Abstract
Backgroud
Efficient communication between the embryo and the endometrium is essential for the successful establishment and maintenance of pregnancy. Uterine-derived extracellular vesicles (EVs) contribute to embryo-maternal communication, supporting early embryonic development. This study aimed to: (i) compare the protein cargo of uterine fluid EVs (UF-EVs) from CYCLIC and PREGNANT heifers; (ii) characterize the protein profile of conditioned medium (CM)-EVs from endometrial explants cultured alone (EXPL) or co-cultured with five d 7 blastocysts (EXPL + EMB) in vitro; and (iii) compare the EV protein cargo between the in vivo and in vitro models (i.e., EXPL vs. CYCLIC and EXPL + EMB vs. PREGNANT).
Results
We identified 1,459 and 1,752 proteins in the UF-EVs of CYCLIC and PREGNANT heifers, respectively. Among these, 12 were exclusive to CYCLIC, and 18 were exclusive to PREGNANT. Among the 1,329 proteins identified in both groups, 16 were differently abundant; ten were more abundant, and six were less abundant in UF-EVs from PREGNANT heifers. In vivo, the changes in UF-EV protein cargo induced by the presence of a blastocyst were related to inflammatory and immune responses, endometrial receptivity, and support of early embryonic development by promoting cell polarity, cell–cell adhesion, and stem cell differentiation. In vitro, we identified 1,501 proteins in the CM-EVs from EXPL, 1,975 in the CM-EVs from EXPL + EMB, and 82 in the CM-EVs from EMB. Additionally, 50 proteins were unique to EXPL + EMB, and another 33 were differentially abundant due to the synergistic interaction between the embryo and the endometrium. These proteins are involved in embryonic development, regulation of stem cell differentiation, establishment and maintenance of cell polarity, interferon tau (IFNT)-mediated cell signaling, endometrial receptivity, and immune modulation. Although there are qualitative and quantitative differences between in vivo and in vitro-derived EVs, UF-EVs from CYCLIC heifers compared to CM-EVs from EXPL, as well as UF-EVs from PREGNANT heifers compared to CM-EVs from EXPL + EMB shared common proteins.
Conclusions
These findings highlight the pivotal role of EVs in embryo-maternal communication, suggesting that their protein cargo may actively contribute to the modulation of the uterine environment to support early embryonic development. Understanding these molecular interactions could provide valuable insights into the mechanisms of implantation and pregnancy establishment.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40104-025-01270-1.
Keywords: Bovine blastocysts, Early pregnancy, Embryonic extracellular vesicles, Embryo-maternal communication, Endometrial explants, Proteomics, Uterine fluid
Introduction
Establishing and maintaining pregnancy requires reciprocal molecular communication between the embryo and the maternal reproductive tract. Despite the advances in assisted reproductive technologies that have made it possible to produce embryos in vitro to the stage of hatching blastocyst, the quality of the resulting embryos is inferior to those derived in vivo [1]. Specifically, embryos generated in vitro exhibit significant alterations in gene expression [2], metabolic changes [3], increased lipid accumulation [4], reduced tolerance to cryopreservation [5], and lower pregnancy success rates [6] when compared to their in vivo counterparts. Furthermore, pregnancy losses in cattle are most common in the pre-implantation window [7]. In both cases, the reduced quality of in vitro-produced embryos and these early pregnancy losses can be attributed to dysfunctions in the interaction between the embryo and the female reproductive tract, as well as the lack of an ideal physiological environment. These findings underscore the critical importance of embryo-maternal communication in the uterus, which regulates both endometrial and embryonic functions to ensure a successful pregnancy.
Although embryo-endometrial interaction is most significant during maternal recognition of pregnancy [8], the early embryo can already modulate gene expression and the proteome of the endometrium, initiating an early embryo-maternal dialogue. Sponchiado et al. [9] demonstrated that physical proximity with the embryo facilitates paracrine regulation of endometrial function, as evidenced by changes in endometrial gene expression in response to the pre-hatching embryo in vivo. Additionally, changes in the metabolite composition of the UF are also observed in response to the pre-hatching bovine embryo [10]. In vitro, d 5 to 9 embryos can induce an anti-inflammatory response in both bovine endometrial epithelial cells (BEEC) and immune cells [11]. Moreover, d 8 embryos have been shown to induce expression changes in interferon-stimulated genes (ISGs) in endometrial explants [12].
Pre-implantation embryos produce a variety of biochemical signals, collectively termed embryotropins, which facilitate communication with the endometrium [13]. Recently, extracellular vesicles (EVs) have emerged as important mediators of maternal-embryonic signaling and as having a role in supporting early embryonic development. EVs are lipid bilayer-delimited nanoparticles actively released by cells into the extracellular space [14]. EVs modulate recipient cell functions and promote intercellular communication by delivering their bioactive cargo, including proteins, lipids, mRNAs, and microRNAs (miRNAs) [15]. Multiple studies have shown that embryos produce and release EVs that can be internalized by endometrial cells [16–18]. Recent findings indicate that EVs secreted by bovine embryos during blastocyst formation (d 5–7) can induce transcriptomic modifications in endometrial cells, particularly by activating interferon tau (IFNT) signaling [19]. Therefore, embryo-derived EVs may play an important role in facilitating embryo-maternal interactions during the pre-implantation period.
Extracellular vesicles are also present in the uterine fluid (UF), also known as histotroph, contributing to the maternal side of this reciprocal communication. Before implantation, the growth and development of the free-floating embryo are supported by the UF [18]. Produced by endometrial glandular cells, the UF also contains a critical mixture of biochemical components, including carbohydrates, lipids, amino acids, and growth factors [19]. UF-EVs have been identified in multiple mammalian species, including cattle [16, 20–23], sheep [18, 24, 25], goats [26], horses [27], mice [28], pigs [29], and humans [30]. These UF-EVs are implicated in several key reproductive processes within the uterus, such as the establishment of endometrial receptivity [21, 31], the elongation and implantation of the conceptus [16, 18, 24], and the regulation of maternal immune responses [22]. The protein [23, 32] and miRNA [21, 33] profiles of UF-EVs undergo dynamic changes throughout the bovine estrous cycle. Additionally, alterations in the miRNA profiles of UF-EVs in the presence of multiple embryos have been described, suggesting that the cargo of these vesicles is influenced by pre-hatching blastocysts [22].
Functionally, UF-EVs are internalized by bovine embryos and modulate their development and quality. Adding UF-EVs to the in vitro culture (IVC) medium significantly increases blastocyst yield [23] and enhances the developmental competence of somatic cell nuclear transfer embryos, as evidenced by improved blastocyst development and hatching [20]. Furthermore, we demonstrated that sequential supplementation of the IVC medium with oviduct fluid-EVs followed by UF-EVs enhances blastocyst quality. This improvement was evidenced by higher survival post-vitrification, increased total cell number, reduced lipid content, and modulation of lipid metabolism-related genes in blastocysts [34], potentially mediated by the miRNA cargo of EVs [33]. These findings emphasize the critical role of maternal UF-EVs in enhancing embryonic development and quality.
In summary, studies on embryo-derived EVs and UF-EVs suggest that the pre-hatching embryo and endometrium actively exchange EVs during pre-implantation. This exchange facilitates embryo-maternal cross-talk, supporting embryo development and endometrial receptivity. As we have recently shown, embryo presence can induce changes in the protein profile of bovine oviductal EVs on d 3.5 of pregnancy, as well as in in vitro-derived oviductal EVs [35]. However, how these signals modulate the protein content of bovine UF-EVs remains a key area of interest that requires further investigation. We hypothesized that the d 7 blastocyst induces specific alterations in the proteomic cargo of UF-EVs, which may influence endometrial receptivity and embryo development. Moreover, local effects of the embryo on the endometrium are challenging to study in vivo and highlight the need for an in vitro model to gain a deeper understanding of the complex maternal-embryonic cross-talk through EVs during the early stages of pregnancy.
Therefore, the present study aimed to: (i) characterize the protein content of UF-EVs from PREGNANT and CYCLIC heifers to identify embryo-induced changes in the UF-EV proteome, and to explore the biological processes potentially modulated by these embryo-maternal communication-driven alterations in UF-EV protein cargo in vivo; (ii) characterize the protein content of conditioned medium (CM)-EVs from endometrial explants cultured alone or co-cultured with d 7 blastocysts, or from d 7 blastocysts cultured alone to identify embryo-induced changes in the CM-EV proteome, and to explore the biological processes potentially modulated by these embryo-maternal communication-driven alterations in CM-EV protein cargo in vitro; and (iii) compare the EV protein profiles between in vivo and in vitro models (i.e., EXPL vs. CYCLIC and EXPL + EMB vs. PREGNANT) to identify shared and distinct features of embryo-maternal communication, and to evaluate the relevance of endometrial explants as a model system for studying EV-mediated signaling.
Materials and methods
Experimental design
The experimental design is shown in Fig. 1. We evaluated the protein content of EVs in four different comparisons:
UF-EVs from non-pregnant heifers (CYCLIC) compared to those from pregnant heifers (PREGNANT).
CM-EVs from endometrial explants cultured alone (EXPL) were compared to those from explants co-cultured with blastocysts (EXPL + EMB) and from blastocysts cultured alone (EMB).
UF-EVs from CYCLIC heifers were compared to EVs from the CM of endometrial explants cultured alone in vitro (EXPL).
UF-EVs from PREGNANT heifers were compared to EVs from the CM of endometrial explants co-cultured with blastocysts (EXPL + EMB) in vitro.
Fig. 1.
Experimental model and group comparisons. Heifers were synchronized and either artificially inseminated or not inseminated. At 7 days post-insemination, heifers were slaughtered and the uterine horn ipsilateral to the corpus luteum was flushed. Pregnancy was confirmed by recovering a blastocyst in inseminated heifers (PREGNANT group, n = 5), whereas the presence of a functional corpus luteum confirmed cyclic status in non‑inseminated heifers (CYCLIC group, n = 5). For the in vitro model, four 8 mm circular endometrial explants were collected from the ipsilateral horn of each CYCLIC heifer and cultured separately in 1 mL of protein-free synthetic oviduct fluid (SOF): two explants were cultured alone (EXPL group, n = 5), and two were co-cultured with five in vitro-produced bovine blastocysts (EXPL + EMB group, n = 5). Additionally, 50 in vitro-produced bovine blastocysts were cultured alone (EMB group, n = 5) in 500 μL of SOF. After 6 h, the conditioned medium (CM) was collected for EV isolation. For each cyclic heifer, CM from their two explants cultured alone was pooled to form one EXPL sample, and CM from their two explants co-cultured with blastocysts was pooled to form one EXPL + EMB sample. Each pool was treated as a single biological replicate per condition per cyclic heifer. The protein content of EVs was analyzed across the following four comparisons: (1) UF‑EVs from CYCLIC versus PREGNANT heifers; (2) CM‑EVs from EXPL, EXPL + EMB, and EMB; (3) UF‑EVs from CYCLIC heifers versus CM‑EVs from EXPL; and (4) UF‑EVs from PREGNANT heifers versus CM‑EVs from EXPL + EMB. Created in BioRender (https://BioRender.com/gizygdh)
In vivo model
Animals
All experimental procedures involving animals were approved by the Animal Research Ethics Committee of University College Dublin and licensed by the Health Products Regulatory Authority, Ireland, in accordance with Statutory Instrument No. 543 of 2012 under Directive 2010/63/EU on the Protection of Animals used for Scientific Purposes.
Crossbred beef heifers (n = 28 predominantly Charolais- and Limousin-cross); 804 ± 135 days old and 602.6 ± 54.3 kg (mean ± standard deviation) were synchronized using an 8-day intravaginal progesterone (P4) device (PRID, 1.55 g P4; Ceva Santé Animale). On the day of PRID insertion, each heifer received a 2-mL intramuscular injection of an analog of gonadotropin-releasing hormone (GnRH; Ovarelin, Ceva Santé Animale, equivalent to 100 μg gonadorelin). On the day before PRID removal, all heifers received a 5-mL intramuscular injection of an analog of prostaglandin F2 alpha (PGF2α; Enzaprost, Ceva Santé Animale, equivalent to 25 mg dinoprost) to induce luteolysis. Estrus was detected in heifers by visual observation performed by trained personnel every 4–6 h, starting 24 h after PRID removal. Heifers were randomly assigned to be either inseminated (n = 15) at detected estrus to generate pregnancies or not inseminated (n = 13) to generate cyclic controls. Heifers were inseminated twice, approximately 12 and 24 h after the onset of estrus. All heifers were slaughtered at a local abattoir approximately 7 d after artificial insemination.
UF collection from PREGNANT and CYCLIC heifers
Reproductive tracts were returned to the laboratory on ice within 3 h of slaughter. The presence of a functional corpus luteum CL was confirmed in all estrus-synchronized heifers by post-mortem ovarian inspection. The uterine horn ipsilateral to the CL was dissected and flushed with 10 mL of phosphate-buffered saline without Ca2+ and Mg2+ (PBS−/−). The presence of a blastocyst in the uterine flushing of inseminated heifers was used to confirm pregnancy (n = 12), while the uterine flushings from non-inseminated heifers were categorized as cyclic (n = 13). All flushings were centrifuged immediately for 7 min at 300 × g and 4 °C to remove cells. The obtained supernatants were then centrifuged for 30 min at 10,000 × g and 4 °C to remove cellular debris and conserved at −80 °C to be later processed for EV isolation. For downstream analyses, five heifers from the 12 confirmed pregnant and five from the 13 non-inseminated heifers were randomly selected to form the PREGNANT and CYCLIC groups, respectively.
In vitro model
Preparation of endometrial explants
Four endometrial explants were obtained from each cyclic heifer, and prepared and cultured as described by Mathew et al. [36]. After flushing, the uterine horn ipsilateral to the CL was longitudinally opened on the anti-mesometrial side to expose the endometrium. Then, an 8-mm biopsy punch was used to dissect completely through the intercaruncular uterine tissue from the anterior portion of the uterine horn. Sterile scissors were then used to dissect the endometrium away from the myometrium. Once dissected, explants from the same animal were washed in Hanks’ Balanced Salt Solution (HBSS; Gibco, ThermoFisher Scientific) containing 1% antibiotic–antimycotic (ABAM; Gibco, 100×). Subsequently, these explants were individually cultured in a 24-well cell culture plate, with the endometrium side facing up, in wells containing 1 mL protein-free synthetic oviduct fluid (SOF) and under 5% CO2 at 38.5 °C in air with maximum humidity for 2 h before use.
Conditioned medium from endometrial explants and blastocysts
Before use, explants obtained as described above were transferred individually to new wells containing 1 mL equilibrated SOF. From the four endometrial explants obtained from each cyclic heifer, two were cultured in medium alone (EXPL), and two were co-cultured with five in vitro-produced bovine blastocysts each (EXPL + EMB) based on Passaro et al. [12], who showed that co-culture with at least five bovine blastocysts was necessary to induce transcriptomic changes in bovine endometrial explants. Also, five groups of 50 in vitro-produced blastocysts were cultured alone (EMB) in 500 μL of SOF each. A preliminary study showed that culturing 5 to 25 blastocysts for 6 h did not produce the minimum particle concentration required for reliable NTA detection (≥ 20 particles/frame). In contrast, 50 embryos consistently yielded sufficient EVs for NTA quantification and downstream analysis [37, 38]. Representative images of the EXPL, EXPL+EMB, and EMB groups are provided in Additional file 1.
All groups were cultured for 6 h at 5% CO2, 38.5 °C, and maximum humidity. A 6-h culture period was selected as it consistently induces embryo responsive alterations in endometrial explants and CM-derived EVs while preserving explant structural and functional integrity [12, 38–41]. After 6 h, the CM from all groups was collected. For each cyclic heifer, CM from their two explants cultured alone was pooled to form one EXPL sample, and CM from their two explants co-cultured with blastocysts was pooled to form one EXPL + EMB sample. A preliminary study demonstrated that pooling the CM from two explants per animal was necessary to achieve the minimum particle concentration required for reliable NTA detection (≥ 20 particles/frame) [37]. Each pool was treated as a single biological sample per condition for each cyclic heifer. CM from all groups were centrifuged for 7 min at 300 × g and 4 °C to remove cells. The obtained supernatant was then centrifuged for 30 min at 10,000 × g and 4 °C to remove cellular debris and the supernatant was stored at −80 °C to be later processed for EV isolation.
To ensure consistency between the in vitro and in vivo models, only explants from the same five CYCLIC heifers selected for the in vivo analyses were used. This resulted in five EXPL and five EXPL + EMB samples derived from these heifers. Additionally, CM from five independent EMB cultures was included in the downstream analyses of the in vitro model.
