Abstract
While there is no conclusive evidence that monosodium glutamate (MSG, a food additive) directly causes liver cancer in humans, certain studies suggest a potential link between MSG-induced liver injury and cancer development. This study aimed to evaluate the protective effect of tannic acid (TA, a natural polyphenol) against MSG-induced hepatotoxicity through the glutathione and thioredoxin systems. Twenty-four rats were randomly divided into control and experimental groups and treated with TA, MSG, and MSG+TA once daily by oral gavage for 21 days. In addition to major oxidative stress indicators (total glutathione; GSH + GSSG and malondialdehyde; MDA), mRNA expression changes and biological activity responses of components of the glutathione and thioredoxin systems were examined in the liver tissues of all animals. The results showed that MSG alone negatively affected both stress indicators and antioxidant system components (glutathione peroxidase; GPx, glutathione reductase; GR, glutathione-S-transferase; GST, and thioredoxin reductase; TrxR) in terms of mRNA expression and biological activity. However, the combination of MSG and TA demonstrated robust antioxidative effects, surpassing the outcomes of MSG treatment. Our results provide new insights into pivotal molecular targets and protective candidates that should be focused on in future in vivo and in vitro HCC research.
Keywords: Monosodium glutamate, Hepatotoxicity, Glutathione, Thioredoxin, Tannic acid
Introduction
Monosodium glutamate (MSG) is a form of glutamic acid, one of the 20 natural amino acids. MSG is a food additive widely used in processed foods or fast food products due to its flavor-enhancing properties (Sukmak et al. 2024; Thongsepee et al. 2024). While regulatory bodies such as the US Food and Drug Administration (FDA) have classified MSG as generally recognized as safe (GRAS), ongoing scientific debate has raised concerns about its potential health effects (Faustman et al. 2021). Recent studies have focused on the long-term consequences that MSG may cause beyond its short-term effects, defined as the MSG symptom complex (Shastri et al. 2023), particularly on liver health (Onaolapo et al. 2016; Shimada et al. 2015). Studies have suggested that MSG significantly increases the levels of critical enzymes that are indicators of liver damage, such as aspartate aminotransferase (AST) and alanine transaminase (ALT), and may cause hepatotoxicity (Banerjee et al. 2021; Sahin et al. 2023). It is also thought that MSG may exacerbate the damage by exhibiting a synergistic effect with other factors, such as lipopolysaccharide (LPS) that can cause liver damage (Asejeje et al. 2023). Given the growing body of literature suggesting a link between MSG and liver dysfunction, there is a critical need for further investigation into the underlying mechanisms of MSG-induced toxicity. Such research could also aid in identifying potential therapeutic agents capable of mitigating these effects.
Polyphenolic compounds, a group of phytochemicals found in many plants, have attracted significant interest in the medical field due to their numerous benefits (Karagaç et al. 2024; Kumar et al. 2023). In addition to their biological properties, their cost-effectiveness and sustainability make these compounds, which include phenolic acids, flavonoids, and tannins, more attractive (Aatif 2023). As research continues, exploring the molecular mechanisms underlying protective effects and the therapeutic potential of natural polyphenolic compounds in medicinal applications is becoming increasingly evident (Karadas et al. 2024). Tannic acid (TA), which can be found in many plants, is the simplest hydrolyzable tannin and is a US Food and Drug Administration (FDA)-approved food additive (Guo et al. 2021). The hydroxyl groups of plant-based polyphenols, such as TA, provide these compounds with hydrogen donor and metal chelating properties, thus making these compounds suitable candidates against oxidative stress-related abnormalities (Varesi et al. 2022). TA is known to have greater antioxidant potential (Dare et al. 2020; Kizir et al. 2023, 2024), superior antibacterial and anti-inflammatory properties (Sahiner et al. 2023), and superior bioavailability and bioaccessibility (Fraga-Corral et al. 2021) compared to other flavonoids, such as gallic acid. Moreover, it has attracted considerable attention in biomaterial development studies because of its ability to form numerous hydrogen bond interactions with a wide variety of molecules (Baldwin & Booth 2022). Such extensive physiological and chemical properties make TA a very strong natural candidate. Recent studies have suggested that TA tends to have a similar impact trend in healing various types of damage that can occur in the liver as a result of different factors (Chu et al. 2016; Yesilkent & Ceylan 2022; Zhang et al. 2017). Research indicates that, by enhancing the cellular antioxidant defense capacity and scavenging free radicals, TA effectively combats MSG-induced oxidative stress (Karagac & Ceylan 2023). Studies using experimental animal models have shown that hepatic enzymes such as AST and ALT and lipid peroxidation are reduced, and glutathione levels are restored with TA (Li et al. 2020; Ozturk et al. 2024). Additionally, the production of pro-inflammatory cytokines that cause hepatic inflammation and fibrosis has also been proven to be reduced in the presence of TA (Chu et al. 2016; Lin et al. 2023). Moreover, by modulating apoptotic pathways, TA exhibits protective effects and supports cell survival mechanisms (Wang et al. 2019). All these reports show that the structural integrity of hepatocytes can be preserved by preventing the factors that cause liver damage with TA. Thus, we hypothesized that these versatile actions position TA as a potent prophylactic agent against MSG exposure-induced alterations in liver tissue.
In the present study, it is aimed to elucidate the molecular mechanisms underlying the protective effects of TA and shed light on potential future therapeutic applications. First, the effects of MSG and TA on healthy rat liver were examined. Subsequently, MSG-induced liver injury model rats were established, and the protective effects of TA on thioredoxin and glutathione systems were investigated.
Materials and methods
Animals and ethics approval
Twenty-four rats (Rattus norvegicus, Sprague–Dawley, male, 180 g ± 10 g) were obtained from the Atatürk University Medical Experimental Application and Research Center (Erzurum, Turkey). The Atatürk University Animal Experiments Local Ethics Committee approved this experimental protocol (protocol no.: 2021–3/63), and all experimental procedures were conducted in compliance with the NIH (National Research Council Committee, 2011) Guide for the Care and Use of Laboratory Animals. Additionally, to improve transparency, the study design and experiments were reported following ARRIVE 2.0 (Animal Research: Reporting of In Vivo Experiments, 2020) guidelines and the 3R/6R principles.
