Abstract
We investigated the relationship between incidence of gastrointestinal tract cancers, metabolic dysfunction-associated steatotic liver disease (MASLD), and alcohol-related steatotic liver disease in diabetic population. A nationwide cohort of 2,616,828 individuals with diabetes under Korean National Health Insurance Service from 2015 to 2016 was divided into four subgroups: no steatosis (group 1), MASLD alone (group 2), MASLD with heavy alcohol intake (group 3), and alcoholic liver disease (group 4). We used fatty liver index to assess the probability of hepatic steatosis using cutoff scores of 30 and 60. We analyzed incidences of esophageal, stomach, colorectal, biliary, and pancreatic cancers until 2022. Compared with group 1 (reference), group 2 showed increased hazard ratios for stomach, colorectal, and biliary cancers, with a decreased hazard ratio for esophageal cancer (adjusted hazard ratio [95% confidence interval]: 1.10 [1.06–1.13], 1.13 [1.10–1.16], 1.10 [1.05–1.16], 0.88 [0.79–0.97], respectively). Probability of hepatic steatosis was positively correlated with all gastrointestinal tract cancers except esophageal cancer in non-drinkers, but only with stomach, colorectal, and biliary cancers in mild drinkers (ptrend < 0.001). In conclusion, MASLD increases gastrointestinal tract cancer risk, except esophageal cancer, in diabetic population. For non or mild drinkers, probability of hepatic steatosis serves as a predictor of gastrointestinal tract cancer risk.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-21005-6.
Keywords: Alimentary tract, Hepatic steatosis, Metabolic dysfunction-associated steatotic liver disease, Gastrointestinal tract cancer, Diabetes
Subject terms: Cancer, Gastroenterology, Oncology
Introduction
Diabetes mellitus is a highly prevalent disease affecting more than 400 million people globally1, with type 2 diabetes increasing by 4.8% annually2. Diabetes is traditionally associated with pancreatic, colorectal, and liver cancers3–6; however, recent epidemiologic analyses suggest an increased risk of other gastrointestinal tract cancers, including stomach and esophageal cancers7,8.
Considering these correlations, identifying specific risk factors in patients with diabetes for different gastrointestinal tract cancers is crucial, with liver steatosis being a significant risk factor. Nonalcoholic fatty liver disease (NAFLD) is strongly associated with diabetes, particularly in patients with type 2 diabetes, with a prevalence as high as 55%9. The pathophysiology of both NAFLD and diabetes is associated with the accumulation of fat in the liver (steatohepatitis) and the development of insulin resistance10. The same pathophysiology may serve as a precipitating factor in other gastrointestinal tract malignancies.
Alcohol consumption increases cancer risk in individuals with diabetes, associated with an increased susceptibility to oral, pharyngeal, colorectal, laryngeal, pancreatic, liver, and lung cancers11,12. Specifically, well-documented mechanisms have elucidated alcohol consumption’s contribution to the development of liver cirrhosis and hepatocellular carcinoma13. Alcohol consumption also increases the risk of esophageal cancer, especially esophageal squamous cell carcinomas14.
The American Association for the Study of Liver Diseases (AASLD) recently reclassified steatotic liver disease (SLD), emphasizing metabolic dysfunction and alcohol as key risk factors15. The AASLD reclassified NAFLD as metabolic dysfunction-associated steatotic liver disease (MASLD), incorporating metabolic syndrome into the criteria by requiring at least one of five cardiometabolic risk factors in the diagnosis. SLD was further categorized into five categories based on alcohol consumption and specific etiology15. The main purpose of this reclassification was to emphasize the subcategories of SLD as a precursor to liver cirrhosis and hepatocellular carcinoma16. As a result, there has been undermined interest in the potential relationship between new subcategories of SLD and other gastrointestinal cancers, despite the shared risk factors such as alcohol consumption and diabetes. Limited studies17,18 has been done to clarify the relationship in the general population, including a Korean nationwide cohort analysis revealing a positive correlation between NAFLD and esophageal, gastric, and colorectal cancers18. Nonetheless, they have not utilized precisely revised AASLD criteria, and the relationship between MASLD and certain gastrointestinal cancers in high-risk populations, such as in diabetic population, requires further analysis.
Therefore, our objective was to investigate the correlation between recently defined MASLD, alcohol-related SLD and the incidence of five major gastrointestinal tract malignancies in a large-scale diabetic cohort. Specifically, we aimed to comprehensively assess the correlation of the newly created MASLD category with gastrointestinal tract cancers and revalidate alcohol dosage-dependent relationship with gastrointestinal tract cancer in SLD.
Results
Characteristics of the study population
Table 1 presents the baseline characteristics of the study population. In the overall population, 60.6% were male, with a mean age of 59.1 ± 12.1 years and a mean body mass index (BMI) of 25.7 ± 3.5 kg/m2. We categorized the participants into four subgroups, groups 1, 2, 3, and 4, each comprising 834,111, 1,120,221, 127,312, and 93,741 participants, respectively. Significant differences were observed in income, smoking status, drinking status, CKD presence, CCI score, diabetes duration, insulin use, BMI, waist circumference, systolic and diastolic blood pressure, serum high-density lipoprotein-cholesterol (HDL-C), serum low-density lipoprotein-cholesterol (LDL-C), serum triglyceride, serum aspartate transaminase, serum alanine transaminase, and serum γ-glutamyl transpeptidase among the groups (p < 0.0001, Table 1). Esophageal cancer had a follow-up of 5.8 ± 0.9 years with 1,989 cases, stomach cancer had 5.8 ± 1.0 years and 20,736 cases, colorectal cancer had 5.8 ± 1.0 years and 27,219 cases, biliary tract cancer had 5.8 ± 0.9 years and 7,054 cases, and pancreatic cancer had 5.8 ± 1.0 years and 15,458 cases.
Table 1.
