Abstract
Background
The worldwide rise in dementia creates an urgent need for screening methods that are both sensitive and easy to administer. Dual-task walking—requiring people to walk while performing a second cognitive or motor task—meets these criteria because it stresses gait and cognition simultaneously, revealing deficits that emerge early in Alzheimer’s disease and Mild Cognitive Impairment (MCI). Although recent studies have explored integrating various gait variables from dual-task assessment with classification models, there remains uncertainty regarding the effective gait variables for inclusion in these models and the selection of the most effective tasks. This study aims to investigate whether incorporating gait variables derived from whole-body movement characteristics improves the performance of classification models and to identify the most effective tasks for inclusion in these models.
Methods
We analyzed data from 36 participants, including 18 cognitively normal individuals and 18 with MCI. Using motion capture technology, gait variables encompassing whole-body movements, including upper body dynamics, were recorded under both normal walking conditions and during dual-task performance. The dual tasks included: (1) Subtracting threes from a given number, (2) Carrying a cup on a tray without moving it, (3) Holding a cup filled with water without spilling it, and (4) Answering verbal questions. Classification models utilized were k-nearest neighbors, random forest, and support vector machines, with performance evaluated by the area under the curve (AUC).
Results
First, we observed that variables related to upper-body motion (i.e., Anterior–Posterior and Medial–Lateral sway) while walking played an important role in the classification models for detecting MCI, particularly during cognitively demanding tasks (subtracting numbers and answering verbal questions) while walking. Second, the tasks carrying a cup on a tray and holding a cup filled with water while walking yielded superior classification model performance to other tasks especially in considering multiple features (AUC = 0.79).
Conclusions
This study underscores the benefits of incorporating gait variables of the upper body to enhance the performance of classification models for MCI detection. These insights could contribute to the development of more precise and practical screening tools for MCI.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-025-06389-4.
Keywords: Gait, Dual task, Alzheimer’s disease, Mild cognitive impairment, Machine learning
Background
The global prevalence of dementia is projected to double every two decades, driving annual costs to approximately USD 2.8 trillion by 2030 [1, 2]. Mild Cognitive Impairment (MCI), a precursor to dementia, is defined as cognitive performance that falls below expectations for an individual’s age and educational background without impairing activities of daily living. A notable portion of MCI patients, over 50%, progress to dementia within five years [3]. Consequently, early diagnosis and intervention are critical for potentially preventing or delaying dementia [4], underscoring the importance of early MCI identification. However, the global rate of MCI diagnosis is disturbingly low, with only 7.9% of anticipated cases being formally recognized [5]. Thus, there is growing interest in developing accurate and easy-to-perform screening tools for the early identification of MCI.
Gait assessment meets these requirements. An expanding body of evidence shows that locomotion recruits executive functions mediated by prefrontal cortical circuits, especially under cognitively demanding tasks [6]. Li et al. framed this “neural-overlap” hypothesis, arguing that dual-task interference arises when motor and cognitive processes compete for common frontal resources [6]. Consistent with this view, dual-task walking—performing an additional cognitive or motor task while walking—has proved more sensitive to MCI than single-task gait assessments [7]. Tasks integrated with walking are diverse [8], such as numerical subtraction [8–11], naming animals [9, 10, 12] and carrying a glass of water [10, 13]. Different tasks recruit distinct cognitive functions [10], with continuous subtraction tasks being linked to working memory and animal naming tasks associated with language fluency [8].
Machine-learning (ML) approaches that combine multiple gait features can further enhance diagnostic accuracy [13–15], yet most pipelines rely almost exclusively on lower-limb parameters. Emerging work has begun to explore upper-body kinematics: Seifallahi et al. measured whole-body joint trajectories with Kinect sensors [16], and Li et al. examined arm motion and gait asymmetry during dual-task walking [17]. The latter found that upper-limb variables were not significantly associated with Montreal Cognitive Assessment (MoCA) scores and, under either dual-task condition, did not improve discrimination between cognitively normal and MCI participants. Thus, while capturing upper-body signals is technically feasible, e.g., Kinect sensors, it remains unclear whether they are effective for classification between cognitively normal and MCI participants. Key open questions concern (i) the role of upper-body features during gait, (ii) task-specific modulation of upper-body features, and (iii) the interpretability of ML models.
To address these gaps, the present study offers three contributions: (i) a direct comparison of four dual-task paradigms—serial-threes subtraction, tray carrying, water carrying, and verbal question answering—within a single experimental protocol; (ii) high-precision optical motion capture that quantifies subtle head movements alongside conventional lower-limb metrics; and (iii) the use of SHapley Additive exPlanations (SHAP) to estimate the individual contribution of each upper-body variable, thereby improving the clinical transparency of the ML classifier. Accordingly, we pursue two objectives: (i) to determine which of the four dual-task paradigms most effectively distinguishes MCI from cognitively normal (CN) older adults, and (ii) to assess the SHAP-derived importance of upper-body motion–related features in the classification model.
