Abstract
Parametric response mapping (PRM) on computed tomography (CT) is an imaging technique for assessing small airway disease (SAD) and emphysema in chronic obstructive pulmonary disease (COPD). This study was aimed to examine the relationship between individual spirometry components decline and PRM-CT-derived data changes over a 6-year observation period in mild and moderate COPD patients using a COPD cohort chronically exposed to dust. This study utilized longitudinal data of the COPD in Dusty Areas (CODA) cohort from 2012 to 2014. COPD was confirmed by a post-bronchodilator forced expiratory volume in one second (FEV1) over forced vital capacity (FVC) value < 0.70. Among the 427 included patients, 106 with 6-year follow-up PRM CT data and having mild (FEV1% of the predicted value [%pred] ≥ 80) or moderate (FEV1%pred 50 to < 80) airflow limitation were analyzed. PRM CT metrics, including percentage of emphysema (PRMemph) and functional small airway disease (PRMfSAD), were also assessed. A positive correlation was found between baseline log-transformed PRMfSAD and PRMemph, which was stronger in patients with moderate airflow limitation. Over the 6-year follow-up, every 10% increase in PRMfSAD was associated with a significant decline in FVC (− 8.0 mL/y, P = 0.016) and FEV1 (− 6.0 mL/y, P = 0.001). This association was only significant in moderate COPD patients (FVC: −26.6 mL/y, P < 0.001; FEV1: −11.8 mL/y, P = 0.002). There was the greater relative contribution of PRMfSAD to lung function decline in moderate COPD compared to mild COPD, suggesting that the utility of PRM CT may differ according to COPD severity even in early stage of COPD.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-21015-4.
Keywords: COPD, Emphysema, Parametric response mapping, Small airway disease
Subject terms: Chronic obstructive pulmonary disease, Image processing
Introduction
Parametric response mapping (PRM) is a developed voxel-wise image analysis technique. It was initially introduced as a biomarker for nonrespiratory diseases, such as hepatocellular carcinoma, breast tumor, and glioma, based on magnetic resonance imaging data1–3. In recent years, modification of the PRM approach can assess changes in image density within respiratory cycle and provides the quantification of functional small airway disease (PRMfSAD) and emphysema (PRMemph) together with spatial information of the extent and location of disease in chronic obstructive pulmonary disease (COPD)4. Distinguishing fSAD from emphysema through PRM CT, which was not detected by traditional CT, promotes understanding disease heterogeneity and phenotype with the direction of COPD progression5,6. Results from SPIROMICS data showed greater variations in PRMfSAD than PRMemph in a 30-day repeatability, while only PRMemph in severe COPD subjects produced significant change, concluding fSAD as a transitional phase from normal lung structure to emphysematous change7.
Given that mild to moderate COPD represent critical transitional phases where early intervention could alter disease trajectory, focused investigation of these groups would be beneficial8. An investigation using a COPDGene cohort showed that PRM-CT could effectively differentiate fSAD from emphysema, revealing that PRMfSAD precedes PRMemph with an increase in COPD severity4. Another study utilizing the same cohort demonstrated a significant correlation between PRMfSAD and FEV₁ decline among patients with COPD GOLD grade 1–4. Further analysis revealed that PRMfSAD was associated with a significantly greater decline in FEV₁ compared to PRMemph in both GOLD 1–2 and GOLD 3–4 groups, with a more pronounced effect observed in the GOLD 1–2 group9. However, this study used only baseline PRM data and patients with mild (GOLD 1) and moderate (GOLD 2) airflow limitation were not analyzed separately despite of different FEV1 decline between GOLD 1 and GOLD 2 COPD9. Thus, current evidence remains unclear regarding how PRM-CT–derived fSAD and emphysema data represent in GOLD 1 and GOLD 2 in each retrospective stage along with their significance for lung function decline.
