Abstract
The cochlear implant (CI) is considered one of the most successful neural prostheses, enabling deaf individuals to achieve intelligible speech perception. However, CI performance remains limited in noise and with complex acoustic scenes, including music and multi-talker speech. One major issue for CIs is the poor electrode-neural interface where electrodes are positioned within the bony cochlea and distant from the auditory nerve fibers. Due to recent advances with microelectrode technologies designed for peripheral nerves, there has been rekindled interest in the auditory nerve implant (ANI), in which a novel prosthesis with a microelectrode array has been developed for direct stimulation of the auditory nerve. Animal studies demonstrate that the ANI achieves substantially lower thresholds and more selective neural activation compared to CI stimulation, which could lead to greater hearing performance. To successfully translate the ANI to patients, the ANI device components need to be further designed for safe and reliable implantation in humans through development of alternative surgical techniques, and validated in chronic animal studies. New stimulation strategies also need to be developed, especially with the potential to insert tens to hundreds of microelectrodes across the spiraling tonotopy of the auditory nerve to activate more spatially and temporally distinct nerve fiber patterns than is possible with the CI. Once in humans, extensive perceptual experiments can be performed with the ANI to characterize thresholds, loudness growth functions, pitch patterns, temporal coding properties, and spectral selectivity, as well as evaluating novel stimulation strategies that will guide the development of the next generation ANI system.
Keywords: Neural prosthesis, Microelectrode array, Electrode-neural interface, Auditory nerve, Cochlear implant, Auditory brainstem implant, Neuromodulation, Hearing loss
Introduction
Hearing loss is the most prevalent sensory disorder, affecting over 466 million individuals, which is more than 5% of the world population according to the World Health Organization. The cochlear implant (CI) has become the standard treatment for severe or profound hearing loss since the U.S. Food and Drug Administration (FDA) approval in the mid-1980s, with approximately one million recipients worldwide and about 65,000 new implantations each year (Zeng 2022). A CI bypasses the non-functioning hair cells in the cochlea and directly stimulates the spiral ganglion neurons (SGNs) with electrical pulses that correspond to the acoustic signal recorded via a microphone. Over the past two-plus decades, advancements in technology, particularly in microelectronics and signal processing informed by significant scientific research in hearing and speech perception, have driven the development of highly effective CIs, particularly for speech in quiet environments (Carlyon and Goehring 2021; Lenarz et al. 2022). Modern CIs integrate multi-channel electrode arrays with advanced audio processing strategies (Clark et al 1987; Wilson et al. 1991; Boyle et al. 2009), enabling the average user to achieve 70–80% accuracy in sentence recognition in quiet environments without lip-reading. Many users can even converse over the phone in quiet settings (Wilson 2000; Skinner et al. 1994). Additionally, CIs offer opportunities for children to acquire language skills (Lenarz et al. 2022; Niparko et al. 2010; Sharma et al. 2002; Holt and Kirk 2005; Harrison et al. 2005), prompting FDA approval for implantation in children as young as 9 months. The standard of care in many countries recommends implanting deaf children from the age of 6 months, some even recommend bilateral implantation (e.g., German Guideline on Cochlear Implantation) (Cochlear implant care 2020). Access to binaural cues has been shown to provide significant benefits, as demonstrated by improved outcomes in individuals with either bilateral implants or bimodal hearing, where residual acoustic hearing is combined with a CI (Tyler et al. 2002; Müller et al. 2002; Litovsky et al. 2006; Blamey et al. 2015; Olson and Shinn 2008; Graaff et al. 2021; Yang and Zeng 2017; Gifford 2020; Hoesel and Tyler 2003).
Limitations of the CI
Despite the remarkable successes of CIs, users still face major limitations such as a restricted dynamic range (the perceived range of loudness with electrical stimulation) (Zeng et al. 2002; Moore 2003), poor perception of pitch and timbre (Oxenham 2008; Moore et al 2005; Gfeller et al. 2002), and dissatisfaction with the unnatural quality of auditory percepts. They also experience significant difficulties recognizing speech in background noise (Müller-Deile et al. 1995; Nelson et al. 2003; Stickney et al. 2004), perceiving tonal languages (Fu et al. 2004; Wei et al. 2004), and enjoying music (Limb and Roy 2014; Dorman et al. 1991; McDermott 2004).
One challenge for CIs is the variability in electrode insertion depth caused by differences in the cochlear duct length (25—45 mm) (Alexiades et al. 2015) among individuals. Such variability prevents some patients from accessing frequency percepts below roughly 600 Hz despite the proven benefits of low-frequency information for word understanding, speech in noise, and melody recognition (Luo and Fu 2006; Gantz et al. 2005). Although attempts have been made to make longer arrays, there remains challenges of safely inserting the electrode array into the decreasing space towards the apex of the cochlea.
A second key bottleneck in CI performance results from the electrode-neuron interface (Zeng 2017). The CI array is immersed in conductive perilymph (cochlear fluids), leading to broad current spread and shunting. Additionally, these electrodes are separated from the targeted SGNs by the poorly conductive bony cochlear wall, the modiolus. As a result, higher current levels are required to activate the SGNs, and overlapping electric fields generated by different electrodes (Kral et al. 1998) reduce spectral resolution, hindering speech perception in noise and music appreciation (Nelson et al. 2003; Fu et al. 1998; Qin and Oxenham 2003; Nelson and Jin 2004). Although innovations like bipolar, partial tripolar, or dynamic current focusing configurations aim to reduce the spread of neural activation (Berenstein et al. 2008; Bierer and Litvak 2016; Zhu et al. 2012; Jong et al. 2019; Donaldson et al. 2011), current CI designs with 12–24 channels still provide only 4–8 effective channels of information (Wilson and Dorman 2008). Research involving normal-hearing listeners and vocoder simulations of a CI suggests that at least ten effective channels are necessary for optimal performance in challenging listening conditions (Fu and Nogaki 2005; Dorman et al. 1998; Shannon et al. 2004), while over 32 channels are required to convey sufficient information for salient pitch perception (Mehta et al. 2020; Mehta and Oxenham 2017). Limited by the functional spectral resolution, audio-processing strategies of the contemporary CI primarily utilize the slow temporal envelope of acoustic signals extracted in different frequency bands to modulate the amplitude or the pulse duration of the electrical pulses. This approach inherently limits the transmission of temporal fine structure in acoustic signals (Loizou 2006; Zeng et al. 2005). Although efforts have been made to convey this information by varying the pulse rates within each channel, evidence remains insufficient to demonstrate that CI users can perceive or effectively utilize such cues (Riss et al. 2016; Nogueira et al. 2009).
CI stimulation also presents unique challenges from a neural coding perspective. Electrical currents directly depolarize auditory nerve fibers introducing abnormal synchronization across nerve populations (Raggio et al 1999; Schreiner and Raggio 1996). The lack of stochastic variability in response timing combined with broad activation (Snyder et al. 2000; Snyder et al. 2004; Bierer and Middlebrooks 2002) can interfere with information transmission to higher auditory centers (Moore and Shannon 2009; Middlebrooks et al. 2005). CI stimulation has been shown to be less effective at activating neurons in the primary auditory cortex that are highly selective to fine spectral structure (Johnson et al. 2016). These effects likely contribute to altered neural computations underlying perception and cognition. Notably, even CI users with good performance in speech tasks may rely on alternative acoustic cues, such as spectral tilts, when spectral peaks (formants) are compromised (Winn and Litovsky 2015; Winn et al. 2012). Studies have also shown increased cognitive demands for CI users (Winn and Teece 2021; O’Neill et al. 2021; Hughes et al. 2018) and abnormal context effects (Stilp 2017; Feng et al 2018; Aravamudhan et al 2005), a process in which the auditory system adapts to the acoustic variability from different environments or speaker characteristics to maintain perceptual constancy (Barlow et al 1961; Dean et al. 2005; Ladefoged and Broadbent 1957; Watkins 1991).
Advancements in alternative hearing prostheses
Neurotrophic agents have been used to promote closer proximity between electrodes and auditory neurons. Previous studies show that such delivery results in rescue or enhanced survival of SGNs over extended cochlear regions, though additional study is needed to achieve targeted, sustained, and safe delivery (Pinyon et al 2014; Leake et al. 2020; Budenz et al. 2012). Alternative prostheses, such as the auditory midbrain implant (AMI) or auditory brainstem implant (ABI), are being developed for patients without functional auditory nerves (Lim and Lenarz 2015; Wong et al. 2019). However, stimulating the central auditory system is challenging. Unlike auditory nerve fibers that map frequencies tonotopically, phase lock to temporal modulations up to 4—6 kHz, and show monotonic responses to increasing loudness (Sumner and Palmer 2012; Evans 1972; Johnson 1980; Taberner and Liberman 2005; Møller 1983; Sachs and Abbas 1974), central neurons encode acoustic features intrinsically. They exhibit sophisticated and diverse frequency receptive field and rate level functions (Syka et al. 2000; Palmer et al. 2013) and transition from phase locking to rate coding above 1000 Hz (Liu et al. 2006; Bartlett and Wang 2007; Lu et al. 2001; Wallace et al. 2007) complicating stimulation strategies.
Researchers are also investigating light-based approaches to achieve more precise spatial activation of auditory nerves. Techniques include infrared lasers (IR), which are hypothesized to alter membrane capacitance (Littlefield and Richter 2021), although the exact mechanism is still not well understood, and optogenetic methods that use light-sensitive proteins to control ion channels (Jeschke and Moser 2015; Keppeler et al 2020). Optogenetic stimulation, in particular, outperforms electric stimulation at medium and high intensities and achieves spectral selectivity comparable to acoustic stimulation at moderate intensities (Dieter et al. 2019). However, these approaches are still in the early stages of development. Optogenetic stimulation requires gene transfer into target cells, raising concerns about unknown long-term effects and sustainability, and the light source is still distant to the target SGNs. IR stimulation may be diffracted by the bony modiolar wall resulting in less focused auditory nerve activation. Substantial technological advancements, as well as rigorous safety and efficacy evaluations, are essential before these methods can transition to clinical applications.
