Abstract
We report a novel post-transcriptional control of the ptsG gene encoding the major glucose transporter IICBGlc. We demonstrate that the level of IICBGlc is markedly reduced when the glycolytic pathway is blocked by a mutation in either the pgi or pfkA gene encoding phosphoglucose isomerase or phosphofructokinase, respectively. This down-regulation of ptsG is not exerted at the transcriptional level. Both northern blot and S1 analyses demonstrate that the mutation dramatically accelerates the degradation of ptsG mRNA. The degradation of ptsG mRNA occurs in wild-type cells when α-methylglucoside, a non- metabolizable analog of glucose, is present in the medium. The addition of any one of the glycolytic intermediates downstream of the block prevents the degradation of ptsG mRNA. The rapid degradation of ptsG mRNA is eliminated when RNase E is thermally inactivated. We conclude that the glycolytic pathway controls ptsG expression by modulating RNase E-mediated mRNA degradation. This is the first instance in which the glycolytic flux has been shown to affect the expression of a specific gene through mRNA stability.
Keywords: glucose transporter/glycolysis/mRNA degradation/PTS/RNase E
Introduction
Glycolysis is a central metabolic pathway that is responsible for the production of numerous intermediary metabolites and energy in cells (Fraenkel, 1996). In bacteria, external glucose is transported into the cells and phosphorylated by the phosphoenolpyruvate (PEP):carbohydrate phosphotransferase system (PTS) (Meadow et al., 1990; Postma et al., 1993; Saier, 1996). The phosphorylated glucose is then converted sequentially to pyruvate by a series of glycolytic enzymes. The PTS consists of two common cytoplasmic proteins, enzyme I and HPr, and an array of sugar-specific enzyme II complexes (EIIs). The glucose-specific EII (glucose transporter) consists of cytoplasmic protein IIAGlc and membrane receptor IICBGlc encoded by crr and ptsG, respectively. The phosphoryl group from PEP is transferred sequentially to enzyme I, to HPr, to EIIs and finally to glucose as it is translocated across the membrane.
In addition to the transport and phosphorylation of sugars, the PTS is involved in a variety of cellular physiological processes acting as a phospho-relay system. For example, IIAGlc regulates both the transport of non-PTS carbohydrates and the activity of adenylate cyclase depending on its phosphorylation state (Meadow et al., 1990; Postma et al., 1993). The former process, called inducer exclusion, is totally responsible for the glucose– lactose diauxie that is a prototype of catabolite repression (Inada et al., 1996; Kimata et al., 1997). A fascinating recent discovery regarding the regulatory function of PTS is that IICBGlc, depending on its phosphorylation state, interacts with Mlc to modulate the cellular localization and activity of this global repressor protein (Lee et al., 2000; Tanaka et al., 2000; Nam et al., 2001).
The expression of the ptsG gene encoding IICBGlc is regulated by at least two global control systems at the transcriptional level (Kimata et al., 1997, 1998; Plumbridge, 1998). First, it is under positive control by cAMP receptor protein (CRP)–cAMP; therefore, ptsG expression is strongly dependent on this complex. Secondly, transcription of ptsG is regulated negatively by a global repressor Mlc. Recent studies have established that external glucose induces ptsG transcription by modulating the Mlc-mediated regulatory pathway (Kimata et al., 1998; Plumbridge, 1998). When glucose is transported into the cell, IICBGlc is dephosphorylated and binds Mlc, resulting in sequestration of Mlc from the cytoplasm to the membrane (Lee et al., 2000; Tanaka et al., 2000; Nam et al., 2001).
While the transcriptional control of ptsG has been well documented, little is known about the post-transcriptional control of ptsG expression. We report here a new finding regarding the regulation of ptsG expression through mRNA stability. During a search for mutations that eliminate the inhibitory effect of glucose on lac operon expression, we found that ptsG expression was reduced dramatically when the glycolytic pathway was blocked. We showed that inhibition of the glycolytic pathway down-regulates ptsG expression by accelerating the degradation of ptsG mRNA. The destabilization of ptsG mRNA was eliminated when any one of the glycolytic intermediates downstream of the block was supplied in the growth medium or when RNase E was inactivated. Thus, the present study established that the flow of glycolysis controls ptsG expression by modulating RNase E- mediated mRNA degradation.
Results
A mutation in the pgi or pfkA gene eliminates glucose effect
The lac operon is expressed to only a small extent in wild-type Escherichia coli cells when both lactose and glucose are present in the growth medium. This is because the transport of glucose into the cells by the PTS decreases the phosporylation state of IIAGlc, which in turn leads to the inhibition of Lac permease by an inducer exclusion mechanism (Inada et al., 1996; Kimata et al., 1997). In order to gain further insight into the mechanism by which glucose affects lac operon expression, we searched for mutations that allow the expression of the lac operon in the presence of glucose. Wild-type cells form a white Lac– colony on LB plates containing X-gal, glucose and lactose. We performed transposon mutagenesis by infecting wild-type W3110 cells with λ phage carrying Tn5 Km (de Lorenzo et al., 1990). Mutants in which the lac operon was significantly expressed were selected as blue Lac+ colonies on the indicator plates. One of the mutants that confer a Lac+ phenotype in the presence of glucose was expected to carry the Tn5 insertion in the lacI gene encoding Lac repressor. Mutation in the crr or ptsG gene should also give a Lac+ phenotype because the IIAGlc/IICBGlc-dependent glucose uptake no longer occurs in crr or ptsG cells. In fact, DNA sequence analysis of the Lac+ colonies revealed that this was exactly the case.
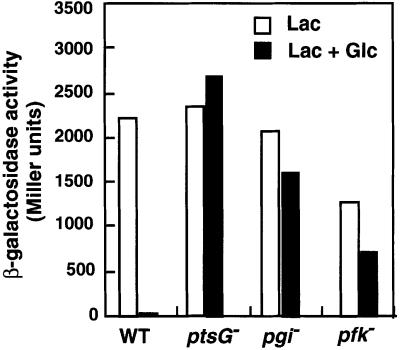
Interestingly, we also obtained Lac+ colonies that carry the Tn5 insertion in the other loci on which we focused in this study. DNA sequencing revealed that some Lac+ colonies carry the Tn5 insertion in either the pgi or pfkA gene encoding phosphoglucose isomerase or major phosphofructokinase (Pfk-1), respectively, both of which are glycolytic enzymes (Figure 1). To confirm that the Lac+ phenotype was due to a single insertion of Tn5 in the respective gene, the kanamycin-resistant phenotype of two representative clones was transferred by P1 transduction to the wild-type PP6 strain. The resulting pgi or pfkA strain was designated KK52 or KK51, respectively. To characterize the pgi and pfkA strains further, the effect of glucose on the β-galactosidase activity in these cells was determined in comparison with wild-type cells (Figure 2). In the presence of both lactose and glucose, the β-galactosidase activity was strongly inhibited through the inducer exclusion mechanism caused by the glucose transport in wild-type cells. On the other hand, β-galactosidase was expressed substantially in pgi and pfkA cells even in the medium with glucose, although its activity in the presence of lactose alone was moderately reduced compared with wild-type cells. pgi and pfkA mutation caused only a moderate reduction in the growth rate in the rich LB medium with or without glucose (data not shown). Thus, pgi and pfkA mutation eliminate the glucose effect, even if not completely. No inhibitory effect of glucose on β-galactosidase activity was observed in ptsG cells, as expected.
