: 0.30–2.36) for HG, and 0.92 (95% CI: 0.49–1.70) for HS.
Table 2.
Pairwise meta-analysis
| Treatment | No. of study | No. of patients (E/C) | WMD (95% CI) | Heterogeneity p value | Heterogeneity I2 (%) |
|---|---|---|---|---|---|
| Operative time | |||||
| KD vs DV | 3 | 76/74 | 41.83 (16.60, 67.07) | 0.07 | 63 |
| HG vs DV | 5 | 369/596 | 4.97 (–6.62, 16.55) | 0.02 | 77 |
| HS vs DV | 4 | 306/453 | 23.58 (15.20, 31.96) | 0.52 | 0 |
| Estimated blood loss | |||||
| KD vs DV | 3 | 76/74 | –7.34 (–34.76, 20.07) | 0.67 | 0 |
| HG vs DV | 5 | 369/596 | 7.29 (–63.26, 77.85) | <0.01 | 90 |
| HS vs DV | 3 | 258/407 | –13.06 (–32.17, 6.06) | 0.03 | 81 |
| Length of stay | |||||
| KD vs DV | 3 | 76/74 | –0.32 (–1.30, 0.67) | 0.14 | 50 |
| HG vs DV | 6 | 388/607 | 0.10 (–0.07, 0.26) | 0.07 | 52 |
| HS vs DV | 2 | 91/89 | 0.20 (–0.25, 0.28) | 0.84 | 0 |
| Console time | |||||
| KD vs DV | 2 | 60/58 | 12.10 (–9.62, 33.82) | 0.66 | 0 |
| HG vs DV | 3 | 66/58 | 6.43 (–9.71, 22.57) | 0.05 | 67 |
| HS vs DV | 3 | 209/207 | 17.78 (3.83, 31.72) | 0.10 | 57 |
| Treatment | No. of study | No. of patients (E/C) | OR (95% CI) | Heterogeneity p value | Heterogeneity I2 (%) |
| Complications | |||||
| KD vs DV | 3 | 76/74 | 1.15 (0.50, 2.63) | 0.77 | 0 |
| HG vs DV | 2 | 49/41 | 0.34 (0.07, 1.52) | 0.58 | 0 |
| HS vs DV | 2 | 166/164 | 0.58 (0.34, 0.98) | 0.3 | 8 |
| Positive surgical margin | |||||
| KD vs DV | 3 | 76/74 | 0.90 (0.45, 1.81) | 0.46 | 0 |
| HG vs DV | 6 | 404/610 | 0.95 (0.68, 1.34) | 0.52 | 0 |
| HS vs DV | 4 | 306/453 | 0.90 (0.65, 1.24) | 0.21 | 34 |
| Urinary incontinence | |||||
| KD vs DV | 1 | 16/16 | 0.47 (0.04, 5.73) | NA | NA |
| HG vs DV | 4 | 230/436 | 0.84 (0.30, 2.36) | 0.07 | 62 |
| HS vs DV | 2 | 80/84 | 0.92 (0.49, 1.70) | 0.56 | 0 |
CI = confidence interval; DV = Da Vinci surgical system; E/C = experimental/control; HG = Hugo robot-assisted surgical system; HS = Hinotori surgical robot system; KD = KangDuo surgical robot system; NA = not applicable; OR = odds ratio; WMD = weighted mean difference.
3.3. Network meta-analysis
3.3.1. Operative time
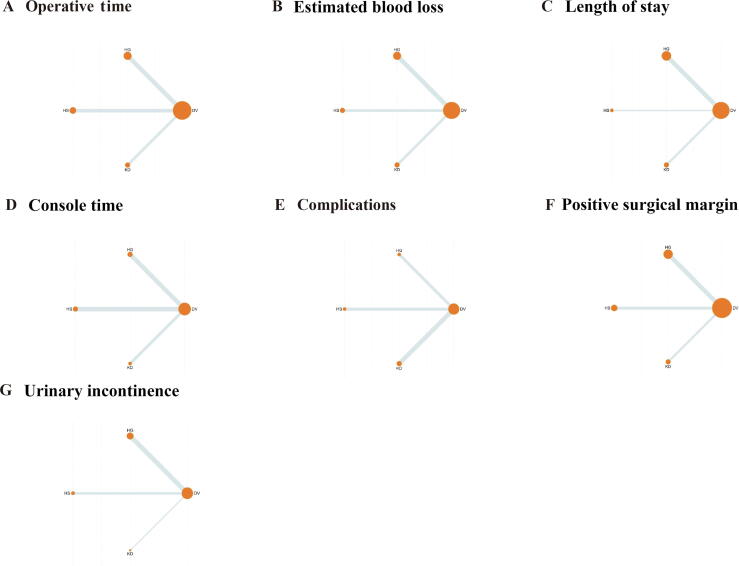
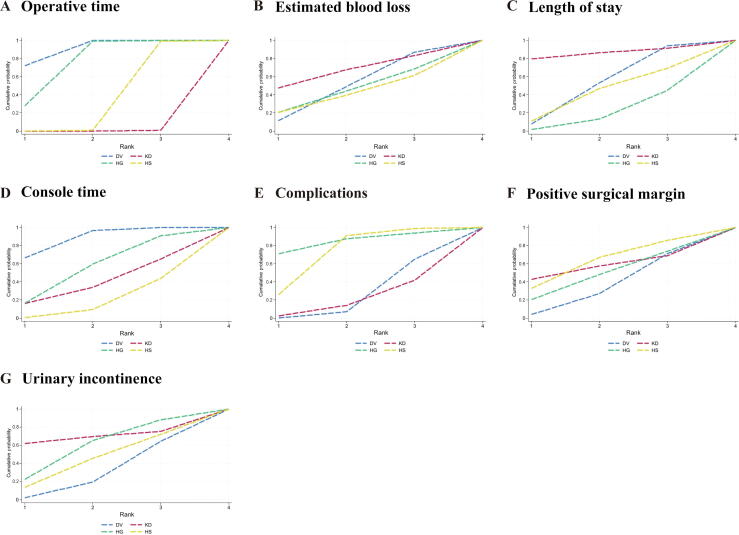
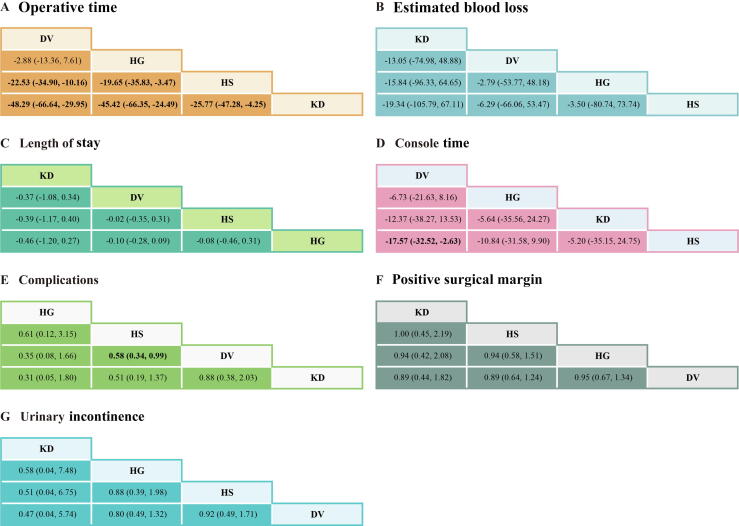
Data on OT were reported in 12 studies, encompassing four robotic systems (DV, HG, HS, and KD; Fig. 3). The NMA revealed that the DV system demonstrated the shortest OT, with the highest SUCRA value (90.7; Table 3 and Fig. 4). Pairwise comparisons illustrated significant advantages for the DV system over both the HS (WMD: –22.53 min; 95% CI: –34.90 to –10.16) and the KD (WMD: –48.29 min; 95% CI: –66.64 to –29.95) system (Fig. 5). While the difference between DV and HG did not reach statistical significance, DV displayed a trend toward shorter OT. OT for the HG system was intermediate, outperforming both HS (WMD: –19.65 min; 95% CI: –35.83 to –3.47) and KD (WMD: –45.42 min; 95% CI: –66.35 to –24.49). In contrast, the HS system demonstrated marginal efficiency gains over KD (WMD: –25.77 min; 95% CI: –47.28 to –4.25), though its advantage over HG was less pronounced. The KD system exhibited the longest OT among all systems.