Baseline characteristics of enrolled NHIS health screening cohort patients.
| Characteristics | No Steatosis (Group 1) |
MASLD (Group 2) |
MetALD (Group 3) |
ALD (Group 4) |
P value |
|---|---|---|---|---|---|
| (n = 834,111) | (n = 1,120,221) | (n = 127,312) | (n = 93,741) | ||
|
Age at enrollment, y a |
61.8 (11.8) |
58.1 (12.1) |
52.9 (10.3) |
55.7 (10.4) |
< 0.0001 |
| < 40 |
28,676 (3.4) |
69,981 (6.3) |
11,290 (8.9) |
5,116 (5.5) |
< 0.0001 |
| 40–64 |
459,333 (55.1) |
711,595 (63.5) |
100,450 (78.9) |
70,944 (75.7) |
|
| ≥ 65 |
346,102 (41.5) |
338,645 (30.2) |
15,572 (12.2) |
17,681 (18.9) |
|
| Sex (male) |
394,254 (47.3) |
720,503 (64.3) |
119,923 (94.2) |
83,989 (89.6) |
< 0.0001 |
|
BMI, mean (SD), kg/m2 |
22.8 (2.3) |
27.0 (3.3) |
26.4 (3.2) |
26.4 (3.4) |
< 0.0001 |
|
Waist circumference, cm a |
79.5 (6.4) |
90.4 (7.7) |
90.0 (7.8) |
90.3 (8.1) |
< 0.0001 |
| Smoking | < 0.0001 | ||||
| Non |
553,191 (66.3) |
583,563 (52.1) |
23,119 (18.2) |
24,495 (26.1) |
|
| Former |
146,947 (17.6) |
262,983 (23.5) |
43,138 (33.9) |
28,981 (30.9) |
|
| Current |
133,973 (16.1) |
273,675 (24.4) |
61,055 (48.0) |
40,265 (43.0) |
|
|
Regular exercise b |
200,123 (24.0) |
222,870 (19.9) |
25,989 (20.4) |
19,324 (20.6) |
< 0.0001 |
|
CCI Score, ≥5 |
162,113 (19.4) |
173,614 (15.5) |
10,152 (8.0) |
20,497 (21.9) |
< 0.0001 |
|
Income, Lowest Q1 |
185,156 (22.2) |
237,849 (21.2) |
23,185 (18.2) |
21,140 (22.6) |
< 0.0001 |
|
SBP, mmHg a |
125.7 (15.0) |
130.1 (14.8) |
131.8 (14.8) |
130.7 (14.9) |
< 0.0001 |
|
DBP, mmHg a |
75.6 (9.5) |
79.5 (9.8) |
82.1 (10.2) |
80.8 (10.1) |
< 0.0001 |
| DM duration | < 0.0001 | ||||
| New onset |
202,110 (24.2) |
372,376 (33.2) |
60,489 (47.5) |
28,701 (30.6) |
|
| < 5 years |
197,471 (23.7) |
320,615 (28.6) |
32,262 (25.3) |
29,426 (31.4) |
|
| < 10 years |
171,170 (20.5) |
208,282 (18.6) |
18,749 (14.7) |
18,836 (20.1) |
|
| ≥ 10 years |
263,360 (31.6) |
218,948 (19.6) |
15,812 (12.4) |
16,778 (17.9) |
|
|
Fasting glucose, mg/dL a |
139.2 (44.6) |
148.5 (46.5) |
154.8 (46.1) |
151.2 (47.9) |
< 0.0001 |
|
Medication for DM |
632,001 (75.8) |
747,845 (66.8) |
66,823 (52.5) |
65,040 (69.4) |
< 0.0001 |
|
OHA, ≥ 3yrs |
196,565 (23.6) |
254,400 (22.7) |
21,331 (16.8) |
23,305 (24.9) |
< 0.0001 |
| Metformin |
568,277 (68.1) |
679,768 (60.7) |
61,806 (48.6) |
59,766 (63.8) |
< 0.0001 |
| Sulfonylurea |
289,342 (34.7) |
370,460 (33.1) |
31,968 (25.1) |
32,984 (35.2) |
< 0.0001 |
| Thiazolidinedione |
67,719 (8.1) |
78,018 (7.0) |
6,883 (5.4) |
7,204 (7.7) |
< 0.0001 |
|
DPP-4 inhibitor |
353,239 (42.4) |
429,610 (38.4) |
37,377 (29.4) |
38,050 (40.6) |
< 0.0001 |
| Meglitinide |
5223 (0.6) |
4281 (0.4) |
224 (0.2) |
351 (0.4) |
< 0.0001 |
|
SGLT-2 inhibitor |
17,844 (2.1) |
32,543 (2.9) |
2,765 (2.2) |
2,784 (3.0) |
< 0.0001 |
| GLP-1 RA |
217 (0.0) |
564 (0.1) |
28 (0.0) |
38 (0.0) |
< 0.0001 |
| Insulin |
75,878 (9.1) |
77,529 (6.9) |
4,443 (3.5) |
6,912 (7.4) |
< 0.0001 |
| CKD |
84,375 (10.1) |
111,944 (10.0) |
4,788 (3.8) |
5,606 (6.0) |
< 0.0001 |
|
eGFR, mL/min/ 1.73m2 a |
88.8 (49.1) |
89.0 (54.9) |
96.0 (63.5) |
93.8 (54.5) |
< 0.0001 |
|
Total Cholesterol, mg/dLa |
177.0 (40.7) |
192.2 (44.8) |
198.7 (44.1) |
188.1 (44.6) |
< 0.0001 |
|
Triglyceride, mg/dL c |
96 (72–128) |
169 (123–237) |
192 (134–283) |
179 (125–265) |
< 0.0001 |
|
HDL-C, mg/dL a |
54.2 (15.3) |
48.5 (13.6) |
52.4 (16.1) |
51.4 (15.8) |
< 0.0001 |
|
LDL-C, mg/dL a |
101.7 (36.5) |
106.2 (39.8) |
104.4 (39.9) |
97.1 (39.8) |
< 0.0001 |
|
AST, IU/L c) |
22 (18–27) |
26 (21–36) |
29 (23–40) |
31 (23–46) |
< 0.0001 |
|
ALT, IU/L c) |
19 (15–26) |
29 (21–44) |
31 (22–46) |
32 (22–49) |
< 0.0001 |
|
r-GTP, IU/L c) |
20 (15–28) |
41 (27–67) |
77 (49–130) |
78 (45–149) |
< 0.0001 |
Unless otherwise noted, presented values are No. (%).
