Abstract
MicroRNAs (miRNAs) are small non-coding RNAs that regulate gene expression and play critical roles in various cellular processes. Increasing evidence suggests that miRNAs are involved in the development and progression of atherosclerosis, which is the leading cause of myocardial infarction and stroke. These molecules influence key pathological mechanisms, including lipid metabolism, endothelial dysfunction, vascular inflammation, and plaque stability. This review summarizes the role of miRNAs in atherosclerosis-induced cardiac and cerebral infarction and explores their potential as therapeutic targets. We discuss emerging miRNA-based interventions, such as miRNA mimics and inhibitors, which offer promising strategies for disease prevention and treatment. Understanding the regulatory functions of miRNAs in cardiovascular and cerebrovascular events may provide new insights for developing innovative therapies aimed at reducing the burden of atherosclerosis-related diseases.
Subject terms: Atherosclerosis, miRNAs
Facts
miRNA affects atherosclerosis by influencing endothelial cell aging, foam cell formation, vascular smooth muscle cell proliferation and migration.
miRNA affects the development process of myocardial infarction by influencing the repair of myocardial cells, the generation of new blood vessels, and inflammatory responses.
miRNA affects cerebral infarction by regulating neuronal apoptosis, glial cells and their inflammatory response, blood-brain barrier and neovascularization recovery.
The miRNA in different cells can be up-regulated or down-regulated by means of chemotherapy, exosome targeted delivery and other means, so as to improve the myocardial infarction and cerebral infarction caused by atherosclerosis.
Introduction
MicroRNAs, also known as miRNAs, were first discovered in 1993 in the nematode Caenorhabditis elegans. They are small non-coding RNAs with a length of approximately 22 nucleotides. MiRNAs originate in the nucleus, where RNA polymerase II transcribes primary miRNA transcripts (pri-miRNAs). Subsequently, they are cleaved into precursor miRNAs by microprocessors and transferred to the cytoplasm for further processing into single-stranded RNAs, known as mature miRNAs. A single miRNA can modulate the pathways of the entire cell by interacting with a wide range of target genes [1]. Although other mechanisms of action have been proposed, most miRNAs function by binding to the 3′ untranslated region (3′ UTR) of the target messenger RNA (mRNA), which is subsequently degraded or translationally repressed by the RNA-induced silencing complex (RISC) [2]. Rather than completely silencing their target genes, this binding typically results in modest downregulation. Since the discovery of miRNAs, their regulatory roles in various diseases have been extensively reported, including cancer [3], immunotherapy [4], glomerulonephritis [5], schizophrenia [6], and diabetes [7], among others. Research has shown that miRNAs can target genes involved in vascular remodeling processes, such as cell proliferation, apoptosis, motility and the production or degradation of the extracellular matrix. These regulatory effects are categorized as either physiological or pathological, encompassing beneficial adaptive responses to changes in hemodynamics, vasoactive substances, or cytokines, as well as maladaptive responses that may contribute to cardiovascular disease (CVD) [8]. Thus, miRNAs have been mechanistically linked to play a critical regulatory role in the development of atherosclerosis (AS) and post-ischemic neovascularization.
Atherosclerotic disease is the leading global cause of mortality and the common pathological basis for major vascular disorders, including myocardial infarction and cerebral infarction. Its hallmark features include lipid deposition, immune cell infiltration, and sustained inflammatory responses. Numerous risk factors contribute to atherosclerosis, such as hypertension, dyslipidemia, and insulin resistance. Current consensus posits that these factors interact with arterial wall cells to drive chronic vascular inflammation, with endothelial cells, macrophages (a subset of leukocytes), and vascular smooth muscle cells (VSMCs) constituting the principal cellular mediators of disease progression. The pathogenesis of atherosclerosis involves multifaceted mechanisms. Early lesions emerge beneath an intact endothelial layer, where low-density lipoprotein (LDL) particles traverse dysfunctional endothelial cells into the subendothelial space. Following extravasation, these particles undergo retention and biochemical modification (e.g., oxidation), triggering pro-inflammatory signaling cascades [9]. Subsequently, upregulated expression of adhesion factors (notably VCAM-1) in endothelial cells promotes monocyte recruitment to the arterial wall, representing one of the earliest events in atherogenesis. Within the subendothelial space, monocytes differentiate into macrophages. These macrophages amplify inflammation by secreting monocyte chemoattractant protein-1 (MCP-1), a potent chemokine that drives further leukocyte infiltration. In the intima of blood vessels, mature macrophages transform into foam cells rich in LDL particles through phagocytosis, which is a sign of early atherosclerosis. Foam cells further accumulate lipids, and eventually release cholesterol through apoptosis or necrosis, or transport cholesterol through membrane transporters, leading to lipid accumulation in plaque [10]. In the intermediate and advanced stages, smooth muscle cells with repair and protective abilities enter the intima and synthesize collagen-rich extracellular matrix components, while proliferating and aggregating in the arterial wall. This excessive repair response strengthens arterial plaque stability to some extent, but it also results in the luminal stenosis and a reduction in blood flow. Smooth muscle cells also transform to other cell types (called phenotypic modulation), exhibiting a variety of cancer-like hallmarks, such as hyperproliferation [11]. As cholesterol deposits and cellular debris progressively accumulate within the intima, atherosclerotic plaques undergo expansion. A subset of these plaques develop structural instability, predisposing them to rupture—an event that triggers vascular occlusion and thrombus formation, constituting the primary driver of mortality in atherosclerosis (AS). The pathogenic cascade originates from endothelial injury, initiating lipid deposition and oxidative modification within the subendothelial space. These pathological changes recruit circulating monocytes (which differentiate into macrophages) and induce vascular smooth muscle cell migration toward the lesion site, ultimately culminating in the formation of complex atherosclerotic plaques [12].
Accumulating evidence has established a robust association between miRNAs and atherosclerotic pathogenesis. miRNAs exert multifaceted regulatory effects on atherogenesis through distinct mechanisms, including modulating endothelial dysfunction [13], attenuating macrophage-mediated intracellular cholesterol accumulation [14], and controlling VSMC proliferation and migratory dynamics [15]. Based on plaque stability, it can be categorized into stable plaques and vulnerable plaques. Vulnerable plaques are a class of plaques that are prone to rupture and ruptured. Once affected by emotions, exercise, temperature, etc., the hemodynamic stressors or the blood flow will impact on the vessel wall, as a result, the lipids and other substances within the plaque will gush out, forming a thrombus that will block the blood vessel. If the thrombus blocks the coronary artery, it will cause acute myocardial infarction; if it blocks the cerebral blood vessels, it will cause cerebral infarction. Stable plaque surface is thicker and not easy to rupture, but if the stable plaque gradually increases, it will also lead to the gradual narrowing of the lumen of the blood vessel, or even completely block the blood vessel, leading to the interruption of blood supply, causing the occurrence of heart infarction, cerebral infarction, and other diseases [16]. This suggests that miRNAs also play different roles in the regulation of heart infarction and cerebral infarction triggered by atherosclerosis.
This review delineates the pivotal regulatory roles of miRNAs in atherosclerosis and its downstream complications, including myocardial infarction and ischemic stroke. miRNAs exert dual regulatory functions throughout disease progression by orchestrating gene expression networks that govern vascular endothelial homeostasis, inflammatory cascades, and plaque stability. Specific miRNA families demonstrate protective effects through suppression of oxidative stress and pro-inflammatory signaling (e.g., NF-κB), whereas others exacerbate atherosclerotic lesion progression by promoting macrophage lipid retention or fibrous cap thinning. Emerging therapeutic strategies leveraging miRNA mimics or inhibitors (e.g., antagomirs) represent novel paradigms for precision modulation of atherogenesis. Furthermore, the tissue specificity and circulatory stability of miRNAs position them as promising diagnostic biomarkers for subclinical atherosclerosis. Collectively, this synthesis advances translational insights into miRNA-based interventions for cardiovascular and cerebrovascular events, while establishing a conceptual framework for developing next-generation therapeutics targeting epigenetic regulatory axes.
miRNA causes Heart and Brain Infarction through Atherosclerosis
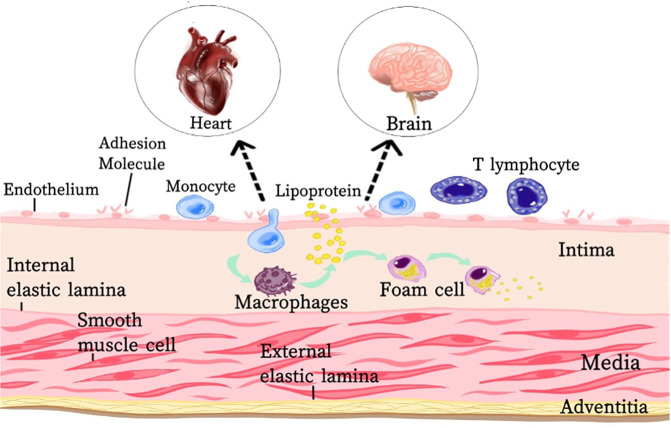
According to recent studies, miRNAs can regulate gene expression changes observed during atherosclerosis, and these miRNAs can be used as biomarkers for early detection of atherosclerosis and targeted therapy. The pathophysiology of AS is modulated by multiple miRNAs, which dichotomously regulate a wide range of biological processes including initiation (endothelial senescence and inflammation), progression (SMC proliferation and migration, increased necrosis of lipid nuclei within plaques, angiogenesis), and endpoint (unstable plaque rupture). In short, with the involvement of miRNAs, endothelial cell function, macrophage recruitment and transformation, and VSMC activity are affected, which in turn promotes or inhibits plaque formation, enlargement, or even rupture, and the formation of thrombus, triggering AMI or cerebral infarction (Fig. 1).
Fig. 1. miRNA causes heart and brain infarction through atherosclerosis.
This figure shows the development process of atherosclerosis and the main participating cells (Endothelial cells damage, vascular smooth muscle cells proliferation and migration, macrophages recruitment). Eventually, plaques are formed in cardiovascular and cerebral vessels, which in turn induce myocardial infarction and cerebral infarction. MiRNAs regulate atherosclerosis by affecting these cells.
miRNAs and endothelial cells
Early atherosclerotic lesions originate from endothelial dysfunction. Emerging research demonstrates that multiple miRNAs regulate endothelial senescence in AS through distinct molecular mechanisms, thereby driving inflammatory cascades (Table 1). Certain miRNAs exhibit pro-atherogenic effects by accelerating endothelial cell senescence and apoptosis, either through repression of anti-aging genes or activation of pro-inflammatory pathways. Among them miR-132/212 promotes AS progression via PTEN suppression, amplifying platelet-derived growth factor BB (PDGF-BB)-induced VSMC proliferation and migration. miR-503-5p, transported via macrophage-derived extracellular vesicles (EVs), suppresses human coronary artery endothelial cell (HCAEC) proliferation/angiogenesis while enhancing human coronary artery smooth muscle cell (HCASMC) migratory capacity. MiR-126-5p in endothelial EVs mediates radiation-induced inflammatory crosstalk to monocytes, increasing monocyte-endothelial adhesion and pro-atherogenic macrophage differentiation. MiR-23a-3p synergistically activates NF-κB and p38/MAPK pathways through TNFAIP3 targeting, concurrently stimulating endothelial apoptosis and inflammasome signaling. This suggests that one miRNA can affect one or even more processes in AS. Some miRNAs, on the other hand, can inhibit the development of atherosclerosis by targeting and repressing genes involved in endothelial cell apoptosis or by suppressing the inflammatory response. Among them, miR-146a-encapsulated liposomes attenuate vascular inflammation in human aortic endothelial cells (HAECs) and VSMCs via ICAM-1 downregulation and monocyte adhesion inhibition. miR-26 exerts dual anti-atherogenic effects by targeting COL1A1, HMGA1/B1, and MALT1 to suppress NF-κB activity, thereby reducing IL-1β, IL-6, and TNF-α production; inhibiting endothelial proliferation and VSMC differentiation through SMAD1/SMAD4 repression, ultimately diminishing plaque burden.
Table 1.
Regulation of atherosclerosis by miRNAs.
| miRNA | target gene | Signaling pathway | Corresponds | Reference |
|---|---|---|---|---|
| miR-125a-5p | RTEF-1 | Pink1-Mfn2-Parkin access road | Inhibition of mitochondrial autophagy | [88] |
| miR-132/212 | Gna12; PTEN | GNA12/PTEN signaling | Exacerbates EC apoptosis; promotes VSMC proliferation and migration | [89] |
| miR-503-5p | Smad7 | Smad7-Smurf1/Smurf2-TGF-β axis | Reduced EC proliferation and angiogenesis and promoted SMC proliferation and migration. | [90] |
| miRNA-130a | PPARγ | PPARγ/NF-κB | promote inflammation | [91] |
| miR-23a-3p | TNFAIP3 | NF-κB and p38/MAPK | Promotes inflammation and endothelial cell apoptosis | [92] |
| miR-92a | SIRT1 and KLF2 | Promotes endothelial cell senescence | [93] | |
| miR-217 | APLNR and VEGFR1 | eNOS Activator Network | exacerbate atherosclerosis | [94] |
| miR-126-5p | promote inflammation | [95] | ||
| miR-652-3p | Ccnd2 | Inhibition of EC proliferation | [96] | |
| miRNA let-7b | HAS-2 | P13K/Akt access | Inhibition of EC apoptosis | [97] |
| miR-181a-5p/3p | TAB2 and NEMO | NF-κB | Reducing inflammation | [98] |
| miR-146a | ICAM-1 | Reducing inflammation | [99] | |
| miR-26 | SMAD1 and SMAD4, etc. | Inhibition of EC growth, angiogenesis and VSMC differentiation | [100] | |
| miR-21-5p | SKP2 | SKP2/EP300/ HMGB1 | Enhances macrophage polarization and promotes inflammation | [101] |
| miR-30b-5p | UBE2D2 | UBE2D2/KAT2B/ HMGB1 | Promote polarization of pro-inflammatory cells and recruitment of macrophages | [102] |
| miR-30a-3p | ABCA1 | Promote foam cell formation | [103] | |
| miR-216a | Smad3 | NF-κB | Facilitated conversion of M2 to M1 | [104] |
| miR-127-3p | SCD-1 | SCD1/UFA | Promote macrophage proliferation | [105] |
| miR-520a-3p | UVRAG | IL4/IL13 | Reduction of macrophage autophagy | [106] |
| miR-146a | IGF2BP1 and HuR | Reduced macrophage migration | [107] | |
| miR-369-3p | GPR91 | Succinate-GPR91-HIF-1α-inflammasome signaling axis | Reduces mitochondrial damage and inflammation | [108] |
| miR-21 | MKK3 | p38-CHOP and JNK signaling | Enhancement of macrophage apoptosis | [109] |
| miR-155-5p | CD36 and Vav3; SOCS1 | KEGG pathway | Inhibition of ox-LDL uptake; increased macrophage cholesterol efflux | [110] |
| miR-382-5p | BMP4 | PPARγ-ABCA1 /ABCG1 | Foam cell formation decreases and cholesterol outflow increases | [111] |
| miR-1914-5p | ICAM, Mac-1, MCP-1 | Inhibition of monocyte recruitment | [112] | |
| miR-140-5p | ROBO4 | ROBO4/VEGF signaling pathway | Inhibition of VSMC apoptosis | [17] |
| miR-214 | Smad7 | TGF-β/Smad pathway | Promotes VSMC proliferation, migration, contraction, hypertrophy and stiffness | [18] |
| miR-92a | KLF4 | Inhibition of VSMC proliferation and migration | [19] | |
| miR-17 | IRF9 | ditto | [113] | |
| miR-146b | Bag1 and Mmp16 | ditto | [114] | |
| miR-214 | NCKAP1 | ditto | [115] | |
| miR-663 | JunB | JunB/myosin light chain 9 | ditto | [116] |
| miR-223-3p | STAT3 | IL-6/STAT3 | Blocking osteogenic transformation and calcification in VSMC | [117] |
| miR-99a-5p | HOXA1 | Inhibition of ASMC proliferation, migration and invasion | [118] | |
| miR-15a-5p /199a-3p | IKKα, IKKβ and p65 | NF-κB | Reduced ox-LDL uptake and inflammation in VSMC. | [20] |
| Let-7d-5p | OLR | NF-κB | Reduced ox-LDL uptake in VSMC and maintained the contractile phenotype | [21] |
miRNAs and macrophages
Atherosclerosis arises from chronic inflammatory processes characterized by macrophage maturation, LDL uptake, and subsequent foam cell formation. The accumulation of cholesterol-laden foam cells within the intima of large arteries marks the onset of early “fatty streak” lesions, which progressively recruit additional cell types and evolve into complex multicellular atherosclerotic plaques. Specific miRNAs exacerbate inflammation and foam cell pathology through distinct mechanisms (Table 1). Certain miRNAs drive macrophage polarization and recruitment via post-translational modifications (e.g., HMGB1 acetylation) mediated by target gene networks, while others impair cholesterol efflux through transcriptional repression of ATP-binding cassette transporters (e.g., ABCA1). Notably, some miRNAs amplify pro-inflammatory M1 macrophage polarization via NF-κB pathway activation, thereby accelerating plaque progression.