Fig. 3.
Network plots: (A) operative time, (B) estimated blood loss, (C) length of stay, (D) console time, (E) complications, (F) positive surgical margin, and (G) urinary incontinence. DV = Da Vinci surgical system; HG = Hugo robot-assisted surgical system; HS = Hinotori surgical robot system; KD = KangDuo surgical robot system.
Table 3.
Results of SUCRA values and rank
| Treatment | SUCRA values/rank |
||||||
|---|---|---|---|---|---|---|---|
| Operative time | Estimated blood loss | Length of stay | Console time | Complications | Positive surgical margin | Urinary incontinence | |
| DV | 90.7/1.3 | 49.1/2.5 | 51.7/2.4 | 87.6/1.4 | 24.4/3.3 | 33.1/3 | 28.9/3.1 |
| KD | 0.3/4 | 66.1/2.0 | 85.8/1.4 | 39.1/2.8 | 19.1/3.4 | 56.7/2.3 | 69.6/1.9 |
| HG | 75.5/1.7 | 44.4/2.7 | 20.0/3.4 | 56.0/2.3 | 84.3/1.5 | 47.9/2.6 | 58/2.3 |
| HS | 33.3/3 | 40.4/2.8 | 42.5/2.7 | 17.8/3.5 | 72.3/1.8 | 62.2/2.1 | 43.5/2.7 |
DV = Da Vinci surgical system; HG = Hugo robot-assisted surgical system; HS = Hinotori surgical robot system; KD = KangDuo surgical robot system; SUCRA = surface under the cumulative ranking curve.
Fig. 4.
SUCRA ranking curves: (A) operative time, (B) estimated blood loss, (C) length of stay, (D) console time, (E) complications, (F) positive surgical margin, and (G) urinary incontinence. DV = Da Vinci Surgical System; HG = Hugo robot-assisted surgical system; HS = Hinotori surgical robot system; KD = KangDuo surgical robot system; SUCRA = surface under the cumulative ranking curve.
Fig. 5.
The league table: (A) operative time, (B) estimated blood loss, (C) length of stay, (D) console time, (E) complications, (F) positive surgical margin, and (G) urinary incontinence. Bold values indicate statistical significance. DV = Da Vinci surgical system; HG = Hugo robot-assisted surgical system; HS = Hinotori surgical robot system; KD = KangDuo surgical robot system.
3.3.2. Estimated blood loss
Eleven studies reported data on estimated blood loss across the four robotic systems (KD, DV, HG, and HS; Fig. 3). Although no statistically significant differences were identified, trend analyses highlighted potential system-specific characteristics. The KD system exhibited the highest SUCRA value for blood loss control (66.1), suggesting a potential role in scenarios requiring meticulous blood management, despite nonsignificant pairwise differences (Table 3, and Fig. 4, Fig. 5).
