Abstract
Dietary proanthocyanidins (PACs) are polyphenols that promote a healthy gut microbiome. PACs are notable for their rich catechol moieties with high affinity for iron, enabling them to interfere with pathogens’ iron uptake. PACs selectively increase the abundance of Akkermansia muciniphila, a symbiont known for supporting metabolic and immune health. We discovered that A. muciniphila MucT utilizes distinct iron-acquisition systems to take up iron sequestered by PACs, supporting its metabolic activity. Integrative proteomics and transcriptomics revealed that A. muciniphila has an active catechol-type siderophore-mediated iron uptake (Fe3+) system, involving membrane ATP-binding cassette transporters and lipocalins. Simultaneously, the expression of ferrous iron (Fe2+) transporters, zinc uptake, and iron storage proteins was upregulated. Administering iron-laden PACs in an iron-depleted medium restored the A. muciniphila growth to levels comparable to those in iron-amended conditions. This was associated with an increased expression of the A. muciniphila siderophore operon and lipocalin genes, indicating that iron-laden PACs are recognized as xenosiderophores to cope with iron depletion. Hence, we identified novel signaling mechanisms for iron acquisition and siderophore uptake regulation in A. muciniphila MucT upon exposure to PACs, enhancing our understanding of the role of dietary PACs in selectively promoting this gut symbiont and potentially outcompeting pathogenic bacteria.
Subject terms: Microbial genetics, Bacterial physiology, Microbiome, Cellular microbiology, Dysbiosis
Dietary proanthocyanidins (PACs) increase the abundance of gut symbiont Akkermansia muciniphila. Here, the authors show that strain MucT employs distinct iron-acquisition systems to take up iron sequestered by PACs, supporting its metabolic activity.
Introduction
Iron is an essential micronutrient for the survival and metabolic processes of prokaryotic and eukaryotic organisms. It acts as a co-factor for enzymes involved in various physiological processes, including DNA synthesis, mitochondrial respiration, xenobiotic metabolism, and cell growth and differentiation1,2. However, iron overload can lead to various harmful effects, including oxidative stress, lipid peroxidation, impaired DNA repair, genomic damage to cells, and excessive inflammation in the host. Gut inflammation can also trigger improper regulatory responses by the host, leading to systemic and cellular iron imbalances. Iron dysregulation and elevated levels of labile reactive iron have been associated with type 2 diabetes, colorectal cancer, and cardiovascular and neurodegenerative diseases3. This imbalance allows unabsorbed iron to accumulate in the colon, fostering pro-inflammatory gut bacteria and pathobionts while concurrently reducing the abundance of beneficial bacteria4,5. Among these beneficial microbes is the mucus-living gut symbiont Akkermansia muciniphila, capable of regulating the host’s metabolism and immune system6–8. Under homeostatic conditions, the host employs mechanisms to limit iron availability in the gut by sequestering it within macrophages9, which is crucial for reducing bacterial proliferation and the virulence of pathobionts. Nevertheless, the acquisition of iron is taxon-specific, and understanding the mechanisms by which symbionts like A. muciniphila acquire iron is instrumental in improving the efficacy of therapeutic strategies for host immunomodulation and iron regulation.
Dietary polyphenols, commonly found in plant-based foods, are recognized for their ability to modulate the gut microbiota and provide health benefits4. Some may bind iron and compete with harmful bacteria for iron uptake, thus preventing opportunistic or pathogenic bacteria colonization and improving the balance of commensal bacteria10. Specifically, flavonoids with a high degree of polymerization, such as proanthocyanidins (PACs), have low bioavailability and reach the colon intactly. Recently, studies in mice have demonstrated that health improvements linked to higher intestinal levels of A. muciniphila can be achieved through the intake of polyphenols, particularly PACs derived from grapes, cranberries, or blueberries11–14. Dietary PACs significantly contribute to lowering inflammatory markers, strengthening the intestinal mucus layer, and supporting the growth of mucin-secreting goblet cells in mice11,15,16. These functions have been associated with an increased abundance of A. muciniphila in the colon. Additional research, especially with mice that lack A. muciniphila, could help clarify the effects of supplementing with this bacterium, both alone and alongside PACs, on intestinal barrier functions and immunomodulation. Likewise, the exact molecular mechanisms through which PACs promote A. muciniphila growth remain unexplored.
PACs are characterized by the presence of catechol structures containing several hydroxyl groups on aromatic rings. These catechol moieties facilitate strong iron-binding for both Fe3+ and Fe2+ ions through cationic interactions17. This structural configuration enables PACs to exhibit strong antimicrobial effects, which are not only achieved by chelating iron but also by interacting with cell membranes through hydrogen bonding and hydrophobic and non-covalent interactions17. Increasing evidence shows that catechol-rich polyphenols attenuate inflammation and influence the gut microbiota positively by scavenging reactive oxygen species (ROS) and chelating iron10,18. These findings highlight the relationship between the microbiome and iron availability. It also stresses the importance of symbiotic bacterial species’ fitness to adapt to and withstand the iron-chelating actions of polyphenols at a molecular and metabolic level. Symbiotic bacteria have evolved a plethora of mechanisms to acquire and solubilize the appropriate amount of iron from both the gut microenvironment and the host’s reserves without exceeding their requirements. Intestinal bacteria employ membrane-embedded transporters for the reduced ferrous form, including metal-regulated transporters and iron storage proteins to supply sufficient iron for survival, metabolism, and replication. Additionally, when iron is scarce, several bacteria produce and release siderophores, which are low molecular weight molecules with high binding affinity for the ferric form of iron19–22. Particularly, pathogenic bacteria rely on endogenous siderophore synthesis for survival and virulence, providing a competitive advantage when thriving in iron-deficient environments23. Some species unable to secrete these ferric siderophores can develop mechanisms to utilize non-self-produced siderophores (xenosiderophores) secreted by other gut microbiota members Like bacterial catechol-type siderophores, PACs show significant stability with Fe3+ metal ion interactions, as each ferric ion can bind to at least three catechol units in different PAC molecules (Fig. S1). Ferrous iron (Fe2+) can oxidize to ferric iron (Fe3+) upon binding to polyphenol catechol and gallate ligands17, as it can also oxidize with increased pH and redox potential in the colonic mucosa24.
Detailed analysis of the genome of the well-studied A. muciniphila strain MucT 25 suggested the presence of various genes coding for ferrous and ferric iron carriers in addition to various genes encoding lipocalins, well-studied proteins with recognition sites for binding catechol-Fe3+ complexes26. A. muciniphila itself does not produce catechol-type siderophores, as it lacks the genes encoding the essential enzymes, isochorismate synthase and isochorismatase, necessary for their synthesis27. The molecular repertoire of A. muciniphila, functionally redundant for ferric and ferrous iron uptake, led us to hypothesize that it might express versatile iron-acquisition mechanisms to support its growth in an iron-limited environment. The structural similarity of PACs’ catechol-Fe3+ complexes with those of heterologous bacterial catechol-Fe3+ (Fig. S1) makes the ferric-xenosiderophores transporter systems an intriguing mechanism for this species to access sources of non-self-produced iron chelators like polyphenols.
In the present study, we used integrative proteomics and transcriptomics to address the impact of polymeric catechol-rich PACs on the molecular regulation of diverse iron-acquisition systems in A. muciniphila strain MucT. We conducted controlled anaerobic fermentations in basal media enriched with catechol-rich PACs. This molecular study sheds light on the potential link between iron sources to the resilience and growth of A. muciniphila. It enhances our understanding of the A. muciniphila iron-uptake strategies and the eco-evolutionary dynamics that enable it to selectively thrive in a polyphenol-enriched media, providing support for a new mechanism by which A. muciniphila can antagonize pathogens.
Results
Catechol-rich PACs significantly shifted the proteome and transcriptome of A. muciniphila
We investigated the A. muciniphila iron acquisition and molecular fitness to dietary PACs by performing transcriptomics and proteomics during its growth on cranberry PACs, grape skin PACs, and Urolithin A. The latter is a microbiota-produced metabolite of ellagitannins and ellagic acid, which are also catechol-rich polyphenols. These compounds have been associated with an increased abundance of A. muciniphila in humans28,29. Fermentations were carried out in parallel bioreactors with controlled anaerobic conditions, stirring rate, and pH levels (see M&M section for details). We monitored carbon source consumption and organic acid production during anaerobic fermentations to determine the impact of polyphenols on A. muciniphila metabolism (Fig. S2). The presence of catechol-rich PACs did not impact the growth curve, carbon source utilization or metabolite production by A. muciniphila. In the presence of cranberry PACs, 97.6 ± 0.2% of the consumed carbon sources were mainly metabolized into propionate and acetate after 44 hours of fermentation, compared to the polyphenol-free medium normalized at 100%. The carbon recovery rate in grape skin PACs was 103.8 ± 08%, while Urolithin A showed a recovery rate of 97.0 ± 1.2%. The acetate-to-propionate ratio was 0.67 ± 0.02 and 0.72 ± 0.01 in cranberry and grape skin PACs, respectively, compared to 0.74 ± 0.03 in the control (Fig. S2 F).
Proteomic and transcriptomic changes in cells grown in basal media-enriched PACs were compared relative to the polyphenol-free medium (vehicle) at early (T12) and mid-logarithmic (T21) growth phases (Fig. S3). A total of 1612 proteins and 2210 unique transcripts were detected and annotated, representing approximately half and two-thirds of the A. muciniphila coding capacity, respectively. Based on the multidimensional scaling plots (MDS) analysis, the omics profile of A. muciniphila underwent significant changes when exposed to catechol-rich PACs relative to the polyphenol-free medium (Fig. S3A). On the contrary, little changes were observed when using Urolithin A, probably because of its less complex chemical structure as a phenolic metabolite. The analysis of differentially abundant proteins (DAPs) revealed that cranberry PACs had the most significant impact on A. muciniphila’s proteome at the early logarithmic phase. At T12, 43.9% of DAPs were observed, and this trend was also evident in the differentially expressed genes (DEGs) during the mid-logarithmic phase. The proportion of significantly regulated transcripts rose from 24.7% at T12 to 42.7% at T21 (Fig. S3C, Supplementary data 1 and 2). Regarding grape skin PACs, 53.6% of differentially expressed genes were observed relative to the polyphenol-free medium at T21. As anticipated, Urolithin A did not trigger the regulation of a high proportion of genes, resulting in only 2.6% and 2.5% of DEGs at T12 and T21, respectively.
