Abstract
PARP-1-deficient mice display a severe defect in the base excision repair pathway leading to radiosensitivity and genomic instability. They are protected against necrosis induced by massive oxidative stress in various inflammatory processes. Mice lacking p53 are highly predisposed to malignancy resulting from defective cell cycle checkpoints, resistance to DNA damage-induced apoptosis as well as from upregulation of the iNOS gene resulting in chronic oxidative stress. Here, we report the generation of doubly null mutant mice. We found that tumour-free survival of parp-1–/–p53–/– mice increased by 50% compared with that of parp- 1+/+p53–/– mice. Tumour formation in nude mice injected with oncogenic parp-1–/–p53–/– fibroblasts was significantly delayed compared with parp-1+/+p53–/– cells. Upon γ-irradiation, a partial restoration of S-phase radiosensitivity was found in parp-1–/–p53–/– primary fibroblasts compared with parp-1+/+p53–/– cells. In addition, iNOS expression and nitrite release were dramatically reduced in the parp-1–/–p53–/– mice compared with parp-1+/+p53–/– mice. The abrogation of the oxydated status of p53–/– cells, due to the absence of parp-1, may be the cause of the delay in the onset of tumorigenesis in parp-1–/–p53–/– mice.
Keywords: cell cycle/γ-irradiation/iNOS expression/knockout mice/nude mice
Introduction
Poly(ADP-ribose) polymerase (PARP-1) is a highly conserved constitutive factor of the DNA damage surveillance network developed by the eukaryotic cell to cope with various environmental and endogenous genotoxic agents (de Murcia and Ménissier de Murcia, 1994). At a site of DNA breakage, the immediate poly(ADP-ribosyl)ation of a limited number of nuclear proteins involved in chromatin architecture and DNA metabolism translates the presence of DNA interruptions into intracellular signals that modulate DNA repair and cell survival. PARP-1 is associated in vivo with XRCC1, a DNA repair protein involved, together with DNA polymerase β and DNA ligase III, in the base excision repair (BER) of DNA (Masson et al., 1998). PARP-1-deficient cells display a severe DNA repair defect that appears to be the primary cause of the observed genomic instability and the cytotoxicity of DNA damaging agents inducing BER (Trucco et al., 1998; Beneke et al., 2000; Dantzer et al., 2000). Accordingly, parp-1–/– mice are hypersensitive to alkylating agents or γ-irradiation (Ménissier de Murcia et al., 1997; Masutani et al., 2000), but paradoxically are not cancer prone.
The knockout strategy has revealed the unexpected instrumental role of PARP-1 in cell death after ischaemia– reperfusion injury (Eliasson et al., 1997), streptozotocin-induced diabetes (Burkart et al., 1999; Masutani et al., 1999) and in different pathologies associated with inflammation (Szabo and Dawson, 1998; Pieper et al., 1999).
We and others (Hassa and Hottiger, 1999; Oliver et al., 1999) have shown recently that parp-1-deficient cells are defective in NF-κB-dependent transcription activation, but not in its nuclear translocation, in response to tumour necrosis factor-α and to lipopolysaccharide (LPS), demonstrating a functional link, in vivo, between PARP-1 and NF-κB. Therefore the complete abrogation of the transcriptional activation of NF-κB leads to a spectacular improvement of the outcome of a systemic inflammatory process in parp-1–/– mice treated with LPS (Oliver et al., 1999).
DNA damage caused by exposure to ionizing radiation, UV light or exogenous chemical mutagens that result in DNA strand breakage, triggers the accumulation of the tumour suppressor p53, which transactivates a number of genes involved in either growth arrest, DNA repair or apoptosis (for review see Lakin and Jackson, 1999; Oren, 1999; Sionov and Haupt, 1999). Mice lacking p53 are prone, very early, to the spontaneous development of malignant lymphomas and sarcomas (Donehower et al., 1992; Jacks et al., 1994). The early carcinogenesis observed in these mice was recently related to a constitutive upregulation of inducible nitric oxide synthase (iNOS) (Ambs et al., 1998a,b).
p53 and PARP-1 are both involved in the cell’s response to genotoxic stress but presumably at different steps. The functional interaction between these two proteins being unknown, here we explore the potential role of PARP-1 in the p53-deficient background. Various cohorts of the four possible homozygous genotypes of both genes were generated by breeding mice mutant for parp-1 and p53. Upon γ-irradiation, a partial restoration of S-phase radiosensitivity was found in primary fibroblasts isolated from parp-1–/–p53–/– mice compared with parp-1+/+p53–/– mice. However, the most striking finding was the gain in tumour-free survival promoted by the genetic deletion of parp-1 in p53-deficient mice. This significant delay in tumour formation was confirmed in nude mice injected with ras-transformed fibroblasts from parp-1–/–p53–/– mice. The retarded initiation in spontaneous tumour formation in double-mutant mice was inversely correlated with the cell’s ability to activate iNOS protein expression and generation of NO-derived oxidative products.
Taken together, our results show that the PARP-1 status dramatically influences tumour latency and cell proliferation, which could lead to new therapeutic approaches for the treatment of human p53 mutant tumours.
Results
Genetic deletion of parp-1 in p53-deficient mice promotes a gain in tumour-free survival
Pregnant or nursing parp-1+/+p53–/– females tend to develop tumours and attempts to use these mice to generate parp-1+/+p53–/– mice have often failed. Therefore, parp-1+/+p53+/– mice were intercrossed to generate parp-1+/+p5+/+ mice and parp-1+/+p53–/– mice. Consistently, the parp-1–/–p53–/– mice were generated from parp-1–/–p53+/– intercrosses although parp-1–/–p53–/– females are generally able to produce progeny. parp-1–/–p53–/– mice were born at the predicted Mendelian frequency indicating that there was no prenatal death of the double mutant.
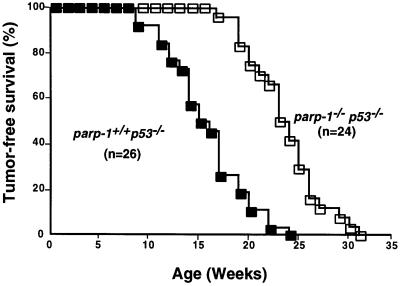
Mice of the four genotypes were observed concurrently to determine the spontaneous rate of tumour development. During the time of the study, no parp-1+/+p53+/+ or parp-1–/–p53+/+ mice died. We found that parp-1–/–p53–/– mice had substantially longer lifespans compared with the parp-1+/+p53–/– control mice (Figure 1A). The average age at which tumour formation occurred in the parp-1+/+p53–/– mice was 15–16 weeks compared with 24 weeks for parp-1–/–p53–/– mice. When they became moribund mice were killed and a detailed histological analysis of the tumour was conducted (Table I). Nearly all the mice had succumbed to tumours although some of them (two parp-1+/+p53–/– and one parp-1–/–p53–/– mice) presented unresolved infections. These cases were not included in Table I.
