Summary
Stable carbon isotope (δ13C) analysis of soil organic matter (SOM) from archaeological contexts is widely employed as a proxy for palaeovegetation and palaeoenvironmental reconstruction. However, the reliability of δ13CSOM as a palaeovegetation proxy is influenced by issues of physico-chemical diagenesis and isotopic fractionation, prompting increased interest in source-specific biomarker approaches. In this study, we present a case study from the Palaeolithic site of Jwalapuram (India), where plant-wax biomarker data indicate the degradation of plant-derived lipids, raising concerns about the reliability of δ13CSOM as a palaeovegetation proxy in this context. To further investigate SOM degradation, topsoil samples from central Andhra Pradesh were analyzed, revealing variable levels of SOM preservation across the region, strongly correlated with sedimentary and vegetation contexts. Our research highlights the importance of integrating source-specific proxies, such as plant-wax biomarkers, with bulk proxies such as δ13CSOM to mitigate preservation biases and ensure the integrity of palaeovegetation reconstructions.
Subject areas: Archeology, Biochemistry, Ecological biochemistry, Paleobiochemistry
Graphical abstract

Highlights
-
•
Bulk δ13CSOM is valuable but diagenesis-sensitive; verify SOM preservation before inference
-
•
Jwalapuram shows multi-cycle SOM degradation; plant-wax ratios reveal microbial impacts
-
•
Combine δ13CSOM with plant-wax biomarkers is essential to verify preservation and sources
-
•
Modern baselines of vegetation + sedimentology enable robust, site-specific reconstructions
Archeology; Biochemistry; Ecological biochemistry; Paleobiochemistry
Introduction
Over the past two decades, there has been a significant surge in scientific investigations exploring the intricate relationship between climate, environmental context, and human activity through time.1,2,3,4 This growing interest has expanded the thematic and disciplinary boundaries of human origins research, fostering multidisciplinary collaborations across fields such as archaeology, palaeoanthropology, ecology, genetics, and the Earth sciences in order to construct comprehensive understandings of long-term human-environment interactions.5,6,7,8 Here, there has been an increasing focus on the importance of developing “on-site” palaeoenvironmental records directly associated with records of human behavior.9,10 Methodologically, various bio-geochemical techniques have been integrated to reconstruct the past climate and environmental changes at different hominin and archaeological sites worldwide.11,12,13 In many instances, the application of these techniques has occurred without the thorough consideration of the specific limitations and interpretive challenges within archaeological settings. One example of this concerns the utilization of stable carbon isotope analysis for palaeoenvironmental reconstruction, particularly focusing on the δ13C value of soil organic matter (SOM), which has been utilized in numerous palaeoanthropological and archaeological studies as a proxy for past vegetation dynamics due to its sensitivity to environmental and climatic changes.14,15,16,17
Bulk δ13CSOM: Simple but limited as a proxy
The use of δ13CSOM as a proxy for palaeovegetation and palaeoclimate is complicated by issues of organic preservation, degradation, and source variability.18 SOM, a complex mixture of organic material from diverse sources, undergoes physical and chemical transformations over time, often altering its isotopic composition and leading to potential misinterpretations of past vegetation and climate.18,19,20,21,22 Global vegetation data show that δ13C values for C3 plants (e.g., trees, shrubs, and herbs) range from −20‰ to −34‰, while C4 plants (e.g., forbs, vines, and tropical grasses) typically range from −10‰ to −14‰.23,24 Ideally, δ13CSOM should reflect the δ13C of its source biomass, serving as a reliable indicator of palaeovegetation. However, in regions with low SOM content, such as tropical and subtropical areas such as India, δ13CSOM often deviates from its source values due to poor preservation caused by warm, humid climates, accelerated decomposition, and intense soil weathering.14,23,25 Anthropogenic land use changes, such as agriculture, deforestation, and mining, further disrupt SOM recycling and introduce fractionation effects.23,26 Studies reveal inconsistencies in the relationship between δ13CSOM and source biomass.18,19,27,28 For example, C4-derived SOM decomposes faster than C3-derived SOM, resulting in decreased δ13CSOM values.19,22,29 Microbial decomposition can also increase δ13CSOM through isotopic fractionation.18,25 Additionally, δ13CSOM tends to increase with increasing soil depth due to diagenetic processes, underscoring the need to evaluate preservation conditions to ensure its reliability as a proxy.18
Beyond bulk δ13CSOM: Source-specific biomarker approaches
Plant-wax biomarkers from archaeological sediments offer a promising solution to address biases associated with SOM preservation and source variability.10,30,31,32,33 These biomarkers, including aliphatic hydrocarbons such as n-Alkanes, n-Alkanols, and n-Alkanoic acids, are derived from vascular plants and are highly resistant to biodegradation.32,34 Long-chain n-Alkanes (C29–C35) and n-Alkanoic acids (C28–C34) are characteristic of terrestrial plants, while short-chain homologues (C17–C21) are associated with algae and microbes.34,35 Mid-chain compounds (C23–C27) are linked to aquatic macrophytes.36,37 Changes in the distribution of these compounds reflect shifts in vegetation composition and climatic conditions.30,31 However, it is essential to club the n-alkanes dataset with compound-specific isotope measurements to confirm sources. Furthermore, biomarker indices, such as the carbon preference index (CPI), odd-over-even chain ratio (OEP), and average chain length (ACL), can assess the source and preservation status of SOM.38,39,40 High CPI, OEP, and ACL values indicate terrestrial plant input and good preservation, while low values suggest microbial or petrogenic origins and poor preservation due to diagenesis or biodegradation.38,39,40,41 These biomarkers provide a reliable means to distinguish between microbial and plant biomass dominance in sediments, ensuring the integrity of palaeoenvironmental reconstructions.
Here, the Jwalapuram Palaeolithic site serves as a case study to highlight how on-site plant-wax biomarkers can address challenges in using bulk δ13CSOM alone for palaeovegetation reconstruction. Our primary objective was to understand the palaeoenvironment of hominin occupation at Jwalapuram using plant-wax biomarkers alongside bulk and compound-specific stable isotopes. However, the multi-proxy data revealed contrasting results, indicating poor SOM preservation and limitations for palaeovegetation reconstruction. We emphasize the necessity of compound-specific analyses, especially plant-wax biomarkers, to accurately distinguish source vegetation signals from diagenetic alterations. This refined understanding significantly improves the reliability of palaeovegetation reconstructions, crucial for both environmental science and archaeological interpretation, where precise vegetation assessments are paramount. Specifically, this study evaluated the accuracy of bulk δ13CSOM alone in reflecting past vegetation at Jwalapuram, considering potential preservation issues, and demonstrated the added value of plant-wax biomarkers in overcoming these limitations.
Study area
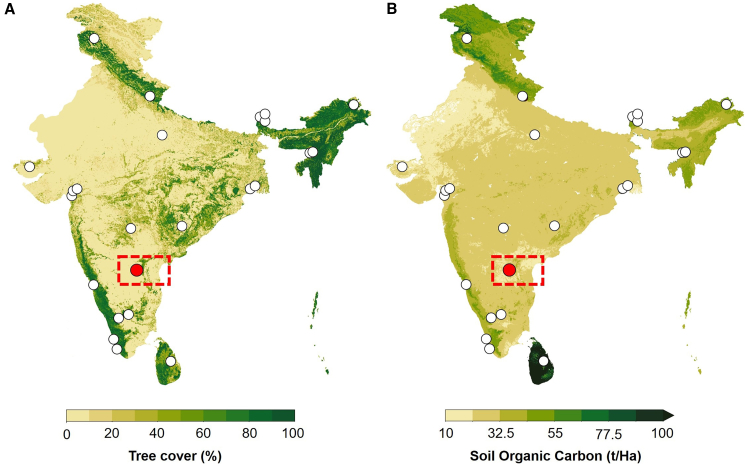
Current work is based on a modern topsoil transect and a palaeolithic site from Andhra Pradesh, southern India. Andhra Pradesh exhibits a tropical climate marked by hot summers with temperatures potentially exceeding 40°C, a monsoon season bringing average rainfall between 560 mm and 1200 mm, and mild winters with temperatures ranging from 15°C to 25°C. This climatic diversity fosters a range of vegetation types across the state. Dry deciduous forests (tropical dry woody-shrubland mosaic), featuring teak and other deciduous trees, are prevalent in areas with moderate rainfall. Regions with higher precipitation in the Eastern Ghats (Nallamala hills) support moist deciduous forests (Moist tropical woodland) with greater species richness. Drier areas, particularly Rayalaseema, are characterized by tropical thorn forests or shrubland (tropical savannah). Regional archaeological dataset shows a wide-scale distribution of palaeolithic across the region, showing a variable palaeoclimatic context. This study aims to explore the relationship between n-Alkane distribution and δ13CSOM in sediment samples from the Palaeolithic site of Jwalapuram (India) to understand preservation status and extent of the biodegradation of SOM, relating to the applicability of δ13CSOM as a palaeovegetation proxy (Figures 1, 2, and 3).
