Abstract
Recent studies have advanced the understanding of the pathogenesis of monoclonal gammopathy of uncertain significance (MGUS), describing the influence of a proinflammatory bone marrow environment on the risk of progression. While albumin is a known inflammatory prognostic biomarker in multiple myeloma, its role in MGUS has not been explored. We conducted a retrospective study to investigate the prognostic value of albumin in MGUS. Eight hundred and thirty-eight patients with MGUS were included: 71 (8.5%) presented hypoalbuminemia (≤3.5 g/dL) at diagnosis. Hypoalbuminemia was more common in men (63.4% vs. 49.3%; P = 0.025), older age (≥70 years: 74.6% vs. 57.2%; P = 0.005), and IgA isotype (33.8% vs. 21.4%; P = 0.018). These patients presented lower hemoglobin levels and higher creatinine values (P < 0.001 and P < 0.001). Serum M protein ≥ 1.5 g/dL (HR 18.9 [95% CI, 1.8–200.8]; P = 0.015) and hypoalbuminemia (HR 14.7 [95% CI, 1.7–124.7]; P = 0.014) were identified as independent prognostic factors for progression. In our series, Mayo Clinic and MGUS-like phenotype models were validated, and the incorporation of hypoalbuminemia into these models helped identify a subgroup of intermediate-risk patients with a higher risk of progression. In conclusion, if confirmed by independent studies, hypoalbuminemia could be integrated into existing prognostic models, improving risk stratification and guiding clinical decision-making.
Subject terms: Myeloma, Myeloma
Introduction
The diagnosis of monoclonal gammopathy of uncertain significance (MGUS) is often incidental and relies on a serum M protein of 3.0 g/dL or less; less than 10% of clonal plasma cells (PC) in the bone marrow (BM) and the absence of CRAB features (hypercalcemia, renal failure, anemia and bone lesions) [1, 2] and SLiM/CRAB criteria. MGUS is the most frequent PC dyscrasia, with a prevalence of 3.2% in people older than 50 years and more than 5.0% in those over 70 years [3–5].
Virtually all cases of multiple myeloma (MM) are preceded by MGUS, although not all MGUS will progress to MM [6–9]. In this context, several prognostic factors for progression to MM or other related disorders have been identified over time and incorporated into various risk models, allowing for a more individualized patient follow-up [10–12]. The Mayo Clinic progression risk model, which is the most widely used, identifies four risk groups based on serum M protein concentration (<1.5 g/dL or ≥1.5 g/dL), immunoglobulin subtype (IgG vs. non-IgG) and free light chain (FLC) ratio (normal vs. abnormal): low, low-intermediate, high-intermediate and high-risk if patients present none, one, two or all three risk factors, respectively [13]. More recently, multiparametric flow cytometry (MFC) has been used to develop an algorithm which classifies patients into three groups, MGUS-like, intermediate, and MM-like phenotypes, based on the percentage of total BM PC and of clonal PC (cPC) within the BM PC compartment, identifying groups of patients with unique clinical outcomes [14]. However, it has not yet been validated specifically in MGUS.
In addition, a better knowledge of the biology of monoclonal gammopathies has led to a better understanding of the pathogenesis of MGUS and its evolution to other entities. In this line, the Iceland screens, treats, or prevents multiple myeloma (iStopMM) project is the largest population-based screening study of MGUS, and is providing valuable knowledge about MGUS. Recently, in the iStopMM project, new reference intervals for FLC and the FLC ratio based on age (over or under 70 years of age) and renal function (estimated glomerular filtration rate (eGFR) over or under 60 mL/min/1.73 m2) [15] have been defined. However, these new intervals have not yet been validated in external cohorts, and it is essential to understand whether they offer any significant improvement in risk stratification when applied to the Mayo Clinic model. Additionally, a proinflammatory BM environment has been shown to contribute to MGUS progression [16–18]. In this context, serum albumin is an established inflammatory biomarker with well-known prognostic value in MM, and its evaluation is simple and widely available in clinical laboratories. However, its potential role in MGUS has not yet been explored.
Based on this background, we conducted a retrospective study of nearly a thousand patients with MGUS over the past 30 years, aiming to identify novel biomarkers of progression, including albumin as a potential inflammatory biomarker, and to validate existing risk models for progression from MGUS to symptomatic-related entities.
Methods
An observational retrospective study was conducted, including 838 patients diagnosed with MGUS and referred to the Hematology Department at the University Hospital of Salamanca between January 1, 1990, and December 31, 2022. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the University Hospital of Salamanca.