3.3.3. Length of hospital stay
Hospital stay data were available from 11 studies (Fig. 3). Differences between robotic systems in the length of stay were minimal, and none reached statistical significance (Fig. 5). Nonetheless, the KD system achieved the highest SUCRA value (85.8), indicating a potential advantage in postoperative recovery. The DV and HS systems followed closely, whereas the HG system exhibited slightly inferior performance (Table 3 and Fig. 4).
3.3.4. Console time
Eight studies reported data on console time (Fig. 3). While some differences were observed, most comparisons did not achieve statistical significance (Fig. 5). The DV system showed relatively favorable performance, with a significantly shorter console time than the HS system (WMD: –17.57 min; 95% CI: –32.52 to –2.63). The DV system also ranked highest in SUCRA values (87.6), underscoring its potential efficiency in surgical operations (Table 3 and Fig. 4).
3.3.5. Complications
Seven studies provided data on postoperative complications (Fig. 3). The HS system demonstrated a significantly lower complication rate than the DV system (OR: 0.58; 95% CI: 0.34–0.99), while other intersystem comparisons did not achieve statistical significance (Fig. 5). Among the systems, HG ranked highest in SUCRA values (84.3), reflecting its potential for minimizing complications. The KD system ranked lowest (SUCRA: 19.1), suggesting weaker performance in this domain (Table 3 and Fig. 4).
3.3.6. Positive surgical margins
Data on PSM were available from 13 studies (Fig. 3). No significant differences were observed between robotic systems in PSM rates (Fig. 5). However, the HS system demonstrated the highest SUCRA value (62.2), indicating relatively stable performance. The KD and HG systems followed closely, while the DV system had the lowest SUCRA value (33.1; Table 3 and Fig. 4).
3.3.7. Urinary incontinence
Urinary incontinence data were reported in seven studies, involving all four robotic systems (HS, KD, DV, and HG; Fig. 3). Although no statistically significant differences were detected, SUCRA rankings revealed system-specific tendencies (Fig. 5). The KD system ranked highest (SUCRA: 69.6), indicating potential superiority in postoperative continence outcomes, followed by HG (SUCRA: 58.0). The DV and HS systems demonstrated lower rankings (SUCRA: 28.9 and 43.5, respectively; Table 3 and Fig. 4).
3.4. Heterogeneity and publication bias
For the pairwise meta-analyses, heterogeneity was primarily assessed using Cochran’s Q test, with p < 0.05 considered indicative of statistically significant heterogeneity. Several outcome comparisons demonstrated significant variability between studies, such as OT for HG versus DV (p = 0.02, I2 = 77%) and estimated blood loss for HS versus DV (p = 0.03, I2 = 81%; Table 2, and Supplementary Fig. 1 and 2). A detailed review of all the included articles did not reveal a single clinical or methodological factor that could definitively account for these differences. Potential contributors include variations in surgeon experience across centers, differences in perioperative management protocols, heterogeneity in patient baseline characteristics, and inconsistencies in outcome measurement or definitions. Although we intended to further explore these factors through subgroup analyses, insufficient and inconsistent reporting across studies precluded such analyses.
The risk of bias was assessed for all the included studies: the two RCTs were evaluated using the Cochrane Risk of Bias tool, while observational studies were assessed with the NOS, yielding scores between 6 and 7, indicative of moderate to high quality. Although most studies were judged to have a low or moderate risk of bias, the performance bias was considered to be of a high risk in the RCTs owing to the nature of surgical interventions. Sensitivity analyses excluding studies at a higher risk of bias produced results consistent with the main analyses; yet, the potential influence of residual heterogeneity cannot be ruled out entirely and is acknowledged explicitly in the limitations (Table 1 and Fig. 1).
The publication bias was assessed using funnel plots for outcomes, which appeared largely symmetrical, indicating no substantial publication bias (Fig. 6). Moreover, the leave-one-out sensitivity analyses indicated that no single study exerted a disproportionate influence on the pooled effect sizes or heterogeneity estimates, suggesting that the observed results were robust and not driven by an isolated outlier (Fig. 7).
Fig. 6.
Funnel plots: (A) operative time, (B) estimated blood loss, (C) length of stay, (D) console time, (E) complications, (F) positive surgical margin, and (G) Urinary Incontinence.
Fig. 7.
Leave-one-out sensitivity analysis for (A) operative time, (B) estimated blood loss, (C) length of stay, (D) console time, (E) complications, (F) positive surgical margin and (G) urinary incontinence. CI = confidence interval.
4. Discussion
In recent years, robot-assisted radical prostatectomy (RARP) has emerged as a mainstream approach for the treatment of PCa globally, marking a significant paradigm shift in surgical oncology. This advanced technique not only offers patients more treatment options, but also demonstrates notable improvements in surgical outcomes, particularly in terms of postoperative recovery, precision of resection, and reduction in complications [[8], [9], [10]]. Owing to its precise operational capabilities, RARP enables complete prostate resection while maximizing the preservation of surrounding nerves and vasculature, thereby reducing the incidence of postoperative urinary incontinence and sexual dysfunction [29]. As RARP has gained widespread adoption, the field of surgical robotics has flourished, with various robotic systems being introduced to the market, including the DV, KD, HG, and HS systems. This competitive landscape reflects both the intense technological race and the growing demand for systems offering higher precision, enhanced functionality, and better cost efficiency. Each of these systems embodies unique features and advantages tailored to different clinical and economic scenarios [27,30,31].