Figure 1.
Map highlights the location of the study area
Map of India showing (A) tree cover (%), highlighting variability in vegetation structure and (B) distribution of Soil Organic Carbon (SOC) concentration (t/Ha) across the region. Note: (a and b) White dots represent published sites with stable carbon isotope data of modern plant and soil from the Indian subcontinent (δ13CSOM data is illustrated in Figure 7); Red dots show the location of Jwalapuram, and the red-dotted rectangle marks the area of the AP topsoil transect.
Figure 2.
Lithological setting and sediment sampling at Jwalapuram, Andhra Pradesh, India
(A) Lithology of JWP2022.
(B) Excavated test-pits of JWP2022. Dark gray area (A) and dotted line (B) represent modern mining and disturbance.
Figure 3.
Sampling sites from the Andhra Pradesh (AP) topsoil transect
(A) Land cover map shows the spatial distribution of AP topsoil transect sampling locations; Major vegetation types observed in the transect area.
(B) Shrubby-grassland.
(C) Shrubland.
(D) Woody-shrubland.
(E) Woodland. Legend: yellow - agricultural land, green - forest, pink - wasteland, blue - waterbody, and red - urban area. Credit: (a) Land cover map is acquired from the open access Bhuvan GIS portal (https://bhuvan-app1.nrsc.gov.in/thematic/thematic/index.php).
Jwalapuram is a renowned Palaeolithic site situated in the northern fringes of the Cuddapah basin in peninsular India42 (Figures 1 and 2). The site has yielded a long record of hominin occupation, spanning from ∼80 ka to 11.3 ka.42,43 Jwalapuram is particularly distinguished by its massive volcanic tephra deposit, belonging to the ∼74 ka Young Toba tuff (YTT) eruption.42 Unfortunately, the archaeological site is currently undergoing heavy mining by villagers and most of the earlier studied archaeological localities are re-buried under the YTT-mixed sediment dump (Figure 2). We therefore sampled a new Palaeolithic locality (JWP2022), located 80 m north of JWP3, which exhibited a lithostratigraphy mirroring that of JWP3/3a44,45,46 (Figure 3). A total of 42 sediment samples were collected from Pre-YTT, YTT, and post-YTT litho-units at 10 cm intervals (Figure 2).
To better understand the SOM preservation at a regional scale, we collected topsoil samples (N = 20) from twenty different locations, creating an east-west transect measuring 250 km across central Andhra Pradesh (AP), southern India (Figure 3). The transect covered all vegetation classes and environmental settings that are currently present in the region (Figure 3). We analyzed topsoil samples from a variety of environmental contexts (represented by dominant vegetation class) (Figures 3 and S3), including shrubland (n = 10), woody-shrubland (n = 4), shrubby-grassland (n = 3), woodland (n = 2), and aquatic contexts (n = 1).
Results
Jwalapuram: Bulk carbon isotopes
The δ13CSOM values of the Jwalapuram samples (n = 42; Figure 2) show high δ13CSOM variability across the sequence (Figure 4; range: −24.1‰ and −14‰). We observe significant differences in δ13CSOM values between multiple litho-units within the Jwalapuram sequence (Figure 4). Pre-YTT (unit – D: −19.6 ± 1.9‰) and YTT (unit – C: −24.7 ± 2.0‰) samples show lower δ13CSOM values than post-YTT samples, ranging between −26.5‰ and −18.3‰, with an average of −23.3‰. Post-YTT (units – A: −20.5 ± 1.2‰ and B: −14.7 ± 0.7‰) samples have relatively higher values than YTT and pre-YTT layers, ranging from −21.4‰ to −14‰. Notably, pre-YTT δ13CSOM values from JWP2247 are similar when compared to pre-YTT samples of JWP2022, with an average δ13CSOM value of −19.7‰.
Figure 4.
Plant-wax biomarker and bulk stable isotope from Jwalapuram
(A) Lithostratigraphy of JWP2022.
(B) Carbon preference index (CPI).
(C) Odd-over-even predominance index.
(D) Average chain length (ACL).
(E) δ13CSOM values of JWP2022 samples. Legend: Pink-line corresponds with the 74 ka YTT event.
Jwalapuram: n-Alkane indices
Here we use CPIC23-C35, OEPC23-C35, and ACLC23-C35 as main indicators to evaluate the degree of SOM degradation. The CPI and OEP indicated the propositional difference between the concentration of odd or even carbon-numbered long-chain n-Alkanes.30,41 Different long-chain n-Alkanes indices such as - CPI, OEP, ACL, and long-chain n-Alkanes Ratios (LAR) ratios allow for determining OM sourcing and preservation.30,41 The OEP values of the Jwalapuram sediment samples are quite low, ranging from 0.4 to 2.6, with an average of 1.6, indicating no differences between the relative concentration of odd and even numbered carbon long-chain n-Alkanes (Figure 4). Furthermore, the average CPI value of samples from Jwalapuram (CPI = 1.5 ± 0.3) is notably low. Lastly, the ACL values of the Jwalapuram samples range between 24.1 and 28.4, suggesting that SOM in the JWP2022 sequence is heavily degraded.48,49,50 In conjunction with OEP, we studied two LAR41 to assess the preservation status of SOM in Jwalapuram (Figure 5). The LAR values of the Jwalapuram (LAR1 = 0.6 ± 0.2 and LAR2 = 0.5 ± 0.1) palaeosol samples also point toward the SOM degradation, as suggested by other n-Alkane indices.
Figure 5.
Correlation between Long-chain n-Alkane ratio (A and B) and odd over even predominance index
Legend: blue – Jwalapuram sediment samples and brown – AP topsoil transect.
Andhra Pradesh topsoil transect: Bulk carbon isotopes
The δ13CSOM values of the topsoil samples from the AP transect show less variability (n = 20; −19.9 ± 2.8‰) compared to their archaeological counterparts (n = 42; −20.8 ± 4‰; Figure 6). Within the modern transect samples, we observed significant variability in δ13CSOM values between different vegetation contexts (Figure 6). Most (n = 10) of the samples were collected from shrubland, which is the predominant vegetation type in the region. The δ13CSOM values of shrubland samples ranged from −22.3‰ to −15.6‰, relatively lower than shrubby-grassland samples but higher than woodland samples. Samples from woodland (−23.8 ± 2.1‰) and woody-shrubland (−20.3 ± 2.4‰) exhibited lower δ13CSOM values compared to shrubby-grassland samples (−16.6 ± 1.3‰).
Figure 6.
Long-chain n-Alkane indices and stable isotope dataset of the AP transect
(A) Carbon preference index.
(B) Average chain length.
(C) Odd-over-even predominance.
(D) δ13CSOM.
Andhra Pradesh topsoil: n-Alkane indices
n-Alkane indices of the AP topsoil transect show a wider variation in n-Alkane distributions (Figure 6). Samples from forested regions (woodland and woody-shrubland) have higher CPI (∼6.4 ± 3.1), OEP (∼11.2 ± 4.8), and ACL (∼30.2 ± 1.2) values in compare to shrubland and grassland samples. Most samples from open contexts (shrubland or grassland) indicate relatively low CPI (1.80 ± 0.6), OEP (3.50 ± 1.1), and ACL (29.6 ± 1.2) values. It is noteworthy that the LAR values of modern topsoil are lower in compared to their palaeosol counterparts, ranging between 0.25 and 0.35 (Figure 5).
Discussion
The variations observed in δ13Csom datasets are not primarily indicative of vegetation changes. Instead, these variations are more likely attributed to the complex processes of SOM degradation and various external influences as evident in the case of Jwalapuram. To understand the limitations and biases related to δ13CSOM interpretations at regional scale, we compiled published δ13C values of modern plants (n = 659) and soil (SOM; n = 453) across the Indian subcontinent (Figure 7). The compiled data reveals that δ13C values of C3 plant ranges from −33.4‰ to −24.5‰, averaging at −29.4‰, while C4 plant δ13C is higher, ranging between −16.6‰ and −9.6‰, with an average of −12.8‰ (Figure 2). However, observed δ13CSOM values of sediment samples exhibit a high degree of variability compared to the input plant values. In C3-dominated landscapes, δ13CSOM values range from −29.1‰ to −18.1‰, with an average of −23.9‰, while mosaic or mixed vegetation areas show even higher variability, ranging between −32.3‰ and −11.4‰, averaging at −21.6‰ (Figure 7). This large variation within δ13CSOM likely reflects its inconsistent relationship with source detritus biomass, primarily due to a constellation of diagenetic effects and resulting isotope fractionation.18,19,27 Similar patterns were observed in the Jwalapuram and the AP topsoil transect δ13CSOM dataset (Figures 4 and 5).
Figure 7.