Integrative proteomics and transcriptomics reveal the role of dietary PACs as xenosiderophores boosting molecular strategies for iron acquisition in A. muciniphila
Genes linked to iron homeostasis were identified as differentially upregulated upon catechol-rich PACs (Figure S3D–G, Supplementary data 3). To determine whether these genes were among the most variable features across a group of samples, we computed each feature’s variance from the proteomic and transcriptomic Log2 count matrices and conducted a hierarchical clustering analysis. Upon integrative analysis of proteomics and transcriptomics, increased regulation of features associated with iron ion and iron-siderophore transport systems was observed among the top 50 genes with the highest variance. Specifically, the expression of two gene clusters ranging from Amuc_1082 to Amuc_1096 and Amuc_1928 to Amuc_1936 were identified as induced in the early logarithmic phase when grown on cranberry PAC-enriched medium, which kept increasing after 21 hours of fermentation (Fig. 1A, B). These genes were also upregulated in A. muciniphila grown in grape skin PAC-enriched media during the mid-logarithmic phase (Supplementary source data 3). The region from Amuc_1082 to Amuc_1096 comprises metal-regulated transporters (Amuc_1082 to Amuc_1084, termed here Iron_cluster_1) and the ferrous iron transport (Feo) system (Amuc_1086 to Amuc_1090, termed here Iron_cluster_2), which are involved in the transport of the reduced ferrous form Fe2+ (Fig. S4). Furthermore, the region of Amuc_1928 to Amuc_1936 comprises the ABC-type siderophore transporter complex YclNOPQ (operon Amuc_1928 to Amuc_1931, termed here Iron_cluster_3) involved in the transport of the oxidized ferric form Fe3+ (Fig. S4). Conditions supplemented with polyphenols demonstrated a reduction in molecular function categories associated with RNA and DNA binding and processing. This suggests that PACs might affect the structural stability of nucleic acids, resulting in altered binding affinities with diverse proteins that are essential for RNA and DNA processing. Recent studies show that proanthocyanidins form strong non-covalent bonds with proteins and DNA, potentially affecting their structure and activity30. Furthermore, the presence of PACs appears to influence the assembly and stability of iron-sulfur (Fe-S) cluster enzymes (see Supplementary data 3).
Fig. 1. Hierarchical clustering analysis of the top 50 proteins and genes with the highest variance in A. muciniphila grown in polyphenol-rich media.
A Heatmap plots illustrate the expression of proteins and B genes across a group of samples of A. muciniphila grown in cranberry PACs (CE), grape skin PACs (GP), urolithin A (Uro) and the control (CT) to early (T12) and mid-logarithmic (T21) phases. Protein and gene counts were Log2 transformed and shown as normalized z-score. Sample names are indicated on the x-axis, and gene names on the right y-axis. Gene sets linked to iron-acquisition systems were identified to be highly variable and upregulated in the proteomics and transcriptomics of CE and GP samples. They are highlighted with a vertical red line on the right. The data are based on the variance calculated from the normalized and Log2-transformed counts of transcripts and proteins. The top 50 features with the highest variance were selected from the proteome (A) and transcriptome (B), and hierarchical clustering was employed to effectively group genes with similar variability patterns, facilitating the identification of those that hold biological significance in the context of iron metabolism. Source data are provided as a Source Data file.
Gene ontology functional classification of DAPs and DEGs uncovers key biological functions contributing to iron ion homeostasis in PAC-induced iron limitation
Iron limitation can lead to an increased iron influx in a cell, induction of iron storage proteins, low activity of non-essential enzymes, and the activation of oxidative stress and DNA repair responses. Here, we screened the activity of iron uptake-related genes potentially contributing to bacterial cell adaptation and survival in iron-limited microenvironments caused by polyphenols. To do so, proteome (DAPs) and transcriptome (DEGs) were ranked according to FDR-adjusted P-values and log2 fold-change (log2 FC) and further functionally annotated against gene ontology (GO) categories.
The early-exponential growth phase was found to be crucial to the adaptation of A. muciniphila to PACs’ iron-chelating actions. It was observed that biological processes and molecular functions involved in iron homeostasis and DNA repair were significantly upregulated in PAC-enriched media during this stage compared to polyphenol-free medium. This phenotype remained enriched even during the mid-logarithmic phase; it was also confirmed by the transcriptomic-based GO annotations (Fig. S5). Among the GO categories, there was an enrichment for iron-acquisition systems, including iron ion homeostasis (GO:0055072), iron ion transport (GO:0006826), siderophore-dependent iron import into cell (GO:0033214), and iron-sulfur cluster binding (GO:0051538). Other enriched molecular functions linked to other metal ion binding were identified. More importantly, the presence of PACs in the media led to the activation of functions related to xenobiotic transmembrane transporter activity (GO:0042910), xenobiotic detoxification by transmembrane export across the plasma membrane (GO:1990961), response to antibiotics (GO:0046677), and response to oxidative stress (GO:0006979). These findings align with the antimicrobial properties of PACs and their ability to interact with bacterial membranes and metals. A. muciniphila seems to have adapted molecular functions linked to antibiotic resistance by utilizing efflux pumps that eliminate unwanted compounds. Additionally, increased functions related to the SOS response were observed, likely prompted by the iron-chelating action of polyphenols and the resulting oxidative stress.
Most upregulated proteins and transcripts associated with iron acquisition mechanisms were reproduced when A. muciniphila was exposed to grape skin PACs (Fig. S5). Likewise, gene ontology analysis identified several protein and gene groups among the PAC and Urolithin A-treated cells that were enriched for processes responsible for efflux and xenobiotic transmembrane transporter activities, response to oxidative stress, and DNA damage stimulus. Nearly all conditions supplemented with polyphenols showed decreased molecular function categories related to RNA/DNA binding and processing.
Weighted gene correlation network analysis (WGCNA) unveils the module’s expression profile of iron-acquisition systems in PAC-treated A. muciniphila and highlights the transcription factor DtxR as an iron homeostasis regulator
We conducted co-expression network analysis by comparing RNA-Seq and proteome data from cells cultivated in polyphenol-enriched and polyphenol-free media during the early and mid-logarithmic growth phases. Then, co-expression networks were constructed based on pairwise correlations between genes responsive to iron restriction using their common expression trends across all samples. The WGCNA, along with the cluster dendrogram, produced distinct color-coded clusters, with each color representing modules of genes that show significant correlation (Fig. 2). Here, we identified eight distinct modules in transcriptomics and nine in proteomics (Fig. 2A and B, respectively). Three gene transcript modules (ME_grey, ME_turquoise and ME_black) were highly expressed in cells grown in PAC-enriched media (Fig. 2A). Likewise, protein modules (ME_green, ME_yellow, ME_red and ME_brown) were positively correlated with PAC-enriched media (Fig. 2B). Modules are clusters of highly interconnected genes or proteins with high correlation coefficients. The module eigengenes were each correlated with distinct treatments as sample-specific eigengene expression profiles. The eigengene is defined as the first principal component of a given module and represents the prevailing pattern of expression observed among a module of correlated genes31.
Fig. 2. Weighted correlation network analysis (WGCNA) of transcriptome and proteome of A. muciniphila grown in polyphenol-rich and unenriched media.
A The expressed genes were clustered in eight modules labeled by different colors, and B The abundant proteins were clustered in nine modules. Each dendrogram branch represents a module, while a leaf stands for one gene or protein. The heatmaps represent module-traits relationships. WGCNA’s Pearson correlation was used to quantify the strength and direction of association between gene modules and traits. The value of the correlation coefficient between the treatments and modules, as sample-specific eigengene expression profiles, is indicated by the color of each cell at the row-column intersection and the scale bar on the right. Source data are provided as a Source Data file.
We delved further into the modules correlated to PAC-specific responses and identified those clustering genes linked to iron homeostasis. We obtained eigengenes data of the corresponding module’s transcript/protein expression profile of A. muciniphila. Subsequently, we created heatmaps displaying the weighted data relative to variance-stabilized transformed TPM values (Transcripts Per Million of aligned reads). The ME_black and ME_brown modules, based on the A. muciniphila’s transcriptome and proteome, represented the primary gene expression modules that characterized the specific response of A. muciniphila to PAC’s iron-chelating action (Fig. 3A, B). The ME_black and ME_brown modules consist of 25 gene transcripts and 104 proteins, respectively, including those involved in transporting ferric iron-siderophore complex and ferrous iron. They also feature lipocalins, iron storage, metal translocating, and iron-sulfur cluster assembly proteins. The cranberry PAC-treated cells showed robust expression of these gene sets at T12, and although to a lesser extent, this phenotype remained enriched at T21 in PAC-treated cells compared to polyphenol-free grown cells.
Fig. 3. Expression patterns of transcriptome and proteome and co-expression gene network of modules significantly correlated with samples of A. muciniphila grown in PAC-enriched media.