As a mature and highly precise platform, the DV robotic system has become the cornerstone of minimally invasive surgery in urology, general surgery, and gynecology thanks to its high‑degree‑of‑freedom robotic arms, 3D high‑definition visualization, and ergonomically stable console that together enable meticulous dissection and suturing within confined anatomical spaces, thereby reducing blood loss, shortening hospital stay, and accelerating functional recovery [32,33]. Supported by extensive multicenter clinical trials and incorporated into leading international guidelines, it remains the preferred modality in tertiary care centers worldwide [34]. The system’s evolution has been marked by distinct generational enhancements: the original Standard/S models (2000–2006) introduced tremor‑filtered 3D optics and wristed EndoWrist instruments, establishing submillimetric precision in tasks such as nerve‑sparing prostatectomy [35,36]; the Si platform (2009) added dual‑console capability for proctoring, real‑time fluorescence (Firefly) imaging for tissue perfusion assessment, and high‑definition endoscopy, markedly increasing both operative flexibility and training efficiency [[37], [38], [39]]; the Xi system (2014) and its cost‑optimized counterpart X (2017) further refined workflow with an overhead boom‑mounted patient cart, universally interchangeable 8-mm arms, laser‑guided patient and port docking, and improved instrument reach for multiquadrant access without redocking—features that have been shown to reduce setup time by up to 20% and enhance surgical ergonomics [40,41]; and most recently, the single‑port (SP) platform (2018) re‑engineered access via a single ∼25-mm cannula housing three elbow‑jointed EndoWrist instruments plus a fully articulating 3D‑HD scope, which, as demonstrated by clinical series, achieves comparable dexterity and oncological outcomes to multiport Xi while offering reduced instrument collisions, shorter docking learning curves, and superior cosmesis in procedures such as transoral and pelvic surgery [42]. Despite these advances, the system’s premium pricing—driven by patented technology and recurring maintenance contracts—remains a significant barrier to adoption in resource‑limited settings [43].
In contrast, the KD system, a Chinese made robotic-assisted surgical device, offers notable advantages in cost and maintenance, presenting a competitive alternative for budget-conscious health care facilities. Studies included in this analysis, such as the study by Dong et al [28], suggest that the KD system costs only 25–30% of the DV system, substantially reducing surgical and hospitalization expenses for patients. This affordability makes KD a viable option for introducing robotic systems in economically constrained areas. However, its shorter development timeline means that its technical maturity and stability require further validation, particularly in complex surgical scenarios.
The HG RAS, developed by Medtronic, boasts a modular design, high-resolution 3D imaging, and flexible configurations, making it suitable for urology, general surgery, and gynecology. It combines strong economic performance with intuitive operation and a short learning curve. Additionally, its lower consumable and maintenance costs appeal to institutions seeking to reduce operational expenses [23]. Despite these advantages, as a relatively new system, HG RAS still lags behind the DV system in technical refinement and the accumulation of clinical data. Its larger modular setup also demands more operating room space, which could be a limiting factor in certain facilities [44].
The HS surgical robot, developed jointly by Kawasaki Heavy Industries (Tokyo, Japan) and Medicaroid, represents the first Japan-made robotic-assisted system designed for minimally invasive surgery. With lower equipment and maintenance costs, it offers a more economical alternative to international systems like DV while addressing the specific anthropometric needs of Asian populations [31]. Optimized sensitivity and ergonomic control further enhance its usability. Nonetheless, similar to the KD and HG systems, the HS system faces challenges in achieving the technical maturity and clinical validation of more established systems.
Taken together, these platforms reflect varying design philosophies, cost structures, and levels of clinical maturity. To help summarize and compare the core attributes of each system, we have outlined, in Table 4, differences in arm architecture, console design, visualization technology, cost levels, spatial requirements, key advantages, and current limitations. This structured overview is intended to provide readers—particularly those involved in institutional planning or surgical training—with a practical reference for evaluating system capabilities in real-world settings. As emerging systems continue to evolve, head-to-head comparisons and long-term clinical data will be critical in validating their performance and supporting informed adoption.
Table 4.
Main architectural and functional differences between the four robotic systems
| System | Manufacturer/country | Arm architecture | Console design | Visualization mode | Cost level | OR space requirement | Key advantages | Main limitations |
|---|---|---|---|---|---|---|---|---|
| Da Vinci | Intuitive Surgical/USA | Integrated multiarm (4 arms); SP: single-port 3 arm | Closed seated console | 3D-HD + Firefly fluorescence | High | Moderate | Technologically mature, extensive clinical validation | High purchase and maintenance cost |
| KangDuo | KangDuo/China | Independent 4-arm setup | Closed seated console | 3D-HD (open architecture) | Low (25–30% of DV) | Slightly compact | Cost effective, accessible in resource-limited settings | Less clinical data, needs further validation |
| Hugo RAS | Medtronic/USA | Modular arms (scalable setup) | Open, standing, modular console | High-resolution 3D-HD | Moderate to low | Large | Flexible configuration, intuitive use, lower upkeep cost | Bulky setup, limited clinical experience so far |
| Hinotori | Medicaroid/Japan and Kawasaki/Japan | Compact multiarm design | Semiclosed seated console | 3D-HD | Moderate to low | Moderate | Ergonomic for Asian body types, affordable maintenance | Still gaining technical maturity and validation |
OR = operating room; RAS = robot-assisted surgery.
Although RAS systems are currently employed in RP for PCa, offering certain advantages in intraoperative precision, postoperative recovery, and complication management, comprehensive evaluations of their safety and reliability remain insufficient. Significant differences in technical characteristics, and intra- and postoperative outcomes exist among these systems; however, the lack of high-quality comparative studies poses challenges for clinicians in selecting the optimal system. To address this gap, the present study, through an NMA, offers the first comprehensive comparison of four robotic systems—DV, KD, HG, and HS—in the clinical outcomes of RP for PCa. The analysis covers key intra- and postoperative parameters, including OT, blood loss, hospital stay, console time, postoperative complications, positive surgical margin rates, and 3-mo postoperative incontinence rates. Our findings not only highlight the strengths and weaknesses of each system, but also provide crucial scientific evidence to guide clinical decision-making in system selection.
