Abstract
Objectives
This study aimed to apply CUBIC tissue clearing combined with three-dimensional (3D) imaging to visualize the pathological architecture of systemic light chain (AL) amyloidosis in the human tongue, assess spatial and microstructural changes associated with amyloid deposition, and explore its potential to enhance early diagnostic accuracy.
Materials and methods
Seven tongue specimens from confirmed AL amyloidosis cases underwent CUBIC processing, multiplex immunofluorescence staining, and 3D imaging. Vascular density, surface area, vessel and muscle fiber diameters were compared between amyloid deposition and non-deposition regions. Ten additional specimens initially negative for AL amyloidosis were re-evaluated using the same procedure, suspected cases were verified by repeat histology and clinical follow-up.
Results
In amyloid deposition regions, vascular density and surface area were (0.14 ± 0.04) μm3 and (0.46 ± 0.11) μm2, showing 50.0% and 33.3% reductions compared to non-deposition regions [(0.28 ± 0.06) μm3 and (0.69 ± 0.12) μm2]. Median vessel diameter in vessels with luminal amyloid deposition was 5.99 μm (IQR: 5.07–7.39), 30.5% smaller than that in vessels without deposition [8.62 μm (IQR: 7.26–11.03)]. Muscle fibers surrounded by amyloid deposits had a diameter of (3.62 ± 0.28)μm, slightly smaller than that of fibers without deposition [(3.70 ± 0.23)] μm. All differences were significant (P < 0.05). Among the ten initially negative cases, five were reclassified as highly suspected and one was confirmed during follow-up.
Conclusions
CUBIC-based 3D imaging enables detailed visualization and quantification of amyloid-associated microstructural changes in tongue tissue, providing deeper insight into disease mechanisms and serving as a valuable complement to conventional histopathology for enhancing early diagnostic accuracy in AL amyloidosis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12903-025-06939-7.
Keywords: Amyloidosis AL, Tissue clearing, Three-dimensional imaging, Tongue, Histopathology, Early diagnosis
Introduction
Systemic light chain (AL) amyloidosis is a rare but often fatal plasma cell disorder characterized by the misfolding of monoclonal κ (kappa) or λ (lambda) light chains into amyloid fibrils. These fibrils accumulate in multiple organs, impairing function and ultimately leading to organ failure and high mortality [1]. The estimated incidence is about 10 cases per million, and AL is the most common type of systemic amyloidosis [2].
The heart and kidneys are the most frequently affected, with reported involvement rates of approximately 77% and 62%, respectively [3, 4]. Cardiac involvement in particular carries a poor prognosis, with median survival less than one year even with treatment [5, 6]. Diagnosis is often delayed due to the insidious onset and non-specific symptoms. The median interval from symptom onset to diagnosis exceeds two years, with nearly one-third of patients consulting five or more physicians before being diagnosed [6, 7]. Such delays have a major impact on prognosis: diagnosis within six months is associated with better survival [8], whereas diagnosis after more than 19 months confers a three to five-fold higher risk of death [9]. These observations highlight the urgent need for earlier and more accurate diagnostic strategies.
Histopathological confirmation of amyloid deposits from affected organs remains the diagnostic gold standard [1]. Cardiac and renal biopsies demonstrate high sensitivity, with reported rates of approximately 97.4% and 95%, respectively [10]. However, these procedures are invasive, technically demanding, and carry risks such as bleeding andperforation;and they may also be contraindicated in patients with coagulopathy or organ dysfunction [11]. By contrast, tongue biopsy, although slightly less sensitive (~ 75%), is minimally invasive, lower risk, and widely used as a practical alternative [12]. Nonetheless, conventional histopathology based on two-dimensional (2D) sections has inherent limitations, including the inability to capture the full three-dimensional architecture of the tissue and susceptibility to sampling bias. Especially in early disease, when deposits are sparse or focal, these shortcomings contribute to false-negative immunofluorescence in more than 30% of cases [13], and may even result in missed diagnoses despite obvious clinical manifestations.
To overcome these limitations, recent advances in whole-mount tissue clearing techniques—particularly Clear, Unobstructed Brain Imaging Cocktails and Computational analysis (CUBIC)—combined with 3D imaging now enable high-resolution volumetric visualization of intact tissues, providing a powerful complement to conventional histopathology [14]. These techniques have shown utility in other protein aggregation disorders, such as Alzheimer’s disease [15], but have not yet been applied to human tongue tissue in AL amyloidosis.
Therefore, this study applied CUBIC combined with 3D imaging to visualize and quantitatively assess the spatial microstructural changes associated with amyloid deposition in tongue tissue from patients with AL amyloidosis,aiming to address the limitations of conventional histopathology, improve early diagnostic accuracy, and establish a methodological foundation for optimizing diagnostic strategies and advancing our understanding of disease mechanisms.
