Abstract
This study reports a novel targeted cancer therapy platform based on luteolin-loaded mesoporous silica nanoparticles functionalized with folic acid (Lu-MSN-FA NPs). MSNs were synthesized via a CTAB-templated sol-gel process employing tetraethylorthosilicate (TEOS) in an ammonium hydroxide-catalyzed system at 45 °C, followed by surfactant removal through acidic methanol reflux. The resulting NPs were surface-modified with (3-aminopropyl) triethoxysilane (APTS) and conjugated with FA using EDC/NHS activation to enable selective receptor-mediated uptake in cancer cells. The characteristics of Lu-MSN-FA NPs were evaluated using dynamic light scattering (DLS) and field-emission scanning electron microscopy (FESEM), revealing uniform, spherical particles with a favorable size for cellular uptake. Luteolin was successfully incorporated into the functionalized MSNs, achieving an encapsulation efficiency of 83.10%. Release assays indicated a controlled and sustained release pattern under experimental conditions. In vitro studies on AGS, HT-29, A2780, and A2058 cell lines revealed concentration-dependent cytotoxicity, with the highest sensitivity observed in A2780 cells. Cytotoxicity assays revealed the concentration-dependent cytotoxic effect of Lu-MSN-FA NPs, particularly on A2780 cells, with an IC50 of 5.7 µg/mL. Annexin V-FITC/PI dual staining and DAPI assays confirmed apoptosis induction. At the same time, real-time PCR demonstrated significant modulation of apoptosis-related genes (caspase 9 and p21) along with reduced expression of the antioxidant enzyme SOD. The Lu-MSN-FA NPs also exhibited robust free radical scavenging activity, underscoring their dual therapeutic potential. These findings indicate the effective formulation and controlled release performance of Lu-MSN-FA NPs, emphasizing their promise as a targeted delivery system for enhanced cancer therapy.
Keywords: Luteolin, Mesoporous silica nanoparticles, Cytotoxicity, Antioxidant capacity, Apoptosis
Introduction
Cancer remains a leading cause of global mortality, with approximately 20 million new cases and 9.7 million deaths in 2022 [1]. New cancer cases are predicted to exceed 35 million by 2050 [2]. This alarming rise highlights the need for more effective and targeted treatments, as conventional therapies like chemotherapy and radiation have significant limitations, including non-specificity, systemic toxicity, and the development of drug resistance. Natural products, such as luteolin, known for their high specificity and low toxicity, present a promising alternative [3]. Many of these compounds target essential pathways in cancer development, thereby preventing drug resistance and enhancing treatment effectiveness [4, 5]. They may enhance traditional therapies, improve patient treatment outcomes, and enhance quality of life.
Cetyltrimethylammonium bromide (CTAB), a cationic surfactant, is extensively utilized to fabricate mesoporous nanoparticles (NPs) for drug delivery applications. As a pore template, CTAB generates mesoporous structures characterized by high surface areas and tunable pore dimensions [6]. Furthermore, it can modify nanocrystalline cellulose, enhancing its drug-binding capacity and sustained release properties. CTAB-based micelles have also been investigated as potential drug carriers in targeted delivery systems [7]. In the synthesis of hydroxyapatite, CTAB influences the size, shape, and surface area of the particles [8]. Ammonium hydroxide (NH4OH) and tetraethyl orthosilicate (TEOS) are commonly employed in conjunction with CTAB for NP synthesis [9]. However, the presence of residual CTAB may pose toxicity risks, necessitating careful removal and consideration of its impact on drug delivery systems [6].
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is a common flavonoid aglycone known for its antioxidant and anti-inflammatory properties [3, 10]. It has demonstrated inhibitory effects across various types of cancer, including breast, colon, lung, gastric, and prostate. Luteolin can inhibit cancer cell proliferation and induce apoptosis by modulating crucial signaling pathways that regulate cancer cell survival, such as the PI3K/Akt, Akt/mTOR, and MAPK pathways [11–13]. Additionally, it reduces cancer cell metastasis by downregulating the expression of matrix metalloproteinases [14]. However, luteolin’s low bioavailability, due to its poor water solubility and rapid metabolism, poses significant challenges to its therapeutic application [15]. In this context, drug delivery systems (DDSs) can significantly enhance the clinical efficacy of compounds like luteolin by improving their bioavailability and solubility. These systems optimize therapeutic outcomes through targeted delivery, sustained release, and reduced side effects [16, 17]. In oncology, NPs have emerged as a valuable DDS, facilitating more effective delivery of therapeutic agents to cancer cells [18, 19]. NPs are nanoscale carriers used in cancer drug delivery. They encapsulate therapeutic agents to improve solubility and stability, effectively targeting tumor sites. Their small size enhances drug accumulation in cancer tissues, reducing systemic toxicity and maximizing treatment efficacy [20].
Among various NPs, mesoporous silica NPs (MSNs) are favored for their high surface area, tunable pore size, and excellent biocompatibility [21]. The porous structure of MSNs enables the effective loading of both hydrophilic and hydrophobic agents. Further, the MSN surface can be modified with functional groups for targeted delivery and controlled release [22]. These NPs also exhibit favorable pharmacokinetics, including prolonged circulation and tumor accumulation, via the enhanced permeability and retention (EPR) effect [23]. Additionally, MSNs are biodegradable, ensuring safe elimination from the body post-therapy.
On the other hand, functionalizing MSNs with folic acid (FA) can significantly enhance targeting, as folate receptors are overexpressed in many cancers but are limited in normal tissues [24]. As a transmembrane glycoprotein, folate receptors play a crucial role in FA uptake, which is essential for DNA synthesis and repair. Many cancer cells, especially in ovarian (A2780), breast, and gastrointestinal tumors (AGS), increase the expression of folate receptors to support rapid proliferation [25]. This allows targeted delivery of MSNs functionalized with FA (MSN-FA), promoting internalization through receptor-mediated endocytosis. Once inside, the MSNs can release therapeutic agents in a controlled manner, potentially improving treatment efficacy and reducing systemic toxicity. This targeted mechanism maximizes the therapeutic payload directly delivered to tumor cells, minimizing off-target effects and systemic toxicity [26]. Concentrating the drug-carrying particles within the tumor microenvironment enhances the treatment’s efficacy while reducing exposure to healthy tissues. This dual advantage, improved selectivity and enhanced cellular uptake, underscores why FA-functionalized MSNs are a promising platform for developing precision cancer therapies [26]. Furthermore, the improved uptake via folate receptor targeting supports more controlled intracellular drug release, which helps achieve sustained therapeutic effects and potentially overcomes issues such as rapid drug metabolism [27]. This innovative approach aligns with current trends in nanomedicine, where receptor-mediated targeting strategies are crucial in advancing personalized and effective cancer treatments [28].
In A2780 cells, folate receptors are known to be highly expressed. This increased expression facilitates the selective uptake of MSN-FA NPs, leading to elevated intracellular levels of luteolin [29]. Once internalized, the release of luteolin initiates apoptosis by activating caspase cascades and upregulating cell cycle inhibitors such as p21 [30]. This is supported by observations of increased caspase-9 (Cas-9) and p21 expression, concurrent with reduced levels of antioxidant enzymes, such as SOD, ultimately resulting in heightened oxidative stress and cell death [31]. In contrast, the composite’s impact on other cell lines can be envisioned through distinct yet interrelated mechanisms. In AGS cells, previous studies have shown that luteolin modulates the PI3K/Akt/mTOR pathway, a critical regulator of cell survival and proliferation, thereby inducing apoptotic cell death [32]. In HT-29 cells (colorectal cancer), similar pathway interference may occur, accompanied by additional effects on signaling molecules associated with cell cycle regulation and metastatic potential, leading to reduced proliferation and enhanced apoptosis [33]. Meanwhile, in A2058 cells (melanoma), luteolin’s inhibitory action on metalloproteinases and its ability to modulate MAPK signaling pathways could be central to impairing cell migration and inducing apoptosis [32].
NP-based drug delivery platforms have exhibited promise in cancer treatment, particularly in overcoming multidrug resistance and enhancing therapeutic efficacy. Superoxide dismutase, an antioxidant enzyme, has been successfully encapsulated within NPs to safeguard against reactive oxygen species and mitigate ischemia-reperfusion injury [34]. Co-delivery systems integrating pharmacological agents and genetic material have demonstrated synergistic effects in cancer therapy [35]. These systems can facilitate the delivery of CRISPR/Cas9 for gene editing and immunotherapeutic applications [36]. Various nanocarriers, including polymers, liposomes, and inorganic materials, have been developed for the co-delivery of multiple therapeutic agents. The regulation of SOD genes holds implications for disease progression and treatment [37].
This study aims to evaluate the efficacy of luteolin-conjugated MSN-FA NPs (Lu-MSN-FA NPs) for targeted cancer therapy. We focused on the AGS, HT-29, A2780, and A2058 cancer cell lines in an in vitro model. By combining the anticancer properties of luteolin with the targeting capabilities of MSN-FA, this approach seeks to enhance therapeutic outcomes while minimizing off-target effects. This research could contribute to the development of innovative nanostructured platforms for precision oncology.
Materials and methods
Chemicals and reagents
The synthesis procedures employed CTAB (Sigma-Aldrich), TEOS (Merck), ammonium hydroxide (Sigma-Aldrich), 3-aminopropyltriethoxysilane (APTS, Sigma-Aldrich), FA (Sigma-Aldrich), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), and N-hydroxysuccinimide (NHS). The solvents used in these procedures included ethanol, dimethyl sulfoxide (DMSO), and methanol. To ensure the reproducibility of our methods, we utilized spectrophotometric reagents, ABTS and DPPH (Sigma-Aldrich), to assess the antioxidant properties.