Experimental protocol
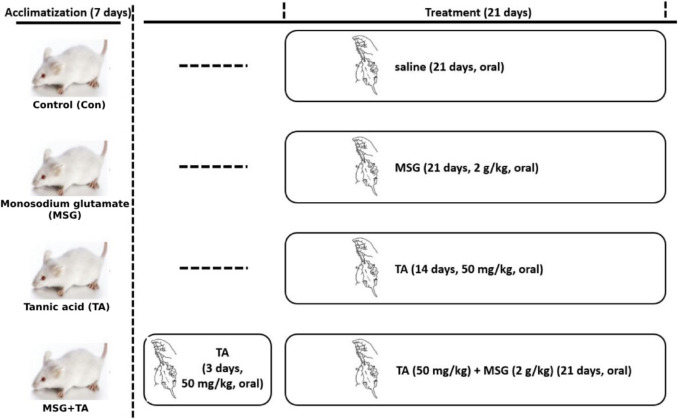
After an acclimatization period, rats were randomly divided into four equal groups (Con, control; MSG, monosodium glutamate; TA, tannic acid; and MSG + TA, combination of monosodium glutamate and tannic acid), each consisting of six animals (Fig. 1). The control group rats were treated with a vehicle (normal saline) for 21 days. Monosodium glutamate (≥ 98.0% purity, Sigma-Aldrich, cat. no. 49621) and tannic acid (≥ 95.0 purity, Thermo Scientific Chemicals, cat. no. 202420050), dissolved in the vehicle and prepared daily, were administered by oral gavage once daily for 21 days at 2 g/kg and 50 mg/kg, respectively. Previous reports indicate that the average daily intake (ADI) of monosodium glutamate (MSG) is around 10 g/day, which is generally considered to have an optimal safety profile. Furthermore, 16.0 mg/kg of body weight consumption has been identified as the no observed adverse effect level (NOAEL) (Beyreuther et al. 2007). Despite the recommended daily limit for adult MSG intake being less than 6 g/day (Thongsepee et al. 2022), several in vivo studies have shown that even lower doses can result in harmful effects, including cardiotoxicity (Hazzaa et al. 2020), renal toxicity (Koohpeyma et al. 2021), neurotoxicity (Hazarika et al. 2022), and hepatotoxicity (Omogbiya et al. 2021). Therefore, rats in the MSG group were orally exposed to 2 g/kg (lower than ADI and NOAEL) of MSG. TA dose was also chosen based on previous research (Biney et al. 2022; Tüzmen et al. 2015). MSG and TA were applied simultaneously to the combined group (MSG + TA) animals for 21 days. To improve the prophylactic effect of the TA, it was administered to the rats in the combined group 1 h before MSG (Al-Jaouni et al. 2019). All groups were housed in plastic cages under standard conditions (free access to diet and tap water, etc.). The rats were euthanized under ketamine/xylazine (3:1) anesthesia, and liver tissues were immediately removed and washed with cold phosphate-buffered saline and kept at − 80 °C for subsequent studies.
Fig. 1.
Experimental protocol of the study
Assessment of oxidative stress indicators
To examine the oxidative stress status after MSG exposure and TA treatment in the liver tissues of untreated and other experimental rat groups, malondialdehyde (MDA; secondary products of lipid peroxidation) levels and total glutathione (GSH + GSSG) contents, which are biomarkers of oxidative stress, were measured. MDA levels in rat liver tissue were measured at wavelengths of 532 nm according to the thiobarbituric acid method as described previously (Suleyman et al. 2009) and presented as nanomoles MDA per mg protein. The reduced glutathione (GSH) quantity in tissue samples was measured at wavelengths of 450 nm as recently described (Kocpinar et al. 2020).
Measurements of the enzyme activities
Total protein was determined by the Bradford method (Bradford 1976) using BSA (bovine serum albumin, ≥ 98.0% purity, Sigma-Aldrich, cat. no. A7906, 1 mg/mL) as a standard. To measure glutathione peroxidase (GPx) enzymatic activity, 100 mg of liver tissues were homogenized (Heidolph Silent Crusher M, Germany) in a buffer containing 1 mM EDTA (ethylenediaminetetraacetic acid, ≥ 98.0% purity, Sigma-Aldrich, cat. no. 798681), 1 mM DTT (dithiothreitol, Sigma Supelco, cat. no. 49018), 1 mM PMSF (phenylmethylsulfonyl fluoride, Roche, cat. no. 10837091001), and 50 mM Tris HCl (Yesilkent & Ceylan 2022). To measure glutathione S-transferase (GST) enzymatic activity, the optical density of 10 µL of supernatant of liver tissue homogenate, 20 mM GSH, and 100 mM phosphate buffer contained mixture was measured at 340 nm after adding 6 mM NADP+ (β-nicotinamide adenine dinucleotide phosphate disodium salt, Roche, cat. no. 10128031001) (Oztay et al. 2020). Glutathione reductase (GR) activity was measured by the modified method of Carlberg and Mannervik (Carlberg & Mannervik 1985) as previously described (Budak et al. 2014). Thioredoxin reductase (TrxR) activity measurement was performed by modifying the method developed by Arner and Holmgren (Arner & Holmgren 2006) as previously described (Kansu et al. 2024).
Quantitative real-time PCR (qPCR) analysis
For relative quantification of target genes mRNA expression, firstly, total RNA was extracted from the rat liver using a commercial total RNA extraction kit (Biorad, Hercules, CA, USA) following the manufacturer’s instruction. Then, the cDNA library was constructed using the iScript cDNA synthesis kit (Biorad, cat. no. 1708891) following the manufacturer’s recommendations. To detect gene expression, pairs of specific primers (Table 1) were designed using the Primer3 (https://bioinfo.ut.ee/primer3-0.4.0/, accessed on 15 June 2024) online tool (Untergasser et al. 2012). The binding specificities of the determined primer sequences were confirmed using the https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 15 June 2024) module. For relative quantification, the SYBR Green-based qPCR assay was performed using SsoAdvanced™ Universal SYBR® Green Supermix (Biorad, cat. no. Biorad, cat. no. 1708891). The 10 µL PCR reaction volume contained cDNA, forward and reverse primers (250–500 nM each), and 1X SsoAdvanced™ Universal SYBR ® Green Supermix (2 ×). Polymerase activation and DNA denaturation occurred for 2 min at 95 °C, amplification for 40 cycles, with denaturation for 15 s at 95 °C, and annealing and extension at 60 °C for 20 s. Gapdh (NM_017008.3) was used as a housekeeping control. The comparative ΔΔCt method (Livak & Schmittgen 2001) was used for the relative quantification of gene expression.
Table 1.
