Abstract
Background
Vinyl acetate (VA) is a synthetic chemical that can be metabolized to form the carcinogen acetaldehyde (AA). This paper summarizes the key evidence relevant to the evaluation of VA’s carcinogenicity.
Methods
We conducted a literature search and reviewed data relevant to the carcinogenicity of VA using a systematic approach. The literature reviewed included epidemiological studies, animal carcinogenicity studies, pharmacokinetic and metabolism studies, as well as studies relevant to the key characteristics of carcinogens.
Results
The body of epidemiological evidence includes several occupational studies with significant limitations and one prospective cohort study that assessed ambient air exposure to VA and breast cancer risk. The evidence from animal carcinogenicity studies is considered strong. VA induced tumors in a number of tissues across different strains of rats and mice in both sexes, via two exposure routes (inhalation and drinking water). Some tumor findings showed dose-related trends and were not limited to site-of-entry tissues. VA’s metabolic link to AA strengthens the evidence by providing biological plausibility: both chemicals induced many of the same DNA adducts, genotoxicity endpoints, and tumor types at many of the same sites. In addition, VA demonstrates three of the ten key characteristics of carcinogens in that it can be metabolically activated to be electrophilic, is genotoxic, and induces cell proliferation.
Conclusion
Our review of VA’s carcinogenicity shows compelling evidence in animal cancer bioassays with supporting mechanistic data, including the formation of reactive compounds and DNA adducts, evidence of genotoxicity including clastogenicity and DNA damage, and the ability to induce cell proliferation and pre-neoplastic lesions. The metabolic link to AA was an important consideration.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12940-025-01218-y.
Keywords: Vinyl acetate, Carcinogenicity, Acetaldehyde, Cancer epidemiology, Animal cancer studies, Key characteristics of carcinogens
Introduction
Vinyl acetate (VA; CAS No: 108-05-4; C4H6O2) is a monocarboxylic unsaturated aliphatic ester (Fig. 1) with a high production volume and is used in many industrial and commercial applications. Its primary use is as a monomer in the production of polymers and copolymers, such as polyvinyl acetate (PVA), ethylene-vinyl acetate (EVA) copolymers, and polyvinyl chloride-acetate copolymers [1, 2]. These VA-based polymers are used in adhesives (as an adhesion/cohesion promoter) and glues, paints, paper coatings, textile and leather finishing, plastics and resins, inks and lacquers, heat-sealing films, pesticides, and cosmetics (e.g., in hairspray) [1–4]. VA is also approved as a modifier for food starch [5]while VA-based polymers (e.g., PVA and VA-vinyl laurate copolymers) have been approved as food additives for use in chewing gum bases, with allowable residual levels of VA to be less than 5 ppm [5–7].
Fig. 1.
Chemical structure of vinyl acetate
VA has been detected in air (e.g., ambient air near emission sources), water (surface water, groundwater, and wastewater effluents), soil, and sediment [2, 3, 8].
Human exposure to VA may occur from occupational sources, or from the environment and use of consumer products. Occupational exposure to VA can occur during the production, transport, or use of VA monomers or VA-based polymers via inhalation or dermal contact [2, 3]. Consumer products that can expose individuals to VA include cigarette and cigar smoke due to the use of PVA or EVA as an adhesive in cigarette paper [9, 10]; microwave-heat-susceptor food packaging [11], carpets [12], building materials [13] and nail polish products [14]. Furthermore, VA was also detected in VA-vinyl laurate copolymers intended for use as a chewing gum base, albeit at low levels (less than 1 ppm) [7].
The International Agency for Research on Cancer (IARC) classified VA as a Group 2B carcinogen (possibly carcinogenic to humans) in 1995 [2]. IARC’s evaluation included consideration of VA’s metabolic link to its metabolite acetaldehyde (AA), an IARC Group 2B carcinogen since 1987 [2, 15]. AA is also “reasonably anticipated to be a human carcinogen” by the National Toxicology Program (NTP) Report on Carcinogens since 1991 [16]. In making the evaluation for VA in 1995, the IARC Working Group considered the facts that (1) VA is rapidly transformed into AA in human blood and animal tissues; (2) there is sufficient evidence in experimental animals for the carcinogenicity of AA; (3) both VA and AA induce nasal cancer in rats after administration by inhalation; and (4) both chemicals are genotoxic in human cells in vitro and in animals. In 2011, the European Chemicals Agency [17] classified VA as a Category 2 carcinogen, “chemicals suspected of causing cancer in humans”. The carcinogenicity of VA has not been reviewed or classified by any major health agencies in the US. Since the 1995 IARC evaluation, new epidemiological studies, animal cancer bioassays, and mechanistic studies on the carcinogenicity of VA have been published.
The Carcinogen Identification Committee (CIC) is a group of scientists designated as the “State’s Qualified Experts” for evaluation of chemical carcinogenicity under California’s Proposition 65. In developing the hazard identification document for VA for evaluation by the CIC, we conducted a comprehensive search of the scientific literature for VA studies relevant to its potential carcinogenicity, and compiled and summarized evidence from human and animal studies, metabolism studies, and mechanistic studies [18]. At their December 19, 2024, meeting, the CIC voted unanimously (11 yes, 0 no) to add VA to the Proposition 65 List for cancer.
Here we summarize the key findings of VA’s carcinogenicity from each evidence stream (human, animal, and mechanistic data). Comparisons between VA and its metabolite AA are made with regard to animal tumors induced, DNA adducts formed, and genotoxicity. We also discuss the potential implication of enzyme polymorphisms associated with reduced ability to detoxify AA for susceptible populations.
Methods
Literature search process
Literature searches were conducted in May 2023 to identify peer-reviewed journal articles, print and digital books, reports, and gray literature that potentially reported toxicological and epidemiological information on the carcinogenicity of VA. We used an approach similar to that recommended in NTP’s Handbook for Preparing Report on Carcinogens Monographs [19]. Primary searches were conducted of major biomedical databases and other data sources. We also considered information submitted by the public during a data call-in period from July–September 2023.
Primary searches for VA were conducted using chemical synonyms in combination with search terms for human cancer studies, animal cancer studies, toxicokinetic studies, and mechanistic studies for genotoxicity and other key characteristics of carcinogens. There were no restrictions in the searches on exposure route or duration of exposure on cancer studies in humans, cancer studies in animals or mechanistic studies, or on publication language. Detailed information on the literature search process is presented in OEHHA (2024) [18] Appendix A.
Literature screening process
Process for human cancer studies, animal cancer studies, and studies on key characteristics of carcinogens and other mechanistic concepts
HAWC (Health Assessment Workspace Collaborative, https://hawcproject.org) [20] was used as a tool to screen and tag the literature. Citations retrieved from the literature searches were uploaded to EndNote libraries, duplicates were removed, then uploaded to HAWC for multi-level screening using specific inclusion and exclusion criteria (see OEHHA 2024, Appendix A) [18].
In Level 1 screening in HAWC, each citation was screened by at least one of the co-authors, based on title and abstract, to eliminate studies or articles that do not contain information on VA and any of the key topics, such as cancer studies in humans or animals, genotoxicity, or other cancer-associated mechanisms. Papers identified for inclusion were tagged in HAWC according to the key topics. In Level 2 screening, full-text papers for all citations that passed the Level 1 screening were screened by at least one of the co-authors, using similar inclusion/exclusion criteria as was used in the Level 1 screening, and tags were updated as needed. If additional relevant studies not cited in the original set of publications (“secondary citations”) were identified, Level 1 and 2 screenings were conducted, and HAWC search results were updated.
Table Builder [20], a web-based application, was applied to systematically extract and analyze the data from included cancer epidemiology studies. Additionally, Table Builder was used as a custom-made database to generate tables in Microsoft Word.
Process for pharmacokinetics and metabolism-related studies
Five Endnote libraries were created, each focusing on a specific topic: vinyl acetate absorption, distribution, metabolism and excretion (ADME); acetaldehyde metabolism; vinyl acetate and acetaldehyde studies; acetaldehyde and cytochrome P450 (CYP450) and other monooxygenase studies; and enzyme polymorphisms. Two libraries (vinyl acetate and acetaldehyde studies and acetaldehyde and CYP450 and other monooxygenase studies) were small and thus screened manually by two of the co-authors.
Sciome Workbench for Interactive computer-Facilitated Text-mining Active Screener (SWIFT Active Screener) (https://www.sciome.com/swift-activescreener/) [21] was used as a tool to facilitate the initial screening of the remaining three libraries related to pharmacokinetics and metabolism (i.e., vinyl acetate ADME, acetaldehyde metabolism, and enzyme polymorphisms). This initial screening in SWIFT-Active Screener allowed for efficient initial literature inclusion and exclusion with the help of artificial intelligence. In each project, two of the co-authors independently completed the screening for a decision to be made on each title and abstract, following predefined inclusion and exclusion criteria.
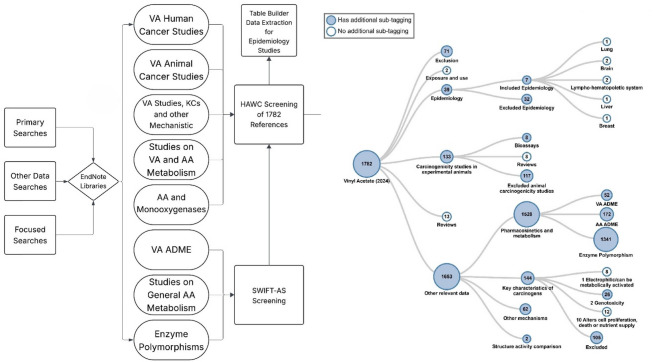
Total references and diagram of the literature search and screening process
Over 1700 references, including peer-reviewed journal articles and government reports, were identified for inclusion through these search strategies. Among these, over 170 references were cited in OEHHA (2024) [18] and a subset of key references are cited in this publication. See Fig. 2 for a summary diagram of the literature search and screening process.
Fig. 2.
Overview of the Literature Search and Screening Process. Eight Endnote libraries were deduplicated and screened in HAWC. Among these eight, three libraries went through initial screening using SWIFT-Active Screener (SWIFT-AS). VA vinyl acetate, AA acetaldehyde, KCs Key Characteristics of Carcinogens, ADME absorption, distribution, metabolism, and elimination
Results
Metabolism of vinyl acetate
The metabolism of VA plays an important role in its carcinogenicity, based on the formation of a carcinogenic metabolite, acetaldehyde (AA), and the generation of electrophilic and genotoxic compounds including DNA and protein adducts, reactive oxygen species (ROS), and alkyl radicals [18]. This section describes the key enzymes and enzymatic reactions, metabolites, and relevant enzyme polymorphisms. For a more detailed summary, see Sect. 5.1 of OEHHA (2024) [18].
