Abstract
In cortex and hippocampus, electrophysiological, molecular, and/or ultrastructural evidence shows that sleep promotes the weakening of most synapses. In primary motor cortex, immediately after training in the complex wheel task, sleep-dependent weakening spares the synapses that potentiated during learning. Together, these results show that sleep can at the same time reduce the cost of synaptic activity and promote memory consolidation. Here we used serial block-face scanning electron microcopy to measure synapse number and size of the axon-spine interface (ASI), an ultrastructural measure of synaptic strength, in the medium size spiny neurons of the mouse dorsomedial (DM) and dorsolateral (DL) striatum. Previous work found that DM is involved in the early phase of motor learning, while DL is engaged later when the task becomes automatic. Four experimental groups were used: mice extensively trained in the complex wheel task for 1 hour (T), untrained awake controls (W), and mice allowed to sleep (S) or sleep deprived (SDep) for 6 hours immediately after training (4–5 male mice/group; at least 401 ASIs/mouse/region). In DM, ASI size increases immediately after skill training in large sets of spines with high plastic potential (with endosomes and without spine apparatus) and, several hours later, the overall number of synapses decreases after sleep but not after sleep deprivation. In DL, the post-training increase in ASI size is restricted to fewer spines and is not followed by sleep-dependent synaptic changes. Thus, post-learning synaptic pruning afforded by sleep may be especially important early in the training, before the task becomes automatic.
Keywords: Synaptic potentiation, synaptic pruning, excitatory synapse, motor learning, serial electron microscopy
Statement of Significance
Sleep promotes the consolidation of motor memories in rodents and humans, but the underlying mechanisms are poorly characterized. In dorsomedial striatum, which is involved in the early phase of learning when movements are imprecise, we find that skill training leads, in most spines, to an increase in the axon-spine interface (ASI), an ultrastructural measure of synaptic strength, and post-learning sleep, but not post-learning sleep deprivation, decreases the number of excitatory synapses. In dorsolateral striatum, which is engaged when the task becomes automatic, the post-training increase in ASI size affects fewer spines and is not followed by sleep-dependent synaptic changes. Synaptic pruning during sleep may therefore be especially important during the early phase of consolidation of a motor skill.
Introduction
Electrophysiological, molecular, and ultrastructural evidence shows that most excitatory synapses in cortex and hippocampus are weaker and/or less numerous after sleep than after waking. This supports the hypothesis that sleep-dependent synaptic weakening benefits the brain by saving energy and avoiding synaptic saturation [1; 2]. In the whole rat cortex and hippocampus, for instance, the synaptic expression of glutamatergic GluA1-containing AMPA receptors is lower after sleep than after waking [3]. In the superficial layers of mouse primary sensory and motor (M1) cortex, serial block-face scanning electron microscopy finds that the size of the axon-spine interface (ASI), an established ultrastructural measure of synaptic strength, is smaller after sleep than after waking in most (~ 80%) excitatory synapses [4]. In M1, a sleep-dependent net decrease in synaptic strength also occurs after motor skill learning and is associated with better performance. Specifically, after an intense, one-hour training in the complex wheel task, performance is consolidated if the mice are allowed to sleep for 5–6 hours post-learning but not if they spend the same time awake [5; 6]. Immediately after training in this task repeated two-photon imaging reveals a net increase in the synaptic expression of GluA1-containing AMPA receptors in the superficial layers of M1 [6]. In the same area, post-learning sleep weakens most synapses but not those (~ 10% of all synapses) that grew stronger after learning. Moreover, sleep-dependent synaptic weakening correlates with improved task performance the next day [6]. In short, sleep-dependent synaptic weakening occurs in the cerebral cortex after motor learning; this process is at the same time broad and selective, allowing to reduce the overall cost of synaptic activity while consolidating the newly acquired motor memory.
In the dorsal striatum the GABAergic medium spiny neurons (MSNs) account for the majority of neurons [7] and the axospinous synapses located on their dendrites account for the majority of striatal synapses [8]. The MSNs project to the output nuclei of the basal ganglia via either a direct pathway targeting substantia nigra pars reticulata and the internal portion of the globus pallidus, or an indirect pathway projecting to the external portion of the globus pallidus. Direct and indirect MSNs are intermingled, present in similar number, and most of their inputs come from the cortex [9]. Like in cortex [10], MSNs activity varies across the sleep/wake cycle and switches, during NREM sleep, between UP states with firing and DOWN states of silence [11]. Unlike among cortical neurons, however, ON/OFF oscillations among striatal neurons are desynchronized, likely because the common input from the cortex is sparse and each cortical axon establishes only 1–2 contacts with individual MSNs [12].
Synaptic potentiation in the striatum can be detected in vivo after learning tasks [13] and the MSNs in dorsomedial (DM) and dorsolateral (DL) striatum undergo distinct plastic changes during motor training that are thought to causally contribute to different phases of skill learning. DM, which receives from prefrontal, retrosplenial, and other associative areas as well as from visual and auditory cortex, is involved in the first phase of motor processing and visuomotor learning while DL, which receives from somatosensory and motor cortex, is mainly engaged later when the skill is perfected and becomes automatic [14]. Supporting this model, mice trained in the rotarod task show electrophysiological changes consistent with synaptic potentiation first in DM and later in DL [15].
Here we used serial block-face scanning electron microscopy to assess the synaptic effects of motor learning and post-learning sleep on MSNs of DM and DL. Synapse number, size of the ASI, and other ultrastructural features were measured in mice that received motor training and untrained controls, as well as in mice that slept or were sleep deprived after training. We tested whether training in the complex wheel task leads to the strengthening of excitatory synapses and is followed by synaptic weakening when mice are allowed to sleep. As in our previous studies, training included 20 trials during one single session, which concentrates most learning in one hour [5; 6].
Materials and methods
Mice and experimental design
Homozygous B6.Cg-Tg(Thy1-YFP)16Jrs/J transgenic male mice (RRID:IMSR_JAX:003709) expressing yellow fluorescent protein (YFP) in a subset of cortical pyramidal neurons were used, as in our previous ultrastructural studies in the cerebral cortex, hippocampus, and cerebellum [4; 16; 17]. Mice were around 1-month old at the time of the experiment. In this mouse strain, the sleep/waking pattern and sleep homeostatic regulation at this age are very similar to those of adult mice [18; 19]. All animal procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and facilities were reviewed and approved by the IACUC of the University of Wisconsin-Madison and were inspected and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Mice were housed in environmentally controlled recording chambers with free access to food and water (12h:12h light-dark cycle; lights on at 8am). Sleep/wake behavior was monitored continuously using infrared cameras (OptiView Technologies). This method estimates total sleep time with a consistent accuracy exceeding 90% [20]. Motor activity was quantified by custom-made video-based motion detection algorithms (Matlab; see details in [21]). Four experimental groups were used: mice awake during the first hour of the light period with no motor training (W, n = 4), mice trained in the complex wheel task during the first hour of the light period (T, n = 4), and mice that were either allowed to sleep (S, n = 5) or sleep deprived (SDep, n = 5) for around 6 hours after training.
