Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) represents a significant and escalating public health challenge, particularly in obese, sedentary populations. Hybrid exercise training, integrating electrical muscle stimulation (EMS) with voluntary muscle contractions, offers a novel, low-impact therapeutic modality; however, its clinical efficacy remains underexplored.
Objectives
This randomized controlled trial investigated the clinical efficacy of hybrid exercise training in improving hepatic steatosis, liver enzyme profiles, systemic inflammation, and metabolic health in sedentary, obese, middle-aged women with NAFLD.
Methods
Thirty women with ultrasound-confirmed grade 2–3 NAFLD were randomized to either six weeks of hybrid exercise training (n = 15) or lifestyle counseling (n = 15). Primary and secondary outcomes included liver steatosis grade, serum liver enzymes (AST, ALT), inflammatory marker IL-6, fasting blood glucose, and anthropometric parameters.
Results
Hybrid exercise training led to significant improvements compared with lifestyle counseling. Steatosis grade decreased markedly (− 0.80 vs. −0.02; p < 0.01; η² = 0.38), accompanied by large reductions in ALT (− 31.86 U/L; p < 0.01; η² = 0.65) and AST (− 27.46 U/L; p < 0.01; η² = 0.61). IL-6 concentrations declined (− 3.0 pg/mL; p < 0.05; η² = 0.42), while anthropometric outcomes improved (body weight − 4.51 kg, BMI − 1.56 kg/m², WHR − 0.042; all p < 0.01; η² ≥ 0.52). Correlation analyses showed that decreases in IL-6 were strongly associated with improvements in ALT (r = − 0.72, p < 0.01) and AST (r = − 0.68, p < 0.01).
Conclusions
Hybrid exercise training is a safe, feasible, and clinically effective intervention for improving liver health and attenuating systemic inflammation in obese women with NAFLD, with strong associations between IL-6 reductions and liver enzyme improvements.
Clinical trial registration
ClinicalTrials.gov, NCT05231564, Registered on 28 February 2022.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13102-025-01349-2.
Keywords: Electrical muscle stimulation, Hybrid training, Non-alcoholic fatty liver disease, Metabolic syndrome, Women's health
Introduction
Non-alcoholic fatty liver disease (NAFLD), closely associated with obesity and metabolic syndrome [1], poses serious risks by contributing to cardiovascular issues, insulin resistance [2], and severe liver disorders such as cirrhosis [3]. Recent data indicate NAFLD affects over 25% of the global population, underscoring the urgency for effective interventions [4]. Often asymptomatic, it’s detected via elevated liver enzymes (e.g., aspartate aminotransferase [AST] and alanine aminotransferase [ALT]) or ultrasound, a non-invasive, cost-effective tool [2–5]. Therefore, effective management strategies are urgently needed to mitigate the progression and complications associated with NAFLD.
Exercise interventions are widely recognized as central to NAFLD management, with proven benefits in improving insulin sensitivity, modulating lipid metabolism, and attenuating systemic inflammation through reductions in pro-inflammatory cytokines such as IL-6 [6, 7]. Despite these benefits, adherence to traditional exercise programs often remains low, particularly among sedentary or obese individuals who face barriers related to motivation, physical limitations, or comorbidities [8, 9]. Hybrid exercise training, which integrates electrical muscle stimulation (EMS) with voluntary contractions, may overcome these barriers by enhancing muscle fiber recruitment while reducing joint stress. This concept was originally introduced by Yanagi et al. [10], who developed the Hybrid Training System combining voluntary contractions against electrically stimulated antagonists. More recently, Matsuse et al. [11] reviewed its applications, emphasizing that this approach enhances muscle activation while minimizing joint stress. This dual activation of fast- and slow-twitch fibers offers restorative potential by improving metabolic efficiency, increasing glucose uptake, and facilitating hepatic recovery in populations with limited exercise adherence [12]– [13]. While electrical muscle stimulation (EMS) has been explored in general populations for metabolic benefits [14, 15], this study is among the first randomized controlled trials to integrate hybrid training—combining agonist, antagonist, and stabilizer stimulation—with a progressive protocol tailored specifically for NAFLD in sedentary, obese women, demonstrating superior outcomes compared to lifestyle counseling alone [14]. Originally developed to counter muscle deterioration in astronauts under microgravity conditions [16], the application of hybrid exercise training in NAFLD management remains underexplored, particularly in structured RCTs targeting sedentary, obese women.
This research investigates the impacts of hybrid exercise training on liver health markers in sedentary, obese, middle-aged women with moderate-to-severe NAFLD. We assessed its impact on liver steatosis (measured via ultrasound), systemic inflammation (IL-6 levels), and various metabolic markers, including anthropometric measures (e.g., body weight, body mass index [BMI], and waist-to-hip ratio [WHR]) and biochemical markers (AST, ALT, and fasting blood sugar [FBS]). We hypothesized that the hybrid exercise group would show statistically significant improvements in these parameters compared to a lifestyle counseling control group. The primary endpoint was liver steatosis reduction; secondary outcomes included changes in liver enzymes, inflammatory markers, and anthropometric data.
