Abstract
Metastatic uveal melanomas are highly resistant to all existing treatments. To identify actionable vulnerabilities, we conducted a CRISPR-Cas9 knockout screen using a library composed of chromatin regulators. We revealed that the lysine methyltransferase, SETDB1, plays a critical role in metastatic uveal melanoma cell proliferation and survival. Functionally, SETDB1 deficiency induces a DNA damage response, senescence-like state and growth arrest. Knockdown of SETDB1 is associated with a decreased expression of genes related to replication and cell cycle. Moreover, deficiency in CDC6, an essential regulator of DNA replication, phenocopies SETDB1 inhibition. Using a pre-clinical model, we further demonstrated that anti-SETDB1 therapy impairs tumor growth in vivo. Therefore, we not only provide evidence that SETDB1 plays a critical role in metastatic uveal melanoma cell growth, but we also identify SETDB1 as a novel relevant therapeutic target for the treatment of metastatic uveal melanoma.
Subject terms: Eye cancer, DNA replication
Introduction
Uveal melanoma is the most common primary intraocular malignancy in adults and a deadly neoplasm. Despite successful treatment of the primary lesion by proton therapy or enucleation, up to 50% of uveal melanoma patients develop metastases, predominantly in the liver (reviewed in [1]). Metastatic uveal melanomas are highly refractory to existing treatments. Recently, tebentafusp (Kimmtrak), a bispecific protein immunotherapy targeting CD3 and the melanoma antigen GP100, has been shown to improve the overall survival of patients with metastatic uveal melanoma [2]. However, tebentafusp treatment is limited to patients with an HLA-A*02:01 haplotype and demonstrated benefit in a small subset of them [3]. To date, ninety percent of patients with metastatic uveal melanoma die within 6 months after diagnosis of metastases, highlighting an unmet clinical need including HLA-independent strategies. The characterization of novel oncogenic molecular mechanisms driving uveal melanoma progression and treatment resistance is essential to improve patients’ survival.
The main oncogenic drivers in uveal melanomas are mutations in the heterotrimeric G-protein alpha subunit GNAQ or its paralog GNA11 (GNAQ/11). Ninety percent of uveal melanomas harbor a mutation in one of these two genes [4]. The most frequent GNAQ and GNA11 mutation is the substitution of glutamate at position 209 by proline or leucine (GNAQ/11Q209P/L) that results in loss of GTPase activity, producing constitutive activation of GNAQ/GNA11. Over the past decade, studies dissecting the molecular mechanisms of uveal melanoma progression have revealed that mutant GNAQ/11 signals through activation of broad downstream signaling modules, including PLCβ-PKC, MEK-ERK, and Hippo-YAP (reviewed in [1]), making GNAQ/11 and/or their downstream pathways attractive targets for anti-uveal melanoma therapies. While drugs targeting these pathways, either alone or in combination, impair uveal melanoma cell growth in vitro, clinical trials have showed limited, if any, efficacy [5]. Thus, advances in the molecular characterization of uveal melanoma over these years have not yet translated into effective therapeutic strategies to prevent or eliminate metastasis. Hence, it remains essential to identify pivotal players in metastatic uveal melanoma proliferation and survival that would be amenable to therapeutic intervention.
Complementary to GNAQ/11 driver mutations, uveal melanoma is characterized by secondary alterations, the most frequent is the loss of the tumor suppressor BRCA1-associated protein-1 (BAP1) gene. BAP1 loss is associated with a high metastatic risk and a poor prognosis [4, 6]. BAP1 is a deubiquitinase with a substrate preference for histone H2A lysine 119 (H2AK119), meaning that BAP1 loss triggers accumulation of H2AK119 mono-ubiquitination, which in turn promotes transcriptional repression [7]. Accumulating evidence indicate that epigenetic changes play important role in cancer progression, but also therapy resistance [8]. Little is known about the mechanisms of epigenetic regulation in uveal melanoma cell biology. A few key roles have been identified for histone deacetylases (HDAC), such as HDAC2 or HDAC4 [9–11], lysine methyltransferases [12, 13], or chromatin remodeling complexes [14, 15] in the pathogenesis of uveal melanoma. While these molecules emerged as promising drug targets in uveal melanoma treatment, none, including the well-known pan-HDAC inhibitors (e.g., vorinostat, entinostat), tested so far showed clinical efficacy [8]. Thus, uncovering epigenetic regulators that can be targeted therapeutically in uveal melanoma is of critical importance.
In order to probe the role of epigenetic-related mechanisms involved in uveal melanoma proliferation and survival, in this study, we performed a CRISPR-Cas9 screen in GNAQQ209P human uveal melanoma cells targeting chromatin modifiers with or without enzymatic activities. We identified the lysine methyltransferase SETDB1, thereby providing the first evidence of SETDB1 implication in uveal melanoma cell proliferation and survival.
Results
A CRISPR-Cas9 screen identifies SETDB1 as a key driver of metastatic uveal melanoma cell growth
To identify actionable vulnerabilities in metastatic uveal melanoma cells, we performed a CRISPR-Cas9 knockout screen targeting ~140 chromatin remodelers with or without enzymatic activity in GNAQQ209P uveal human melanoma cells [16]. Briefly, OMM1.3 uveal melanoma cells, originally derived from liver metastasis and harboring a GNAQQ209P mutation, were engineered to stably express Cas9, transduced with GFP-tagged single-guide RNA (sgRNA) library (3–4 sgRNAs per gene encoded in pLKO.1) and GFP-positive cells were sorted for expansion.
Next, genomic DNA was isolated from cells at day 0, which represents the library distribution prior to the screening selection process, and at day 35, and the abundance of each sgRNA was determined using next-generation sequencing. Analysis of the CRISPR-Cas9 screen dataset with MaGeck software, which calculates a score based on a fold change, revealed depleted (left part of the volcano plot) or enriched (right part of the volcano plot) sgRNA compared to the control condition (Fig. 1A).
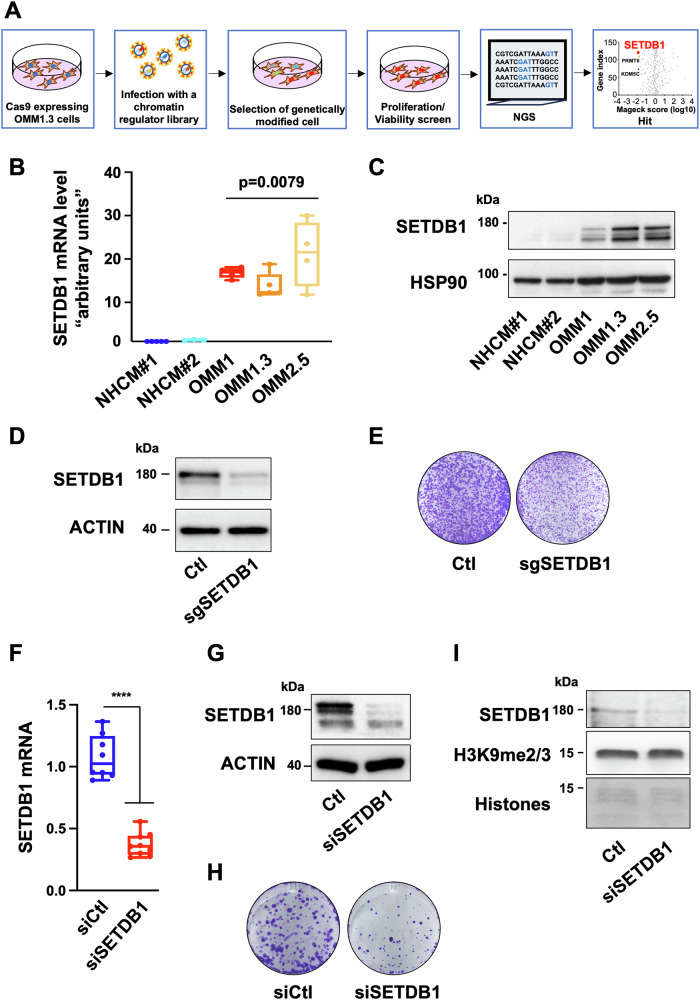
Fig. 1. SETDB1 is critically required for metastatic uveal melanoma cell growth.
A Schematic of the CRISPR-Cas9 chromatin-regulators screen with Log10-transformed MAGeCK robust ranking aggregation (RRA)-scores for either depletion (left) or enrichment (right) of sgRNAs in OMM1.3 cells at D35 compared to D0. B RT-qPCR analysis of SETDB1 in metastatic uveal melanoma cells (OMM1, OMM1.3, OMM2.5) compared to normal human uveal melanocytes from two different patients (NHCM#1 and NHCM#2). Mann–Whitney test was performed for comparison between groups, n = 9. Data are the mean ± SEM. **p = 0.0079. C Immunoblot analysis of SETDB1 in metastatic uveal melanoma cells compared to normal human choroidal melanocytes. HSP90 was used as a loading control. D Immunoblot of SETDB1 in the indicated whole-cell lysates of OMM1.3 infected with control sgRNA (Ctl) or a sgRNA to SETDB1 cell lines. β-Actin was used as a loading control. E OMM1.3 Ctl and pooled SETDB1-KD cells were seeded at the same density and cultured for 10 days. Representative images of three independent experiments are shown. F Representative box and whiskers plots of RT-qPCR analysis of SETDB1 in OMM1.3 cells treated with control siRNA (siCtl) or an siRNA to SETDB1 (siSETDB1) for 48 h. Mann–Whitney test was performed for comparison between groups, n = 5. Data are mean ± SEM. ****p < 0.0001. G Immunoblot analysis to SETDB1 in the indicated whole cell lysates of OMM1.3 cells treated for 6 days. β-Actin was used as a loading control. H OMM1.3 cells treated as in (F) were seeded at the same density and cultured for 10 days. Representative images of three independent experiments are shown. I Immunoblot of SETDB1 and H3K9me2/3 in the indicated chromatin fraction of OMM1.3 cell line (96 h). Histones were used as a loading control.
To identify essential genes involved in cellular proliferation and survival, we have used a “dropout” strategy in which cells with the phenotype of interest are depleted in the screen. This approach is reflected by the number of sgRNAs against these genes being strongly decreased from the population, whereas other sgRNAs are maintained. By focusing our attention on genes whose loss conferred a reduced growth advantage on cells the screen yielded several candidates, with enrichment of factors that mediate histone methylation among the top 10 hits (Table 1). These include the histone demethylases KDM5C and KDM4D, as well as the histone methyltransferases PRMT6 and SETD1B. The lysine methyltransferase SETDB1 [17], that catalyzes the addition of methyl groups to histone H3 at lysine 9 (H3K9) and non-histone proteins, was the most valuable hit using the MaGeck software (Fig. 1A).
Table 1.