The NMA results for OT and console time reveal that the DV system demonstrates the best operational efficiency (SUCRA values: 90.7 and 87.6), significantly outperforming the other systems. This advantage is likely attributable to the DV system’s status as the earliest and most widely utilized robotic platform, with mature technology and extensive clinical experience contributing to its efficiency. In contrast, the KD system showed the longest OT (WMD: 48.29 min; 95% CI: 29.95–66.64), suggesting areas for improvement in system optimization, response speed, and device stability. The HG and HS systems exhibited intermediate OTs, still lagging behind the DV system. These results indicate that the KD system presents substantial room for optimization in terms of operational efficiency and time management, potentially due to factors such as system performance, surgeon proficiency, or equipment limitations. Overall, the DV system stands out for its mature technology, stable performance, and broad clinical application, offering a distinct advantage in OT control. The disparities observed may stem from various factors: first, the structural differences between the DV and KD systems, with the four robotic arms (three for instruments and one for the camera) of the DV system offering greater flexibility and a wider field of view; in contrast, the KD system’s three robotic arms limit operational freedom, although recent updates to the KD system may improve efficiency with the addition of a fourth arm [45]. Moreover, the manual clutch control of the DV system may better align with surgical needs compared with the foot-pedal clutch of the KD system, further enhancing surgical efficiency. Surgeon experience, a critical factor in efficiency, likely also plays a role, as the DV system has been in widespread use for many years, accumulating considerable operational expertise, whereas the other systems, being relatively new, involve steeper learning curves [28]. Surgeons operating with the latter systems may adopt more conservative strategies, which could extend both OT and console times. Thus, future studies should refine the analysis and revisit efficiency disparities after stabilizing the learning curve [15]. Optimizing console time could be crucial for improving overall surgical efficiency and reducing surgeon fatigue, with future research potentially exploring enhancements in user interface design, human-machine collaboration, and intraoperative response time for emerging systems.
Minimal differences were observed among the four robotic systems in key perioperative outcomes such as hospital stay, estimated blood loss, and complication rates, suggesting that all platforms provide comparable short-term safety profiles. This consistency likely reflects the shared advantages of robotic-assisted minimally invasive surgery, namely, enhanced 3D visualization, tremor-filtered precision, and stable instrument control, which form a common technological foundation across systems. Notably, the KD system demonstrated favorable trends in controlling intraoperative blood loss, with a SUCRA value of 66.1. Although the WMD in blood loss between the KD and DV systems was –13.05 ml (95% CI: –74.98 to 48.88), the trend suggests a possible advantage for KD in selected clinical contexts. It is worth emphasizing, however, that this observed difference did not reach statistical significance, and underlying factors—such as case mix and surgical complexity—may have influenced these results [28].
In terms of hospital stay, the KD system again ranked highest, with a SUCRA value of 85.8, outperforming the DV and HS systems, while the HG system ranked lower. This pattern may reflect the KD system’s potential to reduce intraoperative tissue trauma and support faster recovery protocols. Such benefits could be particularly meaningful in resource-limited settings or in cases where early discharge is prioritized. Regarding postoperative complications, the HG system achieved the most favorable SUCRA score (84.3), suggesting potential advantages in safety, possibly due to design-specific features such as modular arm configuration and improved system responsiveness. While the overall differences between platforms remain modest, these individual performance profiles offer insight into potential areas of strength and indicate that further investigation, including direct head-to-head trials, is warranted.
In addition to our comparative efficacy and safety analyses, several included studies have highlighted that each new robotic platform entails its own learning curve. For the KD system KD‑SR‑01, surgeons with extensive DV Si experience (>400 RARP procedures) still required a distinct familiarization phase, with positive margin and continence outcomes plateauing only after large case volumes. The HS system HSRS likewise demonstrated an operative plateau after approximately 50–100 h‑RARP cases, with less experienced surgeons (≤40 prior DV RARPs) showing the greatest initial time reduction within their first 15 HSRS cases, while highly experienced DV users required little additional adaptation. Finally, the HG RAS system achieved proficiency in docking and console times after roughly 22 cases, after which both setup and operative durations closely approximated those of the DV platforms. Taken together, these findings suggest that although prior robotic experience accelerates adoption, each novel system demands its own dedicated learning period, which should be taken into account in future training and comparative studies.
Beyond learning efficiency, technical reliability remains a key consideration in evaluating robotic systems. Among the included studies, only one provided detailed data on intraoperative malfunctions, reporting a markedly higher incidence of technical issues with the HG RAS platform compared with the DV system (20 vs four events). These malfunctions ranged from instrument failures and arm collisions to system power supply errors, several of which required prolonged intervention time exceeding 5 min, thereby disrupting procedural flow and prolonging total OT. In contrast, malfunctions in the DV system were fewer, typically resolved quickly, and had minimal impact on workflow. Although limited to a single study, these findings suggest that technical maturity and system stability still vary between platforms. Therefore, future evaluations should incorporate structured reporting of intraoperative system malfunctions, resolution protocols, and their procedural impact to better inform real-world reliability and system selection. As robotic surgery continues to expand, device-related performance—including not only clinical efficacy, but also engineering robustness—will be increasingly critical to both adoption and long-term sustainability.