Motor training
Mice were trained in a single session of the complex wheel task as described [5; 6]. In short, a modified accelerating rotarod system (EZRod, Omnitech Electronics, Inc.) was equipped with a wheel in which 20 of the original 50 rungs had been removed, to generate 2 identical complex sequences of rungs in one rotation. Mice were handled for at least 3 days before the training session but did not receive any habituation or pretraining using the complex wheel or a regular wheel. The training session included 20 trials (with a 5 min rest after the first 10 trials) and occurred during the first hour of the light period. The session started with the mouse being placed onto the stationary complex wheel, after which acceleration increased until the mouse fell off the wheel (0 to 40 rpm in 10 min; acceleration = 223.3 cm/min2). Time and speed when mice fell off the wheel were recorded and used to assess performance. At the end of the first session mice were sacrificed (T mice) or returned to the home cage and allowed to sleep (S mice), or sleep deprived for 6 hours (SDep mice). Since the mean performance during a session (average of all trials) does not fully capture variability and fatigue, as before [5; 6] we compared performance between the first 3 trials and the best 3 trials.
Staining for Electron Microscopy
Under deep isoflurane anesthesia (3% volume to oxygen) mice were transcardially perfused with normal saline (0.9%, 41°C, 30 secs) followed by 2.5% glutaraldehyde and 4% paraformaldehyde dissolved in 0.1M sodium cacodylate buffer (pH 7.4, 41°C, 10 min). After removal brains were kept in the same fixative overnight at 4°C, and then sliced on a vibratome. The slices (120 μm in thickness) were kept in a cryoprotectant solution until the day of processing. Small blocks of tissue (1mm2) from DM (from bregma, AP 0.49 mm, LL 1.2 mm, DV 2.5 mm) and DL (from bregma, AP 0.49 mm, LL 2.2 mm, DV 2.5 mm) were excised under a stereomicroscope and stained (details in [4; 22]). Staining was performed blind to experimental condition. Briefly, after several rinses in cacodylate buffer, the tissue was 1) incubated with a solution of 1% potassium ferrocyanide/2% osmium tetroxide (in the dark for 1 h on ice); 2) exposed to a solution of 1% thiocarbohydrazide (20 min at room temperature); 3) placed in 2% osmium tetroxide (30 min); 4) incubated with 1% uranyl acetate (2 h at room temperature followed by overnight at 4°C). The next day, the tissue was 1) stained with a solution of lead aspartate (30 min at 60°C, pH 5.5); 2) dehydrated using ice-cold solutions of freshly prepared 35%, 50%, 75%, 80%, 90%, 95%, and 100% ethanol; 3) placed in propylene oxide (2 X 10 min); 4) impregnated with 25%, 50%, 75% Durcupan ACM resin (Electron Microscopy Science) mixed with propylene oxide (2 h each). Finally, the tissue was placed in fresh 100% Durcupan several times, flat embedded with ACLAR embedding film (Electron Microscopy Science) and kept in an oven at 60°C for 48–72 h. After polymerization, the stained tissue was excised under a stereomicroscope and attached on the tip of a metal pin using conductive epoxy resin (Chemtronics) to minimize charging during imaging.
Imaging
Samples were imaged in a ΣIGMA VP field emission scanning electron microscope with the following parameters: aperture 30 µm; high vacuum, acceleration voltage 1.7 kV, image size of 5000 by 5000 pixels, image resolution (xy plane) of 5 nm. One or two stacks of ~700 images each were acquired per animal (~25 X 25 X 35 μm) in DM and DL. Images were Gaussian filtered and automatically aligned using the open-source software Fiji [23]. Ultrathin sections were cut at a nominal thickness of 50 nm. For each stack, the mean actual section thickness was estimated using the cylindrical diameters method [24] and was similar across all groups (DM mean ± std in nm; W = 50.9 ± 0.37; T = 50.96± 0.13; S = 50.67 ± 1.3; SDep =51.07 ± 1.13. DL mean ± std in nm; W = 51.18 ± 0.51; T = 51.39 ± 0.56; S = 51.15 ± 0.72; SDep = 51.04 ± 1.08). Dendritic segments and all their spines were segmented manually in TrakEM2 [25] by three trained annotators who were blind to experimental condition. We randomly selected spiny dendritic segments whose length was at least 8.26 μm (mean ± std in μm; W = 28.58 ± 7.45; T = 26.30 ± 10.00; S = 24.00 ± 8.80; SDep = 25.73 ± 6.94; LME model p = 0.6065) and whose diameter ranged between 0.767 and 1.574 μm. Distribution of dendritic diameters was balanced across experimental groups (mean ± std in μm; W = 1.01 ± 0.13; T = 1.01 ± 0.12; S = 1.03 ± 0.15; SDep = 1.06 ± 0.13; LME model p = 0.5847). In both DM and the DL the target area was located around 150 um below the white matter.
All protrusions were defined as “spines” (as suggested in [26]), including spines with synapses and spines lacking synapses (processes). A synapse was defined by the presence of a presynaptic bouton with at least 2 synaptic vesicles within a 50 nm distance from the cellular membrane facing the spine, a visible synaptic cleft and post-synaptic density (PSD). Branched synapses (housed in 2 or more spines sharing the same spine neck) were counted as two (or more) synapses. In total, 157 dendritic branches were segmented (DM; W = 17, T = 21, S = 24, SDep = 20. DL; W = 17, T = 17, S = 21, SDep = 20). All segmentation data were tested for accuracy and consistency by the same experienced tracers (SSL, CC).
As in previous studies [4; 16] the ASI was traced at the interface between the spine head and the presynaptic terminal or bouton, and computed as described in [27]. Specifically, the region of contact between the two apposed objects was outlined (on the spine head side) in each individual section using the arealist brush suitably set at 1 pixel size. In this way, a quasi two-dimensional sheet-like object representing the interfaced region was created along the z dimension. The total surface area was calculated by computing the smoothed upper bound surface, according to the formula
where n is the number of sections, a and b are the traced elements at the top and bottom of a section k of thickness T, Ps is the smoothed perimeter, and A is the area [25]. The area of the traced element in the section k = 1 and in the section k = n were then subtracted from the smoothed upper bound surface value and the result was divided by 2 to get an approximate value of the apposed surface (AS). In oblique spines the ASI was not segmented because these spines were oriented obliquely or orthogonally to the cutting plane (range 5.1% - 9.0% across groups; Table 1). The presence of the following structures was recorded for each spine: spine apparatus, spinula in the head or neck of the spine, endosomes, coated vesicles, multivesicular bodies (MVBs) and lysosomes.
Table 1.