All patients met the diagnosis criteria proposed in 2014 by the International Myeloma Working Group (IMWG) [1]. Serum M protein was identified and quantified by serum protein electrophoresis. In cases of uncertainty, immunofixation was performed to confirm its presence and to characterize it. Patients with IgM MGUS were excluded as their biology differs from that of non-IgM PC disorders. The serum albumin was determined by spectrophotometry, and hypoalbuminemia was defined as a serum albumin ≤ 3.5 g/dL. MFC was carried out according to EuroFlow recommendations [19] for the diagnosis of PC dyscrasias. The cytogenetic status was assessed by fluorescence in situ hybridization (FISH) in 328 patients. After 2005, FISH was performed in purified CD138 + PC, and the threshold was set at 10% for IGH translocations and 20% for chromosome gains or deletions [20].
Stratification of the patients into different risk groups was performed according to Mayo Clinic and MGUS-like phenotype risk models [13, 14]. The calculator available in www.mgus-like.com was used to assign patients to the distinct prognostic groups defined by the MGUS-like phenotype model. In addition, new reference intervals for FLC and FLC ratio proposed by the iStopMM project were applied to our patient cohort [15].
Time to progression (TTP) was defined as the time from diagnosis to progression to MM or other related diseases, the date of death, or last follow-up.
The significance of differences between the qualitative and quantitative variables was estimated by the chi-square and the ANOVA tests, respectively. The odds ratio (OR) and 95% confidence interval (CI) were calculated using logistic regression. The differences in TTP were defined using the log rank test, and the corresponding hazard ratio (HR) and 95% CI were estimated using Cox regression. Statistical analyses were performed with IBM SPSS Statistics version 28 and Jamovi 2.6.26. Values of P < 0.05 were considered statistically significant.
Results
Baseline characteristics
A total of 838 patients were finally included in the study. The median age at diagnosis was 72 years (range, 28–108 years) and 423 (50.5%) were men. The isotype was IgG in 625 (74.6%) patients, IgA in 188 (22.4%), light chain in 20 (2.4%), and 5 (0.6%) had a biclonal gammopathy. The FLC kappa was the predominant one (66.0%), and the FLC ratio was abnormal in 247 (38.7%) patients of the cohort. Immunoparesis of the uninvolved immunoglobulins (one or both) was present in 234 (27.9%) patients.
BM aspirate/biopsy at diagnosis was performed in 411 (49.0%) patients per local guidelines or as a requirement in clinical research studies. The median PC in BM by morphology was 3% (range, 0–9). The rest of the baseline characteristics of the entire cohort are detailed in Table 1.
Table 1.
Baseline characteristics of the entire cohort.
| Entire cohort (N = 838) | Normal serum albumin (n = 767) | Hypoalbuminemia (n = 71) | P value | |
|---|---|---|---|---|
| Age of diagnosis, median (range) | 72 (28–108) | 71 (28-108) | 79 (48-97) | <0.001 |
| ≥50 years, n (%) | 772 (92.1) | 702 (91.5) | 70 (98.6) | 0.066 |
| ≥70 years, n (%) | 492 (58.7) | 439 (57.2) | 53 (74.6) | 0.005 |
| Gender male, n (%) | 423 (50.5) | 378 (49.3) | 45 (63.4) | 0.025 |
| Isotype, n (%) | ||||
| IgG | 625 (74.6) | 582 (75.9) | 43 (60.6) | 0.012 |
| IgA | 188 (22.4) | 164 (21.4) | 24 (33.8) | 0.018 |
| Light chain | 20 (2.4) | 16 (2.1) | 4 (5.6) | 0.061 |
| Biclonal | 5 (0.6) | 5 (0.6) | 0 (0.0) | 0.495 |
| Kappa FLC, n (%) | 553 (66.0) | 504 (65.7) | 49 (69.0) | 0.574 |
| Immunoparesis, n (%) | 234 (27.9) | 212 (27.6) | 22 (31.0) | 0.548 |
| 1 Ig involved | 168 (20.0) | 150 (19.6) | 18 (25.4) | 0.272 |
| 2 Igs involved | 66 (7.9) | 62 (8.1) | 4 (5.6) | 0.469 |