Fig. 1. Kaplan–Meier plot of tumour incidence in parp-1+/+p53–/– and parp-1–/–p53–/– mice. The percentage of mice remaining tumour free versus mice alive at the outset is plotted against age. The difference was highly significant (p <10–5 by Gehan’s Wilcoxon test and p <10–5 by log-rank test). Note that during the time of study, no parp-1+/+p53+/+ or parp-1–/–p53+/+ mice died.
Table I. Tumor spectrum of parp-1+/+p53–/– and parp-1–/–p53–/– mice.
| Case | Genotype | Histological site | Anatomical site |
|---|---|---|---|
| 1 | parp-1+/+p53–/– | teratoma | ovary |
| 2 | parp-1+/+p53–/– | lymphoma | thymus |
| 3 | parp-1+/+p53–/– | ND | stomach |
| 4 | parp-1+/+p53–/– | osteosarcoma | femur |
| 5 | parp-1+/+p53–/– | lymphoma | thymus |
| 6 | parp-1+/+p53–/– | lymphoma | thymus |
| 7 | parp-1+/+p53–/– | lymphoma | thymus |
| 8 | parp-1+/+p53–/– | lymphoma | hepatic metastasis |
| 9 | parp-1+/+p53–/– | lymphoma | thymus |
| 10 | parp-1+/+p53–/– | lymphoma | thymus |
| 11 | parp-1+/+p53–/– | lymphoma | thymus |
| 1 | parp-1–/–p53–/– | lymphoma | thymus |
| 2 | parp-1–/–p53–/– | lymphoma | thymus |
| 3 | parp-1–/–p53–/– | lymphoma | thymus |
| 4 | parp-1–/–p53–/– | fibrosarcoma | muscle |
| 5 | parp-1–/–p53–/– | osteosarcoma | femur |
| 6 | parp-1–/–p53–/– | adenosarcoma | lung |
| 7 | parp-1–/–p53–/– | lymphoma | thymus |
| 8 | parp-1–/–p53–/– | lymphoma | thymus |
| 9 | parp-1–/–p53–/– | lymphoma | thymus |
| 10 | parp-1–/–p53–/– | angiosarcoma | vessel |
| 11 | parp-1–/–p53–/– | lymphoma | thymus + ovarian metastasis |
| 12 | parp-1–/–p53–/– | epithelioma baso-cellular | skin |
| 13 | parp-1–/–p53–/– | lymphoma | hepatic metastasis |
| 14 | parp-1–/–p53–/– | ND | ? |
| 15 | parp-1–/–p53–/– | lymphoma | thymus |
| 16 | parp-1–/–p53–/– | lymphoma | thymus |
The histological analysis showed that both groups of mice developed predominantly lymphomas, although sarcomas were also observed. Flow cytometric analysis of parp-1+/+p53+/– and parp-1–/–p53+/– thymic lymphomas revealed that they were exclusively of immature T-cell origin (data not shown), as described previously (Jacks et al., 1994). Although the vast majority of p53–/– thymic lymphomas are in the CD4+/CD8+ double positive class, we found some double negative CD4–/CD8– thymic lymphomas in parp-1–/–p53–/– mice. Therefore, the longer latency for tumour formation in the double-mutant animals might be a reflection of resistance of double-positive cells to transformation or tumour development. T-cell lymphomas were also described in PARP-deficient mice in SCID background (Morrison et al., 1997). The tumour spectrum observed in parp-1–/–p53–/– mice is therefore similar to that seen in parp-1+/+p53–/– mice, and is consistent with that reported previously (Jacks et al., 1994) in p53–/– mice. These results suggest that parp-1 does not determine the cell type of the tumour but may contribute to the initiation step of tumorigenesis. To explore this hypothesis, we tested the ability of parp-1+/+p53–/– and parp-1–/–p53–/–-transformed primary fibroblasts to generate tumours in immunocompromised BALB/c nude mice.
Tumour formation is delayed in nude mice injected with ras-transformed fibroblasts from parp-1–/–p53–/–
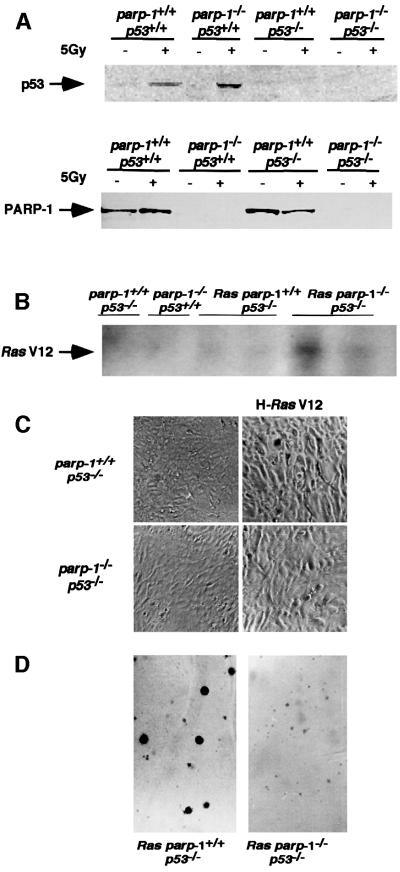
Oncogenic ras efficiently transforms rodent primary cells deficient in p53 or p16 (Tanaka et al., 1994; Serrano et al., 1996). We have introduced an activated ras allele (H-ras V12) into parp-1+/+p53–/– and parp-1–/–p53–/– primary fibroblasts using recombinant replication-deficient retrovirus (Serrano et al., 1997). Before transformation, PARP-1 and p53 expression in mouse embryonic fibroblasts (MEFs) was analysed by western blotting. As shown in Figure 2A, PARP-1 expression was found in parp- 1+/+p53+/+ and in parp-1+/+p53–/– MEFs and 4 h after 5 Gy γ-irradiation, p53 was detectable in parp-1+/+p53+/+ and in parp-1–/–p53+/+ fibroblasts.
Fig. 2. (A) Western blot analysis of PARP-1 and p53 expression in nuclear (p53) or crude (PARP-1) extracts of MEFs of the indicated genotypes. p53 protein was induced after 5 Gy irradiation. (B) Southern blot of HindIII-digested DNA from parp-1+/+p53–/– and parp-1–/–p53–/– MEFs infected or not with H-ras V12 retrovirus, using the ras V12 probe. (C) Morphology of primary and transformed parp-1+/+p53+/+ and parp-1–/–p53 fibroblasts. (D) Anchorage-independent growth of transformed parp-1+/+p53–/– and parp-1–/–p53–/– fibroblasts.