In this context, we hypothesized that PRM-CT-derived data could show distinct associations of lung function decline in COPD patients with GOLD 1 and GOLD 2, facilitating risk stratification and enabling early therapeutic interventions. Therefore, we aimed to examine the changes in lung function and PRM-CT-derived data over a 6-year observation period in patients with mild COPD and moderate COPD. We further assessed the relationship between individual spirometry components decline and PRM-CT-derived data changes over a 6-year observation period, particularly in a COPD cohort chronically exposed to dust.
Results
The baseline characteristics are shown in Table 1. Participants with GOLD 2 are younger, more likely to receive inhaler therapy, exhibit lower lung function, and show a higher percentage of PRMfSAD and PRMemph.
Table 1.
Baseline characteristics (N = 106).
| Overall | GOLD 1 (n = 63) | GOLD 2 (n = 43) |
P-value | |
|---|---|---|---|---|
| Age (years) | 70.9 ± 6.6 | 72.2 ± 5.4 | 69.0 ± 7.6 | 0.013 |
| Sex (male) | 88 (83.0%) | 50 (79.4%) | 38 (88.4%) | 0.342 |
| BMI (kg/m2) | 23.6 ± 3.2 | 24.0 ± 2.9 | 23.1 ± 3.6 | 0.15 |
| Smoking status | 0.2 | |||
| Never | 28 (26.4%) | 20 (31.7%) | 8 (18.6%) | |
| Ever | 78 (73.6%) | 43 (68.3%) | 35 (81.4%) | |
| mMRC grade | ||||
| ≥2 | 39 (36.8%) | 23 (36.5%) | 16 (37.2%) | 1.0 |
| CAT | ||||
| ≥ 10 | 76 (71.7%) | 45 (71.4%) | 31 (72.1%) | 1.0 |
| Exacerbation in previous year | ||||
| ≥ 2 moderate or ≥ 1 severe | 3 (2.8%) | 1 (1.6%) | 2 (4.7%) | 0.736 |
| Charlson comorbidity index | ||||
| ≥2 | 12 (11.3%) | 8 (12.7%) | 4 (9.3%) | 0.818 |
| Inhaler therapy | 16 (15.1%) | 5 (7.9%) | 11 (25.6%) | 0.027 |
| Lung function testing | ||||
| FVC (L) | 3.1 ± 0.7 | 3.2 ± 0.7 | 2.9 ± 0.7 | 0.017 |
| FVC, %pred | 94.8 ± 14.7 | 101.8 ± 12.2 | 84.5 ± 11.6 | < 0.001 |
| FEV1 (L) | 2.0 ± 0.5 | 2.2 ± 0.5 | 1.8 ± 0.4 | < 0.001 |
| FEV1, %pred | 84.1 ± 15.8 | 93.8 ± 12.1 | 69.7 ± 7.3 | < 0.001 |
| FEV1/FVC (%) | 64.8 ± 6.9 | 67.2 ± 5.6 | 61.2 ± 7.2 | < 0.001 |
| PRM value | ||||
| PRMfSAD (%) | 24.3 ± 15.0 | 22.1 ± 14.2 | 27.5 ± 15.7 | 0.065 |
| PRMemph (%) | 4.3 ± 3.9 | 3.6 ± 3.3 | 5.4 ± 4.6 | 0.022 |
BMI = body mass index; mMRC = modified Medical Research Council; CAT = COPD assessment test; FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 s, PRM = parametric response mapping; emph = emphysema; fSAD = functional small airway disease.
The baseline correlation coefficients were − 0.229 between the percentage of predicted value (%pred) of FEV1 and PRMfSAD, − 0.406 between FEV1%pred and PRMemph, − 0.372 between FEV1/forced vital capacity (FVC) and PRMfSAD, and − 0.555 between FEV1/FVC and PRMemph, with P < 0.001 for all analyses. In contrast, FVC was not significantly correlated with either PRMfSAD or PRMemph.
