Abstract
CrkII, a cellular homolog of v-crk, belongs to a family of adaptor proteins that play a central role in signal transduction cascades. We demonstrate that CrkII interacts directly with c-Jun N-terminal kinase 1 (JNK1). A proline-rich sequence of JNK1 is critical for the interaction of the kinase with the N-terminal Src homology 3 (SH3) domain of CrkII. JNK1 is localized with CrkII in membrane ruffles of Crk-overexpressing cells in a Rac1-dependent manner. A JNK1 mutant (K340A) that fails to interact with CrkII is defective in Rac/epidermal growth factor-induced activation, but remains responsive to UVC irradiation. Furthermore, CrkII recruits JNK1 to a p130Cas multiprotein complex where it may be activated through a hematopoietic progenitor kinase 1- and mitogen-activated protein kinase kinase 4-dependent pathway. Together, the results presented here argue for a new mechanism of regulation of the JNK pathway through the CrkII–p130Cas adaptor complex.
Keywords: CrkII/cytoskeleton/JNK1/p130Cas/Rho GTPase
Introduction
Mitogen-activated protein kinases (MAPKs) are serine/threonine kinases that are rapidly activated upon stimulation of diverse cell surface receptors (Cobb and Goldsmith, 1995; Robinson and Cobb, 1997). Members of the MAPK family are involved in several pathways that link extracellular stimuli to the nucleus (Cobb and Goldsmith, 1995; Karin, 1995; Marshall, 1995; Robinson and Cobb, 1997; Lewis et al., 1998; Garrington and Johnson, 1999; Schaeffer and Weber, 1999). The initially identified extracellular responsive kinases (ERKs) control the expression of genes that are essential for many cellular processes, including cell growth and differentiation (Karin, 1995; Marshall, 1995; Robinson and Cobb, 1997; Lewis et al., 1998; Garrington and Johnson, 1999; Schaeffer and Weber, 1999). The stress-activated protein kinases (SAPKs), which include c-Jun N-terminal kinases (JNKs) and p38, are activated both by environmental stresses (UV and γ irradiation, osmotic shock, etc.) and extracellular stimuli, including mitogens such as epidermal growth factor (EGF) or inflammatory cytokines such as tumor necrosis factor α (TNFα) (Ip and Davis, 1998; Davis, 1999). Three mammalian genes have been identified as part of the JNK family (jnk1, jnk2, jnk3). Alternative splicing may generate as many as 10 different isoforms (Gupta et al., 1996). In resting cells, JNK proteins are found in the nucleus as well as in the cytoplasm. Activated JNK phosphorylates transcription factors such as c-jun and activating transcription factor-2 (ATF2) in the nucleus, leading to the transcriptional activation of target genes (Gupta et al., 1996). The cascades that lead to the activation of JNKs are not fully elucidated. However, it is clear that the JNK pathway can be activated by Rac1 and cdc42, two members of the mammalian Rho GTPase family of small G proteins that are also involved in the regulation of the actin cytoskeleton (Coso et al., 1995; Minden et al., 1995; Teramoto et al., 1996; Hall, 1999). Rac1 is involved in the formation of membrane ruffles and lamellipodia, whereas cdc42 is implicated in the formation of filipodia (Ridley et al., 1992; Nobes and Hall, 1995; Tapon and Hall, 1997; Kjoller and Hall, 1999).
The Crk family of adaptor proteins includes CrkI, CrkII, CrkL and v-Crk, each of which contains predominantly Src homology 2 (SH2) and SH3 domains (Matsuda et al., 1992, 1996; Matsuda and Kurata, 1996). The widely expressed CrkII protein contains an N-terminal SH2 domain and two SH3 domains (Matsuda et al., 1992). Despite the lack of an enzymatic kinase domain, CrkII is thought to play a crucial role in growth factor-stimulated signal transduction and regulation of the actin cytoskeleton (Ishiki et al., 1997; Kiyokawa et al., 1997; Feller et al., 1998; Nakashima et al., 1999). Tyrosine-phosphorylated proteins known to be associated with actin stress fiber in adhesion foci, such as p130Cas or paxillin, have been shown to interact with the SH2 domain of CrkII. Moreover, the CrkII N-terminal SH3 domain interacts with several proteins that share a PPxLPxK binding motif, including the nucleotide exchange proteins C3G and Sos (Knudsen et al., 1994, 1995; Feller et al., 1995; Matsuda et al., 1996), DOCK180 (Hasegawa et al., 1996; Kiyokawa et al., 1998b) and the hematopoietic progenitor kinase HPK1 (Ling et al., 1999). Since these different proteins do not interact simultaneously with CrkII, it is likely that distinct combinations of interacting proteins will engage CrkII in various signaling pathways and cellular processes.
The JNK pathway was shown to be activated in v-Crk-transformed cells (Tanaka et al., 1997; Tanaka and Hanafusa, 1998; Mochizuki et al., 2000), and several groups have reported that transient overexpression of CrkII, one of the cellular counterparts of v-Crk, leads to activation of JNK1 (Dolfi et al., 1998; Kiyokawa et al., 1998a). Moreover, activation of JNK1 by CrkII has been shown to be enhanced by p130Cas (Dolfi et al., 1998), another adaptor protein. Since JNK1 contains a PPxIPxK sequence motif reminiscent of the consensus motif found in CrkII (N)SH3 interacting proteins, we have investigated a possible direct interaction between CrkII and JNK1 proteins. We show here that JNK1 interacts with CrkII, and demonstrate that this interaction is a critical step, not only for the activation of JNK1 by CrkII, but also for Rac1-induced activation of JNK1. Our findings also implicate the p130Cas–CrkII complex as a scaffolding interface involved in JNK1 activation.
Results
The CrkII protein interacts with JNK1
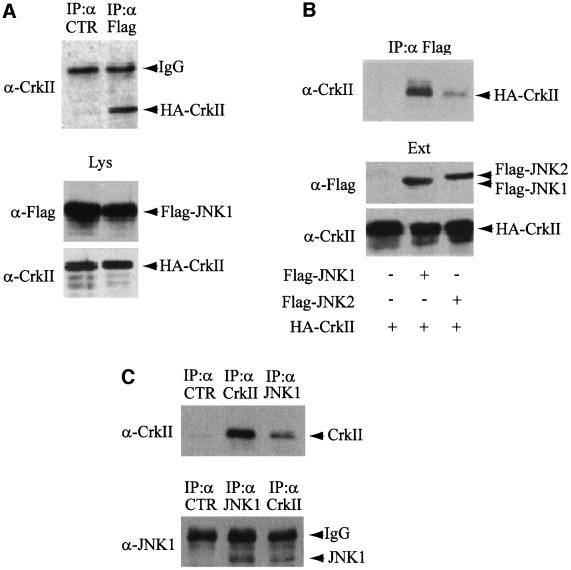
To examine whether the activation of JNK1 by CrkII depends on a direct physical interaction, we prepared hemagglutinin (HA) epitope-tagged CrkII and Flag epitope-tagged JNK1 by translation in vitro using wheat germ lysates (Figure 1A). In vitro products were mixed and analyzed for interaction by co-immunoprecipitation using an unrelated mouse monoclonal antibody as a control (left lane) or an anti-Flag antibody (right lane). CrkII was detected by western blotting only in anti-Flag immunoprecipitates, demonstrating that CrkII and JNK1 interact in vitro. To determine whether the interaction between CrkII and JNK1 also occurred in vivo, we co-transfected expression plasmids encoding HA-CrkII with either Flag-JNK1 or Flag-JNK2 into HeLa cells. Soluble extracts were prepared as described in Materials and methods. Immunoprecipitation by anti-Flag antibodies was followed by gel electrophoresis and western blot analysis with anti-CrkII antibodies. As shown in Figure 1B, CrkII binds to JNK1 in vivo with much higher avidity than to JNK2. In addition, we also observed that endogenous CrkII and JNK1 proteins interact in non-transfected HeLa cells by immunoprecipitating JNK1 with a rabbit anti-JNK1 antibody, and detecting a co-precipitating CrkII protein with an anti-CrkII antibody (Figure 1C, upper panel). The interaction between endogenous CrkII and JNK1 was confirmed by the reciprocal experiment: immunoprecipitation of CrkII using an anti-CrkII mouse monoclonal antibody and detection of a co-precipitating JNK1 protein with a rabbit anti-JNK1 antibody (Figure 1C, lower panel).