This concept paper focuses on the development of an auditory nerve implant (ANI) hearing prosthesis, which involves the placement of a penetrating microelectrode array directly into the auditory nerve to achieve spatially focused stimulation, thereby allowing the transmission of spectrally and temporally specific information in acoustic sounds. For an initial clinical trial for demonstrating proof-of-concept, the ANI will be intended as an alternative to the ABI candidates who still have a functional auditory nerve but cannot sufficiently benefit from a CI. For example, the cochlea may be ossified, scarred, or otherwise occluded (e.g., post-meningitic, fracture, inflammatory) and a CI array cannot be properly inserted. Another example is that CI use is limited by facial nerve or vestibular side effects and/or insufficient hearing performance. Through success in this initial patient cohort and advancements in surgical approaches to the auditory nerve, future ANI studies will broaden the inclusion criteria as appropriate. In this concept paper, we evaluate the feasibility of an ANI from historical context, electrode fabrication, and surgical perspectives, and we comment on key challenges in safety and stimulation efficacy. A primary goal of our translational effort is to implant an ANI device in an initial cohort of deaf patients with a planned 12-month follow-up to assess device safety and stability and to characterize the percepts generated from direct auditory nerve stimulation. Continuation, removal, or re-implantation after 12 months will be determined case-by-case based on risk–benefit considerations, clinical judgment, participant preference, and ongoing monitoring. These first-in-human findings can then guide the development of an optimal ANI design with proper surgical implantation techniques and novel stimulation strategies for evaluation in a multi-site efficacy clinical trial to open the doors for a new type of hearing prosthesis beyond the CI.
ANI concept and design
Prior ANI studies
Direct auditory nerve stimulation was initially explored alongside the development of CIs (House 1976; House and Urban 1973). In 1963, Zoellner and Keidel inserted a 350 µm diameter electrode wire into the cochlea eliciting auditory sensations, although the claimed placement within the modiolus was unconfirmed (Zoellner et al 1963). In 1965, Simmons et. al. implanted a bundle of five 75 µm diameter electrodes with exposed tips linearly spaced 1 mm apart into the auditory nerve through the modiolar wall (Simmons et al. 1965), achieving stable function for over five years with histological evidence of healthy neurons (Simmons et al. 1979). Pitch ranking was possible but highly dependent on the type of stimuli (Simmons et al. 1981).
Despite initial promise, ANIs received far less attention than CIs due to surgical complexity and technological limitations, particularly in the lack of proper microelectrode array technologies suitable for long-term implantation and sufficient spatial coverage of the auditory nerve at that time. The cochlea’s bony structure, though suboptimal for electrode-neuron interfacing, provides a stable and protective cavity for electrodes that reduces movement and minimizes the risk of trauma to the eighth nerve. Its tonotopic organization (Greenwood 1961) with high frequencies mapping to the base and low frequencies mapping to the apex of the cochlea apparently simplifies electrode design. In contrast, the auditory nerve’s location in the narrow internal auditory canal (IAC) near the vestibular and facial nerves may complicate surgical access and long-term electrode placement. Furthermore, the bundled auditory nerve fibers that possibly twist and spiral en route to the brainstem require a three-dimensional array of closely spaced electrodes to sufficiently span the cross-sectional array of the nerve to cover the full range of frequencies encoded by those fibers. It is important to note that even for CIs, tonotopic stimulation is challenging due to the need for current to transmit through the bony modiolar wall to access distant and remaining components of SGNs that are also bundled up and traverse towards the IAC.
Interest in ANIs was renewed with advancements in higher-count electrode array designs and thin-film multichannel electrode technologies. In 1986, Naumann et al. implanted a 12-channel wire-bundle into the auditory nerve via the middle ear and oval window, targeting the nerve as it exits the modiolus into IAC (Naumann et al. 1986). Each electrode wire was arranged to diverge within the auditory nerve for targeted distribution. Although reproducible auditory percepts were achieved, the study was discontinued after 66 days due to possible moisture damage to the receiver. Later, Zappia et al. (1990)demonstrated low thresholds averaging 10 µA for charge-balanced biphasic pulses (200 µs per phase, 400 µs total) using silicon-based microelectrodes in guinea pigs, which were significantly lower than average CI thresholds (Chatterjee 1999). However, they also observed localized SGN loss and vascular injury with chronic implantation.
In 2003, a Utah Electrode Array (UEA; three-dimensional 12-channel penetrating silicon-substrate electrode array) was implanted into the auditory nerve of cats for up to 52 h under anesthesia (Badi et al. 2002; Badi et al. 2003; Hillman et al. 2003). Electrically-evoked auditory brainstem responses (eABRs) confirmed successful activation of the auditory nerve with a median threshold of 15 µA and no obvious activation of vestibular or facial nerves (Badi et al. 2007; Kim et al. 2007). Postoperative imaging showed that the UEAs remained intact and in position with an uncompromised vascular supply. However, long-term stability and biocompatibility were not assessed. In 2007, Middlebrooks and colleagues demonstrated neural responses in the central auditory system using a multisite silicon-substrate array (NeuroNexus, Ann Arbor, Michigan) implanted into the modiolar trunk of the auditory nerve in cats (Middlebrooks and Snyder 2007; Middlebrooks and Snyder 2008; Middlebrooks and Snyder 2010). Their findings highlighted substantially lower thresholds, greater dynamic ranges, access to lower frequencies not typically possible with a CI, and significantly reduced spread of excitation compared to a CI. However, the array’s fragility and challenges in encapsulation and explanation limited its potential for long-term use and clinical translation.
Re-evaluating the feasibility of the ANI
Advances in microelectrode array design, manufacturing, and longevity have addressed many obstacles that previously hindered ANIs. In particular, the novel Utah Slanted Electrode Array (USEA) features a three-dimensional architecture designed to interface with the cross-section of peripheral and cranial nerves (Davis et al. 2016; Wendelken et al. 2017; George et al. 2020a; George et al 2019; Page et al. 2018), making it well-suited for an ANI. The introduction of low impedance arrays through iridium oxide tip metallization processes was a critical breakthrough, allowing effective stimulation within the voltage compliance limits of CIs. Additionally, the development of a helical lead designed for peripheral nerve applications (George et al. 2020a) has successfully passed the stringent lead fatigue tests required by the CI86 standard (Instrumentation A for the A of M, others 2017).
The NeuroPort™ Electrode array (Blackrock Neurotech, Salt Lake City, Utah) is a penetrating microelectrode array that offers up to 100 silicon microneedles arranged with a 400 µm spacing. Each electrode is electrically isolated by glass and is equipped with patterned metal bond pads that connect to a helical wire bundle. The needle tips are metalized with a layer of sputtered iridium oxide film (SIROF) (Negi et al. 2010), providing a highly conductive and biocompatible interface for neural stimulation. The microelectrode array is encapsulated with a Parylene-C layer for insulation and protection (Caldwell et al. 2018; Caldwell et al. 2020; Hsu et al. 2008). Following encapsulation, the Parylene-C layer is selectively etched from the needle tips, exposing the SIROF coating at the active sites (Campbell et al. 1991; Leber et al 2019; Bhandari et al. 2010). This process reduces the impedance of the electrodes to typical values of 5 to 20 kΩ in saline, enabling neural recording and selective low-current neural stimulation (e.g., < 10 µA) (Campbell et al. 1991; Leber et al 2019; Aoyagi et al. 2003; Branner and Normann 2000; Branner et al. 2004; Normann et al 2005; Wark et al. 2013).
The NeuroPort Electrode array has been extensively studied and is cleared for human use in specific applications. In the United States, it is FDA cleared for acute, temporary (< 30 days) recording and monitoring of brain electrical activity. Additionally, the NeuroPort Electrode and its variants have been used in several successful investigational device exemption (IDE) studies in the U.S. and European Union (EU), some lasting over numerous years (Sponheim et al. 2021; Hughes et al. 2021). The array has demonstrated versatility in various applications including stimulation in the somatosensory cortex (Hughes et al. 2021; Flesher et al. 2016) with a more recent major breakthrough of stimulation in peripheral arm nerves (George et al. 2020a; Paskett et al. 2021; George et al. 2020b). For instance, Flesher et al. showed that somatosensory cortex microstimulation evoked tactile sensations (Hughes et al. 2021; Flesher et al. 2016), and Davis et al. and Wendelken et al. reported successful recording and microstimulation in human peripheral nerves to control prostheses (Davis et al. 2016; Wendelken et al. 2017; George et al 2019; Paskett et al. 2021; Page et al. 2021).
Complementing these technical advancements, established intracranial skull base surgical techniques and innovative tools have significantly improved the feasibility of the ANI (Jackler et al 2009; Schwam et al. 2023; Lucas et al. 2023; Kashani et al. 2023). Decades of experience with CIs (Naples and Ruckenstein 2020), along with extensive research on cochlear anatomy, otopathology, physiology, and surgical interventions, provide a robust foundation for optimizing ANI array placement while minimizing damage to surrounding tissues. This surgical precision is crucial for maintaining long-term functionality and patient safety. Collectively, these developments have laid the groundwork for future clinical applications with an ANI.
Proposed ANI system design
The proposed ANI system (Fig. 1) combines advanced CI technology components (i.e., the Sonnet 2 audio processer and the Synchrony 2 implantable stimulator by MED-EL, Innsbruck, Austria), with a novel ANI microelectrode array and cabling (Blackrock Neurotech, Salt Lake City, Utah) to interface directly with the auditory nerve. The ANI array is a modified USEA tailored to the anatomical and functional characteristics of the human auditory nerve. It is comprised of 15 electrodes arranged in a 3 × 5 grid with an electrode-to-electrode spacing of 400 µm (Fig. 2, feature B.1). Electrode length increases linearly from 0.5 mm to 0.9 mm across rows to maximize cross-sectional area coverage of the auditory nerve. A platinum foil handling fin embedded in medical-grade silicone facilitates safe handling and precise placement during surgery (Fig. 2, feature B.2).