Compiled stable carbon isotope data of modern plant and soil samples across the Indian subcontinent
Jwalapuram
δ13CSOM data shows a high degree of variability, ranging from −24.1‰ to −14.0‰ (Figure 4). Assuming δ13CSOM is strongly correlated to palaeovegetation input, the values from Jwalapuram mostly indicate mosaic vegetation throughout the sequence, with proportional shifts in C3 to C4 plant dominance51,52 or stomatal response of C3 plants to increasing aridity in the post-YTT phase (Figure 5).53 The pre-YTT environment appears to have been more humid and C3-dominated compared to the more arid, C4-dominated post-YTT period. This broader trend in δ13CSOM data complements previously published δ13CPC and δ18OPC datasets.45,46 However, the high degree of δ13CSOM variation between different litho-units of the JWP2022 sequence may be due to complexities related to SOM recycling and biodegradation.18,19 Furthermore, the observed smooth temporal pattern of δ13CSOM likely arises because this proxy reflects the bulk isotopic signature of the entire soil organic matter pool, a complex mixture influenced by broad factors such as overall carbon cycling and major vegetation shifts. This bulk measurement inherently averages the isotopic signals from diverse organic sources with varying decomposition rates, potentially masking the more specific and potentially fluctuating preservation patterns of individual plant-derived components. Consequently, the heterogeneity within SOM leads to a bulk isotopic signal that integrates and smooths out variations, resulting in temporal trends that may not capture the finer-scale vegetation dynamics that compound-specific proxies, focusing on individual plant biomarkers, might reveal with their potentially more irregular fluctuations reflecting specific plant responses and preservation.50,54
n-Alkane distribution data and associated indices provide insights into SOM preservation, potentially explaining the higher variability in the δ13CSOM dataset. Key indices such as CPI and OEP reflect the proportion of odd to even carbon-numbered long-chain n-Alkanes.30,38 Higher values indicate odd-numbered n-Alkane dominance, suggesting terrestrial plant sources, while lower values (≤1) imply even-numbered n-Alkane prevalence, pointing to the microbial or mixing of petrogenic components (Figure 4).30,55 ACL helps to differentiate between terrestrial plant, algal, microbial, and petrogenic hydrocarbon sources.32,56 Overall, CPI and OEP values of the Jwalapuram samples are quite low, close to those of petrogenic or highly degraded samples (Figure 4).38 Low ACL values corroborate with the CPI and OEP data, suggesting the same (Figure 4).
The irregular trends observed in n-Alkane indices likely stem from source variability, distinct degradation pathways, or preservation biases, leading to proportional changes in the relative abundance of odd and even chain n-Alkanes within the palaeosol. For instance, while higher plants typically exhibit a strong odd-over-even n-Alkane predominance, increased microbial contributions could shift this toward a CPI closer to 1 or even an even-over-odd preference. Similarly, differential degradation rates of varying n-Alkane chain lengths under fluctuating soil conditions, or selective preservation due to changing depositional environments, can alter their relative proportions, resulting in irregular index trends that do not directly reflect vegetation changes. In the case of Jwalapuram, the presence of proportional differences among these chain lengths (such as a dominance of short over long, or even over odd n-Alkanes) provides critical information for assessing the extent to which the preserved plant-derived lipids have been altered, thus informing the reliability of these molecular proxies for palaeovegetation reconstruction at the site. Our n-Alkane dataset suggests the biochemical degradation of plant-derived lipids, likely caused by microbial activity which is further corroborated by LAR values.
The LAR values of the Jwalapuram samples align with OEP and CPI values, indicating varying degrees of degradation across most samples (Figure 5).41 Some samples exhibit complete degradation, as suggested by their higher LAR values (approaching 1).40,41,57 Correlation data revealed a robust inverse relationship between LARs and OEP, signifying a notable decline in OEP values with increasing LAR values.41 The notable presence of an Unresolved Complex Mixture (UCM) indicates a mixture of hydrocarbons that cannot be separated into distinct peaks, primarily resulting from SOM degradation and petrogenic input (likely from mining and other industrial activities) (Figure S4).9,58,59 The significant presence of UCM masked n-Alkane peaks in chromatograms of several Jwalapuram samples likely represents the degradation of the targeted hydrocarbons and presence of petrogenic compounds.9,58,59 Aforementioned factors have significant impact on δ13CSOM values, could cause preservation bias and lead to mis-interpretation.
Andhra Pradesh topsoil transect
The δ13CSOM and plant-wax biomarker dataset from the AP topsoil transect provides further insights about SOM preservation at a regional scale, highlighting the relationship between SOM preservation, diverse vegetation, and lithological contexts (Figure 3). The δ13CSOM dataset shows significant variability within the studied transect, reflecting structural and compositional variation in local vegetation (Figure 6).18,51 δ13CSOM values of samples from woodland and woody-shrubland are comparatively lower than those of shrubland and grass dominated contexts. δ13CSOM values of samples from shrubland and grassland are highly variable, ranging from −24.1‰ to −14.8‰ (Figure 6). It is important to highlight that most of the samples are collected from mosaic contexts; therefore, variability between δ13CSOM values may be related to varying proportions of shrubs and grasses in the landscape.18,23,52
It is also possible that the relatively quicker decomposition of C4 derived SOM could lead to lower δ13CSOM values, explaining the lower values of some of the grassland and shrubland samples.19,22 It has been demonstrated by other investigators that SOM derived from C4 plants tends to decompose faster than that from C3 plants due to several interrelated factors.19,22 C4 plants generally produce biomass with lower carbon-to-nitrogen (C:N) ratios, which facilitates quicker decomposition as nitrogen is a key nutrient for microbial activity.19,22,23 In addition, the litter from C4 plants often contains lower lignin content and higher concentrations of soluble compounds, making it more accessible to soil microbe activity.60,61,62 It should be noted that CPI (1.8 ± 0.6) and OEP (3.5 ± 1.2) values of topsoil samples from grassland and shrubland are clearly lower than woodland samples (CPI: 6.4 ± 3.5; OEP: 11.2 ± 5.2), suggesting the microbial degradation of SOM in grassland and shrubland samples (Figure 6).30,34,63 LAR values complement the CPI and OEP data, suggesting variable SOM preservation in different vegetation contexts (Figure 5). Overall, LAR values of modern topsoil show better preservation compared to their palaeosol counterparts, ranging between 0.25 and 0.35, reflecting the mosaic vegetation of the region, predominantly characterized by shrubland conditions (Figure 5). However, δ13CSOM values of many samples within the AP topsoil transect do not coincide with their source vegetation, showing an inconsistent relationship likely due to the influence of microbial degradation and SOM recycling. The n-Alkane indices, such as CPI, of those topsoil samples are relatively low (close to 1), showing a predominance of even-chain n-Alkanes, suggesting microbial degradation. This reinforces the importance of the on-site assessment of SOM preservation using plant-wax biomarkers, thereby ensuring the integrity and reliability of δ13CSOM proxies.
Factors behind soil organic matter degradation at Jwalapuram
The degradation of plant-derived lipid is strongly in line with the depositional environment, and relies upon the intricate interactions that occur between lipid molecules, microbial communities (including fungi and bacteria), soil pH level, temperature, oxygen and moisture content.9,31,63,64 Based on these relationships, we suggest some potential reasons that may explain SOM degradation in the Jwalapuram palaeosol and in the AP topsoil transect.
Soil properties
SOM preservation is linked to soil type, mediated by physio-chemical properties that govern microbial activity and organic matter stabilization.65 Clay-rich soils, with their fine texture and limited aeration, generally exhibit enhanced preservation due to protective adsorption and reduced microbial decomposition.66,67 Conversely, sandy soils, with rapid drainage and high oxygen, promote microbial breakdown, leading to lower preservation rates.63,64 Organic-rich soils, especially under anaerobic conditions, offer exceptional preservation due to limited oxygen. Soil pH and mineralogy further modulate microbial communities and organic matter stability, resulting in diverse preservation potentials across soil types.68,69 The Jwalapuram sequence is predominantly characterized by a reddish silty-sand deposit (particularly, unit – B and D), often referred to as red soil46 (Figure 2). These red soils exhibit a notable deficiency in SOM (TOC = 0.05%) and are devoid of essential plant nutrients.70,71 Granulometric factors, such as high soil porosity within these litho-units, contribute to a low moisture retention capacity and poor water-holding ability, which profoundly impact SOM preservation.72 The continuous oxygenation of soil under higher temperatures accelerates the process of SOM decomposition,63,64 particularly in cases of lateritic sandy red soils (unit B).70 Another key litho-type present at Jwalapuram is a brownish-black type (unit A) which is a mixture of red and black soil. These litho-units are also known as vertisols, enriched in calcium-carbonate and lacking in nitrogen and phosphorous.73,74,75 These alkaline litho-units (JWP unit A) are formed under arid conditions, and have a very poor SOM yield (TOC = 0.16%).73,74,75
Similar patterns are also observed in modern topsoil samples from the AP transect (Figure 3). Notably, most of the red topsoil samples (n = 9) from the AP transect have lower CPI (1.4 ± 0.3) and OEP (2.8 ± 0.6) in comparison to other soil types within AP transect and Jwalapuram sequence (i.e., brown sandy-clay and greyish-brown clayey-silt; Figures 4 and 6). It was observed that brown sandy-clay (n = 5) soil samples show better SOM preservation, as reflected by their high CPI (7.2 ± 3.1) and OEP (12.7 ± 4.6) values in comparison to other soil-types (Figure 6). Several studies have demonstrated that clayey soils are effective for SOM preservation due to their large surface area and strong binding capacity, which result in stable organo-mineral complexes that protect organic matter from decomposition.66,67 Their fine texture creates small pores that physically shield organic matter from microbial access, while their higher water-holding capacity limits oxygen availability, further slowing decomposition rates.76 Our geochemical data alongside regional SOC datasets further demonstrate the strong influence of soil properties on SOM preservation in the region.