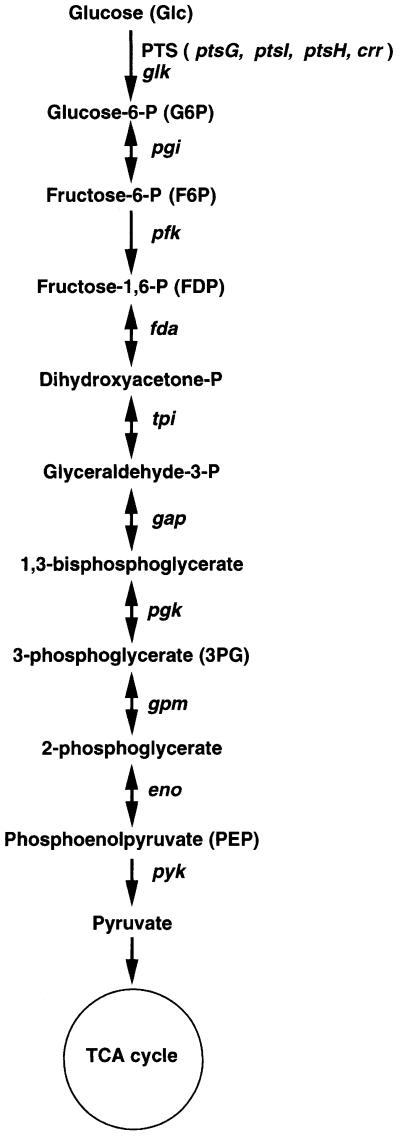
Fig. 1. The glycolytic pathway in E.coli. The phosphoenolpyruvate (PEP) phosphotransferase system (PTS) is the usual route of glucose uptake and phosphorylation in E.coli. Genes of the glycolytic enzymes responsible for each reaction are shown. Glucokinase encoded by glk may act to phosphorylate glucose generated internally (Fraenkel, 1996).
Fig. 2. The effect of glucose on lac operon expression is eliminated in pgi or pfkA mutants. PP6 (wild-type), KK27 (ptsG–), KK52 (pgi–) and KK51 (pfkA–) cells were grown in LB medium with 1% lactose (Lac) or 0.5% glucose (Glc) + 0.5% lactose. Culture samples were taken at an OD600 of 0.6 and β-galactosidase activities were determined.
Expression of IICBGlc is markedly reduced in the pgi or pfkA background
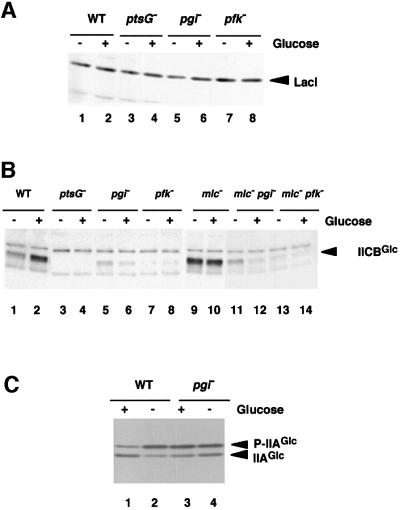
How does mutaion of pgi or pfkA eliminate the inhibitory effect of glucose on lac expression? In principle, this could occur if these mutations reduce the expression of either LacI, IIAGlc or IICBGlc. To test this, we first analyzed the effect of pgi or pfkA mutation on the level of LacI by western blotting using an antibody raised against LacI. As shown in the Figure 3A, LacI expression was not affected significantly by pgi or pfkA mutation either in the presence or absence of glucose. Next, we determined the relative levels of IICBGlc in mutant cells compared with wild-type cells by western blot analysis using anti-IICBGlc antibody. The expression of IICBGlc was significantly induced by glucose in the wild-type cells (Figure 3B, lanes 1 and 2) while no IICBGlc was detected in the ptsG cells (Figure 3B, lanes 3 and 4). Interestingly, the level of IICBGlc was markedly reduced by the pgi or pfkA mutation especially in the presence of glucose (Figure 3B, lanes 5–8). Thus, it is highly possible that the IICBGlc level in these cells is not high enough to transport external glucose sufficiently; therefore, a significant fraction of IIAGlc remains to be phosphorylated. In fact, the phosphorylation state of IIAGlc was hardly affected by glucose in pgi cells (Figure 3C, lanes 3 and 4) while the presence of glucose in the growth medium stimulates dephosphorylation of P-IIAGlc in wild-type cells (Figure 3C, lanes 1 and 2). Figure 3C also indicates that pgi or pfkA mutation does not affect IIAGlc expresssion. Taken together, we conclude that mutation of pgi or pfkA mutation eliminates the effect of glucose on lac expression by reducing the expression of IICBGlc.
Fig. 3. Analyses of the proteins involved in the regulation of lac operon expression. (A) Western blot analysis of LacI. Cells were grown in LB medium with or without 1% glucose. Crude extracts equivalent to OD600 = 0.05 prepared from PP6 (lanes 1 and 2), KK27 (lanes 3 and 4), KK52 (lanes 5 and 6) and KK51 (lanes 7 and 8) were analyzed by western blot using anti-LacI. (B) Western blot analysis of IICBGlc. Cells were grown in LB medium with or without 1% glucose. Crude extracts equivalent to OD600 = 0.01 prepared from PP6 (lanes 1 and 2), KK27 (lanes 3 and 4), KK52 (lanes 5 and 6), KK51 (lanes 7 and 8), KK32 (lanes 9 and 10), KK88 (lanes 11 and 12) and KK87 (lanes 13 and 14) were analyzed by western blot using anti-IICBGlc. (C) Determination of the phosphorylation state of IIAGlc. Cells were grown in LB medium with or without 1% glucose. Samples were prepared from PP6 (lanes 1 and 2) and KK52 (lanes 3 and 4), and analyzed by western blot using anti-IIAGlc as described in Materials and methods.
