Abstract
Background
Anisodus tanguticus, a plateau-dwelling medicinal plant endemic to high-altitude regions, synthesizes pharmaceutically critical tropane alkaloids but faces escalating threats from climate warming. While its unique thermal adaptation mechanisms remain enigmatic, heat shock transcription factors (HSFs) are hypothesized to orchestrate stress resilience in this species.
Results
Here, we present a genome-wide evolutionary and functional dissection of the HSF gene family in A. tanguticus, identifying 20 HSF members (AntHSFs) with distinct structural and regulatory features. Phylogenetic reconstruction classified AntHSFs into three canonical subfamilies, revealing lineage-specific diversification patterns shaped by tandem and segmental duplication events. Conserved motif architectures and DNA-binding domains underscored functional divergence, while synteny analysis highlighted evolutionary constraints and adaptive innovations compared to model species.
The transcript dynamics analysis under heat treatment revealed the dynamic changes in enzyme activity and gene expression of the MDA, GSTT, GSTF, and Cu/ZnSOD genes under different heat-treated times. The AntHSF gene exhibited stage-specific expression patterns consistent with antioxidant enzyme activity, indicating that the AntHSF family plays a critical role in coordinating the antioxidant gene response through temporal regulation networks. Notably, evolutionary analysis and sub-cellular localization revealed that AntHSF7 and AntHSF9 were potentially linked to plateau-specific adaptation.
Conclusion
This study deciphers the evolutionary drivers and functional specialization of HSF genes in A. tanguticus, providing mechanistic insights into its thermal resilience and a genetic roadmap for conserving and improving this climate-vulnerable medicinal species.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-025-07443-4.
Keywords: Anisodus tanguticus, HSF, Transcriptomics, Gene expression, Antioxidant enzyme activity, Heat shock
Background
The accelerating pace of industrialization has precipitated unprecedented increases in greenhouse gas emissions and global temperatures, critically threatening the sustainability of medicinal plant ecosystems. Over the past century, atmospheric CO2 concentrations have surged by nearly 50%, driving a 1.1℃ rise in global average temperatures since the pre-industrial era, with projections indicating a 2.5–4.5℃ increase by the year 2100 under high-emission scenarios [1]. These climatic shifts manifest as habitat alterations marked by rising temperatures, erratic precipitation patterns, and intensified extreme weather events, which collectively destabilize ecological niches. For medicinal plants, particularly those endemic to high-altitude regions, these changes induce range contractions, population fragmentation, and elevated extinction risks [2–4]. Plateau ecosystems, such as the Qinghai-Tibet Plateau, where Anisodus tanguticus thrives, are disproportionately affected due to their low-temperature adaptations and narrow thermal tolerances. Here, endemic species have evolved under stable cold conditions over millennia, rendering them exquisitely sensitive to even marginal temperature increases. For instance, a 2℃ rise in alpine regions can advance phenological stages by 3–4 weeks, desynchronizing plant-pollinator interactions and reducing reproductive success [5]. Concurrently, anthropogenic pressures-overharvesting, grazing, and land-use changes-exacerbate these vulnerabilities, pushing species like A. tanguticus toward ecological tipping points [6, 7].
Central to plant survival under thermal stress are heat shock transcription factors (HSFs), master regulators that activate molecular chaperones (HSPs) to mitigate protein denaturation and maintain cellular homeostasis [8, 9]. The HSF-mediated stress response operates through a hierarchical cascade: upon sensing temperature fluctuations via membrane sensors like histidine kinases, HSFs undergo phosphorylation-dependent activation, enabling their trimerization and nuclear translocation [10]. These activated complexes bind heat shock elements (HSEs; consensus sequence nGAAnnTTCn) in the promoters of HSP genes, triggering the synthesis of chaperones such as HSP70 and HSP90. These chaperones stabilize nascent polypeptides, refold denatured proteins, and target irreparable aggregates for proteasomal degradation, thereby preserving proteostasis under stress [11]. The evolutionary diversification of HSF families across plants reflects their adaptive significance while Arabidopsis thaliana (L.) Heynh harbors 25 HSFs [12], polyploid species like Brassica napus L. (96 HSFs) and Camelina sativa (L.) Crantz (108 HSFs) exhibit expanded repertoires, likely enabling nuanced stress responses in complex environments [13, 14]. However, extremophytes inhabiting thermally volatile niches, such as alpine plants, remain understudied, leaving critical gaps in understanding how HSF evolution underpins ecological resilience. In Solanum lycopersicum L., HSFA2 is directly involved in the activation of protective mechanisms in anthers during heat stress, aiding fruit set under heat treatment conditions [15]. ZmHSF05, a maize heat shock factor, regulates both basal thermotolerance and acquired heat resistance. Overexpression of ZmHSF05 significantly improves plant survival under heat treatment stress by enhancing chlorophyll content [16]. Additionally, OsHSFA2e, which is identified in Oryza sativa L., when overexpressed in A. thaliana, enhances the plant’s heat tolerance [17]. HsfA1a, as a key positive regulator of heat shock response (HSR), can transcriptionally regulate the expression of GST8, MDAR1, and Cu/Zn SOD genes, thereby enhancing antioxidant enzyme activity and detoxifying ROS in tomato anthers under heat stress [18].
Anisodus tanguticus (Maxim.) Pascher, a perennial herb endemic to the Qinghai-Tibet Plateau, epitomizes the convergence of ecological fragility and medicinal indispensability. This species synthesizes tropane alkaloids-notably scopolamine and atropine-which are indispensable in treating neurological disorders, motion sickness, and Parkinson’s disease [19]. Global demand for these compounds exceeds 500 metric tons annually, driving a multimillion-dollar pharmaceutical industry [20]. Yet, A. tanguticus is vanishing from its native range (2,800-4,200 m elevation across Tibet, Qinghai, and Sichuan), with population densities plummeting by 70% since the 1980 s due to overharvesting and habitat fragmentation [7, 21, 22]. Compounding these threats, climate models predict a 3–5℃ temperature rise on the Tibetan Plateau by 2100, exacerbating heat stress in a species already operating at its thermal limits [23]. Field observations reveal that sustained exposure to >30℃ induces leaf necrosis, reduced alkaloid biosynthesis, and reproductive failure in A. tanguticus [24].
Despite the potential significance of HSFs in A. tanguticus’ stress response and growth, the HSF family in its genome has not been previously identified. To fill this knowledge gap, a comprehensive study was carried out. In this research, 20 genes belonging to the AntHSF family were identified. The study further delved into a detailed analysis of the phylogenetic relationships of AntHSF proteins, covering aspects such as chromosomal distribution, gene structure, protein motifs, and both intraspecific and interspecific synteny. RNA-seq and qRT-PCR were employed to identify the expression profiles of AntHSF gene members in different tissues of A. tanguticus and their responses to temperature stress. Additionally, the selected AntHSF proteins were cloned and analyzed.
Overall, this comprehensive exploration of the AntHSF gene family and its expression patterns under high-temperature stress not only laid a solid foundation for understanding the functional properties and expression regulation of AntHSFs during the growth, development, and stress response of A. tanguticus but also provided valuable insights for the selection of A. tanguticus germplasm resources and the optimization of its planting environment. By linking gene family evolution to physiological resilience, we offer actionable insights for conserving A. tanguticus wild populations and engineering heat-resilient cultivars. Future efforts should prioritize in planta CRISPR-based functional studies and field trials to translate these discoveries into sustainable conservation practices, ensuring the persistence of this ecological and pharmaceutical treasure in a warming world.
Materials and methods
Plant materials and growth conditions
Plant materials of Anisodus tanguticus (Maxim.) Pascher were obtained from wild-collected seeds, provided by Chengdu First Pharmaceutical Co. Ltd. (https://www.cddyyy.com/home/about/about_intro). The experimental work was conducted from March to August 2024 in the growth chamber of Chengdu University of Traditional Chinese Medicine, Chengdu, China.
Prior to sowing, seeds were soaked in distilled water at room temperature for 8 h and gently blotted dry on filter paper. They were then immersed in a 400 ppm gibberellin solution, in darkness, for 12 h. Following this treatment, seeds were transferred to a germination box lined with moist cotton. The germination environment included an illumination intensity of approximately 10,000 lux, a 16 h light/8 h dark photoperiod, 60% relative humidity, and a temperature of 22 °C.
When seedlings reached a height of approximately 3 cm, they were transplanted into small pots containing a 3:1:1 (v/v/v) mixture of peat moss, perlite, and vermiculite to ensure adequate drainage and aeration. The seedlings were then moved to the greenhouse, where they continued to grow under controlled conditions. The greenhouse environment was maintained at 75% relative humidity, with day/night temperatures of 25°C/10°C and a 12 h light/12 h dark cycle. Irradiance was provided by LED lamps containing red (R; peak at 630 nm), blue (B; peak at 465 nm), green (G; peak at 550 nm), and white light. The ratio of red to blue (R: B) was 3:1, and the ratio of red to far-red (R: FR) was 10:1 [25]. The LED lamps were positioned 50 cm above the seedling canopy, and irradiance was routinely measured using a quantum sensor (Highpoint, Taiwan, China).
After approximately three months of growth, the plants were subjected to heat treatment at 35 °C, while maintaining the same light and photoperiod conditions. Samples were collected at 0, 4, 6, 12, and 24 h after the onset of heat treatment, with three biological replicates per time point. Whole-plant samples at 0, 4, and 6 h were used for transcriptome analyses, whereas samples at 0, 12, and 24 h were harvested and separated into aboveground and belowground portions for subsequent enzyme activity assays. All samples were immediately frozen in liquid nitrogen and stored at −80°C until further analysis.
