Abstract
Houttuynia cordata essential oil (HCEO) is a promising ingredient for acne-focused phytocosmetics. This in vitro study evaluated its volatile compounds, antimicrobial, antioxidant, wound-healing, anti-melanogenic, and anti-inflammatory properties, along with the stability of topical oil‑in‑water emulsions. GC-MS analysis identified n-decanoic acid (46.21%), β-myrcene (14.082%), and 2-undecanone (11.01%) as the main components. HCEO inhibited the growth of Staphylococcus aureus, Candida albicans, Staphylococcus epidermidis, and Cutibacterium acnes. It displayed antioxidant activity, with IC50 values of 13.25 ± 0.09 mg/mL (DPPH) and 17.44 ± 0.16 mg TEAC/g (FRAP). No cytotoxicity was observed in NIH/3T3 fibroblasts and RAW 264.7 macrophages (up to 500 µg/mL). HCEO enhanced fibroblast migration by 26.94% ± 1.12% at 400 µg/mL. It inhibited melanin synthesis in B16F10 and A375 cells, with IC50 values of 200.61 ± 2.19 and 152.16 ± 1.97 µg/mL, respectively, and tyrosinase activity (IC50 of 48.00 ± 0.01 µg/mL). Additionally, HCEO suppressed nitric oxide production in LPS-stimulated macrophages (IC50 of 1.176 ± 0.084 µg/mL). Oil-in-water emulsions containing HCEO maintained physical stability for eight weeks, indicating suitability for cosmetic product development. These findings suggest HCEO has potential as a natural ingredient for advanced skincare formulations targeting acne and skin inflammation, although further work is needed to optimise loading, assess skin permeation, and confirm efficacy in vivo.
Keywords: Houttuynia cordata essential oil, Antimicrobial, Antioxidant, Wound healing, Anti-melanogenesis, Phytocosmetics
Subject terms: Skin diseases, Biological techniques, Biotechnology, Cell biology, Chemical biology, Microbiology, Molecular biology, Pathogenesis, Inflammation
Introduction
Houttuynia cordata Thunb. is a perennial herb originating from East and Southeast Asia. Traditional medicine has long utilized this plant for its well-known health-promoting effects. Modern research supports broad pharmacological properties of the plant, including antioxidative, anti-inflammatory, antimutagenic, and immunomodulatory activities1,2. These effects are largely attributed to essential oils, flavonoids, alkaloids, and polyphenols present in the plant1–3. The essential oil of H. cordata (HCEO) is characterized by a distinctive aroma, rich in bioactive volatile compounds such as houttuynin, 2-undecanone, and n-decanoic acid. The levels of these compounds are influenced by cultivation and extraction conditions3,4.
HCEO has demonstrated antimicrobial efficacy against various bacteria, including Staphylococcus aureus, Escherichia coli, Cutibacterium acnes, and Candida albicans5. This makes HCEO a potential natural alternative in combating antibiotic resistance5. Cutibacterium acnes and Staphylococcus epidermidis, key contributors to acne vulgaris, affect a high percentage of adolescents and adults6–8. Conventional acne treatments, while effective, often cause side effects such as irritation, dryness, or systemic complications9,10. There is an increasing demand for natural skincare products incorporating plant-derived ingredients. Examples include Aloe vera, Centella asiatica, Melaleuca alternifolia, and Thymus vulgaris, which are commonly used in anti-acne formulations4,11,12. HCEO has gained attention in this context due to its antimicrobial, anti-inflammatory, and sebum-regulating effects, making it particularly suitable for oily and acne-prone skin4,11,12. Korean patents and clinical studies have highlighted its role in reducing sebum and improving skin hydration, leading to its inclusion in toners, creams, and advanced cosmetic formulations11–13.
While previous studies have examined the bioactivities of H. cordata, especially extracts from solvent extraction, none have focused on its essential oil as a potential functional ingredient for cosmetic products. This study, thus bridging traditional herbal knowledge with modern cosmetic science. The study evaluates the antimicrobial, antioxidant, wound healing, anti-melanogenic, and anti-inflammatory properties of the oil. The stability of HCEO in gels and creams is also assessed. The findings suggest that HCEO could serve as a safe and multifunctional ingredient in skincare. The antimicrobial and anti-inflammatory properties of HCEO make it a promising candidate for targeting acne and skin inflammation. The antioxidant potential of HCEO could help combat oxidative stress, a major contributor to skin aging and damage. The wound-healing properties of HCEO indicate potential use in products designed for skin repair and regeneration. In addition, the anti-melanogenic effects of HCEO suggest its applicability in addressing hyperpigmentation and uneven skin tone. Overall, the study highlights the potential of HCEO as a natural, multifunctional ingredient in cosmetic formulations and supports further development towards innovative solutions for diverse skin concerns.
Materials and methods
Plant material and chemicals
The plant material used in this study was Houttuynia cordata Thunb. (Plu Kaow), collected from Phan, Chiang Rai, Thailand, between November and December 2022. H. cordata is widely available across Southeast Asia. It thrives in moist, shaded areas, such as riverbanks and wetlands, and is readily available in local markets for both culinary and medicinal purposes. No specific collection permits were required, as the plant material was sourced from a licensed supplier, and no wild collection was conducted. The sample was authenticated by Mr. Apichart Songsanchun, a botanical scientist at Mae Fah Luang University’s Botanical Garden. The voucher number is MFU20250080. Chemicals employed in the experiments included DPPH, TPTZ, L-DOPA, Trolox (6‑hydroxy‑2,5,7,8‑tetramethylchroman‑2‑carboxylic acid), kojic acid, and mushroom tyrosinase, all purchased from Sigma-Aldrich (St. Louis, MO, USA). Minimum Essential Medium (MEM) and Dulbecco’s Modified Eagle’s Medium (DMEM) were used for cell culture. Additional materials included foetal bovine serum (FBS), L-glutamine, penicillin, streptomycin, and MTT, supplied by ThermoFisher Scientific (Waltham, MA, USA).
Plant material and extraction
The aerial parts of H. cordata were shade-dried for two days to reduce moisture content and then cut into smaller pieces. The plant material was mixed with deionized water at a ratio of 1:10 (w/v) in a 5 L round-bottom flask equipped with magnetic stirring bars (MS-E Series, MTOP, Korea) to prevent bumping during distillation. Hydro-distillation was performed at atmospheric pressure (~ 100 °C) following conventional essential oil extraction protocols for 4 h. After completion, the essential oil layer was carefully collected, and any remaining water was eliminated using anhydrous sodium sulphate. The purified essential oil was stored in a refrigerator. The yield of essential oil was calculated based on the dried weight of H. cordata using the following formula:
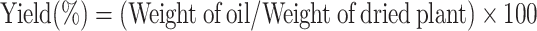 |
Volatile compound analysis by GC-MS
The essential oil was diluted in 99% ethanol at a ratio of 1:100 for analysis. Gas Chromatography-Mass Spectrometry (GC-MS) was performed using an Agilent Technologies Model 7980B Gas Chromatograph equipped with a Triple Quadrupole Mass Selective Detector (Model 7000D). Separation was achieved using an HP-5MS capillary column (30 m x 0.25 mm ID, 0.25 μm film thickness). Helium was used as the carrier gas at a flow rate of 1.2 mL/min, a pressure of 11.01 psi, and an average velocity of 40 cm/sec. The oven temperature was programmed to increase from 50 °C to 325 °C at a rate of 4 °C/min. Electron Ionization (EI) mode was used, with the mass spectrometry detector operating at 70 eV and a scanning range of 40 to 400 amu. The relative content of each compound was determined based on the area of each peak, and identification was aided by mass spectra data from Wiley 11th, NIST 2014, and NIST 2017 libraries.
Antimicrobial activity assessment
The antimicrobial efficacy of H. cordata essential oil (HCEO) was evaluated against three skin-related bacterial species: Staphylococcus aureus (DMST 8013), Staphylococcus epidermidis (DMST 15505), and Cutibacterium acnes (DMST 14916), and the fungus (yeast) Candida albicans (DMST 5815). Specific growth media were used for each organism: nutrient broth (NB) for S. aureus and S. epidermidis, Sabouraud dextrose broth (SDB) for C. albicans, and brain heart infusion (BHI) broth for C. acnes. Incubation was conducted at 37 °C for 24 h (aerobically) for bacteria and fungi, and for 48 h under anaerobic conditions for C. acnes. Microbial suspensions were standardized to a 0.5 McFarland standard, corresponding to an absorbance of 0.08–0.13 at 625 nm and approximately 1.5 × 108 CFU/mL14.