Reduction of ptsG expression in pgi or pfkA cells is independent of both the CRP–cAMP andMlc pathways
The next question is how the pgi or pfkA mutation reduces the expression of IICBGlc. Since the transcription of ptsG is regulated both positively by CRP–cAMP and negatively by Mlc, these mutations could affect the expression of IICBGlc through these two global regulatory pathways. However, the CRP–cAMP system apparently is functional in pgi or pfkA cells because the expression of the lac operon, which is totally dependent on CRP–cAMP, is still significantly expressed in these cells (see Figure 2). This suggests that the reduction of IICBGlc expression in mutant cells may occur independently of CRP–cAMP. We also tested whether pgi or pfkA mutation reduces the expression of IICBGlc by enhancing the action of Mlc. If this were the case, IICBGlc should be highly expressed in the mlc background regardless of whether the pgi or pfkA mutation is present or not. However, expression of IICBGlc in pgi mlc (KK88) or pfkA mlc (KK87) cells was far less than that of the parent mlc cells (Figure 3B, lanes 9–14). Thus, the inhibitory effect of pgi or pfkA mutation on IICBGlc expression is essentially independent of the negative regulation of ptsG transcription by Mlc.
ptsG transcription occurs normally in pgi orpfkA cells
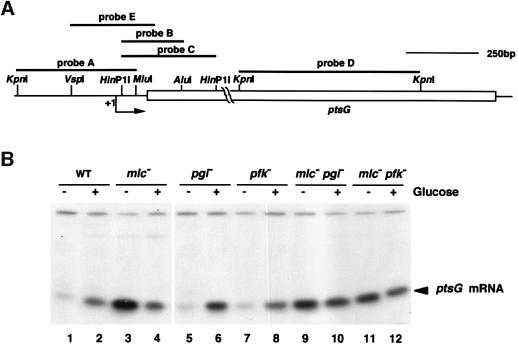
The results presented above raised the question of whether the down-regulation of ptsG by mutation of pgi or pfkA is achieved at the level of transcription. For this, we performed a quantitative S1 analysis using a 5′ DNA probe A (Figure 4A) and determined the relative levels of the 5′ ptsG transcript expressed in various cells. As expected, the abundance of the 5′ ptsG transcript increased upon addition of glucose in wild-type cells (Figure 4B, lanes 1 and 2) while it was highly expressed in the absence of glucose in mlc cells (Figure 4B, lane 3). The level of the 5′ ptsG transcript was reduced moderately in the presence of glucose (Figure 4B, lane 4). This is due to the reduction of both CRP and cAMP levels. Interestingly, ptsG transcription was induced normally by glucose in pgi cells and the relative abundance of the 5′ ptsG transcript was nearly identical to that of wild-type cells (Figure 4B, lanes 5 and 6). Similar results were also observed in pfkA cells although the induced level of the 5′ ptsG transcript was moderately reduced compared with wild-type cells (Figure 4B, lanes 7 and 8). These results indicate that IICBGlc could still modulate the localization of Mlc in response to the availability of glucose although its level is not sufficient to dephosphorylate P-IIAGlc. The 5′ ptsG transcript was constitutively expressed in mlc pgi or mlc pfkA cells (Figure 4B, lanes 9–12). Thus, it is apparent that the 5′ portion of ptsG is transcribed normally in pgi or pfkA cells. These results strongly suggest that mutation of pgi or pfkA affects ptsG expression by some unknown mechanism(s) after transcription initiation.
Fig. 4. Transcription of ptsG occurs normally in pgi or pfkA mutants. (A) Diagram of the ptsG region. The open bar represents the coding region for ptsG. The arrow denotes the start and direction of ptsG transcription. DNA fragments used as probes are shown above the map. The relevant restriction sites are shown. (B) S1 analysis of the 5′ portion of ptsG mRNA. Total RNAs (40 µg) prepared from PP6 (lanes 1 and 2), KK32 (lanes 3 and 4), KK52 (lanes 5 and 6), KK51 (lanes 7 and 8), KK88 (lanes 9 and 10) and KK87 (lanes 11 and 12) were subjected to S1 analysis using32P-labeled probe A.
pgi or pfkA mutation stimulates degradation of ptsG mRNA
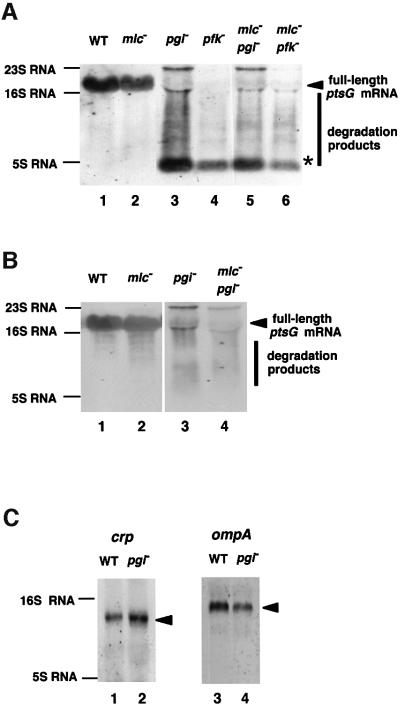
In order to understand the mechanism underlying the down-regulation of ptsG by pgi or pfkA mutation, we analyzed the ptsG mRNA expressed in cells growing in the presence of glucose by northern blotting using DNA probe C (Figure 4A) corresponding to the 5′ portion of the ptsG gene. The full-length ptsG mRNA of ∼1600 nucleotides was expressed in both wild-type and mlc cells (Figure 5A, lanes 1 and 2). Interestingly, pgi or pfkA mutation caused a dramatic decrease in the full-length ptsG mRNA, resulting in an extensive smear together with a short band of ∼100 nucleotides on the northern blot (Figure 5A, lanes 3 and 4), suggesting that ptsG mRNA is degraded rapidly in pgi or pfkA cells. A similar smear of ptsG mRNA was also observed in the mlc pgi or mlc pfkA double mutant (Figure 5, lanes 5 and 6). When probe D corresponding to the 3′ portion of ptsG was used in northern blot analysis, the short band detected by the 5′ probe C was no longer observed while the full-length ptsG mRNA was detected (Figure 5B). This suggests that the short RNA band, which may represent a relatively stable degradation intermediate of ptsG mRNA, may be derived from the 5′ portion of ptsG mRNA. The 3′ end of this RNA was mapped just before the translation initiation codon of ptsG mRNA by S1 mapping experiment using the DNA probe B 32P-labeled at its 3′ end (data not shown).
Fig. 5. The ptsG mRNA is degraded rapidly in pgi or pfkA mutants. (A) Northern blot analysis of the ptsG mRNA with the 5′ DNA probe. Total RNAs (40 µg) prepared from PP6 (lane 1), KK32 (lane 2), KK52 ( lane 3), KK51 (lane 4), KK88 (lane 5) and KK87 (lane 6) grown in LB medium with 1% glucose were subjected to northern blot analysis using fluorescein-labeled probe C. (B) Northern blot analysis of ptsG mRNA with the 3′ DNA probe. Total RNAs (40 µg) prepared from PP6 (lane 1), KK32 (lane 2), KK52 (lane 3) and KK88 (lane 4) grown in LB medium with 1% glucose were subjected to northern blot analysis using fluorescein-labeled probe D. (C) Effect of the pgi mutation on the stability of crp and ompA mRNAs. Total RNAs (40 µg) prepared from PP6 (lanes 1 and 3) and KK52 (lanes 2 and 4) grown in LB medium with 1% glucose were subjected to northern blot analysis using fluorescein-labeled crp (lanes 1 and 2) or ompA (lanes 3 and 4) probes.