Materials and methods
Sample collection
Seventeen patients with suspected systemic AL amyloidosis treated in the Department of Hematology, Beijing Chaoyang Hospital, between October 2023 and December 2024 were enrolled. The study was approved by the Ethics Committee of Beijing Chaoyang Hospital (approval no. 2023–10-25–4), and written informed consent was obtained from all participants. Patient characteristics, including age, sex, inclusion criteria (≥ 2 qualifying clinical or laboratory features), and the interval from clinical eligibility to tongue biopsy, are summarized in Table 1. All biopsies were performed within seven days of eligibility to ensure consistency and relevance for early diagnosis assessment.
Table 1.
Clinical characteristics and timing of tongue biopsy in 17 patients with suspected AL amyloidosis
| Number | Age (years) | Gender | Inclusion criteria met* | Interval from eligibility to biopsy (days) |
|---|---|---|---|---|
| 01 | 65 | Male | ①② | 4 |
| 02 | 58 | Female | ①③ | 6 |
| 03 | 71 | Male | ②③① | 3 |
| 04 | 63 | Female | ①② | 7 |
| 05 | 55 | Male | ②③ | 5 |
| 06 | 68 | Female | ①② | 6 |
| 07 | 60 | Male | ①②③ | 4 |
| 08 | 66 | Female | ②③ | 7 |
| 09 | 73 | Male | ①② | 5 |
| 10 | 59 | Female | ①③ | 6 |
| 11 | 62 | Male | ①② | 3 |
| 12 | 64 | Female | ②③ | 4 |
| 13 | 70 | Male | ②③① | 5 |
| 14 | 57 | Female | ①③ | 5 |
| 15 | 69 | Male | ②③ | 7 |
| 16 | 61 | Female | ①② | 6 |
| 17 | 65 | Male | ②③① | 4 |
*Inclusion criteria: ① Diastolic cardiac dysfunction; ② Unexplained myocardial hypertrophy with NT-pro BNP ≥ 1800 pg/mL; ③ 24-h urinary protein excretion > 3.5 g and abnormal serum free light chain (FLC) κ/λ ratio with monoclonal protein detected by electrophoresis or immune fixation
Specimen processing
Tongue biopsies were obtained from a standardized anatomical site at the apical region, pre-marked with gentian violet to ensure consistency. A tissue block measuring approximately 0.4 × 0.6 × 0.6 cm was excised with a sterile scalpel, and dimensions were measured using a digital Vernier caliper. Each block was longitudinally bisected along the deepest central axis to maintain morphological equivalence. One half (approximately 0.2 × 0.3 × 0.3 cm) was processed by CUBIC tissue clearing and 3D imaging, and the other half (approximately 0.2 × 0.3 × 0.3 cm) was embedded for conventional histopathology.
Specimens for CUBIC preparation were fixed in 4% paraformaldehyde (PFA) at 4 °C for one week, following Matsumoto et al. [14] to ensure uniform fixation, fluorescence compatibility, and antigen preservation. For conventional histopathology, paraffin sections were cut at 3 µm (H&E) and 7 µm (Congo red) to assess tissue morphology, amyloid deposition, and apple-green birefringence under polarized light. Immunofluorescence staining detected κ and λ light chains, while transmission electron microscopy confirmed randomly arranged, non-branching fibrils measuring 8–14 nm in diameter. According to the 2018 International Society of Amyloidosis criteria, specimens fulfilling all pathological requirements were classified as AL amyloidosis (n = 7), whereas those not meeting criteria were designated negative (n = 10).
Optimized CUBIC protocol for whole-mount 3D staining
Tissue blocks for CUBIC processing (approximately 0.2 × 0.3 × 0.3 cm, derived from bisected specimens as described above) were fixed in 4% paraformaldehyde and washed in phosphate-buffered saline (PBS) for 8 h to remove residual fixative. Blocks were then immersed in CUBIC-L [10wt% N-butyldiethanolamine (Tokyo Chemical Industry, B0725) and 10wt% Triton X-100 (Sigma, X-100)] at 45 °C with gentle shaking for 3 days to achieve delipidation and decolorization, followed by an additional 8 h PBS wash to remove Residual CUBIC-L.
For immunostaining, blocks were incubated in 0.5 ml immunostaining buffer (PBS, 0.5% Triton X-100, 0.25% casein; Thermo Scientific, 37,582) containing CD31 antibody (vascular endothelial marker, 1:100; Abcam, ab28364) and Lambda/Kappa-FITC (for λ/κ light chain detection, 1:100; Southern Biotech, 2070–02/2060–02) for 4 days at room temperature with gentle shaking in the dark, then washed in PBS for 8 h. Subsequently, the same buffer was supplemented with DAPI (1:100; Sigma, D9542) for nuclear visualization and spatial reference. Alexa Fluor 546–phalloidin (1:100; Thermo Fisher, A22283) for F-actin labeling to delineate lingual muscle fibers (Fig. 1F), and donkey anti-rabbit IgG Alexa Fluor 647 (1:100; Thermo Scientific, A32795) as the secondary antibody for CD31. Blocks were incubated for 3 days under the same conditions.
