Abstract
Antibody engineering has been widely developed to improve the affinity and activity of therapeutic monoclonal antibodies (mAbs). Conventional single-cell sorting technology is a powerful method for antibody discovery, effectively producing human mAbs; however, it remains expensive and limits the number of cells available for analysis. Furthermore, sorting hundreds of B cells may not yield cells encoding antibodies with high neutralizing potency since several unexpected antibodies, such as autoantibodies and infectivity-enhancing antibodies, can be included in antigen-specific B cells in patients with infectious diseases. The preparation of antibodies for infectious diseases, such as that caused by severe acute respiratory syndrome coronavirus 2 with rapid mutations, requires discovery technology to generate and select diverse human mAb libraries. We present a synthetic chimeric antibody (SynCA) technology that facilitates in vitro reconstitution of diverse mAbs by integrating the bulk sorting of numerous antigen-specific B cells and machine learning. We compared our method, which cloned antibody variable regions from a single cDNA extracted from ∼5000 B cells, with a single-cell sorting method in the convalescent sera of recovered patients with coronavirus disease 2019. The SynCA method enhanced the B cell receptor repertoire diversity in both heavy and light chain genes. Additionally, the random pairing of the heavy and light chain genes reconstituted antibodies in vitro, providing new insights that the nucleotide sequence information from antibody gene regions (DH and JL regions) predicts antibody reconstitution. This method is both cost-effective and rapid, and it can be employed to produce mAbs for therapeutic, diagnostic, and research purposes.
Keywords: BCR repertoire diversity, Bulk sorting, Low cost, Machine learning, Monoclonal antibody discovery, Prediction
Graphical abstract
Highlights
-
•
Low-cost and rapid mAbs discovery method than single-cell sorting technology.
-
•
PCR-based method using bulk sorting enhanced the antibody repertoires.
-
•
Diverse reconstituted mAbs obtained from random pairing of heavy and light chains.
-
•
Antibody gene sequences of the DH and JL regions predict antibody reconstitution.
1. Introduction
Along with developing flow cytometry and single-cell analysis technologies, antibody discovery using single B cells from volunteers infected with infectious diseases is widely performed by mainly biopharmaceutical companies [1]. The therapeutic monoclonal antibodies (mAbs) against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), including REGN17092 (Regeneron) [2], VYD222 (Invivyd) [3], AER-800 (Aerium Therapeutics) [4], AZD3152, and AZD3959 (AstraZeneca) [5,6], remained in development in 2024 and originated from antibody genes of single B cells isolated using the single-cell sorting method [[7], [8], [9], [10]]. Unfortunately, the therapeutic efficacy of the clinically approved mAbs was limited by the rapid emergence of SARS-CoV-2 variants [8,[11], [12], [13], [14]]. Additionally, in patients with coronavirus disease 2019 (COVID-19), several antigen-specific B cells, such as autoantibodies and infectivity-enhancing antibodies, lack neutralizing potency [[15], [16], [17], [18], [19]]. Finding the cell encoding antibody genes with high neutralizing potency from antigen-specific B cells of patients with such diseases may require a large-scale single-cell sorting. Indeed, the mAbs against SARS-CoV-2 were obtained from B cells with high neutralizing potency by screening a large number of B cells (thousands to tens of thousands in scale) [9]. However, the number of single-cell sorting analyses is limited owing to the high cost and time required [1], especially in academia.
Antibody engineering, including modification of the fraction crystallizable (Fc) region of existing mAbs, is widely performed to enhance activity and affinity. An adintrevimab (anti-SARS-CoV-1 mAb) was engineered in the Fc region by multiple rounds of yeast-display affinity maturation to extend the half-life, leading to the discovery of VYD222 [10,11,20]. Furthermore, the cross-recombined antibodies with the heavy and light chain genes from different mAbs were produced to increase the neutralizing potency against SARS-CoV-2 variants [21,22]. Notably, the shuffling of the light chain within the same family resulted in tighter binding than that of the original antibody [22]. Considering that the rapid mutations associated with SARS-CoV-2 evolution have rendered existing therapeutic mAbs insufficiently effective, a simple antibody discovery system, including the random pairing of heavy and light chains, may be useful for generating mAbs quickly against unanticipated infectious diseases.