Determination of enzyme activity of A. tanguticus at high temperature
Samples collected at 0 h, 12 h, and 24 h were used to measure the enzyme activity. The content of Superoxide Dismutase (SOD), Catalase (CAT), Peroxidase (POD), Malondialdehyde (MDA), Hydrogen Peroxide (H2O2) were determined by SOD, CAT, POD, MDA, H2O2 detection kit (Nanjing Ruiyuan Biotechnology, Nanjing, China) following the manufacturer’s instructions, with three biological replicates (independent plant samples). For technical validation, triplicate measurements were performed per biological sample, with mean values ± SD calculated to ensure experimental reproducibility following the manufacturer’s protocols.
RNA-seq library construction and sequencing , data analysis, read mapping
Total RNA from freeze-dried A. tanguticus at 0 h, 4 h, and 6 h was extracted using TRIzol reagent (TIANGEN, Beijing, China). RNA quality was checked with a 2100 bioanalyzer and RNA 6000 Nano LabChip Kit (Agilent Technologies, Santa Clara, USA). RNA samples were used for library construction with an TruSeqTM RNA Sample Preparation Kit (Illumina, California, USA) and sequenced on an OE biotech Co., Ltd. (Shanghai, China)
Raw Illumina sequencing reads (fastq format) were processed with Trimmomatic [26] to remove adapters and filter low-quality/N-containing bases, generating high-quality clean reads. De novo transcriptome assembly was performed using Trinity (version. 2.4) [27] (paired-end mode), followed by gene expression quantification via RSEM (expected maximum RNA-SEQ). Unigenes were functionally annotated by BLASTx (https://blast.ncbi.nlm.nih.gov/) [28], NR (https://www.ncbi.nlm.nih.gov/), UniProt (https://www.uniprot.org/), and KOG databases (E-value ≤ 1e⁻⁵), with top-hit proteins assigned for annotations. GO terms (http://www.geneontology.org/) were mapped through UniProt associations, while KEGG pathways (http://www.genome.jp/kegg/) [29] were annotated based on the sequence alignment of homologs.
Among them, q < 0.005 and |FoldChange| >2 was treated as significantly differentially expressed genes (DEGs). The GO enrichment analysis provided all the GO terms that were significantly enriched with DEGs compared with the genome background using Blast2GO with a false discovery rate ≤ 0.01. The annotations were then refined and enriched using TopGo R package (http://www.bioconductor.org/packages/release/bioc/html/topGO.html). The enrichment of DEGs in KEGG pathways were analyzed using KOBAS software (version 2.0) [30]. The heat maps were drawn using TBtools-II software (version 2.056) [31].
Identification of AntHSF TFs in A. tanguticus
To screen for HSF genes in A. tanguticus, we obtained the whole genome data (login number: CRA005659) from Genome Sequence Archive, Data Center of Beijing Institute of Genomics, Chinese Academy of Sciences (https://bigd.big.ac.cn/). We retrieve the protein sequences of the 25 AtHSFs from the A. thaliana genome database (TAIR) (https://www.arabidopsis.org). Used the A . thaliana HSF amino acid sequence (AtHSFs) as queries, with HMMER3 and HSF-type DBD model (PF00447) (https://www.ebi.ac.uk/interpro/entry/pfam) to search the genome database of A. tanguticus. To ensure functional relevance and verify the integrity of the HSF domain in the identified HSF family members, all putative proteins were rigorously screened using the CD-Search (https://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) and SMART tools (http://smart.embl.de/), requiring unambiguous conservation of DBD structural motifs [32].
We utilized the online tool ProtParam (https://web.expasy.org/protparam/) to predict physicochemical properties of A. tanguticus HSF proteins, including sequence length, molecular weight, theoretical isoelectric point (pI), instability index, aliphatic index, and grand average of hydropathicity (GRAVY).
Subcellular localization of the identified AntHSF family members was predicted using the online websites WoLF PSORT (https://psort.hgc.jp/), and Cell-PLoc (http://www.csbio.sjtu.edu.cn/bioinf/ Cell-PLoc/).
Phylogenetic tree, motif composition and gene structure analysis of AntHSF
Multiple sequence comparison analysis of the A. tanguticus and A. thaliana HSF families was performed using MEGA software (version.11.0.13) (https://www.megasoftware.net/) based on ClustalW default parameters [33].
A comparative phylogenetic tree reconstructing the evolutionary relationship between A. thaliana and A. tanguticus was constructed using the Maximum Likelihood (ML), algorithm in MEGA software (version.11.0.13), and 1000 bootstraps were used to measure the stability of the branch node [34]. Phylogenetic trees were beautified using the online program ITOL (https://itol.embl.de/). A structural map of AntHSF genes was constructed from A. tanguticus genomic data using TBtools-II software (version 2.056) [34].
MEME (https://meme-suite.org/meme/tools/meme) online website was used to analyze the conserved motifs of AntHSF1-20 protein sequences. The number of motifs was set to 10, and other parameters were kept as default values.
To explore the cis-acting elements in the promoter region of AntHSFs family , we extracted the 2,000 bp sequence upstream of each AntHSF gene as the promoter region and submitted it to PlantCARE database (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/) for cis element prediction. Finally, TBtools-II software (version. 2.056) [34] was used to analyze and visualize the structure, conserved motifs, and cis-acting elements of 20 AntHSF genes.
Chromosome mapping, gene duplication and collinearity analysis of AntHSFs
The specific physical information of the AntHSFs gene of A. tanguticus was obtained through the annotation information of the whole genome of A. tanguticus (Kunming Institute of Botany, Chinese Academy of Sciences), and the chromosomal localization map was drawn with TBtools-II software (version. 2.056) [34].
Based on the whole genome sequence, annotation file and protein sequence of A. tanguticus, A.thaliana, C. sativa, Sorghum bicolor (L.) Moench, Vigna unguiculata (L.) Walp, S. lycopersicum, and Zea mays L. TBtools (version. 2.056) was used to analyze the chromosomal intraspecific collinearity of A. tanguticus and six other plants and perform multiple collinearity scans in software kit (Multiple Collinearity Scan toolkit MCScanX) gene duplication and collinear block type. The non-synonymous and synonymous ratios (Ka/Ks) between gene pairs were obtained by TBtools (version. 2.056).
Gene transcription profile in response to heat stress
The transcriptomic abundance of 20 AntHSFs were studied using the transcriptome data of A. tanguticus under temperature stress using standard transcriptome analysis procedures. Transcriptome sequencing analysis of the AntHSF family was performed by TPM levels of AntHSF under heat treatment. All 20 AntHSFs were constitutionally expressed in the samples (TPM >1). The heatmap of AntHSF expression was generated by TBtools (version. 2.056) using TPM values, with data normalized to the average expression level of each gene across all samples and log₂-transformed for visualization [35].
Quantitative real‑time PCR (qRT-PCR) test for candidate genes
Quantitative real time-PCR (qRT-PCR) assays were used to confirm RNA-seq results and analyze target genes expression. Total RNA (2 µg) was used to synthesize cDNA with an RT EasyTM II Kit (Forgene, Chengdu, China). qRT-PCR was performed using ChamQ Universal SYBR qPCR Master Mix (Transgene, Beijing, China). Gene-specific qRT-PCR primers were designed using Primer Premier 5.0 (Premier Biosoft International, Palo Alto, CA, USA), primers are listed in Supplementary Table 3. Data were collected from three biological and three technical replicates, normalized using AntActin (F: TGACGGCGAGGATATTCAGC, R: TGCTCGAGGAGCATCATCAC). The 2−ΔΔCT method was used to analyze the relative transcript levels of the genes [36].
The construction of vector and sub-cellular localization of HSF genes
The cDNA sequences of the AntHSF6, AntHSF7, and AntHSF9 genes were ligated with the linearized vector p121-ncmCherry-RFP (Supplementary Table 3) and transformed into competent Escherichia coli DH5α cells. After the extraction of the cloned plasmid and the transformation of Agrobacterium tumefaciens GV3101, tobacco (Nicotiana benthamiana) transient transformation was conducted. Then the fusion plasmids and control plasmid were transiently expressed in the above-ground, and the transformed N. benthamiana were incubated in the darkness. After 48 h, Red Fluorescent Protein (RFP) fluorescence signals and chloroplasts were observed using confocal microscope (Leica, Germany) [37].
Statistical analysis
Statistical analyses of the data were conducted utilizing Microsoft Excel 2021, GraphPad Prism (version. 9.5.0), and IBM SPSS Statistics (version. 26). Enzyme activity and qRT-PCR data under heat treatments were analyzed using one-way ANOVA, with significant differences identified by the LSD test at p ≤ 0.05. Figures were generated using GraphPad Prism (version. 9.5.0) [38].