Fig. 1. CrkII interacts with JNK1. (A) In vitro interaction. Flag-JNK1 and HA-CrkII were prepared by in vitro translation using wheat germ extracts, mixed, and separated into two aliquots (Lys). A fraction of each aliquot was fractionated on polyacrylamide gel, transferred to a membrane and blotted with either anti-Flag or anti-CrkII antibodies (lower panel). The rest of the two aliquots were immunoprecipitated with an unrelated mouse monoclonal antibody (CTR, left lane) or mouse monoclonal anti-Flag antibody (right lane) and probed with anti-CrkII antibody (upper panel). (B) In vivo interaction. The plasmids encoding HA-CrkII, Flag-JNK1 or Flag-JNK2 were cotransfected into HeLa cells as indicated. The yield of transfected proteins in the extracts (Ext) was determined after immunoblotting with either anti-Flag or anti-CrkII antibodies (lower panel). Interaction was determined by immunoprecipitation using anti-Flag mouse monoclonal antibody. The presence of CrkII in the Flag immunoprecipitates was determined using an anti-CrkII antibody (upper panel). (C) Interaction of the endogenous proteins. The interaction between CrkII and JNK1 was directly addressed with the endogenous proteins after JNK1 or CrkII immunoprecipitation followed by western blotting carried out on HeLa cellular extracts. Immunoprecipitations using an unrelated antibody (CTR) are also presented as negative control.
JNK1 localization to CrkII-induced ruffles is regulated by Rac1 in vivo
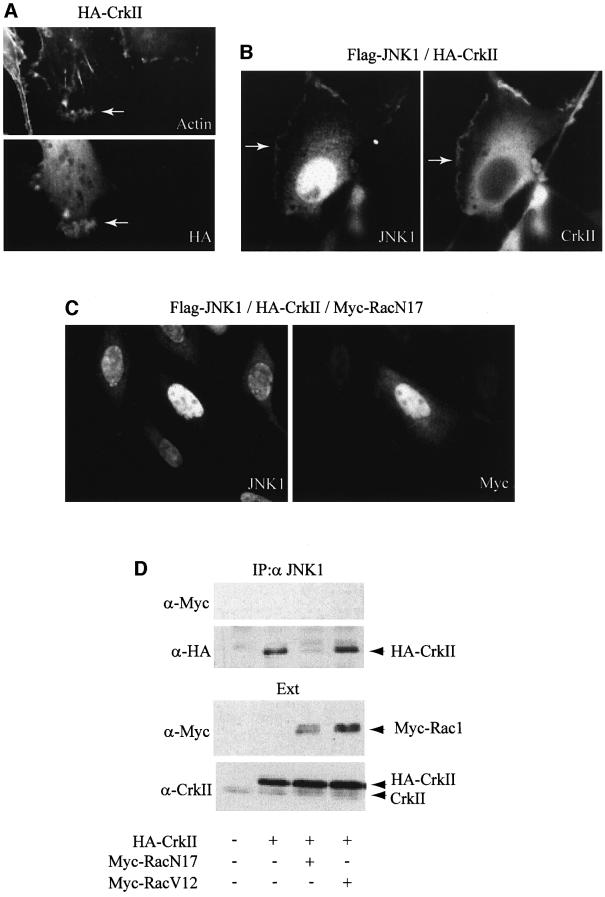
As overexpression of CrkII leads to the formation of membrane ruffles (Dolfi et al., 1998; Hashimoto et al., 1998), we investigated the influence of CrkII overexpression on the intracellular localization of JNK1. As expected, transfection of HeLa cells with an expression vector for CrkII induced strong membrane ruffling in most of the transfected cells (Figure 2A). In contrast, cells transfected with Flag-JNK1 expression vector showed no alteration of the actin cytoskeleton fibers, and transfected JNK1 displayed mostly nuclear localization, with diffuse staining throughout the cytoplasm (data not shown). In cells co-expressing HA-CrkII and Flag-JNK1, and although JNK1 was still detected in the nucleus and the cytoplasm, a fraction of the kinase was recruited to the ruffling structures in which CrkII is also present (Figure 2B). The observation of JNK1 localization to membrane ruffles is very unlikely to be due to a non-specific accumulation of proteins in these structures after CrkII overexpression, since we failed to detect JNK interacting protein-1 (JIP-1), a scaffold protein interacting with JNK, in CrkII-induced membrane ruffles (data not shown). This result suggests that CrkII might recruit JNK1 to ruffling membranes, probably through the direct interaction between CrkII and JNK1. Since Rac1 has been shown to induce membrane ruffling (Ridley et al., 1992; Tapon and Hal, 1997) and to regulate CrkII-induced membrane ruffling (Dolfi et al., 1998), we investigated the effect of blocking Rac1 activity on the localization of JNK1 to membrane ruffles (Figure 2C). Cells transfected with the dominant-negative RacN17 together with CrkII and JNK1 failed to display membrane ruffles, confirming the role of Rac1 activity in CrkII-induced membrane ruffling. Interestingly, we also observed that, in cells transfected with RacN17, overexpressed JNK1 was essentially nuclear (Figure 2C, left) while CrkII displayed a uniformally cytoplasmic localization (data not shown). These observations suggest that CrkII is responsible for the cytoplasmic localization of a fraction of JNK1 by a mechanism dependent upon Rac1 activity.
Fig. 2. CrkII interaction with JNK1, and CrkII-dependent JNK1 localization to ruffles are regulated by Rac1 activity. (A) Overexpression of CrkII induces membrane ruffling. HeLa cells were visualized by indirect immunofluorescence after transfection with HA-CrkII expression vector. Cells were stained using a mouse monoclonal anti-HA. Rhodamine-conjugated phalloidin was used to visualize filamentous actin. Membrane ruffling induced by CrkII is indicated by an arrow. (B) JNK1 localization to ruffles. HeLa cells were visualized by indirect immunofluorescence after cotransfection with Flag-JNK1 and HA-CrkII expression vectors. Cells were stained using a mouse monoclonal anti-CrkII or a polyclonal rabbit anti-JNK1 antibody as indicated. JNK1 localization to ruffles is indicated by an arrow. (C) Effect of Rac1 on JNK1 localization to ruffles. HeLa cells were visualized by indirect immunofluorescence after cotransfection with Flag-JNK1, HA-Crk and Myc-RacN17 expression vectors. Cells were stained using a rabbit polyclonal anti-JNK1 antibody (left panel) or a mouse monoclonal anti-Myc antibody (right panel). Membrane ruffles were absent in all the cells transfected with Myc-RacN17 expression vector. (D) Effect of Rac1 on the CrkII–JNK1 interaction. HeLa cells were transfected with HA-CrkII plus either Myc-RacN17 or Myc-RacV12 expression vectors as indicated. Interaction between JNK1 and transfected HA-CrkII was determined by HA immunoblot on JNK1 immunoprecipitates (top). Myc-Rac1 proteins were not found in the JNK1 immunoprecipitates. Control immunoblots on total protein extracts (Ext) using anti-Myc or anti-CrkII antibodies are presented.