ALD, alcoholic liver disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD, chronic kidney disease; CCI, Charlson comorbidity score; DM, diabetes mellitus; DBP, diastolic blood pressure; DPP-4, dipeptidyl peptidase-4; eGFR, estimated glomerular filtration rate; GLP-1 RA, glucagon like peptide-1 receptor agonist; γ –GTP, γ-glutamyl transpeptidase; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MASLD, metabolic dysfunction-associated steatotic liver disease; MetALD, MASLD with heavy alcohol intake; NHIS, national health insurance service; SBP, systolic blood pressure; SGLT-2, sodium glucose co-transporter 2; TG, triglyceride.
a Age at enrollment, BMI, waist circumference, SBP, DBP, fasting glucose, eGFR, total cholesterol, HDL-C, LDL-C are depicted as mean (standard deviation).
b Regular exercise is defined as vigorous exercise over 3 days per week or moderate-intensity engagement of physical activity over 5 days per week.
c Triglyceride and liver function tests are depicted as median (interquartile range).
Gastrointestinal tract cancer risk according to SLD subtypes
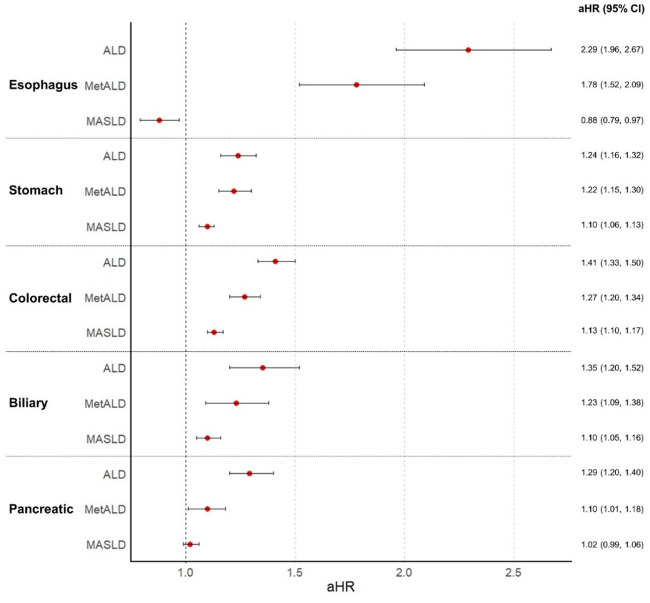
For the main analysis, participants were grouped into four subgroups, as described above, with group 1 as the reference group. Each group was monitored for five subtypes of gastrointestinal tract cancers: esophageal, stomach, colorectal, biliary, and pancreatic. The number of cancer events, incidence rates, and durations in person-years are presented in Supplementary Table S1. Compared with group 1, group 2 showed increased risks of stomach, colorectal, and biliary cancers (aHR [95% CI]: 1.10 [1.06–1.13] for stomach, 1.13 [1.10–1.16] for colorectal, and 1.10 [1.05–1.16] for biliary, respectively); and a decreased risk of esophageal cancer (aHR [95% CI]: 0.88 [0.79–0.97], Fig. 1). The risk of developing gastrointestinal tract cancers compared generally increased from group 2 to group 4 across all types, even after adjusting for confounding variables (p for trend < 0.0001; Fig. 1 and Supplementary Table S1). Subsequent sensitivity analysis by introducing a lag period of 3 years showed similar results, with the increased risk of stomach, colorectal, biliary, and pancreatic cancers in group 2 after adjusting for confounding variables (aHR [95% CI]: 1.11 [1.07–1.15] for stomach, 1.15 [1.11–1.19] for colorectal, 1.07 [1.01–1.14] for biliary, and 1.07 [1.02–1.12], respectively, Supplementary Table S2).
Fig. 1.
Forest plots of hazard ratio between each gastrointestinal cancer and subtypes of Steatotic liver disease. Adjusted hazard ratio (aHR) is depicted for each subtypes of gastrointestinal tract cancer according to SLD status (Adjusted for age, sex, income, smoking status, history of regular exercise, Charlson comorbidity index, fasting glucose, duration of diabetes, use of insulin, medication history of metformin and sulfonylurea, and history of chronic kidney disease). CI, confidence interval; MASLD, metabolic dysfunction-associated steatotic liver disease; metALD, MASLD with heavy alcohol intake; ALD, alcoholic liver disease with metabolic syndrome.
Gastrointestinal tract cancer risk according to probability of hepatic steatosis
To analyze the effect of hepatic steatosis on each gastrointestinal tract cancer, participants were regrouped according to their probability of hepatic steatosis, calculated using the FLI score. Using cutoff scores of 30 and 60, patients were classified into no steatosis (NS), indeterminate steatosis (IS), and probable steatosis (PS) groups. The assigned numbers of participants per group were 834,111 for NS, 714,223 for IS, and 627,051 for PS. Table 2 presents the incidence rate of each gastrointestinal tract cancer, expressed in 1,000 person-years. When adjusted for confounding factors using the multivariate Cox proportional hazards model, the risk of esophagus, stomach, colorectum, and biliary tract cancers positively correlated with the probability of steatosis. The adjusted hazard ratio for IS and PS were 1.01 and 1.16 for esophageal cancer, 1.09 and 1.16 for stomach cancer, 1.12 and 1.22 for colorectal cancer, 1.06 and 1.23 for biliary cancer, and 1.02 and 1.08 for pancreatic cancer after adjusting for confounding variables, respectively (Table 2). Adjusted hazard ratio of all gastrointestinal tract cancers showed positive correlation to probability of hepatic steatosis (all p for trend < 0.05). This correlation was still valid when 3-year lag period was introduced (Supplementary Table S3).