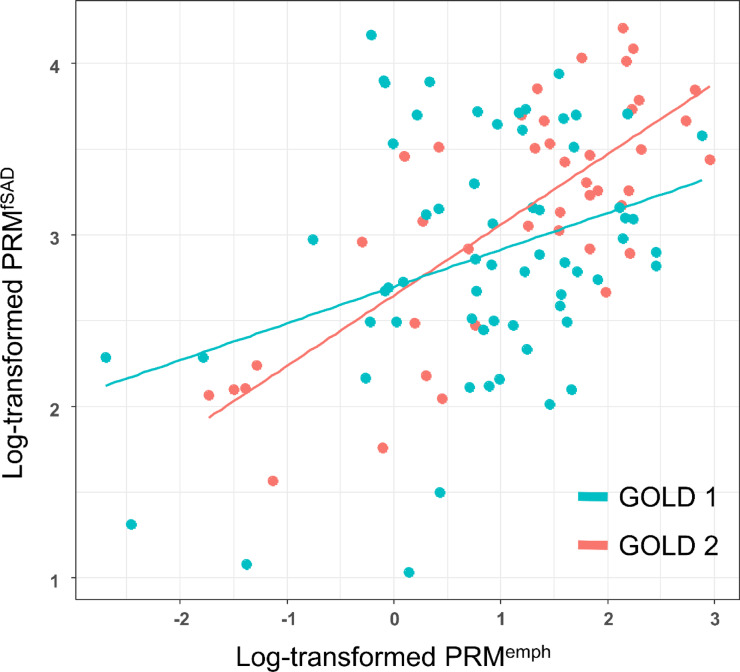
A crude and multivariable adjusted linear regression model showed close relationship between baseline log-transformed PRMfSAD and log-transformed PRMemph (Table 2; Fig. 1). In multivariable adjusted model, the correlation coefficient between these two parameters was 0.307 (P < 0.001). This significance was stronger in GOLD 2 than in GOLD 1.
Table 2.
Correlation between PRMfSAD and PRMemph at baseline using the linear regression model.
| Crude | Multivariable adjusted | |||||
|---|---|---|---|---|---|---|
| Beta | SE | P | Beta | SE | P | |
| All (n = 106) | 0.313 | 0.076 | < 0.001 | 0.307 | 0.053 | < 0.001 |
| GOLD 1 (n = 63) | 0.214 | 0.077 | < 0.001 | 0.184 | 0.091 | 0.049 |
| GOLD 2 (n = 43) | 0.411 | 0.056 | < 0.001 | 0.408 | 0.055 | < 0.001 |
The model was adjusted for age, sex, BMI, smoking, FEV1%pred at baseline, and inhaler therapy at baseline.
Values of PRMemph and PRMfSAD were log-transformed because of the violation of the normality assumption.
PRM = parametric response mapping; fSAD = functional small airway disease; emph = emphysema; FEV1 = forced expiratory volume in 1 s.
Fig. 1.
Correlation between PRMfSAD and PRMemph at baseline. PRM, parametric response mapping; fSAD; functional small airway disease; emph., emphysema.
The average annual decline in FVC was 90.5 mL in GOLD 1 patients and 31.8 mL in GOLD 2 patients. The average annual decline in FEV1 was 60.8 mL in GOLD 1 patients and 23.2 mL in GOLD 2 patients. The annual increase in PRMemph was 0.404% in GOLD 1 and 0.329% in GOLD 2, and the annual increase in PRMfSAD was 0.791% in GOLD 1 and 0.867% in GOLD 2.
The adjusted rates of changes in FVC and FEV1 according to every 10% change of PRMemph and PRMfSAD from baseline are shown in Table 3. During 6-years of follow-up, for every additional 10% of lung affected by PRMfSAD, significant decline in FVC and FEV1 was noted (− 8.0 mL/y per 10% PRMfSAD for FVC with P = 0.016 and − 6.0 mL/y per 10% PRMemph with P = 0.001, respectively). However, this significance only remained in patients with GOLD 2: (− 26.6 mL/y per 10% PRMfSAD for FVC with P < 0.001 and − 11.8 mL/y per 10% PRMfSAD for FEV1 with P = 0.002, respectively).
Table 3.