Before the widespread adoption of single-cell technology-based antibody discovery, a polymerase chain reaction (PCR) method, including classical DNA cloning, was used to generate antibody phage display libraries [23,24]. Repeated rounds (3–7 times) of biopanning, which involves the selection of antibodies with high affinity to the target antigen, is a fundamental step; however, this process is time-consuming and may be a potential cause of selection bias, which depends on the antigen format used [23,25]. The present study improved PCR-based methods without phage display technology, facilitating the generation of human mAb libraries, including mAbs with high affinity or neutralizing potency, from the convalescent sera of patients with COVID-19. The key process involved in our technology is to synthesize a single cDNA from a pool of approximately 5000 memory B cells (Bmem) obtained by bulk sorting (Fig. S1A). No burdensome processes (e.g., generation of hybridoma, biopanning, or single B cell culture) are required, and only classical DNA cloning of antibody genes was included in our method (Fig. S1B). Furthermore, it can be performed at one-tenth the cost of single-cell sorting analysis (calculated by comparing 500 clones using a standard kit for single-cell analysis). As with phage display technology, the original pairs of heavy and light chains naturally occurring in the body are unknown. However, we found that a predictive model involving the nucleotide sequence of complementarity-determining region 3 (CDR3) enables efficient in vitro reconstitution of immunoglobulin G (IgG) antibodies. We termed our method synthetic chimeric antibody (SynCA) technology because it is based on an artificial human-to-human (i.e., random combination of heavy and light chains) chimera. The SynCA method enables the construction of diverse antibody libraries consisting of antigen-specific binding antibodies, including potential therapeutic antibodies, and can be used to generate mAbs for diagnostic and research purposes.
2. Methods
2.1. Study design
We recruited seven convalescent participants with COVID-19 (three with mild symptoms and four with moderate II symptoms) from our COVID-19 cohort [26] at the Juntendo University Hospital in Tokyo, Japan. The participants were adults aged 21–66 years diagnosed with COVID-19 using RT-PCR with a nasopharyngeal swab using LightMix Modular SARS-CoV (COVID-19) N-gene and E-gene assays (Roche Diagnostics, Tokyo, Japan) between August 2020 and July 2021. The severity of COVID-19 was classified into two stages, following the criteria outlined in a previous study [26]: mild (clinical symptoms such as cough and fever without pneumonia according to imaging examination) and moderate II (pneumonia on imaging with respiratory insufficiency and oxygen saturation ≤93 % in a resting state). The following inclusion criteria were applied in the study: (1) patients infected with the SARS-CoV-2 wild-type (WT) strain before the emergence of the B.1.1.7 variant (i.e., August 2020 and March 2021); (2) patients with a serum-neutralizing antibody titer against the WT strain of 30 % or more at the time of discharge (mild: mean = 15.2 ± 4.5 %; moderate II: mean = 44.2 ± 7.4 %); (3) absence of remarkable after-effects, such as cough, headache, and smell/taste disorders; and (4) no gender preference (71.4 % male patients were included). The exclusion criterion was the presence of prolonged infection, as previously described [26]. This study was approved by the Ethics Committee of the Juntendo University School of Medicine (approval number #21–017 and M21–0017). All participants provided written informed consent to use their materials in this study. All procedures were performed in accordance with the principles of the Declaration of Helsinki.
2.2. Sample collection and isolation of PBMCs
Blood samples were collected at outpatient follow-up (1–3 months) after discharge, and peripheral blood mononuclear cells (PBMCs) were isolated as described previously [26]. Briefly, the convalescent sera were collected by BD Vacutainer CPT tubes (BD Biosciences, Franklin Lakes, NJ, USA), maintained at 4 °C, and centrifuged within 1 h for isolating the PBMCs. The obtained PBMCs were immediately frozen and stored in liquid nitrogen until subsequent experiments.