Table 2.
Incidence and risk of Gastrointestinal tract cancers according to probability of hepatic steatosis in diabetic population.
| Probability of hepatic steatosisa |
No. | Events, No. |
PY | IR | HR (95% C.I) | |||
|---|---|---|---|---|---|---|---|---|
| Model 1b | Model 2c | Model 3d | Model 4e | |||||
| Esophagus | ||||||||
| NS | 834,111 | 709 | 4,815,569 | 0.15 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| IS | 714,223 | 684 | 4,145,498 | 0.17 | 1.12 (1.01, 1.25) | 1.03 (0.93, 1.14) | 1.02 (0.92, 1.14) | 1.01 (0.91, 1.13) |
| PS | 627,051 | 596 | 3,637,224 | 0.16 | 1.11 (1.00, 1.24) | 1.20 (1.07, 1.34) | 1.18 (1.05, 1.32) | 1.16 (1.03, 1.30) |
| P for trend | 0.0415 | 0.0027 | 0.0059 | 0.0185 | ||||
| Stomach | ||||||||
| NS | 834,111 | 7,657 | 4,797,490 | 1.60 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| IS | 714,223 | 7,302 | 4,127,616 | 1.77 | 1.11 (1.07, 1.14) | 1.09 (1.05, 1.13) | 1.08 (1.05, 1.12) | 1.09 (1.05, 1.12) |
| PS | 627,051 | 5,777 | 3,623,392 | 1.59 | 1.00 (0.97, 1.03) | 1.17 (1.13, 1.21) | 1.16 (1.12, 1.20) | 1.16 (1.12, 1.21) |
| P for trend | 0.608 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
| Colorectal | ||||||||
| NS | 834,111 | 10,135 | 4,793,395 | 2.11 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| IS | 714,223 | 9,572 | 4,124,301 | 2.32 | 1.01 (1.07, 1.13) | 1.13 (1.10, 1.17) | 1.13 (1.10, 1.16) | 1.12 (1.09, 1.15) |
| PS | 627,051 | 7,512 | 3,620,544 | 2.07 | 0.98 (0.95, 1.01) | 1.25 (1.21, 1.29) | 1.24 (1.20, 1.28) | 1.22 (1.18, 1.26) |
| P for trend | 0.5782 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
| Biliary | ||||||||
| NS | 834,111 | 2,881 | 4,812,640 | 0.60 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| IS | 714,223 | 2,417 | 4,142,978 | 0.58 | 0.98 (0.92, 1.03) | 1.06 (1.01, 1.12) | 1.06 (1.01, 1.12) | 1.06 (1.01, 1.12) |
| PS | 627,051 | 1,756 | 3,635,697 | 0.48 | 0.81 (0.76, 0.86) | 1.22 (1.15, 1.30) | 1.22 (1.15, 1.30) | 1.23 (1.16, 1.31) |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | < 0.0001 | ||||
| Pancreatic | ||||||||
| NS | 834,111 | 6,262 | 4,807,160 | 1.30 | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) | 1 (Ref.) |
| IS | 714,223 | 5,316 | 4,137,957 | 1.28 | 0.99 (0.95, 1.02) | 1.03 (1.00, 1.07) | 1.03 (0.99, 1.07) | 1.02 (0.99, 1.06) |
| PS | 627,051 | 3,880 | 3,631,800 | 1.07 | 0.82 (0.79, 0.86) | 1.10 (1.05, 1.14) | 1.09 (1.05, 1.14) | 1.08 (1.03, 1.13) |
| P for trend | < 0.0001 | < 0.0001 | < 0.0001 | 0.0007 | ||||
PY, observation duration in person-years; IR, incidence rate per 1,000 person-years; aHR, adjusted hazard ratio; CI, confidence interval; Ref., reference.
a Incidence of each subtype of cancer was grouped according to FLI (fatty liver index) score. No steatosis (NS), indeterminate steatosis (IS), and steatosis-probable (PS) group were defined as FLI score 0 to < 30, 30 < to ≤ 60, and > 60, respectively.
b Model 1 was unadjusted.
c Model 2 was adjusted for age, sex.
d Model 3 was adjusted for age, sex, income, smoking status, history of regular exercise, Charlson comorbidity index.
e Model 4 was adjusted for age, sex, income, smoking status, history of regular exercise, Charlson comorbidity index, fasting glucose, duration of diabetes, use of insulin, medication history of metformin or sulfonylurea, and history of chronic kidney disease.
Gastrointestinal tract cancer risk according to steatosis and daily alcohol consumption
Correlation between FLI score and alcohol consumption was significant (r = 0.262, p < 0.001). The combined analysis of the probability of hepatic steatosis and alcohol consumption is presented in Table 3 The association of gastrointestinal tract cancers with the probability of liver steatosis was prominent in non-drinkers and mild-drinkers. In non-drinkers, the risks of gastrointestinal tract cancers, except esophageal cancer, increased with the probability of liver steatosis (adjusted hazard ratio for IS and PS: 1.09 and 1.18 for stomach cancer, 1.14 and 1.27 for colorectal cancer, 1.01 and 1.27 for biliary cancer, and 1.03 and 1.12 for pancreatic cancer, respectively; p for trend < 0.001). In mild drinkers (< 30 g/d for men and < 20 g/d for women), this trend was less evident; however, it was significant in stomach, colorectal, and biliary cancers (adjusted hazard ratio for NS, IS, and PS: 1.09, 1.16, and 1.22 for stomach cancer, 1.16, 1.24, and 1.32 for colorectal cancer, and 1.02, 1.17, and 1.24 for biliary cancer, respectively; p for trend < 0.001, Table 3). In drinkers, neither esophageal nor pancreatic cancer showed significant association with the probability of liver steatosis. Notably, among alcoholics, the risk of esophageal cancer negatively correlated with the probability of liver steatosis (adjusted hazard ratio for NS, IS, and PS: 7.03, 5.45, and 4.27, respectively; p for trend = 0.0215). These trends were concordant even if lag time was increased to 3 years (Supplementary Table S4).