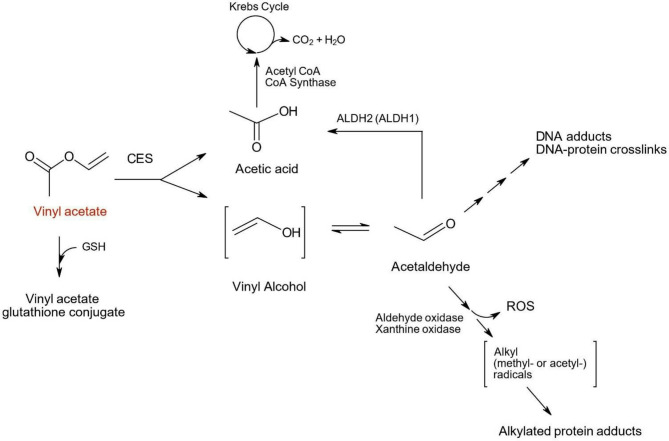
The first step in VA metabolism is its hydrolysis via carboxylesterases (CES), resulting in the formation of acetic acid and vinyl alcohol, an unstable molecule which quickly rearranges to form AA (Fig. 3). The CES-mediated hydrolysis reaction can be inhibited when animals, cells or tissues are treated with the CES inhibitor bis (p-nitrophenyl) phosphate (BNPP) [22, 23].
Fig. 3.
Major metabolic pathways for Vinyl acetate. Vinyl acetate is oxidized to acetic acid and vinyl alcohol via carboxylesterases (CES). The unstable vinyl alcohol is rearranged into acetaldehyde and is further metabolized by ALDH2 to another molecule of acetic acid which is subsequently introduced into the Krebs cycle. Downstream of acetaldehyde, DNA adducts and DNA-protein crosslinks are formed. AA can also be oxidized by aldehyde oxidase and xanthine oxidase. Both enzymatic reactions can form reactive oxygen species (ROS), and xanthine oxidase also produces acetyl and methyl radicals. These radicals can lead to formation of alkylated protein adducts
In a second step, AA is further metabolized to acetic acid by aldehyde dehydrogenases, primarily aldehyde dehydrogenase 2 (ALDH2), a polymorphic enzyme. This reaction releases protons from two sources: the NAD+-dependent ALDH2 oxidation of AA and the disassociation of protons from acetic acid at physiological pH, both of which result in a slight lowering of the intracellular pH [23].
Following AA oxidation by ALDH2, acetic acid undergoes condensation with Coenzyme A (CoA) via acetyl-CoA synthase and is introduced into the tricarboxylic acid (Krebs) cycle for further metabolism [24].
Factors affecting the level of acetaldehyde
ALDH2 plays a key role in the detoxification of AA, based on its high affinity for AA (Km as low as 0.2 µM) [25, 26]. AA is a known carcinogen that is genotoxic in multiple in vitro and in vivo systems, and induces tumors in rodents, including nasal tumors in rats [27, 28] (see also “Comparison of animal carcinogenicity evidence on VA and AA” below).
The metabolic efficiency of ALDH2 is highly impacted by genetic polymorphisms in humans, which can lead to a partial or complete loss-of-function and thus can increase AA levels. Several ALDH2 allelic forms have been examined, with most studies focusing on the rs671 missense variant, NM_000690.4:c.1510G > A, that results in an amino acid change from glutamic acid to lysine (p.Glu504Lys). This allele is commonly denoted as ALDH2*2 and it impairs the enzyme by interfering with its ability to form a tetramer and thus confers a dominant negative phenotype [29]. Thus, individuals that are homozygous wildtype (ALDH2 *1/*1) have full ALDH2 activity; individuals that are heterozygous (ALDH2 *1/*2) have less than half the enzymatic activity, and individuals that are homozygous (ALDH2 *2/*2) for the polymorphism have no enzymatic activity [30]. The ALDH2*2 variant is mainly found in individuals of East Asian descent. Up to 40% of Chinese, Korean, Japanese, or Taiwanese individuals are heterozygous while another 5 to 10% are homozygous for the polymorphism [31]. The rs671 variant has also been reported in some Southeast Asian populations at lower frequencies and is extremely rare in other ethnic groups [31].
The reduced or non-functioning ALDH2 variants can result in significant build-up of AA. A study in healthy male volunteers from Japan showed that AA levels in blood, following ingestion of ethanol (0.4 g/kg of body weight over 10 min), increased from 4.1 µM in subjects with the ALDH2*1/*1 genotype (N = 33) to 23.4 µM in subjects with the heterozygous ALDH2*1/*2 genotype (N = 29), and 79.3 µM in subjects with the homozygous ALDH2*2/*2 genotype (N = 6) [32].
In addition to ALDH2 polymorphisms, repression and/or lack of ALDH2 activity by other factors such as taking pharmaceutical ALDH2 inhibitors (e.g., disulfiram) also leads to increased levels of AA. Increased levels of AA were observed in vitro in human lung cancer cells (A549) with repressed expression of ALDH2 (A549- shALDH2 cells) [33]. Additional experiments highlight the protective role ALDH2 plays, showing that increased AA-induced DNA damage occurred in A549-GFP cells (A549 cells with normal ALDH2 expression) compared to A549-ALDH2 cells that overexpressed ALDH2 [33]. Li et al. (2019) [33] also reported accumulation of AA and increased DNA damage (measured as γH2AX) in vivo in lung tissues from Aldh2-knockout mice compared to wildtype mice. In another study male C57BL/6 wildtype and knockout mice (Aldh2-/-) were administered 125 or 500 ppm of AA for 14 days via inhalation. In Aldh2-/- mice, AA levels in the blood were increased five-fold in the 500-ppm group compared to controls [34].
Reactive metabolites, reactive oxygen species (ROS), alkylated proteins, DNA-protein crosslinks, and DNA adducts
AA that has not been cleared by ALDH2 can be further metabolized by other oxidative enzymes, i.e., xanthine oxidase and aldehyde oxidase. Oxidation of AA via xanthine oxidase leads to the formation of acetyl (methylcarbonyl) radicals and methyl radicals, ROS (e.g., superoxide anion, hydrogen peroxide, and hydroxyl radicals), and alkylated proteins [35–37]. Acetyl radicals were observed in cell-free systems when AA was incubated with xanthine oxidase. Radical formation was dependent on the concentration of added AA, whereas the addition of a hydroxyl radical scavenger (4-pyridyl-1-oxide-N-t-butylnitrone), superoxide dismutase, or catalase inhibited acetyl radical formation and the formation of alkylated protein adducts [35].
Following intragastric administration of AA, methyl radicals were identified in bile samples of bile duct-cannulated male Sprague-Dawley (SD) rats, along with strong evidence indicative of acetyl radical formation [36]. Similarly, production of acetyl and methyl radicals was observed when beef heart submitochondrial particles (derived from the inner mitochondrial membrane) were incubated with AA [36]. These authors also showed, in a cell-free system, that production of acetyl and methyl radicals via xanthine oxidase-catalyzed oxidation of AA was significantly reduced in the presence of the xanthine oxidase inhibitor allopurinol [36].
The oxidation of AA via aldehyde oxidase also generates ROS, including superoxide anion, hydrogen peroxide, and hydroxyl radical [38–40]. Using a cell-free system, the formation of superoxide during the oxidation of AA with aldehyde oxidase from rat liver was observed [38].
Both VA and AA form DNA-protein crosslinks (DPXL) in rodent in vitro systems [41] and both form DNA adducts in rodent in vivo studies [34, 42, 43]. In the Kuykendall study (1993) [41] DPXLs were identified in isolated rat olfactory and respiratory epithelial cells from nasal turbinates following exposure to VA or AA. Increases in AA- and VA-induced DPXLs were time- and dose-dependent. Preincubation with the CES inhibitor BNPP greatly reduced the formation of VA-induced DPXLs in both olfactory and respiratory cells. DNA adducts (primarily N2-ethylidene-deoxyguanosine, or N2-EtD-dG measured in the reduced form as N2-ethyl-dG, or N2-Et-dG) in nasal respiratory and olfactory epithelia were identified by Hsiao et al. (2022) [42] and Liu et al. (2021) [43] following inhalation exposure of rats to isotope-labeled [13C2]-VA monomer and subsequent DNA adduct analysis. Oyama et al. (2010) [34] observed significant increases of the DNA adduct N2-ethylidene-dG (measured in its reduced form as N2-ethyl-dG) in Aldh2 knockout (Aldh2-/-) mice exposed to AA compared to wildtype mice in the nasal epithelium, lung, and dorsal skin, but not in the liver.
Carcinogenicity studies in humans
Epidemiological evidence on VA
There were few epidemiological studies that reported on cancer associated with exposure to VA. One study assessed residential exposure to VA in ambient air (Heck et al. 2024) [44] while the remaining studies assessed occupational exposure. The outcomes studied were lymphohematopoietic cancers [45, 46] brain cancer [47, 48] lung cancer [49], angiosarcoma of the liver [49, 50] and breast cancer [44]. Two studies did not report risk estimates nor enough data for their calculation [49, 51]. The publication by Waxweiler (1981) [51] was excluded from further review because the study population exposed to VA was not clearly identified. There was more than one report from the same study population for lymphohematopoietic cancers [45, 46] and brain cancer [47, 48].
Heck et al. (2024) [44] assessed the association between ambient levels of several air toxics, including VA, and breast cancer risk in the Multiethnic Cohort study. Study participants were 48,665 Californian women residing in the greater Los Angeles area who were followed from 2003 to 2013. Residential addresses geocoded for 1998–2000 and 2001–2003 were linked to the 1999 and 2002 National Air Toxics Assessment (NATA) models, respectively, according to the year 2000 census tracts. VA was not highly correlated with any of the other measured air toxics (range of r2 values: 0.03–0.35 for 1,3-butadiene and methyl isobutyl ketone, respectively). The mean modeled air concentration of VA for the residential census tracts was 8.14 × 10−3 micrograms per cubic meter (µg/m3) (range: 7.93 × 10−7 – 4.87 × 10−2 µg/m3) among all participants and was highest among African Americans compared to Japanese Americans, Latinos, and Whites.
Cox proportional hazards models were used to estimate invasive breast cancer risk per interquartile range (IQR) increase in air toxics exposure lagged by 5 years [44]. The covariates included in all of the adjusted models were race, ethnicity, education, alcohol use, smoking, and several other known risk factors for breast cancer. Among all women, increased risks of invasive breast cancer were observed with VA exposure (hazard ratio (HR), 5.27; 95% CI, 4.14–6.73). Increased risks were also observed in analyses stratified by breast cancer subtypes (hormone receptor-positive or -negative) and race/ethnicity, analyses restricted to non-smokers, and several other sensitivity analyses. The highest adjusted risk estimates were observed in African Americans (HR, 11.30; 95% CI, 7.36–17.35) and in women with hormone receptor-negative tumors (HR, 7.09; 95% CI, 5.18–9.70). Detailed results are presented in Table 1.
Table 1.
