Abstract
Background
C-X-C motif chemokine ligand 10 (CXCL10) is implicated in HIV-associated neuroinflammation, yet its association with HIV-associated neurocognitive disorders (HAND) remains unclear. We conducted a meta-analysis to evaluate cerebrospinal fluid (CSF) CXCL10 differences between people living with HIV (PLWH) with and without HAND and to assess the odds of HAND in relation to high versus low CSF CXCL10 levels.
Methods
PubMed, Embase, and Web of Science were systematically searched. Observational studies reporting CSF CXCL10 levels in adult PLWH with and without HAND were included. Standardized mean difference (SMD) and odds ratio (OR) were pooled using random-effects models accounting for the potential influence of heterogeneity.
Results
Eleven studies involving 1,536 PLWH were included. Compared to those without HAND, PLWH with HAND had significantly higher CSF CXCL10 levels (SMD: 0.56, 95% CI: 0.17–0.96; p < 0.001), with high heterogeneity (I² = 86% and τ² = 0.31). The 95% prediction interval (PI: − 0.78 to 1.91) indicated substantial between-study variability. Subgroup analyses showed significantly greater CXCL10 elevation in studies with ≤ 50% ART coverage (SMD: 0.90 vs. 0.17; p = 0.04) and in those evaluating HIV-associated dementia (SMD: 1.45 vs. 0.37; p < 0.001). Meta-regression did not identify any statistically significant moderators, although ART proportion and CD4 + count explained 29.2% and 25.6% of the variance, respectively. No significant association was found between high CSF CXCL10 and HAND (OR: 1.41, 95% CI: 0.90–2.22; p = 0.13).
Conclusion
Elevated CSF CXCL10 may be associated with HAND in PLWH, particularly among ART-naïve individuals and in more severe cognitive impairment. However, substantial heterogeneity and a wide PI suggest that the strength of this association varies across populations. CSF CXCL10 remains a promising but not definitive biomarker for HAND risk stratification.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12981-025-00804-x.
Keywords: CXCL10, Cerebrospinal fluid, Human immunodeficiency virus, HIV-associated neurocognitive disorders, Meta-analysis
Introduction
HIV-associated neurocognitive disorders (HAND) remain a prevalent and clinically significant complication among people living with HIV (PLWH), even in the era of combination antiretroviral therapy (ART) [1–3]. Despite viral suppression, the prevalence of HAND is estimated to range between 30% and 50% in PLWH, with manifestations spanning from asymptomatic neurocognitive impairment to HIV-associated dementia (HAD) [4, 5]. HAND is associated with reduced quality of life, impaired daily functioning, poor ART adherence, and increased mortality [6, 7]. Multiple risk factors contribute to the development of HAND, including older age, low nadir CD4 + cell count, persistent immune activation, comorbid conditions, and poor CNS penetration of ART regimens [8–10]. Given the multifactorial nature of HAND and its often insidious onset, there is a pressing need for reliable biomarkers that can aid in early detection, risk stratification, and monitoring of disease progression [11].
Among various candidates, chemokines have attracted growing interest due to their role in neuroinflammation and immune cell trafficking within the central nervous system (CNS) [12]. CXCL10, also known as interferon gamma-induced protein 10 (IP-10), is a pro-inflammatory chemokine secreted by astrocytes, microglia, and infiltrating immune cells in response to type I and II interferons [13, 14]. In the CNS, CXCL10 plays a key role in mediating the recruitment of activated T cells across the blood-brain barrier and promoting neuroinflammatory cascades [15, 16]. Elevated levels of CXCL10 in the cerebrospinal fluid (CSF) have been observed in various neuroinflammatory and neurodegenerative diseases, including HAND [16–20]. Mechanistically, sustained CXCL10 elevation may contribute to neuronal dysfunction through excitotoxicity, disruption of synaptic signaling, and glial activation [20, 21]. Although several studies have investigated the association between CSF CXCL10 levels and HAND, their findings remain inconsistent due to variations in patient populations, diagnostic criteria, and assay methods [22, 23]. Therefore, we conducted a systematic review and meta-analysis to quantitatively evaluate the difference in CSF CXCL10 levels between PLWH with and without HAND, and to determine whether elevated CSF CXCL10 is associated with an increased likelihood of HAND.
Methods
This study followed the PRISMA 2020 [24, 25] and Cochrane Handbook guidelines [26] for conducting systematic reviews and meta-analyses, covering study design, data collection, statistical methods, and interpretation of results. The protocol was also registered in PROSPERO under the ID CRD420251074170.
Database search
To identify studies pertinent to this meta-analysis, we searched PubMed, Embase, and Web of Science databases using an extensive array of search terms, which involved the combined terms of (1) CXCL10” OR “CXCL-10” OR “CXCL 10” OR “interferon-inducible protein 10” OR “small inducible cytokine B10” OR “IFN-gamma-inducible protein 10” OR “interferon gamma-induced protein 10”; (2) “human immunodeficiency virus” OR “human immune deficiency virus” OR “human immune-deficiency virus” OR “HIV” OR “acquired immune-deficiency syndrome” OR “acquired immune deficiency syndrome” OR “acquired immunodeficiency syndrome” OR “acquired immuno-deficiency syndrome” OR “AIDS”; and (3) “cognition” OR “cognitive” OR “neurocognitive” OR “neuropsychological” OR “HIV-associated neuro cognitive disorders”. The search was restricted to studies on human subjects and included only full-length articles published in English in peer-reviewed journals. We also manually checked the references of related original and review articles to find additional relevant studies. The search covered all records from database inception up to April 23, 2025. The full search strategy for each database is shown in Supplemental File 1.
Study eligible criteria
We applied the PICOS framework to define the inclusion criteria:
P (patients): Adult (≥ 18 years old) PLWH, regardless of ART status.
I (exposure): High CSF levels of CXCL10 or reported CSF CXCL10 levels (as continuous or categorical variables).
C (comparison): For continuous outcomes: PLWH with HAND versus those without HAND. For dichotomous outcomes: PLWH with low CSF CXCL10 levels versus those with high CSF CXCL10 levels.
O (outcome): The primary outcome was the differences in CSF CXCL10 levels between PLWH with and without HAND, and the secondary outcome was the odds ratios (ORs) with 95% confidence intervals (CIs) for the presence of HAND in relation to high versus low CSF CXCL10 levels.
S (study design): Observational studies (cross-sectional, case-control, or cohort studies), or post-hoc analyses of clinical trials.
We excluded reviews, editorials, other meta-analyses, preclinical studies, studies not in PLWH, studies that involving pediatric patients, studies that did not assess CXCL10 in CSF, did not assess the prevalence of HAND, or those that did not report the data of interest. If studies had overlapping populations, we included the one with the largest sample size in the meta-analysis.