Conversely, protective miRNAs counterbalance these effects by attenuating inflammatory cascades and foam cell dynamics. Key mechanisms include: mitigating macrophage oxidative damage through redox-sensitive gene regulation; enhancing cholesterol efflux via ABCA1/ABCG1 upregulation; and suppressing monocyte-to-macrophage differentiation and recruitment. These regulatory axes collectively modulate the inflammatory microenvironment critical for atherosclerosis pathogenesis.
miRNAs and vascular smooth muscle cells
One of the key events in atherosclerosis is the shift of SMC from a contractile phenotype to a synthetic phenotype. MiRNAs can exert atherosclerosis regulation by accelerating or inhibiting the phenotypic transition (Table 1). Some miRNAs promote SMC proliferation and migration and inhibit SMC apoptosis, thereby accelerating the disease progression. For example, high levels of miR-140-5p promotes VSMC proliferation, migration, and invasion, and inhibits VSMC apoptosis by reducing ROBO4 expression [17]; miR-214 inhibits the level of its target gene Smad7, thereby negatively regulating the TGF-β/Smad pathway. Meanwhile, miR-214 established crosstalk between angiotensin II (Ang II)-induced AT1R signaling and TGF-β-induced TGF-β/Smad signaling. Knocking out miR-214 can inhibit a series of changes induced by Ang II in VSMCs, such as proliferation, migration, swelling, and stiffness [18]. MiR-92a regulates VSMC to a synthetic phenoty through Kruppel-like factor 4 (KLF4) pathway and promotes VSMC proliferation and migration [19]. Some miRNAs can inhibit the transition of VSMC from a contractile to a synthetic phenotype, thereby slowing down the development of atherosclerosis by the mechanism of inhibiting the proliferation and migration of VSMCs, which mechanisms are through modulating of interferon regulatory factors, anti-apoptotic genes, matrix metalloproteinases, and differentiation marker genes. In addition, miR-15a-5p and miR-199a-3p reduced ox-LDL uptake and NF-κB-regulated inflammation in VSMC [20]. Overexpression of let-7d-5p in HAoSMC resulted in a decrease in the number of ox LDL receptor OLR1 on the cell membrane, thereby attenuating pro-inflammatory signaling cascades [21].
miRNA and Myocardial Infarction
Atherosclerotic plaque occlusion or rupture represents the initial pathological event precipitating myocardial infarction. Subsequent thrombosis causes vascular obstruction, compromising systemic circulation and end-organ perfusion. Prolonged ischemia triggers rapid infiltration of neutrophils and inflammatory monocytes into the affected myocardium, resulting in hypoxia-induced cardiomyocyte apoptosis and necrosis. This cellular demise precipitates acute cardiac dysfunction, which may progress to maladaptive ventricular remodeling and ultimately chronic heart failure. Repair of cardiomyocytes, neovascularization, and inhibition of the inflammatory response in a timely manner are the three critical components of this process.
Myocyte Repair
In the adult mammalian heart, the majority of cardiomyocytes exist in a terminally differentiated state with severely restricted proliferative potential, rendering the heart incapable of meaningful regeneration following ischemic injury. Recovery of cardiomyocyte numbers after myocardial infarction can be achieved by inhibiting cardiomyocyte apoptosis, promoting cardiomyocyte proliferation, and mediating progenitor cell differentiation and reprogramming of non-cardiomyocytes (e.g., fibroblasts). The number of miRNAs has been found to be involved in this process (Table 2).
Table 2.
Regulation of infarction by miRNAs.
| miRNA | Target gene | Signaling pathway | Corresponds | Reference |
|---|---|---|---|---|
| miR-103-3p | Hlf | Hlf/Fyco1 | Inhibition of autophagy and promotion of apoptosis in cardiomyocytes | [119] |
| miR-503 | PGC-1β and SIRT3 | Promotes cardiomyocyte death | [120] | |
| miRNA-542-5p | ATG7 | Inhibition of autophagy thereby exacerbating cardiomyocyte damage | [121] | |
| miR-24-3p | HO1 | circCHSY1/miR-24-3p/ HO1 | Aggravated mitochondrial damage in cardiomyocytes | [122] |
| miR-141-3p | CHD8 | CHD8/p21 | Attenuates cardiomyocyte apoptosis | [123] |
| miRNA-146a | NOX4 | NOX4/P38 | Reduction of oxidative stress damage in cardiomyocytes | [124] |
| miR-663b | BCL2L1 | Reduction of cardiomyocyte apoptosis | [125] | |
| miR-450b-5p | ACSL4 | Reduction of cardiomyocyte iron death | [126] | |
| miR-181a | HK2;Adamts1;PDCD4 | Ngal/Aldo-MR; PDCD4/BID | dual role | [27–29] |
| miRNA-21 | Ajuba | ajuba/Isl1 axis access road | Differentiation of BMSCs to cardiomyocyte-like cells | [30] |
| miRNA-29c | PTEN | PTEN/Akt/mTOR signaling pathway | Inhibition of excessive autophagy in cardiomyocytes | [31] |
| miR-126-3p | TSC1 | TSC1/mTORC1 /HIF-1α /VEGFA | Promoted HUVEC proliferation, angiogenesis and migration | [127] |
| miR-543 | COL4A1 | Promotes endothelial cell angiogenesis | [128] | |
| miR-494-3p | Promoting neovascularization after MI | [129] | ||
| miR-486-5p | MMP19 | MMP19/VEGFA cleavage signaling | Increased angiogenic activity of endothelial cells | [130] |
| miR-132-3p | THBS1 | Promoting angiogenesis after MI | [131] | |
| miR-106a | ATG7 | Inhibition of endothelial cell proliferation, autophagy | [32] | |
| miR-409-3p | DNAJB9 | MAPK | Reduced EC proliferation and migration | [33] |
| miR-873-5p | Cab39 | Cab39/AMPK signaling pathway | Inhibition of MSC autophagy leads to MSC cell senescence | [34] |
| miR-155-5p | Cab39 | Cab39/AMPK signaling pathway | Inhibition of MSC Aging | [35] |
| miR-1246 | ELF5 | ELF5/CD31 | Promoting phenotypic transition of fibroblasts to ECs, angiogenesis and proliferation in HCF | [36] |
| miR-1290 | SP1 | SP1/CD31 | Enhanced angiogenesis | [36] |
| miR-214-3p | PTEN | p-AKT signaling pathway | Enhanced endothelial cell migration and reduced cardiomyocyte apoptosis | [37] |
| miRNA-205 | Promotes endothelial cell proliferation and angiogenesis | [38] | ||
| miRNA-24 | eNOs | Increased angiogenesis and induced fibroblast apoptosis | [39] | |
| miR-218-5p or miR-363-3p | p53 and JMY | p53/JMY | Promote the transformation of mesenchymal endothelial cells and inhibit myocardial fibrosis | [42] |
| miR-150 | Sprr1a | Improvement of myocardial fibrosis after MI | [43] | |
| miR-590-3p | ZEB1 | ZEB1-Col1A1/Col3A1 | Inhibition of cardiac fibroblast proliferation, differentiation, migration and collagen synthesis | [44] |
| miR-21 | Jagged1 | TGF-β1/ miR-21/ Jagged1 | Promotes conversion of cardiac fibroblasts to myofibroblasts cells and myocardial fibrosis | [45] |
| miR-133a-3p | LTBP1 and PPP2CA; Ash1l | TGF-β pathway | Inhibition of excessive replacement fibrosis and improvement of cardiac function after MI; inhibition of macrophage M1 polarization and attenuation of cardiac inflammation | [46, 47] |
Among the first identified miRNAs are miR-1, miR-133, and miR-499. In mouse teratoma-derived P19 cells - a well-established in vitro model for cardiomyocyte differentiation - miR-1 overexpression suppresses cardiac lineage commitment through dual mechanisms: enhancing cellular proliferation via upregulation of Hand2 expression, and inhibiting apoptosis by blocking caspase-3 cleavage [22]. MiR-133 can promote cardiac reprogramming through direct inhibition of Snai1 and silencing of fibroblast features to promote cardiac reprogramming [23]. Thus, miR-1 and miR-133 exert opposing regulatory effects on non-cardiomyocyte-to-cardiomyocyte transdifferentiation. Furthermore, these miRNAs demonstrate antagonistic roles in modulating post-ischemic cardiomyocyte apoptosis: elevated miR-1 and/or reduced miR-133 levels promote apoptotic cell death, whereas the inverse expression pattern enhances cell survival. This bidirectional regulation likely occurs through miRNA-mediated post-transcriptional silencing - miR-1 targets heat shock [24]. Mir-499 inhibits cardiomyocyte apoptosis by suppressing the dephosphorylation of dynamic protein-1 (Drp1) mediated by calmodulin phosphatase [25]. MiR-1 and -499 inhibit the proliferation of cardiomyocyte progenitor cells (CMPCs) and enhance the differentiation to cardiomyocytes by decreasing the levels of histone deacetylase 4 and Sox6 protein [26].
Recent advances have led to the identification of numerous miRNA that participate in post-MI cardiomyocyte regulation. Certain miRNAs suppress autophagy while promoting cardiomyocyte apoptosis through multi-pathway targeting of autophagy- and apoptosis-related genes. Conversely, other miRNAs exhibit cardioprotective effects by attenuating apoptotic pathways in cardiomyocytes. Notably, miR-181a-mediated downregulation of hexokinase 2 (HK2) led to increased mitochondrial outer membrane permeability, which resulted in apoptosis of cardiomyocytes after myocardial infarction [27], however, miR-181a directly targeted Adamts1 to regulate the level of lipid transporter protein-2 (Ngal), which improved cardiac function and inactivated the Aldo-MR pathway, proving miR-181a has a cardioprotective effect [28]; it can also play an anti-apoptotic role by targeting PDCD4 to regulate the recruitment of BID to mitochondria [29].This suggests that a miRNA may exert opposite effects through different target genes or pathways, suggesting the limitations of therapy at this target. In addition, miRNA-21 can promote the differentiation of bone marrow mesenchymal stem cells (BMSCs) into cardiomyocytes through the Ajuba/ISL1 signaling pathway [30].miRNA-29c inhibits excessive autophagy in cardiomyocytes through the PTEN/Akt/mTOR axis pathway, thereby attenuating cardiac ischemia/reperfusion injury [31].
Neovascularization
Functional cardiac tissue regeneration necessitates sufficient vascular perfusion to meet the high metabolic demands of cardiomyocytes. Consequently, post- MI neovascularization plays a pivotal role in this reparative process. Emerging evidence indicates that multiple miRNAs modulate angiogenesis through diverse mechanisms, principally by regulating EC proliferation and migration, as well as enhancing stem cell-mediated protection and tissue repair (Table 2).
Some miRNAs promote endothelial cell proliferation by inhibiting target genes and up-regulating pathways such as vascular endothelial growth factor, thus promoting neovascularization after MI; some miRNAs exert inhibitory effects, such as miR-106a attenuates venous endothelial cell proliferation, cell cycle progression, autophagy, and angiogenesis by targeting autophagy-related gene (ATG7) [32], miR-409-3p reduces EC proliferation and migration and inhibits neovascularization and tissue repair by targeting DNAJ homologous subfamily B member 9 (DNAJB9) [33]. In addition, regulation of the transformation of MSCs or fibroblasts to endothelial cells is also a function of miRNAs. For example, miR-873-5p and miR-155-5p inhibit the mitochondrial autophagy pathway in mesenchymal stem cells (MSCs) through the AMPK signaling pathway, and lead to senescence of MSCs by inhibiting the expression of Cab39, which inhibits the vasculature after AMI Generation process [34, 35] miR-1246 and miR-1290 induce upregulation of ELF5 and SP1 by binding to their respective gene promoters. ELF5 and SP1 bind to the CD31 promoter, leading to upregulation of CD31 in human cardiac fibroblasts (HCF) and promoting phenotypic transition from fibroblasts to endothelial cells, angiogenesis, and proliferation in HCF [36]. In fact, cardiomyocyte repair and neovascularization are two closely linked processes, and the same miRNA can act on both cardiomyocytes and neovasculature, but the final biological effects may be the same or opposite. For example, miR-214-3p targeting PTEN can enhance endothelial cell migration by activating the p-AKT pathway, thereby accelerating angiogenesis in hypoxic necrotic areas and reducing myocardial cell apoptosis, thereby reducing infarct size and restoring myocardial function [37]; Adipose tissue-derived mesenchymal stem cells (ADSC)-Exos can reduce cardiomyocyte apoptosis and simultaneously increase angiogenesis through miR-205. Apoptosis while increasing angiogenesis [38]. However, local adenovirus mediated miR-24 targeted administration increases peri infarct angiogenesis and cardiac blood supply, reduces necrotic areas, promotes fibroblast apoptosis, and improves overall cardiac function [39]; However, miR-24 exhibited the opposite effect in endothelial cells by targeting transcription factor GATA2 and the p21-activated kinase PAK4 thereby inducing apoptosis in ECs [40]. This shows that miRNA-24 has different physiological effects on endothelial cells, cardiomyocytes and fibroblasts, so the treatment of MI through miRNA-24 inhibition should be targeted to fibroblasts.
Inflammatory response
Myocardial infarction triggers a robust inflammatory response, which serves as a crucial compensatory mechanism following ischemic injury but may paradoxically contribute to adverse outcomes such as heart failure when dysregulated. Post-infarction, Toll-like receptor (TLR) signaling activation rapidly engages innate immune pathways through upregulated cytokine and chemokine production. Leukocytes infiltrate the infarcted myocardium, where they phagocytose cellular debris while dynamically modulating their own phenotypic and functional states. This inflammatory cascade subsequently recruits and activates mesenchymal repair cells - predominantly myofibroblasts and vascular smooth muscle cells - that secrete extracellular matrix components to preserve left ventricular architecture. However, excessive matrix deposition ultimately results in cross-linked collagen scar formation, compromising cardiac function [41]. Therefore, timely suppression of inflammatory reactions, especially myocardial fibrosis, is crucial for the recovery of MI. Some miRNAs are involved in this process (Table 2), for example, exo-miR-218-5p or exo-miR-363-3p up-regulate cardiac fibroblast p53 and down-regulate JMY expression, which promotes mesenchymal-endothelial transition to inhibit myocardial fibrosis [42]. MiR-150 directly targets the pro-apoptotic small proline-rich protein 1a (Sprr1a) in cardiomyocytes) in cardiomyocytes, thereby ameliorating myocardial fibrosis after MI [43]. MiR-590-3p inhibits the proliferation, differentiation, migration, and collagen synthesis of cardiac fibroblasts by suppressing the expression of ZEB1 [44]. MiR-21 promotes the transformation of cardiac fibroblasts into myofibroblasts and myocardial fibrosis by targeting JAGGED1 [45]. Serum exosomes (IPC-exo) directly target LTBP1 and PPP2CA by transfection of miR-133a-3p to inhibit excessive replacement fibrosis and improve cardiac function after MI, and the mechanism is related to the indirect regulation of the TGF-β pathway [46]; Cardiac tissue-derived EVs (ncEVs) inhibit LPS-induced macrophage M1 polarization, attenuate cardiac inflammation and improve cardiac function, while upregulating their phagocytosis via the regulation of Ash1l pathway by miR-133a-3p [47].
miRNA and Cerebral Infarction
Cerebral infarction, clinically termed ischemic stroke, manifests as a spectrum of neurological symptoms resulting from disrupted cerebral blood supply. This condition arises from diverse cerebrovascular events including cardiac embolism, microvascular occlusion, and atherosclerotic thrombosis [48]. Post-ischemic cerebral hypoxia triggers a cascade of pathological responses - including oxidative stress, inflammatory activation, and cytotoxic edema - culminating in selective neuronal death within the ischemic penumbra [49, 50]. Distinct from myocardial infarction, neuroinflammation constitutes a fundamental pathophysiological component of cerebral infarction progression. Following ischemic stroke, cerebral hypoxia induces both necrotic and reactive oxygen species (ROS)-mediated apoptotic neuronal death, initiating a regulated inflammatory cascade characterized by: chemokine (CCL2, CXCL10) and cytokine release; resident microglial proliferation/activation; and peripheral leukocyte recruitment (monocyte-derived macrophages, neutrophils, and lymphocytes) [51].