Methods
Trial design
This parallel-group randomized clinical trial (RCT) assessed hybrid exercise training in sedentary, obese women with NAFLD. The research was conducted at the Dr. Abedzadeh Ultrasonography Center in Kerman, Iran, in alignment with the ethical principles delineated in the Declaration of Helsinki. The research received approval from the Research Ethics Committee (IR.USB.REC.1398.012) and listed on ClinicalTrials.gov with the registration number NCT05231564. All participants provided written informed consent prior to enrollment. They were informed about the study objectives, procedures, potential risks, benefits, and their right to withdraw at any time without consequence. The consent process was approved by the same Research Ethics Committee. The trial was conducted as planned, with no early termination. As blinding of participants and training personnel was not feasible due to the nature of the exercise intervention, ensuring similarity of interventions was not applicable. However, to minimize measurement bias, the radiologist assessing the ultrasounds was blinded to group allocation, ensuring objective evaluation. The radiologist was not involved in the recruitment or research process. The full trial protocol, including detailed research procedures and statistical analysis plans, is accessible via ClinicalTrials.gov (NCT05231564). No significant methodological changes were made after trial commencement; all procedures followed the pre-registered protocol. Supplementary protocol details can be obtained by contacting the corresponding author.
Participants
The research established a total sample size of 30 participants (15 per group) through Analysis of Covariance (ANCOVA), based on a targeted effect size of 0.8, with 80% statistical power and a significance level (α) of 0.05. This size was determined via an a priori power analysis to guarantee sufficient power for identifying meaningful group differences. The calculation accounted for the anticipated standard deviation of the continuous outcome variable, a key factor for precise sample size determination in randomized controlled trials (RCTs) [17]. The estimate assumed full adherence, consistent with the observed 100% completion rate, and therefore did not include a dropout adjustment. By utilizing ANCOVA, the analysis accounts for baseline measurements, enhancing the precision of the treatment effect estimation [18].
Eligible participants were sedentary women aged 45–65 years, with a BMI of 30 kg/m² or greater, a WHR of 0.85 or greater as these thresholds are consistent with WHO obesity classifications and central adiposity risk criteria, and NAFLD grades 2–3 confirmed via ultrasound imaging [19]. This age range was selected to align with the typical age of NAFLD diagnosis and the menopausal transition, which can influence metabolic health [19, 20]. Participants were enrolled based on an elevated ALT/AST ratio (above 1.0) or elevated liver enzyme levels (AST or ALT above 35 U/L), and blood pressure readings ≥ 130/85 mmHg. Exclusion criteria included viral hepatitis, diabetes, uncontrolled hypertension, hyperthyroidism, coronary artery disease, ethanol consumption, or mobility-limiting conditions. These exclusion criteria were implemented to minimize potential confounding factors and ensure a more homogeneous research population, thereby enhancing the internal validity of the findings (20).
Randomization and allocation
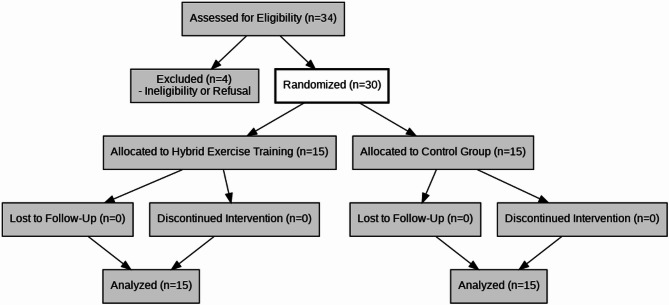
Participants were randomly assigned to research groups utilizing a computer-generated block randomization sequence with a fixed block size of four. Allocation was concealed using sealed opaque envelopes to ensure unbiased group assignments. The random allocation sequence was generated by an independent statistician using a computer-based algorithm. Participant enrollment was conducted by a research coordinator at the Dr. Abedzadeh Ultrasonography Center, who screened and confirmed eligibility based on inclusion/exclusion criteria. Group assignments were performed by a separate research administrator using sealed opaque envelopes to maintain allocation concealment. Figure 1 illustrates participant flow, detailing randomization, allocation, and follow-up compliance.
Fig. 1.
CONSORT Diagram of participant flow (enrollment, allocation, follow-up). This diagram outlines the randomized controlled trial process, illustrating the allocation of participants to either the hybrid exercise training group or the control group, along with follow-up details
Interventions
The hybrid exercise group completed a 6-week program consisting of 2–3 sessions per week, each lasting 45–60 min. The protocol was designed to gradually increase energy expenditure from 500 to 1000 kcal per week. Training was divided into two phases: (i) metabolic training (weeks 1–3), including brisk walking and stationary cycling, and (ii) strength training (weeks 4–6), incorporating body-weight squats, forward lunges, and deadlifts. All sessions were performed with simultaneous EMS (7–85 Hz, 350 µs pulse width), targeting quadriceps, hamstrings, and gluteal muscles, with intensity individually adjusted. Detailed exercise protocols, including EMS parameters and exercise modalities, are provided in Supplementary Tables S1 and S2.
The hybrid training protocol employed EMS on agonist, antagonist, and stabilizer muscles to enhance recruitment and metabolic response while reducing joint stress, consistent with the Hybrid Training System [10, 11]. During metabolic training (walking, cycling), stimulation was applied to the quadriceps (agonists for cycling), hamstrings (antagonists for cycling and walking), and erector spinae (stabilizers for walking). During strength training (squats, lunges, deadlifts), electrodes targeted the quadriceps and hamstrings (agonists/antagonists for squats and lunges) and erector spinae (stabilizers for deadlifts). This strategy optimized activation efficiency and was particularly suited for sedentary individuals with obesity or mobility restrictions. Although initially framed as antagonist-focused stimulation, the protocol combined both agonist and stabilizer activation to augment voluntary contractions. This reflects two established hybrid training strategies: (i) stimulating antagonists during voluntary agonist activity, and (ii) superimposing stimulation on agonists or stabilizers to enhance recruitment. The combined approach was adopted here to maximize activation while ensuring joint safety.