Instrumentation and analytical techniques
A Malvern Zetasizer Nano ZS (Malvern Instruments, UK) was used to measure particle size and surface charge. Morphological analysis was performed using Field Emission Scanning Electron Microscopy (FESEM) on a JEOL JSM-7800 F system (JEOL Ltd., Japan). Fourier-transform infrared (FTIR) spectroscopy was conducted with a Bruker Vertex 70 v spectrometer (Bruker Optics, Germany) to elucidate chemical bonding characteristics. Gene expression analyses were also performed using a Real-Time PCR system (Bio-Rad CFX Connect Real-Time PCR Detection System, Bio-Rad).
Cell lines and culture media
Cancer cell lines (AGS, HT-29, A2780, A2058) and human dermal fibroblast (HDF) cells were obtained from the Institute Pasteur Iran. Sigma-Aldrich supplied Dulbecco’s Modified Eagle Medium (DMEM) and Fetal Bovine Serum (FBS) for cell culture. Cells were maintained and cultured under standard conditions to ensure consistency and reproducibility throughout the experimental procedures.
Apoptosis detection
Cells were stained using an Annexin V‑FITC/propidium iodide (PI) kit to quantify apoptosis. The Annexin V‑FITC/PI reagents were procured from BD Biosciences (BD Pharmingen), which provides high-quality reagents for flow cytometric detection of early and late apoptotic cells. Nuclear staining was performed with 4′,6‑diamidino‑2‑phenylindole (DAPI), purchased from Sigma‑Aldrich.
Synthesis of MSN
To synthesize MSNs, 75 mg of CTAB was dissolved in 30 mL of deionized water at 45 °C. Subsequently, 275 µL of ammonium hydroxide (NH4OH) was added to the solution [26]. Following this, 125 µL of tetraethylorthosilicate (TEOS) was added in a single batch, and the mixture was stirred for 2 h at 300 rpm. The resulting suspension was then centrifuged to isolate the silica NPs. To remove the CTAB surfactant template, the NPs were refluxed in 1% acidic methanol at 60 °C for 4 h. The suspension was again centrifuged and thoroughly washed with methanol multiple times to yield the final MSN product.
To synthesize MSN-FA, 20 mg of MSNs were mixed with 4 mL of ethanol and 50 µL of APTS at room temperature for 24 h. Simultaneously, 30 mg of FA was activated using 9.3 mg of EDC and 7.7 mg of NHS in a 2.7 mL DMSO solution (0.4 mL), stirred in the dark for 24 h. The activated FA was added to the MSN-NH2 solution and stirred overnight in the dark. Finally, the mixture was centrifuged and washed with water and ethanol to yield MSN-FA [38].
Loading of luteolin onto MSN-FA
For drug loading, luteolin (1 mg) was dissolved in deionized water and mixed with the MSN-FA solution, which was stirred for 24 h [39]. Unbound luteolin was removed via dialysis, and the solution was lyophilized and stored at 4 °C. A luteolin standard curve was created to assess encapsulation efficiency using spectrophotometric analysis at 353 nm. Centrifugation isolated the MSN-FA, and free luteolin was quantified in the supernatant. Encapsulation efficiency was calculated using the formula [(total luteolin–free luteolin)/total luteolin] × 100.
NP characterization
The hydrodynamic size and size distribution of Lu-MSN-FA NPs were determined using a Malvern Zetasizer Nano ZS instrument [40]. The NPs were dispersed in deionized water at a concentration of approximately 0.1 mg/mL. Before measurements, the suspension was sonicated in an ultrasonic bath for 5 min to prevent aggregation. All measurements were performed at 25 °C with a fixed scattering angle of 173° (backscattering mode). At least three independent measurements were obtained for each sample to determine the Z-average diameter, polydispersity index (PDI), and zeta potential. The refractive index of silica was taken into account, and the software automatically calculated the particle size distribution using the cumulant analysis method.
Morphological and structural analyses of the NPs were carried out using a JEOL JSM-7500 F FESEM [40]. For sample preparation, a drop of the NP suspension was placed on a clean silicon wafer (alternatively, carbon-coated copper grids were used) and allowed to air-dry. The samples were sputter-coated with a thin (~ 10 nm) layer of gold to enhance electron conductivity. Imaging was performed at an accelerating voltage of 10 kV with a working distance of approximately 4–5 mm. Multiple magnifications were employed to evaluate both the overall size distribution and the detailed morphology of the NPs, verifying their uniform spherical shape and the absence of significant agglomeration.
FTIR analysis was conducted using a Bruker Tensor 27 FTIR spectrometer to confirm the presence of functional groups and the successful conjugation of FA onto the NP surfaces [40, 41]. The lyophilized NP samples were finely powdered and mixed with potassium bromide (KBr) to form a pellet. Spectra were recorded over a broad range of 4000–500 cm⁻¹, with a spectral resolution of 4 cm⁻¹. Each measurement consisted of 32 scans, which were subsequently averaged to enhance the signal-to-noise ratio. The FTIR spectra allowed the identification of characteristic absorption bands: peaks in the region of 1090–1100 cm−1 attributed to Si–O–Si stretching, broad bands around 3300 cm−1 corresponding to O–H stretching, and distinct peaks at approximately 1700 cm−1 from C = O stretching in activated FA. These results confirm both the silica framework of the NPs and the successful surface modification with organic moieties.
Cytotoxicity assay
The cytotoxic effects of Lu-MSN-FA NPs on AGS, HT-29, A2780, and A2058 cancer cell lines, as well as on human dermal fibroblast (HDF) cells, were evaluated using the MTT assay [40]. Cells were seeded in 96-well plates at a density of 1 × 104 cells per well and allowed to adhere for 24 h. After this, fresh medium with varying concentrations of Lu-MSN-FA NPs (7.8, 15.6, 31.2, 62.5, 125, 250, and 500 µg/mL) was added, and the cells were incubated for 48 h. Following incubation, 20 µL of MTT solution was added to each well and left in the dark at 37 °C for 4 hours to allow for formazan crystal formation. After removing the medium, the crystals were dissolved in 200 µL of DMSO, and the absorbance was measured at 570 nm. Cell viability was calculated as a percentage of the control, and IC50 values were determined for each cell line.
Annexin V-FITC/PI assay
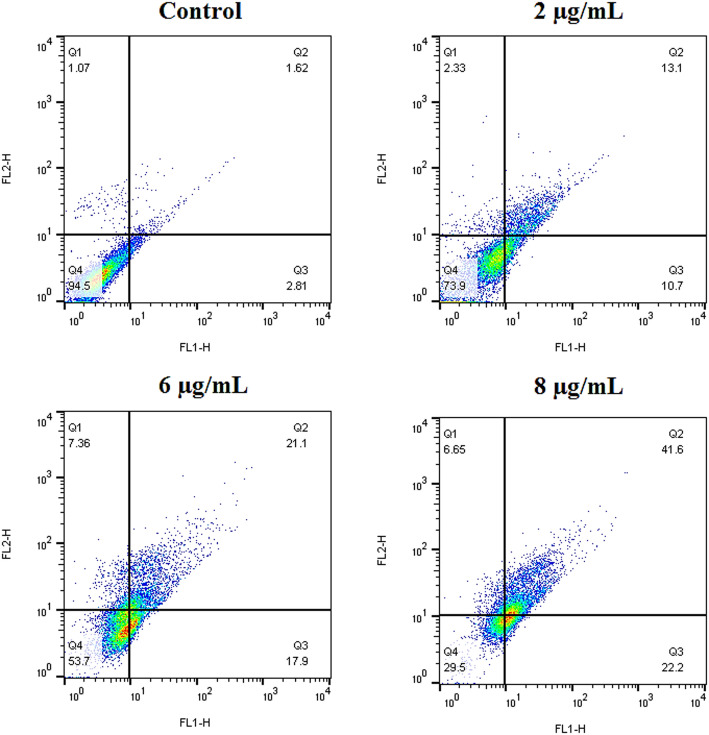
The apoptotic effects of Lu-MSN-FA NPs on A2780 cells were evaluated using the Annexin V-FITC/propidium iodide dual staining assay [40]. Cells were cultured at a density of 1 × 104 cells per well for 24 h and then treated with Lu-MSN-FA NPs at concentrations of 2, 6, and 8 µg/mL for 48 h. Following treatment, the cells were detached using trypsin, washed with PBS, and resuspended in binding buffer (1x) at a concentration of 1 × 106 cells/mL. A 100 µL sample was then incubated with 5 µL of Annexin V-FITC and 5 µL of propidium iodide (PI) at 25 °C for 5 min in the dark. After incubation, the cells were resuspended in 400 µL of binding buffer and analyzed using flow cytometry to assess early apoptotic (Annexin V + and PI-), late apoptotic (Annexin V + and PI+), and necrotic (Annexin V- and PI+) cells. The 6 µg/mL concentration was identified as the IC50, while the 2 and 8 µg/mL concentrations were selected to induce cell death at approximately 25% and 75%, respectively.
DAPI staining
To evaluate apoptosis in A2780 cells that were treated with Lu-MSN-FA NPs at concentrations of 2, 6, and 8 µg/mL for 48 h, we utilized DAPI staining [40]. Following the treatment, cells were rinsed with PBS, fixed in 4% paraformaldehyde for 15 min, and permeabilized using 0.1% Triton X-100 for 10 min. After another wash with PBS, the cells were incubated for 5 min in the dark with 1 µg/mL DAPI, then rewashed and observed under a fluorescence microscope.
Real-time polymerase chain reaction (real-time PCR)
The mRNA expression levels of Cas-9, \SOD, and P21 were analyzed using real-time PCR [31]. Total RNA was extracted from A2780 cells with a QIAGEN kit and quantified via a ThermoFisher Nanodrop ND-1000. The mRNA was converted to complementary DNA (cDNA) using Moloney Murine Leukemia Virus Reverse Transcriptase (Promega). Real-time PCR was conducted with a Corbett thermal cycler, specific primers (Table 1), and Bio-Rad SYBR Green Master Mix. Gene expression levels were normalized using the comparative Ct (cycle threshold) method with GAPDH as the reference gene. Mean Ct values from triplicate samples were analyzed using the 2−ΔΔCt formula.