The repair of neurovascular units after cerebral infarction is mainly through reducing neuronal apoptosis, inhibiting immune-inflammatory response, promoting neovascularization and thus repairing the blood-brain barrier (BBB) (in addition, the glial scar produced after cerebral infarction should be degraded), thus promoting neurovascular regeneration and alleviating cerebral edema and inflammation. Many miRNAs and their target genes participate in multiple pathophysiological processes related to cerebral infarction through different signal transduction pathways.
Apoptosis and the inflammatory response
Inflammation is influenced by signaling pathways associated with multiple factors, including mitogen-activated protein kinase (MAPK), nuclear transcription factor kappa B (NF-κB), and phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT). The activation of the NF-κB pathway is closely related to the development of neuroinflammation [52]. Ischemic reperfusion (I/R) lesions can activate the NF-κB pathway, further promoting the transcription of pro-inflammatory cytokine target genes such as IL-1β, TNF-α, IL-6, and inducing inflammatory responses [53], and many miRNAs have been found to play either positive or negative roles in this neural pathway (Table 3).
Table 3.
miRNA and cerebral infarction.
| miRNA | Target gene | Signaling pathway | Corresponds | Reference |
|---|---|---|---|---|
| miR-155 | MafB | NF-κB pathway | Promotes inflammatory response and apoptosis | [132] |
| miR-155-5p | DUSP14 (also known as MKP6) | NF-κB | Promotes cellular pyroptosis and inflammatory responses | [133] |
| miR-100-5p | TLR7 | NF-κB | Promotion of neuronal apoptosis and activation of the innate immune response | [134] |
| miR-181 | BCL2 and XIAP | NF-κB | Induction of an inflammatory response | [135] |
| miR-19a/b-3p | SIRT1; IGFBP3 | NF-κB | Promoting inflammatory responses | [136] |
| miR-217 | MEF2D | NF-κB; MEF2D /ND6 | Enhances inflammatory response and promotes apoptosis | [137] |
| miR-449c-5p | STAT6 | NF-κB | Activation of microglia M1 phenotype activity enhances inflammatory response | [138] |
| miR-3613-3p | RC3H1 | NF-κB | Promoting microglia M1 polarization and thus neuronal apoptosis | [139] |
| miR-203a-3p/153-3p | SRC | NF-κB; MAPK signaling pathway | Reduced neuronal apoptosis and inflammatory response | [140] |
| miR-203 | MyD88 | NF-κB | reduce inflammation | [141] |
| miR-203-3p | Pde4d | NF-κB | Inhibition of apoptosis, inflammation and oxidative stress | [142] |
| miR-194-5p | GMFB | NF-κB | Promotes cell proliferation and attenuates inflammatory responses | [143] |
| miR-345-3p | TRAF6 | TAK1/p38/NF-κB pathway | Prevention of apoptosis and inflammation | [144] |
| miR-26a | TREM1 | TLR4 / MyD88 / NF-κB pathway | Mitigation of microglia apoptosis and reduction of inflammation | [145] |
| miR-378a-5p | NLRP3 | NF-κB | Inhibits cellular pyroptosis, attenuates neuronal cell damage, and suppresses inflammatory responses | [146] |
| miR-124-3p | TRAF6 | NF-κB | Reduces neuronal cell damage and inflammatory response | [147] |
| miR-124-5p | CYBB | NF-κB | Ditto | [148] |
| miR-5787 | TLR4 | NF-κB | Inhibition of macrophage proliferation and migration migration and inflammatory response | [149] |
| miR-221 | NF-κB | Inhibition of pro-inflammatory responses | [150] | |
| miR-98 | Reduced monocyte infiltration and lowered the proportion of M1 microglia in the affected area, thereby protecting the BBB and attenuating the inflammatory response | [151] | ||
| miR-98 | CCL2, CCL3 | Protection of BBB from monocyte infiltration and prevention of M1 microglia activation | [151] | |
| miR-449c-5p | SIRT1 | SIRT1/FoxO1 pathway | Promotes microglia activation and increases BBB permeability | [152] |
| miR-425-5p | SIRT1 | NF-κB | Promoting microglia inflammatory response and BMEC injury | [153] |
| miR-15a/16-1 | Claudin-5 | Increased peripheral immune cell infiltration, exacerbating BBB leakage | [154] | |
| miR-34a | CYC | Increased disruption of cerebrovascular endothelial cell tight junctions | [155] | |
| miR-29c-5p | LRP6 | LRP6/TJP | Increased BMEC permeability and TJP disruption while enhancing inflammation | [156] |
| miR-29a-3p | SEMA3A | CircSEC11A/ miR-29a-3p/SEMA3A | Promotes BMEC oxidative stress and apoptosis and inhibits cell proliferation | [157] |
| miR-424-5p | FGF2 | FGF2/STAT3 pathway | Promotes BMEC cell injury and exacerbates BBB permeability | [158] |
| miR-33a-5p | XBP1s | DANCR/miR-33a-5p/XBP1s | inhibited proliferation, migration, and angiogenesis in BMESC | [159] |
| miR-671-5p | MMP-9 | NF-κB | Reduces TJP degradation and decreases BBB permeability | [160] |
| miR-23a-5p | TNF | NF-κB | Increased TJP expression and repair of the BBB barrier | [161] |
| miR-92b | NOX4 | Foxo1/miR-92b/ NOX4 | Reducing BBB damage | [162] |
| miR-29b | C1QTNF6 | NF-κB | Reducing BBB destruction and brain damage | [163] |
| miR-539 | SNAI2 | SNAI2/MMP9 | Inhibits vascular endothelial cell proliferation and decreases BBB permeability | [164] |
| miR-150-5p | MLLT1 | circVRK1/ miR-150-5p/ MLLT1 | Reducing BMEC damage | [165] |
| miR-429 | SNAI2 | SNAI2/GSK-3β/β-catenin pathway | Inhibition of angiogenesis in BMEC | [166] |
| miR-181b and miR-486 | PTEN | PTEN /Akt pathway | Promotes proliferation of endothelial cells and thus angiogenesis | [167, 168] |
| miR-185-5p | IGFBP-2 | ADAMTS9-AS2/ miR-185-5p/ IGFBP-2 | Promotes angiogenesis | [169] |
| miR-21-5p | RECK | VEGF/VEGFR | Promote HUVEC proliferation, migration and angiogenesis | [170] |
| miR-142-5p | ADAMTS1 | VEGF/PI3K/AKT | angiogenesis | [76] |
| miR-340-5p | CD147 | Promotes angiogenesis | [171] | |
| miR-210 | Inhibition of apoptosis promotes cell proliferation and migration in HUVESCs, thereby promoting neovascularization | [172, 173] | ||
| miR-126 | PIK3R2 | PI3K/AKT | Promotes angiogenesis | [174] |
| miR-199a-5p | VEGF and BDNF | Promotes angiogenesis and neuronal regeneration | [175] | |
| miR-214-3p | FKBP5 | circ_0007865/miR-214-3p/FKBP5 | Promote growth, proliferation and migration of HBMEC and inhibit apoptosis | [176] |
| miR-6867-5p | TWIST1 | Promotes angiogenesis and decreases BBB permeability | [177] | |
| miR-7 | KLF4/VEGF and ANG-2 | Inhibition of angiogenesis | [77] | |
| miR-203 | SLUG | inhibited the proliferation, invasion and migration of HUVEC | [178] |
Certain microRNAs (miRNAs) promote apoptotic and inflammatory processes through target gene suppression and NF-κB pathway activation, which accelerates pro-inflammatory cytokine release and enhances leukocyte infiltration, thereby exacerbating neuroinflammation. These miRNAs, predominantly expressed in microglia, not only activate NF-κB signaling but also facilitate apoptosis by polarizing microglia toward the pro-inflammatory M1 phenotype while suppressing the anti-inflammatory M2 phenotype. Conversely, other miRNAs exert neuroprotective effects following cerebral infarction by: inhibiting inflammatory cytokine and chemokine release to attenuate apoptosis and inflammation; and suppressing the p38 MAPK/MMP-9 pathway to mitigate acute ischemic brain injury.
Blood-brain barrier and neovascularization
BBB disruption following cerebral infarction primarily results from neuroinflammatory processes, which may precipitate hemorrhagic transformation in ischemic stroke due to degradation of tight junction proteins (TJPs; including claudin, occludin, and ZO-1) between brain microvascular endothelial cells (BMECs). During the acute phase, inflammatory mediators derived from activated brain cells and infiltrating leukocytes exacerbate endothelial injury. Notably, certain upregulated miRNAs amplify BBB damage by both potentiating inflammatory cascades and promoting TJP proteolysis in cerebral endothelial cells. Some miRNAs, on the other hand, are able to promote BBB repair and thus exert a protective effect. By targeting genes or acting on the NF-κB pathway, they attenuate the degradation or increase the expression of TJP in cerebrovascular endothelial endothelial cells, thereby reducing BBB permeability. For example, miR-671-5p targets NF-κB and attenuates TJP degradation by decreasing MMP-9 expression, thereby reducing BBB permeability. In addition, many miRNAs have an active effect on the process of neovascularization after cerebral infarction, and the main mechanism is to enhance the expression of vascular endothelial growth factor (VEGF) and inhibit the expression of endothelial repressor by acting on the target genes, thus promoting the growth and reproduction of endothelial cells, which all negatively affect angiogenesis (Table 3).
Therapeutic approaches for targeting miRNAs
Given the role of miRNAs in the developmental process of atherosclerosis, heart infarction, and cerebral infarction, many ways exert therapeutic effects through the involvement of miRNAs, including chemopharmacological therapies, synthetic specific vectors loaded with or recognizing/targeting miRNAs (e.g., nanoparticles, circulating microparticles, and engineered exosomes), and other regulatory non-coding RNAs (e.g., lncRNAs, circRNAs) regulation, etc.
Treatment of atherosclerosis
Many chemicals are involved in the treatment of miRNA and atherosclerosis, which involves direct activation or inhibition of miRNA expression in target cells by chemicals, modulation of miRNA delivery by exosomes from different sources, and indirect regulation of miRNAs by modulating lncRNAs or circRNAs (Table 4). They directly or indirectly up-regulate or down-regulate miRNAs to ameliorate atherosclerosis and enhance plaque stability. Among them, Salvianolic acid, the active constituent of Salvia miltiorrhiza, demonstrates particular efficacy by differentially regulating gene expression in macrophages and endothelial cells. Specifically, it upregulates let-7g in macrophages while downregulating miR-338-3p in endothelial cells, resulting in inhibition of foam cell formation, protection against endothelial cell apoptosis, and endothelial cell-derived extracellular vesicle-mediated transfer of miR-204-5p to smooth muscle cells (SMCs), which activates endothelial cell autophagy and prevents apoptotic cell death.Anthocyanin-3-O-glucoside miR-204-5p can be simultaneously inhibited in endothelial cells, indicating that the same miRNA may be chemically regulated differently; miR-135a-5p is a direct target of circ_0000231 and also targets CLIC4, astragaloside IV attenuates atherosclerosis by inhibiting circ_0000231; Salvianolic acid B may inhibit the expression of MMP9 and mmp12 in macrophages by upregulating mir-34a-5p, reducing inflammatory cell infiltration and Th1 cell response, and maintaining atherosclerotic plaque stabilization; however, it should be noted that downregulation of miR-34a-5p enhances protection against infarction, suggesting that the medication should be used with attention to the course of atherosclerosis development.
Table 4.
Drug treatment of atherosclerosis.
| Veterinary drug | miRNA | Target gene/signaling pathway | Corresponds | Reference |
|---|---|---|---|---|
| Atorvastatin | miR-26a-5p | PTEN | Enhancement of HUVEC viability, inhibition of apoptosis and migration | [179] |
| Tetrandrine | miR-34a | Wnt5a/Ror2/ABCA1/NF-kB pathway | Promotes cholesterol efflux and inhibits expression of inflammatory factors | [180] |
| Paeonol | miR let-7g | HMGA2/CEBPβ pathway | Inhibits foam cell formation and adipocyte differentiation | [181] |
| Paeonol | miR-338-3p | TET2 | Enhancement of VEC viability and inhibition of apoptosis | [182] |
| Rapamycin | miR-204-5p | Prevention of EC apoptosis and attenuation of SMC calcification | [183] | |
| Rapamycin | miR-155 | Increased autophagic activity in carotid plaques | [184] | |
| Cyanidin-3-O-glucoside (C3G) | miR-204-5p | SIRT1 | Inhibition of HUVEC apoptosis, Inhibition of inflammation | [185] |
| Curcumin | miR-125a-5p | SIRT6/ ABCA1 | Promote cholesterol transport and inhibit the formation of foam cells | [186] |
| Curcumin | miR-124 | lncRNA MIAT/miR-124 | Attenuates ox-LDL-induced cellular inflammation | [187] |
| Yi Mai granule | miR-125a-5p | Mfn2-Parkin access road | Enhanced mitochondrial autophagy in endothelial cells | [88] |
| Hydroxysafflor yellow A | miR-429 | SLCA7A11 | Inhibition of iron death in HUVEC | [188] |
| Aucubin | miR-181a-5p | STING | Inhibition of NF-κB pathway inflammation | [189] |
| astragaloside IV | miR-17-5p | PCSK9/VLDLR signaling pathway | Inhibits vascular inflammation, thereby attenuating endothelial cell damage | [190] |
| astragaloside IV | miR-135a-5p | circ_0000231/miR-135a-5p/ CLIC4 | Inhibition of HUVEC cell damage | [191] |
| Theaflavin | miR-24 | Nrf2/HO-1 signaling pathway | Protection of HUVEC cells from oxidative damage | [192] |
| Notoginsenoside R1 (NGR1) | miR-221-3p | XIST/miR-221-3p/TRAF6 Axis | Promotes HUVEC proliferation and inhibits apoptosis death, inflammation, and oxidative stress | [193] |
| Salvianolic acid B | miR-34a-5p | MMP9 and MMP12 | Reduction of inflammatory cell infiltration and maintenance of atherosclerotic plaque stabilization | [194] |
| dihydromyricetin | miR-21 | DDAH1/ ADMA | Increased endothelial cell NO synthase (eNOS) phosphorylation and NO production | [195] |
| L-Arginine | miR-221 | eNOS | Inhibits oxidized LDL white-induced apoptosis in endothelial cells | [196] |
| bosentan | miR-21 | PDCD4 | Prevents endothelial cell death | [197] |
| Tanshinone IIA | miR-130b | WNT5A | Inhibits foam cell production and inflammation | [198] |
| Tanshinone IIA | miR-375 | KLF4 | Enhancement of macrophage autophagy as well as M2 polarization | [199] |
| Nobiletin | miR-590 | LPL | Reduces lipid accumulation and inflammation | [200] |
| Ojeoksan | miRNA-10a、-126-3p | eNOS and MMP | Increased endothelial cell NO synthase (eNOS) phosphorylation and NO production | [201] |
| ursolic acid | miRNA-21 | PTEN/PI3K | Inhibition of smooth muscle cell proliferation | [202] |
| Diosgenin | miR-19b | ABCA1 | Enhanced macrophage cholesterol efflux | [203] |
| coenzyme Q10 | miR-378 | ATP-binding cassette transporter protein G1 | Enhanced macrophage cholesterol efflux | [204] |
| Tetramethylpyrazine and paeoniflorin combination therapy (TMP-PF) | miR-1268b | circSCRG1/miR-1268b/NR4A1 | Inhibits HUVEC angiogenesis and increases plaque stability | [205] |
An increasing number of studies have been conducted by designing nanoparticles with the ability to load miRNAs (or other substances) and target specific cells. By using click chemistry technology to modify the hyaluronic acid (HA) on the surface of LSS EV, it can specifically bind to the CD44 receptor on the surface of pro-inflammatory macrophages in plaques. As a result,mir-34c-5p in EVs targets the tgf- β -smad3 pathway and promotes the repolarization (reprogramming to M2) of M1 phenotype macrophages [54]. Ultrasound-targeted microvesicle disruption (UTMD) delivery of miR-145a-5p promotes a contractile phenotype in VSMCs [55]. SPION capsule miR-146a is a spherical nucleic acid nanostructure, which can independently enter macrophages and ECs, regulate the NF - κ B pathway and treat atherosclerosis. miR-146a has dual functions of targeting and gene regulation [56]. Using pH low insertion peptide (pHLIP) construct as a vector, miR-33 antisense oligonucleotide was delivered into macrophages, which binds to miR-33 and thus up-regulates the expression of fibrogenic genes, Timp3, and MMP12, and is applied to treat advanced atherosclerosis [57]. Exosome engineered IL-10 mRNA has miR-155 recognition site, when miR-155 is activated, the exosome is delivered to macrophages and acts as an anti-inflammatory [58]. Inhibition of miR-126 by nanoparticles containing miRNA switches inhibits smooth muscle cell division and migration and protects endothelial cells [59]. Co-culture of advanced endothelial progenitor cells (EPC) and circulating particles (MP) improves EPC function through miR-10a, miR-21, miR-126, miR-146a, miR-223 metastasis and IGF-1 expression activation [60]. MiRNAs also play a role in preventing non target cell damage. Delivery of EV based magnetic iBax mRNA and BAX activator BTSA1 effectively promotes the death of receptor aging cells in plaques, while miR-122 overexpressed in liver cells targets BAX mRNA, thereby protecting the liver from potential damage caused by BAX mRNA [61].