Top 10 hits from the CRISPR-Cas9 screen using MAGeCK robust ranking aggregation (RRA)-scores of sgRNAs in OMM1.3 cells at D35 compared to D0.
| id | num | neg|score | neg|p-value | neg|fdr | neg|rank | neg|goodsgrna | neg|lfc | pos|score | pos|p-value | pos|fdr | pos|rank | pos|goodsgrna | pos|lfc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAXX | 4 | 0.00023702 | 0.0010823 | 0.152602 | 1 | 3 | −0.93234 | 0.12217 | 0.20515 | 0.923331 | 26 | 1 | −0.93234 |
| SETDB1 | 4 | 0.013236 | 0.035616 | 0.782619 | 2 | 2 | −0.39488 | 0.71051 | 0.70898 | 0.974234 | 100 | 0 | −0.39488 |
| KDM5C | 4 | 0.014218 | 0.03783 | 0.782619 | 3 | 2 | −0.18554 | 0.13538 | 0.22226 | 0.923331 | 30 | 1 | −0.18554 |
| PRMT6 | 4 | 0.01819 | 0.046837 | 0.782619 | 4 | 2 | −0.37276 | 0.69276 | 0.6914 | 0.974234 | 99 | 0 | −0.37276 |
| CHD1L | 4 | 0.025396 | 0.063406 | 0.782619 | 5 | 1 | −0.53367 | 0.71908 | 0.71756 | 0.974234 | 102 | 0 | −0.53367 |
| MLLT11 | 2 | 0.027284 | 0.04986 | 0.782619 | 6 | 1 | −0.38805 | 0.21259 | 0.22753 | 0.923331 | 44 | 1 | −0.38805 |
| KDM4D | 4 | 0.029838 | 0.073351 | 0.782619 | 7 | 2 | −0.23637 | 0.46253 | 0.49102 | 0.958623 | 68 | 1 | −0.23637 |
| PRDM14 | 4 | 0.030329 | 0.074418 | 0.782619 | 8 | 3 | −0.11042 | 0.29956 | 0.38445 | 0.958623 | 54 | 1 | −0.11042 |
| HIRA | 4 | 0.032562 | 0.079555 | 0.782619 | 9 | 1 | −0.2559 | 0.65485 | 0.65356 | 0.964831 | 95 | 0 | −0.2559 |
| SETD1B | 4 | 0.039688 | 0.094747 | 0.782619 | 10 | 2 | −0.1836 | 0.6482 | 0.64722 | 0.964831 | 93 | 1 | −0.1836 |
We also found approximately a 15-fold higher SETDB1 mRNA level in metastatic uveal melanoma cells as compared to human uveal melanocytes (Fig. 1B), suggesting its upregulation along with tumor transformation and progression. This observation was confirmed at the protein level showing higher SETDB1 expression in metastatic uveal melanoma cells compared to normal uveal melanocytes (Fig. 1C).
Given that SETDB1 was identified as one of the top critical genes for metastatic uveal melanoma cell growth, that its expression is higher in uveal melanoma cells as compared to normal uveal melanocytes and that its role in uveal melanoma biology remained unknown, we further focused our studies on SETDB1.
The functional impact of SETDB1 inhibition on growth was validated by introducing individual sgRNAs. SETDB1 depletion in pooled OMM1.3 metastatic uveal melanoma cells was confirmed by immunoblot (Fig. 1D) and resulted in a substantially decreased cell growth (Fig. 1E). We further validated these results using an siRNA that efficiently downregulated SETDB1 at both the mRNA and protein level (Fig. 1F, G). Likewise, silencing of SETDB1 expression with siRNA resulted in reduced cell growth ability (Fig. 1H). We extended these findings in two other metastatic uveal melanoma cell lines, which harbor typical GNAQ (OMM2.5) or GNA11 (OMM1) hotspot mutations (Supplementary Fig. 1A, B). Our data also showed that SETDB1 knockdown reduced the growth ability of primary uveal melanoma cells that carry a GNAQ mutation and do not express BAP1 (MP46) (Supplementary Fig. 1C). It is noteworthy that an efficient decrease of SETDB1 was observed after 48 h while the growth was affected at a later time, indicating that SETDB1 is a mechanistic driver of growth arrest rather than a downstream consequence. Given that SETDB1 is a histone lysine methyltransferase that mainly catalyses H3K9 di- and tri-methylation [17], we assessed the level of H3K9 di- and tri-methylated histone marks. Unexpectedly, SETDB1 knockdown did not result in global changes in H3K9 di- and tri-methylation, suggesting other compensatory mechanisms (Fig. 1I). Collectively, our data indicate that SETDB1 plays a critical role in metastatic uveal melanoma cell growth but likely acts independently of H3K9 di- and tri-methylation alterations.
SETDB1 regulates DNA replication and genomic integrity
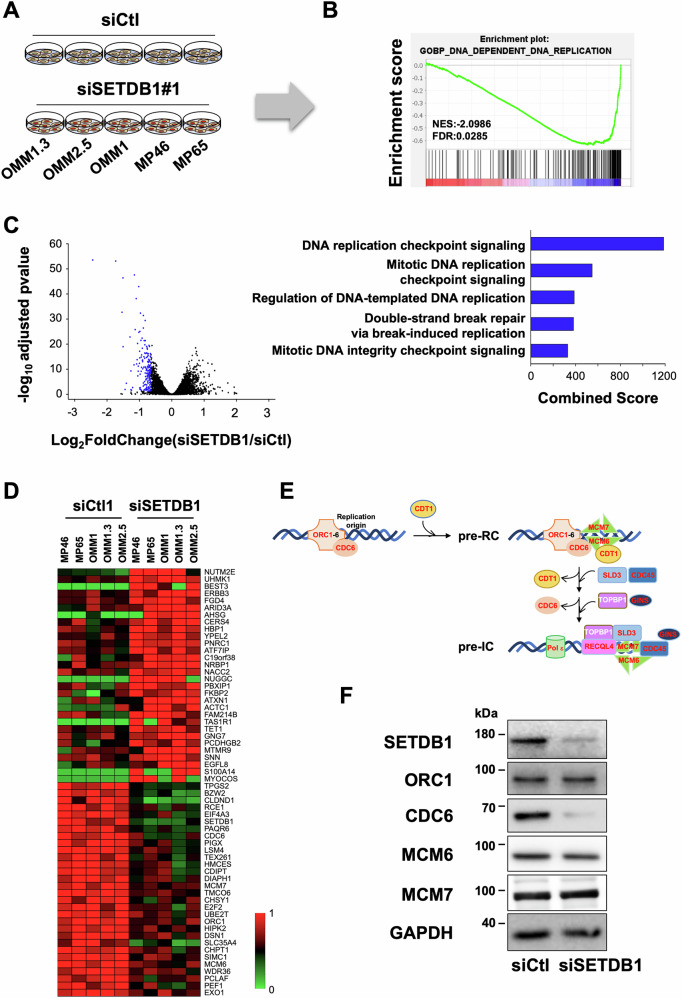
To delineate the mechanisms by which SETDB1 regulates metastatic uveal melanoma cell growth, we profiled the transcriptome of five different uveal melanoma cell lines. Given the molecular distinctions driving the clinical outcome in uveal melanoma, we included both BAP1-positive and BAP1-negative cell lines to ensure that our findings would not be limited to specific BAP1 status and represent a broader spectrum of the disease. Therefore, we have used 3 metastatic BAP1 positive cell lines (OMM1.3, OMM2.5 and OMM1) and 2 primary BAP1 negative cell lines (MP46 and MP65) that were either treated with a control siRNA or with an siRNA directed against SETDB1 (Fig. 2A and Supplementary Fig. 2A). Gene Set Enrichment Analysis (GSEA) uncovered 4 gene sets (out of 10 statistically significant) related to DNA replication, and others related to telomere and cell cycle in SETDB1-KD cells compared to control cells (Fig. 2B and Supplementary Fig. 2B). Moreover, our transcriptomic analyses identified a set of common genes across the five SETDB1-KD cell lines that were significantly downregulated (n = 179) (Table 2) compared to control cells with enrichment of DNA replication biological processes (Fig. 2C). Supporting this, the heatmap revealed several genes implicated in DNA replication origin licensing and assembly of pre-replication complex (ORC1, CDC6, MCM6, MCM7) and cell cycle (E2F2) (Fig. 2D, E). It is worth noting that CDC6 is regulated by E2F proteins [18]. RNA-seq datasets also revealed reduced mRNA level of other components of the pre-initiation complex (CDT1, CDC45, and SLD3/TICRR) in SETDB1-KD cells compared to control cells. Reduction of CDC6 and MCM6 in SETDB1 KD OMM1.3 cells was confirmed at the protein level (Fig. 2F). These data suggest that SETDB1 plays a critical role in uveal melanoma cell growth through regulation of DNA replication.
Fig. 2. SETDB1 regulates DNA replication.
A Schematic representation of the experimental design. B GSEA analysis from OMM1.3 siSETDB1 cells versus OMM1.3 siCtl cells, data queried against the “GOBP” group of gene sets of all genes in the 5 different cell lines upon 48 h of siSETDB1 over siCtl. C Volcano-plot representing -log10(adjusted p-value) as a function of the estimated log2 Fold-Change of all genes in the 5 different cell lines upon 48 h of siSETDB1 over siCtl. Significantly downregulated genes (179 genes) are shown in blue (left). Functional annotation (biological processes) of the 179 genes commonly downregulated after 48 h of siSETDB1 over siCtl in the 5 different cell lines. Categories are ranked by combined score (right). D Heat Map of the top 60 deregulated genes representing relative expression. E Schematic representation of the pre-initiation complex. F Immunoblot of the indicated proteins in whole-cell lysates of OMM1.3 cells treated with a control (siCtl) or SETDB1 siRNA for 48 h. GAPDH was used as a loading control.
Table 2.
Common genes across the five SETDB1-KD cell lines that were significantly downregulated compared to control cells.