Results
Heat treatment affected the oxidase activity of A. tanguticus
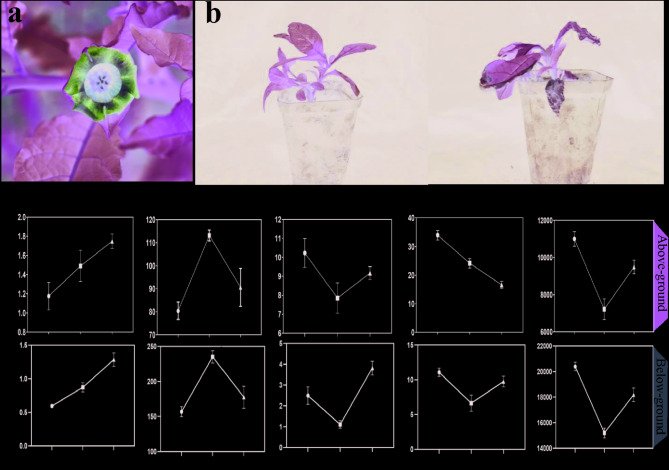
To evaluate the impact of high temperatures on A. tanguticus, we placed the plants into an incubator set continuous high temperature of 35℃. After three days of treatment, wilting was observed in the leaves of the plants (Fig. 1a, b). To clarify how high temperatures affect plants, we further measured the antioxidant enzyme activities in the above-ground and below-ground parts of the plants after 12 h and 24 h heat treatments. In the above-ground, for H2O2 content, there was a continuous increase over time under both 12 h and 24 h heat treatments compared to the control (CK), indicating an accumulation of hydrogen peroxide [39]. Superoxide dismutase (SOD) activity initially rose and then fell after 12 h and 24 h heat treatments, suggesting an initial activation of the antioxidant defense mechanism followed by a possible decline in its effectiveness [40]. Catalase (CAT) activity showed a fluctuating trend with a decrease after 12 h heat treatment and a subsequent increase after 24 h heat treatment. Heat treatment induced progressive H2O2 accumulation, indicative of elevated oxidative stress [41]. Conversely, decreased malondialdehyde (MDA) content suggests mitigated membrane peroxidation, likely due to enhanced ROS scavenging by antioxidant enzymes and adaptive lipid metabolism stabilizing membrane integrity [42]. Peroxidase (POD) activity also presented a fluctuating pattern with a significant drop after 12 h treatment and a subsequent increase after 24 h treatment. In the below-ground, the change of H2O2, SOD and POD content showed a similar pattern in the above-ground but with different magnitudes. CAT and MDA activity first decreased and then increased, showing a V-shaped trend (Fig. 1c).
Fig. 1.
Changes in A. tanguticus after heat treatment. a The flower of A. tanguticus. b Morphological of A. tanguticus after 72 h of 35℃. c Antioxidant enzyme activity indicators of the Above-ground parts and Below-ground of A. tanguticus after 12 h and 24 h of 35℃. The same letter are not significantly different (p ≤ 0.05)
Transcriptomics analysis indicated the crucial role of HSFs under heat stress in A. tanguticus
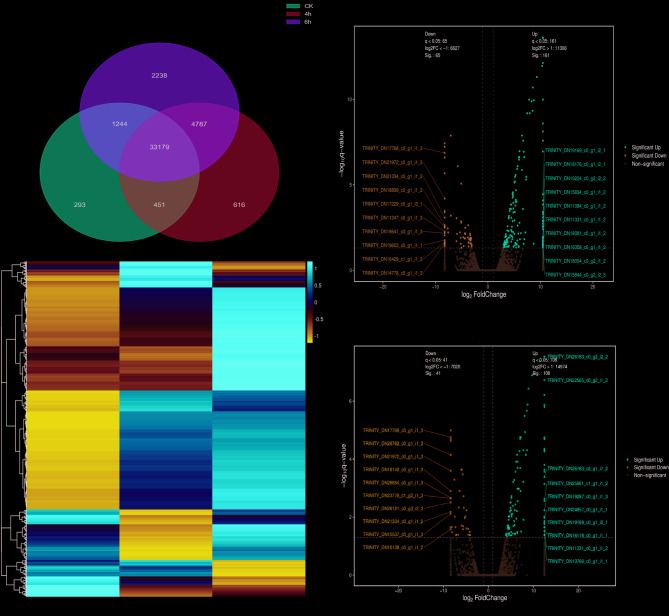
To further elucidate how A. tanguticus alleviates the effects when confronted with high-temperature stress, the transcriptomics analysis was utilized to explore the underlying mechanisms. Considering the dramatical change of antioxidant enzyme content in A. tanguticus plants at 12 h heat treatment, we selected the plants treated with heat for 4 h and 6 h as samples for transcriptome sequencing analysis. Transcriptome analysis revealed 39,025 expressed genes after 4 h of heat treatment, whereas only 24,139 genes exhibited detectable transcriptional activity after 6 h of exposure. At 4 h heat treatment, 161 DEGs were up-regulated, while 65 DEGs were down-regulated. At 6 h, 108 DEGs were up-regulated and 41 DEGs were down-regulated (Fig. 2c). The Venn diagram (Fig. 2a) of differentially expressed genes at 0 h, 4 h, and 6 h after heat treatment highlights the unique and common characteristics of the genes at each time point, providing key insights for a deeper understanding of the dynamic changes in gene expression in plants under heat stress [43].
Fig. 2.
Transcriptome analysis of A. tanguticus responses to Heat treatment. a Venn diagram illustrating the overlap of DEGs under CK and heat treatment 4 h, 6 h. b Heatmap illustrating the hierarchical clustering results of RNA-sequencing (RNA-seq). c Volcano plots depicting the differential expression of genes between CK, 4 h and 6 h heat-treated
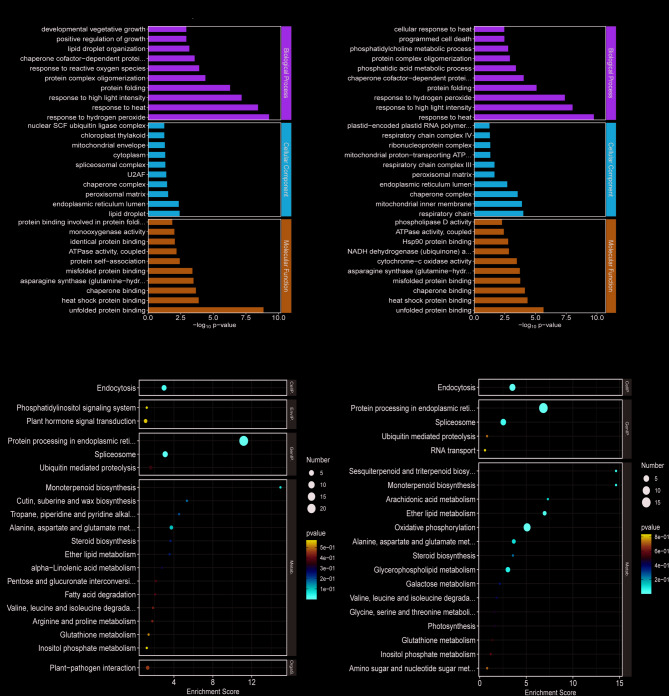
The GO and KEGG functional annotation analysis DEGs was performed. The GO functional annotation of DEGs in the 4 h and 6 h heat treatment groups was highly similar, most of DEGs were significantly enriched in pathways like response to hydrogen peroxide (GO:0042542), response to heat (GO:0009408), response to ROS (GO:0000302), positive regulation of growth (GO:0045927), unblocked protein binding (GO:0051082), and heat shock protein binding (GO:0031072), (Fig. 3a, Supplementary Table 1). These results suggest that heat stress activates coordinated transcriptional responses in A. tanguticus, involved gene expression of hydrogen peroxide response, heat stress, high light intensity response, and ROS response under heat conditions [44]. In the 12 h heat treatment may affect the respiratory chain and chloroplasts of A. tanguticus, enhancing its ability to remove ROS, thereby restoring normal growth and development in heat-stressed plants [44].
Fig. 3.
The enrichment of differential expression genes. a GO enrichment pathway of top 30 term analysis for 4 h and 6 h heat treatments. b KEGG enrichment pathway of top 20 term analysis for 4 h and 6 h heat treatments
In addition, KEGG enrichment analysis revealed that the DEGs were primarily enriched in 70 pathways. In the 4 h and 6 h heat treatments, DEGs were mainly concentrated in metabolism, genetic information processing, environmental information processing, and biological systems. Notably, pathways such as Protein processing in the endoplasmic reticulum (ko04141), Endocytosis (ko04144), and Oxidative phosphorylation (ko00190) were significantly enriched (Fig. 3b, Supplementary Table 2).
Upon further analysis of the enriched ROS response pathways, it was found that the expression of the HSF genes underwent significant changes. Additionally, a deeper examination of the protein processing pathway in the endoplasmic reticulum (ER) from the KEGG database revealed that both HSP and HSF genes exhibited alterations in expression, with |log2FC| >1 (Table 1). This suggested that HSF and HSP may play crucial regulatory roles in responding to heat stress, participating in the regulation of cellular response mechanisms [45], which motivated the indispensability of identifying HSFs family in A. tanguticus plants.
Table 1.