The presence of oncogenic ras detected by Southern blotting with a ras V12 DNA probe was used to determine the transduction efficiency in parp-1+/+p53–/– and parp-1–/–p53–/– primary fibroblasts. As shown in Figure 2B, oncogenic ras was detected in both primary cells. We then tested whether immortalization of both cell lines was achieved by the expression of the H-ras V12 oncogene. Changes in cell morphology were observed after retroviral infection and puromycin selection of pure populations. Both parp-1+/+p53–/– and parp-1–/–p53–/– cells adopted a prominent refractile morphology (Figure 2C) compared with parental lines. Furthermore, both cell lines continued to incorporate bromodeoxyuridine (BrdU) and to proliferate although the rate of proliferation of parp-1–/–p53–/– cells was 25% less than that of parp-1+/+p53–/–cells (data not shown). The tumorigenicity of ras-transduced primary (passage 1) parp-1+/+p53–/– and parp-1–/–p53–/– embryonic fibroblasts was demonstrated by their ability to form colonies in soft agar, a measure of anchorage-independent growth, and to produce tumours when implanted subcutaneously (s.c.) into immunocompromised nude mice. When grown in soft agar, both ras-transduced fibroblasts formed colonies (Figure 2D). Notably, parp-1–/–p53–/– colonies were smaller than parp-1+/+p53–/– colonies, suggesting that transformed parp-1–/–p53–/– cells need a more physiological environment to develop.
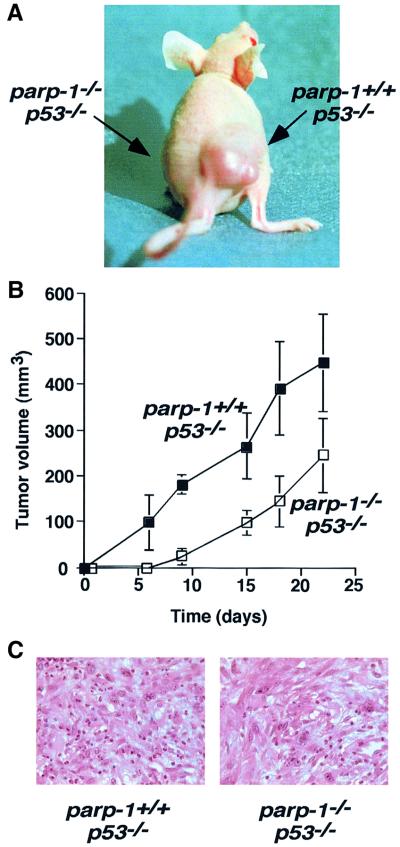
To avoid secondary mutations associated with culture passage, we injected s.c. into BALB/c nude mice primary fibroblasts at passage 1, 36 h after retroviral infection. Eight BALB/c nude mice 6–8 weeks old were injected with 0.5 × 106 transformed parp-1+/+p53–/– cells in the right thigh and the same number of transformed parp-1–/–p53–/– cells in the left thigh. Their tumour-forming ability was evaluated by measurements of tumour growth twice weekly. All the sites injected with transformed parp-1+/+p53–/– developed tumours (8/8), which were visible 15 days after injection. The mice were killed 22–25 days after injection, when they became severely ill. At that time, no tumour (0/8) was detected in the sites injected with transformed parp-1–/–p53–/– cells (Figure 3A).
Fig. 3. (A) Tumour formation in BALB/c nude mice. This representative mouse was inoculated with transformed parp-1+/+p53–/– fibroblasts in the right thigh and transformed parp-1–/–p53–/– fibroblasts in the left thigh. (B) Tumour volume plot after s.c. injection of parp-1+/+p53–/– and parp-1–/–p53–/– transformed fibroblasts mixed in equal volume of Matrigel into Balb/c nude mice. For each genotype, six sites were injected. Each point represents the mean ± SD of measurements. The difference was significant at all data points (p <0.006, Mann–Whitney U-test).
To test whether the modulation of the tumour microenvironment could affect the efficiency of tumour formation in parp-1–/–p53–/– cells, 2 × 106 transformed fibroblasts were mixed with Matrigel before implantation into nude mice. Tumour formations were observed in all cases (6/6), indicating that the stromal microenvironment has significant influence on parp-1–/–p53–/– tumour growth. Although parp-1+/+p53–/– cells formed tumours rapidly, a significant delay was observed in the initiation of parp-1–/–p53–/– tumour formation (Figure 3B), indicating that the loss of PARP-1 expression in a p53–/– background affects the efficiency of tumour formation. We reasoned that the tumour cells might have quite different cell cycle parameters when introduced into a s.c. site. To test this, we isolated cells of explanted tumours from both genotypes and analysed the percentage of cells in S phase following BrdU incorporation. We found that parp-1+/+p53–/– cells proliferate slightly more rapidly than parp–/–p53–/– cells (S phase: 25.9 ± 7% and 21.0 ± 0.4% for parp- 1+/+p53–/– and parp-1–/–p53–/– cells, respectively. This difference could account for reduced progression of the tumour. Moreover, histological analysis of tumours of both genotypes showed poorly differentiated cells without any detectable apoptotic event (Figure 3C).
Partial restoration of the cell cycle G1 arrest following γ-irradiation in double-mutant cells
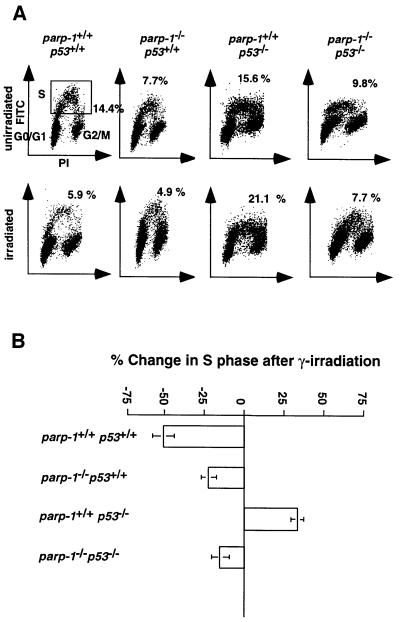
Given the importance of controlling the checkpoints for the prevention of neoplasia, we measured the cell cycle progression through the S phase of asynchronous primary MEFs of the four genotypes 18 h after γ-irradiation (5 Gy). The percentages of S-phase cells were assayed by flow cytometry (Figures 3B and 4A). The p53 protein is a regulator of the G1 checkpoint (Levine, 1997). Irradiation exposure of wild-type as well as parp-1–/– p53+/+ MEFs resulted in a drastic reduction (50.2 ± 6.7 and 25.2 ± 7.4%, respectively) of the number of BrdU-positive S-phase cells. In contrast, the G1/S-phase checkpoint in parp-1+/+p53–/– cells was defective, resulting in an increase (32.8 ± 3.5%) in BrdU-positive cells, as reported previously (Kastan et al., 1992). There was also a marked reduction (14.8 ± 5.6%) in BrdU-positive parp-1–/–p53–/– MEFs after irradiation. Thus, in the absence of PARP-1, a partial restoration of the G1/S-phase checkpoint occurs in p53–/– MEFs that might account for the observed delay in tumorigenesis.