| Ensembl Gene ID | Gene name | Description | Log2 FC (siSETDB1 vs siLuc) | Adjusted p-value (siSETDB1 vs siLuc) |
|---|---|---|---|---|
| ENSG00000080822 | CLDND1 | Claudin domain containing 1 | −2.44067942 | 2.58731E-54 |
| ENSG00000176087 | SLC35A4 | Solute carrier family 35 member A4 | −1.730216179 | 6.70621E-54 |
| ENSG00000136261 | BZW2 | Basic leucine zipper and W2 domains 2 | −1.53357307 | 1.77831E-33 |
| ENSG00000143379 | SETDB1 | SET domain bifurcated histone lysine methyltransferase 1 | −1.507300673 | 3.46712E-47 |
| ENSG00000176720 | BOK | BCL2 family apoptosis regulator BOK | −1.503384978 | 1.02184E-19 |
| ENSG00000197415 | VEPH1 | Ventricular zone expressed PH domain containing 1 | −1.440234166 | 0.011404577 |
| ENSG00000126970 | ZC4H2 | Zinc finger C4H2-type containing | −1.402321242 | 3.63752E-05 |
| ENSG00000108774 | RAB5C | RAB5C, member RAS oncogene family | −1.288855293 | 1.31662E-23 |
| ENSG00000135678 | CPM | Carboxypeptidase M | −1.260576418 | 4.44947E-10 |
| ENSG00000100191 | SLC5A4 | Solute carrier family 5 member 4 | −1.259885791 | 0.014637052 |
| ENSG00000101282 | RSPO4 | R-spondin 4 | −1.24160913 | 0.021345207 |
| ENSG00000236609 | ZNF853 | Zinc finger protein 853 | −1.208217162 | 0.001938456 |
| ENSG00000168061 | SAC3D1 | SAC3 domain containing 1 | −1.163129084 | 1.60468E-12 |
| ENSG00000112576 | CCND3 | Cyclin D3 | −1.152456908 | 2.45482E-48 |
| ENSG00000134779 | TPGS2 | Tubulin polyglutamylase complex subunit 2 | −1.129447327 | 1.53548E-26 |
| ENSG00000163870 | TPRA1 | Transmembrane protein adipocyte associated 1 | −1.097353862 | 7.10679E-39 |
| ENSG00000138111 | MFSD13A | Major facilitator superfamily domain containing 13A | −1.081598123 | 7.26073E-13 |
| ENSG00000198794 | SCAMP5 | Secretory carrier membrane protein 5 | −1.069454475 | 1.43271E-14 |
| ENSG00000141543 | EIF4A3 | Eukaryotic translation initiation factor 4A3 | −1.053239423 | 5.13661E-21 |
| ENSG00000102230 | PCYT1B | Phosphate cytidylyltransferase 1B, choline | −1.046421228 | 4.22045E-08 |
| ENSG00000135097 | MSI1 | Musashi RNA binding protein 1 | −1.037804441 | 5.79642E-10 |
| ENSG00000183624 | HMCES | 5-hydroxymethylcytosine binding, ES cell specific | −1.03418709 | 5.91112E-12 |
| ENSG00000144043 | TEX261 | Testis expressed 261 | −1.018612109 | 1.10457E-43 |
| ENSG00000094804 | CDC6 | Cell division cycle 6 | −1.014134633 | 1.72692E-25 |
| ENSG00000197147 | LRRC8B | Leucine rich repeat containing 8 VRAC subunit B | −0.998151811 | 4.25272E-13 |
| ENSG00000102977 | ACD | ACD shelterin complex subunit and telomerase recruitment factor | −0.990099933 | 6.25433E-18 |
| ENSG00000275591 | XKR5 | XK related 5 | −0.988185209 | 0.002695943 |
| ENSG00000176454 | LPCAT4 | Lysophosphatidylcholine acyltransferase 4 | −0.985862564 | 2.7143E-15 |
| ENSG00000173653 | RCE1 | Ras converting CAAX endopeptidase 1 | −0.984715649 | 7.9172E-26 |
| ENSG00000007062 | PROM1 | Prominin 1 | −0.97961611 | 0.009103299 |
| ENSG00000104369 | JPH1 | Junctophilin 1 | −0.977497844 | 8.80282E-09 |
| ENSG00000180998 | GPR137C | G protein-coupled receptor 137C | −0.974003522 | 0.00222764 |
| ENSG00000178999 | AURKB | Aurora kinase B | −0.966160873 | 4.06418E-33 |
| ENSG00000103502 | CDIPT | CDP-diacylglycerol--inositol 3-phosphatidyltransferase | −0.951329558 | 3.04278E-22 |
| ENSG00000196081 | ZNF724 | Zinc finger protein 724 | −0.950308152 | 0.013396392 |
| ENSG00000127220 | ABHD8 | Abhydrolase domain containing 8 | −0.944454528 | 6.54254E-10 |
| ENSG00000162517 | PEF1 | Penta-EF-hand domain containing 1 | −0.939626338 | 1.33625E-15 |
| ENSG00000172731 | LRRC20 | Leucine rich repeat containing 20 | −0.935106976 | 1.72682E-11 |
| ENSG00000088881 | EBF4 | EBF family member 4 | −0.933391831 | 0.034477292 |
| ENSG00000167700 | MFSD3 | Major facilitator superfamily domain containing 3 | −0.928218276 | 1.883E-16 |
| ENSG00000109079 | TNFAIP1 | TNF alpha induced protein 1 | −0.916384936 | 4.07361E-23 |
| ENSG00000183726 | TMEM50A | Transmembrane protein 50A | −0.909466682 | 2.40125E-14 |
| ENSG00000093009 | CDC45 | Cell division cycle 45 | −0.907262883 | 4.28719E-19 |
| ENSG00000233927 | RPS28 | Ribosomal protein S28 | −0.897534175 | 8.88364E-14 |
| ENSG00000007968 | E2F2 | E2F transcription factor 2 | −0.89724828 | 2.11235E-14 |
| ENSG00000163964 | PIGX | Phosphatidylinositol glycan anchor biosynthesis class X | −0.893049786 | 6.33205E-11 |
| ENSG00000197696 | NMB | Neuromedin B | −0.881664148 | 0.000489433 |
| ENSG00000160767 | FAM189B | Family with sequence similarity 189 member B | −0.878099585 | 3.43974E-32 |
| ENSG00000128408 | RIBC2 | RIB43A domain with coiled-coils 2 | −0.876289923 | 2.7777E-05 |
| ENSG00000131873 | CHSY1 | Chondroitin sulfate synthase 1 | −0.876260038 | 3.45611E-27 |
| ENSG00000130830 | MPP1 | Membrane palmitoylated protein 1 | −0.863737396 | 1.43029E-11 |
| ENSG00000128604 | IRF5 | Interferon regulatory factor 5 | −0.859313035 | 0.027699243 |
| ENSG00000065328 | MCM10 | Minichromosome maintenance 10 replication initiation factor | −0.85800114 | 8.95999E-16 |
| ENSG00000100139 | MICALL1 | MICAL like 1 | −0.850088943 | 3.82816E-22 |
| ENSG00000146094 | DOK3 | Docking protein 3 | −0.849727315 | 0.01351518 |
| ENSG00000099617 | EFNA2 | Ephrin A2 | −0.84965942 | 0.000183769 |
| ENSG00000008118 | CAMK1G | Calcium/calmodulin dependent protein kinase IG | −0.839801505 | 8.3045E-06 |
| ENSG00000109084 | TMEM97 | Transmembrane protein 97 | −0.835177517 | 3.98655E-15 |
| ENSG00000104524 | PYCR3 | Pyrroline-5-carboxylate reductase 3 | −0.833707143 | 3.48264E-17 |
| ENSG00000181991 | MRPS11 | Mitochondrial ribosomal protein S11 | −0.828776036 | 1.70901E-20 |
| ENSG00000197312 | DDI2 | DNA damage inducible 1 homolog 2 | −0.821175254 | 1.12384E-11 |
| ENSG00000131504 | DIAPH1 | Diaphanous related formin 1 | −0.818668953 | 3.61781E-30 |
| ENSG00000141401 | IMPA2 | Inositol monophosphatase 2 | −0.8179472 | 3.21857E-09 |
| ENSG00000007541 | PIGQ | Phosphatidylinositol glycan anchor biosynthesis class Q | −0.815324807 | 3.82816E-22 |
| ENSG00000039068 | CDH1 | Cadherin 1 | −0.813795041 | 0.007133781 |
| ENSG00000136052 | SLC41A2 | Solute carrier family 41 member 2 | −0.809068678 | 0.000110523 |
| ENSG00000006625 | GGCT | Gamma-glutamylcyclotransferase | −0.808252948 | 7.97127E-08 |
| ENSG00000159259 | CHAF1B | Chromatin assembly factor 1 subunit B | −0.803311835 | 4.58958E-17 |
| ENSG00000135622 | SEMA4F | Semaphorin 4F | −0.79905126 | 3.1063E-11 |
| ENSG00000003989 | SLC7A2 | Solute carrier family 7 member 2 | −0.798981174 | 1.70805E-06 |
| ENSG00000111199 | TRPV4 | Transient receptor potential cation channel subfamily V member 4 | −0.795894675 | 0.026723176 |
| ENSG00000111666 | CHPT1 | Choline phosphotransferase 1 | −0.790075556 | 8.37199E-07 |
| ENSG00000132563 | REEP2 | Receptor accessory protein 2 | −0.789760272 | 0.017578955 |
| ENSG00000166803 | PCLAF | PCNA clamp associated factor | −0.787731791 | 2.9663E-10 |
| ENSG00000103018 | CYB5B | Cytochrome b5 type B | −0.782156755 | 6.96841E-07 |
| ENSG00000205309 | NT5M | 5′,3′-nucleotidase, mitochondrial | −0.779227105 | 0.015346667 |
| ENSG00000163888 | CAMK2N2 | Calcium/calmodulin dependent protein kinase II inhibitor 2 | −0.777929574 | 0.004548607 |
| ENSG00000167272 | POP5 | POP5 homolog, ribonuclease P/MRP subunit | −0.776677827 | 8.58558E-11 |
| ENSG00000112039 | FANCE | FA complementation group E | −0.776465629 | 1.15064E-10 |
| ENSG00000165046 | LETM2 | Leucine zipper and EF-hand containing transmembrane protein 2 | −0.775536248 | 0.00139097 |
| ENSG00000254087 | LYN | LYN proto-oncogene, Src family tyrosine kinase | −0.775426645 | 2.03074E-15 |
| ENSG00000267534 | S1PR2 | Sphingosine-1-phosphate receptor 2 | −0.773810704 | 4.2899E-09 |
| ENSG00000146670 | CDCA5 | Cell division cycle associated 5 | −0.773646868 | 3.06923E-17 |
| ENSG00000137404 | NRM | Nurim | −0.772726067 | 8.13588E-20 |
| ENSG00000116791 | CRYZ | Crystallin zeta | −0.772545614 | 0.000174379 |
| ENSG00000102384 | CENPI | Centromere protein I | −0.769337411 | 2.02709E-10 |
| ENSG00000152270 | PDE3B | Phosphodiesterase 3B | −0.764313087 | 4.05836E-07 |
| ENSG00000089063 | TMEM230 | Transmembrane protein 230 | −0.760695783 | 4.69492E-08 |
| ENSG00000187123 | LYPD6 | LY6/PLAUR domain containing 6 | −0.760114435 | 3.36599E-09 |
| ENSG00000117408 | IPO13 | Importin 13 | −0.759202881 | 3.22313E-19 |
| ENSG00000124587 | PEX6 | Peroxisomal biogenesis factor 6 | −0.758743154 | 2.95953E-09 |
| ENSG00000140365 | COMMD4 | COMM domain containing 4 | −0.752311848 | 9.66051E-19 |
| ENSG00000144935 | TRPC1 | Transient receptor potential cation channel subfamily C member 1 | −0.749164557 | 0.011475086 |
| ENSG00000125319 | HROB | Homologous recombination factor with OB-fold | −0.747598102 | 7.23784E-12 |
| ENSG00000170515 | PA2G4 | Proliferation-associated 2G4 | −0.747546163 | 8.61016E-16 |
| ENSG00000107796 | ACTA2 | Actin alpha 2, smooth muscle | −0.742851497 | 1.12389E-05 |
| ENSG00000075218 | GTSE1 | G2 and S-phase expressed 1 | −0.741904138 | 8.50653E-14 |
| ENSG00000169105 | CHST14 | Carbohydrate sulfotransferase 14 | −0.740579126 | 6.41331E-17 |
| ENSG00000276043 | UHRF1 | Ubiquitin like with PHD and ring finger domains 1 | −0.736980602 | 4.32998E-10 |
| ENSG00000167005 | NUDT21 | Nudix hydrolase 21 | −0.735118324 | 6.40005E-08 |
| ENSG00000165806 | CASP7 | Caspase 7 | −0.733408293 | 1.08616E-07 |
| ENSG00000159147 | DONSON | DNA replication fork stabilization factor DONSON | −0.733330798 | 1.79372E-08 |
| ENSG00000137310 | TCF19 | Transcription factor 19 | −0.73287457 | 2.6109E-11 |
| ENSG00000188186 | LAMTOR4 | Late endosomal/lysosomal adaptor, MAPK and MTOR activator 4 | −0.731023031 | 3.67345E-05 |
