Abstract
Hepatocellular carcinoma (HCC) is the most prevalent type of primary liver cancer. Many medications that had been used for a long period to treat the illness were eventually stopped due to negative effects or the development of drug resistance in HCC patients. Canagliflozin (CANA), a sodium-glucose cotransporter 2 (SGLT2) inhibitor, showed in vitro anti-carcinogenic efficacy against various cancer models’ other livers. The current study used rat models to examine canagliflozin’s therapeutic role against experimentally induced HCC. A total of 32 rats were divided into four groups, eight in each: negative control; HCC control: rats were fed a choline-deficient diet (CDD) and subjected to diethyl nitrosamine and thioacetamide (DEN/TAA) injections for 15 weeks; and treated groups: rats were given CANA (10 and 20 mg/kg b.wt.) orally from the 7th week of the experiment till the end. All the measured markers of HCC, liver function, and inflammatory markers were elevated in the HCC control group compared to the negative control (CTRL). Regarding immunohistochemistry, the HCC group showed downregulation in caspase-3 expression and upregulation in PCNA expression. On the other hand, canagliflozin-treated groups showed dose-dependent improvement in the measured parameters associated with HCC. Moreover, canagliflozin therapy ameliorates the histopathological alterations in HCC-induced rats. Taken together, CANA exhibits anti-HCC effects by activating AMP-activated protein kinase (AMPK) and suppressing the HIF-1α/YAP/TAZ pathway, making it a forthcoming therapeutic option for HCC.
Keywords: Hepatocellular carcinoma, Canagliflozin, Diethyl nitrosamine, Thioacetamide
Introduction
Globally, hepatocellular carcinoma (HCC) is one of the main causes of cancer-related mortality (Elleithi et al. 2024). The World Health Organization (WHO) predicts that more than 1 million people would die from HCC worldwide each year by 2030. Approximately 800,000 new cases of primary liver cancer are discovered annually worldwide (Hassan et al. 2024). With 80–90% of all cases, HCC is the most prevalent form of primary liver cancer (Kim 2024). Hepatitis B and hepatitis C virus infections, alcohol consumption, metabolic liver illnesses including diabetes and non-alcoholic fatty liver disease (NAFLD), environmental contaminants, nitrosamine use, and obesity are all strongly associated with chronic liver inflammations, cirrhosis, and HCC development (Afifi et al. 2014; Abd El-Rahman and Fayed 2019). For patients with liver cancer, the prognosis is poor because of the late stage at diagnosis, resistance to current treatment, and high risk of recurrence. Five years following surgery, the recurrence rate is over 50%, and the overall survival rate for individuals with liver cancer is 18% (Sas et al. 2022). Currently, available chemotherapeutic medications are ineffective at controlling the growth of tumors, and drug resistance is widespread. To increase the overall survival rate of patients with HCC, new chemotherapeutic drugs are therefore desperately needed to block HCC at an early stage (Jo et al. 2016).
The precise processes that result in the development of liver oncogenesis are still unknown (Zhang and Zhou 2019). Here, we suggest a signaling strategy where the upstream regulator of Hippo signaling is the cellular energy level. The Hippo pathway has become a crucial tumor suppressor mechanism that influences the growth, regeneration, and carcinogenesis of liver tissue. Briefly, Hippo signaling’s main function is to inhibit its downstream transcriptional coactivators, transcription regulator 1 (TAZ), and Yes-associated protein (YAP), which control cell division and proliferation (Johnson and Halder 2014; Patel et al. 2017). YAP or TAZ, a transcriptional co-activator, has been abnormally activated in a number of human malignancies, including HCC (Zhang and Zhou 2019). Energy stress has been shown in earlier research to limit YAP transcriptional activity and YAP-dependent transformation while inducing YAP cytoplasmic retention. AMP-activated protein kinase (AMPK), the primary metabolic sensor, and the upstream Hippo pathway elements Lats1/Lats2 and angiomotin-like 1 (AMOTL1) are necessary for these effects (DeRan et al. 2014). Since cellular energy and metabolites are necessary for survival and growth, AMPK activation inhibits YAP. In fact, AMPK is a negative regulator of YAP activity. AMPK activation promotes YAP cytoplasm localization, increases YAP phosphorylation, and inhibits YAP target genes (Wang et al. 2015). Furthermore, AMPK and drug resistance are tightly connected, according to several studies (Qin et al. 2019; Zhou et al. 2019). Certain natural products contain bioactive chemicals and tiny molecular inhibitors that activate AMPK, enhancing multidrug resistance (Wang et al. 2016). AMPK activators and chemotherapeutics work well together to limit tumor growth and extend the remission of malignancies of the breast, pancreas, prostate, lung, and ovaries (Wu et al. 2014; Duan et al. 2017). For this reason, AMPK/YAP/TAZ has become a valuable target for cancer treatments.
Cancer models have been shown to overexpress sodium-glucose cotransporter 2 (SGLT2), a critical glucose transporter, which is accompanied by increased glucose uptake in both humans and mice. In vitro and in vivo tumor development can be effectively inhibited by blocking its expression (Basak et al. 2023). Novel SGLT2 inhibitor canagliflozin (CANA) has been shown to decrease patients’ glycemic levels by lowering renal glucose reabsorption (Chao 2014). Notably, in addition to controlling blood sugar levels, pre-clinical research revealed that CANA slowed the progression of lung, pancreatic, and prostate cancer (Villani et al. 2016). By inhibiting the progression of non-alcoholic steatohepatitis (NASH) and inducing apoptosis in HepG2 cells through caspase-3 activation, canagliflozin demonstrated anti-steatotic and anti-inflammatory properties (Jojima et al. 2019). A previous study showed that CANA re-sensitized HCC to cisplatin by triggering ferroptosis through dual effects on glycolysis and glutamine metabolism using cell culture and an in vivo mouse tumor model (Zeng et al. 2023). The effectiveness of CANA against HCC was also investigated by Luo et al. using cell culture and in vivo mouse tumor model. By preventing the accumulation of HIF-1α protein, most likely by targeting the AKT/mTOR pathway, CANA reduced metastasis, angiogenesis, and metabolic reprogramming in HCC (Luo et al. 2021). Simultaneously, Hung et al.’s study, utilizing cell culture and an in vivo mouse tumor model, demonstrated that CANA blocked β-catenin, which could offer new ideas for treating HCC patients who also have diabetes (Hung et al. 2019). Despite the fact that the previously mentioned studies showed that CANA is effective against HCC, no research has examined its effectiveness against HCC caused by CDD/DEN/TAA, which is similar to the course of HCC in humans that results from damage, fibrosis, cirrhosis, and cancer.
Therefore, we conducted this research to assess the potential therapeutic benefit of canagliflozin on CDD/DEN/TAA-induced HCC in rats and explore the role of AMPK/YAP/TAZ pathways that are known to mediate the advancement of liver cancer.
Materials and methods
Experimental animals
One-month-old weaned Wistar rats (50 and 80 g) selected for this investigation were procured from the animal house colony of the National Research Centre (NRC), Giza, Egypt. Rats were kept in cages with enough illumination, a humidity level of 60%, adequate ventilation, and a temperature of 25 ± 0.5 °C. Animals of all groups except the negative control were maintained in clean cages and given a choline-deficient diet (CDD) consisting of 60% corn, 21% soy protein (46%), 15% margarine, and 4% sucrose mix for 4 weeks. This was followed by a standard pellet diet until the end of the experiment, with water provided ad libitum. Experiments using animals were conducted according to the guidelines of the Medical Research Ethics Committee (MREC) at NRC, approval number 13060173.
Drugs and chemicals
Sigma-Aldrich in Germany provided the diethyl nitrosamine (DEN) and thioacetamide (TAA). Tablets of canagliflozin (Invokana; 300 mg) were acquired from “Nile Pharmaceutical Company in Egypt.”
Hepatocarcinogenesis model
Figure 1 summarizes the experimental time line. One-month-old weaned male rats weighing between 50 and 80 g were utilized in the experiment. Rats of the negative control group were kept on standard rat chow all over the experimental period. However, rats of the other groups were accessible to a choline-deficient diet for a duration of 4 weeks, followed by a standard diet until the end of the experiment (Newberne et al. 1982). Following a 4-week dietary regimen, rats received four intraperitoneal (i.p.) diethyl nitrosamine (DEN) 50 mg/kg injections over 8 weeks; each injection was 15 days apart (four strategic intervals) (Kurma et al. 2021). Subsequently, the animals received intraperitoneal injections of thioacetamide (TAA) at a dosage of 100 mg/kg, administered twice weekly for 15 weeks (Ayoub et al. 2022). Treatment of rats was continued for 6 successive weeks. A gross examination of the liver was done at the end of the experimentation.
Fig. 1.
An illustration of the experimental design
Design of experiment
Thirty-two rats were randomly allocated into four groups (8 rats each):
Group I: The negative control rats (CTRL) received saline i.p. all over the experimental period.