Table 1.
Specific primer sequences for real-time PCR
| Primer | Sequence |
|---|---|
| Cas-9 |
F: 5′-CCAGAGATTCGCAAACCAGAGG-3′ R: 5′-GAGCACCGACATCACCAAATCC-3′ |
| SOD |
F 5′- CAGCATGGGTTCCACGTCCA-3′ R 5′- CACATTGGCCACACCGTCCT-3′ |
| P21 |
F 5′- AAGACCATGTGGACCTGTCACTGT-3′ R 5′- GAAGATCAGCCGGCGTTTG-3′ |
| GAPDH |
F 5′- GCAGGGGGGAGCCAAAAGGGT-3′ R 5′- TGGGTGCCAGTGATGGCATGG-3′ |
Antioxidant activity assay
The antioxidant capacity of Lu-MSN-FA NPs was evaluated using the ABTS and DPPH assays [42]. For the ABTS assay, a 7 mM stock solution of ABTS was incubated with 2.45 mM potassium persulfate in the dark for 12 h. After incubation, the solution was diluted to an absorbance of 0.70 at 734 nm. Various Lu-MSN-FA NPs were added, and the absorbance was measured after 6 min. In the DPPH assay, a 0.1 mM DPPH solution in methanol was incubated in the dark for 30 min. Afterward, different concentrations of Lu-MSN-FA NPs were added, and the absorbance was measured at 517 nm. The radical scavenging activity was calculated using the formula: % inhibition = (control absorbance – sample absorbance)/control absorbance × 100.
Statistical analysis
We conducted statistical analyses using SPSS software (version 22). Data normality was evaluated using the Shapiro-Wilk test. A one-way analysis of variance (ANOVA) was employed to compare differences across multiple groups, followed by Tukey’s post hoc test for detailed pairwise comparisons to pinpoint specific group differences. A significance threshold of p < 0.05 was established. Results are presented as means ± standard deviations (SD).
Results
This study aimed to assess the anticancer effects of Lu-MSN-FA NPs on AGS, HT-29, A2780, and A2058 cancer cell lines.
Release profile of Lu-MSN-FA NPs
The release profile of luteolin from the Lu-MSN-FA NPs demonstrated a biphasic pattern. An initial phase of accelerated release was observed during the first 4 h, during which approximately 20–30% of the encapsulated luteolin was released. This was succeeded by a sustained release phase, culminating in an overall cumulative release of roughly 70% over 72 h. These findings suggest that NPs enable the immediate attainment of therapeutic concentrations, followed by prolonged release, which is highly advantageous for targeted cancer therapy, as it helps maintain adequate drug levels over extended periods.
DLS assay
The DLS analysis of Lu-MSN-FA NPs (Fig. 1A) reveals a Z-average particle size of 181.40 nm, which is ideal for cellular uptake and therapeutic delivery. The polydispersity index (PDI) of 0.30 indicates a moderate size distribution, suggesting some heterogeneity due to agglomeration or surface functionalization. The mean intensity diameter (220.46 nm) and mean volume diameter (232.53 nm) are higher than the Z-average size, indicating the presence of larger aggregates. However, the mean number diameter of 53.55 nm suggests a significant number of smaller particles. Additionally, the zeta potential of −21.58 ± 2.29 mV suggests moderate stability in suspension, while the mean electrophoretic mobility of −1.70 ± 0.18 mV confirms the particles’ stability and charge characteristics (Fig. 1B). Overall, Lu-MSN-FA NPs present favorable properties for drug delivery and biomedical applications.
Fig. 1.
Characterization of Lu-MSN-FA NPs. A Dynamic Light Scattering (DLS) analysis reveals a Z-average particle size of 181.40 nm and a polydispersity index (PDI) of 0.30, indicating a moderate size distribution. The mean intensity and volume diameters are 220.46 nm and 232.53 nm, respectively, suggesting larger aggregates are present, while the mean number diameter is 53.55 nm, highlighting a significant population of smaller particles. B The zeta potential is −21.58 ± 2.29 mV, and the mean electrophoretic mobility is −1.70 ± 0.18 mV, indicating moderate stability and suitable charge characteristics for drug delivery. C The FESEM micrograph shows the uniform, spherical shape of Lu-MSN-FA NPs. This means a narrow size distribution and minimal aggregation, suggesting good stability for biomedical applications
FESEM
The FESEM micrograph of Lu-MSN-FA NPs provides a high-magnification view highlighting their morphology and size distribution (Fig. 1C). The NPs are uniform and spherical, displaying a clear and well-defined structure. The image indicates that the Lu-MSN-FA NPs have a narrow size distribution, as evidenced by the consistent appearance of the individual particles. Additionally, the NPs appear well-dispersed, with minimal signs of significant aggregation, which suggests good stability and potential effectiveness for drug delivery applications.
FTIR spectroscopy
As illustrated in Fig. 2, the FTIR analysis of the MSNs revealed a broad peak at 3333.97 cm−1, corresponding to the O–H stretching vibration, indicating the presence of hydroxyl groups on the surface. Peaks at 2925.31 cm−1 and 2851.76 cm−1 are attributed to C–H stretching, indicating the presence of organic functional groups or residual surfactants. The peak at 1621.79 cm−1 is associated with the bending vibrations of adsorbed water, while the peak at 1428.53 cm−1 corresponds to the C–H bending of organic moieties. The strong peak at 1094.41 cm−1 confirms the silica nature of the NPs with Si–O–Si stretching. The peak at 951.65 cm−1 indicates the presence of silanol groups, and the peaks at 808.63 cm−1 and 734.98 cm−1 are associated with Si-O-Si vibrations. Lastly, the peak at 475.52 cm−1 relates to rocking vibrations of Si–O–Si bonds.
Fig. 2.
FTIR spectroscopy analysis of MSN (A), free luteolin (B), and Lu-MSN-FA NPs (C). The spectra reveal the presence of key functional groups, including hydroxyl (O–H), carbonyl (C=O), and aromatic (C=C) groups, confirming the successful functionalization of Lu-MSN-FA NPs and their potential for practical drug delivery applications
Luteoline’s peaks at 3562.77 cm−1, 3505.56 cm−1, 3420.35 cm−1, and 3382.97 cm−1 indicate O–H stretching vibrations from hydroxyl groups. The peak at 3113.28 cm−1 corresponds to aromatic C–H stretching, while peaks at 2847.67 cm−1, 2696.48 cm−1, and 2627.01 cm−1 relate to aliphatic C–H stretching. The carbonyl C=O stretching is at 1746.51 cm−1. Peaks at 1656.36 cm−1, 1612.36 cm−1, and 1502.03 cm−1 indicate C=C stretching in aromatic rings. C–H bending vibrations are observed at 1442.00 cm−1 and 1366.56 cm−1, with C–O–C stretching at 1266.7 cm−1. Peaks at 1163.61 cm−1 and 1120.63 cm−1 correspond to the C–O stretching vibration. The C–H in-plane bending is at 1031.57 cm−1, and out-of-plane bending vibrations appear at 877.5 cm−1, 861.16 cm−1, and 839.11 cm−1, along with additional bending at lower frequencies from 756.53 to 519.4 cm−1.
The FTIR analysis of Lu-MSN-FA NPs reveals a broad peak at 3332.81 cm−1, corresponding to O–H stretching vibrations, indicating the presence of hydroxyl groups on the NP surface. Peaks at 2925.95 cm−1 and 2855.95 cm−1 correspond to asymmetric and symmetric C–H stretching vibrations, suggesting the presence of organic functional groups or residual surfactants. The peak at 1701.31 cm−1 is attributed to C=O stretching from carbonyl groups in FA, while peaks at 1656.13 cm−1 and 1610.15 cm−1 indicate C=C stretching in aromatic rings. Other significant peaks include 1511.90 cm−1 (aromatic C=C stretching), 1437.91 cm−1 (C–H bending), 1356.19 cm−1 (C–N stretching from FA), and 1303.06 cm−1 (C–O–C stretching). A strong peak at 1094.49 cm−1 confirms Si–O–Si asymmetric stretching, indicating the silica nature of the NPs, with additional peaks at 949.54 cm−1 (Si–OH stretching) and 818.16 cm−1 (symmetric Si–O–Si stretching). The peak at 641.09 cm−1 corresponds to out-of-plane bending vibrations of aromatic rings.
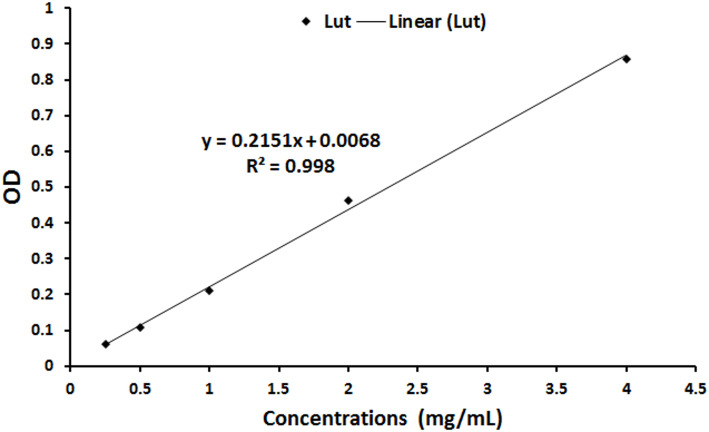
Encapsulation efficacy
The encapsulation efficiency was measured spectrophotometrically at 353 nm, revealing an efficiency of 83.10% (Fig. 3), which indicates the successful incorporation of luteolin into MSN-FA NPs.
Fig. 3.