Study quality evaluation
Two authors independently performed the literature search, study selection, quality assessment, and data extraction. Disagreements were resolved by discussion with the corresponding author. Study quality was assessed using the Newcastle–Ottawa Scale (NOS) [27], which rates selection, control of confounders, and outcome evaluation. Scores range from 1 to 9, with scores of 7 or higher considered good quality.
Data collection
The data collected for analysis included the study details (author, year, location, and design), participant characteristics (diagnosis, number of patients included in each study, mean age, sex distribution, ART status, and blood CD4 + cell count at CSF CXCL10 measurement), CXCL10 assay, methods for the diagnosis of HAND, and number of participants with HAND, and covariates matched or adjusted in when the association between CXCL10 in CSF and HAND was analyzed.
Statistical analysis
The difference between CXCL10 levels in CSF between PLWH with and without HAND was summarized as standardized mean difference (SMD) with 95% CI because various methods were used for measuring CXCL10 [26]. The association between CSF CXCL10 and the presence of HAND in PLWH, compared between LWH with a high versus a low category of CXCL10, was summarized as OR and corresponding 95% CI [26]. ORs and standard errors were directly extracted or calculated from 95% CIs or p values, then log-transformed to stabilize variance and normalize the data [26]. If multiple ORs were reported from different models, we used the one with the most complete adjustment. Heterogeneity was assessed using the Cochrane Q test and I² statistic [28], with a p value < 0.10 suggesting significant heterogeneity and I² values of < 25%, 25–75%, and > 75% indicating low, moderate, and high heterogeneity. τ² (the variance of true effect sizes), and τ (its square root, representing the standard deviation of true effects) were also reported [26]. In addition, 95% prediction intervals (PI) were calculated to estimate the expected range of true effects in future studies [29]. A random-effects model was used to pool the data, accounting for heterogeneity between studies [26]. Sensitivity analyses were performed by removing one study at a time, as well as by including only studies of high quality (NOS ≥ 7). For the primary outcome, predefined subgroup analyses were conducted based on mean ages of the patients, proportion of men, proportion of subjects on ART, blood CD4 + cell count, definition of HAND (HAD only or overall HAND), and methods for the diagnosis of HAND. Medians of continuous variables were used to divide subgroups evenly. Univariate meta-regression analysis was performed to investigate if the association between CSF CXCL10 and HAND was significantly affected by study characteristics in continuous variables, such as sample size, mean age, proportion of men, proportion of subjects on ART, blood CD4 + cell count, or NOS scores [26]. For outcomes involving at least 10 datasets, publication bias was assessed using funnel plots and visual inspection for asymmetry, along with Egger’s test [30]. All analyses were performed using RevMan (Version 5.1; Cochrane Collaboration, Oxford, UK) and Stata (Version 12.0; Stata Corporation, College Station, TX, USA).
Results
Study inclusion
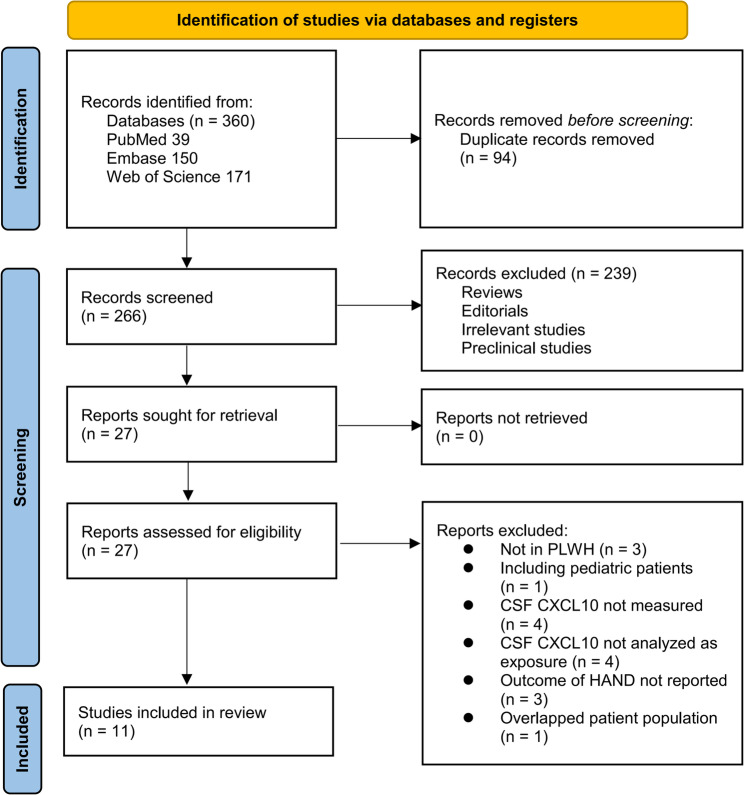
The study selection process is shown in Fig. 1. We first identified 360 records from the three databases. After removing 94 duplicates, 266 articles were screened by title and abstract. Of these, 239 were excluded for not meeting the aims of the meta-analysis. The full texts of the remaining 27 articles were reviewed by two independent authors, and 16 were excluded for various reasons (see Fig. 1). In the end, 11 studies were included in the quantitative analysis [32–46].
Fig. 1.
Flowchart of database search and study inclusion
Summary of study characteristics
Table 1 summarizes the characteristics of the 11 studies included in this meta-analysis [32–46]. One study by Yuan et al. (2013) [32] provided two independent datasets based on ART status—treated and untreated groups—which were included separately in the analysis, resulting in a total of 12 datasets. All studies employed a cross-sectional design and were conducted between 2012 and 2025 across multiple countries, including the United States, China, Brazil, and Romania. Sample sizes ranged from 14 to 405 participants, and a total of 1,536 adult PLWH were included in the meta-analysis, with mean ages spanning from 23.7 to 53.0 years. The proportion of male participants varied from 49.3% to 97.0%. ART coverage among participants ranged widely, from 0% to 100%, and the mean blood CD4 + cells at the time of CSF CXCL10 measuring was 51.9 to 505.0 per µL. CSF CXCL10 was measured using different immunoassay platforms, including Luminex xMAP [32, 35–39, 41], ELISA [33], MesoScale Discovery [37, 39], Bioplex array [31], multiplex bead-based assays [38], and Olink Explore [40]. HAND was diagnosed using established criteria: 3 datasets used the Frascati criteria [35, 36, 39], four datasets used global deficit score (GDS) ≥ 0.5 [33, 37, 38, 41], 3 datasets used the Memorial Sloan Kettering (MSK) scale (score ≥ 0.5) [32, 34], and 2 studies used clinical diagnosis of HIV-associated dementia (HAD) [31, 40]. A total of 665 (43.3%) of the 1,536 participants had HAND. Ten datasets from nine studies [32–39, 43–45] reported the difference in CSF CXCL10 levels between HAND and non-HAND groups, while three studies [35, 37, 41] reported ORs for HAND between high and low CXCL10 level groups. The cutoffs for determining a high CSF CXCL10 levels were defined by the tertile of CXCL10 in one study [35], and by medians in the other two studies [37, 41]. All studies controlled age and sex when the association between CSF CXCL10 and HAND was analyzed. Other key covariates including CD4 + cell count, HIV viral load, and ART status were adjusted or matched to a varying degree among these studies. Study quality was assessed using the NOS (Table 2), with total scores ranging from 6 to 9, indicating moderate to high methodological quality.