Climatic factors
A previous study by Jones46 revealed that the SOM concentration in the Jwalapuram palaeosols is notably low, with organic content ranging from 1.04% to 3.14%. The preservation of SOM appears to be particularly limited in the red soils of arid regions such as Jwalapuram, where elevated temperatures and scarce rainfall accelerate carbon loss, resulting in poor SOM concentrations.73,74 Elevated temperatures can intensify microbial activity, expediting the degradation of organic matter within soils.73,74,77 Changes in precipitation patterns, such as heightened occurrences of heavy rainfall or prolonged droughts, can disrupt soil moisture levels, thus influencing microbial activity and decomposition rates.70,72,74 Scanning Electron Microscopy (SEM) micrographs from the Jwalapuram palaeosol samples indicate microbial activity, manifested in the form of a biofilm developed on different mineral matrices across the JWP2022 sequence (Figure 8). Higher microbial-induced degradation of SOM explains the lower CPI and OEP values of the Jwalapuram palaeosol samples (Figure 4).41,48,49,78
Figure 8.
Scanning electron microscope (SEM) micrograph of microbial film growing on different mineral substrates
(A) Volcanic glass shard.
(B) Feldspar.
(C) Biotite.
(D) Close-up of biofilm on feldspar surface. Micrograph scale is represented by yellow bars at the bottom-right corner.
Modern anthropogenic factors
For nearly 50 years, the residents of Jwalapuram have extensively mined the land by hand to extract tephra deposits.79,80 This activity has created numerous excavations and pits to obtain volcanic ash, which is sold to detergent and cement manufacturers as an abrasive agent.42,79 Over time, this long-term mining has resulted in deep trenches and exposed vertical litho-sections, such as JWP2020/JWP3, leading to the discovery of multiple Palaeolithic sites. However, the extensive mining has likely significantly affected SOM preservation in the area. The repeated removal of surface vegetation destabilizes the underlying soil, increasing runoff and soil loss, especially during rainy periods.26,81,82,83 Additionally, the continuous exoposition of stratified deposits reduces the soil’s water retention capacity, further exacerbating erosion.81,83 This increased erosion disrupts SOM stocks and nutrient cycles.26,83 Particularly during monsoonal showers, exposure of litho-units to oxygen-rich water enhances oxic conditions, allowing aerobic bacteria to degrade plant-wax lipids.84,85,86,87 Research indicates that well-oxygenated soils foster bacteria and filamentous fungi with enzymes capable of breaking down long-chain hydrocarbons.86,87
A significant anthropogenic marker in the region is the prominent presence of UCM in most topsoil and archaeological samples (Figure S4).88 UCM is formed through the degradation of complex organic compounds or the introduction of petrochemical elements, particularly in urban settings.58,59 These mixtures appear as prominent “humps” in chromatograms, representing numerous unresolved compounds.58,59 Activities such as mining, agriculture, and petroleum dumping contribute significantly to UCM formation. Mining introduces organic contaminants from machinery and chemicals used in processing, while agriculture adds persistent agrochemicals.58,59 Observations from modern topsoil transects across Andhra Pradesh indicate widespread anthropogenic alterations to Palaeolithic sites, with poor SOM conditions being a characteristic feature of such disturbed archaeological settings.
Conclusions
Stable carbon isotope analysis (δ13CSOM) is a widely used proxy for studying past human-relevant climate and environments,51,89 but its reliability hinges on understanding how different physio-chemical processes affect δ13CSOM measurements.18 After burial, SOM continues to decompose, leading to multiple cycles of kinetic fractionation, challenging their interpretation for palaeoenvironmental reconstructions.18,90,91 The current case study from Jwalapuram and the AP topsoil transect highlights the nuanced challenges and complexities that are related to such work. In an ideal situation, δ13CSOM should mirror the isotopic composition of the original biomass,51,52,92,93 albeit with a reliable fractionation linked to microbial activity. However, factors such as climate, the sedimentary environment, and taphonomy can have a myriad of influences that significantly alter parameters of interest.18,23,60 To ensure accurate interpretations, researchers should confirm the preservation of plant-derived SOM particularly in more extreme environmental settings.31,41,64,84 At Jwalapuram, the lower concentration of SOM has undergone multiple cycles of degradation. This process is heavily influenced by both sedimentary factors, such as the composition of the surrounding soil, and climatic conditions, including fluctuating rainfall patterns and consistently high temperatures.77,90,94 The application of plant-wax biomarker analysis at the site has shed light on the influence of biotic factors (microbial activity; Figure 8)18,64,85,95 on carbon isotope fractionation and highlights the limitations of this proxy for palaeoenvironmental reconstruction. The n-Alkane ratios and indices for Jwalapuram show evidence of SOM degradation and recycling at the site (Figure 4).31,64,84,95 Therefore, we recommend that the results of δ13CSOM should be combined with plant-wax biomarkers in archaeological research, especially in more extreme environments, in order to verify SOM preservation, source characterization, and diagenesis.
The advent of the plant-wax biomarkers approach is relatively new to archaeological and palaeoanthropological studies, providing significant, new insights about past environments and human-environment interactions. However, to fully leverage this method in archaeological contexts, it is crucial to conduct comprehensive modern baseline studies and detailed on-site vegetation analyses, along with sedimentological assessment (such as pH, grain-size, and weathering).9,30,43,96 These studies are essential for creating a better understanding of SOM preservation and leaf-wax degradation processes specific to the region under investigation.35,97,98 By establishing robust local references and a nuanced understanding of depositional environment, researchers can more accurately interpret biomarker data from archaeological sites, accounting for factors such as differential preservation, microbial activity, and site-specific environmental conditions. This approach would significantly enhance the reliability of palaeoenvironmental reconstructions and their implications for human evolution and archaeological studies.
Limitations of the study
One notable limitation of our study is the absence of CSIA of n-Alkanes from both the Jwalapuram and AP transect samples. It is worth noting that many of the palaeosol samples from Jwalapuram and AP transect exhibit very low n-Alkane concentrations (C29 = ∼5.4 ng/g and C31 = ∼4.7 ng/g), which may not be sufficient for successful CSIA measurements.99 Although current work primarily focuses on SOM preservation, so isotope values are not essential for this phase. However, future research could investigate the reliability of isotope values from degraded samples by comparing data across transects and sequences to assess potential preservation biases. We also recommend considering alternative geochemical proxies such as elemental ratios, weathering indices, and granulometric datasets. Such datasets would provide valuable insights into the factors contributing to soil organic matter degradation and recycling, which are currently lacking in this work.
Resource availability
Lead contact
Additional information: Further information and requests for resources should be directed to, Gopesh Jha (gjha@gea.mpg.de).
Materials availability
This work did not generate new materials.
Data and code availability
-
•
All data associated with the publication are included in this article or provided as a supplemental information. Any additional information required to re-analyze the data reported in this article is available from the lead contact upon request.
-
•
No code generated in this study.
Acknowledgments
This work is funded by the Max Planck Institute of Geoanthropology, Jena, Germany. GJ and KK extend their gratitude to the Archaeological Survey of India (ASI) for supporting this research. GJ thanks Uppu Shivayya and the villagers of Jwalapuram for their invaluable field assistance and Dr. Tejasvi Chauhan for his assistance with data visualization. We would like to thank both reviewers for providing valuable input.