Expression of dominant-negative Rac1 affects the CrkII-dependent recruitment of JNK1 to membrane ruffles and leads to an overall loss of co-localization between CrkII and JNK1, as observed by immunofluorescence. This suggests that the profound reorganization of the actin cytoskeleton resulting from the expression of Rac1N17 is sufficient to affect the CrkII–JNK1 interaction. To test this hypothesis, HeLa cells were transfected with HA-CrkII, Myc-tagged Rac1N17 (a dominant-negative form of Rac1) or Myc-Rac1V12 (a constitutively active form of Rac1) expression vectors. We followed the CrkII–JNK1 interaction by immunoprecipitation using a rabbit polyclonal anti-JNK1 antibody (Figure 2D). While Rac1V12 expression had little effect on the formation of CrkII–JNK1 complexes, Rac1N17 expression resulted in a dramatic reduction in the CrkII–JNK1 association (Figure 2D, upper panel). This effect was not the result of a direct physical interaction between Rac1 and either CrkII or JNK1 since we failed to detect Rac1 in anti-JNK1 (see Figure 2D) or anti-CrkII immunoprecipitates (data not shown). Together, these experiments demonstrate that the in vivo CrkII–JNK1 interaction is dependent on Rac1 activity and suggest that this interaction is very likely to occur predominantly in the membrane ruffles.
CrkII–JNK1 interaction requires CrkII N-terminal SH3 domain
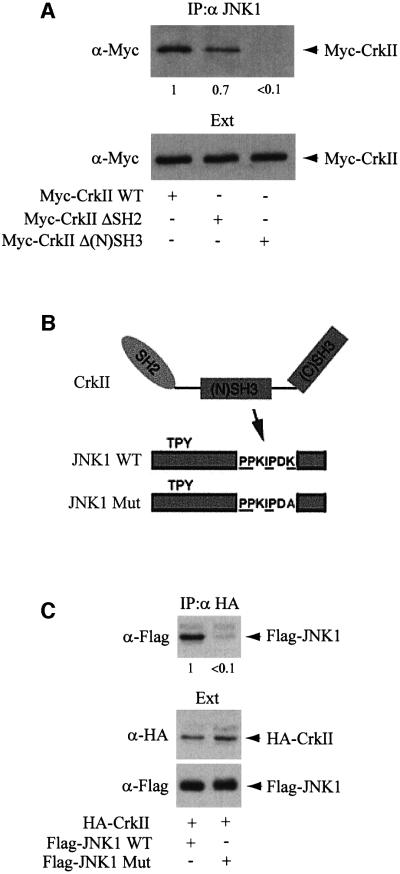
The relatively simple and modular structure of CrkII has been extensively used to analyze the properties of SH2/SH3 domains (Feller et al., 1994, 1995; Knudsen et al., 1995; Matsuda et al., 1996). In order to define more precisely which CrkII domains are responsible for the interaction with JNK1, we used Myc-CrkΔSH2 or Myc-CrkΔ(N)SH3 expression vectors, two CrkII constructs with point mutations in the SH2 domain or the N-terminal SH3 domain of CrkII, respectively (Matsuda et al., 1992). These mutations alter the structure of either the SH2 or the N-terminal SH3 domains of CrkII, and prevent any protein interaction through these respective domains. We transfected the expression vectors for either the wild type or the mutated forms of CrkII tagged with the myc epitope. Immunoprecipitation with anti-JNK1 antibody was followed by gel electrophoresis and western blot analysis using an anti-Myc antibody, allowing comparison of the relative affinities of CrkII wild type, CrkΔSH2, and CrkΔ(N)SH3 for JNK1. As observed in Figure 3A, mutation in the N-terminal SH3 domain resulted in a dramatic decrease in avidity for JNK1, suggesting that the interaction between CrkII and JNK1 may occur through the N-terminal SH3 domain of CrkII. It must be noted, however, that a mutation in the CrkII SH2 domain decreased slightly the avidity of JNK1 for CrkII, suggesting that either this domain directly, or proteins interacting with this domain, such as p130Cas, may contribute to the stabilization of the CrkII–JNK1 complex. Different proteins interacting with the CrkII N-terminal SH3 domain, such as C3G, DOCK180 or HPK1, have been identified (Feller et al., 1995; Matsuda et al., 1996; Kiyokawa et al., 1998a; Ling et al., 1999). These proteins share a PPxLPxK sequence motif (Feller et al., 1995; Knudsen et al., 1995; Matsuda et al., 1996). We noted that JNK1 also contains a very similar sequence in its C-terminal region (PPxIPxK), with an isoleucine replacing the conserved leucine residue. We mutated this sequence in JNK1, by replacing the lysine residue of this motif by an alanine (Figure 3B), since it has been shown that the replacement of the lysine in the PPxLPxK motif dramatically decreases its affinity for the CrkII SH3 domain (Knudsen et al., 1995). As shown in Figure 3C, the interaction between transfected CrkII and the mutated JNK1 was reduced >10-fold, thus confirming that the PPxIPxK sequence was indeed involved in the interaction with CrkII.
Fig. 3. JNK1 interacts with CrkII through CrkII N-terminal SH3 domain. (A) HeLa cells were transfected with Myc-CrkII, Myc-CrkΔSH2 or Myc-CrkΔ(N)SH3 expression vectors as indicated. Interaction between JNK1 and the different forms of CrkII was determined by Myc immunoblot on JNK1 immunoprecipitates. The numbers below the blot give the yield. (B) Schematic diagram showing the features of the JNK1 mutant (depicted as JNK1 Mut). The putative CrkII binding consensus sequence is underlined. (C) JNK Mut does not interact with CrkII. Expression vectors for HA-CrkII, Flag-JNK1 or Flag-JNK1 Mut were transfected into HeLa cells, and the CrkII–JNK1 interaction was assayed by immuno precipitation with anti-HA mouse monoclonal antibody (upper panel) followed by anti-Flag immunoblotting on the CrkII immuno precipitates. Total amounts of HA-CrkII or Flag-JNK1 proteins expressed are shown (lower panels).