Table 1.
Characteristics of the included studies
| Study | Country | Design | Participant characteristics | Sample size | Mean age (years) | Men (%) | On ART (%) | CD4 + cell count (per µL) | CXCL10 assay | Diagnosis of HAND | No. of participants with HAND | Outcomes reported | Variables matched or adjusted |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mehla 2012 | USA | CS | Adults with HIV-1 infection | 14 | 40 | 92.8 | NR | NR | Bioplex array | Clinically diagnosed HAD | 7 | Difference of CXCL10 in CSF | Age, sex, and ethnicity |
| Yuan 2013 treated | China | CS | Adults with HIV-1 infection (clade B/B’) | 46 | 37.6 | 60.9 | 100 | 123 | Luminex xMAP | MSK ≥ 0.5 (mild to severe cognitive impairment | 25 | Difference of CXCL10 in CSF | Age, sex, HIV RNA level, and CD4 + cell counts |
| Yuan 2013 untreated | China | CS | Adults with HIV-1 infection (clade B/B’) | 61 | 39.3 | 72.1 | 0 | 63.9 | Luminex xMAP | MSK ≥ 0.5 (mild to severe cognitive impairment | 39 | Difference of CXCL10 in CSF | Age, sex, HIV RNA level, and CD4 + cell counts |
| Yuan 2015 | China | CS | Adults with HIV-1 infection | 85 | 38 | 69.4 | 43.5 | 51.9 | Luminex xMAP | MSK ≥ 0.5 (mild to severe cognitive impairment | 52 | Difference of CXCL10 in CSF | Age, sex, HIV RNA level, CD4 + cell counts, HAART treatment and duration |
| Schrier 2015 | USA | CS | Adults with HIV initiating or changing ART, undergoing neurocognitive evaluation | 30 | 48 | 97 | 100 | 406 | ELISA | GDS ≥ 0.5 | 10 | Difference of CXCL10 in CSF | Age, sex, CD4/CD8 ratio, myeloid activation (sCD163), and ART status |
| Kallianpur 2019 | USA | CS | HIV-infected adults | 405 | 43 | 81 | 73 | 177 | Luminex xMAP | Frascati criteria | 176 | Difference of CXCL10 in CSF and OR for HAND (T3:T1) | Age, sex, Nadir CD4, ART status, genetic ancestry (PC1/PC2), comorbidity severity |
| Ozturk 2019 | USA | CS | Adults with HIV infection | 41 | 39.8 | 81 | 0 | NR | Luminex xMAP | Frascati criteria | 14 | Difference of CXCL10 in CSF | Age, sex, race, CSF HIV load |
| Burlacu 2020 | Romania | CS | Young adults with chronic HIV infection | 144 | 23.7 | 49.3 | 91.7 | 479 | MesoScale Discovery Multi-spot Assay | GDS ≥ 0.5 | 52 | OR for HAND (median) | Age, sex, plasma viral load |
| De Almeida 2022 | Brazil | CS | Adults with HIV free of CNS opportunistic infections | 60 | 43 | 49 | 80.9 | 369 | Multiplex bead suspension array immunoassays | GDS ≥ 0.5 | 37 | Difference of CXCL10 in CSF | Age, sex, plasma HIV viral load suppression, nadir CD4 count |
| Guha 2023 | USA | CS | Adults with HIV on suppressive ART | 143 | 53 | 85 | 100 | 505 | Meso Scale Discovery platform | Frascati criteria | 74 | Difference of CXCL10 in CSF | Age, sex, and race |
| Hu 2024 | USA | CS | Adults with chronic HIV-1 infection | 144 | 43.4 | 82.8 | 0 | NR | Olink Explore 1536 platform | Clinically diagnosed HAD | 29 | Difference of CXCL10 in CSF | Age and sex |
| Diaz 2025 | USA | CS | Adults with HIV | 363 | 42.5 | 88 | 64 | 484 | Luminex FLEXMAP 3D (Millipore) | GDS ≥ 0.5 | 150 | OR for HAND (median) | Age, sex, education, hypertension, lifetime substance use disorder, BMI, ART status, and head injury |
NR, not reported; USA, United States of America; CS, cross-sectional; PLWH, people living with HIV; HIV, human immunodeficiency virus; HAD, HIV-associated dementia; HAND, HIV-associated neurocognitive disorder; MSK, Memorial Sloan Kettering (neurocognitive scale); GDS, global deficit score; ART, antiretroviral therapy; HAART, highly active antiretroviral therapy; CD4, cluster of differentiation 4 (T-helper cell); CD8, cluster of differentiation 8 (cytotoxic T-cell); OR, odds ratio; CSF, cerebrospinal fluid; CXCL10, C-X-C motif chemokine ligand 10; ELISA, enzyme-linked immunosorbent assay; ADC, AIDS dementia complex; PC1/PC2, principal component 1 and 2 (from genetic ancestry analysis); BMI, body mass index
Table 2.