The spectrophotometric assessment at 353 nm showed an encapsulation efficiency of 83.10% for incorporating luteolin into MSN-FA NPs
Cytotoxicity assay
The cytotoxic effects of Lu-MSN-FA NPs were assessed on various cancer cell lines using the MTT assay (Fig. 4). The results showed concentration-dependent cytotoxicity, with AGS and HT-29 cells exhibiting IC50 values of 14.6 µg/mL and 21.1 µg/mL, respectively. A2058 melanoma cells had an IC50 of 14.04 ± 1.2 µg/mL, while the A2780 ovarian cancer cells were the most sensitive, with an IC50 of 5.7 ± 0.4 µg/mL. In HT-29 cells, concentrations above 7.8 ± 0.71 µg/mL significantly decreased viability (P < 0.05), but other cancer lines showed reduced viability at all tested concentrations (P < 0.001). Normal HDF fibroblast cells demonstrated more resistance, with an IC50 of 363 µg/mL. Lu-MSN-FA NPs displayed selective cytotoxicity towards cancer cell lines, notably affecting A2780 cells.
Fig. 4.
Cell viability results from MTT assays on AGS cells (A), HT-29 (B), A2780 (C), A2058 (D), and HDF (E) cells treated with varying concentrations of MSN-FA NPs. The results indicate concentration-dependent cytotoxicity, with A2780 ovarian cancer cells showing the highest sensitivity (IC50 = 5.7 ± 0.4 µg/mL), while normal HDF fibroblasts exhibited resistance (IC50 = 363 ± 0.8 µg/mL). The data is presented as mean ± standard deviation (SD), and statistical significance is denoted as * P < 0.05 and *** P < 0.001
Annexin V-FITC/PI assay
Figure 5 demonstrates a dose-dependent increase in apoptotic activity in A2780 cells treated with Lu-MSN-FA NPs at 2, 6, and 8 µg/mL concentrations. Untreated control cells had the lowest apoptosis rates (2.81% early, 1.62% late). When treated with a 2 µg/mL concentration, early apoptosis increased by 10.7% and late apoptosis by 13.1%. At a 6 µg/mL concentration, early apoptosis rose to 17.9%, and late apoptosis climbed to 21.1%. Finally, at 8 µg/mL, early apoptosis reached 22.2%, while late apoptosis surged to 41.6%. These results indicate that Lu-MSN-FA NPs effectively induce apoptosis in cancer cells in a concentration-dependent manner.
Fig. 5.
Annexin V-FITC/PI dual staining assay of apoptosis. Results showed a dose-dependent increase in apoptotic activity in A2780 cells following treatment with Lu-MSN-FA NPs. Apoptotic cell percentages for untreated controls were minimal (2.81% early, 1.62% late), while treatment at 2, 6, and 8 µg/mL significantly elevated early (10.7%, 17.9%, 22.2%) and late (13.1%, 21.1%, 41.6%) apoptosis rates, demonstrating effective induction of apoptosis
DAPI staining
The DAPI staining results further confirmed the concentration-dependent cytotoxic effects of Lu-MSN-FA NPs (Fig. 6). The results showed that exposure to concentrations of 2, 6, and 8 µg/mL of Lu-MSN-FA NPs led to a dose-dependent increase in apoptotic A2780 cancer cells. At higher doses of the NPs, the apoptotic cells exhibited nuclear condensation and fragmentation, indicating that the treatment effectively induced apoptosis in a dose-dependent manner. These findings highlight the potential of Lu-MSN-FA NPs as a promising therapeutic approach for treating pancreatic cancer, as the NPs induced significant apoptosis in the tested cancer cell line.
Fig. 6.
DAPI staining results confirm that Lu-MSN-FA NPs exhibit concentration-dependent cytotoxic effects on A2780 cancer cells. Treatment with 2, 6, and 8 µg/mL increased apoptosis, indicated by nuclear condensation and fragmentation, highlighting the potential of Lu-MSN-FA NPs as a therapeutic option for pancreatic cancer
Gene expression assay
The results indicated that treatment with Lu-MSN-FA NPs at a concentration of 6 µg/mL significantly increased the expression of Cas-9 to 2.67 ± 0.3 (Fig. 7A; P < 0.05), suggesting that it promotes apoptosis in cancer cells. Additionally, the cell cycle regulator p21 was upregulated across all concentrations of Lu-MSN-FA NPs (Fig. 7B; P < 0.05), suggesting its involvement in cell cycle arrest and apoptosis. In contrast, the expression of SOD, an antioxidant enzyme, decreased significantly at all concentrations of the NPs (Fig. 7C; P < 0.05). This decrease indicates an increase in oxidative stress, which may contribute to the induction of apoptosis. These findings suggest that Lu-MSN-FA NPs can induce apoptosis in A2780 cells by modulating key genes related to apoptosis, underscoring their potential as a therapeutic approach for ovarian cancer.
Fig. 7.
Real-time PCR assay for mRNA expression of Cas-9, SOD, and p21 in A2780 cells treated with Lu-MSN-FA NPs at 2, 6, and 8 µg/mL. (A) Significant upregulation of Cas-9 (2.67 ± 0.3; P < 0.05) indicates promotion of apoptosis. (B) p21 was upregulated, implicating its role in cell cycle arrest and apoptosis. (C) SOD expression decreased across all concentrations, suggesting increased oxidative stress. These findings highlight the potential of Lu-MSN-FA NPs as a therapeutic approach for ovarian cancer. The data are presented as mean ± standard deviation (SD), and statistical significance is denoted as *P < 0.05
Antioxidant capacity assay
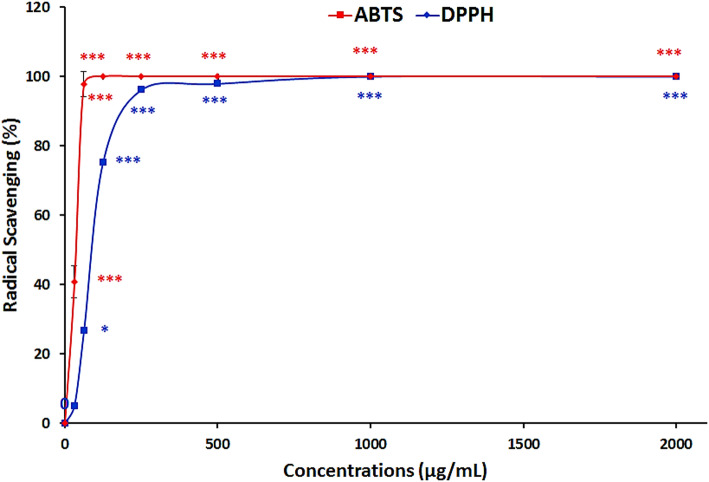
The antioxidant capacity of Lu-MSN-FA NPs was assessed by their ability to scavenge ABTS and DPPH free radicals (Fig. 8). Results showed a significant concentration-dependent increase in scavenging activity for both assays (P < 0.001). The ABTS assay showed complete inhibition at 125 µg/mL, while the DPPH assay showed complete inhibition at 1000 µg/mL. These findings confirm that Lu-MSN-FA NPs possess potent antioxidant properties.
Fig. 8.
Antioxidant capacity assay results for Lu-MSN-FA NPs. Results showed a significant concentration-dependent increase in scavenging activity, with complete inhibition at 125 µg/mL for ABTS and 1000 µg/mL for DPPH, confirming the potent antioxidant properties of Lu-MSN-FA NPs. The data are presented as mean ± standard deviation (SD), and statistical significance is denoted as follows: *P < 0.05 and ***P < 0.001
Discussion
MSNs have emerged as a potent research subject in targeted cancer therapy, owing to their unique structural advantages, enhanced targeting capabilities, improved drug stability and bioavailability, and the ability to overcome conventional delivery limitations [43]. MSNs possess a high surface area and tunable pore sizes, enabling them to load significant amounts of therapeutic agents, such as luteolin, thereby addressing issues such as rapid drug clearance and low bioavailability [44]. Additionally, these NPs can be modified with ligands such as FA, facilitating receptor-mediated endocytosis and thereby improving cellular uptake while minimizing systemic toxicity [45]. Encapsulation within MSNs protects luteolin from premature degradation, enhancing its stability and delivering a higher effective dose to cancer cells [46]. Furthermore, NPs offer a controlled, targeted approach, increasing drug accumulation at the tumor site through the enhanced permeability and retention effect [43]. The ability of NPs to be finely tuned in terms of size, morphology, and surface charge is crucial for designing systems tailored to diverse cancer cell lines [44]. The versatility of NPs allows for the integration of diagnostic, therapeutic, and imaging agents into a single platform, known as theranostics [46].
In this study, we aimed to enhance the therapeutic effects of luteolin, a natural compound with anticancer properties, by delivering it to cancer cells through MSN-FA NPs. MSN-FA NPs are a notable type of NP being explored for cancer treatment due to their high drug-loading capacity and ability to target folate receptors that are overexpressed on cancer cells. This selective targeting renders them a promising candidate for personalized cancer therapeutics. Our dynamic light scattering analysis revealed that Lu-MSN-FA NPs exhibit an optimal size for cellular internalization, with a Z-average diameter of 181.40 nm, moderate colloidal stability, and a polydispersity index indicative of some heterogeneity. These findings align with previous reports on the dimensions of MSN synthesized via a CTAB-templated sol–gel approach [47]. Furthermore, the FESEM images confirmed the uniform, spherical morphology of the NPs, consistent with earlier observations. Our FTIR analysis, revealing characteristic absorption bands corresponding to Si–O–Si stretching and FA conjugation, also compares favorably with the work of Khaled et al. [45] and Shini Feng et al. [48].