Changes in lung function (mL/y) for 6 years per every 10% change of PRMemph and PRMfSAD from baseline by GOLD classification at the baseline.
| Crude | ||||
|---|---|---|---|---|
| PRMemph | FVC change | P | FEV1 change | P |
| All (n = 106) | 30.2 (− 9.1, 69.4) | 0.13 | −6.2 (− 29.9, 17.6) | 0.608 |
| GOLD 1 (n = 63) | 2.5 (− 61.6, 66.6) | 0.937 | 3.0 (− 38.3, 44.3) | 0.884 |
| GOLD 2 (n = 43) | 38.3 (− 10.4, 86.9) | 0.119 | −11.2 (− 38.6, 16.2) | 0.413 |
| PRMfSAD | FVC change | P | FEV1 change | P |
|---|---|---|---|---|
| All (n = 106) | −9.0 (− 15.5, − 2.4) | 0.008 | −6.0 (− 9.9, − 2.2) | 0.003 |
| GOLD 1 (n = 63) | −4.0 (− 10.9, 2.9) | 0.249 | −3.9 (− 8.3, 0.1) | 0.083 |
| GOLD 2 (n = 43) | −16.7 (− 28.6, − 4.9) | 0.006 | −8.9 (− 15.5, − 2.5) | 0.008 |
| Adjusted | ||||
|---|---|---|---|---|
| PRMemph | FVC change | P | FEV1 change | P |
| All (n = 106) | 22.9 (− 16.1, 61.9) | 0.247 | −6.7 (− 29.1, 15.7) | 0.553 |
| GOLD 1 (n = 63) | −3.6 (− 68.9, 61.8) | 0.913 | −1.5 (− 15.1, 42.1) | 0.944 |
| GOLD 2 (n = 43) | 39.5 (− 18.1, 97.0) | 0.172 | −1.9 (− 30.7, 26.9) | 0.895 |
| PRMfSAD | FVC change | P | FEV1 change | P |
|---|---|---|---|---|
| All (n = 106) | −8.0 (− 14.5, − 1.6) | 0.016 | −6.0 (− 9.6, − 2.4) | 0.001 |
| GOLD 1 (n = 63) | −5.6 (− 12.7, 1.5) | 0.119 | −4.2 (− 8.9, 0.4) | 0.074 |
| GOLD 2 (n = 43) | −26.6 (− 40.8, − 12.4) | < 0.001 | −11.8 (− 18.9, − 4.7) | 0.002 |
*Adjusted for age, sex, BMI, smoking, FEV1%pred at baseline, and inhaler therapy at baseline.
FVC = forced vital capacity; FEV1 = forced expiratory volume in 1 s; %pred = % of the predicted value; emph = emphysema; fSAD = functional small airway disease; BMI = body mass index.
An additional subgroup analyses based on age and smoking status are revealed in S1 Table. Change in lung function for FVC and FEV1 was not significantly associated with change of PRMemph in ever smokers but this association was significant in never smokers. In contrast, the increase in PRMfSAD showed a significant association with FEV1 decline irrespective of smoking status.
Discussion
In this study, fSAD change measured using PRM CT over 6 years was associated with a longitudinal decline in FVC and FEV1, especially for COPD patients with GOLD 2, whereas the change in PRMemph was not associated with changes in the lung function in GOLD 1 and GOLD 2. The findings of this study indicate that change in the lung function in GOLD 2 is related to the progression of small airway disease rather than emphysema. Our another findings with baseline values are consistent with those of previous studies, wherein positive correlations between the baseline values of PRMemph and PRMfSAD 4,7,10 and negative correlations between baseline PRM metrics and baseline lung function6 were noted. We not only confirmed these findings but extended the relationships on the longitudinal aspects, particularly in populations at risk of dust-related COPD.