Treatment of heart attacks
As previously discussed, numerous pharmacological interventions delay atherosclerotic plaque formation and progression, while preventing plaque rupture - a critical event leading to vascular occlusion and subsequent infarction - represents another key therapeutic strategy. Many drug therapies exert therapeutic effects on heart attack by directly affecting miRNAs or indirectly regulating miRNAs through lncRNAs and circRNAs (Table 5). Exosomes derived from serum, cardiac tissue, adipose tissue, macrophages, MSCs, and other sources play a role in repairing post-infarction damage by transfecting miRNAs, and therefore therapeutically significant exosomes can be targeted to target cells by means of physical techniques or chemical agents. For example, miR-132-3p was enriched in M2 macrophage-derived exosomes (M2-exos), which were translocated into endothelial cells to promote post-MI angiogenesis by directly targeting THBS1. Pretreatment of exosomes from MSCs with vericiguat (MSC(VER)-Exo), applied to cardiac fibroblasts, inhibited fibroblast proliferation, migration, and pro-fibroblast gene expression, thereby attenuating cardiac fibrosis, in which miR-1180-3p, which targets ETS1, plays an effective role in anti-fibroblasts [62]. MiR-125b is a cardiac infarction miR-125b is an important miRNA molecule in infarction. Targeted delivery of MSC membrane ligands and miR-125b to the inflamed region of AMI using ultrasound-targeted microbubble disruption (UTMD) prevents cardiomyocyte death and inhibits the growth of fibroblasts [63]. In addition, precise cardiac-specific genome editing can be performed with combining extracellular vesicles (EV) with cardiac-targeting peptides (T). RNP complexes of single guide RNA targeting miR-34a were loaded into EV, which inhibited miR-34a expression and attenuated apoptosis of cardiomyocytes [64]. C166-derived EV was also an effective deliverer of miRNA combinations in vitro and in vivo, delivering miR-148a-3p to fibroblasts and induces reprogramming of fibroblasts into cardiac muscle cells by targeting Mdfic [65]. Intramyocardial injection of cardiopulmonary progenitor (CPP) cells improves cardiac function by promoting cardiomyocyte proliferation and vascularization via exosomes of CPPs (CPPs-Exo). It has been shown that high expression of miR-27b-3p and its target gene, Sik1, in CPPs-Exo affects the transcriptional activity of CREB1 [66].
Table 5.
Drugs for the treatment of myocardial infarction.
| Veterinary drug | miRNA | Target gene/signaling pathway | Corresponds | Reference |
|---|---|---|---|---|
| Carvedilol | miR-125b-5p | circ_NFIX/ miR-125b-5p/ TLR4 | Protection of H9c2 cells from H2O2-induced cellular dysfunction | [206] |
| quercetin | miR-221 | circPAN3/ miR-221/PTEN | Reduction of cardiomyocyte apoptosis | [207] |
| Qili Qiangxin Capsule (QLQX) | miR133a | GRP78, IRE1, ATF6 and XBP1 | Reduced cardiomyocyte apoptosis | [208] |
| Astragaloside IV | miR-411 | HIF-1α | Improves heart function and promotes neovascularization | [209] |
| atorvastatin (ATV) | miR-139-3p | Stat1 pathway | Promoting macrophage polarization and cardiac repair | [210] |
| ginsenoside Rg3 | miR-128-3p | MDM4 | Inhibited cardiomyocyte apoptosis and oxidative stress | [211] |
| Melatonin | miR-200b-3p | HMGB1 | Promotes H9c2 cell proliferation and inhibit apoptosis in ischemic environment | [212] |
| EEpigallocatechin gallate | miR-450b-5p | ACSL4 | Mitigating AMI-induced iron death | [213] |
| IFN-γ-Exo | miR-21 | BTG2 | Promotes angiogenesis and reduces apoptosis | [214] |
| take care of the heart’s health (TCM) | miR-146a-5p | IRAK1 / NF-κB p65 | Reduces cardiomyocyte apoptosis and inflammation | [215] |
| Tanshinone IIA (Tan IIA) | miR-499-5p | PTEN | Promotes proliferation and migration of HUVEC and angiogenesis | [216] |
| propofol | miR-206 | MALAT1/miR-206/ATG3 | Protects cardiomyocytes from I/R damage | [217] |
| Danhong Injection (DHI) | miR-125b | p53 myocardial apoptosis pathway | Attenuating apoptosis in the heart after MI | [218] |
| Photobiomodulation (PBM) | miR-136-5p | Ino80/ miR-136-5p | Promote cardiomyocyte proliferation | [219] |
Treatment of cerebral infarction
Extracellular vesicles containing superparamagnetic iron oxide nanoparticles (SPION-EX) can effectively penetrate the BBB and enter neurons in brain tissue, improving mitochondrial function of post-stroke neurons through the miR-1228-5 p/TRAF 6/NOX 1 signaling pathway [67]. MiR-21-5p is transferred from ADSC exosomes to microglia to promote M2 polarization and alleviate inflammation through the PIK3R1/PI3K/AKT cell pathway [68]. MSC-derived exosomes (Hypo-Exo) cultured under hypoxic conditions containing miR-214-3p promote angiogenesis through the PTEN/Akt pathway [69]. A probe with aggregation-induced emission (AIE) properties (i.e., TTCP) that efficiently labels EC-EV, which carries miRNA-155-5p is taken up by astrocytes and promotes neurological restoration by targeting the c-Fos/AP-1 pathway [70]. By pre-treating astrocytes with berberine, it is possible to induce the release of extracellular vesicles (BBR exos) carrying miR-182-5p from damaged neurons, and Rac 1 inhibits neuroinflammation and improves expression of brain injury after cerebral [71]. The delivery of miR-124 through Ca-MOF nano delivery system can significantly promote the internalization of miR-124 in neural stem cells (NSCs) and promote the differentiation of NSCs into mature neurons [72]. Circ-Rps5 modified ADSC exosomes improve cerebral infarction by alleviating neuronal damage and converting microglia from M1 phenotype to M2 phenotype in the hippocampus, which mechanism is to downregulate miR-124-3p, thereby upregulating SIRT7 [73]. Circ-Rps5 modified ADSC exosomes improve cerebral infarction by alleviating neuronal damage and converting microglia from M1 phenotype to M2 phenotype in the hippocampus. The mechanism is to downregulate miR-124-3p, thereby upregulating SIRT7 [74]. Nanoparticles coated with peptide nucleic acid (PNA) or phosphorothioate (PS) anti-miRs-141-3p probes reduce the production of the pro-inflammatory cytokine TNF-α [75].
Some chemical drugs are also involved in the treatment of cerebral infarction through miRNAs (Table 6). In addition, electroacupuncture has been found to be a useful therapy for cerebral infarction, and several studies have elucidated its mechanism of treating diseases by regulating miRNA. For example, electroacupuncture therapy upregulated the expression of miR-142-5p and inhibited its target gene ADAMTS1, thereby promoting the VEGF/PI3K/AKT/eNOS pathway, which led to a reduction in the infarct area [76]. Reduced miR-7 expression after electroacupuncture deregulated the downstream target genes KLF4/VEGF and ANG-2, thereby promoting post-infarction angiogenesis [77]. Acupuncture treatment upregulated the expression of miR-34c-5p and enhanced cellular autophagy, which was beneficial to cerebral infarction treatment, and its combination with the autophagy agonist RAPA enhanced the therapeutic effect [78]. Pretreatment of MSC with lithium altered the EV secretion pattern, and Li-EV inhibited the NF-κB signaling pathway by targeting TLR4 through the delivery of miR-1906, resulting in a reduction of inflammation levels [79]. Nespas expression was significantly elevated after transcranial focused ultrasound stimulation (tFUS) treatment, which down-regulated miR-383-3p expression, deregulated its inhibition of the target gene SHP2, and inhibited the production of pro-inflammatory cytokines in microglia [80].
Table 6.
Drug treatment of cerebral infarcts.
| Veterinary drug | MiRNA | Target gene/signaling pathway | Corresponds | Reference |
|---|---|---|---|---|
| Tanshinone IIA | miR-124-5p | FoxO1 | Inhibition of inflammatory response and neuronal apoptosis | [220] |
| berberine (medicine) | miR-377-3p | METTL3/ NEAT1/miR-377-3p | Neuroprotective effects | [221] |
| Gualou Guizhi decoction | miRNA210 | HIF/VEGF | Enhanced angiogenesis | [222] |
| dexmedetomidine | miR-665 | ROCK2 and NF-κB p65 | Reduces inflammation and apoptosis | [223] |
| Ginseng Yangrong decoction (GSYRD) | miRNA-210 | HIF/VEGF/Notch signaling pathway | Promotion of neovascularization and cerebral protection after ischemic brain injury | [224] |
| Gastrodin (GAS) | miR-22-3p | lncRNA NEAT1/miR-22-3p | Attenuates I/R-induced inflammation in neuronal cells | [225] |
| Qingda granule (QDG) | miR-137 | lncRNA GAS5/miR-137 | neuroprotection | [226] |
The core pathophysiological basis of myocardial infarction and cerebral infarction is the same, that is, atherothrombosis leads to acute obstruction of the main blood supply artery or its branches, which directly leads to blood flow interruption and insufficient oxygen supply. Interventions targeting these upstream Pro thrombotic and vascular disease miRNAs (such as antagonists or mimics) can theoretically reduce the risk of myocardial infarction and cerebral infarction at the same time, and play a role in preventing the occurrence of disease. After myocardial infarction and cerebral infarction, cells in the heart and brain are subjected to ischemia / reperfusion injury. At this time, broad-spectrum neuroprotective miRNA drugs that can alleviate ischemia / reperfusion injury (such as targeting inflammation, oxidative stress, cell death, repair remodeling, etc.) can be developed, so as to reduce organ damage and promote tissue repair. It should be noted that although the target pathways have similarities, the final effect still needs to be fully verified in specific cell types of their respective organs to avoid off target effects. At the same time, the location of cerebral infarction is different from that of myocardial infarction. Due to the existence of blood-brain barrier, targeted drug delivery after cerebral infarction is more difficult, which should also be noted.
Challenges and Development Strategies
This review aims to provide new therapeutic approaches for cerebral infarcts and infarcts caused by atherosclerosis. However, we found that there are many challenges in targeting miRNAs for cerebral and cardiac infarction. MiRNAs are small non-coding RNAs with specificity, which can regulate gene expression by degrading or inhibiting the translation of multiple target mRNAs, with a low precision, and the principle of action of miRNAs in cardiac and cerebral infarction is complex and diverse, different miRNAs may exert different effects at different pathological stages. Therefore, there is a need to utilize tissue-specific promoters or enhancers to drive miRNA expression in specific tissues, or to design synthetic miRNAs to be active only in specific cell types, e.g., by binding to cell-type-specific RNA-binding proteins, to avoid potential side effects [81].
Second, the delivery system for miRNA therapy is an important challenge. Due to the molecular nature of miRNAs, their stability and targeting in vivo is limited. Researchers are exploring a variety of delivery systems, such as nanoparticles, liposomes, polymers, and other novel delivery vehicles, to improve the delivery efficiency and targeting of miRNA. For example, low molecular weight heparin-modified nanocomplexes have been used to improve miRNA delivery in infarcted regions, showing favorable therapeutic effects. Alternatively, viral vectors, such as adenovirus or lentivirus, are utilized for tissue-specific delivery, addressing both immunogenicity and toxicity. Exosomes also have potential as natural delivery vectors and can be utilized for their natural intercellular communication [82].
In addition, in terms of safety, miRNA modulation may affect multiple physiological pathways, and therefore, it is necessary to evaluate long-term safety, including potential off target effects and side effects. Rigorous in vitro and in vivo toxicity tests, including cytotoxicity, immune response, and genotoxicity, are required during experimentation and translation, as well as the use of bioinformatics tools to predict off-target effects of miRNAs and validate them in preclinical studies.
In recent studies, some new targets involved in atherosclerosis have been found. For example, Apelin/APJ mediates endoplasmic reticulum autophagy by upregulating SEC62 as an endoplasmic reticulum autophagy receptor protein, and upregulating the expression of adhesion molecules ICAM-1/VCAM-1, thereby promoting monocyte adhesion to vascular endothelium [83]; CYSLTR2 and P2RY6 are potential endogenous receptors for C16:0 ceramide induced inflammasome activation in endothelial cells and macrophages, increasing the risk of atherosclerosis [84]; Liver kinase B1 (LKB1) inhibits the phenotypic transformation of vascular smooth muscle cells by activating SIRT6, thereby delaying the onset of atherosclerosis [85]; Targeting the PGAM5-ANGPT3 signaling axis by inhibiting PGAM5 can reduce inflammation and improve macrophage lipid metabolism [86]. However, the research on the targeting effect of miRNAs on these proteins and potential therapeutic options are still blank.
In addition, in addition to the mainstream cells involved in atherosclerosis (endothelial cells, macrophages, vascular smooth muscle cells), other immune cells (such as proinflammatory T cells) may also have therapeutic targets. A study showed that PD-1 monoclonal antibody can inhibit plaque activation and proinflammatory PD-1 positive T cell function, thereby alleviating atherosclerosis, suggesting that we can also explore the targets of miRNAs in T lymphocytes [87].
In conclusion, although targeted miRNA therapies show great potential in heart and brain infarction, their clinical application still needs to overcome multiple challenges such as delivery system, specificity and safety. Future studies should continue to explore in depth the mechanism of miRNA action in cardiovascular and cerebrovascular diseases and develop more efficient and safe therapeutic strategies.
Conclusion
This review organizes the involvement of miRNAs in atherosclerosis and the resulting heart and brain infarcts, including the three main types of cells in atherosclerosis (ECs, macrophages, and VSMCs), and the development of heart infarcts (apoptosis of cardiomyocytes, angiogenesis, etc.) versus brain infarcts (neuroinflammatory response, BBB repair, etc.) after plaque rupture. In addition, various therapeutic means for targeting miRNAs, such as drug therapy, engineered exosomes, acupuncture therapy, etc., are also highlighted and summarized. MiRNAs, as a key gene expression regulator, play an important role in the development and progression of atherosclerosis. By targeting specific miRNAs, the expression of atherosclerosis-related genes can be effectively regulated, thus achieving the purpose of treating cerebral and cardiac infarction. miRNAs’ strategy of treating cerebral and cardiac infarction by regulating atherosclerosis not only reveals the molecular mechanism of disease occurrence, but also provides new targets and ideas for clinical treatment. In the future, with the in-depth study of miRNA function and the continuous progress of technical means, targeted miRNA therapy is expected to become an important means in the field of CVD treatment, which will bring more effective therapeutic choices and better quality of life for patients.