Primer sets used in qPCR. F, forward; R, reverse; Tm, melting temperature
| Gene symbol | Accession ID | Sequence | Tm (°C) |
|---|---|---|---|
|
Gpx_F Gpx _R |
NM_030826.4 | 5′-TCGGACATCAGGAGAATGG-3′ | 59.57 |
| 5′-AGGTAAAGAGCGGGTGAGC-3′ | 59.44 | ||
|
Gst_F Gst_R |
NM_001010921.1 | 5′-TTCTGACCCCTTTCCCTCTG-3′ | 59.67 |
| 5′-TGGCTGGCTTTCTCTGACTG-3′ | 59.97 | ||
|
Gr_F Gr_R |
NM_031632.1 | 5′-TGTGGTGGTGAGCAGAAAGA-3′ | 60.26 |
| 5′-TCCTGGTATGGGACAGCATC-3′ | 59.95 | ||
|
Txnrd_F Txnrd_R |
NM_022584.3 | 5′-AAGCCGTGCAAAACCATGTG-3′ | 59.97 |
| 5′-ACCGTGAACTGTGTGCTCGT-3′ | 60.04 | ||
|
Gapdh_F Gapdh_R |
NM_017008.3 | 5′-AAACCCATCACCATCTTCCA-3′ | 60.17 |
| 5′-ATACTCAGCACCAGCATCACC-3′ | 60.16 |
Statistical analysis
Statistical comparison of data obtained from measurements made in triplicate (for each animal and sample) was evaluated with one-way ANOVA and Tukey’s post hoc test using Prism (GraphPad Software, San Diego, CA) software. Significant difference between groups (compared to the control group) are indicated with asterisks. The statistically significant differences are presented as follows: nsp > 0.05 (not significant), *p < 0.05 (significant), **p < 0.01 (very significant), *** or ****p < 0.001 or 0.0001 (extremely significant).
Results
Effects of MSG and TA administration on liver MDA and total GSH content
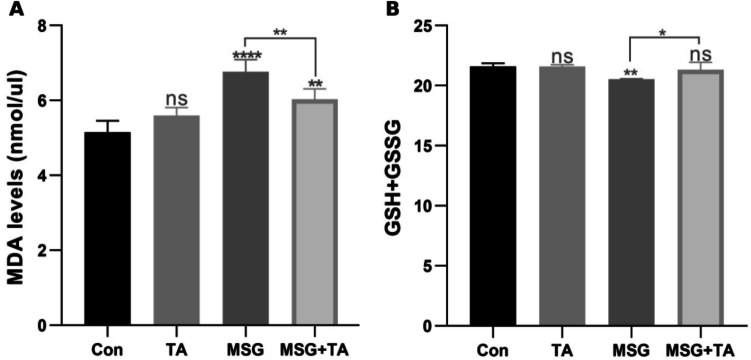
To explore the effects of MSG and TA on the redox balance in rat liver, lipid peroxidation and total glutathione contents were analyzed. First, the concentration of MDA was measured in the livers of rats treated with MSG, both alone and combined with TA. As shown in Fig. 2A, a significant increase in MDA levels was observed in the livers of rats in the MSG-only group compared to the control group. In contrast, TA administration alone did not result in a significant increase in MDA levels. Additionally, TA effectively reduced the increase in lipid peroxidation induced by MSG. The results presented here clearly show that the MSG exposure may cause lipid peroxidation, which is induced by an excess of ROS in the rat liver. When examining GSH levels, it was found that MSG treatment alone significantly decreased total GSH content, while TA alone did not have an effect on GSH levels (Fig. 2B). Moreover, the simultaneous administration of TA with MSG reversed the reduction in GSH levels caused by MSG. Our results have evidence of the disruption of glutathione homeostasis after MSG exposure. However, the results presented here revealed that TA treatment enabled the stabilization of glutathione depletion.
Fig. 2.
Malondialdehyde (MDA) levels and total glutathione (GSH + GSSG) content in the rat liver tissues. MDA levels in liver tissues (A) and comparison of total GSH content (B). ns represents p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 vs control group. The data are shown as mean ± SEM (n = 5)
Glutathione system response in liver tissue after MSG and TA administration
The effects of MSG and TA on antioxidant system components were studied at both gene and protein levels. As illustrated in Fig. 3A and B, the marked decrease in Gpx mRNA expression caused by MSG was countered by TA treatment. A similar pattern was observed in GPx enzyme activity. The decline in enzyme activity induced by MSG was significantly restored by TA, matching the control group’s levels. Gst mRNA expression was notably reduced by MSG exposure, but TA alone did not impact it. Moreover, the combination of MSG and TA significantly mitigated the severe reduction in gene expression compared to MSG alone (Fig. 3C). It was also found that GST enzymatic activity significantly decreased following MSG administration (Fig. 3D). A similar result was observed for glutathione reductase. Gr mRNA expression, which was suppressed by MSG exposure alone, rebounded with TA supplementation (Fig. 3E). Additionally, GR enzyme activity was significantly reduced after MSG exposure. However, TA supplementation steadily increased enzyme activity (Fig. 3F). These findings suggest that MSG may increase cellular oxidative stress by suppressing the antioxidant defense system, and TA may significantly alleviate these adverse effects both at the gene level and in terms of enzyme activity, thus renormalizing the cellular redox level.
Fig. 3.
Effects of MSG and TA on the mRNA expression and specific activities of glutathione metabolism members in the rat liver tissues. The relative mRNA expressions of Gpx, glutathione peroxidase (A), Gst, glutathione S-transferase (C), and Gr, glutathione reductase (E). The enzymatic activities of GPx (B), GST (D), and GR (F) after saline and TA, MSG, and MSG + TA treatment. ns represents p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 vs control group. The data are shown as mean ± SEM (n = 5)
Thioredoxin system response in liver tissue after MSG and TA administration
Lastly, the levels of thioredoxin reductase mRNA and its enzyme activity were assessed. MSG exposure significantly suppressed Txnrd mRNA expression. However, the co-administration of TA with MSG reversed this reduction in gene expression (Fig. 4A). Similar results were observed for thioredoxin reductase activity. Enzyme activity, significantly suppressed by MSG, rebounded with TA treatment (Fig. 4B). These findings indicate that MSG may trigger oxidative stress by suppressing the expression and activity of thioredoxin reductase, an important antioxidant enzyme in the liver. However, TA treatment reverses this suppression, strengthens the antioxidant capacity of the liver, and suggests that it may be an effective candidate for protection against MSG-induced damage.
Fig. 4.