Our data showed that the change of PRM CT-measured fSAD rather than emphysema was more closely related to the longitudinal lung function decline in mild-to-moderate COPD. The fSAD pattern, rather than the emphysema pattern, might more appropriately reflect the disease progression in patients with early COPD10–12. In addition, in our explorative analysis, longitudinal changes in PRMfSAD explained a greater proportion of the variance in lung function decline compared to baseline PRM metrics or changes in emphysema: specifically, the partial R² for fSAD change was approximately 8.8% for FEV₁ decline and 5.8% for FVC decline. SAD manifests in the early stages of COPD and becomes increasingly prevalent with progression to more severe COPD13. Thus, certain injuries such as those caused by smoking could lead to airway remodeling, mucous plugging, and immune cell infiltration, which may aggravate COPD and affect bacterial colonization. These processes may predispose individuals to the progression of SAD and development of emphysema13. In a COPDGene study, relative contribution to the decline in the lung function was more prominent with PRMfSAD than PRMemph 9. In more severe lung obstruction, PRMfSAD reaches a plateau around 40–50%, whereas PRMemph continuously increases to over 20% of the lung volume4. Furthermore, the decline in PRMfSAD with an increase in PRMemph suggests progression to a more chronic COPD status7. In summary, fSAD presents in the early course of COPD whereas emphysema is a major component in the later course of COPD.
Notably, our data found that a significant association between decline in FEV1 and FVC and PRMfSAD was only observed in COPD patients with GOLD 2. In the COPDGene study, the intensity of relative contribution of PRMfSAD to decline in the lung function was more prominent in patients with GOLD 1–2 than in those with GOLD 3–49. We extended these meaningful associations with lung function decline and change of PRMfSAD over 6 years. Furthermore, our data showed that this association was evident only in COPD patients with GOLD 2 and there was no significant association between lung function decline and PRMfSAD change in patients with GOLD (1) There are couple of explanations for this finding. First, the small airway disease extent between GOLD 1 and 2 could be different. Previous cross-sectional study showed that the reductions in the number of terminal and respiratory bronchioles were 29 and 41% respectively in GOLD 1 patients and the reductions were 40 and 53% respectively in GOLD 2 patients compared with controls14. Another study showed that the presence of SAD assessed by the impulse oscillometry system was reported to be significantly higher in patients with GOLD 2 than in those with GOLD 115. Similarly, the change in fSAD tended to be relatively greater compared to the decline in lung function in GOLD 2 than in GOLD 1 in our study. Second, as a significant portion of the exhaled volume of lung function is influenced by the larger airways, calculation of FEV1 using spirometry could not detect relatively smaller SAD change in GOLD 1 than GOLD (2) Indeed, The difference in GOLD 1 and 2 is also corroborated by another study wherein the expiratory flow limitation appeared first in GOLD 216.
Our study also found a significant association between longitudinal increase in PRMfSAD and decrease in FVC, which might reflect the progression of air trapping. In a recent cross-sectional studies based on the COPDGene data, air trapping assessed using PRMfSAD at the end of full expiratory effort was negatively correlated with FVC17. Another study also showed that PRMfSAD was mainly associated with total lung capacity alveolar volume and residual volume which are measures of pulmonary air trapping6.
PRM CT has shown its potential in distinguishing COPD phenotypes and visualizing disease progression4,6,18. By integrating pulmonary function testing, PRM CT could have synergistic impact in understanding the heterogeneity of COPD6,18. Since small airway disease is one of the earliest features of COPD progression, PRM CT could complement pulmonary function testing by detecting early disease onset, might otherwise be overlooked when relying solely on the conventional FEV1/FVC cut-off ratio of 0.709,14. In the COPDGene study, GOLD 0 subjects with 48.5% of current smokers, defined as by postbronchodilator FEV1/FVC greater than or equal to 0.70 at baseline visit and FEV1% predicted greater than or equal to 80, with 48.5% of current smokers, already showed the significant association of PRMfSAD but not PRMemph with FEV1 decline9. Additionally, in a study that combined PRM CT metrics with histological findings, small airway disease was observed in mild-to-moderate COPD without evidence of emphysema14. Notably, this small airway disease is closely associated with emphysema progression. when there was no emphysema at baseline19. Therefore, early detection of small airway disease or emphysema using PRM CT shows promise for enhancing disease monitoring, identifying at-risk individuals, and enabling timely interventions to prevent COPD progression.