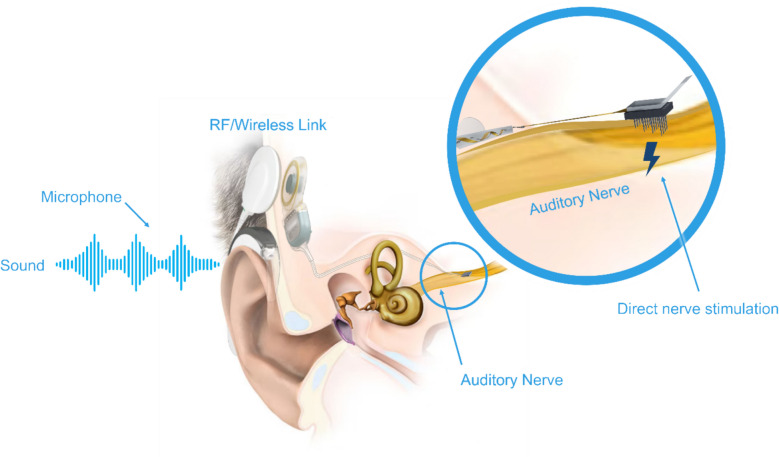
Fig. 1.
Concept of the auditory nerve implant (ANI) system consisting of an audio processor and cochlear implant simulator (MED-EL, Innsbruck, Austria) integrated with the ANI array (Blackrock Neurotech, Salt Lake City, Utah), which is placed directly into the auditory nerve medial to its exit from the cochlea. Image courtesy of Blackrock Neurotech and MED-EL. This schematic portrays the concept of the ANI system in which individual components are not drawn to scale or do not represent the final design that will be implanted in humans, and the placement along the nerve is expected to be closer to the cochlea within the IAC
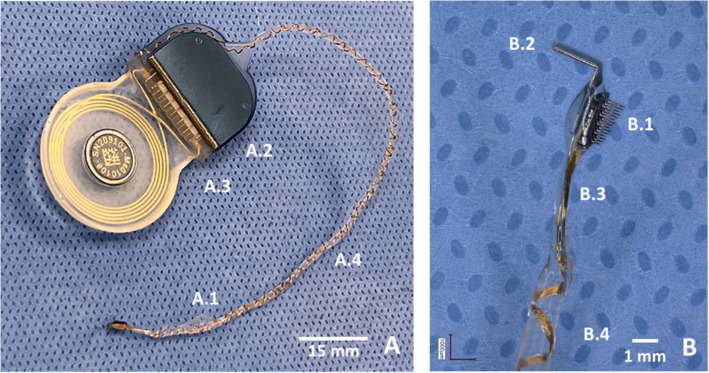
Fig. 2.
The components of the ANI system. (A) Backside of the integrated device, showing the silicone wing (A.1) for surgical handling, a MED-EL Synchrony 2 cochlear stimulator (A.2 and A.3), and the helical lead (A.4) for enhanced mechanical stability. (B) Close-up of the ANI array, highlighting its 3 × 5 microelectrode configuration (B.1), platinum handling fin for surgical manipulation (B.2), bendable plastic section of the wire bundle (B.3), and the beginning of the helical lead (B.4)
The wire bundle connecting the electrode array to the stimulator is constructed from the same gold alloy wire used in the NeuroPort Electrode and is overmolded in medical-grade silicone. Most of the wire bundle is coiled into a helix (Fig. 2, feature A.4 and B.4). This helical design improves the flexibility and minimizes the risk of wire breakage from bending fatigue or surgical handling. These improvements have been demonstrated previously through innovative development and testing in median and ulnar nerve stimulation in arm amputees for control of a robotic arm (George et al 2019; George et al. 2020b; Duncan et al. 2019). A short, uncoiled segment of the lead extends from the edge of the electrode array before the start of the helical coil. This unique portion creates a bendable, plastic section that retains its orientation and shape, allowing for precise positioning of the array on the auditory nerve during surgery (Fig. 2, feature B.3). To further assist surgeons during placement, a silicone handling wing is molded into the helical coil just after the beginning of the helix to enhance torsional control (Fig. 2, feature A.1). Both the silicone handling wing and the platinum handling fin are innovations that were created based on numerous cadaver studies performed by the co-authors of this paper that greatly facilitate the surgical positioning and implantation of the electrode array into the auditory nerve. Finally, the helical lead is precision molded allowing for press fitting of the lead into the mastoid bone groove.
The initial ANI device (Fig. 2) has been developed with available technologies and materials that have already been extensively used safely in humans (i.e., Blackrock NeuroPort Electrode and MED-EL Synchrony 2 components). The rationale for using available technologies is to enable safe and expedient translation to patients so that critical aspects of device design and function can be examined. The MED-EL Synchrony 2 stimulator has 12 stimulation channels and electrodes, therefore, a 3 × 5 electrode array was used in this first device design (with three inactive electrodes). This design will allow for examination and rigorous psychophysical testing of chronic microelectrode stimulation of the auditory nerve in awake and responding patients. Additionally, this design will hopefully allow for open-set speech recognition for these patients. Future ANI systems will have increased channel counts as well as more effective stimulation strategies specific to microstimulation of the auditory nerve based on results from this first-in-human study.
Surgical considerations for ANI implantation
Our team has carefully evaluated multiple surgical approaches for placing the ANI array in the auditory nerve. We evaluated standard skull base approaches to the IAC and vestibulocochlear nerve bundle, including translabyrinthine, retrosigmoid, retrolabyrinthine, and middle fossa approaches, as well as endoscopic-assisted approaches. This research progressed to fabrication of prototype electrode arrays and surgical placement of electrodes in more than 40 auditory nerves in human cadaver studies, which will be reported upon separately. Subsequently, intraoperative acute experiments were performed with human subjects under Ethics Committee approval (Clinical Trial NCT06306534) in Hannover, Germany. Patients requiring vestibular schwannoma resection with auditory nerve preservation gave informed consent to have an ANI array briefly implanted in the auditory nerve. Brief pulse trains of electrical stimulation were presented on a subset of the implanted electrodes, and the resulting eABRs were recorded. These results will also be reported in a future publication.
As the auditory nerve exits the cochlea, it converges with the vestibular nerve in the IAC to form a nerve bundle (cranial nerve VIII) that is immediately next to the facial nerve (cranial nerve VII). Thus, insertion in the lateralmost part of the auditory nerve, proximal to the cochlea, is more likely to result in auditory-specific stimulation compared to a medial IAC or cerebellopontine angle insertion. The translabyrinthine approach provides access to the auditory nerve bundle within the lateral IAC while also affording a natural bony support for the nerve during electrode insertion. The precision-molded helical lead can be press fit and securely anchored in precisely sized and oriented grooves in the mastoid bone, while packing materials can be placed around the inserted array to minimize the effects of cerebrospinal fluid (CSF) pulsations in the area for increased device stability. This approach allows for accommodation of anatomical variations across patients and facilitates optimal placement of the electrode array for the initial human pilot study. Based on our observations, the translabyrinthine approach will be used for the first-in-human scientific study with the ANI in deaf patients. Once the auditory nerve is exposed, the implantable stimulator is placed in a standard skull bed (same as with a CI), and the lead is fixed in the mastoid grooves. Then, the electrode array is positioned above and inserted into auditory nerve. After insertion of the electrode array, packing materials are added to the cavity, and the final aspects of the surgery are completed.
Future directions
The successful clinical translation of an ANI will require a multidisciplinary collaboration among engineers, clinicians, physiologists, and experts in speech and hearing to evaluate the device’s safety and efficacy. Future research and development should prioritize the following key areas:
Ensuring long-term safety and durability of the electrode array
A comprehensive pre-clinical testing plan will evaluate electrode impedance, structural integrity, and material degradation over an extended equivalent lifetime. Long-term stimulation testing under conditions exceeding typical clinical requirements, such as various pulse waveforms, higher currents, and higher pulse rates, is essential to ensure device durability. The Parylene-C encapsulation layer, a vital biocompatible component, must demonstrate minimal degradation with over 90% of electrode sites maintaining stable impedances. Additionally, monitoring for particulates or signs of corrosion is necessary to confirm that materials, including iridium and tip metallization materials, are not released during stimulation.
The lead is designed to achieve an optimal balance between flexibility and durability, facilitating surgical handling, reducing tethering forces, and ensuring secure anchoring. Mechanical and electrical reliability will be validated through rigorous benchtop testing. This includes bend and elongation assessments for mechanical resilience, continuity testing for electrical integrity, and insulation evaluations to confirm dielectric robustness based on the ANSI/AAMI CI86 regulatory guidelines (Instrumentation A for the A of M, others 2017). Additionally, biocompatibility testing, chemical characterization, sterilization validation, and electromagnetic compatibility testing according to ISO 14708–7 will be a part of the final verification and validation of the ANI.