2.3. Bmem sorting by flow cytometry
The antibody staining protocol has been described previously [26]. Briefly, PBMCs were incubated with His-tagged SARS-CoV-2 S-RBD recombinant protein [WT strain (#17862; Cell Signaling Technology, Danvers, MA, USA; 1:50 dilution), B.1.1.7 N501Y strain (#10730-CV; R&D Systems, Minneapolis, MN, USA; 1:12.5 dilution), and B.1.617 strain (#10846-CV; R&D Systems; 1:12.5 dilution)] for 30 min on ice. After washing the cells, fluorescence-conjugated antibody cocktail staining was performed for 30 min on ice. The following antibodies were used: anti-human CD3 (SK7), CD4 (SK3), CD8 (RPA-T8), CD56 (B159), CD11b (D12), CD19 (HIB19), CD27 (O323), and IgG (G18-145), all from BD Biosciences; FITC-conjugated His-tag antibody from Abcam (Cambridge, UK); and anti-human CD27 from BioLegend (San Diego, CA, USA). The anti-human CD19 and CD27 antibodies were diluted 1:100, while the others were diluted 1:200. The SARS-CoV-2 S-RBD binding Bmem were defined as CD3−CD4−CD8−CD56−CD11b−CD19+CD27+IgG+His-tag+ live cells. Dead cells were excluded using propidium iodide (#P4864; Sigma-Aldrich, St Louis, MO, USA). All cells were sorted using a fluorescence-activated cell sorter, Aria II (BD Biosciences). All experiments were analyzed using the FlowJo software, version 10.6.1 (TreeStar, Ashland, OR, USA).
For bulk sorting, approximately 5000 Bmem were sorted into a 1.5-ml tube containing Buffer RLT (Qiagen, Hilden, Germany) with 2-mercaptoethanol (Sigma-Aldrich) and RNasin® Ribonuclease Inhibitor (Promega Corp., Madison, WI, USA). For single-cell sorting, a cell was sorted into a 96-well plate containing 4 μL/well of 8U RNAsin (Promega Corp.), 4U of recombinant RNase inhibitor (Takara Bio, Shiga, Japan), 10 mM DTT (Promega Corp.), and 10 × phosphate-buffered saline (Nacalai Tesque, Kyoto, Japan) as described previously [26]. All samples were frozen in liquid nitrogen immediately and stored at −80 °C until the following experiment.
2.4. Construction of human IgG antibody expression vector
Total RNA was extracted from Bmem using an RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. The full-length cDNA ligated known cDNA sequence (RNA oligo sequence) at the 5ʹ end was obtained by RNA ligase-mediated and oligo-capping rapid amplification of cDNA ends (RACE) method using a GeneRacer™ Kit (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer's instructions. The PCR was performed following the KOD FX Neo DNA polymerase guidelines (TOYOBO, Osaka, Japan) with the GeneRacer™ 5ʹ primer and each gene-specific 3ʹ primer (i.e., IgG1, IgGκ, and IgGλ). This study modified the GeneRacer™ 5ʹ nested primer and gene-specific 3ʹ primers with each restriction enzyme sequence (i.e., Bgl II and Xho I, respectively) for the Gateway® cloning system. In the experiments for different PCR conditions, we used two other PCR enzymes (KOD-Plus-Neo; TOYOBO; and Takara Ex Taq DNA polymerase; Takara Bio). The purified PCR product was ligated into a pENTR™ vector (pENTR-3 × HA-MCS gifted by Dr. T. Shimazaki) using a DNA Ligation kit (#6023; Takara Bio) and then transformed into Escherichia coli DH5α competent cells (#9057; Takara Bio) with overnight incubation at 37 °C. Several colonies picked up and purified their DNA with a QIAprep Spin Miniprep Kit (Qiagen, #27106) after overnight incubation at 37 °C. The IgG gene sequence within the plasmid was determined by Sanger sequencing (Azenta Life Science, Tokyo, Japan), and the V (F + ORF+in-frame P), D (F + ORF), J (F + ORF), and C gene sequences were obtained from the International Immunogenetics Information System (IMGT) database via NCBI IgBlast (v1.22.0; http://www.ncbi.nlm.nih.gov/igblast). The expression site (IgG gene sequence) was transferred into the destination vector (pCAGGS-RfA, gifted by Dr. J. Tsuyama) by the LR Clonase™ enzyme reaction using Gateway technology (Life Technologies, Carlsbad, CA, USA).
2.5. Diversity analyses
The Shannon diversity index is effective for examining species with few clonotypes in the same specimen. A Gini coefficient (ranging between 0 and 1) estimates the evenness of wealth distribution in the economy and biology: 0 means a perfectly even distribution, and 1 means less evenness [[27], [28], [29]]. These indices were calculated using the vegan package (v2.6−4) and the ineq package (v0.2-13) of the statistical programming language R, version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
2.6. Reconstitution of human IgG antibody
Expi293F™ cells were transiently transfected using the Expi293™ Expression System Kit (#A14635; Thermo Fisher Scientific) according to the manufacturer's instructions. Briefly, following 24 h of cell culture with a 125-ml flask scale, the expression vector, including heavy (i.e., IgG1) and light (i.e., IgGκ or IgGλ) chains, were transfected at a ratio of 1:1, and the culture supernatant was collected after 4 days. The level of human IgG antibody in the conditioned media was determined using a Human Immunoglobulin Quantification Kit (#RDB-3257; RD-Biotech, Besançon, France) according to the manufacturer's instructions.