Epidemiological studies of VA and cancer (presented newest to oldest)
| Reference, study design, location, year | Population, exposure assessment details | Cancer site/type | Results | Comments | ||
|---|---|---|---|---|---|---|
| Exposure category or level | Risk estimate (95% CI) | Exposed cases or deaths | ||||
| General population study | ||||||
|
Heck et al. (2024) [44] Prospective cohort Los Angeles, CA 1993–2013 |
48,665 women enrolled in the Multiethnic Cohort Ambient air toxics modeled with NATA and HAPEM; linked to geocoded addresses by census tract |
Breast | HR, per IQR increase in VA, 5-year exposure lagging | All models adjusted for: age at entry, race and ethnicity, BMI, family history of breast cancer, age at first live birth, age at menarche, number of children, menopausal status, hormone replacement therapy, physical activity, energy intake, alcohol use, smoking, education, neighborhood SES | ||
| All women | 5.27 (4.14–6.73) | NR | ||||
| Non-smokers | 4.82 (3.59–6.48) | NR | ||||
| Hormone receptor-negative (ER- & PR-) | 7.09 (5.18–9.70) | NR | ||||
| Hormone receptor-positive (ER+ & PR+) | 4.77 (3.70–6.15) | NR | ||||
| African Americans | 11.30 (7.36–17.35) | NR | ||||
| Japanese Americans | 3.81 (2.33–6.24) | NR | ||||
| Latinos | 4.69 (3.32–6.62) | NR | ||||
| Whites | 5.71 (4.04–8.06) | NR | ||||
| Non-movers only | 6.76 (4.63–9.87) | NR | ||||
| Movers only | 5.28 (4.14–6.73) | NR | ||||
| Occupational studies | ||||||
|
Lewis & Rampala (2003) [50] Case-cohort Louisville, Kentucky 1942–2002 |
6,076 employees at PVC & nitrile rubber copolymer plant. Analysis limited to 1,817 white men hired before 1967 & worked ≥ 1 year Chemical exposure estimates ranked for each job, building, & year |
Liver cancer (angio-sarcoma) | OR, ever exposed | [1.0 (0.994–1.005)] a | NR |
Model adjusted for vinyl chloride exposure Limitations: Cases co-exposed to several other chemicals (e.g., vinyl chloride, vinylidene chloride, acrylonitrile, 1,3-butadiene, styrene). No quantitative exposure data or measurements of VA exposure. |
|
Ott et al. (1989) [45] Nested case-control West Virginia 1940–1978 |
29,139 men employed at UCC facilities Chemical exposure estimates based on work activity, area, & production. |
Non-lymphocytic leukemia | OR, ever exposed | 0.5 (NR) | 2 |
Same analysis and study population as a pre-publication report (Union Carbide 1989) Limitations: crude categorization of exposure, lack of adjustment for co-exposures (e.g., vinyl chloride, acetaldehyde, acrylonitrile, benzene, 1,3-butadiene, dioxane) & confounders, few exposed cases. |
| Lymphocytic leukemia | OR, ever exposed | 1.8 (NR) | 2 | |||
| Multiple myeloma | OR, ever exposed | 1.6 (NR) | 3 | |||
| Non-Hodgkin’s lymphoma | OR, ever exposed | 1.2 (NR) | 7 | |||
|
Leffingwell et al. (1983) [48] Nested case-control Texas City, Texas 1950–1977 |
Male employees at UCC chemicals & plastics plant Exposures estimated by which departments used, produced, or redistributed chemicals. Company records verified by NIOSH/OSHA researchers. |
Brain cancer (glioma) | OR, years of exposure (excluding maintenance workers) |
Same study population as Austin and Schnatter (1983) Limitations: workers exposed to multiple chemicals that were not adjusted for in analyses (e.g., vinyl chloride, acetaldehyde, diethanolamine, methyl isobutyl ketone). No quantitative measurements. |
||
| 0–14 years | 3.1 (0.8–12.05) b | 5 | ||||
| 15 + years | 2.74 (0.57–13.2) b | 5 | ||||
| Ever exposure | 3.3 (0.84–12.88) b | 6 | ||||
| OR, years of exposure (including maintenance workers) | ||||||
| 0–14 years | 2.67 (0.85–8.38) b | 11 | ||||
| 15 + years | 1.89 (0.62–5.75) b | 11 | ||||
| Ever exposure | 2.47 (0.88–6.94) b | 13 | ||||
|
Austin and Schnatter (1983) [47] Case-control Texas City, Texas 1941–1977 |
White male employees at a UCC chemical plant. Industrial hygienists identified principal chemicals used/produced in each department at any time in history of plant |
Brain cancer (glioma + meningioma) | OR, ever exposed | Limitations: cannot accurately determine exposures to specific chemicals for approx. half of employees because they worked in maintenance and were highly mobile. Workers exposed to multiple chemicals that were not adjusted for in analyses (e.g., vinyl chloride, benzene, ethylene oxide) | ||
| Control 1 | [1.75 (0.42–7.3)] a | 5 | ||||
| Control 2 | [1.64 (0.39–6.98)] a | 5 | ||||
| Hourly Control 1 | [1.64 (0.39–6.98)] a | 5 | ||||
| Hourly Control 2 | [1.70 (0.39–7.36)] a | 5 | ||||
| Brain cancer (glioma) | OR, ever exposed | |||||
| Control 1 | [2.19 (0.49–9.76)] a | 5 | ||||
| Control 2 | [2.05 (0.45–9.31)] a | 5 | ||||
| Hourly Control 1 | [2.05 (0.45–9.31)] a | 5 | ||||
| Hourly Control 2 | [2.13 (0.46–9.83)] a | 5 | ||||
|
Waxweiler et al. (1981) [49] Retrospective cohort and nested case-control designs Kentucky 1942–1973 |
4,806 white male employees at chemicals plant Doses expressed as “dose units” calculated by multiplying exposure ranking assigned to each employee based on job history & task by number of calendar days worked. |
Lung cancer |
Did not report risk estimates. Reported only cumulative dose difference (observed minus expected) in lung cancer cases. Subgroup with large-cell undifferentiated lung cancer had slightly higher cumulative exposure than expected. Observed doses were lower in cases compared to non-cases for all lung cancer and adenocarcinoma and large-cell undifferentiated cancers combined. |
Limitations: workers exposed to multiple chemicals that were not adjusted for in analyses (e.g., vinyl chloride, acrylonitrile, 1,3-butadiene). No quantitative exposure data or measurements. | ||
BMI Body mass index, CI Confidence interval, ER Estrogen receptor, HAPEM Hazardous Air Pollutant Exposure Model, HR Hazard ratio, IQR Interquartile range, NATA National Air Toxics Assessment, NR Not reported, NOx Nitrogen oxides, OR Odds ratio, PR Progesterone receptor, PVC Polyvinyl chloride, SES Socioeconomic status, UCC Union Carbide Corporation
aOR and 95% CI calculated from data reported in publication [indicated by brackets] (see Appendix B of OEHHA 2024 for details)
b90% CIs reported in publication
There were several strengths of this study, including large sample size, prospective cohort study design, multiethnic population, its use of a detailed questionnaire that collected data on multiple covariates and detailed residential histories available for residents who lived in California during the study period [44]. This analysis was well-powered to study air toxics because it was conducted in an urban setting with high traffic and industrial pollutant levels. A unique feature was the inclusion of neighborhoods comprised primarily of historically marginalized racial and ethnic groups that incurred higher pollution burden.
For interpreting the exposure assessment, there are several notable issues. Most importantly, there was some imprecision and uncertainty in the NATA-modeled air toxics exposure estimates at the census tract level [52]. Having more localized measurements at individuals’ residences or personal monitoring to account for exposures acquired outside of one’s residential neighborhood likely would have enhanced the precision of the exposure assessment. Additionally, the list of chemicals assessed was not exhaustive, and it is possible the chemicals studied are correlated with unmeasured chemicals. Finally, exposures occurring earlier in life outside of the study period were not accounted for [44].
The remainder of the studies were conducted in workers but were considered less informative. In all of these studies, exposure was assessed by combining employee work history with chemical exposure estimates for the manufacturing plant. In brief, ever having been exposed to VA was reported to be associated with an increased risk of brain cancer (though not statistically significant) [47, 48] and lymphohematopoietic cancers (though confidence intervals were not reported) [45], but no associations were observed for lung cancer or liver angiosarcoma. Interpretation of each of these studies was limited by small numbers of exposed cases, confidence intervals that were wide or lacked statistical significance or were not reported, and crude methods for assessing exposure to VA. For example, most studies assessed only ‘ever exposure’ to VA without further information on intensity, frequency, or duration of exposure. Although the epidemiological studies were conducted in populations potentially highly exposed occupationally to VA, co-exposure to multiple chemicals, including carcinogens (e.g., vinyl chloride, 1,3-butadiene, and acrylonitrile), was another challenge to interpreting these data. Co-exposures were generally not accounted for in statistical analysis; thus, it is difficult to attribute any cancer outcomes in these workers specifically to exposure to VA.
Details of study design and epidemiological findings for these studies are presented in Table 1.
Epidemiological evidence on AA
AA has been classified by IARC as a Group 2B carcinogen since 1999 based on sufficient evidence in experimental animals [28]while since 2009, “acetaldehyde associated with consumption of alcoholic beverages” has been classified as a Group 1 carcinogen based on sufficient evidence in humans, particularly cancers of the esophagus and the upper aerodigestive tract [53]. In making this determination, IARC noted that the primary carcinogenic compound in alcoholic beverages, ethanol, is converted into AA, which is oxidized to acetate via ALDH, and that there is sufficient evidence showing that humans who are ALDH2-deficient have an increased risk for developing alcohol-related cancers [53].
Animal carcinogenicity evidence on VA
A total of 24 animal bioassays investigating the carcinogenicity of VA have been reported by several laboratories from the US [54, 55], Japan, [56, 57] and Europe, including the Hazleton Laboratories in the UK [58–61] and the Ramazzini Institute in Italy [62–64]. Sixteen of these studies were conducted in five strains of rats, and eight studies were conducted in three strains of mice. The strains and species include male and female SD rats, SD derived Crl: CD(SD)BR rats, Fischer 344 (F344) rats, F344/DuCrj rats, Wistar rats, Swiss mice, Swiss-derived Crl: CD-1(ICR)BR mice, and Crj: BDF1 mice. Among the 24 studies, there were four inhalation studies and 20 drinking water studies.
In rats, ten studies started treatment when the animals were 6 weeks of age or older, with exposure durations of 100 or 104 weeks. In addition, six studies started exposures during the preconception or in utero period, with exposures continuing after birth until 104 weeks of age, and animals in these studies are referred to as male or female offspring (or F1) rats (See Supplementary Table 1). In mice, six studies started treatment when the animals were 6 weeks of age or older, with exposure durations of 78 or 104 weeks. Two studies of F1 mice started exposures in utero and continued after birth until 78 weeks of age (See Supplementary Table 2). Study design and tumor incidences from each animal study are shown in Supplementary Tables 1 and 2, and additional details can be found in OEHHA (2024) [18].