| ENSG00000189057 | FAM111B | FAM111 trypsin like peptidase B | −0.729814217 | 3.26705E-06 |
| ENSG00000113119 | TMCO6 | Transmembrane and coiled-coil domains 6 | −0.729753081 | 3.77511E-08 |
| ENSG00000132016 | BRME1 | Break repair meiotic recombinase recruitment factor 1 | −0.729700409 | 0.001873868 |
| ENSG00000160753 | RUSC1 | RUN and SH3 domain containing 1 | −0.728484775 | 5.50404E-17 |
| ENSG00000166508 | MCM7 | Minichromosome maintenance complex component 7 | −0.728365624 | 2.96948E-16 |
| ENSG00000221955 | SLC12A8 | Solute carrier family 12 member 8 | −0.726823055 | 0.039726441 |
| ENSG00000109519 | GRPEL1 | GrpE like 1, mitochondrial | −0.725689313 | 6.80513E-09 |
| ENSG00000123685 | BATF3 | Basic leucine zipper ATF-like transcription factor 3 | −0.72458263 | 0.001522053 |
| ENSG00000148110 | MFSD14B | Major facilitator superfamily domain containing 14B | −0.722394583 | 1.8746E-13 |
| ENSG00000177917 | ARL6IP6 | ADP ribosylation factor like GTPase 6 interacting protein 6 | −0.718700533 | 1.42364E-08 |
| ENSG00000180228 | PRKRA | Protein activator of interferon induced protein kinase EIF2AK2 | −0.716677371 | 2.02939E-12 |
| ENSG00000136574 | GATA4 | GATA binding protein 4 | −0.711352562 | 0.007185606 |
| ENSG00000077152 | UBE2T | Ubiquitin conjugating enzyme E2 T | −0.709554446 | 1.08386E-14 |
| ENSG00000183010 | PYCR1 | Pyrroline-5-carboxylate reductase 1 | −0.708477376 | 3.11792E-10 |
| ENSG00000146648 | EGFR | Epidermal growth factor receptor | −0.70834571 | 0.001003247 |
| ENSG00000085840 | ORC1 | Origin recognition complex subunit 1 | −0.707703656 | 1.16587E-11 |
| ENSG00000064393 | HIPK2 | Homeodomain interacting protein kinase 2 | −0.706969488 | 1.76206E-14 |
| ENSG00000141542 | RAB40B | RAB40B, member RAS oncogene family | −0.703840844 | 0.001296392 |
| ENSG00000143590 | EFNA3 | Ephrin A3 | −0.703826254 | 0.004199258 |
| ENSG00000105676 | ARMC6 | Armadillo repeat containing 6 | −0.702557762 | 2.88567E-20 |
| ENSG00000130734 | ATG4D | Autophagy related 4D cysteine peptidase | −0.700560962 | 7.30284E-14 |
| ENSG00000176974 | SHMT1 | Serine hydroxymethyltransferase 1 | −0.700145922 | 2.87823E-09 |
| ENSG00000168792 | ABHD15 | Abhydrolase domain containing 15 | −0.699505212 | 7.30284E-14 |
| ENSG00000163577 | EIF5A2 | Eukaryotic translation initiation factor 5A2 | −0.698832403 | 5.90333E-07 |
| ENSG00000149636 | DSN1 | DSN1 component of MIS12 kinetochore complex | −0.69800473 | 1.72175E-09 |
| ENSG00000054277 | OPN3 | Opsin 3 | −0.697429021 | 0.005367454 |
| ENSG00000158109 | TPRG1L | Tumor protein p63 regulated 1 like | −0.69576735 | 7.88947E-12 |
| ENSG00000007255 | TRAPPC6A | Trafficking protein particle complex subunit 6A | −0.69333341 | 0.003909706 |
| ENSG00000186318 | BACE1 | Beta-secretase 1 | −0.693003562 | 1.59805E-10 |
| ENSG00000123444 | KBTBD4 | Kelch repeat and BTB domain containing 4 | −0.691858123 | 1.14532E-08 |
| ENSG00000129173 | E2F8 | E2F transcription factor 8 | −0.691368268 | 1.53483E-07 |
| ENSG00000116337 | AMPD2 | Adenosine monophosphate deaminase 2 | −0.688901876 | 7.65844E-20 |
| ENSG00000034152 | MAP2K3 | Mitogen-activated protein kinase kinase 3 | −0.688418607 | 7.14337E-12 |
| ENSG00000188312 | CENPP | Centromere protein P | −0.687348522 | 1.41132E-07 |
| ENSG00000101412 | E2F1 | E2F transcription factor 1 | −0.685900049 | 1.57498E-11 |
| ENSG00000178752 | ERFE | Erythroferrone | −0.685630344 | 8.2682E-07 |
| ENSG00000167513 | CDT1 | Chromatin licensing and DNA replication factor 1 | −0.684859075 | 4.78247E-12 |
| ENSG00000076248 | UNG | Uracil DNA glycosylase | −0.684741771 | 9.43088E-09 |
| ENSG00000143416 | SELENBP1 | Selenium binding protein 1 | −0.684686569 | 3.17263E-08 |
| ENSG00000144554 | FANCD2 | FA complementation group D2 | −0.684496681 | 2.65731E-14 |
| ENSG00000167524 | RSKR | Ribosomal protein S6 kinase related | −0.684436037 | 0.040929878 |
| ENSG00000170085 | SIMC1 | SUMO interacting motifs containing 1 | −0.684107509 | 4.2041E-08 |
| ENSG00000160233 | LRRC3 | Leucine rich repeat containing 3 | −0.681849404 | 1.74181E-07 |
| ENSG00000157927 | RADIL | Rap associating with DIL domain | −0.67922331 | 2.07827E-05 |
| ENSG00000136463 | TACO1 | Translational activator of cytochrome c oxidase I | −0.678764049 | 0.000179835 |
| ENSG00000136146 | MED4 | Mediator complex subunit 4 | −0.677778412 | 2.74481E-12 |
| ENSG00000274641 | H2BC17 | H2B clustered histone 17 | −0.676395464 | 1.28072E-07 |
| ENSG00000140534 | TICRR | TOPBP1 interacting checkpoint and replication regulator | −0.675488889 | 3.02285E-10 |
| ENSG00000076003 | MCM6 | Minichromosome maintenance complex component 6 | −0.675329486 | 1.48125E-10 |
| ENSG00000187741 | FANCA | FA complementation group A | −0.672648133 | 1.15152E-09 |
| ENSG00000276368 | H2AC14 | H2A clustered histone 14 | −0.672072742 | 2.72213E-06 |
| ENSG00000106236 | NPTX2 | Neuronal pentraxin 2 | −0.671365 | 0.034171406 |
| ENSG00000111331 | OAS3 | 2′-5′-oligoadenylate synthetase 3 | −0.669574335 | 0.000154628 |
| ENSG00000180011 | ZADH2 | Zinc binding alcohol dehydrogenase domain containing 2 | −0.66559808 | 9.40295E-07 |
| ENSG00000134987 | WDR36 | WD repeat domain 36 | −0.664728619 | 3.40613E-09 |
| ENSG00000116685 | KIAA2013 | KIAA2013 | −0.66440737 | 1.57824E-10 |
| ENSG00000131153 | GINS2 | GINS complex subunit 2 | −0.663138222 | 3.08693E-10 |
| ENSG00000165480 | SKA3 | Spindle and kinetochore associated complex subunit 3 | −0.661381525 | 1.68765E-09 |
| ENSG00000070366 | SMG6 | SMG6 nonsense mediated mRNA decay factor | −0.660870628 | 2.32961E-09 |
| ENSG00000100401 | RANGAP1 | Ran GTPase activating protein 1 | −0.660517742 | 6.83745E-16 |
| ENSG00000169884 | WNT10B | Wnt family member 10B | −0.657739715 | 7.54512E-06 |
| ENSG00000004864 | SLC25A13 | Solute carrier family 25 member 13 | −0.657514506 | 5.50404E-17 |
| ENSG00000179532 | DNHD1 | Dynein heavy chain domain 1 | −0.657360294 | 0.000903431 |
| ENSG00000169679 | BUB1 | BUB1 mitotic checkpoint serine/threonine kinase | −0.65732404 | 1.12205E-15 |
| ENSG00000092853 | CLSPN | Claspin | −0.657027473 | 8.82631E-11 |
| ENSG00000175175 | PPM1E | Protein phosphatase, Mg2 + /Mn2+ dependent 1E | −0.656944979 | 0.009390568 |
| ENSG00000128973 | CLN6 | CLN6 transmembrane ER protein | −0.656174514 | 1.45612E-13 |
| ENSG00000132646 | PCNA | Proliferating cell nuclear antigen | −0.654414489 | 2.43078E-10 |
| ENSG00000136982 | DSCC1 | DNA replication and sister chromatid cohesion 1 | −0.653987621 | 3.70426E-09 |
| ENSG00000102387 | TAF7L | TATA-box binding protein associated factor 7 like | −0.652644895 | 0.028474932 |
| ENSG00000160957 | RECQL4 | RecQ like helicase 4 | −0.652136684 | 2.0202E-12 |
| ENSG00000160117 | ANKLE1 | Ankyrin repeat and LEM domain containing 1 | −0.651964708 | 0.012275559 |
| ENSG00000127564 | PKMYT1 | Protein kinase, membrane associated tyrosine/threonine 1 | −0.650369204 | 1.59377E-11 |
| ENSG00000116199 | FAM20B | FAM20B glycosaminoglycan xylosylkinase | −0.650171171 | 2.25423E-05 |
| ENSG00000148985 | PGAP2 | Post-GPI attachment to proteins 2 | −0.650053227 | 4.6646E-10 |
SETDB1 inhibition triggers DNA damage and senescence-like phenotypes
DNA replication takes place in the S phase of the cell cycle. To further analyze the S phase entry, OMM1.3 proliferating cells treated with control siRNA or siRNA against SETDB1 were stained for incorporated EdU against total DNA content using Hoechst 33342. SETDB1-KD confirmed by immunoblot (Supplementary Fig. 3A), displayed increased percentage of cells in G0/G1 phase and reduced percentage of cells in late S phase upon flow cytometry analyses and quantification (Supplementary Fig. 3B–D).
Given that accurate and complete DNA replication is critical for achieving genome integrity and cell survival, and that eukaryotic cells progressing through S phase with a reduced number of licensed origins are more vulnerable to replication stress and DNA damage, we hypothesized that SETDB1 downregulation would promote DNA damage. To address this point, we analyzed phosphorylated CHK2 and H2AX (γ-H2AX), canonical markers of DNA damage and checkpoint activation. We also performed staining of 53BP1, a marker of DNA double-strand breaks and an important component of the DNA damage response [19].
Immunoblot of OMM1.3 cells treated with siRNA to SETDB1 showed enhanced phosphorylation of CHK2 (Fig. 3A). Moreover, as shown by immunofluorescence analyses, SETDB1-KD enhanced γ-H2AX and 53BP1 foci formation compared to the control cells (Fig. 3B, C). Enhanced phosphorylation of CHK2 as well as an increased γ-H2AX and 53BP1 staining, were also observed when expression of SETDB1 was reduced using the CRISPR-Cas9 approach as compared to cells infected with control sgRNA (Supplementary Fig. 4A–D). Together, these data indicate that SETDB1 KD is sufficient to drive the DNA damage response. Persistent DNA damage is well known to promote a senescent phenotype [20]. Thus, we then conducted β-Galactosidase (SA-βGal) staining at pH6 to measure senescence. Our data demonstrated SA-βGal staining in OMM1.3 treated with SETDB1 siRNA compared to control cells (Fig. 3D) as well as using a sgRNA against SETDB1 (Supplementary Fig. 4E). SA-βGal staining following SETDB1 KD by siRNA was confirmed in OMM1 cells (Supplementary Fig. 5A, B). In addition, time course analysis showed that SETDB1 KD caused enhanced p21 expression another marker of the senescence state and of growth arrest (Fig. 3E) that is observed later on. Collectively, our findings indicate that SETDB1 expression may sustain DNA replication and prevent DNA damage to overcome the process of senescence and favor uveal melanoma cell growth.