The agar well diffusion method was employed for antimicrobial assessment. Sterile agar plates were prepared using nutrient agar (NA) for S. aureus and S. epidermidis, Sabouraud dextrose agar (SDA) for C. albicans, and brain heart infusion agar (BHA) for C. acnes. Each plate was seeded with 100 µL of standardized microbial suspension, and wells with a diameter of 6 mm were punched into the agar. Each well was filled with 0.3 mg of HCEO. Ampicillin (10 µg/disc) served as the positive control, while Dimethyl sulfoxide (DMSO) was used as the negative control to solubilize HCEO. Inhibition zone diameters were measured after incubation under strain-specific conditions, and triplicate assays were performed14.
Determination of MIC, MBC, and MFC
The minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), and minimum fungicidal concentration (MFC) were determined using a modified version of the standard method15. Microbial cultures were adjusted to the McFarland 0.5 scale (1.5 × 108 CFU/mL). HCEO was dissolved in DMSO to prepare a stock solution of 200 mg/mL, ensuring solubility and enabling a wide range of two-fold serial dilutions. Serial dilutions were prepared in sterile broth media, generating nine final testing concentrations. The formula used for calculation was:
 |
Where, C1 = concentration of stock solution, V1 = volume of stock used, C2 = desired concentration, V2 = total volume.
A volume of 1 mL of microbial suspension was added to each tube, followed by incubation at 37 °C. Staphylococcus aureus, Staphylococcus epidermidis, and Candida albicans were incubated for 24 h, while Cutibacterium acnes required 48 h under anaerobic conditions. After incubation, the solutions were plated on solid agar and incubated again under the same conditions. The lowest concentration that halted microbial growth was recorded as the MIC. The highest dilution preventing colony formation on agar was recorded as the MBC for S. aureus, S. epidermidis, and C. acnes, and as MFC for C. albicans.
Antioxidant activity
The DPPH radical scavenging assay and the ferric reducing antioxidant power (FRAP) assay were used in this study16. These methods evaluate the free radical neutralization and electron-donating capacity of Houttuynia cordata essential oil, respectively. For the DPPH assay, a 0.1 mM solution of DPPH in ethanol (modified from the original use of methanol) was mixed with H. cordata essential oil and incubated in the dark for 30 min. Absorbance was measured at 517 nm using a microplate reader (SPECTROstar Nano, BMG Labtech, UK), with DMSO serving as the blank. The percentage of radical scavenging was calculated, and the IC50 value, indicating the concentration required to inhibit 50% of radicals, was derived from a Trolox standard curve17.
In the FRAP assay, the essential oil (diluted 1:10 in DMSO) was added to freshly prepared FRAP reagent (300 mM acetate buffer pH 3.6, 10 mM TPTZ in 40 mM HCl, and 20 mM ferric chloride in a 10:1:1 ratio). After 30 min at 37 °C, absorbance was read at 593 nm. Antioxidant capacity was quantified as mg Trolox equivalent antioxidant capacity (TEAC) per gram of extract18. All assays were conducted in triplicate using independent sample preparations.
Cell survival
The fibroblast cell line NIH/3T3 (ATCC CRL-1658) and the macrophage cell line RAW 264.7 (ATCC TIB-71) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with high glucose, supplemented with 10% foetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin, and maintained at 37 °C in a humidified atmosphere with 5% CO219. Cells were passaged using 0.25% trypsin solution when needed. Exponentially growing cells (at approximately 80% confluence) were seeded into 96-well plates at 1 × 10^4 cells per well and allowed to adhere to the plate for 24 h.
Cell viability was assessed using the MTT assay [3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyl-2 H-tetrazolium bromide], with modifications for a 96-well format based on an established method20. The assay quantifies cellular metabolic activity, as active cells reduce MTT into purple formazan crystals. For the experiment, H. cordata essential oil was initially dissolved in 100% DMSO at a 1:10 ratio and then diluted with culture medium to maintain a final DMSO concentration of 1%. Serial dilutions of the essential oil were prepared in medium containing 1% DMSO, resulting in concentrations ranging from 25 to 500 µg/mL. The 1% DMSO medium was used as the negative control, as preliminary testing confirmed it was non-toxic to the cells. Cells were exposed to these treatments for 24 h, the logarithmic (log) growth phase during which they exhibit high metabolic activity, active proliferation, and optimal response to external stimuli or test compounds at 37 °C under a 5% CO2 atmosphere. After treatment, 50 µL of MTT working solution (0.5 mg/mL) prepared in serum-free medium (pH 7.4) was added to each well, and the cells were incubated for 4 h Afterward, the medium was removed, and the formazan crystals were solubilized in 100 µL of DMSO with 25 µL of Sorensen’s glycine buffer. The absorbance of the soluble formazan was measured at 570 nm using a microplate reader (SPECTROstar Nano, BMG Labtech, UK). All experiments were conducted in triplicate to ensure accuracy and reproducibility.
In vitro wound healing
An in vitro wound healing assay was performed to investigate the effect of H. cordata essential oil on fibroblast cell migration, a key factor in wound repair and tissue regeneration21,22. The NIH/3T3 fibroblast cells were seeded at a density of 5 × 104 cells per well into 24-well plates. After the cells reached approximately 80% confluence, a wound was created by manually scratching the cell monolayer using a sterile cell scratcher. The cells were then treated with various concentrations of the test compounds. For negative controls, serum-free DMEM was used. The samples were prepared by diluting with culture medium containing 0.5% DMSO to achieve final concentrations of 0.1, 0.2, 0.3, and 0.4 mg/mL. Each concentration of the essential oil was tested in triplicate. Images of the wound area were captured at 0, 24, 48, and 72 h post-treatment using an inverted microscope (CKX41SF, Olympus Corporation, Japan) under 10 times magnification. The extent of wound closure was quantified by measuring the remaining wound area at each time point, and results were expressed as a percentage of the original wound area. This assay allowed for the assessment of the essential oil’s potential to enhance cell migration and promote wound healing during the log phase of cells, and extend to the delayed effect until 72 h of treatment.
Anti-melanogenesis
Melanogenesis is the process by which melanin is produced in the skin, and its overproduction can lead to hyperpigmentation and related disorders. Inhibiting melanogenesis, particularly through the inhibition of tyrosinase activity, is a crucial strategy for treating hyperpigmentation23. To evaluate the anti-melanogenic potential of HCEO, an in vitro mushroom tyrosinase inhibition assay was conducted. Tyrosinase, a key enzyme in melanin biosynthesis, was inhibited using a modified method as described in the literature24.