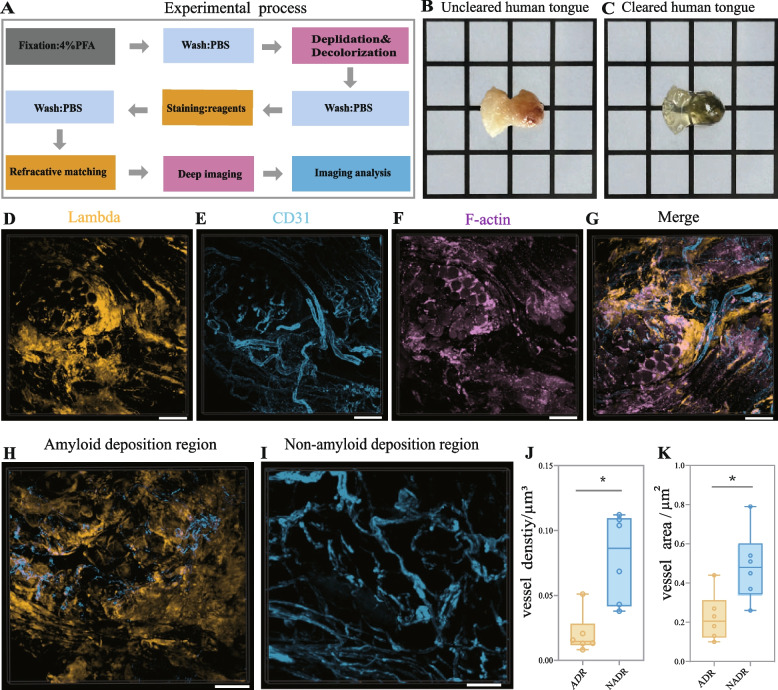
Fig. 1.
3D structural mapping and vascular alterations in AL amyloidosis of the human tongue. A Workflow of CUBIC tissue clearing protocol. B Tongue specimen before CUBIC processing. C The Same specimen after CUBIC processing, showing optical transparency. D–F Confocal optical slices showing amyloid deposits (D, yellow), CD31-labeled blood vessels (E, blue), and F-actin-labeled muscle fibers (F, magenta). G Merged image showing spatial co-localization of amyloid deposits (yellow), blood vessels (blue), and muscle fibers (magenta). H–I Representative views of amyloid deposition region (ADR) and non-amyloid deposition region (NADR). J–K Quantitative comparisons of vascular density (J) and surface area (K) between ADR and NADR (*P < 0.005). Scale bars: 100 μm. Abbreviations: CUBIC, Clear Unobstructed Brain Imaging Cocktails; ADR, amyloid deposition region; NADR, non-amyloid deposition region.
After a final 8 h PBS wash, blocks were transferred to RIMS solution [20 g Histodenz™ (Sigma, D2158) in 15 ml 0.2 × PBS] and maintained in the dark on a shaker until optically transparent. as defined by CUBIC protocols [14]. Block transparency was assessed every 2 days and considered complete when black printed text placed beneath the tissue was clearly visible under bright-field illumination.
Image data obtaining and analysis
Cleared human tongue tissues were positioned upright on glass bottom culture dishes (NEST) and imaged using both a Zeiss Z1 LightSheet microscope with 10X lenses and a Nikon C2 confocal microscope with 20X and 10X lenses. Tile scans were stitched using Nikon software.
The imaging analysis was performed with Imaris (10.0.1, Bitplane),.and the Concrete analysis were as follows: The CD31 signal, FITC signal and F-actin signal were used to generate a surface of the blood vessels, amyloid deposits and lingual muscular fibers separately for quantitative analysis. Under the 3D surface module, according to the signals from CD31, FITC, DAPI to determine vascular volume, amyloid deposits volume, and cleared tissue volume. The dimensions of lingual muscular fiber cells and their nuclei were measured under the Slice module. Amyloid deposits, blood vessels, lingual muscles were analyzed individually. The snapshot and animation functions were used to capture images and videos, respectively.
Statistics analysis
Quantitative data were analyzed using the unpaired Student’s t-test or Mann Whitney U test, as appropriate. Statistical analyses were conducted with GraphPad Prism 10.0 and SPSS 26.0. Data are presented as mean ± standard deviation or median (P25, P75). A p-value < 0.05 was considered statistically significant.
Screening and pathological re-evaluation of highly suspected AL amyloidosis cases
To verify compatibility between CUBIC processing and conventional histopathology, tongue specimens previously diagnosed as AL amyloidosis–positive or –negative by conventional histopathology were subjected to CUBIC clearing and reprocessed for paraffin embedding and standard staining. All positive cases remained positive and all negative cases remained negative, confirming that CUBIC did not interfere with subsequent histopathological assessment [16].