Fig. 3. SETDB1 knockdown triggers DNA damage and senescence-like phenotypes.
A Immunoblot to SETDB1, CHK2, and P-CHK2 in lysates of OMM1.3 cells treated with control siRNA or siRNA to SETDB1 for 48 h. β-Actin is used as a loading control. B OMM1.3 cells treated with control siRNA or siRNA to SETDB1 for 72 h were analyzed by immunofluorescence for H2AX phosphorylated on Ser139 (γH2AX) or 53BP1. Cell nuclei were counterstained with Hoechst. Representative fluorescence images are shown. Bar = 20 µM. C Representative box and whiskers plots of quantification of γH2AX (left, n = 3. p-value was derived from Welch’s t-test. *p = 0.012) or 53BP1 (right, n = 3. p-value was derived from Welch’s t-test. *p = 0.038) foci number per nucleus. D Senescence-associated β-galactosidase (SA-β-Gal) staining of OMM1.3 cells treated with control siRNA or siRNA to SETDB1 for 96 h, Bar = 20 µM (left) and representative box and whiskers plots of quantification with the percentage of SA-β-Gal positive cells relative to the total number of cells (right) n = 3. p-value was derived from Welch’s t-test. ***p = 0.0007. E Immunoblot to SETDB1 and p21 of OMM1.3 cells treated with control siRNA or siRNA to SETDB1 for 24, 48, and 72 h. β-Actin was used as a loading control. * Indicates non-specific band.
CDC6 depletion phenocopies SETDB1 knockdown
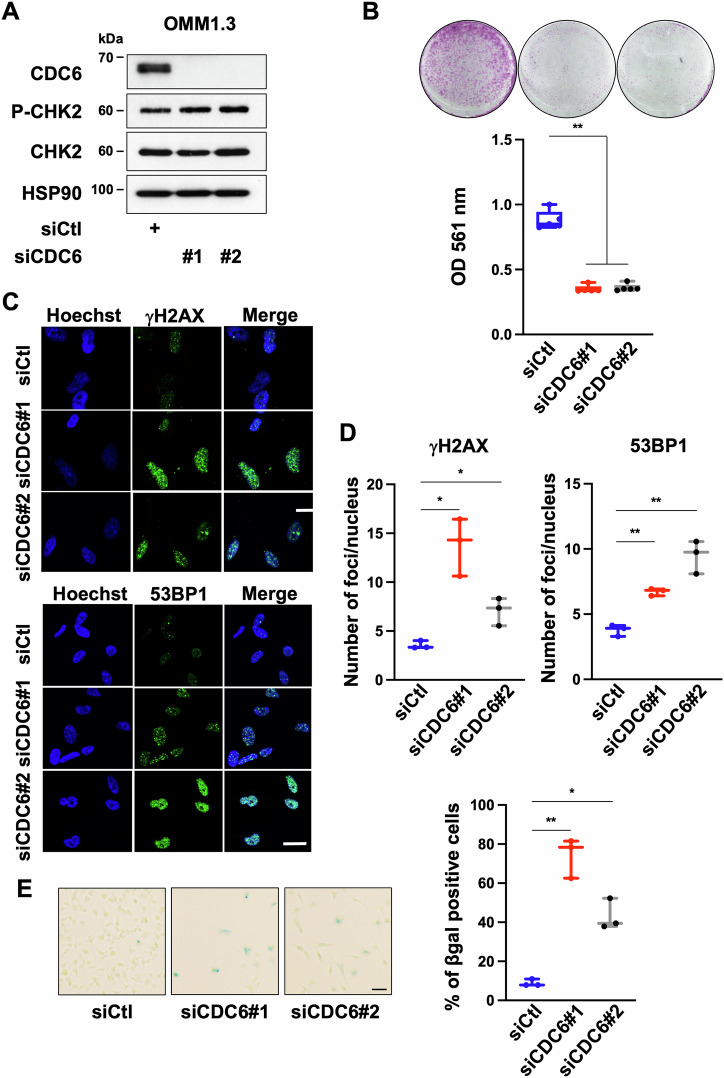
Cell division cycle 6 (CDC6) and Minichromosome Maintenance Complex Component 6 (MCM6), that are downregulated in cells with reduced SETDB1 level, are essential regulators of DNA replication in eukaryotic cells and they play critical roles in the establishment and maintenance of the cell replication program [21]. However, OMM1.3 and OMM2.5 cells treated with siRNA against MCM6 did not display reduced growth potential (Supplementary Fig. 6A–D). In contrast, CDC6 depletion with two different siRNAs reduced cell growth capacity of OMM1.3 cells (Fig. 4A, B). This finding was extended to OMM2.5 cells treated with the CDC6 siRNAs, which also exhibited reduced cell growth capacity (Supplementary Fig. 7A, B). These data prompted us to focus our attention on CDC6. As performed for SETDB1, we analyzed phosphorylated CHK2, H2AX (γ-H2AX) and 53BP1. Immunoblot of OMM1.3 cells treated with siRNAs to CDC6 showed enhanced phosphorylation of CHK2 (Fig. 4A) and immunofluorescence revealed that CDC6 KD activated the DNA damage response as illustrated by enhanced γ-H2AX and 53BP1 staining compared to control cells (Fig. 4C, D). SA-βGal activity was also detected in cells treated with CDC6 siRNAs (Fig. 4E). Altogether, our findings demonstrate the critical role of CDC6 in DNA replication, but also in DNA repair and senescence and suggest that CDC6 function downstream SETDB1 in uveal melanoma cell growth.
Fig. 4. CDC6 knockdown induces growth arrest and senescence-like features.
A Immunoblot to CDC6, CHK2, and P-CHK2 in lysates of OMM1.3 cells treated with a control siRNA or with two different siRNA to CDC6 for 72 h. HSP90 was used as a loading control. B OMM1.3 cells treated with a control siRNA (siCtl) or siRNA to CDC6 were seeded at the same density and cultured for 10 days (top), representative box and whiskers plots of crystal violet quantification at OD 561 nm (bottom). Mann–Whitney test was performed for comparison between groups, n = 5. Data are mean ± SEM. **p = 0.0079. C OMM1.3 cells treated for 72 h with control siRNA or siRNA to CDC6 were analyzed by immunofluorescence for H2AX phosphorylated on Ser139 (γH2AX) or 53BP1. Cell nuclei were counterstained with Hoechst. Representative fluorescence images are shown. Bar = 20 µM. D Representative box and whiskers plots of quantification of γH2AX (left, n = 3. p-value was derived from Welch’s t-test. *p = 0.025, *p = 0.041) or 53BP1 (right, n = 3. p-value was derived from Welch’s t-test. **p = 0.013, **p = 0.009) foci number per nucleus. E SA-β-Gal staining of OMM1.3 cells treated with control siRNA or siRNA to CDC6 for 6 days. Bar=20 µM (left) and representative box and whiskers plots of quantification with the percentage of SA-β-Gal positive cells relative to the total number of cells (right) n = 3. p-value was derived from Welch’s t-test. **p = 0.0068, *p = 0.0139.
Anti-SETDB1 therapy reduces uveal melanoma cell growth
Our data point towards SETDB1 as a potential relevant therapeutic target in metastatic uveal melanomas. Mithramycin A, an antitumor antibiotic used in phase II clinical trials for the treatment of patients with a broad range of malignancies (ClinicalTrials.gov: NCT01624090), has been demonstrated to inhibit SETDB1 expression [22]. Mithramycin A is reported to impair SETDB1 expression by blocking binding of the SP-1 transcription factor at the SETDB1 promoter [23]. We tested the effect of Mithramycin A on different GNAQ/11-mutated human metastatic uveal melanoma cells in vitro. OMM1.3 cells exposed to increasing concentration of Mithramycin A showed reduced cell growth ability (Fig. 5A). Similar observations were performed in two other human metastatic uveal cell lines, OMM2.5 and OMM1, demonstrating that the growth defects were not restrain to a unique cell line (Fig. 5A). Interestingly, a more direct SETDB1 inhibitor (SETDB1i), which prevents the interaction of SETDB1 with histones, has been recently reported [24]. Increasing concentration of SETDB1i also caused reduced cell growth ability of the different human metastatic uveal melanoma cell lines (Supplementary Fig. 8A). Therefore, our data show that a panel of human metastatic uveal melanoma cells are sensitive to two different SETDB1 inhibitors.
Fig. 5. Anti-SETDB1 therapy reduces metastatic uveal melanoma cell proliferation and survival.
A OMM1.3, OMM2.5, and OMM1 metastatic uveal melanoma cells were seeded at low density and cultured for 96 h in absence or presence of increasing concentration of Mithramycin A. Representative images of three independent experiments are shown. B Immunoblot analysis of metastatic uveal melanoma cells exposed to Mithramycin A (15 and 30 nM) for 72 h with the indicated antibodies. HSP90 was used as a loading control. C Immunoblot to cleaved PARP in control OMM1.3 cells and OMM1.3 cells treated with Mithramycin A (15 and 30 nM) for 72 h. β-Actin was used as a loading control. D Analysis of apoptosis in control OMM1.3 cells and OMM1.3 cells treated with Mithramycin A at the indicated concentrations for 96 h. Annexin V diagram and quantification of the percentage of late apoptotic cells using the Annexin V assay, n = 3. p-value was derived from Welch’s t-test. *p = 0.0399.
Both Mithramycin A and SETDB1i reduced SETDB1 and CDC6 protein levels (Fig. 5B and supplementary Fig. 8B), in agreement with CDC6 being regulated by SETDB1. It is worth noting that Mithramycin A and SETDB1i did not induce SA-βGal staining. Instead, they both promoted apoptosis as illustrated by annexin V/PI labelling and the detection of cleaved PARP, a well-studied caspase 3 substrate. It is also worth noting that apoptosis was most visible at the highest inhibitor concentration (Fig. 5C, D and Supplementary Fig. 8C, D).