Another critical consideration for long-term safety is the biological response to chronic implantation, particularly the risk of tissue encapsulation and neural loss due to micromotion of rigid electrodes (Woeppel et al. 2021; Polikov et al. 2005; Salatino et al. 2017; Christensen et al. 2014; Biran et al. 2007; Biran et al. 2005). This tissue response has been well-documented in long-term cortical (Szymanski et al 2021) and peripheral nerve (Christensen et al. 2014) clinical investigations with the UEA, where micro-movements between the array and neural tissue can provoke glial scarring, fibrotic encapsulation, cause electrode migration, and potentially isolate the device from nearby neurons (Biran et al. 2007; Kim et al. 2004). However, unlike peripheral nerve implants, where substantial displacement may occur in the surrounding muscle activity that might generate mechanical loadings on the device, the ANI is implanted within the IAC, a constrained bony structure that offers mechanical isolation. Additionally, the simulator and lead are anchored to the skull, substantially limiting large movement of the array relative to the nerve, while still having enough flexibility in the lead to allow the array to accommodate slight movements of the nerve, such as during vessel pulsations. This anatomical context, more akin to that of cortical applications, is expected to minimize micromotion-induced injury. While some degree of encapsulation is anticipated, long-term studies of cortical UEA implants have shown that stimulation and recording can be maintained for several years despite the presence of a tissue response or damage to the electrode arrays (Sponheim et al. 2021; Hughes et al. 2021; Woeppel et al. 2021; Bjånes et al. 2025; Patrick-Krueger et al. 2024; Greenspon et al. 2024). These observations provide encouraging evidence for the chronic viability of rigid electrode arrays in anatomically protected environments such as the IAC.
Refining surgical techniques and pre-clinical testing
Continuing to refine minimally invasive and precise surgical methods is critical for reducing complications and ensuring the long-term stability of the electrode array. Cadaver studies have already provided valuable insights into the anatomical dimensions and placement requirements for the ANI array and the lead. Further cadaver experiments are being pursued by the University of Minnesota and Hannover Medical School in Germany to identify and develop less invasive surgical approaches to the auditory nerve that can be more widely implementable by the otology field. Additionally, co-authors at the University of Utah have developed a feline model and have successfully implanted ANI arrays into auditory nerves via a transbullar approach (Thomas et al. 2024). These feline surgical methods and techniques closely simulate the translabyrinthine approach used in humans. Pre-clinical validation in several animal models at the University of Utah and University of Minnesota is an ongoing effort to demonstrate the safety and efficacy of ANI implantation and stimulation for human use.
Auditory nerve degeneration is a critical consideration for candidate selection and outcome prediction with ANI. Long-term sensorineural hearing loss (SNHL) is frequently accompanied by an associated loss of auditory nerve fibers (Nadol et al. 1989), resulting in reduced nerve diameter and neural density, and the extent of degeneration can vary considerably across individuals (Herman and Angeli 2011; Naguib et al. 2017; Reimann et al. 2024; Nadol and Xu 1992; Zimmermann et al. 1995). This variability can influence ANI efficacy and patient outcomes. Nevertheless, clinical experience with CIs suggest that many individuals retain sufficient residual fibers to support functional stimulation (Kamakura and Nadol 2016; Cheng and Svirsky 2021; Seyyedi et al. 2014). To mitigate the risk associated with degeneration, preoperative imaging, such as high-resolution T2-weighted MRI with multiplanar reconstruction, can aid in assessing nerve integrity and guiding candidacy decisions. When a sufficient quantity of auditory-nerve fibers remains, the USEA could, in principle, offer design flexibility (e.g., electrode length and density) to better match smaller nerves. For individuals with severe degeneration, an ABI may be more appropriate. Additionally, neuroprotective strategies such as neurotrophin delivery or chronic low-level electrical stimulation are under active investigation and may help preserve or partially restore neural populations and promote long-term survival of SGNs (Leake et al. 2020; Leake et al. 2013; Shepherd et al. 2005; Shepherd et al. 2008). These strategies could potentially be used alongside the ANI.
Optimizing stimulation strategies
Direct stimulation of the auditory nerve has been shown to provide lower thresholds and more selective neural activation, but maximizing these potential benefits requires experimental testing and validation. Studies in animals and humans measuring evoked compound action potentials (eCAPs) and eABRs can help characterize nerve activation, establish safe stimulation ranges, and lead to improved stimulation strategies that avoid activation of nearby structures like the facial nerve. Current spread and channel interaction may also be assessed. Recording neural responses in the inferior colliculus or auditory cortex to ANI stimulation in animal models can be a useful approach for quantifying thresholds, dynamic ranges, frequency activation patterns, and phase-locking properties, as well as comparing neural patterns with acoustic responses to guide parameter optimization such as pulse rate, pulse width, current levels, and multi-polar stimulation patterns. These various approaches can lead to more effective stimulation strategies for activating the central neurons and for providing higher fidelity transmission of hearing information to the brain.
ANI introduces potential challenges that can be distinct from CIs. Although the three-dimensional array designs of the ANI can access more spatially distinct fibers spanning a broader frequency range compared to the CI, the frequency-to-place mapping in the spiraling auditory nerves may not fully follow the monotonic gradient observed along the cochlea. Consequently, spatially adjacent sites can, especially at higher currents, produce frequency crossover, activating neurons representing disparate frequencies, which can distort intended spectral features and alter loudness growth. In addition, frequency maps can vary across patients depending on the array placement. Identifying the individual frequency map and employing appropriate, low-current stimulation to minimize unwanted frequency crossover are critical for stimulation strategies. Importantly, despite these complexities, direct nerve access with ANI retains a more peripheral, tonotopically grounded interface than auditory brainstem or midbrain implants, and thus may offer better hearing outcomes when it is appropriately mapped and fitted.
In addition, action potentials primarily initiate on the central axon of the SGNs with ANI stimulation, which results in shorter latency but increased neural synchrony (Kiang and Moxon 1972; Hartmann et al. 1984). Since spiking timing across neural population carries stimulus information (e.g. mean and standard deviation of first spike timing are correlated with sound loudness) (Huet et al. 2018; Heil and Irvine 1997; Rubinstein and Hong 2003), this timing change could alter neural coding and contribute to steep loudness growth and limited dynamic range under electrical stimulation (Hong and Rubinstein 2003; Zeng et al. 1998). The loss of spontaneous activity in deafferented nerves (Johnson and Kiang 1976) further limits the independence of spike trains across fibers, thereby reducing stochastic resonance (Rubinstein and Hong 2003), a nonlinear mechanism to improve signal detection. Strategies like adding Gaussian noise (Matsuoka et al. 2000) or using high pulse rates (Hong and Rubinstein 2003) show limited potential in CI research, but they may show promise with ANI stimulation. Moreover, delivering spectro-temporal fine structure information demands coordinated pulse trains across electrode sites that may be possible with ANI stimulation. Both physiological and modeling studies may provide insights that help overcome these challenges and guide sound coding and stimulation strategy development for the ANI.
Looking forward, while increasing intracochlear contact density has not yielded significant improvements in speech outcomes (Fishman et al. 1997), largely due to current spread, direct nerve access via ANI may allow more effective use of higher-density stimulation. In principle, this could better enable transmission of spectrotemporal fine structure in complex sounds (speech, music), potentially improving sound quality, aesthetic perception, and speech understanding in noise. The USEA platform has already been demonstrated to scale toward high-density versions (HD-USEA) with up to four times more channels, and further advances in microfabrication, electronics miniaturization, and packaging may enable even greater scaling (Wark et al. 2013). These innovations could expand the number and diversity of evoked percepts, but will also pose challenges for psychophysical fitting, coding strategies, and power management. Similar to CIs, the relationship between ANI channel count and perceptual benefit is unlikely to be linear, with potential plateaus (Fishman et al. 1997). Accordingly, future clinical and psychophysical studies will be needed to determine the optimal balance among electrode density, coding approaches, and user outcomes for ANI.
Finally, a scientific study involving chronic implantation of an ANI in human patients would provide a unique opportunity to characterize important perceptual effects and capabilities of different stimulation patterns, as well as hearing performance for varying stimulation strategies. These and future studies will help guide the development of a new generation of auditory prostheses that have the potential to push hearing performance beyond the traditional CI.
Acknowledgements
N/A
Abbreviations
- CI
Cochlear implant
- ANI
Auditory nerve implant
- eCAP
Evoked compound action potential
- eABR
Electrically-evoked auditory brainstem response
- SGN
Spiral ganglion neuron
- IAC
Internal auditory canal
- CSF
Cerebrospinal fluid
- UEA
Utah Electrode Array
- USEA
Utah Slanted Electrode Array
- IDE
Investigational device exemption
- SIROF
Sputtered iridium oxide film
Authors’ contributions
LF, the corresponding author, coordinated the manuscript structure and led drafting and revisions. HHL, TL, and FS provided the overall vision and conceptual framework for the project, and contributed to manuscript organization and revisions. KD, IS, LR, and MA contributed to sections of the initial draft. MA, TL, RS, and AS contributed to content related to surgical procedures. LR, ML, FS, JC, and RF contributed to sections and figures addressing electrodes, including manufacturing considerations. KH, SS, IH, and CB contributed to device design and provided engineering review of the processor. LAJ, GMG, MA, DJW, HH, and WMT contributed content related to large-animal models for evaluating device efficacy and safety. AJO and WN contributed to sections on cochlear implant research. All authors reviewed and approved the final manuscript.