The assay was carried out in a 96-well microplate using L-DOPA (L-3,4-dihydroxyphenylalanine) as the substrate. Kojic acid, a well-known tyrosinase inhibitor, served as a positive control. The essential oil was dissolved in 25% DMSO with a phosphate buffer solution (50 mM, pH 6.8). In each well, 140 µL of the buffer was mixed with different non-cytotoxic concentrations of HCEO. To this mixture, 10 µL of mushroom tyrosinase (50 U/mL) was added, and the plate was incubated for 10 min. After incubation, 50 µL of 0.95 mM L-DOPA was introduced to initiate the reaction, and the plate was incubated for another 10 min. The absorbance was measured at 475 nm using a microplate reader (SPECTROstar Nano, BMG Labtech, UK), which reflects the formation of dopachrome, an intermediate in melanin synthesis. A blank containing all components except L-DOPA was used to account for background interference, while the control group received 25% DMSO instead of the essential oil. The percentage of tyrosinase inhibition was calculated using the following formula:
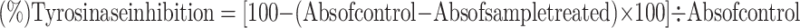 |
In addition to tyrosinase inhibition, the effect of H. cordata essential oil on cellular melanin production was evaluated as per previous research25. The assay was performed on B16F10 melanoma cells and A375 human melanoma cells, which are commonly used in studies on melanogenesis. These cells were seeded at densities of 3 × 104 cells/well and 5 × 104 cells/well, respectively, in 96-well plates. After 48 h, the cells were treated with varying concentrations of the essential oil for 24 and 48 h to examine the response to the test compounds. Following incubation, the medium was removed, and 100 µL of 1 M NaOH containing 10% DMSO was added to each well to solubilize the melanin within the cells. The plates were incubated at 80◦C for 90 min, after which the absorbance was measured at 490 nm using a microplate reader. Kojic acid, a standard compound known for its anti-melanogenic activity, was used as the reference for comparing melanin content26. The percentage inhibition of melanin production was calculated using the following formula:
 |
Anti-inflammatory
The anti-inflammatory activity of H. cordata essential oil was evaluated by measuring the reduction of nitrite release in LPS-stimulated RAW 264.7 macrophage cells, using a method adapted from previous studies17,27,28. RAW 264.7 cells were seeded in a 96-well plate at a density of 1 × 105 cells per well. The cells were pre-treated with varying concentrations of H. cordata essential oil for 1 h, followed by the induction of inflammation through the addition of lipopolysaccharide (LPS, 1 µg/mL). After 24 h of incubation, the cell supernatant was collected, and the nitrite levels, indicative of nitric oxide (NO) production, were measured using the Griess reaction.
Cell supernatant (25 µL) was incubated with sulphanilamide (25 µL) in the dark for 10 min, followed by the addition of N-(1-naphthyl) ethylenediamine (25 µL) in 0.5 N HCl (NED reagent). After an additional 10 min of incubation, the absorbance was measured at 540 nm using a microplate reader (Thermo Scientific). A nitrite standard curve was used to quantify NO levels, and the percentage of inhibition of nitrite production was calculated relative to the LPS-treated control.
Formulation of gel and emulsion containing HCEO
Gel and cream base formulations were selected as prototypes for developing anti-acne preparations incorporating HCEO (Table 1). The gel formula included sodium carboxymethyl cellulose dispersed in aqueous disodium EDTA, humectants like glycerine and 1,3-propanediol, and phenoxyethanol as a preservative. The cream formulation employed xanthan gum as a thickening agent27, and its oil phase consisted of squalane, isononyl isononanoate, and caprylic/capric triglycerides.
Table 1.
Composition of gel and emulsion containing H. cordata essential oil.
| Phase | Ingredient | Gel % (w/w) | Cream % (w/w) |
|---|---|---|---|
| A | Distilled water | q.s. 100 | q.s. 100 |
| Sodium carboxymethyl cellulose | 2 | - | |
| Xanthan gum | - | 1 | |
| Glycerine | 3 | 3 | |
| 1,3 propanediol | 3 | 3 | |
| Disodium EDTA | 0.1 | 0.1 | |
| B | Squalane | - | 5 |
| Isononyl isononanoate | - | 5 | |
| Caprylic/capric triglyceride | - | 3 | |
| Sodium polyacryloyidimethyl taurate and Hydrogenated polydecene and trideceth-10 | - | 3 | |
| C | Phenoxyethanol | 0.8 | 0.8 |
| D | H. cordata essential oil (2% in EtOH) | 0.5 | 0.5 |
To emulsify the cream, a pre-blended mixture of sodium polyacryloyl-dimethyl taurate, hydrogenated polydecene, and trideceth-10 was used as an oil-in-water emulsifier. For both the gel and cream, HCEO was first dissolved in 95% ethanol at a concentration of 2% (w/w) before being incorporated into the base formulations. This ensured uniform distribution of the essential oil and allowed for the evaluation of its physical stability within the formulations.
Gel and cream base formulations were selected as prototypes for developing anti-acne preparations incorporating HCEO. The gel formula included sodium carboxymethyl cellulose dispersed in aqueous disodium EDTA, humectants like glycerine and 1,3-propanediol, and phenoxyethanol as a preservative. The cream formulation employed xanthan gum as a thickening agent27, and its oil phase consisted of squalane, isononyl isononanoate, and caprylic/capric triglycerides. To emulsify the cream, a pre-blended mixture of sodium polyacryloyl-dimethyl taurate, hydrogenated polydecene, and trideceth-10 was used as an oil-in-water emulsifier. For both the gel and cream, HCEO was first dissolved in 95% ethanol at a concentration of 2% (w/w) before being incorporated into the base formulations. This ensured uniform distribution of the essential oil and allowed for the evaluation of its physical stability within the formulations.
The physical and chemical stability of the formulations was tested under accelerated conditions by alternating heating at 40 °C for 24 h and cooling at 4 °C for 24 h as one cycle; the test was conducted for six cycles. Both the base formulations and those containing HCEO were analysed for comparison. Stability indicators included changes in pH, measured with an emulsion probe pH meter (S220 SevenCompact pH/Ion Meter, METTLER TOLEDO, USA), and physical characteristics such as phase separation and odour changes, which were manually inspected. Colour stability was assessed using a colorimeter (UltraScan VIS), recording L*, a*, b*, and ΔE values. The viscosity measurements were taken after each cycle using a viscometer (Brookfield, RVDV2T Extra, USA) to assess the rheological properties of the formulations.
Statistical analysis
All data were expressed as mean ± standard deviation (SD) based on triplicate experiments. The Shapiro-Wilk test was applied to assess normality. Statistical significance for comparisons between two groups was determined using a T-test with SPSS software, version 22.0 (SPSS Inc., Chicago, IL, USA). The multiple group comparisons were performed using one‑way ANOVA followed by Tukey’s post hoc test. A p-value of less than 0.05 (p < 0.05) is considered statistically significant.
Results and discussion
Yield of HCEO
The dried plant of H. cordata yielded a pale-yellow oil with a fishy odour, with an extraction yield of 0.035 ± 0.23% (v/w). Previous studies on essential oil yields from fresh aerial parts of H. cordata have reported ranges from 0.015% to 0.06–0.14% (v/w) using hydro-distillation for 3 h17,27. Whole fresh plants yielded a clear, colourless to pale-yellow oil at a rate of 0.057% (v/w) when extracted at 100 °C for 3 h28. The yield of H. cordata essential oil has been reported to range from 0.03% to 0.06%. Seasonal variations significantly influence yield, with plants harvested in summer yielding 0.044 ± 0.029%, while those harvested in autumn showed a slightly higher yield of 0.036 ± 0.028%29. Several factors, including plant parts, collection season, extraction method, and environmental conditions, can impact essential oil yields29,30.
Volatile compound profile of HCEO by GC-MS
The chemical composition of essential oil is highly dependent on extraction conditions. Temperature plays a critical role in influencing the volatility and stability of aromatic compounds. While higher temperatures may enhance yield, they can also promote the degradation or rearrangement of thermally sensitive constituents. Similarly, pressure modulates boiling points, with advanced vacuum distillation allowing extraction at lower temperatures to preserve thermolabile compounds. In this study, hydro-distillation at atmospheric pressure was selected as the practical and scalable extraction protocol. The volatile compound profile of H. cordata essential oil was analysed by GC-MS, identifying a total of 27 compounds (Table 2). The predominant compound was n-decanoic acid (capric acid), which constituted 46.21% of the oil. Followed by β-Myrcene (14.082%), 2-undecanone (11.01%), 3-oxodecanal (4.51%), decanoic acid, ethyl ester (4.06%), dodecanoic acid (2.05%), α-selinene (1.96%), and caryophyllene oxide (1.69%). Odour-contributing terpenoids included n-nonanol (1.10%), n-decanol (0.75%), caryophyllene (0.45%), linalool (0.13%), and β-pinene (0.08%).
Table 2.