Selective effect of pgi or pfkA mutation on mRNA stability
To examine whether mutation of pgi or pfkA affects the expression and stability of other mRNAs, we performed northern blot analysis with the crp and lac mRNAs. We found that the stability of crp mRNA was not significantly affected by the pgi mutation (Figure 5C, left). Similarly, the pgi mutation did not significantly affect the stability and/or expression of lac mRNA (data not shown). Because mutation of pgi or pfkA caused a moderate reduction in the growth rate and some mRNAs such as ompA are known to be destabilized when the growth rate is reduced (Nilsson et al., 1984; Vytvytska et al., 1998), it is interesting to examine how mutation of pgi or pfkA affect the expression and stability of ompA mRNA. No dramatic degradation of ompA mRNA was observed in pgi cells, although the level of ompA mRNA was reduced moderately (Figure 5C, right). We conclude that the effect of mutation of pgi or pfkA on mRNA stability is selective rather than universal.
Half-life of ptsG mRNA is reduced dramatically in pgi cells
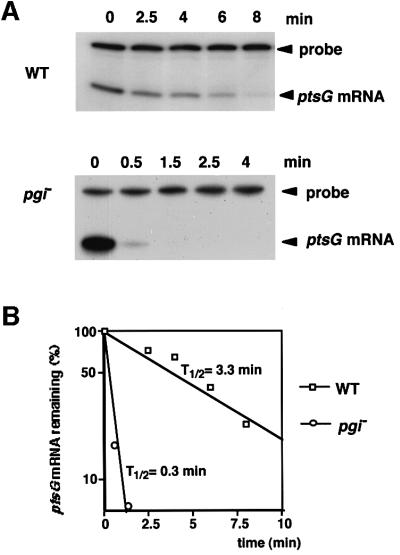
The results of northern blotting strongly suggest that the stability of ptsG mRNA must decrease significantly in pgi or pfkA cells. To evaluate the effect of mutation of pgi or pfkA on the stability of the ptsG mRNA more quantitatively, the chemical decay rate of the ptsG mRNAs in both wild-type and pgi cells was determined. Cells were grown to exponential phase in the presence of glucose, and rifamipicin was added to prevent further initiation of transcription. Cellular RNAs were isolated at various times after the addition of rifampicin, and these RNA samples were subjected to S1 nuclease assay using DNA probe E. As shown in Figure 6A, a dramatic increase in the decay of ptsG mRNA was observed in pgi cells. The radioactivity of the S1-resistant DNA bands was quantified to determine the rate of mRNA degradation (Figure 6B). The half-life of ptsG mRNA was estimated to be ∼3.3 min in wild-type cells, while it was reduced to ∼0.3 min in pgi cells. The reduction of the half-life of the 5′-most segment of ptsG mRNA in pgi cells was less significant when the S1 nuclease assay was performed using DNA probe A (data not shown). This is because the short degradation intermediate of ptsG mRNA produced in pgi cells was relatively stable.
Fig. 6. Effect of the pgi mutation on ptsG mRNA stability analyzed by S1 assay. (A) ptsG mRNA decay in wild-type (upper) and pgi– (lower) cells. PP6 and KK52 cells were grown in LB medium with 1% glucose to OD600 = 0.6 and treated with 0.2 mg/ml rifampicin. Cellular RNAs were prepared at the indicated times after the addition of rifampicin. Total RNAs of PP6 (40 µg) or KK52 (100 µg) were subjected to S1 analysis using 32P-labeled probe E at its 5′ end. The exposure of the gel for autoradiography was much longer in pgi than in WT cells. (B) Semi-logarithmic plots of the radioactivity of S1-resistant DNA versus time. The S1-resistant DNA bands were quantified using the Bioimage Analyzer BAS2000 (Fuji). The half-life (t1/2) of ptsG mRNA was determined based on these plots.
Exogenous glycolytic intermediates stabilize ptsG mRNA in the pgi or pfkA background
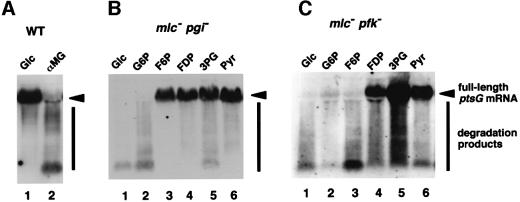
Because the block of the glycolytic pathway is expected to produce a variety of metabolic stresses, there are several possible factors that lead to the rapid degradation of ptsG mRNA in pgi or pfkA cells. For example, the missing protein, phosphoglucose isomerase or phosphofructokinase, in the mutant cells could be involved in the stabilization of ptsG mRNA. Secondly, the accumulation of glucose-6-phosphate (G6P) or fructose-6-phosphate (F6P) due to the mutation of pgi or pfkA could produce a signal for the destabilization of ptsG mRNA. Thirdly, deficiency of a single or several glycolysis intermediates after the block point could be responsible for the destabilization of ptsG mRNA. Finally, inhibition of the glycolytic pathway should affect downstream metabolic pathways such as the tricarboxylic acid (TCA) cycle, which may produce a signal for the destabilization of ptsG mRNA. To test these possibilities, we first examined the effect of α-methylglucoside on the stability of ptsG mRNA in wild-type cells. The normal glycolytic pathway would be inhibited in the presence of α-methylglucoside because this non-metabolizable glucose analog is transported and phosphorylated by the glucose PTS but not metabolized further. We found that the degradation of ptsG mRNA was stimulated by adding α-methylglucoside to wild-type cells as in the case of pgi or pfkA cells (Figure 7A). This suggests that the lack of phosphoglucose isomerase or phosphofructokinase itself is not responsible for the rapid degradation of ptsG mRNA. We then examined whether the addition of any of the glycolytic intermediates to the growth medium affects the stability of ptsG mRNA in mlc pgi or mlc pfkA double mutant cells (Figure 7B and C). The addition of F6P stabilized the ptsG mRNA in pgi but not in pfkA cells, while the addition of fructose-1,6-diphosphate (FDP) stabilized the ptsG mRNA in both cell types. These results suggest that accumulation of G6P or F6P may not be responsible for the destabilization of ptsG mRNA. Interestingly, all intermediates tested except G6P could restore the stability of ptsG mRNA in pgi cells (Figure 7B). Similarly, intermediates downstream of FDP could stabilize the ptsG mRNA in the mlc pfkA double mutant cells (Figure 7C). It should be noted that pyruvate, which is located the most downstream of the glycolytic pathway could stabilize the ptsG mRNA in the mutant cells. Taken together, we conclude that deficiency of any of the glycolysis intermediates may not be responsible for the destabilization of ptsG mRNA in pgi or pfkA cells.