Energy expenditure during sessions was continuously monitored with RS400 cardiac devices to confirm adherence to prescribed intensity levels [20]. Detailed EMS parameters, including pulse shapes (square/sinusoidal), durations (unlimited or connected/disconnected), and targeted muscles (e.g., agonists like quadriceps, antagonists like hamstrings, stabilizers like erector spinae), are outlined in Supplementary Tables S1 (metabolic phase) and S2 (strength phase). Gluteal muscles were engaged indirectly via hamstring and erector spinae stimulation during exercises like deadlifts and squats, optimizing recruitment while minimizing joint stress, as per the Hybrid Training System [10, 11]. The control group attended weekly lifestyle counseling sessions (45–60 min) emphasizing ≥ 150 min/week of moderate exercise, Mediterranean dietary principles, and stress management, with a minimum attendance rate of 85% required.
Outcome measurements
Anthropometric data, such as body weight, BMI, and WHR, were collected following WHO guidelines. A Seca 755 scale (Seca GmbH, Hamburg, Germany) was used to measure stature and body mass, while waist circumference was taken horizontally midway between the iliac crest and the inferior edge of the lowest detectable rib. Hip circumference was recorded at the point of maximum buttock protrusion [21]. Blood pressure was measured in line with European Blood Pressure Association (EBPA) standards [18], using a Riester barometer (model Precisa N 1360, Germany) to reduce variability between observers.
Biochemical markers (AST, ALT, IL-6, FBS) were assessed through venipuncture post-12-hour fast. Liver enzymes (AST/ALT) were evaluated using Pars Azmoun assay kits (Iran). The levels of IL-6 were quantified with Carmania Pars Gene kits (Iran). FBS levels were measured utilizing the Wiener® Liquid AA enzymatic glucose assay (Wiener Lab, Argentina), which utilizes an enzymatic oxidation method for glucose determination.
Liver steatosis was graded using Siemens Color Doppler ultrasound (Germany) based on established criteria [22], including the presence of hepatic brightness, blurring of hepatic vessels, and increased echogenicity relative to the renal cortex. Steatosis was graded on a scale of 0 to 3, where:
Grade 0: Absence of steatosis.
Grade 1: Mild steatosis with slight hepatic brightness.
Grade 2: Moderate steatosis with distinct hepatic brightness and blurring of vessels.
Grade 3: Severe steatosis with marked hepatic brightness, significant blurring of vessels, and posterior attenuation.
Lifestyle factors were evaluated using the Physical Activity Readiness Questionnaire (PAR-Q) and the Simple Lifestyle Index Questionnaire (SLIQ) to assess baseline physical activity levels and general lifestyle habits, ensuring participant eligibility and providing descriptive data for the research population. Participants reported no regular physical activity in the previous 6 months, consistent with sedentary classification. Dietary intake was tracked via 4-day diaries (three weekdays and one weekend day) [23]. Daily energy consumption was calculated using the EVIDENT II program [12, 20]. To minimize potential confounding factors, participants were advised to retain unvarying caloric intake and activity levels throughout the research interval. No standardized dietary intervention was implemented, which may have influenced fasting blood glucose outcomes. All outcome measurements were performed at baseline and after six weeks of intervention to assess changes within and between groups. No modifications were made to the primary or secondary outcomes after the trial began.
Statistical analyses
Statistical analyses utilized means ± standard deviations (SD) to summarize continuous variables (e.g., age, BMI, liver enzyme levels) and percentages for categorical variables (e.g., NAFLD grades). The Shapiro-Wilk test checked data normality. Pre-intervention group comparisons were examined with independent samples t-tests for continuous variables and chi-square tests for categorical ones. Post-intervention group comparisons were conducted employing analysis of covariance (ANCOVA), with baseline values as covariates. Effect sizes for ANCOVA were reported as partial eta-squared (η²), interpreted as small (η² ≥ 0.01), medium (η² ≥ 0.06), or large (η² ≥ 0.14) [24]. Cohen’s d was calculated for pairwise comparisons (mean difference divided by pooled SD), with thresholds of small (d ≥ 0.2), medium (d ≥ 0.5), and large (d ≥ 0.8) [25]. Paired t-tests assessed within-group changes in continuous variables over time. Hepatic steatosis was evaluated via the Wilcoxon signed-rank test to determine within-group changes and the Mann-Whitney U test for between-group comparisons. Spearman’s rho (ρ) evaluated correlations between systemic inflammation (IL-6 levels) and liver enzymes (AST/ALT). Levene’s test confirmed the homogeneity of variance.
To address the research objectives, which included evaluating the effect of hybrid exercise training on liver steatosis, inflammation, and various metabolic markers, the following statistical methods were employed. While this research was primarily hypothesis-driven, aiming to test specific pre-defined outcomes, it also included an exploratory component to investigate potential secondary effects, given the limited existing research on hybrid exercise training in NAFLD. To reduce the risk of Type II errors in this hypothesis-driven trial with a small sample, no correction for multiple comparisons was applied, though this increases the potential for false positives [17]. This decision was made in consideration of the potential for Type II errors (false negatives) that can arise from overly conservative correction methods in research with small sample sizes. However, we acknowledge that the lack of adjustment increases the risk of Type I errors (false positives), particularly in the exploratory analyses. Therefore, results from secondary outcomes and correlations should be interpreted with caution and regarded as preliminary, warranting further investigation in larger, more definitive trials with appropriate adjustments for multiple comparisons. This approach is consistent with the research design and short duration, focusing on targeted outcomes related to liver health and metabolic improvements. Data processing was executed utilizing SPSS version 24.0 (IBM Corp., Armonk, NY, USA), with statistical significance set at a two-tailed p < 0.05 (95% confidence level). No interim analyses or stopping guidelines were employed due to the short intervention duration and low-risk nature of the study.