Furthermore, surgeon ergonomics and system responsiveness—though less often quantified—are equally vital to overall platform reliability. Subtle command‑to‑motion delays, imprecise motion scaling at the extremes of instrument reach, and unfamiliar clutch or foot‑pedal controls can increase cognitive load and physical strain, especially during lengthy or complex cases. To address these aspects, we propose that future studies incorporate objective latency measurements (eg, real‑time command‑to‑instrument response logging), motion‑capture analyses to assess instrument trajectory smoothness, and validated ergonomic workload assessments (such as NASA‑TLX or the Surgical Task Load Index) [46,47]. By systematically monitoring these parameters alongside clinical outcomes, investigators can more comprehensively evaluate both the engineering robustness and the user experience of emerging robotic platforms, ultimately guiding improvements in hardware design, software optimization, and surgeon training.
Finally, positive surgical margin rates and postoperative incontinence are key indicators of long-term outcomes in PCa radical surgery. The study revealed minimal differences between the robotic systems in these areas, with no statistically significant findings. This suggests that all systems—DV, KD, HG, and HS—offer comparable tumor excision thoroughness and preservation of sphincter function, likely due to the shared benefits of robotic technology, such as enhanced magnification, precise anatomical dissection, and meticulous protection of critical structures during surgery.
4.1. Limitations and future directions
While our NMA offers important early insights into the comparative performance of emerging robotic platforms, several limitations should be acknowledged with due modesty. First, the overall number of studies and their sample sizes were relatively small, which may have limited statistical power for outcomes that did not reach significance. Second, notable clinical and methodological heterogeneity was observed across some outcomes, likely reflecting differences in patient demographics, tumor stage, surgical complexity, surgeon experience, perioperative protocols, and outcome definitions. Although a random-effect model was applied to account for between-study variability, this approach does not remove heterogeneity, and any residual variability may still influence the interpretation and generalizability of pooled estimates. We intended to explore potential sources of heterogeneity through subgroup analyses; however, incomplete and inconsistent reporting of key effect modifiers—such as tumor stage, extent of lymphadenectomy, and platform generation—precluded such analyses, meaning that the pooled “average effect” estimates should be interpreted with caution. Third, reporting on the specific generation of the DV system was incomplete in many studies (see Table 1), preventing meaningful subgroup analyses of the S, Si, Xi, or SP platforms. Fourth, postoperative sexual function data were sparse and inconsistent—only two studies (by Olsen et al [25] and Ou et al [22]) reported erectile outcomes using different follow-up intervals and assessment tools—so we could not pool these measures. Fifth, while we focused on hard clinical endpoints, subtle yet critical factors such as instrument latency, motion-scaling precision, and ergonomic strain—especially during prolonged or complex cases—remain under-reported and may influence surgeon cognitive load, fatigue, and system preference. Finally, direct head-to-head trials remain scarce. We therefore encourage future large-scale, multicenter randomized studies with matched cohorts, standardized reporting of platform generations and functional outcomes (eg, International Index of Erectile Function for sexual function), structured monitoring of technical malfunctions and ergonomic metrics, and comprehensive long-term assessments of oncological efficacy, quality of life, and cost effectiveness to validate and extend our conclusions. These considerations, while reflecting the nascent state of comparative robotic research, do not undermine our key findings but rather identify clear pathways for further strengthening the evidence base.
5. Conclusions
In this NMA of RP for PCa, we compared perioperative and early functional outcomes across four robotic platforms—the DV, KD, HG, and HS systems. Our pooled estimates demonstrate that all systems achieved comparable safety and efficacy profiles, with no statistically significant differences in OT, estimated blood loss, length of hospital stay, overall complication rates, 3‑mo continence recovery, or positive surgical margin rates. These findings suggest that each platform can be considered a viable option for minimally invasive prostatectomy, and that choice of system may be guided primarily by institutional resources, surgeon experience, and patient‑specific factors rather than by intrinsic performance disparities.
Author contributions: Li Yang had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: J.-W. Yang, L. Yang.
Acquisition of data: J.-W. Yang, Wang, Chen.
Analysis and interpretation of data: Zhao, Wan.
Drafting of the manuscript: J.-W. Yang, Chai, L. Yang.
Critical revision of the manuscript for important intellectual content: Chai, L. Yang.
Statistical analysis: Chai, L. Yang.
Obtaining funding: J.-W. Yang, Zhao, Wan.
Administrative, technical, or material support: Chai, L. Yang.
Supervision: Chai, L. Yang.
Other: Chai, L. Yang.
Financial disclosures: Li Yang certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: This work was supported by Gansu Provincial Natural Science Foundation (grant number 25JRRA556), Gansu Provincial Key Development Program (Social Development Sector; grant number 24YFFA049), and Cuiying Student Research Program of Lanzhou University Second Hospital (grant number CYXZ2024-20).
Data sharing statement: The data necessary for the study are included within the article. Additional data and materials can be made available upon request from the corresponding author.
Associate Editor: Roderick C.N. van den Bergh
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.euros.2025.09.012.
Contributor Information
Jin Chai, Email: 1832918404@qq.com.