A Heatmaps and module eigengenes (ME) generated from weighted correlation network analysis (WGCNA) of transcriptome and B proteome significantly positively correlated with PAC-treated samples at early (T12) and mid-logarithmic phase (T21). The heatmaps display the weighted relative variance-stabilizing transformed TPM values (Transcripts Per Million of aligned reads) and eigengene expression profile of the ME_black (A) and ME_brown (B) modules based on transcriptome and proteome of A. muciniphila grown in basal medium enriched either with cranberry PACs (CE), grape skin PACs (GP), urolithin A (Uro) or the vehicle (CT). At the bottom of the heatmaps are plotted the ME values (y-axis) and the sample type (x-axis), which are color-coded as treatment-time points. The ME scale on the right indicates the increased (red) and decreased (blue) expression of multiple module genes. ME_black and ME_brown modules are clustering gene sets linked to iron-acquisition systems that are highlighted by vertical lines on the right y-axis of the heatmaps. C The correlation and co-expression gene network of the ME_black module. Twenty-five genes with edge weight >0.1 are visualized using VisANT. Each node represents a responsive gene while connecting lines (edges) represent co-expression correlations. The thickness of connecting lines is proportional to the correlation weight. Fifteen genes were identified to be linked to iron homeostasis and were clustered into two subnetworks shown as grey and pink nodes. The grey cluster (Amuc_1928 to Amuc_1935) encodes ferric siderophore transporters, including the ABC transporter complex YclNOPQ, and the pink cluster encodes ferrous iron transporters (Amuc_1082 to Amuc_1091). The diamond shape represents a transcription factor identified within the co-expressed genes. Amuc_1082 is a transcription regulator (TR) encoding an iron-dependent repressor, DtxR family 1 HTH_DTXR. This TR shows high connectivity with several genes, including those belonging to the ferrous iron transporter’s cluster. Genes annotated as DNA repair protein RecN (Amuc_0877), hypothetical protein (Amuc_1900), and alanine--glyoxylate aminotransferase family protein (Amuc_0703) are highly correlated with the TR Amuc_1082. Source data are provided as a Source Data file.
A transcription gene expression network was created using the WGCNA outcome from an iron-limited response-specific module (Fig. 3C). The network highlights two clusters related to iron-acquisition systems: the Feo system and YclNOPQ operon (Iron_cluster_2 and Iron_cluster_3), colored in grey and pink, respectively. Notably, these two highly expressed clusters in PAC-treated samples display a strong correlation. The network underscores a transcription factor (TF) encoding an iron-dependent repressor DtxR (Diphtheria toxin repressor) family (Amuc_1082) as potentially playing a crucial role in leading this regulation. This DtxR homolog (Amuc_1082) has a predicted helix-turn-helix motif and shares considerable homology with the iron/manganese repressor family (PFam PF01325.25). The Amuc_1082 gene is located within the Iron Cluster 1 gene cluster (Fig. S4) and its expression is regulated by iron, showing a profile similar to that of Amuc_0486 (predicted to code for Iron Uptake Regulator, Fur), as seen in the transcriptome data (Fig. 5), which strongly suggests a role in iron regulation. However, further verification and analysis are required to ascertain the nature of the interactions.
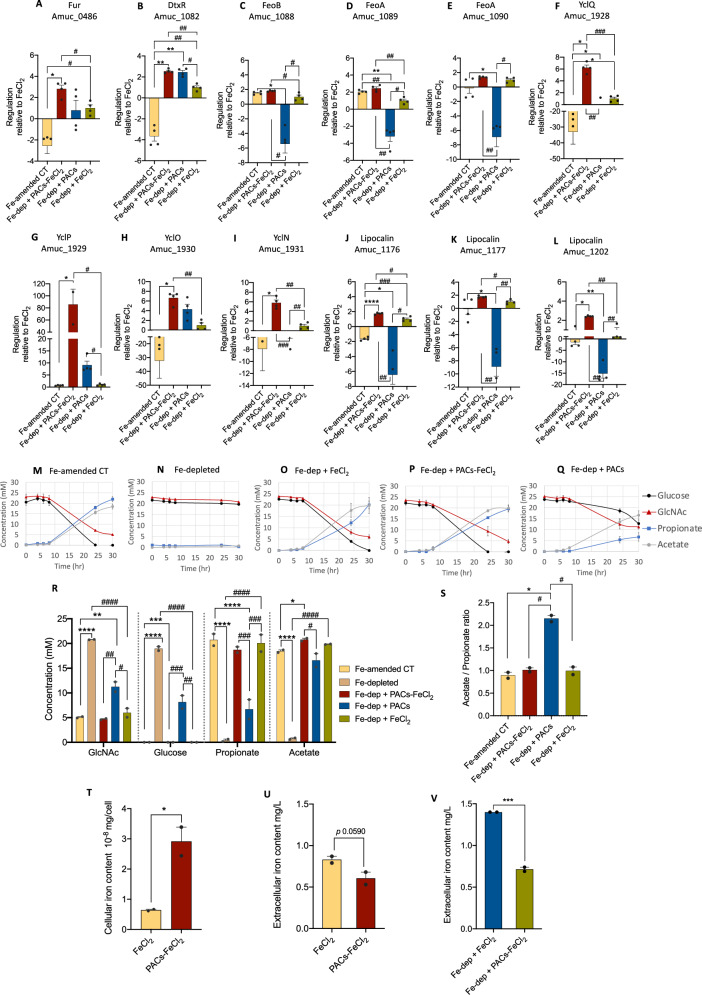
Fig. 5. Impact of iron-laden PACs on A. muciniphila’s metabolism and expression of xenosideophore and ferrous iron uptake systems.
A Cells were grown in iron-depleted media with 2,2’dipyridyl (Fe-dep) and iron-amended medium (CT) for 30 hr. After 8 hr, Fe-dep media were supplemented either with FeCl2 (Fe-dep + FeCl2), iron-laden PACs (Fe-dep + PACs-FeCl2), or iron-deficient PACs (Fe-dep + PACs). Analysis of the ferrous iron transport (Feo) system and the xenosiderophore uptake strategies, including the YclNOPQ operon and lipocalins, was performed by qRT-PCR after 24 hr of growth. The data were normalized relative to the reference gene RpoD. A) Iron-dependent repressor (DtxR); B Ferric uptake regulator (Fur); C–E Feo-related genes; F xenosiderophore substrate-binding protein, yclQ; G ATP-type xenosiderophore binding protein, yclP; H ABC-type transporter permease protein,yclO; I ABC-type transporter permease protein yclN; J–L bacterial lipocalins; M–Q Curves of carbon sources uptake (Glucose and GlcNAc) and short-chain fatty acid (SCFA) production (propionate and acetate); R Carbon source and SCFA concentrations after 30 hr of fermentation; S Propionate/ Acetate ratio; T Iron levels in cells grown in media supplemented with iron (FeCl2) and iron-laden PACs (PACs-FeCl2) after 30 hr; U Extracellular iron levels in media supplemented with iron (FeCl2) and iron-laden PACs (PACs-FeCl2) after 30 hr; V Extracellular iron levels in Dip-induced iron-depleted media supplemented with iron (Fe-dep + FeCl2) and iron-laden PACs (Fe-dep + PACs-FeCl2) after 30 hr. Data represent two independent experiments performed in duplicate. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001 compared to CT control and #, P < 0.05; ##, P < 0.01; ###, P < 0.001; ####, P < 0.0001 compared to Fe-dep + FeCl2 by two-tailed paired T-test. Inductively Coupled Plasma Mass Spectrometry (ICP-MS) was used to quantify both extra- and cellular iron levels; the bar graphs represent the mean ± standard error of the mean (SEM), with statistical significance indicated as *P < 0.05; ***P < 0.001. Source data are provided as a Source Data file.
In-silico regulatory motifs identification of correlated iron homeostasis-related genes reveals the Iron Uptake Regulator Fur, response regulator OmpR, and sigma factors as potential candidate TFs increased in PAC-treated A. muciniphila
To determine whether the gene sets that were functionally correlated and co-expressed were also co-regulated, we further searched in-silico for regulatory motifs in the promoter regions of iron homeostasis-related genes (IHRGs) (Supplementary note). Top-five candidate motifs from promoter regions of functional co-expressed genes significantly matched known prokaryotic motifs leading regulatory TFs of metal iron and zinc homeostasis (Fig. S6, Table S 1, 2). Specifically, the motifs M_IHRG2 and M_IHRG4 concurrently matched with 11 known binding motifs for the ferric uptake regulator, Fur, from different species, indicating they may potentially serve as binding sites for the Fur TF in A. muciniphila (encoded by Amuc_0486). This TF is involved in iron homeostasis, employing iron as a cofactor to bind the operator of the iron transport operon32. Likewise, the predicted motif M_IHRG3 was closely related to extra-cytoplasmic function (ECF) Sigma factor PvdS, which regulates cellular response to iron ions. Furthermore, two of the identified matches for the motifs M_IHRG2 and M_IHRG5 were linked to zinc homeostasis (Zn2+), revealing as potential candidate TFs, the zinc uptake regulation protein Zur, also part of the Fur TF family, and the RNA polymerase sigma-54 factor RpoN (Amuc_0021). We evaluated the expression of these Iron-responsive TFs in A. muciniphila cells grown in PAC-enriched and unenriched media (Fig. S7A). At the early logarithmic phase, the expression of the Fur gene (Amuc_0486) was increased in PAC-treated cells. At the same time, the expression of the putative DxtR gene (Amuc_1082) was enriched in cells exposed to cranberry PACs at both early and mid-logarithmic phases (Fig. S7B). Strikingly, a remarkable pattern of upregulated sigma factors belonging to the RpoN and ECF families (Sigma-70, σ70) was also observed in the cells grown in cranberry and grape skin PACs compared to the polyphenol-free control (Fig. S7B). This outcome coincides with the significant role the σ70 factors play in coordinating the activation of genes responsible for stress responses, morphological development, and ancillary metabolism, including iron uptake33. Likewise, OmpR regulators appear to be involved in A. muciniphila cells’ response to environmental stimuli caused by the PACs in the medium (Fig. S7B). OmpRs are response regulators of two-component signal transduction systems (TCSs), which coordinate the transcription of porins and membrane-associated transporters involved in the efflux of molecules across the outer membrane34. Employing methods such as deleting relevant TFs, analyzing chromatin immunoprecipitation (ChIP) targets, and using ChIP coupled to protein-DNA interactions or next-generation sequencing analyses (ChIP-seq)32 might provide more insights into how transcriptional activity of iron acquisition systems is regulated in A. muciniphila.