Following validation, ten cases initially reported as negative by conventional histopathology were re-evaluated using the CUBIC protocol with immunofluorescence staining for λ and κ light chains and whole-tissue confocal imaging. Cases were classified as CUBIC/3D-positive or -negative according to a semi-quantitative 0–3 + scale [17]. Staining intensity ≥ 2 + with a 3D distribution pattern consistent with amyloid deposition was defined as positive, whereas < 2 + intensity or non-specific background staining was considered negative.
Specimens screened positive by CUBIC were subsequently processed for conventional histopathology. After immersion in 10 mL 1 × PBS for 8 h at room temperature until opaque, tissues were paraffin-embedded and sectioned at 3 μm for hematoxylin and eosin (H&E) and 7 μm for Congo red. H&E sections were stained with hematoxylin (10 min), differentiated in 1% acid alcohol (5 s), blued in alkaline water, counterstained with eosin (3 min), dehydrated, cleared, and mounted. Congo red sections were stained with commercial Congo red solution (30 min), differentiated in alkaline alcohol (1%NaOH in 80% ethanol, 8 s under microscopic control), counterstained with hematoxylin (1–2 min), dehydrated, and mounted.
All slides were reviewed independently by two experienced pathologists. Discrepancies were resolved by joint review; if consensus could not be reached, a third senior pathologist was consulted and the final diagnosis determined by majority opinion. Amyloid deposits were identified as homogeneous, structureless, eosinophilic material on H&E, brick-red deposits on Congo red and apple-green birefringence under polarized light. Cases showing these features together with relevant clinical manifestations were designated as highly suspected AL amyloidosis. Such cases were closely monitored, and those subsequently meeting International Society of Amyloidosis (ISA) criteria were classified as confirmed AL amyloidosis.
Results
3D structural mapping and vascular alterations in AL amyloidosis of the human tongue
Human tongue tissue specimens fixed in 4% paraformaldehyde were processed using the CUBIC tissue clearing technology, including delipidation, decolorization, immunofluorescence labeling, and refractive index matching (Fig. 1A). These steps rendered the originally opaque tissues optically transparent (Fig. 1B, 1C), enabling high-resolution 3D imaging. All seven specimens were confirmed as AL amyloidosis by conventional histopathology. Multiplex immunofluorescence staining targeted κ or λ light chains, CD31 (vascular endothelial marker), F-actin (muscle fiber marker), and DAPI (for visualizing cell nuclei and facilitating tissue orientation). Confocal imaging with 3D reconstruction revealed the spatial distribution of amyloid deposits (Fig. 1D), muscle fibers (Fig. 1E), and vasculature (Fig. 1F), with regional co-localization among these structures (Fig. 1G). These composite images provided an integrated 3D representation of the pathological architecture in AL amyloidosis.
To assess vascular involvement, vascular parameters were compared between amyloid deposition regions (ADR) and non-amyloid deposition regions (NADR) (Fig. 1H, 1I). Vascular density in ADR was (0.14 ± 0.04) μm3, about 50% lower than in NADR (0.28 ± 0.06) μm3 (Fig. 1J). Similarly, vascular surface area in ADR was (0.46 ± 0.11) μm2, representing a 53% reduction compared with NADR (0.69 ± 0.12) μm2 (Fig. 1K). Both differences were statistically significant (P < 0.05). These findings demonstrate substantial vascular loss within amyloid-deposition regions, suggesting that amyloid accumulation contributes to vascular rarefaction and local micro-environmental disruption.
3D analysis of spatial relationships between amyloid deposits and blood vessels
3D imaging revealed three principal patterns of amyloid–vessel interaction: circumferential deposits surrounding the vessel wall (Fig. 2A, B; Supplementary Videos 1,2); luminal accumulation forming hollow or mass-like structures that narrowed or occluded the lumen (Fig. 2E–H; Supplementary Videos 3,4); and discontinuous deposits at vessel bifurcations or within the lumen (Fig. 2C, D). The median diameter of vessels with luminal amyloid deposition was [5.99 μm (IQR: 5.07–7.39)], which was 30.5% smaller than that of vessels without deposition [8.62 μm (IQR: 7.26–11.03)] (Fig. 2I P < 0.001). These findings indicate that AL amyloid preferentially involves small-caliber vessels, where luminal narrowing or occlusion may impair perfusion, limit nutrient delivery, and contribute to local tissue injury.
Fig. 2.