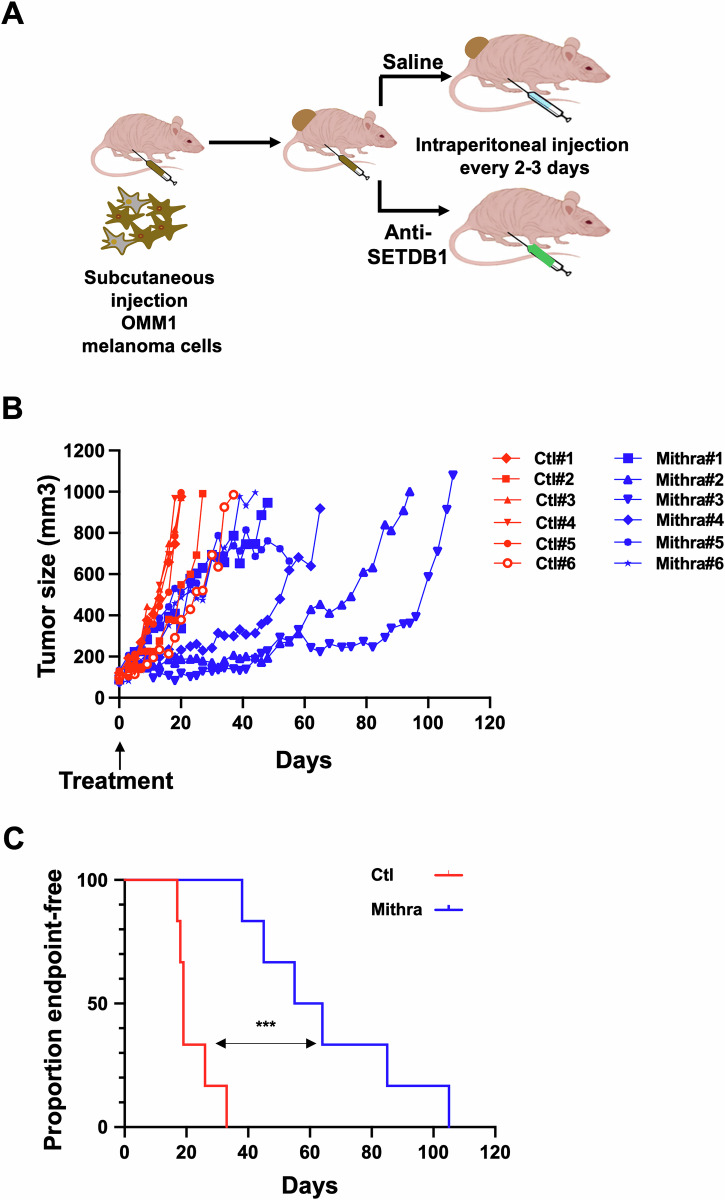
These data indicate that SETDB1 represents a promising anti-metastatic uveal melanoma therapy. To address this point, we next investigated the therapeutic relevance of inhibiting SETDB1 on tumor growth in vivo. Given that Mithramycin A works at nanomolar concentration compared to SETDB1i and that it has been assessed in clinical trials, we pursued in vivo studies with Mithramycin A. Notably, no toxicity was observed in normal uveal melanocytes or fibroblasts (Supplementary Fig. 9A, B). Both male and female mice were used in these experiments since uveal melanoma incidence is similar in men and women. OMM1 cells were subcutaneously injected into NSG immunodeficient mice and when the tumors were palpable (approximately 100 mm3), mice were injected intraperitoneally every 3 days with Mithramycin A (1 mg/kg) (Fig. 6A). When the tumor reached ~1 cm3, the mouse was sacrificed. Our data show that Mithramycin A strongly impaired uveal melanoma growth as illustrated by reduced tumor volume compared to the vehicle control group (Fig. 6B). Moreover, treatment with Mithramycin A resulted in a significant survival advantage until the ethical endpoint was reached compared to vehicle-treated mice (Fig. 6C) and no metastases were observed in this experiment.
Fig. 6. SETDB1 inhibition has anti-tumoral effect in vivo.
A Schematic of in vivo experiment where NSG mice were injected subcutaneously with OMM1 cells. When the tumor reached 100 mm3, mice began a treatment regimen of vehicle (PBS) or Mithramycin A via intraperitoneal injection (1 mg/kg) three times per week. B Plot showing tumor volume for individual mice bearing a OMM1 cell xenograft treated with PBS (Control mice in red) or with Mithramycin A (Mithramycin A-treated mice in blue). C Kaplan–Meier analyses of tumor growth to ethical endpoint for mice bearing the OMM1 cell xenografts showing time to 0.8 cm3 in vehicle (red) or 1 mg/kg Mithramycin A (blue) treated mice. Log-rank (Mantel–Cox) test was performed for comparison between groups. ***p = 0.0005.
Altogether, our findings demonstrate that SETDB1 critically supports metastatic uveal melanoma progression in vivo, and establish SETDB1 as a promising effective therapeutic strategy in these often untreatable tumors.
Discussion
We hereby present a chromatin-focused CRISPR-Cas9 screen to identify factors that play a critical role in proliferation and survival of metastatic uveal melanoma cells, and identified the lysine methyltransferase SETDB1. Our screen also revealed additional candidates. In our context, the histone demethylase KDM5C that removes the active H3K4me3 marks and the histone methyltransferase PRMT6 adding the repressive H3R2me2a marks could contribute to tumor suppressor gene silencing. The histone demethylase KDM4D targeting the repressive H3K9me2/H3K9me3 marks and the histone methyltransferase SETD1B, which deposits activating H3K4me3 marks might activate oncogenes [25, 26]. These regulators might create aberrant chromatin landscape in uveal melanoma cells in which their roles remain to be elucidated, thus representing a new area for future investigation.
SETDB1 has been reported to be upregulated in a variety of tumors, such as human skin melanomas, and promotes cancer development [27]. Previous work also showed that SETDB1 regulates the proliferation of different tumor cells in vitro and in vivo [28–32]. Aligned with that, our findings show a high expression of SETDB1 protein in human uveal melanoma cells compared to normal uveal melanocytes. We also found that SETDB1 knockdown in uveal melanomas activates a DNA damage response, associated with a senescence-like state and a growth arrest. This senescence-like state has been shown to be overcame by SETDB1 overexpression in the study of Ceol and collaborators, most likely through a different mechanism that we observed [27]. Indeed, among the list of genes downregulated in SETDB1 overexpressing melanomas that they have identified, none are deregulated in our transcriptomic data. These data indicate that SETDB1 plays a critical role in growth of uveal melanoma cells, likely by overcoming the process of senescence, a major barrier against tumor progression, most likely in a context-dependent function.
SETDB1 is amplified in different types of cancers, including breast, ovarian, bladder, and cutaneous melanomas, in which it can also be found mutated [27, 31, 33]. We found a higher SETDB1 level in metastatic uveal melanoma cells compared to normal choroidal melanocytes, yet analysis of public datasets revealed no alteration in copy number or mutations in SETDB1 in uveal melanomas. However, increased SETDB1 expression could occur through other mechanisms, including chromosomal translocation, single-nucleotide polymorphism in regulatory regions and mutation or activation of upstream signaling pathways. How SETDB1 expression is regulated in uveal melanoma remains to be determined.
A metastasis-promoting role for SETDB1 has also been reported in different cancer types, such as in colorectal cancer, and in cutaneous melanomas [29, 34] in which high SETDB1 expression was detected at the invasive front [28]. Supporting this, in cutaneous malignant melanomas, SETDB1 regulates the expression of thrombospondin-1, known to stimulate metastasis formation [28]. Our data did not show any change in the motile ability of SETDB1 knocked-down uveal melanoma cells (not shown). In contrast, our transcriptomic analysis highlighted downregulation of factors involved in the assembly of the replication initiation machinery in SETDB1-deficient cells. Among them, reduced CDC6 and MCM6 expression was validated at the protein level, but only CDC6 knockdown impaired metastatic uveal melanoma cell growth. The effect of MCM6 reduction is likely to be offset by the high abundance of MCM proteins in contrast to CDC6, which is an essential and rate-limiting factor [35, 36]. High CDC6 expression is associated with enhanced malignant behavior of cancer cells [37, 38] and drug resistance [39–41]. Upon CDC6 inhibition, uveal melanoma cells exhibited signs of DNA damage and senescent phenotypes. Growth inhibition and senescence in response to CDC6 inhibition have been evoked in other cancers [42, 43]. Altogether, our observations suggest that SETDB1 can mediate its effect in part through CDC6.
Hence, both SETDB1 and CDC6 might represent new prognostic biomarkers and new potential therapeutic targets in uveal melanomas.
How SETDB1 inhibits the expression of these factors remains to be elucidated. It is known to catalyze the repressive H3K9me3 mark and mediate gene repression. However, we observed no difference in overall H3K9me2/3 levels after SETDB1 knockdown in OMM1.3 uveal melanoma cells. We cannot rule out the existence of compensation mechanisms by other H3K9 methyltransferases for the regulation of overall H3K9me3 metabolism in metastatic uveal melanoma cells. Indeed, H3K9me3 is catalyzed by two enzymatic systems, SUV39H and SETDB1/ESET1 (SET domain bifurcated) [44]. Another possibility is that SETDB1 regulates a transcriptional program independently of its H3K9me3 activity. This is reminiscent of other studies demonstrating that SETDB1 functions through methylation of non-histone proteins such as p53 or AKT [45, 46].
Regardless of the precise mechanisms of SETDB1 activity, given that SETDB1 inhibition strongly reduced the growth capacity of metastatic uveal melanoma cells, suggests that SETDB1 is a highly relevant therapeutic target for the treatment of this tumor type.
This is evidenced by in vitro effect of Mithramycin A and SETDB1i, two reported SETDB1 inhibitors. It is noteworthy that Mithramycin A or SETDB1i does not induce senescence but instead apoptosis. This might be related to the levels of p53-p21 pathway activation. Importantly, in a pre-clinical model, Mithramycin A strongly reduced metastatic uveal melanoma progression and robustly extended mouse lifespan.
In sum, our findings demonstrate that SETDB1 inhibition represent a novel and valid therapeutic option for the treatment of metastatic uveal melanomas.
Material and Methods
Cell cultures
Human uveal melanoma cell lines OMM1.3 (GNAQQ209P) [47] and OMM2.5 (GNAQQ209P) [47] were grown in RPMI supplemented with 10% FBS and 5% penicillin/Streptomycin antibiotic at 37 °C in a humidified atmosphere containing 5% CO2 [48]. OMM1 (GNA11Q209L) was grown in Gibco DMEM supplemented with 10% FBC, 5% penicillin/Streptomycin, 1% Sodium pyruvate 100 mM, 1% MEM essential vitamin mixture and 1% NEAA mixture and 1% HEPES Buffer solution. No mutation is reported for BAP1, SF3B1, or EIF1AX in these cell lines. MP46 (GNAQQ209L) and MP65 (GNA11Q209L) were grown in RPMI supplemented with 10% FBS and 5% penicillin/Streptomycin antibiotic at 37 °C in a humidified atmosphere containing 5% CO2. MP46 and MP65 are BAP1-deficient, no mutation for SF3B1 or EIF1AX in these cell lines are reported. Melanocytes were isolated from the healthy part of the choroid of two donor eyeballs (NHCM#1 and NHCM#2). Cell lines are regularly tested for mycoplasma and are mycoplasma-free. They were authenticated through short tandem repeat (STR) profiling.
Biochemicals
Mithramycin A was from Santa Cruz (sc-200909) and SETDB1i was from MedChem Express (HY-141539).
Pooled CRISPR library details
The custom oligonucleotide library with sgRNAs targeting ~140 human chromatin regulators genes (3–4 sgRNAs per target) was used as previously described [16]. To ensure library diversity, colonies were collected from 15 bacterial plates after transformation of 10-beta electrocompetent (New England Biolabs). The pool of plasmids was prepared for infection using an endotoxin-free Maxi prep kit (Qiagen).
CRISPR-Cas9 screen
Human OMM1.3 uveal melanoma cells were first infected with the lentiCas9-Hygro (Addgene # 104995) and selected with hygromycin (200 μg/mL). Cells were then infected with the sgRNA library at a low MOI (< 1) to ensure a single sgRNA vector per cell. After 4 days of infection, cells were analyzed by flow cytometry and <20% of cells were EGFP-positive, corresponding to single vector copy. EGFP-positive cells were expanded for 10 days. A fraction of cells was collected at day 0 to ensure a proper coverage of sgRNAs. Medium was changed every 3 days. At day 35, cells from all conditions were collected and genomic DNA was extracted. Since melanin pigment may interfere with DNA-and/or RNA-based molecular profiling [49], we purified the samples using the OneStepTM PCR inhibitor Removal Kit (Zymo Research). The integrated sgRNAs were then amplified by PCR with primers containing multiplexing barcodes and adaptors and sequenced on the Illumina NextSeq500. Hits were selected based on the log2 fold change of sgRNA reads at day 35. Analyses and plots of the sequencing data were conducted using Prism 6 software (GraphPad Software). Data were analysed using the software Mageck, which calculates a score based on a fold change where either sgRNA is depleted or enriched compared to the control condition.
mRNA preparation and real-time/quantitative PCR
The mRNAs were prepared using TRIzol (Fisher Scientific,15596026 T) according to a standard procedure. RT-qPCR was performed using SYBR® Green I (Fisher Scientific, 4368708) and Multiscribe Reverse Transcriptase (Applied Biosystems) and subsequently monitored using the ABI Prism 7900 Sequence Detection System (Applied Biosystems, Foster City, CA) as previously reported [50]. The detection of the RPLP0 mRMA was used to normalize the results. Primer sequences for each cDNA were designed using either Primer bank (https://pga.mgh.harvard.edu/primerbank/). Sequences are available upon request.