Methods
Participants
In this study, 71 individuals were recruited through local recruiting agencies or advertisements in the community in Ibaraki, Japan. We applied the following exclusion criteria for subjects with MCI: a diagnosis of dementia, diagnosis of MCI not attributed to Alzheimer’s disease, or any other disease or disability that would interfere with neuropsychological examination. All examinations were conducted in Japanese. MCI attributed to Alzheimer’s disease was assessed with the National Institute on Aging and Alzheimer’s Association (NIA-AA) core clinical criteria for MCI [18] and the Alzheimer’s Disease Neuroimaging Initiative (ADNI) criteria for MCI [19]. Three psychiatrists (authors T.A., K.N., and M.O.), who are experts in dementia and were blinded to participants’ behavioral data, examined each case in terms of the clinical record, as well as the cognitive and clinical measures, and they confirmed the diagnoses.
Experimental procedure
Participants performed a baseline single-task trial (simple walking) for three specific purposes: (i) to create a reference against which the classification performance of each dual-task could be compared; (ii) to calculate cognitive cost, defined as the relative change from baseline speed; and (iii) to quantify, via SHAP, the extent to which baseline gait contributes to prediction accuracy in multivariate models. They then completed dual-task trials (walking for nine meters at their self-selected speed while performing the following additional tasks: 1) Subtracting by threes from a given number (‘subtraction’), 2) Carrying a cup on a tray without moving it (‘tray’), 3) Holding a cup filled with water without spilling it (‘water’), and 4) Answering verbal questions (‘question’). Throughout these tasks, participants proceeded at their natural walking pace. Each task was repeated across three trials, with data captured at a 120 Hz sampling frequency utilizing the OptiTrack Motive motion capture system. For more details on the tasks and data processing, please see the Supplementary Materials.
Data analysis and gait variables
We applied two inclusion criteria: (1) each participant produced at least 10 heel-strikes across the three trials of every task, and (2) baseline walking velocity was recorded.
After screening, 28 CN and 18 MCI participants met these criteria. To eliminate group imbalances, we drew a simple random sample of 18 CN participants (matched for sex with the MCI group), yielding a final cohort of 36 participants (18 CN and 18 MCI) for all subsequent analyses.
We evaluated 15 gait variables derived from dual-task gait assessments alongside standard gait speed to elucidate the movement characteristics associated with MCI. Heel strike was defined as the time frame at which the vertical coordinate of the heel marker reached a local minimum, in accordance with kinematic event-detection procedures validated in previous work [20]. Each gait variable was obtained by calculating the mean of the values for each trial. These variables were analyzed as follows, with data processing and computations executed in MATLAB R2022a.
Gait Speed: This metric encompassed mean speed and speed variability, focusing on the waist back marker for each trial. Mean speed was determined by calculating the mean of the speeds across trials, and variability was assessed using a coefficient of variation for steps:
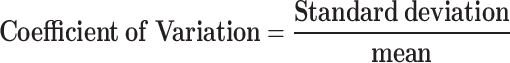 |
1 |
Step Width: Analysis of step width included mean width and its variability, defining step width as the distance between right and left heels at each heel contact in the mediolateral direction. The mean for each step was calculated, and variability was determined using the coefficient of variation. Height normalization was applied to mitigate height-related discrepancies.
Step Length: Calculations for step length involved mean length, variability, and the Symmetry Index (SI). Step length was gauged by the heel marker distance at each heel contact in the anterior-posterior direction, with height normalization applied. The SI was introduced to examine walking asymmetry [21]:
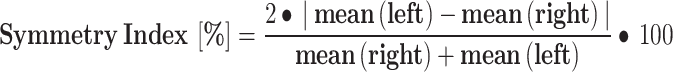 |
2 |
Step Time: Metrics for step time included the mean time, variability, and SI, based on the intervals between ground contacts of the heel markers. The mean time per step and its variability were calculated, along with the SI to assess step timing symmetry.
Cognitive Cost: We regard the change in gait speed under dual-task conditions compared to normal walking as cognitive cost following [10]. It was quantified using the designated formula, with mean values computed for each trial:
 |
3 |
Anterior-Posterior and Medial-Lateral Sway: To quantify head sway during walking, we first calculated the position of the Head Top marker relative to the Waist Back marker at each frame. For each step-to-step interval, we decomposed the resulting head-to-waist vector into two orthogonal components: Mediolateral (ML) axis, perpendicular to the direction of progression, representing side-to-side movement; and Anteroposterior (AP) axis, parallel to the direction of progression, representing forward–backward movement. Within each step interval, sway amplitude was quantified as the range of motion along each axis, calculated as the difference between the maximum and minimum values of the relative coordinate (Max – Min). This yielded one ML range and one AP range per step, referred to as MLS and APS, respectively in the following. To characterize overall sway behavior, we then computed the mean and variability of these ranges across all intervals. Higher mean values indicate greater typical sway excursions, whereas higher variability suggests less consistent head stabilization. This approach aligns with recent gait analysis research [22].