Integrative enrichment analysis of the transcriptome and proteome of iron acquisition systems reveals significant upregulation of the YclNOPQ operon, Feo system, and iron storage proteins in PAC-treated cells
Gene ontology and variability analysis of proteomics and transcriptomics unveiled that regulating the xenosiderophore transporter complex encoded by the yclNOPQ operon plays a crucial role in A. muciniphila’s molecular setting when exposed to catechol-rich PACs. Specifically, this operon includes a periplasmic substrate-binding protein (YclQ, Amuc_1928), ATP-binding protein (YclP, Amuc_1929), and permease proteins (YclO - Amuc_1930 and YclN - Amuc_1931). We then defined targeted gene sets linked to iron ion and siderophore uptake to rank and calculate their enrichment scores across all individual samples (Table S3). By conducting an enrichment analysis (GSEA) of differentially expressed proteins and transcripts, it was observed that the features encoding the transport systems for catechol-type xenosiderophores, as well as bacterial lipocalins, were upregulated upon exposure of A. muciniphila to PACs as indicated by positive enrichment scores (Fig. 4). The upregulation of the yclNOPQ operon is evidently more pronounced early than at the mid-logarithmic phase upon exposure to cranberry PACs. Still, grape skin PACs also induced these ferric-catecholate uptake systems during the mid-logarithmic growth phase. Additionally, ferrous iron uptake systems, specifically the Feo system, were upregulated in cranberry and grape skin PAC-grown cells. The transcription of iron storage proteins, representing another strategy of bacteria to buffer the low extracellular concentration of iron, was also upregulated. We observed that the expression of rubrerythrin family proteins (Amuc_2056, Amuc_2057, Amuc_2072), flavodoxin (Amuc_1899), and ferredoxin (Amuc_1922) was increased when A. muciniphila was cultured in polyphenol-rich media.
Fig. 4. Gene Set Enrichment Analysis (GSEA) of transcriptomics and proteomics linked to iron-acquisition systems and catechol-type siderophores transport in A. muciniphila grown in polyphenol-rich media.
The genes assigned to different iron-acquisition systems were submitted to GSEA and were ranked by measuring their significant differential expression between the groups of samples. The GSEA enrichment score indicates how much a feature in a specific gene set or operon is up- or down-regulated together in a sample. The dot plots display the enrichment scores of A transcripts and B proteins categorized in four color-coded sets on the x-axis and organized by groups on the y-axis. Dot color represents the degree of differential enrichment (average expression) of the normalized enrichment score (NES). The NES score was computed through a systematic assessment of marker sets intricately involved in iron acquisition systems based on a ranked list generated from differential expression analysis of normalized transcript and protein data. If the NES value is high, it means that the genes in the set were ranked higher in the transcriptome and proteome data. Conversely, if the NES value is low, it means that the genes in the set tend to be ranked lower. Enrichment analysis was performed by Hypergeometric testing with Benjamini-Hochberg procedure for false discovery rate (FDR) correction. Source data are provided as a Source Data file.
A. muciniphila uptakes iron-laden PACs as xenosiderophores and enhances growth and molecular strategies for iron acquisition in iron-deficient conditions
To validate the molecular response of A. muciniphila under iron-starved conditions, we next conducted controlled growing fermentations by introducing to media 10 µM of 2,2’ dipyridyl (Dip), a metal-chelating agent that binds free iron. We evaluated the significance of A. muciniphila’s iron acquisition mechanisms with and without PACs. We used qRT-PCR to assess the expression of the Feo system, xenosiderophore operon, lipocalins, and the TFs identified to be involved in iron uptake regulation, Fur and DtxR. Our study showed that Dip-induced iron-depleted medium limited A. muciniphila’s survival. Yet, the addition of 7.5 µM of FeCl2 rescued its growth, indicating the essential role of iron in this process (Fig. 5). After 24 hours of incubation, the expression levels of the Feo system genes (Amuc_1088, Amuc_1089 and Amuc_1090) were significantly increased, which is consistent with its essential function in capturing iron to supply bacterial needs and support growth (Fig. 5C–E).
We investigated A. muciniphila’s ability to access iron when bound to polyphenols by introducing a solution containing iron-laden PACs (catecholates) to the iron-depleted medium after eight hours of incubation. Bacteria can utilize iron bound to catecholate through enzymatic degradation of catechol-rich PACs or by utilizing xenosiderophore uptake systems35. We focused on the latter mechanism by determining the expression of the siderophore transporter operon yclNOPQ and lipocalin genes, both of which recognize catechol-Fe3+ complexes. Our study showed that the growth of A. muciniphila was restored by adding iron-laden PACs, similar to that of the iron-amended medium, with comparable levels of acetate and propionate production (Fig. 5S). In line with the omics data, the expression of the yclNOPQ operon (Amuc_1928 to Amuc_1931) was significantly increased in cells grown in the medium supplemented with iron-laden PACs compared to the iron-supplemented media (Fig. 5F–I). The xenosiderophore substrate-binding protein gene, yclQ, exhibited a 6.22 ± 0.37-fold increase in iron-laden PAC-treated cells compared to FeCl2 (P < 0.001). In contrast, it showed a stunning 33.03 ± 7.55-fold decrease in cells grown in the iron-amended medium. The gene for the ATP-type xenosiderophore binding protein, yclP, demonstrated a very substantial upregulation of 85.6 ± 25.4-fold in iron-laden PAC-treated cells relative to FeCl2-treated cells. Similarly, the genes for xenosiderophore permeases, yclO and yclN, showed significant upregulation by 6.6 ± 0.62 and 5.8-fold ± 0.52, respectively. Furthermore, the expression of the genes for lipocalins encoded by Amuc_1176, Amuc_1177, and Amuc_1202 significantly increased by 1.7 ± 0.06, 1.7 ± 0.08, and 2.4 ± 0.04-fold, respectively, compared to the control. All these transcriptional outcomes are consistent with the increased upregulation of the two putative iron regulators, Fur (Amuc_0486) and DtxR (Amuc_1082), as both might act as sensors of cytosolic iron concentrations and function by binding to their target promoters as a complex with ferrous iron (Fig. 5A, B). After 24 hours of administering iron-laden PACs, the Amuc_1082 gene exhibited a 2.6 ± 0.1-fold increase (P < 0.01), while the Amuc_0486 gene demonstrated a 2.8 ± 0.36-fold increase (P < 0.05) compared to FeCl2-treated cells. Interestingly, both regulators were significantly downregulated in cells grown in the regular iron-amended medium by 3.7 ± 0.47-fold and 2.6 ± 0.71-fold for the putative DtxR and Fur genes, respectively. We further measured the cellular and extracellular iron content by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) after submitting A. muciniphila to iron-laden PACs in either basal media or iron-depleted media for 30 hours of growth. The outcome demonstrates that exposure of A. muciniphila to PACs led to significant changes in cellular iron levels compared to the PAC-free control (Fe-dep + FeCl2) (Fig. 5, TV). Specifically, the intracellular iron content in PAC-treated cells was 4.6 times higher (Fe-dep + FeCl2-PACs: 2.92e-08 ± 0.475 mg/cell) than in the PAC-free treatment (Fe-dep + FeCl2: 6.39e-09 ± 0.029 mg/cell). Likewise, cells grown in iron-laden PACs supplemented medium (Fe-dep + FeCl2-PACs) showed a decrease in extracellular iron levels after 30 hours of growth compared to cells in media supplemented solely with iron (Fe-dep+ FeCl2) (Fig. 5V). This is consistent with the increased intracellular iron content observed in cells grown with Fe-dep + FeCl2-PACs. These significant findings highlight the crucial role of the iron regulators in activating the xenosiderophore uptake systems in A. muciniphila when facing iron starvation and provide further evidence that A. muciniphila can cope with iron deficiency by recognizing iron-catechol-rich PAC complexes as xenosiderophores.
Discussion
Our research has uncovered the versatility of A. muciniphila MucT in thriving in low-iron PAC-enriched environments. We demonstrated that this gut symbiont employs distinct mechanisms to acquire ferrous and ferric iron ions, which enables it to remain resilient and metabolically active in the presence of dietary PACs. Our study emphasizes the significance of A. muciniphila’s ability to acquire iron bound to PACs’ catechol moieties because of their structural homology to bacterial catechol-type xenosiderophores (Fig. S1). The A. muciniphila yclNOPQ operon is predicted to encode the transport of bacterial ferric-catecholate siderophores. However, here, we demonstrate that its expression is upregulated when iron-loaded PACs are provided. We propose that xenosiderophore transporters encoded by yclNOPQ enable A. muciniphila to utilize catechol-type xenosiderophores produced by other members of the gut microbiota. This mechanism may lead to reduced iron availability in the gut, allowing A. muciniphila to compete with siderophore-dependent opportunistic pathogens and gain an advantageous position for persistence in the colon. Engineered xenosiderophores and probiotics with effective iron uptake systems have been used for similar purposes19,20,35. We hypothesize that polymeric PACs are transported via the same mechanism due to their abundance of catechol moieties, which allow them to chelate iron. This mechanism is crucial for bacteria to colonize the colon and adapt to fluctuating iron environmental conditions, as demonstrated in murine models36,37. This function has been observed in Escherichia coli strains, which possess genetic machinery for siderophore-mediated iron acquisition. Siderophore uptake systems enable these strains to outcompete mutants that lack siderophore receptors, allowing colonization and persistence in the intestinal tract37.
We identified the xenosiderophore-transporter encoded by the yclNOPQ operon among the most highly variable features in both the proteome and transcriptome of A. muciniphila grown in basal media enriched with PACs but not with the phenolic metabolite Urolithin A. Urolithin A did not have a significant effect on the response of A. muciniphila to iron-restricting conditions. Only a tiny percentage of the transcriptome (2.62% and 2.53% at early and mid-logarithmic phases, respectively) showed differential expression compared to cells grown in a polyphenol-free medium. The features modulated in Urolithin A-treated cells were primarily associated with xenobiotic transmembrane transport activity and antibiotic resistance, which aligns with the antimicrobial properties of Urolithin A against harmful and, in this case, also symbiotic microorganisms38,39. Despite its simpler chemical structure than polymeric PACs, Urolithin A still features a 1-benzopyran moiety with a ketone group on the C ring. This structural characteristic may enable it to bind metals and interact with cell membranes (Fig. S1). However, catechol-containing PACs have a higher affinity for Fe3+ due to the strong electron density generated by the two oxygen atoms40. Here, we demonstrated that A. muciniphila uses PACs as iron-chelating xenosiderophores, but these compounds may be expelled from the cells after iron is reduced. Although we did not assess the degradation of PACs in the current study, prior research has shown that A. muciniphila MucT 4,41,42 does not have a direct role in PACs metabolism, contrary to suggestions for other phenolic categories like ellagitannins and quercetin28. Indeed, apart from the siderophore-dependent iron import, xenobiotic detoxification and transmembrane transporter activities stood out as significant functions enriched in PAC-treated cells. Furthermore, mechanisms linked to other metal ion uptake, including zinc, copper, cobalt, magnesium, and manganese, were also activated in A. muciniphila grown in PAC-enriched media. This is congruent with the fact that PACs also function as metallophores since they can bind copper, manganese, and zinc43–45. The latter was backed by elevated cellular zinc levels in cells cultivated in PAC-supplemented media, corresponding with a notable decrease in zinc levels in the growth media (Fig. S9). Intriguingly, A. muciniphila supplementation has also been demonstrated to shield against the detrimental effects of heavy metals by repairing colon mucosal damage and reducing ROS production in mice46.