In summary, VA exposure increased the incidences of a number of tumor types across different strains of rats and mice. Statistically significant increases in tumors of the respiratory, digestive, endocrine, reproductive, and immune systems were observed in treated animals compared with the controls using the Fisher’s exact pairwise test. Some tumors occurred in multiple dose groups, with significant dose-related trends. Examples include forestomach squamous cell carcinomas in male F1 SD rats (control, 0/107; 1000 ppm, 6/83**; 5000 ppm, 7/53***; Fisher pairwise comparison with control p < 0.01 and p < 0.001, respectively; Exact trend test, p < 0.01) and female F1 SD rats (control, 0/99; 1000 ppm, 3/87; 5000 ppm, 4/57*, Fisher pairwise comparison with control, p < 0.05; Exact trend test, p < 0.05), where VA was administered in the drinking water (Suppl. Table 1). In male F1 Swiss mice, VA administered via drinking water significantly increased forestomach acanthomas by pairwise comparison and had a significant dose-related trend (control, 0/38; 1000 ppm, 1/37; 5000 ppm, 8/49**, Fisher pairwise comparison with control, p < 0.01; Exact trend test, p < 0.01) (Suppl. Table 2). Among the VA treatment-related tumors observed, several types were considered rare (i.e., occurring at a rate less than 1% in appropriate historical controls) [65]. Table 2 provides a comprehensive overview of tumor findings from the rat and mouse carcinogenicity studies.
Table 2.
Summary of tumor findings from animal studies of vinyl acetate, with statistically significant findings in bold
| Species & strain System |
Rats | Mice | |||||
|---|---|---|---|---|---|---|---|
| SD [62] | SD derived Crl: CD(SD)BR [58, 60] | F344 [54, 55] | F344/DuCrj [56, 57] | Wistar [63] | Swiss [64] | Crj: BDF1 [56, 57] | |
| Respiratory |
Nasal cavity SCP (M)r, SCC (M r, F 2, r), carcinoma in situ (M)r, SCP, SCC and carcinoma in situ combined (M) r, Larynx SCC (F) r |
Pharynx carcinoma (M) 2 | Lung adenoma (F) 2 | Larynx SCC (M) r | |||
| Digestive |
Oral cavity and lips SCC (M, F), Tongue SCC (F) r, Forestomach SCC (M, F) r, Pancreatic Islet cell adenoma (M), Pancreatic exocrine adenoma (M) r |
Liver hepatocellular adenoma (F) |
Oral cavity and lips SCC (M, F) r, SCP (M) r, SCC and SCP combined (M) r |
Oral cavity and lips SCC (M, F), Tongue SCC (F), Esophagus SCC (M 2, F), Forestomach SCC (M, F) 2, Pancreatic exocrine adenoma (M) |
Oral cavity SCC (M, F), Tongue SCC (M 2, F), Esophagus SCC (M, F), Esophagus acanthoma (F) 2, Forestomach SCC (F), Forestomach acanthoma (M, F) |
Oral cavity and lips SCC (M, F) r, SCP (M, F) 2, r, SCC and SCP combined (M, F) r, Esophagus SCC (M) r, Forestomach SCC (M r, F2, r), SCP (M, F) r, SCC and SCP combined (M r, F 2, r) | |
| Endocrine | Adrenal gland pheochromo-blastoma (F) | Pituitary adenoma (F), Thyroid C-cell tumors (adenoma, adenoma and carcinoma combined) (F) | Thyroid C-cell tumors (adenoma, adenoma and carcinoma combined) (F) | Adrenal gland pheochromo-blastoma (M), pheochromo-cytoma (F) | |||
| Reproductive |
Uterine adenocarcinoma (F)r, Uterine endometrial stromal polyp (F) |
Testicular interstitial cell tumors (M) |
Uterine adenocarcinoma (F), Uterine fibrosarcoma (F) 2 |
Uterine leiomyosarcoma (F) | |||
| Immune | Lymphomas and leukemias of the hemolympho-reticular tissues (F) | Spleen malignant lymphoma (F) | |||||
| Auditory | Zymbal gland carcinoma (F) | ||||||
| Integumentary | Mammary gland adenocarcinoma (F) 2 | Mammary gland liposarcoma (F) 2 | |||||
r rare tumor, M Male, F Female, SCC Squamous cell carcinoma, SCP Squamous cell papilloma
1 The 104-week inhalation studies in male and female Crl:CD-1(ICR)BR mice [58, 61] did not identify any treatment-related tumors and are not included in this table
2 Tumor incidences with an apparent statistically significant trend, driven by increases (statistically non-significant) of tumors only at the high dose in comparison to controls. These findings are not in bold
The strength of evidence from VA animal carcinogenicity studies is strong. Notably, when comparing tumor sites across different species and strains, tumor findings were observed in multiple organs and systems in both rats and mice. Some target tumor sites or types were consistently found across different strains of rats or mice. For example, VA induced tumors in the oral cavity, pancreas, and adrenal glands in both SD and Wistar rats [62, 63]. Tumors of the oral cavity, esophagus, and forestomach occurred in both Swiss and Crj: BDF1 mice [56, 57, 64]. In several studies, VA induced tumors in multiple organ systems. For example, in F344, F344/DuCrj, and Wistar rats and in Swiss mice, tumors were observed in at least three organ systems. Moreover, consistent findings of certain tumor types were observed in multiple strains and in both sexes. For example, oral cavity tumors were seen in male and female SD, F344/DuCrj, and Wistar rats, and in male and female Swiss and Crj: BDF1 mice [56, 57, 62–64]. Additionally, some of the tumors occurred in tissues distant to the site of entry and were not part of the gastrointestinal or respiratory tract for exposures administered orally or via inhalation, respectively. The distal tumor sites in rat drinking water studies include the uterus, thyroid gland, pancreas, and adrenal glands, while the distal tumor sites in mouse drinking water studies include the uterus, mammary glands, Zymbal glands, and spleen.
Comparison of animal carcinogenicity evidence on VA and AA
NTP (2021) [16] and IARC (1999) [28] reviewed and summarized the evidence for the animal carcinogenicity of AA. In inhalation studies, AA induced tumors of the respiratory tract, including adenocarcinomas and squamous cell carcinomas of the nasal mucosa in rats and laryngeal carcinomas in hamsters. In drinking water studies [66], administration of AA increased the tumor incidence of hemolymphoreticular tissues (leukemia and lymphoma combined), benign tumors of the pancreas (islet-cell adenoma), bone osteosarcomas and nasal cavity carcinomas in male rats, and benign mammary gland tumors (fibroma or fibroadenoma) in female rats. Increased incidences of tumors observed at other sites occurred only at one of the lower doses tested [16, 66].
In animal studies, most target tumor sites associated with AA administration overlapped with those associated with VA administration (Table 3). From inhalation studies, nasal tumors were observed in rats exposed to either VA or AA. Laryngeal tumors were observed in rats exposed to VA, and in hamsters exposed to AA. From drinking water studies, exposure to either VA or AA induced tumors in hemolymphoreticular tissues, pancreas, and mammary glands in rats. In addition, VA exposure has been shown to induce tumors in additional tissues in the respiratory, digestive, endocrine and auditory systems, such as the tongue, esophagus, forestomach, pituitary gland, thyroid gland, and adrenal glands. These findings are presented in Table 2.
Table 3.
Comparison of tumor sites between vinyl acetate and acetaldehyde
| Route | Vinyl Acetate | Acetaldehyde |
|---|---|---|
| Inhalation |
• Nasal tumors in rats • Laryngeal tumors in rats |
• Nasal tumors in rats • Laryngeal tumors in hamsters |
| Drinking water |
• Hemolymphoreticular cancer (leukemia and lymphoma combined) in rats • Pancreatic tumors (islet cell adenoma and exocrine adenoma) in rats • Mammary gland tumors in rats (adenocarcinoma) and mice (liposarcoma) • And several other sites, e.g., tongue, esophagus, forestomach, pituitary gland, thyroid gland, adrenal glands, Zymbal glands (see Table 2 for details) |
• Hemolymphoreticular cancer (leukemia and lymphoma combined) in rats • Pancreatic tumors (islet-cell adenoma) in rats • Mammary gland tumors (benign fibroma or fibroadenoma) in rats • Nasal cavity tumors (carcinoma) in rats • Bone tumors (osteosarcoma) in rats |
Tumor sites that were observed with both chemicals are shown in bold
Evidence from data related to the key characteristics (KCs) of carcinogens
We used the KCs of carcinogens approach [67] to systematically identify, organize, and summarize information on mechanisms of carcinogenesis for VA. Data for three of the 10 KCs, namely KCs 1, 2, and 10, were identified and summarized here. Overall, mechanistic data support the observations that VA can be metabolically activated to an electrophilic chemical (AA) and form DNA adducts, can cause genotoxicity including clastogenicity and DNA damage, and can induce cell proliferation and pre-neoplastic lesions such as hyperplasia and dysplasia.
KC1. Is electrophilic or can be metabolically activated
Although few studies have explicitly focused on VA exposures and the associated DNA adducts, much is known about DNA adducts from studies of direct AA exposure (as opposed to AA produced by VA metabolism). AA has been shown to bind to DNA in studies in rodents in vivo, human and other mammalian cells in vitro, and in cell-free systems [27, 28]. This robust body of KC1 evidence is summarized in Table 4.
Table 4.
Shared genotoxicity endpoints between VA and AA
| Endpoint | Vinyl Acetate | Acetaldehydea |
|---|---|---|
| Micronuclei formation (MN) | MN in human cells in vitro [68, 69] and rats in vivo [70] | MN in human and rat cells in vitro and mice in vivo |
| Chromosomal aberrations (CAs) | CAs in exposed humans [71], human cells in vitro [72–74], and rodents in vivo [75] | CAs in human and rat cells in vitro and rats in vivo |
| Sister chromatid exchange (SCE) | SCE in human [73, 76, 77] and animal cells in vitro [73] and rodents in vivo [78] | SCE in human and hamster cells in vitro and mice in vivo |
| DNA adducts | Formation of DNA adducts in rats in vivo (e.g., N2- Ethyl-dG, N2-propano-dG) [42, 43] | Formation of DNA adducts in exposed humans, human and rodent cells in vitro, rodents in vivo (e.g., N2- Ethyl-dG, N2-propano-dG) |
| DNA-DNA and DNA-protein crosslinks | Increases in DNA-DNA or DNA-protein crosslinks in human [79] and rodent cells in vitro [41] and in an acellular system [80] | Increases in DNA-protein crosslinks in human and rodent cells in vitro and in an acellular system |
| DNA breaks | No data | DNA strand breaks in human cells in vitro |
| Mutagenicity |
• Mutations at thymidine kinase locus in human [68] and mouse cells in vitro [81] • Did not induce mutations at HPRT locus in human cells in vitro [68] • Did not induce mutations in S. typhimurium or E. coli [82–94] |
• Mutations at thymidine kinase locus in human and mouse cells in vitro • Mutations at HPRT locus in human cells in vitro • Did not induce mutations in S. typhimurium or E. coli (although positive in one E. coli study) • Mutations at TP53 locus in human cells in vitro • Mutations in fungus |
Two recent in vivo studies of VA conducted in rats have demonstrated that administration of [13C2]-VA via inhalation results in the formation of DNA adducts in the nasal respiratory and olfactory epithelia and in peripheral blood mononuclear cells [42, 43].