Results
All 30 enrolled participants (15 per group) completed the 6-week trial, with no dropouts (Fig. 1).
Patient characteristics
Baseline demographic and clinical characteristics were comparable between groups, with no statistically significant differences (all p > 0.05). Both groups included sedentary, obese women of similar age, BMI, and hepatic steatosis grade distribution. Energy intake remained stable throughout the study in both groups. Compliance with the protocol was high: 95% in the hybrid exercise group (11.4 of 12 sessions attended) and 100% in the control group (6 of 6 lifestyle counseling sessions attended) (Table 1).
Table 1.
Baseline demographics, compliance, energy intake, and ultrasound results. Baseline demographics, adherence rates, energy intake, and hepatic steatosis distribution in the hybrid exercise and control groups. Data are presented as mean ± sd for continuous variables and percentage (n) for categorical variables
| Demographic feature | Group | |
|---|---|---|
| Hybrid exercise (n = 15) | Control (n = 15) | |
| Age (year) a | 53 ± 4.99 | 51 ± 4.69 |
| Gender | woman | woman |
| Height (cm) a | 171.12 ± 3.61 | 168.18 ± 4.19 |
| Training compliance | ||
| Exercise energy expenditure (kcal/week)a | 1000 ± 4.6 | n.a. |
| Exercise Duration (min)a | 51 ± 8.7 | n.a. |
| Energy intake, kcal/day | ||
| Pre-interventiona | 1768 ± 189 | 1731 ± 166 |
| Post-interventiona | 1788 ± 165 | 1814 ± 158 |
| Hepatic Steatosis | ||
| Grade 1b | 0 | 0 |
| Grade 2b | 66.7 (10) | 66.7 (10) |
| Grade 3b | 33.3 (5) | 33.3 (5) |
| Adherence Rate c | 95% | 100% |
a Data are given as mean ± standard deviation
b Data are given as Percentage (Number) of participants
c Adherence rate = (attended sessions / planned) × 100%
Effects of hybrid training on anthropometry
The hybrid exercise group demonstrated significant improvements in anthropometric indices compared with controls (Table 2). Mean body weight decreased by − 4.51 kg (95% CI: −5.8, − 3.2; p = 0.01, η² = 0.52), corresponding to a large effect size (Cohen’s d = 1.2). BMI decreased by − 1.56 kg/m² (95% CI: −1.9, − 1.2; p < 0.01, η² = 0.67), also with a large effect size (d = 1.3). WHR declined significantly in the hybrid group (− 0.042; 95% CI: −0.05, − 0.03; p = 0.006, η² = 0.31), whereas no meaningful changes were observed in the control group. Building on these anthropometric improvements, biochemical markers further demonstrated the efficacy of hybrid exercise training.
Table 2.
Pre- and post-intervention outcomes with effect sizes in sedentary obese women with NAFLD. Baseline and post-intervention values for anthropometric measures, liver enzymes, fasting blood sugar, IL-6, and hepatic steatosis grades in the hybrid exercise and control groups. Between-group differences were assessed using ANCOVA with baseline values as covariates. Effect sizes are reported as partial η² for ANCOVA models and cohen’s d for within-group comparisons. Significant results (p < 0.05) denote improvements in favor of the hybrid exercise group
| Outcome | Group | Pre-Intervention (Mean ± SD) | Post-Intervention (Mean ± SD) | Adjusted Mean Difference (95% CI) | Partial η² | Cohen’s d | p-value |
|---|---|---|---|---|---|---|---|
| Body Weight (kg) | Hybrid Exercise | 91.82 ± 4.64 | 87.31 ± 5.61 | −4.51 (− 5.8, − 3.2) | 0.52 | 1.2 | 0.01* |
| Control | 90.47 ± 6.43 | 91.27 ± 6.12 | 0.80 (− 0.6, 2.2) | 0.03 | 0.1 | 0.46 | |
| BMI (kg/m²) | Hybrid Exercise | 31.36 ± 1.19 | 29.80 ± 1.21 | −1.56 (− 1.9, − 1.2) | 0.67 | 1.3 | < 0.01* |
| Control | 32.08 ± 3.06 | 32.43 ± 3.41 | 0.35 (− 0.1, 0.8) | 0.02 | 0.1 | 0.14 | |
| WHR | Hybrid Exercise | 1.28 ± 0.01 | 1.238 ± 0.05 | −0.042 (− 0.05, − 0.03) | 0.31 | 0.9 | 0.006* |
| Control | 1.33 ± 0.09 | 1.34 ± 0.10 | 0.01 (− 0.02, 0.04) | 0.01 | 0.1 | 0.92 | |
| AST (U/L) | Hybrid Exercise | 86.69 ± 5.70 | 59.23 ± 6.22 | −27.46 (− 30.1, − 24.8) | 0.61 | 1.4 | < 0.01* |
| Control | 84.22 ± 5.11 | 88.28 ± 6.82 | 4.06 (1.5, 6.6) | 0.05 | 0.2 | 0.51 | |
| ALT (U/L) | Hybrid Exercise | 86.15 ± 9.37 | 54.29 ± 5.48 | −31.86 (− 34.9, − 28.8) | 0.65 | 1.6 | < 0.01* |
| Control | 90.98 ± 5.70 | 93.82 ± 5.61 | 2.84 (− 0.2, 5.9) | 0.04 | 0.2 | 0.36 | |