Summary of ultrastructural measures in the dorsomedial and dorsolateral striatum
| DM | DL | |||||||
|---|---|---|---|---|---|---|---|---|
| W | T | S | SDep | W | T | S | SDep | |
| Total N of dendrites | 17 | 21 | 24 | 20 | 17 | 17 | 21 | 20 |
| Total N of spines | 1325 | 1348 | 1417 | 1565 | 1714 | 1445 | 1681 | 1711 |
| Total N of spines with synapse (some incomplete, oblique) | 1283 | 1313 | 1370 | 1493 | 1616 | 1393 | 1606 | 1644 |
|
Total N spines with synapse
with measured ASI |
1164 | 1147 | 1215 | 1293 | 1306 | 1154 | 1313 | 1314 |
| Total N shaft synapses | 213 | 197 | 324 | 303 | 278 | 309 | 269 | 284 |
| ASI (µm2, mean±std) range (µm2) | 0.133±0.153 0.005–1.436 |
0.143±0.168 0.006–1.631 |
0.146±0.168 0.006–1.672 |
0.131±0.151 0.005–1.188 |
0.137±0.150 0.008–1.174 |
0.143±0.155 0.007–1.487 |
0.138±0.152 0.006–1.174 |
0.146±0.153 0.005–1.403 |
| Dendrite diameter (µm, mean±std) | 1.01±0.14 | 1.04±0.11 | 1.03±0.11 | 1.07±0.13 | 1.02±0.14 | 0.98±0.12 | 1.03±0.20 | 1.06±0.13 |
| Dendrite length (µm, mean±std) | 27.3±5.6 | 23.6±10.6 | 24.8±8.4 | 27.1±5.2 | 29.9±8.9 | 29.6±8.3 | 23.1±9.4 | 24.4±8.3 |
| Spine density (with/without synapse) by dendrite surface area (#/µm2, mean±std) | 0.89±0.17 | 0.84±0.12 | 0.75±0.13 | 0.88±0.13 | 1.05±0.14 | 0.96±0.18 | 1.09±0.26 | 1.04±0.20 |
|
Process density (spines without synapse) by dendrite surface area (#/µm2, mean±std) |
0.03±0.02 | 0.02±0.02 | 0.02±0.02 | 0.04±0.02 | 0.06±0.04 | 0.03±0.01 | 0.05±0.03 | 0.04±0.02 |
| Synapse density (excitatory + inhibitory) by dendrite surface area (#/µm2, mean±std) | 0.86±0.16 | 0.82±0.13 | 0.72±0.12 | 0.83±0.13 | 0.99±0.15 | 0.93±0.19 | 1.04±0.24 | 1.00±0.20 |
|
Excitatory synapse density by dendrite surface area (#/µm2, mean±std) |
0.86±0.16 | 0.82±0.13 | 0.72±0.12 | 0.83±0.13 | 0.97±0.16 | 0.92±0.18 | 1.04±0.25 | 1.00±0.20 |
|
Inhibitory synapse density by dendrite surface area (#/µm2, mean±std) |
0.003±0.006 | 0.005±0.010 | 0.006±0.010 | 0.013±0.016 | 0.017±0.018 | 0.014±0.015 | 0.005±0.012 | 0.006±0.010 |
| Shaft synapse density by dendrite suface area (#/µm2, mean±std) | 0.147±0.068 | 0.131±0.055 | 0.169±0.089 | 0.161±0.051 | 0.169±0.054 | 0.200±0.062 | 0.188±0.077 | 0.179±0.079 |
|
Oblique spines
% of synapses per mouse (mean±std) |
5.1±1.7% | 6.4±1.2% | 5.2±2.0% | 5.9±1.4% | 9.0±1.8% | 8.4±2.7% | 7.3±2.4% | 7.7±1.2% |
| Perforated spines % of synapses per mouse (mean±std) | 2.9±1.6% | 2.4±0.8% | 2.4±0.5% | 2.0±1.3% | 3.2±0.9% | 3.3±0.7% | 4.1±1.8% | 3.6±1.2% |
| Spines with spine apparatus % of spines with synapse per mouse (mean±std) | 31.7±5.1% | 27.0±3.4% | 28.9±4.4% | 32.1±5.4% | 34.6±2.9% | 32.6±6.6% | 41.3±9.4% | 40.3±8.7% |
| Spines with endosome/s % of spines with synapse per mouse (mean±std) | 65.5±6.5% | 58.5±5.1% | 70.2±5.5% | 73.8±6.9% | 65.5±7.9% | 67.8±5.4% | 67.7±5.7% | 65.8±5.1% |
| Synapses with coated vesicle % of spines with synapse per mouse (mean±std) | 3.0±2.5% | 3.1±2.2% | 5.3±1.1% | 4.6±2.0% | 2.7±1.1% | 1.8±0.6% | 5.8±2.1% | 3.4±2.2% |
| Synapses with spinula % of spines with synapse per mouse (mean±std) | 1.7±0.9% | 0.9±0.5% | 1.1±1.3% | 2.0±1.6% | 3.9±1.7% | 4.1±1.3% | 3.1±2.1% | 5.0±2.1% |
| Synapses with MVB % of spines with synapse per mouse (mean±std) | 2.2±1.7% | 1.3±0.7% | 1.1±0.7% | 1.2±0.9% | 0.6±0.5% | 1.0±0.7% | 0.9±0.7% | 0.9±0.5% |
| Synapses with lysosome % of spines with synapse per mouse (mean±std) | 6.8±1.1% | 3.6±0.7% | 6.4±3.0% | 5.7±3.1% | 3.5±0.6% | 3.8±0.4% | 5.4±2.4% | 3.5±1.2% |
| Branched synapses % of spines with synapse per mouse (mean±std) | 23.6±8.2% | 24.9±3.0% | 24.0±4.1% | 31.2±6.7% | 35.9±5.4% | 30.0±0.6% | 35.9±6.0% | 31.0±3.0% |
Statistical analysis
Statistical analysis was performed using linear mixed effects (LME) models that include both random and fixed effects [28]. The random effects in LME models are used to account for potential correlations among datapoints due to having multiple measurements from a single unit (in the current study, multiple dendrites per mouse and multiple synapses per dendrite). The method is similar to a repeated measures ANOVA but is more flexible to handle missing data and unbalanced designs (e.g., differing numbers of synapses per dendrite, or dendrites per mouse). An LME model has the following form:
where
and
For the LME model, y is the vector of response variables (e.g., ASI or synapse density), u is a vector of random effects (independent and normally distributed with mean zero and covariance Σ), and β is the vector of fixed effects. For response variables measured on individual synapses, the model includes random intercepts for both dendrite and mouse, while response variables measured on dendrites only include random intercepts for mouse. Design matrices Z and X link the response variables to the random and fixed effects, and ϵ is the residual error, assumed to be independent and normally distributed with constant variance σ2. Maximum likelihood estimates of model parameters are computed using numerical optimization methods, implemented in R by the lmer() function of the lme4 package [29]. Model assumptions (normality and constant variance) were assessed visually using diagnostic plots, and transformations (log or square root) were applied as appropriate. Null hypothesis significance testing (e.g., testing for a group effect) was performed by fitting a reduced model with the effect of interest removed and using an asymptotic likelihood ratio test (LRT) to compare the full and reduced models. For significant (p < 0.05) or trending (0.05 ≤ p < 0.1) results, Cohen’s f2 was used as a measure of effect size between the full and reduced models. When appropriate, post-hoc tests were performed using the glht() function of the multcomp package in R, with p-values adjusted for multiple comparisons using a single-step method [30], and effect sizes measured using Cohen’s d (mean difference divided by residual standard error from the LME model).
For the analysis of organelles, the proportion of spines with the organelle of interest is computed for each dendrite, and then used as the response variable in a dendrite-level LME. An alternative analysis utilized generalized linear mixed effect models (GLME) to fit a synapse-level model where the response variable is the presence or absence of the organelle for each spine (0 for absent, 1 for present). The models were fit using the glmer() function in the lme4 package using the “binomial” family, and null hypothesis significance testing was performed using an LRT in the same sequence as describe above for LME models. The results of the GLME analysis were qualitatively similar to the LME analysis and are not presented here.
Results
We used 4 experimental groups: mice without training and awake for one hour starting at light onset (W, n = 4 mice), mice trained in the complex wheel task for one hour starting at light onset (T, n = 4), and mice that were either allowed to sleep (S, n = 5) or sleep deprived (SDep, n = 5) for approximately 6 hours after training (Fig. 1A). To reduce variability in prior sleep/wake history, only mice that were spontaneously awake for at least 75% of the last 3 hours of the dark period were selected for the experiment and assigned randomly to one of the groups. All T, S and SDep mice were learners and improved their performance to a similar extent (% increase, first 3 trials vs best 3 trials, mean ± stdev, all mice = 196 ± 102; T = 220 ± 59; S = 153 ± 94; SDep = 219 ± 136; no significant group differences based on single-factor ANOVA, p = 0.544). S mice were mostly asleep after training (mean ± stdev, % of time asleep in the last 6 hours = 69 ± 10; last hour = 87 ± 7). SDep mice were kept awake with gentle handling without exposure to novel objects to limit further learning after motor training.