Our study had several limitations. First, this COPD in Dusty Area (CODA) cohort consisted of COPD patients living in dusty areas near cement plants in South Korea. Although selection bias may have influenced our results, environmental exposure could have increased the detection of small airway disease in this population. Second, comparing changes in PRM CT metrics with individuals who have normal lung function was not feasible in this study; therefore, additional research involving participants with normal lung function is needed. Third, the present study analyzed 6-year follow-up data and contained loss to follow-up, which led to differences in baseline characteristics between participants with available follow-up data and those without (S2 Table). The included group had a significantly higher proportion of males and ever-smokers, whereas the excluded group exhibited a higher prevalence of comorbidities. These differences could raise the possibility that patients with more severe conditions might have been preferentially lost to follow-up, potentially introducing selection bias and affecting the generalizability of the findings. Fourth, this study lacks lung volume measurements (e.g., total lung capacity, residual volume) for emphysema quantification, diffusion capacity data, and small airway function tests (e.g., impulse oscillometry).
In conclusion, this longitudinal cohort study of patients with GOLD 1 and 2 COPD showed a positive relationship between baseline PRMfSAD and PRMemph. Over a 6-year follow-up period, the longitudinal decline in FVC and FEV₁ correlated with PRM CT-assessed fSAD, with a more pronounced association observed in GOLD 2 patients compared to those with GOLD 1. However, changes in PRMemph were not significantly associated with lung function decline in either GOLD group. The stronger contribution of PRMfSAD to lung function decline in GOLD 2 suggests that the utility of PRM CT may differ according to COPD severity even in early stage of COPD, making it particularly valuable for identifying patients at higher risk of rapid disease progression who could benefit from earlier therapeutic interventions. Future studies should further investigate the role of PRM CT in personalized COPD management strategies.
Materials and methods
Data source and patient selection
This study utilized data of the CODA cohort from 2012 to 2014. The CODA cohort is a longitudinal observational study conducted in six cities (Gangneung, Donghae, Samcheok, Yeongheung, Danyang, and Jecheon) of Kangwon and Chungbuk provinces, South Korea, with cement plants, with 20,000 residents. Participants in the CODA cohort study comprised individuals, with either airflow limitation or normal spirometry, who agreed to participate in the study. Airflow limitation was confirmed by a post-bronchodilator FEV1 over FVC value < 0.70 in spirometry20. The detailed methods adopted in the CODA study have been previously described21,22.
The patient selection process is depicted in Fig. 2. As this study is a longitudinal observational study rather than a randomized controlled trial, a formal sample size calculation was not performed. The final sample size was determined based on the availability of patients with complete 6-year follow-up PRM CT data, ensuring that all eligible participants were included in the analysis. Of a total 504 patients in the CODA cohort, 77 were excluded for lung surgery (n = 4), CT quantification error (n = 8), co-existed lung disease (n = 10), severe lung parenchymal distortion by tuberculosis sequelae and pneumoconiosis with progressive massive fibrosis (n = 30), and GOLD 3–4 (n = 25). Among the remaining 427 patients, 106 having 6-year follow-up PRM CT data were analyzed.
Fig. 2.
Patient selection process. COPD, chronic obstructive pulmonary disease; CODA, COPD in Dusty Areas; CT, computed tomography; FEV1; forced expiratory volume in one second; PRM, parametric response mapping.
Lung function testing
Spirometry was performed using an Easy One Kit (NDD, Zurich, Switzerland). To check bronchodilator reversibility, spirometry was performed before bronchodilation and at 15 min after inhaling 400 mcg of salbutamol. Calibration and quality control were performed following the standardization criteria of the American Thoracic Society and European Respiratory Society23. FEV1 (L) and FEV1%pred, FVC (L) and FVC %pred, and the ratio of FEV1/FVC (%) were obtained from both pre- and postbronchodilator tests. Patients were further categorized into GOLD 1 and 2 by post-bronchodilator FEV1%pred20: GOLD 1 (FEV1%pred ≥ 80) and GOLD 2 (FEV1%pred 50 to < 80).