Funding
NIH UG3 NS107688, NSF UtB DGE 1734815, German Hearing4All, DFG Cluster of Excellence EXC 2177/1, Minnesota Lions Hearing Foundation, The Hamilton and Mildred Haley Kellogg Charitable Trust, MED-EL, Blackrock Neurotech, Feinstein Institutes for Medical Research (Internal Funding), WVU (Startup Funding).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alexiades G, Dhanasingh A, Jolly C. Method to estimate the complete and two-turn cochlear duct length. Otol Neurotol. 2015;36(5):904–7. [DOI] [PubMed] [Google Scholar]
- Aoyagi Y, Stein RB, Branner A, Pearson KG, Normann RA. Capabilities of a penetrating microelectrode array for recording single units in dorsal root ganglia of the cat. J Neurosci Methods. 2003;128(1–2):9–20. [DOI] [PubMed] [Google Scholar]
- Aravamudhan R, Lotto AJ. Phonetic context effects in adult listeners with cochlear implants. J Acoust Soc Am. 2005;118(3_Supplement):1962–3. [Google Scholar]
- Badi AN, Hillman T, Shelton C, Normann RA. A technique for implantation of a 3-dimensional penetrating electrode array in the modiolar nerve of cats and humans. Arch Otolaryngol Head Neck Surg. 2002;128(9):1019–25. [DOI] [PubMed] [Google Scholar]
- Badi AN, Kertesz TR, Gurgel RK, Shelton C, Normann RA. Development of a novel eighth-nerve intraneural auditory neuroprosthesis. Laryngoscope. 2003;113(5):833–42. [DOI] [PubMed] [Google Scholar]
- Badi AN, Owa AO, Shelton C, Normann RA. Electrode independence in intraneural cochlear nerve stimulation. Otol Neurotol. 2007;28(1):16–24. [DOI] [PubMed] [Google Scholar]
- Barlow HB, others. Possible principles underlying the transformation of sensory messages. Sens Commun. 1961;1(01):217–33.
- Bartlett EL, Wang X. Neural representations of temporally modulated signals in the auditory thalamus of awake primates. J Neurophysiol. 2007;97(2):1005–17. [DOI] [PubMed] [Google Scholar]
- Berenstein CK, Mens LH, Mulder JJ, Vanpoucke FJ. Current steering and current focusing in cochlear implants: comparison of monopolar, tripolar, and virtual channel electrode configurations. Ear Hear. 2008;29(2):250–60. [DOI] [PubMed] [Google Scholar]
- Bhandari R, Negi S, Solzbacher F. Wafer-scale fabrication of penetrating neural microelectrode arrays. Biomed Microdevices. 2010;12:797–807. [DOI] [PubMed] [Google Scholar]
- Bierer JA, Litvak L. Reducing channel interaction through cochlear implant programming may improve speech perception: current focusing and channel deactivation. Trends Hear. 2016;20:2331216516653389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer JA, Middlebrooks JC. Auditory cortical images of cochlear-implant stimuli: dependence on electrode configuration. J Neurophysiol. 2002;87(1):478–92. [DOI] [PubMed] [Google Scholar]
- Biran R, Martin DC, Tresco PA. Neuronal cell loss accompanies the brain tissue response to chronically implanted silicon microelectrode arrays. Exp Neurol. 2005;195(1):115–26. [DOI] [PubMed] [Google Scholar]
- Biran R, Martin DC, Tresco PA. The brain tissue response to implanted silicon microelectrode arrays is increased when the device is tethered to the skull. J Biomed Mater Res A. 2007;82A(1):169–78. [DOI] [PubMed] [Google Scholar]
- Bjånes DA, Kellis S, Nickl R, Baker B, Aflalo T, Bashford L, et al. Quantifying physical degradation alongside recording and stimulation performance of 980 intracortical microelectrodes chronically implanted in three humans for 956–2130 days. Acta Biomater. 2025;198:188–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blamey PJ, Maat B, Başkent D, Mawman D, Burke E, Dillier N, et al. A retrospective multicenter study comparing speech perception outcomes for bilateral implantation and bimodal rehabilitation. Ear Hear. 2015;36(4):408–16. [DOI] [PubMed] [Google Scholar]
- Boyle PJ, Büchner A, Stone MA, Lenarz T, Moore BC. Comparison of dual-time-constant and fast-acting automatic gain control (AGC) systems in cochlear implants. Int J Audiol. 2009;48(4):211–21. [DOI] [PubMed] [Google Scholar]
- Branner A, Normann RA. A multielectrode array for intrafascicular recording and stimulation in sciatic nerve of cats. Brain Res Bull. 2000;51(4):293–306. [DOI] [PubMed] [Google Scholar]
- Branner A, Stein RB, Fernandez E, Aoyagi Y, Normann RA. Long-term stimulation and recording with a penetrating microelectrode array in cat sciatic nerve. IEEE Trans Biomed Eng. 2004;51(1):146–57. [DOI] [PubMed] [Google Scholar]
- Budenz CL, Pfingst BE, Raphael Y. The use of neurotrophin therapy in the inner ear to augment cochlear implantation outcomes. Anat Rec. 2012;295(11):1896–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell R, Sharma R, Takmakov P, Street MG, Solzbacher F, Tathireddy P, et al. Neural electrode resilience against dielectric damage may be improved by use of highly doped silicon as a conductive material. J Neurosci Methods. 2018;293:210–25. [DOI] [PubMed] [Google Scholar]
- Caldwell R, Street MG, Sharma R, Takmakov P, Baker B, Rieth L. Characterization of Parylene-C degradation mechanisms: in vitro reactive accelerated aging model compared to multiyear in vivo implantation. Biomaterials. 2020;232:119731. [DOI] [PubMed] [Google Scholar]
- Campbell PK, Jones KE, Huber RJ, Horch KW, Normann RA. A silicon-based, three-dimensional neural interface: manufacturing processes for an intracortical electrode array. IEEE Trans Biomed Eng. 1991;38(8):758–68. [DOI] [PubMed] [Google Scholar]
- Carlyon RP, Goehring T. Cochlear implant research and development in the twenty-first century: a critical update. J Assoc Res Otolaryngol. 2021;22(5):481–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M. Effects of stimulation mode on threshold and loudness growth in multielectrode cochlear implants. J Acoust Soc Am. 1999;105(2):850–60. [DOI] [PubMed] [Google Scholar]
- Cheng YS, Svirsky MA. Meta-analysis—correlation between spiral ganglion cell counts and speech perception with a cochlear implant. Audiol Res. 2021;11(2):220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MB, Pearce SM, Ledbetter NM, Warren DJ, Clark GA, Tresco PA. The foreign body response to the Utah slant electrode array in the cat sciatic nerve. Acta Biomater. 2014;10(11):4650–60. [DOI] [PubMed] [Google Scholar]
- Clark GM, Blamey P, Brown A, Gusby P, Dowell R, Franz B, et al. The University of Melbourne–nucleus multi-electrode cochlear implant. Adv Otorhinolaryngol. 1987;38:V–IX. [PubMed]
- Cochlear implant care. German Society of Otorhinolaryngology, Head and Neck Surgery. 2020.
- Davis TS, Wark HA, Hutchinson D, Warren DJ, O’neill K, Scheinblum T, et al. Restoring motor control and sensory feedback in people with upper extremity amputations using arrays of 96 microelectrodes implanted in the median and ulnar nerves. J Neural Eng. 2016;13(3):036001. [DOI] [PubMed] [Google Scholar]
- De Graaff F, Eikelboom RH, Sucher C, Kramer SE, Smits C. Binaural summation, binaural unmasking and fluctuating masker benefit in bimodal and bilateral adult cochlear implant users. Cochlear Implants Int. 2021;22(5):245–56. [DOI] [PubMed] [Google Scholar]
- de Jong MA, Briaire JJ, van der Woude SF, Frijns JH. Dynamic current focusing for loudness encoding in cochlear implants: a take-home trial. Int J Audiol. 2019;58(9):553–64. [DOI] [PubMed] [Google Scholar]
- Dean I, Harper NS, McAlpine D. Neural population coding of sound level adapts to stimulus statistics. Nat Neurosci. 2005;8(12):1684–9. [DOI] [PubMed] [Google Scholar]
- Dieter A, Duque-Afonso CJ, Rankovic V, Jeschke M, Moser T. Near physiological spectral selectivity of cochlear optogenetics. Nat Commun. 2019;10(1):1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson GS, Dawson PK, Borden LZ. Within-subjects comparison of the HiRes and Fidelity120 speech processing strategies: speech perception and its relation to place-pitch sensitivity. Ear Hear. 2011;32(2):238–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman MF, Basham K, McCandless G, Dove H. Speech understanding and music appreciation with the Ineraid cochlear implant. Hear J. 1991;44(6):34–7. [Google Scholar]
- Dorman MF, Loizou PC, Fitzke J, Tu Z. The recognition of sentences in noise by normal-hearing listeners using simulations of cochlear-implant signal processors with 6–20 channels. J Acoust Soc Am. 1998;104(6):3583–5. [DOI] [PubMed] [Google Scholar]
- Duncan CC, Kluger DT, Davis TS, Warren DJ, Page DM, Hutchinson DT, et al. Selective decrease in allodynia with high-frequency neuromodulation via high-electrode-count intrafascicular peripheral nerve interface after brachial plexus injury. Neuromodulation: Technology at the Neural Interface. 2019;22(5):597–606. [DOI] [PubMed] [Google Scholar]
- Evans EF. The frequency response and other properties of single fibres in the guinea-pig cochlear nerve. J Physiol. 1972;226(1):263–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Oxenham AJ. Effects of spectral resolution on spectral contrast effects in cochlear-implant users. J Acoust Soc Am. 2018;143(6):EL468–73. [DOI] [PMC free article] [PubMed]
- Fishman KE, Shannon RV, Slattery WH. Speech recognition as a function of the number of electrodes used in the SPEAK cochlear implant speech processor. J Speech Lang Hear Res. 1997;40(5):1201–15. [DOI] [PubMed] [Google Scholar]