Effects of MSG and TA on the mRNA expression and specific activities of thioredoxin metabolism members in the rat liver tissues. The relative mRNA expressions of Txnrd; thioredoxin reductase (A). The enzymatic activities of TrxR (B) after saline, TA, MSG, and MSG + TA treatment. ns represents p > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 vs control group. The data are shown as mean ± SEM (n = 5)
Discussion
As the world continues to grow, it is not surprising that the demand for food production will increase. In parallel with the global population growth, food safety and sustainability concerns are increasing (Oluwole et al. 2023). In addition to meeting the increasing demand, food additives, frequently preferred in the food industry to improve the texture of foods and extend their shelf life, raise concerns about their health effects (Muncke et al. 2020). Therefore, to overcome these concerns, it is necessary to determine sustainable food systems that encourage healthy dietary habits. MSG, which is widely used, especially in processed foods today, is an additive that is associated with various health problems. In addition to the metabolic and neurological effects of MSG consumption, it is suggested that it can affect the functions of critical organs, such as the kidneys and liver (Maulina et al. 2024; Subramanian et al. 2023; Yoshida et al. 2024). Recent studies have suggested that MSG consumption may cause changes in liver function, particularly through oxidative stress. Recent studies indicate that TA exhibits potent antioxidant activity through multiple mechanisms (Jing et al. 2022; Tong et al. 2021). TA can protect DNA and proteins from oxidative damage by directly scavenging hydroxyl radicals and reactive oxygen species such as hydrogen peroxide by donating electrons or hydrogen atoms (Wang et al. 2020). In addition, TA upregulates endogenous antioxidant enzymes mostly through the activation of transcription factors such as Nrf2 that bind to antioxidant response elements (ARE) in gene promoters (Jin et al. 2020; Li et al. 2020). TA can further reduce ROS production by inhibiting pro-oxidant enzymes, such as xanthine oxidase and NADPH oxidase (Azimullah et al. 2023). Lastly, TA helps maintain intracellular glutathione balance by reducing its consumption or supporting its regeneration (Laskar et al. 2023). Due to these properties, TA is seen as an ideal candidate to reduce MSG-induced cellular oxidative damage by strengthening antioxidant defenses.
Cells constantly face the challenge of managing oxidants, which can originate from environmental and endogenous factors (Demir et al. 2024; Pizzino et al. 2017). However, their antioxidant networks balance both the production and elimination of reactive oxygen species (ROS). This situation may vary depending on the state of the cell. For example, while ROS elimination is desired for the maintenance of life in healthy cells, the opposite is aimed at in cancer cells to promote ROS production. In this way, the growth and proliferation of tumor cells can be prevented (Nakamura & Takada 2021; Zhou et al. 2014). Glutathione (GSH) and thioredoxin (TRX) systems are essential antioxidant defense mechanisms that maintain cellular redox balance by neutralizing ROS (Ren et al. 2017). The GSH system uses glutathione, a tripeptide composed of glutamate, cysteine, and glycine, as a reducing agent to convert excess ROS into non-toxic compounds, thereby preserving cellular homeostasis (Cassier-Chauvat et al. 2023). The balance between GSH and GSSG is commonly used to assess oxidative stress and the overall redox state within cells, tissues, and bodily fluids. A lower GSH/GSSG ratio indicates heightened oxidative stress, which may lead to a range of diseases and health conditions (Arauz et al. 2016; Nuhu et al. 2020). The TRX system, which includes the redox-active protein Trx and its associated reductase (TrxR), plays a central role in regulating cellular redox balance by catalyzing the reduction of disulfide bonds in target proteins (Drechsel & Patel 2010; Muri & Kopf 2023). As a result, both systems, which are interconnected, ensure tightly regulated redox balance through cross-talk mechanisms. Studies have shown that dysregulation of these antioxidant systems leads to imbalances in several cellular mechanisms, particularly oxidative stress, and contributes to the development of distinct features in various cancers, including hepatocellular carcinoma (HCC) (Abdel-Hamid et al. 2018; Jaganjac et al. 2020; McLoughlin et al. 2019). Recent reports indicate that these systems are upregulated in HCC to help cancer cells cope with oxidative stress induced by their altered metabolism (Dong et al. 2022; Lee et al. 2019). The upregulation of antioxidant systems in cancer cells helps prevent apoptosis and enhances drug resistance, thereby supporting their survival. Furthermore, the increased activity of antioxidant systems in HCC is thought to be a potential therapeutic target. Indeed, inhibiting key components of the GSH and TRX systems, such as GR and TrxR, could increase ROS levels in cancer cells to lethal levels, leading to cell death (Salmain et al. 2023; Xu et al. 2022). However, this suggests that an opposite modulation is required for healthy cells. Control of ROS balance may help protect healthy cells from DNA damage, activation of oncogenic signaling pathways, and mutagenesis, processes that threaten cell viability (Cai et al. 2024). Therefore, fully understanding the roles of the GSH and TRX systems in HCC and identifying the factors leading to their dysregulation is crucial for developing effective therapeutic strategies against liver cancer formation and progression.
In the context of HCC, dysregulation of the GSH and TRX systems is a common adaptive response to abnormal oxidative stress, which disrupts healthy cell metabolism (Brahma et al. 2021). It is thought that the pro-oxidant effect caused by MSG will increase oxidative stress and trigger a faster and more destructive progression of such a scenario. Total glutathione and malondialdehyde levels, which are critical markers of cellular redox balance and are associated with lipid peroxidation, can provide insight into the performance of the antioxidant defense system. Many studies have reported that low GSH and elevated MDA levels correlate with impaired antioxidant metabolism (Chaves et al. 2019; Sahin et al. 2023). In this context, experimental studies conducted with models that have caused hepatic injury with MSG show that MSG causes liver deficits through the disruption of the glutathione system (Moldovan et al. 2023; Shukry et al. 2020).
Previous studies have demonstrated acute inhibition or depletion of TrxR sensitizes rat cells to ROS such as hydrogen peroxide (Stancill et al. 2019, 2020). Studies conducted by creating genetic inhibition and/or ablation models for hepatospecific TrxR1 signaling have reported significant alterations in the antioxidant response pathway (Cebula et al. 2015; Suvorova et al. 2009). These previous studies suggest that TrxR is necessary for the cell defense against ROS. When the current literature was examined, no study was found on the MSG-TrxR-liver axis. When evaluated from this perspective, we think that the current study is the first report investigating the effect of MSG on TrxR in liver tissue. The results presented in this study indicate that long-term MSG exposure may disrupt the usual regulation of both GSH and TrxR system components, suppressing ROS neutralization and increasing the likelihood of HCC development (Fig. 5).
Fig. 5.
Simplified scheme of the study. Oxidative modulation in liver tissue after MSG exposure and TA treatment. MSG triggers hepatotoxicity by disrupting oxidative modulation in liver tissues. TA restores GST, GPx, GR, and TRXR gene expression and biological activities, promotes lipid peroxidation reduction, and increases glutathione levels
Conclusion
The current tendency in protecting healthy cells against HCC development is towards using treatment strategies such as preventing oxidative stress, which directly affects cell viability. Although the mechanisms behind MSG-induced liver injury are becoming clearer, effectively addressing and mitigating its harmful effects continues to be a major challenge in nutrition-related toxicity, with many aspects still to be uncovered. The results presented in this study show that MSG is an actor that hurts both glutathione and thioredoxin systems. However, further in-depth research, interdisciplinary collaboration, and human studies are vital to improving quality of life, clarifying effective dosages, and optimizing bioavailability. In particular, detailed dose–response investigations are needed to determine the optimal and safe concentrations for biological efficacy. Additionally, studies focusing on the bioavailability, metabolism, and tissue distribution of tannic acid are critical to fully understand its in vivo effectiveness and to guide its potential clinical application.