To differentiate between DNA adducts caused by VA treatment and adducts caused by other (e.g., endogenous) sources of AA, rats were treated with [13C2]-VA (50, 200, or 400 ppm) via inhalation for 6 hr [43]. The VA-derived DNA adduct [13C2]-N 2-ethyl-2’- deoxyguanosine (N2-Ethyl-dG) was detected in nasal respiratory and olfactory epithelia in a dose-dependent manner. [13C4]−1,N2-propano-dG (N2-propano-dG) adducts were also detected in the respiratory epithelia of rats exposed to 400 ppm [13C2]-VA, although these adducts were present at lower levels than [13C2]-N2-Ethyl-dG adducts in these animals. Furthermore, low amounts of [13C2]-N2-Ethyl-dG adducts in the peripheral blood mononuclear cells were detected in all VA-treated groups, indicating systemic effects of VA exposure, beyond the nasal epithelia.
In another study using lower VA exposures over multiple days, [13C2]-N2-Ethyl-dG adducts were detected in the nasal respiratory and olfactory epithelia of rats treated with [13C2]-VA (10 or 50 ppm) via inhalation for 6 h/day for 14 days [42]. Low amounts of [13C2]-N2-Ethyl-dG adducts were also detected in one of three pooled samples of peripheral blood mononuclear cells of rats exposed to 50 ppm [13C2]-VA. No VA-derived adducts were detected in the liver, brain, or bone marrow of exposed rats.
KC2. Is genotoxic
IARC concluded that both “vinyl acetate and acetaldehyde are genotoxic in human cells in vitro and in animals in vivo.” [2] Overall, there are many studies reporting chromosomal effects of VA, and a few studies reporting DNA damage and mutagenicity associated with exposure to VA.
A number of in vitro studies in human cells and in vivo studies in rodents have reported increases in chromosomal effects following treatment with VA, including increases in micronuclei formation (MN) [68–70], chromosomal aberrations (CAs) [72–75] and sister chromatid exchange (SCE) [73, 76–78]. In addition, one in vitro study of VA in animal cells reported an increase in SCE [73] while a small study in exposed humans, published in Russian, reported increased levels of CAs in the lymphocytes of PVA manufacturing workers [71].
A few studies have reported that VA induced DNA damage. As previously described under KC1, two studies reported the formation of DNA adducts in rats in vivo [42, 43]. In addition, increases in DNA-DNA or DNA-protein crosslinks were observed in a study of human cells in vitro [79]a study of rodent cells in vitro [41]and an acellular system following treatment with VA [80].
Additionally, VA induced mutations at the thymidine kinase (TK) locus in human TK6 lymphoblastoid cells in vitro [68] and in a mouse lymphoma cell line [81]. However, VA did not induce mutations in bacteria, or at the HPRT (hypoxanthine-guanine phosphoribosyltransferase) locus in human TK6 cells [68]. Some in vitro studies found that VA is genotoxic at non-cytotoxic concentrations [41, 68, 69, 73, 74].
Overall, the genotoxicity findings for VA are consistent with and supported by findings from studies of its metabolite, AA (Table 4).
KC10. Alters cell proliferation, cell death or nutrient supply
VA has been shown to increase cellular proliferation, hyperplasia, or dysplasia in rodents. These effects were observed in both inhalation and oral exposure studies and findings were predominantly observed in the upper respiratory and digestive tracts. In male rats, increased cell proliferation was observed in the nasal respiratory and olfactory epithelia after a single 6 h inhalation exposure, and in the nasal olfactory epithelium after twenty repeated 6 h exposures over 4 weeks [95]. Cell proliferation of the oral cavity was increased in rats and mice exposed to VA via drinking water [96]. Tissue concordance between tumors and hyperplasia/dysplasia was observed for several sites in some long-term cancer bioassays of VA. For example, in female rats, hyperplasia and tumors were observed in the nasal cavity, esophagus, and thyroid tissues [55, 63]. In male mice, dysplasia and tumors were observed in the esophagus [64]. In another study of male mice, hyperplasia and tumors were observed in the oral cavity and the esophagus [56]. Finally, in female mice, hyperplasia and tumors were observed in the oral cavity and forestomach [56].
Discussion
This work is the most recent comprehensive review of VA’s carcinogenicity, including all evidence streams (human, animal, and mechanistic data). There were few epidemiological studies reporting on VA exposure and cancer. All studies except one were conducted in workers, who were co-exposed to many known and suspected carcinogens. There were some elevated risk estimates in the occupational studies, but these studies all had issues with quality, and chance, bias and confounding could not be ruled out. The only informative study assessed the association between ambient levels of several air toxics, including VA, and breast cancer risk in the Multiethnic Cohort study in Los Angeles, CA [44]. This study found an increased risk of breast cancer with exposure to VA in all women, as well as in analyses stratified by breast cancer subtype, race/ethnicity, non-smokers, and other subgroups. The CIC noted several strengths of the study, particularly the large population-based multiethnic prospective cohort that is unlikely to have been biased by significant selection bias, and the use of a detailed questionnaire to collect data on covariates. The CIC also noted that although residual confounding may be possible, it would likely affect the entire cohort (i.e., non-differential) and bias the results towards the null. Limitations of the study included some inherent imprecision in modeled air toxics exposure estimation and that exposures occurring earlier in life were not accounted for. However, the study also had access to detailed residential history for estimating ambient air exposure, and risk estimates remain elevated in subgroup analyses in movers and non-movers alike and even higher in the non-movers. The results on breast cancer risk reported in Heck et al. (2024) [44] may be biologically plausible considering the metabolic links between vinyl acetate, acetaldehyde, and ethanol. For example, IARC concluded that there is sufficient evidence in humans that consumption of alcoholic beverages causes breast cancer [97].
Compelling evidence for VA’s carcinogenicity stems from the large number of animal cancer bioassays, including several studies that were published after IARC’s 1995 classification. Based on our review, exposure to VA induced statistically significant treatment-related tumors in 13 rat studies and 5 mouse studies. These studies were conducted across multiple strains, in both sexes, and via inhalation or drinking water exposure routes. Occurrence of several tumor types were statistically significant by pairwise comparisons and with significant dose-related trends. For example, dose-related trends were observed for nasal tumors and forestomach carcinomas of rats, and forestomach acanthomas of mice. While some researchers have proposed a “threshold” hypothesis, some VA-induced tumors were significantly increased at the low- or mid-dosed group (e.g., thyroid gland C-cell tumors or malignant lymphoma of the spleen). In addition, tumors were not restricted to site of entry and included distal sites (e.g., tumors of the uterus, thyroid gland, pancreas, and adrenal glands in drinking water studies). Several of the tumors are considered rare, such as squamous cell carcinoma of the oral cavity, esophagus, and forestomach in rats and mice, and uterine adenocarcinoma in rats. While some of the animal tumors induced by VA occurred in organs that do not have a human counterpart, (i.e., forestomach and Zymbal gland), there is no evidence to suggest that VA is acting via a species-specific mechanism in these organs, and thus these findings are considered relevant to humans and to contribute to the evidence of cancer hazard. For example, IARC has reviewed the issue of rodent forestomach tumors, and concluded the following: “While humans do not have a forestomach, they do have a comparable epithelial tissue in the oral cavity and the upper two-third of the esophagus. Thus, in principle, carcinogens targeting the forestomach squamous epithelium in rodents are relevant for humans. Also, the target tissue for carcinogens may differ between experimental animals and humans, and a forestomach carcinogen in rodents may target a different tissue in humans” [98]. Moreover, the IARC Preamble states that “the inference of potential carcinogenic hazard to humans does not imply tumor site concordance across species” [99].
Challenges to the interpretation of the animal tumor data were raised and some were discussed at the CIC meeting, including the instability of VA in the dosing solution, criticism on pathological diagnosis of tumors, and lack of histopathological peer review for some of the studies conducted by the Ramazzini Institute. Overall, these challenges did not lead to significant uncertainties in the overall weight of the evidence from animal studies. For example, VA instability in solution would lead to lower overall exposures of the animals to VA. However, despite dosing at concentrations possibly lower than the targeted dose, increased tumor incidences were observed, demonstrating that VA can induce tumors, perhaps at doses lower than reported. Criticisms on the pathology review for some of the studies by the Ramazzini Institute were allayed by reviews from the NTP and US EPA [100, 101]. Specifically, independent pathology assessments by a Pathology Working Group jointly-sponsored by the NTP and the US EPA [100] have found high-agreement in diagnosing solid tumors with the Ramazzini Institute’s evaluations. US EPA (2010) [101] issued a decision that the agency would continue to consider solid tumor data from the Ramazzini Institute in their Integrated Risk Information System (IRIS) assessments. With regard to the VA studies, at their 2024 meeting, the CIC noted that the Ramazzini Institute tumor findings [62–64] in the oral studies were reasonably consistent with those from the Japanese studies [56, 57] and even the U.S. studies [54, 55]. Such consistency can be seen in Table 2.
The metabolic link between VA and AA plays a significant role in the carcinogenicity of VA. CES metabolize VA to AA, an electrophilic and reactive compound that can react with DNA and proteins to form DNA adducts and DPXLs. AA is oxidized by ALDH2, but can undergo further metabolism by other enzymes, such as xanthine oxidase and aldehyde oxidase. These downstream reactions can form ROS and acetyl and methyl radicals. In individuals with a reduced capacity for metabolic clearance of AA (e.g., due to a polymorphism), AA levels can build up in the body, likely increasing the risk of cancer for these individuals. While no epidemiology studies were identified regarding the effect of ALDH2 polymorphisms on the potential cancer risk caused by VA, many studies have investigated the associations between alcohol consumption, ALDH2 polymorphisms and risks of various cancer types, and the potential gene-environment interaction between ALDH2 polymorphisms and alcohol consumption. Carrying the ALDH2*2 allele, especially the homozygous genotype, has been associated with an increased risk of multiple cancers, including oropharyngolaryngeal, esophageal, gastric, colon, lung, head and neck cancers [102–107]. In general, ALDH2 polymorphisms are associated with increases in cancer risk, shorter time to tumor recurrence, and higher mortality compared to wildtype ALDH2 when taking into account similar levels of alcohol consumption [108]. Based on the prevalence of the ALDH2*2 variant in certain East Asian ethnic groups (Chinese, Japanese, Korean, and Taiwanese) as well as 2020 California census data [109] and 2020 United States census data [110], we estimate that at least one million Californians and three million Americans have reduced ALDH2 activity. Worldwide, roughly 8% or 560 million people are carriers of ALDH2*2111, 112. Other variants of ALDH2 with reduced expression and enzyme activity have been detected in other ethnic groups (e.g. ALDH2*4 and ALDH2*6 in ~ 5–10% of Latinos) [111, 112] and account for an additional 120 million people worldwide that may be more susceptible to carcinogenic effects induced by VA.