Author contributions
Conceptualization: G.J., P.R., and M.P.; methodology: G.J., D.K.J., R.P., J.I., and PR.; investigation: G.J., V.V., P.R., K.K., and M.P.; writing – original draft: G.J. writing – review and editing: G.J., V.V., D.K.J, R.P., J.I., R.P.A., N.B., R.R., K.K., P.R., and M.P.; supervision: P.R. and M.P.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Dichloromethane (DCM), HPLC grade, 40°C boiling point, 3.1 polarity index | Millipore | DX0831 |
| Methanol (MeOH), HPLC grade, 64.7°C boiling point, 5.1 polarity index | Millipore | MX0488 |
| Hexane (HEX), HPLC grade, 69°C boiling point, 0.1 polarity index | Millipore | 100795 |
| Supelco C8 - C40 Alkanes Calibration Standard in dichloromethane | Merck | 40147-U |
| 2M hydrochloric acid (HCl), ACS reagent | Millipore | 320331 |
| USGS40 L-glutamic acid | RIL-USGS | N/A |
| USGS60 Caffeine | RIL-USGS | N/A |
| IAEA C-6 | IAEA | https://analytical-reference-materials.iaea.org/iaea-c6 |
| IAEA N2 | IAEA | https://analytical-reference-materials.iaea.org/iaea-n-2 |
| UREA | IAEA | https://analytical-reference-materials.iaea.org/iaea-310 |
| Tin Capsules NA2000 | Fisher Scientific | 25208000 |
| Buchi Quartz Ottawa sand, 0.3-0.9 | Fisher Scientific | BUCHI 037689 |
| 100- to 200-mesh silica gel, high-purity grade (Davisil Grade 923), pore size 30 Å | Millipore | 214477 |
| 14.6-cm (5 3/4-inch) and 22.9-cm (9-inch) borosilicate glass Pasteur pipets | VWR | N/A |
| Glass wool | Fisher Scientific | N/A |
| Combusted glassware (1.5ml GC vials, 4ml vials and GC inserts) | VWR | N/A |
| Others | ||
| Pressurized speed extractor: Büchi Speed Extractor E-916; with extraction apparatus | Buchi | E-916 |
| BUCHI SyncorePlus Polyvap Parallel Evaporator System | Buchi | 11SQP2S000121 |
| Gas Chromatography-Mass Spectrometer (GC-MS) | Agilent | 5977C |
| ConFlo IV Thermo® Scientific Isotope Mass Ratio Spectrometer (IRMS) coupled with a Thermo Scientific FLASH 2000 HT Elemental Analyzer | Thermo Scientific | 11206175 |
| Nitrogen Gas Generator | Peak | N/A |
| Micro-balance | Sartorius | N/A |
| Software and algorithms | ||
| Mass Hunter Workstation | Agilent Technologies | https://www.agilent.com/en/products/softwareinformatics/masshunter-suite/masshunterquantitative-analysis |
| NIST Mass Spectra Database v.14 | NIST | https://chemdata.nist.gov/ |
| IsoDat 3.0 | Thermo Scientific | https://www.thermofisher.com/es/es/home/technical-resources/software-downloads.html |
Method details
Field sampling
Jwalapuram is a well-known Palaeolithic site that was extensively excavated between 2003 and 2009. As part of our ongoing research, we revisited the key locality of JWP3, which has since been completely destroyed due to modern tephra mining activities. To continue our investigation, we identified a new Palaeolithic locality, designated JWP2022, situated approximately 80 metres north of JWP3. This site exhibits a lithostratigraphy that closely resembles that of JWP3/3a. JWP2022 comprises a large mining trench measuring approximately 15 metres by 7 metres, which was excavated by tephra miners in 2021. At this site, we laid out two geological trenches: Trench I (1 m × 1 m × 2.8 m) and Trench II (1 m × 1 m × 2.2 m). Prior to sample collection, one metre of exposed surface sediment was removed to access undisturbed layers. A total of 40 sediment samples were collected at 10cm interval, with each sample weighing approximately 150 grams. All permits were obtained for sediments sampling from concerning institution like – ASI-Delhi (No.T-17012/1/2021-EE), JNU-Delhi, and MSU-Baroda.”
In addition, to establish a regional environmental baseline, modern topsoil samples were collected from 20 locations along a west-east transect extending from the interior of the Cuddapah Basin to the eastern coastal region (range – 250 km). These samples were collected from undisturbed areas, purposefully located away from human settlements to minimize contamination. All samples were carefully stored in ziplocked bags and sun-dried at the field base camp to reduce excess moisture. In April 2022, the samples were transported to the Max Planck Institute for Geoanthropology in Jena. Upon arrival, they were reopened, oven-dried at 40°C for 48 hours, and subsequently homogenized in preparation for geochemical analysis.
Plan-wax lipid extraction and chromatography
We employed the Total Lipid Extraction (TLE) and separation methodology as described by Patalano et al.9 and Jha et al.33 Initially, approximately ∼70g of dried and homogenized sediments underwent extraction using a Büchi (E-916) Pressurized Speed Extractor. The extraction utilized a 9:1 (v/v) Dichloromethane (DCM):Methanol (MeOH) solvent mixture under conditions of 100°C and 103 bar, conducted in three 10-minute cycles. The solvent carrying the total lipid extract (TLE) was then concentrated to approximately 1ml using a Büchi Syncore-Plus evaporator, followed by further evaporation to dryness with N2 gas. Subsequently, the TLE was fractionated into three fractions through silica-gel (100–200 mesh) column chromatography, which was activated at 150°C for 24 hours. Elution of the fractions was achieved using hexanes (F1), DCM (F2), and MeOH (F3) solvents. The F1 fractions, enriched with n-Alkanes, were concentrated to 0.5ml under high purity N2 gas and transferred into pre-baked 1.5ml Agilent vials for Gas Chromatography (GC) analysis.
The analysis of n-Alkane samples was performed using a GC system (Agilent 7890B) equipped with an Agilent HP-5 capillary column (30m length x 250μm film thickness x 0.25mm column diameter) and an injector in split/split-less mode, coupled to an Agilent 5977A Series Mass Selective Detector at the Max Planck Institute of Geoanthropology (MPI-GEA) in Germany. Samples were injected in splitless mode at 250°C, with the GC oven programmed from 60°C (1-minute hold) to 180°C at a rate of 6°C/min, followed by an increase to 310°C at 10°C/min (12-minute hold). Helium served as the carrier gas at a constant flow rate of 1.3 ml/min. The Mass Spectrometer (MS) source operated at 230°C with an electron ionization (EI) mode set to 70 eV, scanning the mass-to-charge ratio range of m/z 50-550. Individual n-Alkane compounds were identified based on retention times obtained from Supelco (C8-C40 Alkane) standards. For quantitative analysis, we prepared 8-point calibration curves (concentration vs peak area) for individual homologs of Supelco (C8-C40) at various dilutions: 0.5 ng/μl, 1 ng/μl, 3 ng/μl, 7 ng/μl, 10 ng/μl, 15 ng/μl, 20 ng/μl, and 30 ng/μl. The slope and intercept of these calibrations were subsequently utilized to determine the absolute concentration of the samples. This refined methodology ensures accurate extraction, separation, and analysis of n-Alkanes from sediment samples, providing reliable data for further interpretation and study.
n-Alkane data analysis
Variation in n-Alkane composition and distribution pattern offers clues about the preservation of organic matter and its potential source. Different n-Alkane indices were calculated using absolute concentration of long-chain n-Alkanes (refer to SI geochemical dataset), with aim to assess intensity of biodegradation (kinetic effect) of SOM in sediments from Palaeolithic and modern context. Here we use carbon preference index (CPI), odd over even ratio (OEP), and average chain length (ACL) as main indicators to evaluate degree of OM degradation.33,37,39 The CPI and OEP indicated the propositional difference between the concentration of odd or even carbon-numbered long-chain n-Alkanes. Higher CPI or OEP values indicating a dominance of odd-numbered n-Alkanes which is often associated with terrestrial plant sources. Conversely, lower CPI or OEP (close to 1 or lesser than 1) values suggest dominance of even-numbered n-Alkanes, typically linked to microbial or petrogenic sources. Further, ACL allow us to differentiate terrestrial higher plant sources from algal or microbial origins and to discern petrogenic hydrocarbon inputs.
CPI, OEP, and ACL for the n-Alkanes are defined as:
| (Equation 1) |
| (Equation 2) |
| (Equation 3) |
Furthermore, we also combine different long-chain n-Alkane ratio (LAR) with CPI, OEP, and ACL, to ensure the chemotaxonomic representation. Decreasing LAR values are strongly correlated to reducing predominance of odd over even long-chain n-Alkanes.40 Thus, LARs are as sensitive as CPI or ACL, considered as a reliable indicator of biodegradation and post-sedimentary alteration.
Here we use two LAR indices:
| (Equation 4) |
| (Equation 5) |
TOC and δ13CSOM analysis of bulk soil
Here we measured δ13CSOM using methods suggested by Jha et al.33 Sediment samples (∼1g) underwent decarbonation with 2M HCl, followed by neutralization using Milli-Q water. After neutralization, the samples were dried for 24 hours at 40°C and then re-homogenized. The pre-treated samples were then packed into tin capsules for analysis using a Thermo Scientific Flash 2000 Elemental Analyzer coupled with a Thermo Delta V Advantage Mass Spectrometer. Carbon isotope ratios (13C/12C) were determined by measuring CO2 evolution during combustion relative to reference gas pulses. Calibration and reproducibility were ensured by using IAEA/USGS standards (USGS40, USGS60, IAEA C6, and IAEA N2). Duplicate measurements were conducted to confirm sample homogeneity and reproducibility (refer to SI geochemical dataset).