Fig. 7. Effect of the addition of metabolic intermediates or analogs on the stability of ptsG mRNA. PP6 (A), KK88 (B) and KK87 (C) cells were grown in LB medium with 1% of the following compounds: Glc, glucose; G6P, glucose-6-phosphate; αMG, α-methylglucoside; F6P, fructose-6-phosphate; FDP, fructose-1,6-diphosphate; 3PG, 3-phosphoglycerate; Pyr, pyruvate. Total RNAs (40 µg) were subjected to northern blotting with32P-labeled probe C.
The rapid degradation of ptsG mRNA in pgi or pfkA cells is prevented by inactivation of RNase E
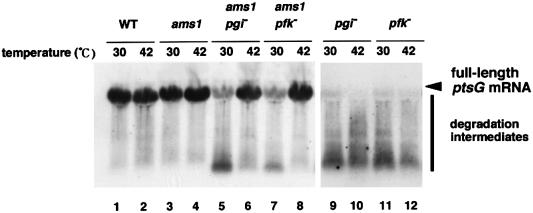
Because RNase E is the major ribonuclease responsible for general mRNA turnover (Kushner, 1996; Cohen and McDowall, 1997), we examined the effect of inactivation of RNase E on ptsG mRNA degradation. For this, we constructed three isogenic strains carrying a temperature-sensitive rne allele: KK65 (ams-1), KK69 (pgi ams-1) and KK67 (pfkA ams-1). Cells were grown at 30°C to mid-logarithmic phase in the presence of glucose and the temperature was shifted to 42°C to inactivate RNase E. Total RNAs were isolated before and 10 min after the temperature shift, and RNA samples were subjected to northern blot analysis using DNA probe C. The temperature shift from 30 to 42°C moderately increased the level of ptsG mRNA in ams-1 cells, presumably due to the inactivation of RNase E (Figure 8, lanes 3 and 4), but not in wild-type cells (Figure 8, lanes 1 and 2). Marked degradation of ptsG mRNA was observed at 30°C in cells carrying both ams-1 and pgi, or pfkA ams-1 mutations (Figure 8, lanes 5 and 7) as in the case of pgi cells (Figure 8, lane 9). However, the rapid degradation of ptsG mRNA in the ams-1 cells was inhibited when RNase E was inactivated by shifting to 42°C (Figure 8, lanes 6 and 8). The temperature shift from 30 to 42°C did not affect the degradation pattern of ptsG mRNA when cells carry only the pgi or pfkA mutation (Figure 8, lanes 9–12). These findings indicate that RNase E is responsible for the rapid degradation of ptsG mRNA when the glycolytic pathway is blocked.
Fig. 8. Inactivation of RNase E stabilizes ptsG expression in pgi or pfkA mutants. PP6 (lanes 1 and 2), KK65 (lanes 3 and 4), KK69 (lanes 5 and 6), KK67 (lanes 7 and 8), KK52 (lanes 9 and 10) and KK51 (lanes 11 and 12) were grown in LB medium with 1% glucose (Glc) at 30°C. At OD600 = 0.5, the temperature was shifted to 42°C (lanes 2, 4, 6, 8, 10 and 12) or kept at 30°C (lanes 1, 3, 5, 7, 9 and 11). Cellular RNAs were prepared after the incubation was continued for 10 min at the indicated temperatures. Total RNAs (40 µg) were subjected to northern blotting with 32P-labeled probe C.
Discussion
The PTS catalyzes the transport and phosphorylation of a number of carbohydrates in enteric bacteria and the phosphorylated sugars are metabolized by a series of chain reactions in which the glycolytic pathway always acts as a trunk route (Meadow et al., 1990; Postma et al., 1993; Saier, 1996). Thus, both the PTS and glycolysis play a central role in intermediary sugar metabolism. The expression of genes encoding various PTS components appears to be regulated in a complex and dynamic fashion to meet various physiological requirements. Transcription of several PTS genes including ptsG has been shown to be under the control of both CRP–cAMP and Mlc (Kimata et al., 1997, 1998; Plumbridge, 1998). However, little is known about post-transcriptional regulation of PTS genes. We have demonstrated here that the expression of ptsG encoding a major glucose transporter is regulated at the level of mRNA degradation in response to the glycolytic flux. Our findings can be summarized as follows: (i) block of the glycolytic pathway causes a dramatic reduction in the level of IICBGlc; (ii) this effect is independent of both CRP–cAMP and Mlc regulatory circuits and not exerted at the step of transcription initiation; (iii) the reduction in the IICBGlc level is due to a dramatic increase in the degradation rate of ptsG mRNA; (iv) α-methylglucoside, a non-metabolizable glucose analog, causes rapid degradation of ptsG mRNA in wild-type cells; (v) the presence of any glycolysis intermediates downstream of the block in growth medium could stabilize the ptsG mRNA in the pgi or pfkA mutant cells; and (vi) the destabilization of ptsG mRNA no longer occurs when RNase E is thermally inactivated in cells carrying a temperature-sensitive rne allele. These results led us to conclude that the block of the glycolytic pathway prevents ptsG expression by accelerating the degradation of ptsG mRNA and that RNase E is responsible for the rapid degradation of ptsG mRNA. Thus, we demonstrate a clear link between the carbon flux in central metabolism and the stability of a specific mRNA.
The degradation of mRNA is an important step for controlling gene expression because it determines, in part, the cellular concentration of mRNA and therefore the capacity for protein synthesis (Belasco, 1993; Grunberg-Manago, 1999; Rauhut and Klug, 1999). The longevity of bacterial mRNAs varies among transcripts and the rate of degradation of individual mRNAs can vary in response to changes in the environmental conditions either selectively or pleiotropically (Belasco, 1993). The best known example of this is the growth-rate dependent regulation of certain mRNA species in E.coli. It is well known that the long-lived ompA mRNA encoding the major outer membrane protein A decreases under conditions of slow cell growth (Nilsson et al., 1984; Vytvytska et al., 1998). The initial cleavage in the 5′-untranslated region of the ompA mRNA during slow growth was attributed to RNase E (Lundberg et al., 1990). Another environmental signal that affects mRNA stability in E.coli cells is oxygen availability. A general mRNA stabilization coupled with a decrease in RNA synthesis occurs during anaerobic slow growth, suggesting that oxygen availability somehow regulates RNases (Georgellis et al., 1993). Any environmental stimulus such as growth rate or oxygen availability should affect the flux in various metabolic pathways. However, little is known about whether a specific change in a certain metabolic pathway affects the stability of individual mRNAs. We showed here that ptsG mRNA becomes extremely unstable when glycolysis is blocked. Although the inhibition of the glycolytic pathway by pgi or pfkA mutation caused a moderate reduction in the growth rate, the dramatic effect on mRNA stability apparently is rather selective for ptsG mRNA because the stability of other mRNAs, including that for ompA, was not significantly affected by pgi or pfkA mutation. The present study has given a clear example where genetic and/or nutritional alterations in the central metabolism cause a dramatic change in stability of a specific mRNA to regulate its expression.