| AST/ALT ratio | Hybrid Exercise | 1.01 ± 0.09 | 1.09 ± 0.07 | 0.08 (0.05, 0.11) | 0.45 | 1.0 | 0.02* |
| Control | 0.93 ± 0.08 | 0.94 ± 0.09 | 0.01 (− 0.02, 0.04) | 0.02 | 0.1 | 0.81 | |
| FBS (mg/dL) | Hybrid Exercise | 115 ± 4 | 114 ± 4 | −2 (− 2.4, − 1.6) | 0.06 | −0.3 | 0.10 |
| Control | 112 ± 4 | 113 ± 4 | 2 (1.6, 2.4) | 0.06 | 0.3 | 0.10 | |
| IL-6 (pg/mL) | Hybrid Exercise | 19.3 ± 0.8 | 16.3 ± 1.2 | −3.0 (− 3.4, − 2.6) | 0.42 | 1.1 | 0.03* |
| Control | 19.8 ± 0.3 | 20.3 ± 0.7 | 0.5 (0.1, 0.9) | 0.06 | 0.3 | 0.73 | |
| Liver Steatosis Grade | Hybrid Exercise | 2.33 ± 0.48 | 1.53 ± 0.51 | −0.80 (− 1.0, − 0.6) | 0.38 | 1.0 | 0.003* |
| Control | 2.31 ± 0.48 | 2.29 ± 0.59 | −0.02 (− 0.2, 0.2) | 0.01 | 0.1 | 0.17 |
Notes: Data presented as Mean ± SD unless otherwise indicated. Adjusted Mean Difference calculated via ANCOVA with baseline values as covariates (95% confidence intervals). Partial η² values ≥ 0.14 denote large effects. Cohen’s d values ≥ 0.8 indicate large effects. *p < 0.05 considered statistically significant
Abbreviations: BMI = Body Mass Index; WHR = Waist-to-Hip Ratio; AST = Aspartate Transaminase; ALT = Alanine Transaminase; FBS = Fasting Blood Sugar; IL-6 = Interleukin-6
Effects of hybrid training on liver enzymes and steatosis
Significant reductions in liver enzymes and steatosis grade were observed in the hybrid exercise group compared with controls (Table 2). ALT decreased by − 31.86 U/L (95% CI: −34.9, − 28.8; p < 0.01, η² = 0.65, d = 1.6) and AST by − 27.46 U/L (95% CI: −30.1, − 24.8; p < 0.01, η² = 0.61, d = 1.4). In contrast, the control group showed no improvement, with small, non-significant increases in both enzymes. In addition, the AST/ALT ratio, a clinical indicator of liver function, improved significantly in the hybrid exercise group (from 1.01 ± 0.09 to 1.09 ± 0.07, adjusted difference 0.08 [95% CI: 0.05–0.11], p = 0.02, η²=0.45, d = 1.0), indicating reduced hepatocellular injury relative to controls, consistent with improved liver function. Complementing these liver enzyme changes, systemic inflammation was also attenuated.
Ultrasound assessments confirmed reductions in hepatic steatosis, aligning with biochemical improvements. Liver steatosis grade declined from 2.33 ± 0.48 to 1.53 ± 0.51 in the hybrid group, compared with minimal change in the control group (2.31 ± 0.48 to 2.29 ± 0.59). The between-group adjusted mean difference was − 0.80 grades (95% CI: −1.0, − 0.6; p = 0.003, η² = 0.38, d = 1.0), reflecting a robust treatment effect.
Inflammatory marker responses
IL-6 levels decreased significantly in the hybrid exercise group (− 3.0 pg/mL; 95% CI: −3.4, − 2.6; p = 0.03, η² = 0.42, d = 1.1), while the control group exhibited a slight, non-significant increase (+ 0.5 pg/mL).
Correlation analyses
Given non-normality (Shapiro-Wilk p < 0.05), Spearman’s rho was used. Spearman correlation analysis revealed strong inverse associations between reductions in IL-6 levels and improvements in both ALT (rho = − 0.72, p < 0.01) and AST (rho = − 0.68, p < 0.01; Fig. 2), suggesting that reduced systemic inflammation may be associated with improved liver health. These findings should be interpreted cautiously due to the small sample size and observational nature of the analysis.
Fig. 2.
Spearman correlation between IL-6 reduction and liver enzyme improvements. A scatter plot showing the Spearman correlation between reductions in IL-6 levels and liver enzyme (AST and ALT) improvements following hybrid exercise training. The trend suggests a link between systemic inflammation reduction and liver function improvement
Discussion
Hybrid exercise training significantly reduced liver steatosis (− 0.8 grades, p < 0.01) and IL-6 levels (− 3.0 pg/mL, p < 0.05) in sedentary, obese women with NAFLD, supporting its efficacy in promoting liver health. Our results align with those reported by Kawaguchi et al. [7], who reported similar reductions in steatosis and liver enzymes following hybrid protocols, as well as with Oh et al. [13], who observed improved insulin sensitivity after hybrid exercise training—consistent with our results showing enhanced metabolic markers. This approach may improve adherence in high-risk groups, as evidenced by our 95% compliance rate (Table 1).