Li Yang, Email: ery_yangli@lzu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Liu X., Jiang H. The global, regional, and national prostate cancer burden and trends from 1990 to 2021, results from the global burden of disease study 2021. Front Public Health. 2025;13 doi: 10.3389/fpubh.2025.1553747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornford P., van den Bergh R.C.N., Briers E., et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer—2024 update. Part I: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2024;86:148–163. doi: 10.1016/j.eururo.2024.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Sekhoacha M., Riet K., Motloung P., Gumenku L., Adegoke A., Mashele S. Prostate cancer review: genetics, diagnosis, treatment options, and alternative approaches. Molecules. 2022;27:5730. doi: 10.3390/molecules27175730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeeri M.N.E., Awies M., Constantinou C. Prostate cancer, pathophysiology and recent developments in management: a narrative review. Curr Oncol Rep. 2024;26:1511–1519. doi: 10.1007/s11912-024-01614-6. [DOI] [PubMed] [Google Scholar]
- 5.Rawla P. Epidemiology of prostate cancer. World J Oncol. 2019;10:63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmberg L., Garmo H., Andersson S.-O., et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2024;391:1362–1364. doi: 10.1056/NEJMc2406108. [DOI] [PubMed] [Google Scholar]
- 7.Abou Heidar N.F., Ayoub C.H., Abou Mrad A., Abdul Khalek J., Tamim H., El-Hajj A. Robotic-assisted radical prostatectomy is pushing the boundaries: a national survey of frailty using the national surgical quality improvement program. Ther Adv Urol. 2023;15 doi: 10.1177/17562872231177780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gandaglia G., Montorsi F., Karakiewicz P.I., Sun M. Robot-assisted radical prostatectomy in prostate cancer. Future Oncol. 2015;11:2767–2773. doi: 10.2217/fon.15.169. [DOI] [PubMed] [Google Scholar]
- 9.Wang J., Hu K., Wang Y., et al. Robot-assisted versus open radical prostatectomy: a systematic review and meta-analysis of prospective studies. J Robot Surg. 2023;17:2617–2631. doi: 10.1007/s11701-023-01714-8. [DOI] [PubMed] [Google Scholar]
- 10.Scarcella S., Castellani D., Gauhar V., et al. Robotic-assisted versus open simple prostatectomy: Results from a systematic review and meta-analysis of comparative studies. Investig Clin Urol. 2021;62:631–640. doi: 10.4111/icu.20210297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shamseer L., Moher D., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350 doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J.P.T., Altman D.G., Gøtzsche P.C., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells G., Shea B., O’Connell D., et al. The Ottawa Hospital Research Institute; 2000. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 14.Sasaki Y., Kusuhara Y., Oyama T., et al. Radical prostatectomy using the Hinotori robot-assisted surgical system: Docking-free design may contribute to reduction in postoperative pain. Int J Med Robot Comput Assist Surg. 2024;20:e2648. doi: 10.1002/rcs.2648. [DOI] [PubMed] [Google Scholar]
- 15.Nakayama A., Izumi K., Ikezoe E., et al. Robot-assisted radical prostatectomy using the novel hinotoriTM surgical robot system: initial experience and operation learning curve at a single institution. Transl Cancer Res. 2024;13:57–64. doi: 10.21037/tcr-23-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohjimoto Y., Yamashita S., Iwagami S., Muraoka S., Wakamiya T., Hara I. hinotoriTM vs. da Vinci®: propensity score-matched analysis of surgical outcomes of robot-assisted radical prostatectomy. J Robot Surg. 2024;18:130. doi: 10.1007/s11701-024-01877-y. [DOI] [PubMed] [Google Scholar]
- 17.Tsujioka H., Setoguchi K., Nirazuka A., et al. Comparison of robot-assisted laparoscopic prostatectomy using the made-in-Japan robotic system Hinotori versus Da Vinci: a propensity score-matched analysis. Int J Med Robot Comput Assist Surg. 2024;20 doi: 10.1002/rcs.70013. [DOI] [PubMed] [Google Scholar]
- 18.Antonelli A., Veccia A., Malandra S., et al. Intraoperative performance of DaVinci versus Hugo RAS during radical prostatectomy: focus on timing, malfunctioning, complications, and user satisfaction in 100 consecutive cases (the COMPAR-P trial) Eur Urol Open Sci. 2024;63:104–112. doi: 10.1016/j.euros.2024.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bravi C.A., Balestrazzi E., De Loof M., et al. Robot-assisted radical prostatectomy performed with different robotic platforms: first comparative evidence between Da Vinci and HUGO robot-assisted surgery robots. Eur Urol Focus. 2024;10:107–114. doi: 10.1016/j.euf.2023.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Brime Menendez R., García Rojo E., Hevia Palacios V., et al. Da Vinci vs. Hugo RAS for robot-assisted radical prostatectomy: a prospective comparative single-center study. World J Urol. 2024;42:336. doi: 10.1007/s00345-024-05045-7. [DOI] [PubMed] [Google Scholar]
- 21.Gandi C., Marino F., Totaro A., et al. Perioperative outcomes of robotic radical prostatectomy with HugoTM RAS versus daVinci surgical platform: propensity score-matched comparative analysis. J Clin Med. 2024;13:3157. doi: 10.3390/jcm13113157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou H.-C., Marian L., Li C.-C., et al. Robot-assisted radical prostatectomy by the Hugo robotic-assisted surgery (RAS) system and the da Vinci system: a comparison between the two platforms. Cancers. 2024;16:1207. doi: 10.3390/cancers16061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sighinolfi M.C., Messina L.A., Stocco M., et al. Cost analysis of new robotic competitors: a comparison of direct costs for initial hospital stay between Da Vinci and Hugo RAS for radical prostatectomy. J Robot Surg. 2024;18:251. doi: 10.1007/s11701-024-01930-w. [DOI] [PubMed] [Google Scholar]