Performance screening across models and tasks
We compared three algorithms—k-nearest neighbors (KNN), random forest (RF), and support vector machines (SVM)—on four dual-task gait conditions, following the multimodal behavioral study by Yamada et al., 2021 [23].
The three models bring complementary inductive biases: instance‑based (KNN), ensemble tree‑based (RF) and kernel‑based (SVM) learners jointly span linear and non‑linear decision boundaries while remaining data‑efficient for the modest sample sizes typical in clinical gait research.
All classifiers were implemented in scikit-learn v1.3.2. Features had been z-standardized upstream. To minimize redundancy, whenever two features showed |r| >0.70 (Pearson) we retained the one with the higher univariate ROC-AUC.
Because CN cases outnumbered MCI cases in the raw data, we performed a single, fixed random under-sampling of cognitively normal (CN) cases before cross-validation (seed = 42), yielding a balanced cohort of 18 MCI and 18 CN participants. Model performance was measured as the mean ROC-AUC over 20 repeats of 10-fold stratified cross-validation (200 train/test splits) using RepeatedStratifiedKFold. Hyperparameters were tuned inside each training fold via nested 5-fold grid search.
For context, we also trained a baseline logistic-regression model using MMSE-J, age, sex, and education with the identical CV procedure.
For the MMSE assessment, the Japanese version (MMSE-J) was administered following the manufacturer’s instructions (Nihon Bunka Kagakusha, Tokyo, Japan; PAR, Inc., Lutz, FL, USA).
Feature-importance analysis with SHAP
Interpretability was assessed with SHAP (SHapley Additive exPlanations, v0.44.0) applied to the RF models [24]. Inspired by cooperative game theory’s Shapley Values, SHAP offers a framework to quantify each feature’s contribution to the model’s predictive accuracy, elucidating the relative importance of various features in distinguishing between MCI and CN states. Tree-SHAP gives exact, consistent attributions with low computational cost, whereas kernel-SHAP for SVM/KNN is approximate [25]; hence we limited the explanation step to RF. For each task we computed participant-level SHAP values, averaged the absolute magnitudes across the 20 × 10 folds, and visualized features with bar plots. This quantified the contribution of upper-body variables to the MCI-vs-CN discrimination. Further implementation details are provided in the Supplementary Materials.
Use of AI-assisted technologies
In the preparation of this manuscript, ChatGPT (Models GPT-4 and 4o) was utilized to enhance the language quality and improve overall readability. The authors carefully reviewed and edited the content following its use to ensure accuracy, clarity, and adherence to academic standards.
Results
Table 1 presents the demographic and cognitive profiles of the participants. Comparative analysis revealed significant differences in Logical Memory (immediate and delayed) and Clinical Dementia Rating, including the sum of boxes. Gait variables in each group and task were summarized in Table 2.
Table 1.
Participant demographics and cognitive status
| Variables | CN (n = 18) | MCI (n = 18) | p-value |
|---|---|---|---|
| Age in years (SD) | 69.7 (5.9) | 72.2 (4.9) | 0.1848 |
| Years of education (SD) | 14.3 (2.2) | 14.0 (0.0) | 0.7552 |
| female, n (%) | 10 (55.6) | 8 (44.4) | 0.1155 |
| Height [cm] (SD) | 157.6 (9.3) | 158.1 (8.0) | 0.8576 |
| Logical Memory – immediate (SD) | 12.0 (3.6) | 7.1 (2.8) | < 10 −4 |
| Logical Memory – delayed (SD) | 9.7 (3.4) | 4.7 (2.6) | < 10 −4 |
| Mini-Mental State Examination (SD) | 28.0 (1.3) | 27.3 (1.7) | 0.1607 |
| Frontal Assessment Battery (SD) | 14.1 (1.8) | 14.1 (2.4) | 1.0000 |
| Trail Making Test part A (SD) | 33.4 (12.0) | 36.8 (9.0) | 0.3535 |
| Trail Making Test part B (SD) | 91.3 (57.4) | 109.8 (58.7) | 0.3474 |
| Clock Drawing Test (SD) | 6.6 (1.2) | 6.5 (0.9) | 0.7627 |
| Clinical Dementia Rating (SD) | 0.0 (0.0) | 0.5 (0.0) | < 10 −6 |
| Clinical Dementia Rating - sum of boxes (SD) | 0.0 (0.0) | 0.7 (0.3) | < 10 −6 |
| Medial Temporal Lobe Atrophy (SD) | 0.8 (0.6) | 1.0 (0.8) | 0.4553 |
Values were analyzed using t-tests or chi-square tests where applicable. Logical Memory-immediate and Logical Memory-delayed refer to immediate and delayed recall of Logical Memory Story A from the Wechsler Memory Scale-Revised. The total score ranges are as follows: Logical Memory (immediate and delayed), 0 to 25; Mini-Mental State Examination, 0 to 30; Frontal Assessment Battery, 0 to 18; Trail Making Test (parts A and B), 0 to 300; Clock Drawing Test, 0 to 7; Clinical Dementia Rating, 0 to 3; Clinical Dementia Rating - sum of boxes, 0 to 18. Medial Temporal Lobe Atrophy was expressed as a Z-score relative to cognitively healthy adults by using a stand-alone, voxel-based specific regional analysis system for AD [26]
Table 2.