How does the inhibition of the glycolytic pathway enhance the degradation of ptsG mRNA? We showed that RNase E is certainly responsible for the rapid degradation of ptsG mRNA. Our data indicate that neither accumulation nor shortage of any of the intermediate metabolites of glycolysis is responsible for the rapid degradation of ptsG mRNA by RNase E. It is likely that inhibition of the glycolytic pathway may somehow produce a signal to stimulate the RNase E-dependent degradation of ptsG mRNA by affecting downstream metabolic pathways such as the TCA cycle. How the inhibition of the glycolytic pathway activates RNase E-mediated degradation of ptsG mRNA remains to be resolved.
RNase E encoded by rne/ams has an important role in the degradation of a variety of mRNAs in E.coli cells (Cohen and McDowall, 1997; Ow et al., 2000). It has been established that RNase E exists as a macromolecular complex called a degradosome, a multienzyme RNA degradation complex that also contains PNPase, RhlB helicase, enolase and other minor components (Carpousis et al., 1994; Py et al., 1994, 1996; Miczak et al., 1996). PNPase, one of two known 3′ to 5′ exonucleases, is believed to have an important role in degrading products generated by RNase E. The role of RhlB, a member of the DEAD-box family of ATP-dependent RNA helicases, would be to unwind the secondary structure to permit access by RNase E and/or PNPase (Coburn and Mackie, 1999). The role of enolase in RNA degradation remains a completely mystery. It would be interesting to test whether any of these components of the degradosome plays some role in the regulation of the RNase E-mediated degradation of ptsG mRNA.
The pathway for mRNA degradation apparently involves multiple steps and additional trans-acting factors. For example, host factor I (Hfq), originally recognized for its function in phage Qβ replication, binds to ompA mRNA in a growth rate-dependent fashion and stimulates its decay by interfering with ribosome binding (Vytvytska et al., 1998, 2000). Another example is CsrA, originally identified as a negative regulator of glycogen biosynthesis genes such as glgC and now known to be involved in pleiotropic regulation of central carbohydrate metabolism (Liu et al., 1997; Romeo, 1998). CsrA has been shown to enhance the decay of glgC mRNA by acting as an RNA-binding protein (Liu and Romeo, 1997). It will be interesting to determine whether Hfq and/or CsrA are involved in the regulation of the RNase E-mediated degradation of ptsG mRNA.
What is the physiological relevance of the modulation of ptsG mRNA stability by the central carbon flux? One situation in which the glycolysis flux decreases would be shortage of carbohydrate in the growth medium. In fact, we observed that the degradation of ptsG mRNA is partially stimulated when wild-type cells are forced to use only amino acids as carbon source (our unpublished results). The reduced stability of ptsG mRNA along with the negative regulation of ptsG transcription by Mlc is useful to prevent unnecessary synthesis of transporter protein when its substrate is absent. On the other hand, the stabilization of ptsG mRNA certainly allows the cells to synthesize IICBGlc rapidly when ptsG transcription is induced through inactivation of Mlc in response to glucose availability.
The discovery of a close link between central metabolism and mRNA stability has raised a number of questions and opened up new avenues for future studies. How does inhibition of the glycolytic pathway produce a signal that leads to the rapid degradation of ptsG mRNA mediated by RNase E? Is the regulation of ptsG mRNA degradation mediated by trans-acting factors such as Hfq and CsrA/CsrB? Are there any other mRNAs that are destabilized by the inhibition of the glycolytic pathway? What are the structural determinants of ptsG mRNA stability recognized by RNase E and/or some other factors? Does the inhibition of the glycolytic pathway affect the level of the components of the degradosome? Do mutations in other glycolytic genes affect the stability of ptsG mRNA? Many of these questions would be testable experimentally. Regarding the last question, it should be noted that mutation of ts8, an allele of fda encoding another glycolytic enzyme fructose-1,6-diphosphate aldolase, is reported to inhibit stable RNA synthesis at the non-permissive temperature (Singer et al., 1991). It will certainly be interesting to test the fda mutation on ptsG mRNA.
Materials and methods
Bacterial strains and growth conditions
The E.coli K-12 strains used in this study are listed in Table I. PP6 was used as a parent wild-type strain. IT1320 (W3110 pgi::Tn5) and IT1165 (W3110 pfkA::Tn5) were obtained by random insertion of Tn5 from W3110. The pgi::Tn5 region of IT1320 or the pfkA::Tn5 region of IT1165 was transferred to PP6 by P1 transduction to obtain KK52 and KK51, respectively. The mlc::Tn10 region of KK32 (PP6 mlc1157::Tn10) was transferred to KK52 and KK51 by P1 transduction to obtain KK88 and KK87, respectively. The zce-726::Tn10 region of GW10 (Wachi et al., 1997) was transferred to PP6, KK52 and KK51 by P1 transduction to obtain KK64, KK68 and KK66, respectively. The ams-1 zce-726::Tn10 region of GW20 (Wachi et al., 1997) was transferred to PP6, KK52 and KK51 by P1 transduction to obtain KK65, KK69 and KK67, respectively. Cells were grown aerobically at 37°C in Luria–Bertani broth (LB) medium (Miller, 1972). Antibiotics were used at the following concentrations: ampicillin (50 µg/ml), kanamycin (50 µg/ml), tetracycline (15 µg/ml) and chloramphenicol (15 µg/ml). Bacterial growth was monitored by determining the optical density at 600 nm.
Table I. Bacterial strains used in this study.
| Strain/plasmid | Relevant genotype and property | Source |
|---|---|---|
| PP6 | wild type | Kimata et al. (1998) |
| IT1165 | W3110 pfkA::Tn5 | this study |
| IT1320 | W3110 pgi::Tn5 | this study |
| IT1168 | W3110 ptsG::Tn5 | Kimata et al. (1997) |
| KK27 | PP6 ptsG::Tn5 | Tanaka et al. (2000) |
| KK32 | PP6 mlc1157::Tn10 | Kimata et al. (1998) |
| KK51 | PP6 pfkA::Tn5 | this study |
| KK52 | PP6 pgi::Tn5 | this study |
| KK87 | PP6 pfkA::Tn5 mlc1157::Tn10 | this study |
| KK88 | PP6 pgi::Tn5 mlc1157::Tn10 | this study |
| GW10 | W3110 zce-726::Tn10 | Wachi et al. (1997) |
| GW20 | W3110 ams1 zce-726::Tn10 | Wachi et al. (1997) |
| KK64 | PP6 zce-726::Tn10 | this study |
| KK65 | PP6 ams1 zce-726::Tn10 | this study |
| KK66 | PP6 pfkA::Tn5 zce-726::Tn10 | this study |
| KK67 | PP6 pfkA::Tn5 ams1 zce-726::Tn10 | this study |
| KK68 | PP6 pgi::Tn5 zce-726::Tn10 | this study |
| KK69 | PP6 pgi::Tn5 ams1 zce-726::Tn10 | this study |
β-galactosidase assay
β-galactosidase activity was determined according to the method of Miller (1972).