CT and quantitative image analysis
CT scan was performed at maximal inspiration and expiration, with patients exhaling to residual volume in the supine position using a dual-source CT scanner (Somatom Definition, Siemens Healthcare, Forchheim, Germany for the CODA cohort) with the following parameters: 140 kVp, 100 mA, and 09 − 1 beam pitch24. All CT scan images were obtained without the use of contrast medium. CT images were reconstructed at a 0.6 mm slice thickness using a B30f kernel. Quantitative CT analysis was performed using an automatic segmentation software (Aview, Coreline Soft, Seoul, Korea). Whole-lung images were extracted from the chest wall, mediastinum, and large airways. Lung segmentation was visually checked. Inspiration and expiration CT images were registered using a nonrigid method. In PRM, the lung parenchyma is classified as normal lung parenchyma (normal), fSAD, or emphysema: (1) PRMemph was defined as voxels with densities less than − 950 HU on inspiration CT and less than − 856 HU on expiration CT; (2) PRMfSAD was defined as voxels with densities greater than or equal to − 950 HU on inspiration CT and less than − 856 HU on expiration CT; and (3) PRMnormal was defined as voxels ≥–950 HU on inspiration CT and ≥ − 856 HU on expiratory CT4. The PRM CT values are presented as a percentage of the total lung volume.
Other variables
Other variables collected included age, sex, body mass index (kg/m2), smoking status (never or ever), the modified Medical Research Council dyspnea scale, patient-reported COPD assessment test score, history of exacerbation within the previous year (moderate: ≥2 events, where a moderate event is defined as the use of antibiotics or steroids; severe: ≥1 event, where a severe event is defined as an hospitalization or emergency room visit due to respiratory symptoms), Charlson comorbidity index, and inhaler therapy (long-acting muscarinic antagonist, long-acting beta2-agonist, inhaled corticosteroid, LAMA/LAMA combination, and inhaled corticosteroid/long-acting beta2-agonist combination).
Statistical analysis
All statistical analyses were performed using R software, version 4.3.2 for Windows (R Development Core Team). Statistical significance was set at P < 0.05. Comparisons were performed using two sample t-tests for continuous variables and chi-squared test for categorical variables. Correlation analysis was performed to identify any relationship between PRMemph and PRMfSAD at baseline. A linear regression model was adopted to study the relationship between PRMemph and PRMfSAD at baseline, and the values were log-transformed because of the violation of normality. To calculate changes in the lung function according to potential predictors (PRMemph and PRMfSAD), multivariable linear regression model was employed. The outcome of change in FEV1, FVC, and FEV1/FVC% for each individual was calculated by subtracting the baseline value from the value at 6-year follow-up and dividing by the time between visits. The model was adjusted for age, sex, body mass index, smoking, FEV1%pred at baseline, and inhaler therapy at baseline. In addition, subgroup analyses were performed based on the FEV1%pred, categorized as either GOLD 1 or 2. Another subgroup analysis was conducted according to age and smoking status.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
TK: Writing the original draft, Methodology, formal analysis, and investigation. WJK and HYP: Writing, review, editing, supervision, and project administration. YK, SHB, SHS, HYL, YI, and YC: Validation. All the authors discussed the results and approved the final version of the manuscript.
Funding
This research was supported by Institute for Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) (No.RS-2022-II221196, Regional strategic Industry convergence security core talent training business). This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI23C1234).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
The study design and methods were approved by the Institutional Review Board (IRB) of Kangwon National University Hospital (IRB No. KNUH 2012-06-007) and the study was conducted following the tenets of the Declaration of Helsinki. Informed consent was waived by the ethics committee of Kangwon National University Hospital because of the retrospective nature of the study and the analysis used anonymous clinical data.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Taeyun Kim and Youlim Kim have contributed equally as first authors.