- Flesher SN, Collinger JL, Foldes ST, Weiss JM, Downey JE, Tyler-Kabara EC, et al. Intracortical microstimulation of human somatosensory cortex. Sci Transl Med. 2016;8(361):361ra141-361ra141. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Nogaki G. Noise susceptibility of cochlear implant users: the role of spectral resolution and smearing. J Assoc Res Otolaryngol. 2005;6(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Shannon RV, Wang X. Effects of noise and spectral resolution on vowel and consonant recognition: acoustic and electric hearing. J Acoust Soc Am. 1998;104(6):3586–96. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Hsu CJ, Horng MJ. Effects of speech processing strategy on Chinese tone recognition by Nucleus-24 cochlear implant users. Ear Hear. 2004;25(5):501–8. [DOI] [PubMed] [Google Scholar]
- Gantz BJ, Turner C, Gfeller KE, Lowder MW. Preservation of hearing in cochlear implant surgery: advantages of combined electrical and acoustical speech processing. Laryngoscope. 2005;115(5):796–802. [DOI] [PubMed] [Google Scholar]
- George JA, Page DM, Davis TS, Duncan CC, Hutchinson DT, Rieth LW, et al. Long-term performance of Utah slanted electrode arrays and intramuscular electromyographic leads implanted chronically in human arm nerves and muscles. J Neural Eng. 2020a;17(5):056042. [DOI] [PubMed] [Google Scholar]
- George JA, Davis TS, Brinton MR, Clark GA. Intuitive neuromyoelectric control of a dexterous bionic arm using a modified Kalman filter. J Neurosci Methods. 2020b;330:108462. [DOI] [PubMed] [Google Scholar]
- George JA, Kluger DT, Davis TS, Wendelken SM, Okorokova EV, He Q, et al. Biomimetic sensory feedback through peripheral nerve stimulation improves dexterous use of a bionic hand. Sci Robot. 2019;4(32):eaax2352. [DOI] [PubMed] [Google Scholar]
- Gfeller K, Witt S, Mehr MA, Woodworth G, Knutson J. Effects of frequency, instrumental family, and cochlear implant type on timbre recognition and appraisal. Ann Otol Rhinol Laryngol. 2002;111(4):349–56. [DOI] [PubMed] [Google Scholar]
- Gifford RH. Bilateral cochlear implants or bimodal hearing for children with bilateral sensorineural hearing loss. Curr Otorhinolaryngol Rep. 2020;8:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspon CM, Valle G, Shelchkova ND, Hobbs TG, Verbaarschot C, Callier T, et al. Evoking stable and precise tactile sensations via multi-electrode intracortical microstimulation of the somatosensory cortex. Nat Biomed Eng. 2024;9(6):935–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood DD. Critical bandwidth and the frequency coordinates of the basilar membrane. J Acoust Soc Am. 1961;33(10):1344–56. [Google Scholar]
- Harrison RV, Gordon KA, Mount RJ. Is there a critical period for cochlear implantation in congenitally deaf children? Analyses of hearing and speech perception performance after implantation. Dev Psychobiol. 2005;46(3):252–61. [DOI] [PubMed] [Google Scholar]
- Hartmann R, Topp G, Klinke R. Discharge patterns of cat primary auditory fibers with electrical stimulation of the cochlea. Hear Res. 1984;13(1):47–62. [DOI] [PubMed] [Google Scholar]
- Heil P, Irvine DRF. First-spike timing of auditory-nerve fibers and comparison with auditory cortex. J Neurophysiol. 1997;78(5):2438–54. [DOI] [PubMed] [Google Scholar]
- Herman B, Angeli S. Differences in cochlear nerve cross-sectional area between normal hearing and postlingually deafened patients on MRI. Otolaryngol Head Neck Surg. 2011;144(1):64–6. [DOI] [PubMed] [Google Scholar]
- Hillman T, Badi AN, Normann RA, Kertesz T, Shelton C. Cochlear nerve stimulation with a 3-dimensional penetrating electrode array. Otol Neurotol. 2003;24(5):764–8. [DOI] [PubMed] [Google Scholar]
- Holt RF, Kirk KI. Speech and language development in cognitively delayed children with cochlear implants. Ear Hear. 2005;26(2):132–48. [DOI] [PubMed] [Google Scholar]
- Hong RS, Rubinstein JT. High-rate conditioning pulse trains in cochlear implants: dynamic range measures with sinusoidal stimuli. J Acoust Soc Am. 2003;114(6):3327–42. [DOI] [PubMed] [Google Scholar]
- House WF. Cochlear implants. Ann Otol Rhinol Laryngol. 1976;85(3_suppl):3–3. [PubMed] [Google Scholar]
- House WF, Urban J. Long term results of electrode implantation and electronic stimulation of the cochlea in man. Ann Otol Rhinol Laryngol. 1973;82(4):504–17. [DOI] [PubMed] [Google Scholar]
- Hsu JM, Rieth L, Normann RA, Tathireddy P, Solzbacher F. Encapsulation of an integrated neural interface device with parylene c. IEEE Trans Biomed Eng. 2008;56(1):23–9. [DOI] [PubMed] [Google Scholar]
- Huet A, Desmadryl G, Justal T, Nouvian R, Puel JL, Bourien J. The interplay between spike-time and spike-rate modes in the auditory nerve encodes tone-in-noise threshold. J Neurosci. 2018;38(25):5727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SE, Hutchings HA, Rapport FL, McMahon CM, Boisvert I. Social connectedness and perceived listening effort in adult cochlear implant users: a grounded theory to establish content validity for a new patient-reported outcome measure. Ear Hear. 2018;39(5):922–34. [DOI] [PubMed] [Google Scholar]
- Hughes CL, Flesher SN, Weiss JM, Downey JE, Boninger M, Collinger JL, et al. Neural stimulation and recording performance in human sensorimotor cortex over 1500 days. J Neural Eng. 2021;18(4):045012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Instrumentation A for the A of M, others. Cochlear implant systems: Requirements for safety, functional verification, labeling and reliability reporting. ANSI/AAMI CI86. 2017.
- Jackler RK. Atlas of skull base surgery and neurotology (2nd ed.). Thieme. 2009;
- Jeschke M, Moser T. Considering optogenetic stimulation for cochlear implants. Hear Res. 2015;322:224–34. [DOI] [PubMed] [Google Scholar]
- Johnson DH. The relationship between spike rate and synchrony in responses of auditory-nerve fibers to single tones. J Acoust Soc Am. 1980;68(4):1115–22. [DOI] [PubMed] [Google Scholar]
- Johnson DH, Kiang N. Analysis of discharges recorded simultaneously from pairs of auditory nerve fibers. Biophys J. 1976;16(7):719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Della Santina CC, Wang X. Selective neuronal activation by cochlear implant stimulation in auditory cortex of awake primate. J Neurosci. 2016;36(49):12468–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamakura T, Nadol JB. Correlation between word recognition score and intracochlear new bone and fibrous tissue after cochlear implantation in the human. Hear Res. 2016;339:132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani RG, Kocharyan A, Claussen AD, Gantz BJ, Hansen MR. Middle cranial fossa approach for sporadic vestibular schwannoma. Otolaryngol Clin North Am. 2023;56(3):495–507. [DOI] [PubMed] [Google Scholar]
- Keppeler D, Schwaerzle M, Harczos T, Jablonski L, Dieter A, Wolf B, et al. Multichannel optogenetic stimulation of the auditory pathway using microfabricated LED cochlear implants in rodents. Sci Transl Med. 2020;12(553):eabb8086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiang NY, Moxon EC. Physiological considerations in artificial stimulation of the inner ear. Ann Otol Rhinol Laryngol. 1972;81(5):714–30. [DOI] [PubMed] [Google Scholar]
- Kim YT, Hitchcock RW, Bridge MJ, Tresco PA. Chronic response of adult rat brain tissue to implants anchored to the skull. Biomaterials. 2004;25(12):2229–37. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Badi AN, Normann RA. Selective activation of cat primary auditory cortex by way of direct intraneural auditory nerve stimulation. Laryngoscope. 2007;117(6):1053–62. [DOI] [PubMed] [Google Scholar]
- Kral A, Hartmann R, Mortazavi D, Klinke R. Spatial resolution of cochlear implants: the electrical field and excitation of auditory afferents. Hear Res. 1998;121(1–2):11–28. [DOI] [PubMed] [Google Scholar]
- Ladefoged P, Broadbent DE. Information conveyed by vowels. J Acoust Soc Am. 1957;29(1):98–104. [DOI] [PubMed] [Google Scholar]
- Leake PA, Stakhovskaya O, Hetherington A, Rebscher SJ, Bonham B. Effects of brain-derived neurotrophic factor (BDNF) and electrical stimulation on survival and function of cochlear spiral ganglion neurons in deafened, developing cats. J Assoc Res Otolaryngol. 2013;14(2):187–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leake PA, Akil O, Lang H. Neurotrophin gene therapy to promote survival of spiral ganglion neurons after deafness. Hear Res. 2020;394:107955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber M, Körner J, Reiche CF, Yin M, Bhandari R, Franklin R, et al. Advances in penetrating multichannel microelectrodes based on the utah array platform. Neural Interface Front Appl. 2019;1–40. [DOI] [PubMed]
- Lenarz T, Büchner A, Illg A. Cochlear implantation: concept, results outcomes and quality of life. Laryngo-Rhino-Otol. 2022;101(S 01):S36-78. [DOI] [PubMed] [Google Scholar]
- Lim HH, Lenarz T. Auditory midbrain implant: research and development towards a second clinical trial. Hear Res. 2015;322(Apr):212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limb CJ, Roy AT. Technological, biological, and acoustical constraints to music perception in cochlear implant users. Hear Res. 2014;308:13–26. [DOI] [PubMed] [Google Scholar]
- Litovsky RY, Johnstone PM, Godar SP. Benefits of bilateral cochlear implants and/or hearing aids in children: Beneficios de los implantes cocleares bilaterales y/o auxiliares auditivos en niños. Int J Audiol. 2006;45(sup1):78–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield PD, Richter C. Near-infrared stimulation of the auditory nerve: a decade of progress toward an optical cochlear implant. Laryngoscope Investig Otolaryngol. 2021;6(2):310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LF, Palmer AR, Wallace MN. Phase-locked responses to pure tones in the inferior colliculus. J Neurophysiol. 2006;95(3):1926–35. [DOI] [PubMed] [Google Scholar]