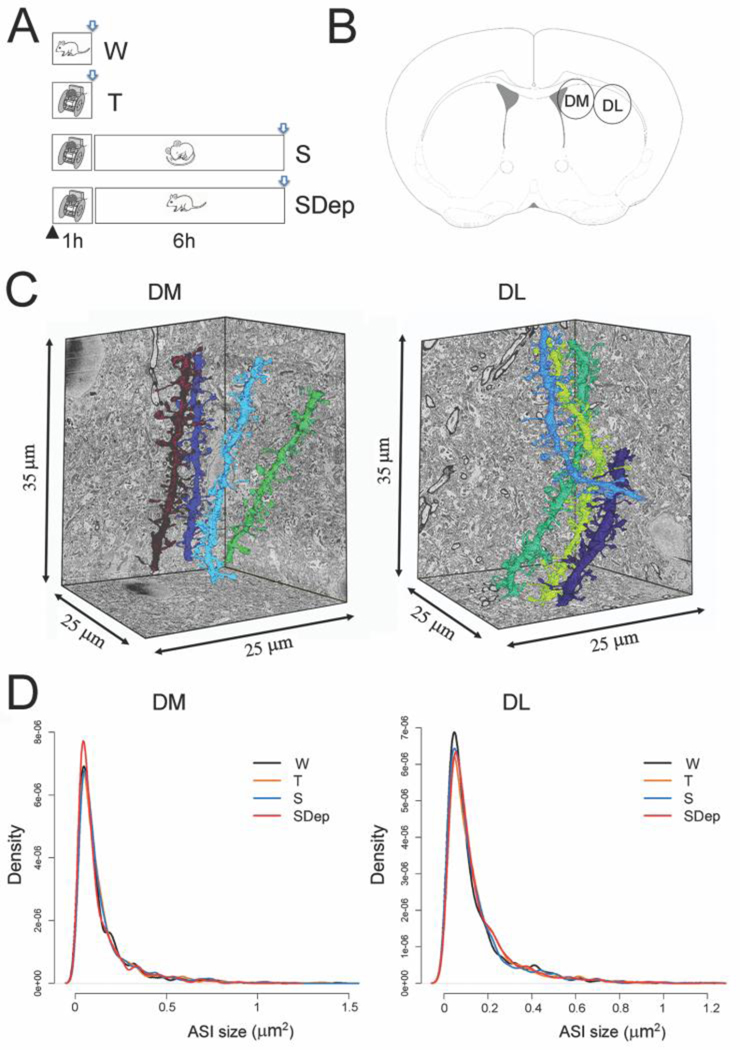
Figure 1. Experimental design.
A, the four experimental groups: W, one hour of waking; T, one hour of motor training; S, 6 hours of sleep after motor training; SDep, 6 hours of sleep deprivation after motor training. The black arrowhead indicates the onset of the light period. Brain collection (open arrows) occurred around one hour after lights on for W and T mice and around 7 hours after lights on for S and SDep mice. B, schematic representation of a coronal section of the mouse brain with circles indicating the areas of DM and DL used for ultrastructural analysis. C, 3D reconstruction of several dendritic branches in representative stacks of DM and DL. D, log-normal distribution of ASI sizes in the 4 experimental groups, shown separately for DM and DL.
In each mouse we used serial block-face scanning electron microscopy to acquire stacks of ~700 images (~25 X 25 X 35 μm), one in DM and one in DL (Fig. 1B, C). Spiny dendritic segments were randomly selected within each stack and all their protrusions, also called spines (see Methods), were manually segmented by trained annotators blind to experimental condition. The length and diameter of the dendrites were balanced across groups (Table 1). Overall, 157 dendritic branches, all spiny (Suppl. Fig. 1 and 2), were segmented (4 to 8 dendrites/mouse in both DM and DL) for a total of 12206 spines. Of these spines, 11718 had a synapse, and in 9906 of them the ASI could be fully traced and measured (N of ASIs, T = 2301; W = 2470; S = 2528; SDep = 2607; at least 401 ASIs/mouse) (Table 1). For each spine, the presence/absence of several organelles was also recorded, including spine apparatus, spinulae in the head or neck of the spine, endosomes (tubules, small uncoated vesicles, large coated or uncoated vesicles), lysosomes, and multivesicular bodies (Fig. 2).
Figure 2. Spine organelles.

Examples of organelles in the reconstructed spines, including lysosome (A), spine apparatus and endosome (B), coated vesicle (B,C), and multivesicular body (MVB)(C). D, a spinula is shows in 3 consecutive sections, and in the 3D reconstruction. In each image the ASI is indicated in red and the spine head in green. In D, part of the reconstructed dendritic shaft is also shown in green.
Ultrastructural differences between DL and DM.
A direct comparison of ultrastructural parameters in DM and DL revealed several differences. At the dendrite level, the average spine density (number of spines per dendrite surface area) and the average density of excitatory synapses were higher in DL than DM in all four groups (Table 1, Suppl. Table 1). Also higher in DL than DM were the density of branched spines (sharing the same neck), the density of processes (spines lacking a synapse; DL > DM in 3 of the 4 groups), and the density of shaft synapses, which include inhibitory, modulatory, and some excitatory (thalamic) synapses [31; 32]. Dendrites in DL also had higher proportion of perforated spines (with a discontinuity in the post-synaptic density), as well as and of spines containing a spine apparatus or spinula. By contrast, the proportion of spines containing lysosomes was higher in DM than in DL. At the spine level, on the other hand, DM and DL shared many of the same properties. Specifically, the distribution of ASI size was log-normal in both DM and DL, as in cortex [4] (Fig. 1D), and ASI size was generally similar in DL compared to DM except in the SD group (DL>DM; Suppl. Table 1). In both DM and DL the presence of any organelle inside the spine significantly increased the size of the ASI, while branched spines had significantly smaller ASI (Suppl. Table 2). Generally, these effects were not group specific (Suppl. Table 2). In summary, we found that the dendrites in DL are more complex than in DM: they harbor more spines and more branched spines as well as more synapses, including excitatory synapses on spines and shaft synapses. The proportion of perforated synapses, which are rare (~ 3% of all synapses) is also higher in DL. DM and DL are instead similar at the synapse level: they have similar average ASI size and most of their synapses have a small or medium size ASI, with very few large ASIs (log-normal distribution). Moreover, in both regions the presence of any organelle inside the spine is associated with larger synapses (larger ASI).
Effects of training.