Apart from employing ferric-siderophore uptake mechanisms, bacteria utilize various means to obtain Fe2+ and Fe3+ in a free form. Notably, the Feo system, broadly distributed in Gram-negative species, facilitates the acquisition of ferrous iron, which, unlike ferric iron, is often found in a free form that bacteria can readily access47. Alternatively, metalloproteins and iron-storage proteins like bacterioferritin can effectively incorporate intracellular free iron and ensure it is available for different cellular processes1,48. Importantly, the clustered genes encoding for FeoB (Amuc_1088), FeoA (Amuc_1089), and FeoA-like protein (Amuc_1090), and iron storage proteins were found to be significantly upregulated in PAC-treated cells. Our findings align with a previous study demonstrating the ability of A. muciniphila to thrive in human milk, which is a highly iron-restricted environment due to the presence of lactoferrin49. Human breast milk contains high concentrations of lactoferrin (3–7 g/L), a glycoprotein with iron-chelating capabilities50,51. This enables it to effectively bind ferrous, ferric, and siderophore-bound iron and restrict their availability for bacterial growth, thereby exerting a bacteriostatic function. Thus, the low iron level in human milk (0.4 mg/L) is further made less accessible to microbes due to lactoferrin52. In this context, the Feo system and the iron-storage flavodoxin protein (Amuc_1899, 17-fold) were also significantly upregulated in A. muciniphila MucT during growth on human milk oligosaccharides (HMO) compared to mucus49. These also corroborate recent work demonstrating the upregulation of iron uptake-related genes in human-associated Akkermansia strains, including A. muciniphila MucT and A. biwaensis CSUN-19 grown in basal media enriched with the HMO 2′-fucosyllactose53. In A. muciniphila MucT, genes related to siderophore uptake, such as the substrate-binding protein (YclQ, Amuc_1928) and ABC-binding transporter (YclP, Amuc_1929), as well as rubrerythrin (Amuc_2072), were found to be upregulated when grown in 2′-fucosyllactose53. In our study, we could further identify key transcription factors, Fur and DtxR, as potentially coordinating the expression of the genes responsible for the Feo system and ferric-siderophore ABC-transporter complex (encoded by part of the yclNOPQ operon) that were functionally co-expressed in PAC-treated cells. Both TFs work as regulatory repressors and activators of proteins responsible for synthesizing and transporting siderophores54. For instance, the DtxR TF has been characterized as a master regulator of the expression of more than 50 genes involved in iron metabolism in other bacteria55 and might govern the iron starvation response in A. muciniphila. Yet, further experimental research is necessary to validate whether these and the other inferred TFs might participate in the direct regulation of iron homeostasis-related genes32.
We replicated an increase in the expression of the xenosiderophore transport operon and the putative Fur/DtxR TFs when introducing iron-laden PACs into iron-deficient media supplemented with 2,2’dipyridyl. This resulted in a boost in the growth and metabolism of A. muciniphila, similar to what was observed in iron-amended media. An increased cellular iron content was consistent with the significant upregulation of the xenosiderophore transport yclNOPQ operon (Fig. 5). Based on these findings, we propose that ferric-PAC catecholates are taken up as xenosiderophores by A. muciniphila and that the Fur/DtxR TFs may modulate the expression of iron-acquisition systems (Fig. 6). The significant increase of lipocalins in PAC-treated cells also strengthens this notion. Lipocalins are small extracellular proteins that can bind to iron-laden catecholate-siderophores through simple ionic and cation-π interactions26. They are known to be expressed in response to environmental stress and play a critical role in capturing and neutralizing antimicrobials in the bacterial environment and regulating cellular homeostasis56. It is noteworthy that human cells employ comparable mechanisms to control the proliferation of bacterial pathogens by releasing analogous lipocalin proteins, namely siderocalin (Scn) or lipocalin 2 (Lcn2). Human cells specifically bind various siderophores from the catechol-type family and effectively halt the spread of pathogens that rely on corresponding catecholates for iron uptake57. In the bacterial context, the selective transporter mechanisms of ferric-catecholate allow A. muciniphila to utilize iron and could serve as a defense strategy against PACs as antimicrobial agents by sequestering them.
Fig. 6. Proposed model of iron acquiring systems in A. muciniphila grown in PAC-enriched medium.
Iron acquisition involves the uptake of ferrous iron via the Feo (ferrous iron transport) system or ferric iron bound to siderophores and lipocalins. The expression of proteins involved in xenosiderophore and iron uptake is controlled by the concentration of intracellular Fe2+. This is transcriptionally regulated by the ferric uptake regulator (Fur) protein and the diphtheria toxin repressor (DtxR). The Fur (Amuc_0486) and DtxR (Amuc_1082) have similar physiological roles as iron homeostasis regulators via their DNA-binding domain’s way of functioning. When iron concentrations are high, iron-loaded Fur/DtxR binds to DNA and represses transcription of iron uptake systems (OFF). When iron is scarce, unloaded Fur/DtxR does not bind to DNA, and gene expression is activated (ON). When A. muciniphila experiences PAC-induced iron limitation, it increases (ON) the xenosiderophore uptake operon and lipocalins to sequester more iron. Iron is internalized by binding to specific outer membrane receptors (OMR, Amuc_0336) and transporters. Iron-laden PACs (catecholates) are recognized as xenosiderophores and transported into the periplasm. Once in the periplasm, a periplasmic substrate-binding protein (PBP, Amuc_1928) captures the catecholate, and with the help of ATP-binding cassette (ABC) transporter (Amuc_1929) and permeases (Amuc_1930 and Amuc_1931), this is delivered to the cytoplasm to satisfy iron requirements. Here, iron is liberated from the Fe3+-PAC complex through reduction to Fe2+ by the action of ferric reductases, and the PACs are expelled from the cells via efflux pumps. They could be recycled to capture more iron. Lipocalins (Blc, Amuc_1177 and Amuc_1202) are expressed as a response to starvation and stress, and they also sense ferric-laden PACs. Catecholate-type siderophores are highly hydrophobic, facilitating lipocalins exposed on the outer membrane to sequester ferric-PAC complexes through hydrophobic interactions. Furthermore, ferrous iron diffuses freely through porins into the periplasm, where FeoB takes up iron. The expression of FeoB (Amuc_1088) and FeoA (Amuc_1089 and Amuc_1090) is also potentially coordinated by the Fur/DtxR iron-dependent regulators. This graph was created through BioRender.
In our investigation, we examined the impact of PACs on the growth and metabolism of A. muciniphila under iron-depleted conditions with the goal of determining if the background of the PACs extract could activate the xenosiderophore-acquiring system and detect any iron traces in the solution. Our results revealed a noteworthy decrease in the expression of the Feo system, lipocalins, and the xenosiderophore operon compared to the iron-laden PAC-supplemented medium. While the growth of A. muciniphila showed a slight enhancement, as indicated by increased acetate production, propionate was negatively affected, with a higher acetate-to-propionate ratio of 2.2 ± 0.07 compared to 1.0 ± 0.09 in iron-amended conditions. This suggests that the iron level in the PACs extract was insufficient to fully support the metabolism of A. muciniphila. Specifically, it was inadequate to sustain the essential role of Fe-S-dependent enzymes that facilitate pyruvate metabolism to acetyl-CoA and further succinate and propionate (Figure S9). The Fe-S clusters are essential cofactors in various redox enzymes and reactions, including the TCA cycle pathway58,59. Many gut bacteria produce acetate and propionate using the regular glycolytic pathway, which involves pyruvate metabolism; hence, low iron concentrations can disturb the generation of these metabolites. Our findings are consistent with previous studies on animal models and in vitro colon fermentations that mimicked iron deficiency59,60. These studies demonstrated reduced metabolic activity, resulting in lower energy production and a slowdown of bacterial growth and propionate levels via the methylmalonyl CoA pathway.
This study expanded our understanding of how A. muciniphila MucT modifies its molecular iron-acquiring strategies in response to dietary catechol-rich PACs acting as xenosiderophores. Our findings emphasize the roles that the siderophore uptake complex and lipocalins might play in iron-depletion adaptation in A. muciniphila when facing polyphenol-rich environments. Interestingly, after studying the distribution of the most relevant genes involved in iron acquisition systems in other Akkermansia strains, we found that genes attributed to Iron Cluster 3 (ABC transporter complex YcINOPQ; Amuc_1926 to Amuc_1934) seem exclusive to A. muciniphila strains. In contrast, genes linked to Iron Clusters 1 (transmembrane ion movement; Amuc_1082 to Amuc_1084) and 2 (Feo system; iron ion binding and Fe2+ transport; Amuc_1088 to Amuc_1090) are conserved across different strains of A. muciniphila and A. massiliensis. This suggests that xenosiderophore acquisition mechanisms (complex YcINOPQ) of A. muciniphila MucT might be crucial for its adaptation to changing iron levels, which has been shown to be vital for the protective qualities of probiotic species. Previous research has shown that in a mouse model of Salmonella-induced colitis, probiotic E. coli Nissle strains possessing strong iron acquisition mechanisms effectively resisted pathogen infection, while mutants without these systems failed to limit pathogen dissemination61. Bifidobacteria are also shown to antagonize enteropathogenic infection by sequestering iron by means of ferrous and specific ferric iron operons, thus inhibiting pathogen adhesion to intestinal epithelial cells in vitro19,20. Similar suggestions have been made regarding the benefits of A. muciniphila supplementation in counteracting colorectal tumorigenesis induced by dietary iron overload7. However, in some human association studies, the relative abundance of Akkermansia spp. was found to be positively correlated with some disease phenotypes, notably neurological disorders62. Unfortunately, no causality studies have been reported, and some of the initial associations could be attributed to confounding factors6.