Volatile compounds in H. cordata essential oil.
| Retention time (min) | LRI | LRI (lit) | Identified compounds | Relative percentage |
|---|---|---|---|---|
| 7.99 | 961.55 | 98031 | β-Pinene | 0.077 |
| 8.36 | 968.07 | 98531 | β-Myrcene | 14.082 |
| 12.40 | 1063.03 | 109932 | Linalool | 0.126 |
| 13.34 | 1093.5 | 1388.9033 | n-Nonanol | 1.096 |
| 14.50 | 1127.14 | n-Decanol | 0.747 | |
| 17.20 | 1204.85 | 128432 | Bornyl acetate | 1.175 |
| 17.46 | 1212.53 | 129334 | 2-Undecanone | 11.007 |
| 18.46 | 1242.08 | 1186.2533 | Methyl caprate | 0.701 |
| 20.78 | 1311.34 | n-Decanoic acid | 46.21 | |
| 21.50 | 1333.33 | 142032 | Caryophyllene | 0.453 |
| 22.83 | 1374.07 | α -Guaiene | 1.256 | |
| 23.47 | 1394.14 | γ-Selinene | 1.396 | |
| 23.65 | 1399.69 | 3-Oxodecanal | 4.506 | |
| 23.83 | 1405.51 | α-Selinene | 1.961 | |
| 24.31 | 1421.09 | 2,4-Di-tert-butylphenol | 1.410 | |
| 26.01 | 1476.23 | Dodecanoic acid | 2.054 | |
| 26.25 | 1484.03 | Spathulenol | 0.611 | |
| 26.41 | 1489.22 | 150531 | Caryophyllene oxide | 1.687 |
| 26.78 | 1502.03 | 1232.2234 | Decanoic acid, ethyl ester | 4.064 |
| 32.43 | 1699.28 | 2-Ethylhexyl salicylate | 0.643 | |
| 33.43 | 1737.02 | Hexahydrofarnesyl acetone | 0.745 | |
| 35.44 | 1813.59 | n-Hexadecanoic acid methyl ester | 0.815 | |
| 35.96 | 1834.19 | Isophytol | 0.931 | |
| 38.94 | 1954.56 | Nonyl decanoate | 1.394 | |
| 39.38 | 1972.71 | Methyl linoleate | 0.764 | |
| 39.53 | 1978.91 | Methyl linolenate | 1.373 | |
| 39.82 | 1991.03 | 212934 | Phytol | 1.311 |
*Components are recorded as per their order of elution from an HP-5MS column; LRILit = linear retention index from the literature.
In a previous study, 2-undecanone was primarily identified in the underground parts of H. cordata35. Essential oils extracted from these underground parts contained 2-undecanone (23.96%) and β-myrcene (14.29%) as the major components17. In contrast, essential oils from the leaves were characterized by higher concentrations of β-myrcene (30.8%) and 2-undecanone (19.7%)28. Similarly, findings from fresh plants in Lamphun, Thailand, reported β-myrcene (44.22%) and 2-undecanone (21.98%) as the dominant compounds28. Both dry and fresh plants showed 2-undecanone (44.92%), β-myrcene (17.68%), and β-pinene (9.95%) as the most abundant constituents30. The previous research of HC from Payao, Thailand reported the main constituents were 2-undecanone (48.61%), β-myrcene (11.94%), (Z)-β-ocimene (11.59%), 2-decanone (4.99%), and γ-terpinene (2.62%)27.
Essential oils, derived from aromatic plants, are complex mixtures that can exhibit antioxidant and anti-inflammatory properties. Recent pharmacological studies suggest that components of HCEO possess anti-inflammatory and antibacterial effects17,28. Specifically, 1-decanol has demonstrated antifungal activity36, linalool exhibits antimicrobial and antioxidant properties, and nonanal has been found to have anti-inflammatory and antibacterial effects37. Notably, the underground parts of H. cordata are richer in compounds with anti-inflammatory and antibacterial properties, while the above-ground parts are more associated with flavour and antioxidant properties31,37.
Antimicrobial activity of HCEO
The antimicrobial activity of HCEO is summarized in Table 3. No inhibition zones were observed in the DMSO negative control, confirming that the antimicrobial activity was attributable to HCEO. At a concentration of 0.3 mg, HCEO demonstrated inhibitory effects against Candida albicans (16.0 ± 1.6 mm), Staphylococcus aureus (15.9 ± 0.9 mm), Staphylococcus epidermidis (15.5 ± 0.4 mm), and Cutibacterium acnes (11.6 ± 0.6 mm). In comparison, the positive control ampicillin showed inhibition zones of 10.3 ± 0.6 mm for C. albicans, 26.9 ± 0.2 mm for S. aureus, 12.1 ± 3.3 mm for S. epidermidis, and 24.1 ± 3.0 mm for C. acnes. HCEO demonstrated higher inhibitory activity than ampicillin, particularly against C. albicans, with negligible differences observed against S. epidermidis.
Table 3.
Antimicrobial activity of H. cordata essential oil against microorganisms.
| Test strains | DMSO | Ampicillin | H. cordata essential oil | ||
|---|---|---|---|---|---|
| (mm)* | (mm)* | (mm)* | MIC (µg/mL) |
MBC/MFC (µg/mL) |
|
| Staphylococcus aureus | ND | 26.9 ± 0.2a | 15.9 ± 0.9b | 3.125 | 6.25 |
| Staphylococcus epidermidis | ND | 12.1 ± 3.3a | 15.5 ± 0.4a | 12.5 | 25.0 |
| Cutibacterium acnes | ND | 24.1 ± 3.0a | 11.6 ± 0.6b | 12.5 | 25.0 |
| Candida albicans | ND | 10.3 ± 0.6b | 16.0 ± 1.6a | 3.125 | 6.25 |
*Inhibition zone, values are means of triplicate determination (n = 3) ± standard deviations; ND, no zone of inhibition was detected. Different letters indicate a significant difference in the clear zone diameter between Ampicillin and the H. cordata essential oil at a significance level of p < 0.05.
HCEO shows inhibitory effects against various microorganisms, including β-hemolytic Streptococcus, S. aureus, Pseudomonas aeruginosa, and Escherichia coli17. These findings are consistent with the antimicrobial activity observed with Origanum vulgare L. essential oil, which showed inhibition zones ranging from 16.0 to 32.0 mm against S. epidermidis and C. acnes10. HCEO from the underground parts exhibited significant antibacterial activity, with inhibition zones greater than 10.0–15.0 mm, and antifungal activity against C. albicans, producing an inhibition zone of 20.0 mm. It also showed inhibition zones of 11.0 mm and 13.0 mm against C. kefyr and S. aureus, respectively. The minimum inhibitory concentration (MIC) of HCEO from the underground parts against bacterial strains ranged from 0.52 to 1.04 µg/mL and from 2.08 to 16.66 µg/mL for fungal strains37.
In contrast, HCEO from the aerial parts exhibited lower activity against two fungal strains, with inhibition zones between 5.0 and 10.0 mm. This is less than the inhibition zone observed with the same aerial part of HCEO against C. albicans (16.0 mm). The lowest concentration of HCEO required to prevent visible microbial growth (MIC) was 3.125 µg/mL for S. aureus and C. albicans, and 12.5 µg/mL for C. acnes and S. epidermidis. The minimum bactericidal concentration (MBC) was 6.25 µg/mL for S. aureus, 25.0 µg/mL for C. acnes and S. epidermidis, and the minimum fungicidal concentration (MFC) of 6.25 µg/mL for C. albicans.
Hydro-distillation of whole H. cordata has also shown potential for inhibiting S. aureus, with MIC values between 0.0625 and 4.0 mg/mL. This activity is attributed to compounds such as α-pinene, β-pinene, limonene, n-decanal, and α-terpineol, which exhibit antimicrobial and antifungal properties. Moreover, n-decanoic acid has been reported to cause membrane rupture in yeast, leading to rapid cell death29. HCEO containing aldehydes like decanal, dodecanal, and decanoyl acetaldehyde has also been noted for its antibacterial activity38.
Antioxidant activities of HCEO
The antioxidant activities of HCEO were evaluated using several assays, including DPPH radical scavenging, ferric-reducing antioxidant power (FRAP), and tyrosinase inhibition (Table 4). HCEO demonstrated DPPH radical scavenging activity with an IC50 value of 13.25 ± 0.09 mg/mL, while Trolox, a standard antioxidant, exhibited a significantly lower IC50 value of 0.008 ± 0.00 mg/mL. In the FRAP assay, the antioxidant capacity of HCEO was measured at 17.44 ± 0.16 mg Trolox equivalent antioxidant capacity (TEAC) per gram.
Table 4.