In vitro embryo production
Embryos were produced in vitro as previously described by Rizos et al. [1]. Briefly, bovine immature cumulus-oocyte complexes (COCs) were obtained by aspirating follicles from the ovaries of mature heifers slaughtered at a local abattoir. After selection, COCs were matured during 24 h in groups of 50/well in 500 µL maturation medium (TCM-199) supplemented with 10% of fetal calf serum (FCS) and 10 ng/mL epidermal growth factor (EGF) at 38.5 °C under an atmosphere of 5% CO2 in air with maximum humidity. Matured COCs were fertilized with frozen-thawed sperm from a bull of proven fertility at a concentration of 1× 106 sperm/mL. Gametes were co-incubated in 500 µL of fertilization medium for 18–20 h at 38.5 °C, 5% CO2 in air with Maximum humidity. Presumptive zygotes were denuded by vortexing and cultured in 500 µL of SOF supplemented with 5% of EV-depleted FCS (dFCS) at 38.5 °C, under 5% CO2, 5% O2, and 90% N2 with maximum humidity. The dFCS was produced in our laboratory according to the protocol used by Leal et al. [34]. Briefly, heat-inactivated FCS (56 °C for 30 min) was ultra-centrifuged at 100,000 × g for 18 h at 4 °C using an Optima-L-100XP Beckman Coulter ultracentrifuge. The supernatant (dFCS) was collected, aliquoted, and stored at −20 °C for later use. Embryos of excellent or good quality [42] were recovered 7 d after fertilization at the blastocyst stage for subsequent use.
EV isolation
Extracellular vesicles were isolated from the UF of five animals per group (n = 5 CYCLIC and n = 5 PREGNANT) and from five CM samples per group (n = 5 EXPL, n = 5 EXPL + EMB, and n = 5 EMB) following the protocol previously reported by our group [35].
This protocol is based on size-exclusion chromatography (SEC) using PURE-EV® columns (HansaBioMed Life Sciences) [21], an effective method for separating EVs from circulating proteins without altering their structure or function [43], followed by ultrafiltration using Vivaspin® Turbo 15 centrifugal concentrator (Sartorius, 100 K MWCO PES). Briefly, after discarding the buffer provided within the SEC column, the column was washed with 30 mL of PBS−/−, and then either UF (≈ 2 mL) or CM (≈ 2 mL) fluid samples were loaded onto the top of the SEC column. Once the sample was entirely within the column, 11 mL of PBS−/−was loaded, preventing the column from drying out. The EVs were collected in the 2.5 mL fraction after discarding the first 3 mL fraction. Subsequently, the 2.5 mL EV fraction was concentrated by ultrafiltration for 30 min at 2,000 × g and 4 °C, resulting in a final volume of 100 µL of concentrated EVs to be used later for EV characterization and proteomic analysis.
EV characterization
Following the Minimal Information for Studies of Extracellular Vesicles 2018 guidelines [44], EVs from UF and CM were characterized using flow cytometry (FC), nanoparticle tracking analysis (NTA), and transmission electron microscopy (TEM) as previously described by Mazzarella et al. [35].
Flow cytometry
The analyses were conducted utilizing the high-sensitive flow cytometer CytoFLEX S (Beckman Coulter), equipped with violet (405 nm), blue (488 nm), yellow (561 nm), and red (638 nm) lasers. Recombinant EVs expressing green fluorescent protein (GFP, SAE0193, Merck) were used to verify the accuracy of the flow cytometer for EV detection and counting. The optical configuration was optimized to use side scatter (SSC) information from the 405-nm laser (vSCC). Both forward scatter (FSC) and SSC were set to logarithmic scale, with the fluorescence channels also adjusted to logarithmic scale. The analysis was restricted to events with FSC and SSC characteristics specific to EVs. Samples were analyzed using the low flow speed setting (10 μL/min) with a minimum acquisition of 10 × 103 events per sample. Distilled water (filtered through a 0.1-μm filter) was used as the sheath fluid, and 0.1-μm-filtered phosphate-buffered saline (PBS) was employed to detect background noise. Two-minute washing steps with 0.1-μm-filtered distilled water were conducted between EV samples as described in Barranco et al. [45]. 10 μL EV sample was incubated with CellTrace CFSE (Thermofisher) for 30 min in darkness at 37 °C, a non-fluorescent probe that becomes fluorescent hydrolysis by active esterases present only in functional intact membrane structures, to discriminate intact EVs from membrane fragments. Tetraspanin antibodies anti-CD63-FITC and anti-CD81-APC (REA, Miltenyi Biotec) and anti-CD44-PerCP (Biolegend), with cross-reactivity with bovine species were used (30 min, RT in darkness), following the International Society of Extracellular Vesicles recommendations (MIFlowCyt-EV) [46].
Nanoparticle tracking analysis
The concentration and size distribution of EVs were analyzed using a NanoSight LM-10 system equipped with a CCD video camera and particle-tracking software NTA 3.1 Build 3.1.45 (NanoSight Ltd.). Five µL of UF-EVs or CM-EVs solution obtained after SEC were diluted in (1:10) with PBS-/-. PBS-/- was used as a negative control. The NTA measurement conditions were detection thresholds 2 to 3, camera level 13, temperature 22 °C, and measurement time 60 s. Three recordings were performed for each sample.
Transmission electron microscopy
Transmission electron microscopy was exclusively employed to confirm the successful isolation of EVs following the established protocol. For that, 5 µL of UF-EVs or CM-EVs solution obtained after SEC were diluted (1:5) with PBS-/- to perform the negative staining of EVs. A carbon-coated collodion 400 mesh nickel grid (Gilder) was floated for 2 min and stained with 2% uranyl acetate (Electron Microscopy Sciences) for 1 min for the negative staining. Grids were visualized in a JEOL JEM 1400 Flash electron microscope (operating at 100 kV). Micrographs were taken with a Gatan OneView digital camera at various magnifications.
Proteomic analyses
Proteomic analysis was conducted as previously described by Mazzarella et al. [35].
In solution digestion
Protein was extracted in a sample containing 7 mol/L urea, 2 mol/L Thiourea, 4% CHAPS, and 5 mmol/L DTT, then digested following filter-aided sample preparation protocol described by Wisniewski et al. [47] with minor modifications. Trypsin, used to generate peptides through specific cleavage, was added at a trypsin: protein ratio of 1:20, and the mixture was incubated overnight at 37 °C, dried out in an RVC2 25 speedvac concentrator (Christ), and resuspended in 0.1% formic acid (FA). Peptides were desalted and resuspended in 0.1% FA using C18 stage tips (Millipore).
Mass spectrometry analysis
Samples were analyzed in a timsTOF Pro with PASEF (Bruker Daltonics) coupled online to an Evosep ONE liquid chromatograph (Evosep). A total of 200 ng were directly loaded onto the Evosep ONE and resolved using the 60 samples-per-day protocol. Protein identification and quantification were carried out using the label-free quantification (LFQ) method integrated into MaxQuant 1.6.17.0 software. Searches were carried out against a database consisting of Bos taurus entries from UniProt Swissprot + TrEMBL (downloaded on April 6, 2022), consisting of 117,111 entries. Carbamidomethylation of cysteines was set as a fixed modification, and oxidation of methionine and N-terminal acetylation of proteins were set as variable modifications. Two missed cleavages were allowed for trypsin digestion. Precursor and fragment tolerances of 20 ppm and 0.05 Da were considered for the searches, respectively.
A 1% false discovery rate (FDR) was applied at both the peptide-spectrum match (PSM) and protein levels. Only proteins identified with at least two peptides at FDR < 1% were considered for further analysis. Proteins were considered ‘identified’ when detected in at least three out of five samples in each experimental group and were considered ‘exclusive’ when detected in at least three out of five samples within one group and not detected in any sample within the other. The quantitative analysis of the in vitro model was performed in pairs: EXPL vs. EMB, EMB vs. EXPL + EMB, and EXPL vs. EXPL + EMB. Initially, differentially abundant proteins (DAPs) were identified for each comparison. Then, to isolate proteins specifically associated with embryo-maternal interactions, those proteins also differentially abundant in the EXPL vs. EMB comparison, where no embryo-maternal interaction occurs, were excluded. Subsequently, to exclude proteins whose differential abundance arises solely from the individual effects of the explant (EXPL) or the embryo (EMB), only proteins differentially abundant in both the EXPL vs. EXPL + EMB and EMB vs. EXPL + EMB comparisons were considered. The differential abundance of these common proteins was attributed to the synergistic effects of the co-culture of explants with embryos (EXPL + EMB), reflecting embryo-maternal communication, and was subsequently used for downstream analysis. The strategy used to identify differentially abundant proteins associated with embryo-maternal interactions in vitro is illustrated in Additional file 2.
Statistical and bioinformatics analysis
EV characterization
In vivo and in vitro data were tested for outliers using the ROUT test and for normality using the Shapiro–Wilk test. Normality was confirmed, and in vivo, data were analyzed using Student’s t-test, while in vitro data were analyzed using one-way ANOVA followed by Tukey’s test. Statistical analyses were performed using GraphPad Prism 10. For all analyses, P ≤ 0.05 was considered significant.
Proteomics
Protein abundance data were analyzed using Student’s t-test. For all analyses, P ≤ 0.05 was considered significant for further analyses and discussion. Peak area data were transformed using log2 for graphical representation. Principal component analysis (PCA) was generated by Metaboanalyst 6.0 (https://www.metaboanalyst.ca). Venn diagrams were constructed using Venny 2.1 (https://bioinfogp.cnb.csic.es/tools/venny/). Molecular function, biological processes, cellular processes, protein class, and identification of biological pathways of the proteins were evaluated using the PANTHER 18.0 Classification System (https://PANTHERdb.org/) with Bos taurus as the selected organism [48]. Metascape Membership tool v3.5.20240101 (https://metascape.org) was used to identify significant enrichment (P ≤ 0.05) matching the term “embryo development” [49].
Results
EV characterization
EVs isolated from both in vivo and in vitro models were characterized regarding their size and concentration using NTA, their EV marker expression (CD63, CD81, and CD44) through FC, and their morphology by TEM.
In vivo model
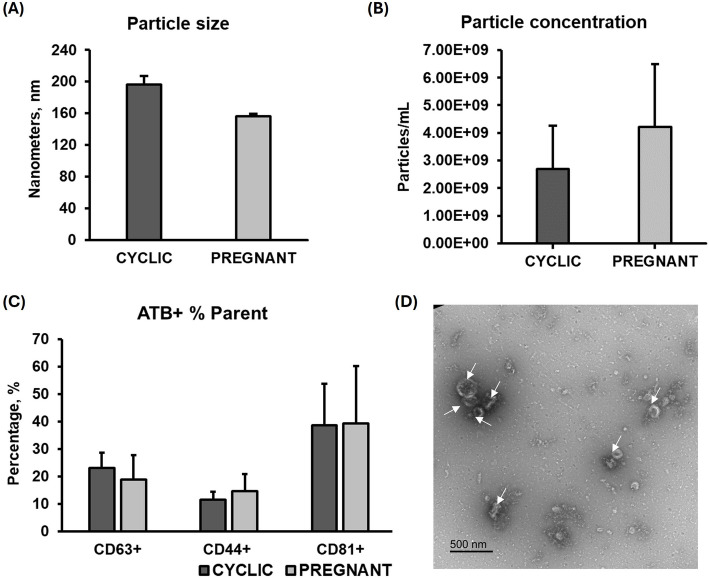
The NTA analysis demonstrated no significant difference in particle size between the CYCLIC (196 ± 11.25 nm) and PREGNANT (156.4 ± 2.7 nm) groups (Fig. 2A), nor in particle concentration (CYCLIC: 2.70 × 109 ± 1.55 × 109 particles/mL and PREGNANT: 4.21 × 109 ± 2.29 × 109 particles/mL; Fig. 2B). The NTA negative control showed zero particles per frame (Additional file 3A). The presence of EVs was corroborated by FC, which identified CD63, CD81, and CD44 positive events in both groups (Fig. 2C). No differences in the percentage of CD63⁺ (CYCLIC: 23% ± 5.66% and PREGNANT: 19% ± 8.91%), CD44⁺ (CYCLIC: 12% ± 2.90% and PREGNANT: 15% ± 6.13%), and CD81⁺ (CYCLIC: 39% ± 15.11% and PREGNANT: 39% ± 20.83%) EVs among the groups were observed. TEM images displayed cup-shaped particles with sizes characteristic of EVs in the UF (Fig. 2D), while no particles were observed in the negative control (Additional file 3B). Thus, we confirmed the presence of EVs in the UF of both CYCLIC and PREGNANT heifers and validated the effectiveness of the isolation protocol.
Fig. 2.
Characterization of uterine fluid extracellular vesicles (UF-EVs). Nanoparticle tracking analysis indicated no difference in particle size (A) or concentration (B) between the groups CYCLIC and PREGNANT. C Flow cytometry detected the presence of CD63, CD81, and CD44 markers in both groups. D Transmission electron microscopy images of UF-EVs, representative of both groups, revealed cup-shaped particles with sizes characteristic of EVs. Error bars represent the standard error of the mean. White arrows indicate EVs
In vitro model
The NTA analysis indicated no significant differences in particle size among the EXPL, EXPL + EMB, and EMB groups (EXPL: 169 ± 8.78 nm, EXPL + EMB: 179 ± 16.02 nm, EMB: 159 ± 6.10 nm; Fig. 3A). Particle concentration was lower in the CM from EMB (EXPL: 1.23 × 109 ± 1.27 × 108 particles/mL, EXPL + EMB: 1.60 × 109 ± 1.82 × 108 particles/mL and EMB: 2.84 × 108 ± 6.28 × 107 particles/mL; Fig. 3B). The NTA negative control showed zero particles per frame (Additional file 3A). EV presence was confirmed via FC, identifying the CD63, CD81, and CD44 markers across all three groups (Fig. 3C). A significant difference in the percentage of CD63⁺ and CD44⁺ EVs among the groups was observed (P = 0.0001 and P = 0.0038, respectively). The EMB group exhibited a higher percentage of CD63⁺ EVs (43% ± 2.11%) compared to EXPL (12% ± 2.63%) and EXPL + EMB (16% ± 1.41%). Similarly, EMB group showed a higher percentage of CD44⁺ EVs (21% ± 0.90%) than EXPL (8% ± 1.68%) and EXPL + EMB (12% ± 2.13%). Regarding CD81⁺ EVs, no differences were found between the groups (EXPL: 68% ± 3.87%, EXPL + EMB: 70% ± 2.45% and EMB: 60% ± 3.11%). TEM images showed cup-shaped particles with sizes characteristic of EVs in the CM from EXPL, EXPL + EMB, and EMB groups (Fig. 3D–F), while no particles were observed in the negative control (Additional file 3B). Therefore, we confirmed the presence of EVs in the CM and validated the effectiveness of the isolation protocol.
Fig. 3.
Characterization of conditioned medium (CM) extracellular vesicles (EVs). Nanoparticle tracking analysis revealed no significant differences in particle size (A) among the explants cultured alone (EXPL), explants co-cultured with embryos (EXPL + EMB), and embryos cultured alone (EMB) groups, while particle concentration was lower in the CM from EMB in comparison to EXPL and EXPL + EMB (B). Flow cytometry confirmed the presence of CD63, CD81, and CD44 markers in all groups (C). Transmission electron microscopy images showed cup-shaped particles with sizes characteristic of EVs in the CM from EXPL (D), EXPL + EMB (E), and EMB (F). Error bars represent the standard error of the mean. White arrows indicate EVs. Different letters indicate significant differences (P ≤ 0.05)
Qualitative and quantitative characterization of proteins
In vivo model
We identified 1,459 proteins in the UF-EVs of CYCLIC and 1,752 proteins in the UF-EVs from PREGNANT heifers (Additional files 4A and 4B, respectively). Of these, 1,329 proteins were commonly identified between the two groups, whereas 12 were exclusive to UF-EVs from CYCLIC and 18 were exclusive to UF-EVs from PREGNANT heifers (Fig. 4A). Additionally, the PCA plot (Fig. 4B) from the DAPs revealed two distinct clusters of CYCLIC and PREGNANT heifers. Among the 1,329 identified in both groups, 16 proteins were differentially abundant (P ≤ 0.05; Additional file 4C): six proteins were less abundant, and 10 were more abundant in PREGNANT vs. CYCLIC heifers (Fig. 4C and D).
Fig. 4.
Protein profile of uterine fluid extracellular vesicles (UF-EVs) from CYCLIC and PREGNANT heifers. A The table indicates the number of proteins identified in each group, and the Venn diagram represents the 1,329 proteins common to both, the 12 proteins exclusively detected in CYCLIC and 18 proteins exclusively detected in PREGNANT heifers. B Principal Component Analysis of differentially abundant proteins. C Ten proteins were overabundant in UF-EVs from PREGNANT compared to CYCLIC heifers. D Six proteins were less abundant in UF-EVs from PREGNANT compared to CYCLIC heifers. Proteins were considered ‘identified’ if detected in at least three out of five replicates and considered ‘exclusive’ if detected in at least three out of five replicates within one group but absent in all samples of other groups. Error bars represent the standard error of the mean. P ≤ 0.05 was considered as significant
Among the top 30 most abundant proteins in CYCLIC heifers (Table 1A), 19 were ribosomal proteins (RPS8, RPL18, RPL7A, RPL7, RPL6, RPL19, RPS6, RPL36, RPS9, E1BK63, RPLP0, RPS2, RPL31, RPL24, RPL13A, RPS16, RPL10A, RPS25 and RPL27). Similarly, in PREGNANT heifers (Table 1B), 18 of the 30 most abundant proteins were ribosomal proteins (PIK3C2A, RPS2, RPS24, RPS4, RPS6, RPS8, RPS9, RPLP0, Q862L6, RPL13A, RPL31, RPL36, RPL6, RPL7A, RPL10A, RPL18, RPL19, and RPL7). The top 100 most abundant proteins in UF-EVs from CYCLIC and PREGNANT heifers are available in Additional file 4D.