The Total Organic Carbon (TOC) content was determined using a regression equation derived from the relationship between the carbon amount of the standard and the combined peak areas of mass-to-charge (m/z) signals 44, 45, and 46. We employed the slope (m) and intercept (c) of the least-squares fit to estimate the TOC percentage in the sediment samples, following the methodologies outlined by Jha et al.33 This approach ensures a robust and accurate quantification of organic carbon content in the analysed sediments, providing valuable insights into the organic matter composition and potential paleoenvironmental indicators.
Quantification and statistical analysis
Raw datasets were processed using vendor software. Plant-wax n-Alkane distributions and concentrations were analyzed in Mass Hunter Workstation (Agilent Technologies) with spectral matching against the NIST Mass Spectral Library v.14. δ13CSOM and TOC data were curated in Isodat 3.0 (Thermo Scientific). All datasets were summarized in Microsoft Excel, where we computed descriptive statistics (mean, standard deviation, minimum, and maximum). Ranges for the n-Alkane indices were calculated in Excel using the equations provided above. We performed exploratory linear regressions among n-Alkane indices to assess pairwise relationships; these outputs informed interpretation but were not included in the final results because they were not central to our objectives.
Published: September 27, 2025
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2025.113656.
Supplemental information
References
- 1.Lieberman B., Gordon E. Bloomsbury Publishing; 2021. Climate Change in Human History: Prehistory to the Present. [Google Scholar]
- 2.Xu C., Kohler T.A., Lenton T.M., Svenning J.-C., Scheffer M. Future of the human climate niche. Proc. Natl. Acad. Sci. USA. 2020;117:11350–11355. doi: 10.1073/pnas.1910114117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carleton W.C., Collard M. Recent major themes and research areas in the study of human-environment interaction in prehistory. Environ. Archaeol. 2020;25:114–130. [Google Scholar]
- 4.Davis D.S. Studying human responses to environmental change: Trends and trajectories of archaeological research. Environ. Archaeol. 2020;25:367–380. [Google Scholar]
- 5.Kerr S. The future of archaeology, interdisciplinarity and global challenges. Antiquity. 2020;94:1337–1348. [Google Scholar]
- 6.Lombardo V., Karatas T., Gulmini M., Guidorzi L., Angelici D. Transdisciplinary approach to archaeological investigations in a semantic web perspective. Web. 2022;14:361–383. [Google Scholar]
- 7.Yılmaz D. In: Transdisciplinarity. Rezaei N., editor. Springer; 2022. Archaeology as an Interdisciplinary Science at the Cross-Roads of Physical, Chemical, Biological, and Social Sciences: New Perspectives and Research; pp. 435–455. [Google Scholar]
- 8.Morley M.W., Moffat I., Kotarba-Morley A.M., Hernandez V.C., Zerboni A., Herries A.I.R., Joannes-Boyau R., Westaway K. Why the geosciences are becoming increasingly vital to the interpretation of the human evolutionary record. Nat. Ecol. Evol. 2023;7:1971–1977. doi: 10.1038/s41559-023-02215-5. [DOI] [PubMed] [Google Scholar]
- 9.Patalano R., Roberts P., Boivin N., Petraglia M.D., Mercader J. Plant wax biomarkers in human evolutionary studies. Evol. Anthropol. 2021;30:385–398. doi: 10.1002/evan.21921. [DOI] [PubMed] [Google Scholar]
- 10.Jha D.K., Sanyal P., Philippe A. Multi-proxy evidence of Late Quaternary climate and vegetational history of north-central India: Implication for the Paleolithic to Neolithic phases. Quat. Sci. Rev. 2020;229 [Google Scholar]
- 11.Marean C.W., Anderson R.J., Bar-Matthews M., Braun K., Cawthra H.C., Cowling R.M., Engelbrecht F., Esler K.J., Fisher E., Franklin J., et al. A new research strategy for integrating studies of paleoclimate, paleoenvironment, and paleoanthropology. Evol. Anthropol. 2015;24:62–72. doi: 10.1002/evan.21443. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A., Arrowsmith R., Behrensmeyer A.K., Campisano C., Feibel C., Fisseha S., Johnson R., Kubsa Bedaso Z., Lockwoodt C., Mbua R., et al. Understanding paleoclimate and human evolution through the Hominin Sites and Paleolakes Drilling Project. Sci. Dril. 2009;8:60–65. [Google Scholar]
- 13.Sandweiss D.H., Kelley A.R. Archaeological contributions to climate change research: The archaeological record as a paleoclimatic and paleoenvironmental archive. Annu. Rev. Anthropol. 2012;41:371–391. [Google Scholar]
- 14.Soldatova E., Krasilnikov S., Kuzyakov Y. Soil organic matter turnover: Global implications from δ13C and δ15N signatures. Sci. Total Environ. 2024;912 doi: 10.1016/j.scitotenv.2023.169423. [DOI] [PubMed] [Google Scholar]
- 15.Tankersley K.B., Conover D.G., Lentz D.L., Callihan A., Weakley J., Hassett I., Platt E., Laiveling A., Bradford E. The impact of maize (Zea mays) on the stable carbon isotope values of archaeological soil organic matter. J. Archaeol. Sci. Rep. 2019;24:324–329. [Google Scholar]
- 16.Hartman G. Reconstructing Mid-Pleistocene paleovegetation and paleoclimate in the Golan Heights using the δ13C values of modern vegetation and soil organic carbon of paleosols. J. Hum. Evol. 2011;60:452–463. doi: 10.1016/j.jhevol.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Vancampenhout K., Wouters K., Caus A., Buurman P., Swennen R., Deckers J. Fingerprinting of soil organic matter as a proxy for assessing climate and vegetation changes in last interglacial palaeosols (Veldwezelt, Belgium) Quat. Res. 2008;69:145–162. [Google Scholar]
- 18.Wynn J.G. Carbon isotope fractionation during decomposition of organic matter in soils and paleosols: Implications for paleoecological interpretations of paleosols. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007;251:437–448. [Google Scholar]
- 19.Wynn J.G., Bird M.I. C4-derived soil organic carbon decomposes faster than its C3 counterpart in mixed C3/C4 soils. Glob. Chang. Biol. 2007;13:2206–2217. [Google Scholar]
- 20.Ankit Y., Muneer W., Gaye B., Lahajnar N., Bhattacharya S., Bulbul M., Jehangir A., Anoop A., Mishra P.K. Apportioning sedimentary organic matter sources and its degradation state: inferences based on aliphatic hydrocarbons, amino acids and δ15N. Environ. Res. 2022;205 doi: 10.1016/j.envres.2021.112409. [DOI] [PubMed] [Google Scholar]
- 21.Blagodatskaya E., Yuyukina T., Blagodatsky S., Kuzyakov Y. Turnover of soil organic matter and of microbial biomass under C3–C4 vegetation change: consideration of 13C fractionation and preferential substrate utilization. Soil Biol. Biochem. 2011;43:159–166. [Google Scholar]
- 22.Da J., Li G.K., Ji J. Overestimate of C4 plant abundance caused by soil degradation-induced carbon isotope fractionation. Geophys. Res. Lett. 2021;48 [Google Scholar]
- 23.Rao Z., Guo W., Cao J., Shi F., Jiang H., Li C. Relationship between the stable carbon isotopic composition of modern plants and surface soils and climate: A global review. Earth Sci. Rev. 2017;165:110–119. [Google Scholar]
- 24.Diefendorf A.F., Mueller K.E., Wing S.L., Koch P.L., Freeman K.H. Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc. Natl. Acad. Sci. USA. 2010;107:5738–5743. doi: 10.1073/pnas.0910513107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang G., Feng X., Han J., Zhou L., Tan W., Su F. Paleovegetation reconstruction using δ 13 C of soil organic matter. Biogeosciences. 2008;5:1325–1337. [Google Scholar]
- 26.Punia A., Bharti R. Loss of soil organic matter in the mining landscape and its implication to climate change. Arab. J. Geosci. 2023;16 [Google Scholar]
- 27.Wynn J.G., Bird M.I., Wong V.N.L. Rayleigh distillation and the depth profile of 13C/12C ratios of soil organic carbon from soils of disparate texture in Iron Range National Park, Far North Queensland, Australia. Geochim. Cosmochim. Acta. 2005;69:1961–1973. [Google Scholar]