Availability of data and Materials
From Pubmed Data base.
Author contributions
J. Wang and YH. Li wrote the manuscript. HX. Wang drew the tables. Q. Meng and PY. Li drew figures. YQ. Wang, K. Wang and SM. Yang revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Open Project Program of State Key Laboratory of Frigid Zone Cardiovascular Diseases (SKLFZCD), Harbin Medical University (HDHY2024007); National Natural Science Foundation of China (82370291, 82401019); Qingdao Science and Technology Benefiting the People Demonstration Project (24-1-8-smjk-7-nsh); Major Basic Research Projects in Shandong Province (ZR2024ZD46), Taishan Scholar Distinguished Expert, Guiding Fund of Government’s Science and Technology (YDZX2021004); China Postdoctoral Science Foundation (2024M761553); Shandong Provincial Natural Science Foundation (ZR2024QH184); Qingdao Natural Science Foundation (24-4-4-zrjj-33-jch) and Qingdao Postdoctoral Applied Research Project (QDBSH20240201020).
Data availability
From Pubmed Data base.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Consent for publication Not applicable
These authors contributed equally: Jie Wang, Yinghui Li, Haoxuan Wang.
Contributor Information
YuQin Wang, Email: yqWang530@126.com.
Kun Wang, Email: wangk696@qdu.edu.cn.
SuMin Yang, Email: SMinYangFY@126.com.
References
- 1.Diener C, Keller A, Meese E. Emerging concepts of miRNA therapeutics: from cells to clinic. Trends Genet. 2022;38:613–26. [DOI] [PubMed] [Google Scholar]
- 2.Bartel DP. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell. 2004;116:281–97. [DOI] [PubMed] [Google Scholar]
- 3.Mishra S, Yadav T, Rani V. Exploring miRNA based approaches in cancer diagnostics and therapeutics. Crit Rev Oncol Hematol. 2016;98:12–23. [DOI] [PubMed] [Google Scholar]
- 4.Dosil SG, Rodríguez-Galán A, Sánchez-Madrid F, Fernández-Messina L. MicroRNAs in T Cell-Immunotherapy. Int J Mol Sci. 2022;24:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Selvaskandan H, Pawluczyk I, Barratt J. Clinical application of microRNAs in glomerular diseases. Nephrol Dial Transpl. 2023;38:1375–84. [DOI] [PubMed] [Google Scholar]
- 6.Zhang HC, Du Y, Chen L, Yuan ZQ, Cheng Y. MicroRNA schizophrenia: Etiology, biomarkers and therapeutic targets. Neurosci Biobehav Rev. 2023;146:105064. [DOI] [PubMed] [Google Scholar]
- 7.Hathaway QA, Pinti MV, Durr AJ, Waris S, Shepherd DL, Hollander JM. Regulating microRNA expression: at the heart of diabetes mellitus and the mitochondrion. Am J Physiol Heart Circ Physiol. 2018;314:H293–h310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons GH, Dzau VJ. The Emerging Concept of Vascular Remodeling. N Engl J Med. 1994;330:1431–8. [DOI] [PubMed] [Google Scholar]
- 9.Gimbrone MA, García-Cardeña G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circulation Res. 2016;118:620–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabas I, Bornfeldt KE. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circulation Res. 2016;118:653–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan H, Ho SE, Xue C, Cui J, Johanson QS, Sachs N, et al. Atherosclerosis Is a Smooth Muscle Cell–Driven Tumor-Like Disease. Circulation. 2024;149:1885–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Zhao Y, Suguro S, Suguro R. MicroRNAs Regulate Function in Atherosclerosis and Clinical Implications. Oxid Med Cell Longev 2023;2023:2561509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhaolin Z, Jiaojiao C, Peng W, Yami L, Tingting Z, Jun T, et al. OxLDL induces vascular endothelial cell pyroptosis through miR-125a-5p/TET2 pathway. J Cell Physiol. 2019;234:7475–91. [DOI] [PubMed] [Google Scholar]
- 14.Xu Y, Xu Y, Zhu Y, Sun H, Juguilon C, Li F, et al. Macrophage miR-34a Is a Key Regulator of Cholesterol Efflux and Atherosclerosis. Mol Ther. 2020;28:202–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao L, Wang B, Sun L, Sun B, Li Y. Association of miR-192-5p with Atherosclerosis and its Effect on Proliferation and Migration of Vascular Smooth Muscle Cells. Mol Biotechnol. 2021;63:1244–51. [DOI] [PubMed] [Google Scholar]
- 16.Rozhkov AN, Shchekochikhin DY, Ashikhmin YI, Mitina YO, Evgrafova VV, Zhelankin AV, et al. The Profile of Circulating Blood microRNAs in Outpatients with Vulnerable and Stable Atherosclerotic Plaques: Associations with Cardiovascular Risks. Non-Coding RNA. 2022;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Y, Li Y, Peng H, Zhao Y. miR-140-5p regulates vascular smooth muscle cell viability, migration and apoptosis by targeting ROBO4 gene expression in atherosclerosis. Mol Med Rep. 2021;23:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Li H, Xing W, Li J, Du R, Cao D, et al. Vascular smooth muscle cell-specific miRNA-214 knockout inhibits angiotensin II-induced hypertension through upregulation of Smad7. Faseb J. 2021;35:e21947. [DOI] [PubMed] [Google Scholar]
- 19.Jiang F, Zhang B, Zhang X, Zhang R, Lu Q, Shi F, et al. miRNA‑92a inhibits vascular smooth muscle cell phenotypic modulation and may help prevent in‑stent restenosis. Mol Med Rep. 2023;27:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.González-López P, Álvarez-Villarreal M, Ruiz-Simón R, López-Pastor AR, de Ceniga MV, Esparza L, et al. Role of miR-15a-5p and miR-199a-3p in the inflammatory pathway regulated by NF-κB in experimental and human atherosclerosis. Clin Transl Med. 2023;13:e1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vartak T, Giardini E, Kelly D, Moran B, Kennedy C, Barry M, et al. Induction of let-7d-5p miRNA modulates aortic smooth muscle inflammatory signaling and phenotypic switching. Atherosclerosis. 2024;395:117573. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Yuan Y, He X, Xia X, Mo X. MicroRNA-1 upregulation promotes myocardiocyte proliferation and suppresses apoptosis during heart development. Mol Med Rep. 2017;15:2837–42. [DOI] [PubMed] [Google Scholar]
- 23.Muraoka N, Yamakawa H, Miyamoto K, Sadahiro T, Umei T, Isomi M, et al. MiR-133 promotes cardiac reprogramming by directly repressing Snai1 and silencing fibroblast signatures. Embo j. 2014;33:1565–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu C, Lu Y, Pan Z, Chu W, Luo X, Lin H, et al. The muscle-specific microRNAs miR-1 and miR-133 produce opposing effects on apoptosis by targeting HSP60, HSP70 and caspase-9 in cardiomyocytes. J Cell Sci. 2007;120:3045–52. [DOI] [PubMed] [Google Scholar]
- 25.Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. [DOI] [PubMed] [Google Scholar]
- 26.Sluijter JP, van Mil A, van Vliet P, Metz CH, Liu J, Doevendans PA, et al. MicroRNA-1 and -499 regulate differentiation and proliferation in human-derived cardiomyocyte progenitor cells. Arterioscler Thromb Vasc Biol. 2010;30:859–68. [DOI] [PubMed] [Google Scholar]
- 27.Kim KW, Kim SW, Lim S, Yoo KJ, Hwang KC, Lee S. Neutralization of hexokinase 2-targeting miRNA attenuates the oxidative stress-induced cardiomyocyte apoptosis. Clin Hemorheol Microcirc. 2021;78:57–68. [DOI] [PubMed] [Google Scholar]
- 28.Garg A, Foinquinos A, Jung M, Janssen-Peters H, Biss S, Bauersachs J, et al. MiRNA-181a is a novel regulator of aldosterone-mineralocorticoid receptor-mediated cardiac remodelling. Eur J Heart Fail. 2020;22:1366–77. [DOI] [PubMed] [Google Scholar]
- 29.Zhu J, Wang Q, Zheng Z, Ma L, Guo J, Shi H, et al. MiR-181a protects the heart against myocardial infarction by regulating mitochondrial fission via targeting programmed cell death protein 4. Sci Rep. 2024;14:6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang W, Zhao L, Xue S, Liu J, Shan J. MiRNA-21 promotes differentiation of bone marrow mesenchymal stem cells into cardiomyocyte-like cells by regulating the Ajuba/Isl1 axis pathway. Arch Med Sci. 2022;18:1672–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li T, Gu J, Yang O, Wang J, Wang Y, Kong J. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal miRNA-29c Decreases Cardiac Ischemia/Reperfusion Injury Through Inhibition of Excessive Autophagy via the PTEN/Akt/mTOR Signaling Pathway. Circ J. 2020;84:1304–11. [DOI] [PubMed] [Google Scholar]
- 32.Bai G, Yang J, Liao W, Zhou X, He Y, Li N, et al. MiR-106a targets ATG7 to inhibit autophagy and angiogenesis after myocardial infarction. Anim Model Exp Med. 2024;7:408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bestepe F, Fritsche C, Lakhotiya K, Niosi CE, Ghanem GF, Martin GL, et al. Deficiency of miR-409-3p improves myocardial neovascularization and function through modulation of DNAJB9/p38 MAPK signaling. Mol Ther Nucleic Acids. 2023;32:995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu W, Du W, Duan R, Liu Y, Zong B, Jin X, et al. miR-873-5p Suppression Reinvigorates Aging Mesenchymal Stem Cells and Improves Cardiac Repair after Myocardial Infarction. ACS Pharm Transl Sci. 2024;7:743–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong Y, He H, Jiang G, Zhang H, Tao W, Ding Y, et al. miR-155-5p inhibition rejuvenates aged mesenchymal stem cells and enhances cardioprotection following infarction. Aging Cell. 2020;19:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Chen L, Feng Z, Chen W, Yan S, Yang R, et al. EPC-Derived Exosomal miR-1246 and miR-1290 Regulate Phenotypic Changes of Fibroblasts to Endothelial Cells to Exert Protective Effects on Myocardial Infarction by Targeting ELF5 and SP1. Front Cell Dev Biol 2021;9:647763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu W, Wang Q, Zhang J, Sun L, Hong X, Du W, et al. Exosomes derived from mir-214-3p overexpressing mesenchymal stem cells promote myocardial repair. Biomater Res. 2023;27:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang T, Li T, Niu X, Hu L, Cheng J, Guo D, et al. ADSC-derived exosomes attenuate myocardial infarction injury by promoting miR-205-mediated cardiac angiogenesis. Biol Direct. 2023;18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meloni M, Marchetti M, Garner K, Littlejohns B, Sala-Newby G, Xenophontos N, et al. Local inhibition of microRNA-24 improves reparative angiogenesis and left ventricle remodeling and function in mice with myocardial infarction. Mol Ther. 2013;21:1390–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, et al. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–30. [DOI] [PubMed] [Google Scholar]
- 41.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ke X, Yang R, Wu F, Wang X, Liang J, Hu X, et al. Exosomal miR-218-5p/miR-363-3p from Endothelial Progenitor Cells Ameliorate Myocardial Infarction by Targeting the p53/JMY Signaling Pathway. Oxid Med Cell Longev 2021;2021:5529430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawaguchi S, Moukette B, Sepúlveda MN, Hayasaka T, Aonuma T, Haskell AK, et al. SPRR1A is a key downstream effector of MiR-150 during both maladaptive cardiac remodeling in mice and human cardiac fibroblast activation. Cell Death Dis. 2023;14:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan X, Pan J, Wen L, Gong B, Li J, Gao H, et al. MiR-590-3p regulates proliferation, migration and collagen synthesis of cardiac fibroblast by targeting ZEB1. J Cell Mol Med. 2020;24:227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou XL, Xu H, Liu ZB, Wu QC, Zhu RR, Liu JC. miR-21 promotes cardiac fibroblast-to-myofibroblast transformation and myocardial fibrosis by targeting Jagged1. J Cell Mol Med. 2018;22:3816–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang N, Hou YB, Cui TH, Yu JM, He SF, Zhu HJ. Ischemic-Preconditioning Induced Serum Exosomal miR-133a-3p Improved Post-Myocardial Infarction Repair via Targeting LTBP1 and PPP2CA. Int J Nanomed. 2024;19:9035–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi S, Liu X, Geng X, Meng Q, Gao M, Wang E, et al. Neonatal heart tissue-derived EVs alleviate adult ischemic cardiac injury via regulating the function of macrophages and cardiac regeneration in murine models. Int Immunopharmacol 2024;143:113251. [DOI] [PubMed] [Google Scholar]
- 48.Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 49.Yang K, Zeng L, Ge A, Wang S, Zeng J, Yuan X, et al. A systematic review of the research progress of non-coding RNA in neuroinflammation and immune regulation in cerebral infarction/ischemia-reperfusion injury. Front Immunol. 2022;13:930171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim JE, Lee RP, Yazigi E, Atta L, Feghali J, Pant A, et al. Soluble PD-L1 reprograms blood monocytes to prevent cerebral edema and facilitate recovery after ischemic stroke. Brain Behav Immun. 2024;116:160–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory Mechanisms in Ischemic Stroke: Focus on Cardioembolic Stroke, Background, and Therapeutic Approaches. Int J Mol Sci. 2020;21:6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang J, Zheng Y, Luo Y, Du Y, Zhang X, Fu J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/ TLR4/ NF-κB pathways in BV2 cells. Mol Immunol. 2019;116:29–37. [DOI] [PubMed] [Google Scholar]
- 53.Xu X, Piao HN, Aosai F, Zeng XY, Cheng JH, Cui YX, et al. Arctigenin protects against depression by inhibiting microglial activation and neuroinflammation via HMGB1/TLR4/NF-κB and TNF-α/TNFR1/NF-κB pathways. Br J Pharm. 2020;177:5224–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li C, Fang F, Wang E, Yang H, Yang X, Wang Q, et al. Engineering extracellular vesicles derived from endothelial cells sheared by laminar flow for anti-atherosclerotic therapy through reprogramming macrophage. Biomaterials. 2025;314:122832. [DOI] [PubMed] [Google Scholar]
- 55.Wu Y, Deng C, Xu J, Wang W, Chen Y, Qin X, et al. Enhanced Local Delivery of microRNA-145a-5P into Mouse Aorta via Ultrasound-Targeted Microbubble Destruction Inhibits Atherosclerotic Plaque Formation. Mol Pharm. 2023;20:1086–95. [DOI] [PubMed] [Google Scholar]