| Serum M protein, g/L (mean ± SD) | 0.8 ± 0.5 | 0.8 ± 0.5 | 0.8 ± 0.6 | 0.153 |
| ≥1.5 g/L, n (%) | 73 (8.7) | 67 (8.7) | 6 (8.5) | 0.935 |
| FLC ratio (mean ± SD) | 2.9 ± 8.4 | 2.9 ± 8.6 | 2.9 ± 6.0 | 0.462 |
| Abnormal FLC ratio, n (%) | 247/638 (38.7) | 225/586 (38.4) | 22/52 (42.3) | 0.579 |
| Hb, g/dL (mean ± SD) | 13.7 ± 1.9 | 13.9 ± 1.8 | 11.8 ± 1.8 | <0.001 |
| Serum creatinine, mg/dL (mean ± SD) | 1.0 ± 0.5 | 1.0 ± 0.4 | 1.4 ± 0.9 | <0.001 |
| eGFR, ml/min (mean ± SD) | 73.6 ± 27.2 | 74.9 ± 26.9 | 59.4 ± 27.1 | <0.001 |
| PC in BM by morphology (mean ± SD) | 3.2 ± 2.4 | 3.2 ± 2.4 | 3.7 ± 2.6 | 0.153 |
| ≥5% PC in BM, n (%) | 114/433 (26.3) | 104/403 (25.8) | 10/30 (33.3) | 0.366 |
| Clonal PC in BM by MFC (mean ± SD) | 60.7 ± 33.9 | 61.2 ± 33.8 | 54.4 ± 35.4 | 0.170 |
| ≥95% clonal PC in BM by MFC, n (%) | 58/411 (14.1) | 55/384 (14.3) | 3/27 (11.1) | 0.643 |
| Cytogenetic abnormalities, n (%) | ||||
| t(11;14) | 32/230 (13.9) | 30/212 (14.2) | 2/18 (11.1) | 0.732 |
| High riska | 14/253 (5.5) | 14/233 (6.0) | 0/20 (0.0) | 0.259 |
| Progression, n (%) | 43 (5.1) | 37 (4.8) | 6 (8.5) | 0.185 |
Captions: BM bone marrow, eGFR estimated glomerular filtration rate, FLC free light chain, Hb hemoglobin, Ig immunoglobulin, MFC multiparametric flow cytometry, PC plasma cell, SD standard deviation.
aHigh-risk cytogenetics were considered t(4;14), t(14;16) and del17p.
The bold values represent statistically significant results.
Seventy-one (8.5%) patients presented hypoalbuminemia at diagnosis. Comparing with those with normal serum albumin, we observed than hypoalbuminemia was significantly more frequent in older patients, with 74.6% of those affected being 70 years or older, compared to 57.2% of the patients with normal serum albumin (OR 2.4 [95% CI, 1.4–4.3]; P = 0.005). Likewise, hypoalbuminemia was more common in men (OR 1.8 [95% CI, 1.1-2.9]; P = 0.025). The IgA isotype was more prevalent among patients with hypoalbuminemia (33.8% vs. 21.4%; OR 1.9 [95% CI, 1.1–3.2]; P = 0.018). In contrast, the IgG isotype was less prevalent in patients with low serum albumin levels (60.6% vs. 75.9%; OR 2.0 [1.2–3.3]; P = 0.012). These patients presented significantly higher serum creatinine values (1.4 ± 0.9 mg/dL) and lower hemoglobin values (11.8 ± 1.8 g/dL) than patients with normal serum albumin (creatinine: 1.0 ± 0.4 mg/dL, P < 0.001; hemoglobin: 13.8 ± 1.8 g/dL, P < 0.001). No differences were observed in paraprotein values, abnormal FLC ratio, immunoparesis, BM infiltration, or cytogenetic abnormalities.
Risk of progression
With a median follow-up of 7.0 years (range, 2.0–31.0 years), progression to other related diseases occurred in 48 (5.7%) patients. Thirty-nine (81.2%) patients progressed to MM; 4 (8.3%) to smoldering MM (SMM); 2 (4.2%) to lymphoproliferative disorders; 2 (4.2%) to AL amyloidosis, and 1 (2.1%) patient progressed to light chain deposition disease. Neither light chain, defined as abnormal FLC ratio with elevation of the involved FLC without evidence of heavy chain M protein, nor biclonal MGUS patients progressed.
We analyzed if hypoalbuminemia was associated with higher risk of progression in our series, and indeed, it resulted in almost five times the risk increased (HR 4.8 [95% CI, 2.2–10.4]; P < 0.001). In addition a univariate analysis was done to confirm other predictors of progression and serum M protein (HR 9.0 [95% CI, 5.1–15.9]; P < 0.001), an abnormal FLC ratio (HR 5.1 [95% CI, 2.2–12.1]; P < 0.001), the immunoparesis of the 2 uninvolved immunoglobins (HR 2.7 [95% CI, 1.2–6.0]; P = 0.015), the presence of ≥5% PC by morphology (HR 7.5 [95% CI, 3.5–15.9]; P < 0.001) and ≥95% cPC within the PC compartment in BM by MFC (HR 5.9 [95% CI, 2.8–12.2]; P < 0.001) were associated with a higher risk of progression.