Description and expression of genes in A. tanguticus involved in the response to reactive oxygen species signaling pathway. (q-value < 0.05 & |log2FC| >1.0)
| GENE ID | Description (based on A. thaliana orthologs) | Expression level | ||
|---|---|---|---|---|
| CK | 4 h | 6 h | ||
| TRINITY_DN27637_c0_g1_i12_2 | HSF-type DNA-binding | 2.87 | 4.17 | 5.35 |
| TRINITY_DN29818_c0_g1_i1_1 | HSF-type DNA-binding | 3.59 | 6.6 | 12.99 |
| TRINITY_DN11119_c0_g1_i1_3 | Hsp20 protein | 11.4 | 1591.99 | 3661.31 |
| TRINITY_DN21375_c0_g1_i2_3 | Hsp20 protein | 15.3 | 8699.3 | 11161.8 |
| TRINITY_DN23706_c0_g2_i1_1 | Hsp20 protein | 2.1 | 4.01 | 6.78 |
| TRINITY_DN28800_c1_g1_i5_1 | Hsp20 protein | 5.08 | 148.62 | 196.49 |
| TRINITY_DN30060_c0_g1_i2_1 | Hsp20 protein | 17.2 | 792.2 | 1478.02 |
| TRINITY_DN31975_c0_g2_i1_1 | Hsp20 protein | 3.42 | 562.34 | 1488.89 |
| TRINITY_DN33130_c0_g2_i1_1 | Hsp20 protein | 4.43 | 164.51 | 385.39 |
| TRINITY_DN33363_c3_g6_i1_1 | Hsp20 protein | 37.25 | 1668.35 | 3195.79 |
| TRINITY_DN34036_c0_g1_i2_1 | Hsp20 protein | 33.97 | 1019.95 | 1602.12 |
| TRINITY_DN26133_c1_g2_i1_3 | Hsp70 protein | 50.44 | 190.71 | 241.57 |
| TRINITY_DN28073_c1_g1_i1_2 | Hsp70 protein | 14.03 | 88.93 | 108.24 |
| TRINITY_DN29261_c2_g1_i3_2 | Hsp70 protein | 301.99 | 904.01 | 1215.2 |
| TRINITY_DN29512_c0_g1_i3_2 | Hsp70 protein | 142.67 | 191.19 | 279.48 |
| TRINITY_DN31400_c0_g1_i2_1 | Hsp70 protein | 3.15 | 7.1 | 14.96 |
| TRINITY_DN31400_c0_g5_i1_1 | Hsp70 protein | 2.4 | 6.99 | 11.17 |
| TRINITY_DN34494_c1_g1_i1_1 | Hsp70 protein | 3.11 | 171.08 | 315.3 |
| TRINITY_DN26168_c0_g3_i1_3 | Hsp90 protein | 187.05 | 442.76 | 582.91 |
| TRINITY_DN28902_c1_g1_i3_1 | Hsp90 protein | 64.97 | 367.7 | 621.24 |
| TRINITY_DN28975_c0_g1_i2_2 | Hsp90 protein | 25.58 | 79.7 | 83.16 |
| TRINITY_DN29368_c1_g2_i3_2 | Hsp90 protein | 31.08 | 102.66 | 157.93 |
| TRINITY_DN32674_c0_g2_i8_1 | Hsp90 protein | 4.7 | 239.51 | 533.43 |
| TRINITY_DN34470_c0_g1_i1_1 | Hsp90 protein | 14.99 | 48.69 | 78.99 |
| TRINITY_DN22670_c0_g1_i1_2 | Peroxidase | 3.82 | 6.73 | 12.34 |
| TRINITY_DN24436_c0_g1_i1_1 | Peroxidase | 15.89 | 38.42 | 83.29 |
| TRINITY_DN25548_c0_g2_i2_3 | Peroxidase | 86.84 | 103.25 | 149.12 |
| TRINITY_DN28387_c0_g1_i1_1 | Peroxidase | 2.54 | 6.36 | 12.25 |
| TRINITY_DN29352_c0_g1_i9_2 | Peroxidase | 156.21 | 337.12 | 389.37 |
Genome-wide identification of the HSF genes in A. tanguticus
Combined HMMER 3 (PF00447 DBD model ) and BLASTP (E-value ≤ 1e⁻¹⁰, score ≥ 100) searches using A. thaliana AtHSFs as queries identified 23 candidate AntHSFs in A. tanguticu [46]. Following the CD-Search/SMART validation for DBD conservation and the removal of redundant sequences, a total of 20 non-redundant AntHSF genes (AntHSF1-20) were identified (Supplementary Table 3).
The genomic and amino acid sequences of AntHSF genes were listed in Supplementary Table 3. Then, the analysis of each AtHSF proteins, including chromosomal location, protein length, isoelectric point (pI), subcellular prediction show that most of the proteins are expressed in the nucleus and cytoplasm, and transmembrane structural domain, were conducted (Supplementary Table 4). The total length of AntHSF protein ranges from 122 to 501 amino acids, and the molecular weight ranges from 14.09 to 55.87 kDa. In addition, the pIs ranges from 4.75 to 9.25, with an average of 5.998, the average of GRAVY is −0.7012, indicating that these proteins were hydrophilic (Supplementary Table 4) [47].
Multiple sequence alignment and phylogenetic classification of AntHSFs
To investigate the evolutionary relationships among the AntHSF family members, the ML phylogenetic tree was constructed using MEGA, including the amino acid sequences of 20 AntHSF and 25 AtHSF (A. thaliana) proteins [48]. Based on the established classification of the A. thaliana HSF family, the AntHSFs were categorized into three main classes: HSFA, HSFB, and HSFC (Fig. 4a). The phylogenetic tree classifies HSFs into three major classes: HSFA, HSFB, and HSFC. The HSFA class contains 13 members, which are further divided into subgroups A1 to A8. The HSFB class contains 6 members, classified into 4 subgroups: B1 to B4. The HSFC class includes a single member. The classification of AntHSF validates the diversity of protein structures and suggests that the different members of the subfamily might have different regulatory functions [9]. This underlines the potential for AntHSF proteins to play different roles in coordinating plant responses and adapting to various environmental signals. Among the 20 AntHSFs proteins, we identified complete or nearly complete conserved sequences (Fig. 4b) [49].
Fig. 4.
Phylogenetic analysis and sequence alignment of AntHSF protein. a Phylogenetic tree showing the relationship and classification of HSF proteins in A. tanguticus and A.thaliana, with different colors representing different classifications. b Alignment of the amino acid sequences of AntHSF proteins
Analysis of gene structure in AntHSFs
The gene structure of AntHSF indicates that HSF genes within the same group generally possess a comparable number of introns in their structure (Supplementary Fig. 1). Conserved motif analysis via MEME identified 10 distinct motifs (motif1–motif10) in AntHSF proteins, revealing conserved structural diversity within the HSF family (Supplementary Fig. 1) [10]. As expected, the same subgroups of AntHSF showed highly consistent conserved motifs. Motifs 1, 2, and 3 are present in most genes, while motif 6 was exclusive to the HSFB class. Motifs 7, 8, 9, and 10 were specific to the HSFA class. Notably, the HSFC class contains only motifs 1, 2, 3, and 4, which might be associated with the absence of certain domains [50]. Promoter analysis (2,000 bp upstream of ATG) of AntHSF genes using PlantCARE identified 38 cis-acting elements, categorized into abiotic and biotic stresses, phytohormones responsive and plant growth and development (Fig. 5, Supplementary Table 5). Interestingly, almost all promoter regions of AntHSF genes contained numerous light-responsive elements, indicating a synergistically coordinated with the responses to other environmental factors [51]. The promoter regions of AntHSF5 and AntHSF7 contained 12 light-responsive elements, suggesting a more sensitive response to the light. The number of hormone-responsive motifs in the promoter of AntHSF8 and AntHSF17, in response to the methyl jasmonate acid and abscisic acid, respectively, suggests a possible gene activation by these two hormones [52].
Fig. 5.
Number of each cis-acting element in the promoter region of AntHSF genes. The color intensity of grids correlates positively with the number of cis-elements
Chromosomal localization and collinearity analysis of AntHSF genes
The chromosomal mapping revealed conserved collinearity and physical distribution patterns among AntHSF genes [53]. The chromosomal distribution map was created based on the position information of the family members, and it showed that the AntHSF genes were unevenly distributed across 14 chromosomes and relatively dispersed. Notably, the AntHSF genes within the same subfamily were in proximity at the same or adjacent chromosome (Supplementary Fig. 2) [54].
To explore the evolutionary relationships of AntHSF among diverse species, the synteny analysis was constructed that included three dicotyledonous plants (A. thaliana, C. sativa, S. lycopersicum) and three monocotyledonous plants (V. unguiculata, S. bicolor, Z. mays) (Fig. 6). It was found that the AntHSF gene family exhibited a strong syntenic relationship with the HSF genes of dicotyledonous plants, with C. sativa showing the highest level of synteny, followed by A. thaliana and S. lycopersicum. The analysis showed that A. thaliana exhibited 75% synteny with A. tanguticus. In contrast, A. tanguticus demonstrates only 15% synteny with Z. mays, highlighting the more distant evolutionary relationship between dicots and monocots (Fig. 6). Although these three monocotyledonous plants are heat-tolerant [55], the collinearity between their HSF families and that of A. tanguticus was extremely low.
Fig. 6.
Collinearity analysis of HSF genes between AntHSF and other plant species. Visualization of gene collinearity among AntHSF and three dicotyledon plants (A. thaliana, Solanum lycopersicum, Camelina sativa), and three monocotyledon plants (Vigna unguiculata, Sorghum bicolor, Zea mays). The gray lines represent large-scale collinear blocks of the genome between AntHSF and other plants, while the red lines indicate homologous relationships of HSF genes
To investigate the evolutionary constraints on the HSF genes, we calculated the Ka/Ks ratios for the gene pairs identified with segmental duplication and collinearity [56]. Excluding genes with unavailable data (Supplementary Table 6), the Ka/Ks ratios was < 1. This indicated that the HSF gene family likely experienced significant purifying selection during its evolution [16].