Table 3.
Risk of Gastrointestinal tract cancers according to FLI score and degree of alcohol consumption in diabetic population.
| Probability of hepatic steatosisa |
aHR (95% C.I.)b |
P-for trend |
P-for interaction |
|||
|---|---|---|---|---|---|---|
| Non- drinkers |
Mild | Heavy | Alcoholic | |||
| Esophagus | 0.1895 | |||||
| NS | 1 (Ref.) | 1.92 (1.63, 2.27) | 3.59 (2.77, 4.66) | 7.03 (4.83, 10.21) | < 0.0001 | |
| IS | 1.00 (0.84, 1.19) | 1.76 (1.48, 2.08) | 3.08 (2.45, 3.87) | 5.45 (3.96, 7.49) | < 0.0001 | |
| PS | 1.14 (0.92, 1.40) | 1.83 (1.53, 2.19) | 2.98 (2.41, 3.68) | 4.27 (3.22, 5.66) | < 0.0001 | |
|
P for trend |
0.3002 | 0.5619 | 0.2221 | 0.0215 | ||
| Stomach | 0.4751 | |||||
| NS | 1 (Ref.) | 1.09 (1.03, 1.15) | 1.20 (1.07, 1.35) | 1.37 (1.09, 1.72) | < 0.0001 | |
| IS | 1.09 (1.04, 1.13) | 1.16 (1.10, 1.22) | 1.30 (1.19, 1.42) | 1.37 (1.16, 1.61) | < 0.0001 | |
| PS | 1.18 (1.12, 1.24) | 1.22 (1.16, 1.29) | 1.31 (1.22, 1.42) | 1.30 (1.14, 1.47) | 0.0075 | |
|
P for trend |
< 0.0001 | 0.0001 | 0.2193 | 0.5906 | ||
| Colorectal | 0.0005 | |||||
| NS | 1 (Ref.) | 1.16 (1.11, 1.22) | 1.43 (1.29, 1.58) | 1.51 (1.24, 1.85) | < 0.0001 | |
| IS | 1.14 (1.10, 1.18) | 1.24 (1.18, 1.29) | 1.38 (1.28, 1.50) | 1.50 (1.30, 1.75) | < 0.0001 | |
| PS | 1.27 (1.21, 1.32) | 1.32 (1.26, 1.39) | 1.42 (1.33, 1.53) | 1.48 (1.33, 1.66) | < 0.0001 | |
|
P for trend |
< 0.0001 | < 0.0001 | 0.8864 | 0.8385 | ||
| Biliary | 0.0058 | |||||
| NS | 1 (Ref.) | 1.02 (0.93, 1.12) | 1.19 (0.96, 1.47) | 1.29 (0.83, 2.00) | 0.1247 | |
| IS | 1.01 (0.94, 1.08) | 1.17 (1.07, 1.27) | 1.48 (1.26, 1.73) | 1.37 (0.99, 1.88) | < 0.0001 | |
| PS | 1.27 (1.17, 1.37) | 1.24 (1.12, 1.37) | 1.20 (1.03, 1.41) | 1.53 (1.21, 1.94) | 0.6531 | |
|
P for trend |
< 0.0001 | 0.0006 | 0.7114 | 0.428 | ||
| Pancreatic | 0.0758 | |||||
| NS | 1 (Ref.) | 1.05 (0.98, 1.11) | 1.23 (1.07, 1.41) | 1.53 (1.19, 1.98) | < 0.0001 | |
| IS | 1.03 (0.98, 1.08) | 1.05 (0.99, 1.11) | 1.22 (1.09, 1.35) | 1.18 (0.96, 1.46) | 0.0056 | |
| PS | 1.12 (1.06, 1.18) | 1.08 (1.01, 1.15) | 1.14 (1.03, 1.25) | 1.30 (1.12, 1.52) | 0.2206 | |
|
P for trend |
0.0003 | 0.488 | 0.2661 | 0.4927 | ||
aHR, adjusted hazard ratio; CI, confidence interval; FLI, fatty liver index, Ref., reference.
a Incidence of each subtype of cancer was grouped according to FLI (fatty liver index) score. No steatosis (NS), indeterminate steatosis (IS), and steatosis-probable (PS) group were defined as FLI score 0 to < 30, 30 < to ≤ 60, and > 60, respectively.
b Adjusted for age, sex, income, smoking status, history of regular exercise, Charlson comorbidity index, fasting glucose, duration of diabetes, use of insulin, medication history metformin or sulfonylurea, and history of chronic kidney disease.
In contrast, the amount of alcohol consumption positively correlated with the risks of all gastrointestinal tract cancers (Supplementary Tables S5 and S6), which was concordant regardless of the probability of liver steatosis. Significant dose-response relationship was observed in esophageal, stomach, and colorectal cancers (all p for trend < 0.01). In pancreatic cancer, significant dose-response relationship was observed in only NS and IS groups. For biliary cancer, this relationship was significant only in the IS group (p for trend < 0.05; Table 3). Interaction between FLI score and alcohol intake were significant for the development of colorectal and biliary cancers (p for interaction = 0.0005 and 0.0058, respectively), while no interaction was observed in development of esophageal or stomach cancer (p for interaction: 0.1895 and 0.4751, respectively); marginal significance was observed for development of pancreatic cancer (p for interaction = 0.0758, Table 3).
Discussion
To our knowledge, this is the first study to comprehensively explore the association between SLD subtypes and various gastrointestinal tract malignancies in patients with diabetes. MASLD is associated with an elevated risk of stomach, colorectal, and biliary malignancies and a reduced risk of esophageal cancer. Higher probability of hepatic steatosis correlates with increased risks of esophageal, stomach, colorectal, and biliary tract cancers, especially in those with lower alcohol intake. Overall, there is a dose-dependent positive correlation between alcohol consumption and the risk of all gastrointestinal tract cancers, particularly in esophageal and colorectal cancers. This is consistent with earlier studies that confirmed the dose-response relationship between alcohol intake and the incidence of early-onset colorectal cancer19 and esophageal squamous cell carcinoma (ESCC)20.