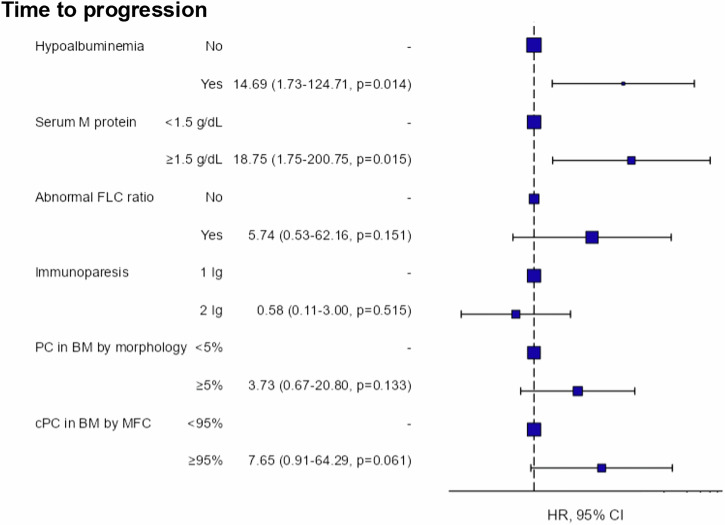
The multivariate analysis (Fig. 1) confirmed that hypoalbuminemia was an independent marker for progression (HR 14.7 [95% CI, 1.7–124.7]; P = 0.014), as well as serum M protein ≥ 1.5 g/dL (HR 18.8 [95% CI, 1.8–200.8]; P = 0.015).
Fig. 1. Multivariant analysis of the risk of progression in the entire cohort.
Captions: BM bone marrow, CI confidence interval, cPC clonal plasma cell, FLC free light chain, HR hazard ratio, Ig immunoglobulin, MFC multiparametric flow cytometry, PC plasma cell.
The same analyses were also performed using continuous variables, yielding identical results; therefore, dichotomized variables were considered for easier interpretation.
Progression risk models
Firstly, the Mayo Clinic progression risk model was applied in 638 (76.1%) patients. Two hundred and eighty-seven (45.0%) were classified as low-risk; 244 (38.2%) as low-intermediate risk; 103 (16.2%) as high-intermediate risk, and 4 (0.6%) were allocated to the high-risk groups.
The model was validated in our series, revealing four groups with different risk of progression. Considering the low-risk group as a reference (median TTP: not reached) (Fig. 2), low-intermediate risk (median TTP: not reached, HR 3.8 [95% CI, 1.2–11.9]; P = 0.023), high-intermediate risk (median TTP: not reached, HR 8.7 [95% CI, 2.7–28.5]; P < 0.001) and high-risk groups (median TTP: 7 years, HR 68.1 [95% CI, 15.0–308.9]; P < 0.001) had significantly higher risk of progression.
Fig. 2. Time to progression in the global cohort according to Mayo Clinic risk model.
Captions: CI confidence interval, HR hazard ratio.
Secondly, the MGUS-like phenotype risk model was applied in the 411 (49.0%) patients in whom a BM aspirate/biopsy had been performed at diagnosis (Fig. 3). Two hundred and ninety (70.6%) patients had a MGUS-like phenotype, 121 (29.4%) patients had an intermediate phenotype, and none of the patients presented with a MM-like phenotype. Significant statistically differences in TTP were observed between MGUS-like and intermediate phenotypes (median TTP 31.0 years vs. not reached, respectively; HR 4.0 [95% CI, 1.9–8.5]; P < 0.001).
Fig. 3. Time to progression in the global cohort according to the MGUS-like phenotype model.
Captions: CI confidence interval, HR hazard ratio, MGUS monoclonal gammopathy of uncertain significance.
Considering that hypoalbuminemia was found to be an independent factor for progression, we analyzed the impact of hypoalbuminemia in the different Mayo Clinic prognostic groups (Supplementary Table S1). No patient in the low-risk group with hypoalbuminemia progressed. Of the remaining groups, 1 (5.3%) patient of the 19 low-intermediate risk patients progressed; 3 (20.0%) of the 15 high-intermediate risk patients and the only one high-risk patient with hypoalbuminemia progressed. Within the high-intermediate risk group, patients with hypoalbuminemia had a higher risk of progression than those with normal serum albumin (median TTP: 7.0 years vs. not reached, HR 5.2 [95% CI, 1.2–22.2]; P = 0.027) (Fig. 4A). In the remaining groups, hypoalbuminemia did not contribute significant differences in TTP. Since hypoalbuminemia had an impact on TTP only in the Mayo Clinic high-intermediate risk group, a new prognostic classification model for MGUS is proposed with 5 groups of patients (Fig. 5A): the 4 classical groups (low, low-intermediate, high-intermediate, and high risk) and a new subgroup, high-intermediate with hypoalbuminemia. This new subgroup of patients would have a similar risk of progression to the high-risk group (HR 0.9 [95% CI, 0.2–5.9]; P = 0.952).
Fig. 4. Impact of hypoalbuminemia on risk of progression.