3D imaging of amyloid deposits and vascular alterations in human tongue tissue with AL amyloidosis. A Perivascular amyloid deposition. B Magnified view of the boxed area in (A). C Segmental intravascular amyloid deposition. D 3D rendering of the segmental pattern shown in (C). E Intraluminal amyloid deposits without vascular lumen obstruction. F Magnified view of the boxed area in (E). G Intraluminal amyloid deposits causing partial or complete obstruction of the vascular lumen. H Magnified view of the boxed region in (G) showing complete obstruction of the vascular lumen. I–K Schematic illustrations corresponding to (C), (E), and (G), respectively. L Quantitative comparison showing that vessels with intraluminal amyloid deposits have significantly smaller luminal diameters than those without amyloid deposits (****P < 0.001). Scale bars: 50 μm (A–D, F); 70 μm (E, H); 100 μm (G).
3D analysis of amyloid deposition and lingual muscle fiber atrophy
3D imaging revealed two predominant patterns of amyloid deposition around lingual muscle fibers: sleeve-like encasement, mainly in longitudinal sections (Fig. 3A, B), and circumferential encasement, commonly in cross-sections (Fig. 3C). In severe cases, individual muscle fibers were entirely surrounded by amyloid deposition. Quantitative analysis showed a slight but statistically significant reduction in muscle fiber diameter within amyloid deposition regions (3.62 ± 0.28μm) compared with non-deposition regions (3.70 ± 0.23 μm); (P < 0.05, Fig. 3D). These results suggest that amyloid accumulation is associated with localized muscle atrophy, potentially contributing to the restricted tongue mobility observed in patients with lingual AL amyloidosis.
Fig. 3.
Three-dimensional imaging and analysis of amyloid deposits surrounding lingual muscle fibers in AL amyloidosis. A Confocal optical slice (longitudinal view) showing “sleeve-like” amyloid deposits encasing muscle fibers. B Corresponding 3D rendering confirming longitudinal amyloid encasement. C Cross-sectional view illustrating circumferential amyloid encasement. D Quantitative comparison of muscle fiber diameters between amyloid deposition regions (ADR) and non- amyloid deposition regions (NADR). *P < 0.05. Scale bars: 100 μm. Abbreviations: ADR, amyloid deposition region; NADR, non-amyloid deposition region.
Validation and application of CUBIC for screening suspected AL amyloidosis cases
To assess compatibility with conventional histopathology, CUBIC was applied to tongue specimens from both confirmed AL amyloidosis and cases previously reported as negative by conventional histopathology. After tissue clearing, samples were restored and processed for routine histological sectioning and staining (see Materials and Methods). In confirmed AL amyloidosis cases, immunofluorescence staining revealed λ orκ light chain amyloid deposits (Fig. 4A); H&E staining showed amorphous, eosinophilic extracellular material (Fig. 4B); Congo red staining presented brick-red deposits (Fig. 4C); and polarized light confirmed apple-green birefringence (Fig. 4D). In negative cases, no amyloid features were identified on any staining modality (Fig. 4E–H). These results demonstrate that CUBIC is compatible with conventional histopathology and does not interfere with routine pathological staining.
Fig. 4.
Validation of CUBIC compatibility with conventional histopathology and its application in screening suspected AL amyloidosis cases. A–D Confirmed AL amyloidosis: A Immunofluorescence staining shows amyloid deposits (dark green). B H&E staining shows homogeneous eosinophilic material. C Congo red staining reveals brick-red deposits. D Polarized light confirms apple-green birefringence. E–H Negative AL amyloidosis: E Immunofluorescence staining shows no amyloid deposits. F–H No amyloid pathological features shown on H&E staining, Congo red staining, or polarized light microscopy. I–L Suspected AL amyloidosis identified by CUBIC re-evaluation: I Immunofluorescence staining shows amyloid deposits (dark green). J–L H&E staining, Congo red staining, and polarized light show typical amyloid pathological features. Scale bars: 100 μm.
Based on this validation, CUBIC was then applied to re-evaluate ten cases previously diagnosed as negative by conventional pathology. According to predefined immunofluorescence staining grading criteria (see Materials and Methods), five cases were classified as CUBIC-positive and the remaining five as CUBIC-negative. The CUBIC-positive cases showed amyloid deposits on immunofluorescence staining (Fig. 4I), corroborated by corresponding H&E, Congo red, and polarized light features (Fig. 4J–L). Notably, one patient subsequently developed clinical manifestations consistent with AL amyloidosis and responded well to, therapy. while the remaining four are asymptomatic but under close surveillance for potential progression. To our knowledge, this is the first study to apply CUBIC for screening suspected AL amyloidosis, highlighting its potential as a novel tool for early detection.
Discussion
This study represents, to our knowledge, the first to apply CUBIC-based tissue clearing with three-dimensional imaging to tongue tissue in systemic AL amyloidosis. The method provided volumetric visualization of amyloid deposits, demonstrating vascular rarefaction, preferential involvement of small-caliber vessels, and associated muscle fiber atrophy. Notably, CUBIC detected amyloid in five of ten cases previously judged negative by conventional histopathology, including one that later progressed to clinically evident AL amyloidosis. These results support three-dimensional pathology as a complementary tool to conventional assessment, withthe potential to improve early diagnostic accuracy in suspected but histologically inconclusive cases.