Authors contributions
MAAA, MSK, and ENY performed the experiments, acquired and analyzed the data. HC designed the study and wrote the manuscript. All authors have read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Data availability
All source data for this work (or generated in this study) are available upon reasonable request.
Declarations
Ethics approval
All of the experimental procedures were performed under the guidelines outlined by the National Research Council’s Guide for the Care and Use of Laboratory Animals and were approved by the Atatürk University Local Ethics Council for Animal Experiments (protocol no.: 2021–3/63).
Consent to participate
This article does not contain any studies involving human participants performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aatif M (2023) Current understanding of polyphenols to enhance bioavailability for better therapies. Biomedicines 11(7):2078. 10.3390/biomedicines11072078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Hamid NM, Mahmoud TK, Abass SA, El-Shishtawy MM (2018) Expression of thioredoxin and glutaredoxin in experimental hepatocellular carcinoma-Relevance for prognostic and diagnostic evaluation. Pathophysiology 25(4):433–438. 10.1016/j.pathophys.2018.08.008 [DOI] [PubMed] [Google Scholar]
- Al-Jaouni S, Abdul-Hady S, El-Bassossy H, Salah N, Hagras M (2019) Ajwa nanopreparation prevents doxorubicin-associated cardiac dysfunction: effect on cardiac ischemia and antioxidant capacity. Integr Cancer Ther 18:1534735419862351. 10.1177/1534735419862351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arauz J, Ramos-Tovar E, Muriel P (2016) Redox state and methods to evaluate oxidative stress in liver damage: from bench to bedside. Ann Hepatol 15(2):160–173. 10.5604/16652681.1193701 [DOI] [PubMed] [Google Scholar]
- Arner ES, Holmgren A (2006) The thioredoxin system in cancer. Semin Cancer Biol 16(6):420–426. 10.1016/j.semcancer.2006.10.009 [DOI] [PubMed] [Google Scholar]
- Asejeje FO, Gabriel GO, Abiola MA (2023) Monosodium glutamate aggravates lipopolysaccharide-induced liver injury via inflammation and oxidative stress in rats. Nutrire 48(1). 10.1186/s41110-023-00188-w
- Azimullah S, Meeran MFN, Ayoob K, Arunachalam S, Ojha S, Beiram R (2023) Tannic acid mitigates rotenone-induced dopaminergic neurodegeneration by inhibiting inflammation, oxidative stress, apoptosis, and glutamate toxicity in rats. Int J Mol Sci 24(12):9876. 10.3390/ijms24129876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin A, Booth BW (2022) Biomedical applications of tannic acid. J Biomater Appl 36(8):1503–1523. 10.1177/08853282211058099 [DOI] [PubMed] [Google Scholar]
- Banerjee A, Mukherjee S, Maji BK (2021) Monosodium glutamate causes hepato-cardiac derangement in male rats. Human Exp Toxicol 40(12_suppl):S359–S369. 10.1177/09603271211049550 [DOI] [PubMed] [Google Scholar]
- Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, Heinemann U, Kempski O, Stehle P, Steinhart H, Walker R (2007) Consensus meeting: monosodium glutamate - an update. Eur J Clin Nutr 61(3):304–313. 10.1038/sj.ejcn.1602526 [DOI] [PubMed] [Google Scholar]
- Biney RP, Djankpa FT, Osei SA, Egbenya DL, Aboagye B, Karikari AA, Ussif A, Wiafe GA, Nuertey D (2022) Effects of in utero exposure to monosodium glutamate on locomotion, anxiety, depression, memory and KCC2 expression in offspring. Int J Dev Neurosci 82(1):50–62. 10.1002/jdn.10158 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. 10.1006/abio.1976.9999 [DOI] [PubMed] [Google Scholar]
- Brahma MK, Gilglioni EH, Zhou L, Trepo E, Chen P, Gurzov EN (2021) Oxidative stress in obesity-associated hepatocellular carcinoma: sources, signaling and therapeutic challenges. Oncogene 40(33):5155–5167. 10.1038/s41388-021-01950-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budak H, Gonul N, Ceylan H, Kocpinar EF (2014) Impact of long term Fe(3)(+) toxicity on expression of glutathione system in rat liver. Environ Toxicol Pharmacol 37(1):365–370. 10.1016/j.etap.2013.12.007 [DOI] [PubMed] [Google Scholar]
- Cai H, Meng Z, Yu F (2024) The involvement of ROS-regulated programmed cell death in hepatocellular carcinoma. Crit Rev Oncol Hematol 197:104361. 10.1016/j.critrevonc.2024.104361 [DOI] [PubMed] [Google Scholar]
- Carlberg I, Mannervik B (1985) Glutathione reductase. Methods Enzymol 113:484–490. 10.1016/s0076-6879(85)13062-4 [DOI] [PubMed] [Google Scholar]
- Cassier-Chauvat C, Marceau F, Farci S, Ouchane S, Chauvat F (2023) The glutathione system: a journey from cyanobacteria to higher eukaryotes. Antioxidants (Basel) 12(6):1199. 10.3390/antiox12061199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebula M, Schmidt EE, Arner ES (2015) TrxR1 as a potent regulator of the Nrf2-Keap1 response system. Antioxid Redox Signal 23(10):823–853. 10.1089/ars.2015.6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves NA, Alegria TGP, Dantas LS, Netto LES, Miyamoto S, BoniniDomingos CR, da Silva DGH (2019) Impaired antioxidant capacity causes a disruption of metabolic homeostasis in sickle erythrocytes. Free Radical Biol Med 141:34–46. 10.1016/j.freeradbiomed.2019.05.034 [DOI] [PubMed] [Google Scholar]
- Chu X, Wang H, Jiang YM, Zhang YY, Bao YF, Zhang X, Zhang JP, Guo H, Yang F, Luan YC, Dong YS (2016) Ameliorative effects of tannic acid on carbon tetrachloride-induced liver fibrosis in vivo and in vitro. J Pharmacol Sci 130(1):15–23. 10.1016/j.jphs.2015.12.002 [DOI] [PubMed] [Google Scholar]
- Dare RG, Nakamura CV, Ximenes VF, Lautenschlager SOS (2020) Tannic acid, a promising anti-photoaging agent: evidences of its antioxidant and anti-wrinkle potentials, and its ability to prevent photodamage and MMP-1 expression in L929 fibroblasts exposed to UVB. Free Radical Biol Med 160:342–355. 10.1016/j.freeradbiomed.2020.08.019 [DOI] [PubMed] [Google Scholar]
- Demir Y, Öztürk N, Isiyel M, Ceylan H (2024) Effects of carnosic and usnic acid on pentose phosphate pathway enzymes: an experimental and molecular docking study. Chemistryselect 9(27). 10.1002/slct.202401067