A Mayo Clinic high-intermediate risk group and B intermediate group of the MGUS-like phenotype model. Captions: CI confidence interval, HR hazard ratio.
Fig. 5. Time to progression in the global cohort.
A Mayo Clinic risk model including patients of high-intermediate risk with hypoalbuminemia and B MGUS-like phenotype model including patients with intermediate group and hypoalbuminemia. Captions: CI confidence interval, HR hazard ratio, MGUS monoclonal gammopathy of uncertain significance.
The role of hypoalbuminemia in the MGUS-like phenotype model was also investigated (Supplementary Table S2). Of the patients with hypoalbuminemia, 1 (4.5%) of the 22 patients in the MGUS-like group and 2 (33.3%) of the 6 patients in the intermediate group progressed, respectively. Patients with hypoalbuminemia in the intermediate group had a risk of progression up to 5 times higher (median TTP: 9 years) than those with normal serum albumin levels at diagnosis (median TTP: not reached, HR 4.5 [95% CI, 1.1–20.2]; P = 0.049) (Fig. 4B). Similarly, another new prognostic model based on the MGUS-like phenotype model is proposed, adding a new subgroup of patients to the already known (Fig. 5B): those with intermediate behavior and hypoalbuminemia.
New reference intervals for free light chains
In the entire cohort, 247 patients out of 638 (38.7%) with FLC assessment had abnormal FLC ratio. To explore if new intervals of reference identified by the iStopMM might change the consideration of having abnormal FLC ratio, these new intervals were applied in our series, and 216 (33.9%) patients presented abnormal FLC ratio. Among the cases considered abnormal under the previous ranges, 85 (34.4%) patients would now be classified as having a normal FLC ratio. On the contrary, 54 (13.8%) patients previously consider having normal FLC ratio, after applying new reference intervals resulted in abnormal FLC ratio (Supplementary Fig. S1).
In addition, the Mayo Clinic prognostic stratification model was applied according to the new FLC intervals: 309 (48.4%) patients were classified as low risk, 231 (36.2%) as low-intermediate risk, 94 (14.7%) as high-intermediate risk, and 4 (0.6%) as high risk.
When compared to previous reference intervals (Supplementary Fig. S2): 32 (11.1%) low-risk patients were reclassified as low-intermediate risk. In the low-intermediate risk group, 54 (22.1%) patients were reclassified as low risk and 21 (8.6%) as high-intermediate risk. In the high-intermediate risk group, 30 (29.1%) patients were reclassified as low-intermediate risk and 1 (0.1%) patient as high risk. One (25.0%) patient in the high-risk group was reclassified as high-intermediate risk.
However, despite the re-stratifications using the new intervals proposed by the Icelandic project, no major differences in the risk of progression were observed compared to the same groups evaluated using the old intervals (Supplementary Fig. S3).
Discussion
To our knowledge, this is the first study to analyze the impact of hypoalbuminemia on the risk of progression of MGUS and to demonstrate that it is an independent prognostic factor for progression. Hypoalbuminemia (≤3.5 g/dL) is not very common in MGUS patients since it was present in 8.5% of patients at diagnosis of this entity. It was associated with known poor prognostic factors of monoclonal gammopathies, such as older age, male sex, IgA isotype, lower hemoglobin levels, and higher creatinine values, and it was identified as an independent biomarker of progression in MGUS patients. Low serum albumin levels significantly increased the risk of progression from the earliest stages of monoclonal gammopathies. The presence of hypoalbuminemia at diagnosis of MGUS increased almost 5-fold the probability of progression to symptomatic disease compared to normal serum albumin. Of note, the incorporation of hypoalbuminemia to the Mayo Clinic risk model allowed us to identify a new subset of patients, those with high-intermediate risk and hypoalbuminemia, who presented a similar risk of progression to the high-risk group. Accordingly, we propose to manage patients identified as high-intermediate risk who also present with hypoalbuminemia as high-risk. Likewise, in the MGUS-like phenotype model, among patients with an intermediate phenotype, hypoalbuminemia was associated with a fivefold increase in the risk of progression. These results may reflect an underlying biological substrate, as some studies have shown that the proinflammatory environment of the BM and its interactions with surrounding elements contribute to the progression of MGUS to more advanced diseases. Cenzano et al. [17], in their analysis of BM changes during the transition from premalignant stages to MM, observed an increase in the angiogenic profile of endothelial cells and an inflammatory phenotype of mesenchymal cells (with increased release of cytokines, interferon…), which could complement the value the Mayo score, primarily focused on tumor burden.