We tested the effects of training by comparing the W and T mice whose brains were collected at the same time of day, one hour after the beginning of the light period, after having spent awake that hour either without (W) or with motor training (T). Statistical analysis was performed separately in DM and DL using LME models with group (W/T) as fixed effect, and mouse and dendrite as random effects (see Methods). We first considered all spines and found no main effect of group on synapse density or ASI, either in DM or in DL (DM: synapse density, p = 0.3997; ASI, p = 0.2937; DL: synapse density, p = 0.4979; ASI, p = 0.2853) (Fig. 3A). Next, we focused on subgroups of spines defined by the presence of the spine apparatus, a specialized form of the smooth endoplasmic reticulum (SER), or endosomes (tubules and vesicles), which are non-SER elements. In spines with a spine apparatus (~ 36.7% of all spines, Table 1) no differences in ASI size between W and T mice were found in either DM or DL (DM, p = 0.4380; DL, p = 0.5974). By contrast, in DM, spines without a spine apparatus had a significantly larger ASI after training as compared to no training (T vs W = ASI, p = 0.0160, f2 = 0.005), and DL showed a trend in the same direction (DL, T vs W = ASI, p = 0.0808, f2 = 0.007) (Fig. 3B). Moreover in DM, but not DL, spines with endosomes (~ 69.7% of all spines, Table 1) showed a trend increase in ASI size after training relative to no training (DM, p = 0.0847, f2 = 0.003; DL p = 0.3846) (Fig. 3C), while no differences were present in spines without endosomes (DM, p = 0.2189; DL, p = 0.5532). In DM, 58.3% of spines with endosomes also lacked a spine apparatus (56.5– 60.2% across groups), and 59.2% of spines without a spine apparatus contained endosomes (49.0–66.4% across groups), and similar overlaps were found in DL (51.8% of spines with endosomes lacked spine apparatus; 61.7% of spines without spine apparatus contained endosomes), suggesting robust but incomplete overlap between the two subsets of spines. When we tested the effects of training in the subset (38.2%) of spines that have endosomes and lack a spine apparatus, we found a significant increase in ASI after training in both DM and DL (DM, p = 0.0172, f2 = 0.008; T > 1h; DL, p = 0.0241, f2 = 0.005; T > 1h). Altogether, these results suggest that training increases ASI size in a large subset of spines, specifically those with high potential to grow, i.e. spines that do not already have a spine apparatus and/or have recycling endosomes. These effects involve more spines and are stronger in DM, but to some extent also occur in DL.
Figure 3. Effects of training on ASI size.
ASI size in all spines (A), spines without a spine apparatus (B), spines with endosomes (C), and spines with endosomes without a spine apparatus (D) in DM (left) and DL (right). In all panels each dot represents one spine. W, one hour of waking without training; T, one hour of training. In the plots, the thick black line indicates the median.
Effects of post-training sleep and sleep deprivation.
To test for the effects of sleep and sleep deprivation after training, statistical analysis was performed in the three groups of trained mice. LME models were run again separately in DM and DL, with group (T/S/SDep) as fixed effect, and mouse and dendrite as random effects (see Methods). In DM, there was a significant effect of group on the density of excitatory synapses (p = 0.0307, f2 = 0.18) (Fig. 4A). Specifically, based on post-hoc comparisons, the density of excitatory synapses in S was lower than in the other groups (vs T, p = 0.0292, d = 0.83; vs SDep, p = 0.0162, d = 0.89), with no significant differences between T and SDep (p = 0.9782). Given the group effect on excitatory synapse density, we then analyzed the cumulative ASI per dendrite (mean ASI * synapse density) in addition to the mean ASI, and found no effect of group (ASI p = 0.1781; cumulative ASI p = 0.396) (Fig. 4B,C). On average, however, the cumulative ASI in the S group reached the lowest level, ~ 86.2% of the value after training (mean ± sd, µm2: T 0.1176 ± 0.0216; S 0.1014 ± 0.0187; SDep 0.1082 ± 0.0333) (Fig. 4C). The decrease in cumulative ASI in the S group relative to training was larger (S = 83.8% of T) in the spines most affected by training (with endosomes and without spine apparatus) (mean ± sd, µm2: T 0.0832 ± 0.0210; S 0.0697 ± 0.0130; SDep 0.0772 ± 0.0221) (Fig. 4D), and smaller (S = 91.8% of T) in the remaining spines (mean ± sd, µm2: T 0.1392 ± 0.0364; S 0.1277 ± 0.0323; SDep 0.1306 ± 0.0434).
Figure 4. Effects of post-training sleep and sleep deprivation.
A, group effect on the density of excitatory synapses in DM, with lowest density in S. B, distribution of ASI in each group in DM. C, D, distribution of cumulative ASI for all synapses (C) and synapses with endosome and without spine apparatus (D). Each dot represents one ASI (B) or one dendrite (A,C,D). E-H, same as in A-D but for DL. In the plots, the thick black line indicates the median.
In DL, we found no significant effect of group on synapse density, ASI or cumulative ASI (mean ASI * synapse density) (T/S/SDep: synapse density, p = 0.193; ASI, p = 0.167; cumulative ASI, p = 0.296) (Fig. 4E-H). Like in DM, however, the cumulative ASI was lowest in the S group when considering the subset of spines with endosomes and without spine apparatus (T 0.0889 ± 0.0207; S 0.0830 ± 0.0276; SD 0.0890 ± 0.0255).
Next, we tested for the effects of sleep and sleep deprivation on the proportion of spines containing organelles, as well as the proportion of perforated and branched spines (Suppl. Table 3). In DM, there was a significant effect for endosomes, with higher percentage of spines with endosomes in both S and SDep relative to W and T (Fig. 5A). In DL, the percentage of spines with coated vesicles was higher in S relative to the other 3 groups (Fig. 5B).
Figure 5. Group effects on spine organelles and processes.
Proportion of spines with endosomes (A) and with coated density (B), and process density (C) in each group in DM. D-F, as in A-C but for DL. In all panels each dot represents one dendrite. In the plots, the thick black line indicates the median.
We also analyzed the density the spines lacking a synapse, called processes. In DM a trend effect of group was found (p = 0.071, X = 7.03, f2 = 0.12). Further exploring the trend effect with post-hoc tests, the process density in SDep was significantly greater than in T (p = 0.0182, Z = 2.923, d = 0.95), with no other significant pair-wise comparisons (Fig. 5C). In DL there was no effect of group on process density (p = 0.153. X = 5.27, f2 = 0.07)
Lastly, we analyzed symmetric synapses on spines and shaft synapses. For the former, LME models were not reliable due to a large number of dendrites with zero symmetric synapses. For the ASI on shaft synapses, we found no significant group effect in either DL (p = 0.2851, X = 3.79, f2 = 0.01) or DM (p = 0.1673, X = 5.06, f2 = 0.02).
Discussion
Here we used serial electron microscopy to assess the synaptic effects of learning, post-learning sleep and post-learning sleep deprivation in the medial and lateral parts of the mouse dorsal striatum. By first comparing DM and DL across all experimental groups, we found that the two areas differ from each other in several basic anatomical features. Then, we found that in DM, skill training increases ASI size in large sets of spines with high plastic potential (with endosomes and without spine apparatus) and, several hours later, sleep leads to a decrease in the density of excitatory synapses that does not happen if the mice are forced to stay awake. In DL, the post-training increase in ASI size is restricted to fewer spines and is not followed by sleep-dependent synaptic pruning.
We are now aware of previous studies that compared DM and DL at the ultrastructural level. In doing so, we found that dendrites in DL contain more spines and more excitatory synapses. In each dendrite, there is a higher proportion of spines containing a spine apparatus or spinula in DL than DM (but the opposite is true for lysosomes), as well as more perforated synapses and more branched spines. Spinulae are short (< 500nm) protrusions that from the spine extend into, and are surrounded by, a presynaptic element. They may help spines to find new presynaptic partners and/or promote the formation of new synapses [33]. Shaft synapses are also more numerous in DL than DM. The general features of excitatory synapses, on the other hand, are similar between the two regions, including the log-normal distribution of ASI size, i.e. most excitatory synapses in DM and DL are small or medium size, like in cortex [4], and unlike in the CA1 region of the hippocampus (bimodal distribution, [16]) and the cerebellum (unimodal distribution, [17]). The average size of the ASI is also similar in DM and DL and several general “rules” apply to both regions, including that the smallest spines do not contain any organelle while the largest spines have a spine apparatus, and that the presence of any organelle is associated with an increase in ASI size. In the future, it will be important to test whether the anatomical differences described here are confirmed in larger samples and, if so, whether they reflect, or contribute to, the differential role of DM and DL in motor learning.