RNA-sequencing
Reads were preprocessed to remove adapter and low-quality sequences (Phred quality score below 20). After this preprocessing, reads shorter than 40 bases were discarded for further analysis. These preprocessing steps were performed using cutadapt version 1.10. Reads were mapped to rRNA sequences using bowtie version 2.2.8 and reads mapping to rRNA sequences were removed for further analysis. Reads were mapped onto the hg38 assembly of Homo sapiens genome using STAR version 2.5.3a. Gene expression quantification was performed from uniquely aligned reads using htseq-count version 0.6.1p1, with annotations from Ensembl version 99 and “union” mode. Only non-ambiguously assigned reads have been retained for further analyses. Read counts have been normalized across samples with the median-of-ratios method [51]. Differential gene expression analysis was performed using the methodology implemented in the Bioconductor package DESeq2 version 1.16.1 [52]. P-values were adjusted for multiple testing by the method proposed by Benjamini and Hochberg [53]. Deregulated genes were defined as genes with log2(foldchange) ≥ 0.65 or ≤ −0.65 and adjusted P-value ≤ 0.05.
Transient transfection of siRNA and infection of shRNA
Briefly, a single pulse of 50 nM of control siRNA, siRNA to SETDB1 (Sigma SASI_Hs02_00344324) or siRNA to CDC6 (Sigma SASI_Hs01_00047246 and SASI_Hs01_00047247) was administered to the cells at 50% confluency through transfection with 5 µl of LipofectamineTM RNAiMAX in Opti-MEM medium (Invitrogen, San Diego, CA, USA) as described [54].
Cell cycle analysis
The Click-iT Plus EdU Alexa Fluor 647 Flow Cytometry Assay Kit (Invitrogen C10634) was used for detection of replicating OMM1.3 cells based on incorporation of 2 μM EdU (5-ethynyl 2′-deoxyuridine) into newly synthesized DNA for 2 h followed by its recognition with azide dyes via copper-mediated “click” reaction, according to the manufacturer’s protocol. DAPI and EdU (C10634, Invitrogen) double staining was used to measure DNA content in live cells by flow cytometry.
Whole cell protein extractions and chromatin fractionation
Cells were washed with PBS and lysed on ice for 5 min in NP40 buffer (50 mM Tris pH 7.5, 1% NP40, 150 mM NaCl, 10% Glycerol, 1 mM EDTA) supplemented with protease and phosphatase inhibitors (Roche). Lysates were centrifuged at 15,000 rpm for 15 min and the protein concentration was quantified using BCA (Pierce). Chromatin fractionation performed as described [55]. All lysates were freshly prepared and supplemented with Laemmli loading buffer with subsequent boiling for immunoblotting.
Immunoblot assays
Briefly, cell lysates (30 µg) were separated using SDS-PAGE, transferred onto a PVDF membrane as previously described [56] and subsequently exposed to the appropriate antibodies, anti-SETDB1 (VMA00243; 1/1000) from Biorad, anti-p21 (2947; 1/1000), anti-CDC6 (3387; 1/1000), anti-phospho CHEK2 (2197; 1/1000), anti-CHEK2 (6334; 1/1000), and anti-PARP (9542, 1/1000), from Ozyme, anti MCM6 (ab201683; 1/1000) from Abcam, anti-β-ACTIN (sc-47778; 1/1000), anti-GAPDH (sc-47724; 1/1000) and anti-HSP90 (sc-13119; 1/1000) from Santa Cruz Biotechnology, H3K9me2/3 (5327; 1/1000) from Cell Signaling Technology. The proteins were visualized using the ECL system (Amersham). The immunoblots shown are representative of at least 3 independent experiments.
Cell growth assay
Human uveal melanoma cells were seeded onto six-well plates at low density, allowed to adhere overnight and cultured as indicated. Then, the colonies were stained with 0.04% crystal violet/2% ethanol in PBS for 30 min. Photographs of the stained colonies were captured. Crystal violet was then solubilized and growth was monitored by measuring the absorbance at 561 nm as previously reported [57]. Photographs of the stained colonies were captured. The assay was performed in triplicate.
Immunofluorescence staining
Immunofluorescence experiments were carried out as previously described [58]. Briefly, cells grown on glass coverslips were fixed in 4% formaldehyde in PBS supplemented with 0.1% Triton ×-100 for 15 min at room temperature prior to permeabilization in 0.1% Triton ×-100 for 10 min. After 1 h of blocking with 1% BSA in PBS containing 0.1% Tween 20, the cells were stained overnight at 4 °C in a humidified chamber in a blocking solution with antibodies to γ-H2AX (1/500, Abcam ab11174), 53BP1 (1/50, Bethyl, IHC-00001). Primary antibody detection was achieved via incubation with anti-rabbit or anti-mouse Alexa Fluor 594- or 488-conjugated secondary antibodies (Invitrogen) for 90 min at room temperature. The slides were mounted in DAKO mounting medium supplemented with Hoechst (1/1000, Invitrogen, #H3570) and examined using a 40× oil immersion objective with a NIKON AR1 confocal microscope. Representative experiments are shown.
Animal experimentation
Animal experiments were performed in accordance with French law and approved by a local institutional ethical committee. The animals were maintained on a 12-h light/dark cycle in a temperature-controlled facility at 22 °C and provided free access to food (standard laboratory chow diet). Human OMM1 melanoma cells (5 × 106 cells) were subcutaneously inoculated into 8-wk-old male and female immune-deficient Nod scid gamma (NSG) mice (Janvier Laboratory). When the tumors became palpable, mice received intraperitoneal injection of Mithramycin (1 mg/kg) 3 times per week dissolved in phosphate buffered saline (PBS). Overall, Mithramycin A was well tolerated, with only transient weight loss in some mice that resolved after treatment discontinuation for one or two rounds. Control mice were injected with PBS alone. The tumor growth curves were determined after measuring the tumor volume using the equation V = (L × W2)/2 as previously reported [59]. Mice were randomly assigned to the different treatment groups.
Statistics
No data were excluded from the analyses. Investigators were not blinded. No statistical methods were used to determine the sample size. Sample size was determined to be adequate based on the magnitude and consistency of measurable differences between groups. The statistical analyses were performed by GraphPad Prism 6 (GraphPad Software). Statistical significance between groups was determined using GraphPad Prism as indicated in the legends. *p-value ≤ 0.05; **p-value ≤ 0.01; ***p-value ≤ 0.001; ****p-value ≤ 0.0001.
Supplementary information
Acknowledgements
This work was supported by the French government, INSERM, La Ligue Nationale contre le cancer, INCA PLBio to CB (INCA-16070), Labex Signalife, and La Ville de Nice. IK acknowledges the financial support provided by Région Provence-Alpes-Côte d’Azur “Emplois jeunes doctorants”. IK was also a recipient from La Ligue Nationale contre le cancer. TS acknowledges the financial support provided by La Fondation pour la Recherche Médicale (FRM ARF201809006989), the Fondation de France (00120250/WB-2021-33281) and the Fondation ARC (PJA 20191209328). VN acknowledges the financial support provided by La Ligue contre le cancer CD94/Val de Marne (research subventions 2023–2024). The author thanks Nicolas Nottet for sequencing analyses using the MAGeCK method. AEA and EB were supported by a Melanoma Research Alliance Team Science Award.
Author contributions
CB, RB, and TS: Conceptualization; data curation; formal analysis; supervision; funding acquisition; writing–original draft; writing–review and editing. IK, CP, YC, KB, MD, CH, FS: performed the experiments. SH: data curation. MI, DH, SC: Software; methodology. JCV, SNE, AM, SL, JPC, CM, SB: scientific discussions. AEA, ID, EB, and VN: scientific discussions–review and editing.
Data availability
The RNA-sequencing data generated and/or analyzed during this study have been deposited in the NCBI Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) under the SuperSeries GSE302422.
Competing interests
AE Aplin has ownership interest in patent number 9880150 and has a pending patent, PCT/US22/76492. The other authors declare no conflicts of interest.
Ethics approval and consent to participate
Animal experiments were performed in accordance with French law and approved by a local institutional ethical committee.
Footnotes
Edited by Jean-Christophe Marine
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Robert Ballotti, Corine Bertolotto, Thomas Strub.
Contributor Information
Corine Bertolotto, Email: Corine.Bertolotto@univ-cotedazur.fr.
Thomas Strub, Email: thomas.strub@univ-cotedazur.fr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41419-025-08084-z.