Group II: served as (HCC group).
Group III and IV: the rats were orally administered canagliflozin at doses of (10 and 20 mg/kg/day, orally) (Mabrouk Gabr and Hassan 2020) for 6 weeks after induction of hepatocarcinogenesis.
Sacrification and biological sample collection
Blood samples were taken from the tail vein of rat under light phenobarbital anesthesia. Centrifugation was utilized to separate the sera, which were then used for the biochemical analysis. The liver was extracted, saline-washed, blot-dried, and divided into three pieces. One slice was then submerged in 10% buffered formalin for immunohistochemical and histological examination. In 10% w/v phosphate-buffered saline, another slice was homogenized, and the supernatant was used for the ELISA test and the assessment of oxidative stress biomarkers. The remainder was frozen for PCR analysis at − 80 °C.
Liver injury indicators
By utilizing enzymatic colorimetric techniques, the liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined using Biodiagnostic kits, Egypt, Catalog No AL 10 31 (45) and AS 10 61 (45), respectively. Gamma-glutamyl transferase (GGT) level “(Cat# EZ009LQ)” was determined colorimetrically using kits manufactured by Biodiagnostic kits, Giza, Egypt.
Analyzing the liver’s oxidative stress and antioxidant levels
Following the manufacturer’s instructions, kits from “Bio-diagnostic, Egypt” were used to evaluate the liver levels of “reduced glutathione (GSH), superoxide dismutase (SOD), and malondialdehyde (MDA)” (Cat# GR2511, SD2521, MD2529).
Inflammatory indicators
STAT3, liver signal transducer, and activator of transcription 3 (Cat# SL1672Ra) and p-STAT-3 (Cat# SLD1757Ra) levels were determined with the ELISA technique using Sunlong Biotech Co., Ltd., China kits.
HCC markers
Serum alpha-fetoprotein (AFP) (Cat# SL0042Ra), alpha-l-fucosidase (AFU) (Cat# SL0044Ra), carcinoembryonic antigen (CEA) (Cat# SL1244Ra), and transforming growth factor β1 (TGF-β1) (Cat# SL0705Ra) were determined by the Elisa technique using Sunlong Biotech Co., Ltd., China kits.
Measurement of SIRT1, Nrf2, AMPK, and p-AMPK levels
Following the manufacturer’s instructions and using Sunlong Biotech Co., Ltd., China kits, the levels of “Nrf2 (NF-E2-related factor 2) (Cat# SL0985Ra), AMPK (AMP-activated protein kinase) (Cat# SL1695Ra), SIRT1 (Sirtuin 1) (Cat# SL1254Ra), and p-AMPK (Cat# SL0570Ra)” in liver tissues were assessed using the ELISA technique.
Measurement of YAP-1, TAZ, and HIF-1α levels
YAP-1 (Cat# SL1731Ra), TAZ (China; Cat# SLD1758Ra), and HIF-1α (China; Cat# SL0357Ra) were assayed using Sunlong Biotech Co., Ltd., China kits.
Quantitative real-time PCR to measure “AMPK and HIF-1α” gene expression
After 30 mg of tissue sample was added to 600 µl of RLT buffer with 10 µl β mercaptoethanol per 1 ml, the “QIAamp RNeasy Mini kit (Qiagen, Germany, GmbH)” was used to extract RNA from the tissue samples. Adapter sets that are connected to the “Qiagen tissue Lyser clamps” were used to insert the tubes and homogenize the samples. A high-speed (30 Hz) shaking step was used for 2 min in order to cause disruption. To purify total RNA from animal tissues, 70% ethanol was added to the cleaned lysate using the “QIAamp RNeasy Mini kit (Qiagen, Germany, GmbH)” method. Note: DNase digestion was performed on the column to eliminate any leftover DNA. “Metabion (Germany)” supplied the oligonucleotide primers, which are listed in Table 1. The 25 µl reaction contained 12.5 µl of the 2 × QuantiTect SYBR Green PCR Master Mix (Qiagen, Germany, GmbH), 0.25 µl of RevertAid Reverse Transcriptase (200 U/µL) (Thermo Fisher), 0.5 µl of each primer at a concentration of 20 pmol, 8.25 µl of water, and 3 µl of RNA template. To perform the reaction, a “Stratagene MX3005P real-time PCR device” was used. Utilizing the “StratageneMX3005P program,” the amplification curves and ct values were acquired. The variance in gene expression on the RNA of the different samples was evaluated by comparing each sample’s CT with that of the positive control group using the following ratio: 2-ΔΔct, in line with Yuan et al. (Yuan et al. 2006) “ΔΔCt” technique. Nevertheless, “ΔCt reference − ΔCt target” equals ΔΔCt.
Table 1.
A list of primers for qPCR
Histopathological analysis
Livers were routinely processed after being taken from the various experimental groups. Hematoxylin and Eosin (H&E) (Bancroft and Gamble 2008) were used to stain the 5-micron-thick paraffin-embedded blocks so they could be examined histopathologically using “a light microscope (Olympus BX50, Japan).”
Classification of histopathological alterations
The following criteria were used to grade the histological changes in the livers: (0) indicated no changes, and (+ +), (+ + +), and (+ + + +) indicated mild, moderate, and severe changes appropriately (Arsad et al. 2014).
Immunohistochemical analysis
After cutting liver tissue sections onto sticky slides, deparaffinizing them, and rehydrating them with distilled water, a heat-induced epitope retrieval step was carried out. The tissue sections were then incubated for an hour at room temperature with “primary anti-caspase-3 (at a dilution of 1:200, sc-7272; Santa Cruz Biotechnology, Inc., Heidelberg, Germany) and anti-PCNA (at a dilution of 1:1000, 10,205–2-AP; Proteintech, Germany).” In accordance with the manufacturer’s instructions, the color was developed after washing using an HRP-labeled detection kit (BioSB, USA). Myer hematoxylin was used as a counterstain. By avoiding incubation with primary antibodies, negative control slides were acquired. The mean area % for each group was used to quantify positive expressiveness.
Statistical approach
Before the statistical analysis, data values were checked for normality using the Shapiro test, and heteroscedasticity was checked using the Brown-Forsythe test. The data were presented as means ± SEM. Data were processed by one-way ANOVA followed by the Tukey as a post-hoc test. In contrast, non-parametric values were presented as median ± interquartile range and analyzed by the Kruskal–Wallis test followed by Dunn’s test; additionally, the exact p-value between each compared pairwise group is stated above the pairwise bar. GraphPad Prism software (version 10, San Diego, CA, USA) was utilized to establish the represented graphs. The significance level was set to be p ≤ 0.05 for all statistical tests.
Results
Liver enzyme activity: ALT, AST, and GGT levels
In the HCC group, ALT, AST, and GGT levels were significantly elevated compared to the control group, indicating severe liver injury. ALT levels in the HCC group increased to 479.7% of the control group (p ≤ 0.05). Treatment with canagliflozin (Cana) at 10 mg/kg and 20 mg/kg reduced ALT levels to 57.5% and 46.7% of the HCC group, respectively, with the high dose showing a more significant reduction (p ≤ 0.05). AST levels in the HCC group rose to 485.6% of the control group (p ≤ 0.05), while treatment with Cana at 10 mg/kg and 20 mg/kg reduced AST levels to 69.1% and 50.8% of the HCC group, respectively (p ≤ 0.05). Similarly, GGT levels in the HCC group increased to 678.3% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced GGT levels to 75.2% and 55.8% of the HCC group, respectively (p ≤ 0.05). Despite significant improvements, none of the treated groups fully normalized to control levels (Fig. 2).
Fig. 2.
Assessment of canagliflozin effect on liver function: “ALT, AST, and GGT” in HCC rats. A “Serum ALT” (U/L). B “Serum AST” (U/L). C “Serum GGT” (U/L). Data are presented as mean ± SEM. The exact p-value between each compared pairwise group is stated above the pairwise bar. HCC, hepatocellular carcinoma; Cana, canagliflozin
Oxidative stress and antioxidant markers: MDA, SOD, and GSH levels
Liver MDA levels, a marker of oxidative stress, were significantly elevated in the HCC group, reaching 890.4% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced MDA levels to 48.2% and 13.9% of the HCC group, respectively, with the high dose showing near normalization to control levels (p ≤ 0.05). SOD activity, an antioxidant enzyme, was significantly reduced in the HCC group to 34.9% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg increased SOD activity to 176.3% and 258.5% of the HCC group, respectively, with the high dose restoring levels close to normal (p ≤ 0.05). Similarly, GSH levels in the HCC group were reduced to 42.2% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg increased GSH levels to 125.7% and 200.9% of the HCC group, respectively, with the high dose showing significant improvement (p ≤ 0.05) (Fig. 3).
Fig. 3.