Although MGUS is a common entity in people over 50 years, only about 1% of patients per year progress to symptomatic disease [21–24]. Our results are also consistent with previous studies that identified different risk factors for the progression of MGUS to other disorders, particularly to MM. Kyle et al. [25] reported the natural history of 1384 patients with MGUS in Minnesota with long-term follow-up and found that the initial concentration of serum M protein and the FLC ratio were the most important predictors for progression. Additionally, the isotype and the low concentration of the two uninvolved immunoglobulins were described as risk factors. Beyond these serum factors, the percentage of PC in the BM of patients with MGUS has also been correlated with the risk of progression. In this regard, a percentage ≥ 5% of PC in BM by morphology increased the risk of progression 2-fold over those with a lower infiltration, as described by Cesana et al. [26]. Similar results were reported by Pérez-Persona et al. [27], who additionally showed that the presence of ≥95% cPC within the PC compartment in BM proved to be an independent risk factor for progression.
These risk factors for progression have been used to develop different prognostic staging models to adapt MGUS patient’s follow-up and guide clinical decision making [28–32]. One of the most widely used models over time has been the Mayo Clinic risk model [13]. The distribution across risk groups and the observed progression rates in our series are consistent with those reported by the Mayo Clinic, supporting the representativeness of our cohort and validating this prognostic model.
Recently, Burgos et al. [14] have used the percentage of PC in BM and the percentage of cPC within the PC compartment in BM by MFC to identify patients with monoclonal gammopathies that presented different clinical behavior. The model has been applied in MM, smoldering MM, and AL amyloidosis, but not in MGUS patients. In our cohort, this model identified nearly one-third of patients with an intermediate phenotypic profile who did not exhibit typical MGUS behavior. Therefore, the MGUS-like phenotype model could complement the Mayo Clinic model in tailoring the follow-up of patients with MGUS.
Furthermore, it is noteworthy that hypoalbuminemia could potentially worsen the prognosis of subgroup of patients of high-intermediate risk (Mayo Clinic model) and intermediate profile (MGUS-like model), resembling high-risk patients with a median TTP ranging from 7 to 9 years. In this context, we propose adapting the follow up of this particularly at-risk subgroup to that of high-risk patients. However, hypoalbuminemia was only assessed at the time of MGUS diagnosis. It may be beneficial to incorporate hypoalbuminemia, alongside other established prognostic factors, into follow-up assessments in order to dynamically re-evaluate the risk of progression.
Reference ranges for serum FLC kappa (3.3–19.4 mg/L), FLC lambda (5.7–26.3 mg/L), and the FLC ratio (0.26–1.65) were defined in a small cohort of 282 patients with normal renal function [33]. However, new reference intervals have been proposed by the iStopMM project based on age and renal function [15, 34]. When comparing both in our series, FLC ratio was abnormal in 39% of patients using the classic thresholds versus 33% using the new reference intervals. Despite this, the risk of progression did not change substantially when applying new reference intervals, based on the follow-up data available so far.
A limitation of the present study is its retrospective, single-center design, and the consequent lack of some clinical and/or laboratory data of the patients. In addition, only MGUS patients with follow-up by the hematologist were included, so those who died early due to competing risks may have been missed. Hypoalbuminemia may also reflect unmeasured confounding factors, such as comorbidities, chronic diseases, or frailty. Moreover, low serum albumin levels were relatively infrequent in the cohort, and very few of these patients progressed, resulting in wide CI, which is one of the main limitations of the study. A higher-than-expected number of bone marrow studies were performed due to the inclusion of patients in biological studies. Also, the follow-up for patients diagnosed in recent years is still relatively short. Nevertheless, our study identifies novel prognostic factors for progression, contributing to a more individualized approach in the management of patients with MGUS. It would also be interesting to further explore inflammatory biomarkers (such as CRP, ferritin, and IL-6) to investigate a potential association between a pro-inflammatory state and low serum albumin levels.
In conclusion, albumin is an easily accessible serum inflammatory biomarker with prognostic value in patients with MGUS and could complement existing risk models. Notably, hypoalbuminemia may help identify a subset of patients at increased risk of progression, allowing for more tailored monitoring and decision making. This is the first study to evaluate the prognostic impact of hypoalbuminemia in patients with MGUS; therefore, validation in larger and independent cohorts is essential.
Supplementary information
Acknowledgements
The authors would like to thank the “Fundación para el Desarrollo de la Hematología y Hemoterapia de Salamanca” for the financial support.
Author contributions
E.A., V.G.-C. and M.-V.M. conceived the study idea; E.A. and B.P. provided the study materials; E.A., V.G.-C., B.P. and M.-V.M. had full access to all the study data; E.A., V.G.-C., B.P. and M.-V.M. analyzed and interpreted the data and wrote the original draft of the manuscript. The rest of the authors (P.B., C.A., B.R.-B., N.C.G. and N.P.) treated the patients and reviewed, edited, and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Data availability
Due to the sensitive nature of the data, information created and/or analyzed during the current study is available from the first and corresponding authors.