Structural alterations observed in tongue tissue are likely to account for its clinical manifestations. Impaired vascular perfusion may contribute to mucosal ulceration and hypogeusia, while amyloid-related myofiber encasement and atrophy could underlie restricted tongue mobility [18].
Cardiac involvement is the most common and adverse manifestation of systemic AL amyloidosis, affecting up to 77% of cases [3]. It often presents with exertional dyspnea, peripheral edema, orthostatic hypotension, arrhythmias, and echocardiographic features of restrictive cardiomyopathy [19–21]. These structural and functional abnormalities suggest that similar pathological processes may occur in the heart—an organ central to prognosis but with incompletely understood mechanisms of amyloid-related injury [22]. Such processes may include amyloid infiltration impairing myocardial contractility and microvascular involvement that compromises perfusion [23].
Given the partial similarity in the muscular and vascular architecture of the tongue and heart [18, 19], tongue biopsy offers a minimally invasive, well-tolerated alternative with a low complication rate, whereas myocardial biopsy carries greater procedural risks and may be contraindicated in late-stage disease [11, 12]. The pathological alterations identified in tongue may therefore provide histopathological clues to analogous myocardial pathology, prompting closer cardiac monitoring and timely intervention to prevent irreversible damage and improve prognosis [18, 24, 25]. Thus, the tongue may serve as an accessible surrogate for high-risk cardiac assessment and a practical window into systemic amyloid pathology.
This study also revealed a significant reduction in vascular distribution within amyloid-deposited regions of the tongue compared with non-deposited areas, suggesting that local vascular obstruction and degeneration may result from amyloid accumulation. Such vascular compromise is likely to impair nutrient and oxygen delivery, leading to tissue ischemia and functional deterioration [26]. Comparable vascular involvement has been implicated in the pathogenesis of renal and other systemic manifestations of AL amyloidosis [27], supporting the hypothesis that similar microvascular pathology may underlies dysfunction across multiple organs and thereby contributes to disease progression and prognosis. Consequently, integrating vascular architectural changes and amyloid burden into a quantitative grading system, as demonstrated in renal amyloidosis [28], may provide a histopathological basis for disease staging and serve as a reference for both monitoring progression and evaluating prognosis. Particularly when direct sampling of critical organs is not impractical.
Early and accurate diagnosis of systemic AL amyloidosis remains challenging but is essential for guiding treatment and improving outcomes. A critical dilemma arises when conventional histopathology yields negative or inconclusive results in patients who nonetheless present with evident clinical symptoms strongly suggestive of AL amyloidosis. This issue is particularly critical in cardiac involvement, where diagnostic delays frequently result in irreversible myocardial damage and reduced survival [9, 25]. In this study, CUBIC-based 3D imaging offers a valuable supplementary strategy for patients with strong clinical suspicion but negative initial histopathology. By enabling full-volume visualization of biopsy specimens, it enhances detection of sparse or spatially amyloid deposits that may be missed or overlooked by conventional histopathology analysis [13]. Clinically, integrating this approach into diagnostic workflows may reduce missed diagnoses, expedite evaluation in suspected cases, and facilitate timely intervention. This is particularly important in patients at high risk of cardiac involvement, where early identification can improve treatment efficacy, prevent irreversible damage, and enhance survival [24].
Despite the novel insights and clinical relevance of this study, A few limitations should be noted. The small sample size and limited follow-up period restricted longitudinal assessment. Moreover, the current CUBIC workflow is time-consuming (approximately three weeks) and requires high-resolution confocal microscopy, which may limit its feasibility in routine clinical practice.
To address these challenges, we plan to expand the sample size through multicentre collaboration and extend follow-up to enable longitudinal outcome assessment. Building on the renal amyloid quantification framework proposed by Fan.Y et al. [29]. We will develop a standardized 3D grading system for tongue AL amyloidosis that integrates vascular and amyloid deposition metrics to support disease staging and monitoring. In parallel, the implementation of an AI-assisted platform for automated detection and quantification may further improve diagnostic efficiency and facilitate broader clinical adoption in the future [30].
Conclusion
This study presents the first 3D pathological characterization of AL amyloidosis in human tongue using the CUBIC technique combined with 3D imaging. The findings demonstrate that amyloid deposition disrupts both vascular and muscular architecture, yielding novel insights into disease mechanisms and clinical manifestations. Importantly, this approach improves detection in clinically suspected cases where conventional histopathology may be inconclusive, underscoring its potential value as a supplementary tool for early and accurate diagnosis.
Supplementary Information
Acknowledgements
Not Applicable.