Assessment of canagliflozin effect on oxidative stress markers: “MDA, GSH, and SOD” in HCC rats. A “Liver MDA” (nmol/g protein). B “Liver GSH” (µmol/g protein). C “Liver SOD” (U/mg protein) activity. Data are presented as mean ± SE. The exact p-value between each compared pairwise group is stated above the pairwise bar. HCC, hepatocellular carcinoma; Cana, canagliflozin
Tumor markers: AFP, AFU, and CEA levels
Serum AFP, AFU, and CEA levels were significantly elevated in the HCC group, reflecting tumor progression. AFP levels in the HCC group increased to 1051.8% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced AFP levels to 52.7% and 21.2% of the HCC group, respectively, with the high dose showing a more significant reduction (p ≤ 0.05). AFU levels in the HCC group rose to 983.1% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced AFU levels to 31.3% and 24.3% of the HCC group, respectively (p ≤ 0.05). Similarly, CEA levels in the HCC group increased to 388.3% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced CEA levels to 40.8% and 31.6% of the HCC group, respectively (p ≤ 0.05). None of the treated groups fully normalized to control levels (Fig. 4).
Fig. 4.
Assessment of canagliflozin effect on HCC markers: AFP, AFU, and CEA in HCC rats. A AFP (pg/ml). B AFU (U/L). C CEA (Pg/ml). Data are presented as mean ± SEM. The exact p-value between each compared pairwise group is stated above the pairwise bar. HCC, hepatocellular carcinoma; Cana, canagliflozin
Hypoxia and fibrosis markers: YAP1, TAZ, and TGF-β1
YAP1 levels in the HCC group increased to 423.1% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced YAP1 levels to 51.9% and 25.6% of the HCC group, respectively (p ≤ 0.05). TAZ levels in the HCC group increased to 303.2% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced TAZ levels to 64.3% and 36.8% of the HCC group, respectively (p ≤ 0.05). TGF-β1 levels in the HCC group increased to 985.5% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced TGF-β1 levels to 53.9% and 30.9% of the HCC group, respectively (p ≤ 0.05) (Fig. 5).
Fig. 5.
Assessment of canagliflozin effect on YAP1, TAZ, and TGF-β1 levels in HCC rats. A YAP-1 (Pg/mg protein). B TAZ (Pg/mg protein). C TGF-β1 (ng/ml). Data are presented as mean ± SEM. The exact p-value between each compared pairwise group is stated above the pairwise bar. HCC, hepatocellular carcinoma; Cana, canagliflozin
Energy metabolism markers: AMPK, P-AMPK, AMPK mRNA relative expression, and P-AMPK/AMPK ratio
Liver AMPK levels in the HCC group were significantly reduced to 17.7% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg increased AMPK levels to 355.5% and 480.0% of the HCC group, respectively (p ≤ 0.05). P-AMPK levels in the HCC group were reduced to 41.2% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg increased P-AMPK levels to 148.6% and 201.7% of the HCC group, respectively (p ≤ 0.05). The P-AMPK/AMPK ratio in the HCC group was 2.7, compared to 1.36 in the control group. Treatment with Cana at 10 mg/kg and 20 mg/kg restored the ratio to 1.33 and 1.36, respectively, indicating normalization of energy metabolism (p ≤ 0.05) (Fig. 6).
Fig. 6.
Assessment of canagliflozin effect on AMPK and p-AMPK levels in HCC rats. A AMPK (ng/mg protein). B p-AMPK (Pg/mg protein) and C AMPK mRNA relative expression. Data are presented as mean ± SEM. The exact p-value between each compared pairwise group is stated above the pairwise bar. HCC, hepatocellular carcinoma; Cana, canagliflozin
In the HCC group, AMPK mRNA relative expression and liver AMPK protein levels were significantly reduced compared to the control group, while HIF-1α mRNA relative expression and liver HIF-1α protein levels were markedly elevated, indicating disrupted energy metabolism and increased hypoxia signaling. AMPK mRNA expression in the HCC group decreased to 25.2% of the control group (p ≤ 0.05). Treatment with canagliflozin (Cana) at 10 mg/kg and 20 mg/kg increased AMPK mRNA expression to 51.9% and 67.0% of the control group, respectively, with the high dose showing a more significant improvement (p ≤ 0.05) (Fig. 6).
Antioxidant and stress response markers: SIRT1 and Nrf2
Liver SIRT1 levels in the HCC group increased to 312.5% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced SIRT1 levels to 60.9% and 41.1% of the HCC group, respectively (p ≤ 0.05). Nrf2 levels in the HCC group increased to 271.2% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced Nrf2 levels to 66.6% and 51.6% of the HCC group, respectively (p ≤ 0.05). Despite significant improvements, neither marker fully normalized to control levels (Fig. 7).
Fig. 7.
Assessment of canagliflozin effect on SIRT1 and Nrf2 levels in HCC rats. A SIRT1 (ng/mg protein). B Nrf2 (Pg/mg protein). Data are presented as mean ± SEM. The exact p-value between each compared pairwise group is stated above the pairwise bar. HCC, hepatocellular carcinoma; Cana, canagliflozin
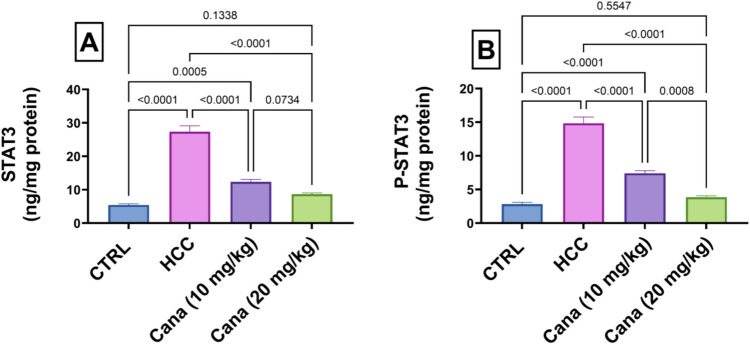
Inflammatory markers: STAT3, P-STAT3, and P-STAT3/STAT3 ratio
Liver STAT3 levels in the HCC group increased to 506.1% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced STAT3 levels to 45.2% and 31.6% of the HCC group, respectively (p ≤ 0.05). P-STAT3 levels in the HCC group increased to 526.4% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced P-STAT3 levels to 49.9% and 25.8% of the HCC group, respectively (p ≤ 0.05). The P-STAT3/STAT3 ratio in the HCC group was 1.02, compared to 0.52 in the control group. Treatment with Cana at 10 mg/kg and 20 mg/kg restored the ratio to 0.55 and 0.54, respectively, indicating normalization of inflammatory signaling (p ≤ 0.05) (Fig. 8).
Fig. 8.
Assessment of canagliflozin effect on liver STAT3 and p-STAT3 in HCC rats. A STAT3 (ng/mg protein). B p-STAT3 (ng/mg protein). Data are presented as mean ± SEM. The exact p-value between each compared pairwise group is stated above the pairwise bar. HCC, hepatocellular carcinoma; Cana, canagliflozin
Effects of canagliflozin on HIF-1α mRNA expression and HIF-1α protein in HCC
Liver HIF-1α protein levels in the HCC group increased to 549.2% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced HIF-1α levels to 71.7% and 21.1% of the HCC group, respectively (p ≤ 0.05). Similarly, HIF-1α mRNA expression in the HCC group increased to 831.7% of the control group (p ≤ 0.05). Treatment with Cana at 10 mg/kg and 20 mg/kg reduced HIF-1α mRNA expression to 76.8% and 43.2% of the HCC group, respectively (p ≤ 0.05) (Fig. 9).
Fig. 9.
Assessment of canagliflozin effect on liver HIF-1α and HIF-1α gene expressions in HCC rats. A HIF-1α. B HIF-1α gene expressions. Data are presented as mean ± SEM. The exact p-value between each compared pairwise group is stated above the pairwise bar. HCC, hepatocellular carcinoma; Cana, canagliflozin
Gross liver findings
During necropsy, each rat group’s liver was examined for any obvious abnormalities. It was revealed that the liver of the control group showed a normal appearance. Contrarily, the liver of the HCC-induced control group was pale, exhibiting irregularities and nodules, with the presence of lumps inside the liver tissue. The liver of animals treated with CANA revealed rough fibrotic surface with more improvement especially in the high-dose group (Fig. 10).
Fig. 10.
Macroscopic appearance of the liver of rats
Results of histopathology
The normal group’s liver displayed typical hepatic parenchyma, including normal portal regions, blood sinusoids, hepatocytes, and central veins (Fig. 11a), while the liver from the HCC group revealed HCC lesions in the form of acinus-like structure with minimal atypia among all hepatic lobules, together with severe interlobular reaction in the form of congestion, fibrosis, and leucocytic cell infiltrations (Fig. 11b). Livers from the CANA (10 mg/kg) group showed the absence of HCC lesions with mild interlobular reaction (Fig. 11c), while the liver from the CANA (20 mg/kg) group revealed the absence of HCC lesions with no interlobular reaction (Fig. 11d). Histopathological grading of lesions in liver sections is listed in Table 2.