Table 1.
Top 30 most abundant proteins identified in vivo
| Protein accession | Symbol | Description | Peak area (log2) |
|---|---|---|---|
| (A) Top 30 most abundant proteins in UF-EVs from CYCLIC heifers | |||
| A0A4W2FD79 | ZNF638 | Zinc finger protein 638 | 23.70 |
| E1BAU6 | INPP5E | Inositol polyphosphate-5-phosphatase E | 22.73 |
| A0A4W2EXX8 | RPS8 | 40S ribosomal protein S8 | 22.22 |
| A0A4W2EFE8 | RPL18 | Ribosomal protein L18 | 21.88 |
| A0A4W2GZL4 | IL1RAP | Interleukin 1 receptor accessory protein | 21.66 |
| Q2TBQ5 | RPL7A | 60S ribosomal protein L7a | 21.60 |
| A0A4W2GAA4 | RPL7 | Ribosomal protein L7 | 21.60 |
| A0A4W2DYQ2 | ACTB | Actin beta | 21.51 |
| A0A4W2GH10 | ANPEP | Aminopeptidase | 21.50 |
| A0A4W2HYA4 | EZR | Ezrin | 21.42 |
| A0A4W2E7H4 | RPL6 | 60S ribosomal protein L6 | 21.39 |
| A0A4W2E0U9 | RPL19 | Ribosomal protein L19 | 21.23 |
| A0A4W2EMD8 | RPS6 | 40S ribosomal protein S6 | 21.06 |
| A0A4W2D0E5 | RPL36 | 60S ribosomal protein L36 | 21.02 |
| A0A4W2D2Y6 | RPS9 | 40S ribosomal protein S9 | 21.02 |
| E1BK63 | ─ | Ribosomal protein L15 | 21.02 |
| A0A4W2DVZ1 | TUBB | Tubulin beta chain | 20.99 |
| A0A4W2H475 | RPLP0 | 60S acidic ribosomal protein P0 | 20.98 |
| Q28042 | OVGP1 | Oviduct-specific glycoprotein (Fragment) | 20.90 |
| F1MQ37 | MYH9 | Myosin heavy chain 9 | 20.89 |
| A0A452DIA7 | RPS2 | 40S ribosomal protein S2 | 20.87 |
| A0A4W2CVQ1 | ANXA2 | Annexin | 20.82 |
| A0A4W2FQP8 | RPL31 | 60S ribosomal protein L31 | 20.79 |
| A0A4W2F392 | RPL24 | Ribosomal protein L24 | 20.79 |
| A0A4W2CJG3 | RPL13A | 60S ribosomal protein L13a | 20.76 |
| A0A4W2HV66 | RPS16 | Ribosomal protein S16 | 20.76 |
| P09487 | ALPL | Alkaline phosphatase, tissue-nonspecific isozyme | 20.74 |
| A0A4W2HK63 | RPL10A | Ribosomal protein | 20.69 |
| A0A4W2EYV4 | RPS25 | 40S ribosomal protein S25 | 20.67 |
| A0A4W2CDD8 | RPL27 | 60S ribosomal protein L27 | 20.67 |
| (B) Top 30 most abundant proteins in UF-EVs from PREGNANT heifers | |||
| A0A4W2FD79 | ZNF638 | Zinc finger protein 638 | 23.45 |
| E1BAU6 | INPP5E | Inositol polyphosphate-5-phosphatase E | 22.51 |
| A0A4W2EXX8 | RPS8 | 40S ribosomal protein S8 | 22.19 |
| A0A4W2GH10 | ANPEP | Aminopeptidase | 22.10 |
| A0A4W2DYQ2 | ACTB | Actin beta | 21.93 |
| A0A4W2EFE8 | RPL18 | Ribosomal protein L18 | 21.72 |
| A0A4W2GAA4 | RPL7 | Ribosomal protein L7 | 21.67 |
| A0A4W2C549 | LGALS3BP | Galectin-3-binding protein | 21.46 |
| A0A4W2DVZ1 | TUBB | Tubulin beta chain | 21.34 |
| F1MZ85 | VCAN | Versican core protein | 21.28 |
| Q2TBQ5 | RPL7A | 60S ribosomal protein L7a | 21.22 |
| A0A4W2E7H4 | RPL6 | 60S ribosomal protein L6 | 21.19 |
| A0A4W2CVQ1 | ANXA2 | Annexin | 21.15 |
| F1MQ37 | MYH9 | Myosin heavy chain 9 | 21.13 |
| A0A4W2E0U9 | RPL19 | Ribosomal protein L19 | 21.10 |
| Q862L6 | ─ | 60S ribosomal protein L12 (Fragment) | 21.05 |
| A0A4W2HYA4 | EZR | Ezrin | 21.03 |
| A0A452DIA7 | RPS2 | 40S ribosomal protein S2 | 21.03 |
| A0A4W2GZL4 | IL1RAP | Interleukin 1 receptor accessory protein | 21.00 |
| A0A4W2H475 | RPLP0 | 60S acidic ribosomal protein P0 | 21.00 |
| A0A4W2D2Y6 | RPS9 | 40S ribosomal protein S9 | 20.97 |
| A0A4W2EMD8 | RPS6 | 40S ribosomal protein S6 | 20.80 |
| A0A4W2CJG3 | RPL13A | 60S ribosomal protein L13a | 20.80 |
| P09487 | ALPL | Alkaline phosphatase, tissue-nonspecific isozyme | 20.79 |
| A0A4W2F5Z1 | RPS24 | 40S ribosomal protein S24 | 20.74 |
| A0A4W2HK63 | RPL10A | Ribosomal protein | 20.73 |
| P79103 | RPS4 | 40S ribosomal protein S4 | 20.68 |
| A0A4W2FQP8 | RPL31 | 60S ribosomal protein L31 | 20.68 |
| A0A4W2C999 | PIK3C2A | 40S ribosomal protein S13 | 20.64 |
| A0A4W2D0E5 | RPL36 | 60S ribosomal protein L36 | 20.49 |
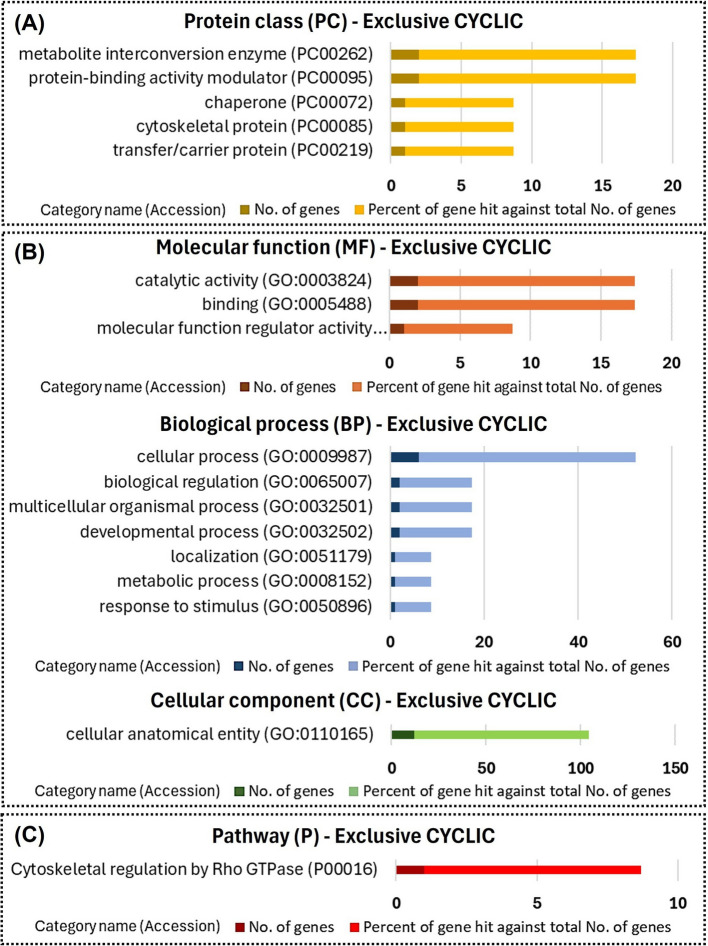
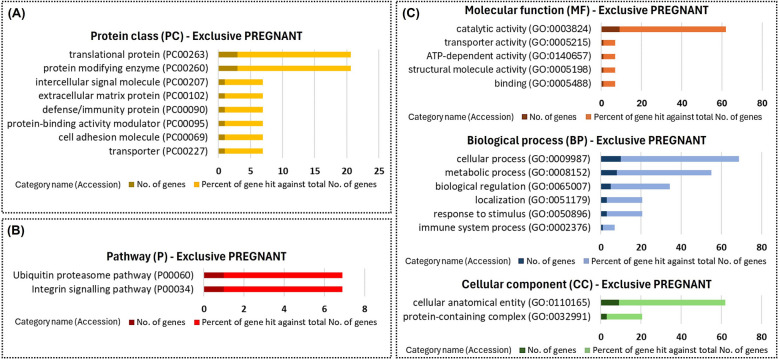
Functional enrichment using the PANTHER database indicated that among proteins exclusive to UF-EVs of CYCLIC heifers were proteins with enzymes related catalytic activity (Fig. 5A and B), and proteins involved in the cytoskeletal regulation pathway (Fig. 5C). Among the proteins exclusive to UF-EVs in PREGNANT heifers were translational proteins and protein-modifying enzymes (Fig. 6A). These proteins were also involved in biological processes including cellular and metabolic activities (Fig. 6C) and were components of pathways such as the integrin signaling pathway (Fig. 6B).
Fig. 5.
Functional enrichment of the proteins exclusive to uterine fluid extracellular vesicles of CYCLIC heifers. Protein class (A), Gene Ontology (B), and pathways (C) identified using the PANTHER 18.0 Classification System (https://pantherdb.org/). Darker bars indicate the number of genes associated with each category name, while lighter bars represent the percentage of these genes relative to the total number of genes in that category
Fig. 6.
Functional enrichment of the proteins exclusive to uterine fluid extracellular vesicles of PREGNANT heifers. Protein class (A), Gene Ontology (B), and pathways (C) identified using the PANTHER 18.0 Classification System (https://pantherdb.org/). Darker bars indicate the number of genes associated with each category name, while lighter bars represent the percentage of these genes relative to the total number of genes in that category
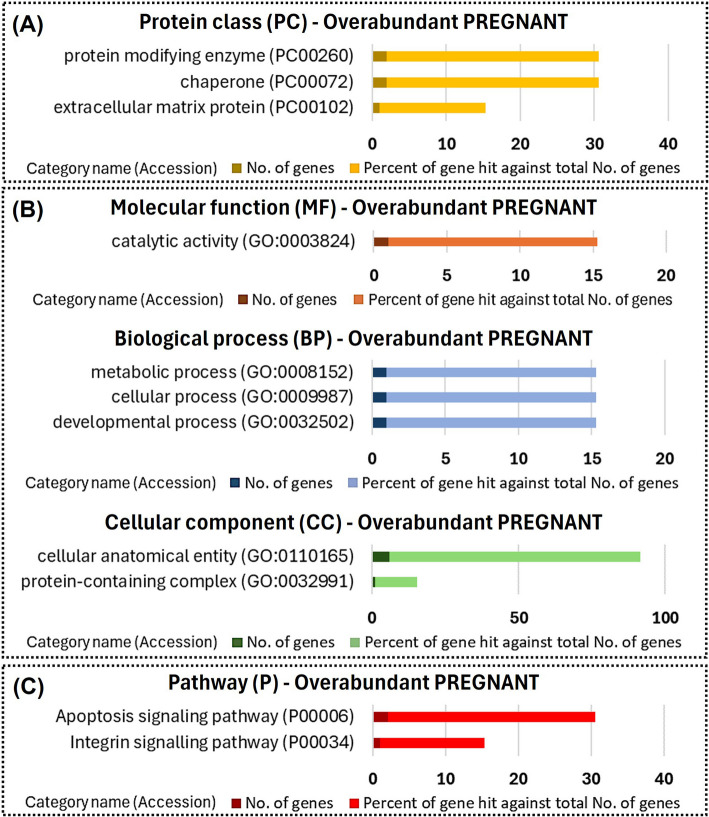
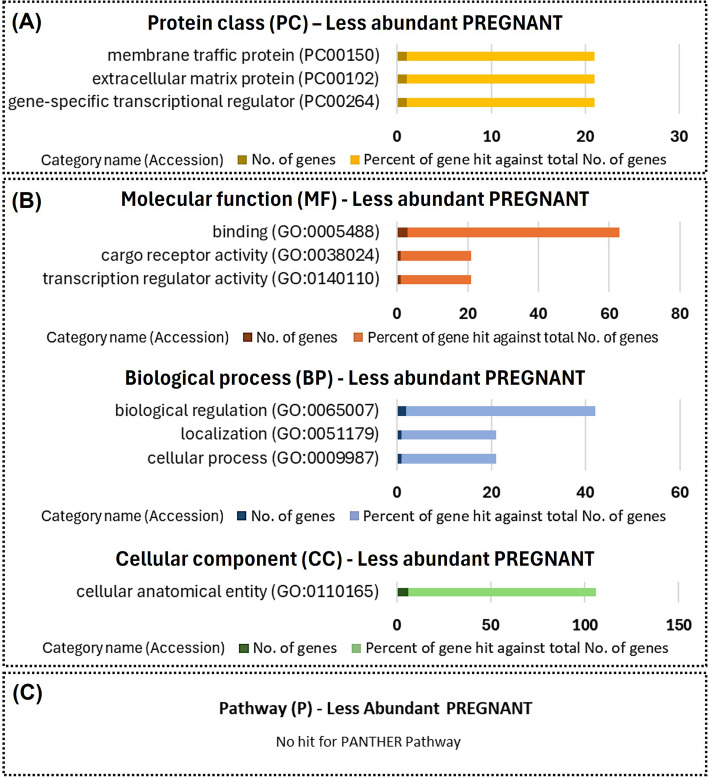
Overabundant proteins in PREGNANT heifers compared to CYCLIC heifers were related to protein-modifying enzymes (Fig. 7A). The less abundant proteins were associated with membrane trafficking, the extracellular matrix, and transcriptional regulation (Fig. 8A). Overabundant proteins were mainly associated with metabolic, cellular, and developmental processes, and were involved in pathways such as apoptosis and integrin signaling (Fig. 7B and C). Additionally, the less abundant proteins were related to biological regulation and cellular processes (Fig. 8B). No pathways were identified as modulated by the less abundant proteins in PREGNANT heifers (Fig. 8C).
Fig. 7.
Functional enrichment of proteins overabundant in uterine fluid extracellular vesicles from PREGNANT versus CYCLIC heifers. Protein class (A), Gene Ontology (B) and pathways (C) identified using the PANTHER 18.0 Classification System (https://pantherdb.org/). Darker bars indicate the number of genes associated with each category name, while lighter bars represent the percentage of these genes relative to the total number of genes in that category
Fig. 8.
Functional enrichment of proteins less abundant in uterine fluid extracellular vesicles from PREGNANT versus CYCLIC heifers. Protein class (A), gene ontology (B), and pathways (C) identified using the PANTHER 18.0 Classification System (https://pantherdb.org/). Darker bars indicate the number of genes associated with each category name, while lighter bars represent the percentage of these genes relative to the total number of genes in that category
In vitro model
We identified 1,501 proteins in the CM-EVs from EXPL, 1,975 from EXPL + EMB, and 82 from EMB (Fig. 9A, Additional files 5A, 5B and 5C, respectively). Of these, 66 proteins were commonly identified among the three groups, 1,145 were common to EXPL and EXPL + EMB but not to EMB (Fig. 9B). Two proteins were unique to EXPL (ANKRD44 and ATP2A1), and one was unique to EMB (ITFG1). Additionally, 50 proteins were identified as being exclusively present in the EXPL + EMB group (Fig. 9B, red box) when there is an interaction between the endometrium and the embryo in vitro.
Fig. 9.
Protein profile of in vitro-derived extracellular vesicles (EVs). EVs were isolated from conditioned medium (CM) following the culture of endometrial explants in the absence (EXPL) or presence (EXPL + EMB) of blastocysts, or from blastocysts cultured alone (EMB). A Table representing the 1,501 proteins identified in the CM-EVs from ExpL, 1,975 proteins identified in ExpL + Emb, and 82 proteins identified in EMB. B Venn diagram represents the number of proteins associated with CM-EVs from EXPL, EXPL + EMB, and EMB. The red box indicates the list of the 50 proteins identified as only present when there is an interaction between the endometrium and the embryo in vitro. Proteins were considered ‘identified’ if detected in at least three out of five replicates and considered ‘exclusive’ if detected in at least three out of five replicates within one group but absent in all samples of other groups
Among the top 30 most abundant proteins in EXPL (Table 2A) and EXPL + EMB (Table 2B) were proteins such as zinc finger protein 638 (ZNF638), histones (H4 and H2B), inositol polyphosphate-5-phosphatase E (INPP5E), actins (ACTB, and ACTC1), tubulins (TUBB, TUBA1D and TUBB4B) and heat shock protein (HSP90AA1 and HSPA8). In EMB, the most abundant proteins include inositol polyphosphate-5-phosphatase E (INPP5E), zinc finger proteins (ZNF638), and keratins (KRT76, KRT18, KRT75, and KRT8) (Table 2C). The top 100 most abundant proteins in CM-EVs from EXPL, EXPL + EMB, and EMB are available in Additional file 5D.