The enhanced cytotoxicity observed in the A2780 cell line (IC₅₀ of 5.7 µg/mL) is comparable to studies demonstrating the anticancer effects of luteolin-loaded systems [49]. Our apoptosis analyses, using Annexin V-FITC/PI and DAPI staining, demonstrated dose-dependent increases in early and late apoptosis, corroborating previous research that indicates luteolin induces apoptosis through the upregulation of caspase pathways [50]. Additionally, the gene expression data, which showed elevated Cas-9 and p21 levels alongside decreased SOD expression, align with the molecular mechanisms described in prior studies [32]. Moreover, the antioxidant capacity assays confirmed the NPs’ ability to scavenge free radicals. The in vitro drug release study demonstrated a biphasic release pattern, characterized by an initial burst release followed by sustained release over 72 h. This release profile aligns with the kinetics reported for other NP-based drug delivery systems, such as those described by Yu Dang et al. [18] and Chettupalli et al. [51]. The initial rapid release facilitates the achievement of therapeutic drug levels. In contrast, the subsequent sustained release prolongs the therapeutic efficacy. These findings underscore the potential of Lu-MSN-FA NPs as a promising therapeutic strategy for ovarian cancer, combining effective drug delivery with significant anticancer activity.
The improved anticancer effect of Lu-MSN-FA NPs comes from the combined benefits of better drug delivery, targeted cellular uptake, and prolonged biological activity [52]. This differentiates them from free luteolin and other incomplete formulations, such as MSN, Lu-MSN, MSN-FA, or uncoated FA [49]. In its unbound state, free luteolin exhibits anticancer capabilities but is constrained by inadequate solubility and accelerated metabolism [53]. MSN and MSN-FA (without Lu) are generally bioinert, exhibiting negligible cytotoxicity, and function as carriers for targeting cancer cells due to the overexpression of folate receptors [26]. The Lu-MSN formulation enhances luteolin’s solubility and stability, improving cytotoxic efficacy [54]. Nevertheless, without a targeting ligand, the NPs rely exclusively on the enhanced permeability and retention effect for tumor accumulation, potentially resulting in suboptimal selectivity [55]. The composite benefits from receptor-mediated cellular internalization when luteolin is encapsulated within MSN-NPs, further functionalized with FA [56]. Cancer cells frequently overexpress folate receptors to satisfy their elevated demand for folate [57]. This selective uptake substantially enhances the intracellular concentration of luteolin in tumor cells, as evidenced by notably lower half-maximal inhibitory concentration values compared to free luteolin or non-targeted formulations [49].
Free luteolin, a potent antioxidant, can scavenge free radicals, but its rapid metabolism may diminish its therapeutic potential [42]. Encapsulation into NP formulations stabilizes luteolin and preserves its antioxidant activity, allowing it to maintain robust radical-scavenging capacities [40]. This is significant as reducing oxidative stress can modulate redox-sensitive pathways in cancer cells, promoting apoptosis and cell cycle arrest [49]. FA may also enhance the cellular uptake and retention of the NP system, ensuring luteolin’s antioxidant and cytotoxic effects are effectively delivered to the tumor site [49]. The Lu-MSN-FA NPs overcome limitations of free luteolin, such as low water solubility and rapid in vivo clearance [46]. They induce cytotoxicity through pathways such as caspase activation and exert antioxidant effects to modulate cellular redox states, thereby creating a robust anticancer mechanism [49]. Formulations lacking one or more components do not achieve the same synergistic enhancement, underscoring the necessity of each element in the composite [49]. Although free luteolin possesses inherent pro-apoptotic characteristics, mediated by its capacity to modulate pivotal signaling cascades like PI3K/Akt, MAPK, and caspase activation, it is hindered by poor water solubility and rapid metabolic clearance. These limitations restrict its cellular internalization, resulting in diminished intracellular concentrations and ultimately reducing its overall ability to induce apoptosis in cancer cells compared to its NP-encapsulated form [11–13].
MSNs and their FA-functionalized counterparts primarily serve as inert delivery vehicles. They do not inherently exhibit substantial apoptotic activity but can facilitate drug delivery [58]. FA functionalization enhances cellular uptake through receptor-mediated endocytosis in cancer cells; however, without a cytotoxic payload, such as luteolin, it induces minimal apoptotic effects [59]. Encapsulation of luteolin within MSNs enhances its stability and solubility, resulting in improved cytotoxic and pro-apoptotic effects compared to free luteolin [60]. However, without a targeting ligand, the selective accumulation in cancer cells is less efficient, and drug release is predominantly governed by passive mechanisms, which may result in a suboptimal apoptotic response [61]. Luteolin-loaded, FA-functionalized MSN enhances the formulation by targeting overexpressed folate receptors on cancer cells and providing a controlled release of luteolin [49]. This selective uptake increases the intracellular concentration of luteolin in tumor cells while sparing normal cells [49]. The MSN matrix ensures effective doses are delivered over prolonged periods, leading to a significantly higher level of apoptosis, as evidenced by flow cytometry assays showing a dose-dependent increase in both early and late apoptosis, as well as upregulation of apoptotic markers detected by real-time PCR [49, 62]. FA does not directly induce cell death but functions as a targeting ligand. Its primary role is to enhance the selectivity of the delivery system, rather than acting as an agent to induce apoptosis [63].
Luteolin is an herbal secondary metabolite used in traditional Chinese medicine to treat inflammatory disorders, hypertension, and certain types of cancer [64]. Biochemically, it is an antioxidant that scavenges free radicals, chelates metal ions, inhibits pro-oxidant enzymes, and induces antioxidant enzymes. Furthermore, luteolin has a favorable safety profile with few side effects. Its oral LD50 is over 2500 mg/kg in mice and 5000 mg/kg in rats, equivalent to 219.8−793.7 mg/kg in humans [65]. In addition, luteolin possesses anticancer properties by inhibiting tumor cell proliferation, metastasis, invasion, and angiogenesis. It achieves this through the suppression of kinases, induction of apoptosis, regulation of the tumor cell cycle, and reduced transcription factors [39]. In vitro, luteolin inhibits tumor cell proliferation with IC50 values ranging from 3 to 50 µM [66]. Animal studies indicate that a diet with luteolin at 50–200 ppm significantly decreases tumor volume [67]. Previous studies have demonstrated the anticancer properties of luteolin against various cancers, including colon, lung, prostate, gastric, glioblastoma, liver, and breast cancer [68]. In colon cancer, luteolin inhibits metastasis by downregulating the expression of MMP-2 and MMP-9 [69]. Additionally, it induces apoptosis and cell cycle arrest by affecting Wnt/β-catenin signaling and modulating the levels of cyclin D1, Bcl-2, Bax, and caspase-3 [70]. In lung cancer, luteolin promotes apoptosis and inhibits migration by activating the MEK/ERK pathway and inducing endoplasmic reticulum (ER) stress [71]. It also suppresses tumor growth by downregulating the LIMK1 signaling pathway in xenograft models [72]. Luteolin inhibits cell viability and angiogenesis for prostate cancer by targeting the VEGFR-2-regulated AKT/ERK pathway [73]. In gastric cancer, it reduces tumor growth by downregulating VEGF-A and the cMet/Akt/ERK signaling pathways while also inducing apoptosis [74, 75]. Luteolin inhibits cell migration and the epithelial-mesenchymal transition (EMT) process in glioblastoma by modulating the PI3K/Akt signaling pathway [76]. In liver cancer, the AMPK signaling pathway is involved [77]. Lastly, luteolin inhibits cell proliferation in breast cancer and induces apoptosis by targeting EGFR signaling and modulating various apoptotic pathways [78]. These findings underscore luteolin’s potential as a therapeutic agent in cancer treatment, due to its action on multiple molecular targets and signaling pathways.
Despite the promising anticancer effects of luteolin, its clinical application is hindered by low bioavailability. Luteolin is rapidly absorbed from the intestine, achieving peak plasma concentrations within 1 to 1.1 h [79]. However, its metabolism primarily transforms it into glucuronide and sulfate conjugates [80]. This rapid conversion, low water solubility, and extensive pre-systemic metabolism significantly limit its bioavailability, resulting in quick excretion and diminished therapeutic effectiveness [81]. To address these challenges, nano-delivery systems have been developed to improve luteolin’s poor water solubility and bioavailability, which limit its therapeutic efficacy in cancer treatment. Various formulations, including MPEG-PCL micelles, FA-modified micelles, phytosomes, zinc oxide NPs, and elastic liposomes, have shown promise in enhancing luteolin’s solubility and targeting capabilities. For example, luteolin-loaded MPEG-PCL micelles have demonstrated increased cytotoxicity and apoptosis in glioblastoma cells, while FA-modified micelles further enhance these effects in vivo [82]. In breast cancer studies, luteolin-loaded phytosomes have improved the efficacy of doxorubicin, and zinc oxide NPs have demonstrated significant anticancer activity against MCF-7 cells [83]. Additionally, elastic liposomes have facilitated better luteolin transdermal delivery and cellular uptake [84]. Her-2-PLGA NPs have been shown to significantly increase luteolin uptake in gastric cancer research, thereby inhibiting cancer cell proliferation and migration [85].
The mentioned nanocarrier systems can enhance the bioavailability and targeting of luteolin. However, they face stability, delivery efficiency, and potential toxicity issues, which limit their treatment effectiveness. Furthermore, MSNs have shown promising results in cancer therapy due to their tunable properties and ability to improve drug delivery. Despite MSNs’ potential, these carriers have not previously been investigated for their ability to deliver luteolin in cancer treatment. Investigating MSNs could leverage their advantages to enhance the bioavailability and efficacy of luteolin, contributing to more effective cancer therapies. MSNs have emerged as versatile carriers in various cancer treatment strategies, addressing challenges such as poor drug solubility, multidrug resistance (MDR), and non-specificity of traditional therapies [86]. MSNs facilitate targeted drug delivery, enhance the solubility of hydrophobic anticancer agents such as camptothecin (CPT) and paclitaxel (PTX), and significantly improve their cytotoxic effects [87]. They can be functionalized to control drug release in response to specific tumor environments, making them suitable for photodynamic therapy (PDT) [88] and sonodynamic therapy (SDT) [89]. In PDT, MSNs can enhance the efficacy of photosensitizers, while in SDT, they improve tissue penetration and ROS generation for tumor destruction.