Fig. 11.

a–d Hepatocellular photomicrographs from several experimental groups stained with H&E X200 reveal: “a Normal group (0), b HCC group with HCC lesions in the form of acinus-like structure (arrow) (+ + +) among all hepatic lobules, c CANA (10 mg/kg) group with bridging fibrosis and formation of cirrhotic nodules (+), and d CANA (20 mg/kg) group with normal hepatic lobules with no interlobular reaction (0).” E Box and whiskers plot represents the median ± interquartile range and analyzed by the Kruskal–Wallis test followed by Dunn’s test. The exact p-value between each compared pairwise group is stated above the pairwise bar. HCC, hepatocellular carcinoma; Cana, canagliflozin
Table 2.
Grading of histopathological lesions in the liver
| Groups | Lesion grades | |||
|---|---|---|---|---|
| 0 (negative) | + (mild) | + + (moderate) | + + + + (severe) | |
| CTRL | √ | |||
| HCC | √ | |||
| CANA (10 mg/kg) | √ | |||
| CANA (20 mg/kg) | √ | |||
Histopathological lesion scoring
Immunohistochemical staining
By immunohistochemistry, staining of the CANA (10 mg/kg) group, and the HCC group tissues using PCNA-specific monoclonal antibody, the expression of PCNA was found. The nucleus’s PCNA-positive cell staining was brown-yellow and appeared finely granular. PCNA expression was strong in the control positive group and mild in CANA (10 mg/kg) (Fig. 12a–d). The brown granules that were caspase-3-positive cells were primarily seen in the portal region surrounding the hepatocytes’ cytoplasm; however, there were also some nuclear expressions. Caspase-3 expression was mild in the HCC group and strong in the CANA (10 mg/kg) group (Fig. 13a–d).
Fig. 12.

a–d Expression of proliferating cell nuclear antigen (PCNA) in liver tissues from different experimental groups showing the nucleus’s PCNA-positive cell staining was brown-yellow and appeared finely granular. a Normal group (0), b HCC group (+ + +), c CANA (10 mg/kg) group (+), and d CANA (20 mg/kg) group (0). E Box and whiskers plot represents the median ± interquartile range and analyzed by the Kruskal–Wallis test followed by Dunn’s test. The exact p-value between each compared pairwise group is stated above the pairwise bar. HCC, hepatocellular carcinoma; Cana, canagliflozin
Fig. 13.

Expression of caspase-3 in liver tissues from different experimental groups showing: the brown granules that were Caspase-3 positive cells were primarily expressed in the portal region surrounding the hepatocytes’ cytoplasm, with minimal expression in the nucleus. a. Normalgroup (0), b. HCC group (0), c. CANA (10 mg/kg) group (+), d. CANA (20 mg/kg) group (++). E. Box and Whiskers plot represents median ± interquartile range and analyzed by Kruskal–Wallis test followed by Dunn’s test. The exact p-value between each compared pairwise group is stated above the pairwise bar. HCC: Hepatocellular carcinoma; Cana: Canagliflozin
Discussion
Numerous studies have looked into developing HCC animal models to lower “the time-to-death ratio” and restrict the carcinogen doses administered. Thus, we employed a two-stage paradigm in the current investigation that involved a single dosage of a genotoxic diethylnitrosamine (DEN) for initiation and the non-genotoxic compound thioacetamide (TAA) as a cancer promoter, following a choline-deficient diet (CDD) (Kimura et al. 2013). The gene expression patterns of chemically derived HCC models are similar to the poor prognosis linked to human HCC (Singh et al. 2018; Rahman et al. 2019). The CDD was employed in our study’s hepatocarcinogenesis model. Deficit of choline causes lipid peroxidation, mitochondrial malfunction, overexpression of cytochrome P450 2E1 (CYP2E1), and increased generation of reactive oxygen species (ROS) (Lima et al. 2008). When cytochrome P450 bioactivates DEN, mutagenic DNA adducts are formed. Additionally, reactive oxygen species (ROS) are formed, launching oxidation stress, DNA damage, and, ultimately, the promotion of hepatocarcinogenesis (Valko et al. 2006). Additionally, cytochrome P450 enzymes bioactivate TAA to become TA sulfoxide (TAASO) and then TAASO2, which are hazardous substances that increase ROS formation, lipid peroxidation, cytotoxicity, and mitochondrial damage. Eventually, this results in apoptosis, centrilobular necrosis, and the development of HCC (Rekha et al. 2008; Ramadan et al. 2018). Consequently, the current study’s goal is to determine whether CANA may treat CDD/DEN/TAA-induced HCC in a rat animal model.
Current investigation revealed a substantial increase in the serum GGT, AST, and ALT levels in the HCC group, which highlighted the extent of hepatocyte necrosis (Abdel-Rahman et al. 2022; Nyblom et al. 2004). This increase could be the result of hepatic necrosis and the consequent release of these enzymes from cancerous cells or irreparably damaged hepatic cells into circulation (Abdo et al. 2015). Treatment with CANA, especially at a dose of 20 mg/kg, normalized the aberrant serum levels. Canagliflozin normalized liver enzyme activity which was corroborated by Wang’s study (Wang et al. 2021).
Furthermore, the transformation of liver injury to HCC in our investigation was demonstrated by increased tumor markers (AFP, AFU, CEA, TGF-β1, YAP, TAZ, and HIF-1α) and a histological analysis of the liver that showed an increase in the size and quantity of tumor nodules in the HCC group. Alpha-fetoprotein (AFP) is frequently used for HCC diagnosis, screening, and treatment monitoring (Sauzay et al. 2016). Hepatocytes showed evidence of AFP gene reactivation in the early stages of hepatocarcinogenesis; cytoplasmic AFP promotes the growth of cancerous liver cells (Lu et al. 2016). According to the current study, the HCC group showed an increase in serum AFP, which aligns with those of Zhang et al. (2016) who showed elevated levels of AFP in HCC rats. Furthermore, the present study showed that the HCC group had marked elevated levels of alpha-l-fucosidase (AFU), which is consistent with previous findings (Abdallah and Khattab 2004). AFU is a significant HCC biomarker since it is raised in HCC patients (Mohamed et al. 2019). Additionally, Moriwaki et al. (2007) have revealed that there is an increase in AFU enzyme levels as a result of the fucosylation of sugar proteins during the course of hepatocarcinogenesis. Additionally, carcinoembryonic antigen (CEA) was assayed in our study, which is used for the clinical diagnosis of gastrointestinal cancer (Yang et al. 2018); besides, certain patients with a poor prognosis for HCC have a higher CEA level (Yoshikawa et al. 2017). CANA-treated groups demonstrated a significant decrease in AFP, AFU, and CEA levels. In line with our findings, a mouse model of diabetes and non-alcoholic steatohepatitis-related hepatocarcinogenesis, Jojima et al. (Jojima et al. 2019) showed that CANA prevents carcinogenesis. All of these results point to the effectiveness of canagliflozin treatment for anti-hepatocarcinogenesis, as it decreased the levels of tumor markers: AFP, AFU, and CEA.
Solid tumors, such as HCC, frequently experience oxygen deprivation, which stimulates hypoxia-inducible factor-1 alpha (HIF-1α) to increase angiogenesis and the creation of vascular endothelial growth factor (VEGF) (Carbajo-Pescador et al. 2013). Furthermore, the transforming growth factor β1 (TGF-β1) level is highly expressed in cancerous cells during hypoxia through HIF-1α, according to Deng (Deng et al. 2013). The HCC group in the present study showed an increase in HIF-1α and TGF-β1 levels. Tamim et al.’s study (Tamim et al. 2024) found that the HCC group caused by DEN had higher levels of TGF-β1 and HIF-1α, which is consistent with these findings. Additionally, the Hippo pathway’s major downstream terminal effectors are YAP and its homolog transcriptional co-activator TAZ (Lei et al. 2008). In malignancies, YAP/TAZ are moved to the nucleus and attach to TEAD (TEA domain) proteins, which leads to the expansion of organ size through cell proliferation, apoptosis prevention, and progenitor/stem cell amplification (Cai et al. 2010; Hong and Guan 2012). Overexpression of the YAP/TAZ genes has been found in liver cancer patients (Pan 2010). Interestingly, treatment with CANA led to a significant drop in hypoxia and fibrosis markers levels, which included AFP, AFU, and TGF-β1.
Furthermore, AMPK, “a cellular energy stress sensor” that is triggered by elevated AMP levels, has the ability to regulate cellular metabolism and work in tandem with available energy to coordinate cell development. Active AMPK is lower in the tumor compared to the surrounding tissue in patients with liver cancer (Lee et al. 2012). Inhibition of YAP by AMPK has been documented (Zhao et al. 2007). AMPK is essential for YAP suppression because it mediates YAP phosphorylation, specifically on Ser 94, and prevents the formation of the YAP-TEAD complex (Li et al. 2010). Current findings revealed that CANA increased energy metabolism markers: AMPK, P-AMPK, AMPK mRNA relative expression, and P-AMPK/AMPK ratio.