Table 2.
Top 30 most abundant proteins in vitro
| Protein accession | Symbol | Description | Peak area (log2) |
|---|---|---|---|
| (A) Top 30 most abundant proteins in CM-EVs from EXPL | |||
| A0A4W2FD79 | ZNF638 | Zinc finger protein 638 | 23.77 |
| A0A4W2HPP0 | ─ | Uncharacterized protein | 23.23 |
| P62803 | ─ | Histone H4 | 23.23 |
| A0A4W2HHA6 | ─ | Histone H2B | 23.10 |
| E1BAU6 | INPP5E | Inositol polyphosphate-5-phosphatase E | 23.03 |
| A0A4W2DYQ2 | ACTB | Actin beta | 22.94 |
| A0A3Q1MDT7 | ─ | Histone H4 | 22.68 |
| Q3ZC07 | ACTC1 | Actin, alpha cardiac muscle 1 | 22.06 |
| A0A4W2DVZ1 | TUBB | Tubulin beta chain | 21.51 |
| A0A4W2H221 | FGA | Fibrinogen alpha chain | 21.25 |
| P10096 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | 21.24 |
| F1MGU7 | FGG | Fibrinogen gamma-B chain | 21.23 |
| F6S1Q0 | KRT18 | Keratin 18 | 21.18 |
| A0A452DIF5 | H1-2 | H1.2 linker histone, cluster member | 21.15 |
| A0A4W2F0F2 | TMPRSS6 | Sulfurtransferase | 21.14 |
| Q76LV2 | HSP90AA1 | Heat shock protein HSP 90-alpha | 20.88 |
| A0A452DJ66 | TUBA1D | Tubulin alpha chain | 20.81 |
| Q3MHM5 | TUBB4B | Tubulin beta-4B chain | 20.77 |
| A0A4W2GV41 | CLTC | Clathrin heavy chain | 20.45 |
| A0A4W2CTC9 | A2M | Uncharacterized protein | 20.36 |
| A0A4W2FJT1 | HSPA8 | Heat shock protein family A (Hsp70) member 8 | 20.32 |
| A0A4W2CIB6 | VIM | Vimentin | 20.16 |
| A0A4W2G1A4 | KRT76 | Keratin, type II cytoskeletal 2 oral-like | 20.15 |
| F1MU12 | KRT8 | Keratin, type II cytoskeletal 8 | 20.08 |
| A0A4W2HN26 | FLNA | Filamin A | 19.98 |
| F1MD77 | LAMC1 | Laminin subunit gamma 1 | 19.95 |
| A0A4W2EG96 | VCP | 15S Mg(2+)-ATPase p97 subunit | 19.85 |
| E9RHW1 | HSPB1 | Heat shock 27 kDa protein | 19.85 |
| A0A4W2C164 | ─ | Uncharacterized protein | 19.83 |
| F1MZ85 | VCAN | Versican core protein | 19.79 |
| (B) Top 30 most abundant proteins in CM-EVs from EXPL + EMB | |||
| A0A4W2FD79 | ZNF638 | Zinc finger protein 638 | 23.25 |
| A0A4W2DYQ2 | ACTB | Actin beta | 22.91 |
| A0A4W2HPP0 | ─ | Uncharacterized protein | 22.80 |
| P62803 | ─ | Histone H4 | 22.62 |
| Q3ZC07 | ACTC1 | Actin, alpha cardiac muscle 1 | 22.47 |
| A0A4W2HHA6 | ─ | Histone H2B | 22.34 |
| E1BAU6 | INPP5E | Inositol polyphosphate-5-phosphatase E | 22.17 |
| A0A3Q1MDT7 | ─ | Histone H4 | 21.94 |
| Q76LV2 | HSP90AA1 | Heat shock protein HSP 90-alpha | 21.61 |
| A0A4W2DVZ1 | TUBB | Tubulin beta chain | 21.50 |
| P10096 | GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | 21.30 |
| A0A4W2H221 | FGA | Fibrinogen alpha chain | 21.15 |
| F1MGU7 | FGG | Fibrinogen gamma-B chain | 21.14 |
| F6S1Q0 | KRT18 | Keratin 18 | 21.10 |
| A0A452DJ66 | TUBA1D | Tubulin alpha chain | 21.07 |
| A0A4W2CGL9 | SMC3 | Chondroitin sulfate proteoglycan 6 | 21.01 |
| A0A4W2F0F2 | TMPRSS6 | Sulfurtransferase | 20.97 |
| Q3MHM5 | TUBB4B | Tubulin beta-4B chain | 20.81 |
| A0A452DIF5 | H1-2 | H1.2 linker histone, cluster member | 20.74 |
| A0A4W2GV41 | CLTC | Clathrin heavy chain | 20.70 |
| A0A4W2BUC1 | COPG1 | Coatomer subunit gamma | 20.47 |
| A0A4W2FJT1 | HSPA8 | Heat shock protein family A (Hsp70) member 8 | 20.42 |
| A7MBJ5 | CAND1 | Cullin-associated NEDD8-dissociated protein 1 | 20.38 |
| F1MU12 | KRT8 | Keratin, type II cytoskeletal 8 | 20.32 |
| A0A4W2CIB6 | VIM | Vimentin | 20.29 |
| A0A4W2HN26 | FLNA | Filamin A | 20.20 |
| A0A4W2CTC9 | A2M | Uncharacterized protein | 20.13 |
| A0A4W2I521 | EPHX2 | Epoxide hydrolase 2 | 20.12 |
| A0A4W2HR21 | SPTAN1 | Spectrin alpha, non-erythrocytic 1 | 20.07 |
| F1MD77 | LAMC1 | Laminin subunit gamma 1 | 20.07 |
| (C) Top 30 most abundant proteins in CM-EVs from EMB | |||
| E1BAU6 | INPP5E | Inositol polyphosphate-5-phosphatase E | 23.98 |
| A0A4W2FD79 | ZNF638 | Zinc finger protein 638 | 22.64 |
| A0A4W2G1A4 | KRT76 | Keratin, type II cytoskeletal 2 oral-like | 21.13 |
| A0A4W2CGL9 | SMC3 | Chondroitin sulfate proteoglycan 6 | 20.73 |
| A0A3Q1M4X6 | SMUG1 | Single-strand selective monofunctional uracil DNA glycosylase | 20.00 |
| F6S1Q0 | KRT18 | Keratin 18 | 19.12 |
| A0A4W2ENX3 | KRT75 | Keratin 75 | 17.83 |
| F1MF78 | SYNE2 | Spectrin repeat containing nuclear envelope protein 2 | 17.50 |
| A0A4W2GNU2 | ASNA1 | ATPase ASNA1 | 17.38 |
| A0A4W2D966 | VPS13D | Vacuolar protein sorting 13 homolog D | 17.04 |
| A0A4W2H231 | DSP | Desmoplakin | 16.90 |
| A0A4W2HA98 | CEP135 | Centrosomal protein 135 | 16.87 |
| A0A4W2CM56 | ─ | Aldo | 16.85 |
| F1MU12 | KRT8 | Keratin, type II cytoskeletal 8 | 16.79 |
| A0A3Q1M1M7 | JUP | Junction plakoglobin | 16.68 |
| A0A4W2DYQ2 | ACTB | Actin beta | 16.55 |
| F1MIW8 | DSG1 | Desmoglein-1 | 16.54 |
| A0A4W2E085 | SRSF3 | Serine and arginine rich splicing factor 3 | 16.46 |
| A0A4W2BWI2 | SEC16A | Protein transport protein sec16 | 16.42 |
| A0A4W2GAK0 | SYNE1 | Spectrin repeat containing nuclear envelope protein 1 | 16.26 |
| A0A4W2ECV0 | AIDA | Axin interactor, dorsalization associated | 16.25 |
| Q0MRP5 | LZ | Lysozyme | 16.17 |
| A0A4W2E9X9 | SPATS2L | Uncharacterized protein | 15.83 |
| A0A4W2FQ19 | UPK1B | Tetraspanin | 15.80 |
| A0A4W2GW83 | ALB | Albumin | 15.70 |
| V6F7W7 | UBE4A | Ubiquitin conjugation factor E4 A | 15.60 |
| A0A4W2H672 | MELTF | Melanotransferrin | 15.56 |
| A0A4W2HUQ2 | ATP5MF | ATP synthase membrane subunit f | 15.39 |
| P08728 | KRT19 | Keratin, type I cytoskeletal 19 | 15.33 |
| A0A4W2IEJ4 | MYOF | Myoferlin | 15.29 |
CM-EVs Conditioned medium extracellular vesicles, EXPL Endometrial explants cultured alone, EXPL+EMB Endometrial explants co-cultured with blastocysts, EMB Blastocysts cultured alone
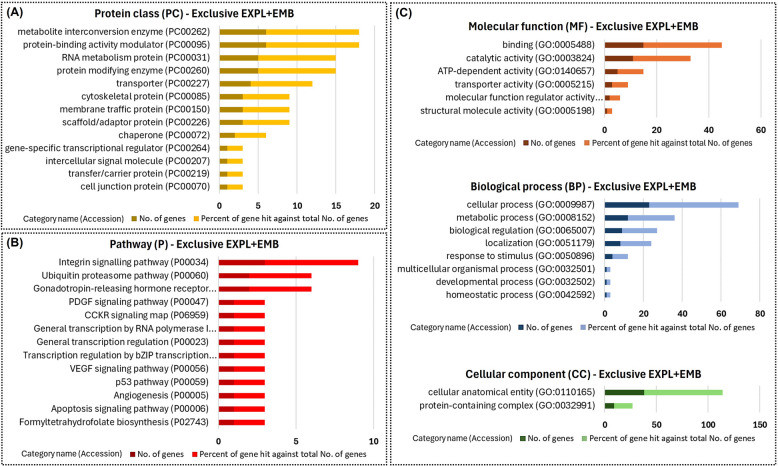
Functional enrichment using the PANTHER database showed that the identified proteins were involved in different GO biological processes and pathways. The 50 proteins identified as being exclusively present in the EXPL + EMB group were mainly metabolite interconversion enzymes, protein modifying enzymes, RNA metabolism proteins, and metabolite interconversion enzymes (Fig. 10 A). These proteins participate in the cellular and metabolic process and biological regulation of pathways such as gonadotropin-releasing hormone receptors and integrin signaling (Fig. 10B and C).
Fig. 10.
Functional enrichment of proteins exclusive to CM-EVs from EXPL + EMB. Fifty proteins were identified exclusively in the conditioned medium extracellular vesicles (CM-EVs) from endometrial explants co-cultured with blastocysts (EXPL + EMB). Protein class (A), pathways (B), and Gene Ontology (C) identified using the PANTHER 18.0 Classification System (https://pantherdb.org/). Darker bars indicate the number of genes associated with each category name, while lighter bars represent the percentage of these genes relative to the total number of genes in that category
In the quantitative analyses, we identified 33 differentially abundant proteins resulting from the synergistic effect between the embryo and blastocyst in vitro (Table 3). The quantitative analysis of the in vitro model was performed in pairs: EXPL vs. EMB, EMB vs. EXPL + EMB, and EXPL vs. EXPL + EMB. Once the differentially expressed proteins between these comparisons were identified, differentially abundant proteins not resulting from the embryo-maternal interaction (EXPL vs. EMB) were disregarded, leaving 33 differentially abundant proteins due to embryo-maternal interaction (Table 3).
Table 3.
Differentially abundant proteins due to embryo–maternal interaction in vitro
| Accession | Symbol | Description | EXPL vs. EMB (P-value) | EXPL vs. EXPL + EMB (P-value) | EMB vs. EXPL + EMB (P-value) | EXPL vs. EMB | EXPL vs. EXPL + EMB | EMB vs. EXPL + EMB |
|---|---|---|---|---|---|---|---|---|
| A0A4W2EGQ6 | ARPC2 | Arp2/3 complex 34 kDa subunit | 0.0799 | 0.0435 | 0.0002 | 0 | 1 | 1 |
| A0A4W2F3T9 | LRRC17 | Armadillo repeat containing 10 | 0.0773 | 0.0180 | 0.0002 | 0 | 1 | 1 |
| A0A4W2F6C3 | SURF4 | Surfeit locus protein 4 | 0.2357 | 0.0371 | 0.0002 | 0 | 1 | 1 |
| A5D7E8 | PDIA3 | Protein disulfide-isomerase | 0.2348 | 0.0048 | 0.0003 | 0 | 1 | 1 |
| Q9TU47 | EIF6 | Eukaryotic translation initiation factor 6 | 0.1417 | 0.0139 | 0.0003 | 0 | 1 | 1 |
| A0A4W2GKH0 | POLR2H | DNA-directed RNA polymerases I, II, and III subunit RPABC3 | 0.0631 | 0.0139 | 0.0003 | 0 | 1 | 1 |
| Q3MHK9 | FSCN1 | Fascin | 0.0594 | 0.0424 | 0.0004 | 0 | 1 | 1 |
| A0A4W2DIA0 | ERAP2 | Aminopeptidase | 0.2546 | 0.0224 | 0.0004 | 0 | 1 | 1 |
| A0A4W2DCF6 | PDCD10 | Programmed cell death 10 | 0.1014 | 0.0262 | 0.0004 | 0 | 1 | 1 |
| A0A4W2CF85 | CAPNS1 | Calcium-activated neutral proteinase small subunit | 0.0512 | 0.0279 | 0.0005 | 0 | 1 | 1 |
| A0A4W2EYS9 | OSBPL9 | Oxysterol-binding protein | 0.1473 | 0.0094 | 0.0006 | 0 | 1 | 1 |
| A5PKD6 | GNB4 | G protein subunit beta 4 | 0.3466 | 0.0030 | 0.0009 | 0 | 1 | 1 |
| A0A4W2FR72 | RAB25 | RAB25, member RAS oncogene family | 0.3466 | 0.0313 | 0.0011 | 0 | 1 | 1 |
| A0A4W2HBQ0 | YWHAH | Tyrosine 3-monooxygenase/tryptophan 5- monooxygenase activation protein eta | 0.0545 | 0.0438 | 0.0022 | 0 | 1 | 1 |
| A0A452DI24 | GDI2 | Rab GDP dissociation inhibitor | 0.2967 | 0.0121 | 0.0032 | 0 | 1 | 1 |
| V6F832 | CRYAB | Alpha(B)-crystallin | 0.2347 | 0.0374 | 0.0033 | 0 | 1 | 1 |
| Q1RMW9 | GOLPH3 | Golgi phosphoprotein 3 | 0.1872 | 0.0433 | 0.0040 | 0 | 1 | 1 |
| Q2T9M8 | SNAP23 | Synaptosomal-associated protein | 0.1281 | 0.0364 | 0.0046 | 0 | 1 | 1 |
| P37980 | PPA1 | Inorganic pyrophosphatase | 0.3466 | 0.0179 | 0.0050 | 0 | 1 | 1 |
| P07857 | SCP2 | Sterol carrier protein 2 | 0.0877 | 0.0395 | 0.0053 | 0 | 1 | 1 |
| Q2KIW9 | CMPK1 | UMP-CMP kinase | 0.3466 | 0.0103 | 0.0055 | 0 | 1 | 1 |
| A0A4W2DWW9 | ATE1 | Arginyl-tRNA–protein transferase 1 | 0.1411 | 0.0310 | 0.0066 | 0 | 1 | 1 |
| A0A4W2DB49 | NAP1L1 | Nucleosome assembly protein 1 Like 1 | 0.0536 | 0.0441 | 0.0068 | 0 | 1 | 1 |
| A0A4W2HIZ5 | FKBP9 | Peptidylprolyl isomerase | 0.3466 | 0.0357 | 0.0074 | 0 | 1 | 1 |
| A8KC77 | EIF5 | eIF5 protein (Fragment) | 0.3466 | 0.0211 | 0.0091 | 0 | 1 | 1 |
| A0A4W2I2Z9 | GSK3A | [Tau protein] kinase | 0.3466 | 0.0392 | 0.0116 | 0 | 1 | 1 |
| A0A4W2C0I1 | ─ | Elongation factor 1-alpha | 0.1449 | 0.0434 | 0.0119 | 0 | 1 | 1 |
| P21856 | GDI1 | Rab GDP dissociation inhibitor alpha | 0.3466 | 0.0332 | 0.0129 | 0 | 1 | 1 |
| F1N1F9 | SOX17 | SRY-box transcription factor 17 | 0.2611 | 0.0318 | 0.0134 | 0 | 1 | 1 |
| A0A4W2E056 | FAT2 | FAT atypical cadherin 2 | 0.3466 | 0.0323 | 0.0153 | 0 | 1 | 1 |
| E1BB38 | SRP72 | Signal recognition particle subunit SRP72 | 0.0579 | 0.0326 | 0.0270 | 0 | 1 | 1 |
| Q3SZX8 | CCDC25 | Coiled-coil domain-containing protein 25 | 0.3466 | 0.0496 | 0.0270 | 0 | 1 | 1 |
| A0A4W2H076 | STAG1 | Stromal antigen 1 | 0.3466 | 0.0252 | 0.0340 | 0 | 1 | 1 |
EXPL Endometrial explants cultured alone, EXPL + EMB Endometrial explants co-cultured with blastocysts, EMB Blastocysts cultured alone. 0, No significant change, 1, Significant change (P < 0.05)
Thirty-three proteins were differentially abundant in conditioned medium extracellular vesicles (CM-EVs) resulting from the synergistic effect of embryo–maternal interaction in the in vitro model
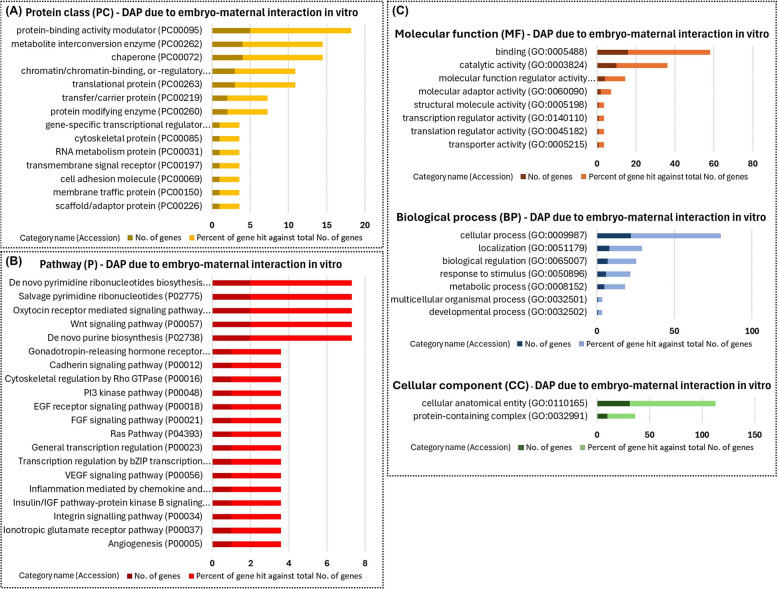
The PANTHER database was utilized to investigate the GO biological processes and pathways of these proteins. The proteins resulting from the synergistic effect between the embryo and blastocyst in vitro were primarily protein-binding modulators, metabolite interconversion enzymes, and chaperones (Fig. 11A). These proteins were associated with binding functions and processes such as biological regulation and response to stimuli (Fig. 11C). Additionally, they were involved in pathways such as Wnt signaling, PI3 kinase, and Ras (Fig. 11B).