- 24.Ragavan N., Bharathkumar S., Chirravur P., Sankaran S. Robot-assisted laparoscopic radical prostatectomy utilizing Hugo RAS platform: initial experience. J Endourol. 2023;37:147–150. doi: 10.1089/end.2022.0461. [DOI] [PubMed] [Google Scholar]
- 25.Olsen R.G., Karas V., Bjerrum F., et al. Skills transfer from the DaVinci® system to the HugoTM RAS system. Int Urol Nephrol. 2024;56:389–397. doi: 10.1007/s11255-023-03807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen C., Yan W., Chen S., et al. Robot-assisted radical prostatectomy with the KangDuo surgical system versus the da Vinci Si system: a prospective, double-center, randomized controlled trial. Eur Urol Focus. 2024;10:1019–1026. doi: 10.1016/j.euf.2024.05.023. [DOI] [PubMed] [Google Scholar]
- 27.Fan S., Hao H., Chen S., et al. Robot-assisted laparoscopic radical prostatectomy using the KangDuo surgical robot system vs the da Vinci Si robotic system. J Endourol. 2023;37:568–574. doi: 10.1089/end.2022.0739. [DOI] [PubMed] [Google Scholar]
- 28.Dong J., Ji R., Cui L., et al. Feasibility, safety and effectiveness of robot-assisted radical prostatectomy with a new robotic surgical system: a prospective, controlled, randomized clinical trial. BMC Cancer. 2024;24:1194. doi: 10.1186/s12885-024-12855-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyberg M., Hugosson J., Wiklund P., et al. Functional and oncologic outcomes between open and robotic radical prostatectomy at 24-month follow-up in the Swedish LAPPRO trial. Eur Urol Oncol. 2018;1:353–360. doi: 10.1016/j.euo.2018.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soputro N.A., Olivares R. Current urological applications of the HugoTM RAS system. World J Urol. 2023;41:2555–2561. doi: 10.1007/s00345-023-04538-1. [DOI] [PubMed] [Google Scholar]
- 31.Miyake H., Fujisawa M. Early experience and future prospects regarding use of newly developed surgical robot system, Hinotori, in the field of urologic cancer surgery. Int J Clin Oncol. 2024;29:640–646. doi: 10.1007/s10147-024-02503-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsuda S., Oleynikov D., Gould J., et al. SAGES TAVAC safety and effectiveness analysis: da Vinci® surgical system (Intuitive Surgical, Sunnyvale, CA) Surg Endosc. 2015;29:2873–2884. doi: 10.1007/s00464-015-4428-y. [DOI] [PubMed] [Google Scholar]
- 33.Miyamura H., Takada K., Ohwaki A., et al. Initial experience and surgical outcomes of robotic-assisted total hysterectomy using the da Vinci SP surgical system. Asian J Endosc Surg. 2024;17 doi: 10.1111/ases.13298. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen T.T., Basilius J., Ali S.N., Dobbs R.W., Lee D.I. Single-port robotic applications in urology. J Endourol. 2023;37:688–699. doi: 10.1089/end.2022.0600. [DOI] [PubMed] [Google Scholar]
- 35.Rivero-Moreno Y., Echevarria S., Vidal-Valderrama C., et al. Robotic surgery: a comprehensive review of the literature and current trends. Cureus. 2013;15 doi: 10.7759/cureus.42370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marino M.V., Shabat G., Gulotta G., Komorowski A.L. From illusion to reality: a brief history of robotic surgery. Surg Innov. 2018;25:291–296. doi: 10.1177/1553350618771417. [DOI] [PubMed] [Google Scholar]
- 37.Crusco S., Jackson T., Advincula A. Comparing the da Vinci Si single console and dual console in teaching novice surgeons suturing techniques. JSLS. 2014;18 doi: 10.4293/JSLS-D-13-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Landry D., Quinn K.R., Helmer S.D., Sanchez N.C. Is two better than one? A retrospective study on colorectal surgery outcomes using the Da Vinci® dual-console robot. Kans J Med. 2022;15:418–421. doi: 10.17161/kjm.vol15.18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lue J.R., Pyrzak A., Allen J. Improving accuracy of intraoperative diagnosis of endometriosis: role of firefly in minimal access robotic surgery. J Minimal Access Surg. 2016;12:186–189. doi: 10.4103/0972-9941.158969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu D.Y., Chang Y.W., Lee H.Y., et al. Detailed comparison of the da Vinci Xi and S surgical systems for transaxillary thyroidectomy. Medicine (Baltimore) 2021;100 doi: 10.1097/MD.0000000000024370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrell A.L.G., Morrell-Junior A.C., Morrell A.G., et al. The history of robotic surgery and its evolution: when illusion becomes reality. Rev Colégio Bras Cir. 2021;48 doi: 10.1590/0100-6991e-20202798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Celotto F., Ramacciotti N., Mangano A., et al. Da Vinci single-port robotic system current application and future perspective in general surgery: a scoping review. Surg Endosc. 2024;38:4814–4830. doi: 10.1007/s00464-024-11126-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rabaza J. Is the Caribbean ready for robotics? Int J Surg Lond Engl. 2019;72S:3–5. doi: 10.1016/j.ijsu.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 44.Ngu J.C.-Y., Lin C.C.-W., Sia C.J.-Y., Teo N.-Z. A narrative review of the Medtronic Hugo RAS and technical comparison with the Intuitive da Vinci robotic surgical system. J Robot Surg. 2024;18:99. doi: 10.1007/s11701-024-01838-5. [DOI] [PubMed] [Google Scholar]
- 45.Xu L., Li X., Fan S., et al. Analysis of KangDuo-SR-1500 and KangDuo-SR-2000 robotic partial nephrectomy from an operative and ergonomic perspective: a prospective controlled study in porcine models. J Robot Surg. 2024;18:26. doi: 10.1007/s11701-023-01770-0. [DOI] [PubMed] [Google Scholar]
- 46.Bell S.W., Kong J.C.H., Clark D.A., et al. The National Aeronautics and Space Administration‐task load index: NASA‐TLX: evaluation of its use in surgery. Anz J Surg. 2022;92:3022–3028. doi: 10.1111/ans.17830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson M.R., Poolton J.M., Malhotra N., Ngo K., Bright E., Masters R.S.W. Development and validation of a surgical workload measure: the Surgery Task Load Index (SURG-TLX) World J Surg. 2011;35:1961–1969. doi: 10.1007/s00268-011-1141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.