Moreover, prior research has demonstrated that regular exercise can modulate systemic inflammation by reducing pro-inflammatory cytokines such as IL-6, which supports our observed correlations between IL-6 reduction and improvements in liver enzymes [6, 7]. The strong correlations between IL-6 reduction and improvements in liver enzymes (ALT: rho = − 0.72; AST: rho = − 0.68) suggest a possible association between decreased systemic inflammation and enhanced liver function. However, causality cannot be inferred due to the small sample size, lack of adjustment for multiple comparisons, and the observational nature of the analysis. These findings are preliminary and warrant confirmation in larger trials with robust statistical controls. Collectively, these findings highlight the capability of hybrid exercise training as a low-impact exercise strategy, particularly beneficial for individuals with restricted mobility or those who lack motivation for conventional exercise regimens.
Hybrid exercise training, which combines EMS with voluntary contractions, offers unique advantages by enhancing muscle fiber recruitment and promoting beneficial metabolic adaptations. This dual approach preferentially engages fast-twitch muscle fibers, which are critical for metabolic efficiency, as evidenced by prior research [13, 26, 27]. This may also involve modulation of both pro- and anti-inflammatory properties of IL-6 depending on exercise modality and duration [14, 27]. The intervention activates molecular pathways such as high-threshold motor unit recruitment, stimulation of mitochondrial biogenesis, and modulation of inflammatory pathways through myokines such as IL − 6 and IL − 15 [13, 26, 27]. These processes probably contribute to the noted enhancements in liver health and metabolic function in individuals with NAFLD. The large effect sizes observed (e.g., η²=0.65 for ALT, Table 2) surpass those reported in prior exercise studies for NAFLD [6], underscoring the potential of hybrid training to enhance adherence and efficacy in high-risk groups, as evidenced by our 95% compliance rate (Table 1). Moreover, hybrid exercise training enhances muscle metabolism by upregulating glucose transporter 1 (GLUT-1) and GLUT-4 expression, thereby increasing glucose uptake and improving insulin sensitivity [28, 29]. Notably, EMS at frequencies below 100 Hz stimulates anaerobic glycolysis, delays fatigue onset, and has demonstrated benefits for insulin resistance, body composition, and lipid metabolism in middle-aged individuals [12, 15, 30].
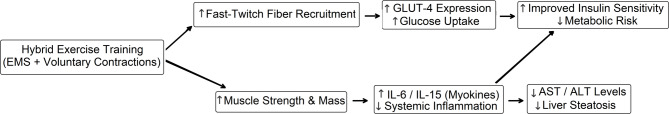
The mechanical and metabolic demands of hybrid exercise training promote muscle growth and reduce the risk of sarcopenia, a significant concern in middle-aged populations [16]. Simultaneous activation of agonist − antagonist muscle groups minimizes mechanical strain, reducing the risk of orthopedic injuries, and making it suitable for individuals with obesity, osteoarthritis, or mobility limitations who may find conventional resistance training challenging [31, 32]. However, the lack of menopausal status tracking important modifier of metabolic and hepatic outcomes interpretability in middle-aged women. Consistent with these benefits, our findings of reduced AST and ALT levels, decreased liver steatosis grades, and lower IL-6 levels underscore hybrid exercise training’s potential in regulating metabolic and inflammatory pathways (Table 2). These improvements are likely mediated by enhanced glucose uptake via upregulated GLUT-4 expression and reduced systemic inflammation [28, 33]. The improved AST/ALT ratio (0.08, p = 0.02, Table 2) complements individual enzyme reductions, suggesting comprehensive liver recovery via reduced inflammation, as evidenced by strong correlations with IL-6 (r = − 0.68 for AST, r = − 0.72 for ALT). This aligns with mechanisms such as myokine modulation (e.g., IL-6, IL-15) and enhanced glucose uptake [27, 28]. The interconnected nature of these mechanisms is visually represented in Fig. 3.
Fig. 3.
Conceptual framework illustrating the effects of hybrid exercise training on metabolic and inflammatory pathways in women with NAFLD. Hybrid exercise training, combining electrical muscle stimulation (EMS) with voluntary contractions, activates fast-twitch muscle fibers and enhances muscle strength. These neuromuscular adaptations upregulate glucose transporter expression (GLUT-4), increasing glucose uptake and improving insulin sensitivity. Concurrently, myokine release (e.g., IL-6, IL-15) contributes to reduced systemic inflammation. These combined effects result in lowered liver enzyme levels (AST, ALT), decreased hepatic steatosis, and improved metabolic profiles in sedentary, obese women with non-alcoholic fatty liver disease (NAFLD)
However, despite these metabolic benefits, fasting blood sugar levels remained unchanged, indicating that exercise alone may be insufficient for comprehensive glycemic control without concurrent dietary modifications. This could be due to the transient nature of exercise-induced insulin sensitivity, which is known to diminish post-exercise [34], highlighting the need for integrated dietary strategies in future research. Moreover, these findings have notable clinical implications for NAFLD management. Hybrid exercise training presents a safe and effective intervention for sedentary, obese individuals, improving liver enzymes, reducing steatosis, and attenuating inflammation (Results Section, Paragraphs 2), thus presenting a viable complement to multidisciplinary clinical management programs [15].
Despite these encouraging findings, some limitations should be acknowledged. The six-week intervention period precludes conclusions about long-term sustainability. The unchanged fasting blood glucose levels (Table 2) highlight the need for integrated dietary interventions alongside exercise to achieve comprehensive metabolic control, as exercise-induced insulin sensitivity may be transient [34]. Additionally, the exclusive inclusion of middle-aged women restricts generalizability across sexes and broader age groups. Future research should examine longer interventions, combine hybrid training with dietary strategies, and incorporate advanced imaging (e.g., FibroScan, elastography) for more sensitive hepatic assessment. The integration of wearable technology to support adherence and individualized prescription, as well as investigation of patient-reported outcomes such as quality of life, will be critical for translation into clinical practice.