- Loizou PC. Speech processing in vocoder-centric cochlear implants. Cochlear Brainstem Implants. 2006;64:109–43. [DOI] [PubMed] [Google Scholar]
- Lu T, Liang L, Wang X. Temporal and rate representations of time-varying signals in the auditory cortex of awake primates. Nat Neurosci. 2001;4(11):1131–8. [DOI] [PubMed] [Google Scholar]
- Lucas JC, Fan CJ, Jacob JT, Babu SC. Retrosigmoid approach for sporadic vestibular schwannoma. Otolaryngol Clin North Am. 2023;56(3):509–20. [DOI] [PubMed] [Google Scholar]
- Luo X, Fu QJ. Contribution of low-frequency acoustic information to Chinese speech recognition in cochlear implant simulations. J Acoust Soc Am. 2006;120(4):2260–6. [DOI] [PubMed] [Google Scholar]
- Matsuoka A, Abbas P, Rubinstein J, Miller C. The neuronal response to electrical constant-amplitude pulse train stimulation: additive Gaussian noise. Hear Res. 2000;149(1–2):129–37. [DOI] [PubMed] [Google Scholar]
- McDermott HJ. Music perception with cochlear implants: a review. Trends in Amplification. 2004;8(2):49–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AH, Oxenham AJ. Vocoder simulations explain complex pitch perception limitations experienced by cochlear implant users. J Assoc Res Otolaryngol. 2017;18(6):789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta AH, Lu H, Oxenham AJ. The perception of multiple simultaneous pitches as a function of number of spectral channels and spectral spread in a noise-excited envelope vocoder. J Assoc Res Otolaryngol. 2020;21(1):61–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC, Snyder RL. Auditory prosthesis with a penetrating nerve array. J Assoc Res Otolaryngol. 2007;8:258–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC, Snyder RL. Intraneural stimulation for auditory prosthesis: modiolar trunk and intracranial stimulation sites. Hear Res. 2008;242(1–2):52–63. [DOI] [PubMed] [Google Scholar]
- Middlebrooks JC, Snyder RL. Selective electrical stimulation of the auditory nerve activates a pathway specialized for high temporal acuity. J Neurosci. 2010;30(5):1937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlebrooks JC, Bierer JA, Snyder RL. Cochlear implants: the view from the brain. Curr Opin Neurobiol. 2005;15(4):488–93. [DOI] [PubMed] [Google Scholar]
- Møller AR. Frequency selectivity of phase-locking of complex sounds in the auditory nerve of the rat. Hear Res. 1983;11(3):267–84. [DOI] [PubMed] [Google Scholar]
- Moore BC. Coding of sounds in the auditory system and its relevance to signal processing and coding in cochlear implants. Otol Neurotol. 2003;24(2):243–54. [DOI] [PubMed] [Google Scholar]
- Moore DR, Shannon RV. Beyond cochlear implants: awakening the deafened brain. Nat Neurosci. 2009;12(6):686–91. [DOI] [PubMed] [Google Scholar]
- Moore BC, Carlyon RP. Perception of pitch by people with cochlear hearing loss and by cochlear implant users. In: Pitch: Neural coding and perception. New York: Springer New York ; 2005. p. 234–77.
- Müller J, Schon F, Helms J. Speech understanding in quiet and noise in bilateral users of the MED-EL COMBI 40/40+ cochlear implant system. Ear Hear. 2002;23(3):198–206. [DOI] [PubMed] [Google Scholar]
- Müller-Deile J, Schmidt B, Rudert H. Effects of noise on speech discrimination in cochlear implant patients. Ann Otol Rhinol Laryngol Suppl. 1995;166:303–6. [PubMed] [Google Scholar]
- Nadol JB, Xu WZ. Diameter of the cochlear nerve in deaf humans: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1992;101(12):988–93. [DOI] [PubMed] [Google Scholar]
- Nadol JB, Young YS, Glynn RJ. Survival of spiral ganglion cells in profound sensorineural hearing loss: implications for cochlear implantation. Ann Otol Rhinol Laryngol. 1989;98(6):411–6. [DOI] [PubMed] [Google Scholar]
- Naguib NNN, Hey C, Shaaban MS, Elabd AM, Hassan HHM, Gruber-Rouh T, et al. Assessment of the cochlear nerve to facial nerve size ratio using MR multiplanar reconstruction of the internal auditory canal in patients presenting with acquired long-standing hearing loss. Br J Radiol. 2017;90(1073):20160870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naples JG, Ruckenstein MJ. Cochlear implant. Otolaryngol Clin North Am. 2020;53(1):87–102. [DOI] [PubMed] [Google Scholar]
- Naumann H, Zwicker E, Scherer H, Seifert J, Leysieffer H, Zollner M. Erfahrungen mit der Implantation einer Mehrkanalelektrode in den Nervus acusticus. Laryngo-Rhino-Otol. 1986;65(03):118–22. [PubMed] [Google Scholar]
- Negi S, Bhandari R, Rieth L, Solzbacher F. In vitro comparison of sputtered iridium oxide and platinum-coated neural implantable microelectrode arrays. Biomed Mater. 2010;5(1):015007. [DOI] [PubMed] [Google Scholar]
- Nelson PB, Jin SH. Factors affecting speech understanding in gated interference: cochlear implant users and normal-hearing listeners. J Acoust Soc Am. 2004;115(5):2286–94. [DOI] [PubMed] [Google Scholar]
- Nelson PB, Jin SH, Carney AE, Nelson DA. Understanding speech in modulated interference: cochlear implant users and normal-hearing listeners. J Acoust Soc Am. 2003;113(2):961–8. [DOI] [PubMed] [Google Scholar]
- Niparko JK, Tobey EA, Thal DJ, Eisenberg LS, Wang NY, Quittner AL, et al. Spoken language development in children following cochlear implantation. JAMA. 2010;303(15):1498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira W, Litvak L, Edler B, Ostermann J, Büchner A. Signal processing strategies for cochlear implants using current steering. EURASIP J Adv Signal Process. 2009;2009(1):531213. [Google Scholar]
- Normann RA, McDonnall D, Clark GA, Stein RB, Branner A. Physiological activation of the hind limb muscles of the anesthetized cat using the utah slanted electrode array. In: Proceedings 2005 IEEE International Joint Conference on Neural Networks, 2005. IEEE; 2005. p. 3103–8.
- O’Neill ER, Basile JD, Nelson P. Individual hearing outcomes in cochlear implant users influence social engagement and listening behavior in everyday life. J Speech Lang Hear Res. 2021;64(12):4982–99. [DOI] [PubMed] [Google Scholar]
- Olson AD, Shinn JB. A systematic review to determine the effectiveness of using amplification in conjunction with cochlear implantation. J Am Acad Audiol. 2008;19(9):657–71. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ. Pitch perception and auditory stream segregation: implications for hearing loss and cochlear implants. Trends Amplif. 2008;12(4):316–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page DM, George JA, Kluger DT, Duncan C, Wendelken S, Davis T, et al. Motor Control and Sensory Feedback Enhance Prosthesis Embodiment and Reduce Phantom Pain After Long-Term Hand Amputation. Front Hum Neurosci. 2018;21(12):352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page DM, George JA, Wendelken SM, Davis TS, Kluger DT, Hutchinson DT, et al. Discriminability of multiple cutaneous and proprioceptive hand percepts evoked by intraneural stimulation with Utah slanted electrode arrays in human amputees. J Neuroeng Rehabil. 2021;18(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer AR, Shackleton TM, Sumner CJ, Zobay O, Rees A. Classification of frequency response areas in the inferior colliculus reveals continua not discrete classes. J Physiol. 2013;591(16):4003–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskett MD, Brinton MR, Hansen TC, George JA, Davis TS, Duncan CC, et al. Activities of daily living with bionic arm improved by combination training and latching filter in prosthesis control comparison. J NeuroEngineering Rehabil. 2021;18:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick-Krueger KM, Burkhart I, Contreras-Vidal JL. The state of clinical trials of implantable brain–computer interfaces. Nat Rev Bioeng. 2024;3(1):50–67. [Google Scholar]
- Pinyon JL, Tadros SF, Froud KE, Y. Wong AC, Tompson IT, Crawford EN, et al. Close-Field Electroporation Gene Delivery Using the Cochlear Implant Electrode Array Enhances the Bionic Ear. Sci Transl Med. 2014;6(233):233ra54. [DOI] [PubMed]
- Polikov VS, Tresco PA, Reichert WM. Response of brain tissue to chronically implanted neural electrodes. J Neurosci Methods. 2005;148(1):1–18. [DOI] [PubMed] [Google Scholar]
- Qin MK, Oxenham AJ. Effects of simulated cochlear-implant processing on speech reception in fluctuating maskers. J Acoust Soc Am. 2003;114(1):446–54. [DOI] [PubMed] [Google Scholar]
- Raggio MW, Schreiner CE. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation. III. Activation patterns in short- and long-term deafness. J Neurophysiol. 1999;82(6):3506–26. [DOI] [PubMed] [Google Scholar]
- Reimann K, Klose U, Ehrenpfordt U, Thangavelu K, Schulze M. Detection of reduced diameter of the cochlear nerve in long-term deaf patients quantified with semiautomatic measurement of nerve cross-sectional area using 3T MRI data. Otology & Neurotology Open. 2024;4(1):e047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riss D, Hamzavi JS, Blineder M, Flak S, Baumgartner WD, Kaider A, et al. Effects of stimulation rate with the FS4 and HDCIS coding strategies in cochlear implant recipients. Otol Neurotol. 2016;37(7):882–8. [DOI] [PubMed] [Google Scholar]
- Rubinstein JT, Hong R. Signal coding in cochlear implants: exploiting stochastic effects of electrical stimulation. Ann Otol Rhinol Laryngol. 2003;112(9_suppl):14–9. [DOI] [PubMed] [Google Scholar]