Differences in the expression of HSF gene family members in A. tanguticus
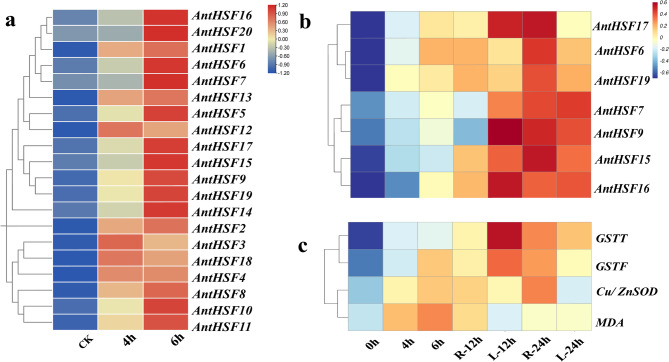
Based on the RNA-seq data, the expression profiles of AntHSF genes with heat-treated were analyzed (Fig. 7a). It was showed that certain genes, including AntHSF3, AntHSF12 and AntHSF18, might show rapid upregulation within 1–3 h of heat treatment, indicating their role in immediate stress signaling. Other genes, like AntHSF7 and AntHSF20, might exhibit delayed activation, peaking after 6 h, suggesting involvement in prolonged stress adaptation or recovery. Part of AntHSF genes, including AntHSF1, AntHSF2 and AntHSF13, maintained high expression throughout the treatment.
Fig. 7.
Expression profile of AntHSF genes and Antioxidant enzyme activity gene under heat stress in A. tanguticus plants. a The expression profile of AntHSF gene under 4 h and 6 h heat stress. b qRT-PCR analysis of AntHSF gene expression at 0 h, 4 h, 6 h, 12 h, 24 h in A. tanguticus plants subjected to heat stress. c qRT-PCR analysis of Antioxidant enzyme activity gene expression at 0 h, 4 h, 6 h, 12 h, 24 h of A. tanguticus plants at heat treatment. “R” represents root, and “L” represents leaf
To further investigate the expression pattern of HSFs under heat-treated in A. tanguticus, we selected seven genes from the AntHSFs family (AntHSF6, AntHSF7, AntHSF9, AntHSF15, AntHSF16, AntHSF17, and AntHSF19) based on the results of the cis-acting element analysis (Supplementary Table 5). The results demonstrated that in seedling stage (Fig. 7b), the seven HSF genes exhibited different response variations in different tissues under heat stress. Among them, in seedling stage (Fig. 7b), the below-ground part, except for AntHSF15 gene, the expression levels of other genes were downregulated after short-term heat treatment (12 h) and up-regulated after extended heat treatment (24 h). The expression of AntHSF15 gene in above-ground part was up-regulated according to the increase of heat treatment time. In the below-ground part, gene expression levels of AntHSF6, AntHSF9, AntHSF15, AntHSF16 and AntHSF17 were up-regulated after 12 h heat treatment, and down-regulated under 24 h heat treatment, and AntHSF6 expression level changed the most. The AntHSF7 gene showed an upward trend with the extension of processing time. The expression trend of AntHSF19 gene after heat stress was opposite to that of other genes. The expression level of AntHSF19 gene decreased in 12 h and increased after 24 h heat treatment. On the whole, all the seven genes were able to respond to high temperature stress, and showed different trend in the above-ground part, suggesting that they may play a dynamic role in the regulation of high temperature stress.
Through the combined analysis of enzyme activity and transcriptomic GO enrichment results, we found that the HSF gene may play a crucial role in regulating key enzyme genes. To underly the potential of AntHSF, the qRT-PCR was conducted to analyze the expression levels of MDA, GSTT, GSTF, and Cu/ZnSOD genes at 0, 4, 6, 12, and 24 h, which further supported the dynamic changes in enzyme activity and gene expression [18, 57]. The expression of GSTT and GSTF genes rapidly increased from 0 to 12 h, showing a parallel trend with the decrease in MDA enzyme content (Fig. 1c). From 12 to 24 h, these genes continued to be highly expressed, maintaining elevated levels, which supports the further reduction of MDA gene (Fig. 7c). The Cu/ZnSOD gene was significantly upregulated from 0 to 12 h, which correlated strongly with the increase in SOD activity, confirming its transcriptional regulation as a core mechanism for early ROS clearance (Fig. 7c) [44]. From 12 to 24 h, the expression level decreased, consistent with the decline in SOD activity, possibly due to a negative feedback regulation triggered by the reduced H₂O₂ levels (Fig. 7c) [41].
Notably, the expression of HSF genes exhibited a distinct stage-specific coordinated pattern: during the early stress phase (0–12 h), AntHSF6, AntHSF15, AntHSF16, and AntHSF17 (Fig. 7b) were upregulated in synchrony with GSTT and GSTF; during the later recovery phase (12–24 h), the expression of AntHSF6, AntHSF9, AntHSF15, AntHSF16, AntHSF17, and AntHSF19 stabilized, aligning with the steady-state levels of SOD activity and the expression trend of GSTF (Fig. 7b), suggesting that the HSF family may coordinate the temporal response of antioxidant enzyme genes through a stage-specific regulatory network [12].
The subcellular localization of AntHSF6, AntHSF7 and AntHSF9
In conjunction with the qRT-PCR experimental results, the significant differences in expression levels of AntHSF6 and AntHSF7 suggest that these two genes may play pivotal roles in cellular response processes (Fig. 7b-c). Additionally, we discovered that AntHSF9 was potentially involved in the oxidative enzyme activity pathway, implying its possible participation in cellular oxidative stress responses (Supplementary Table 2). Based on these findings, we conducted subcellular localization experiments for the three genes AntHSF6, AntHSF7, and AntHSF9 to elucidate their specific distributions and functions within the cell (Fig. 8). The experimental results revealed that the RFP fusion protein of AntHSF6 was predominantly localized in the nucleus, with a minor distribution on the plasma membrane. The AntHSF7 protein was primarily localized in the endoplasmic reticulum, suggesting the participation in biological processes related to oxidative stress, particularly in the endoplasmic reticulum region of the cytoplasm. In contrast, the AntHSF9 protein was mainly distributed in the nucleus, indicating that the AntHSF9 gene may be involved in the regulation of gene expression within the nucleus.
Fig. 8.
The Subcellular localization analysis of AntHSF6, AntHSF7 and AntHSF9 protein
Discussion
As a clinically essential anesthetic species endemic to vulnerable plateau ecosystems, A. tanguticus faces unique challenges under global climate change scenarios. Our integrated physiological, transcriptomic, and evolutionary analyses reveal multilayered adaptation mechanisms that enable this medicinal plant to withstand thermal stress while maintaining essential metabolic functions. The observed responses span from rapid antioxidant system modulation to evolutionary innovations in heat shock transcription factor (HSF) regulation, painting a comprehensive picture of alpine plant thermo-tolerance.
Hierarchical oxidative stress management system
The physiological cascade observed under heat treatment demonstrates A. tanguticus’ sophisticated oxidative damage control strategy. The initial surge in superoxide dismutase (SOD) activity (peaking at 12 h) followed by subsequent decline mirrors the biphasic ROS neutralization pattern reported in Rhodiola crenulate (Hook. f. & Thomson) H. Ohba, another high-altitude species [58]. This transient activation suggests strategic resource allocation-immediate superoxide radical conversion prioritizes prevention of cellular macromolecule damage, while later phase shifts to hydrogen peroxide management through coordinated catalase (CAT) and peroxidase (POD) activities (Fig. 1c).
Notably, the progressive decline in malondialdehyde (MDA) content despite sustained H2O2 accumulation (Fig. 1c) indicates two distinct protective mechanisms: (1) Primary antioxidant enzyme-mediated ROS quenching, which limits oxidative damage, and (2) Secondary membrane stabilization through enhanced lipid metabolism. These regulatory mechanisms may attenuate lipid peroxidation cascades, thus effectively limiting MDA accumulation even under sustained in the presence of elevated ROS levels. This dual strategy aligns with recent findings in Hippophae rhamnoides L., where heat-acclimated plants showed increased phospholipid biosynthesis gene expression [59].
The dynamic expression patterns of GSTT, GSTF, and Cu/ZnSOD genes highlight a layered system for managing oxidative stress. GSTT and GSTF are rapidly upregulated during the early stages (0 to 12 h), reducing oxidative damage as indicated by the decrease in MDA levels [18, 35, 57]. Their continued high expression from 12 to 24 h suggests sustained detoxification efforts to maintain cellular homeostasis. Cu/ZnSOD, upregulated from 0 to 12 h, plays a key role in scavenging superoxide radicals, with its expression and activity decreasing as oxidative stress subsides [60]. This negative feedback mechanism ensures ROS levels are tightly controlled, preventing overexpression of antioxidants [61]. Together, these genes form an integrated response to oxidative stress, balancing ROS levels to protect cells and restore homeostasis [39]. Future research should explore the molecular mechanisms regulating these genes and their interaction with other cellular pathways [44].
Transcriptional reprogramming and HSF network dynamics
The transcriptomic landscape under heat treatment reveals A. tanguticus’ evolutionary optimization for thermal responsiveness. Of the 2,347 DEGs identified, 68.3% showed immediate response within 4 h (Fig. 2c and Supplementary Table 7), contrasting with the delayed activation patterns observed in lowland species [62]. This rapid genomic mobilization likely reflects adaptation to the extreme diurnal temperature fluctuations characteristic of plateau environments [6].
Central to this response is the HSF regulatory network. Our identification of 20 AntHSF genes (Supplementary Table 3) represents the first comprehensive characterization in A. tanguticus, revealing both conserved and novel features compared to model species. While the gene count is lower than in A. thaliana (25) or S. lycopersicum (24), we calculated the Ka/Ks ratio for all of the identified gene pairs and found that all of the values were less than one, indicating that the HSF gene family might have experienced strong purifying selection pressure during evolution [63]. (Supplementary Table 6). Phylogenetic analysis positions AntHSFs within clade-specific clusters (Fig. 4), with AntHSF7/9/15 forming a distinct branch potentially associated with alpine adaptation-a pattern reminiscent of H. tibetana Schltdl HSF diversification [64].