Our data support previous research indicating a heightened susceptibility to digestive tract malignancies in individuals with NAFLD17,21,22. This association may stem from hormonal and metabolic dysregulation linked to obesity. Increased accumulation of fat leads to insulin resistance, which consequently contributes to chronic inflammation and cytokine activation throughout the body23. Adipokines and pro-inflammatory cytokines associated with gastrointestinal tract cancer, such as leptin, adiponectin, plasminogen activator inhibitor-1, resistin-1, interleukin-6, tumor necrosis factor-α, and chemokine macrophage chemoattractant protein-1, are thought to contribute to these systemic alterations24.
However, only esophageal cancer demonstrated a lower risk in patients with MASLD (Fig. 1). This may due to ESCC’s negative correlation with BMI and its high prevalence, which accounts for 96.9% of all esophageal cancer cases in Korea25,26. Therefore, the results of this study are likely influenced by the risk factors associated with ESCC rather than those associated with esophageal adenocarcinoma (EAC), which is positively associated with obesity, diabetes, and gastroesophageal reflux disease27. Previous meta-analyses of ESCC have shown a linear inverse correlation between ESCC and obesity,28 possibly attributed to other residual confounding factors such as smoking. A cohort study found that only smokers had a significant inverse correlation with obesity, whereas a combined analysis that included former or never smokers found no such association26. Several mechanisms have been proposed for this inverse correlation between MASLD and ESCC, including metformin reducing tumor angiogenesis, enhanced immunomodulation by CD8 + lymphocytes, activation of 5’-adenosine monophosphate activated protein kinase, and suppression of mTOR signaling29.
Another finding was the negative correlation between esophageal cancer risk and the probability of hepatic steatosis in heavy drinkers (Table 3). This contrasts with previous findings, such as a similar Korean NHIS cohort study from 2009 to 2012 demonstrating a dose-response relationship between the FLI and esophageal cancer incidence30. The explanation for these contradictory results is unclear, possibly owing to poor mechanistic knowledge between ESCC and steatohepatitis or an inadequate sample size.
Subgroup analysis, stratified by the FLI (an indicator of steatosis likelihood) and alcohol consumption, demonstrated a stronger positive correlation between hepatic steatosis and gastrointestinal tract cancer risk in groups with lower alcohol consumption. This correlation was not observed in heavy drinkers or alcoholic patients, suggesting that the severity of liver steatosis may need to be evaluated differently based on alcohol consumption when assessing cancer risk in each gastrointestinal organ. This suggests that overall alcohol intake plays a more important role in cancer development than the probability of steatosis.
There were several limitations associated with this investigation. First, a recent study suggested discrepancies between values of self-reported alcohol intake and values reported by semi-quantitative alcohol intake measurements using patients’ hair, urine, and serum,31 implying that additional verification methods may be needed to confirm true daily alcohol intake value of individuals. Cancer incidence was reported based on the ICD-10 code entry, thereby lacking histological, imaging, or cancer stage data for individual patients. However, the national health insurance system in Korea, which provides significant insurance coverage (up to 95%) for cancer patients, supports the accuracy of cancer diagnoses by requiring extensive scrutiny for ICD-10 code entry32. Furthermore, prevalent incidence of the ESCC subtype over EAC25 in Korean population reduces potential confounding effects generated by the EAC subtype. Furthermore, since this study was confined to a single nationwide cohort, external validation by additional cohorts from other ethnic groups would be required for generalization.
In conclusion, MASLD is associated with a high risk of gastrointestinal tract malignancy. Probability of hepatic steatosis is associated with an increased risk of stomach, colorectal, and biliary.
cancers. In non-drinkers or mild drinkers, the probability of hepatic steatosis increases the risks of gastrointestinal tract cancers. These findings highlight the importance of carefully assessing the potential of gastrointestinal tract cancer in individuals with diabetes, metabolic syndrome, or steatotic liver disease. Although concurrent MASLD reduces the incidence of esophageal cancer, further research is necessary to fully elucidate this relationship.
Methods
Study design and population
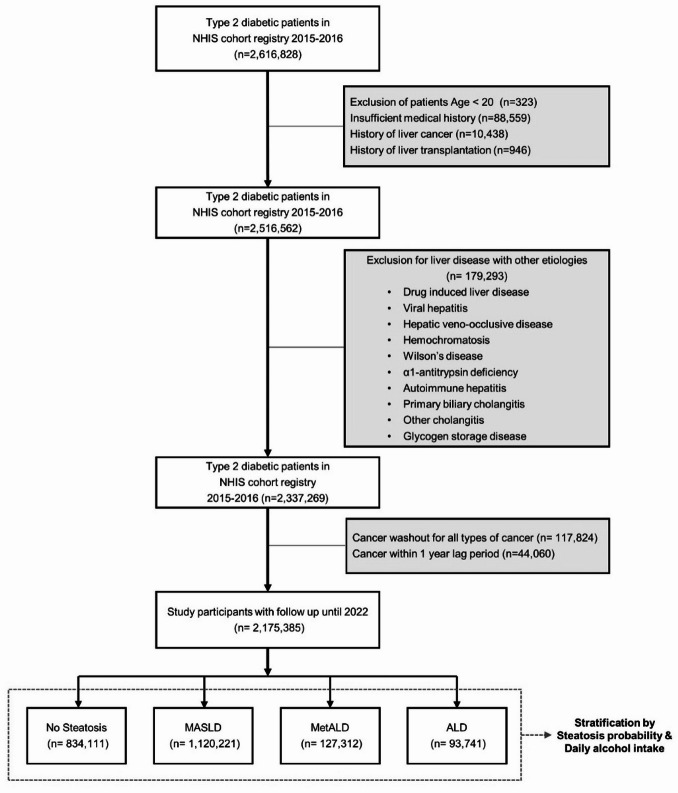
This study used a retrospective cohort from the National Health Insurance Service (NHIS) healthcare screening database, following methodologies outlined in previous literature17. 2,616,828 individuals with type 2 diabetes who received NHIS health checkups from January 1st, 2015 to December 31, 2016 was included for analysis. Excluded individuals were 323 under 20 years old, 88,559 with insufficient medical history, 10,438 with liver cancer, 946 with a history of liver transplantation. Additionally, a total of 179,293 individuals with liver diseases of other etiologies were excluded, based by the identification of the ICD-10 diagnostic code including drug-induced liver disease (K71), viral hepatitis (B15–B19, B00.8, B25.1), hepatic veno-occlusive disease (I82), hemochromatosis (E83.1), Wilson’s disease (E83.0), α1-antitrypsin deficiency (E88.0), autoimmune hepatitis (K75.4), primary biliary cholangitis (K74.3, K74.4), other cholangitis (K83), or glycogen storage disease (E74). Sufficient medical history was defined as having ICD-10 diagnostic codes for each subtype of cancer with date of the diagnosis, along with demographic information, anthropometric measurements, and results of laboratory tests. After implementing a washout period for all cancers, 129,919 patients were excluded, followed by a 1-year lag that excluded 44,060 individuals. The remaining 2,175,385 individuals were included in the analysis and followed up until December 31, 2022. Figure 2 illustrates the study flow and participant overview.