Antioxidant, anti-tyrosinase, and anti-inflammatory activities of H. cordata essential oils.
| Sample | DPPH (IC50, mg/mL) |
FRAP (mg TEAC/g) |
Tyrosinase inhibition (IC50, mg/mL) |
Anti-inflammatory activity (µg/mL) |
|---|---|---|---|---|
| H. cordata essential oils | 13.25 ± 0.09 | 17.44 ± 0.16 | 48.00 ± 0.10 | 1.176 ± 0.084 |
| Trolox | 0.008 ± 0.00 | - | - | - |
| Kojic acid | - | - | 5.60 ± 0.30 | - |
The antioxidant properties of HCEO are attributed to several of its compounds, such as β-myrcene and α-pinene30. In previous studies, HCEO showed DPPH radical scavenging activity with an IC50 value of 20.88 ± 0.19 mg/mL39. Additionally, HCEO has been reported to inhibit oxidative activity40. This contrasts with the essential oil of Ocimum basilicum, which displayed a lower DPPH radical scavenging ability, with an IC50 value of 26.53 mg/mL41. The antioxidant activity of HCEO may be influenced by its phenolic content, as phenolic compounds are known for their free radical scavenging properties due to their hydroxyl groups. However, the antioxidant and anti-inflammatory effects of HCEO can vary depending on factors such as harvesting time, climatic and agronomic conditions, the development stage of the plant, the plant part used, and the extraction method2. While HCEO does not exhibit as high antioxidant potential as purified standards like Trolox or stronger essential oils, its antioxidant activity is comparable to prior studies on HCEO and remains sufficient for use in topical antioxidant protection, especially when paired with its antimicrobial and anti-inflammatory properties.
Cell survival
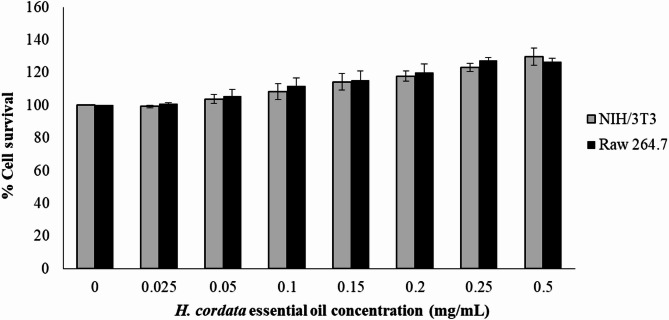
In cell survival assays using NIH/3T3 fibroblast cells and RAW 264.7 macrophage cells, cell viability was assessed by comparing the absorbance of treated cells to that of untreated control cells, which were set at 100% viability. The results showed that cells treated with HCEO exhibited a higher percentage of viability compared to untreated controls. This suggests that HCEO does not exhibit toxicity to NIH/3T3 fibroblast cells or RAW 264.7 macrophage cells at concentrations up to 500 µg/mL after 24 h of exposure (Fig. 1).
Fig. 1.
The effect of the H. cordata essential oil on the percentage of the cell survival of NIH/3T3 fibroblast cells and Raw 264.7 macrophage cells after 24 h of exposure.
Previous research has indicated that extracts from H. cordata can positively influence fibroblast cell viability. For instance, ethanol extracts of H. cordata enhanced cell viability in human fibroblasts (NHDF) at concentrations ranging from 25 mg/mL to 400 mg/mL without exhibiting toxicity to RAW 264.7 macrophages. In contrast, aqueous extracts reduced cell viability at 250 mg/mL in fibroblast cells and at 62.5 mg/mL in RAW 264.7 macrophages42.
Furthermore, H. cordata extracts prepared with 70% ethanol demonstrated cytotoxicity at 100 µg/mL in 3T3-L1 fibroblast cells37. Another study reported that H. cordata extract was non-toxic to RAW 264.7 macrophages within the concentration range of 1 µg/mL to 100 µg/mL43. Although the current findings suggest that HCEO is likely safe for use in fibroblast cell cultures, there is limited information specifically regarding the cytotoxicity of this essential oil in fibroblast cells. Therefore, the cell survival results were compared with data from crude extracts of H. cordata and other essential oils to provide context for the safety profile of HCEO and to support its potential applications.
Comparatively, other essential oils have shown varied effects on cell viability. For example, B. morelensis essential oil at 10 µg did not affect cell viability44. Eucalyptus globulus essential oil at 0.01% did not reduce fibroblast cell viability after 48 h of treatment45. The essential oil of E. dysenterica exhibited cytotoxicity with an IC50 of 542.2 µg/mL on L292 cells but was safe for RAW 264.7 macrophages at 292.0 µg/mL46. Similarly, essential oils from lemongrass, ginger, black pepper, and long pepper were reported as non-toxic to fibroblast cells at concentrations of 25 µg/mL, 125 µg/mL, 125 µg/mL, and 250 µg/mL, respectively47.
Wound healing activity of HCEO
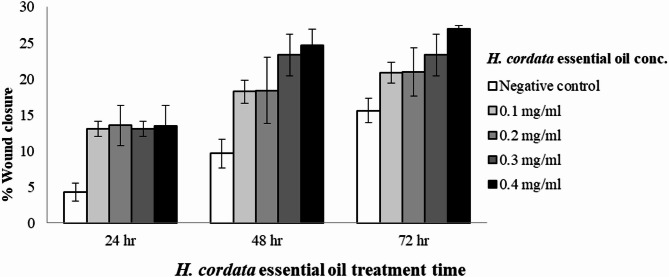
The wound healing assay results, illustrated in Fig. 2, demonstrate that HCEO, under the non-cytotoxic range (< 0.5 mg/mL), effectively promotes cell migration in fibroblast cells. The percentage of wound healing was calculated by comparing the area of cell migration to that of the negative control, which is cell culture medium without treatment (DMEM). Treatment with HCEO led to a significant increase in wound closure in a dose-dependent manner, with concentrations ranging from 0.05 to 0.4 mg/mL showing enhanced cell migration compared to the control. The effect of the treatment was also time-dependent; notably, at the delayed affected time of 72 h, the highest concentration tested (0.4 mg/mL) resulted in a maximum wound closure of 26.94%.
Fig. 2.
In vitro wound healing activity of H. cordata essential oil.
These findings are consistent with previous studies that have reported similar effects. For example, H. cordata extract was shown to promote cell migration at a dose of 40 µg/mL48. The essential oil from B. morelensis facilitated fibroblast migration, achieving complete wound closure after 48 h at a concentration of 0.01 mg/mL44. In comparison, E. dysenterica essential oil demonstrated complete wound closure in a scratch assay model at an IC50 concentration of 542.2 µg/mL46.
In vitro and cellular anti-melanogenesis of HCEO
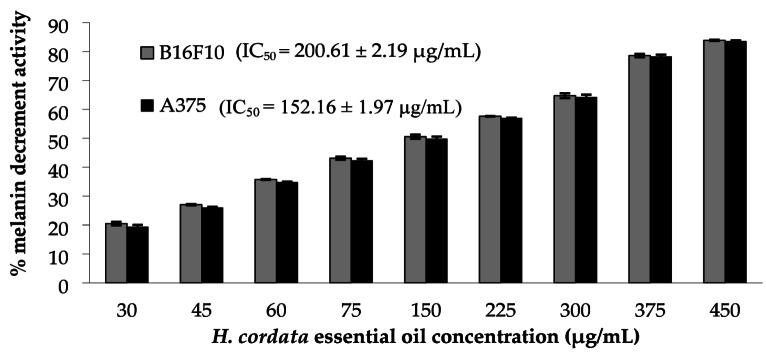
The anti-melanogenic activity of HCEO at the non-cytotoxic concentration (< 0.5 mg/mL) was assessed by measuring mushroom tyrosinase inhibition and melanin content in B16F10 and A375 melanoma cells. As shown in Fig. 3, HCEO demonstrated tyrosinase inhibitory activity with an IC50 value of 48.00 ± 0.1 µg/mL. While the positive control kojic acid consistently shows an IC50 of 5.6 ± 0.3 µg/mL, demonstrating stronger direct inhibition but also with known risks of irritation and long-term safety concerns in cosmetics. Although the tyrosinase inhibitory activity of HCEO is less potent than kojic acid, its broader multifunctional profile (antimicrobial, antioxidant, anti-inflammatory) and natural origin make it a promising candidate for safer skin-brightening formulations with additional dermal benefits.