Fig. 4. (A) Representative flow cytometry scatter plots of MEFs nuclei 18 h after irradiation (5 Gy), plotted as increasing fluorescence of propidium iodide (PI; x-axis) versus increasing FITC fluorescence obtained with an anti-BrdU–FITC-conjugated antibody (y-axis). Gating for S-phase cells is shown in the panel from unirradiated wild-type mice (upper left). (B) Graph representing the mean ± SD change in the percentage of S-phase cells after irradiation (n = 3–6).
Increased genomic instability in primary fibroblasts from double-mutant mice
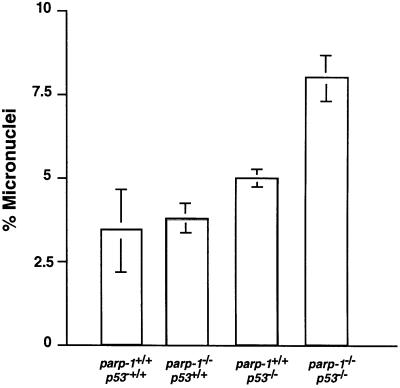
Genomic instability often correlates with a susceptibility to mutation and consequently with a propensity to carcinogenesis. It has been reported previously that both PARP-1 (Ménissier de Murcia et al., 1997; Wang et al., 1997; Trucco et al., 1998; Simbulan-Rosenthal et al., 1999) and p53 act to prevent genomic instability (Harvey et al., 1993; Lee et al., 1994). During their in vitro passaging, the tumorigenic parp-1+/+p53–/– and parp-1–/–p53–/– MEFs expressing H-ras V12 might have sustained genetic changes such as chromosomal abnormalities. This genomic instability might well predispose to carcinogenesis. Both primary parp-1+/+p53–/– and parp-1–/–p53–/– populations contained a mixture of near-diploid (65%) and near-tetraploid (35%) cells. The majority (70%) of tumorigenic parp-1+/+p53–/– and parp-1–/–p53–/– cells expressing H-ras V12 are near tetraploid. Since micronuclei represent extranuclear chromosomal fragments that are not incorporated into the nucleus during mitosis, they are considered as biomarkers of chromosomal instability. Spontaneous primary binucleated fibroblasts containing micronuclei were enumerated from four different genotypes. As shown in Figure 5, the percentage of parp-1–/–p53+/+ (3.3 ± 0.4%) and parp+/+p53–/– (4.5 ± 0.1%) MEFs containing micronuclei was increased by a factor of 1.4–1.9 compared with the wild-type cells (2.3 ± 1.3%), whereas double-mutant MEFs containing micronuclei (7.1 ± 2.1%) increased 3-fold in comparison with wild-type controls (p ≤0.005). These results further indicated that (i) the lack of both PARP-1 and p53 had a synergic effect on genomic stability and (ii) the observed tumorigenicity did not seem to be correlated with the level of spontaneous genomic instability.
Fig. 5. Micronuclei formation in primary MEFs at passage 2 isolated from mice of the indicated genotypes. A total of 700–1000 binucleated cells in each genotype was counted.
Reduction of iNOS expression and nitrite, and nitrate release in double-mutant mice
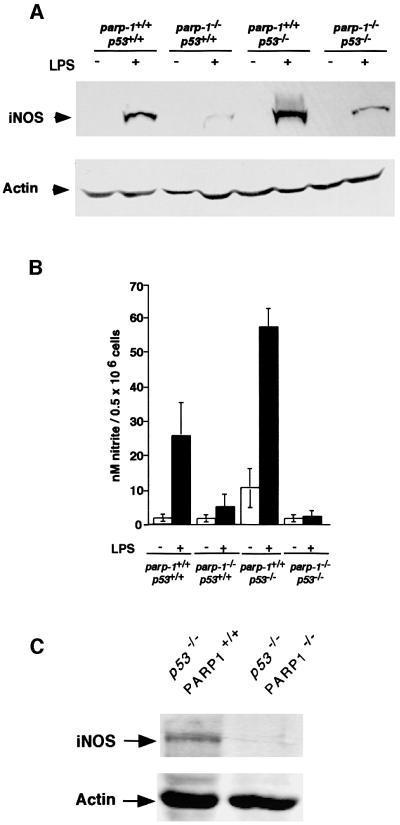
iNOS expression is deregulated in both mouse models: downregulation in parp-1–/– mice (Oliver et al., 1999) and upregulation in p53–/– mice (Ambs et al., 1998a,b). Macrophages being the main source of iNOS, we determined the basal and LPS-induced iNOS expression and NO release in macrophages of the four genotypes. Mice were treated with LPS (20 mg/kg i.p.) and 18 h later, the iNOS protein levels were determined in peritoneal macrophages by western blotting (Figure 5A). Following LPS treatment, iNOS expression was increased in macrophages taken from parp-1+/+p53+/+mice, but not from parp-1–/–p53+/+ mice, as expected (Oliver et al., 1999). In contrast, iNOS protein levels were increased in parp-1+/+ p53–/– cells, as reported previously (Ambs et al., 1998b). A dramatic reduction of iNOS expression was also observed in parp-1–/–p53–/– cells. This differential expression of iNOS in all four genotypes, was reflected in measurements of nitrite plus nitrate release in the supernatant of cultured primary macrophages stimulated with LPS (5 µg/ml) (Figure 6B). Because parp-1+/+ p53–/– and parp-1–/–p53–/– mice spontaneously developed predominantly thymic lymphomas, we sought to determine whether the deregulated iNOS expression was specific to macrophages or whether this occurred in thymocytes as well. To test this hypothesis, we performed western blot analysis on cellular extracts from parp-1+/+ p53–/– and parp-1–/–p53–/– thymus. The results showed that iNOS is downregulated in parp-1–/–p53–/– compared with parp-1+/+p53–/– thymocytes (Figure 6C). Therefore, the downregulation of iNOS in parp-1–/–p53–/– mice, accompanied by a drop in the oxidative status, may contribute to the delayed tumour initiation observed in double-mutant mice, by decreasing the mutagenic potential of NO (Caulfield et al., 1998).
Fig. 6. (A) Western blot analysis of iNOS expression in crude extracts of peritoneal macrophages from mice of the genotypes indicated, 18 h after LPS treatment. Expression of actin is shown as a loading control. (B) Nitrite release in primary cultured murine macrophages from mice of the indicated genotype treated or not with 5 µg/ml LPS for 24 h. Each value represents the mean ± SD of three independent experiments. (C) iNOS expression in crude extracts of splenocytes derived from parp-1+/+p53–/– and parp-1–/–p53–/– mice.