Competing interests
E.A. has received honoraria from Jonhson & Jonhson. V.G.-C. has received honoraria from Jonhson & Jonhson and Celgene; reports research funding from Jonhson & Jonhson; and received honoraria for consulting and advisory roles for Prothena and Jonhson & Jonhson. B.P. has received honoraria from Jonhson & Jonhson, Amgen, Sanofi, Pfizer, Bristol Myers Squibb/Celgene and Aptitude Health. C.A. has received honoraria from The Binding Site. B.R.-B. has received a speaker’s fees from Jonhson & Jonhson and Amgen. N.C.G. has received honoraria from Sanofi and Amgen. N.P. has received honoraria for consulting or advisory roles from Amgen, Celgene, Jonhson & Jonhson, Takeda, The Binding Site, GlaxoSmithKline, and Sanofi. M.-V.M. has received honoraria derived from lectures and advisory board activities from Jonhson & Jonhson, Bristol Myers Squibb/Celgene, Amgen, Takeda, AbbVie, Sanofi, Oncopeptides, Adaptive, Roche, Pfizer, Regeneron, GlaxoSmithKline, Bluebird Bio, and Sea-Gen. For the remaining authors, no relevant conflicts of interest were declared.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the University Hospital of Salamanca, with a waiver of informed consent, and conducted in accordance with the Declaration of Helsinki.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41408-025-01371-0.
References
- 1.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–48. 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 2.Rajkumar SV, Lacy MQ, Kyle RA. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma. Blood Rev. 2007;21:255–65. 10.1016/j.blre.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, et al. Prevalence of monoclonal gammopathy of undetermined significance. New Engl J Med. 2006;354:1362–9. 10.1056/NEJMoa054494. [DOI] [PubMed] [Google Scholar]
- 4.Wadhera RK, Rajkumar SV. Prevalence of monoclonal gammopathy of undetermined significance: a systematic review. Mayo Clin Proc. 2010;85:933–42. 10.4065/mcp.2010.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rögnvaldsson S, Love TJ, Thorsteinsdottir S, Reed ER, Óskarsson JÞ, Pétursdóttir Í, et al. Iceland screens, treats, or prevents multiple myeloma (iStopMM): a population-based screening study for monoclonal gammopathy of undetermined significance and randomized controlled trial of follow-up strategies. Blood Cancer J. 2021;11:1–13. 10.1038/s41408-021-00480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kyle RA. Monoclonal gammopathy of undetermined significance. Am J Med. 1978;64:814–26. 10.1016/0002-9343(78)90522-3. [DOI] [PubMed] [Google Scholar]
- 7.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ. Long-term follow-up of 241 patients with monoclonal gammopathy of undetermined significance: the original mayo clinic series 25 years later. Mayo Clin Proc. 2004;79:859–66. 10.4065/79.7.859. [DOI] [PubMed] [Google Scholar]
- 8.Dhodapkar MV. MGUS to myeloma: a mysterious gammopathy of underexplored significance. Blood. 2016;128:2599–606. 10.1182/blood-2016-09-692954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7. 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kyle RA, Rajkumar SV. Monoclonal gammopathy of undetermined significance and smouldering multiple myeloma: emphasis on risk factors for progression. Br J Haematol. 2007;139:730–43. 10.1111/j.1365-2141.2007.06873.x. [DOI] [PubMed] [Google Scholar]
- 11.Ravindran A, Lackore KA, Glasgow AE, Drake MT, Hobbs MA, Kourelis T, et al. Monoclonal gammopathy of undetermined significance: indications for prediagnostic testing, subsequent diagnoses, and follow-up practice at Mayo Clinic. Mayo Clin Proc. 2020;95:944–54. 10.1016/j.mayocp.2019.12.033. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Hsu SH, Wang R, Theprungsirikul P, Neparidze N, Chang SH, et al. Associations between patient characteristics and progression to multiple myeloma among patients with monoclonal gammopathy of undetermined significance: a systematic review. Clin Lymphoma Myeloma Leuk. 2025;25:e222–31. 10.1016/j.clml.2024.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajkumar SV, Kyle RA, Therneau TM, Melton LJ, Bradwell AR, Clark RJ, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005;106:812–7. 10.1182/blood-2005-03-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burgos L, Tamariz-Amador LE, Puig N, Cedena MT, Guerrero C, Jelínek T, et al. Definition and clinical significance of the monoclonal gammopathy of undetermined significance–like phenotype in patients with monoclonal gammopathies. JCO. 2023;41:3019–31. 10.1200/JCO.22.01916. [DOI] [PubMed] [Google Scholar]