Additionally, MSNs are utilized in chemotherapy to co-deliver anticancer drugs and MDR inhibitors, significantly enhancing treatment efficacy without systemic toxicity. Their application extends to radiotherapy, where they enable the delivery of targeted alpha radiation, thereby minimizing damage to healthy tissues. In immunotherapy, MSNs enhance the delivery of cancer vaccines and adjuvants, activating T cells and improving immune responses [90]. Furthermore, they serve as carriers for gene therapy, allowing for the efficient delivery of therapeutic nucleic acids to silence oncogenes [91]. Overall, MSNs represent a promising platform for advancing cancer treatment through their multifunctional capabilities and targeted delivery systems.
Luteolin-loaded NPs have demonstrated promising in vitro anticancer effects but face several challenges in vivo settings. These include concerns related to biodistribution, pharmacokinetics, stability, aggregation, potential toxicity, accumulation, immunogenicity, and off-target impacts [49, 92]. In a living organism, NPs often experience rapid clearance by the mononuclear phagocyte or reticuloendothelial system, limiting their accumulation at tumor sites [92]. Researchers are investigating approaches such as surface PEGylation or stealth coatings to evade immune recognition and extend circulation time [93]. Aggregation can impair targeting capabilities and release profiles. Strategies to address this include optimizing surface properties, utilizing biocompatible stabilizers, and incorporating responsive coatings [94]. Overcoming these limitations may involve modifying the composition or structure to promote biodegradability or using alternative materials that are fully metabolized and cleared from the body [95].
In summary, this study provides insights into the cytotoxic effects and induction of apoptosis by luteolin-loaded MSN-FA NPs using in vitro models; however, several limitations should be noted. The reliance on cell culture systems does not accurately reflect the physiological complexities of whole organisms, leaving unanswered questions about biodistribution, pharmacokinetics, immune responses, and systemic toxicity. Additionally, the lack of structural characterization via scanning transmission electron microscopy (STEM) and X-ray diffraction (XRD) is a methodological constraint, as these analyses are essential for validating NP properties that influence therapeutic performance. Furthermore, without in vivo validation, potential off-target effects and metabolic pathways remain unassessed. Future research should focus on comprehensive material characterization using STEM/XRD and preclinical testing in relevant animal models to establish structure-activity relationships and ensure safety for therapeutic development.
Affordability and budget-friendliness
The MSN-FA platform exhibits notable economic advantages compared to lipid-based or polymeric nanocarriers, with core materials such as TEOS and CTAB being commercially available at industrial-scale prices. While demonstrating significantly lower precursor costs compared to synthetic polymer synthesis, the translational economics require careful evaluation. Multistep functionalization processes introduce additional production costs compared to bare MSNs; however, emerging flow chemistry approaches show potential for reducing this gap [96]. Crucially, the system’s thermal stability and room-temperature storage capability substantially lower cold-chain logistics costs, a key translational advantage over biological carriers that require stringent temperature control. Current industrial initiatives to automate template removal and FA conjugation could reduce batch-to-batch variability while achieving favorable projected scale-up costs at metric ton production levels [97]. These combined factors position MSN-FA NPs as a clinically viable platform, pending GMP-compliant manufacturing validation, particularly for oral or topical formulations, where silica’s regulatory precedence offers a faster clinical pipeline entry compared to novel nanomaterials.
Conclusion
In conclusion, Lu-MSN-FA NPs exhibit significant cytotoxicity and antioxidant capacity against AGS, HT-29, A2780, and A2058 cancer cell lines, with ovarian cancer cells showing the highest sensitivity. The effective encapsulation of luteolin leads to a concentration-dependent induction of apoptosis, as demonstrated by Annexin V-FITC/PI and DAPI staining. Additionally, these NPs modulate key apoptosis-related genes, such as caspase 9 and p21, and induce oxidative stress by reducing SOD expression. Although the results are promising, the study’s limitations include reliance on in vitro assays, with a need for in vivo models to assess long-term effects and safety. Future research should also explore combining these NPs with other treatments to enhance efficacy across various cancer types.
Acknowledgements
This work was supported by Islamic Azad University, Mashhad, Iran, and is appreciated by the author.
Author contributions
Maryam Hosseini and Mozhgan Soltani: Investigation, Methodology and Writing-Original draft. Masoud Homayouni and Bita Behboodian: Supervision, Data curation, Conceptualization Software, Validation, and Writing Reviewing.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All institutional and national guidelines for the care and use of laboratory animals were followed.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu Y, Zheng Z. Understanding the global cancer statistics 2022: growing cancer burden. Sci China Life Sci. 2024;67(10):2274–6. [DOI] [PubMed] [Google Scholar]
- 2.Chen J, Cui Y, Deng Y, Xiang Y, Chen J, Wang Y, et al. Global, regional, and national burden of cancers attributable to particulate matter pollution from 1990 to 2019 and projection to 2050: worsening or improving? J Hazard Mater. 2024;477:135319. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y, Shi R, Wang X, Shen H-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr Cancer Drug Targets. 2008;8(7):634–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tewari D, Patni P, Bishayee A, Sah AN, Bishayee A, editors. Natural products targeting the PI3K-Akt-mTOR signaling pathway in cancer: a novel therapeutic strategy. Semin Cancer Biol. 2022. [DOI] [PubMed]
- 5.Haider T, Pandey V, Banjare N, Gupta PN, Soni V. Drug resistance in cancer: mechanisms and tackling strategies. Pharmacol Rep. 2020;72(5):1125–51. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho GC, Marena GD, Karnopp JCF, Jorge J, Sábio RM, Martines MAU, et al. Cetyltrimethylammonium bromide in the synthesis of mesoporous silica nanoparticles: general aspects and in vitro toxicity. Adv Colloid Interface Sci. 2022;307:102746. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RD, Raghav N. Differential effect of surfactants tetra-n-butyl ammonium bromide and N-cetyl-N, N, N-trimethyl ammonium bromide bound to nano-cellulose on binding and sustained release of some non-steroidal anti-inflammatory drugs. Int J Biol Macromol. 2020;164:2745–52. [DOI] [PubMed] [Google Scholar]
- 8.Monteiro APF, Idczak G, Tilkin RG, Vandeberg RM, Vertruyen B, Lambert SD, et al. Evaluation of hydroxyapatite texture using CTAB template and effects on protein adsorption. Surf Interface. 2021;27:101565. [Google Scholar]
- 9.Shende RA, Chaudhari BP. Robust optimization and characterization of MCM-41 nanoparticle synthesis using modified sol-gel method. ChemSelect. 2023;8(11):e202204968. [Google Scholar]
- 10.Imran M, Rauf A, Abu-Izneid T, Nadeem M, Shariati MA, Khan IA, et al. Luteolin, a flavonoid, as an anticancer agent: a review. Biomed Pharmacother. 2019;112:108612. [DOI] [PubMed] [Google Scholar]
- 11.Lu X, Li Y, Li X, Aisa HA. Luteolin induces apoptosis in vitro through suppressing the MAPK and PI3K signaling pathways in gastric cancer. Oncol Lett. 2017;14(2):1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raina R, Pramodh S, Rais N, Haque S, Shafarin J, Bajbouj K, et al. Luteolin inhibits proliferation, triggers apoptosis and modulates Akt/mTOR and MAP kinase pathways in HeLa cells. Oncol Lett. 2021;21(3):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee W-J, Wu L-F, Chen W-K, Wang C-J, Tseng T-H. Inhibitory effect of Luteolin on hepatocyte growth factor/scatter factor-induced HepG2 cell invasion involving both MAPK/ERKs and PI3K–Akt pathways. Chemico-Biological Interactions. 2006;160(2):123–33. [DOI] [PubMed] [Google Scholar]
- 14.Velmurugan BK, Lin J-T, Mahalakshmi B, Chuang Y-C, Lin C-C, Lo Y-S, et al. Luteolin-7-O-glucoside inhibits oral cancer cell migration and invasion by regulating matrix metalloproteinase-2 expression and extracellular signal-regulated kinase pathway. Biomolecules. 2020;10(4):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyashita A, Ito J, Parida IS, Syoji N, Fujii T, Takahashi H, et al. Improving water dispersibility and bioavailability of Luteolin using microemulsion system. Sci Rep. 2022;12(1):11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin S-Y, Zhang A-Q, Cheng S-X, Rong L, Zhang X-Z. Drug self-delivery systems for cancer therapy. Biomaterials. 2017;112:234–47. [DOI] [PubMed] [Google Scholar]
- 17.Khan MI, Hossain MI, Hossain MK, Rubel M, Hossain K, Mahfuz A, et al. Recent progress in nanostructured smart drug delivery systems for cancer therapy: a review. ACS Appl Bio Mater. 2022;5(3):971–1012. [DOI] [PubMed] [Google Scholar]