Discussion
PARP-1 activity now appears to be a pivotal determinant in the mechanism of oxidant-induced cell death (Ha and Snyder, 1999). Recent studies from several laboratories have implicated PARP-1 in the pathogenesis of various diseases, such as myocardial ischaemia–reperfusion injury (Thiemermann et al., 1997; Zingarelli et al., 1998; Pieper et al., 2000), endotoxic shock (Hassa and Hottiger, 1999; Oliver et al., 1999), arthritis (Szabo et al., 1998), type-1 diabetes (Burkart et al., 1999; Masutani et al., 1999) and brain ischaemia (Eliasson et al., 1997). The inactivation of parp-1 in genetically engineered animals confers protection against the diseases mentioned above. Significant protection against oxidant-induced tissue damage can also be achieved with pharmacological PARP-1 inhibitors (Szabo and Dawson, 1998; Szabo et al., 1998). Various mechanisms have been proposed to explain the role of PARP-1 in the pathogenicity of these diseases. The most prevalent explanation is the so-called ‘PARP suicide pathway’: the oxygen-derived free radicals, NO and peroxynitrite are generated during inflammation and induce DNA single-stranded breaks, which in turn overactivate PARP-1 (Berger, 1985). This excessive activation leads to intracellular NAD+ and ATP depletion resulting in mitochondrial free radical generation and cell necrosis (Ha and Snyder, 1999; Pieper et al., 1999). We and others have reported previously a functional link between PARP-1 and the transcription factor NF-κB in a model of endotoxic shock (Hassa and Hottiger, 1999; Oliver et al., 1999). PARP-1-deficient mice are extremely resistant to death induced by LPS because NF-κB-dependent transcription is impaired, consequently the in vivo release of inflammatory mediators as well as iNOS expression are downregulated during endotoxic shock. PARP-1 appears to promote inflammation by co-activativation of NF-κB-dependent transcription and by mediating the cytotoxicity of NO derivatives.
The data presented in this study reveal a new role for PARP-1 in tumorigenesis that, again, appears to be linked to the regulatory properties of the cell’s oxidative status. We have demonstrated that spontaneous tumour development in p53–/– mice is significantly delayed in the absence of PARP-1, resulting in increased survival of parp-1–/– p53–/– mice. Furthermore, tumour formation in nude mice showed that PARP-1 inactivation interfered with the initiation step of tumorigenesis. While the injection of 0.5 × 106 ras-transformed cells generated tumours at 15 days post-injection, parp-1–/–p53–/– ras-transformed cells failed to generate any visible tumour, except when mixed with Matrigel. Even then tumour initiation was clearly delayed compared with that generated with single p53 mutant cells (Figure 2C). Matrigel is a solubilized basement membrane preparation whose major components are laminin, collagen IV, heparan sulfate, proteoglycans and multiple growth factors. Matrigel could supply effective support for the attachment of transformed cells and a higher concentration of survival factors providing optimal conditions for the proliferation of ras-transformed parp-1–/–p53–/– cells. It is conceivable that in the absence of PARP-1, ras-transformed p53–/– cells were unable to proliferate and develop tumours, explaining the observed delay in tumorigenesis in double-mutant mice.
Although the double-mutant mice exhibited more spontaneous genomic instability compared with parp-1+/+ p53–/– mice, they did not exhibit an acceleration of tumour formation. In contrast, genomic instability inversely correlates with tumour development maybe through the induction of various cell cycle checkpoints. Consistent with these results, PARP-1-deficient mice did not show any particular predisposition to develop tumours but displayed genomic instability (Figure 5; Ménissier de Murcia et al., 1997; Wang et al., 1997; Trucco et al., 1998; Simbulan-Rosenthal et al., 1999). Genomic instability per se may therefore not be sufficient for tumorigenesis.
Among alternative mechanisms that could be responsible for the delay in tumour formation in p53-deficient mice, we examined the epistatic relationship between PARP-1 and p53 in response to γ-irradiation through S-phase progression. Radioresistant-DNA synthesis is one of the p53–/– fibroblast phenotypes (Harvey et al., 1993), whereas parp-1–/– fibroblasts exhibit normal G1/S checkpoint. parp-1–/–p53–/– cells showed a reproducible partial restoration of the G1/S checkpoint that might account for the delay in tumorigenesis observed in vivo. However, the molecular mechanism underlying this restoration remains unknown.
NO is synthesized from l-arginine by a family of enzymes called NO synthases (NOSs). The inducible isoform of NOS (iNOS or NOS2) generates NO for longer periods of time and at rates several orders of magnitude greater than the constitutive isoforms (Nicolson et al., 1993). High levels of NO and peroxynitrite formed from their interaction with the superoxide anion have been shown to induce damage to membranes, oxidation of intracellular proteins, DNA single-stranded breaks, nitrosative deamination and DNA base oxidative damage. High NO concentrations are elevated in ulcerative colitis (Singer et al., 1996) and other chronic inflammatory conditions, such as chronic hepatitis and Helicobacter pylori gastritis (Mannick et al., 1996), that predispose individuals to cancer. An abundant expression of NOS, as well as NOS activity, has been positively correlated with the degree of malignancy in human ovarian and uterine cancers (Thomsen et al., 1994), central nervous system tumours (Cobbs et al., 1995) and breast cancer (Thomsen et al., 1995). Numerous studies in animal models have also provided direct evidence for a stimulatory role of NO in tumour progression. In a rat colonic adenocarcinoma model showing iNOS expression in the tumour vasculature, treatment with NG-nitro-l-arginine methyl ester (l-NAME), a potent NOS inhibitor, reduces NO production and tumour growth (Kennovin et al., 1994). Similarly, engineered expression of murine iNOS in a human colonic adenocarcinoma cell line resulting in continuous, moderate levels of NO production in vitro, was associated with increased tumour growth and vascularity in vivo following transplantation in nude mice (Jenkins et al., 1995). The exposure of cells to high concentrations of NO results in wild-type p53 accumulation, which in turn mediates a trans-repression of iNOS gene expression in a regulatory negative feedback loop (Forrester et al., 1996). In line with this, p53–/– constitutively upregulates the iNOS gene, leading to sustained production of NO in some cell types (Ambs et al., 1998b). This excessive NO production leading to radical-induced DNA damage as well as inhibition of DNA repair enzymes, may promote the lymphomagenesis observed in these mice. The data presented here show a dramatic reduction in iNOS expression and NO production in the macrophages of parp-1–/–p53–/– mice upon LPS stimulation. Thus, the absence of PARP-1 downregulates the iNOS gene expression in p53-deficient mice, reducing the high levels of NO observed in these mice. The decreased carcinogenic potential of NO in parp-1–/–p53–/– mice could therefore explain the delay in tumorigenicity. In line with this, it is interesting to notice that the disruption of the gene encoding p66shc, an enzyme involved in oxidative stress response, confers a heightened cellular resistance to agents that cause oxidative damage and a 30% increase in lifespan (Migliaccio et al., 1999).