Fig. 11.
Functional enrichment of differentially abundant proteins due to embryo–maternal interaction in vitro. Thirty-three proteins were differentially abundant in conditioned medium extracellular vesicles (CM-EVs) resulting from the synergistic effect of embryo–maternal interaction in the in vitro model. Protein class (A), pathways (B), and Gene Ontology (C) identified using the PANTHER 18.0 Classification System (https://pantherdb.org/). Darker bars indicate the number of genes associated with each category name, while lighter bars represent the percentage of these genes relative to the total number of genes in that category
Comparison of in vivo model and in vitro model
To investigate the distinctions and similarities in the embryo-maternal communication between in vivo and in vitro models, we conducted a comparative analysis using UF-EVs from CYCLIC heifers with CM-EVs from EXPL, and UF-EVs from PREGNANT heifers with CM-EVs from EXPL + EMB.
The analysis of the UF-EVs from CYCLIC heifers versus CM-EVs from EXPL (Fig. 12A) revealed 125 proteins exclusive to CYCLIC heifers, 106 unique to EXPL, and 1,019 proteins shared between them, among which 328 (32.2%) were identified as differentially abundant proteins. Similarly, in the comparison of UF-EVs from PREGNANT heifers with CM-EVs from EXPL + EMB (Fig. 12B), we identified 108 proteins exclusive to PREGNANT heifers, 195 unique to EXPL + EMB, and 1,373 proteins in common. Among these, 560 proteins (40.8%) were identified as differentially abundant proteins. Additionally, 10 proteins exclusively identified in PREGNANT when compared with CYCLIC heifers were also present in the EXPL + EMB group in vitro (Fig. 12B, right table).
Fig. 12.
Comparison of protein identification between in vivo and in vitro models. A Venn diagram showing the proteins found in uterine fluid extracellular vesicles (UF-EVs) from CYCLIC heifers versus conditioned medium extracellular vesicles (CM-EVs) from endometrial explants cultured alone in vitro (EXPL). B Venn diagram showing the proteins in UF-EVs from PREGNANT heifers versus CM-EVs from endometrial explants cultured with blastocysts (EXPL + EMB) in vitro. Additionally, it identifies 10 proteins exclusively present in UF-EVs from PREGNANT heifers that were also detected in CM-EVs from EXPL + EMB. Proteins were considered ‘exclusive’ if detected in at least three out of five replicates within one group but absent in all samples of other groups
Functional enrichment analysis using PANTHER revealed distinct GO biological processes when comparing proteins identified in UF-EVs from CYCLIC heifers and those in the CM-EVs from EXPL. Proteins exclusive to UF-EVs from CYCLIC heifers predominantly included metabolite interconversion enzymes, protein-binding activity modulators, membrane traffic proteins, and proteins modifying enzymes (Additional file 6A). Proteins unique to CM-EVs from EXPL were primarily metabolite interconversion enzymes, protein modifying enzymes, and RNA metabolism proteins (Additional file 6B). Equally abundant proteins consisted mainly of metabolite interconversion enzymes, protein modifying enzymes, and cytoskeletal proteins (Additional file 6C). Differentially abundant proteins were largely translational proteins, metabolite interconversion enzymes, and protein modifying enzymes (Additional file 6D). Both equally abundant and differentially abundant proteins contribute to binding, cellular, metabolic, and biological regulation, as well as to response to stimulus.
Proteins found exclusively in UF-EVs from PREGNANT heifers were mainly protein-binding activity modulators and metabolite interconversion enzymes (Additional file 7A). Proteins unique to CM-EVs from EXPL + EMB primarily included cytoskeletal proteins, protein modifying enzymes, and metabolite interconversion enzymes (Additional file 7B). Equally abundant proteins predominantly consisted of membrane traffic proteins, protein modifying enzymes, and metabolite interconversion enzymes (Additional file 7C). Differentially abundant proteins were largely protein-modifying enzymes, translational proteins, and metabolite interconversion enzymes (Additional file 7D). Both equally abundant and differentially abundant proteins contribute to the response to stimulus, localization, biological regulation, metabolic process, and cellular process.
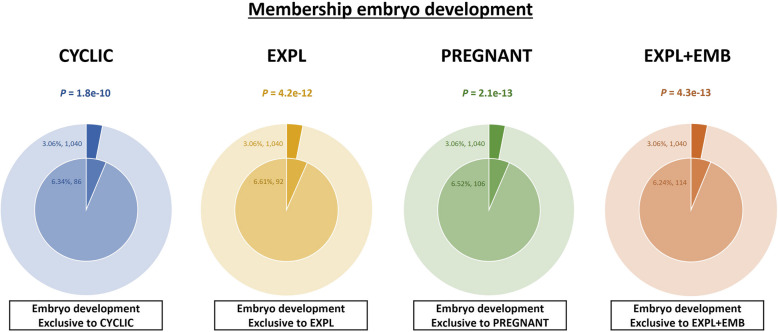
Furthermore, functional membership analysis for proteins matching the term “embryo development” was conducted using proteins identified in both in vivo and in vitro models (Fig. 13). CM-EVs from EXPL + EMB had a higher number of proteins associated with embryo development (n = 114), followed by UF-EVs from PREGNANT heifers (n = 106). In UF-EVs from CYCLIC heifers, 86 proteins related to “embryo development” were identified, and 92 proteins related to embryo development were identified in the CM-EVs from EXPL. Additionally, the EXPL + EMB and PREGNANT groups showed lower P-values for this membership (4.3 × 10–13 and 2.1 × 10–13, respectively), indicating stronger statistical significance compared to CYCLIC (1.8 × 10–10) and EXPL (4.2 × 10–12).
Fig. 13.
Functional membership analysis for proteins matching with “embryo development” term. Proteins identified in the uterine fluid extracellular vesicles from CYCLIC (blue pie chart) and PREGNANT heifers (green pie chart) and in the conditioned medium extracellular vesicles from explants cultured alone (EXPL; yellow pie chart) and explants co-cultured with embryos (EXPL + EMB; orange pie chart). The outer pie chart illustrates the count and proportion of proteins within the background dataset that were affiliated with the “embryo development” membership, whereas the inner pie chart presents the count and proportion of proteins in the specific input gene list associated with this membership. The p-value positioned above the pie charts indicates a statistically significant enrichment of the membership across all groups
Discussion
To the best of our knowledge, this study is the first to characterize embryo-induced changes in the protein cargo of bovine uterine EVs on d 7 of pregnancy, conducted both in vivo and in vitro. These changes in protein content reflect embryo-maternal signaling through EVs and provide important insight into how these EVs may modulate key pathways involved in early pregnancy. In vivo, the blastocyst induces changes in UF-EV protein cargo related to inflammatory and immune responses, endometrial receptivity, and early embryonic development by promoting cell polarity, cell-cell adhesion, and stem cell differentiation. In vitro, embryo-induced alterations in CM-EVs include changes in proteins involved in embryonic development, regulation of stem cell differentiation, establishment and maintenance of cell polarity, IFNT-mediated cell signaling, endometrial receptivity, and immune modulation.
Moreover, EVs derived from the UF and CM exhibit distinct protein profiles, likely driven by different stimuli under in vitro and in vivo conditions, yet explants may offer a starting point for studying maternal-embryonic communication due to preserved characteristics among the models.
Uterine and embryonic EVs
EVs have been recognized as constituents of the bovine UF [21]. In addition, embryonic EVs have been detected in spent culture media [50]. UF-EVs cargo, including miRNAs [21, 33] and proteins [16, 23, 32], have been investigated during the estrous cycle, with miRNA content changes also observed in the presence of multiple d 7 embryos [22]. Unlike prior studies, we identified the changes in the protein cargo of UF-EVs resulting from the presence of a single embryo at a more precise time-point by using synchronized heifers instead of abattoir-derived tissues. Moreover, although the protein cargo of UF-EVs has been analyzed in other ruminant species, such as sheep [18, 24] and goats [26], these studies focused on d 10 to 16, while we examined the changes occurring during the early pre-implantation period (d 7) of pregnancy.
In vitro, EVs from BEEC monolayer cultures have been shown to mediate embryo-maternal communication and enhance embryo development [51]; however, their cargo remains largely unexplored. Unlike previous studies, we employed endometrial explants to investigate embryo-maternal communication via EV protein content. Explants preserve both cellular and extracellular architecture, facilitating communication between different populations of endometrial cells. Studies have demonstrated that a 6-h incubation period is sufficient to induce significant changes in response to the embryo while preserving the structural and functional integrity of the explants [12, 39]. Additionally, previous studies have employed endometrial explants to investigate immunity and inflammation [36], IFNT modulation, and conceptus origin (in vivo vs. in vitro), sex [36], and size [52] effect on the endometrium.
Moreover, Passaro et al. [12] reported that d 8 embryos induced upregulation of ISG expression in endometrial explants, and that blastocyst-conditioned medium alone induces a response comparable to that seen with direct blastocyst-endometrium contact [12], suggesting that embryotrophins, including EVs, mediate this communication. EVs generated by in vitro-produced bovine blastocysts have been documented by several groups [53]. Most studies investigating the cargo of embryonic EV have focused on their miRNA profiles under different oxygen tensions [54], varying embryo viability [55], or developmental origin (in vitro vs. in vivo) [56, 57], with more recent attention given to their DNA content [58]. In contrast, we investigated the protein cargo of blastocyst-derived EVs in the context of embryo-maternal communication in vitro.
In vivo model: embryo-induced alteration in UF-EVs
UF-EVs in early embryonic development
Extracellular matrix (ECM) components, cytoskeleton reorganization, and adhesion proteins are crucial for maintaining pluripotency and embryonic stem cell differentiation, thereby influencing blastocyst formation [59]. For example, trophoblast invasion and adhesion may depend on LAMB1, an ECM component overabundant in UF-EVs from PREGNANT heifers. Through interactions with other ECM components, LAMB1 uses a high-affinity receptor to interact with cells, promoting cell adhesion, motility, and organization throughout embryonic development [60]. In mice, LAMB1 knockout results in peri-implantation lethality around d 5.5 due to the failure of endoderm differentiation and impaired implantation [61]. Additionally, LAMB1 expression is elevated in human blastocyst trophectoderm during implantation [62] and in bovine conceptus during elongation [62]. These findings suggest that UF-EVs may modulate ECM components on d 7 blastocysts, potentially facilitating cell differentiation and blastocyst progression.
The CRB2 protein, exclusive to UF-EVs from PREGNANT heifers, is a critical member of the Crumbs cell polarity complex family, playing an essential role in early embryonic development. This transmembrane protein controls epithelial cell polarity and cell-cell adhesion, and its deficiency has been associated with embryonic lethality in mice [63]. The asymmetric localization of Crumbs in mouse blastomeres is essential for lineage development, preserving the polarity of epithelial cells and helping the polar signals to be transmitted at close junctions and the apical membrane [64]. Furthermore, the CRB2 gene is upregulated in pig embryos [65], and single-cell gene expression studies of bovine blastocysts have revealed an overexpression of CRB2 in specific trophectoderm subpopulations [66], highlighting its importance in embryonic development and lineage specification. CRB2 also contributes to Hippo signaling, a regulator of cell fate determination and blastocyst formation [67]. The exclusive presence of CRB2 in UF-EVs from PREGNANT heifers suggests that it may represent a maternal response induced by the embryo’s presence, possibly influencing embryonic cell polarity, cell–cell adhesion, and lineage specification.
UF-EVs in inflammatory and immune response
Several proteins exclusively identified in UF-EVs from PREGNANT are predicted to participate in the immune response, notably UFL1. UFL1 maintains cell homeostasis and the inflammatory response by controlling nuclear factor-κB (NF-κB) signaling [68]. The overexpression of UFL1 in bovine mammary epithelial cells and bovine ovarian granulosa cells reduces inflammatory response and decreases cell damage [68]. This overexpression also inhibited the activation of the TLR4/NF-κB pathway and mitigated lipopolysaccharide-induced endoplasmic reticulum stress, apoptosis, autophagy, and oxidative stress [69, 70]. Conceptus presence also modulates UFL1 expression in the endometrium of pregnant swine, with significantly higher gene expression observed during allogeneic pregnancies (resulting from embryo transfer) compared to hemi-allogeneic pregnancies (resulting from artificial insemination) [71]. The exclusive presence of UFL1 in UF-EVs from PREGNANT heifers strongly suggests that these vesicles actively modulate endometrial function by delivering UFL1 and other regulatory proteins, shaping the local inflammatory response and enhancing endometrial receptivity to the embryo.
UF-EVs in endometrial receptivity
During the estrous cycle and pregnancy, the endometrium undergoes substantial functional alterations and remodeling necessary for successful implantation and placentation [72]. In our results, ECM-related proteins were modulated by the embryo presence and are associated with ECM organization, remodeling, and cell adhesion processes that could modulate uterine receptivity. COL1A2 is related to ECM organization within the pregnant endometrium [73], suggesting that the embryo could modulate ECM components, mainly during bovine maternal recognition of pregnancy (MRP). Indeed, COL1A2 facilitates bidirectional communication between the conceptus and endometrium in bovine during MRP [74]. Additionally, COL1A2 overexpression was observed in bovine endometrial epithelial cells treated with UF-EVs from d 17 to 20 of pregnancy [75].
Moreover, the ECM component LAMC1, involved in adhesion, migration, differentiation, and invasiveness, particularly in tumor cells [76], may also play a role in the endometrium. In mice, inhibition of Lamc1 negatively affects embryo implantation [77], while in ewes, LAMC1 expression increases in the endometrium on d 17 of pregnancy [78]. Similarly, in pregnant mares, LAMC1 shows higher relative expression in the endometrium on d 11 of pregnancy [79]. Although previous research indicated that changes in the distribution pattern of ECMs in the bovine endometrium are more evident by d 14 of pregnancy [72], our findings suggest that such modulation may initiate as early as d 7. Hence, in the embryo’s presence, COL1A2 and LAMC1 within UF-EVs may mediate endometrial ECM remodeling and assist endometrial receptivity.
UF-EVs as biomarkers of developmental competence
Proteins that may signal developmental competence were identified in UF-EVs from PREGNANT heifers. Heat shock proteins (HSPs) maintain intracellular homeostasis by regulating protein folding and exhibiting anti-apoptotic effects [80]. HSPA2, an HSPA (HSP70) family member, is also associated with cancer cell growth, survival, and male fertility, notably through its role in spermatid-specific chromatin remodeling during sperm cell differentiation and maturation [80]. Its deficiency is associated with male infertility and predicts failure in assisted reproductive technologies [80]. Although HSPA2 role beyond spermatogenesis is unclear, recent studies have identified HSPA2 in the bovine UF proteome during the periovulatory period of the estrous cycle [81], during pregnancy at d 10 and 13 [82], as well as in UF-EVs during peri-implantation periods (d 17, 20, and 22) [16] and in OF-EVs at d 3.5 of pregnancy [35].