PACs might serve as promising dietary therapeutics by reducing iron availability and selectively depleting siderophore-dependent pathogens without precluding the benefits of A. muciniphila in the host35. This symbiont can adapt to the antimicrobial properties of PACs and utilize them as iron carriers, enabling it to thrive in conditions of iron limitation. This capability might also help it to grow when plant catecholate siderophores are employed as nutritional iron chelators in health conditions associated with iron imbalances. Nevertheless, the findings of this study must be considered in light of certain limitations that could be addressed in future research. Firstly, the used cranberry polyphenolic extract did not only contain PACs, as the remaining 15% consisted of flavonols, phenolic acids, and anthocyanins63. Secondly, it is crucial to comprehend the intricate role of the microbiome in regulating iron levels and its impacts on the host. Investigating how A. muciniphila’s iron ion and xenosiderophore uptake systems relate to the host immune system and microbiome modulation could lead to new treatment avenues for diseases related to iron imbalance. Knockout studies on essential genes related to iron metabolism in A. muciniphila will offer insights into the extent of the contribution of iron acquisition systems and their associated benefits for the host. A. muciniphila is a promising next-generation beneficial microbe6 with intricate iron sequestering systems that may promote its competitive edge, reducing gut pathogens. Dietary polyphenols will enhance this competitive potential and, in that way, promote the intestinal levels of A. muciniphila. Hence, developing dietary products with effective catechol-type xenosiderophores could be an attractive option for therapeutic applications seeking to improve gut health via A. muciniphila-mediated iron acquisition.
Methods
Growth conditions
The strain A. muciniphila MucT (ATCC BAA-835) was pre-cultured anaerobically at 37 °C using a basal medium64. The basal medium was composed (per liter of deionized water) of 0.4 g KH2PO4, 0.669 g Na2HPO4*2H2O, 0.3 g NH4Cl, 0.3 g NaCl, 0.1 g MgCl2*6H2O, 1 mM L-threonine, 0·5 mg resazurin, 4 g NaHCO3, 0·25 g Na2S.7–9H2O, 1 ml acid trace mineral solution, and 1 ml alkaline trace element solution. The acid mineral solution contained 50 mM HCl, 1 mM H3BO3, 0.5 mM MnCl2*4H2O, 7.5 mM FeCl2*4H2O, 0.5 mM CoCl2, 0.1 mM NiCl2, 0.5 mM ZnCl2, and 0.1 mM CuCl2*2H2O. The alkaline trace element solution contained 10 mM NaOH, 0.1 mM Na2SeO3, 0.1 mM Na2WO4, and 0.1 mM Na2MoO4. The basal medium was autoclaved and supplemented with 1% (v/v) of filter-sterilized vitamin solution (11 g L−1 CaCl2, 20 mg L−1 biotin, 200 mg L−1 nicotinamide, 100 mg L−1 p-aminobenzoic acid, 200 mg L−1 thiamine, 100 mg L−1 pantothenic acid, 500 mg L−1 pyridoxamine, 100 mg L-1 vitamin B12, and 100 mg L−1 riboflavin), and 5% (v/v) of reducing solution (80 g L−1 NaHCO3, 20 mL L−1 Na2S*9H2O solution (240 g/L) and 10 g L−1 cysteine-HCl). Furthermore, N-acetylglucosamine (GlcNAc) and glucose were added as carbon sources in equimolar amounts to 25 mM (Sigma-Aldrich). Incubations were performed in serum bottles sealed with butyl-rubber stoppers provided by a gas phase of 182 kPa (1·8 atm) N2/CO2 (80: 20, v/v).
Anaerobic fermentations
To conduct the fermentation experiments, vessels were set at 800 mL of the basal medium mentioned earlier. The media were then supplemented with a filter-sterilized aqueous solution of either cranberry (Vaccinium macrocarpon Aiton) proanthocyanidins (PACs) extract (75% of PACs as characterized previously63) (CE), grape skin (Vitis vinifera) PACs (composed of 70% PACs) (GP), or the vehicle (1:5 dimethyl sulfoxide (DMSO): deionized water) (CT). All the PAC extracts were added to the media at 1% (v/v) and a final concentration of 600 mg L−1. Furthermore, we evaluated Urolithin A (Sigma-Aldrich) (Uro), a gut microbial metabolite of ellagitannins, which are also catechol-rich polyphenols. We added this metabolite to the media to achieve a final concentration of 6.85 mg L−1 (30 µM equivalent). Symrise Canada (Champlain, Québec) provided the hydroethanolic cranberry extract, while Silvateam provided the grape skin extract, Tan’Activ U (Italy, www.silvateam.com). The basal media used in this study, either polyphenol-enriched or un-enriched, had a final concentration of 7.5 µM of ferrous chloride as a source of iron.
Three bioreactors (DasGip, Eppendorf, Germany) were used to conduct parallel fermentations. Each experiment consisted of a negative control (bacteria-free polyphenol-enriched media), a positive control (vehicle) and the target (polyphenol-enriched media). A continuous stream of N2/CO2 mixed gas with a ratio of 80% to 20% was utilized to maintain anaerobic conditions. The pH level was set at 6.8, the temperature at 37 °C, and the stirring rate was maintained at 100 rpm. Each bioreactor was inoculated with 1% (v/v) of pre-grown cultures of A. muciniphila. The fermentations lasted for 48 hours, during which samples were taken at different time points to measure bacterial growth and perform HPLC analysis, RNA sequencing, and proteomics. Before being analyzed, these samples were stored appropriately at −20 °C (HPLC) or −80 °C (transcriptomics and proteomics). Fermentation processes and the analyses were run in triplicates.
High-performance liquid chromatography for short-chain fatty acids analysis
During incubation, samples were collected at various intervals to analyze the fermentation products. Samples were prepared by centrifuging 1 mL of the bacterial culture at 12,000 × g for 10 min. The supernatants were mixed with crotonate (30 mM) and used as an internal standard. Standard curves of short-chain fatty acids (SCFAs), including lactate, formate, acetate, propionate, butyrate, and succinate, along with the monosaccharides GlcNAc, and glucose, were prepared using three different concentrations (10, 20 and 30 mM). Substrate conversion and product formation were measured using a Shimadzu LC_2030C plus equipped with a refractive index detector and a Shodex SH1011 column. The samples were run twice; first, the column was maintained at 45 °C with a pump flow of 1.0 mL/min, and second, the column was kept at 30 °C with a pump flow of 0.9 mL/min. In both cases, the eluent used was 0.01 N H2SO4.
Protein extraction, sample preparation and nLC-MS/MS analysis
Akkermansia muciniphila MucT was grown in basal medium supplemented with either cranberry PACs (600 mg L−1), grape skin PACs (600 mg L−1), the microbial phenolic metabolite Urolithin A (6.85 mg L−1) or the vehicle. After 12 and 21 h incubation, that is, early and mid-logarithmic growth phase, 4 mL of cells were pelleted at 10.000 × g for 1 min at 4 °C and re-suspended in Protein LoBind® Tubes containing 200 µl ice-cold 100 mM Tris buffer pH 8.0. Pellets were washed twice in 200 µl ice-cold 100 mM Tris buffer pH 8.0, and centrifuged repeatedly for 1 min at 10.000 x g until the supernatant was clear. Subsequently, cells were dissolved in 100 μl in low-binding Eppendorf tubes and lysed by sonication. For this, a Qsonic sonicator equipped with an MS-72 probe was used. The suspensions were submitted to four rows of 45 seconds with an amplitude of 40% 15 s ON, 15 s OFF, placing the samples on ice between rows. The protein concentration of cell extracts was measured using the Pierce™ BCA Protein Assay Kit.
To prepare the cell extracts for nLC-MS/MS analysis, they were diluted to a concentration of 1 μg/μl protein using 100 mM Tris pH 8. A sample volume of 60 μl was taken, which underwent a series of steps, including reduction, unfolding, alkylation, and trypsin digestion as described in Supplementary methods. Peptide samples were measured by nLC–MS/MS with a nLC1000 and Orbitrap Exploris 480 mass spectrometer65.
Proteome data analysis
Data pre-processing was performed using MaxQuant66 and Perseus software version 2.0.7.067. The peptides count dataset and the differentially abundant proteins (DAPs) of A. muciniphila grown in PAC-enriched media relative to the polyphenol-free medium for each time point (T12 and T21) were assessed in R (see supplementary methods). A < 5% FDR threshold (q-value < 0.05) was used to determine statistical significance of normalized Log2FC-transformed data. Samples were analyzed in triplicates.
RNA isolation and sequencing
Samples of 10 mL were taken at the early and mid-exponential growth phase, after 12 and 21 h of fermentation from A. muciniphila grown in basal medium supplemented with cranberry PACs, grape skin PACs, Urolithin A and CT. Cells were pelleted at 5000 × g for 30 min at 4 °C and stored at −80 °C. Samples were thawed on ice, and total RNA was extracted using the TRIZOLTM method combined with further RNA purification using the RNeasy Mini Kit (Qiagen, #74104), as described previously68. During the RNA purification, samples were treated with an on-column DNase digestion using the RNase-free DNase I recombinant enzyme (Roche Diagnostics, Germany). The RNA samples were assessed for concentration and quality using Qubit RNA BR assay (Invitrogen) and Bioptic Qsep100 (GC biotech, Waddinxveen, the Netherlands), respectively. The removal of rRNA and sequencing were done at Novogene (Novogene Europe Laboratory: Cambridge, UK) using Illumina NovaSeq-PE150. Triplicates for samples taken after 12 and 21 h of fermentation from polyphenol-rich (CE, GP, Uro) and un-enriched media (CT) were analyzed.