2.7. Determining the original pair of IgG genes
Single-cell RT-PCR was performed as previously described [26,30]. The PCR product was purified using the QIAquick PCR Purification Kit (Qiagen) and sequenced by Sanger sequencing using the reverse primers used for nested PCR (Azenta Life Science). All the primers used are described in the previous study [26]. The IgG gene sequence was determined using the IMGT database as described above.
2.8. Detection of autoantibodies
Conditioned media, including reconstituted human IgG antibodies, were obtained using Expi293™ cell culture, as described above. Conditioned medium was sent to Filgen Biosciences (Nagoya, Japan). The protein array was performed using the RayBio® Human Autoimmune Disease IgG Autoantibody Detection Array G1 kit (RayBiotech Inc., Peachtree Corners, GA, USA).
2.9. In vitro neutralization assay
Neutralizing activity against the SARS-CoV-2 delta variant was measured using a SARS-CoV-2 neutralizing antibody detection kit (B.1.617.2 Variant, Delta; AdipoGen Life Sciences, San Diego, CA, USA) according to the manufacturer's instructions, as described previously [26]. Absorbance was measured using a Multiskan Fc with an incubator (Thermo Fisher Scientific). All samples were frozen once after collection in duplicate.
2.10. Construction of the random forest classifier
To generate a random forest classifier for the in vitro reconstitution of human IgG antibodies, we divided the SynCA clones into four groups depending on their concentration. The categories were based on the 25th (0 ng/ml), 50th (598.2 ng/ml), and 75th (10501.4 ng/ml) percentile of the concentration of 189 clones. Non indicated a concentration of 10 ng/ml or less, low indicated a concentration ranging from 10 to 598.2 ng/ml, middle indicated a concentration ranging from 598.2 to 10501.4 ng/ml, and high indicated a concentration of 10501.4 ng/ml or more. A random forest model was generated using the random forest package (v4.7–1.1). The following parameters were determined as predictive signatures: whether the heavy and light chains were from the same donor (i.e., identical), disease severity of the donor in each heavy and light chain (H-severity and L-severity), gene sequence in each region (VH, VDH, DH, DJH, H-CDR3, CH, VL, VJL, JL, L-CDR3, and CL), each somatic hypermutation (H-SHM and L-SHM), and CDR3 length (H-length and L-length). All predictive models were trained using 20 rounds of 10-fold cross-validation. To calculate the median area under the receiver operating characteristic curve (AUROC), 95 % confidence interval (CI), and variable importance (mean decrease in Gini), 100 independent training runs were conducted using different random seeds, as described previously [31].
2.11. Validation of prediction
To validate the predictive model for the reconstitution of human IgG antibodies, we generated new SynCA clones (n = 6 per category) based on the variable importance in each classification. In terms of the decision whether or not to reconstitute (i.e., low vs. non, middle vs. non, and high vs. non), the DH region was selected as the most important variable. L-CDR3 was the most important variable in classifying the concentration-dependent groups (i.e., middle vs. low, high vs. low, and high vs. middle). However, we selected the JL region to investigate the effect of a specific region within CDR3. The predictive “non” category was reconstituted with a focus on the DH region. The predictive “low” and “middle plus high (i.e., ≥middle)” categories were reconstituted with a focus on the JL region. The sequences in the DH and JL regions partially overlapped, particularly among the low, middle, and high categories. The JL region in the predictive “non” category and the DH region in the predictive “low” and “≥middle” categories were selected sequences belonging to the “non,” “low,” and “middle” groups where possible, respectively. The level of human IgG antibody in the conditioned media was measured using a human immunoglobulin quantification kit (#RDB-3257; RD-Biotech) according to the manufacturer's instructions.
3. Data quantification and statistical analysis
All statistical analyses were performed using the statistical programming language R version 4.3.3 (2024-02-29; R Foundation for Statistical Computing). Statistical significance was determined using the Wilcoxon signed-rank test and Wilcoxon rank-sum test with the Benjamini–Hochberg method. P-values below 0.05 were considered statistically significant.