Crk–JNK1 interaction is a critical step for CrkII-, EGF- or Rac1-induced JNK1 activation
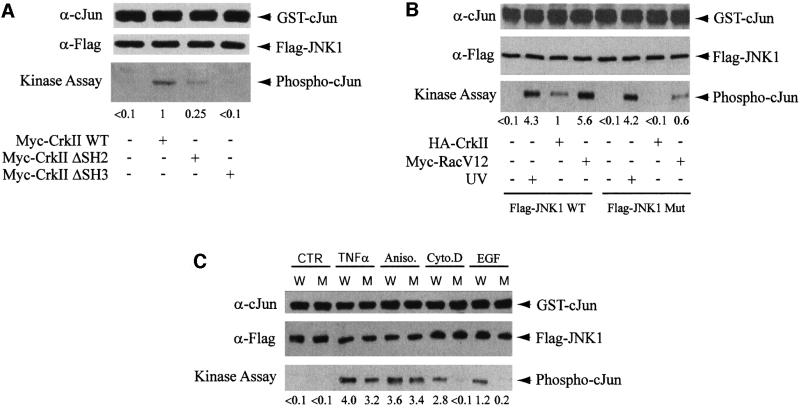
In order to investigate the role of the interaction between CrkII and JNK1 in JNK1 activation, we first compared the capacity of CrkII, CrkΔSH2 and CrkΔ(N)SH3 to activate JNK1. A kinase assay analysis was performed after transfection of JNK1 plus either form of CrkII expression vector. JNK1 activity was measured in JNK1 immunoprecipitates with glutathione S-transferase (GST)–c-Jun (1–79) as the substrate. The phosphorylation level of the substrate was assayed after gel fractionation and transfer to a membrane by immunodetection with anti-phospho-c-Jun antibody. We observed that CrkII overexpression was sufficient to activate JNK1, but this activation is reduced by ∼4-fold if CrkII is mutated in its SH2 domain, and by >10-fold if its N-terminal SH3 domain is mutated (Figure 4A). These results are consistent with previous data obtained using a different cellular model (Dolfi et al., 1998). This observation raises the possibility that the lack of JNK1 activation through CrkΔ(N)SH3 overexpression could be due to the inability of CrkΔ(N)SH3 to interact with JNK1. In order to test directly the latter possibility, we compared the kinase activities of JNK1 and JNK1 Mut, the mutant form of JNK1 that does not interact with CrkII, by kinase assay. The activation of the mutated JNK1 by CrkII or Rac1V12 was much weaker than the activation of its wild-type counterpart (Figure 4B). In contrast, the mutated JNK1 was still fully activated by UVC irradiation, to the same extent as wild-type JNK1 protein. These results clearly show that the interaction between CrkII and JNK1 is a critical step not only for CrkII-induced JNK1 activation, but also for Rac1-induced JNK1 activation. In addition, using a JNK kinase assay, we compared the activation profiles of transfected wild-type or mutant JNK by various agonists known to activate the JNK pathway (Figure 4C). We observed that the activation of the wild-type and mutant kinases by TNFα or anisomycin were rather comparable, suggesting that these agonists were able to activate JNK1 independently of the interaction between CrkII and JNK1. In contrast, activation of JNK1 by exposure to cytochalasin D or EGF was strongly inhibited when the CrkII–JNK1 interaction was prevented. Together, these results suggest that the interaction between JNK1 and CrkII is essential for CrkII- and Rac1-induced JNK1 activation. Moreover, these results are in agreement with previous reports demonstrating that Rac1 was necessary for EGF, but not for TNFα or anisomycin-induced JNK1 activation (Coso et al., 1995; Minden et al., 1995).
Fig. 4. The importance of the interaction between JNK1 and CrkII on JNK1 activation by CrkII, Rac1 and EGF. (A) Myc-CrkΔSH2 and Myc-CrkΔ(N)SH3 fail to activate JNK1. HeLa cells were transfected with Myc-CrkII, Myc-CrkΔSH2 or Myc-CrkΔ(N)SH3 expression vectors as indicated. Activation of JNK1 by the different forms of CrkII was determined by a quantitative JNK1 kinase assay. (B) JNK Mut activation by CrkII and Rac1 is impaired. HeLa cells were transfected with HA-CrkII and Flag-JNK1 WT or Flag-JNK1 Mut expression vectors plus Myc-RacV12, an expression vector for an activated form of Rac1. In two lanes, HeLa cells were irradiated with 80 J/m2 UVC for 15 min. JNK kinase assay was performed after anti-Flag immunoprecipitation of cellular extracts by incubating immunoprecipitated JNK1 in the presence of GST–c-Jun (1–79). A fraction of the immunoprecipitates was immunoblotted with anti-c-Jun and anti-Flag antibodies, and the rest was subjected to an in vitro kinase assay. JNK1 kinase activity was detected using mouse monoclonal anti-phospho-c-Jun antibody, and quantified. (C) Activation of JNK1 and JNK1 Mut by various agonists. HeLa cells were transfected with expression vectors for Flag-JNK1 (W) or Flag-JNK1 Mut (M), and either left untreated (CTR) or stimulated for 20 min with 100 ng/ml TNFα, 15 ng/ml anisomycin, 2 µg/ml cytochalasin D or 20 ng/ml EGF before protein extraction. JNK kinase assay was performed as in (B).
p130Cas–CrkII is a scaffolding module for JNK1 and its upstream kinases
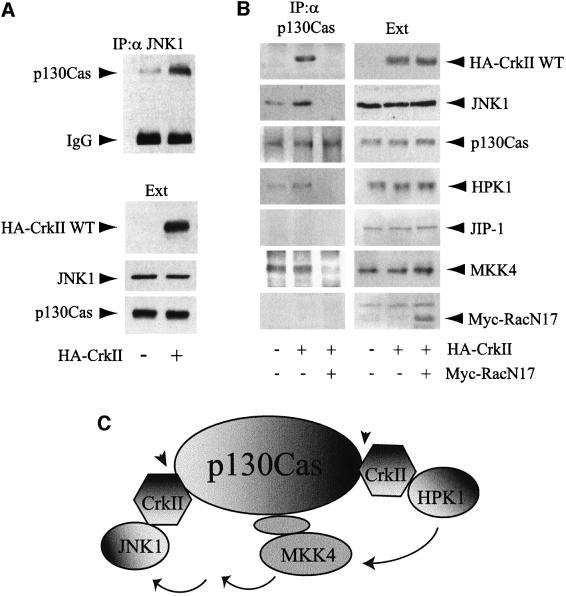
As CrkII is an adaptor protein that lacks any kinase domain, it is very likely that its interaction with JNK1 is not directly responsible for JNK1 activation by phosphorylation. Rather, it could recruit a fraction of cellular JNK1 to structures where it could then be activated by upstream kinases. As p130Cas, an adaptor protein containing several phosphorylatable tyrosines, is known to interact with the SH2 domain of CrkII, we tested whether CrkII could recruit JNK1 to a multiprotein complex containing p130Cas. Indeed, co-immunoprecipitation analysis revealed that CrkII strongly increased the amounts of p130Cas found in JNK1 immunoprecipitates (Figure 5A). This result was confirmed by the reciprocal immunoprecipitation of p130Cas (Figure 5B). Moreover, since p130Cas is known to contain multiple CrkII binding sites (Sakai et al., 1994), we analyzed whether CrkII overexpression could favor the recruitment of other signaling molecules to p130Cas. The presence of HPK1 in this module was assessed since HPK1 has been shown to interact with CrkII through its N-terminal SH3 domain and is able to activate JNK1 (Ling et al., 1999). As seen in Figure 5B, the amount of HPK1 in p130Cas immunoprecipitates was increased by CrkII overexpression. Interestingly, MKK4, which has been shown to lie upstream of JNK1 in the HPK1 signaling pathway (Ling et al., 1999), was also detected in p130Cas immunoprecipitates, but its interaction was not enhanced by CrkII overexpression, suggesting that the presence of MKK4 in the p130Cas–CrkII module was independent of CrkII. We were unable to detect either mixed lineage kinase 3 (MLK3) (data not shown) or JIP-1, the scaffolding protein specific for the MLK→MKK7→JNK pathway (Dickens et al., 1997; Whitmarsh et al., 1998), in the p130Cas immunoprecipitates even after CrkII overexpression. These data suggest that the p130Cas–CrkII module could favor the assembly of the postulated CrkII→HPK1→ MKK4→JNK1 signaling pathway (Ling et al., 1999).