Fig. 3.
Percentage of melanin decrement activity of H. cordata essential oil against B16F10 and A375 melanoma cell lines.
In the cellular assays, HCEO was tested for its impact on melanin production in B16F10 and A375 melanoma cell lines. The results, shown in Fig. 3, indicate that HCEO significantly reduced melanin content in a dose-dependent manner, with IC50 values of 200.61 ± 2.19 µg/mL for B16F10 cells and 152.16 ± 1.97 µg/mL for A375 cells. Essential oils often contain terpenes such as α-pinene, β-pinene, α-terpineol, β-humulene, and β-myrcene, which have been previously reported to exhibit anti-tyrosinase activities49–52. Previous studies have highlighted that lime mint essential oil (0.5 mg/mL) and its major constituent, β-caryophyllene, inhibit melanogenesis in B16-F10 cell lines53. Pomelo peel essential oil exhibited a dose-dependent inhibition of tyrosinase activity, resulting in 48.28% inhibition of in vitro melanin synthesis at a concentration of 50 µg/mL54.
Anti-inflammatory activity of HCEO
The anti-inflammatory potential of HCEO was evaluated by measuring nitrite release in RAW 264.7 macrophage cells. The IC50 value of HCEO was found to be 1.176 ± 0.084 µg/mL, indicating a significant anti-inflammatory effect (Table 4). This suggests that HCEO could be a promising candidate for use as an anti-inflammatory ingredient. Due to limited data specifically addressing the anti-inflammatory effects of HCEO, the results were compared with those from crude extracts of H. cordata and other essential oils. Previous research has shown that H. cordata extract and its volatile oil significantly suppress lipopolysaccharide (LPS)-stimulated nitric oxide (NO) production42,55. For example, H. cordata extract exhibited an IC50 for NO production inhibition of less than 20 µg/mL, and both fresh and dried extracts of H. cordata have been reported to inhibit NO production55. Moreover, essential oils from other sources, such as E. dysenterica, have demonstrated anti-inflammatory effects by inhibiting NO production, with concentrations ≤ 292 µg/mL being safe for skin applications. Terpenes, which are common components of essential oils, are well-known for their anti-inflammatory properties13. Future studies should incorporate a positive control, such as Dexamethasone or Indomethacin, and also investigate the anti-inflammatory activity of major constituents identified in HCEO to provide a more comprehensive analysis.
Stability testing of gel and emulsion formulations
The stability of gel and emulsion formulations containing HCEO was evaluated by examining pH, viscosity, and physical appearance over time and under various storage conditions. Figure 4 shows the physical characteristics of both cream and gel formulations before and after the stability tests. Initial tests showed that both the gel and cream base formulations, along with their essential oil-containing variants, maintained clarity with no colour change. Colour measurements, including L*, a*, b*, and ΔE, were recorded using a colorimeter (Table 5). The incorporation of HCEO imparted a distinctive herbal fragrance to both the gel and cream, which slightly faded but still had an herbal scent throughout the testing period. The pH of the cream formulations remained stable, ranging from 5.5 to 6.0 on day 0, indicating that the essential oil did not significantly affect the pH.
Fig. 4.
Physical characteristics after different stability testing conditions. (A) shows the physical appearance of the gel base formulation after testing, (B) shows the physical appearance of the H. cordata essential oil-containing gel after testing. (C) shows the physical appearance of the cream base formulation after testing, (D) shows the physical appearance of the H. cordata essential oil-containing cream after testing.
Table 5.
Colour stability of gel and cream base formulas and formulas containing H. cordata essential oil.
| L* | a* | b* | ΔE | ||||
|---|---|---|---|---|---|---|---|
| Base | H. cordata | Base | H. cordata | Base | H. cordata | ||
| Gel | |||||||
| D0 | 33.80 ± 0.15 | 33.26 ± 0.71 | 0.57 ± 0.64 | 0.33 ± 0.51 | 1.03 ± 1.19 | 0.78 ± 1.94 | 0.94 ± 0.55 |
| D7 | 32.13 ± 0.09 | 33.63 ± 0.23 | 0.16 ± 0.03 | 0.05 ± 0.11 | −1.45 ± 0.02 | −1.64 ± 0.03 | 1.52 ± 0.16 |
| D14 | 32.61 ± 0.09** | 0.06 ± 0.02 | −1.39 ± 0.04 | 32.86 ± 0.05 | 0.12 ± 0.03 | −1.79 ± 0.02 | 0.48 ± 0.08 |
| D21 | 31.39 ± 0.41 | 0.03 ± 0.05 | −0.85 ± 0.06 | 33.04 ± 0.33 | 0.21 ± 0.16 | −1.71 ± 0.11 | 1.78 ± 0.21 |
| Cream | |||||||
| D0 | 0.51 ± 0.53 | −0.31 ± 0.10 | −0.19 ± 0.23 | 70.23 ± 0.13 | −0.47 ± 0.25 | −0.74 ± 0.27 | 0.79 ± 0.19 |
| D7 | 71.86 ± 0.02** | 70.09 ± 0.07** | −0.68 ± 0.02 | −0.65 ± 0.01 | −0.24 ± 0.02 | −0.71 ± 0.03 | 1.82 ± 0.07 |
| D14 | 68.66 ± 1.05** | −0.61 ± 0.04** | −1.28 ± 0.16 | 70.49 ± 0.01 | −0.67 ± 0.02 | −1.13 ± 0.01 | 1.63 ± 1.06 |
| D21 | 67.48 ± 0.01** | 70.29 ± 0.06** | −0.51 ± 0.02 | −0.59 ± 0.02 | −0.51 ± 0.02 | −1.39 ± 0.01 | 2.82 ± 0.06 |
Gel formulation stability
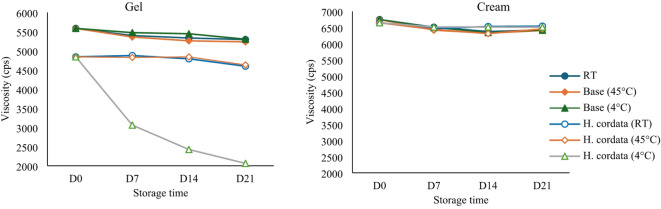
The viscosity of the gel base formula showed only minor decreases after 4 weeks (28 days), regardless of storage conditions (room temperature, heat, and cool). The essential oil-containing gel had a viscosity of 4,596 ± 153.57 cPs at room temperature and 4,629 ± 90.02 cPs under heat conditions (Fig. 5). However, under cool conditions (4 °C), there was a notable decrease in viscosity to 2,062 ± 4.04 cPs.
Fig. 5.
Viscosity stability of oil in water emulsion and gel preparation and formulas containing H. cordata essential oil at different storage temperatures; room temperature (27–29 °C), heating at 40 °C, and cooling at 4 °C.
The significant drop in viscosity of gel formulations at 4 °C is primarily due to reduced hydration and solubility of carboxymethyl cellulose (CMC) at low temperatures. Cold conditions limit water mobility, decreasing polymer swelling and weakening the gel network. In addition, ethanol, used to dissolve the essential oil, can further destabilize the system by reducing CMC solubility at low temperatures, leading to partial polymer collapse or phase separation. To mitigate this effect, more cold-stable gelling agents (e.g., xanthan gum, HEC) or polymer blends (e.g., CMC with xanthan) can be used. Adding humectants like glycerine may also help maintain polymer hydration and stabilize viscosity under cold storage.
Cream formulation stability
For the cream formulations, both the base and essential oil-containing variants demonstrated stable viscosities across different storage conditions. The base cream had an initial viscosity of 6,744 ± 71.11 cPs and maintained viscosities of 6,419 ± 84.79 cPs at room temperature, 6,405 ± 65.16 cPs under heat, and 6,413 ± 56.19 cPs under cool conditions (Fig. 5). Similarly, the cream formulation containing HCEO showed consistent viscosity measurements of 6,539 ± 28.10 cPs at room temperature, 6,453 ± 58.97 cPs under heat, and 6,509 ± 48.88 cPs under cool conditions.