These and previous results (Oliver et al., 1999) from this laboratory are in full agreement with previously published data showing the attenuation or abrogation of established inflammatory diseases (Thiemermann et al., 1997; Szabo and Dawson, 1998; Pieper et al., 1999; Jijon et al., 2000) and a lack of tumorigenicity caused by the pharmacological inactivation of PARP-1 in vivo (Tseng et al., 1987; Bauer et al., 1996). It has also been shown recently that inhibition of PARP-1 by overexpression in HeLa cells of a trans-dominant-negative mutant dramatically reduces tumour formation of these cells injected into nude mice (Hans et al., 1999). The suggested mechanism was an increase in apoptosis due to the expression of a dominant-negative PARP mutant. In our model, this increase in apoptosis did not occur. The absence of PARP-1 failed to activate a p53-independent apoptotic pathway, suggesting that other mechanisms could be implicated in the observed interference with tumorigenesis.
The present results suggest a possible therapeutic approach to human cancers, most of which are p53 mutated. It is possible that in specific tumours expressing functional p53, inactivation of PARP-1 may sensitize tumour cells to therapeutic irradiation. In addition, therapeutic targeting of PARP-1 may improve the clinical outcome by retarding the growth of tumours expressing mutant p53. Such an approach would have to be carefully tailored in order to avoid toxicity derived from DNA repair inhibition in surrounding tissues.
Materials and methods
Generation of parp-1–/–p53–/– mice
parp-1–/– and p53–/– mice were described previously (Jacks et al., 1994; Ménissier de Murcia et al., 1997) and both were in the mixed genetic background C57BL/6 × 129Sv. parp-1–/– females were bred with a p53+/– male to generate parp-1+/–p53+/– mice, which were intercrossed to generate parp-1+/+p53+/– and parp-1–/–p53+/– progeny. parp-1+/+p53+/– and parp-1–/–p53+/– mice were then intercrossed to generate mice of the four genotypes: parp-1+/+p53+/+, parp-1–/–p53+/+, parp-1+/+p53–/– and parp-1–/–p53–/–.
Cell culture and cell cycle analysis
MEFs were derived from day 13.5 embryos as described (Serrano et al., 1997). parp-1+/+p53–/– and parp-1–/–p53–/– were obtained from intercrosses of parp-1+/+p53+/– and parp-1–/–p53+/–, respectively. MEFs were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Sigma) supplemented with 10% fetal calf serum (FCS) and 0.05 mg/ml gentamicin at 37°C with 5% CO2. For cell cycle analysis, primary MEFs at passage 2 were grown in 75 cm2 flasks at a density of 106 cells/flask in normal DMEM, irradiated with a 60Co γ-ray (5 Gy) source and cultured for 18 h. Then, the medium of the irradiated and non-irradiated MEFs was supplemented with 10 µM BrdU. After 30 min of BrdU labelling, cells were harvested, fixed in 70% ethanol, and analysed using a FACScan and the Cellquest program as described previously (Ménissier de Murcia et al., 1997). DNA content was revealed using propidium iodide and DNA synthesis by staining with V-fluorescein-5-isothiocyanate (FITC)-conjugated anti-BrdU antibody (Pharmingen). At least 104 cells were analysed per sample.
Tumorigenicity assay
Passage-1 MEFs were infected with an H-ras V12 oncogene-expressing retrovirus (kindly provided by M.Serrano, CNBC, CSIC, Madrid, Spain) as described previously (Serrano et al., 1997). Thirty-six hours after infection, cells were trypsinized, washed twice in phosphate-buffered saline (PBS) and 0.5 × 106 cells were injected s.c. into 6- to 8-week-old female BALB/c nude mice (Iffa-Credo, L’Arbresle, France). In some experiments, 2 × 106 transformed cells mixed in equal volume of cold Matrigel (Becton Dickinson, Bedford, MA, USA) were injected. Tumour volume (V) was determined twice a week by calliper measurement of the length (L) and width (W) as V = (L × W2)/2 (Soengas et al., 1999). Tumour cells were re-isolated by mincing the tumour and incubation in collagenase for 4 h. After washing in PBS, the cells were replated in DMEM supplemented with 10% FCS.
Soft agar assays
A bottom layer of 0.8% agar in DMEM was first placed on to 6 cm dishes. Ras-transformed parp-1+/+p53+/– and parp-1–/–p53+/– fibroblasts (5 × 103 cells) were seeded in 0.3% top agar containing DMEM and 30% FCS. Colonies were counted after 3 weeks.
Micronuclei assay
Primary MEFs at passage 2 were seeded on coverslips the day before treatment with cytochalasin B (6 µg/ml) (Fenech and Morley, 1985), and 24 h later micronuclei were determined as described previously (Trucco et al., 1998).
Western blot analysis
Total or nuclear (Velasco et al., 1997) proteins were separated by 10% SDS–PAGE, transferred to a nitrocellulose membrane and probed with either anti-iNOS (Sigma), anti-p53 (pAb 421) or anti-PARP-1 (VIC-5) antibodies. Bound antibodies were revealed with goat anti-rabbit or sheep anti-mouse–horseradish peroxidase and blots were developed using the Renaissance blotting detection system (New England Nuclear, Boston, MA).
Southern blot analysis
Genomic DNA (15 µg) from retroviral-infected MEFs was restricted with HindIII, electrophoresed on a 1% agarose gel and transferred to a nitrocellulose sheet (Amersham) according to the manufacturer’s protocol. The radiolabelled mouse oncogenic ras cDNA excised by HindIII from the retroviral pLPCX ras plasmid (Morgenstern and Land, 1990; Serrano et al., 1997) was used as a probe to hybridize the membrane. The hybridized filter was exposed to X-ray film.
Nitrite release
Nitrite release in the culture medium of murine macrophages was determined by the Griess reaction as described previously (Szabo et al., 1994), following stimulation in vitro with 1 µg/ml LPS for 24 h.
Acknowledgments
Acknowledgements
We are indebted to C.Waltzinger for FACScan assistance, to Professor P.Chambon for constant support, to the Centre Paul Strauss (Strasbourg) for radiation experiments and to Dr S.Natarajan-Amé for critical reading of the manuscript. C.C. was supported by a postdoctoral fellowship from EGIDE and by the Centre National de la Recherche Scientifique (CNRS). This work was supported by grants from Association pour la Recherche contre le Cancer, Ligue contre le Cancer, Electricité de France, Commissariat à l’Energie Atomique and Fondation pour la Recherche Médicale, and to F.J.O. from Fondo de Investigaciones Sanitarias 00/0948, Spanish Ministry of Health.