Contributor Information
Woo Jin Kim, Email: pulmo2@kangwon.ac.kr.
Hye Yun Park, Email: hyeyunpark@skku.edu.
References
- 1.Choi, S. J. et al. Parametric response mapping of dynamic CT for predicting intrahepatic recurrence of hepatocellular carcinoma after conventional transcatheter arterial chemoembolization. Eur. Radiol.26, 225–234 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Moffat, B. A. et al. Functional diffusion map: a noninvasive MRI biomarker for early stratification of clinical brain tumor response. Proc. Natl. Acad. Sci. U S A. 102, 5524–5529 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamstra, D. A. et al. Functional diffusion map as an early imaging biomarker for high-grade glioma: correlation with conventional radiologic response and overall survival. J. Clin. Oncol.26, 3387–3394 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Galbán, C. J. et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat. Med.18, 1711–1715 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang, J. M. et al. Topologic parametric response mapping identifies tissue subtypes associated with emphysema progression. Acad. Radiol.31, 1148–1159 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pompe, E. et al. Parametric response mapping on chest computed tomography associates with clinical and functional parameters in chronic obstructive pulmonary disease. Respir Med.123, 48–55 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boes, J. L. et al. Parametric response mapping monitors Temporal changes on lung CT scans in the subpopulations and intermediate outcome measures in COPD study (SPIROMICS). Acad. Radiol.22, 186–194 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fazleen, A., Wilkinson, T. & Early COPD: current evidence for diagnosis and management. Ther. Adv. Respir Dis.14, 1753466620942128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt, S. P. et al. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am. J. Respir Crit. Care Med.194, 178–184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoff, B. A. et al. CT-Based local distribution metric improves characterization of COPD. Sci. Rep.7, 2999 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pompe, E. et al. Parametric response mapping adds value to current computed tomography biomarkers in diagnosing chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med.191, 1084–1086 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Vasilescu, D. M. et al. Noninvasive imaging biomarker identifies small airway damage in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit Care Med.200, 575–581 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higham, A., Quinn, A. M., Cançado, J. E. D. & Singh, D. The pathology of small airways disease in COPD: historical aspects and future directions. Respir Res.20, 49 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koo, H. K. et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study. Lancet Respir Med.6, 591–602. 10.1016/s2213-2600(18)30196-6 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Crisafulli, E. et al. Prevalence of Small-Airway dysfunction among COPD patients with different GOLD stages and its role in the impact of disease. Respiration93, 32–41 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Gennimata, S. A. et al. Pathophysiology of evolution of small airways disease to overt COPD. Copd7, 269–275 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Comellas, A. P. et al. RV versus FRC CT assessment of functional small airways disease in smokers with and without COPD. Am. J. Respir. Crit. Care Med. (2023). [DOI] [PMC free article] [PubMed]
- 18.Almeida, S. D. et al. Capturing COPD heterogeneity: anomaly detection and parametric response mapping comparison for phenotyping on chest computed tomography. Front. Med. (Lausanne). 11, 1360706. 10.3389/fmed.2024.1360706 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pompe, E. et al. Progression of emphysema and small airways disease in cigarette smokers. Chronic Obstr. Pulm Dis.8, 198–212. 10.15326/jcopdf.2020.0140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agustí, A. et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Eur. Respir. J.61, 2300239 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee, H. et al. Inflammatory biomarkers and radiologic measurements in never-smokers with COPD: A cross-sectional study from the CODA cohort. Chron. Respir Dis.15, 138–145. 10.1177/1479972317736293 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong, Y. et al. Methodology of an observational cohort study for subjects with chronic obstructive pulmonary disease in dusty areas near cement plants. J. Pulm Respir Med.4, 169 (2014). [Google Scholar]
- 23.Miller, M. R. et al. Standardisation of spirometry. Eur. Respir. J.26, 319–338 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Do, A. R. et al. Genome-wide association study of airway wall thickening in a Korean chronic obstructive pulmonary disease cohort. Genes (Basel). 13. 10.3390/genes13071258 (2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.