Abbreviations
- 3D
Three-dimensional
- AL
Amyloid light-chain
- CUBIC
Clear, unobstructed brain/body imaging cocktails and computational analysis
- IF
Immunofluorescence
- ADR
Amyloid deposition region
- NADR
Non-amyloid deposition region
- DAPI
4′,6-Diamidino-2-phenylindole
- BNP
Brain natriuretic peptide
- FLC
Free light chain
- PBS
Phosphate-buffered saline
- PFA
Paraformaldehyde
- H&E
Hematoxylin and eosin
Authors’ contributions
Zuomin Wang and Yongcun Qu conceived the project and supervised the overall study design. Liqiong Wu performed the experiments, including sample preparation, CUBIC tissue clearing and immunostaining, with assistance from Fanyuan Sun, Si Xu, Xiaoli Gao, Jing Chen, Zheng Zhang, and Zhiqiang Liu. Liqiong Wu also conducted three-dimensional imaging and data acquisition, with technical support from Fanyuan Sun. Zheng Zhang contributed to image post-processing and data quantification. Data analysis was performed by Liqiong Wu and Fanyuan Sun under the supervision of Yongcun Qu. Liqiong Wu wrote the manuscript and prepared Figs. 1, 2, 3 and 4. Liqiong Wu and Fanyuan Sun contributed equally to this work. All authors read and approved the final manuscript.
Funding
This work was supported by the Beijing Natural Science Foundation Haidian Original Innovation Joint Fund (Grant No. L232104).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Beijing Chaoyang Hospital (Approval No. 2023–10-25–4). Informed consent was obtained from all individual participants included in the study. This study was conducted in accordance with the principles of the Declaration of Helsinki and relevant institutional guidelines.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Liqiong Wu and Fanyuan Sun contributed equally to this work.
Contributor Information
Yongcun Qu, Email: quyongcun@cupes.edu.cn.
Zuomin Wang, Email: wzuomin@sina.cn, Email: kokorora@sina.com.
References
- 1.Gertz MA. Immunoglobulin light chain amyloidosis: 2024 update on diagnosis, prognosis, and treatment. Am J Hematol. 2024;99(2):309–24. 10.1002/ajh.27177. [DOI] [PubMed] [Google Scholar]
- 2.Kumar N, Zhang NJ, Cherepanov D, et al. Global epidemiology of amyloid light-chain amyloidosis. Orphanet J Rare Dis. 2022;17(1):278. 10.1186/s13023-022-02414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wechalekar AD, Fontana M, Quarta CC, Liedtke M. AL Amyloidosis for cardiologists: awareness, diagnosis, and future prospects: JACC: Cardio Oncology state-of-the-art review. JACC Cardio Oncol. 2022;4(4):427–41. 10.1016/j.jaccao.2022.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shafqat A, Elmaleh H, Mushtaq A, et al. Renal AL amyloidosis: updates on diagnosis, staging, and management. J Clin Med. 2024;13(6):1744. 10.3390/jcm13061744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staron A, Zheng L, Doros G, et al. Marked progress in AL amyloidosis survival: a 40-year longitudinal natural history study. Blood Cancer J. 2021;11(8):139. 10.1038/s41408-021-00529-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustine JN, Staron A, Mendelson L, et al. Predictors of treatment response and survival outcomes in patients with advanced cardiac AL amyloidosis. Blood Adv. 2023;7(20):6080–91. 10.1182/bloodadvances.2023010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh A, Szabo A, Lian Q, et al. Timing and co-occurrence of symptoms prior to a diagnosis of light chain (AL) amyloidosis. Blood Cancer J. 2024;14(1):61. 10.1038/s41408-024-01040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulman A, Connors LH, Weinberg J, et al. Patient outcomes in light chain (AL) amyloidosis: the clock is ticking from symptoms to diagnosis. Eur J Haematol. 2020;105(4):495–501. 10.1111/ejh.13472. [DOI] [PubMed] [Google Scholar]
- 9.Oubari S, Naser E, Papathanasiou M, et al. Impact of time to diagnosis on Mayo stages, treatment outcome, and survival in patients with AL amyloidosis and cardiac involvement. Eur J Haematol. 2021;107(4):449–57. 10.1111/ejh.13681. [DOI] [PubMed] [Google Scholar]
- 10.Chee CE, Lacy MQ, Dogan A, Zeldenrust SR, Gertz MA. Pitfalls in the diagnosis of primary amyloidosis. Clin Lymphoma Myeloma Leuk. 2010;10(3):177–80. 10.3816/CLML.2010.n.027. [DOI] [PubMed] [Google Scholar]