- Dong G, Ye X, Wang S, Li W, Cai R, Du L, Shi X, Li M (2022) Au-24 as a potential thioredoxin reductase inhibitor in hepatocellular carcinoma cells. Pharmacol Res 177:106113. 10.1016/j.phrs.2022.106113 [DOI] [PubMed] [Google Scholar]
- Drechsel DA, Patel M (2010) Respiration-dependent H2O2 removal in brain mitochondria via the thioredoxin/peroxiredoxin system. J Biol Chem 285(36):27850–27858. 10.1074/jbc.M110.101196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustman C, Aaron D, Negowetti N, Leib EB (2021) Ten years post-GAO assessment, FDA remains uninformed of potentially harmful GRAS substances in foods. Crit Rev Food Sci Nutr 61(8):1260–1268. 10.1080/10408398.2020.1756217 [DOI] [PubMed] [Google Scholar]
- Fraga-Corral M, Otero P, Cassani L, Echave J, Garcia-Oliveira P, Carpena M, Chamorro F, Lourenco-Lopes C, Prieto MA, Simal-Gandara J (2021) Traditional applications of tannin rich extracts supported by scientific data: chemical composition, bioavailability and bioaccessibility. Foods 10(2):251. 10.3390/foods10020251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z, Xie W, Lu J, Guo X, Xu J, Xu W, Chi Y, Takuya N, Wu H, Zhao L (2021) Tannic acid-based metal phenolic networks for bio-applications: a review. J Mater Chem B 9(20):4098–4110. 10.1039/d1tb00383f [DOI] [PubMed] [Google Scholar]
- Hazarika I, Mukundan GK, Sundari PS (2022) Neuroprotective effect of hydrocotyle sibthorpioides against monosodium glutamate-induced excitotoxicity. Nat Prod Res 36(23):6156–6159. 10.1080/14786419.2022.2057493 [DOI] [PubMed] [Google Scholar]
- Hazzaa SM, El-Roghy ES, AbdEldaim MA, Elgarawany GE (2020) Monosodium glutamate induces cardiac toxicity via oxidative stress, fibrosis, and P53 proapoptotic protein expression in rats. Environ Sci Pollut Res Int 27(16):20014–20024. 10.1007/s11356-020-08436-6 [DOI] [PubMed] [Google Scholar]
- Jaganjac M, Milkovic L, Sunjic SB, Zarkovic N (2020) The NRF2, Thioredoxin, and glutathione system in tumorigenesis and anticancer therapies. Antioxidants (Basel) 9(11):1151. 10.3390/antiox9111151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Xue Y, Xue Y, Han X, Song Q, Zhang J, Li Z, Cheng J, Guan S, Sun S, Chu L (2020) Tannic acid ameliorates arsenic trioxide-induced nephrotoxicity, contribution of NF-kappaB and Nrf2 pathways. Biomed Pharmacother 126:110047. 10.1016/j.biopha.2020.110047 [DOI] [PubMed] [Google Scholar]
- Jing W, Xiaolan C, Yu C, Feng Q, Haifeng Y (2022) Pharmacological effects and mechanisms of tannic acid. Biomed Pharmacother 154:113561. 10.1016/j.biopha.2022.113561 [DOI] [PubMed] [Google Scholar]
- Kansu G, Ozturk N, Karagac MS, Yesilkent EN, Ceylan H (2024) The interplay between doxorubicin chemotherapy, antioxidant system, and cardiotoxicity: unrevealing of the protective potential of tannic acid. Biotechnol Appl Biochem. 10.1002/bab.2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karadas H, Tosun H, Ceylan H (2024) Identification of dilated cardiomyopathy-linked key genes by bioinformatics methods and evaluating the impact of tannic acid and monosodium glutamate in rats. Biotechnol Appl Biochem. 10.1002/bab.2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagaç MS, Yesilkent EN, Kizir D, Öztürk N, Isiyel M, Karadas H, Tosun H, Karaman M, Ceylan H, Demir Y (2024) Esculetin improves inflammation of the kidney via gene expression against doxorubicin-induced nephrotoxicity in rats: in vivo and in silico studies. Food Biosci 62:105159. 10.1016/j.fbio.2024.105159 [Google Scholar]
- Karagac MS, Ceylan H (2023) Neuroprotective potential of tannic acid against neurotoxic outputs of monosodium glutamate in rat cerebral cortex. Neurotox Res 41(6):670–680. 10.1007/s12640-023-00667-y [DOI] [PubMed] [Google Scholar]
- Kizir D, Karaman M, Ceylan H (2023) Tannic acid may ameliorate doxorubicin-induced changes in oxidative stress parameters in rat spleen. Naunyn-Schmiedebergs Arch Pharmacol 396(12):3605–3613. 10.1007/s00210-023-02563-w [DOI] [PubMed] [Google Scholar]
- Kizir D, Karaman M, Demir Y, Ceylan H (2024) Effect of tannic acid on doxorubicin-induced cellular stress: expression levels of heat shock genes in rat spleen. Biotechnol Appl Biochem 71(6):1339–1345. 10.1002/bab.2633 [DOI] [PubMed] [Google Scholar]
- Kocpinar EF, Baltaci NG, Ceylan H, Kalin SN, Erdogan O, Budak H (2020) Effect of a prolonged dietary iron intake on the gene expression and activity of the testicular antioxidant defense system in rats. Biol Trace Elem Res 195(1):135–141. 10.1007/s12011-019-01817-0 [DOI] [PubMed] [Google Scholar]
- Koohpeyma F, Siri M, Allahyari S, Mahmoodi M, Saki F, Dastghaib S (2021) The effects of L-carnitine on renal function and gene expression of caspase-9 and Bcl-2 in monosodium glutamate-induced rats. BMC Nephrol 22(1):162. 10.1186/s12882-021-02364-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Nirmal P, Kumar M, Jose A, Tomer V, Oz E, Proestos C, Zeng M, Elobeid T, Sneha K, Oz F (2023) Major phytochemicals: recent advances in health benefits and extraction method. Molecules 28(2):887. 10.3390/molecules28020887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskar P, Dhasmana A, Kotnala S, Jaggi M, Yallapu MM, Chauhan SC (2023) Glutathione-responsive tannic acid-assisted FRET nanomedicine for cancer therapy. Pharmaceutics 15(5):1326. 10.3390/pharmaceutics15051326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Xu IM, Chiu DK, Leibold J, Tse AP, Bao MH, Yuen VW, Chan CY, Lai RK, Chin DW, Chan DF, Cheung TT, Chok SH, Wong CM, Lowe SW, Ng IO, Wong CC (2019) Induction of oxidative stress through inhibition of thioredoxin reductase 1 is an effective therapeutic approach for hepatocellular carcinoma. Hepatology 69(4):1768–1786. 10.1002/hep.30467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Liu P, Xue Y, Liang Y, Shi J, Han X, Zhang J, Chu X, Chu L (2020) Tannic acid attenuates hepatic oxidative stress, apoptosis and inflammation by activating the Keap1-Nrf2/ARE signaling pathway in arsenic trioxide-toxicated rats. Oncol Rep 44(5):2306–2316. 10.3892/or.2020.7764 [DOI] [PubMed] [Google Scholar]