Mean and standard deviation of gait parameters focused on this study
| Gait variable | type | Group | Walking test condition [mean(SD)] | |||
|---|---|---|---|---|---|---|
| subtraction | tray | water | question | |||
| Gait speed | mean (m/s) | CN | 1.31 (0.15) | 1.12 (0.24) | 1.13 (0.22) | 1.19 (0.23) |
| MCI | 1.23 (0.17) | 1.04 (0.18) | 1.02 (0.19) | 1.07 (0.20) | ||
| variability (CV, %) | CN | 3.70 (1.25) | 8.74 (6.55) | 9.05 (7.03) | 6.81 (3.69) | |
| MCI | 4.36 (2.03) | 7.49 (3.27) | 14.05 (20.11) | 8.19 (4.35) | ||
| Step width (Normalized by subject height) | mean | CN | 5.17 (1.75) | 5.21 (1.69) | 5.31 (2.06) | 5.26 (2.08) |
| MCI | 5.87 (2.19) | 6.03 (2.45) | 6.47 (2.75) | 6.56 (2.69) | ||
| variability (CV, %) | CN | 31.12 (11.88) | 35.11 (10.56) | 32.24 (16.05) | 31.05 (15.33) | |
| MCI | 23.31 (10.96) | 28.74(12.34) | 26.18 (13.63) | 26.12 (15.24) | ||
| Step length (Normalized by subject height) | mean | CN | 41.67 (3.37) | 37.45 (5.03) | 38.22 (4.61) | 39.56 (4.73) |
| MCI | 39.63 (3.64) | 36.09 (3.99) | 35.97 (5.14) | 37.11 (5.81) | ||
| variability (CV, %) | CN | 5.00 (1.37) | 7.21 (4.20) | 7.85 (3.84) | 6.87 (2.60) | |
| MCI | 6.20 (3.37) | 7.16 (1.88) | 11.01 (10.93) | 8.35 (4.33) | ||
| SI | CN | 2.93 (2.32) | 3.95 (2.90) | 4.91 (3.76) | 3.35 (3.09) | |
| MCI | 3.13 (2.89) | 5.28 (3.99) | 7.02 (6.17) | 5.63 (4.47) | ||
| Step time | mean (ms) | CN | 493.28 (36.91) | 536.13 (70.28) | 538.29 (65.10) | 529.93 (53.52) |
| MCI | 506.81 (43.66) | 556.53 (66.82) | 550.30 (56.43) | 544.19 (52.32) | ||
| variability (CV, %) | CN | 4.87 (2.81) | 6.69 (4.21) | 7.45 (4.93) | 6.67 (3.65) | |
| MCI | 4.81 (1.48) | 6.84 (3.03) | 9.14 (11.82) | 6.27 (2.19) | ||
| SI | CN | 1.72 (1.67) | 1.34 (1.34) | 2.23 (1.90) | 1.87 (1.79) | |
| MCI | 1.20 (1.04) | 2.03 (1.85) | 3.06 (5.59) | 1.78 (1.96) | ||
| Cognitive cost | mean (%) | CN | 2.97 (7.90) | 17.16 (17.30) | 17.32 (9.51) | 12.89 (9.91) |
| MCI | 3.08 (8.23) | 17.90 (12.66) | 19.14 (13.70) | 15.23 (15.00) | ||
| APS | mean (cm) | CN | 2.63 (0.86) | 2.49 (0.48) | 3.36 (0.91) | 3.57 (1.06) |
| MCI | 2.25 (0.48) | 2.29 (0.40) | 2.86 (0.73) | 2.91 (0.84) | ||
| variability (CV, %) | CN | 32.80 (8.40) | 34.18 (9.86) | 35.47 (12.72) | 35.26 (16.37) | |
| MCI | 30.76 (8.04) | 33.92 (10.48) | 35.38 (8.59) | 33.93 (11.81) | ||
| MLS | mean (cm) | CN | 2.20 (1.11) | 1.82 (0.71) | 2.84 (1.11) | 3.19 (1.16) |
| MCI | 2.24 (0.80) | 1.84 (0.59) | 2.36 (1.09) | 2.54 (1.20) | ||
| variability (CV, %) | CN | 29.94 (8.05) | 38.55 (11.26) | 33.34 (11.52) | 31.42 (7.26) | |
| MCI | 27.62 (8.33) | 33.15 (7.33) | 37.82 (10.77) | 33.85 (7.74) | ||
Based on AUC values, we examined effective feature variables to classify participants into CN and MCI. Based on MMSE (a representative non-motor feature; AUC = 0.63) or normal walk velocity in single-task trials (AUC = 0.60), AUC was at most 0.63 (Table 3). AUC value was improved if we used a set of non-motor features for classification (i.e., Baseline model [see Methods for the details]).