Fig. 5. p130Cas–CrkII as a scaffolding interface for JNK1 that is regulated by Rac1. (A) CrkII overexpression increases the p130Cas–JNK1 interaction. HeLa cells were transfected with HA-CrkII as indicated. The concentrations of transfected HA-CrkII, endogenous p130Cas and JNK1 proteins in the extracts (Ext) were determined after immunoblotting with either anti-HA or polyclonal antibodies for p130Cas and JNK1. Interaction between endogenous forms of p130Cas and JNK1 was determined by immunoprecipitation using anti-JNK1 rabbit polyclonal antibody. The presence of p130Cas in the JNK1 immunoprecipitates was determined using an anti-p130Cas antibody. (B) p130Cas acts as a Rac1-dependent scaffold for JNK1. HeLa cells were either non-transfected or transfected with expression vectors for HA-CrkII or HA-CrkII+Rac1N17. The interaction between p130Cas and several proteins was assayed by immunoprecipitation using a rabbit polyclonal anti-p130Cas antibody followed by western blotting with the corresponding antibodies (left panels). Total amounts of proteins in the extracts (Ext) are shown (right panels). (C) Schematic diagram illustrating the putative role of p130Cas–CrkII as a scaffolding complex for the CrkII→HPK1→MKK4→JNK1 signaling pathway. Several CrkII molecules may interact simultanously with p130Cas since p130Cas is known to contain multiple CrkII binding motifs. Protein–protein interactions that we found to be regulated by Rac1 (see also Figure 2D) are indicated by arrowheads.
We next analyzed the role of Rac1 in the assembly of the p130Cas–CrkII scaffolding module. As shown in Figure 5B, overexpression of Rac1N17 abolished the interaction between p130Cas and CrkII, JNK1 or HPK1, and decreased the binding of MKK4. This result suggests that Rac1 activity is essential for the maintenance of the p130Cas–CrkII scaffolding module. In agreement with these findings, it has been shown that Rac1N17 blocks JNK activation by p130Cas–CrkII (Dolfi et al., 1998). A schematic model illustrating how p130Cas–CrkII could act as a scaffolding module for the specific signaling pathway involving HPK1, MKK4 and JNK1 is depicted in Figure 5C.
Discussion
The actin cytoskeleton has long been considered as a complex and static multiprotein structure. The discovery of cytoskeletal dynamics, such as the formation of actin stress fibers or membrane ruffling and its regulation by Rho GTPases, has led to the identification of links between signaling pathways and the cytoskeleton (Ridley et al., 1992; Coso et al., 1995; Minden et al., 1995; Nobes and Hall, 1995; Teramoto et al., 1996; Tapon and Hall, 1997; Hall, 1999; Kjoller and Hall, 1999). The same Rho GTPases were shown to activate the SAPKs (Coso et al., 1995; Minden et al., 1995; Teramoto et al., 1996; Hall, 1999). Concomitant with these studies, several groups have reported that CrkII overexpression, an SH2/SH3 adaptor protein localized to focal adhesion and membrane ruffles, activated the JNK pathway (Tanaka et al., 1997; Dolfi et al., 1998; Mochizuki et al., 2000). Moreover, v-Crk, an oncogenic protein originally identified in CT10 chicken tumor virus, has also been shown to activate JNK1 (Tanaka et al., 1997; Tanaka and Hanafusa, 1998) by at least two independent pathways, one involving Ras and a second dependent on Rac1 (Mochizuki et al., 2000).
In the present study we have attempted to unravel how CrkII activates the JNK pathway. CrkII is an adaptor protein known to interact with several proteins through either its SH2 domain or SH3 domains. We show here that CrkII can interact directly with JNK1. However, co-immunoprecipitation and immunofluorescence data suggest that only a fraction of CrkII interacts with JNK1 and, conversely, only a minor fraction of JNK1 can be found associated with CrkII in membrane ruffles. This observation suggests that different pools of JNK1 are physically separated in the cell in order to respond to various stimuli, thus allowing the kinase to interact with several different partners in distinct signaling pathways. Indeed, we could show that JNK1 activation by EGF, but not by UV irradiation, TNFα or anisomycin, was affected by preventing the interaction between CrkII and JNK1. Moreover, the CrkII binding domain found in JNK1 is not conserved in JNK2, and CrkII interacts only weakly with JNK2, suggesting that differential requirement for CrkII may contribute to define distinct stress signaling pathways involving JNK1 or JNK2. However, since a residual interaction was still observed between the mutant JNK1 and CrkII, we can not exclude the possibility that CrkII domains other than the N-terminal SH3 domain could form weaker interactions with JNK1 or JNK2.
The data presented here strongly support the hypothesis that the CrkII–JNK1 interaction is functionally linked to JNK1 activation by CrkII. (i) By using CrkΔ(N)SH3, a CrkII mutant protein harboring single mutations that alter the structure of the N-terminal SH3 domain, we showed that JNK1 interacts with CrkII through its N-terminal SH3 domain. We could demonstrate that while wild-type CrkII activates JNK1, CrkΔ(N)SH3 overexpression failed to do so. (ii) As mutation in the CrkII N-terminal SH3 domain could also affect other signaling pathways, we directly addressed the role of CrkII–JNK1 interaction in JNK1 activation by mutating a lysine residue in a proline-rich cluster of JNK1 that shares high homology with the CrkII N-terminal SH3 binding domain motif. Indeed, this lysine to alanine substitution strongly decreased both the CrkII–JNK1 interaction and CrkII-induced JNK activation. As we could also demonstrate that the activation of JNK1 by CrkII is concomitant with the appearance of N-terminally phosphorylated c-Jun in the nucleus (data not shown), it is very likely that CrkII regulates the transcription of genes dependent upon the activity of c-Jun. However, we can not exclude the possibility that JNK1 also phosphorylates cytoplasmic proteins.