- Lin YH, Lin YC, Hou YT (2023) Prospective application of tannic acid in acetaminophen (APAP)-induced acute liver failure. Int J Mol Sci 25(1):317. 10.3390/ijms25010317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25(4):402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Maulina N, Zachreini I, Gholib G, Suwandi A, Akmal M (2024) Black garlic exhibited hepatoprotective effect against monosodium glutamate-induced hepatotoxicity in animal model. Narra J 4(2):e799. 10.52225/narra.v4i2.799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLoughlin MR, Orlicky DJ, Prigge JR, Krishna P, Talago EA, Cavigli IR, Eriksson S, Miller CG, Kundert JA, Sayin VI, Sabol RA, Heinemann J, Brandenberger LO, Iverson SV, Bothner B, Papagiannakopoulos T, Shearn CT, Arner ESJ, Schmidt EE (2019) TrxR1, Gsr, and oxidative stress determine hepatocellular carcinoma malignancy. Proc Natl Acad Sci USA 116(23):11408–11417. 10.1073/pnas.1903244116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan OL, Vari CE, Tero-Vescan A, Cotoi OS, Cocuz IG, Tabaran FA, Pop R, Fulop I, Chis RF, Lungu IA, Rusu A (2023) Potential defence mechanisms triggered by monosodium glutamate sub-chronic consumption in two-year-old Wistar rats. Nutrients 15(20):4436. 10.3390/nu15204436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muncke J, Andersson AM, Backhaus T, Boucher JM, Carney Almroth B, Castillo Castillo A, Chevrier J, Demeneix BA, Emmanuel JA, Fini JB, Gee D, Geueke B, Groh K, Heindel JJ, Houlihan J, Kassotis CD, Kwiatkowski CF, Lefferts LY, Maffini MV, …. Scheringer M (2020) Impacts of food contact chemicals on human health: a consensus statement. Environ Health: A Global Access Sci Source 19(1):25. 10.1186/s12940-020-0572-5 [DOI] [PMC free article] [PubMed]
- Muri J, Kopf M (2023) The thioredoxin system: Balancing redox responses in immune cells and tumors. Eur J Immunol 53(1):e2249948. 10.1002/eji.202249948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H, Takada K (2021) Reactive oxygen species in cancer: current findings and future directions. Cancer Sci 112(10):3945–3952. 10.1111/cas.15068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council Committee (2011) Guide for the care and use of laboratory animals, 8th edn. Washington, DC. National Academies Press. 10.17226/12910
- Nuhu F, Gordon A, Sturmey R, Seymour AM, Bhandari S (2020) Measurement of glutathione as a tool for oxidative stress studies by high performance liquid chromatography. Molecules 25(18):4196. 10.3390/molecules25184196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluwole O, Ibidapo O, Arowosola T, Raji F, Zandonadi RP, Alasqah I, Lho LH, Han H, Raposo A (2023) Sustainable transformation agenda for enhanced global food and nutrition security: a narrative review. Front Nutr 10:1226538. 10.3389/fnut.2023.1226538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omogbiya AI, Ben-Azu B, Eduviere AT, Eneni AO, Nwokoye PO, Ajayi AM, Umukoro S (2021) Monosodium glutamate induces memory and hepatic dysfunctions in mice: ameliorative role of Jobelyn((R)) through the augmentation of cellular antioxidant defense machineries. Toxicol Res 37(3):323–335. 10.1007/s43188-020-00068-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaolapo OJ, Onaolapo AY, Akanmu MA, Gbola O (2016) Evidence of alterations in brain structure and antioxidant status following ‘low-dose’ monosodium glutamate ingestion. Pathophysiology 23(3):147–156. 10.1016/j.pathophys.2016.05.001 [DOI] [PubMed] [Google Scholar]
- Oztay F, Tunali S, Kayalar O, Yanardag R (2020) The protective effect of vitamin U on valproic acid-induced lung toxicity in rats via amelioration of oxidative stress. J Biochem Mol Toxicol 34(12):e22602. 10.1002/jbt.22602 [DOI] [PubMed] [Google Scholar]
- Ozturk N, Ceylan H, Demir Y (2024) The hepatoprotective potential of tannic acid against doxorubicin-induced hepatotoxicity: insights into its antioxidative, anti-inflammatory, and antiapoptotic mechanisms. J Biochem Mol Toxicol 38(8):e23798. 10.1002/jbt.23798 [DOI] [PubMed] [Google Scholar]
- Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, Squadrito F, Altavilla D, Bitto A (2017) Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017:8416763. 10.1155/2017/8416763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Zou L, Zhang X, Branco V, Wang J, Carvalho C, Holmgren A, Lu J (2017) Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system. Antioxid Redox Signal 27(13):989–1010. 10.1089/ars.2016.6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin B, AcikelElmas M, BingolOzakpinar O, Arbak S (2023) The effects of apocynin on monosodium glutamate induced liver damage of rats. Heliyon 9(7):e17327. 10.1016/j.heliyon.2023.e17327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahiner M, Yilmaz AS, Demirci S, Sahiner N (2023) Physically and chemically crosslinked, tannic acid embedded linear PEI-based hydrogels and cryogels with natural antibacterial and antioxidant properties. Biomedicines 11(3):706. 10.3390/biomedicines11030706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmain M, Gaschard M, Baroud M, Lepeltier E, Jaouen G, Passirani C, Vessieres A (2023) Thioredoxin reductase and organometallic complexes: a pivotal system to tackle multidrug resistant tumors? Cancers 15(18):4448. 10.3390/cancers15184448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shastri M, Raval DM, Rathod VM (2023) Monosodium glutamate (MSG) symptom complex (Chinese restaurant syndrome): nightmare of Chinese food lovers! J Assoc Physicians India 71(6):11–12. 10.5005/japi-11001-0264 [DOI] [PubMed] [Google Scholar]
- Shimada A, Baad-Hansen L, Castrillon E, Ghafouri B, Stensson N, Gerdle B, Ernberg M, Cairns B, Svensson P (2015) Differential effects of repetitive oral administration of monosodium glutamate on interstitial glutamate concentration and muscle pain sensitivity. Nutrition 31(2):315–323. 10.1016/j.nut.2014.07.011 [DOI] [PubMed] [Google Scholar]