Table 3.
AUC comparisons across conditions
| type | condition | AUC |
|---|---|---|
| Baseline | Normal walk velocity | 0.60 |
| Baseline (non-motor features) | MMSE | 0.63 |
| Baseline model | 0.77 | |
| Single feature | APS mean (subtraction) | 0.70 |
| MLS mean (subtraction) | 0.69 | |
| Step width variability (tray) | 0.69 | |
| Step width variability (water) | 0.68 | |
| APS mean (question) | 0.68 | |
| Multiple features | subtraction | 0.75 |
| tray | 0.79 | |
| water | 0.79 | |
| question | 0.78 |
For single features, only the top five with the highest AUC values are displayed
The current study then focused on a single motor feature in each dual-task trial. Table 3 listed the five largest AUC values. Upper body movements (i.e., APS and MLS) showed equivalent AUC values to step width variability, indicating the effectiveness of upper body movements as motor features in classifying participants into CN and MCI.
Additionally, we examined AUC values for each dual‑task trial when multiple motor features were combined. The ‘tray’ and ‘water’ tasks yielded the highest classification performance, each reaching an AUC of 0.79. The model based on the ‘question’ task achieved an AUC of 0.78, while the ‘subtraction’ task attained an AUC of 0.75. All dual‑task models outperformed classification based solely on the MMSE score (AUC = 0.63), and three of the four tasks (tray, water, and question) also surpassed the performance of the baseline model that combines MMSE with demographic variables (AUC = 0.77).
The models that yielded the most substantial classification performance differed across tasks. Random forest classifiers were selected for the ‘tray’, ‘water’, and ‘question’ tasks, whereas an SVM with a radial basis function (RBF) kernel proved optimal for the ‘subtraction’ task.
Finally, we investigated the motor features that contribute to the classification of participants into CN and MCI groups using SHAP values. Figure 1 presents the mean absolute SHAP values of the features derived from the random forest models that achieved the highest AUC for each task. A higher SHAP value indicates a greater impact on model prediction. The results demonstrated that upper-body-related features, particularly the mean values of Anterior-Posterior Sway (APS) and Mediolateral Sway (MLS), play a significant role—especially in tasks involving cognitive demands, such as the question and subtraction tasks.
Fig. 1.
Feature Importance in Random Forest Model (SHAP Values). Higher SHAP values indicate greater contributions to classify participants into CN and MCI
Discussion
In our investigation, we aimed to identify the dual-task walking task that most effectively distinguishes between MCI and CN states using motion-capture data, thereby facilitating early detection of MCI. Our analysis yielded two pivotal findings. First, variables related to upper-body motion— Mediolateral Sway (MLS) and Anterior-Posterior Sway (APS)—played an important role in our classification models, particularly in tasks related to cognitive ability such as the subtraction and question tasks. Second, the dual-task trials that required participants to maintain postural stability while carrying an object—the ‘tray’ and ‘water’ tasks—produced the highest overall performance (AUC = 0.79 each), outperforming both the baseline model that combined MMSE with demographic variables (AUC = 0.77) and the MMSE score alone (AUC = 0.63). The verbal question task also performed well (AUC = 0.78), and the subtraction task reached an AUC of 0.75. These insights are crucial for developing practical, accurate diagnostic tools and point to targeted interventions that could enhance early diagnostic processes and therapeutic strategies.
Upper-body–related features dominated the best single-feature results. Particularly during cognitively demanding tasks (subtraction and question), the mean values of APS and MLS achieved AUCs comparable to—or higher than—traditional gait measures such as step-width variability (Table 3). Even in multivariate machine-learning models, APS and MLS retained substantial influence—particularly during cognitively demanding tasks (subtraction and question). For the question task, the highest SHAP mean importance was observed for APS mean, followed by MLS variability; for subtraction, APS mean ranked first, followed by MLS mean. Of the 16 gait variables incorporated, eight were calculated solely from heel markers, whereas APS and MLS captured information unique to head and waist motion. These gait variables were calculated from head and waist markers and utilizes an approach from a recent study using motion capture [22]. Of the 16 gait variables incorporated in these models, 8 were derived only from heel markers. On the other hand, APS and MLS measures derived from head and waist markers may provide different insights than the features calculated from heel markers. Although many past studies applying machine learning to dual-task gait tests for MCI classification have focused mainly on lower-limb variables such as stride length and gait velocity [13–15], our findings suggest that upper-body kinematics are also informative. Recent research highlights the importance of kinematic parameters pertaining to the upper body, advocating for their inclusion as a complementary aspect of gait analysis in some neurological disorders [27]. Comprehensive evaluation of both lower and upper body dynamics may lead to the discovery of more discriminative gait variables in MCI in the future.