- 56.Bai Q, Xiao Y, Hong H, Cao X, Zhang L, Han R, et al. Scavenger receptor-targeted plaque delivery of microRNA-coated nanoparticles for alleviating atherosclerosis. Proc Natl Acad Sci USA. 2022;119:e2201443119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang X, Rotllan N, Canfrán-Duque A, Sun J, Toczek J, Moshnikova A, et al. Targeted Suppression of miRNA-33 Using pHLIP Improves Atherosclerosis Regression. Circ Res. 2022;131:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bu T, Li Z, Hou Y, Sun W, Zhang R, Zhao L, et al. Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics. 2021;11:9988–10000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lockhart JH, VanWye J, Banerjee R, Wickline SA, Pan H, Totary-Jain H. Self-assembled miRNA-switch nanoparticles target denuded regions and prevent restenosis. Mol Ther. 2021;29:1744–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alexandru N, Andrei E, Niculescu L, Dragan E, Ristoiu V, Georgescu A. Microparticles of healthy origins improve endothelial progenitor cell dysfunction via microRNA transfer in an atherosclerotic hamster model. Acta Physiol (Oxf). 2017;221:230–49. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Wang C, Hu W, Bu T, Sun W, Zhou T, et al. Targeted elimination of senescent cells by engineered extracellular vesicles attenuates atherosclerosis in ApoE(-/-) mice with minimal side effects. Theranostics. 2023;13:5114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li C, Zheng C, Pu Y, Zhou H, Li Y, Wang W, et al. Vericiguat enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction through microRNA-1180-3p/ETS1 pathway. Cell Signal. 2025;125:111512. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z, Chen J, Wang J, Xu M, Yang H, Yang H, et al. MSCs biomimetic ultrasonic phase change nanoparticles promotes cardiac functional recovery after acute myocardial infarction. Biomaterials. 2025;313:122775. [DOI] [PubMed] [Google Scholar]
- 64.Mun D, Kang JY, Kim H, Yun N, Joung B. Small extracellular vesicle-mediated CRISPR-Cas9 RNP delivery for cardiac-specific genome editing. J Control Release. 2024;370:798–810. [DOI] [PubMed] [Google Scholar]
- 65.Sun H, Wang X, Pratt RE, Dzau VJ, Hodgkinson CP. C166 EVs potentiate miR cardiac reprogramming via miR-148a-3p. J Mol Cell Cardiol. 2024;190:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiao YY, Xia LX, Jiang WJ, Qin JF, Zhao LX, Li Z, et al. Cardiopulmonary progenitors facilitate cardiac repair via exosomal transfer of miR-27b-3p targeting the SIK1-CREB1 axis. Cell Prolif. 2024;57:e13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu WJ, Wei H, Cai LL, Xu YH, Du R, Zhou Q, et al. Magnetic targeting enhances the neuroprotective function of human mesenchymal stem cell-derived iron oxide exosomes by delivering miR-1228-5p. J Nanobiotechnology. 2024;22:665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu C, Yin T, Zhang M, Li Z, Xu B, Lv H, et al. Function of miR-21-5p derived from ADSCs-exos on the neuroinflammation after cerebral ischemia. J Stroke Cerebrovasc Dis 2024;33:107779. [DOI] [PubMed] [Google Scholar]
- 69.Wu Q, Wu JH, Ye ZY, She W, Peng WJ, Zhang HX, et al. Exosomes from Hypoxia-treated Mesenchymal Stem Cells: Promoting Neuroprotection in Ischemic Stroke Through miR-214-3p/PTEN Mechanism. Mol Neurobiol. 2024;61:7611–26. [DOI] [PubMed] [Google Scholar]
- 70.Gao X, Gao H, Yue K, Cao X, Yang E, Zhang Z, et al. Observing Extracellular Vesicles Originating from Endothelial Cells in Vivo Demonstrates Improved Astrocyte Function Following Ischemic Stroke via Aggregation-Induced Emission Luminogens. ACS Nano. 2023;17:16174–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ding W, Gu Q, Liu M, Zou J, Sun J, Zhu J. Astrocytes-derived exosomes pre-treated by berberine inhibit neuroinflammation after stroke via miR-182-5p/Rac1 pathway. Int Immunopharmacol. 2023;118:110047. [DOI] [PubMed] [Google Scholar]
- 72.Yang H, Han M, Li J, Ke H, Kong Y, Wang W, et al. Delivery of miRNAs through Metal-Organic Framework Nanoparticles for Assisting Neural Stem Cell Therapy for Ischemic Stroke. ACS Nano. 2022;16:14503–16. [DOI] [PubMed] [Google Scholar]
- 73.Yang H, Tu Z, Yang D, Hu M, Zhou L, Li Q, et al. Exosomes from hypoxic pre-treated ADSCs attenuate acute ischemic stroke-induced brain injury via delivery of circ-Rps5 and promote M2 microglia/macrophage polarization. Neurosci Lett. 2022;769:136389. [DOI] [PubMed] [Google Scholar]
- 74.Kim M, Lee Y, Lee M. Hypoxia-specific anti-RAGE exosomes for nose-to-brain delivery of anti-miR-181a oligonucleotide in an ischemic stroke model. Nanoscale. 2021;13:14166–78. [DOI] [PubMed] [Google Scholar]
- 75.Dhuri K, Vyas RN, Blumenfeld L, Verma R, Bahal R. Nanoparticle Delivered Anti-miR-141-3p for Stroke Therapy. Cells. 2021;10:1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Si SH, Song SZ, Xie XY, Zhang TT, Kui XF, Yang XH, et al. Electroacupuncture promotes angiogenesis by regulating miR-142-5p and activating ADAMTS1/PI3K/AKT pathway in ischemic stroke rats. Zhen Ci Yan Jiu. 2024;49:787–96. [DOI] [PubMed] [Google Scholar]
- 77.Yu Q, Shu S, Ju XY, Peng W, Ren XQ, Si SH, et al. Electroacupuncture Promotes Angiogenesis in Mice with Cerebral Ischemia by Inhibiting miR-7. Chin J Integr Med. 2024;30:543–50. [DOI] [PubMed] [Google Scholar]
- 78.Lu XY, Lv QY, Li QL, Zhang H, Chen CT, Tian HM. Impact of acupuncture on ischemia/reperfusion injury: Unraveling the role of miR-34c-5p and autophagy activation. Brain Res Bull. 2024;215:111031. [DOI] [PubMed] [Google Scholar]
- 79.Haupt M, Zheng X, Kuang Y, Lieschke S, Janssen L, Bosche B, et al. Lithium modulates miR-1906 levels of mesenchymal stem cell-derived extracellular vesicles contributing to poststroke neuroprotection by toll-like receptor 4 regulation. Stem Cells Transl Med. 2021;10:357–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hong Z, Zuo Z, Zhao Y, Ai Y, Zhang L, Li L, et al. Transcranial focused ultrasound stimulation alleviates NLRP3-related neuroinflammation induced by ischemic stroke via regulation of the Nespas/miR-383-3p/SHP2 pathway. Int Immunopharmacol. 2025;144:113680. [DOI] [PubMed] [Google Scholar]
- 81.Baldán Á, Fernández-Hernando C. Truths and controversies concerning the role of miRNAs in atherosclerosis and lipid metabolism. Curr Opin Lipido. 2016;27:623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wei Q, Xiao Y, Du L, Li Y. Advances in Nanoparticles in the Prevention and Treatment of Myocardial Infarction. Molecules. 2024;29:2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Z, Cheng J, Zhou Q, Wu LL, Chen JW, Duan XN, et al. SEC62-dependent ER-phagy contributes to apelin-13/APJ-induced monocyte-vascular endothelial cell adhesion in atherosclerosis pathogenesis. Acta Pharm Sin. 2025;46:1652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang S, Lin H, Wang J, Rui J, Wang T, Cai Z, et al. Sensing ceramides by CYSLTR2 and P2RY6 to aggravate atherosclerosis. Nature. 2025;641:476–85. [DOI] [PubMed] [Google Scholar]
- 85.Chen K, Deng Z, Zhu C, Zhang Q, Chen R, Li T, et al. LKB1 delays atherosclerosis by inhibiting phenotypic transformation of vascular smooth muscle cells. Int J Cardiol. 2024;394:131363. [DOI] [PubMed] [Google Scholar]
- 86.Hurt-Camejo E. Unveiling the PGAM5-ANGPTL3 Axis: Implications for Atherosclerosis Progression and Therapy. JACC Basic Transl Sci. 2025;10:674–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fan L, Liu J, Hu W, Chen Z, Lan J, Zhang T, et al. Targeting pro-inflammatory T cells as a novel therapeutic approach to potentially resolve atherosclerosis in humans. Cell Res. 2024;34:407–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kong Z, Sun P, Lu Y, Yang Y, Min DY, Zheng SC, et al. Yi Mai granule improve energy supply of endothelial cells in atherosclerosis via miRNA-125a-5p regulating mitochondrial autophagy through Pink1-Mfn2-Parkin pathway. J Ethnopharmacol. 2024;319:117114. [DOI] [PubMed] [Google Scholar]
- 89.Guo B, Zhuang TT, Li CC, Li F, Shan SK, Zheng MH, et al. MiRNA-132/212 encapsulated by adipose tissue-derived exosomes worsen atherosclerosis progression. Cardiovasc Diabetol. 2024;23:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Y, Xu Z, Wang X, Zheng J, Peng L, Zhou Y, et al. Extracellular-vesicle containing miRNA-503-5p released by macrophages contributes to atherosclerosis. Aging (Albany NY). 2021;13:12239–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu F, Liu Y, Du Y, Li Y. MiRNA-130a promotes inflammation to accelerate atherosclerosis via the regulation of proliferator-activated receptor γ (PPARγ) expression. Anatol J Cardiol. 2021;25:630–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo J, Mei H, Sheng Z, Meng Q, Véniant MM, Yin H. Hsa-miRNA-23a-3p promotes atherogenesis in a novel mouse model of atherosclerosis. J Lipid Res. 2020;61:1764–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Loyer X, Potteaux S, Vion AC, Guérin CL, Boulkroun S, Rautou PE, et al. Inhibition of microRNA-92a prevents endothelial dysfunction and atherosclerosis in mice. Circ Res. 2014;114:434–43. [DOI] [PubMed] [Google Scholar]
- 94.de Yébenes VG, Briones AM, Martos-Folgado I, Mur SM, Oller J, Bilal F, et al. Aging-Associated miR-217 Aggravates Atherosclerosis and Promotes Cardiovascular Dysfunction. Arterioscler Thromb Vasc Biol. 2020;40:2408–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Choi YY, Kim A, Lee Y, Lee YH, Park M, Shin E, et al. The miR-126-5p and miR-212-3p in the extracellular vesicles activate monocytes in the early stage of radiation-induced vascular inflammation implicated in atherosclerosis. J Extracell Vesicles. 2023;12:e12325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang R, Hu Z, Cao Y, Li H, Zhang H, Su W, et al. MiR-652-3p inhibition enhances endothelial repair and reduces atherosclerosis by promoting Cyclin D2 expression. EBioMedicine. 2019;40:685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhu L, Li Q, Qi D, Niu F, Li Q, Yang H, et al. Atherosclerosis-associated endothelial cell apoptosis by miRNA let7-b-mediated downregulation of HAS-2. J Cell Biochem. 2020;121:3961–72. [DOI] [PubMed] [Google Scholar]
- 98.Su Y, Yuan J, Zhang F, Lei Q, Zhang T, Li K, et al. MicroRNA-181a-5p and microRNA-181a-3p cooperatively restrict vascular inflammation and atherosclerosis. Cell Death Dis. 2019;10:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bonacini M, Rossi A, Ferrigno I, Muratore F, Boiardi L, Cavazza A, et al. miR-146a and miR-146b regulate the expression of ICAM-1 in giant cell arteritis. J Autoimmun. 2024;144:103186. [DOI] [PubMed] [Google Scholar]
- 100.Chen W, Wu X, Hu J, Liu X, Guo Z, Wu J, et al. The translational potential of miR-26 in atherosclerosis and development of agents for its target genes ACC1/2, COL1A1, CPT1A, FBP1, DGAT2, and SMAD7. Cardiovasc Diabetol. 2024;23:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu J, Liu T, Xie W, Zhuo Y, Feng Y. Ox-LDL promotes M1-like polarization of macrophages through the miR-21-5p/SKP2/EP300 pathway. J Biochem Mol Toxicol. 2024;38:e23516. [DOI] [PubMed] [Google Scholar]
- 102.Qi X, Wang H, Xia L, Lin R, Li T, Guan C, et al. miR-30b-5p releases HMGB1 via UBE2D2/KAT2B/HMGB1 pathway to promote pro-inflammatory polarization and recruitment of macrophages. Atherosclerosis. 2021;324:38–45. [DOI] [PubMed] [Google Scholar]
- 103.Chen X, Chen S, Pang J, Huang R, You Y, Zhang H, et al. Hepatic steatosis aggravates atherosclerosis via small extracellular vesicle-mediated inhibition of cellular cholesterol efflux. J Hepatol. 2023;79:1491–501. [DOI] [PubMed] [Google Scholar]
- 104.Yang S, Li J, Chen Y, Zhang S, Feng C, Hou Z, et al. MicroRNA-216a promotes M1 macrophages polarization and atherosclerosis progression by activating telomerase via the Smad3/NF-κB pathway. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1772–81. [DOI] [PubMed] [Google Scholar]
- 105.Liu Y, Wu Y, Wang C, Hu W, Zou S, Ren H, et al. MiR-127-3p enhances macrophagic proliferation via disturbing fatty acid profiles and oxidative phosphorylation in atherosclerosis. J Mol Cell Cardiol. 2024;193:36–52. [DOI] [PubMed] [Google Scholar]
- 106.Qi JR, Zhao DR, Zhao L, Luo F, Yang M. MiR-520a-3p Inhibited Macrophage Polarization and Promoted the Development of Atherosclerosis via Targeting UVRAG in Apolipoprotein E Knockout Mice. Front Mol Biosci. 2020;7:621324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nguyen MA, Karunakaran D, Geoffrion M, Cheng HS, Tandoc K, Perisic Matic L, et al. Extracellular Vesicles Secreted by Atherogenic Macrophages Transfer MicroRNA to Inhibit Cell Migration. Arterioscler Thromb Vasc Biol. 2018;38:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rawal S, Randhawa V, Rizvi SHM, Sachan M, Wara AK, Pérez-Cremades D, et al. miR-369-3p ameliorates diabetes-associated atherosclerosis by regulating macrophage succinate-GPR91 signaling. Cardiovasc Res. 2024;120:1693–712. 10.1093/cvr/cvae102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Canfrán-Duque A, Rotllan N, Zhang X, Fernández-Fuertes M, Ramírez-Hidalgo C, Araldi E, et al. Macrophage deficiency of miR-21 promotes apoptosis, plaque necrosis, and vascular inflammation during atherogenesis. EMBO Mol Med. 2017;9:1244–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rachmawati E, Sargowo D, Rohman MS, Widodo N, Kalsum U. miR-155-5p predictive role to decelerate foam cell atherosclerosis through CD36, VAV3, and SOCS1 pathway. Noncoding RNA Res. 2021;6:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu Y, Sun Y, Lin X, Zhang D, Hu C, Liu J, et al. Perivascular adipose-derived exosomes reduce macrophage foam cell formation through miR-382-5p and the BMP4-PPARγ-ABCA1/ABCG1 pathways. Vasc Pharm. 2022;143:106968. [DOI] [PubMed] [Google Scholar]
- 112.Toriuchi K, Kihara T, Aoki H, Kakita H, Takeshita S, Ueda H, et al. Monocyte-Derived miRNA-1914-5p Attenuates IL-1β-Induced Monocyte Adhesion and Transmigration. Int J Mol Sci. 2023;24:2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li W, Deng P, Wang J, Li Z, Zhang H. MiR-17 Knockdown Promotes Vascular Smooth Muscle Cell Phenotypic Modulation Through Upregulated Interferon Regulator Factor 9 Expression. Am J Hypertens. 2020;33:1119–26. [DOI] [PubMed] [Google Scholar]
- 114.Sun D, Xiang G, Wang J, Li Y, Mei S, Ding H, et al. miRNA 146b-5p protects against atherosclerosis by inhibiting vascular smooth muscle cell proliferation and migration. Epigenomics. 2020;12:2189–204. [DOI] [PubMed] [Google Scholar]