Promoter analysis uncovered an exceptional density of stress-responsive cis-elements (Fig. 5), including 12.3 elements/gene versus 8.7 in A. thaliana HSF promoters [65]. This enrichment extends beyond heat-responsive motifs to include ABA-responsive elements (ABREs) and drought-inducible sequences (Supplementary Table 5), suggesting A. tanguticus employs combinatorial stress signaling strategies. The co-occurrence of light-responsive elements (G-box, Sp1) in 85% of AntHSF promoters implies photoperiodic regulation of thermo-tolerance-a crucial adaptation for high UV environments [66].
Functional specialization of key AntHSF members
The average GRAVY value of −0.7012 indicates that these AntHSF proteins are hydrophilic, which is consistent with their probable role as transcription factors that interact with water-soluble molecules such as RNA and other regulatory proteins [67]. When compared to other heat shock factors (HSF) from different species, the hydrophilic nature of AntHSF proteins is similar to that observed in plant HSFs, which are typically involved in the regulation of stress response genes [68]. This is in contrast to transcription factors like the bZIP family, which are more hydrophobic and often associate with membranes [69].
The dual regulatory features of AntHSF7 enriched light-responsive cis-elements in its promoter and endoplasmic reticulum (ER) localization suggest a unique adaptive mechanism integrating environmental sensing and organelle-specific stress management (Figs. 5 and 8). The significant upregulation of AntHSF7 under heat treatment (Fig. 7) implies its potential role in ER-associated protein quality control, possibly through coordination with heat-induced unfolded protein response (UPR) pathways. The light-responsive elements (e.g., G-box, Sp1) may enable cross-talk between photoperiodic signals and thermal adaptation, allowing A. tanguticus to synchronize heat shock responses with high-light conditions prevalent in plateau ecosystems. This dual localization and regulatory architecture positions AntHSF7 as a key integrator of abiotic stressors, potentially modulating ROS signaling and chaperone networks at the ER-membrane interface during combined light/heat stress [70]. This is consistent with findings in S. lycopersicum, where heat stress activates HsfA1a, which directly regulates the transcription of small heat shock proteins (sHSPs) in the endoplasmic reticulum [71].
The phylogenetic clustering of AntHSF6 with AntHSF7 despite their distinct chromosomal loci suggests divergent evolution following gene duplication, potentially driven by sub-functionalization or neo-functionalization. Such divergence is further supported by their differential expression dynamics under heat treatment. Unlike canonical heat shock factors (HSFs) that rapidly activate during early stress, AntHSF6 exhibits delayed induction, peaking at 12 h in the above-ground before returning to baseline by 24 h (Fig. 7b). This delayed response implies a specialized role in prolonged heat adaptation, possibly coordinating late-stage protective mechanisms, such as cellular repair or secondary stress signaling. The transient expression spike aligns with its nuclear localization, typical of HSFs regulating heat-responsive genes, while its dual localization to the membrane hints at unconventional roles, such as membrane-associated signaling or stress perception (Fig. 8), which resemble the osmosensory functions of SlHSFA3 in tomato [72].
Besides, the AntHSF9 (GENE ID TRINITY_DN27637_c0_g1_i12_2), which is nuclear localization and persistent upregulation position as a transcriptional orchestrator. Its enrichment in “oxidase activity” pathways (Fig. 3; Table 1) suggest dual roles in ROS signaling and proteostasis. The identified SUMOylation site (Lys152) may enable stress-dependent subnuclear repositioning, as reported for OsHSFA2d in rice [17].
Evolutionary context and comparative genomics
The AntHSF family’s compact size (20 genes) compared to other angiosperms reflects both evolutionary history and environmental pressures (Fig. 6). Whole-genome duplication (WGD) analysis dates the last polyploidization event to ~ 800 MYA, preceding the Tibetan Plateau uplift [73]. Unlike C. sinensis, which expanded HSF genes through recent WGD [14], A. tanguticus retained only 32% of ancestral HSF paralogs, indicating stringent selection.
Collinearity analysis reveals 68% synteny with dicot HSFs versus 22% with monocots (Fig. 6), consistent with molecular dating placing A. tanguticus divergence after monocot-dicot split [50]. The several pairs of tandem - duplicated HSF genes in A. tanguticus that exhibit signs of positive selection, particularly in their DNA-binding domains, which may enhance their ability to recognize promoters under varying thermal conditions [53]. The stage-specific adaptation strategies have critical implications for conservation. Seedlings’ vulnerability during early suppression phases (0–12 h) coincides with peak UV-B radiation in Tibetan summer afternoons [24], creating synergistic stress. Mature plants’ delayed response paradigm suggests inherent resilience but risks metabolic imbalance during prolonged heatwaves. The observed trade-off between AntHSF16-mediated growth regulation and stress responses (Fig. 7) echoes recent findings in Quinoa [74], highlighting universal challenges in balancing productivity and stress tolerance.
While identifying core AntHSF networks, our study underscores several research frontiers:1) CRISPR-Cas9 validation. Prioritizing AntHSF7/9 for knockout/overexpression studies to dissect Light-Heat or ABA-ROS cross-talk; 2) Epigenetic regulation. Investigating DNA methylation patterns in HSF promoters across developmental stages; 3) Ecological genomics. Population-level analysis of HSF allelic variation along altitudinal gradients. The conservation of AntHSF functional domains (Supplementary Table 5) with medicinal species like Hyoscyamus niger L. [75]. opens possibilities for horizontal gene transfer studies.
Conclusions
In this study, the comprehensive genome-wide identification of HSF gene family members of A. tanguticus were conducted, and a total of 20 AntHSF genes were identified. Based on the phylogenetic relationships, these genes were divided into 3 major groups, and the physicochemical properties, phylogeny, gene structure and conserved motifs of these HSF proteins were analyzed, thereby laying a foundation for further comprehension of the evolutionary relationships of AntHSF gene families. In addition, through representative RNA-seq and qRT-PCR, the expression pattern and expression profile of AntHSFs under temperature stress were investigated, and 7 highly induced genes under temperature stress were selected (AntHSF6, AntHSF7, AntHSF9, AntHSF15, AntHSF16, AntHSF17, AntHSF19). Based on subcellular localization analyses, AntHSF6 (nuclear/membrane) and AntHSF7 (endoplasmic reticulum) are pivotal in cellular stress responses, while AntHSF9 (nuclear) likely regulates oxidative enzyme activity and gene expression.
This study provided solid and valuable information for understanding the classification and function of AntHSFs transcription factors, laid a foundation for further research on the function and regulatory mechanism of key genes under temperature stress of A. tanguticus, and provided candidate heat-resistant genes for the improvement of A. tanguticus varieties.
Supplementary Information
Supplementary Material 1. Figure S1: The analysis of the exon-intron structure, conserved motifs; Figure S2: Distribution of AntHSF in A. tanguticus on chromosomes; Figure S3: Statistical analysis of qRT-PCR validation for AntHSF gene expression; Figure S4: Statistical analysis of qRT-PCR validation for GSTT, GSTF, Cu/Zn SOD, MDA expression; Table S1:Functional classification of DEGs based on the GO dataset; Table S2: KEGG enrichment of DEGs under normal and heat treatment; Table S3: Primer sequences used in this study; Table S4: The amino acid information of AntHSF genes family identified in A. tanguticus; Table S5:The detailed functional annotations of cis-elements in promoter region of AntHSFs; Table S6: Segmentally duplicated AntHSF gene pairs; Table S7: Differentially Expressed Genes from RNA-seq; Table S8: The sequence of HSF transcriptional factors in A. tanguticus.
Acknowledgements
We extend their appreciation to the Researchers Supporting State Key Laboratory of Southwestern Chinese Medicine.We really appreciated the supports from Chengdu First Pharmaceutical.
Authors’ contributions
Z.H.L. carried out the design of this research work and writing this manuscript; Q.Z. and L.Q.F. curated the data; F.Q.L. and Y.X. performed the formal analysis and validated the results; Z.Q.L. developed the methodology; F.W. administered the project; Y.T.M., T.Z and B.J.X. supervised the study; Z.H.L. and B.J.X. wrote the main manuscript text; T.Z. and B.J.X. reviewed and edited the manuscript.
Funding
This study was supported by the Xinglin Talent Program of Chengdu University of TCM (No. MPRC2021030 and QJRC2024043).
Data availability
The sequencing data generated in this study are available under NCBI BioProject accession PRJNA1183605, which includes the BioSample accessions SAMN44835069, SAMN44835070, and SAMN44835071.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tao Zhou, Email: 364462907@qq.com.
Yuntong Ma, Email: mayuntong@cdutcm.edu.cn.
Binjie Xu, Email: binjiexu@outlook.com.