- 28.Patalano R., Arthur C., Carleton W.C., Challis S., Dewar G., Gayantha K., Gleixner G., Ilgner J., Lucas M., Marzo S., et al. Ecological stability of Late Pleistocene-to-Holocene Lesotho, southern Africa, facilitated human upland habitation. Commun. Earth Environ. 2023;4:129. [Google Scholar]
- 29.Sreemany A., Bera M.K. Is it C4 or ‘C3 in disguise’? Disentangling the ambiguity in palaeovegetation estimation. J. Quat. Sci. 2022;37:1069–1082. [Google Scholar]
- 30.Berke M.A. In: Vertebrate Paleobiology and Paleoanthropology. Croft D.A., Su D.F., Simpson S.W., editors. Springer International Publishing; 2018. Reconstructing terrestrial paleoenvironments using sedimentary organic biomarkers. [DOI] [Google Scholar]
- 31.Inglis G.N., Bhattacharya T., Hemingway J.D., Hollingsworth E.H., Feakins S.J., Tierney J.E. Biomarker approaches for reconstructing terrestrial environmental change. Annu. Rev. Earth Planet Sci. 2022;50:369–394. [Google Scholar]
- 32.Eglinton T.I., Eglinton G. Molecular proxies for paleoclimatology. Earth Planet Sci. Lett. 2008;275:1–16. [Google Scholar]
- 33.Jha D.K., Patalano R., Ilgner J., Achyuthan H., Alsharekh A.M., Armitage S., Blinkhorn J., Boivin N., Breeze P.S., Devra R., et al. Preservation of plant-wax biomarkers in deserts: implications for Quaternary environment and human evolutionary studies. J. Quat. Sci. 2024;39:349–358. [Google Scholar]
- 34.Eglinton G., Hamilton R.J. The distribution of alkanes. Chemical Plant Taxonomy. 1963;187:217. [Google Scholar]
- 35.Bush R.T., McInerney F.A. vol. 2010. 2010. Variation in N-Alkane Distributions of Modern Plants: Questioning Applications of N-Alkanes in Chemotaxonomy and Paleoecology. (AGU Fall Meeting Abstracts). PP21C-1704. [Google Scholar]
- 36.Ficken K.J., Li B., Swain D.L., Eglinton G. An n-alkane proxy for the sedimentary input of submerged/floating freshwater aquatic macrophytes. Org. Geochem. 2000;31:745–749. [Google Scholar]
- 37.Chikaraishi Y., Naraoka H. Compound-specific δD-δ13C analyses of n-alkanes extracted from terrestrial and aquatic plants. Phytochemistry. 2003;63:361–371. doi: 10.1016/s0031-9422(02)00749-5. [DOI] [PubMed] [Google Scholar]
- 38.Marzi R., Torkelson B.E., Olson R.K. A revised carbon preference index. Org. Geochem. 1993;20:1303–1306. [Google Scholar]
- 39.Li C., Ma S., Xia Y., He X., Gao W., Zhang G. Assessment of the relationship between ACL/CPI values of long chain n-alkanes and climate for the application of paleoclimate over the Tibetan Plateau. Quat. Int. 2020;544:76–87. [Google Scholar]
- 40.Häggi C., Zech R., McIntyre C., Zech M., Eglinton T.I. On the stratigraphic integrity of leaf-wax biomarkers in loess paleosols. Biogeosciences. 2014;11:2455–2463. [Google Scholar]
- 41.Buggle B., Wiesenberg G.L.B., Glaser B. Is there a possibility to correct fossil n-alkane data for postsedimentary alteration effects? Appl. Geochem. 2010;25:947–957. [Google Scholar]
- 42.Petraglia M.D., Ditchfield P., Jones S., Korisettar R., Pal J.N. The Toba volcanic super-eruption, environmental change, and hominin occupation history in India over the last 140,000 years. Quat. Int. 2012;258:119–134. [Google Scholar]
- 43.Jha G., Costa M., Tsoupra A., Dias C.B., Kwiecien O., Longman J., Breitenbach S.F.M., Ditchfield P., Jha D.K., Rudd R., et al. Seasonally-resolved stratigraphy at Jwalapuram India shows regional surface warming after the Toba volcanic super-eruption. PNAS Nexus. 2025;4 doi: 10.1093/pnasnexus/pgaf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petraglia M., Korisettar R., Boivin N., Clarkson C., Ditchfield P., Jones S., Koshy J., Lahr M.M., Oppenheimer C., Pyle D., et al. Middle Paleolithic Assemblages from the Indian Subcontinent Before and After the Toba Super-Eruption. Science. 2007;317:114–116. doi: 10.1126/science.1141564. [DOI] [PubMed] [Google Scholar]
- 45.Haslam M., Clarkson C., Petraglia M., Korisettar R., Jones S., Shipton C., Ditchfield P., Ambrose S.H. The 74 ka Toba super-eruption and southern Indian hominins: Archaeology, lithic technology and environments at Jwalapuram Locality 3. J. Archaeol. Sci. 2010;37:3370–3384. [Google Scholar]
- 46.Jones S.C. Palaeoenvironmental response to the ∼74 ka Toba ash-fall in the Jurreru and Middle Son valleys in southern and north-central India. Quat. Res. 2010;73:336–350. [Google Scholar]
- 47.Haslam M., Clarkson C., Roberts R.G., Bora J., Korisettar R., Ditchfield P., Chivas A.R., Harris C., Smith V., Oh A., et al. A southern Indian Middle Palaeolithic occupation surface sealed by the 74 ka Toba eruption: Further evidence from Jwalapuram Locality 22. Quat. Int. 2012;258:148–164. [Google Scholar]
- 48.Wang B., Yang J., Jiang H., Zhang G., Dong H. Chemical composition of n-alkanes and microbially mediated n-alkane degradation potential differ in the sediments of Qinghai-Tibetan lakes with different salinity. Chem. Geol. 2019;524:37–48. [Google Scholar]
- 49.Hsu B.-M., Chen J.S., Huang T.Y., Hussain B., Chao W.C., Fan C.W. Short-term microbial effects on n-alkane during the early phase degradation and consequential modification of biomarkers in a lowland subtropical rainforest in southern Taiwan: A litterbag experiment. J. Environ. Manage. 2023;326 doi: 10.1016/j.jenvman.2022.116780. [DOI] [PubMed] [Google Scholar]
- 50.Thomas C.L., Jansen B., Van Loon E.E., Wiesenberg G.L.B. Transformation of n-alkanes from plant to soil: a review. Soil. 2021;7:785–809. doi: 10.5194/soil-7-785-2021. Preprint at. [DOI] [Google Scholar]
- 51.Meyers P.A. Preservation of elemental and isotopic source identification of sedimentary organic matter. Chem. Geol. 1994;114:289–302. [Google Scholar]
- 52.O’Leary M.H. Carbon isotopes in photosynthesis. Bioscience. 1988;38:328–336. [Google Scholar]
- 53.Hare V.J., Loftus E., Jeffrey A., Ramsey C.B. Atmospheric CO2 effect on stable carbon isotope composition of terrestrial fossil archives. Nat. Commun. 2018;9 doi: 10.1038/s41467-017-02691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Award T., November R., December A. Plant-derived triterpenoid biomarkers and their applications in paleoenvironmental reconstructions: chemotaxonomy, geological alteration, and vegetation reconstruction. Res. Organic Geochem. 2019;35:11–35. [Google Scholar]
- 55.Zhang Z., Zhao M., Eglinton G., Lu H., Huang C.Y. Leaf wax lipids as paleovegetational and paleoenvironmental proxies for the Chinese Loess Plateau over the last 170 kyr. Quat. Sci. Rev. 2006;25:575–594. [Google Scholar]
- 56.Poynter J., Eglinton G. vol. 116. Scientific Results; 1990. Molecular composition of three sediments from hole 717c: The Bengal fan; pp. 155–161. (Proceedings of the Ocean Drilling Program). [Google Scholar]
- 57.Bliedtner M., Schäfer I.K., Zech R., Von Suchodoletz H. Leaf wax n-alkanes in modern plants and topsoils from eastern Georgia (Caucasus) - Implications for reconstructing regional paleovegetation. Biogeosciences. 2018;15:3927–3936. [Google Scholar]
- 58.Jeon S.-K., Kwon D., Lee S. Identification of weathered multiple petroleum products in contaminated soils by characterizing unresolved complex mixture hump in gas chromatograph data. Sci. Total Environ. 2017;607–608:42–52. doi: 10.1016/j.scitotenv.2017.06.251. [DOI] [PubMed] [Google Scholar]
- 59.Farrington J.W., Quinn J.G. “Unresolved complex mixture”(UCM): a brief history of the term and moving beyond it. Mar. Pollut. Bull. 2015;96:29–31. doi: 10.1016/j.marpolbul.2015.04.039. [DOI] [PubMed] [Google Scholar]
- 60.Hobbie E.A., Werner R.A. Intramolecular, Compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: a review and synthesis. New Phytol. 2004;161:371–385. doi: 10.1111/j.1469-8137.2004.00970.x. [DOI] [PubMed] [Google Scholar]
- 61.Wedin D.A., Tieszen L.L., Dewey B., Pastor J. Carbon isotope dynamics during grass decomposition and soil organic matter formation. Ecology. 1995;76:1383–1392. [Google Scholar]