SGLT2 is overexpressed in many kinds of cancer, providing the glucose that cancer cells need to meet their high energy requirements (Scafoglio et al. 2015). One of the potential molecular mechanisms is that it prevents the renal tubules from re-absorbing glucose, which lowers the amount of glucose needed for tumor cell development and metabolism (Dutka et al. 2022), so preventing tumor cell growth and proliferation. SGLT2 suppression consistently inhibited the growth of tumors in mice (Nasiri et al. 2019). There is an increasing evidence that SGLT2 inhibition may reduce the growth of carcinomas that express SGLT2, including breast (Zhou et al. 2020), cervical (Xie et al. 2020), hepatocellular (Shiba et al. 2018), prostate, and lung carcinomas (Villani et al. 2016). According to Kaji et al., by inhibiting the uptake of glucose, the formation of lactate, and the synthesis of intracellular ATP, canagliflozin prevents the growth of HepG2 cells (Kaji et al. 2018). AMPK activation and decreased ATP synthesis result from the inhibition of mitochondrial complex I by canagliflozin, a strong SGLT2 inhibitor (Hawley et al. 2016). Inhibiting mTOR through AMPK activation results in cell cycle arrest and the production of apoptosis (Motoshima et al. 2006). By suppressing forkhead box A1 and sonic hedgehog signaling, activated AMPK prevents the growth of tumor cells and triggers apoptosis (Xie et al. 2020). Canagliflozin, through AMPK activation and mTOR inhibition, downregulates HIF-1α, hence inhibiting the proliferation of non-small cell lung cancer (NSCLC) and improving the efficacy of radiation therapy (Biziotis et al. 2023).
In line with previous studies, CANA therapy, particularly high doses, showed a marked decrease in HIF-1α, TGF-β1, and YAP/TAZ levels and exhibited a marked increase in AMPK and p-AMPK levels and expression. The idea that CANA may also inhibit intra-tumor neovascularization supports our findings (Polet and Feron 2013). These findings, with the previous findings related to AFP, CEA, and AFU, confirmed that CANA treatment has anti-tumor and anti-angiogenic effects.
Further, canagliflozin suppressed oxidative stress and enhanced the levels of antioxidant markers: MDA, SOD, and GSH, evidenced by measuring lipid peroxide as MDA is one of the most prominent indicators of oxidation stress (Elbaset et al. 2023). Our findings showed a notable increase in MDA and a large decrease in GSH and SOD, the antioxidants essential for MDA scavenging. The decline in the cellular antioxidants is indicative of the oxidative toxic impact of DEN/TAA. This result was corroborated by Zhang et al.’s study (Zhang et al. 2016) which discovered that DEN can stimulate HCC progression via interacting with macromolecules such as DNA, lipids, anti-oxidative enzymes, and enzymes involved in the DNA repair pathway. Furthermore, TAA biotransformation produces extremely reactive compounds that bind covalently to liver macromolecules, initiating hepatic damage and inhibiting SOD activities and GSH content (Elbaset et al. 2024). HCC rats exhibited notably reduced hepatic MDA levels and increased GSH content and SOD activity following CANA administration. The findings of this study may indicate that CANA can lessen hepatic oxidative damage and, in turn, lessen HCC progression. The antioxidant properties of CANA have been proven in multiple studies using experimental disease models (Kabil and Mahmoud 2018; Hasan et al. 2020).
In 55% of HCC patients, sirtuin 1 (SIRT1) is overexpressed, which is linked to a greater tumor grade and a lower chance of survival. Due to SIRT1’s ability to modify the activity of proteins essential for carcinogenesis, it promotes both drug resistance and tumor growth and progression (Chen et al. 2012; Ling et al. 2017). The deacetylase SIRT1 is dependent on nicotinamide adenine dinucleotide (NAD). Because cancer cells undergo metabolic alterations, they need extra NAD (as a redox partner) in cellular energy metabolism processes. Important biological functions required for the proliferation of cancer cells, such as “transcription, cell-cycle progression, anti-apoptosis, and DNA repair,” are regulated by NAD-dependent enzymes like SIRT1 (Garten et al. 2015; Revollo et al. 2004). According to our data, the HCC group’s SIRT1 levels significantly increased. These results were consistent with the Chen et al. study (Chen et al. 2020), which discovered that SIRT1 was substantially more in HCC tissues compared to nearby normal tissues. Meanwhile, treatment with canagliflozin restored the elevated Sirt1 to normal levels.
Nrf2 regulates and encodes antioxidant proteins through its interaction with the antioxidant-responsive element (ARE) (Elbaset et al. 2024). Nrf2 normally binds with “the Kelch-like ECH-associated protein-1 (Keap1)” in the cytoplasm (Raslan et al. 2021). Upon exposure to DEN/TAA free radicals, Nrf2 is liberated from Keap1 and moves into the nucleus, triggering the antioxidant genes (Rotblat et al. 2012; Abdel-Rahman et al. 2024). It is significant that prior research has demonstrated the crucial function of the NRF2/KEAP1 signaling pathway in carcinogenesis and the relationship between redox and metabolism in cancer (Hayes and Dinkova-Kostova 2014), providing additional evidence that Nrf2 acts as an oncogene to encourage tumor cell motility and invasion, potentially by boosting the tumor’s resistance to oxidative stress. It is increasingly evident that the NRF2/KEAP1 pathway contributes significantly to the metabolic reprogramming of cancer cells by means of a transcriptional program that promotes cancer cell proliferation and malignant development. Furthermore, Nrf2 promotes intermediate metabolism by means of glutaminolysis (Romero et al. 2017), which leads to an imbalance in metabolic activities, including nucleotide (Mitsuishi et al. 2012) and amino acid biosynthesis (DeNicola et al. 2015). Our findings showed that Nrf2 plays an oncogenic role in the formation of liver cancer since the HCC group had elevated Nrf2 levels, which were reversed to normal levels in the groups receiving CANA treatment.
Signal transducer and activator of transcription 3 (STAT3) is a versatile transcription factor that is intimately linked to the transformation, survival, invasion, proliferation, and metastasis of tumor cells as well as the tumor inflammatory response (Tolomeo and Cascio 2021). Numerous cytokines, growth factors, hormones, and hepatitis virus proteins all contribute to the hepatic activation of STAT3. Among these, IL-6 and IL-22 are two strong inducers of hepatocyte STAT3 activation (Wang et al. 2011). The hyperactivity of STAT3 in HCC cells has been demonstrated to enhance the malignant biological behavior of tumor cells by promoting their invasion, metastasis, and proliferation while also preventing their apoptosis (Yuen et al. 2010). According to Li et al. (2006) RNA interference on STAT3 dramatically decreased tumor volume and suppressed cell proliferation in mice. Furthermore, STAT3 activation can trigger the production of numerous downstream genes that impact the pathogenesis of various malignancies, including Bcl-2 (Levy and Darnell 2002). Canagliflozin treatment significantly decreased STAT3 and p-STAT3 levels, which increased markedly in the HCC group. According to a prior study, conditional deletion of STAT3 in hepatocytes stops the formation of liver tumors caused by a single injection of DEN (He et al. 2010) which confirmed our findings.
Canagliflozin may mitigate the hyperinsulinemic state and diminish the related risk factors for liver carcinogenesis by lowering blood glucose levels and enhancing insulin sensitivity. Decreased insulin transmission may consequently diminish the proliferative signals that promote cancer in the liver. Canagliflozin has demonstrated the capacity to diminish systemic inflammation, maybe via its influence on metabolic regulation. The medicine may diminish inflammatory cytokine cascades (e.g., TNF-α, IL-6) by reducing glucose levels and enhancing insulin sensitivity, so mitigating liver damage and fibrosis, which elevate the risk of liver cancer. Furthermore, enhanced glucose metabolism may diminish the liver’s exposure to advanced glycation end-products (AGEs), which are byproducts of non-enzymatic glucose metabolism that provoke inflammation and tissue damage. Canagliflozin’s capacity to decrease blood glucose levels may diminish the production of reactive oxygen species (ROS) and oxidative injury to hepatic cells, therefore alleviating a principal mechanism that fosters carcinogenesis (DeFronzo et al. 2015; Wanner et al. 2016; Mohamed et al. 2016; Davidson 2019).
Both an increase in cell proliferation and a decrease in cell death can lead to the growth of tumors. Dysregulation of apoptosis (programmed cell death) is believed to contribute to cancer progression (Wyllie 1997). Apoptotic cell death is initiated and carried out by members of the caspase family of cellular proteases. An important protease in the apoptotic process is caspase-3 (McIlwain et al. 2013). Immune expression of the cell proliferation marker, proliferating cell nuclear antigen (PCNA), and caspase-3 showed that the HCC group had significantly higher expression of PCNA and significantly lower expression of caspase-3. After CANA therapy, PCNA expression decreased, and caspase-3 expression increased. Comparable results were reported in a study by Jojima et al. (Jojima et al. 2019) that showed canagliflozin activated caspase-3 to induce apoptosis in HepG2 cells.