References
- 1.Chen J, Yang Y, Sun H. Advances in the studies of responses of alpine plants to global warming*. Chin J Appl Environ Biology. 2012;17:435–46. 10.3724/SP.J.1145.2011.00435. [Google Scholar]
- 2.Zhan P, Wang F, Xia P, Zhao G, Wei M, Wei F, et al. Assessment of suitable cultivation region for Panax notoginseng under different climatic conditions using maxent model and high-performance liquid chromatography in China. Ind Crops Prod. 2022;176:114416. 10.1016/j.indcrop.2021.114416. [Google Scholar]
- 3.Mahmoodi S, Heydari M, Ahmadi K, Khwarahm NR, Karami O, Almasieh K, et al. The current and future potential geographical distribution of Nepeta Crispa Willd., an endemic, rare and threatened aromatic plant of Iran: implications for ecological conservation and restoration. Ecol Indic. 2022;137:108752. 10.1016/j.ecolind.2022.108752. [Google Scholar]
- 4.Wen J, Zhou L, Liu L, He Y. Analysis of the impact of climate change on the distribution and active compound content of the plateau medicinal plant Nardostachys jatamansi (D. Don) DC. Ind Crops Prod. 2022;187:115438. 10.1016/j.indcrop.2022.115438. [Google Scholar]
- 5.Yang L, Min X, Wei Z, Liu N, Li J, Zhang Y, et al. Genome-wide identification and expression analysis of the Dof transcription factor in annual alfalfa medicago polymorpha. Plants. 2023;12:1831. 10.3390/plants12091831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma L, Sun X, Kong X, Galvan JV, Li X, Yang S, et al. Physiological, biochemical and proteomics analysis reveals the adaptation strategies of the alpine plant potentilla saundersiana at altitude gradient of the Northwestern Tibetan plateau. J Proteomics. 2015;112:63–82. 10.1016/j.jprot.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Zheng W, Wang L, Meng L, Liu J. Genetic variation in the endangered Anisodus tanguticus (solanaceae), an alpine perennial endemic to the qinghai-tibetan plateau. Genetica. 2008;132:123–9. 10.1007/s10709-007-9154-5. [DOI] [PubMed] [Google Scholar]
- 8.Westerheide D, Raynes S, Powell R, Xue C, Uversky BN. HSF transcription factor family, heat shock response, and protein intrinsic disorder. Curr Protein Pept Sci. 2012;13:86–103. 10.2174/138920312799277956. [DOI] [PubMed] [Google Scholar]
- 9.Scharf K-D, Berberich T, Ebersberger I, Nover L. The plant heat stress transcription factor (hsf) family: structure, function and evolution. Biochim Biophys Acta BBA - Gene Regul Mech. 2012;1819:104–19. 10.1016/j.bbagrm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Nover L, Scharf K-D, Gagliardi D, Vergne P, Czarnecka-Verner E, Gurley WB. The Hsf world: classification and properties of plant heat stress transcription factors. Cell Stress Chaperones. 1996;1:215. 10.1379/1466-1268(1996)001%3C0215:thwcap%3E2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Y, Wang Y, Xu F, Song C, Yang X, Zhang Z, et al. Small HSPs play an important role in crosstalk between HSF-HSP and ROS pathways in heat stress response through transcriptomic analysis in lilies (Lilium longiflorum). BMC Plant Biol. 2022;22:202. 10.1186/s12870-022-03587-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swindell WR, Huebner M, Weber AP. Transcriptional profiling of Arabidopsis heat shock proteins and transcription factors reveals extensive overlap between heat and non-heat stress response pathways. BMC Genomics. 2007;8:125. 10.1186/1471-2164-8-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu X, Huang C, Zhang L, Liu H, Yu J, Hu Z, et al. Systematic analysis of Hsf family genes in the Brassica napus genome reveals novel responses to heat, drought and high CO2 stresses. Front Plant Sci. 2017;8:1174. 10.3389/fpls.2017.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Zhang M, Hou X, Xiao B, Gao Y. Identification of the CsFtsH genes from camellia sinensis reveals its potential role in leaf color phenotype. Gene. 2024;927:148672. 10.1016/j.gene.2024.148672. [DOI] [PubMed] [Google Scholar]
- 15.Giorno F, Wolters-Arts M, Grillo S, Scharf K-D, Vriezen WH, Mariani C. Developmental and heat stress-regulated expression of HsfA2 and small heat shock proteins in tomato anthers. J Exp Bot. 2010;61:453–62. 10.1093/jxb/erp316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li G, Zhang H, Shao H, Wang G, Zhang Y, Zhang Y, et al. ZmHsf05, a new heat shock transcription factor from Zea mays L. improves thermotolerance in Arabidopsis thaliana and rescues thermotolerance defects of the Athsfa2 mutant. Plant Sci. 2019;283:375–84. 10.1016/j.plantsci.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Yokotani N, Ichikawa T, Kondou Y, Matsui M, Hirochika H, Iwabuchi M, et al. Expression of rice heat stress transcription factor OsHsfA2e enhances tolerance to environmental stresses in transgenic Arabidopsis. Planta. 2008;227:957–67. 10.1007/s00425-007-0670-4. [DOI] [PubMed] [Google Scholar]
- 18.Xie D-L, Huang H-M, Zhou C-Y, Liu C-X, Kanwar MK, Qi Z-Y, et al. HsfA1a confers pollen thermotolerance through upregulating antioxidant capacity, protein repair, and degradation in Solanum lycopersicum L. Hortic Res. 2022;9:uhac163. 10.1093/hr/uhac163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xia Q, Pingcuo R, Yang C, Xiong W, Peng X, Xia J, et al. A review on the chemical properties, plant sources, anti-shock effects, pharmacokinetics, toxicity, and clinical applications of anisodamine. Chem Biodivers. 2024;21:e202301477. 10.1002/cbdv.202301477. [DOI] [PubMed] [Google Scholar]
- 20.Ganaie MM, Reshi ZA, Verma V, Raja V. Family solanaceae: taxonomy and modern trends. Ann Plant Sci. 2018;7:2403–14. 10.21746/aps.2018.7.9.1. [Google Scholar]
- 21.He T, Jia JF. Breaking dormancy in seeds of Anisodus tanguticus: an endangered medicinal herb of high altitude in the qinghai-tibet plateau. Seed Sci Technol. 2009;37:229–31. 10.15258/sst.2009.37.1.26. [Google Scholar]
- 22.Wang B, Chen C, Xiao Y, Chen K, Wang J, Wang L, et al. A core root bacteria contribute to plant growth and anisodine accumulation of Anisodus tanguticus. BMC Plant Biol. 2023;23:655. 10.1186/s12870-023-04690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuang X, Jiao JJ. Review on climate change on the Tibetan plateau during the last half century. J Geophys Res Atmos. 2016;121:3979–4007. 10.1002/2015JD024728. [Google Scholar]
- 24.Chen C, Wang B, Li J, Xiao Y, Chen K, Liu N, et al. Predicting potential and quality distribution of Anisodus tanguticus (maxim.) pascher under different climatic conditions in the qinghai–tibet plateau. Front Plant Sci. 2024. 10.3389/fpls.2024.1369641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou T, Chang F, Li X, Yang W, Huang X, Yan J, et al. Role of auxin and gibberellin under low light in enhancing saffron corm starch degradation during sprouting. Int J Biol Macromol. 2024;279:135234. 10.1016/j.ijbiomac.2024.135234. [DOI] [PubMed] [Google Scholar]
- 26.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30:2114–20. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol. 2011;29:644–52. 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 29.Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008;36 suppl1:D480–4. 10.1093/nar/gkm882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, et al. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39 suppl2:W316–22. 10.1093/nar/gkr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corley SM, MacKenzie KL, Beverdam A, Roddam LF, Wilkins MR. Differentially expressed genes from RNA-seq and functional enrichment results are affected by the choice of single-end versus paired-end reads and stranded versus non-stranded protocols. BMC Genomics. 2017;18:399. 10.1186/s12864-017-3797-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, et al. CDD: ncbi’s conserved domain database. Nucleic Acids Res. 2015;43:D222–6. 10.1093/nar/gku1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall BG. Building phylogenetic trees from molecular data with MEGA. Mol Biol Evol. 2013;30:1229–35. 10.1093/molbev/mst012. [DOI] [PubMed] [Google Scholar]
- 34.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13:1194–202. 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad S, Khan K, Saleh IA, Okla MK, Alaraidh IA, AbdElgawad H, et al. TALE gene family: identification, evolutionary and expression analysis under various exogenous hormones and waterlogging stress in cucumis sativus L. BMC Plant Biol. 2024;24:564. 10.1186/s12870-024-05274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong K, Deng L, Zhao J, Yan X, Sun T, Li J, et al. A novel near-infrared fluorescent probe for highly selective recognition of hydrogen sulfide and imaging in living cells. RSC Adv. 2018;8:23924–9. 10.1039/C8RA03457E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Zhang J, Wang S, Liu Y, Yang M, Huang Y. Genome-wide identification of the pyrus R2R3-MYB gene family and PhMYB62 regulation analysis in Pyrus hopeiensis flowers at low temperature. Int J Biol Macromol. 2024;257:128611. 10.1016/j.ijbiomac.2023.128611. [DOI] [PubMed] [Google Scholar]
- 38.Peng M, Liu Z, Chen X, Xiao Y, Wang S, Yan Z, et al. LED blue light enhances the accumulation and synthesis of steroidal alkaloids in Fritillaria unibracteata Hsiao et K. C. Hsia in vitro. Ind Crops Prod. 2024;216:118836. 10.1016/j.indcrop.2024.118836. [Google Scholar]
- 39.Gong M, Chen B, o., Li Z-G, Guo L-H. Heat-shock-induced cross adaptation to heat, chilling, drought and salt stress in maize seedlings and involvement of H2O2. J Plant Physiol. 2001;158:1125–30. 10.1078/0176-1617-00327. [Google Scholar]
- 40.Alscher RG, Erturk N, Heath LS. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot. 2002;53:1331–41. 10.1093/jexbot/53.372.1331. [PubMed] [Google Scholar]