Study quality evaluation via the Newcastle-Ottawa scale
| Studies | Adequate definition of cases | Representativeness of cases | Selection of controls | Definition of controls | Control for age and sex | Control for other confounders | Exposure ascertainment | Same methods for events ascertainment | Non-response rates | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Mehla 2012 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 6 |
| Yuan 2013 treated | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Yuan 2013 untreated | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Yuan 2015 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Schrier 2015 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Kallianpur 2019 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Ozturk 2019 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Burlacu 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| De Almeida 2022 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Guha 2023 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Hu 2024 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Diaz 2025 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
Difference of CSF CXCL10 between PLWH with and without HAND
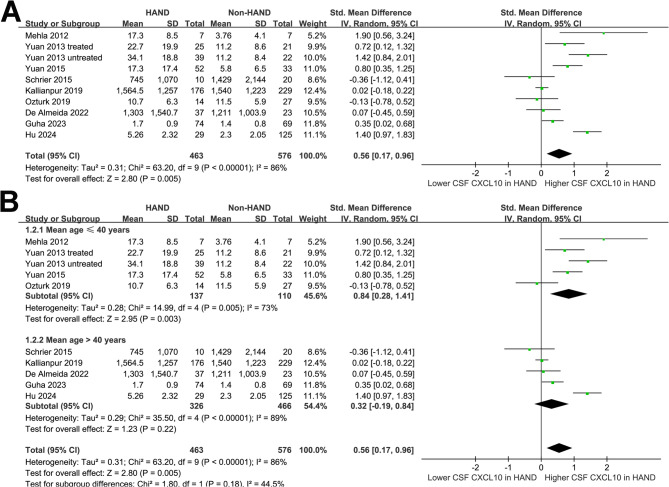
The pooled results of ten datasets from nine studies [32–39, 43–45] showed that overall PLWH with HAND were associated with a higher CSF level of CXCL10 (SMD: 0.56, 95% CI: 0.17 to 0.96, p < 0.001; Fig. 2A) with significant heterogeneity (p for Cochrane Q test = 0.005, I2 = 86%). The estimated between-study variance was τ² = 0.31 (τ = 0.56). The 95% PI ranged from − 0.78 to 1.91, suggesting that while the average effect was positive, true effects in future studies may plausibly vary from negligible to strongly positive. Sensitivity analyses were performed by removing one dataset at a time, and the results remained stable (SMD: 0.44 to 0.65, p all < 0.05; I2: 80–87%; Supplemental Table 1). No single study adequately explained the observed heterogeneity. Specifically, sensitivity analysis limited to studies with high quality (NOS ≥ 7) [33–39, 43–45]showed consistent results (SMD: 0.49, 95% CI: 0.10 to 0.88, p < 0.001; I2 = 86%).
Fig. 2.
Forest plots for the meta-analysis of the difference of CSF CXCL10 level between PLWH with and without HAND; A, overall meta-analysis; and B, subgroup analysis according to the mean age of the population;
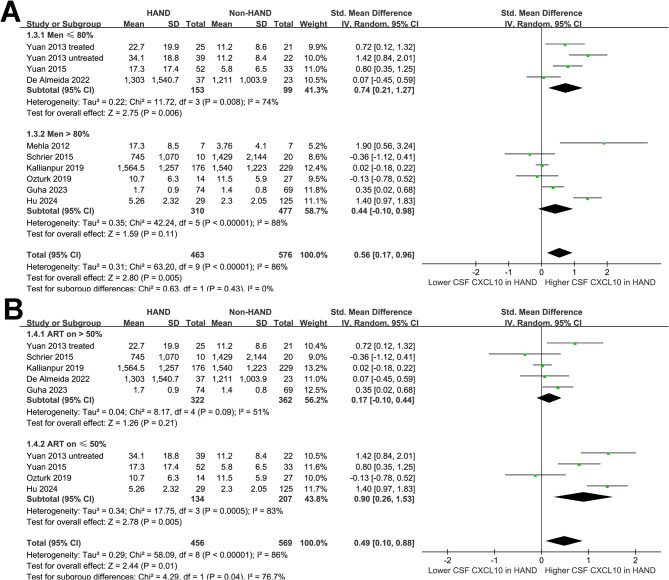
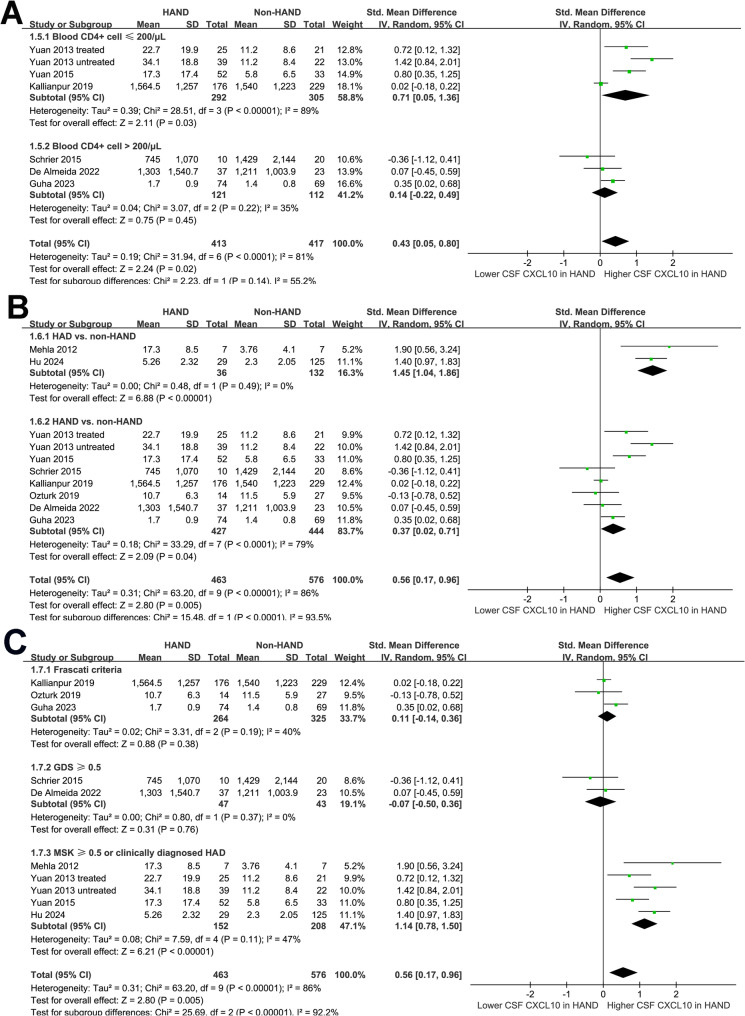
Subgroup analyses indicated that the results were not significantly modified by mean ages of the participants (p for subgroup difference = 0.18; Fig. 2B) or the proportions of men (p for subgroup difference = 0.43; Fig. 3A). A more remarkable increase of CSF level of CXCL10 in HAND was observed in studies with ≤ 50% participants on ART compared to those with > 50% participants on ART (SMD: 0.90 versus 0.17, p for subgroup difference = 0.04; Fig. 3B). The results were not statistically different between participants with mean CD4 + cell count ≤ or > 200 per µL (SMD: 0.71 versus 0.14, p for subgroup difference = 0.14; Fig. 4A). Interestingly, a more remarkable increase of CSF CXCL10 was observed in studies evaluating HAD as compared to overall HAND (SMD: 1.45 versus 0.37, p for subgroup difference < 0.001; Fig. 4B). Similarly, a more remarkable increased of CSF CXCL10 was observed in studies with HAND diagnosed by MSK ≥ 0.5 or clinically diagnosed HAD as compared to HAND diagnosed with Frascati criteria or GDS ≥ 0.5 (SMD: 1.14 vs. 0.11 and − 0.07, p for subgroup difference < 0.001; Fig. 4C).
Fig. 3.
Forest plots for the subgroup analyses of the difference of CSF CXCL10 level between PLWH with and without HAND; A, subgroup analysis according to the proportion of men; and B, subgroup analysis according to the proportion of patients on ART;
Fig. 4.