Although optimal tasks for distinguishing MCI remain undefined, existing literature hints that tasks with increased cognitive demands may improve discrimination sensitivity [7]. Hunter et al. claimed that the selected task should sufficiently challenge individuals, pushing them to operate near their capacity limits [10]. They also suggest a framework for evaluating the complexity of tasks, indicating that tasks demanding greater cognitive effort, such as serial subtraction of sevens, are more challenging than simpler tasks like naming animals or carrying a glass of water. Interestingly, the subtraction-by‐three dual task yielded the lowest classification accuracy of all conditions (AUC = 0.75), suggesting that the cognitive load imposed by subtracting consecutive threes may lie outside the optimal “sweet spot” proposed by Hunter et al.—that is, the task might be either too demanding (causing many participants to abandon the calculation altogether) or insufficiently demanding for higher-functioning participants, thereby reducing between-group variance. Future studies should therefore consider parametrically titrating the subtraction interval (e.g., subtracting sevens, fives, or alternating intervals) and dynamically adjusting the starting value to better match individual capacity. Such calibration could help identify a level of difficulty that maximizes discriminatory power while minimizing floor- and ceiling-effects. Moreover, an inspection of the SHAP-derived feature importance revealed different predictor profiles for motor-dominant tasks (tray, water) versus cognition-dominant tasks (question, subtraction) (Table 3). This finding implies that, beyond overall difficulty, the intrinsic nature of a task—motor coordination versus executive processing—modulates which gait features carry the most diagnostic signal. Consequently, Hunter’s framework may benefit from incorporating task modality (motor, cognitive, or hybrid) alongside complexity, as both dimensions appear to influence model performance and the physiological signatures leveraged for classification [10].
The current insights into the efficacy of using whole-body gait features and a task answering verbal questions in MCI detection could guide the development of future monitoring tools for early cognitive decline signs. Advances in technology have broadened the scope for capturing comprehensive movement data through wearable devices. Previous research efforts are focused on obtaining gait metrics via insoles [28] and monitoring head motion measurement with earphone-type wearables [29]. The future development of a model that integrates whole-body movement data collected from these diverse wearable technologies holds promise for significantly improving the detection of MCI.
Moreover, the dual task scenario of carrying things or thinking while walking, are common situations encountered in everyday life. Recent studies have pointed to the possibility of identifying MCI from spoken data of questions automatically collected in everyday life [30, 31]. By integrating the identification of particular questions within these dialogues with gait metrics obtained from wearable technology, it may be possible to enhance the sensitivity of MCI detection. This integrated approach, leveraging daily-life activities and advanced wearable technology, could pave the way for more effective early diagnosis and intervention strategies for cognitive decline.
This study primarily concentrated on classification models for distinct tasks. However, models that amalgamate multiple tasks or behavioral data beyond dual tasks could achieve superior performance. Hunter et al. indicated that different tasks engage varied cognitive domains [10], with serial subtraction tasks linked to working memory and animal naming tasks associated with language fluency [8]. This diversity suggests that some individuals may find specific tasks more challenging than others. Previous studies have shown that aggregating multiple types of data collected from everyday activities could detect MCI and AD more accurately than single data modalities [23]. Future research could explore multimodal models incorporating behavioral data from a range of daily activities, including dual tasks, speech, and drawing, to achieve more precise MCI classification.
We acknowledge a limitation of our study: the collection of gait variables was conducted in a laboratory setting, which, due to its controlled nature, may have influenced the behavior of participants. Consequently, to foster the development of applications that perform effectively in real-world contexts, it is essential to identify and analyze dual-task activities under everyday conditions. In addition, although our participants generated an average of 19.0–21.6 steps per task (see Supplementary Materials for details), the literature offers no universal consensus on the minimum number of strides required for reliable gait variability metrics. Some high-sensitivity parameters (e.g., stride velocity variability) may require longer walking bouts. Therefore, future studies should incorporate longer or continuous recordings to ensure the robustness of these measures. Finally, to explore whether upper-body (trunk) sway metrics contributed critically to classification, we retrained all models after excluding every sway-related feature. The resulting AUCs differed by ≤ 0.06 from the original models, and none of the paired contrasts were significant (all p ≥ 0.12, Wilcoxon signed-rank test; full results in Supplementary Table S4 and Figure S1). These findings indicate that the dual-task gait paradigm preserves its diagnostic value even without explicit measures of trunk sway. Nevertheless, the modest cohort size (n ≈ 36) limits our power to detect subtle incremental effects. Future studies with larger samples and tasks designed to elicit more pronounced upper-body sway—such as uneven-surface walking, heavier load carrying, or sudden perturbations—are warranted to fully delineate the contribution of trunk dynamics.
