Abstract
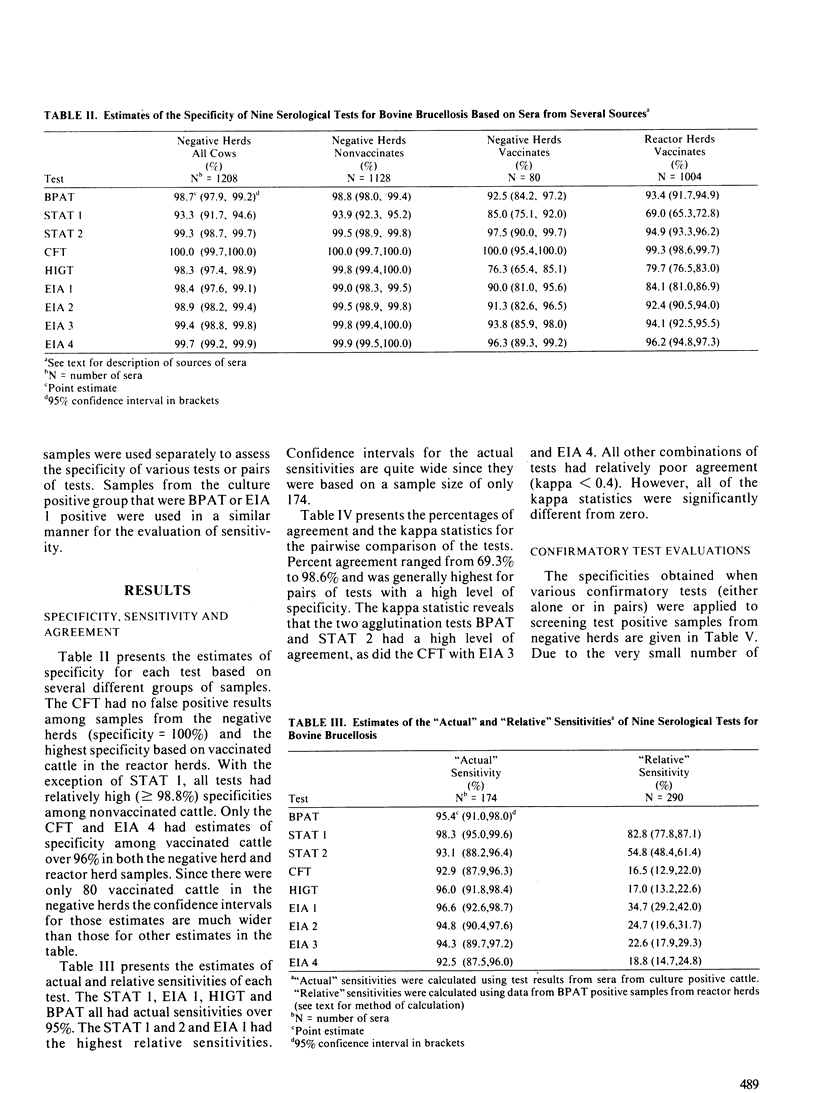
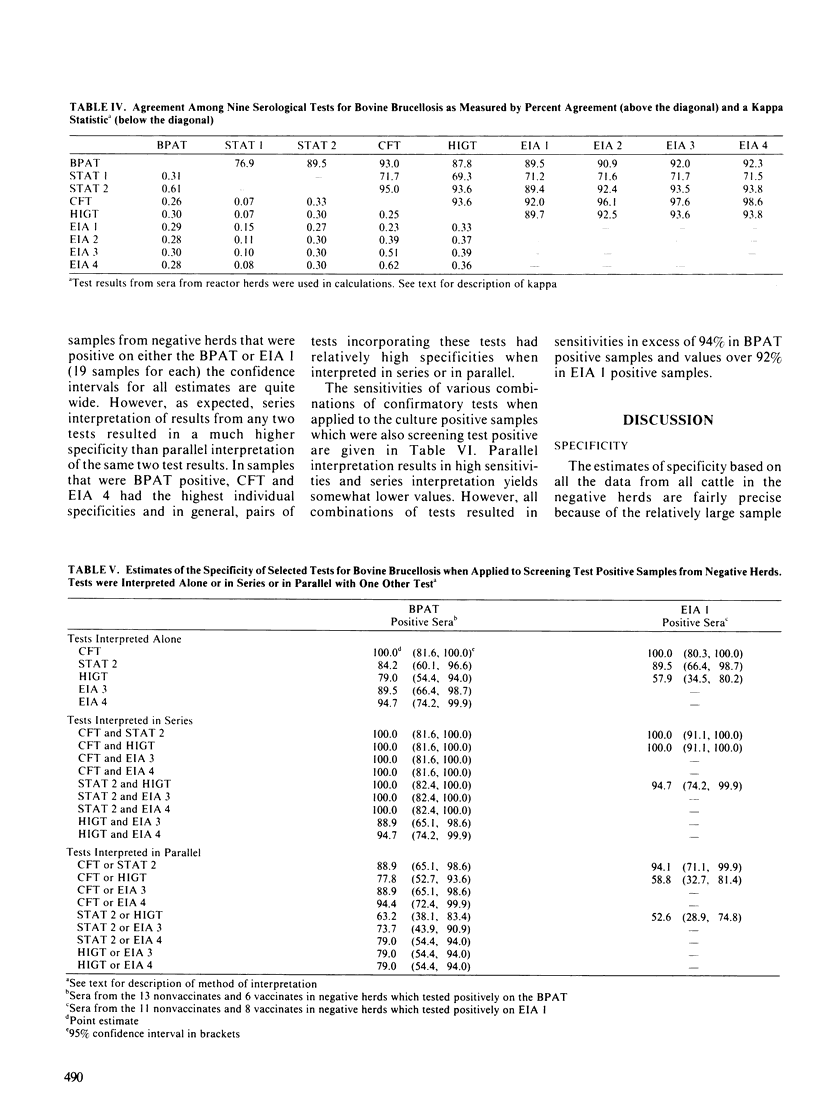
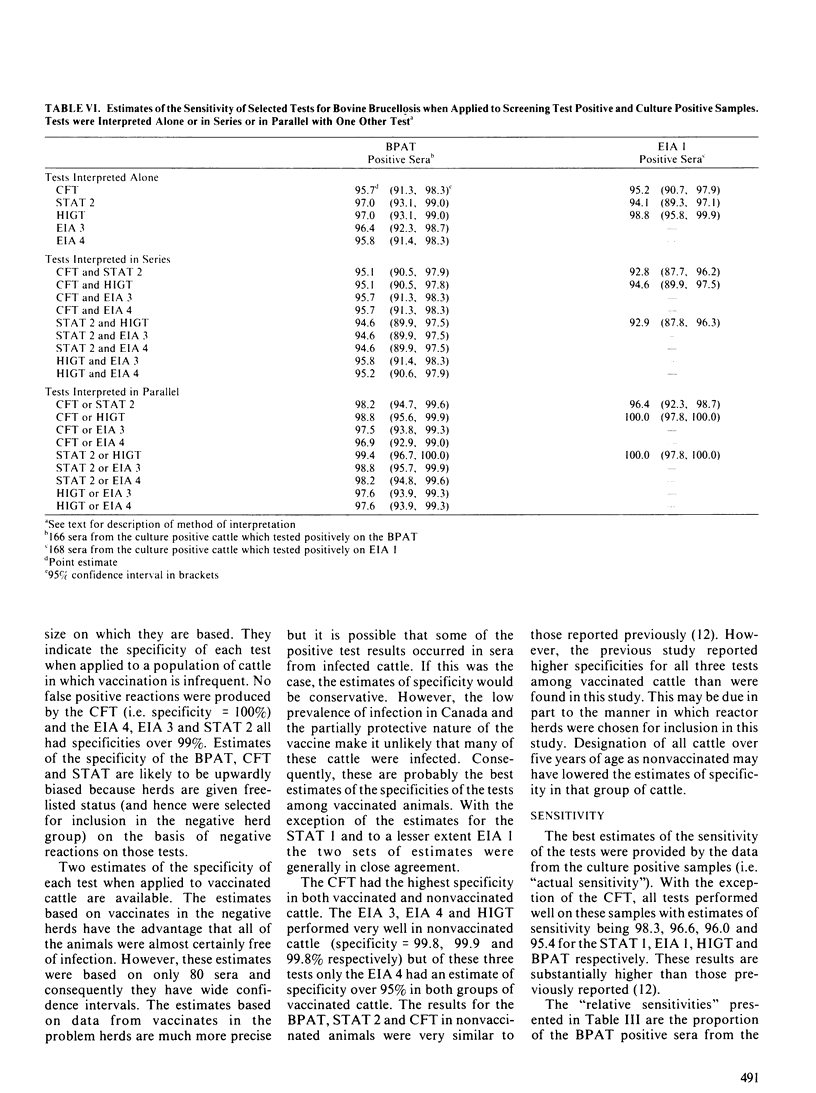
Five serological assays: the buffered plate antigen test, the standard tube agglutination test, the complement fixation test, the hemolysis-in-gel test and the indirect enzyme immunoassay were diagnostically evaluated. Test data consisted of results from 1208 cattle in brucellosis-free herds, 1578 cattle in reactor herds of unknown infection status and 174 cattle from which Brucella abortus had been cultured. The complement fixation test had the highest specificity in both nonvaccinated and vaccinated cattle. The indirect enzyme immunoassay, if interpreted at a high threshold, also exhibited a high specificity in both groups of cattle. The hemolysis-in-gel test had a very high specificity when used in nonvaccinated cattle but quite a low specificity among vaccinates. With the exception of the complement fixation test, all tests had high sensitivities if interpreted at the minimum threshold. However, the sensitivities of the standard tube agglutination test and indirect enzyme immunoassay, when interpreted at high thresholds