Forest plots for the subgroup analyses of the difference of CSF CXCL10 level between PLWH with and without HAND; A, subgroup analysis according to the mean plasma CD4 + cell count; B, subgroup analysis according to the outcome of HAND; and C, subgroup analysis according to the methods for the diagnosis of HAND;
Based on the results presented in Table 3, the univariate meta-regression analyses did not identify any statistically significant moderators for the difference in CSF CXCL10 levels between individuals with and without HAND. Specifically, variables such as sample size (p = 0.55), mean age (p = 0.30), percentage of male participants (p = 0.98), proportion on ART (p = 0.12), blood CD4 + cell count (p = 0.13), and study quality (NOS score, p = 0.24) were not significantly associated with the effect size (SMD) of CXCL10 differences. Among these, ART proportion and blood CD4 + count explained the highest proportion of between-study variance (adjusted R² = 29.2% and 25.6%, respectively), although these results did not reach statistical significance. Overall, the findings suggest that the heterogeneity in CXCL10 differences across studies could not be fully explained by the examined covariates, while variations of participants on ART and blood CD4 + cell count may explain part of the heterogeneity.
Table 3.
Results of univariate meta-regression analysis for the difference of CXCL10 in CSF in subjects with and without HAND
| Variables | SMD for the difference of CXCL10 in CSF in subjects with and without HAND | |||
|---|---|---|---|---|
| Coefficient | 95% CI | P values | Adjusted R2 | |
| Sample size | -0.0013 | -0.0061 to 0.0035 | 0.55 | -12.0% |
| Mean age (years) | -0.054 | -0.165 to 0.057 | 0.30 | 0.3% |
| Men (%) | 0.00051 | -0.0416 to 0.0426 | 0.98 | -17.3% |
| On ART (%) | -0.0080 | -0.0019 to 0.0028 | 0.12 | 29.2% |
| CD4 + cell count (per µL) | -0.0020 | -0.0050 to 0.0009 | 0.13 | 25.6% |
| NOS | -0.57 | -1.39 to 0.25 | 0.24 | 8.4% |
SMD, standardized mean difference; CI, confidence interval; ART, antiretroviral therapy; CD4, cluster of differentiation 4; NOS, Newcastle–Ottawa Scale
OR for the association between a high CXCL10 in CSF and HAND
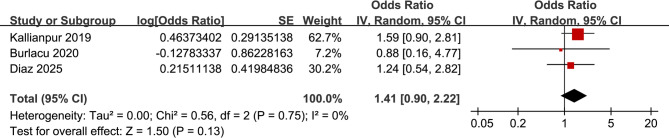
Further meta-analysis involving three studies [35, 37, 41] failed to show that a high CSF CXCL10 was significantly associated with the presence of HAND in PLWH (OR: 1.41, 95% CI: 0.90 to 2.22, p = 0.13; Fig. 5) with no significant heterogeneity (p for Cochrane Q test = 0.75; I2 = 0%).
Fig. 5.
Forest plots for the association between patients with a high CSF CXCL10 level and HAND;
Publication bias
Funnel plots for the meta-analysis comparing the CSF CXCL10 between PLWH with and without HAND are shown in Fig. 6. The plots appeared symmetrical, suggesting a low risk of publication bias. Egger’s test also showed no evidence of publication bias (p = 0.29). The publication bias underlying the meta-analysis evaluating the OR for the association between a high CXCL10 in CSF and HAND was unable to determine because only three studies were included.
Fig. 6.
Funnel plot for publication bias assessment. The horizontal axis represents the standardized mean difference (SMD) for CSF CXCL10, and the vertical axis represents the standard error (SE) of each study. Each dot corresponds to a single study. Asymmetry may indicate potential small-study effects or publication bias
Discussion
This meta-analysis provides quantitative evidence supporting a significant association between elevated CSF CXCL10 levels and HAND in PLWH. Based on data from 12 datasets across 11 studies involving 1,536 adult PLWH, the pooled results demonstrated that individuals with HAND had significantly higher CSF CXCL10 levels compared to those without HAND. Although the effect size was moderate, the finding remained robust across sensitivity analyses, including restriction to high-quality studies. However, significant heterogeneity was observed, prompting further exploration of contributing factors. In addition, pooled analysis of three datasets assessing the odds of HAND in relation to high versus low CSF CXCL10 failed to reach statistical significance (OR: 1.36, p = 0.13), suggesting that while CXCL10 elevation correlates with HAND, its predictive utility as a categorical biomarker remains uncertain and requires further validation.
The observed association between increased CXCL10 levels in CSF and HAND aligns with the understanding of HAND as a neuroinflammatory disorder. CXCL10, induced by interferons and other inflammatory signals, facilitates the recruitment of CXCR3-positive immune cells such as activated T cells and monocytes across the blood-brain barrier [42, 43]. In the context of chronic HIV infection, sustained CXCL10 production may amplify glial activation, perpetuate a proinflammatory CNS milieu, and disrupt neuronal homeostasis [49–51]. Experimental studies have shown that elevated CXCL10 can impair synaptic function, induce excitotoxicity, and contribute to neuronal apoptosis [47, 48]. Additionally, CXCL10 may interact with other soluble neurotoxic mediators such as TNF-α, IL-6, MCP-1 (CCL2), interferon-γ, and soluble CD14, which together amplify glial activation, disrupt neuronal signaling, and contribute to synaptic injury in the context of HIV-associated neuroinflammation [49, 50]. Given its cellular sources and immune-modulating properties, CXCL10 is both a marker and mediator of CNS immune activation, making it a biologically plausible candidate biomarker for HAND [22]. However, the exact molecular pathways underlying the association between CXCL10 and HAND remain to be determined in future studies.