Overall, the stability tests indicate that HCEO did not adversely affect the physical properties of the gel and cream formulations. While the gel formulation exhibited some viscosity changes under cool conditions, the cream formulations remained stable in terms of viscosity, appearance, and texture. These results suggest that the essential oil can be effectively incorporated into gel and cream formulations without compromising their stability. Further studies might explore alternative solubilizers or gelling agents to mitigate viscosity changes in gels under varying conditions.
Limitations and future directions
The present study was confined to in vitro assays, which may not fully reflect biological responses in complex physiological systems1,55. The absence of in vivo validation limits confirmation of efficacy, safety, and pharmacokinetic behaviour under realistic conditions3,55. No permeation or bioavailability assessments were performed, leaving uncertainty regarding dermal absorption and retention of active constituents12,20. The dosing parameters remain unoptimised for achieving maximal therapeutic benefit with minimal adverse effects10,47. Future investigations should include in vivo validation in relevant animal models3,55, together with permeation and pharmacokinetic studies to determine systemic exposure12,20. Additional work on dosing regimens, formulation strategies, and delivery systems will be essential to enhance therapeutic potential10,47. Further mechanistic studies of HCEO and its major compounds identified from this study are required to clarify the pathways underlying the observed antimicrobial, antioxidant, anti‑melanogenic, and anti‑inflammatory effects1,37,49 guiding the development of these bioactive agents for skincare applications.
Conclusions
This study explored the biological properties and formulation stability of Houttuynia cordata essential oil (HCEO), highlighting its potential as a multifunctional ingredient for skincare applications. HCEO exhibited antimicrobial, antioxidant, wound-healing, anti-melanogenic, and anti-inflammatory activities, making it provide promising in vitro evidence for addressing various skin concerns, including acne, hyperpigmentation, and oxidative stress. No cytotoxic effects were observed on fibroblasts and macrophages at relevant concentrations, supporting its safety for topical use. In terms of formulation stability, the oil-in-water cream containing HCEO demonstrated excellent stability under various storage conditions, whereas the gel formulation showed sensitivity to cold storage, an issue that could be addressed through the incorporation of alternative gelling agents or humectants in future formulations. Although the efficacy of HCEO in some assays did not surpass that of standard reference compounds (e.g., Trolox for antioxidant activity or kojic acid for tyrosinase inhibition), its broad spectrum of bioactivities positions it as a promising natural ingredient for multifunctional skincare products. Overall, this study provides promising in vitro evidence supporting the potential use of HCEO in the development of safe and effective phytocosmetic formulations, particularly for acne-prone and hyperpigmented skin. Future research should focus on optimizing formulations, conducting in vivo studies, and exploring long-term safety to further validate its potential in the cosmetic industry.
Acknowledgements
We would like to thank the Scientific & Technological Instruments Center, Mae Fah Luang University. Sarita Sangthong received funding supported by National Science, Research and Innovation Fund (NSRF), Thailand through Fundamental Fund 2022 (NRIIS number 166695). The research collaboration was funded by the Reinventing University has received funding support from the Office of the Permanent Secretary of the Ministry of Higher Education, Science, Research and Innovation, Thailand. Lutfun Nahar gratefully acknowledges the support from the European Regional Development Fund (Project ENOCH #CZ.02.1.01/0.0/0.0/16_019/0000868), and the Czech Science Foundation (Project #23–05474 S).
Author contributions
S.S. Conceptualization, investigation, validation, and Writing: original draft preparation, review and final editing; P.C. Writing: review and final editing; P.P. Writing: review and final editing; B.S. Writing: review and final editing; M.S. Writing: review and final editing; T.T. Writing: review and final editing; K.C. GC-MS analysis, Writing: review and final editing; H.A. Collection of resources and data analysis; S.D.S. Writing: review and final editing. L.N. Conceptualization, investigation, validation, and Writing: original draft preparation, review and final editing; All authors reviewed the final version.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sarita Sangthong, Email: sarita.san@mfu.ac.th.
Hesamoddin Arabnozari, Email: hesamarabnozari@yahoo.com.
Lutfun Nahar, Email: profnahar@outlook.com.
References
- 1.Laldinsangi, C. Therapeutic potential of Houttuynia cordata: a current review. Heliyon8, e10386 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rafiq, S., Hao, H., Ijaz, M. & Raza, A. Pharmacological effects of Houttuynia cordata thunb (H. cordata): a comprehensive review. Pharmaceuticals15, 1079 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu, H. M., Liang, Y. Z., Yi, L. Z. & Wu, X. J. Anti-inflammatory effect of Houttuynia cordata injection. J. Ethnopharmacol.104, 245–249 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luo, Q. et al. Comprehensive assessment of Houttuynia cordata Thunb., an important medicinal plant and vegetable. Agronomy12 (10), 2582 (2022). [Google Scholar]
- 5.Wu, Z. et al. Houttuynia cordata thunb: an ethnopharmacological review. Front. Pharmacol.12, 714694 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhate, K. & Williams, H. C. Epidemiology of acne vulgaris. Br. J. Dermatol.168, 474–485 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Williams, H. C., Dellavalle, R. P. & Garner, S. Acne vulgaris. Lancet379, 361–372 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Tanghetti, E. A. The role of inflammation in the pathology of acne. J. Clin. Aesthet. Dermatol.6, 27–35 (2013). [PMC free article] [PubMed] [Google Scholar]
- 9.Rivers, J. K. The role of cosmeceuticals in antiaging therapy. Skin. Ther. Lett.13, 5–9 (2008). [PubMed] [Google Scholar]
- 10.Taleb, M. H. et al. Origanum vulgare L. essential oil as a potential anti-acne topical nanoemulsion - in vitro and in vivo study. Molecules23, 2164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anurukvorakun, O. & Numnim, S. Development and clinical efficacy evaluation of facial toner containing Houttuynia cordata thunb. Cosmetics10, 133 (2023). [Google Scholar]
- 12.Nguyen, M. H. et al. Response surface methodology for aqueous two-phase system extraction: an unprecedented approach for specific flavonoid-rich extraction of Houttuynia cordata Thunb. Leaves towards acne treatment. Heliyon10, e25245 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim, H. J., Lee, H. J. & Lim, M. H. Comparison of antioxidant and anti-inflammatory activity of Korean Houttuynia cordata Thunb. Extracts. J. Korean Appl. Sci. Technol.8, 217–227 (2021). [Google Scholar]
- 14.Srisayam, M., Puengtang, C., Srisopa, A. & Chodnakarin, A. Biological activities, phenolic and vitamin C contents from Syzygium cumini (L.) extract. J. Sci. Technol.7, 103–113 (2022). [Google Scholar]
- 15.Kowalska-Krochmal, B. & Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens10, 165 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tok, N. C., Jain, K. K., Sahu, N. P., Varghese, T. & Daniel, N. Evaluation of antioxidative and biological activity of Houttuynia cordata extracts. Int. J. Sci. Res. Publ. 7, 22 (2017). [Google Scholar]
- 17.Verma, R. S. et al. Chemical composition and allelopathic, antibacterial, antifungal, and antiacetylcholinesterase activity of fish-mint (Houttuynia cordata Thunb.) from India. Chem. Biodivers.14, 1700189 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Kumar, V., Mathela, C. S., Kumar, M. & Tewari, G. Antioxidant potential of essential oils from some Himalayan Asteraceae and lamiaceae species. Med. Drug Discov. 1, 100004 (2019). [Google Scholar]