In rats, SGLT2 inhibitors like canagliflozin have demonstrated advantageous benefits in diminishing hepatic steatosis, inflammation, and fibrosis. These are all essential antecedents to the progression of HCC. Given that metabolic disorders such as insulin resistance, hyperglycemia, and non-alcoholic fatty liver disease (NAFLD) are pivotal to the advancement of human HCC, the results reported in rats (e.g., enhanced glucose metabolism and diminished liver fat) may be pertinent for human therapeutic applications. Nonetheless, the specific metabolic pathways may vary marginally among species, and the severity of HCC in humans is affected by various factors, including genetic predisposition, viral infections (such as hepatitis B or C), and environmental influences, which may not be entirely mirrored in rat models (Xu et al. 2022).
Rats treated with SGLT2 inhibitors have demonstrated reduced liver inflammation and fibrosis, which are critical components in the progression from liver disease to HCC. These findings are relevant to humans, as chronic inflammation and liver fibrosis are major risk factors for HCC. In humans, inflammation from conditions like NAFLD and NASH (non-alcoholic steatohepatitis) plays a key role in driving tumorigenesis (Shiba et al. 2018).
SGLT2 inhibitors, such as canagliflozin, have demonstrated a reduction in oxidative stress in animal models, a critical element in hepatic injury and carcinogenesis. Given that oxidative stress has a role in DNA damage and cancer in both rats and humans, this phenomenon is very pertinent. Mitigating oxidative stress may potentially decelerate the advancement of liver disease and diminish the probability of HCC occurrence in humans. Although oxidative stress significantly impacts both rats and people, the regulatory mechanisms of oxidative damage may vary, necessitating long-term investigations in humans to validate these effects (Davidson 2019).
The results from rat models offer a promising basis for investigating the possible application of SGLT2 inhibitors, such as canagliflozin, in halting the advancement of liver disease and mitigating the risk of HCC. The decrease in hepatic fat, inflammation, fibrosis, and oxidative stress in rats are mechanisms that are significantly pertinent to human liver disease. Nonetheless, despite the encouraging benefits, the translation of findings from animal models to human treatment necessitates additional clinical research to validate their efficacy and safety in human populations. Future research must meticulously evaluate human-specific aspects, including genetic variations and the multifactorial characteristics of HCC. Human clinical trials and longitudinal investigations are necessary to verify the efficacy of SGLT2 inhibitors in mitigating HCC risk or enhancing outcomes in individuals with liver disease.
Conclusion
The current study showed that canagliflozin effectively lessens liver injuries and oxidative stress, which occur in HCC in rats after exposure to CDD, DEN, and TAA. The canagliflozin doses of 10 and 20 mg/kg were observed to have considerably reduced the elevated liver enzymes of ALT, AST, and GGT, along with an improvement in liver function. Correspondingly, oxidative stress markers-MDA were considerably decreased, accompanied by elevated levels of antioxidant markers such as SOD and GSH, which significantly improved upon the possible damage caused by oxidative stress.
In addition, treatment with canagliflozin led to a significant drop in tumor marker levels, which included AFP, AFU, and CEA, potentially influencing the tumor during development. Also, a modulation was demonstrated in significant pathways, such as HIF-1α or YAP1, TAZ. Expression of TGF-β1, a discovery related to the progression and development of cancer, is in altered expression.
Moreover, canagliflozin showed strong beneficial effects on energy demand and inflammatory response, evidenced by AMPK and STAT3 signal transduction pathways. The excellent improvements in biochemical and histopathological parameters point to the therapeutic potential of canagliflozin with respect to liver injury and HCC. Eventually, the notable enhancements in biochemical and histological measurements demonstrated the therapeutic potential of canagliflozin for liver damage and HCC.
Author contributions
HMF, MAE, and RFA: Conceptualization and methodology. RFA, HMF, MAE, GFA, ZAE, MMA and FAI: Animal experiment and Biochemical analysis. SSA: Histopathological and immunohistochemical experiments. MAE: Data analysis. OAS, AND SSM: Supervision. HMF, RFA, MAM, and GFA: Writing, reviewing and editing. The authors declare that all data were generated in-house and that no paper mill was used.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors are grateful to the National Research Centre-Egypt for financing research project #13060173.
Data availability
All source data for this work (or generated in this study) are available upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abd El-Rahman SS, Fayed HM (2019) Targeting AngII/AT1R signaling pathway by perindopril inhibits ongoing liver fibrosis in rat. J Tissue Eng Regen Med 13(12):2131–2141 [DOI] [PubMed] [Google Scholar]
- Abdallah IZ, Khattab HA (2004) Protective role of lycopene against diethylnitrosamine induced experimental hepatocarcinogenesis. The Egyptian Journal of Hospital Medicine 16(1):1–13 [Google Scholar]
- Abdel-Rahman RF, Fayed HM, Ogaly HA, Hussein RA, Raslan MA (2022) Phytoconstituents of Sansevieria suffruticosa N.E.Br. leaves and its hepatoprotective effect via activation of the NRF2/ARE signaling pathway in an experimentally induced liver fibrosis rat model. Chem Biodivers 19(4):e202100960 [DOI] [PubMed] [Google Scholar]
- Abdel-Rahman RF, Elbaset MA, Fayed HM, Esatbeyoglu T, Afifi SM, Diab RA (2024) Reno-protective effect of Roflumilast against kidney injury induced by ischemia/reperfusion in rats: evidence from biochemical and histological investigations. Pharmacol Res - Rep 2:100014 [Google Scholar]
- Abdo W, Hirata A, Shukry M, Kamal T, Abdel-Sattar E, Mahrous E et al (2015) Calligonum comosum extract inhibits diethylnitrosamine-induced hepatocarcinogenesis in rats. Oncol Lett 10(2):716–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afifi N, Yassin N, Fawzy R, Rahman A, Aiad A, Afifi N, et al (2014) Fayed. Hepatoprotective effect of artichoke extract against pre-cancerous lesion of experimentally induced hepatocellular carcinoma in rats 1097–8135
- Arsad S, Esa N, Hamzah H (2014) Histopathologic changes in liver and kidney tissues from male Sprague Dawley rats treated with Rhaphidophora decursiva (Roxb.) Schott extract. J Cytol Histol S 4(1):1–6 [Google Scholar]
- Ayoub IM, El-Baset MA, Elghonemy MM, Bashandy SAE, Ibrahim FAA, Ahmed-Farid OAH et al (2022) Chemical profile of Cyperus laevigatus and its protective effects against thioacetamide-induced hepatorenal toxicity in rats. Molecules 27(19):6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft JD, Gamble M (2008) Theory and practice of histological techniques: Elsevier health sciences
- Banni M, Messaoudi I, Said L, El Heni J, Kerkeni A, Said K (2010) Metallothionein gene expression in liver of rats exposed to cadmium and supplemented with zinc and selenium. Arch Environ Contam Toxicol 59(3):513–519 [DOI] [PubMed] [Google Scholar]
- Basak D, Gamez D, Deb S (2023) SGLT2 inhibitors as potential anticancer agents. Biomedicines 11(7);1867. 10.3390/biomedicines11071867 [DOI] [PMC free article] [PubMed]
- Biziotis OD, Tsakiridis EE, Ali A, Ahmadi E, Wu J, Wang S et al (2023) Canagliflozin mediates tumor suppression alone and in combination with radiotherapy in non-small cell lung cancer (NSCLC) through inhibition of HIF-1α. Mol Oncol 17(11):2235–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D (2010) The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev 24(21):2383–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbajo-Pescador S, Ordoñez R, Benet M, Jover R, García-Palomo A, Mauriz JL et al (2013) Inhibition of VEGF expression through blockade of Hif1α and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br J Cancer 109(1):83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao EC (2014) SGLT-2 inhibitors: a new mechanism for glycemic control. Clin Diabetes 32(1):4–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H-C, Jeng Y-M, Yuan R-H, Hsu H-C, Chen Y-L (2012) SIRT1 promotes tumorigenesis and resistance to chemotherapy in hepatocellular carcinoma and its expression predicts poor prognosis. Ann Surg Oncol 19:2011–2019 [DOI] [PubMed] [Google Scholar]
- Chen Z, He M, Chen J, Li C, Zhang Q (2020) Long non-coding RNA SNHG7 inhibits NLRP3-dependent pyroptosis by targeting the miR-34a/SIRT1 axis in liver cancer. Oncol Lett 20(1):893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JA (2019) SGLT2 inhibitors in patients with type 2 diabetes and renal disease: overview of current evidence. Postgrad Med 131(4):251–260 [DOI] [PubMed] [Google Scholar]