Previous studies found that the early phase of motor learning is associated with the occurrence of plastic changes in DM, while DL shows changes only after a few days. The exact extent and timing of DM and DL engagement are debated, however, and likely vary depending on task and learning protocol (e.g.[34; 35]). In the rotarod task, increased firing is observed early in DM, after one session of 10 trials, while changes in the NMDA/AMPA ratio occur late in DL, after 3 daily sessions of 10 trials each [15]. Also, training in the reaching task strengthens a subset of synaptic connections between M1 and DL only after several days of practice [36]. A recent study found that perfecting a multi-step sequence learning task over thousands of trials requires DL. That study was able to detect sequences of firing in MSNs that were “played” online during repeated learning, as well as “replayed” offline especially at sleep onset [37]. Long-term skill learning spanning several days is also associated with complex changes in coordination between M1 and DL as measured electrophysiologically. For instance, theta coherence seems to increase during training in early trials, while in late trials it decreases during training and increases offline, during periods of NREM sleep [38].
We don’t know of any previous study that examined the role of DM and DL in the complex wheel task. This task is more complex than the rotarod task, suggesting that it may take even longer for DL to be engaged when “canonical” training sessions with 10 trials are used. However, our daily training session included 20 trials. This was done on purpose to maximize learning in one session and examine the effects of sleep immediately afterwards. Indeed, we previously found that mice master the complex wheel task quite well after 20 trials, with only some residual improvement in performance occurring after the second day [5; 6]. Thus, in the current study we expected to see plastic changes in DM, but we were open to the possibility that DL would also show changes, which is what we found. Specifically, when compared to mice awake for one hour without training, trained mice showed no significant differences in the average synapse density or the mean ASI size, either in DM or in DL. However, in DM, training resulted in an increase in ASI size in a large subset of spines (~ 65–70% of all spines), those without a spine apparatus, and a trend towards larger ASI after training was also present in the large subset of spines that contain endosomes (~ 60–65% of all spines). Spines may contain flat tubules that are a direct continuation of the SER present in the dendritic shaft and the spine apparatus is a specialized form of the SER often present in large mushroom spines. DL showed only a trend for larger ASI size in spines with a spine apparatus, and no effect of training in spines with endosomes. However, when we focused on the large subset of spines that have endosomes and lack a spine apparatus, we found a significant increase in ASI size after training in both DM and DL. In short, effects of training are more obvious in DM, but they are also present in DL, presumably because our mice are in an intermediate phase of learning when DL starts to be engaged. It is possible that the effects of training become more obvious in DL only late, when the mice are reaching top levels of performance.
The spine apparatus consists of a stack of two or more flat cisternae [39] intercalated with F-actin and synaptopodin, an F-actin bundling protein that serves as a specific marker for the spine apparatus and is required for its formation. The spine apparatus has been implicated in the buffering of calcium inside the spine, as well as in the synthesis of receptors and proteins of the post-synaptic density [40; 41]. Spines can also contain several non-SER elements including uncoated large and small vesicles, coated vesicles and coated pits, tubules, multivesicular bodies, and lysosomes that together form the so-called non-SER endosomal compartment [42]. In this compartment, a distinction can be made between constructive elements, mainly pits, vesicles and tubules, and degradative elements, including multivesicular bodies containing vesicles in a dark matrix and secondary lysosomes with a homogenous dark interior. Degradative elements are involved in digesting intracellular organelles and occur rarely only in some large spines [39; 43]. LTP induction can cause a large increase in the incidence of constructive non-SER elements, mainly endosomes (tubules and vesicles), inside the small spines, presumably to support synaptogenesis [43; 44]. Our results point to specific effects of training in the subset of spines that have endosomes and lack spine apparatus. These findings are consistent with the idea that after training plastic changes are more likely to occur in spines that have high potential to grow because they do not already have a spine apparatus (i.e. they are not already very large) and/or because they have recycling endosomes.
The spines with larger ASI after training (with endosomes and without spine apparatus) represent a large subset of spines (38.2%), suggesting that early motor training has broad effects. Previous experiments also found that more than 50% of neurons in DM and DL show changes in firing and glutamatergic transmission after early and late training, respectively [15; 45]. In our experimental conditions, the changes caused by training are more likely to have occurred in corticostriatal [46] rather than thalamostriatal synapses [8; 47], because only the former show short-term facilitation and a high NMDA to AMPA ratio, two features that likely promote the induction of long-term potentiation [48]. Moreover, calcium imaging shows that D1 positive (direct) neurons are engaged earlier during multi-day training, both in the rotarod task [49] and in a treadmill task [50]. Thus, our training protocol may have affected mostly MSNs belonging to the direct pathway. This remains a speculation, however, because D1 positive and D2 positive (indirect) neurons share similar somatodendritic morphology [8], have similar spine density [51; 52; 53; 54; 55], and receive converging cortical and thalamic inputs [8] in similar proportion [55], thus cannot be resolved with our morphological analysis.
We found that, in DM, training is followed after several hours by a significant overall decrease in the density of excitatory synapses that occurs only if the mouse is allowed to sleep. We previously found that sleep-dependent synaptic pruning with no changes in ASI occurs in the cerebellum, where it affects specifically the branched synapses [17]. Branched synapses are on average smaller and have fewer AMPA receptors than other synapses, thus they may be more susceptible to disappear after several hours of sleep because they lack the strong cytoskeleton network that usually anchor the receptors to the membrane [17]. Like in the cerebellum, branched synapses in the dorsal striatum are on average smaller than other synapses. In DM, we also found that an average of 24% of all spines had branched synapses after post-learning sleep, compared to an average of 31% after post-learning sleep deprivation. Although not significant, this difference suggests that branched synapses are in general at higher risk for sleep-dependent down-selection.
The molecular mechanisms mediating sleep-dependent synaptic pruning are not known. A well characterized form of striatal long-term depression active in both direct and indirect MSNs is mediated by the activation of metabotropic mGluR5 glutamatergic receptors. However, this mechanism is presynaptic and facilitated, in the indirect pathway, by dopamine [31] whose levels in the striatum are low during sleep [56]. A more likely candidate is a recently discovered robust form of long-term synaptic depression mediated by nitric oxide released by low threshold spike interneurons. This mechanism is postsynaptic and broad, affecting corticostriatal and thalamostriatal synapses in direct and indirect neurons [57]. It was suggested that this form of synaptic depression may be important for attenuating the strength of inactive synapses and keeping overall synaptic strength within acceptable bound, specifically to unlearn previously rewarded associations and acquire new ones [31]. Our mice were not rewarded, but their task involved learning a new sequence of uneven steps. There is some evidence that low threshold spike interneurons are recruited when mice learn new sequential stepping patterns [58]. Moreover, one study found that the inhibitory control of corticostriatal integration is region-specific, with parvalbumin interneurons efficiently controlling MSNs in DL, and low threshold spike interneurons, which release GABA and somatostatin in addition to nitric oxide, controlling MSNs in DM [59]. Somatostatin positive interneurons in cortex are involved in the generation of OFF periods during NREM sleep [60]. Future studies should test whether low threshold spike interneurons are specifically active in sleep, and more specifically in DM.