Another factor that could impact endogenous levels of AA is the enzyme activity and affinity of CES. Rapid VA metabolism via CES, coupled with slow clearance, could lead to higher endogenous AA levels. Several polymorphisms of CES1 and CES2 have been identified in human populations [113–115]with some resulting in decreased enzyme activity while others not. However, we were not able to find specific information on the impact of CES polymorphisms on the metabolism of VA. Hence, a conclusion regarding the effect of CES polymorphisms on VA metabolism cannot be drawn, and it remains a topic for future studies.
Other mechanistic data, organized by the key characteristics of carcinogens, provide some insight as to VA’s possible carcinogenicity mechanisms. In addition to being metabolically activated to electrophilic AA and other reactive radicals, VA forms DNA adducts, causes genotoxicity including clastogenicity and DNA damage, and induces cell proliferation and pre-neoplastic lesions such as hyperplasia and dysplasia. Some researchers have suggested that intracellular acidification may be linked to cellular proliferation following VA exposure [23, 24, 116]. However, this hypothesis has not been verified to date, as there are no studies demonstrating VA decreasing cellular pH while increasing mitogenic cell proliferation.
In summary, our review shows compelling evidence in animal cancer bioassays with supporting mechanistic data, including the formation of reactive species, DNA adducts, its genotoxicity, and the ability to induce cell proliferation and pre-neoplastic lesions. Moreover, susceptible individuals with a non-functioning or reduced-functioning ALDH2 may have higher levels of AA and thus may have a greater risk of cancer.
Supplementary Information
Acknowledgements
The authors thank Ms. Nancy Firchow for assistance with the literature searches, and Dr. Sarah Park and Dr. Jennifer Hsieh for their thoughtful review and helpful input on this work.
Disclaimer
The views expressed are those of the authors and do not necessarily represent those of the Office of Environmental Health Hazard Assessment (OEHHA), the California Environmental Protection Agency, or the State of California.
Abbreviations
- μg
Microgram
- μM
Micromolar
- AA
Acetaldehyde
- ADME
Absorption, distribution, metabolism and elimination
- ALDH2
Aldehyde dehydrogenase 2
- BMI
Body mass index
- BNPP
Bis (p-nitrophenyl) phosphate
- CA
Chromosomal aberration
- CES
Carboxylesterase
- CI
Confidence interval
- CIC
Carcinogen Identification Committee
- CoA
Coenzyme A
- CYP450
Cytochrome P450
- DNA
Deoxyribonucleic acid
- DPXL
DNA-protein crosslink
- ER
Estrogen receptor
- EVA
Ethylene-vinyl acetate
- F0
Parental generation
- F1
First filial generation
- F344
Fischer 344
- HAPEM
Hazardous Air Pollutant Exposure Model
- HAWC
Health Assessment Workspace Collaborative
- HPRT
Hypoxanthine-guanine phosphoribosyl transferase
- HR
Hazard ratio
- hr
Hour
- IARC
International Agency for Research on Cancer
- IQR
Interquartile range
- KC
Key characteristic
- Km
Michaelis constant
- m
Meter
- MN
Micronuclei
- N2-Etd-dG
N2-ethylidene-2’-deoxyguanosine or N2-ethylidene-dG
- N2-Ethyl-dG
N2-ethyl-2’-deoxyguanosine
- NATA
National Air Toxics Assessment
- NOx
Nitrogen oxides
- NR
Not reported
- NTP
National Toxicology Program
- OEHHA
Office of Environmental Health Hazard Assessment
- OR
Odds ratio
- ppm
Parts per million
- PR
Progesterone receptor
- PVA
Polyvinyl acetate
- PVC
Polyvinyl chloride
- ROS
Reactive oxygen species
- SCE
Sister chromatid exchange
- SD
Sprague Dawley
- SES
Socioeconomic status
- SWIFT
Sciome Workbench for Interactive computer-Facilitated Text-mining
- TK
Thymidine kinase
- UCC
Union Carbide Corporation
- US
United States
- US EPA
United States Environmental Protection Agency
- VA
Vinyl acetate
Authors’ contributions
KL, KR, FCT, VC, GO, NG, SE, MSS and MS contributed to the conception and design of the work. KL, KR, FCT, VC, GO, NG, SEE contributed to the literature search and screening using HAWC.KL, KR, FCT, VC, GO, NG, SE, IA, MSS and MS drafted and contributed to revisions of the manuscript. KL, KR, FCT, VC, GO, NG, SE, IA, MSS and MS each contributed substantially to the interpretation of data. Specifically, GO, NG, MSS and MS contributed to the interpretation of epidemiological data; KL, VC, SE, IA, MSS and MS contributed to the interpretation of animal toxicological data; KR, FCT, VC, MSS and MS contributed to the interpretation of data relevant to pharmacokinetics and metabolism; VC, GO, SE, IA, MSS and MS contributed to the interpretation of data relevant to the key characteristics of carcinogens. All authors have approved the submitted version (and any substantially modified version that involves the author’s contribution to the study).All authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Funding
None.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Clinical trial number
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.US EPA. Chemical Data Reporting, https://www.epa.gov/chemical-data-reporting/access-chemical-data-reporting-data#2020 (2020).
- 2.IARC. Dry cleaning, Some Chlorinated Solvents and Other Industrial Chemicals. Review. 1995. Lyon, France: International Agency for Research on Cancer.
- 3.ATSDR. Toxicological profile for vinyl acetate draft for public comment. US Department of Health and Human Services; 2023.
- 4.Carthew P, Griffiths H, Keech S, et al. Safety assessment for hair-spray resins: risk assessment based on rodent inhalation studies. Inhal Toxicol. 2002;14:401–16. 10.1080/08958370252871023. Article. [DOI] [PubMed] [Google Scholar]
- 5.US FDA. Food Additive Status List, https://www.fda.gov/food/food-additives-petitions/food-additive-status-list (2023).
- 6.US FDA. GRAS Notification for the Intended Use of Vinyl acetate-Vinyl laurate Copolymers (VINNPAS® B 500/20 BL and VINNAPAS® B 500/40 VL) in Chewing Gum Base, https://www.fda.gov/food/gras-notice-inventory/agency-response-letter-gras-notice-no-grn-000606 (2015).
- 7.US FDA. GRAS notification for the intended use of vinyl Acetate-Vinyl laurate copolymer (5 to 40% VL) in chewing gum base, https://www.fda.gov/media/135363/download (2019).
- 8.Rago R, Peters AR. Indoor air background levels of volatile organic compounds and air-Phase petroleum hydrocarbons in office buildings and schools. Groundw Monit Remediation. 2021;41:27–47. [Google Scholar]
- 9.Coggins CR, Jerome AM, Lilly PD, et al. A comprehensive toxicological evaluation of three adhesives using experimental cigarettes. Inhal Toxicol. 2013;25(Suppl 2):6–18. 10.3109/08958378.2013.854430. [DOI] [PubMed] [Google Scholar]
- 10.Xu A, Fan Z, Chen Z, et al. Simultaneous determination of Furan and vinyl acetate in vapor phase of mainstream cigarette smoke by GC-MS. Acad Bras Cienc. 2017;89:383–90. 2017/05/12. [DOI] [PubMed] [Google Scholar]
- 11.McNeal TP, Hollifield HC. Determination of volatile chemicals released from microwave-heat-susceptor food packaging. J AOAC Int. 1993;76:1268–75. 1993/11/01. [PubMed] [Google Scholar]
- 12.Hodgson AT, Wooley JD, Daisey JM. Emissions of volatile organic compounds from new carpets measured in a large-scale environmental chamber. Air Waste Manage Assoc. 1993;43:316–24. 1993/03/01. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Gou R, Zhang S, et al. Comprehensive theoretical study on safety performance and mechanical properties of 3-nitro-1,2,4-triazol-5-one (NTO)-based polymer-bonded explosives (PBXs) via molecular dynamics simulation. J Mol Model. 2022;28:406. 10.1007/s00894-022-05393-4. [DOI] [PubMed] [Google Scholar]
- 14.DTSC. Laboratory study of chemicals in nail products. Department of Toxic Substances Control; 2023.
- 15.IARC. Overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1 to 42 1987. Lyon, France: International Agency for Research on Cancer. [PubMed]
- 16.NTP. 15th Report on Carcinogens. 2021. Research Triangle Park, North Carolina US Department of Health and Human Services.
- 17.ECHA. Opinion proposing harmonised classification and labelling at Community level of vinyl acetate. 2011.
- 18.OEHHA. Evidence on the carcinogenicity of vinyl acetate. Office of Environmental Health Hazard Assessment; 2024.
- 19.NTP. Handbook for Preparing Report on Carcinogens Monographs. 2015.