- 62.Henn M.R., Chapela I.H. Differential C isotope discrimination by fungi during decomposition of C3-and C4-derived sucrose. Appl. Environ. Microbiol. 2000;66:4180–4186. doi: 10.1128/aem.66.10.4180-4186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eglinton G., Logan G.A. Molecular preservation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1991;333:315–328. doi: 10.1098/rstb.1991.0081. [DOI] [PubMed] [Google Scholar]
- 64.Killops S.D., Killops V.J. John Wiley & Sons; 2013. Introduction to Organic Geochemistry. [Google Scholar]
- 65.Wu M.S., West A.J., Feakins S.J. Tropical soil profiles reveal the fate of plant wax biomarkers during soil storage. Org. Geochem. 2019;128:1–15. [Google Scholar]
- 66.Kögel-Knabner I., Amelung W. Soil organic matter in major pedogenic soil groups. Geoderma. 2021;384 [Google Scholar]
- 67.Wiseman C.L.S., Püttmann W. Interactions between mineral phases in the preservation of soil organic matter. Geoderma. 2006;134:109–118. [Google Scholar]
- 68.Li Q., Wang L., Fu Y., Lin D., Hou M., Li X., Hu D., Wang Z. Transformation of soil organic matter subjected to environmental disturbance and preservation of organic matter bound to soil minerals: a review. J. Soils Sediments. 2023;23:1485–1500. [Google Scholar]
- 69.Lützow M.V., Kögel-Knabner I., Ekschmitt K., Matzner E., Guggenberger G., Marschner B., Flessa H. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions–a review. Eur. J. Soil Sci. 2006;57:426–445. [Google Scholar]
- 70.Vijayalakshmi K., Vittal K.P.R., Rao U.M.B. Minimal irrigation on small agricultural watersheds with red soils in the semi-arid tropics of Andhra Pradesh, India. Agric. Water Manag. 1989;16:279–291. [Google Scholar]
- 71.Patel A., Singh S., Babu A., Verma S., Singh S.K. Effect of monthly rainfall distribution on physico-chemical properties and availability of nutrients in upland red soil of Mirzapur. J. Pharmacogn. Phytochem. 2018;7:424–429. [Google Scholar]
- 72.Cotrufo M.F., Lavallee J.M. Soil organic matter formation, persistence, and functioning: A synthesis of current understanding to inform its conservation and regeneration. Adv. Agronomy. 2022;172:1–66. [Google Scholar]
- 73.Hidalgo C., Merino A., Osorio-Hernández V., Etchevers J.D., Figueroa B., Limon-Ortega A., Aguirre E. Physical and chemical processes determining soil organic matter dynamics in a managed vertisol in a tropical dryland area. Soil Tillage Res. 2019;194 [Google Scholar]
- 74.Srinivasan R., Hegde R., Srinivas S., Niranjana K., Vasundhara R., Lalitha M., Kalaiselvi B., Maddileti N. Assessment of soil organic carbon stock in major land uses of arid-region of Andhra Pradesh, India. Clim. Chang. and Environ. Sustain. 2020;8:171–180. [Google Scholar]
- 75.Sharma K.L., Grace J.K., Mishra P.K., Venkateswarlu B., Nagdeve M.B., Gabhane V.V., Sankar G.M., Korwar G.R., Chary G.R., Rao C.S., et al. Effect of soil and nutrient-management treatments on soil quality indices under cotton-based production system in rainfed semi-arid tropical vertisol. Commun. Soil Sci. Plant Anal. 2011;42:1298–1315. [Google Scholar]
- 76.Islam M.R., Singh B., Dijkstra F.A. Stabilisation of soil organic matter: Interactions between clay and microbes. Biogeochemistry. 2022;160:145–158. [Google Scholar]
- 77.Conant R.T. Temperature and soil organic matter decomposition rates–synthesis of current knowledge and a way forward. Glob. Chang. Biol. 2011;17:3392–3404. [Google Scholar]
- 78.Zhou W., Zheng Y., Meyers P.A., Jull A.J.T., Xie S. Postglacial climate-change record in biomarker lipid compositions of the Hani peat sequence, Northeastern China. Earth Planet Sci. Lett. 2010;294:37–46. [Google Scholar]
- 79.Jones S.C. Vertebrate Paleobiology and Paleoanthropology. Dordrecht: Springer Netherlands; 2007. The Toba supervolcanic eruption: Tephra-fall deposits in India and paleoanthropological implications; pp. 173–200. [DOI] [Google Scholar]
- 80.Korisettar R. Holocene Climate Change and Environment. Elsevier; 2021. Late Quaternary geoarchaeological and palaeoenvironmental aspects of Kurnool Basin in the Indian Peninsula; pp. 575–610. [DOI] [Google Scholar]
- 81.Lal R. Tillage effects on soil degradation, soil resilience, soil quality, and sustainability. Soil Tillage Res. 1993;27:1–8. [Google Scholar]
- 82.Obalum S.E., Chibuike G.U., Peth S., Ouyang Y. Soil organic matter as sole indicator of soil degradation. Environ. Monit. Assess. 2017;189:176. doi: 10.1007/s10661-017-5881-y. [DOI] [PubMed] [Google Scholar]
- 83.Berhe A.A., Harden J.W., Harte J., Torn M.S. Soil degradation and global change: role of soil erosion and deposition in carbon sequestration. UC Berkeley: University of California International and Area Studies; 2005. [Google Scholar]
- 84.Li Z., Sun Y., Nie X. Biomarkers as a soil organic carbon tracer of sediment: Recent advances and challenges. Earth Sci. Rev. 2020;208 [Google Scholar]
- 85.Leahy J.G., Colwell R.R. Microbial degradation of hydrocarbons in the environment. Microbiol. Rev. 1990;54:305–315. doi: 10.1128/mr.54.3.305-315.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brittingham A., Hren M.T., Hartman G. Microbial alteration of the hydrogen and carbon isotopic composition of n-alkanes in sediments. Org. Geochem. 2017;107:1–8. [Google Scholar]
- 87.Nguyen Tu T.T., Egasse C., Anquetil C., Zanetti F., Zeller B., Huon S., Derenne S. Leaf lipid degradation in soils and surface sediments: A litterbag experiment. Org. Geochem. 2017;104:35–41. [Google Scholar]
- 88.Sojinu O.S., Shittu A.O. Higher plants n-alkane profiles as indicators of anthropogenic environmental. J. Chem. Society Nigeria. 2018;43:1–14. [Google Scholar]
- 89.Klein R.G. Stable carbon isotopes and human evolution. Proc. Natl. Acad. Sci. USA. 2013;110:10470–10472. doi: 10.1073/pnas.1307308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wagai R. Linking temperature sensitivity of soil organic matter decomposition to its molecular structure, accessibility, and microbial physiology. Glob. Chang. Biol. 2013;19:1114–1125. doi: 10.1111/gcb.12112. [DOI] [PubMed] [Google Scholar]
- 91.Wang G., Jia Y., Li W. Effects of environmental and biotic factors on carbon isotopic fractionation during decomposition of soil organic matter. Sci. Rep. 2015;5 doi: 10.1038/srep11043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kohn M.J. Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo)climate. Proc. Natl. Acad. Sci. USA. 2010;107:19691–19695. doi: 10.1073/pnas.1004933107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ehleringer J.R., Cerling T.E. C3 and C4 photosynthesis. Encyclopedia of Global Environmental Change. 2002;2:186–190. [Google Scholar]
- 94.Li H. Temperature sensitivity of SOM decomposition is linked with a K-selected microbial community. Glob. Chang. Biol. 2021;27:2763–2779. doi: 10.1111/gcb.15593. [DOI] [PubMed] [Google Scholar]
- 95.Li G. Microbial production of long-chain n-alkanes: Implication for interpreting sedimentary leaf wax signals. Org. Geochem. 2018;115:24–31. [Google Scholar]
- 96.Stefanović M., Šajnović A., Kašanin-Grubin M., Vergari F., Troiani F., Moreno-de-las-Heras M., Gallart F., Desloges J., Jovančićević B. Impact of weathering processes on n-alkane pattern in badlands. Catena. 2023;231 [Google Scholar]
- 97.Schäfer I.K. Leaf waxes in litter and topsoils along a European transect. Soil. 2016;2:551–564. [Google Scholar]
- 98.Bush R.T., McInerney F.A. Leaf wax n-alkane distributions in and across modern plants: Implications for paleoecology and chemotaxonomy. Geochim. Cosmochim. Acta. 2013;117:161–179. [Google Scholar]
- 99.Pedentchouk N., Turich C. Carbon and Hydrogen Isotopic Compositions of N-Alkanes as a Tool in Petroleum Exploration. Geological Society, London, UK; 2018. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data associated with the publication are included in this article or provided as a supplemental information. Any additional information required to re-analyze the data reported in this article is available from the lead contact upon request.
-
•
No code generated in this study.