Conclusions
Hybrid exercise training safely and effectively improves hepatic steatosis, liver enzyme profiles, systemic inflammation, and body composition in sedentary obese women with NAFLD. These results support its use as a complementary strategy within multidisciplinary NAFLD management programs. The observed associations between IL-6 reductions and liver enzyme improvements suggest a potential mechanistic link, though these findings are preliminary and require further investigation to establish causality.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary Material 3: Supplementary table S1: Initial 3-week metabolic training plan. Supplementary table S2: Subsequent 3-week strength training plan.
Acknowledgements
The authors would like to express their gratitude for the invaluable contributions made by the Dr. Abedzadeh Ultrasonography Center and the research team’s organization, which provided essential resources, including equipment and personnel.
Abbreviations
- NAFLD
Non-alcoholic fatty liver disease
- EMS
Electrical muscle stimulation
- BMI
Body mass index
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- IL-6
Interleukin-6
- FBS
Fasting blood sugar
- WHR
Waist-to-hip ratio
- RCT
Randomized controlled trial
- SPSS
Statistical package for the social sciences
Author contributions
• Niloofar Zareie Mohammadzadeh: Conceptualization, Data Collection, Data Visualization, Writing – Original Draft. • Mohammadreza Rezaeipour: Methodology, Supervision, Formal Analysis, Writing – Review & Editing, Project Administration.
Funding
No funding was obtained for this study.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request, subject to ethical approval and participant confidentiality. Data containing sensitive or identifying information will be shared in accordance with institutional and national ethical guidelines to ensure participant privacy is fully protected.
Declarations
Ethics approval and consent to participate
The study was approved by the Research Ethics Committee of the University of Sistan and Baluchestan (Approval Code: IR.USB.REC.1398.012). Written informed consent was obtained from all participants prior to their enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American association for the study of liver diseases. Hepatology. 2018;67(1):328–57. 10.1002/hep.29367 [DOI] [PubMed] [Google Scholar]
- 2.Veloz MG, Agarwal K, Chamley M, Ajaz S. Lack of awareness of a NAFLD pandemic in a high risk group. Is it time to act in primary care? J Hepatol. 2022;77:S171. 10.1016/S0168-8278(22)00721-8 [Google Scholar]
- 3.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell. 2021;184(10):2537–64. 10.1016/j.cell.2021.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 5.Chartampilas E. Imaging of nonalcoholic fatty liver disease and its clinical utility. Horm (Athens). 2018;17(1):69–81. 10.1007/s42000-018-0012-x [DOI] [PubMed] [Google Scholar]
- 6.Ibrahim AA, Abdelbasset WK. The role of physical exercise in treating people with non-alcoholic fatty liver disease. J Adv Pharm Educ Res. 2020;10(2):64–70. [Google Scholar]
- 7.Kawaguchi T, Shiba N, Maeda T, Matsugaki T, Takano Y, Itou M, et al. Hybrid training of voluntary and electrical muscle contractions reduces steatosis, insulin resistance, and IL-6 levels in patients with NAFLD: a pilot study. J Gastroenterol. 2011;46:746–57. 10.1007/s00535-011-0378-x [DOI] [PubMed] [Google Scholar]
- 8.Frith J, Day CP, Robinson L, Elliott C, Jones DE, Newton JL. Potential strategies to improve uptake of exercise interventions in non-alcoholic fatty liver disease. J Hepatol. 2010;52(1):112–6. 10.1016/j.jhep.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 9.Cerqueira MS, Rolnick N, Vieira WHB. Letter to the editor concerning the article: the effectiveness of blood-flow restricted resistance training in the musculoskeletal rehabilitation of patients with lower limb disorders: A systematic review and meta-analysis. Clin Rehabil. 2021;35(10):1500–2. 10.1177/02692155211011929 [DOI] [PubMed] [Google Scholar]
- 10.Yanagi T, Shiba N, Maeda T, Iwasa K, Umezu Y, Tagawa Y, Matsuo S, Nagata K, Yamamoto T, Basford JR. Agonist contractions against electrically stimulated antagonists. Arch Phys Med Rehabil. 2003;84(6):843–8. 10.1016/s0003-9993(02)04948-1 [DOI] [PubMed] [Google Scholar]
- 11.Matsuse H, Tajima H, Baba E, Iwanaga S, Omoto M, Hashida R, Nago T, Shiba N. Hybrid training system consisting of synchronized neuromuscular electrical stimulation for voluntary exercise using an articular motion sensor. Kurume Med J. 2024;70(34):83–9. 10.2739/kurumemedj.MS7034006 [DOI] [PubMed] [Google Scholar]
- 12.Fisher G, Windham ST, Griffin P, Warren JL, Gower BA, Hunter GR. Associations of human skeletal muscle fiber type and insulin sensitivity, blood lipids, and vascular hemodynamics in a cohort of premenopausal women. Eur J Appl Physiol. 2017;117:1413–22. 10.1007/s00421-017-3634-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gondin J, Guette M, Ballay Y, Martin A. Electromyostimulation training effects on neural drive and muscle architecture. Med Sci Sports Exerc. 2005;37(8):1291–9. 10.1249/01.mss.0000175090.49048.41 [DOI] [PubMed] [Google Scholar]