Subgroup analyses provided important insights into sources of variability. First, the association between elevated CSF CXCL10 and HAND was more pronounced in studies where ≤ 50% of participants were on ART compared to those with > 50% ART coverage, with a statistically significant subgroup difference. This finding supports the hypothesis that uncontrolled HIV replication and associated immune activation in ART-naïve individuals may lead to greater CXCL10-mediated neuroinflammation [51]. Similarly, although not statistically significant, studies involving participants with lower CD4 + cell counts (≤ 200/µL) showed a larger effect size than those with higher counts, suggesting a possible contribution of immune suppression to neuroinflammatory burden [52]. Notably, studies that specifically evaluated HAD, the most severe form of HAND, demonstrated a much stronger association compared to studies evaluating broader HAND definitions, with a significant subgroup difference. This gradient supports a dose-response relationship between CXCL10 elevation and neurocognitive severity. Subgroup analysis further revealed that the magnitude of CSF CXCL10 elevation varied substantially depending on the diagnostic criteria for HAND. A more pronounced increase was observed in studies using MSK ≥ 0.5 or clinical HAD definitions compared with those applying Frascati or GDS ≥ 0.5 thresholds. This pattern is biologically plausible, as MSK/HAD definitions typically capture individuals with more advanced neurocognitive impairment, who may present with greater neuroinflammatory activity. In contrast, the Frascati nosology incorporates both neuropsychological and functional assessments, whereas the GDS algorithm is purely psychometric and may classify milder forms of impairment [53, 54]. These differences highlight how diagnostic heterogeneity influences pooled estimates and should be considered when interpreting biomarker associations across the HAND spectrum. To further explore these patterns, univariate meta-regression was performed. While none of the study-level variables reached statistical significance, the proportion of participants on ART and average CD4 + cell count explained 29.2% and 25.6% of the between-study variance, respectively. These findings reinforce the role of systemic immune status and treatment history in shaping CNS immune activation [55, 56]. However, the lack of statistically significant moderators suggests that other unmeasured factors, such as duration of HIV infection, CNS viral load, ART CNS penetrance, or host genetic susceptibility, may also contribute to observed heterogeneity. The possible influences of these factors on the risk of HAND and their interaction with CSF CXCL10 level remain to be determined in future prospective studies.
The secondary analysis examining the association between high versus low CSF CXCL10 and the odds of HAND yielded nonsignificant results. This likely reflects both the small number of included datasets (n = 3) and variation in the methods used to dichotomize CXCL10 levels (e.g., tertiles vs. medians). Although the pooled OR exceeded 1.3, the confidence interval crossed unity, and statistical power was limited. As such, these findings should be interpreted as preliminary and hypothesis-generating rather than conclusive.
This meta-analysis has several strengths. It is the first, to our knowledge, to systematically and quantitatively synthesize the available evidence linking CSF CXCL10 to HAND in PLWH. An up-to-date and comprehensive literature search was performed across multiple databases, and all included studies employed standardized HAND diagnostic criteria. The inclusion of subgroup and meta-regression analyses allowed us to explore potential modifiers of the association, and sensitivity analyses confirmed the robustness of primary findings. However, limitations must be acknowledged. First, the observational and cross-sectional design of included studies precludes causal inference and limits assessment of temporal relationships. Second, significant heterogeneity was observed in the primary analysis, and while subgroup and meta-regression identified potential influences, residual confounding likely remains. In addition, the subgroup and meta-regression analyses were based on study-level data rather than individual patient data, and limited datasets were included for these analyses. Therefore, the findings should be interpreted with caution. Sensitivity analyses confirmed that no individual study disproportionately influenced the pooled estimate, indicating that heterogeneity reflects broader methodological and clinical differences rather than a single outlier. Cohorts restricted to HAD tended to yield larger effect sizes, while differences in diagnostic approaches (Frascati vs. GDS vs. MSK or clinical diagnosis), sample handling, and assay platforms may also have contributed. Taken together, these findings suggest that although CSF CXCL10 is elevated in HAND overall, variability across studies underscores the importance of harmonized case definitions and standardized biomarker protocols in future research. Third, the relatively small number of studies reporting ORs limited our ability to evaluate the prognostic utility of CXCL10 as a binary biomarker. Fourth, variations in laboratory assays and thresholds for defining high CXCL10 levels may reduce comparability across studies. Finally, the findings may not be generalizable to pediatric populations, women, or PLWH in low-resource settings, as most included participants were adults from high-income countries with male predominance.
Despite these limitations, our findings have important clinical implications. Elevated CSF CXCL10 may serve as a biomarker of HAND risk, particularly in ART-naïve individuals or those with advanced immunosuppression. Given the modest effect size and significant heterogeneity, CSF CXCL10 should not be used in isolation for diagnosis but may have value as part of a biomarker panel. From a pathophysiological standpoint, our results underscore the relevance of chronic CNS immune activation in HAND and support continued exploration of CXCL10 as a therapeutic target [13]. Longitudinal studies are needed to establish the temporal relationship between CXCL10 levels and cognitive decline, while standardized assay protocols would enhance future comparability.
Conclusions
In conclusion, this meta-analysis demonstrates that CSF CXCL10 levels are significantly elevated in PLWH with HAND, with stronger associations observed in ART-naïve individuals and in those with more severe neurocognitive impairment. Nonetheless, substantial heterogeneity and a wide PI indicate that the strength of this association varies considerably across studies. Although evidence from categorical comparisons for predictive utility remains limited, CSF CXCL10 may be a promising biomarker of HAND-related neuroinflammation. Further longitudinal and mechanistic studies with harmonized diagnostic criteria and standardized biomarker protocols are warranted to validate its role in HAND pathogenesis and its potential application in early diagnosis and therapeutic monitoring.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
Qingqing Gu and Yifa Zhang conceived the study. Qingqing Gu and Shanshan Wang performed database search, literauture review, study screening, and inclusion. Qingqing Gu, Lin Zhu, Qingyuan Sun, and Yifa Zhang performed data extraction and statistical analysis. Qingqing Gu, Shanshan Wang, and Yifa Zhang interpreted the results. Qingqing Gu drafted the manuscript. All authors revised the manuscript and approved the submission.
Funding
This study is supported by the Project of Clinical Analysis of HIV/AIDS Combined with Pulmonary Tuberculosis Co-Infection in Lianyungang City (zd202006).
Data availability
The authors confirm that the data supporting the findings of this study are available within the article. Further inquiries can be directed to the corresponding author.