- 19.Yilmaz, Ş. et al. Calcium hypochlorite on mouse embryonic fibroblast cells (NIH3T3) in vitro cytotoxicity and genotoxicity: MTT and comet assay. Mol. Biol. Rep.47, 5377–5383 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Pavelkova, R., Matouskova, P., Hoova, J., Porizka, J. & Marova, I. Preparation and characterization of organic UV filters based on combined PHB/liposomes with natural phenolic compounds. Biotechnol. Rep. 100021 (2020). [DOI] [PubMed]
- 21.Stamm, A. et al. In vitro wound healing assays – State of the Art. BioNanoMaterials17, 79–87 (2016). [Google Scholar]
- 22.Wang, X., Decker, C. C., Zechner, L., Krstin, S. & Wink, M. Vitro wound healing of tumor cells: Inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacol. Toxicol.20, 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, K., Huh, Y. J. & Lim, K. M. Anti-pigmentary natural compounds and their mode of action. Int. J. Mol. Sci.22, 6206 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akaberi, M., Emami, S. A., Vatani, M. & Tayarani-Najaran, Z. Evaluation of antioxidant and anti-melanogenic activity of different extracts of aerial parts of N. sintenisii in murine melanoma B16F10 cells. Iran. J. Pharm. Res.17, 225–235 (2018). [PMC free article] [PubMed] [Google Scholar]
- 25.Sangthong, S., Promputtha, I., Pintathong, P. & Chaiwut, P. Chemical constituents, antioxidant, anti-tyrosinase, cytotoxicity, and anti-melanogenesis activities of Etlingera elatior (Jack) leaf essential oils. Molecules27, 3469 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lorençoni, M. F. et al. Chemical composition and anti-inflammatory activity of essential oil and ethanolic extract of Campomanesia phaea (O. Berg.) landrum leaves. J. Ethnopharmacol.252, 112562 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Khamsan, S. et al. Chemical compositions and anticancer activity of essential oil from Houttuynia cordata thunb. Int. J. Sci.7, 23–31 (2020). [Google Scholar]
- 28.Wigraiboon, S., Nomura, N. & Whangchai, N. Effect of essential oils from Houttuynia cordata Thunb. Supplemented diets on growth performance and immune response of hybrid red tilapia (Oreochromis mossambicus Linn. X Oreochromis niloticus Linn). Int. J. Fish. Aquat.4, 677–684 (2016). [Google Scholar]
- 29.Lu, H. M., Wu, X., Liang, Y. & Zhang, J. Variation in chemical composition and antibacterial activities of essential oils from two species of Houttuynia thunb. Chem. Pharm. Bull.54, 936–940 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Pan, X. et al. Comparison of essential oils of Houttuynia cordata Thunb. From different processing methods and harvest seasons based on GC-MS and chemometric analysis. Int. J. Anal. Chem. 8324169 (2021). [DOI] [PMC free article] [PubMed]
- 31.Kupska, M., Wasilewski, T., Jędrkiewicz, R., Gromadzka, J. & Namieśnik, J. Determination of terpene profiles in potential superfruits. Int. J. Food Prop.19, 2726–2738 (2016). [Google Scholar]
- 32.Pokajewicz, K. et al. Comparative evaluation of the essential oil of the new Ukrainian Lavandula angustifolia and Lavandula x intermedia cultivars grown on the same plots. Molecules27, 2152 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan, M., Khan, M., Abdullah, M. M. S., Alkhathlan, H. Z. & Saeed, M. Characterization of secondary metabolites of leaf and stem essential oils of Achillea fragrantissima from central region of Saudi Arabia. Arab. J. Chem.13, 5254–5261 (2020). [Google Scholar]
- 34.Dai, D. N., Thang, T. D., Ogunmoye, A. O., Eresanya, O. I. & Ogunwande, I. A. Chemical constituents of essential oils from the leaves of Tithonia diversifolia, Houttuynia cordata and Asarum glabrum grown in Vietnam. Am. J. Essent. Oil. 2, 17–211 (2015). [Google Scholar]
- 35.Marques, F. M. et al. Vitro anti-inflammatory activity of terpenes via suppression of superoxide and nitric oxide generation and the NF-κB signalling pathway. Inflammopharmacol27, 281–289 (2019). [DOI] [PubMed] [Google Scholar]
- 36.Fu, J., Dai, L., Lin, Z. & Lu, H. Houttuynia cordata thunb: a review of phytochemistry, pharmacology, and quality control. Chin. Med.4, 3015 (2013). [Google Scholar]
- 37.Zhang, W., Lu, F., Pan, S. & Tan, S. Antioxidant activity of essential oils from Houttuynia cordata and their main components. Food Chem.135, 1313–1320 (2012). [Google Scholar]
- 38.Jin, J. W., Koh, Y. W., Yun, K. W., Kim, K. J., Je, S. H., Im, S. B., … Seo, K. S.Cell viability and hair growth effect on 3T3-L1 cells of ethanol extract from Calendula officinalis L. flower, Phellinus linteus fruit body and Houttuynia cordata Thunb. whole plant. Korean J. Medicinal Crop Sci. 25(6), 404–410 (2017).
- 39.Kim, S. Y., Kim, S. M. & Park, S. J. Antioxidant activities of natural essential oils derived from Korean endemic plants. J. Agri Life Environ. Sci.31, 17–25 (2019). [Google Scholar]
- 40.Sekita, Y. et al. Anti-bacterial and anti-inflammatory effects of ethanol extract from Houttuynia cordata poultice. Biosci. Biotechnol. Biochem.80, 1205–1213 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Miguel, M. G. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules15, 9252–9287 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phorsi, S. et al. Anti-aging, anti-acne, and cytotoxic activities of Houttuynia cordata extracts and phytochemicals analysis by LC-MS/MS. Cosmetics9, 136 (2022). [Google Scholar]
- 43.Lim, J. H., Kin, S. H., Lee, M. K. & Kim, S. Evaluation of the anti-inflammatory effect and non-toxicity of Houttuynia cordata extract on RAW 264.7 macrophages. J. Ethnopharmacol.269, 113750 (2021).33359856 [Google Scholar]
- 44.Salas-Oropeza, J. et al. Essential oil of Bursera morelensis promotes cell migration on fibroblasts: in vitro assays. Molecules28, 6258 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khazerei, H., Shamsdin, S. A. & Zamamni, M. Vitro cytotoxicity and apoptotic assay of Eucalyptus globulus essential oil in colon and liver cancer cell lines. J. Gastrointest. Cancer. 53, 363–369 (2021). [DOI] [PubMed] [Google Scholar]
- 46.Mazutti da Silva, S. M. et al. D. O. Wound healing effect of essential oil extracted from Eugenia dysenterica DC (Myrtaceae) leaves. Molecules24, 2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ngamdokmai, N. et al. Effects of essential oils and some constituents from ingredients of anti-cellulite herbal compress on 3T3-L1 adipocytes and rat aortae. Pharmaceuticals14, 253 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Subhawa, S., Chewonarin, T. & Banjerdpongchai, R. The effects of Houttuynia cordata thunb and Piper ribesioides wall extracts on breast carcinoma cell proliferation, migration, invasion and apoptosis. Molecules25, 1196 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pillaiyar, T., Manickam, M. & Jung, S. H. Downregulation of melanogenesis: drug discovery and therapeutic options. Drug Discov Today. 22, 282–298 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Chao, W. W., Su, C. C., Peng, H. Y. & Chou, S. T. Melaleuca quinquenervia essential oil inhibits α-melanocyte-stimulating hormone-induced melanin production and oxidative stress in B16 melanoma cells. Pharmaceuticals34, 191–201 (2017). [DOI] [PubMed] [Google Scholar]
- 51.Kumar, K. J. S. et al. Essential oils of Alpinia nantoensis retard forskolin-induced melanogenesis via ERK1/2-mediated proteasomal degradation of MITF. Plants9, 1672 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matsuura, R., Ukeda, H. & Sawamura, M. Tyrosinase inhibitory activity of citrus essential oils. J. Agric. Food Chem.54, 2309–2313 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Yang, C. H. et al. Inhibition of melanogenesis by β-caryophyllene from lime mint essential oil in mouse B16 melanoma cells. Int. J. Cosmet. Sci.37, 550–554 (2015). [DOI] [PubMed] [Google Scholar]
- 54.He, W., Li, X., Peng, Y., He, X. & Pan, S. Antioxidant and anti-melanogenic properties of essential oil from Peel of pomelo Cv. Guan Xi Molecules. 24, 242 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li, W. et al. Houttuynia cordata Thunb. Volatile oil exhibited anti-inflammatory effects in vivo and inhibited nitric oxide and tumor necrosis factor-α production in LPS-stimulated mouse peritoneal macrophages in vitro. Phytother Res.27, 1629–1639 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.