- 14.Sanchez MJ, Chen X, Smith JD, Lee HJ, Kim Y, Park S, et al. Effects of neuromuscular electrical stimulation on glycemic control: a systematic review and meta-analysis. Front Endocrinol. 2023;14:1222532. 10.3389/fendo.2023.1222532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paillard T. Combined application of neuromuscular electrical stimulation and voluntary muscular contractions. Sports Med. 2008;38:161–77. 10.2165/00007256-200838020-00005 [DOI] [PubMed] [Google Scholar]
- 16.Herrick RE, Borghi-Silva A, Collins JL, McConnell AK, Maffiuletti NA, Malhotra A, et al. Neuromuscular electrical stimulation as a potential countermeasure for skeletal muscle atrophy and weakness during human spaceflight. Front Physiol. 2015;6:356. 10.3389/fphys.2015.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren Y, Jia Y, Yang M, Yao M, Wang Y, Mei F, et al. Sample size calculations for randomized controlled trials with repeatedly measured continuous variables as primary outcomes need improvements: a cross-sectional study. J Clin Epidemiol. 2024;166:111235. 10.1016/j.jclinepi.2023.111235 [DOI] [PubMed] [Google Scholar]
- 18.Van DTI, Askie L, Vandermeer B, Ellenberg S, Fernandes RM, Saloojee H, et al. Standard 4: determining adequate sample sizes. Pediatrics. 2012;130(Suppl 3):138–45. 10.1542/peds.2012-0055G [DOI] [PubMed] [Google Scholar]
- 19.Das K. Non-alcoholic fatty liver disease: a clinical update. J Clin Transl Hepatol. 2016;4(2):130–7. 10.14218/JCTH.2016.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasudevan S, et al. Confounding factors in metabolic syndrome studies: a review of exclusion criteria. Metab Syndr Relat Disord. 2024;22(1):12–20. 10.1089/met.2023.0123 [Google Scholar]
- 21.Mehri F, Rahbar A, Talebi Ghane E, Panahi A, Esfahani M. Investigating hyponatremia status and Interleukin 6 concentration and their possible relationship in COVID-19 patients compared to healthy people. Avicenna J Med Biochem. 2023;11(2):123–8. 10.34172/ajmb.2445 [Google Scholar]
- 22.Ferraioli G, Soares Monteiro L. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol. 2019;25(40):6053–62. 10.3748/wjg.v25.i40.6053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rezaeipour M, Nychyporuk VI. The effect of waterinmotion (WiM) aquatic exercise on weight loss and metabolic profiles in sedentary obese elderly men. Hormozgan Med J. 2021;25(2):65–70. 10.34172/hmj.2021.05 [Google Scholar]
- 24.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 25.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. 10.3389/fpsyg.2013.00863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson AG, Kokkonen J, Arnall DA. Twenty minutes of passive stretching lowers glucose levels in an at-risk population: an experimental study. J Physiother. 2011;57(3):173–8. 10.1016/S1836-9553(11)70038-8 [DOI] [PubMed] [Google Scholar]
- 27.Pedersen BK. Muscles and their myokines. J Exp Biol. 2011;214(2):337–46. 10.1242/jeb.048074 [DOI] [PubMed] [Google Scholar]
- 28.Gurudut P, Rajan AP. Immediate effect of passive static stretching versus resistance exercises on postprandial blood sugar levels in type 2 diabetes mellitus: a randomized clinical trial. J Exerc Rehabil. 2017;13(5):581–7. 10.12965/jer.1735032.516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh S, Maruyama T, Eguchi K, Shida T, Arai E, Isobe T, et al. Therapeutic effect of hybrid training of voluntary and electrical muscle contractions in middle-aged obese women with nonalcoholic fatty liver disease: a pilot trial. Ther Clin Risk Manag. 2015;11:371–80. 10.2147/TCRM.S75109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crouter S, Albright C, Bassett DR Jr. Accuracy of Polar S410 heart rate monitor to estimate energy cost of exercise. Med Sci Sports Exerc. 2004;36(8):1433–9. 10.1249/01.MSS.0000135794.01507.48 [DOI] [PubMed] [Google Scholar]
- 31.n der Scheer JW, Goosey-Tolfrey VL, Valentino SE, Davis GM, Ho CH. Functional electrical stimulation cycling exercise after spinal cord injury: a systematic review of health and fitness-related outcomes. J Neuroeng Rehabil. 2021;18(1):99. 10.1186/s12984-021-00882-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watanabe K, Yoshida T, Ishikawa T, Kawade S, Moritani T. Effect of the combination of whole-body neuromuscular electrical stimulation and voluntary exercise on metabolic responses in human. Front Physiol. 2019;10:291. 10.3389/fphys.2019.00291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemmler W, Teschler M, Bebenek M, von Stengel S. (Very) high creatine kinase concentration after exertional whole-body electromyostimulation application: health risks and longitudinal adaptations. Wien Med Wochenschr. 2015;165:427–35. 10.1007/s10354-015-0394-1 [DOI] [PubMed] [Google Scholar]
- 34.Kemmler W, Von Stengel S, Schwarz J, Mayhew JL. Effect of whole-body electromyostimulation on energy expenditure during exercise. J Strength Cond Res. 2012;26(1):240–5. 10.1519/JSC.0b013e31821a3a11 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 3: Supplementary table S1: Initial 3-week metabolic training plan. Supplementary table S2: Subsequent 3-week strength training plan.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request, subject to ethical approval and participant confidentiality. Data containing sensitive or identifying information will be shared in accordance with institutional and national ethical guidelines to ensure participant privacy is fully protected.