- de Lima VM, Oliveira CP, Alves VA, Chammas MC, Oliveira EP, Stefano JT et al (2008) A rodent model of NASH with cirrhosis, oval cell proliferation and hepatocellular carcinoma. J Hepatol 49(6):1055–1061 [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Ferrannini E, Groop L, Henry RR, Herman WH, Holst JJ et al (2015) Type 2 diabetes mellitus. Nat Rev Dis Primers 1(1):15019 [DOI] [PubMed] [Google Scholar]
- Deng B, Zhu JM, Wang Y, Liu TT, Ding YB, Xiao WM et al (2013) Intratumor hypoxia promotes immune tolerance by inducing regulatory T cells via TGF-β1 in gastric cancer. PLoS ONE 8(5):e63777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNicola GM, Chen PH, Mullarky E, Sudderth JA, Hu Z, Wu D et al (2015) NRF2 regulates serine biosynthesis in non-small cell lung cancer. Nat Genet 47(12):1475–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRan M, Yang J, Shen C-H, Peters Eric C, Fitamant J, Chan P et al (2014) Energy stress regulates Hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep 9(2):495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan W, Chen K, Jiang Z, Chen X, Sun L, Li J et al (2017) Desmoplasia suppression by metformin-mediated AMPK activation inhibits pancreatic cancer progression. Cancer Lett 385:225–233 [DOI] [PubMed] [Google Scholar]
- Dutka M, Bobiński R, Francuz T, Garczorz W, Zimmer K, Ilczak T et al (2022) SGLT-2 inhibitors in cancer treatment-mechanisms of action and emerging new perspectives. Cancers (Basel) 14(23):5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaset MA, Mohamed B, Moustafa PE, Mansour DF, Afifi SM, Esatbeyoglu T et al (2023) Erythropoietin suppresses the hepatic fibrosis caused by thioacetamide: role of the PI3K/Akt and TLR4 signaling pathways. Oxid Med Cell Longev 2023:5514248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaset MA, Mohamed B, Hessin A, Abd El-Rahman SS, Esatbeyoglu T, Afifi SM et al (2024) Nrf2/HO-1, NF-κB and PI3K/Akt signalling pathways decipher the therapeutic mechanism of pitavastatin in early phase liver fibrosis in rats. J Cell Mol Med 28(3):e18116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleithi Y, El-Gayar A, Amin MN (2024) Autophagy modulation attenuates sorafenib resistance in HCC induced in rats. Cell Death Dis 15(8):595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Schuster S, Penke M, Gorski T, De Giorgis T, Kiess W (2015) Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat Rev Endocrinol 11(9):535–546 [DOI] [PubMed] [Google Scholar]
- Hasan R, Lasker S, Hasan A, Zerin F, Zamila M, Chowdhury FI et al (2020) Canagliflozin attenuates isoprenaline-induced cardiac oxidative stress by stimulating multiple antioxidant and anti-inflammatory signaling pathways. Sci Rep 10(1):14459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan HM, Hamdan AM, Alattar A, Alshaman R, Bahattab O, Al-Gayyar MMH (2024) Evaluating anticancer activity of emodin by enhancing antioxidant activities and affecting PKC/ADAMTS4 pathway in thioacetamide-induced hepatocellular carcinoma in rats. Redox Rep 29(1):2365590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Ford RJ, Smith BK, Gowans GJ, Mancini SJ, Pitt RD et al (2016) The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 65(9):2784–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39(4):199–218 [DOI] [PubMed] [Google Scholar]
- He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T et al (2010) Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell 17(3):286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Yang QC, Zhou Q, Zhu H, Niu WY, Feng J et al (2014) Effects of varying degrees of intermittent hypoxia on proinflammatory cytokines and adipokines in rats and 3T3-L1 adipocytes. PLoS ONE 9(1):e86326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W, Guan KL (2012) The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol 23(7):785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung M-H, Chen Y-L, Chen L-J, Chu P-Y, Hsieh F-S, Tsai M-H et al (2019) Canagliflozin inhibits growth of hepatocellular carcinoma via blocking glucose-influx-induced β-catenin activation. Cell Death Dis 10(6):420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo W, Yu ES, Chang M, Park HK, Choi HJ, Ryu JE et al (2016) Metformin inhibits early stage diethylnitrosamine-induced hepatocarcinogenesis in rats. Mol Med Rep 13(1):146–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R, Halder G (2014) The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discovery 13(1):63–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jojima T, Wakamatsu S, Kase M, Iijima T, Maejima Y, Shimomura K et al (2019) The SGLT2 inhibitor canagliflozin prevents carcinogenesis in a mouse model of diabetes and non-alcoholic steatohepatitis-related hepatocarcinogenesis: association with SGLT2 expression in hepatocellular carcinoma 20(20) [DOI] [PMC free article] [PubMed]
- Kabil SL, Mahmoud NM (2018) Canagliflozin protects against non-alcoholic steatohepatitis in type-2 diabetic rats through zinc alpha-2 glycoprotein up-regulation. Eur J Pharmacol 828:135–145 [DOI] [PubMed] [Google Scholar]
- Kaji K, Nishimura N, Seki K, Sato S, Saikawa S, Nakanishi K et al (2018) Sodium glucose cotransporter 2 inhibitor canagliflozin attenuates liver cancer cell growth and angiogenic activity by inhibiting glucose uptake. Int J Cancer 142(8):1712–1722 [DOI] [PubMed] [Google Scholar]
- Kim DY (2024) Changing etiology and epidemiology of hepatocellular carcinoma: Asia and worldwide. J Liver Cancer 24(1):62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Fujii Y, Yamamoto R, Yafune A, Hayashi SM, Suzuki K et al (2013) Involvement of multiple cell cycle aberrations in early preneoplastic liver cell lesions by tumor promotion with thioacetamide in a two-stage rat hepatocarcinogenesis model. Exp Toxicol Pathol 65(7–8):979–988 [DOI] [PubMed] [Google Scholar]
- Kurma K, Manches O, Chuffart F, Sturm N, Gharzeddine K, Zhang J et al (2021) DEN-induced rat model reproduces key features of human hepatocellular carcinoma. Cancers (Basel) 13(19):4981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Wong LL, Tse EY, Liu HF, Leong VY, Lee JM et al (2012) AMPK promotes p53 acetylation via phosphorylation and inactivation of SIRT1 in liver cancer cells. Cancer Res 72(17):4394–4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH et al (2008) TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol 28(7):2426–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DE, Darnell JE Jr (2002) Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol 3(9):651–662 [DOI] [PubMed] [Google Scholar]
- Li WC, Ye SL, Sun RX, Liu YK, Tang ZY, Kim Y et al (2006) Inhibition of growth and metastasis of human hepatocellular carcinoma by antisense oligonucleotide targeting signal transducer and activator of transcription 3. Clin Cancer Res 12(23):7140–7148 [DOI] [PubMed] [Google Scholar]
- Li Z, Zhao B, Wang P, Chen F, Dong Z, Yang H et al (2010) Structural insights into the YAP and TEAD complex. Genes Dev 24(3):235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling S, Li J, Shan Q, Dai H, Lu D, Wen X et al (2017) USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway. Mol Oncol 11(6):682–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Zhu M, Li W, Lin B, Dong X, Chen Y et al (2016) Alpha fetoprotein plays a critical role in promoting metastasis of hepatocellular carcinoma cells. J Cell Mol Med 20(3):549–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Sun P, Zhang X, Lin G, Xin Q, Niu Y et al (2021) Canagliflozin modulates hypoxia-induced metastasis, angiogenesis and glycolysis by decreasing HIF-1α protein synthesis via AKT/mTOR pathway. Int J Mol Sci 22(24):13336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabrouk Gabr N, Hassan MI (2020) A comparative study of canagliflozin (INVOKANA) on type-I and type-II diabetes mellitus on adult male Albino rat. Al-Azhar Medical Journal 49(1):15–32 [Google Scholar]
- McCrimmon RJ, Fan X, Cheng H, McNay E, Chan O, Shaw M et al (2006) Activation of AMP-activated protein kinase within the ventromedial hypothalamus amplifies counterregulatory hormone responses in rats with defective counterregulation. Diabetes 55(6):1755–1760 [DOI] [PubMed] [Google Scholar]
- McIlwain DR, Berger T, Mak TW (2013) Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol 5(4):a008656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuishi Y, Taguchi K, Kawatani Y, Shibata T, Nukiwa T, Aburatani H et al (2012) Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell 22(1):66–79 [DOI] [PubMed] [Google Scholar]
- Mohamed J, Nazratun Nafizah AH, Zariyantey AH, Budin SB (2016) Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J 16(2):e132–e141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed NZ, Aly HF, moneim El-Mezayen HA, El-Salamony HE (2019) Effect of co-administration of bee honey and some chemotherapeutic drugs on dissemination of hepatocellular carcinoma in rats. Toxicol Rep 6:875–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriwaki K, Noda K, Nakagawa T, Asahi M, Yoshihara H, Taniguchi N et al (2007) A high expression of GDP-fucose transporter in hepatocellular carcinoma is a key factor for increases in fucosylation. Glycobiology 17(12):1311–1320 [DOI] [PubMed] [Google Scholar]