Interestingly, higher gene expression of HSPA2 has been observed in bovine blastocysts compared to degenerate embryos [83], suggesting its involvement in embryo quality. Of note, other genes encoding proteins overabundant in UF-EVs from PREGNANT heifers (TTLL12 and PSMB2) have been associated with embryo quality and oocyte competence. Although their functions are not well characterized in reproduction, the TTLL12 gene was found to be increased in a competent blastocyst that resulted in pregnancy [84], while the PSMB2 gene has been linked to the competence of bovine oocytes [85]. These findings highlight the potential of UF-EV proteins as biomarkers of embryonic developmental competence; however, the specific roles of this protein in reproductive processes remain to be fully elucidated and warrant further investigation.
In vitro model: embryo-induced alteration in CM-EVs
CM-EVs in early embryonic development
Embryo-induced alteration in CM-EVs may contribute to embryonic development by supporting stem cell differentiation and establishment and maintenance of cell polarity. Notably, NELFB is critical for cell proliferation and survival; its loss causes inner cell mass deficiency and embryonic lethality during mice implantation, while in porcine, reduced NELFB gene expression lowers blastocyst formation by impairing embryonic genome activation [86]. Similarly, disrupting THO proteins, such as THOC5, leads to early embryonic lethality in mice [87]. THOC5 is needed to export mRNAs encoding essential pluripotency factors (Nanog, Sox2, and Klf4), and its knockout affects stem cell maintenance, differentiation, and proliferation during mouse blastocyst development [88]. Thus, the delivery of these proteins through EVs to the embryo may enhance embryo progression.
Furthermore, CXADR and CLDN7, exclusive to the EXPL + EMB group, support tight junction integrity during early embryonic development, which is essential for maintaining cell polarity and fate specification, guiding morphogenesis through mechanotransduction and signaling networks [89]. CXADR knockdown impairs mice implantation capacity due to defective trophoblast development [90], while in porcine, CXADR knockdown embryos fail to reach the blastocyst stage, and those that do exhibit incomplete expansion [91]. Similarly, CLDN7 knockdown in porcine embryos also reduces blastocyst formation and the number of cells [92]. Furthermore, CLDN7 also contributes to cell–cell adhesion in murine uterine epithelial cells, where it localizes to the cell base and lateral plasma membrane [93]. These findings underscore the importance of these proteins in both embryonic morphogenesis and the maintenance of endometrial stability during early development.
CM-EVs in embryotrophin-mediated responses
In ruminants, IFNT, the most well-characterized embryotrophic factor secreted by trophoblastic cells, supports pregnancy establishment through its antiluteolytic effect and gene stimulation in the endometrium [94]. IFNT within EVs has been reported in ovine conceptus-derived EVs during d 15 and 17 of pregnancy [95] and in bovine UF-EVs on d 17, 20, and 22 of pregnancy [16], coinciding with the period around the peak of IFNT production [96]. We have not detected the presence of IFNT in UF-EVs in vivo, nor in CM-EVs in vitro, as early as d 7 of pregnancy. Nevertheless, previous studies indicated that the secretion of IFNT by bovine pre-hatching blastocysts, as well as EVs derived from d 5 to 7 embryos, induces the expression of ISGs in endometrial cells [9, 19].
Consistent with prior studies, we identified changes in CM-EVs exclusive to EXP + EMB likely associated with the IFNT effects. CM-EVs from the EXP + EMB group contain proteins encoded by the IFNT-dependent genes SCRN1, TUBA1A, MLEC, SCP2, and ERAP2 [97], as well as EIF2AK2, GNB4, PPA1, which are both IFNT-dependent and conceptus-induced genes [36, 97]. EIF2AK2 regulates protein synthesis to mediate inflammatory responses [98] and may influence STAT1 transcription, a key IFN signaling factor [99]. Additionally, the EIF2AK2 gene is overexpressed in bovine oviductal epithelial cells in the embryo’s presence [100], overabundant in pregnant ewes endometrium during the peri-implantation [101], but reduced in the endometrium of subfertile cows in early pregnancy [102], suggesting its role in proper endometrial function. Although further research is needed to elucidate the specific roles of these proteins in the endometrium, our findings strongly suggest that the presence of SCRN1, TUBA1A, MLEC, EIF2AK2, ERAP2, GNB4, PPA1, and SCP2 within EVs reflects active embryo-maternal communication mediated by embryotropins, potentially orchestrated through IFNT signaling pathways.
CM-EVs in endometrial receptivity and immune response
Several proteins exclusive to the EXPL + EMB group are implicated in endometrial receptivity. For example, LCN2 is mainly associated with tissue rearrangement and pregnancy in the female reproductive system [103]. In mares, LCN2 is upregulated in the endometrium at the site of conceptus implantation, likely driven by the conceptus, and related to endometrial innate immune responses [104]. In bovine, LCN2 levels in UF-EVs increase during the estrous cycle (d 0–16) and are also related to immune modulation [23]. Furthermore, LCN2 is upregulated in the bovine endometrium in response to pre-implantation factors [73], suggesting its modulation by the embryo-maternal communication. Therefore, LCN2 EV cargo may modulate endometrial receptivity by regulating inflammatory responses and facilitating embryo development in the uterus.
Moreover, Prostaglandin E Synthase 2 (PTGES2), exclusive to CM-EVs from EXP + EMB, may also play a significant role in uterine receptivity and conceptus development. Although our results did not detect PTGES2 in vivo within UF-EVs, the PTGES2 gene is upregulated in the oviduct of pregnant cows [105] and is expressed in the bovine endometrium on d 7 of pregnancy [9]. PTGES2 catalyzes the synthesis of PGE2 from PGH2 in the endometrium [106]. Therefore, we propose that the delivery of PTGES2 via EVs may enhance these processes by facilitating PGE2 production in the bovine endometrium. Notably, PGE2 plays a critical role in supporting pre-implantation embryo development, luteotrophic signaling, and modulating endometrial receptivity and immune tolerance [107–110].
In vivo vs. in vitro models
EVs derived from in vivo and in vitro conditions exhibit distinct qualitative and quantitative differences in their protein cargo. Endometrial explants were employed as an experimental model to approximate in vitro conditions as closely as possible to the in vivo uterine environment. In contrast to two-dimensional monocultures, explants preserve the native three-dimensional architecture and cellular heterogeneity of the endometrium [39], thereby offering a more physiologically relevant platform for investigating embryo-maternal interactions. Although this model presents inherent limitations, such as a restricted culture window and the number of embryos required per experimental condition, it effectively served our objective of capturing the early interaction between d 7 blastocysts and maternal tissue in vitro. As discussed above, both models show embryo-induced modifications in EV cargo that potentially support early embryonic development, endometrial receptivity, and immune response, yet these modifications involve different sets of proteins.
In the context of embryonic development, UF-EV cargo changes are associated with the regulation of embryonic cell polarity, cell–cell adhesion, and lineage specification through proteins like LAMB1 and CRB2. In contrast, changes in the in vitro model are associated with stem cell differentiation and establishment and maintenance of cell polarity through proteins including NELFB, THOC5, CXADR, and CLDN7. Moreover, changes in the EV protein cargo due to embryo-maternal communication facilitated by embryotrophins, likely via IFNT signaling, were detected only in CM-EVs, which may be attributed to signal amplification in the in vitro system due to the presence of multiple embryos, whereas in vivo, such changes might be less detectable at early stages of pregnancy. These differences in EV protein cargo, likely driven by distinct stimuli in in vitro and in vivo conditions, suggest that embryo-maternal communication employs unique molecular signals in each model while promoting similar developmental outcomes.
Despite these distinctions, it is noteworthy that 59.2% of proteins identified in both the PREGNANT and EXPL + EMB were equally abundant in both in vivo and in vitro models. These proteins are involved in pathways critical to embryo development, including Wnt (SFRP1, PPP2CA, GNB1, GNG2, PRKCI, GSK3B, GNB2, PPP2R5D, CSNK1A1, CSNK2A1, CDH1, and HDAC1), Ras (RPS6KA1, RPS6KA3, PAK2, RAC1, RHOA, GSK3B, CDC42, MAPK1, RAC1, STAT1, STAT3, and KRAS), and PI3K (GNAI1, GNB1, GNB2, YWHAZ, GSK3B, and KRAS) [111–113]. SFRP1 influences cell differentiation and growth during embryogenesis, ensuring proper embryonic modeling during early development [114]; while GSK3B and CSNK1A1 are related to proper cell differentiation [115]. Furthermore, GSK3B gene expression is positively correlated with successful embryo development [116]. In addition, HDAC1 regulates gene expression via epigenetic modifications, balancing cell proliferation and differentiation during embryonic development [117]. Similarly, STAT1 and STAT3 are involved in the regulation of cell proliferation and differentiation and are also involved in IFN-mediated cell signaling and immune responses [118]. These results highlight that essential signaling pathways for embryo development are present both in vivo and in vitro conditions.
On the other hand, 108 proteins were exclusive to UF-EVs from PREGNANT heifers compared to the EXPL + EMB group, including 11 (NF2, TSC1, SPINT2, BYSL, TFEB, ZNF358, TERF2, TULP3, VPS52, MARCKS, and CRB2) associated with embryo development, as indicated by Metascape tool. NF2 regulates the Hippo pathway during pre-implantation, controlling cell fate specification in mouse and bovine embryos [119], with its inhibition disrupting trophectoderm and inner cell mass segregation [120]. BYSL supports mice blastocyst formation by activation of the translational machinery [121]. Moreover, although its role remains unclear, MARCKS gene expression is associated with embryo competence [84]. Additionally, CRB2, related to cell polarity and cell–cell adhesion as previously discussed, was also exclusively detected in UF-EVs from PREGNANT heifers compared to CYCLIC ones. In this sense, EVs from in vivo conditions may offer greater benefits to embryonic development. Indeed, recent studies have indicated that EVs from the UF from cyclic cows and BEEC sources exert different effects on in vitro embryo development, with UF-EVs demonstrating superior effects on embryo quality [51]. Therefore, EVs derived from in vivo conditions, particularly UF-EVs from PREGNANT heifers, likely provide better support for embryonic development due to their distinct and specialized protein cargo.
Conclusion
In conclusion, this study characterized specific protein signatures of UF-EVs from PREGNANT heifers, which is likely due to the interactions established between the endometrium and the d 7 blastocyst. The blastocyst presence induces changes in UF-EVs protein cargo that are related to the regulation of inflammatory and immune responses, cell adhesion, polarity, and stem cell differentiation, potentially enhancing endometrial receptivity and supporting early embryonic development. Additionally, CM-EVs from endometrial explants cultured alone show different protein profiles compared to those co-cultured with d 7 blastocysts, as well as from the CM-EVs of d 7 blastocysts cultured alone. Embryo-induced alterations in CM-EVs are involved in embryonic development, regulation of stem cell differentiation, establishment and maintenance of cell polarity, IFNT-mediated cell signaling, endometrial receptivity, and immune modulation. Moreover, although uterine EVs from in vivo and in vitro sources exhibit distinct protein profiles, the preserved characteristics support the use of explants as a starting point for studying maternal-embryonic communication, with the acknowledgment that endometrium and embryos encounter unique in vitro conditions and stimuli compared to the maternal reproductive tract. Altogether, our findings reveal that the embryo-induced changes in the protein cargo of uterine EVs, observed in in vivo and in vitro models, position these EVs as key modulators of embryo-maternal communication, playing a pivotal role in supporting pre-implantation embryo development.
Supplementary Information
Additional file 1. Experimental setup of the in vitro model. Extracellular vesicles (EVs) were isolated from the conditioned medium (CM) collected from three in vitro experimental groups: (1A–B) endometrial explants cultured alone (EXPL), (2A–B) endometrial explants co-cultured with blastocysts (EXPL + EMB), and (3A) blastocysts cultured alone (EMB).
Additional file 2. Strategy for quantitative analysis for in vitro comparison. Venn diagrams illustrate the identification of differentially abundant proteins (DAPs) between EXPL vs. EMB, EMB vs. EXPL + EMB, and EXPL vs. EXPL + EMB. The green circle represents DAPs in EXPL vs. EMB comparison.
Additional file 3. Negative controls for the characterization of extracellular vesicles (EVs) from uterine fluid (UF) and conditioned medium (CM). Phosphate‑buffered saline without calcium and magnesium (PBS−/−) was used as the negative control for both nanoparticle tracking analysis (NTA) and transmission electron microscopy (TEM). A. NTA of PBS−/−, showing 0 particles per frame. B. TEM images of PBS−/−, showing no vesicle-like structures.
Additional file 4. Proteomic profile of uterine fluid extracellular vesicles (UF-EVs). A. All 1459 proteins present in UF-EVs from CYCLIC heifers. B. All 1329 proteins present in UF-EVs from PREGNANT heifers. C. Differentially abundant proteins in UF-EVs from CYCLIC compared to PREGNANT heifers. D. Top 100 most abundant proteins in UF-EVs from CYCLIC and PREGNANT heifers.
Additional file 5. Proteomic profile of conditioned medium extracellular vesicles (CM-EVs). A. All 1501 proteins present in CM-EVs from explants cultured alone. B. All 1975 proteins present in CM-EVs from explants cocultured with embryos. C. All 82 proteins present in CM-EVs from embryos cultured alone. D. Top 100 most abundant proteins in CM-EVs from EXPL, EXPL+EMB and EMB.
Additional file 6. Functional enrichment of proteins identified in uterine fluid extracellular vesicles (UF-EVs) from CYCLIC heifers compared to conditioned medium extracellular vesicles (CM-EVs) from endometrial explants cultured alone (EXPL). A. Proteins (n =125) exclusive to UF-EVs from CYCLIC. B. Proteins (106) exclusive to CM-EVs form EXPL. C. Proteins (n = 691) equally abundant among CYCLIC vs. EXPL. D. Proteins (n = 328) differentially abundant among CYCLIC vs. EXPL.
Additional file 7. Functional enrichment of proteins identified in uterine fluid extracellular vesicles (UF-EVs) from PREGNANT heifers compared to conditioned medium extracellular vesicles (CM-EVs) from endometrial explants co-cultured with blastocysts (EXPL + EMB). A. Proteins (n =108) exclusive to UF-EVs from PREGNANT. B. Proteins (n = 195) exclusive to CM-EVs form EXPL + EMB. C. Eight hundred and thirteen proteins (n =813) equally abundant among PREGNANT vs. EXPL + EMB. D. Proteins (n = 560) differentially abundant among PREGNANT vs. EXPL + EMB.
Acknowledgements
We thank the staff of the Laboratory of Assisted Reproduction and Pre-implantation Embryology at INIA-CSIC (Spain) and the laboratory of Professor Patrik Lonergan at UCD (Ireland) for their support. We also thank the electron microscopy service at the National Center for Biotechnology (CNB-CSIC-Spain) for the TEM images taken. Special thanks are extended to local slaughterhouses for providing access to the biological material (ovaries) used in the present study.
Abbreviations
- BEEC
Bovine endometrial epithelial cells
- CL
Corpus luteum
- CM
Conditioned medium
- CM-EVs
Conditioned medium extracellular vesicles
- COCs
Cumulus-oocyte complexes
- DAPs
Differentially abundant proteins
- dFCS
EV-depleted fetal calf serum
- EMB
Blastocysts cultured alone
- EVs
Extracellular vesicles
- EXPL
Endometrial explants cultured alone
- EXPL + EMB
Endometrial explants co-cultured with blastocysts
- FA
Formic acid
- FC
Flow cytometry
- FCS
Fetal calf serum
- FDR
False discovery rate
- FSC
Forward scatter
- HSPs
Heat shock proteins
- IFNT
Interferon tau
- ISGs
Interferon-stimulated genes
- IVC
In vitro culture
- LFQ
Label-free quantification
- miRNAs
MicroRNAs
- MRP
Maternal recognition of pregnancy
- NTA
Nanoparticle tracking analysis
- PBS−/−
Phosphate-buffered saline without Ca2+ and Mg2+
- PBS
Phosphate-buffered saline
- PCA
Principal component analysis
- PGF2α
Prostaglandin F2 alpha
- PRID
Progesterone-releasing intravaginal device
- PSM
Peptide-spectrum match
- PTGES2
Prostaglandin E synthase 2
- SEC
Size-exclusion chromatography
- SOF
Synthetic oviduct fluid
- SSC
Side scatter
- TEM
Transmission electron microscopy
- UF
Uterine fluid
- UF-EVs
Uterine fluid extracellular vesicles
Authors’ contributions
Conceptualization: DR, PL, RM, JMS, BFF. Methodology: DR, PL. Investigation: RM, JMS, BFF, SGE, MM. Flow cytometry and proteomic data curation: AÁB, EG, JMFP, MA, FE. Formal analysis: RM, MA. Supervision: BFF, DR, MEG. Resources: DR, PL. Writing – original draft: RM. Writing – review and editing: RM, JMS, SGE, MM, AÁB, EG, JMFP, MA, FE, MEG, PL, DR, BFF.