Transcriptome data analysis
The initial step in analyzing RNA-seq data involved filtering and trimming the sequence reads, followed by quality control and mapping to the reference genome of A. muciniphila (GCF_000020225.1_ASM2022v1). Subsequently, reads per gene quantification and clustering analyses were performed. The fully assembled genome and annotation files (in FASTA and GTF format) were downloaded from the National Center of Biological Information (NCBI; https://www.ncbi.nlm.nih.gov). Details of the Quality check and processing of RNA-seq raw read data and analysis of differentially expressed genes (DEGs) are described in the Supplementary methods. The P-values were calculated using the Wald test, with Benjamini and Hochberg method correction (BH-adjusted P values). The q-value cut-off was set at 0.05. Genes with a higher number of reads (fold2-change cut-off of 1.5) compared to the CT were considered upregulated, while genes with a lower number of reads were considered downregulated.
Expression patterns of proteome and transcriptome and interaction network by Weighted Gene Correlation Network Analysis (WGCNA)
An association analysis of omics data was carried out to further investigate the expression profiles of iron-acquisition systems in A. muciniphila. For this purpose, a Seurat object containing both proteome and transcriptome data assays was generated using the Seurat package in R. Next, the dataset was transposed and prepared for the WGCNA. Co-expression networks were constructed using the WGCNA R software package (v1.47)31. The expression profiles of modules of interest were plotted in heatmaps. Furthermore, the generated module-linked networks were exported and visualized using visANT69.
In silico identification of motifs and regulatory transcription factors of iron-acquisition systems
To identify over-represented motifs in the transcriptome associated with iron acquisition and xenosiderophore transporters that were significantly regulated in A. muciniphila, we utilized in silico motif-discovery software. Firstly, we considered the availability of start codons as a factor in analyzing functional genes. To determine the transcription’s start site (TSS) region, we extended the sequences from the genomic coordinate region, and the query sequences in FASTA format were used as input data. The Neural Network Promoter Prediction (BDGP: NNPP version 2.2) tool was used to retrieve the sequences upstream from the TSS region. These sequences were assumed to contain the potential core promoter. The minimum standard predictive promoter score was set to a default cut-off value of 0.8. This was done to eliminate any zero counts by 80% from the query sequence before the transformation. Regions with multiple TSS were evaluated by selecting the prediction with the highest score, which was deemed accurate. The upstream sequence regions were imported and analyzed using the MEME algorithm, 5.1.1 version, via a web server (http://meme-suite.org/tools/meme) (see Supplementary methods).
Effects of iron-laden PACs on iron-depleted growth assay
The cells were grown overnight in the basal medium supplemented with 7.5 μM FeCl2. They were then subcultured (1% v/v) into a fresh iron-limited basal medium supplemented with 10 µM of 2,2’ dipyridyl (Dip) to remove any available iron. The incubation was allowed to proceed anaerobically for 8 hours. After that, the media were supplemented with either 7.5 μM FeCl2, PACs (600 mg/L) or ion-laden PACs (1% v/v). To obtain ion-laden PACs, sterile 750 µM FeCl2 was incubated with filter-sterilized PAC solution (600 mg/ml) at a 1:1 (v/v) ratio overnight at 4 °C. The resulting solution, rich in catecholates, was then added to iron-depleted media at 1% (v/v), resulting in a final concentration of (7.5 µM FeCl2 + 600 mg/L PACs). The specifics of the iron-binding capacity and saturation percentage of the iron-laden PACs solution are detailed in the Supplementary methods. Aliquots of 4 ml of cell cultures were harvested for RNA isolation and SCFA measurements after 24 and 30 hours of incubation. Experiments were performed in duplicate. Iron and zinc concentrations in cells and growth media were assessed using Inductively Coupled Plasma Mass Spectrometry (ICP-MS; see Supplementary methods).
Quantification of mRNA levels of targeted iron-acquiring mechanisms
Total RNA from cell cultures was extracted and purified using the TRIZOLTM reagent method, and further RNA purification was done using the RNeasy Mini Kit (Qiagen). Maxima H minus First Strand cDNA synthesis kit (Thermo-Fisher Scientific, #K1681) was used to generate cDNA. An additional genomic DNA elimination followed by first-strand cDNA synthesis was performed as per the manufacturer’s recommendation. Real-time PCR was performed using a Sensi Mix SYBR No-ROX kit (Bioline, Alphen aan den Rijn, The Netherlands), and data was acquired in a CFX384 thermal cycler (Bio-Rad Laboratories). Each qPCR reaction consisted of 5 μL of SYBR mix, 0.4 μL of 5 μM Forward and Reverse primers, 3.2 μL of DNase/RNase-free water, and 1 μL of cDNA. The following conditions were used: 95 °C for 10 min for hot-start polymerase activation, followed by 35 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min, and a melting curve stage as default setting from 60 °C to 95 °C. Each sample was tested in duplicate. The custom-designed primer pairs used for qRT-PCR amplification are presented in Table S10. Sequences of internal reference genes, gyrA and rpoD, were submitted to stability validation by NormFinder software70. The gene expression of the samples was then normalized against rpoD as the most stable reference gene and then calculated using the comparative Ct method (2−DDCt).
Statistical analysis
All experiments were repeated at least three times, and the data are presented as the mean ± standard error. Enrichment analysis for proteomics and transcriptomics was performed by Hypergeometric testing with Benjamini-Hochberg procedure for false discovery rate (FDR) correction using GSEA in R. A two-tailed Student’s t-test was applied to the fold changes in qRT-PCR-measured mRNA levels data using GraphPad Prism 8.0 software. Unless otherwise stated,*P < 0.05; **P < 0.01; ***P < 0.001; ns, not statistically significant. P-values were adjusted with the False Discovery Rate (FDR) method for multiple comparisons.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary File
Source data
Acknowledgements
The authors thank Silvateam (San Michele Mondovì CN – Italy) and Symrise Canada for providing the grape skin and cranberry PACs utilized in the current study. We also thank Dr. Janneke Elzinga for her assistance in preparing the samples for ICP-MS analysis. This work was supported by the Soehngen Institute of Anaerobic Microbiology (SIAM) Gravitation (Grant 024.002.002) of the Netherlands Organization for Scientific Research (NWO) to WMdV.
Author contributions
MCRD, HT, YD, and WMdV conceived and designed the study. MCRD performed the experiments. SB contributed to the proteomics methodology. MCRD performed bioinformatics and analyzed the data. WMdV supervised all the research. MCRD drafted the manuscript, and all the authors contributed to reviewing and editing the final manuscript.
Peer review
Peer review information
Nature Communications thanks Marco Ventura, Wenhan Zhuand the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The study’s RNA raw sequencing data have been deposited in the BioProject Database of the National Centre for Biotechnology Information (NCBI) database under accession code PRJNA1163526. MS raw data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD06744771. The metadata linking the omics sequences with the appropriate sample ID in this study are provided in Supplementary Data 4. Data supporting the findings of this study, including the main and Supplementary Figs., are available within the paper provided as Source Data file [and its supplementary information files]. Source data are provided with this paper.
Competing interests
YD holds an NSERC-Symrise Industrial Chair on the prebiotic effects of fruit and vegetable polyphenols. WMdV is an inventor of patent applications regarding the use of Akkermansia and other intestinal bacteria. It is also a co-founder, shareholder, and SAB member of The Akkermansia Company SA and Caelus Health BV. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-64477-w.
References
- 1.Frawley, E. R. & Fang, F. C. The ins and outs of bacterial iron metabolism: Bacterial iron effux transporters. Mol. Microbiol.93, 609–616 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ward, D. M. & Cloonan, S. M. Mitochondrial iron in human health and disease. Annu. Rev. Physiol.81, 453–482 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holbein, B. E. & Lehmann, C. Dysregulated iron homeostasis as common disease etiology and promising therapeutic target. Antioxidants12, 671 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodríguez-Daza, M. C. et al. Polyphenol-mediated gut microbiota modulation: toward prebiotics and further. Front. Nutr.8, 689456 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Hul, M. et al. What defines a healthy gut microbiome?. Gut73, 1893–1908 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cani, P. D., Depommier, C., Derrien, M., Everard, A. & De Vos, W. M. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol.19, 625–637 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Liu, C. et al. Dietary iron modulates gut microbiota and induces SLPI secretion to promote colorectal tumorigenesis. Gut Microbes15, 2221978 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Vello, P. et al. The lipooligosaccharide of the gut symbiont Akkermansia muciniphila exhibits a remarkable structure and TLR signaling capacity. Nat. Commun.15, 8411 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nairz, M., Theurl, I., Swirski, F. K. & Weiss, G. “Pumping iron”—how macrophages handle iron at the systemic, microenvironmental, and cellular levels. Pflüg. Arch. - Eur. J. Physiol.469, 397–418 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarano, A. et al. The Chelating ability of plant polyphenols can affect iron homeostasis and gut microbiota. Antioxidants12, 630 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Daza, M.-C. et al. Wild blueberry proanthocyanidins shape distinct gut microbiota profile and influence glucose homeostasis and intestinal phenotypes in high-fat high-sucrose fed mice. Sci. Rep.10, 2217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anhê, F. F. et al. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut64, 872–883 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Zhang, L. et al. Grape proanthocyanidin-induced intestinal bloom of Akkermansia muciniphila is dependent on its baseline abundance and precedes activation of host genes related to metabolic health. J. Nutr. Biochem.56, 142–151 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roopchand, D. E. et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet–induced metabolic syndrome. Diabetes64, 2847–2858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Redondo-Castillejo, R. et al. Proanthocyanidins: Impact on gut microbiota and intestinal action mechanisms in the prevention and treatment of metabolic syndrome. Int. J. Mol. Sci.24, 5369 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med.23, 107–113 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Perron, N. R. & Brumaghim, J. L. A Review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys.53, 75–100 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Li, Y., Feng, X., Zheng, X., Zhu, J. & Gu, Q. Food-borne polyphenols: A biocompatible anchor recuperating iron homeostasis. Food Front. fft2.244 10.1002/fft2.244 (2023).