- 41.Luna CM, Pastori GM, Driscoll S, Groten K, Bernard S, Foyer CH. Drought controls on H2O2 accumulation, catalase (CAT) activity and CAT gene expression in wheat. J Exp Bot. 2005;56:417–23. 10.1093/jxb/eri039. [DOI] [PubMed] [Google Scholar]
- 42.Slimen IB, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia. 2014;30:513–23. 10.3109/02656736.2014.971446. [DOI] [PubMed] [Google Scholar]
- 43.Mangelsen E, Kilian J, Harter K, Jansson C, Wanke D, Sundberg E. Transcriptome analysis of high-temperature stress in developing barley caryopses: early stress responses and effects on storage compound biosynthesis. Mol Plant. 2011;4:97–115. 10.1093/mp/ssq058. [DOI] [PubMed] [Google Scholar]
- 44.Medina E, Kim S-H, Yun M, Choi W-G. Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants. 2021. 10.3390/plants10020371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li B, Gao K, Ren H, Tang W. Molecular mechanisms governing plant responses to high temperatures. J Integr Plant Biol. 2018;60:757–79. 10.1111/jipb.12701. [DOI] [PubMed] [Google Scholar]
- 46.Xiong R, Peng Z, Zhou H, Xue G, He A, Yao X, et al. Genome-wide identification, structural characterization and gene expression analysis of the WRKY transcription factor family in pea (Pisum sativum L). BMC Plant Biol. 2024;24:113. 10.1186/s12870-024-04774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ouzounis CA, Coulson RM, Enright AJ, Kunin V, Pereira-Leal JB. Classification schemes for protein structure and function. Nat Rev Genet. 2003;4:508–19. 10.1038/nrg1113. [DOI] [PubMed] [Google Scholar]
- 48.Zhao K, Dang H, Zhou L, Hu J, Jin X, Han Y, et al. Genome-wide identification and expression analysis of the HSF gene family in Poplar. Forests. 2023. 10.3390/f14030510. [Google Scholar]
- 49.Zhang J, Liu B, Li J, Zhang L, Wang Y, Zheng H, et al. Hsf and Hsp gene families in populus: genome-wide identification, organization and correlated expression during development and in stress responses. BMC Genomics. 2015;16:181. 10.1186/s12864-015-1398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Song X, Liu G, Duan W, Liu T, Huang Z, Ren J, et al. Genome-wide identification, classification and expression analysis of the heat shock transcription factor family in Chinese cabbage. Mol Genet Genomics. 2014;289:541–51. 10.1007/s00438-014-0833-5. [DOI] [PubMed] [Google Scholar]
- 51.Chow C-N, Chiang-Hsieh Y-F, Chien C-H, Zheng H-Q, Lee T-Y, Wu N-Y, et al. Delineation of condition specific cis- and trans-acting elements in plant promoters under various endo- and exogenous stimuli. BMC Genomics. 2018;19:85. 10.1186/s12864-018-4469-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rerksiri W, Zhang X, Xiong H, Chen X. Expression and promoter analysis of six heat stress-inducible genes in rice. Sci World J. 2013;2013(1):397401. 10.1155/2013/397401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y-X, Wang M, Wu X-Y, Zhou Y-N, Qiu J, Cai X, et al. The chromosome-level genome assembly of an endangered herb Bergenia scopulosa provides insights into local adaptation and genomic vulnerability under climate change. Gigascience. 2024;13:giae091. 10.1093/gigascience/giae091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao Z, Zhang Z, Jiang J, Pan L, Zhang J, Cui X, et al. Complete mitochondrial genome of melia Azedarach L., reveals two conformations generated by the repeat sequence mediated recombination. BMC Plant Biol. 2024;24:645. 10.1186/s12870-024-05319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, Shi X, Chen S, Ma C, Xu S. Evolutionary origin, gradual accumulation and functional divergence of heat shock factor gene family with plant evolution. Front Plant Sci. 2018;9:71. 10.3389/fpls.2018.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao K, Dang H, Zhou L, Hu J, Jin X, Han Y, et al. Genome-wide identification and expression analysis of the HSF gene family in Poplar. Forests. 2023;14:510. 10.3390/f14030510. [Google Scholar]
- 57.Zhang H, Yang J, Li W, Chen Y, Lu H, Zhao S, et al. PuHSFA4a enhances tolerance to excess zinc by regulating reactive oxygen species production and root development in Populus. Plant Physiol. 2019;180:2254–71. 10.1104/pp.18.01495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiang H-M, Chen H-C, Wu C-S, Wu P-Y, Wen K-C. Rhodiola plants: chemistry and biological activity. J Food Drug Anal. 2015;23:359–69. 10.1016/j.jfda.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan Y, Wu LJ, Yu ZL. Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis fisch). Plant Growth Regul. 2006;49:157–65. 10.1007/s10725-006-9101-y. [Google Scholar]
- 60.Ji HS, Bang SG, Ahn M-A, Kim G, Kim E, Eom SH, et al. Molecular cloning and functional characterization of heat stress-responsive superoxide dismutases in Garlic (Allium sativum L). Antioxidants. 2021. 10.3390/antiox10050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin H, Chen Q, Yi M. Effects of short-term heat stress on oxidative damage and responses of antioxidant system in lilium longiflorum. Plant Growth Regul. 2008;54:45–54. 10.1007/s10725-007-9227-6. [Google Scholar]
- 62.Reddy AR, Chaitanya KV, Vivekanandan M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J Plant Physiol. 2004;161(11):1189–202. 10.1016/j.jplph.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 63.Khan N, Hu C, Khan WA, Wang W, Ke H, Huijie D, et al. Genome-wide identification, classification, and expression pattern of homeobox gene family in Brassica rapa under various stresses. Sci Rep. 2018;8:16265. 10.1038/s41598-018-34448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rejeb IB, Pastor V, Mauch-Mani B. Plant responses to simultaneous biotic and abiotic stress: molecular mechanisms. Plants. 2014;3:458–75. 10.3390/plants3040458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pirkkala L, Nykänen P, Sistonen L. Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J. 2001;15:1118–31. 10.1096/fj00-0294rev. [DOI] [PubMed] [Google Scholar]
- 66.Huang Y, An J, Sircar S, Bergis C, Lopes CD, He X, et al. HSFA1a modulates plant heat stress responses and alters the 3d chromatin organization of enhancer-promoter interactions. Nat Commun. 2023;14:469. 10.1038/s41467-023-36227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, Liu H, Ma X, Zhao L, He F, Li M, et al. Genome-wide identification and expression analysis of the class III peroxidase gene (PRXIII) family in medicago sativa L. and its function in the abiotic stress response. BMC Plant Biol. 2025;25:443. 10.1186/s12870-025-06470-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kan W, Gao Y, Zhu Y, Wang Z, Yang Z, Cheng Y, et al. Genome-wide identification and expression analysis of TaFDL gene family responded to vernalization in wheat (Triticum aestivum L). BMC Genomics. 2025;26:255. 10.1186/s12864-025-11436-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H, Li L, ShangGuan G, Jia C, Deng S, Noman M, et al. Genome-wide identification and expression analysis of bZIP gene family in Carthamus tinctorius L. Sci Rep. 2020;10:15521. 10.1038/s41598-020-72390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.ShuangShuang ZHANG, JianXiang LIU, SunJie LU. Heat shock transcription factor HSFA1d is involved in ER stress response in Arabidopsis. Sci Sin Vitae. 2016;46:441–8. 10.1360/N052016-00102. [Google Scholar]
- 71.Löchli K, Torbica E, Haile-Weldeslasie M, Baku D, Aziz A, Bublak D, et al. Crosstalk between endoplasmic reticulum and cytosolic unfolded protein response in tomato. Cell Stress Chaperones. 2023;28:511–28. 10.1007/s12192-022-01316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Z, Zhang L, Wang A, Xu X, Li J. Ectopic overexpression of SlHsfA3, a heat stress transcription factor from tomato, confers increased thermotolerance and salt hypersensitivity in germination in transgenic Arabidopsis. PLoS ONE. 2013;8:e54880. 10.1371/journal.pone.0054880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zuntini AR, Carruthers T, Maurin O, Bailey PC, Leempoel K, Brewer GE, et al. Phylogenomics and the rise of the angiosperms. Nature. 2024;629:843–50. 10.1038/s41586-024-07324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tashi G, Zhan H, Xing G, Chang X, Zhang H, Nie X, et al. Genome-wide identification and expression analysis of heat shock transcription factor family in Chenopodium Quinoa Willd. Agronomy. 2018. 10.3390/agronomy8070103. [Google Scholar]
- 75.Joshi KDA, Vyas A. Phyto-chemical and pharmacological profiles of hyoscyamus niger Linn (parasika yavani) – a review. Pharma Sci Monit. 2015;6:153–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1. Figure S1: The analysis of the exon-intron structure, conserved motifs; Figure S2: Distribution of AntHSF in A. tanguticus on chromosomes; Figure S3: Statistical analysis of qRT-PCR validation for AntHSF gene expression; Figure S4: Statistical analysis of qRT-PCR validation for GSTT, GSTF, Cu/Zn SOD, MDA expression; Table S1:Functional classification of DEGs based on the GO dataset; Table S2: KEGG enrichment of DEGs under normal and heat treatment; Table S3: Primer sequences used in this study; Table S4: The amino acid information of AntHSF genes family identified in A. tanguticus; Table S5:The detailed functional annotations of cis-elements in promoter region of AntHSFs; Table S6: Segmentally duplicated AntHSF gene pairs; Table S7: Differentially Expressed Genes from RNA-seq; Table S8: The sequence of HSF transcriptional factors in A. tanguticus.
Data Availability Statement
The sequencing data generated in this study are available under NCBI BioProject accession PRJNA1183605, which includes the BioSample accessions SAMN44835069, SAMN44835070, and SAMN44835071.