Funding
This research was supported by research projects: PID2019‑111641RB‑I00 and PID2023-149027OB-I00 funded by MCIN/AEI/10.13039/501100011033/ to DR and PRE2020‑094452 to RM.
Data availability
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (https://proteomecentral.proteomexchange.org) via the iProX partner repository [122, 123] with the dataset identifier PXD053670.
Declarations
Ethics approval and consent to participate
All experimental procedures involving animals were approved by the Animal Research Ethics Committee of University College Dublin and licensed by the Health Products Regulatory Authority, Ireland, in accordance with Statutory Instrument No. 543 of 2012 under Directive 2010/63/EU on the Protection of Animals used for Scientific Purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any conflict of interest.
Contributor Information
Dimitrios Rizos, Email: drizos@inia.csic.es.
Beatriz Fernandez-Fuertes, Email: beatriz.fernandez@inia.csic.es.
References
- 1.Rizos D, Ward F, Duffy P, Boland MP, Lonergan P. Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: Implications for blastocyst yield and blastocyst quality. Mol Reprod Dev. 2002;61:234–48. [DOI] [PubMed] [Google Scholar]
- 2.Lonergan P. Temporal divergence in the pattern of messenger RNA expression in bovine embryos cultured from the zygote to blastocyst stage in vitro or in vivo. Biol Reprod. 2003;69:1424–31. [DOI] [PubMed] [Google Scholar]
- 3.Khurana NK, Niemann H. Energy metabolism in preimplantation bovine embryos derived in vitro or in vivo. Biol Reprod. 2000;62:847–56. [DOI] [PubMed] [Google Scholar]
- 4.Rizos D, Fair T, Papadopoulos S, Boland MP, Lonergan P. Developmental, qualitative, and ultrastructural differences between ovine and bovine embryos produced in vivo or in vitro. Mol Reprod Dev. 2002;62:320–7. [DOI] [PubMed] [Google Scholar]
- 5.Rizos D, Clemente M, Bermejo-Alvarez P, De La Fuente J, Lonergan P, Gutiérrez-Adán A. Consequences of in vitro culture conditions on embryo development and quality. Reprod Domest Anim. 2008;43:44–50. [DOI] [PubMed] [Google Scholar]
- 6.Pontes JHF, Nonato-Junior I, Sanches BV, Ereno-Junior JC, Uvo S, Barreiros TRR, et al. Comparison of embryo yield and pregnancy rate between in vivo and in vitro methods in the same Nelore (Bos indicus) donor cows. Theriogenology. 2009;71:690–7. [DOI] [PubMed] [Google Scholar]
- 7.Reese ST, Franco GA, Poole RK, Hood R, Fernadez Montero L, Oliveira Filho RV, et al. Pregnancy loss in beef cattle: A meta-analysis. Anim Reprod Sci. 2020;212:106251. [DOI] [PubMed] [Google Scholar]
- 8.Binelli M, Silva FACC, Rocha CC, Martins T, Sponchiado M, Van Hoeck V, et al. Endometrial receptivity in cattle: the mutual reprogramming paradigm. Anim Reprod. 2022;19:e20220097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sponchiado M, Gomes NS, Fontes PK, Martins T, del Collado M, Pastore ADA, et al. Pre-hatching embryo-dependent and -independent programming of endometrial function in cattle. PLoS ONE. 2017;12:e0175954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sponchiado M, Gonella-Diaza AM, Rocha CC, Turco EG Lo, Pugliesi G, Leroy JLMR, et al. The pre-hatching bovine embryo transforms the uterine luminal metabolite composition in vivo. Sci Rep. 2019;9:8354. [DOI] [PMC free article] [PubMed]
- 11.Talukder AK, Yousef MS, Rashid MB, Awai K, Acosta TJ, Shimizu T, et al. Bovine embryo induces an anti-inflammatory response in uterine epithelial cells and immune cells in vitro: possible involvement of interferon tau as an intermediator. J Reprod Dev. 2017;63:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passaro C, Tutt D, Mathew DJ, Sanchez JM, Browne JA, Boe-Hansen GB, et al. Blastocyst-induced changes in the bovine endometrial transcriptome. Reproduction. 2018;156:219–29. [DOI] [PubMed] [Google Scholar]
- 13.Wydooghe E, Vandaele L, Heras S, De Sutter P, Deforce D, Peelman L, et al. Autocrine embryotropins revisited: how do embryos communicate with each other in vitro when cultured in groups? Biol Rev. 2017;92:505–20. [DOI] [PubMed] [Google Scholar]
- 14.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, et al. Reassessment of exosome composition. Cell. 2019;177:428-445.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kusama K, Nakamura K, Bai R, Nagaoka K, Sakurai T, Imakawa K. Intrauterine exosomes are required for bovine conceptus implantation. Biochem Biophys Res Commun. 2018;495:1370–5. [DOI] [PubMed] [Google Scholar]
- 17.Giacomini E, Vago R, Sanchez AM, Podini P, Zarovni N, Murdica V, et al. Secretome of in vitro cultured human embryos contains extracellular vesicles that are uptaken by the maternal side. Sci Rep. 2017;7:5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burns GW, Brooks KE, Spencer TE. Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol Reprod. 2016;94:56. [DOI] [PubMed] [Google Scholar]
- 19.Aguilera C, Velásquez AE, Gutierrez-Reinoso MA, Wong Y Sen, Melo-Baez B, Cabezas J, et al. Extracellular vesicles secreted by pre-hatching bovine embryos produced in vitro and in vivo alter the expression of IFNtau-stimulated genes in bovine endometrial cells. Int J Mol Sci. 2023;24:7438. [DOI] [PMC free article] [PubMed]
- 20.Qiao F, Ge H, Ma X, Zhang Y, Zuo Z, Wang M, et al. Bovine uterus-derived exosomes improve developmental competence of somatic cell nuclear transfer embryos. Theriogenology. 2018;114:199–205. [DOI] [PubMed] [Google Scholar]
- 21.Hamdi M, Cañon-Beltrán K, Mazzarella R, Cajas YN, Leal CL, Gutierrez-Adan A, et al. Characterization and profiling analysis of bovine oviduct and uterine extracellular vesicles and their miRNA cargo through the estrous cycle. FASEB J. 2021;35:e22000. [DOI] [PubMed] [Google Scholar]
- 22.Kusama K, Rashid MB, Kowsar R, Marey MA, Talukder AK, Nagaoka K, et al. Day 7 embryos change the proteomics and exosomal micro-RNAs content of bovine uterine fluid: involvement of innate immune functions. Front Genet. 2021;12:676791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piibor J, Waldmann A, Dissanayake K, Andronowska A, Ivask M, Prasadani M, et al. Uterine fluid extracellular vesicles proteome is altered during the estrous cycle. Mol Cell Proteomics. 2023;22:100642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiz-González I, Xu J, Wang X, Burghardt RC, Dunlap KA, Bazer FW. Exosomes, endogenous retroviruses and toll-like receptors: Pregnancy recognition in ewes. Reproduction. 2015;149:281–91. [DOI] [PubMed] [Google Scholar]
- 25.Burns G, Brooks K, Wildung M, Navakanitworakul R, Christenson LK, Spencer TE. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS ONE. 2014;9:e90913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Y, Liu G, Zang X, Hu Q, Zhou C, Li Y, et al. Differential expression pattern of goat uterine fluids extracellular vesicles miRNAs during peri-implantation. Cells. 2021;10:2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudolf Vegas A, Hamdi M, Podico G, Bollwein H, Fröhlich T, Canisso IF, et al. Uterine extracellular vesicles as multi-signal messengers during maternal recognition of pregnancy in the mare. Sci Rep. 2022;12:15616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan Q, Shi S, Liang J, Zhang X, Cao D, Wang Z. MicroRNAs in small extracellular vesicles indicate successful embryo implantation during early pregnancy. Cells. 2020;9:645. [DOI] [PMC free article] [PubMed]
- 29.Bidarimath M, Khalaj K, Kridli RT, Kan FWK, Koti M, Tayade C. Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: A new paradigm for conceptus-endometrial cross-talk. Sci Rep. 2017;7:40476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poh QH, Rai A, Salamonsen LA, Greening DW. Omics insights into extracellular vesicles in embryo implantation and their therapeutic utility. Proteomics. 2023;23:e2200107. [DOI] [PubMed] [Google Scholar]
- 31.Ibañez-Perez J, Díaz-Nuñez M, Clos-García M, Lainz L, Iglesias M, Díez-Zapirain M, et al. microRNA-based signatures obtained from endometrial fluid identify implantative endometrium. Hum Reprod. 2022;37:2375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piibor J, Dissanayake K, Midekessa G, Andronowska A, Kavak A, Waldmann A, et al. Characterization of bovine uterine fluid extracellular vesicles proteomic profiles at follicular and luteal phases of the oestrous cycle. Vet Res Commun. 2023;47:885–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazzarella R, Cañón-Beltrán K, Cajas YN, Hamdi M, González EM, da Silveira JC, et al. Extracellular vesicles-coupled miRNAs from oviduct and uterus modulate signaling pathways related to lipid metabolism and bovine early embryo development. J Anim Sci Biotechnol. 2024;15:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leal CLV, Cañón-Beltrán K, Cajas YN, Hamdi M, Yaryes A, Millán de la Blanca MG, et al. Extracellular vesicles from oviductal and uterine fluids supplementation in sequential in vitro culture improves bovine embryo quality. J Anim Sci Biotechnol. 2022;13:116. [DOI] [PMC free article] [PubMed]
- 35.Mazzarella R, Sánchez JM, Fernandez-Fuertes B, Egido SG, McDonald M, Álvarez-Barrientos A, et al. Embryo-induced changes in the protein profile of bovine oviductal extracellular vesicles. Mol Cell Proteomics. 2025;24:100935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathew DJ, Sánchez JM, Passaro C, Charpigny G, Behura SK, Spencer TE, et al. Interferon tau-dependent and independent effects of the bovine conceptus on the endometrial transcriptome. Biol Reprod. 2019;100:365–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachurski D, Schuldner M, Nguyen P, Malz A, Reiners KS, Grenzi PC, et al. Extracellular vesicle measurements with nanoparticle tracking analysis – An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J Extracell Vesicles. 2019;8:1596016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mazzarella R, Gascón Collado D, Jin I, Beltran-Breña P, Fernandez-Fuertes B, Sánchez JM, et al. Characterization of extracellular vesicles secreted by preimplantation bovine embryos cultured in vitro. Proceedings of the 16th International Congress of the Spanish Society for Animal Reproduction (AERA), 20-22 October 2022, Leén, Spain. 2022;57(S5):131.
- 39.Borges ÁM, Healey GD, Sheldon IM. Explants of intact endometrium to model bovine innate immunity and inflammation ex vivo. Am J Reprod Immunol. 2012;67:526–39. [DOI] [PubMed] [Google Scholar]
- 40.Bridi A, Sangalli JR, Nociti RP, Dos Santos AC, Alves L, Bastos NM, et al. Small extracellular vesicles derived from the crosstalk between early embryos and the endometrium potentially mediate corpus luteum function. Biol Reprod. 2025;112:54–69. [DOI] [PubMed] [Google Scholar]
- 41.De la Fuente Toro D, Mazzarella R, Mateos A, Guisado Egidio S, Almpanis V, González EM, et al. Extracellular vesicles secreted by bovine endometrial and oviductal explants. Proceedings of the 16th International Congress of the Spanish Society for Animal Reproduction (AERA), 20-22 October 2022, Leén, Spain. Reprod Domest Ani. 2022;57(S5):141.
- 42.Barfield J, Demetrio DGB. Considerations for evaluating in vitro-produced bovine embryos. Man Int Embryo Transf Soc Proced Manag Sanit risks. 5th ed. Savoy, IL: International Embryo Transfer Society. 2020. p. 150–60.
- 43.Monguió-Tortajada M, Morón-Font M, Gámez-Valero A, Carreras-Planella L, Borràs FE, Franquesa M. Extracellular-vesicle isolation from different biological fluids by size-exclusion chromatography. Curr Protoc Stem Cell Biol. 2019;49:e82. [DOI] [PubMed] [Google Scholar]
- 44.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barranco I, Padilla L, Parrilla I, Álvarez-Barrientos A, Pérez-Patiño C, Peña FJ, et al. Extracellular vesicles isolated from porcine seminal plasma exhibit different tetraspanin expression profiles. Sci Rep. 2019;9:11584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welsh JA, Van Der Pol E, Arkesteijn GJA, Bremer M, Brisson A, Coumans F, et al. MIFlowCyt-EV: a framework for standardized reporting of extracellular vesicle flow cytometry experiments. J Extracell Vesicles. 2020;9:1713526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–62. [DOI] [PubMed] [Google Scholar]
- 48.Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, et al. PANTHER: A library of protein families and subfamilies indexed by function. Genome Res. 2003;13:2129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10:1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mellisho EA, Velásquez AE, Nuñez MJ, Cabezas JG, Cueto JA, Fader C, et al. Identification and characteristics of extracellular vesicles from bovine blastocysts produced in vitro. PLoS ONE. 2017;12:e0178306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aguilera C, Wong YS, Gutierrez-Reinoso MA, Velásquez AE, Melo-Báez B, Cabezas J, et al. Embryo-maternal communication mediated by extracellular vesicles in the early stages of embryonic development is modified by in vitro conditions. Theriogenology. 2024;214:43–56. [DOI] [PubMed] [Google Scholar]
- 52.Sánchez JM, Mathew DJ, Behura SK, Passaro C, Charpigny G, Butler ST, et al. Bovine endometrium responds differentially to age-matched short and long conceptuses. Biol Reprod. 2019;101:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mazzarella R, Cajas YN, Gonzalez Martínez ME, Rizos D. Extracellular vesicles: emerging paradigms in bovine embryo-maternal communication. Anim Reprod. 2024;21:e20240065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andrade GM, Bomfim MM, del Collado M, Meirelles FV, Perecin F, da Silveira JC. Oxygen tension modulates extracellular vesicles and its miRNA contents in bovine embryo culture medium. Mol Reprod Dev. 2019;86:1067–80. [DOI] [PubMed] [Google Scholar]
- 55.Mellisho EA, Briones MA, Velásquez AE, Cabezas J, Castro FO, Rodríguez-Álvarez L. Extracellular vesicles secreted during blastulation show viability of bovine embryos. Reproduction. 2019;158:477–92. [DOI] [PubMed] [Google Scholar]
- 56.Bridi A, Andrade GM, del Collado M, Sangalli JR, de Ávila ACFCM, Motta IG, et al. Small extracellular vesicles derived from in vivo‐ or in vitro‐produced bovine blastocysts have different miRNAs profiles—Implications for embryo‐maternal recognition. Mol Reprod Dev. 2021;88:628–43. [DOI] [PubMed] [Google Scholar]
- 57.Lange-Consiglio A, Lazzari B, Pizzi F, Idda A, Cremonesi F, Capra E. Amniotic microvesicles impact hatching and pregnancy percentages of in vitro bovine embryos and blastocyst microRNA expression versus in vivo controls. Sci Rep. 2020;10:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caamaño D, Cabezas J, Aguilera C, Martinez I, Wong YS, Sagredo DS, et al. DNA content in embryonic extracellular vesicles is independent of the apoptotic rate in bovine embryos produced in vitro. Animals. 2024;14:1041. [DOI] [PMC free article] [PubMed]
- 59.Bloor DJ, Metcalfe AD, Rutherford A, Brison DR, Kimber SJ. Expression of cell adhesion molecules during human preimplantation embryo development. Mol Hum Reprod. 2002;8:237–45. [DOI] [PubMed] [Google Scholar]
- 60.Klaffky E, Williams R, Yao C-C, Ziober B, Kramer R, Sutherland A. Trophoblast-specific expression and function of the integrin α7 subunit in the peri-implantation mouse embryo. Dev Biol. 2001;239:161–75. [DOI] [PubMed] [Google Scholar]
- 61.Smyth N, Vatansever SH, Murray P, Meyer M, Frie C, Paulsson M, et al. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribeiro ES, Greco LF, Bisinotto RS, Lima FS, Thatcher WW, Santos JE. Biology of preimplantation conceptus at the onset of elongation in dairy cows. Biol Reprod. 2016;94:97. [DOI] [PubMed] [Google Scholar]
- 63.Xiao Z, Patrakka J, Nukui M, Chi L, Niu D, Betsholtz C, et al. Deficiency in crumbs homolog 2 ( Crb2) affects gastrulation and results in embryonic lethality in mice. Dev Dyn. 2011;240:2646–56. [DOI] [PubMed] [Google Scholar]
- 64.Jiang X, An W, Yang X, Lin J, Ma S, Wang D, et al. Asymmetric distribution of CRUMBS polarity complex proteins from compacted 8-cell to blastocyst stage during mouse preimplantation development. Gene Expr Patterns. 2018;27:93–8. [DOI] [PubMed] [Google Scholar]