- 19.Vazquez-Gutierrez, P. et al. Bifidobacteria strains isolated from stools of iron deficient infants can efficiently sequester iron. BMC Microbiol.15, 3 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vazquez-Gutierrez, P., De Wouters, T., Werder, J., Chassard, C. & Lacroix, C. High iron-sequestrating bifidobacteria inhibit enteropathogen growth and adhesion to intestinal epithelial cells in vitro. Front. Microbiol.7, (2016). [DOI] [PMC free article] [PubMed]
- 21.Zhu, W. et al. Xenosiderophore utilization promotes Bacteroides thetaiotaomicron resilience during colitis. Cell Host Microbe27, 376–388.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pi, H. & Helmann, J. D. Sequential induction of Fur-regulated genes in response to iron limitation in Bacillus subtilis. Proc. Natl. Acad. Sci.114, 12785–12790 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khasheii, B., Mahmoodi, P. & Mohammadzadeh, A. Siderophores: Importance in bacterial pathogenesis and applications in medicine and industry. Microbiol. Res.250, 126790 (2021). [DOI] [PubMed] [Google Scholar]
- 24.Romanowski, K. et al. Prevention of siderophore- mediated gut-derived sepsis due to P. aeruginosa can be achieved without iron provision by maintaining local phosphate abundance: role of pH. BMC Microbiol.11, 212 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Passel, M. W. J. et al. The Genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS ONE6, e16876 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golonka, R., Yeoh, B. S. & Vijay-Kumar, M. The iron tug-of-war between bacterial siderophores and innate immunity. J. Innate Immun.11, 249–262 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patzer, S. I. & Braun, V. Gene cluster involved in the biosynthesis of griseobactin, a catechol-peptide siderophore of Streptomyces sp. ATCC 700974. J. Bacteriol.192, 426–435 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henning, S. M. et al. Pomegranate ellagitannins stimulate the growth of Akkermansia muciniphila in vivo. Anaerobe43, 56–60 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Singh, A. et al. Direct supplementation with Urolithin A overcomes limitations of dietary exposure and gut microbiome variability in healthy adults to achieve consistent levels across the population. Eur. J. Clin. Nutr.76, 297–308 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, J. et al. The interaction of proanthocyanidins with DNA molecules studied by atomic force microscopy and spectroscopic method. Ultramicroscopy230, 113393 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Langfelder, P. & Horvath, S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinforma.9, 559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seo, S. W. et al. Deciphering Fur transcriptional regulatory network highlights its complex role beyond iron metabolism in Escherichia coli. Nat. Commun.5, 4910 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paget, M. S. & Helmann, J. D. The σ70 family of sigma factors. Genome Biol.4, 203 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimada, T., Takada, H., Yamamoto, K. & Ishihama, A. Expanded roles of two-component response regulator OmpR in Escherichia coli: genomic SELEX search for novel regulation targets. Genes Cells20, 915–931 (2015). [DOI] [PubMed] [Google Scholar]
- 35.Luscher, A. et al. Plant-derived catechols are substrates of TonB-dependent transporters and sensitize Pseudomonas aeruginosa to siderophore-drug conjugates. mBio13, e01498-22 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pi, H. et al. Role of catecholate siderophores in gram-negative bacterial colonization of the mouse gut. PLoS ONE7, e50020 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres, A. G. et al. In vivo bioluminescence imaging of Escherichia coli O104:H4 and role of aerobactin during colonization of a mouse model of infection. BMC Microbiol.12, 112 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giménez-Bastida, J. A. et al. Urolithins, ellagitannin metabolites produced by colon microbiota, inhibit Quorum Sensing in Yersinia enterocolitica: Phenotypic response and associated molecular changes. Food Chem.132, 1465–1474 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Mousavi, S., Weschka, D., Bereswill, S. & Heimesaat, M. M. Preclinical evaluation of oral urolithin-a for the treatment of acute campylobacteriosis in Campylobacter jejuni infected microbiota-depleted IL-10−/− mice. Pathogens10, 7 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu, Z. D. & Hider, R. C. Design of clinically useful iron(III)-selective chelators. Med. Res. Rev.22, 26–64 (2002). [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Daza, M. C. Prebiotic-like effects of berry polyphenols on the gut microbiota: Akkermansia muciniphila and its molecular adaptation mechanisms to phenolics. (Laval University, Quebec, QC, Canada, 2020).
- 42.Rodríguez-Daza, M. C. & De Vos, W. M. Polyphenols as drivers of a homeostatic gut microecology and immuno-metabolic traits of Akkermansia muciniphila: From mouse to man. Int. J. Mol. Sci.24, 45 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghssein, G. & Ezzeddine, Z. The key element role of metallophores in the pathogenicity and virulence of Staphylococcus aureus: A Review. Biology11, 1525 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lakey-Beitia, J., Burillo, A. M., La Penna, G., Hegde, M. L. & Rao, K. S. Polyphenols as potential metal chelation compounds against Alzheimer’s disease. j. Alzheimers Dis.82, S335–S357 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinreb, O., Amit, T. & Youdim, M. B. H. A novel approach of proteomics and transcriptomics to study the mechanism of action of the antioxidant–iron chelator green tea polyphenol (-)-epigallocatechin-3-gallate. Free Radic. Biol. Med.43, 546–556 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Xie, S. et al. Indispensable role of melatonin, a scavenger of reactive oxygen species (ROS), in the protective effect of Akkermansia muciniphila in cadmium-induced intestinal mucosal damage. Free Radic. Biol. Med.193, 447–458 (2022). [DOI] [PubMed] [Google Scholar]
- 47.Lau, C. K. Y., Krewulak, K. D. & Vogel, H. J. Bacterial ferrous iron transport: the Feo system. FEMS Microbiol. Rev.40, 273–298 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Rivera, M. Mobilization of iron stored in bacterioferritin, a new target for perturbing iron homeostasis and developing antibacterial and antibiofilm molecules. J. Inorg. Biochem.247, 112306 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kostopoulos, I. et al. Akkermansia muciniphila uses human milk oligosaccharides to thrive in the early life conditions in vitro. Sci. Rep.10, 14330 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kell, D. B., Heyden, E. L. & Pretorius, E. The Biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol.11, 1221 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Czosnykowska-Łukacka, M., Orczyk-Pawiłowicz, M., Broers, B. & Królak-Olejnik, B. Lactoferrin in human milk of prolonged lactation. Nutrients11, 2350 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friel, J., Qasem, W. & Cai, C. Iron and the Breastfed Infant. Antioxidants7, 54 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Padilla, L. et al. Mechanism of 2′-fucosyllactose degradation by human-associated Akkermansia. J. Bacteriol.206, e00334-23 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kramer, J., Özkaya, Ö & Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol.18, 152–163 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brune, I. et al. The DtxR protein acting as dual transcriptional regulator directs a global regulatory network involved in iron metabolism of Corynebacterium glutamicum. BMC Genomics7, 21 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Halfawy, O. M. et al. Antibiotic capture by bacterial lipocalins uncovers an extracellular mechanism of intrinsic antibiotic resistance. mBio8, e00225–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dauner, M. & Skerra, A. Scavenging bacterial siderophores with engineered lipocalin proteins as an alternative antimicrobial strategy. ChemBioChem21, 601–606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saini, A., Mapolelo, D. T., Chahal, H. K., Johnson, M. K. & Outten, F. W. SufD and SufC ATPase activity are required for iron acquisition during in vivo Fe-S cluster formation on SufB. Biochemistry49, 9402–9412 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis, J. P. & Gui, Q. Iron deficiency modulates metabolic landscape of bacteroidetes promoting its resilience during inflammation. Microbiol. Spectr.11, e04733–22 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parmanand, B. A. et al. A decrease in iron availability to human gut microbiome reduces the growth of potentially pathogenic gut bacteria; an in vitro colonic fermentation study. J. Nutr. Biochem.67, 20–27 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deriu, E. et al. Probiotic bacteria reduce Salmonella typhimurium intestinal colonization by competing for iron. Cell Host Microbe14, 26–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma, X., Liu, Q. & Yang, G. The multifaceted roles of Akkermansia muciniphila in neurological disorders. Trends Neurosci. S0166223625000797 10.1016/j.tins.2025.04.004 (2025). [DOI] [PubMed]
- 63.Lessard-Lord, J. et al. Short term supplementation with cranberry extract modulates gut microbiota in human and displays a bifidogenic effect. Npj Biofilms Microbiomes10, 18 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Derrien, M., Vaughan, E. E., Plugge, C. M. & De Vos, W. M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol.54, 1469–1476 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Feng, Y. et al. Comparative genomics and proteomics of Eubacterium maltosivorans: functional identification of trimethylamine methyltransferases and bacterial microcompartments in a human intestinal bacterium with a versatile lifestyle. Environ. Microbiol.24, 517–534 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cox, J. et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteom.13, 2513–2526 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods13, 731–740 (2016). [DOI] [PubMed] [Google Scholar]
- 68.Zoetendal, E. G. et al. Isolation of RNA from bacterial samples of the human gastrointestinal tract. Nat. Protoc.1, 954–959 (2006). [DOI] [PubMed] [Google Scholar]
- 69.Hu, Z., Snitkin, E. S. & DeLisi, C. VisANT: an integrative framework for networks in systems biology. Brief. Bioinform.9, 317–325 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Andersen, C. L., Jensen, J. L. & Ørntoft, T. F. Normalization of real-time quantitative reverse transcription-pcr data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res.64, 5245–5250 (2004). [DOI] [PubMed] [Google Scholar]
- 71.Perez-Riverol, Y. et al. The PRIDE database at 20 years: 2025 update. Nucleic Acids Res53, D543–D553 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary File
Data Availability Statement
The study’s RNA raw sequencing data have been deposited in the BioProject Database of the National Centre for Biotechnology Information (NCBI) database under accession code PRJNA1163526. MS raw data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD06744771. The metadata linking the omics sequences with the appropriate sample ID in this study are provided in Supplementary Data 4. Data supporting the findings of this study, including the main and Supplementary Figs., are available within the paper provided as Source Data file [and its supplementary information files]. Source data are provided with this paper.