- 18.Dang Y, Guan J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Materials in Medicine. 2020;1:10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dristant U, Mukherjee K, Saha S, Maity D. An overview of polymeric nanoparticles-based drug delivery system in cancer treatment. Technol Cancer Res Treat. 2023;22:15330338231152083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Avramović N, Mandić B, Savić-Radojević A, Simić T. Polymeric nanocarriers of drug delivery systems in cancer therapy. Pharmaceutics. 2020;12(4):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li T, Shi S, Goel S, Shen X, Xie X, Chen Z, et al. Recent advancements in mesoporous silica nanoparticles towards therapeutic applications for cancer. Acta Biomater. 2019;89:1–13. [DOI] [PubMed] [Google Scholar]
- 22.Feng Y, Panwar N, Tng DJH, Tjin SC, Wang K, Yong K-T. The application of mesoporous silica nanoparticle family in cancer theranostics. Coord Chem Rev. 2016;319:86–109. [Google Scholar]
- 23.Taleghani AS, Nakhjiri AT, Khakzad MJ, Rezayat SM, Ebrahimnejad P, Heydarinasab A, et al. Mesoporous silica nanoparticles as a versatile nanocarrier for cancer treatment: a review. J Mol Liq. 2021;328:115417. [Google Scholar]
- 24.Mishra S, Manna K, Kayal U, Saha M, Chatterjee S, Chandra D, et al. Folic acid-conjugated magnetic mesoporous silica nanoparticles loaded with quercetin: a theranostic approach for cancer management. RSC Adv. 2020;10(39):23148–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frigerio B, Bizzoni C, Jansen G, Leamon CP, Peters GJ, Low PS, et al. Folate receptors and transporters: biological role and diagnostic/therapeutic targets in cancer and other diseases. J Exp Clin Cancer Res. 2019. 10.1186/s13046-019-1123-1. [DOI] [PMC free article] [PubMed]
- 26.Khosravian P, Shafiee Ardestani M, Khoobi M, Ostad SN, Dorkoosh FA, Akbari Javar H, et al. Mesoporous silica nanoparticles functionalized with folic acid/methionine for active targeted delivery of docetaxel. Onco Targets Ther. 2016;9:7315–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hong L, Li W, Li Y, Yin S. Nanoparticle-based drug delivery systems targeting cancer cell surfaces. RSC Adv. 2023;13(31):21365–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iranshahy M, Hanafi-Bojd MY, Aghili SH, Iranshahi M, Nabavi SM, Saberi S, et al. Curcumin-loaded mesoporous silica nanoparticles for drug delivery: synthesis, biological assays and therapeutic potential – a review. RSC Adv. 2023;13(32):22250–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nosrati S, Javid H, Amiri H, Jafari N, Hashemy SI. Investigating the anticancer effects of chitosan-NLC-folate nanohybrid loaded with auraptene on A2780 ovarian cancer cells. Naunyn Schmiedebergs Arch Pharmacol. 2025;398(2):1895–903. [DOI] [PubMed] [Google Scholar]
- 30.Chiang C-T, Way T-D, Lin J-K. Sensitizing HER2-overexpressing cancer cells to luteolin-induced apoptosis through suppressing p21WAF1/CIP1 expression with rapamycin. Mol Cancer Ther. 2007;6(7):2127–38. [DOI] [PubMed] [Google Scholar]
- 31.Saxena P, Selvaraj K, Khare SK, Chaudhary N. Superoxide dismutase as multipotent therapeutic antioxidant enzyme: role in human diseases. Biotechnol Lett. 2022;44(1):1–22. [DOI] [PubMed] [Google Scholar]
- 32.Singh D, Shukla G. The multifaceted anticancer potential of luteolin: involvement of NF-κB, AMPK/mTOR, PI3K/Akt, MAPK, and Wnt/β-catenin pathways. Inflammopharmacology. 2025;33(2):505–25. [DOI] [PubMed] [Google Scholar]
- 33.Lim DY, Jeong Y, Tyner AL, Park JH. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G66-75. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y-P, Chen C-T, Hung Y, Chou C-M, Liu T-P, Liang M-R, et al. A new strategy for intracellular delivery of enzyme using mesoporous silica nanoparticles: superoxide dismutase. J Am Chem Soc. 2013;135(4):1516–23. [DOI] [PubMed] [Google Scholar]
- 35.Yang Z, Gao D, Cao Z, Zhang C, Cheng D, Liu J, et al. Drug and gene co-delivery systems for cancer treatment. Biomater Sci. 2015;3(7):1035–49. [DOI] [PubMed] [Google Scholar]
- 36.Xu X, Liu C, Wang Y, Koivisto O, Zhou J, Shu Y, et al. Nanotechnology-based delivery of CRISPR/Cas9 for cancer treatment. Adv Drug Deliv Rev. 2021;176:113891. [DOI] [PubMed] [Google Scholar]
- 37.Carvalho BG, Vit FF, Carvalho HF, Han SW, de la Torre LG. Recent advances in co-delivery nanosystems for synergistic action in cancer treatment. J Mater Chem B. 2021;9(5):1208–37. [DOI] [PubMed] [Google Scholar]
- 38.Tonbul H, Sahin A, Tavukcuoglu E, Ultav G, Akbas S, Aktas Y, et al. Folic acid decoration of mesoporous silica nanoparticles to increase cellular uptake and cytotoxic activity of doxorubicin in human breast cancer cells. J Drug Deliv Sci Technol. 2021;63:102535. [Google Scholar]
- 39.Singh Tuli H, Rath P, Chauhan A, Sak K, Aggarwal D, Choudhary R, et al. Luteolin, a potent anticancer compound: from chemistry to cellular interactions and synergetic perspectives. Cancers. 2022;14(21):5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dang H, Meng MHW, Zhao H, Iqbal J, Dai R, Deng Y, et al. Luteolin-loaded solid lipid nanoparticles synthesis, characterization, & improvement of bioavailability, pharmacokinetics in vitro and vivo studies. J Nanopart Res. 2014;16(4):2347. [Google Scholar]
- 41.Yang C, Gao S, Kjems J. Folic acid conjugated chitosan for targeted delivery of siRNA to activated macrophages in vitro and in vivo. J Mater Chem B. 2014;2(48):8608–15. [DOI] [PubMed] [Google Scholar]
- 42.Popov AM, Osipov AN, Korepanova EA, Krivoshapko ON, Artyukov AA, Klimovich AA. A study of the antioxidant and membranotropic activities of Luteolin using different model systems. Biophysics. 2016;61(6):843–50. [Google Scholar]
- 43.Rosenholm JM, Mamaeva V, Sahlgren C, Lindén M. Nanoparticles in targeted cancer therapy: mesoporous silica nanoparticles entering preclinical development stage. Nanomed (Lond). 2012;7(1):111–20. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Shang J, Tang X, Xu X. Tfr aptamer-functionalized MSNs for enhancing targeted cellular uptake and therapy of cancer cells. ACS Omega. 2023;8(51):48975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.AbouAitah K, Swiderska-Sroda A, Farghali AA, Wojnarowicz J, Stefanek A, Gierlotka S, et al. Folic acid-conjugated mesoporous silica particles as nanocarriers of natural prodrugs for cancer targeting and antioxidant action. Oncotarget. 2018;9(41):26466–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dutta Gupta Y, Mackeyev Y, Krishnan S, Bhandary S. Mesoporous silica nanotechnology: promising advances in augmenting cancer theranostics. Cancer Nanotechnol. 2024;15(1):9. [Google Scholar]
- 47.Islam S, Bakhtiar H, Alshoaibi A, Haider Z, Riaz S, Naseem S. Fast responsive thermally stable silica microspheres for sensing evaluation: sol–gel approach. J Sol-Gel Sci Technol. 2020;96(3):614–26. [Google Scholar]
- 48.Feng S, Zhang H, Xu S, Zhi C, Nakanishi H, Gao X-D. Folate-conjugated, mesoporous silica functionalized boron nitride nanospheres for targeted delivery of doxorubicin. Materials Science and Engineering: C. 2019;96:552–60. [DOI] [PubMed] [Google Scholar]
- 49.Mod Razif MRF, Chan SY, Chew Y-L, Hassan M, Ahmad Hisham S, Abdul Rahman S, et al. Recent developments in luteolin-loaded nanoformulations for enhanced anti-carcinogenic activities: insights from in vitro and in vivo studies. Sci. 2024;6(4):68. [Google Scholar]
- 50.Zhang H, Li X, Zhang Y, Luan X. Luteolin induces apoptosis by activating Fas signaling pathway at the receptor level in laryngeal squamous cell line Hep-2 cells. Eur Arch Otorhinolaryngol. 2014;271(6):1653–9. [DOI] [PubMed] [Google Scholar]
- 51.Chettupalli AK, Bukke SPN, Rahaman SA, Unnisa A, Adepu M, Kavitha M, et al. Ritonavir loaded solid lipid nanoparticles for oral drug delivery and bioavailability enhancement. Discover Appl Sci. 2025;7(1):58. [Google Scholar]
- 52.Li H, Wang Y, Tang Q, Yin D, Tang C, He E, et al. The protein corona and its effects on nanoparticle-based drug delivery systems. Acta Biomater. 2021;129:57–72. [DOI] [PubMed] [Google Scholar]
- 53.Prasher P, Sharma M, Singh SK, Gulati M, Chellappan DK, Zacconi F, et al. Luteolin: a flavonoid with a multifaceted anticancer potential. Cancer Cell Int. 2022;22(1):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ansari MJ, Abdullah A, Abdulaziz AI, Mohammed AF, Bader A, Saad A, et al. Formulation, characterization, in vitro and in vivo evaluations of self-nanoemulsifying drug delivery system of Luteolin. J Taibah Univ Sci. 2020;14(1):1386–401. [Google Scholar]
- 55.Wu J. The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J Pers Med. 2021;11(8):771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fernández M, Javaid F, Chudasama V. Advances in targeting the folate receptor in the treatment/imaging of cancers. Chem Sci. 2018;9(4):790–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez T, Muminovic M, Nano O, Vulfovich M. Folate receptor alpha—a novel approach to cancer therapy. Int J Mol Sci. 2024;25(2):1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Djayanti K, Maharjan P, Cho KH, Jeong S, Kim MS, Shin MC, et al. Mesoporous silica nanoparticles as a potential nanoplatform: therapeutic applications and considerations. Int J Mol Sci. 2023;24(7):6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gorobets MG, Toroptseva AV, Abdullina MI, Pokrovsky VS, Khachatryan DS, Bychkova AV. Folic acid conjugated with serum albumin for nano- and submicron delivery systems for applications in therapy and diagnostics. Explor Drug Sci. 2025;3:1008101. [Google Scholar]