- 65.Teixeira SA, Marques DBD, Costa TC, Oliveira HC, Costa KA, Carrara ER, et al. Transcription landscape of the early developmental biology in pigs. Animals. 2021;11:1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Negrón-Pérez VM, Zhang Y, Hansen PJ. Single-cell gene expression of the bovine blastocyst. Reproduction. 2017;154:627–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Z, Guan K-L. Hippo signaling in embryogenesis and development. Trends Biochem Sci. 2021;46:51–63. [DOI] [PMC free article] [PubMed]
- 68.Xie Z, Fang Z, Pan Z. Ufl1/RCAD, a Ufm1 E3 ligase, has an intricate connection with ER stress. Int J Biol Macromol. 2019;135:760–7. [DOI] [PubMed] [Google Scholar]
- 69.Li C, Li L, Chen K, Wang Y, Yang F, Wang G. UFL1 alleviates lipopolysaccharide-induced cell damage and inflammation via regulation of the TLR4/NF-κB pathway in bovine mammary epithelial cells. Oxid Med Cell Longev. 2019;2019:6505373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang X, Li C, Wang Y, Li L, Han Z, Wang G. UFL1 alleviates LPS-induced apoptosis by regulating the NF-κB signaling pathway in bovine ovarian granulosa cells. Biomolecules. 2020;10:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martinez CA, Alvarez-Rodriguez M, Rodriguez-Martinez H. A decreased expression of interferon stimulated genes in peri-implantation endometrium of embryo transfer recipient sows could contribute to embryo death. Animal. 2022;16:100590. [DOI] [PubMed] [Google Scholar]
- 72.Yamada O, Todoroki JI, Takahashi T, Hashizume K. The dynamic expression of extracellular matrix in the bovine endometrium at implantation. J Vet Med Sci. 2002;64:207–14. [DOI] [PubMed] [Google Scholar]
- 73.Wonfor RE, Creevey CJ, Natoli M, Hegarty M, Nash DM, Rose MT. Interaction of preimplantation factor with the global bovine endometrial transcriptome. PLoS ONE. 2020;15:e0242874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mamo S, Mehta JP, Forde N, McGettigan P, Lonergan P. Conceptus-endometrium crosstalk during maternal recognition of pregnancy in cattle. Biol Reprod. 2012;87:6. [DOI] [PubMed] [Google Scholar]
- 75.Nakamura K, Kusama K, Ideta A, Kimura K, Hori M, Imakawa K. Effects of miR-98 in intrauterine extracellular vesicles on maternal immune regulation during the peri-implantation period in cattle. Sci Rep. 2019;9:20330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ye GX, Qin Y, Wang S, Pan DB, Xu SQ, Wu CJ, et al. Lamc1 promotes the Warburg effect in hepatocellular carcinoma cells by regulating PKM2 expression through AKT pathway. Cancer Biol Ther. 2019;20:711–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cao D, Liang J, Feng F, Shi S, Tan Q, Wang Z. MiR-183 impeded embryo implantation by regulating Hbegf and Lamc1 in mouse uterus. Theriogenology. 2020;158:218–26. [DOI] [PubMed] [Google Scholar]
- 78.Wang Y, Wang C, Hou Z, Miao K, Zhao H, Wang R, et al. Comparative analysis of proteomic profiles between endometrial caruncular and intercaruncular areas in ewes during the peri-implantation period. J Anim Sci Biotechnol. 2013;4:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klohonatz KM, Cameron AD, Hergenreder JR, da Silveira JC, Belk AD, Veeramachaneni DNR, et al. Circulating miRNAs as potential alternative cell signaling associated with maternal recognition of pregnancy in the mare. Biol Reprod. 2016;95:124. [DOI] [PubMed] [Google Scholar]
- 80.Scieglinska D, Krawczyk Z. Expression, function, and regulation of the testis-enriched heat shock HSPA2 gene in rodents and humans. Cell Stress Chaperones. 2015;20:221–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mahé C, Marcelo P, Tsikis G, Tomas D, Labas V, Saint-Dizier M. The bovine uterine fluid proteome is more impacted by the stage of the estrous cycle than the proximity of the ovulating ovary in the periconception period. Theriogenology. 2023;198:332–43. [DOI] [PubMed] [Google Scholar]
- 82.Forde N, McGettigan PA, Mehta JP, O’Hara L, Mamo S, Bazer FW, et al. Proteomic analysis of uterine fluid during the pre-implantation period of pregnancy in cattle. Reproduction. 2014;147:575–87. [DOI] [PubMed] [Google Scholar]
- 83.Zhang B, Peñagaricano F, Driver A, Chen H, Khatib H. Differential expression of heat shock protein genes and their splice variants in bovine preimplantation embryos. J Dairy Sci. 2011;94:4174–82. [DOI] [PubMed] [Google Scholar]
- 84.Rabaglino MB, Salilew-Wondim D, Zolini A, Tesfaye D, Hoelker M, Lonergan P, et al. Machine-learning methods applied to integrated transcriptomic data from bovine blastocysts and elongating conceptuses to identify genes predictive of embryonic competence. FASEB J. 2023;37:e22809. [DOI] [PubMed] [Google Scholar]
- 85.Mourot M, Dufort I, Gravel C, Algriany O, Dieleman S, Sirard M-A. The influence of follicle size, FSH-enriched maturation medium, and early cleavage on bovine oocyte maternal mRNA levels. Mol Reprod Dev. 2006;73:1367–79. [DOI] [PubMed] [Google Scholar]
- 86.Amleh A, Nair SJ, Sun J, Sutherland A, Hasty P, Li R. Mouse cofactor of BRCA1 (Cobra1) is required for early embryogenesis. PLoS ONE. 2009;4:e5034. [DOI] [PMC free article] [PubMed]
- 87.Mancini A, Niemann-Seyde SC, Pankow R, El Bounkari O, Klebba-Färber S, Koch A, et al. THOC5/FMIP, an mRNA export TREX complex protein, is essential for hematopoietic primitive cell survival in vivo. BMC Biol. 2010;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang L, Miao Y-L, Zheng X, Lackford B, Zhou B, Han L, et al. The THO complex regulates pluripotency gene mRNA export and controls embryonic stem cell self-renewal and somatic cell reprogramming. Cell Stem Cell. 2013;13:676–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Canse C, Yildirim E, Yaba A. Overview of junctional complexes during mammalian early embryonic development. Front Endocrinol (Lausanne). 2023;14:1150017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jeong Y, Ock SA, Yoo JG, Yu DY, Choi I. The Cxadr–Adam10 complex plays pivotal roles in tight junction integrity and early trophoblast development in mice. Mol Reprod Dev. 2019;86:1628–38. [DOI] [PubMed] [Google Scholar]
- 91.Kwon JW, Kim NH, Choi I. CXADR is required for AJ and TJ assembly during porcine blastocyst formation. Reproduction. 2016;151:297–304. [DOI] [PubMed] [Google Scholar]
- 92.Gao D, Xu T, Qi X, Ning W, Ren S, Ru Z, et al. CLAUDIN7 modulates trophectoderm barrier function to maintain blastocyst development in pigs. Theriogenology. 2020;158:346–57. [DOI] [PubMed] [Google Scholar]
- 93.Dong G, Sun R, Zhang R, Qin Y, Lu C, Wang X, et al. Preimplantation triclosan exposure alters uterine receptivity through affecting tight junction protein. Biol Reprod. 2022;107:349–57. [DOI] [PubMed] [Google Scholar]
- 94.Hansen TR, Sinedino LDP, Spencer TE. Paracrine and endocrine actions of interferon tau (IFNT). Reproduction. 2017;154:F45-59. [DOI] [PubMed] [Google Scholar]
- 95.Nakamura K, Kusama K, Bai R, Sakurai T, Isuzugawa K, Godkin JD, et al. Induction of IFNT-stimulated genes by conceptus-derived exosomes during the attachment period. PLoS ONE. 2016;11:e0158278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Forde N, Lonergan P. Interferon-tau and fertility in ruminants. Reproduction. 2017;154:F33-43. [DOI] [PubMed] [Google Scholar]
- 97.Chaney HL, Grose LF, Charpigny G, Behura SK, Sheldon IM, Cronin JG, et al. Conceptus-induced, interferon tau-dependent gene expression in bovine endometrial epithelial and stromal cells. Biol Reprod. 2021;104:669–83. [DOI] [PubMed] [Google Scholar]
- 98.Williams BRG. Signal Integration via PKR. Sci STKE. 2001;2001:re2–re2. [DOI] [PubMed]
- 99.Wong AHT, Ning Tam NW, Yang YL, Cuddihy AR, Li S, Kirchhoff S, et al. Physical association between STAT1 and the interferon-inducible protein kinase PKR and implications for interferon and double-stranded RNA signaling pathways. EMBO J. 1997;16:1291–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmaltz-Panneau B, Cordova A, Dhorne-Pollet S, Hennequet-Antier C, Uzbekova S, Martinot E, et al. Early bovine embryos regulate oviduct epithelial cell gene expression during in vitro co-culture. Anim Reprod Sci. 2014;149:103–16. [DOI] [PubMed] [Google Scholar]
- 101.Zhao H, Sui L, Miao K, An L, Wang D, Hou Z, et al. Comparative analysis between endometrial proteomes of pregnant and non-pregnant ewes during the peri-implantation period. J Anim Sci Biotechnol. 2015;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walker CG, Littlejohn MD, Mitchell MD, Roche JR, Meier S. Endometrial gene expression during early pregnancy differs between fertile and subfertile dairy cow strains. Physiol Genomics. 2012;44:47–58. [DOI] [PubMed] [Google Scholar]
- 103.Krizanac M, Mass Sanchez PB, Weiskirchen R, Schröder SK. Overview of the expression patterns and roles of Lipocalin 2 in the reproductive system. Front Endocrinol (Lausanne). 2024;15:1320097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hayes MA, Quinn BA, Lillie BN, Côté O, Bienzle D, Waelchli RO, et al. Changes in various endometrial proteins during cloprostenol-induced failure of early pregnancy in mares. Anim Reprod Sci. 2012;9:723–41. [Google Scholar]
- 105.Mazzarella R, Bastos NM, Bridi A, del Collado M, Andrade GM, Pinzon J, et al. Changes in oviductal cells and small extracellular vesicles miRNAs in pregnant cows. Front Vet Sci. 2021;8:639752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Waclawik A, Rivero-Muller A, Blitek A, Kaczmarek MM, Brokken LJS, Watanabe K, et al. Molecular cloning and spatiotemporal expression of prostaglandin F synthase and microsomal prostaglandin E synthase-1 in porcine endometrium. Endocrinology. 2006;147:210–21. [DOI] [PubMed] [Google Scholar]
- 107.Arosh JA, Banu SK, McCracken JA. Novel concepts on the role of prostaglandins on luteal maintenance and maternal recognition and establishment of pregnancy in ruminants. J Dairy Sci. 2016;99:5926–40. [DOI] [PubMed] [Google Scholar]
- 108.Scenna FN, Edwards JL, Rohrbach NR, Hockett ME, Saxton AM, Schrick FN. Detrimental effects of prostaglandin F2α on preimplantation bovine embryos. Prostaglandins Other Lipid Mediat. 2004;73:215–26. [DOI] [PubMed] [Google Scholar]
- 109.Kennedy TG, Gillio-Meina C, Phang SH. Prostaglandins and the initiation of blastocyst implantation and decidualization. Reproduction. 2007;134:635–43. [DOI] [PubMed] [Google Scholar]
- 110.Baratelli F, Lin Y, Zhu L, Yang S-C, Heuzé-Vourc’h N, Zeng G, et al. Prostaglandin E2 induces FOXP3 gene expression and T regulatory cell function in human CD4+ T cells. J Immunol. 2005;175:1483–90. [DOI] [PubMed] [Google Scholar]
- 111.Hayat R, Manzoor M, Hussain A. Wnt signaling pathway: a comprehensive review. Cell Biol Int. 2022;46:863–77. [DOI] [PubMed] [Google Scholar]
- 112.Riley JK, Carayannopoulos MO, H. Wyman A, Chi M, Ratajczak CK, Moley KH. The PI3K/Akt pathway is present and functional in the preimplantation mouse embryo. Dev Biol. 2005;284:377–86. [DOI] [PubMed] [Google Scholar]
- 113.Lu CW, Yabuuchi A, Chen L, Viswanathan S, Kim K, Daley GQ. Ras-MAPK signaling promotes trophectoderm formation from embryonic stem cells and mouse embryos. Nat Genet. 2008;40:921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–46. [DOI] [PubMed] [Google Scholar]
- 115.Denicol AC, Dobbs KB, McLean KM, Carambula SF, Loureiro B, Hansen PJ. Canonical WNT signaling regulates development of bovine embryos to the blastocyst stage. Sci Rep. 2013;3:1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aparicio IM, Garcia-Herreros M, Fair T, Lonergan P. Identification and regulation of glycogen synthase kinase-3 during bovine embryo development. Reproduction. 2010;140:83–92. [DOI] [PubMed] [Google Scholar]
- 117.Brunmeir R, Lagger S, Seiser C. Histone deacetylase HDAC1/HDAC2-controlled embryonic development and cell differentiation. Int J Dev Biol. 2009;53:275–89. [DOI] [PubMed] [Google Scholar]
- 118.Walker CG, Meier S, Littlejohn MD, Lehnert K, Roche JR, Mitchell MD. Modulation of the maternal immune system by the pre-implantation embryo. BMC Genomics. 2010;11:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sharma J, Antenos M, Madan P. A comparative analysis of Hippo signaling pathway components during murine and bovine early mammalian embryogenesis. Genes (Basel). 2021;12:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cockburn K, Biechele S, Garner J, Rossant J. The Hippo pathway member Nf2 is required for inner cell mass specification. Curr Biol. 2013;23:1195–201. [DOI] [PubMed] [Google Scholar]
- 121.Adachi K, Soeta-Saneyoshi C, Sagara H, Iwakura Y. Crucial role of Bysl in mammalian preimplantation development as an integral factor for 40S ribosome biogenesis. Mol Cell Biol. 2007;27:2202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen T, Ma J, Liu Y, Chen Z, Xiao N, Lu Y, et al. iProX in 2021: connecting proteomics data sharing with big data. Nucleic Acids Res. 2022;50:D1522–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ma J, Chen T, Wu S, Yang C, Bai M, Shu K, et al. iProX: an integrated proteome resource. Nucleic Acids Res. 2019;47:D1211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Experimental setup of the in vitro model. Extracellular vesicles (EVs) were isolated from the conditioned medium (CM) collected from three in vitro experimental groups: (1A–B) endometrial explants cultured alone (EXPL), (2A–B) endometrial explants co-cultured with blastocysts (EXPL + EMB), and (3A) blastocysts cultured alone (EMB).
Additional file 2. Strategy for quantitative analysis for in vitro comparison. Venn diagrams illustrate the identification of differentially abundant proteins (DAPs) between EXPL vs. EMB, EMB vs. EXPL + EMB, and EXPL vs. EXPL + EMB. The green circle represents DAPs in EXPL vs. EMB comparison.
Additional file 3. Negative controls for the characterization of extracellular vesicles (EVs) from uterine fluid (UF) and conditioned medium (CM). Phosphate‑buffered saline without calcium and magnesium (PBS−/−) was used as the negative control for both nanoparticle tracking analysis (NTA) and transmission electron microscopy (TEM). A. NTA of PBS−/−, showing 0 particles per frame. B. TEM images of PBS−/−, showing no vesicle-like structures.
Additional file 4. Proteomic profile of uterine fluid extracellular vesicles (UF-EVs). A. All 1459 proteins present in UF-EVs from CYCLIC heifers. B. All 1329 proteins present in UF-EVs from PREGNANT heifers. C. Differentially abundant proteins in UF-EVs from CYCLIC compared to PREGNANT heifers. D. Top 100 most abundant proteins in UF-EVs from CYCLIC and PREGNANT heifers.
Additional file 5. Proteomic profile of conditioned medium extracellular vesicles (CM-EVs). A. All 1501 proteins present in CM-EVs from explants cultured alone. B. All 1975 proteins present in CM-EVs from explants cocultured with embryos. C. All 82 proteins present in CM-EVs from embryos cultured alone. D. Top 100 most abundant proteins in CM-EVs from EXPL, EXPL+EMB and EMB.
Additional file 6. Functional enrichment of proteins identified in uterine fluid extracellular vesicles (UF-EVs) from CYCLIC heifers compared to conditioned medium extracellular vesicles (CM-EVs) from endometrial explants cultured alone (EXPL). A. Proteins (n =125) exclusive to UF-EVs from CYCLIC. B. Proteins (106) exclusive to CM-EVs form EXPL. C. Proteins (n = 691) equally abundant among CYCLIC vs. EXPL. D. Proteins (n = 328) differentially abundant among CYCLIC vs. EXPL.
Additional file 7. Functional enrichment of proteins identified in uterine fluid extracellular vesicles (UF-EVs) from PREGNANT heifers compared to conditioned medium extracellular vesicles (CM-EVs) from endometrial explants co-cultured with blastocysts (EXPL + EMB). A. Proteins (n =108) exclusive to UF-EVs from PREGNANT. B. Proteins (n = 195) exclusive to CM-EVs form EXPL + EMB. C. Eight hundred and thirteen proteins (n =813) equally abundant among PREGNANT vs. EXPL + EMB. D. Proteins (n = 560) differentially abundant among PREGNANT vs. EXPL + EMB.
Data Availability Statement
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (https://proteomecentral.proteomexchange.org) via the iProX partner repository [122, 123] with the dataset identifier PXD053670.