- 11.Soares SM, Fervenza FC, Lager DJ, Gertz MA, Cosio FG, Leung N. Bleeding complications after transcutaneous kidney biopsy in patients with systemic amyloidosis: single-center experience in 101 patients. Am J Kidney Dis. 2008;52(6):1079–83. 10.1053/j.ajkd.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Zhang CL, Feng J, Cao XX, et al. Selection of biopsy site for patients with systemic amyloidosis. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2016;38(6):706–9. 10.3881/j.issn.1000-503X.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury R, Shah S, Latcha S, Lobato L. Kidney disease in systemic amyloidosis: a review of amyloid, amyloid serum A protein, leukocyte chemotactic factor 2, and transthyretin amyloid. Kidney360. 2024;5(12):1925–37. 10.34067/KID.0000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsumoto K, Mitani TT, Horiguchi SA, et al. Advanced cubic tissue clearing for whole-organ cell profiling. Nat Protoc. 2019;14(12):3506–37. 10.1038/s41596-019-0240-9. [DOI] [PubMed] [Google Scholar]
- 15.Liebmann T, Renier N, Bettayeb K, et al. Three-dimensional study of Alzheimer’s disease hallmarks using the iDISCO clearing method. Cell Rep. 2016;16(4):1138–52. 10.1016/j.celrep.2016.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nojima S, Susaki EA, Yoshida K, et al. CUBIC pathology: three-dimensional imaging for pathological diagnosis. Sci Rep. 2017;7(1):9269. 10.1038/s41598-017-09117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jennette JC, Olson JL, Silva FG, D’Agati VD, editors. Heptinstall’s Pathology of the Kidney. 8th ed. Philadelphia: Wolters Kluwer; 2022. [Google Scholar]
- 18.Tao Y, Qiu X, Ye F, Liao Z, Wu P. Amyloidosis of the tongue: a rare case report. Braz J Otorhinolaryngol. 2023;89(4):101286. 10.1016/j.bjorl.2023.101286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckberg G, et al. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–87. [DOI] [PubMed] [Google Scholar]
- 20.Stelmach-Gołdyś A, Zaborek-Łyczba M, Łyczba J, et al. Physiology, diagnosis and treatment of cardiac light chain amyloidosis. J Clin Med. 2022;11(4):911. 10.3390/jcm11040911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palladini G, Milani P, Merlini G. Novel strategies for the diagnosis and treatment of cardiac amyloidosis. Expert Rev Cardiovasc Ther. 2015;13:1195–211. [DOI] [PubMed] [Google Scholar]
- 22.Escher F, Senoner M, Doerler J, et al. When and how do patients with cardiac amyloidosis die. Clin Res Cardiol. 2020;109:78–88. 10.1007/s00392-019-01490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Migrino RQ, Harmann L, Christenson R, Hari P. Clinical and imaging predictors of 1-year and long-term mortality in light chain (AL) amyloidosis: a 5-year follow-up study. Heart Vessels. 2014;29(6):793–800. 10.1007/s00380-013-0419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picken MM. Modern approaches to the treatment of amyloidosis: the critical importance of early detection in surgical pathology. Adv Anat Pathol. 2013;20(6):424–39. 10.1097/PAP.0b013e3182a92dc3. [DOI] [PubMed] [Google Scholar]
- 25.Hester LL, Gifkins DM, Bellew KM, et al. Diagnostic delay and characterization of the clinical prodrome in AL amyloidosis among 1523 US adults diagnosed between 2001 and 2019. Eur J Haematol. 2021;107(4):428–35. 10.1111/ejh.13679. [DOI] [PubMed] [Google Scholar]
- 26.Seal J, Gewertz BL, Janczyk RJ, et al. Vascular dysfunction in ischemia-reperfusion injury. Ann Vasc Surg. 2005;19(4):572–84. 10.1007/S10016-005-4616-7. [DOI] [PubMed] [Google Scholar]
- 27.Bohle A, Wehrmann M, Eissele R, von Gise H, Mackensen-Haen S, Müller C. The long-term prognosis of AA and AL renal amyloidosis and the pathogenesis of chronic renal failure in renal amyloidosis. Pathol Res Pract. 1993;189(3):316–31. 10.1016/s0344-0338(11)80516-9. [DOI] [PubMed] [Google Scholar]
- 28.Rubinstein S, Cornell RF, Du L, et al. Novel pathologic scoring tools predict end-stage kidney disease in light chain AL amyloidosis. Amyloid. 2017;24(3):205–11. 10.1080/13506129.2017.1360272. [DOI] [PubMed] [Google Scholar]
- 29.Fan Y, Liang D, Rui H, et al. Using digital whole-slide images to evaluate renal amyloid deposition and its association with clinical features and outcomes of AL amyloidosis. J Nephrol. 2021;34(5):1747–56. 10.1007/s40620-020-00948-1. [DOI] [PubMed] [Google Scholar]
- 30.Yu KH, Zhang C, Berry GJ, et al. Predicting non-small cell lung cancer prognosis by fully automated microscopic pathology image features. Nat Commun. 2016;7:12474. 10.1038/ncomms12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.