were comparable to that of the complement fixation test. A kappa statistic was used to measure the agreement between the various tests. In general the kappa statistics were quite low, suggesting that the various tests may detect different antibody isotypes. There was however, good agreement between the buffered plate antigen test and standard tube agglutination test (the two agglutination tests evaluated) and between the complement fixation test and the indirect enzyme immunoassay when interpreted at a high threshold. With the exception of the buffered plate antigen test, all tests were evaluated as confirmatory tests by estimating their specificity and sensitivity on screening-test positive samples.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erdreich L. S., Lee E. T. Use of relative operating characteristic analysis in epidemiology. A method for dealing with subjective judgement. Am J Epidemiol. 1981 Nov;114(5):649–662. doi: 10.1093/oxfordjournals.aje.a113236. [DOI] [PubMed] [Google Scholar]
- Malkin K. L., Tailyour J. M., Bhatia T. R., Archibald R. M., Dorward W. J. A serological survey for brucellosis in Canadian swine. Can J Comp Med. 1968 Oct;32(4):598–599. [PMC free article] [PubMed] [Google Scholar]
- Martin S. W. The evaluation of tests. Can J Comp Med. 1977 Jan;41(1):19–25. [PMC free article] [PubMed] [Google Scholar]
- Ruckerbauer G. M., Garcia M. M., Rigby C. E., Robertson F. J., Samagh B. S., Stemshorn B. W. An hemolysis-in-gel test for bovine brucellosis. Dev Biol Stand. 1984;56:513–520. [PubMed] [Google Scholar]
- Stemshorn B. W., Forbes L. B., Eaglesome M. D., Nielsen K. H., Robertson F. J., Samagh B. S. A comparison of standard serological tests for the diagnosis of bovine brucellosis in Canada. Can J Comp Med. 1985 Oct;49(4):391–394. [PMC free article] [PubMed] [Google Scholar]
- Stemshorn B. W., Nielsen K. H., Samagh B. S., Forbes L. B., Ingram D. G. Evaluation of an enzyme-labeled antiglobulin test for anti-Brucella immunoglobulin G among 3 cattle populations. Am J Vet Res. 1980 Nov;41(11):1779–1784. [PubMed] [Google Scholar]
- Stiller J. M., Nielsen K. H. Affinity purification of bovine antibodies to Brucella abortus Lipopolysaccharide. J Clin Microbiol. 1983 Feb;17(2):323–326. doi: 10.1128/jcm.17.2.323-326.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]