- Motoshima H, Goldstein BJ, Igata M, Araki E (2006) AMPK and cell proliferation–AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 574(Pt 1):63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiri AR, Rodrigues MR, Li Z, Leitner BP, Perry RJ (2019) SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer & Metabolism 7:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberne PM, Camargo JL, Clark AJ (1982) Choline deficiency, partial hepatectomy, and liver tumors in rats and mice. Toxicol Pathol 10(2):95–106 [DOI] [PubMed] [Google Scholar]
- Nyblom H, Berggren U, Balldin J, Olsson R (2004) High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol 39(4):336–339 [DOI] [PubMed] [Google Scholar]
- Pan D (2010) The Hippo signaling pathway in development and cancer. Dev Cell 19(4):491–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SH, Camargo FD, Yimlamai D (2017) Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology 152(3):533–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polet F, Feron O (2013) Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Intern Med 273(2):156–165 [DOI] [PubMed] [Google Scholar]
- Qin Y, Sekine I, Hanazono M, Morinaga T, Fan M, Takiguchi Y et al (2019) AMPK activation induced in pemetrexed-treated cells is associated with development of drug resistance independently of target enzyme expression. Mol Oncol 13(6):1419–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, Al-Ghamdi SA, Alharbi KS, Beg S, Sharma K, Anwar F et al (2019) Ganoderic acid loaded nano-lipidic carriers improvise treatment of hepatocellular carcinoma. Drug Deliv 26(1):782–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan A, Afifi N, Yassin NZ, Abdel-Rahman RF, Abd El-Rahman SS, Fayed HM (2018) Mesalazine, an osteopontin inhibitor: the potential prophylactic and remedial roles in induced liver fibrosis in rats. Chem Biol Interact 289:109–118 [DOI] [PubMed] [Google Scholar]
- Raslan M, Abdel Rahman R, Fayed H, Ogaly H, Fikry R (2021) Metabolomic profiling of sansevieria trifasciata hort ex. Prain leaves and roots by HPLC-PAD-ESI/MS and its hepatoprotective effect via activation of the NRF2/ARE signaling pathway in an experimentally induced liver fibrosis rat model. Egypt J Chem 64(11):6647–71 [Google Scholar]
- Rekha RD, Amali AA, Her GM, Yeh YH, Gong HY, Hu SY et al (2008) Thioacetamide accelerates steatohepatitis, cirrhosis and HCC by expressing HCV core protein in transgenic zebrafish Danio rerio. Toxicology 243(1–2):11–22 [DOI] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S-i (2004) The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem 279(49):50754–63 [DOI] [PubMed] [Google Scholar]
- Romero R, Sayin VI, Davidson SM, Bauer MR, Singh SX, LeBoeuf SE et al (2017) Keap1 loss promotes Kras-driven lung cancer and results in dependence on glutaminolysis. Nat Med 23(11):1362–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotblat B, Melino G, Knight RA (2012) NRF2 and p53: Januses in cancer? Oncotarget 3(11):1272–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sas Z, Cendrowicz E, Weinhäuser I, Rygiel TP (2022) Tumor microenvironment of hepatocellular carcinoma: challenges and opportunities for new treatment options. Int J Mol Sci 23(7):3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauzay C, Petit A, Bourgeois AM, Barbare JC, Chauffert B, Galmiche A et al (2016) Alpha-foetoprotein (AFP): a multi-purpose marker in hepatocellular carcinoma. Clin Chim Acta 463:39–44 [DOI] [PubMed] [Google Scholar]
- Scafoglio C, Hirayama BA, Kepe V, Liu J, Ghezzi C, Satyamurthy N et al (2015) Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci 112(30):E4111–E4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba K, Tsuchiya K, Komiya C, Miyachi Y, Mori K, Shimazu N et al (2018) Canagliflozin, an SGLT2 inhibitor, attenuates the development of hepatocellular carcinoma in a mouse model of human NASH. Sci Rep 8(1):2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Singh M, Yadav E, Falls N, Komal U, Dangi DS et al (2018) Amelioration of diethylnitrosamine (DEN)-induced hepatocellular carcinogenesis in animal models via knockdown oxidative stress and proinflammatory markers by Madhuca longifolia embedded silver nanoparticles. RSC Adv 8(13):6940–6953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamim YM, Nagy AA, Abdellah AM, Osman AH, Ismail AFM (2024) Anticancer effect of propranolol on diethylnitrosamine-induced hepatocellular carcinoma rat model. Fundam Clin Pharmacol 38(4):742–757 [DOI] [PubMed] [Google Scholar]
- Tolomeo M, Cascio A (2021) The multifaced role of STAT3 in cancer and its implication for anticancer therapy. Int J Mol Sci 22(2):603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact 160(1):1–40 [DOI] [PubMed] [Google Scholar]
- Villani LA, Smith BK, Marcinko K, Ford RJ, Broadfield LA, Green AE et al (2016) The diabetes medication canagliflozin reduces cancer cell proliferation by inhibiting mitochondrial complex-I supported respiration. Molecular Metabolism 5(10):1048–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lafdil F, Kong X, Gao B (2011) Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci 7(5):536–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Xiao Z-D, Li X, Aziz KE, Gan B, Johnson RL et al (2015) AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol 17(4):490–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Liu P, Chen Q, Deng S, Liu X, Situ H et al (2016) Targeting AMPK signaling pathway to overcome drug resistance for cancer therapy. Curr Drug Targets 17(8):853–864 [DOI] [PubMed] [Google Scholar]
- Wang S, Ding Y, Dong R, Wang H, Yin L, Meng S (2021) Canagliflozin improves liver function in rats by upregulating asparagine synthetase. Pharmacology 106(11–12):606–615 [DOI] [PubMed] [Google Scholar]
- Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M et al (2016) Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med 375(4):323–334 [DOI] [PubMed] [Google Scholar]
- Wu W-D, Hu Z-M, Shang M-J, Zhao D-J, Zhang C-W, Hong D-F et al (2014) Cordycepin down-regulates multiple drug resistant (MDR)/HIF-1α through regulating AMPK/mTORC1 signaling in GBC-SD gallbladder cancer cells. Int J Mol Sci 15(7):12778–12790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie AH (1997) Apoptosis and carcinogenesis. Eur J Cell Biol 73(3):189–197 [PubMed] [Google Scholar]
- Xie Z, Wang F, Lin L, Duan S, Liu X, Li X et al (2020) An SGLT2 inhibitor modulates SHH expression by activating AMPK to inhibit the migration and induce the apoptosis of cervical carcinoma cells. Cancer Lett 495:200–210 [DOI] [PubMed] [Google Scholar]
- Xu Z, Hu W, Wang B, Xu T, Wang J, Wei D (2022) Canagliflozin ameliorates nonalcoholic fatty liver disease by regulating lipid metabolism and inhibiting inflammation through induction of autophagy. Yonsei Med J 63(7):619–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Luo Y, Hu S, Li Y, Liu Q (2018) Value of combined detection of serum carcino-embryonic antigen, carbohydrate antigen 19–9 and cyclooxygenase-2 in the diagnosis of colorectal cancer. Oncol Lett 16(2):1551–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M, Morine Y, Ikemoto T, Imura S, Higashijima J, Iwahashi S et al (2017) Elevated preoperative serum CEA level is associated with poor prognosis in patients with hepatocellular carcinoma through the epithelial-mesenchymal transition. Anticancer Res 37(3):1169–1175 [DOI] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN (2006) Statistical analysis of real-time PCR data. BMC Bioinformatics 7(1):85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen JW, Poon LS, Chan AS, Yu FW, Lo RK, Wong YH (2010) Activation of STAT3 by specific Galpha subunits and multiple Gbetagamma dimers. Int J Biochem Cell Biol 42(6):1052–1059 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Jiang H, Zhang X, Xu J, Wu X, Xu Q et al (2023) Canagliflozin reduces chemoresistance in hepatocellular carcinoma through PKM2-c-Myc complex-mediated glutamine starvation. Free Radical Biol Med 208:571–586 [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhou D (2019) Role of the transcriptional coactivators YAP/TAZ in liver cancer. Curr Opin Cell Biol 61:64–71 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yang J, Wang J (2016) Modulatory effect of luteolin on redox homeostasis and inflammatory cytokines in a mouse model of liver cancer. Oncol Lett 12(6):4767–4772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J et al (2007) Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21(21):2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wang Q, Zhang H, Li Y, Xie S, Xu M (2019) YAP inhibition by nuciferine via AMPK-mediated downregulation of HMGCR sensitizes pancreatic cancer cells to gemcitabine. Biomolecules 9(10):620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhu J, Yu S-J, Ma H-L, Chen J, Ding X-F et al (2020) Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed Pharmacother 132:110821 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All source data for this work (or generated in this study) are available upon reasonable request.