Western blotting
Bacterial cells grown in LB medium containing appropriate antibiotic(s) were harvested at OD600 = 0.6 and suspended in 100 µl of SDS–PAGE loading buffer (62.5 mM Tris–HCl pH 6.8, 2% SDS, 10% glycerol, 5% β-mercaptoethanol, 0.1% bromophenol blue). The sample was heated at 100°C for 5 min. The indicated amounts of total cellular proteins were subjected to 12% acrylamide–0.1% SDS gel electrophoresis and transferred to an Immobilon membrane (Millipore). The polypeptides detected by the antibodies were visualized by the enhanced chemiluminescence (ECL) system (Pharmacia). The anti-LacI, anti-IIBGlc and anti-IIAGlc polyclonal antibodies have been described previously (Tanaka et al., 1999, 2000; Abo et al., 2000).
Determination of the phosphorylation state of IIAGlc
The phosphorylation state of IIAGlc in cells was determined by the alkali-freeze method (Takahashi et al., 1998). Cells were grown to an OD600 of 0.6. Culture samples of 0.2 ml were taken and immediately killed by adding with 20 µl of 10 M NaOH followed by vigorous vortexing for 10 s. Then, 180 µl of 3 M sodium acetate pH 5.2 and 1 ml of ethanol were added. The mixture was chilled at –70°C for 10 min and centrifuged at 12 000 g for 10 min at 4°C. The resulting pellet was rinsed with 70% ethanol and dissolved in 100 µl of SDS sample buffer (2% SDS, 5% 2-mercaptoethanol, 62.5 mM Tris–HCl pH 8.0, 10% glycerol, 0.1% bromophenol blue). Then 20 µl of sample was subjected to SDS– 12% polyacrylamide gel electrophoresis and analyzed by western blotting using polyclonal anti-IIAGlc antibody.
S1 nuclease analysis
The S1 nuclease assay was performed as described previously (Aiba et al., 1981). Total cellular RNAs were isolated from cells grown in LB medium to an OD600 of 0.6. The 411 bp KpnI–MluI DNA fragment (probe A) 32P-labeled at the MluI 5′ end was used as a DNA probe to determine the relative level of the 5′ most portion of ptsG mRNA (Figure 4). The 283 bp VspI-PG3 (PG3; GGAGCACTCTCAATTATGTTTAAGAATGC) DNA fragment (probe E) 32P-labeled at the PG3 5′ end, obtained by PCR, was used for determination of the half-life of ptsG mRNA. The 204 bp HinP1I–AluI DNA fragment (probe B) 32P-labeled at the HinP1I 3′ end was used to determine the 3′ end of the short degradation intermediate of ptsG mRNA. The DNA fragments were obtained by PCR amplification, except probes A and B, which were derived from purified plasmid pIT499 (Kimata et al., 1997). The DNA probe and total RNA were hybridized and treated with 40 U of S1 nuclease at 37°C for 15 min. The reaction products were analyzed on an 8% polyacrylamide–8 M urea gel.
Northern blot analysis
The total RNAs were resolved by 1.0% agarose gel electrophoresis in the presence of formamide and blotted onto Hybond-N+ membrane (Amersham Pharmacia Biotech) as described (Sambrook et al., 1989). The DNA probes were labeled with fluorescein-11-dUTP by random priming (Amersham Pharmacia Biotech). The 571 bp KpnI–KpnI fragment (probe C) corresponding to the 3′ portion of the ptsG structural gene and the 326 bp HinP1I–HinP1I fragment (probe E) containing the 5′ portion of the ptsG gene were used as the 3′ and 5′ DNA probes, respectively. The 725 bp NruI–BamHI DNA fragment containing the ompA structural gene and the 738 bp HindIII–EcoRV DNA fragment containing the crp gene were used as probes for ompA and crp mRNAs, respectively. The DNA fragments used were obtained by PCR amplification. The membrane was hybridized with the probes and washed. The signals were produced by addition of the anti-fluorescein alkaline phosphatase conjugate and CDP-Star (Amersham Pharmacia Biotech) to the membrane and visualized by exposure to films.
Acknowledgments
Acknowledgements
We thank Dr M.Wachi (Tokyo Institute of Technology) for providing strains GW10 and GW20. This work was supported by Grants-in-Aid from Ministry of Education, Science, Sports and Culture of Japan.
References
- Abo T., Inada,T., Ogawa,K. and Aiba,H. (2000) SsrA-mediated tagging and proteolysis of LacI and its role in regulation of the lac operon. EMBO J., 19, 3762–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Adhya,S. and de Crombrugghe,B. (1981) Evidence for two functional gal promoters in intact Escherichia coli cells. J. Biol. Chem., 256, 11905–11910. [PubMed] [Google Scholar]
- Belasco J. (1993) mRNA degradation in proprokaryotic cells: an overview. In Belasco,J. and Brawerman,G. (eds), Control of Messenger RNA Stability. Academic Press, New York, NY, pp. 3–12.
- Carpousis A.J., Van Houwe,G., Ehretsmann,C. and Krisch,H.M. (1994) Copurification of E.coli RNAase E and PNPase: evidence for a specific association between two enzymes important in RNA processing and degradation. Cell, 76, 889–900. [DOI] [PubMed] [Google Scholar]
- Coburn G.A. and Mackie,G.A. (1999) Degradation of mRNA in Escherichia coli: an old problem with some new twists. Prog. Nucleic Acid Res. Mol. Biol., 62, 55–108. [DOI] [PubMed] [Google Scholar]
- Cohen S.N. and McDowall,K.J. (1997) RNase E: still a wonderfully mysterious enzyme. Mol. Microbiol., 23, 1099–1106. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Herrero,M., Jakubzik,U. and Timmis,K.N. (1990) Mini-Tn5 transposon derivatives for insertion mutagenesis, promoter probing and chromosomal insertion of cloned DNA in gram-negative eubacteria. J. Bacteriol., 172, 6568–6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D.G. (1996) Glycolysis. In Neidhardt,F.C. (ed.), Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. ASM Press, Washington, DC, pp. 189–205.