- 60.Lai Z, Pang Y, Zhou Y, Chen L, Zheng K, Yuan S, et al. Luteolin as an adjuvant effectively enhanced the efficacy of adoptive tumor-specific CTLs therapy. BMC Cancer. 2025;25(1):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eryilmaz IE, Colakoglu Bergel C, Arioz B, Huriyet N, Cecener G, Egeli U. Luteolin induces oxidative stress and apoptosis via dysregulating the cytoprotective Nrf2-Keap1-Cul3 redox signaling in metastatic castration-resistant prostate cancer cells. Mol Biol Rep. 2024;52(1):65. [DOI] [PubMed] [Google Scholar]
- 62.Ibrahim MAI, Othman R, Chee CF, Ahmad Fisol F. Evaluation of Folate-functionalized nanoparticle drug delivery systems—effectiveness and concerns. Biomedicines. 2023;11(7):2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Almomen A, Alhowyan A. A comprehensive study on Folate-Targeted mesoporous silica nanoparticles loaded with 5-Fluorouracil for the enhanced treatment of gynecological cancers. J Funct Biomater. 2024;15(3):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55(6):481–504. [DOI] [PubMed] [Google Scholar]
- 65.Aziz N, Kim M-Y, Cho JY. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. 2018;225:342–58. [DOI] [PubMed] [Google Scholar]
- 66.Seelinger G, Merfort I, Wölfle U, Schempp CM. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008;13(10):2628–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Selvendiran K, Koga H, Ueno T, Yoshida T, Maeyama M, Torimura T, et al. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: an implication for the antitumor potential of flavonoids. Cancer Res. 2006;66(9):4826–34. [DOI] [PubMed] [Google Scholar]
- 68.Çetinkaya M, Baran Y. Therapeutic potential of luteolin on cancer. Vaccines. 2023;11(3):554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pandurangan A, Dharmalingam P, Sadagopan S, Ganapasam S. Luteolin inhibits matrix metalloproteinase 9 and 2 in azoxymethane-induced colon carcinogenesis. Hum Exp Toxicol. 2014;33(11):1176–85. [DOI] [PubMed] [Google Scholar]
- 70.Pandurangan AK, Dharmalingam P, Sadagopan SKA, Ramar M, Munusamy A. Luteolin induces growth arrest in colon cancer cells through involvement of Wnt/β-catenin/GSK-3β signaling. J Environ Pathol Toxicol Oncol. 2013. 10.1615/JEnvironPatholToxicolOncol.2013007522. [DOI] [PubMed] [Google Scholar]
- 71.Park S-H, Park HS, Lee JH, Chi GY, Kim G-Y, Moon S-K, et al. Induction of endoplasmic reticulum stress-mediated apoptosis and non-canonical autophagy by Luteolin in NCI-H460 lung carcinoma cells. Food Chem Toxicol. 2013;56:100–9. [DOI] [PubMed] [Google Scholar]
- 72.Zhang M, Wang R, Tian J, Song M, Zhao R, Liu K, et al. Targeting LIMK1 with luteolin inhibits the growth of lung cancer in vitro and in vivo. J Cell Mol Med. 2021;25(12):5560–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pratheeshkumar P, Son Y-O, Budhraja A, Wang X, Ding S, Wang L, et al. Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. PLoS ONE. 2012;7(12):e52279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu X, Li Y, Xiao X, Li X. Inhibitory effects of luteolin on human gastric carcinoma xenografts in nude mice and its mechanism. Zhonghua Yi Xue Za Zhi. 2013;93(2):142–6. [PubMed] [Google Scholar]
- 75.Lu J, Li G, He K, Jiang W, Xu C, Li Z, et al. Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. J Transl Med. 2015;13(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cheng W-Y, Chiao M-T, Liang Y-J, Yang Y-C, Shen C-C, Yang C-Y. Luteolin inhibits migration of human glioblastoma U-87 MG and T98G cells through downregulation of Cdc42 expression and PI3K/AKT activity. Mol Biol Rep. 2013;40:5315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hwang J-T, Park OJ, Lee YK, Sung MJ, Hur HJ, Kim MS, et al. Anti-tumor effect of Luteolin is accompanied by AMP-activated protein kinase and nuclear factor-κB modulation in HepG2 hepatocarcinoma cells. Int J Mol Med. 2011;28(1):25–31. [DOI] [PubMed] [Google Scholar]
- 78.Lee E-J, Oh S-Y, Sung M-K. Luteolin exerts anti-tumor activity through the suppression of epidermal growth factor receptor-mediated pathway in MDA-MB-231 ER-negative breast cancer cells. Food Chem Toxicol. 2012;50(11):4136–43. [DOI] [PubMed] [Google Scholar]
- 79.Chen T, Li L-P, Lu X-Y, Jiang H-D, Zeng S. Absorption and excretion of luteolin and apigenin in rats after oral administration of chrysanthemum morifolium extract. J Agric Food Chem. 2007;55(2):273–7. [DOI] [PubMed] [Google Scholar]
- 80.Kaci H, Bodnárová S, Fliszár-Nyúl E, Lemli B, Pelantová H, Valentová K, et al. Interaction of luteolin, naringenin, and their sulfate and glucuronide conjugates with human serum albumin, cytochrome P450 (CYP2C9, CYP2C19, and CYP3A4) enzymes and organic anion transporting polypeptide (OATP1B1 and OATP2B1) transporters. Biomed Pharmacother. 2023;157:114078. [DOI] [PubMed] [Google Scholar]
- 81.Bangar SP, Kajla P, Chaudhary V, Sharma N, Ozogul F. Luteolin. A flavone with myriads of bioactivities and food applications. Food Biosci. 2023;52:102366. [Google Scholar]
- 82.Zheng S, Cheng Y, Teng Y, Liu X, Yu T, Wang Y, et al. Application of luteolin nanomicelles anti-glioma effect with improvement in vitro and in vivo. Oncotarget. 2017;8(37):61146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sabzichi M, Hamishehkar H, Ramezani F, Sharifi S, Tabasinezhad M, Pirouzpanah M, et al. Luteolin-loaded phytosomes sensitize human breast carcinoma MDA-MB 231 cells to doxorubicin by suppressing Nrf2 mediated signalling. Asian Pac J Cancer Prev. 2014;15(13):5311–6. [DOI] [PubMed] [Google Scholar]
- 84.Altamimi MA, Hussain A, AlRajhi M, Alshehri S, Imam SS, Qamar W. Luteolin-loaded elastic liposomes for transdermal delivery to control breast cancer: in vitro and ex vivo evaluations. Pharmaceuticals. 2021;14(11):1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ding J, Li Q, He S, Xie J, Liang X, Wu T, et al. Luteolin-loading of Her-2-poly (lactic-co-glycolic acid) nanoparticles and proliferative inhibition of gastric cancer cells via targeted regulation of forkhead box protein O1. J Cancer Res Ther. 2020;16(2):263–8. [DOI] [PubMed] [Google Scholar]
- 86.Moreira AF, Dias DR, Correia IJ. Stimuli-responsive mesoporous silica nanoparticles for cancer therapy: a review. Microporous Mesoporous Mater. 2016;236:141–57. [Google Scholar]
- 87.Hussain A. Hydrophobic drug release studies from the core/shell magnetic mesoporous silica nanoparticles and their anticancer application: drug release studies from magnetic mesoporous nanoparticles and their anticancer application. Proc Pakistan Acad Sciences: B Life Environ Sci. 2021;58(1):77–88. [Google Scholar]
- 88.Bayir S, Barras A, Boukherroub R, Szunerits S, Raehm L, Richeter S, et al. Mesoporous silica nanoparticles in recent photodynamic therapy applications. Photochem Photobiol Sci. 2018;17:1651–74. [DOI] [PubMed] [Google Scholar]
- 89.Ho Y-J, Wu C-H, Jin Q-f, Lin C-Y, Chiang P-H, Wu N, et al. Superhydrophobic drug-loaded mesoporous silica nanoparticles capped with β-cyclodextrin for ultrasound image-guided combined antivascular and chemo-sonodynamic therapy. Biomaterials. 2020;232:119723. [DOI] [PubMed] [Google Scholar]
- 90.Mody KT, Popat A, Mahony D, Cavallaro AS, Yu C, Mitter N. Mesoporous silica nanoparticles as antigen carriers and adjuvants for vaccine delivery. Nanoscale. 2013;5(12):5167–79. [DOI] [PubMed] [Google Scholar]
- 91.Heidari R, Assadollahi V, Khosravian P, Mirzaei SA, Elahian F. Engineered mesoporous silica nanoparticles, new insight nanoplatforms into effective cancer gene therapy. Int J Biol Macromol. 2023. 10.1016/j.ijbiomac.2023.127060. [DOI] [PubMed] [Google Scholar]
- 92.Mills JA, Liu F, Jarrett TR, Fletcher NL, Thurecht KJ. Nanoparticle based medicines: approaches for evading and manipulating the mononuclear phagocyte system and potential for clinical translation. Biomaterials Sci. 2022;10(12):3029–53. [DOI] [PubMed] [Google Scholar]
- 93.Hussain Z, Khan S, Imran M, Sohail M, Shah SWA, de Matas M. PEGylation: a promising strategy to overcome challenges to cancer-targeted nanomedicines: a review of challenges to clinical transition and promising resolution. Drug Deliv Transl Res. 2019;9(3):721–34. [DOI] [PubMed] [Google Scholar]
- 94.Shi L, Zhang J, Zhao M, Tang S, Cheng X, Zhang W, et al. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale. 2021;13(24):10748–64. [DOI] [PubMed] [Google Scholar]
- 95.Kučuk N, Primožič M, Knez Ž, Leitgeb M. Sustainable biodegradable biopolymer-based nanoparticles for healthcare applications. Int J Mol Sci. 2023;24(4):3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu SH, Mou CY, Lin HP. Synthesis of mesoporous silica nanoparticles. Chem Soc Rev. 2013;42(9):3862–75. [DOI] [PubMed] [Google Scholar]
- 97.Singh B, Na J, Konarova M, Wakihara T, Yamauchi Y, Salomon C, et al. Functional mesoporous silica nanomaterials for catalysis and environmental applications. Bull Chem Soc Jpn. 2020;93(12):1459–96. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.