- Shukry M, El-Shehawi AM, El-Kholy WM, Elsisy RA, Hamoda HS, Tohamy HG, Abumandour MM, Farrag FA (2020) Ameliorative effect of graviola (Annona muricata) on mono sodium glutamate-induced hepatic injury in rats: antioxidant, apoptotic, anti-inflammatory, lipogenesis markers, and histopathological studies. Animals (Basel) 10(11):1996. 10.3390/ani10111996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancill JS, Broniowska KA, Oleson BJ, Naatz A, Corbett JA (2019) Pancreatic beta-cells detoxify H(2)O(2) through the peroxiredoxin/thioredoxin antioxidant system. J Biol Chem 294(13):4843–4853. 10.1074/jbc.RA118.006219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancill JS, Happ JT, Broniowska KA, Hogg N, Corbett JA (2020) Peroxiredoxin 1 plays a primary role in protecting pancreatic beta-cells from hydrogen peroxide and peroxynitrite. Am J Physiol Regul Integr Comp Physiol 318(5):R1004–R1013. 10.1152/ajpregu.00011.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A, Tamilanban T, Sekar M, Begum MY, Atiya A, Ramachawolran G, Wong LS, Subramaniyan V, Gan SH, Mat Rani NNI, Wu YS, Chinni SV, Fuloria S, Fuloria NK (2023) Neuroprotective potential of Marsilea quadrifolia Linn against monosodium glutamate-induced excitotoxicity in rats. Front Pharmacol 14:1212376. 10.3389/fphar.2023.1212376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukmak M, Kyaw TS, Nahok K, Sharma A, Silsirivanit A, Lert-Itthiporn W, Japrung D, Pinlaor S, Anutrakulchai S, Selmi C, Slupsky CM, Hammock BD, Cha’on U (2024) Urinary metabolic profile and its predictive indexes after MSG consumption in rat. PLoS ONE 19(9):e0309728. 10.1371/journal.pone.0309728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleyman H, Cadirci E, Albayrak A, Polat B, Halici Z, Koc F, Hacimuftuoglu A, Bayir Y (2009) Comparative study on the gastroprotective potential of some antidepressants in indomethacin-induced ulcer in rats. Chem Biol Interact 180(2):318–324. 10.1016/j.cbi.2009.03.002 [DOI] [PubMed] [Google Scholar]
- Suvorova ES, Lucas O, Weisend CM, Rollins MF, Merrill GF, Capecchi MR, Schmidt EE (2009) Cytoprotective Nrf2 pathway is induced in chronically txnrd 1-deficient hepatocytes. PLoS ONE 4(7):e6158. 10.1371/journal.pone.0006158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongsepee N, Martviset P, Chantree P, Sornchuer P, Sangpairoj K, Prathaphan P, Ruangtong J, Hiranyachattada S (2022) Daily consumption of monosodium glutamate pronounced hypertension and altered renal excretory function in normotensive and hypertensive rats. Heliyon 8(10):e10972. 10.1016/j.heliyon.2022.e10972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongsepee N, Himakhun W, Kankul K, Martviset P, Chantree P, Sornchuer P, Ruangtong J, Hiranyachattada S (2024) Monosodium glutamate altered renal architecture and modulated expression of NMDA-R, eNOS, and nNOS in normotensive and hypertensive rats. Food Chem Toxicol 189:114763. 10.1016/j.fct.2024.114763 [DOI] [PubMed] [Google Scholar]
- Tong Z, He W, Fan X, Guo A (2021) Biological function of plant tannin and its application in animal health. Front Vet Sci 8:803657. 10.3389/fvets.2021.803657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tüzmen MN, Yücel NC, Kalburcu T, Demiryas N (2015) Effects of curcumin and tannic acid on the aluminum- and lead-induced oxidative neurotoxicity and alterations in NMDA receptors. Toxicol Mech Methods 25(2):120–127. 10.3109/15376516.2014.997947 [DOI] [PubMed] [Google Scholar]
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, Rozen SG (2012) Primer3–new capabilities and interfaces. Nucleic Acids Res 40(15):e115. 10.1093/nar/gks596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varesi A, Chirumbolo S, Campagnoli LIM, Pierella E, Piccini GB, Carrara A, Ricevuti G, Scassellati C, Bonvicini C, Pascale A (2022) The role of antioxidants in the interplay between oxidative stress and senescence. Antioxidants (Basel) 11(7):1224. 10.3390/antiox11071224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Xiao H, Zhu Y, Liu S, Yuan Z, Wu J, Wen L (2019) Tannic acid induces the mitochondrial pathway of apoptosis and S phase arrest in porcine intestinal IPEC-J2 cells. Toxins 11(7):397. 10.3390/toxins11070397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ji M, Zhai H, Liang Y (2020) Electron donating capacities of DOM model compounds and their relationships with chlorine demand, byproduct formation, and other properties in chlorination. Chemosphere 261:127764. 10.1016/j.chemosphere.2020.127764 [DOI] [PubMed] [Google Scholar]
- Xu Z, Xu J, Sun S, Lin W, Li Y, Lu Q, Li F, Yang Z, Lu Y, Liu W (2022) Mecheliolide elicits ROS-mediated ERS driven immunogenic cell death in hepatocellular carcinoma. Redox Biol 54:102351. 10.1016/j.redox.2022.102351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilkent EN, Ceylan H (2022) Investigation of the multi-targeted protection potential of tannic acid against doxorubicin-induced kidney damage in rats. Chem Biol Interact 365:110111. 10.1016/j.cbi.2022.110111 [DOI] [PubMed] [Google Scholar]
- Yoshida S, Chao H, Takumi A, Kohmura M (2024) Monosodium glutamate added to food does not induce damage to the pancreas nor aggravate diabetes due to enhancement of oxidative stress. J Biochem Mol Toxicol 38(11):e23859. 10.1002/jbt.23859 [DOI] [PubMed] [Google Scholar]
- Zhang J, Song Q, Han X, Zhang Y, Zhang Y, Zhang X, Chu X, Zhang F, Chu L (2017) Multi-targeted protection of acetaminophen-induced hepatotoxicity in mice by tannic acid. Int Immunopharmacol 47:95–105. 10.1016/j.intimp.2017.03.027 [DOI] [PubMed] [Google Scholar]
- Zhou D, Shao L, Spitz DR (2014) Reactive oxygen species in normal and tumor stem cells. Adv Cancer Res 122:1–67. 10.1016/B978-0-12-420117-0.00001-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All source data for this work (or generated in this study) are available upon reasonable request.