Conclusions
This study demonstrates that combining whole-body movement metrics with cognitively and physically demanding dual-task gait tests can discriminate individuals with MCI from CN peers more accurately than traditional screening alone. Whereas the MMSE or normal walking speed achieved only modest classification power (AUC ≤ 0.63), models derived from dual-task trials—especially those that required carrying a tray or cup of water, or answering everyday questions while walking—raised the AUC to 0.78–0.79. Crucially, the random forest and SVM classifiers identified upper-body sway parameters (mean anterior–posterior and mediolateral sway) as key contributors, underscoring that subtle postural adjustments, not just spatiotemporal gait measures, reveal early cognitive decline.
These findings suggest a practical pathway toward earlier, ecologically valid MCI detection: brief, easy-to-administer walking tasks that mirror real-world multitasking, paired with wearable motion sensors and machine-learning analytics. By capturing the interplay between motor control and cognition, such protocols can complement—or in some settings surpass—conventional neuropsychological tests, enabling clinicians to flag at-risk adults sooner and intervene while neuroplasticity remains favorable.
Future work should validate these results in larger, more diverse cohorts and track participants longitudinally to confirm predictive value for conversion to dementia. Integrating additional contextual data (e.g., home activity patterns) and refining feature-selection strategies may further boost performance. Ultimately, the multimodal, dual-task approach outlined here holds promise for scalable community screening and personalized monitoring in the pre-clinical stages of cognitive impairment.
Supplementary Information
Acknowledgements
The authors thank all the study participants.
Abbreviations
- MCI
Mild cognitive impairment
- CN
Cognitively normal
- AUC
Area under the curve
- NIA-AA
National Institute on Aging and Alzheimer’s Association
- ADNI
Alzheimer’s disease neuroimaging initiative
- APS
Anterior-posterior sway
- MLS
Medial-lateral sway
- SVM
Support vector machines
- ROC
Receiver operating characteristic
- MMSE
Mini-mental state examination
- SHAP
SHapley Additive exPlanations
- RBF
Radial basis function
Authors’ contributions
Study concept and design: RK, KT, YY, and MK; Acquisition of data: YY, KS, MK, MN, MH, MO, KN, TA; Analysis and interpretation of data: RK, KT, YY, and MK; Drafting of the manuscript: RK and KT; Critical review of the manuscript for important intellectual content: KT, YY, KS, MK, MN, MH, MO, KN, TA. All authors revised and approved the final version of the paper.
Funding
This work was supported by the Japan Society for the Promotion of Science, KAKENHI [grant number 19H01084, 21K12153, and 24K02840].
Data availability
The datasets presented in this article are not readily available because they contain information that could compromise research participant privacy/consent. Anonymized data that support the findings of this study may be made available upon reasonable request for academic purposes.
Declarations
Ethics approval and consent to participate
The study was conducted under the approval of the Ethics Committee, University of Tsukuba Hospital (H29-065), and it followed the ethical code for research with humans as stated in the Declaration of Helsinki. All participants provided written informed consent to participate in the study.
Consent for publication
Not applicable.
Competing interests
Tetsuaki Arai received payment for lectures from Eisai.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Prince M, Wimo A, Guerchet Maëlenn, Ali G-C, Wu Y-T. Matthew Prina. World Alzheimer Report 2015 The global impact of dementia: An analysis of prevalence, incidence, cost and trends. 2015.
- 2.Simon L. Chloé Benoist, Wendy Weidner. World Alzheimer Report 2023 Reducing dementia risk: never too early, never too late. 2023.