- 115.Afzal TA, Luong LA, Chen D, Zhang C, Yang F, Chen Q, et al. NCK Associated Protein 1 Modulated by miRNA-214 Determines Vascular Smooth Muscle Cell Migration, Proliferation, and Neointima Hyperplasia. J Am Heart Assoc. 2016;5:e004629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li P, Zhu N, Yi B, Wang N, Chen M, You X, et al. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circ Res. 2013;113:1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Han Y, Zhang J, Huang S, Cheng N, Zhang C, Li Y, et al. MicroRNA-223-3p inhibits vascular calcification and the osteogenic switch of vascular smooth muscle cells. J Biol Chem. 2021;296:100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Han Z, Guan Y, Liu B, Lin Y, Yan Y, Wang H, et al. MicroRNA-99a-5p alleviates atherosclerosis via regulating Homeobox A1. Life Sci. 2019;232:116664. [DOI] [PubMed] [Google Scholar]
- 119.Xue P, Liu Y, Wang H, Huang J, Luo M. miRNA-103-3p-Hlf regulates apoptosis and autophagy by targeting hepatic leukaemia factor in heart failure. ESC Heart Fail. 2023;10:3038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun P, Wang C, Mang G, Xu X, Fu S, Chen J, et al. Extracellular vesicle-packaged mitochondrial disturbing miRNA exacerbates cardiac injury during acute myocardial infarction. Clin Transl Med. 2022;12:e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang F, Min X, Hu SY, You DL, Jiang TT, Wang L, et al. Hypoxia/reoxygenation-induced upregulation of miRNA-542-5p aggravated cardiomyocyte injury by repressing autophagy. Hum Cell. 2021;34:349–59. [DOI] [PubMed] [Google Scholar]
- 122.Tan J, Min J, Jiang Y, Liu S, Ke M, Wang Z, et al. CircCHSY1 protects hearts against ischaemia/reperfusion injury by enhancing heme oxygenase 1 expression via miR-24-3p. Cardiovasc Res. 2024;120:1924–38. [DOI] [PubMed] [Google Scholar]
- 123.Yao B, Wan X, Zheng X, Zhong T, Hu J, Zhou Y, et al. Critical roles of microRNA-141-3p and CHD8 in hypoxia/reoxygenation-induced cardiomyocyte apoptosis. Cell Biosci. 2020;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xiao L, Gu Y, Ren G, Chen L, Liu L, Wang X, et al. miRNA-146a Mimic Inhibits NOX4/P38 Signalling to Ameliorate Mouse Myocardial Ischaemia Reperfusion (I/R) Injury. Oxid Med Cell Longev. 2021;2021:6366254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu F, Zhang X, Sun C, Xu W, Xia J. Downregulation of miRNA-663b protects against hypoxia-induced injury in cardiomyocytes by targeting BCL2L1. Exp Ther Med. 2020;19:3581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu Q, Li Y, Zhang N, Lu J, Gan X, Chen L, et al. Silencing of lncRNA NEAT1 alleviates acute myocardial infarction by suppressing miR-450-5p/ACSL4-mediated ferroptosis. Exp Cell Res. 2024;442:114217. [DOI] [PubMed] [Google Scholar]
- 127.Duan S, Wang C, Xu X, Zhang X, Su G, Li Y, et al. Peripheral Serum Exosomes Isolated from Patients with Acute Myocardial Infarction Promote Endothelial Cell Angiogenesis via the miR-126-3p/TSC1/mTORC1/HIF-1α Pathway. Int J Nanomed. 2022;17:1577–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang M, Liu X, Jiang M, Li J, Tang Y, Zhou L. miR-543 in human mesenchymal stem cell-derived exosomes promotes cardiac microvascular endothelial cell angiogenesis after myocardial infarction through COL4A1. IUBMB Life. 2021;73:927–40. [DOI] [PubMed] [Google Scholar]
- 129.Liu H, Zhang Y, Yuan J, Gao W, Zhong X, Yao K, et al. Dendritic cell‑derived exosomal miR‑494‑3p promotes angiogenesis following myocardial infarction. Int J Mol Med. 2021;47:315–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li Q, Xu Y, Lv K, Wang Y, Zhong Z, Xiao C, et al. Small extracellular vesicles containing miR-486-5p promote angiogenesis after myocardial infarction in mice and nonhuman primates. Sci Transl Med. 2021;13:eabb0202. [DOI] [PubMed] [Google Scholar]
- 131.Guo H, Li Z, Xiao B, Huang R. M2 macrophage-derived exosomes promote angiogenesis and improve cardiac function after myocardial infarction. Biol Direct. 2024;19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang L, Liu C, Huang C, Xu X, Teng J. miR-155 Knockdown Protects against Cerebral Ischemia and Reperfusion Injury by Targeting MafB. Biomed Res Int. 2020;2020:6458204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shi Y, Li Z, Li K, Xu K. miR-155-5p accelerates cerebral ischemia-reperfusion inflammation injury and cell pyroptosis via DUSP14/ TXNIP/NLRP3 pathway. Acta Biochim Pol. 2022;69:787–93. [DOI] [PubMed] [Google Scholar]
- 134.Xin D, Li T, Zhao Y, Guo X, Gai C, Jiang Z, et al. MiR-100-5p-rich small extracellular vesicles from activated neuron to aggravate microglial activation and neuronal activity after stroke. J Nanobiotechnology. 2024;22:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu LJ, Ouyang YB, Xiong X, Stary CM, Giffard RG. Post-stroke treatment with miR-181 antagomir reduces injury and improves long-term behavioral recovery in mice after focal cerebral ischemia. Exp Neurol. 2015;264:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zhou F, Wang YK, Zhang CG, Wu BY. miR-19a/b-3p promotes inflammation during cerebral ischemia/reperfusion injury via SIRT1/FoxO3/SPHK1 pathway. J Neuroinflammation. 2021;18:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shi L, Tian Z, Fu Q, Li H, Zhang L, Tian L, et al. miR-217-regulated MEF2D-HDAC5/ND6 signaling pathway participates in the oxidative stress and inflammatory response after cerebral ischemia. Brain Res. 2020;1739:146835. [DOI] [PubMed] [Google Scholar]
- 138.Zhang S, Sun WC, Liang ZD, Yin XR, Ji ZR, Chen XH, et al. LncRNA SNHG4 Attenuates Inflammatory Responses by Sponging miR-449c-5p and Up-Regulating STAT6 in Microglial During Cerebral Ischemia-Reperfusion Injury. Drug Des Devel Ther. 2020;14:3683–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang M, Wu Q, Tang M, Chen Z, Wu H. Exosomal Mir-3613-3p derived from oxygen-glucose deprivation-treated brain microvascular endothelial cell promotes microglial M1 polarization. Cell Mol Biol Lett. 2023;28:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li Y, Peng B, Li Y, Huang A, Peng Y, Yu Q, et al. MiR-203a-3p/153-3p improves cognitive impairments induced by ischemia/reperfusion via blockade of SRC-mediated MAPK signaling pathway in ischemic stroke. Chem Biol Interact. 2022;358:109900. [DOI] [PubMed] [Google Scholar]
- 141.Zhong H, Chen H, Gu C. Sevoflurane Post-treatment Upregulated miR-203 Expression to Attenuate Cerebral Ischemia-Reperfusion-Induced Neuroinflammation by Targeting MyD88. Inflammation. 2020;43:651–63. [DOI] [PubMed] [Google Scholar]
- 142.Deng Y, Sun S. Runx1 promotes neuronal injury in ischemic stroke through mediating miR-203-3p/Pde4d axis. Brain Inj. 2024;38:1035–45. [DOI] [PubMed] [Google Scholar]
- 143.Qian Y, Tang B, Zhang H, Yang H. Highly-expressed circ_0129657 inhibits proliferation as well as promotes apoptosis and inflammation in HBMECs after oxygen-glucose deprivation via miR-194-5p/GMFB axis. Autoimmunity. 2023;56:2201405. [DOI] [PubMed] [Google Scholar]
- 144.Wei Q, Tu Y, Zuo L, Zhao J, Chang Z, Zou Y, et al. MiR-345-3p attenuates apoptosis and inflammation caused by oxidized low-density lipoprotein by targeting TRAF6 via TAK1/p38/NF-kB signaling in endothelial cells. Life Sci. 2020;241:117142. [DOI] [PubMed] [Google Scholar]
- 145.Xu D, Guo Q. miR-26a Improves Microglial Activation and Neuronal Apoptosis in a Rat Model of Cerebral Infarction by Regulating the TREM1-TLR4/MyD88/NF-κB Axis. Dev Neurosci. 2024;46:221–36. [DOI] [PubMed] [Google Scholar]
- 146.Sun R, Liao W, Lang T, Qin K, Jiao K, Shao L, et al. Astrocyte-derived exosomal miR-378a-5p mitigates cerebral ischemic neuroinflammation by modulating NLRP3-mediated pyroptosis. Front Immunol. 2024;15:1454116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheng Z, Li X, Ye X, Yu R, Deng Y. Purpurogallin Reverses Neuronal Apoptosis and Enhances “M2” Polarization of Microglia Under Ischemia via Mediating the miR-124-3p/TRAF6/NF-κB Axis. Neurochem Res. 2023;48:375–92. [DOI] [PubMed] [Google Scholar]
- 148.Wu Y, Yao J, Feng K. miR-124-5p/NOX2 Axis Modulates the ROS Production and the Inflammatory Microenvironment to Protect Against the Cerebral I/R Injury. Neurochem Res. 2020;45:404–17. [DOI] [PubMed] [Google Scholar]
- 149.Bao Z, Zhang S, Li X. MiR-5787 Attenuates Macrophages-Mediated Inflammation by Targeting TLR4/NF-κB in Ischemic Cerebral Infarction. Neuromolecular Med. 2021;23:363–70. [DOI] [PubMed] [Google Scholar]
- 150.Shan Y, Hu J, Lv H, Cui X, Di W. miR-221 Exerts Neuroprotective Effects in Ischemic Stroke by Inhibiting the Proinflammatory Response. J Stroke Cerebrovasc Dis. 2021;30:105489. [DOI] [PubMed] [Google Scholar]
- 151.Bernstein DL, Zuluaga-Ramirez V, Gajghate S, Reichenbach NL, Polyak B, Persidsky Y, et al. miR-98 reduces endothelial dysfunction by protecting blood-brain barrier (BBB) and improves neurological outcomes in mouse ischemia/reperfusion stroke model. J Cereb Blood Flow Metab. 2020;40:1953–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang D, Pan N, Jiang C, Hao M. LncRNA SNHG8 sponges miR-449c-5p and regulates the SIRT1/FoxO1 pathway to affect microglia activation and blood-brain barrier permeability in ischemic stroke. J Leukoc Biol. 2022;111:953–66. [DOI] [PubMed] [Google Scholar]
- 153.Tian J, Liu Y, Wang Z, Zhang S, Yang Y, Zhu Y, et al. LncRNA Snhg8 attenuates microglial inflammation response and blood-brain barrier damage in ischemic stroke through regulating miR-425-5p mediated SIRT1/NF-κB signaling. J Biochem Mol Toxicol. 2021;35:e22724. [DOI] [PubMed] [Google Scholar]
- 154.Ma F, Sun P, Zhang X, Hamblin MH, Yin KJ. Endothelium-targeted deletion of the miR-15a/16-1 cluster ameliorates blood-brain barrier dysfunction in ischemic stroke. Sci Signal. 2020;13:eaay5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hu H, Hone EA, Provencher EAP, Sprowls SA, Farooqi I, Corbin DR, et al. MiR-34a Interacts with Cytochrome c and Shapes Stroke Outcomes. Sci Rep. 2020;10:3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dai Q, Sun J, Dai T, Xu Q, Ding Y. miR-29c-5p knockdown reduces inflammation and blood-brain barrier disruption by upregulating LRP6. Open Med (Wars). 2022;17:353–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhou Z, Hu Q, Guo H, Wang X. CircSEC11A knockdown alleviates oxidative stress and apoptosis and promotes cell proliferation and angiogenesis by regulating miR-29a-3p/SEMA3A axis in OGD-induced human brain microvascular endothelial cells (HBMECs). Clin Hemorheol Microcirc. 2023;84:247–62. [DOI] [PubMed] [Google Scholar]
- 158.Xie L, Zhao H, Wang Y, Chen Z. Exosomal shuttled miR-424-5p from ischemic preconditioned microglia mediates cerebral endothelial cell injury through negatively regulation of FGF2/STAT3 pathway. Exp Neurol. 2020;333:113411. [DOI] [PubMed] [Google Scholar]
- 159.Zhang M, Tang M, Wu Q, Wang Z, Chen Z, Ding H, et al. LncRNA DANCR attenuates brain microvascular endothelial cell damage induced by oxygen-glucose deprivation through regulating of miR-33a-5p/XBP1s. Aging (Albany NY). 2020;12:1778–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Deng L, Zhang J, Chen S, Wu Y, Fan X, Zuo T, et al. miR-671-5p Upregulation Attenuates Blood-Brain Barrier Disruption in the Ischemia Stroke Model Via the NF-кB/MMP-9 Signaling Pathway. Mol Neurobiol. 2023;60:3824–38. [DOI] [PubMed] [Google Scholar]
- 161.Pan JJ, Qi L, Wang L, Liu C, Song Y, Mamtilahun M, et al. M2 Microglial Extracellular Vesicles Attenuated Blood-brain Barrier Disruption via MiR-23a-5p in Cerebral Ischemic Mice. Aging Dis. 2024;15:1344–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shen J, Li G, Zhu Y, Xu Q, Zhou H, Xu K, et al. Foxo1-induced miR-92b down-regulation promotes blood-brain barrier damage after ischaemic stroke by targeting NOX4. J Cell Mol Med. 2021;25:5269–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li G, Ma X, Zhao H, Fan J, Liu T, Luo Y, et al. Long non-coding RNA H19 promotes leukocyte inflammation in ischemic stroke by targeting the miR-29b/C1QTNF6 axis. CNS Neurosci Ther. 2022;28:953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li H, Han G, He D, Wang Y, Lin Y, Zhang T, et al. miR-539 Targeting SNAI2 Regulates MMP9 Signaling Pathway and Affects Blood-Brain Barrier Permeability in Cerebrovascular Occlusive Diseases: A Study Based on Head and Neck Ultrasound and CTA. J Health Eng. 2021;2021:5699025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Tan L, Wang L, Liu J, Yu Y. Circvrk1 downregulation attenuates brain microvascular endothelial cell damage induced by oxygen-glucose deprivation through modulating the miR-150-5p/MLLT1 axis. Exp Brain Res. 2023;241:781–91. [DOI] [PubMed] [Google Scholar]
- 166.Sun Y, Ding S, Fan Y, Shen F, Dong Q, Zhao B, et al. MiR-429 Inhibits the Angiogenesis of Human Brain Microvascular Endothelial Cells through SNAI2-Mediated GSK-3β/β-Catenin Pathway. Comput Math Methods Med. 2021;2021:6753926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xue LX, Shu LY, Wang HM, Lu KL, Huang LG, Xiang JY, et al. miR-181b promotes angiogenesis and neurological function recovery after ischemic stroke. Neural Regen Res. 2023;18:1983–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bao H, Mao S, Hu X, Li L, Tao H, Zhou J, et al. Exosomal miR-486 derived from bone marrow mesenchymal stem cells promotes angiogenesis following cerebral ischemic injury by regulating the PTEN/Akt pathway. Sci Rep. 2024;14:18086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Feng N, Wang Z, Wu Y, Zheng H, Jiang X, Wang Z, et al. ADAMTS9-AS2 Promotes Angiogenesis of Brain Microvascular Endothelial Cells Through Regulating miR-185-5p/IGFBP-2 Axis in Ischemic Stroke. Mol Neurobiol. 2022;59:2593–604. [DOI] [PubMed] [Google Scholar]
- 170.Hu H, Hu X, Li L, Fang Y, Yang Y, Gu J, et al. Exosomes Derived from Bone Marrow Mesenchymal Stem Cells Promote Angiogenesis in Ischemic Stroke Mice via Upregulation of MiR-21-5p. Biomolecules. 2022;12:883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Xu C, Yu H, Chen B, Ma Y, Lv P. Serum Exosomal mir-340-5p Promotes Angiogenesis in Brain Microvascular Endothelial Cells During Oxygen-Glucose Deprivation. Neurochem Res. 2022;47:907–20. [DOI] [PubMed] [Google Scholar]