The sleep-dependent decrease in excitatory synapse density in DM was not accompanied by a statistically significant decrease in cumulative ASI per dendrite. Given that cumulative ASI was actually lower in post-training sleep compared to after training and after post-training sleep deprivation, the lack of statistical significance may be due to the limited number of dendrites (cumulative ASI is measured at the dendrite level). Moreover, sleep-dependent pruning likely affected the smallest synapses, as suggested by the fact that the mean ASI (as opposed to the cumulative ASI) was as high or slightly higher in post-training sleep compared to the other groups, while the percentage of branched synapses was lower. The pruning of the synapses with the smallest ASI after sleep may thus have limited impact on the cumulative ASI.
It should also be noted that skill learning in DM led to an increase of ASI size in a large subset of synapses, especially those with the highest plastic potential, i.e. those with endosomes and without a spine apparatus. In primary motor cortex, by contrast, we found that ~ 10% of all synapses acquired more receptors (“learned”) after motor training, and were then spared from sleep-dependent synaptic weakening [6]. A direct comparison is difficult because the study in cortex used a molecular marker of synaptic strength that could be quantified in the same synapses before and after training with two-photon imaging, while the current analysis in the striatum involves two independent groups of mice and uses a structural marker of synaptic strength. Notwithstanding these issues, one way to interpret these results is that endosomes, which are present in most spines in both cortex and dorsal striatum, confer synapses with the ability to potentiate during learning and to weaken during sleep. When the percentage of learned synapses is relatively small, as in cortex, the net effect of post-learning sleep on synapse (ASI) size is more obvious, while when a large fraction of synapses is affected by learning, as in dorsal striatum, the weakening effect of sleep is mainly confined to removing the weakest spines.
Supplementary Material
Acknowledgements.
Supported by NIH grants R01NS131389 (CC, GT), Department of Defense PR230899 (CC), Department of Defense W911NF1910280 (CC, GT).
Footnotes
Disclosure Statement. The authors declare no competing financial or non-financial interests.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- [1].Tononi G, and Cirelli C, Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81 (2014) 12–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cirelli C, and Tononi G, The why and how of sleep-dependent synaptic down-selection. Semin Cell Dev Biol 125 (2022) 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vyazovskiy V, Cirelli C, Pfister-Genskow M, Faraguna U, and Tononi G, Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nature neuroscience 11 (2008) 200–208. [DOI] [PubMed] [Google Scholar]
- [4].de Vivo L, Bellesi M, Marshall W, Bushong EA, Ellisman MH, Tononi G, and Cirelli C, Ultrastructural evidence for synaptic scaling across the wake/sleep cycle. Science 355 (2017) 507–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nagai H, de Vivo L, Bellesi M, Ghilardi MF, Tononi G, and Cirelli C, Sleep Consolidates Motor Learning of Complex Movement Sequences in Mice. Sleep 40 (2017) zsw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Miyamoto D, Marshall W, Tononi G, and Cirelli C, Net decrease in spine-surface GluA1-containing AMPA receptors after post-learning sleep in the adult mouse cortex. Nature communications 12 (2021) 2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Graveland GA, and DiFiglia M, The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain research 327 (1985) 307–11. [DOI] [PubMed] [Google Scholar]
- [8].Huerta-Ocampo I, Mena-Segovia J, and Bolam JP, Convergence of cortical and thalamic input to direct and indirect pathway medium spiny neurons in the striatum. Brain structure & function 219 (2014) 1787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gerfen CR, and Surmeier DJ, Modulation of striatal projection systems by dopamine. Annu Rev Neurosci 34 (2011) 441–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Steriade M, Timofeev I, and Grenier F, Natural waking and sleep states: a view from inside neocortical neurons. Journal of neurophysiology 85 (2001) 1969–85. [DOI] [PubMed] [Google Scholar]
- [11].Mahon S, Vautrelle N, Pezard L, Slaght SJ, Deniau JM, Chouvet G, and Charpier S, Distinct patterns of striatal medium spiny neuron activity during the natural sleep-wake cycle. The Journal of neuroscience : the official journal of the Society for Neuroscience 26 (2006) 12587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mizrahi-Kliger AD, Kaplan A, Israel Z, and Bergman H, Desynchronization of slow oscillations in the basal ganglia during natural sleep. Proceedings of the National Academy of Sciences of the United States of America 115 (2018) E4274–E4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Perrin E, and Venance L, Bridging the gap between striatal plasticity and learning. Curr Opin Neurobiol 54 (2019) 104–112. [DOI] [PubMed] [Google Scholar]
- [14].Yin HH, and Knowlton BJ, The role of the basal ganglia in habit formation. Nat Rev Neurosci 7 (2006) 464–76. [DOI] [PubMed] [Google Scholar]
- [15].Yin HH, Mulcare SP, Hilario MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, and Costa RM, Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nature neuroscience 12 (2009) 333–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Spano GM, Banningh SW, Marshall W, de Vivo L, Bellesi M, Loschky SS, Tononi G, and Cirelli C, Sleep Deprivation by Exposure to Novel Objects Increases Synapse Density and Axon-Spine Interface in the Hippocampal CA1 Region of Adolescent Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience 39 (2019) 6613–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Loschky SS, Spano GM, Marshall W, Schroeder A, Nemec KM, Schiereck SS, de Vivo L, Bellesi M, Banningh SW, Tononi G, and Cirelli C, Ultrastructural effects of sleep and wake on the parallel fiber synapses of the cerebellum. Elife 11 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nelson AB, Faraguna U, Zoltan JT, Tononi G, and Cirelli C, Sleep Patterns and Homeostatic Mechanisms in Adolescent Mice. Brain sciences 3 (2013) 318–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cirelli C, and Tononi G, Effects of sleep and waking on the synaptic ultrastructure. Philos Trans R Soc Lond B Biol Sci 375 (2020) 20190235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Maret S, Faraguna U, Nelson AB, Cirelli C, and Tononi G, Sleep and waking modulate spine turnover in the adolescent mouse cortex. Nature neuroscience 14 (2011) 1418–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bellesi M, Pfister-Genskow M, Maret S, Keles S, Tononi G, and Cirelli C, Effects of sleep and wake on oligodendrocytes and their precursors. The Journal of neuroscience : the official journal of the Society for Neuroscience 33 (2013) 14288–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wilke SA, Antonios JK, Bushong EA, Badkoobehi A, Malek E, Hwang M, Terada M, Ellisman MH, and Ghosh A, Deconstructing complexity: serial block-face electron microscopic analysis of the hippocampal mossy fiber synapse. The Journal of neuroscience : the official journal of the Society for Neuroscience 33 (2013) 507–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, and Cardona A, Fiji: an open-source platform for biological-image analysis. Nature methods 9 (2012) 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fiala JC, and Harris KM, Cylindrical diameters method for calibrating section thickness in serial electron microscopy. J Microsc 202 (2001) 468–72. [DOI] [PubMed] [Google Scholar]
- [25].Cardona A, Saalfeld S, Schindelin J, Arganda-Carreras I, Preibisch S, Longair M, Tomancak P, Hartenstein V, and Douglas RJ, TrakEM2 software for neural circuit reconstruction. PloS one 7 (2012) e38011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Holtmaat A, and Svoboda K, Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci 10 (2009) 647–58. [DOI] [PubMed] [Google Scholar]