References
- Ambs S. et al. (1998a) p53 and vascular endothelial growth factor regulate tumor growth of NOS2-expressing human carcinoma cells. Nature Med., 4, 1371–1376. [DOI] [PubMed] [Google Scholar]
- Ambs S., Ogunfusika,M.O., Merriam,W.G., Bennett,W.P., Billiar,T.R. and Harris,C.C. (1998b) Up-regulation of inducible nitric oxide synthase expression in cancer-prone p53 knockout mice. Proc. Natl Acad. Sci. USA, 95, 8823–8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer P.I. et al. (1996) Modification of growth related enzymatic pathways and apparent loss of tumorigenicity of a ras-transformed bovine endothelial cell line by treatment with 5-iodo-6-amino-1,2-benzopyrone (INH2BP). Int. J. Oncol., 8, 239–252. [DOI] [PubMed] [Google Scholar]
- Beneke R., Geisen,C., Zevnik,B., Bauch,T., Muller,W.U., Kupper,J.H. and Moroy,T. (2000) DNA excision repair and DNA damage-induced apoptosis are linked to poly(ADP-ribosyl)ation but have different requirements for p53. Mol. Cell. Biol., 20, 6695–6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger N.A. (1985) Poly(ADP-ribose) in the cellular response to DNA damage. Radiat. Res., 101, 4–15. [PubMed] [Google Scholar]
- Burkart V., Wang,Z.Q., Radons,J., Heller,B., Herceg,Z., Stingl,L., Wagner,E.F. and Kolb,H. (1999) Mice lacking the poly(ADP-ribose) polymerase gene are resistant to pancreatic β-cell destruction and diabetes development induced by streptozocin. Nature Med., 5, 314–319. [DOI] [PubMed] [Google Scholar]
- Caulfield J.L., Wishnok,J.S. and Tannenbaum,S.R. (1998) Nitric oxide-induced deamination of cytosine and guanine in deoxynucleosides and oligonucleotides. J. Biol. Chem., 273, 12689–12695. [DOI] [PubMed] [Google Scholar]
- Cobbs C.S., Brenman,J.E., Aldape,K.D., Bredt,D.S. and Israel,M.A. (1995) Expression of nitric oxide synthase in human central nervous system tumors. Cancer Res., 55, 727–730. [PubMed] [Google Scholar]
- Dantzer F., de la Rubia,G., Ménissier-de Murcia,J., Hostomsky,Z., de Murcia,G. and Schreiber,V. (2000) Base excision repair is impaired in mammalian cells lacking poly(ADP-ribose) polymerase-1. Biochemistry, 39, 7559–7569. [DOI] [PubMed] [Google Scholar]
- de Murcia G. and Ménissier de Murcia,J. (1994) Poly(ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem. Sci., 19, 172–176. [DOI] [PubMed] [Google Scholar]
- Donehower L.A., Harvey,M., Slagle,B.L., McArthur,M.J., Montgomery,C.A.,Jr, Butel,J.S. and Bradley,A. (1992) Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature, 356, 215–221. [DOI] [PubMed] [Google Scholar]
- Eliasson M.J. et al. (1997) Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nature Med., 3, 1089–1095. [DOI] [PubMed] [Google Scholar]
- Fenech M. and Morley,A.A. (1985) Measurement of micronuclei in lymphocytes. Mutat. Res., 147, 29–36. [DOI] [PubMed] [Google Scholar]
- Forrester K. et al. (1996) Nitric oxide-induced p53 accumulation and regulation of inducible nitric oxide synthase expression by wild-type p53. Proc. Natl Acad. Sci. USA, 93, 2442–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha H.C. and Snyder,S.H. (1999) Poly(ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc. Natl Acad. Sci. USA, 96, 13978–13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hans M.A., Muller,M., Meyer-Ficca,M., Burkle,A. and Kupper,J.H. (1999) Overexpression of dominant negative PARP interferes with tumor formation of HeLa cells in nude mice: evidence for increased tumor cell apoptosis in vivo. Oncogene, 18, 7010–7015. [DOI] [PubMed] [Google Scholar]
- Harvey M. et al. (1993) In vitro growth characteristics of embryo fibroblasts isolated from p53-deficient mice. Oncogene, 8, 2457–2467. [PubMed] [Google Scholar]
- Hassa P.O. and Hottiger,M.O. (1999) A role of poly(ADP-ribose) polymerase in NF-κB transcriptional activation. Biol. Chem., 380, 953–959. [DOI] [PubMed] [Google Scholar]
- Jacks T., Remington,L., Williams,B.O., Schmitt,E.M., Halachmi,S., Bronson,R.T. and Weinberg,R.A. (1994) Tumor spectrum analysis in p53-mutant mice. Curr. Biol., 4, 1–7. [DOI] [PubMed] [Google Scholar]
- Jenkins D.C. et al. (1995) Roles of nitric oxide in tumor growth. Proc. Natl Acad. Sci. USA, 92, 4392–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jijon H.B., Churchill,T., Malfair,D., Wessler,A., Jewell,L.D., Parsons,H.G. and Madsen,K.L. (2000) Inhibition of poly(ADP-ribose) polymerase attenuates inflammation in a model of chronic colitis. Am. J. Physiol. Gastrointest. Liver Physiol., 279, G641–G651. [DOI] [PubMed] [Google Scholar]
- Kastan M.B., Zhan,Q., el-Deiry,W.S., Carrier,F., Jacks,T., Walsh,W.V., Plunkett,B.S., Vogelstein,B. and Fornace,A.J.,Jr (1992) A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell, 71, 587–597. [DOI] [PubMed] [Google Scholar]
- Kennovin G.D., Stratford,M.R.L. and Flitney,F.W. (1994) Inducible nitric oxide synthase is expressed in tumor-associated vasculature: inhibition retards tumor growth in vivo. In Moncada S., Feelisch,M., Busse,R. and Higgs,E.A. (eds), Biology of Nitric Oxide, Part 4: Enzymology, Biochemistry and Immunology. Portland Press, London, UK, pp. 473–479.