Declarations
Ethics approval and consent to participate
Institutional Review Board approval was not required because this is a meta-analysis.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Diaz MM. Update on neurological complications of HIV. Curr Opin HIV AIDS. 2025;20(4):337–43. 10.1097/coh.0000000000000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olajide T, Ogungbemi E, Olajide G, Ogundijo D, Osakuade O, Moshood F. HIV-associated neurocognitive disorders in Africa: challenges, peculiarities, and future directions. AIDS Res Ther. 2024;21(1):88. 10.1186/s12981-024-00677-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou C, Wei J, Zhang H, Li H. Evolving strategies in the diagnosis and treatment of HIV-associated neurocognitive disorders. Rev Neurosci. 2025. 10.1515/revneuro-2025-0004. [DOI] [PubMed] [Google Scholar]
- 4.Flatt A, Gentry T, Kellett-Wright J, Eaton P, Joseph M, Urasa S, et al. Prevalence and 1-year incidence of HIV-associated neurocognitive disorder (HAND) in adults aged ≥ 50 years attending standard HIV clinical care in kilimanjaro, Tanzania. Int Psychogeriatr. 2023;35(7):339–50. 10.1017/s1041610221000156. [DOI] [PubMed] [Google Scholar]
- 5.Saloner R, Cysique LA. HIV-Associated neurocognitive disorders: A global perspective. J Int Neuropsychol Soc. 2017;23(9–10):860–9. 10.1017/s1355617717001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nweke M, Mshunqane N, Govender N, Akinpelu AO, Ukwuoma M. Impact of HIV-associated cognitive impairment on functional independence, frailty and quality of life in the modern era: a meta-analysis. Sci Rep. 2022;12(1):6470. 10.1038/s41598-022-10474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsegaw M, Andargie G, Alem G, Tareke M. Screening HIV-associated neurocognitive disorders (HAND) among HIV positive patients attending antiretroviral therapy in South wollo, Ethiopia. J Psychiatr Res. 2017;85:37–41. 10.1016/j.jpsychires.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 8.Cross S, Önen N, Gase A, Overton ET, Ances BM. Identifying risk factors for HIV-associated neurocognitive disorders using the international HIV dementia scale. J Neuroimmune Pharmacol. 2013;8(5):1114–22. 10.1007/s11481-013-9505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Namagga JK, Rukundo GZ, Voss JG. Prevalence and risk factors of HIV-Associated neurocognitive disorders in rural Southwestern Uganda. J Assoc Nurses AIDS Care. 2019;30(5):531–8. 10.1097/jnc.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu Q, Ye Q, Chen D, Li X. Neurocognitive function and its influencing factors in people living with HIV/AIDS. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2024;49(12):1902–8. 10.11817/j.issn.1672-7347.2024.230555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams ME, Naudé PJW, van der Westhuizen FH. Proteomics and metabolomics of HIV-associated neurocognitive disorders: A systematic review. J Neurochem. 2021;157(3):429–49. 10.1111/jnc.15295. [DOI] [PubMed] [Google Scholar]
- 12.Bhol NK, Bhanjadeo MM, Singh AK, Dash UC, Ojha RR, Majhi S, et al. The interplay between cytokines, inflammation, and antioxidants: mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed Pharmacother. 2024;178:117177. 10.1016/j.biopha.2024.117177. [DOI] [PubMed] [Google Scholar]
- 13.Bufi AA, Di Stefano J, Papait A, Silini AR, Parolini O, Ponsaerts P. The central role of CXCL10-CXCR3 signaling in neuroinflammation and neuropathology. Cytokine Growth Factor Rev. 2025. 10.1016/j.cytogfr.2025.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Watson AES, Goodkey K, Footz T, Voronova A. Regulation of CNS precursor function by neuronal chemokines. Neurosci Lett. 2020;715:134533. 10.1016/j.neulet.2019.134533. [DOI] [PubMed] [Google Scholar]
- 15.Elemam NM, Talaat IM, Maghazachi AA. CXCL10 chemokine: A critical player in RNA and DNA viral infections. Viruses. 2022;14(11). 10.3390/v14112445. [DOI] [PMC free article] [PubMed]
- 16.Zhang W, Xiao D, Mao Q, Xia H. Role of neuroinflammation in neurodegeneration development. Signal Transduct Target Ther. 2023;8(1):267. 10.1038/s41392-023-01486-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blandford SN, Fudge NJ, Moore CS. CXCL10 is associated with increased cerebrospinal fluid immune cell infiltration and disease duration in multiple sclerosis. Biomolecules. 2023;13(8). 10.3390/biom13081204. [DOI] [PMC free article] [PubMed]
- 18.Da Silva SJ, Cabral-Castro MJ, Faria LC, Rosadas C, de Araújo MFL, Dutra ACS, et al. CXCL-10 in cerebrospinal fluid detects neuroinflammation in HTLV-1-Associated myelopathy with high accuracy. Viruses. 2025;17(1). 10.3390/v17010089. [DOI] [PMC free article] [PubMed]
- 19.Zhou F, Sun Y, Xie X, Zhao Y. Blood and CSF chemokines in alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Alzheimers Res Ther. 2023;15(1):107. 10.1186/s13195-023-01254-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon HS, Koh SH. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl Neurodegener. 2020;9(1):42. 10.1186/s40035-020-00221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koper OM, Kamińska J, Sawicki K, Kemona H. CXCL9, CXCL10, CXCL11, and their receptor (CXCR3) in neuroinflammation and neurodegeneration. Adv Clin Exp Med. 2018;27(6):849–56. 10.17219/acem/68846. [DOI] [PubMed] [Google Scholar]
- 22.Lei J, Yin X, Shang H, Jiang Y. IP-10 is highly involved in HIV infection. Cytokine. 2019;115:97–103. 10.1016/j.cyto.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Mejías-Trueba M, Saborido-Alconchel A, Serna-Gallego A, Trujillo-Rodríguez M, Muñoz-Muela E, Llaves-Flores S, et al. Plasma concentrations of IL-6, MIP-1β, IP-10, and PTX-3 as predictors of the immunological response to antiretroviral treatment in people with HIV. Front Immunol. 2024;15:1447926. 10.3389/fimmu.2024.1447926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.2. The Cochrane Collaboration. 2021;www.training.cochrane.org/handbook