Fig. 2.
Flowchart of patient enrollment process of the NHIS cohort.
Data acquisition
Standardized self-reported questionnaires were used to examine an individual’s health behavior, which included demographic information (age, sex, and income status), smoking behaviors, alcohol intake, and the existence of regular exercise. Anthropometric measurements included height, weight, blood pressure, and waist circumference. The laboratory tests included measurements of serum fasting glucose, total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and estimated glomerular filtration rate (eGFR). Blood tests were conducted following a minimum 8-hour period of fasting. The NHIS diagnostic code data were used to identify comorbidities such as diabetes mellitus (DM) and chronic kidney disease (CKD).
Operational criteria
Each patient’s cancer subtype was identified by searching for registration of differential co-payment registrations in health insurance with concurrent corresponding ICD-10 diagnostic codes of the following: C15 for esophageal cancer, C16 for stomach cancer, C18 – C20 for colorectal cancer, C23 and C24 as for biliary cancer, and C25 for pancreatic cancer. Fatty liver index (FLI) score, as described by Bedogni et al.,33 was used to stratify the probability of hepatic steatosis. The no steatosis (NS), indeterminate steatosis (IS), and probable steatosis (PS) groups were defined by FLI scores of 0–30, 30–60, and > 60, respectively. SLD was defined as an FLI score ≥ 30, whereas MASLD was identified based on at least one adult criterion as per AASLD definitions. For primary analysis, patients were categorized into the following subgroups by their disease status: group 1 (no steatosis, FLI score 0 to < 30), group 2 (MASLD alone), group 3 (MASLD with heavy alcohol intake, metALD, 30–60 g/d for men and 20–50 g/d for women), and group 4: alcoholic liver disease (ALD, alcohol intake ≥ 60 g/d for men and ≥ 50 g/d for women) with metabolic syndrome. In subgroup analysis, patients were grouped into four categories according to their daily alcohol intake: non-drinker (0 g/d), mild-drinker (< 30 g/d for men and < 20 g/d for women), heavy-drinker (30–59 g/d for men and 20–49 g/d for women), and alcoholic (≥ 60 g/d for men and ≥ 50 g/d for women). Supplementary data provides detailed definitions for chronic kidney disease (CKD), type 2 diabetes mellitus, and metabolic syndrome.
Ethics approval
The study protocol was approved by the Institutional Review Board of Seoul National University Bundang Hospital (X-2410-932-902), complied with the 1964 Declaration of Helsinki with its subsequent revisions, and other ethical standards of a similar nature. The retrospective nature of the study waived the requirement for informed consent.
Statistical analysis
We applied χ2-test to identify differences in the proportion of categorical variables, whereas analysis of variance (ANOVA) was applied to evaluate variations between the means of continuous variables. Kruskal–Wallis test was used to evaluate variables with non-nominal distributions. Multivariate Cox proportional hazard regression was used to estimate adjusted hazard ratios (aHR) and 95% confidence intervals (CI) for each type of gastrointestinal tract cancer. The analysis considered variables including age, sex, income, smoking status, regular exercise history, Charlson comorbidity Index (CCI), fasting glucose levels, diabetes duration, insulin use, metformin or sulfonylurea history, and CKD history. Analysis was performed using SAS version 9.4 (SAS Institute, Cary, NC), with a two-sided p-value < 0.05 considered statistically significant.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing.
Author contributions
S.G.I. analyzed the data and drafted the manuscript, tables, and figures, and approved the final version to be published. C.M.S. conceptualized and designed the study, drafted the manuscript and revised the manuscript, and approved the final version. K.H. conceptualized and designed the study, acquired the data, analyzed the data, and supervised statistical analyses. J.H.J. acquired the data, analyzed the data, and provided technical support. J.C., H.J., H.L., E.H.J., S.J.K., J.H.L., and Y.J.C. provided critical review of the manuscript, and approved the final version. D.H.L. supervised overall study, revised the draft, and approved the final version. All authors fulfill the ICMJE criteria for authorship.
Data availability
The data that support the findings of this study are available National Health Insurance Sharing Service (NHISS, https://nhiss.nhis.or.kr/), Republic of Korea. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of NHISS upon reasonable request. Requests for data access should be directed to Prof. Kyungdo Han (hkd917@naver.com).
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Cheol Min Shin, Email: scm6md@gmail.com, Email: brightsky@snu.ac.kr.
Kyungdo Han, Email: hkd917@naver.com, Email: hkd@ssu.ac.kr.