- Georgellis D., Barlow,T., Arvidson,S. and von Gabain,A. (1993) Retarded RNA turnover in Escherichia coli: a means of maintaining gene expression during anaerobiosis. Mol. Microbiol., 9, 375–381. [DOI] [PubMed] [Google Scholar]
- Grunberg-Manago M. (1999) Messenger RNA stability and its role in control of gene expression in bacteria and phages. Annu. Rev. Genet., 33, 193–227. [DOI] [PubMed] [Google Scholar]
- Inada T., Kimata,K. and Aiba,H. (1996) Mechanism responsible for glucose–lactose diauxie in Escherichia coli: challenge to the cAMP model. Genes Cells, 1, 293–301. [DOI] [PubMed] [Google Scholar]
- Kimata K., Takahashi,H., Inada,T., Postma,P. and Aiba,H. (1997) cAMP receptor protein–cAMP plays a crucial role in glucose–lactose diauxie by activating the major glucose transporter gene in Escherichia coli. Proc. Natl Acad. Sci. USA, 94, 12914–12919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata K., Inada,T., Tagami,H. and Aiba,H. (1998) A global repressor (Mlc) is involved in glucose induction of the ptsG gene encoding major glucose transporter in Escherichia coli. Mol. Microbiol., 29, 1509–1519. [DOI] [PubMed] [Google Scholar]
- Kushner S.R. (1996) mRNA decay. In Neidhardt,F.C. (ed.), Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. ASM Press, Washington, DC, pp. 849–860.
- Lee S.J., Boos,W., Bouche,J.P. and Plumbridge,J. (2000) Signal transduction between a membrane-bound transporter, PtsG and a soluble transcription factor, mlc, of Escherichia coli. EMBO J., 19, 5353–5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg U., von Gabain,A. and Melefors,Ö. (1990) Cleavages in the 5′ region of the ompA and bla mRNA control stability: studies with an E.coli mutant altering mRNA stability and a novel endoribonuclease. EMBO J., 9, 2731–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.Y. and Romeo,T. (1997) The global regulator CsrA of Escherichia coli is a specific mRNA-binding protein. J. Bacteriol., 179, 4639–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.Y., Gui,G., Wei,B., Preston,J.F., Oakford,L., Yuksel,U., Giedroc,D.P. and Romeo,T. (1997) The RNA molecule CsrB binds to the global regulatory protein CsrA and antagonizes its activity in Escherichia coli. J. Biol. Chem., 272, 17502–17510. [DOI] [PubMed] [Google Scholar]
- Meadow N.D., Fox,D.K. and Roseman,S. (1990) The bacterial phosphoenolpyruvate:glycose phosphotransferase system. Annu. Rev. Biochem., 59, 497–542. [DOI] [PubMed] [Google Scholar]
- Miczak A., Kaberdin,V.R., Wei,C.L. and Lin-Chao,S. (1996) Proteins associated with RNase E in a multicomponent ribonucleolytic complex. Proc. Natl Acad. Sci. USA, 93, 3865–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. (1972) Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Nam T.W. et al. (2001) The Escherichia coli glucose transporter enzyme IICB(Glc) recruits the global repressor Mlc. EMBO J., 20, 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson G., Belasco,J.G., Cohen,S.N. and von Gabain,A. (1984) Growth-rate dependent regulation of mRNA stability in Escherichia coli. Nature, 312, 75–77. [DOI] [PubMed] [Google Scholar]
- Ow M.C., Liu,Q. and Kushner,S.R. (2000) Analysis of mRNA decay and rRNA processing in Escherichia coli in the absence of RNase E-based degradosome assembly. Mol. Microbiol., 38, 854–866. [DOI] [PubMed] [Google Scholar]
- Plumbridge J. (1998) Expression of ptsG, the gene for the major glucose PTS transporter in Escherichia coli, is repressed by Mlc and induced by growth on glucose. Mol. Microbiol., 29, 1053–1063. [DOI] [PubMed] [Google Scholar]
- Postma P.W., Lengeler,J.W. and Jacobson,G.R. (1993) Phospho enolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev., 57, 543–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py B., Causton,H., Mudd,E.A. and Higgins,C.F. (1994) A protein complex mediating mRNA degradation in Escherichia coli. Mol. Microbiol., 14, 717–729. [DOI] [PubMed] [Google Scholar]
- Py B., Higgins,C.F., Krisch,H.M. and Carpousis,A.J. (1996) A DEAD-box RNA helicase in the Escherichia coli RNA degradosome. Nature, 381, 169–172. [DOI] [PubMed] [Google Scholar]
- Rauhut R. and Klug,G. (1999) mRNA degradation in bacteria. FEMS Microbiol. Rev., 23, 353–370. [DOI] [PubMed] [Google Scholar]
- Romeo T. (1998) Global regulation by the small RNA-binding protein CsrA and the non-coding RNA molecule CsrB. Mol. Microbiol., 29, 1321–1330. [DOI] [PubMed] [Google Scholar]
- Saier M.H. Jr, Ramseier,T.M. and Reizer,J. (1996) Regulation of carbon utilization. In Neidhardt,F.C. (ed.), Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. ASM Press, Washington, DC, pp. 1325–1343.
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Singer M., Rossmiessl,P., Cali,B.M., Liebke,H. and Gross,C.A. (1991) The Escherichia coli ts8 mutation is an allele of fda, the gene encoding fructose-1,6-diphosphate aldolase. J. Bacteriol., 173, 6242–6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Inada,T., Postma,P. and Aiba,H. (1998) CRP down-regulates adenylate cyclase activity by reducing the level of phosphorylated IIA(Glc), the glucose-specific phosphotransferase protein, in Escherichia coli. Mol. Gen. Genet., 259, 317–326. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Kimata,K., Inada,T., Tagami,H. and Aiba,H. (1999) Negative regulation of the pts operon by Mlc: mechanism underlying glucose induction in Escherichia coli. Genes Cells, 4, 391–399. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Kimata,K. and Aiba,H. (2000) A novel regulatory role of glucose transporter of Escherichia coli: membrane sequestration of a global repressor Mlc. EMBO J., 19, 5344–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytvytska O., Jakobsen,J.S., Balcunaite,G., Andersen,J.S., Baccarini,M. and von Gabain,A. (1998) Host factor I, Hfq, binds to Escherichia coli ompA mRNA in a growth rate-dependent fashion and regulates its stability. Proc. Natl Acad. Sci. USA, 95, 14118–14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vytvytska O., Moll,I., Kaberdin,V.R., von Gabain,A. and Blasi,U. (2000) Hfq (HF1) stimulates ompA mRNA decay by interfering with ribosome binding. Genes Dev., 14, 1109–1118. [PMC free article] [PubMed] [Google Scholar]
- Wachi M., Umitsuki,G. and Nagai,K. (1997) Functional relationship between Escherichia coli RNase E and the CafA protein. Mol. Gen. Genet., 253, 515–519. [DOI] [PubMed] [Google Scholar]