- Sachs MB, Abbas PJ. Rate versus level functions for auditory-nerve fibers in cats: tone-burst stimuli. J Acoust Soc Am. 1974;56(6):1835–47. [DOI] [PubMed] [Google Scholar]
- Salatino JW, Ludwig KA, Kozai TDY, Purcell EK. Glial responses to implanted electrodes in the brain. Nat Biomed Eng. 2017;1(11):862–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner CE, Raggio MW. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation. II. Repetition rate coding. J Neurophysiol. 1996;75(3):1283–300. [DOI] [PubMed] [Google Scholar]
- Schwam ZG, Cosetti MK, Wanna GB. Translabyrinthine approach for sporadic vestibular schwannoma. Otolaryngol Clin North Am. 2023;56(3):483–93. [DOI] [PubMed] [Google Scholar]
- Seyyedi M, Viana LM, Nadol JB. Within-subject comparison of word recognition and spiral ganglion cell count in bilateral cochlear implant recipients. Otol Neurotol. 2014;35(8):1446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon R, Fu QJ, Galvin IJ. The number of spectral channels required for speech recognition depends on the difficulty of the listening situation. Acta Otolaryngol (Stockh). 2004;124:50–4. [DOI] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23(6):532–9. [DOI] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol. 2005;486(2):145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd RK, Coco A, Epp SB. Neurotrophins and electrical stimulation for protection and repair of spiral ganglion neurons following sensorineural hearing loss. Hear Res. 2008;242(1–2):100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons FB, Epley JM, Lummis RC, Guttman N, Frishkopf LS, Harmon LD, et al. Auditory nerve: electrical stimulation in man. Science. 1965;148(3666):104–6. [DOI] [PubMed] [Google Scholar]
- Simmons FB, Mathews RG, Walker MG, White RL. A functioning multichannel auditory nerve stimulator a preliminary report on two human volunteers. Acta Oto-Laryngol. 1979;87(3–6):170–5. [DOI] [PubMed] [Google Scholar]
- Simmons F, Walker M, White R, Mathews R. Pitch correlates of direct auditory nerve electrical stimulation. Ann Otol Rhinol Laryngol. 1981;90(2_suppl2):15–8. [DOI] [PubMed] [Google Scholar]
- Skinner MW, Clark GM, Whitford LA, Seligman PM, Staller SJ, Shipp DB, et al. Evaluation of a new spectral peak coding strategy for the Nucleus 22 channel cochlear implant system. Otol Neurotol. 1994;15:15–27. [PubMed] [Google Scholar]
- Snyder RL, Vollmer M, Moore CM, Rebscher SJ, Leake PA, Beitel RE. Responses of inferior colliculus neurons to amplitude-modulated intracochlear electrical pulses in deaf cats. J Neurophysiol. 2000;84(1):166–83. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Bierer JA, Middlebrooks JC. Topographic spread of inferior colliculus activation in response to acoustic and intracochlear electric stimulation. J Assoc Res Otolaryngol. 2004;5:305–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim C, Papadourakis V, Collinger JL, Downey J, Weiss J, Pentousi L, et al. Longevity and reliability of chronic unit recordings using the Utah, intracortical multi-electrode arrays. J Neural Eng. 2021;18(6):066044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickney GS, Zeng FG, Litovsky R, Assmann P. Cochlear implant speech recognition with speech maskers. J Acoust Soc Am. 2004;116(2):1081–91. [DOI] [PubMed] [Google Scholar]
- Stilp CE. Acoustic context alters vowel categorization in perception of noise-vocoded speech. J Assoc Res Otolaryngol. 2017;18:465–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner CJ, Palmer AR. Auditory nerve fibre responses in the ferret. Eur J Neurosci. 2012;36(4):2428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J, Popelář J, Kvašňák E, Astl J. Response properties of neurons in the central nucleus and external and dorsal cortices of the inferior colliculus in guinea pig. Exp Brain Res. 2000 5;133(2):254–66. [DOI] [PubMed] [Google Scholar]
- Szymanski LJ, Kellis S, Liu CY, Jones KT, Andersen RA, Commins D, et al. Neuropathological effects of chronically implanted, intracortical microelectrodes in a tetraplegic patient. J Neural Eng. 2021;18(4):0460b9. [DOI] [PubMed]
- Taberner AM, Liberman MC. Response properties of single auditory nerve fibers in the mouse. J Neurophysiol. 2005;93(1):557–69. [DOI] [PubMed] [Google Scholar]
- Thomas WM, Zuniga SA, Sondh I, Leber M, Solzbacher F, Lenarz T, et al. Development of a feline model for preclinical research of a new translabyrinthine auditory nerve implant. Front Neurosci. 2024;18:1308663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler RS, Parkinson AJ, Wilson BS, Witt S, Preece JP, Noble W. Patients utilizing a hearing aid and a cochlear implant: speech perception and localization. Ear Hear. 2002;23(2):98–105. [DOI] [PubMed] [Google Scholar]
- Van Hoesel RJM, Tyler RS. Speech perception, localization, and lateralization with bilateral cochlear implants. J Acoust Soc Am. 2003;113(3):1617–30. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Anderson LA, Palmer AR. Phase-locked responses to pure tones in the auditory thalamus. J Neurophysiol. 2007;98(4):1941–52. [DOI] [PubMed] [Google Scholar]
- Wark H, Sharma R, Mathews K, Fernandez E, Yoo J, Christensen B, et al. A new high-density (25 electrodes/mm2) penetrating microelectrode array for recording and stimulating sub-millimeter neuroanatomical structures. J Neural Eng. 2013;10(4):045003. [DOI] [PubMed] [Google Scholar]
- Watkins AJ. Central, auditory mechanisms of perceptual compensation for spectral-envelope distortiona). J Acoust Soc Am. 1991;90(6):2942–55. [DOI] [PubMed] [Google Scholar]
- Wei CG, Cao K, Zeng FG. Mandarin tone recognition in cochlear-implant subjects. Hear Res. 2004;197(1–2):87–95. [DOI] [PubMed] [Google Scholar]
- Wendelken S, Page DM, Davis T, Wark HA, Kluger DT, Duncan C, et al. Restoration of motor control and proprioceptive and cutaneous sensation in humans with prior upper-limb amputation via multiple Utah Slanted Electrode Arrays (USEAs) implanted in residual peripheral arm nerves. J Neuroengineering Rehabil. 2017;14:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Dorman MF. Cochlear implants: a remarkable past and a brilliant future. Hear Res. 2008;242(1–2):3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BS, Finley CC, Lawson DT, Wolford RD, Eddington DK, Rabinowitz WM. Better speech recognition with cochlear implants. Nature. 1991;352(6332):236–8. [DOI] [PubMed] [Google Scholar]
- Wilson BS. Cochlear implant technology. In: Niparko JK, Kirk KI, Mellon NK, Robbins AM, Tucci DL, Wilson BS, editors. Cochlear Implants: Principles & Practices. Philadelphia: Lippincott Williams & Wilkins; 2000. p. 109-19.
- Winn MB, Litovsky RY. Using speech sounds to test functional spectral resolution in listeners with cochlear implants. J Acoust Soc Am. 2015;137(3):1430–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MB, Teece KH. Listening effort is not the same as speech intelligibility score. Trends Hear. 2021;25:23312165211027688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MB, Chatterjee M, Idsardi WJ. The use of acoustic cues for phonetic identification: effects of spectral degradation and electric hearing. J Acoust Soc Am. 2012;131(2):1465–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woeppel K, Hughes C, Herrera AJ, Eles JR, Tyler-Kabara EC, Gaunt RA, et al. Explant Analysis of Utah Electrode Arrays Implanted in Human Cortex for Brain-Computer-Interfaces. Front Bioeng Biotechnol. 2021;7(9):759711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Kozin ED, Kanumuri VV, Vachicouras N, Miller J, Lacour S, et al. Auditory Brainstem Implants: Recent Progress and Future Perspectives. Front Neurosci. 2019;29(13):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HI, Zeng FG. Bimodal benefits in Mandarin-speaking cochlear implant users with contralateral residual acoustic hearing. Int J Audiol. 2017;56(sup2):S17-22. [DOI] [PubMed] [Google Scholar]
- Zappia JJ, Hetke JF, Altschuler RA, Niparko JK. Evaluation of a silicon-substrate modiolar eighth nerve implant in a guinea pig. Otolaryngol Head Neck Surg. 1990;103(4):575–82. [DOI] [PubMed] [Google Scholar]
- Zeng FG. Challenges in improving cochlear implant performance and accessibility. IEEE Trans Biomed Eng. 2017;64(8):1662–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng FG. Celebrating the one millionth cochlear implant. JASA Express Lett. 2022;2(7). [DOI] [PubMed]
- Zeng FG, Galvin JJ, Zhang C. Encoding loudness by electric stimulation of the auditory nerve. NeuroReport. 1998;9(8):1845–8. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Grant G, Niparko J, Galvin J, Shannon R, Opie J, et al. Speech dynamic range and its effect on cochlear implant performance. J Acoust Soc Am. 2002;111(1):377–86. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Nie K, Stickney GS, Kong YY, Vongphoe M, Bhargave A, et al. Speech recognition with amplitude and frequency modulations. Proc Natl Acad Sci U S A. 2005;102(7):2293–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Tang Q, Zeng FG, Guan T, Ye D. Cochlear-implant spatial selectivity with monopolar, bipolar and tripolar stimulation. Hear Res. 2012;283(1–2):45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann CE, Burgess BJ, Nadol JB. Patterns of degeneration in the human cochlear nerve. Hear Res. 1995;90(1–2):192–201. [DOI] [PubMed] [Google Scholar]
- Zoellner F, WD K. Transmission of hearing by electrical stimulation of the acoustic nerve.(preliminary report). Arch Ohren Nasen Kehlkopfheilkd. 1963;181:216–23. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.