- 15.Einarsson Long T, Rögnvaldsson S, Thorsteinsdottir S, Sverrisdottir I, Eythorsson E, Indridason O, et al. Revised definition of free light chains in serum and light chain monoclonal gammopathy of undetermined significance: results of the ISTOPMM study. Blood. 2023;142:535 10.1182/blood-2023-188547. [Google Scholar]
- 16.Gámez B, Edwards CM. Contributions of the bone microenvironment to monoclonal gammopathy of undetermined significance pathogenesis. Curr Osteoporos Rep. 2018;16:635–41. 10.1007/s11914-018-0479-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cenzano I, Cocera M, Bantan A, Larrayoz M, Vilas-Zornoza A, San-Martin P, et al. Comprehensive characterization of the bone marrow microenvironment transcriptional remodeling in the progression from MGUS to smoldering and multiple Myeloma. Blood. 2023;142:88 10.1182/blood-2023-178534. [Google Scholar]
- 18.Saade C, Ghobrial IM. Updates on mechanisms of disease progression in precursor myeloma: Monoclonal gammopathy of undermined significance and smoldering myeloma. La Presse Méd. 2025;54:104268. 10.1016/j.lpm.2025.104268. [DOI] [PubMed] [Google Scholar]
- 19.Flores-Montero J, Sanoja-Flores L, Paiva B, Puig N, García-Sánchez O, Böttcher S, et al. Next Generation Flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia. 2017;31:2094–103. 10.1038/leu.2017.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ross FM, Avet-Loiseau H, Ameye G, Gutierrez NC, Liebisch P, O’Connor S, et al. Report from the European Myeloma Network on interphase FISH in multiple myeloma and related disorders. Haematologica. 2012;97:1272–7. 10.3324/haematol.2011.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–9. 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 22.Kyle RA, Vincent Rajkumar S. Monoclonal gammopathies of undetermined significance. Best Pract Res Clin Haematol. 2005;18:689–707. 10.1016/j.beha.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Kyle RA, Kumar S. The significance of monoclonal gammopathy of undetermined significance. Haematologica. 2009;94:1641–4. 10.3324/haematol.2009.013961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Visram A, Larson D, Norman A, Dispenzieri A, Murray D, Kyle R, et al. Comparison of progression risk of monoclonal gammopathy of undetermined significance by method of detection. Blood. 2025;145:325–33. 10.1182/blood.2024025415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kyle RA, Larson DR, Therneau TM, Dispenzieri A, Kumar S, Cerhan JR, et al. Long-term follow-up of monoclonal gammopathy of undetermined significance. N Engl J Med. 2018;378:241–9. 10.1056/NEJMoa1709974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cesana C, Klersy C, Barbarano L, Nosari AM, Crugnola M, Pungolino E, et al. Prognostic factors for malignant transformation in monoclonal gammopathy of undetermined significance and smoldering multiple Myeloma. JCO. 2002;20:1625–34. 10.1200/JCO.2002.20.6.1625. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Persona E, Vidriales MB, Mateo G, García-Sanz R, Mateos MV, De Coca AG, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007;110:2586–92. 10.1182/blood-2007-05-088443. [DOI] [PubMed] [Google Scholar]
- 28.Kyle RA, Durie BGM, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010;24:1121–7. 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bianchi G, Kyle RA, Colby CL, Larson DR, Kumar S, Katzmann JA, et al. Impact of optimal follow-up of monoclonal gammopathy of undetermined significance on early diagnosis and prevention of myeloma-related complications. Blood. 2010;116:2019–25. 10.1182/blood-2010-04-277566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berenson JR, Anderson KC, Audell RA, Boccia RV, Coleman M, Dimopoulos MA, et al. Monoclonal gammopathy of undetermined significance: a consensus statement. Br J Haematol. 2010;150:28–38. 10.1111/j.1365-2141.2010.08207.x. [DOI] [PubMed] [Google Scholar]
- 31.Kyle RA, Rajkumar SV. Management of monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM). Oncology. 2011;25:578–86. [PMC free article] [PubMed] [Google Scholar]
- 32.Van De Donk NWCJ, Palumbo A, Johnsen HE, Engelhardt M, Gay F, Gregersen H, et al. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: recommendations from the European Myeloma Network. Haematologica. 2014;99:984–96. 10.3324/haematol.2013.100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437–44. [PubMed] [Google Scholar]
- 34.Long TE, Indridason OS, Palsson R, Rognvaldsson S, Love TJ, Thorsteinsdottir S, et al. Defining new reference intervals for serum free light chains in individuals with chronic kidney disease: results of the iStopMM study. Blood Cancer J. 2022;12:133. 10.1038/s41408-022-00732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the sensitive nature of the data, information created and/or analyzed during the current study is available from the first and corresponding authors.