References
- 1.Glovaci, D., Fan, W. & Wong, N. D. Epidemiology of diabetes mellitus and cardiovascular disease. Curr. Cardiol. Rep.21, 21 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Ingelfinger, J. R. & Jarcho, J. A. Increase in the incidence of diabetes and its implications. N Engl. J. Med.376, 1473–1474 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Lawler, T. et al. Type 2 diabetes and colorectal cancer risk. JAMA Netw. Open.6, e2343333 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng, L., Gui, Z., Zhao, L., Wang, J. & Shen, L. Diabetes mellitus and the incidence of colorectal cancer: an updated systematic review and meta-analysis. Dig. Dis. Sci.57, 1576–1585 (2012). [DOI] [PubMed] [Google Scholar]
- 5.El-Serag, H. B., Tran, T. & Everhart, J. E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology126, 460–468 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Cui, Y. & Andersen, D. K. Diabetes and pancreatic cancer. Endocr. Relat. Cancer. 19, F9–F26 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Cheung, K. S. Big data approach in the field of gastric and colorectal cancer research. J. Gastroenterol. Hepatol.39 (6), 1027–1032 (2024). [DOI] [PubMed] [Google Scholar]
- 8.Xu, B., Zhou, X., Li, X., Liu, C. & Yang, C. Diabetes mellitus carries a risk of esophageal cancer: a meta-analysis. Med. (Baltim).96, e7944 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol.71, 793–801 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Vanni, E. et al. From the metabolic syndrome to NAFLD or vice versa? Dig. Liver Dis.42, 320–330 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Bagnardi, V. et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br. J. Cancer. 112, 580–593 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng, R. W. et al. Global prevalence, clinical characteristics, surveillance, treatment allocation, and outcomes of alcohol-associated hepatocellular carcinoma.. Clin. Gastroenterol. Hepatol.22 (12), 2394–2402 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Grewal, P. & Viswanathen, V. A. Liver cancer and alcohol. Clin. Liver Dis.16, 839–850 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Yu, X., Chen, J., Jiang, W., Zhang, D. & Alcohol Alcoholic beverages and risk of esophageal cancer by histological type: a dose-response meta-analysis of observational studies. Alcohol Alcohol. 55, 457–467 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol.79, 1542–1556 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Phoolchund, A. G. S. & Khakoo, S. I. MASLD and the development of HCC: pathogenesis and therapeutic challenges. Cancers (Basel)2024, 16 (2024). [DOI] [PMC free article] [PubMed]
- 17.Lee, J. M. et al. The association between nonalcoholic fatty liver disease and esophageal, stomach, or colorectal cancer: National population-based cohort study. PLoS One15(1), e0226351 (2020). [DOI] [PMC free article] [PubMed]
- 18.Lee, H., Lee, H. W., Kim, S. U. & Chang Kim, H. Metabolic Dysfunction-Associated fatty liver disease increases colon cancer risk: A nationwide cohort study. Clin. Transl Gastroenterol.13 (1), e00435 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin, E. H. et al. Sex and Tumor-Site differences in the association of alcohol intake with the risk of Early-Onset colorectal cancer. J. Clin. Oncol.41, 3816–3825 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandeya, N. et al. Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology136, 1215–1224 (2009). e1211-1212. [DOI] [PubMed] [Google Scholar]
- 21.Parizadeh, S. M. et al. Association between non-alcoholic fatty liver disease and colorectal cancer. Expert Rev. Gastroenterol. Hepatol.13, 633–641 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Allen, A. M., Hicks, S. B., Mara, K. C., Larson, J. J. & Therneau, T. M. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity - A longitudinal cohort study. J. Hepatol.71 (6), 1229–1236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, S. et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res.68 (1), 323–328 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto, K., Ikeya, T., Okuyama, S., Fukuda, K. & Kobayashi, D. The association between non-alcoholic fatty liver disease (with or without metabolic syndrome) and extrahepatic cancer development. J. Gastroenterol. Hepatol.36, 1971–1978 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Jung, H. K. et al. Treatment pattern and overall survival in esophageal cancer during a 13-year period: a nationwide cohort study of 6,354 Korean patients. PLoS One15(4), e0231456 (2020). [DOI] [PMC free article] [PubMed]
- 26.Lindkvist, B. et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: a prospective study of 580,000 subjects within the Me-Can project. BMC Cancer. 14, 103 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dixon, J. L. et al. Association between diabetes and esophageal cancer, independent of obesity, in the united States veterans affairs population. Dis. Esophagus. 29, 747–751 (2016). [DOI] [PubMed] [Google Scholar]
- 28.Smith, M. et al. Esophageal cancer and body mass index: results from a prospective study of 220,000 men in China and a meta-analysis of published studies. Int. J. Cancer. 122, 1604–1610 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Tseng, C. H. Metformin and esophageal cancer risk in Taiwanese patients with type 2 diabetes mellitus. Oncotarget8 (12), 18802–18810 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon, Y. J. et al. Metabolic dysfunction-associated steatotic liver disease and risk of esophageal cancer in patients with diabetes mellitus: a nationwide cohort study. Dis. Esophagus. 37 (8), doae029 (2024). [DOI] [PubMed] [Google Scholar]
- 31.Staufer, K. et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed non-alcoholic fatty liver disease. J. Hepatol.77 (4), 918–930 (2022). [DOI] [PubMed] [Google Scholar]
- 32.Choi, D. W. et al. Cancer care patterns in South korea: types of hospital where patients receive care and outcomes using National health insurance claims data. Cancer Med.12 (13), 14707–14717 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bedogni, G. et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol.6, 33 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available National Health Insurance Sharing Service (NHISS, https://nhiss.nhis.or.kr/), Republic of Korea. Restrictions apply to the availability of these data, which were used under license for this study. Data are available from the authors with the permission of NHISS upon reasonable request. Requests for data access should be directed to Prof. Kyungdo Han (hkd917@naver.com).