- Lakin N.D. and Jackson,S.P. (1999) Regulation of p53 in response to DNA damage. Oncogene, 18, 7644–7655. [DOI] [PubMed] [Google Scholar]
- Lee J.M., Abrahamson,J.L., Kandel,R., Donehower,L.A. and Bernstein,A. (1994) Susceptibility to radiation-carcinogenesis and accumulation of chromosomal breakage in p53 deficient mice. Oncogene, 9, 3731–3736. [PubMed] [Google Scholar]
- Levine A.J. (1997) p53, the cellular gatekeeper for growth and division. Cell, 88, 323–331. [DOI] [PubMed] [Google Scholar]
- Mannick E.E. et al. (1996) Inducible nitric oxide synthase, nitrotyrosine and apoptosis in Helicobacter pylori gastritis: effect of antibiotics and antioxidants. Cancer Res., 56, 3238–3243. [PubMed] [Google Scholar]
- Masson M., Niedergang,C., Schreiber,V., Muller,S., Menissier-de Murcia,J. and de Murcia,G. (1998) XRCC1 is specifically associated with poly(ADP-ribose) polymerase and negatively regulates its activity following DNA damage. Mol. Cell. Biol., 18, 3563–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutani M. et al. (1999) Poly(ADP-ribose) polymerase gene disruption conferred mice resistant to streptozotocin-induced diabetes. Proc. Natl Acad. Sci. USA, 96, 2301–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masutani M., Nozaki,T., Nakamoto,K., Nakagama,H., Suzuki,H., Kusuoka,O., Tsutsumi,M. and Sugimura,T. (2000) The response of Parp knockout mice against DNA damaging agents. Mutat. Res., 462, 159–166. [DOI] [PubMed] [Google Scholar]
- Ménissier de Murcia J. et al. (1997) Requirement of poly(ADP-ribose) polymerase in recovery from DNA damage in mice and in cells. Proc. Natl Acad. Sci. USA, 94, 7303–7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio E., Giorgio,M., Mele,S., Pelicci,G., Reboldi,P., Pandolfi,P.P., Lanfrancone,L. and Pelicci,P.G. (1999) The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature, 402, 309–313. [DOI] [PubMed] [Google Scholar]
- Morgenstern J.P. and Land,H. (1990) A series of mammalian expression vectors and characterisation of their expression of a reporter gene in stably and transiently transfected cells. Nucleic Acids Res., 18, 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison C., Smith,G.C., Stingl,L., Jackson,S.P., Wagner,E.F. and Wang,Z.Q. (1997) Genetic interaction between PARP and DNA-PK in V(D)J recombination and tumorigenesis. Nature Genet., 17, 479–482. [DOI] [PubMed] [Google Scholar]
- Nicolson A.G., Haites,N.E., McKay,N.G., Wilson,H.M., MacLeod,A.M. and Benjamin,N. (1993) Induction of nitric oxide synthase in human mesangial cells. Biochem. Biophys. Res. Commun., 193, 1269–1274. [DOI] [PubMed] [Google Scholar]
- Oliver F.J., Ménissier-de Murcia,J., Nacci,C., Decker,P., Andriantsitohaina,R., Muller,S., de la Rubia,G., Stoclet,J.C. and de Murcia,G. (1999) Resistance to endotoxic shock as a consequence of defective NF-κB activation in poly(ADP-ribose) polymerase-1 deficient mice. EMBO J., 18, 4446–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M. (1999) Regulation of the p53 tumor suppressor protein. J. Biol. Chem., 274, 36031–36034. [DOI] [PubMed] [Google Scholar]
- Pieper A.A., Verma,A., Zhang,J. and Snyder,S.H. (1999) Poly(ADP-ribose) polymerase, nitric oxide and cell death. Trends Pharmacol. Sci., 20, 171–181. [DOI] [PubMed] [Google Scholar]
- Pieper A.A., Walles,T., Wei,G., Clements,E.E., Verma,A., Snyder,S.H. and Zweier,J.L. (2000) Myocardial postischemic injury is reduced by polyADPribose polymerase-1 gene disruption. Mol. Med., 6, 271–282. [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Lee,H., Chin,L., Cordon-Cardo,C., Beach,D. and DePinho,R.A. (1996) Role of the INK4a locus in tumor suppression and cell mortality. Cell, 85, 27–37. [DOI] [PubMed] [Google Scholar]
- Serrano M., Lin,A.W., McCurrach,M.E., Beach,D. and Lowe,S.W. (1997) Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell, 88, 593–602. [DOI] [PubMed] [Google Scholar]
- Simbulan-Rosenthal C.M. et al. (1999) Chromosomal aberrations in PARP(–/–) mice: genome stabilization in immortalized cells by reintroduction of poly(ADP-ribose) polymerase cDNA. Proc. Natl Acad. Sci. USA, 96, 13191–13196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I.I., Kawka,D.W., Scott,S., Weidner,J.R., Mumford,R.A., Riehl,T.E. and Stenson,W.F. (1996) Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology, 111, 871–885. [DOI] [PubMed] [Google Scholar]
- Sionov R.V. and Haupt,Y. (1999) The cellular response to p53: the decision between life and death. Oncogene, 18, 6145–6157. [DOI] [PubMed] [Google Scholar]
- Soengas M.S., Alarcon,R.M., Yoshida,H., Giaccia,A.J., Hakem,R., Mak,T.W. and Lowe,S.W. (1999) Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science, 284, 156–159. [DOI] [PubMed] [Google Scholar]
- Szabo C. and Dawson,V.L. (1998) Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol. Sci., 19, 287–298. [DOI] [PubMed] [Google Scholar]
- Szabo C., Southan,G.J. and Thiemermann,C. (1994) Beneficial effects and improved survival in rodent models of septic shock with S-methylisothiourea sulfate, a potent and selective inhibitor of inducible nitric oxide synthase. Proc. Natl Acad. Sci. USA, 91, 12472–12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C., Virag,L., Cuzzocrea,S., Scott,G.S., Hake,P., O’Connor,M.P., Zingarelli,B., Salzman,A. and Kun,E. (1998) Protection against peroxynitrite-induced fibroblast injury and arthritis development by inhibition of poly(ADP-ribose) synthase. Proc. Natl Acad. Sci. USA, 95, 3867–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N. et al. (1994) Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell, 77, 829–839. [DOI] [PubMed] [Google Scholar]
- Thiemermann C., Bowes,J., Myint,F.P. and Vane,J.R. (1997) Inhibition of the activity of poly(ADP ribose) synthetase reduces ischemia-reperfusion injury in the heart and skeletal muscle. Proc. Natl Acad. Sci. USA, 94, 679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen L.L., Lawton,F.G., Knowles,R.G., Beesley,J.E., Riveros-Moreno,V. and Moncada,S. (1994) Nitric oxide synthase activity in human gynecological cancer. Cancer Res., 54, 1352–1354. [PubMed] [Google Scholar]
- Thomsen L.L., Miles,D.W., Happerfield,L., Bobrow,L.G., Knowles,R.G. and Moncada,S. (1995) Nitric oxide synthase activity in human breast cancer. Br. J. Cancer, 72, 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucco C., Oliver,F.J., de Murcia,G. and Ménissier-de Murcia,J. (1998) DNA repair defect in poly(ADP-ribose) polymerase-deficient cell lines. Nucleic Acids Res., 26, 2644–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A. Jr, Lee,W.M., Jakobovits,E.B., Kirsten,E., Hakam,A., McLick,J., Buki,K. and Kun,E. (1987) Prevention of tumorigenesis of oncogene-transformed rat fibroblasts with DNA site inhibitors of poly(ADP ribose) polymerase. Proc. Natl Acad. Sci. USA, 84, 1107–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco M., Diaz-Guerra,M.J., Martin-Sanz,P., Alvarez,A. and Bosca,L. (1997) Rapid up-regulation of IκBβ and abrogation of NF-κB activity in peritoneal macrophages stimulated with lipopolysaccharide. J. Biol. Chem., 272, 23025–23030. [DOI] [PubMed] [Google Scholar]
- Wang Z.Q., Stingl,L., Morrison,C., Jantsch,M., Los,M., Schulze-Osthoff,K. and Wagner,E.F. (1997) PARP is important for genomic stability but dispensable in apoptosis. Genes Dev., 11, 2347–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingarelli B., Salzman,A.L. and Szabo,C. (1998) Genetic disruption of poly(ADP-ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ. Res., 83, 85–94. [DOI] [PubMed] [Google Scholar]