Recent evidence argues for a role of the docking protein p130Cas in the regulation of CrkII function and the activation of JNK1 by CrkII (Dolfi et al., 1998; Zhu et al., 1998). The p130Cas–CrkII interaction has also been shown to regulate cell migration (Klemke et al., 1998). Crk proteins interact with tyrosine-phosphorylated p130Cas through their SH2 domain, and a number of putative CrkII binding sites have been characterized in p130Cas (Sakai et al., 1994). We found that CrkΔ(N)SH2, a CrkII mutant protein harboring a single mutation that alters the structure of its SH2 domain, fails to interact with p130Cas. This mutant displays a slightly decreased avidity for JNK1, yet fails to activate JNK1 as well as wild-type CrkII, as previously reported (Dolfi et al., 1998). This observation suggests that, in addition to the CrkII–JNK1 association, an interaction involving the CrkII SH2 is important for CrkII-dependent JNK1 activation. Indeed, we found that CrkII recruits JNK1 to p130Cas-containing complexes, providing a possible explanation for the previously described enhancement of CrkII-dependent activation of JNK1 by p130Cas (Dolfi et al., 1998). Previous work from Dolfi et al. suggests that overexpression of CasΔSD, a p130Cas mutant that fails to interact with CrkII, inhibits integrin-dependent but not EGF-dependent activation of JNK (Dolfi et al., 1998). Therefore, it is likely that the p130Cas–CrkII complex identified here plays a role in separating integrin-dependent and growth factor-dependent signaling pathways. We show here that preventing the interaction between CrkII and JNK1 blocks EGF-dependent activation of JNK1. As CrkII has been shown to interact directly with the cytoplasmic tail of the EGF receptor (Hashimoto et al., 1998), it is possible that the K340A JNK1 mutation affects the EGFR–CrkII–JNK1 pathway rather than the one involving p130Cas–CrkII. Moreover, we could demonstrate that p130Cas interacts with MKK4, one of the known upstream kinases of JNK1. Furthermore, CrkII overexpression also enhances the interaction between HPK1, which has been shown to activate JNK1 through a MKK4-dependent signaling pathway (Ling et al., 1999), and the p130Cas–CrkII complex. Our data suggest that the complex between p130Cas and several CrkII molecules can be considered as a scaffolding structure, allowing the specific activation of JNK1 through the HPK1→MKK4→ JNK1 signaling pathway. Such a signaling pathway has been proposed, and MEK kinase 1 (MEKK1) has been postulated to lie between HPK1 and MKK4 in this cascade (Ling et al., 1999). Hence, it will be of interest to test whether MEKK1 or a related kinase also interacts with the p130Cas–CrkII interface. In addition, further work is required to investigate whether the recruitment of these kinases to the p130Cas–CrkII complex is sufficient for their activation, or whether the recruitment of the kinases to a close vicinity only facilitates the linear propagation of signals originated upstream. Our data are in agreement with a recent report showing that JNK1 co-localizes with focal adhesion kinase (FAK) and p130Cas in focal contacts of cells plated on fibronectin (Almeida et al., 2000). The authors proposed that the FAK–p130Cas complex creates an adaptor platform linking the extracellular matrix to scaffolding proteins which recruits Ras, Rac1, Pak1, MKK4 and JNK1. This recruitment would then trigger the activation of a survival signaling pathway when serum is absent (Almeida et al., 2000). Our study clearly places CrkII as a major constituent of this platform. A well studied scaffolding molecule for JNK1 is JIP-1, which has been shown to interact with HPK1, MLK3, MKK7 and JNK1 (Dickens et al., 1997; Whitmarsh et al., 1998; Yasuda et al., 1999). Even though the JIP-1 and p130Cas–CrkII interfaces appear to share some functional similarities, they could physically separate two signaling pathways specific for JNK1, since, apart from HPK1, the kinases involved in these pathways are distinct.
Several lines of evidence link Rac1 function to the p130Cas–CrkII interface. First, Rac1, like CrkII and p130Cas, induces membrane ruffling by overexpression (Ridley et al., 1992). Moreover, p130Cas–CrkII-dependent induction of membrane ruffles or cell migration requires Rac1 (Dolfi et al., 1998; Klemke et al., 1998), suggesting that these molecules share common mechanisms to regulate the cytoskeletal network. Secondly, activation of JNK1 by CrkII or p130Cas–CrkII is blocked by Rac1N17 (Dolfi et al., 1998), a dominant-negative mutant form of Rac1, indicating that Rac1 activity is required to achieve full activation of JNK1 through p130Cas–CrkII. The characterization of a direct interaction between CrkII and JNK1 allowed us to investigate the role of Rac1 in CrkII-induced JNK activation through an original angle. We could demonstrate that CrkII overexpression is sufficient to recruit a fraction of JNK1 to membrane ruffles, where CrkII and p130Cas are also known to localize (Dolfi et al., 1998), through a Rac1-dependent mechanism. Importantly, our data strongly suggest that Rac1 participates in the establishment and/or maintenance of the p130Cas–CrkII scaffolding interface. Rac1N17 overexpression affects the network of protein– protein interactions at the level of p130Cas–CrkII. This may account for the previously shown inhibition by Rac1N17 of the p130Cas–CrkII-dependent JNK1 activation (Dolfi et al., 1998). Moreover, we showed that JNK1(K340A), a JNK1 protein mutated on the CrkII binding site, is partially refractory to activation by constitutively active Rac1. A likely explanation for this finding is that JNK1 activation through constitutively active Rac1 occurs predominantly at the level of the p130Cas–CrkII scaffolding interface, from which JNK1(K340A) would be excluded. Therefore, our results represent the first step towards understanding the requirement for Rac1 in p130Cas–CrkII-dependent signaling pathways.
In conclusion, our results argue for a role of the p130Cas–CrkII interface as a scaffolding complex regulated by Rac1, which is involved in a specific signaling pathway leading to JNK1 activation. These findings suggest that different routes leading to JNK activation may be physically separated by different scaffolding proteins, such as JIP-1 or the p130Cas–CrkII complex. The sequestration of kinase cascades could, in turn, allow cells to modulate their responses to various stimuli.
Materials and methods
Cell culture
HeLa cells were grown at 37°C in 7% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 7% fetal calf serum (FCS). When indicated, HeLa cells were rinsed twice in phosphate-buffered saline (PBS), and then irradiated with UVC (80 J/m2) in a minimal volume of PBS before culture medium was re-added.
Expression vectors, transfection and reporter assays
pcDNA3 mammalian expression vector containing HA-CrkII cDNA, and pcDNA3 containing Flag-JNK1 or Flag-JNK2 cDNAs, were kindly provided by Dr M.Anafi and Dr R.Davis, respectively. PEXV-Myc-RacV12 and pEXV-Myc-RacN17 expression vectors were kindly provided by Dr A.Hall. PCAGGS mammalian expression vectors containing either Myc-CrkII, Myc-CrkII R38V (CrkIIΔSH2) or Myc-CrkII W169L [CrkIIΔ(N)SH3] cDNAs were kindly provided by Dr M.Matsuda.
HeLa cells were transfected by the calcium phosphate method, using the empty pcDNA3 vector as a control. After overnight transfection, cells were rinsed twice in PBS before addition of fresh medium, and collected after 40 h.
Antibodies
The preparation of mouse monoclonal antibody to phosphorylated c-Jun has been described previously (Lallemand et al., 1998). In brief, phospho-specific mouse monoclonal c-Jun antibody was raised against a peptide corresponding to mouse c-Jun amino acids 57–68 with a phosphorylated serine at position 63.
Rabbit polyclonal antibodies against HA epitope, JNK1, HPK1, MKK4, JIP-1 and p130Cas were from Santa Cruz Biotechnology. Mouse monoclonal antibody against c-Myc (Ab-1) was from Calbiochem. M2 mouse monoclonal antibody against Flag epitope was from Sigma. Mouse monoclonal antibody against CrkII was from Transduction Laboratories.
Western blotting, immunoprecipitation and transfections
Cell extracts were prepared by rinsing cultures grown on Petri dishes with cold PBS. Cells were harvested with a rubber policeman and centrifuged. The supernatant was removed and immunoprecipitation buffer (20 mM Tris pH 7.4, 150 mM NaCl, 1 mM MgCl2, 10% glycerol, 1% NP-40, 0.5 µg/ml each of leupeptin, aprotinin and pepstatin A, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride; all reagents from Sigma) was then added directly to the pellet. Homogenization was obtained by pipetting up and down before centrifugation at 14 000 g for 10 min. Protein concentration was determined using the Bradford assay. Cell extracts were used at this step for whole-cell extract immunoblotting. For immunoprecipitation, 100 µg of proteins were pre-cleared for 30 min with immunoprecipitation buffer-equilibrated protein A–Sepharose (Amersham) or protein G–agarose (Pierce) beads, depending on the antibody used. After centrifugation, the extract was incubated at 4°C for 2 h with the appropriate antibodies, followed by 1 h incubation in the presence of the beads. Beads were then washed extensively with immunoprecipitation buffer and eluted with Laemmli buffer.