- 172.Xu SY, Zeng CL, Ni SM, Peng YJ. The Angiogenesis Effects of Electro-acupuncture Treatment via Exosomal miR-210 in Cerebral Ischemia-Reperfusion Rats. Curr Neurovasc Res. 2022;19:61–72. [DOI] [PubMed] [Google Scholar]
- 173.Xu SY, Ni SM, Zeng CL, Peng YJ. Electro-acupuncture Promotes Angiogenesis via Exosomal miR-210 in the Hypoxia-induced HUVECs Mediated HIF-1α/VEGF/Notch 1 Signal Pathway. Curr Neurovasc Res. 2022;19:406–17. [DOI] [PubMed] [Google Scholar]
- 174.Zhang Y, Yang Q, Cheng H, Zhang Y, Xie Y, Zhang Q. Extracellular vesicles derived from endothelial progenitor cells modified by Houshiheisan promote angiogenesis and attenuate cerebral ischemic injury via miR-126/PIK3R2. Sci Rep. 2024;14:28166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhuge L, Fang Y, Jin H, Li L, Yang Y, Hu X, et al. [Chinese medicine Buyang Huanwu decoction promotes neurogenesis and angiogenesis in ischemic stroke rats by upregulating miR-199a-5p expression]. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:687–96. [DOI] [PMC free article] [PubMed]
- 176.Liu J, Zhang H, Di K, Hou L, Yu S. Circular noncoding RNA circ_0007865, serves as a competing endogenous RNA, targeting the miR-214-3p/FKBP5 axis to regulate oxygen-glucose deprivation-induced injury in brain microvascular endothelial cells. Neuroreport. 2022;33:163–72. [DOI] [PubMed] [Google Scholar]
- 177.Yan G, Zhao H, Hong X. LncRNA MACC1-AS1 attenuates microvascular endothelial cell injury and promotes angiogenesis under hypoxic conditions via modulating miR-6867-5p/TWIST1 in human brain microvascular endothelial cells. Ann Transl Med. 2020;8:876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li Y, Bi W, Han B, Yuan T, Shi L, Liu Y, et al. MiR-203 Targets to the 3’-UTR of SLUG to Suppress Cerebral Infarction-Induced Endothelial Cell Growth and Motility. Evid Based Complement Altern Med. 2021;2021:5597567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jia Z, An L, Lu Y, Xu C, Wang S, Wang J, et al. Oxidized Low Density Lipoprotein-Induced Atherogenic Response of Human Umbilical Vascular Endothelial Cells (HUVECs) was Protected by Atorvastatin by Regulating miR-26a-5p/Phosphatase and Tensin Homolog (PTEN). Med Sci Monit. 2019;25:9836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.El Zouka Y, Sheta E, Abdelrazek Salama M, Selima E, Refaat R, Salaheldin Abdelhamid Ibrahim S. Tetrandrine ameliorated atherosclerosis in vitamin D3/high cholesterol diet-challenged rats via modulation of miR-34a and Wnt5a/Ror2/ABCA1/NF-kB trajectory. Sci Rep. 2024;14:21371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yan J, Yang Y, Liu Y, Shi X, Wu H, Dai M. MicroRNA let-7g links foam cell formation and adipogenic differentiation: A key regulator of Paeonol treating atherosclerosis-osteoporosis. Phytomedicine. 2024;126:155447. [DOI] [PubMed] [Google Scholar]
- 182.Yu Y, Yan R, Chen X, Sun T, Yan J. Paeonol suppresses the effect of ox-LDL on mice vascular endothelial cells by regulating miR-338-3p/TET2 axis in atherosclerosis. Mol Cell Biochem. 2020;475:127–35. [DOI] [PubMed] [Google Scholar]
- 183.Tian Z, Ning H, Wang X, Wang Y, Han T, Sun C. Endothelial Autophagy Promotes Atheroprotective Communication Between Endothelial and Smooth Muscle Cells via Exosome-Mediated Delivery of miR-204-5p. Arterioscler Thromb Vasc Biol. 2024;44:1813–32. [DOI] [PubMed] [Google Scholar]
- 184.Ma J, Yang S, Ma A, Pan X, Wang H, Li N, et al. Expression of miRNA-155 in carotid atherosclerotic plaques of apolipoprotein E knockout (ApoE(-/-)) mice and the interventional effect of rapamycin. Int Immunopharmacol. 2017;46:70–74. [DOI] [PubMed] [Google Scholar]
- 185.Wang Z, Zhang M, Wang Z, Guo Z, Wang Z, Chen Q. Cyanidin-3-O-glucoside attenuates endothelial cell dysfunction by modulating miR-204-5p/SIRT1-mediated inflammation and apoptosis. Biofactors. 2020;46:803–12. [DOI] [PubMed] [Google Scholar]
- 186.Tan C, Zhou L, Wen W, Xiao N. Curcumin promotes cholesterol efflux by regulating ABCA1 expression through miR-125a-5p/SIRT6 axis in THP-1 macrophage to prevent atherosclerosis. J Toxicol Sci. 2021;46:209–22. [DOI] [PubMed] [Google Scholar]
- 187.Ouyang S, Zhang O, Xiang H, Yao YH, Fang ZY. Curcumin improves atherosclerosis by inhibiting the epigenetic repression of lncRNA MIAT to miR-124. Vascular. 2022;30:1213–23. [DOI] [PubMed] [Google Scholar]
- 188.Rong J, Li C, Zhang Q, Zheng G, Fan W, Pan Z, et al. Hydroxysafflor yellow A inhibits endothelial cell ferroptosis in diabetic atherosclerosis mice by regulating miR-429/SLC7A11. Pharm Biol. 2023;61:404–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu Y, Zhang Y, Zhu H, Shen W, Chen Z, Bai J, et al. Aucubin administration suppresses STING signaling and mitigated high-fat diet-induced atherosclerosis and steatohepatosis in LDL receptor deficient mice. Food Chem Toxicol. 2022;169:113422. [DOI] [PubMed] [Google Scholar]
- 190.Qin HW, Zhang QS, Li YJ, Li WT, Wang Y. [Molecular mechanism of astragaloside IV against atherosclerosis by regulating miR-17-5p and PCSK9/VLDLR signal pathway]. Zhongguo Zhong Yao Za Zhi. 2022;47:492–8. [DOI] [PubMed]
- 191.Shao X, Liu Z, Liu S, Lin N, Deng Y. Astragaloside IV alleviates atherosclerosis through targeting circ_0000231/miR-135a-5p/CLIC4 axis in AS cell model in vitro. Mol Cell Biochem. 2021;476:1783–95. [DOI] [PubMed] [Google Scholar]
- 192.Zeng J, Deng Z, Zou Y, Liu C, Fu H, Gu Y, et al. Theaflavin alleviates oxidative injury and atherosclerosis progress via activating microRNA-24-mediated Nrf2/HO-1 signal. Phytother Res. 2021;35:3418–27. [DOI] [PubMed] [Google Scholar]
- 193.Zhao J, Cui L, Sun J, Xie Z, Zhang L, Ding Z, et al. Notoginsenoside R1 alleviates oxidized low-density lipoprotein-induced apoptosis, inflammatory response, and oxidative stress in HUVECS through modulation of XIST/miR-221-3p/TRAF6 axis. Cell Signal. 2020;76:109781. [DOI] [PubMed] [Google Scholar]
- 194.Jin Z, Zhao H, Luo Y, Li X, Cui J, Yan J, et al. Identification of core genes associated with the anti-atherosclerotic effects of Salvianolic acid B and immune cell infiltration characteristics using bioinformatics analysis. BMC Complement Med Ther. 2022;22:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yang D, Yang Z, Chen L, Kuang D, Zou Y, Li J, et al. Dihydromyricetin increases endothelial nitric oxide production and inhibits atherosclerosis through microRNA-21 in apolipoprotein E-deficient mice. J Cell Mol Med. 2020;24:5911–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang H, Wang L, Peng F, Wang X, Gong H. L-Arginine Ameliorates High-Fat Diet-Induced Atherosclerosis by Downregulating miR-221. Biomed Res Int. 2020;2020:4291327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xu X, Zhao Z, Li G. The Protective Effect of Bosentan against Atherosclerosis in Apolipoprotein E-Deficient Mice Is Mediated by miRNA-21. Biomed Res Int. 2019;2019:8348430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yuan L, Li Q, Zhang Z, Liu Q, Wang X, Fan L. Tanshinone IIA inhibits the adipogenesis and inflammatory response in ox-LDL-challenged human monocyte-derived macrophages via regulating miR-130b/WNT5A. J Cell Biochem. 2020;121:1400–8. [DOI] [PubMed] [Google Scholar]
- 199.Chen W, Li X, Guo S, Song N, Wang J, Jia L, et al. Tanshinone IIA harmonizes the crosstalk of autophagy and polarization in macrophages via miR-375/KLF4 pathway to attenuate atherosclerosis. Int Immunopharmacol. 2019;70:486–97. [DOI] [PubMed] [Google Scholar]
- 200.He PP, Shen QQ, Wen M, Zou JQ, Wang Y, Yang JX, et al. Nobiletin reduces LPL-mediated lipid accumulation and pro-inflammatory cytokine secretion through upregulation of miR-590 expression. Biochem Biophys Res Commun. 2019;508:97–101. [DOI] [PubMed] [Google Scholar]
- 201.Han BH, Seo CS, Yoon JJ, Kim HY, Ahn YM, Eun SY, et al. The Inhibitory Effect of Ojeoksan on Early and Advanced Atherosclerosis. Nutrients. 2018;10:1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Jiang Q, Han Y, Gao H, Tian R, Li P, Wang C. Ursolic acid induced anti-proliferation effects in rat primary vascular smooth muscle cells is associated with inhibition of microRNA-21 and subsequent PTEN/PI3K. Eur J Pharm. 2016;781:69–75. [DOI] [PubMed] [Google Scholar]
- 203.Lv YC, Yang J, Yao F, Xie W, Tang YY, Ouyang XP, et al. Diosgenin inhibits atherosclerosis via suppressing the MiR-19b-induced downregulation of ATP-binding cassette transporter A1. Atherosclerosis. 2015;240:80–89. [DOI] [PubMed] [Google Scholar]
- 204.Wang D, Yan X, Xia M, Yang Y, Li D, Li X, et al. Coenzyme Q10 promotes macrophage cholesterol efflux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1-signaling pathway. Arterioscler Thromb Vasc Biol. 2014;34:1860–70. [DOI] [PubMed] [Google Scholar]
- 205.Yuan R, Xin Q, Ma X, Yu M, Miao Y, Chen K, et al. Identification of a Novel Angiogenesis Signalling circSCRG1/miR-1268b/NR4A1 Pathway in Atherosclerosis and the Regulatory Effects of TMP-PF In Vitro. Molecules. 2023;28:1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang X, Sun Q, Hu W. Carvedilol Protects Against the H2O2-induced Cell Damages in Rat Myoblasts by Regulating the Circ_NFIX/miR-125b-5p/TLR4 Signal Axis. J Cardiovasc Pharm. 2021;78:604–14. [DOI] [PubMed] [Google Scholar]
- 207.Farazi MM, Rostamzadeh F, Jafarinejad-Farsangi S, Moazam Jazi M, Jafari E, Gharbi S. CircPAN3/miR-221/PTEN axis and apoptosis in myocardial Infarction: Quercetin’s regulatory effects. Gene. 2024;909:148316. [DOI] [PubMed] [Google Scholar]
- 208.Ji XD, Yang D, Cui XY, Lou LX, Nie B, Zhao JL, et al. Mechanism of Qili Qiangxin Capsule for Heart Failure Based on miR133a-Endoplasmic Reticulum Stress. Chin J Integr Med. 2024;30:398–407. [DOI] [PubMed] [Google Scholar]
- 209.Yang L, Liu N, Yang Y. Astragaloside IV-induced BMSC exosomes promote neovascularization and protect cardiac function in myocardial infarction mice via the miR-411/HIF-1α axis. J Liposome Res. 2024;34:452–63. [DOI] [PubMed] [Google Scholar]
- 210.Ning Y, Huang P, Chen G, Xiong Y, Gong Z, Wu C, et al. Atorvastatin-pretreated mesenchymal stem cell-derived extracellular vesicles promote cardiac repair after myocardial infarction via shifting macrophage polarization by targeting microRNA-139-3p/Stat1 pathway. BMC Med. 2023;21:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhou X, Xia X. Ginsenoside Rg3 improves microcystin-induced cardiotoxicity through the miR-128-3p/MDM4 axis. Drug Chem Toxicol. 2024;47:682–92. [DOI] [PubMed] [Google Scholar]
- 212.Liu ZH, Wu F, Ren K, Huo JL. Melatonin attenuates inflammation and cardiac dysfunction in myocardial infarction by regulating the miRNA-200b-3p/high mobility group box chromosomal protein 1 axis. J Physiol Pharmacol. 2023;74:377–87. [DOI] [PubMed]
- 213.Yu Q, Zhang N, Gan X, Chen L, Wang R, Liang R, et al. EGCG attenuated acute myocardial infarction by inhibiting ferroptosis via miR-450b-5p/ACSL4 axis. Phytomedicine. 2023;119:154999. [DOI] [PubMed] [Google Scholar]
- 214.Zhang J, Lu Y, Mao Y, Yu Y, Wu T, Zhao W, et al. IFN-γ enhances the efficacy of mesenchymal stromal cell-derived exosomes via miR-21 in myocardial infarction rats. Stem Cell Res Ther. 2022;13:333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Xiong Y, Tang R, Xu J, Jiang W, Gong Z, Zhang L, et al. Tongxinluo-pretreated mesenchymal stem cells facilitate cardiac repair via exosomal transfer of miR-146a-5p targeting IRAK1/NF-κB p65 pathway. Stem Cell Res Ther. 2022;13:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wang X, Wu C. Tanshinone IIA improves cardiac function via regulating miR-499-5p dependent angiogenesis in myocardial ischemic mice. Microvasc Res. 2022;143:104399. [DOI] [PubMed] [Google Scholar]
- 217.Jing H, Wang C, Zhao L, Cheng J, Qin P, Lin H. Propofol protects cardiomyocytes from hypoxia/reoxygenation injury via regulating MALAT1/miR-206/ATG3 axis. J Biochem Mol Toxicol. 2021;35:e22880. [DOI] [PubMed] [Google Scholar]
- 218.Li SN, Liu ZH, Zhou MX, Liu WH, Lai XL, Li P, et al. Danhong Injection Up-regulates miR-125b in Endothelial Exosomes and Attenuates Apoptosis in Post-Infarction Myocardium. Chin J Integr Med. 2023;29:1099–110. [DOI] [PubMed] [Google Scholar]
- 219.Gao X, Li H, Zhang W, Wang X, Sun H, Cao Y, et al. Photobiomodulation Drives MiR-136-5p Expression to Promote Injury Repair after Myocardial Infarction. Int J Biol Sci. 2022;18:2980–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Su W, Lv M, Wang D, He Y, Han H, Zhang Y, et al. Tanshinone IIA Alleviates Traumatic Brain Injury by Reducing Ischemia‒Reperfusion via the miR-124-5p/FoxO1 Axis. Mediators Inflamm. 2024;2024:7459054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hu J, Duan H, Zou J, Ding W, Wei Z, Peng Q, et al. METTL3-dependent N6-methyladenosine modification is involved in berberine-mediated neuroprotection in ischemic stroke by enhancing the stability of NEAT1 in astrocytes. Aging (Albany NY). 2024;16:299–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhang Y, Cao Y, Li Y, Xiao L, Xu W, Xu W, et al. Gualou Guizhi decoction promotes therapeutic angiogenesis via the miR210/HIF/VEGF pathway in vivo and in vitro. Pharm Biol. 2023;61:779–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Liu Q, Wu J, Lai S, Li G. MiR-665 Participates in the Protective Effect of Dexmedetomidine in Ischemic Stroke by ROCK2/NF-κB Axis. Neurochem Res. 2022;47:2064–75. [DOI] [PubMed] [Google Scholar]
- 224.Liang C, Zhang T, Shi XL, Jia L, Wang YL, Yan CH. Modified Renshen Yangrong decoction enhances angiogenesis in ischemic stroke through promotion of MicroRNA-210 expression by regulating the HIF/VEGF/Notch signaling pathway. Brain Behav. 2021;11:e2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang HS, Ouyang B, Ji XY, Liu MF. Gastrodin Alleviates Cerebral Ischaemia/Reperfusion Injury by Inhibiting Pyroptosis by Regulating the lncRNA NEAT1/miR-22-3p Axis. Neurochem Res. 2021;46:1747–58. [DOI] [PubMed] [Google Scholar]
- 226.Zhang L, Cai Q, Lin S, Chen B, Jia B, Ye R, et al. Qingda granule exerts neuroprotective effects against ischemia/reperfusion-induced cerebral injury via lncRNA GAS5/miR-137 signaling pathway. Int J Med Sci. 2021;18:1687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
From Pubmed Data base.
From Pubmed Data base.