- 20.Shapiro AJ, Antoni S, Guyton KZ, et al. Software tools to facilitate systematic review used for cancer hazard identification. Environ Health Perspect. 2018;126:104501. 2018/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard BE, Phillips J, Tandon A, et al. SWIFT-Active screener: accelerated document screening through active learning and integrated recall Estimation. Environ Int. 2020;138:105623. 10.1016/j.envint.2020.105623. 2020/03/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogdanffy MS, Manning LA, Sarangapani R. High-affinity nasal extraction of vinyl acetate vapor is carboxylesterase dependent. Inhal Toxicol. 1999;11:927–41. 10.1080/089583799196718. [DOI] [PubMed] [Google Scholar]
- 23.Bogdanffy MS. Vinyl acetate-induced intracellular acidification: implications for risk assessment. Toxicol Sci. 2002;66:320–6. 10.1093/toxsci/66.2.320. Article. [DOI] [PubMed] [Google Scholar]
- 24.Bogdanffy MS, Valentine R. Differentiating between local cytotoxicity, mitogenesis, and genotoxicity in carcinogen risk assessments: the case of vinyl acetate. Toxicol Lett. 2003;140–141:83–98. 10.1016/s0378-4274(02)00504-0. [DOI] [PubMed] [Google Scholar]
- 25.Ginsberg G, Smolenski S, Hattis D, et al. Population distribution of aldehyde dehydrogenase-2 genetic polymorphism: implications for risk assessment. Regul Toxicol Pharmacol. 2002;36:297–309. 2002/12/11. [DOI] [PubMed] [Google Scholar]
- 26.Rashkovetsky LG, Maret W, Klyosov AA. Human liver aldehyde dehydrogenases: new method of purification of the major mitochondrial and cytosolic enzymes and re-evaluation of their kinetic properties. Biochim Biophys Acta. 1994;1205:301–7. 10.1016/0167-4838(94)90249-6. [DOI] [PubMed] [Google Scholar]
- 27.Albertini RJ. Vinyl acetate monomer (VAM) genotoxicity profile: relevance for carcinogenicity. Crit Rev Toxicol. 2013;43:671–706. 10.3109/10408444.2013.827151. [DOI] [PubMed] [Google Scholar]
- 28.IARC. Re-evaluation of some organic chemicals, hydrazine, and hydrogen peroxide (Part 1, part 2, part 3). France: International Agency for Research on Cancer: Lyon; 1999. [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Q, Weiner H, Crabb DW. The mutation in the mitochondrial aldehyde dehydrogenase (ALDH2) gene responsible for alcohol-induced Flushing increases turnover of the enzyme tetramers in a dominant fashion. J Clin Invest. 1996;98:2027–32. 10.1172/JCI119007. 1996/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai CL, Yao CT, Chau GY, et al. Dominance of the inactive Asian variant over activity and protein contents of mitochondrial aldehyde dehydrogenase 2 in human liver. Alcohol Clin Exp Res. 2014;38:44–50. 2013/08/06. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Borinskaya S, Yoshimura K, et al. Refined geographic distribution of the Oriental ALDH2*504Lys (nee 487Lys) variant. Ann Hum Genet. 2009;73:335–45. 10.1111/j.1469-1809.2009.00517.x. 2009/05/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizoi Y, Yamamoto K, Ueno Y, et al. Involvement of genetic polymorphism of alcohol and aldehyde dehydrogenases in individual variation of alcohol metabolism. Alcohol Alcoholism. 1994;29:707–10. [PubMed] [Google Scholar]
- 33.Li K, Guo W, Li Z, et al. ALDH2 repression promotes lung tumor progression via accumulated acetaldehyde and DNA damage. Neoplasia. 2019;21:602–14. 10.1016/j.neo.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oyama T, Nagayoshi H, Matsuda T, et al. Effects of acetaldehyde inhalation in mitochondrial aldehyde dehydrogenase deficient mice (Aldh2-/-). Front Biosci. 2010;2:1344–54. 10.2741/e194. [DOI] [PubMed] [Google Scholar]
- 35.Albano E, Clot P, Comoglio A, et al. Free radical activation of acetaldehyde and its role in protein alkylation. FEBS Lett. 1994;348:65–9. 10.1016/0014-5793(94)00549-4. [DOI] [PubMed] [Google Scholar]
- 36.Nakao LS, Kadiiska MB, Mason RP, et al. Metabolism of acetaldehyde to Methyl and acetyl radicals: in vitro and in vivo electron paramagnetic resonance spin-trapping studies. Free Radic Biol Med. 2000;29:721–9. 10.1016/s0891-5849(00)00374-9. [DOI] [PubMed] [Google Scholar]
- 37.Puntarulo S, Cederbaum AI. Chemiluminescence from acetaldehyde oxidation by Xanthine oxidase involves generation of and interactions with hydroxyl radicals. Alcohol Clin Exp Res. 1989;13:84–90. 10.1111/j.1530-0277.1989.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 38.Mira L, Maia L, Barreira L, et al. Evidence for free radical generation due to NADH oxidation by aldehyde oxidase during ethanol metabolism. Arch Biochem Biophys. 1995;318:53–8. 1995/04/01. [DOI] [PubMed] [Google Scholar]
- 39.Shaw S, Jayatilleke E. The role of aldehyde oxidase in ethanol-induced hepatic lipid peroxidation in the rat. Biochem J. 1990;268:579–83. 1990/06/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shaw S, Jayatilleke E. Ethanol-induced iron mobilization: role of acetaldehyde-aldehyde oxidase generated superoxide. Free Radic Biol Med. 1990;9:11–7. 10.1016/0891-5849(90)90044-j. 1990/01/01. [DOI] [PubMed] [Google Scholar]
- 41.Kuykendall JR, Taylor ML, Bogdanffy MS. Cytotoxicity and DNA-protein crosslink formation in rat nasal tissues exposed to vinyl acetate are carboxylesterase-mediated. Toxicol Appl Pharmacol. 1993;123:283–92. 10.1006/taap.1993.1247. [DOI] [PubMed] [Google Scholar]
- 42.Hsiao YC, Liu CW, Hoffman G, et al. Molecular dosimetry of DNA adducts in rats exposed to vinyl acetate monomer. Toxicol Sci. 2022;185:197–207. 10.1093/toxsci/kfab140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu CW, Hsiao YC, Hoffman G, et al. LC-MS/MS analysis of the formation and loss of DNA adducts in rats exposed to vinyl acetate monomer through inhalation. Chem Res Toxicol. 2021;34:793–803. 10.1021/acs.chemrestox.0c00404. [DOI] [PubMed] [Google Scholar]
- 44.Heck JE, He D, Wing SE, et al. Exposure to outdoor ambient air toxics and risk of breast cancer: the multiethnic cohort. Int J Hyg Environ Health. 2024;259:114362. 2024/04/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ott MG, Teta MJ, Greenberg HL. Lymphatic and hematopoietic tissue cancer in a chemical manufacturing environment. Am J Ind Med. 1989;16:631–43. 10.1002/ajim.4700160603. [DOI] [PubMed] [Google Scholar]
- 46.UnionCarbide. Lymphatic and Hematopoietic Tissue Cancer in a Chemical Manufacturing Environment. 1989. [DOI] [PubMed]
- 47.Austin SG, Schnatter AR. A case control study of chemical exposures and brain tumors in petrochemical workers. J Occup Med. 1983;25:313–20. Article. [PubMed] [Google Scholar]
- 48.Leffingwell SS, Waxweiler R, Alexander V. Case-control study of gliomas of the brain among workers employed by a Texas city, Texas chemical plant. Neuroepidemiology. 1983;2:179–95. 10.1159/000110523. [Google Scholar]
- 49.Waxweiler RJ, Smith AH, Falk H, et al. Excess lung cancer risk in a synthetic chemicals plant. Environ Health Persp. 1981;41:159–65. 10.1289/ehp.8141159. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis R, Rempala GA, Case-Cohort. Study of angiosarcoma of the liver and brain cancer at a polymer production plant. J Occup Environ Med. 2003;45:538–45. 10.1097/01.jom.0000063616.37065.06. [DOI] [PubMed] [Google Scholar]
- 51.Waxweiler RJ. Epidemiologic problems associated with exposure to several agents. Environ Health Persp. 1981;42:51–6. 10.1289/ehp.814251. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.US EPA. Technical Support Document EPA’s 2011 National-scale Air Toxics Assessment. 2015.
- 53.IARC. Personal Habits and Indoor Combustions. Review. 2012. Lyon, France: International Agency for Research on Cancer.
- 54.Lijinsky W, Reuber MD. Chronic toxicity studies of vinyl acetate in Fischer rats. Toxicol Appl Pharmacol. 1983;68:43–53. 10.1016/0041-008x(83)90353-8. [DOI] [PubMed] [Google Scholar]
- 55.EPL. Bioassay of Vinyl Acetate in F344 Rats Pathology Report Addendum. October 1982 1982.
- 56.Umeda Y, Matsumoto M, Yamazaki K, et al. Carcinogenicity and chronic toxicity in mice and rats administered vinyl acetate monomer in drinking water. J Occup Health. 2004;46:87–99. 10.1539/joh.46.87. [DOI] [PubMed] [Google Scholar]
- 57.JBRC. Report on carcinogenicity studies on vinyl acetate orally administered (mixed in drinking water) to rats and mice (Main report, tables, figures, photographs, and appendices 1–3). Japan Bioassay Research Center; 1995.
- 58.Owen PE. Vinyl acetate: 104 week inhalation combined chronic toxicity and carcinogenicity study in the rat and mouse. Hazelton UK; 1988.
- 59.Shaw DC. Vinyl Acetate: 104 Week Oral (Drinking Water) Combined Chronic Toxicity and Carcinogenicity Study in the Rat Following In Utero Exposure. 1988. Hazleton UK.
- 60.Bogdanffy MS, Dreef-van der Meulen HC, Beems RB, et al. Chronic toxicity and oncogenicity inhalation study with vinyl acetate in the rat and mouse. Fundam Appl Toxicol. 1994;23:215–29. 10.1006/faat.1994.1100. [DOI] [PubMed] [Google Scholar]
- 61.Bogdanffy MS, Tyler TR, Vinegar MB, et al. Chronic toxicity and oncogenicity study with vinyl acetate in the rat: in utero exposure in drinking water. Fundam Appl Toxicol. 1994;23:206–14. 10.1006/faat.1994.1099. Article. [DOI] [PubMed] [Google Scholar]
- 62.Minardi F, Belpoggi F, Soffritti M, et al. Results of long-term carcinogenicity bioassay on vinyl acetate monomer in Sprague-Dawley rats. Ann N Y Acad Sci. 2002;982:106–22. 10.1111/j.1749-6632.2002.tb04927.x. [DOI] [PubMed] [Google Scholar]
- 63.Belpoggi F, Soffritti M, Minardi F, et al. Results of a long-term carcinogenicity bioassay on vinyl acetate monomer in Wistar rats. Eur J Oncol. 2002;7:279–93. Article. [DOI] [PubMed] [Google Scholar]
- 64.Maltoni C, Ciliberti A, Lefemine G, et al. Results of a long-term experimental study on the carcinogenicity of vinyl acetate monomer in mice. Ann N Y Acad Sci. 1997;837:209–38. 10.1111/j.1749-6632.1997.tb56876.x. [DOI] [PubMed] [Google Scholar]
- 65.Haseman JK. Statistical support of the proposed National toxicology program protocol. Toxicol Pathol. 1983;11:77–82. 10.1177/019262338301100113. 1983/01/01. [DOI] [PubMed] [Google Scholar]
- 66.Soffritti M, Belpoggi F, Esposti DD, et al. Consequences of exposure to carcinogens beginning during developmental life. Basic Clin Pharmacol Toxicol. 2008;102:118–24. 10.1111/j.1742-7843.2007.00200.x. [DOI] [PubMed] [Google Scholar]
- 67.Smith MT, Guyton KZ, Gibbons CF, et al. Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis. Environ Health Perspect. 2016;124:713–21. 2015/11/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Budinsky R, Gollapudi B, Albertini RJ, et al. Nonlinear responses for chromosome and gene level effects induced by vinyl acetate monomer and its metabolite, acetaldehyde in TK6 cells. Environ Mol Mutagen. 2013;54(20130828):755–68. 10.1002/em.21809. [DOI] [PubMed] [Google Scholar]
- 69.Mäki-Paakkanen J, Norppa H. Induction of micronuclei by vinyl acetate in mouse bone marrow cells and cultured human lymphocytes. Mutat Res. 1987;190:41–5. 10.1016/0165-7992(87)90080-7. [DOI] [PubMed] [Google Scholar]
- 70.NTP. G04: in vivo micronucleus summary data of vinyl acetate in Fischer 344 rats. Research Triangle Park, North Carolina: US Department of Health and Human Services; 2017. [Google Scholar]
- 71.Shirinian G, Arutyunyan R. Study of levels of cytogenetic changes in manufacture of polyvinylacetate. Biol J Armen. 1980;23:748–52. [Google Scholar]
- 72.Mustonen R, Kangas J, Vuojolahti P, et al. Effects of phenoxyacetic acids on the induction of chromosome aberrations in vitro and in vivo. Mutagenesis. 1986;1:241–5. 10.1093/mutage/1.4.241. 1986/07/01. [DOI] [PubMed] [Google Scholar]
- 73.Norppa H, Tursi F, Pfäffli P, et al. Chromosome damage induced by vinyl acetate through in vitro formation of acetaldehyde in human lymphocytes and Chinese hamster ovary cells. Cancer Res. 1985;45:4816–21. [PubMed] [Google Scholar]
- 74.Jantunen K, Mäki-Paakkanen J, Norppa H. Induction of chromosome aberrations by styrene and vinylacetate in cultured human lymphocytes: dependence on erythrocytes. Mutat Res. 1986;159:109–16. 10.1016/0027-5107(86)90119-3. [DOI] [PubMed] [Google Scholar]
- 75.Nersesyan A, Kukumadzhyan V, Zil’fyan V. Evaluation of activity of some chemical substances being in use in the industry of Armenia. Biol Zh Arm. 1990;9:796–7. [Google Scholar]
- 76.He SM, Lambert B. Induction and persistence of SCE-inducing damage in human lymphocytes exposed to vinyl acetate and acetaldehyde in vitro. Mutat Res. 1985;158:201–8. 10.1016/0165-1218(85)90086-2. [DOI] [PubMed] [Google Scholar]
- 77.Sipi P, Järventaus H, Norppa H. Sister-chromatid exchanges induced by vinyl esters and respective carboxylic acids in cultured human lymphocytes. Mutat Res. 1992;279:75–82. 10.1016/0165-1218(92)90248-x. [DOI] [PubMed] [Google Scholar]
- 78.Takeshita T, Iijima S, Makoto H. Vinyl Acetate-induced sister chromatid exchanges in murine bone marrow cells. Proc Jpn Acad Ser B Phys Biol Sci. 1986;62:239–42. [Google Scholar]
- 79.Lambert B, Chen Y, He SM, et al. DNA cross-links in human leucocytes treated with vinyl acetate and acetaldehyde in vitro. Mutat Res. 1985;146:301–3. 10.1016/0167-8817(85)90072-0. [DOI] [PubMed] [Google Scholar]
- 80.Kuykendall JR, Bogdanffy MS. Reaction kinetics of DNA-histone crosslinking by vinyl acetate and acetaldehyde. Carcinogenesis. 1992;13:2095–100. 10.1093/carcin/13.11.2095. [DOI] [PubMed] [Google Scholar]
- 81.Kirby P. Mouse lymphoma mutagenesis assay with 40171 (ML-NCI 78). Microbiological Associates; 1983.