Immunoblotting was carried out according to standard procedures. Proteins were separated by SDS–PAGE and transferred to nitrocellulose. The membrane was then blocked with PBS/0.1% Tween-20/10% FCS and incubated with the various antibodies. Enhanced chemiluminescence (ECL or ECLPlus) reagents (Amersham) were used for detection.
Immunofluorescence
HeLa cells were plated on glass coverslips and grown for 48 h prior to staining, allowing transfection when needed. Cells were then rinsed twice in PBS before fixation in 2% paraformaldehyde for 25 min. They were then permeabilized with 0.1% Triton X-100 in PBS for 25 min. After washing in PBS supplemented with 0.05% Tween-20 (PBST), cells were incubated for 1 h at room temperature with the appropriate antibodies in PBST supplemented with 10% FCS. Coverslips were then rinsed twice with PBST, and incubated for 1 h at room temperature with the secondary antibodies (fluorescein-coupled sheep anti-mouse IgG or Texas red-coupled donkey anti-rabbit IgG; Amersham) in PBST plus 10% FCS. After one rinse in PBST containing 4′,6-diamidino-2-phenylindole (DAPI) and one rinse in distilled water, the coverslips were mounted with an anti-bleaching glycerol mixture (Citifluor Ltd). Samples were viewed using a Zeiss Axiophot epifluorescence microscope with a 100× immersion oil objective. The images, given by the Hamamatsu C4880 cooled CCD camera connected to the Serie 150/151 hardware from Imaging Technology Inc., were acquired by the Khoros software package from Khoral Research Inc. In order to correct an uneven illumination from the mercury lamp, a shading correction was applied to the images.
JNK kinase assay
JNK1 activity was measured by immunocomplex kinase assay. HeLa cells transfected with Flag-JNK1 expression vector were lysed, and 250 µg of total proteins were analyzed for exogenous JNK1 activity by Flag immunoprecipitation of the cell lysates using protein G–agarose beads, followed (after extensive washing of the beads with immunoprecipitation buffer) by incubation with GST–c-Jun (amino acids 1–79) as a substrate in a kinase buffer (25 mM Tris pH 7.5, 5 mM glycerol phosphate, 2 mM dithiothreitol, 0.1 mM sodium vanadate, 10 mM MgCl2). The kinase reactions were carried out in the presence of 100 µM ATP at 30°C for 30 min. c-Jun phosphorylation was selectively detected by western immunoblotting using a specific c-Jun antibody recognizing phosphorylation of c-Jun at serine 63 before chemiluminescent detection (ECLPlus; Amersham). In parallel with the kinase assays, equal aliquots of the cell lysates were analyzed to determine the expression levels of the transfected proteins.
Acknowledgments
Acknowledgements
We are grateful to D.Lallemand for the preparation of the Phospho63-c-Jun monoclonal antibody. We thank R.Davis for providing the expression vectors for Flag-JNK1 and Flag-JNK2, A.Hall for the expression vectors for the mutant forms of Rac1, and M.Anafi for the expression vectors encoding HA-CrkII proteins. We also thank S.Garbay for help with the image acquisition system used for immunofluorescence. We are grateful to D.J.Philpott and J.Weitzman for critical reading of the manuscript. This work was supported by grants from the Association pour la Recherche contre le Cancer (ARC), the Ligue National contre le Cancer-Ile de France, the EEC Biomed Training Mobility Programs and fellowship by the Ministry of Education and Research (MESR) and the Pasteur-Weizmann Foundation to S.E.G.
References
- Almeida E.A., Ilic,D., Han,Q., Hauck,C.R., Jin,F., Kawakatsu,H., Schlaepfer,D.D. and Damsky,C.H. (2000) Matrix survival signaling. From fibronectin via focal adhesion kinase to c-jun NH2-terminal kinase. J. Cell Biol., 149, 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb M.H. and Goldsmith,E.J. (1995) How MAP kinases are regulated. J. Biol. Chem., 270, 14843–14846. [DOI] [PubMed] [Google Scholar]
- Coso O.A., Chiariello,M., Yu,J.C., Teramoto,H., Crespo,P., Xu,N., Miki,T. and Gutkind,J.S. (1995) The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell, 81, 1137–1146. [DOI] [PubMed] [Google Scholar]
- Davis R.J. (1999) Signal transduction by the c-Jun N-terminal kinase. Biochem. Soc. Symp., 64, 1–12. [DOI] [PubMed] [Google Scholar]
- Dickens M., Rogers,J.S., Cavanagh,J., Raitano,A., Xia,Z., Halpern,J.R., Greenberg,M.E., Sawyers,C.L. and Davis,R.J. (1997) A cytoplasmic inhibitor of the JNK signal transduction pathway. Science, 277, 693–696. [DOI] [PubMed] [Google Scholar]
- Dolfi F., Garcia-Guzman,M., Ojaniemi,M., Nakamura,H., Matsuda,M. and Vuori,K. (1998) The adaptor protein Crk connects multiple cellular stimuli to the JNK signaling pathway. Proc. Natl Acad. Sci. USA, 95, 15394–15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller S.M., Ren,R., Hanafusa,H. and Baltimore,D. (1994) SH2 and SH3 domains as molecular adhesives: the interactions of Crk and Abl. Trends Biochem. Sci., 19, 453–458. [DOI] [PubMed] [Google Scholar]
- Feller S.M., Knudsen,B. and Hanafusa,H. (1995) Cellular proteins binding to the first Src homology 3 (SH3) domain of the proto-oncogene product c-Crk indicate Crk-specific signaling pathways. Oncogene, 10, 1465–1473. [PubMed] [Google Scholar]
- Feller S.M., Posern,G., Voss,J., Kardinal,C., Sakkab,D., Zheng,J. and Knudsen,B.S. (1998) Physiological signals and oncogenesis mediated through Crk family adapter proteins. J. Cell Physiol., 177, 535–552. [DOI] [PubMed] [Google Scholar]
- Garrington T.P. and Johnson,G.L. (1999) Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr. Opin. Cell Biol., 11, 211–218. [DOI] [PubMed] [Google Scholar]
- Gupta S., Barrett,T., Whitmarsh,A.J., Cavanagh,J., Sluss,H.K., Derijard,B. and Davis,R.J. (1996) Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J., 15, 2760–2770. [PMC free article] [PubMed] [Google Scholar]
- Hall A. (1999) Signal transduction pathways regulated by the Rho family of small GTPases. Br. J. Cancer, 80, 25–27. [PubMed] [Google Scholar]
- Hasegawa H., Kiyokawa,E., Tanaka,S., Nagashima,K., Gotoh,N., Shibuya,M., Kurata,T. and Matsuda,M. (1996) DOCK180, a major CRK-binding protein, alters cell morphology upon translocation to the cell membrane. Mol. Cell. Biol., 16, 1770–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y., Katayama,H., Kiyokawa,E., Ota,S., Kurata,T., Gotoh,N., Otsuka,N., Shibata,M. and Matsuda,M. (1998) Phosphorylation of CrkII adaptor protein at tyrosine 221 by epidermal growth factor receptor. J. Biol. Chem., 273, 17186–17191. [DOI] [PubMed] [Google Scholar]
- Ip Y.T. and Davis,R.J. (1998) Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr. Opin. Cell Biol., 10, 205–219. [DOI] [PubMed] [Google Scholar]