- 27.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2010;http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Spineli LM, Pandis N. Prediction interval in random-effects meta-analysis. Am J Orthod Dentofac Orthop. 2020;157(4):586–8. 10.1016/j.ajodo.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehla R, Bivalkar-Mehla S, Nagarkatti M, Chauhan A. Programming of neurotoxic cofactor CXCL-10 in HIV-1-associated dementia: abrogation of CXCL-10-induced neuro-glial toxicity in vitro by PKC activator. J Neuroinflammation. 2012;9:239. 10.1186/1742-2094-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan L, Qiao L, Wei F, Yin J, Liu L, Ji Y, et al. Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J Neurovirol. 2013;19(2):144–9. 10.1007/s13365-013-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schrier RD, Hong S, Crescini M, Ellis R, Pérez-Santiago J, Spina C, et al. Cerebrospinal fluid (CSF) CD8 + T-cells that express interferon-gamma contribute to HIV associated neurocognitive disorders (HAND). PLoS ONE. 2015;10(2):e0116526. 10.1371/journal.pone.0116526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan L, Liu A, Qiao L, Sheng B, Xu M, Li W, et al. The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. Biomed Res Int. 2015;2015:506872. 10.1155/2015/506872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallianpur AR, Gittleman H, Letendre S, Ellis R, Barnholtz-Sloan JS, Bush WS,et al. Cerebrospinal Fluid Ceruloplasmin, Haptoglobin, and Vascular Endothelial Growth Factor Are Associated with Neurocognitive Impairment in Adults with HIV Infection.Mol Neurobiol. 2019;56(5):3808-18. 10.1007/s12035-018-1329-9 [DOI] [PMC free article] [PubMed]
- 36.Ozturk T, Kollhoff A, Anderson AM, Christina Howell J, Loring DW, Waldrop-Valverde D, et al. Linked CSF reduction of phosphorylated tau and IL-8 in HIV associated neurocognitive disorder. Sci Rep. 2019;9(1):8733. 10.1038/s41598-019-45418-2 8733 [DOI] [PMC free article] [PubMed]
- 37.Burlacu R, Umlauf A, Marcotte TD, Soontornniyomkij B, Diaconu CC, Bulacu-Talnariu A, et al. Plasma CXCL10 correlates with HAND in HIV-infected women. J Neurovirol.2020;26(1):23–31. 10.1007/s13365-019-00785-4 [DOI] [PMC free article] [PubMed]
- 38.De Almeida SM, Rotta I, Tang B, Umlauf A, Vaida F, Cherner M, et al. Higher Cerebrospinal Fluid Soluble Urokinase-type Plasminogen Activator Receptor, But Not Interferon γ-inducible Protein 10, Correlate With Higher Working Memory Deficits. J Acquir Immune Defic Syndr. 2022;90(1):106 – 14. d10.1097/qai.0000000000002924. [DOI] [PMC free article] [PubMed]
- 39.Guha D, Misra V, Yin J, Horiguchi M, Uno H, Gabuzda D. Vascular injury markers associated with cognitive impairment in people with HIV on suppressive antiretroviral therapy. AIDS. 2023;37(14):2137-47. 10.1097/QAD.0000000000003675. [DOI] [PMC free article] [PubMed]
- 40.Hu Z, Cinque P, Dravid A, Hagberg L, Yilmaz A, Zetterberg H, et al. Changes in cerebrospinal fluid proteins across the spectrum of untreated and treated chronic HIV-1 infection. PLoS Pathog. 2024;20(9):e1012470. 10.1371/journal.ppat.1012470. [DOI] [PMC free article] [PubMed]
- 41.Diaz MM, Kamalyan L, Al-Rousan T, Breton J, Franklin DR, Jr., Umlauf A, et al.Cerebrospinal fluid biomarkers of inflammation and immune activation associated with neurocognitive impairment among US Latinos with HIV. AIDS. 2025;39(7):838 – 47. 10.1097/qad.0000000000004143. [DOI] [PMC free article] [PubMed]
- 42.Liu M, Guo S, Hibbert JM, Jain V, Singh N, Wilson NO, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011;22(3):121 – 30. 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed]
- 43.Turner MD, Nedjai B, Hurst T, Pennington DJ. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta. 2014;1843(11):2563-82. 10.1016/j.bbamcr.2014.05.014. [DOI] [PubMed]
- 44.Yin X, Wang Z, Wu T, Ma M, Zhang Z, Chu Z, et al. The combination of CXCL9, CXCL10 and CXCL11 levels during primary HIV infection predicts HIV disease progression. J Transl Med. 2019;17(1):417. 10.1186/s12967-019-02172-3. [DOI] [PMC free article] [PubMed]
- 45.Anderson AM, Kundu S, Tang B, Vaida F, Okwuegbuna O, McClernon D, et al. Cerebrospinal fluid CXCL10 is associated with the presence of low level CSF HIV during suppressive antiretroviral therapy. J Neuroimmunol. 2021;353:577493. 10.1016/j.jneuroim.2021.577493. [DOI] [PMC free article] [PubMed]
- 46.Valverde-Villegas JM, de Medeiros RM, Ellwanger JH, Santos BR, Melo MG, Almeida SEM, et al. High CXCL10/IP-10 levels are a hallmark in the clinical evolution of the HIV infection. Infect Genet Evol. 2018;57:51 – 8. 10.1016/j.meegid.2017.11.002. [DOI] [PubMed]
- 47.Sui Y, Potula R, Dhillon N, Pinson D, Li S, Nath A, et al. Neuronal apoptosis is mediated by CXCL10 overexpression in simian human immunodeficiency virus encephalitis.Am J Pathol. 2004;164(5):1557-66. 10.1016/s0002-9440(10)63714-5. [DOI] [PMC free article] [PubMed]
- 48.Cho J, Nelson TE, Bajova H, Gruol DL. Chronic CXCL10 alters neuronal properties in rat hippocampal culture. J Neuroimmunol. 2009;207(1–2):92–100. 10.1016/j.jneuroim.2008.12.007. [DOI] [PMC free article] [PubMed]
- 49.Tousi NS, Buck DJ, Curtis JT, Davis RL. α-Synuclein potentiates interleukin-1β-induced CXCL10 expression in human A172 astrocytoma cells. Neurosci Lett. 2012;507(2):133-6. 10.1016/j.neulet.2011.12.001. [DOI] [PMC free article] [PubMed]
- 50.Lawrence JM, Schardien K, Wigdahl B, Nonnemacher MR. Roles of neuropathology-associated reactive astrocytes: a systematic review. Acta Neuropathol Commun. 2023;11(1):42. 10.1186/s40478-023-01526-9. [DOI] [PMC free article] [PubMed]
- 51.Bandera A, Taramasso L, Bozzi G, Muscatello A, Robinson JA, Burdo TH, et al. HIV-Associated Neurocognitive Impairment in the Modern ART Era: Are We Close to Discovering Reliable Biomarkers in the Setting of Virological Suppression? Front Aging Neurosci. 2019;11:187. 10.3389/fnagi.2019.00187. [DOI] [PMC free article] [PubMed]
- 52.Hong S, Banks WA. Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun. 2015;45:1–12. 10.1016/j.bbi.2014.10.008. [DOI] [PMC free article] [PubMed]
- 53.Carey CL, Woods SP, Rippeth JD, Gonzalez R, Moore DJ, Marcotte TD, et al. Initial validation of a screening battery for the detection of HIV-associated cognitive impairment.Clin Neuropsychol. 2004;18(2):234 – 48. 10.1080/13854040490501448. [DOI] [PubMed]
- 54.Blackstone K, Moore DJ, Franklin DR, Clifford DB, Collier AC, Marra CM, et al.Defining neurocognitive impairment in HIV: deficit scores versus clinical ratings.Clin Neuropsychol. 2012;26(6):894–908. 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed]
- 55.Kompella S, Al-Khateeb T, Riaz OA, Orimaye SO, Sodeke PO, Awujoola AO, et al.HIV-Associated Neurocognitive Disorder (HAND): Relative Risk Factors. Curr Top Behav Neurosci. 2021;50:401 – 26. 10.1007/7854_2020_131. [DOI] [PubMed]
- 56.Adhikary K, Banerjee A, Sarkar R, Banerjee R, Chowdhury SR, Ganguly K, et al.HIV-associated neurocognitive disorders (HAND): Optimal diagnosis, antiviral therapy,pharmacological treatment, management, and future scopes. J Neurol Sci. 2025;470:123410. 10.1016/j.jns.2025.123410. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article. Further inquiries can be directed to the corresponding author.