References
- 1.Pandiani C, Béranger GE, Leclerc J, Ballotti R, Bertolotto C. Focus on cutaneous and uveal melanoma specificities. Genes Dev. 2017;31:724–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nathan P, Hassel JC, Rutkowski P, Baurain JF, Butler MO, Schlaak M, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2021;385:1196–206. [DOI] [PubMed] [Google Scholar]
- 3.Hassel JC, Piperno-Neumann S, Rutkowski P, Baurain JF, Schlaak M, Butler MO, et al. Three-year overall survival with tebentafusp in metastatic uveal melanoma. N Engl J Med. 2023;389:2256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson AG, Shih J, Yau C, Gibb EA, Oba J, Mungall KL, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell. 2017;32:204–20.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvajal RD, Sacco JJ, Jager MJ, Eschelman DJ, Olofsson BR, Harbour JW, et al. Advances in the clinical management of uveal melanoma. Nat Rev Clin Oncol. 2023;20:99–115. [DOI] [PubMed]
- 6.Harbour JW, Onken MD, Roberson EDO, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330:1410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheuermann JC, Alonso AG, de A, Oktaba K, Ly-Hartig N, McGinty RK, et al. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strub T, Ballotti R, Bertolotto C. The “ART” of epigenetics in melanoma: from histone “alterations, to resistance and therapies.” Theranostics. 2020;10:1777–97. [DOI] [PMC free article] [PubMed]
- 9.Levidou G, Gajdzis P, Cassoux N, Donizy P, Masaoutis C, Gajdzis M, et al. Histone deacetylase (HDAC)-1, -2, -4, and -6 in uveal melanomas: associations with clinicopathological parameters and patients’ survival. Cancers (Basel). 2021;13:4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Souri Z, Wierenga APA, Mulder A, Jochemsen AG, Jager MJ. HLA expression in uveal melanoma: an indicator of malignancy and a modifiable immunological target. Cancers. 2019;11:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuznetsoff JN, Owens DA, Lopez A, Rodriguez DA, Chee NT, Kurtenbach S, et al. Dual screen for efficacy and toxicity identifies HDAC inhibitor with distinctive activity spectrum for BAP1-mutant uveal melanoma. Mol Cancer Res. 2020;19:1–9. [DOI] [PMC free article] [PubMed]
- 12.Gu X, Hua Y, Yu J, Yang L, Ge S, Jia R, et al. Epigenetic drug library screening reveals targeting DOT1L abrogates NAD+ synthesis by reprogramming H3K79 methylation in uveal melanoma. J Pharm Anal. 2023;13:24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herwig-Carl MC, Sharma A, Tischler V, Pelusi N, Loeffler KU, Holz FG, et al. Mass spectrometry-based profiling of histone post-translational modifications in uveal melanoma tissues, human melanocytes, and uveal melanoma cell lines - A pilot study. Invest Ophthalmol Vis Sci. 2024;65:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rago F, Elliott G, Li A, Sprouffske K, Kerr G, Desplat A, et al. The discovery of SWI/SNF chromatin remodeling activity as a novel and targetable dependency in uveal melanoma. Mol Cancer Ther. 2020;19:2186–95. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Wu S. RUVBL1-modulated chromatin remodeling alters the transcriptional activity of oncogenic CTNNB1 in uveal melanoma. Cell Death Discov. 2023;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strub T, Ghiraldini FG, Carcamo S, Li M, Wroblewska A, Singh R, et al. SIRT6 haploinsufficiency induces BRAF(V600E) melanoma cell resistance to MAPK inhibitors via IGF signalling. Nat Commun. 2018;9:3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strepkos D, Markouli M, Klonou A, Papavassiliou AG, Piperi C. Histone methyltransferase SETDB1: a common denominator of tumorigenesis with therapeutic potential. Cancer Res. 2021;81:525–34. [DOI] [PubMed] [Google Scholar]
- 18.Yan Z, Degregori J, Shohet R, Leone G, Stillman B, Nevins JR, et al. Cdc6 is regulated by E2F and is essential for DNA replication in mammalian cells. Proc Natl Acad Sci USA. 1998;95:3603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bártová E, Legartová S, Dundr M, Suchánková J. A role of the 53BP1 protein in genome protection: structural and functional characteristics of 53BP1-dependent DNA repair. Aging (Albany NY). 2019;11:2488–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodier F, Coppé JP, Patil CK, Hoeijmakers WAM, Muñoz DP, Raza SR, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fragkos M, Ganier O, Coulombe P, Méchali M. DNA replication origin activation in space and time. Nat Rev Mol Cell Biol. 2015;16:360–74. [DOI] [PubMed] [Google Scholar]
- 22.Ryu H, Lee J, Hagerty SW, Soh BY, McAlpin SE, Cormier KA, et al. ESET/SETDB1 gene expression and histone H3 (K9) trimethylation in Huntington’s disease. Proc Natl Acad Sci USA. 2016;103:19176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleiman SF, Langley BC, Basso M, Berlin J, Xia L, Payappilly JB, et al. Mithramycin Is A Gene-selective Sp1 inhibitor that identifies a biological intersection between cancer and neurodegeneration. J Neurosci. 2011;31:6858–70. [DOI] [PMC free article] [PubMed]
- 24.Guo Y, Mao X, Xiong L, Xia A, You J, Lin G, et al. Structure-guided discovery of a potent and selective cell-active inhibitor of SETDB1 tudor domain. Angew Chem Int Ed Engl. 2021;60:8760–5. [DOI] [PubMed] [Google Scholar]
- 25.Zhao S, Allis CD, Wang GG. The language of chromatin modification in human cancers. Nat Rev Cancer. 2021;21:413–30. [DOI] [PMC free article] [PubMed]
- 26.Guccione E, Bassi C, Casadio F, Martinato F, Cesaroni M, Schuchlautz H, et al. Methylation of histone H3R2 by PRMT6 and H3K4 by an MLL complex are mutually exclusive. Nature. 2007;449:933–7. [DOI] [PubMed] [Google Scholar]
- 27.Ceol CJ, Houvras Y, Jane-Valbuena J, Bilodeau S, Orlando DA, Battisti V, et al. The histone methyltransferase SETDB1 is recurrently amplified in melanoma and accelerates its onset. Nature. 2011;471:513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orouji E, Federico A, Larribère L, Novak D, Lipka DB, Assenov Y, et al. Histone methyltransferase SETDB1 contributes to melanoma tumorigenesis and serves as a new potential therapeutic target. Int J Cancer. 2019;145:3462–77. [DOI] [PubMed] [Google Scholar]
- 29.Shi X, Tasdogan A, Huang F, Hu Z, Morrison SJ, DeBerardinis RJ. The abundance of metabolites related to protein methylation correlates with the metastatic capacity of human melanoma xenografts. Sci Adv. 2017;3:eaao5268. [DOI] [PMC free article] [PubMed]
- 30.Spyropoulou A, Gargalionis A, Dalagiorgou G, Adamopoulos C, Papavassiliou KA, Lea RW, et al. Role of histone lysine Methyltransferases SUV39H1 and SETDB1 in gliomagenesis: modulation of cell proliferation, migration, and Colony formation. Neuromolecular med. 2014;16:70–82. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Paredes M, de Paz AM, Simó-Riudalbas L, Sayols S, Moutinho C, Moran S, et al. Gene amplification of the histone methyltransferase SETDB1 contributes to human lung tumorigenesis. Oncogene. 2014;33:2807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong CM, Wei L, Law CT, Ho DWH, Tsang FHC, Au SLK, et al. Up-regulation of histone methyltransferase SETDB1 by multiple mechanisms in hepatocellular carcinoma promotes cancer metastasis. Hepatology. 2015;63:474–87. [DOI] [PubMed] [Google Scholar]
- 33.Atlas NCG. Genomic classification of cutaneous melanoma. Cell. 2015;161:1681–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li W, Yang X, Liu X, Deng H, Li W, He X, et al. SETDB1 confers colorectal cancer metastasis by regulation of WNT/β-catenin signaling. Biochim Biophys Acta (BBA) - Gen Subj. 2023;1867:130377. [DOI] [PubMed] [Google Scholar]
- 35.Cook JG, Park CH, Burke TW, Leone G, DeGregori J, Engel A, et al. Analysis of Cdc6 function in the assembly of mammalian prereplication complexes. Proc Natl Acad Sci. 2002;99:1347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadav AK, Polasek-Sedlackova H. Quantity and quality of minichromosome maintenance protein complexes couple replication licensing to genome integrity. Commun Biol. 2024;7:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liontos M, Koutsami M, Sideridou M, Evangelou K, Kletsas D, Levy B, et al. Cancer, Deregulated overexpression of hCdt1 and hCdc6 promotes malignant behavior. Cancer Res. 2007;67:10899–909. [DOI] [PubMed] [Google Scholar]
- 38.Ohta S, Koide M, Tokuyama T, Yokota N, Nishizawa S, Namba H. Cdc6 expression as a marker of proliferative activity in brain tumors. Oncol Rep. 2001;8:1063–6. [DOI] [PubMed] [Google Scholar]
- 39.Chen S, Chen X, Xie G, He Y, Yan D, Zheng D, et al. Cdc6 contributes to cisplatin-resistance by activation of ATR-Chk1 pathway in bladder cancer cells. Oncotarget. 2016;7:40362–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao B, Zhang J, Chen X, Xu H, Huang B, Huang B. Mir-26b inhibits growth and resistance to paclitaxel chemotherapy by silencing the CDC6 gene in gastric cancer. Arch Med Sci. 2019;15:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He Y, Yan D, Zheng D, Hu Z, Li H, Li J. Cell division cycle 6 promotes mitotic slippage and contributes to drug resistance in paclitaxel-treated cancer cells. PLoS ONE. 2016;11:e0162633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mourkioti I, Polyzou A, Veroutis D, Theocharous G, Lagopati N, Gentile E, et al. A GATA2-CDC6 axis modulates androgen receptor blockade-induced senescence in prostate cancer. J Exp Clin Cancer Res. 2023;42:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu X, Liu Y, Yin L, Peng Y, Peng Y, Gao Y, et al. Radiation-promoted CDC6 protein stability contributes to radioresistance by regulating senescence and epithelial to mesenchymal transition. Oncogene. 2019;38:549–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Husmann D, Gozani O. Histone lysine methyltransferases in biology and disease. Nat Struct Mol Biol. 2019;26:880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo J, Dai X, Laurent B, Zheng N, Gan W, Zhang J, et al. AKT methylation by SETDB1 promotes AKT kinase activity and oncogenic functions. Nat Cell Biol. 2019;21:226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fei Q, Shang K, Zhang J, Chuai S, Kong D, Zhou T, et al. Histone methyltransferase SETDB1 regulates liver cancer cell growth through methylation of p53. Nat Commun. 2015;6:8651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen P, Murray T, Uno T, Salgaller M, Reddy R, Ksander B. Expression of MAGE genes in ocular melanoma during progression from primary to metastatic disease. Clin Exp Metastasis. 1997;15:509–18. [DOI] [PubMed] [Google Scholar]
- 48.Griewank KG, Yu X, Khalili J, Sozen MM, Stempke-Hale K, Bernatchez C, et al. Genetic and molecular characterization of uveal melanoma cell lines. Pigment Cell Melanoma Res. 2012;25:182–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lagonigro MS, De Cecco L, Carninci P, Di Stasi D, Ranzani T, Rodolfo M, et al. CTAB-urea method purifies RNA from melanin for cDNA microarray analysis. Pigment Cell Res. 2004;17:312–5. [DOI] [PubMed] [Google Scholar]
- 50.Pandiani C, Strub T, Nottet N, Cheli Y, Gambi G, Bille K, et al. Single-cell RNA sequencing reveals intratumoral heterogeneity in primary uveal melanomas and identifies HES6 as a driver of the metastatic disease. Cell Death Differ. 2021;28:1990–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anders S, Wolfgang H. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. JSTOR. 1995;57:289–300. [Google Scholar]
- 54.Leclerc J, Garandeau D, Pandiani C, Gaudel C, Bille K, Nottet N, et al. Lysosomal acid ceramidase ASAH1 controls the transition between invasive and proliferative phenotype in melanoma cells. Oncogene. 2019;38:1282–95. [DOI] [PubMed] [Google Scholar]
- 55.Vardabasso C, Gaspar-Maia A, Hasson D, Punzeler S, Valle-Garcia D, Straub T, et al. Histone variant H2A.Z.2 mediates proliferation and drug sensitivity of malignant melanoma. Mol Cell. 2015;59:75–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bellini L, Strub T, Habel N, Pandiani C, Marchetti S, Martel A, et al. Endoplasmic reticulum stress mediates resistance to BCL-2 inhibitor in uveal melanoma cells. Cell Death Discov. 2020;6:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Proteau S, Krossa I, Husser C, Guéguinou M, Sella F, Bille K, et al. LKB1-SIK2 loss drives uveal melanoma proliferation and hypersensitivity to SLC8A1 and ROS inhibition. EMBO Mol Med. 2023;15:e17719. [DOI] [PMC free article] [PubMed]
- 58.Bourseguin J, Bonet C, Renaud E, Pandiani C, Boncompagni M, Giuliano S, et al. FANCD2 functions as a critical factor downstream of MiTF to maintain the proliferation and survival of melanoma cells. Sci Rep. 2016;6:36539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohanna M, Cerezo M, Nottet N, Bille K, Didier R, Beranger G, et al. Pivotal role of NAMPT in the switch of melanoma cells toward an invasive and drug-resistant phenotype. Genes Dev. 2018;32:448–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-sequencing data generated and/or analyzed during this study have been deposited in the NCBI Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) under the SuperSeries GSE302422.