- Ishiki M., Sasaoka,T., Ishihara,H., Imamura,T., Usui,I., Takata,Y. and Kobayashi,M. (1997) Evidence for functional roles of Crk-II in insulin and epidermal growth factor signaling in Rat-1 fibroblasts overexpressing insulin receptors. Endocrinology, 138, 4950–4958. [DOI] [PubMed] [Google Scholar]
- Karin M. (1995) The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem., 270, 16483–16486. [DOI] [PubMed] [Google Scholar]
- Kiyokawa E., Mochizuki,N., Kurata,T. and Matsuda,M. (1997) Role of Crk oncogene product in physiologic signaling. Crit. Rev. Oncog., 8, 329–342. [DOI] [PubMed] [Google Scholar]
- Kiyokawa E., Hashimoto,Y., Kobayashi,S., Sugimura,H., Kurata,T. and Matsuda,M. (1998a) Activation of Rac1 by a Crk SH3-binding protein, DOCK180. Genes Dev., 12, 3331–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyokawa E., Hashimoto,Y., Kurata,T., Sugimura,H. and Matsuda,M. (1998b) Evidence that DOCK180 up-regulates signals from the CrkII–p130Cas complex. J. Biol. Chem., 273, 24479–24484. [DOI] [PubMed] [Google Scholar]
- Kjoller L. and Hall,A. (1999) Signaling to Rho GTPases. Exp. Cell Res., 253, 166–179. [DOI] [PubMed] [Google Scholar]
- Klemke R.L., Leng,J., Molander,R., Brooks,P.C., Vuori,K. and Cheresh,D.A. (1998) CAS/Crk coupling serves as a ‘molecular switch’ for induction of cell migration. J. Cell Biol., 140, 961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen B.S., Feller,S.M. and Hanafusa,H. (1994) Four proline-rich sequences of the guanine-nucleotide exchange factor C3G bind with unique specificity to the first Src homology 3 domain of Crk. J. Biol. Chem., 269, 32781–32787. [PubMed] [Google Scholar]
- Knudsen B.S., Zheng,J., Feller,S.M., Mayer,J.P., Burrell,S.K., Cowburn,D. and Hanafusa,H. (1995) Affinity and specificity requirements for the first Src homology 3 domain of the Crk proteins. EMBO J., 14, 2191–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemand D., Ham,J., Garbay,S., Bakiri,L., Traincard,F., Jeannequin,O., Pfarr,C.M. and Yaniv,M. (1998) Stress-activated protein kinases are negatively regulated by cell density. EMBO J., 17, 5615–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis T.S., Shapiro,P.S. and Ahn,N.G. (1998) Signal transduction through MAP kinase cascades. Adv. Cancer Res., 74, 49–139. [DOI] [PubMed] [Google Scholar]
- Ling P., Yao,Z., Meyer,C.F., Wang,X.S., Oehrl,W., Feller,S.M. and Tan,T.H. (1999) Interaction of hematopoietic progenitor kinase 1 with adapter proteins Crk and CrkL leads to synergistic activation of c-Jun N-terminal kinase. Mol. Cell. Biol., 19, 1359–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C.J. (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell, 80, 179–185. [DOI] [PubMed] [Google Scholar]
- Matsuda M. and Kurata,T. (1996) Emerging components of the Crk oncogene product: the first identified adaptor protein. Cell Signal, 8, 335–340. [DOI] [PubMed] [Google Scholar]
- Matsuda M., Tanaka,S., Nagata,S., Kojima,A., Kurata,T. and Shibuya,M. (1992) Two species of human CRK cDNA encode proteins with distinct biological activities. Mol. Cell. Biol., 12, 3482–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Ota,S., Tanimura,R., Nakamura,H., Matuoka,K., Takenawa,T., Nagashima,K. and Kurata,T. (1996) Interaction between the amino-terminal SH3 domain of CRK and its natural target proteins. J. Biol. Chem., 271, 14468–14472. [DOI] [PubMed] [Google Scholar]
- Minden A., Lin,A., Claret,F.X., Abo,A. and Karin,M. (1995) Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell, 81, 1147–1157. [DOI] [PubMed] [Google Scholar]
- Mochizuki N., Ohba,Y., Kobayashi,S., Otsuka,N., Graybiel,A.M., Tanaka,S. and Matsuda,M. (2000) Crk activation of JNK via C3G and R-Ras. J. Biol. Chem., 275, 12667–12671. [DOI] [PubMed] [Google Scholar]
- Nakashima N., Rose,D.W., Xiao,S., Egawa,K., Martin,S.S., Haruta,T., Saltiel,A.R. and Olefsky,J.M. (1999) The functional role of CrkII in actin cytoskeleton organization and mitogenesis. J. Biol. Chem., 274, 3001–3008. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1995) Rho, rac and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell, 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Paterson,H.F., Johnston,C.L., Diekmann,D. and Hall,A. (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell, 70, 401–410. [DOI] [PubMed] [Google Scholar]
- Robinson M.J. and Cobb,M.H. (1997) Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol., 9, 180–186. [DOI] [PubMed] [Google Scholar]
- Sakai R., Iwamatsu,A., Hirano,N., Ogawa,S., Tanaka,T., Mano,H., Yazaki,Y. and Hirai,H. (1994) A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J., 13, 3748–3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer H.J. and Weber,M.J. (1999) Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol., 19, 2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S. and Hanafusa,H. (1998) Guanine-nucleotide exchange protein C3G activates JNK1 by a ras-independent mechanism. JNK1 activation inhibited by kinase negative forms of MLK3 and DLK mixed lineage kinases. J. Biol. Chem., 273, 1281–1284. [DOI] [PubMed] [Google Scholar]
- Tanaka S., Ouchi,T. and Hanafusa,H. (1997) Downstream of Crk adaptor signaling pathway: activation of Jun kinase by v-Crk through the guanine nucleotide exchange protein C3G. Proc. Natl Acad. Sci. USA, 94, 2356–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N. and Hall,A. (1997) Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr. Opin. Cell Biol., 9, 86–92. [DOI] [PubMed] [Google Scholar]
- Teramoto H., Coso,O.A., Miyata,H., Igishi,T., Miki,T. and Gutkind,J.S. (1996) Signaling from the small GTP-binding proteins Rac1 and Cdc42 to the c-Jun N-terminal kinase/stress-activated protein kinase pathway. A role for mixed lineage kinase 3/protein-tyrosine kinase 1, a novel member of the mixed lineage kinase family. J. Biol. Chem., 271, 27225–27228. [DOI] [PubMed] [Google Scholar]
- Whitmarsh A.J., Cavanagh,J., Tournier,C., Yasuda,J. and Davis,R.J. (1998) A mammalian scaffold complex that selectively mediates MAP kinase activation. Science, 281, 1671–1674. [DOI] [PubMed] [Google Scholar]
- Yasuda J., Whitmarsh,A.J., Cavanagh,J., Sharma,M. and Davis,R.J. (1999) The JIP group of mitogen-activated protein kinase scaffold proteins. Mol. Cell. Biol., 19, 7245–7254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Goh,E.L., LeRoith,D. and Lobie,P.E. (1998) Growth hormone stimulates the formation of a multiprotein signaling complex involving p130Cas and CrkII. Resultant activation of c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK). J. Biol. Chem., 273, 33864–33865. [DOI] [PubMed] [Google Scholar]