- [27].Bellesi M, de Vivo L, Tononi G, and Cirelli C, Effects of sleep and wake on astrocytes: clues from molecular and ultrastructural studies. BMC biology 13 (2015) 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Laird NM, and Ware JH, Random effects models for longitudinal data. Biometrics 38 (1982) 963–74. [PubMed] [Google Scholar]
- [29].Bates D, Maechler M, Bolker B, and Walker S, Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67 (2015) 1–48. [Google Scholar]
- [30].Bretz F, Hothorn T, and Westfall P, Multiple Comparisons using R, CRC Press, 2011. [Google Scholar]
- [31].Shen W, Zhai S, and Surmeier DJ, Striatal synaptic adaptations in Parkinson’s disease. Neurobiol Dis 167 (2022) 105686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Smith AD, and Bolam JP, The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci 13 (1990) 259–65. [DOI] [PubMed] [Google Scholar]
- [33].Zaccard CR, Gippo I, Song A, Geula C, and Penzes P, Dendritic spinule-mediated structural synaptic plasticity: Implications for development, aging, and psychiatric disease. Front Mol Neurosci 16 (2023) 1059730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Villet M, Reynaud-Bouret P, Poitreau J, Baldi J, Jaffard S, James A, Muzy A, Kartsaki E, Scarella G, Sargolini F, and Bethus I, Coding Dynamics of the Striatal Networks During Learning. eNeuro 11 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mastrorilli V, Centofante E, Antonelli F, Rinaldi A, and Mele A, The neural substrate of spatial memory stabilization depends on the distribution of the training sessions. Proceedings of the National Academy of Sciences of the United States of America 119 (2022) e2120717119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hwang FJ, Roth RH, Wu YW, Sun Y, Kwon DK, Liu Y, and Ding JB, Motor learning selectively strengthens cortical and striatal synapses of motor engram neurons. Neuron 110 (2022) 2790–2801 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Thompson E, Rollik L, Waked B, Mills G, Kaur J, Geva B, Carrasco-Davis R, George T, Domine C, William Dorrell W, and Stephenson-Jones M, Replay of procedural experience is independent of the hippocampus. bioRxiv 10.1101/2024.06.05.597547 (2024). [DOI] [Google Scholar]
- [38].Lemke SM, Ramanathan DS, Darevksy D, Egert D, Berke JD, and Ganguly K, Coupling between motor cortex and striatum increases during sleep over long-term skill learning. Elife 10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Spacek J, and Harris KM, Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. The Journal of neuroscience : the official journal of the Society for Neuroscience 17 (1997) 190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ostroff LE, Cain CK, Bedont J, Monfils MH, and Ledoux JE, Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proceedings of the National Academy of Sciences of the United States of America 107 (2010) 9418–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chirillo MA, Waters MS, Lindsey LF, Bourne JN, and Harris KM, Local resources of polyribosomes and SER promote synapse enlargement and spine clustering after long-term potentiation in adult rat hippocampus. Sci Rep 9 (2019) 3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cooney JR, Hurlburt JL, Selig DK, Harris KM, and Fiala JC, Endosomal compartments serve multiple hippocampal dendritic spines from a widespread rather than a local store of recycling membrane. The Journal of neuroscience : the official journal of the Society for Neuroscience 22 (2002) 2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Kulik YD, Watson DJ, Cao G, Kuwajima M, and Harris KM, Structural plasticity of dendritic secretory compartments during LTP-induced synaptogenesis. Elife 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Park M, Salgado JM, Ostroff L, Helton TD, Robinson CG, Harris KM, and Ehlers MD, Plasticity-induced growth of dendritic spines by exocytic trafficking from recycling endosomes. Neuron 52 (2006) 817–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Costa RM, Cohen D, and Nicolelis MA, Differential corticostriatal plasticity during fast and slow motor skill learning in mice. Curr Biol 14 (2004) 1124–34. [DOI] [PubMed] [Google Scholar]
- [46].Hintiryan H, Foster NN, Bowman I, Bay M, Song MY, Gou L, Yamashita S, Bienkowski MS, Zingg B, Zhu M, Yang XW, Shih JC, Toga AW, and Dong HW, The mouse cortico-striatal projectome. Nature neuroscience 19 (2016) 1100–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Mandelbaum G, Taranda J, Haynes TM, Hochbaum DR, Huang KW, Hyun M, Umadevi Venkataraju K, Straub C, Wang W, Robertson K, Osten P, and Sabatini BL, Distinct Cortical-Thalamic-Striatal Circuits through the Parafascicular Nucleus. Neuron 102 (2019) 636–652 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ding J, Peterson JD, and Surmeier DJ, Corticostriatal and thalamostriatal synapses have distinctive properties. The Journal of neuroscience : the official journal of the Society for Neuroscience 28 (2008) 6483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Liang B, Zhang L, Zhang Y, Werner CT, Beacher NJ, Denman AJ, Li Y, Chen R, Gerfen CR, Barbera G, and Lin DT, Striatal direct pathway neurons play leading roles in accelerating rotarod motor skill learning. iScience 25 (2022) 104245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cataldi S, Lacefield C, Shashaank N, Kumar G, Boumhaouad S, and Sulzer D, Decreased Dorsomedial Striatum Direct Pathway Neuronal Activity Is Required for Learned Motor Coordination. eNeuro 9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, and Surmeier DJ, Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nature neuroscience 9 (2006) 251–9. [DOI] [PubMed] [Google Scholar]
- [52].Gagnon D, Petryszyn S, Sanchez MG, Bories C, Beaulieu JM, De Koninck Y, Parent A, and Parent M, Striatal Neurons Expressing D(1) and D(2) Receptors are Morphologically Distinct and Differently Affected by Dopamine Denervation in Mice. Sci Rep 7 (2017) 41432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Toy WA, Petzinger GM, Leyshon BJ, Akopian GK, Walsh JP, Hoffman MV, Vuckovic MG, and Jakowec MW, Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiol Dis 63 (2014) 201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Suarez LM, Solis O, Carames JM, Taravini IR, Solis JM, Murer MG, and Moratalla R, L-DOPA treatment selectively restores spine density in dopamine receptor D2-expressing projection neurons in dyskinetic mice. Biol Psychiatry 75 (2014) 711–22. [DOI] [PubMed] [Google Scholar]
- [55].Fieblinger T, Graves SM, Sebel LE, Alcacer C, Plotkin JL, Gertler TS, Chan CS, Heiman M, Greengard P, Cenci MA, and Surmeier DJ, Cell type-specific plasticity of striatal projection neurons in parkinsonism and L-DOPA-induced dyskinesia. Nature communications 5 (2014) 5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Dong H, Wang J, Yang YF, Shen Y, Qu WM, and Huang ZL, Dorsal Striatum Dopamine Levels Fluctuate Across the Sleep-Wake Cycle and Respond to Salient Stimuli in Mice. Front Neurosci 13 (2019) 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Rafalovich IV, Melendez AE, Plotkin JL, Tanimura A, Zhai S, and Surmeier DJ, Interneuronal Nitric Oxide Signaling Mediates Post-synaptic Long-Term Depression of Striatal Glutamatergic Synapses. Cell Rep 13 (2015) 1336–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Nakamura T, Nagata M, Yagi T, Graybiel AM, Yamamori T, and Kitsukawa T, Learning new sequential stepping patterns requires striatal plasticity during the earliest phase of acquisition. The European journal of neuroscience 45 (2017) 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fino E, Vandecasteele M, Perez S, Saudou F, and Venance L, Region-specific and state-dependent action of striatal GABAergic interneurons. Nature communications 9 (2018) 3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Funk CM, Peelman K, Bellesi M, Marshall W, Cirelli C, and Tononi G, Role of Somatostatin-Positive Cortical Interneurons in the Generation of Sleep Slow Waves. The Journal of neuroscience : the official journal of the Society for Neuroscience 37 (2017) 9132–9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.