- 3.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. 2006;367:1262–70. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen J, Langerman H. Alzheimer’s disease – why we need early diagnosis. Degener Neurol Neuromuscul Dis. 2019;9:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattke S, Jun H, Chen E, Liu Y, Becker A, Wallick C. Expected and diagnosed rates of mild cognitive impairment and dementia in the U.S. Medicare population: observational analysis. Alzheimers Res Ther. 2023;15:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li KZH, Bherer L, Mirelman A, Maidan I, Hausdorff JM. Cognitive involvement in balance, gait and dual-tasking in aging: a focused review from a neuroscience of aging perspective. Front Neurol. 2018. 10.3389/fneur.2018.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahureksa L, Najafi B, Saleh A, Sabbagh M, Coon D, Mohler MJ, et al. The impact of mild cognitive impairment on gait and balance: a systematic review and meta-analysis of studies using instrumented assessment. Gerontology. 2017;63:67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2011;35:715–28. [DOI] [PubMed] [Google Scholar]
- 9.Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: the effect of dual-task challenges across the cognitive spectrum. Gait Posture. 2012;35:96–100. [DOI] [PubMed] [Google Scholar]
- 10.Hunter SW, Divine A, Frengopoulos C, Montero Odasso M. A framework for secondary cognitive and motor tasks in dual-task gait testing in people with mild cognitive impairment. BMC Geriatr. 2018;18:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montero-Odasso M, Casas A, Hansen KT, Bilski P, Gutmanis I, Wells JL, et al. Quantitative gait analysis under dual-task in older people with mild cognitive impairment: a reliability study. J Neuroeng Rehabil. 2009;6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Yang Q, Tian C, Zeng J, Yang M, Li J, et al. A dual-task gait test detects mild cognitive impairment with a specificity of 91.2%. Front Neurosci. 2023;16:1100642. [DOI] [PMC free article] [PubMed]
- 13.Taylor ME, Delbaere K, Mikolaizak AS, Lord SR, Close JCT. Gait parameter risk factors for falls under simple and dual task conditions in cognitively impaired older people. Gait Posture. 2013;37:126–30. [DOI] [PubMed] [Google Scholar]
- 14.Boettcher LN, Hssayeni M, Rosenfeld A, Tolea MI, Galvin JE, Ghoraani B. Dual-Task gait assessment and machine learning for Early-detection of cognitive decline. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:3204–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghoraani B, Boettcher LN, Hssayeni MD, Rosenfeld A, Tolea MI, Galvin JE. Detection of mild cognitive impairment and alzheimer’s disease using dual-task gait assessments and machine learning. Biomed Signal Process Control. 2021;64:102249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seifallahi M, Galvin JE, Ghoraani B. Detection of mild cognitive impairment using various types of gait tests and machine learning. Front Neurol. 2024;15:1354092. [DOI] [PMC free article] [PubMed]
- 17.Li Z, Zhu J, Liu J, Shi M, Liu P, Guo J, et al. Using dual-task gait to recognize alzheimer’s disease and mild cognitive impairment: a cross-sectional study. Front Hum Neurosci. 2023;17:1284805. [DOI] [PMC free article] [PubMed]
- 18.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer’s disease neuroimaging initiative (ADNI). Neurology. 2010;74:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fellin RE, Rose WC, Royer TD, Davis IS. Comparison of methods for kinematic identification of footstrike and toe-off during overground and treadmill running. J Sci Med Sport. 2010;13:646–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viteckova S, Kutilek P, Svoboda Z, Krupicka R, Kauler J, Szabo Z. Gait symmetry measures: a review of current and prospective methods. Biomed Signal Process Control. 2018;42:89–100. [Google Scholar]
- 22.Kadirvelu B, Gavriel C, Nageshwaran S, Chan JPK, Nethisinghe S, Athanasopoulos S, et al. A wearable motion capture suit and machine learning predict disease progression in Friedreich’s ataxia. Nat Med. 2023;29:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamada Y, Shinkawa K, Kobayashi M, Caggiano V, Nemoto M, Nemoto K, et al. Combining multimodal behavioral data of gait, speech, and drawing for classification of Alzheimer’s disease and mild cognitive impairment. J Alzheimers Dis. 2021;84:315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundberg SM, Erion G, Chen H, DeGrave A, Prutkin JM, Nair B, et al. From local explanations to global understanding with explainable AI for trees. Nat Mach Intell. 2020;2:56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aas K, Jullum M, Løland A. Explaining individual predictions when features are dependent: more accurate approximations to Shapley values. Artif Intell. 2021;298:103502. [Google Scholar]
- 26.Matsuda H, Mizumura S, Nemoto K, Yamashita F, Imabayashi E, Sato N, et al. Automatic voxel-based morphometry of structural MRI by SPM8 plus diffeomorphic anatomic registration through exponentiated lie algebra improves the diagnosis of probable Alzheimer disease. Am J Neuroradiol. 2012;33:1109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley C, Alcock L, McArdle R, Rehman R, Del Din S, Mazzà C, et al. The role of movement analysis in diagnosing and monitoring neurodegenerative conditions: insights from gait and postural control. Brain Sci. 2019;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin F, Wang A, Zhuang Y, Tomita MR, Xu W. Smart insole: a wearable sensor device for unobtrusive gait monitoring in daily life. IEEE Trans Ind Inform. 2016;12:2281–91. [Google Scholar]
- 29.Yamanobe Y, Fujioka M, Ohashi M, Ozawa H. Potential usefulness of tracking head movement via a wearable device for equilibrium function testing at home. J Med Syst. 2022;46:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada Y, Shinkawa K, Kobayashi M, Nishimura M, Nemoto M, Tsukada E, et al. Tablet-based automatic assessment for early detection of alzheimer’s disease using speech responses to daily life questions. Front Digit Health. 2021;3:653904. [DOI] [PMC free article] [PubMed]
- 31.Yamada Y, Shinkawa K, Nemoto M, Nemoto K, Arai T. A mobile application using automatic speech analysis for classifying Alzheimer’s disease and mild cognitive impairment. Comput Speech Lang. 2023;81:101514. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this article are not readily available because they contain information that could compromise research participant privacy/consent. Anonymized data that support the findings of this study may be made available upon reasonable request for academic purposes.