- 82.Brams A, Buchet JP, Crutzen-Fayt MC, et al. A comparative study, with 40 chemicals, of the efficiency of the Salmonella assay and the SOS chromotest (kit procedure). Toxicol Lett. 1987;38:123–33. 10.1016/0378-4274(87)90120-2. 1987/09/01. [DOI] [PubMed] [Google Scholar]
- 83.Bartsch H. Predictive value of mutagenicity tests in chemical carcinogenesis. Mutat Res. 1976;38:177–90. Article. [DOI] [PubMed] [Google Scholar]
- 84.Bartsch H, Malaveille C, Barbin A, et al. Mutagenic and alkylating metabolites of halo-ethylenes, chlorobutadienes and Dichlorobutenes produced by rodent or human liver tissues - Evidence for oxirane formation by P450-linked microsomal mono-oxygenases. Arch Toxicol. 1979;41:249–77. 10.1007/BF00296896. Article. [DOI] [PubMed] [Google Scholar]
- 85.Emmert B, Bünger J, Keuch K, et al. Mutagenicity of cytochrome P450 2E1 substrates in the Ames test with the metabolic competent S. typhimurium strain YG7108pin3ERb5. Toxicology. 2006;228:66–7620060822. 10.1016/j.tox.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 86.Florin I, Rutberg L, Curvall M, et al. Screening of tobacco smoke constituents for mutagenicity using the ames’ test. Toxicology. 1980;15:219–32. 10.1016/0300-483x(80)90055-4. 1980/01/01. [DOI] [PubMed] [Google Scholar]
- 87.IARC. Screening tests in chemical carcinogenesis. Lyon, France: International Agency for Research on Cancer; 1976. [Google Scholar]
- 88.JETOC. Vinyl acetate mutagenicity in bacterial test. Japan Chemical Industry Ecology- Toxicology and Information Center; 2004.
- 89.Jung R, Engelhart G, Herbolt B, et al. Collaborative study of mutagenicity with Salmonella typhimurium TA102. Mutat Res. 1992;278:265–70. Article. 10.1016/S0165-1218(10)80006-0. [DOI] [PubMed] [Google Scholar]
- 90.Lijinsky W, Andrews AW. Mutagenicity of vinyl compounds in Salmonella typhimurium. Teratog Carcinog Mutagen. 1980;1:259–67. 10.1002/tcm.1770010303. [DOI] [PubMed] [Google Scholar]
- 91.McCann J, Choi E, Yamasaki E, et al. Detection of carcinogens as mutagens in the salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci USA. 1975;72:5135–9. 1975/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muller W, Engelhart G, Herbold B, et al. Evaluation of mutagenicity testing with Salmonella typhimurium TA102 in three different laboratories. Environ Health Persp Suppl. 1993;101:33–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.NTP. G06: Ames summary data. Research Triangle Park, North Carolina: US Department of Health and Human Services; 2017. [Google Scholar]
- 94.Watanabe K, Sasaki T, Kawakami K. Comparisons of chemically-induced mutation among four bacterial strains, Salmonella typhimurium TA102 and TA2638, and Escherichia coli WP2/pKM101 and WP2 uvrA/pKM101: collaborative study III and evaluation of the usefulness of these strains. Mutat Res. 1998;416:169–81. 10.1016/S1383-5718(98)00085-0. Article. [DOI] [PubMed] [Google Scholar]
- 95.Bogdanffy MS, Gladnick NL, Kegelman T, et al. Four-week inhalation cell proliferation study of the effects of vinyl acetate on rat nasal epithelium. Inhal Toxicol. 1997;9:331–50. 10.1080/089583797198178. Article. [Google Scholar]
- 96.Valentine R, Bamberger JR, Szostek B, et al. Time- and concentration-dependent increases in cell proliferation in rats and mice administered vinyl acetate in drinking water. Toxicol Sci. 2002;67:190–7. 10.1093/toxsci/67.2.190. [DOI] [PubMed] [Google Scholar]
- 97.IARC. Alcohol Consumption and Ethyl Carbamate. Review. 2010. Lyon, France: International Agency for Research on Cancer.
- 98.IARC. Predictive value of rodent forestomach and gastric neuroendocrine tumours in evaluating carcinogenic risks to Humans. Report no. 39, 2003. Lyon, France.
- 99.IARC. IARC monographs on the identification of carcinogenic hazards to humans preamble. Lyon, France: International Agency for Research on Cancer; 2019. [Google Scholar]
- 100.NTP. Summary Report of the National Toxicology Program and Environmental Protection Agency-Sponsored. Review of Pathology Materials from Selected Ramazzini Institute Rodent Cancer Bioassays, https://ntp.niehs.nih.gov/sites/default/files/ntp/about_ntp/partnerships/international/summarypwg_report_ri_bioassays.pdf (2011).
- 101.US EPA. Update on Ramazzini Institute Data in IRIS Assessments. https://www.epa.gov/iris/update-ramazzini-institute-data-iris-assessments (2010, accessed November 19, 2024).
- 102.Gao CM, Takezaki T, Wu JZ, et al. Polymorphisms of alcohol dehydrogenase 2 and aldehyde dehydrogenase 2 and colorectal cancer risk in Chinese males. World J Gastroenterol. 2008;14:5078–83. 10.3748/wjg.14.5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kang SJ, Shin CM, Sung J, et al. Association between ALDH2 polymorphism and gastric cancer risk in terms of alcohol consumption: A Meta-Analysis. Alcohol Clin Exp Res. 2021;45(20201216):6–14. 10.1111/acer.14508. [DOI] [PubMed] [Google Scholar]
- 104.Yokoyama A, Omori T. Genetic polymorphisms of alcohol and aldehyde dehydrogenases and risk for esophageal and head and neck cancers. Jpn J Clin Oncol. 2003;33:111–21. 10.1093/jjco/hyg026. [DOI] [PubMed] [Google Scholar]
- 105.Yokoyama A, Muramatsu T, Ohmori T, et al. Alcohol-related cancers and aldehyde dehydrogenase-2 in Japanese alcoholics. Carcinogenesis. 1998;19:1383–7. 10.1093/carcin/19.8.1383. [DOI] [PubMed] [Google Scholar]
- 106.Marchitti SA, Brocker C, Stagos D, et al. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4:697–720. 2008/07/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang H, Zhou Y, Zhou Z, et al. A novel polymorphism rs1329149 of CYP2E1 and a known polymorphism rs671 of ALDH2 of alcohol metabolizing enzymes are associated with colorectal cancer in a Southwestern Chinese population. Cancer Epidem Biomar. 2009;18:2522–7. 10.1158/1055-9965.Epi-09-0398. [DOI] [PubMed] [Google Scholar]
- 108.Zhang H, Fu L. The role of ALDH2 in tumorigenesis and tumor progression: targeting ALDH2 as a potential cancer treatment. Acta Pharm Sinica B. 2021;11:1400–11. 10.1016/j.apsb.2021.02.008. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.U.S. Census Bureau. Asian Alone by Selected Groups. https://data.census.gov/table/ACSDT1Y2023.B02015?q=Race%20and%20Ethnicity&g=040XX00US06 (2020, accessed April 8, 2025).
- 110.U.S. Census Bureau. Selected Population Profile in the United States. https://data.census.gov/table?q=United+States&t=-04&g=010XX00US (2020, accessed April 8, 2025).
- 111.Chen CH, Kraemer BR, Mochly-Rosen D. ALDH2 variance in disease and populations. Dis Model Mech. 2022;15(20220624). 10.1242/dmm.049601. [DOI] [PMC free article] [PubMed]
- 112.Chen CH, Ferreira JCB, Joshi AU et al. Novel and prevalent non-East Asian ALDH2 variants; Implications for global susceptibility to aldehydes’ toxicity. EBioMedicine 2020; 55: 102753. 2020/05/1410.1016/j.ebiom.2020.102753. [DOI] [PMC free article] [PubMed]
- 113.Cha YJ, Jeong HE, Shin JG, et al. Genetic polymorphisms of the carboxylesterase 1 (CES1) gene in a Korean population. Transl Clin Pharmacol. 2014;22:30–4. 10.12793/tcp.2014.22.1.30. Article. [Google Scholar]
- 114.Chen F, Zhang B, Parker RB, et al. Clinical implications of genetic variation in carboxylesterase drug metabolism. Expert Opin Drug Metab Toxicol. 2018;14:131–14220180104. 10.1080/17425255.2018.1420164. [DOI] [PubMed] [Google Scholar]
- 115.Marsh S, Xiao M, Yu J, et al. Pharmacogenomic assessment of carboxylesterases 1 and 2. Genomics. 2004;84:661–8. 10.1016/j.ygeno.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 116.Nakamoto T, Wagner M, Melvin JE, et al. Vinyl acetate induces intracellular acidification in mouse oral buccal epithelial cells. Toxicol Lett. 2005;158:116–21. 10.1016/j.toxlet.2005.03.002. Article. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.