4. Results
4.1. SynCA method enhances the BCR repertoire diversity
We initially sorted specific Bmem binding to recombinant SARS-CoV-2 S-RBD WT, N501Y, and L452R proteins from the PBMCs of recovered participants infected with the WT, B.1.1.7, and B.1.167 strains, respectively, using flow cytometry (Fig. S1A). A key aspect of the SynCA method is the synthesis of a single cDNA from a pool of approximately 5000 Bmem by bulk sorting. The gating strategy for sorting Bmem is shown in Fig. S2. The proportions of Bmem binding to each SARS-CoV-2 S-RBD protein were tiny (0.027–0.128 % in total cells; Fig. S2B). More than 10 IgG heavy and more than 10 light chain gene sequences were obtained from one cDNA via an optimized cloning strategy using the RACE method and gateway technology (Fig. S3; see Methods).
To evaluate the diversity of the clones obtained, we compared the B cell receptor (BCR) repertoire of each heavy and light chain gene using SynCA and single-cell sorting methods in an identical donor. The distribution of the heavy and light (IgGλ) chain gene families of three participants infected with the WT or variant strains was visualized on a pie chart (Fig. 1A and B, S4A, and S4B). The distribution in the single-cell sorting method was skewed toward specific families; however, many families were identified using the SynCA method. To quantify the BCR repertoire diversity, we compared the Shannon diversity and Gini coefficients between the two methods. The Shannon diversity indices of the heavy chain, particularly in the JH region, were significantly higher when using the SynCA method than those obtained when using the single-cell sorting method (P = 0.031; Fig. 1C). The Vλ/κ and Jλ/κ region indices were also higher when the SynCA method was used than those obtained using the single-cell sorting method (P = 0.039 and 0.008, respectively; Fig. 1D). The inequality scores obtained using the Gini coefficient of the JH, Vλ/κ, and Jλ/κ regions were significantly decreased when the SynCA method was used compared with those obtained using the single-cell sorting method (P = 0.031, 0.039, and 0.008, respectively; Fig. 1E and F). We then evaluated SHM in heavy and light chain genes and compared them to the IMGT germline. The number of SHMs in the heavy chain gene using the SynCA method was significantly lower than that obtained using the single-cell sorting method (P < 0.005; Fig. 1G). No significant differences were found in the SHM of the light chain genes (Fig. 1G). The CDR3 lengths in the heavy and light chain genes are shown in Fig. 1H and I. These results suggest that our SynCA method enhanced the BCR repertoire diversity in each heavy and light chain gene.
Fig. 1.
Enhancing BCR repertoire diversities in the SynCA method
(A and B) Pie charts showing the distribution of the heavy (A) and light (B) chain (IgGλ) gene families in mild and moderate II patients infected with the WT strain (n = 3). The number in each inner circle indicates the number of clones evaluated in the single-cell sorting and SynCA methods. Same colors depict identical sequences.
(C and D) Graphs showing the Shannon diversity scores of each VDJ region in heavy (C) and light (D) chains (heavy chain: n = 6; and light chain: n = 8). Statistical significance was determined using the Wilcoxon rank-sum test (P < 0.05).
(E and F) Graphs showing the Gini coefficient scores of each VDJ region in heavy (E) and light (F) chains (heavy chain: n = 6; and light chain: n = 8). Statistical significance was determined using the Wilcoxon rank-sum test (P < 0.05).
(G) Graph showing the number of SHMs in heavy and light chains compared with germline IMGT sequences. Statistical significance was determined using the Wilcoxon rank-sum test (P < 0.05).
(H and I) Graph showing the distribution of CDR3 length in heavy (H) and light (I) chains.
The distribution of clones in single-cell sorting and SynCA (shown in panels G, H, and I) was as follows: heavy chain, n = 179 and 130, and light chain, n = 320 and 361, respectively.
Abbreviations: BCR, B cell receptor; PBMCs, peripheral blood mononuclear cells; SynCA, synthetic chimeric antibody method; SARS-CoV2, severe acute respiratory syndrome coronavirus 2; WT, wild-type; FACS, fluorescence-activated cell sorting; VDJ, variable, diversity, and joining; SHM, somatic hypermutation; IMGT, International Immunogenetics Information System; CDR3, complementarity-determining region 3.
4.2. BCR repertoire diversities are increased mainly via the PCR step
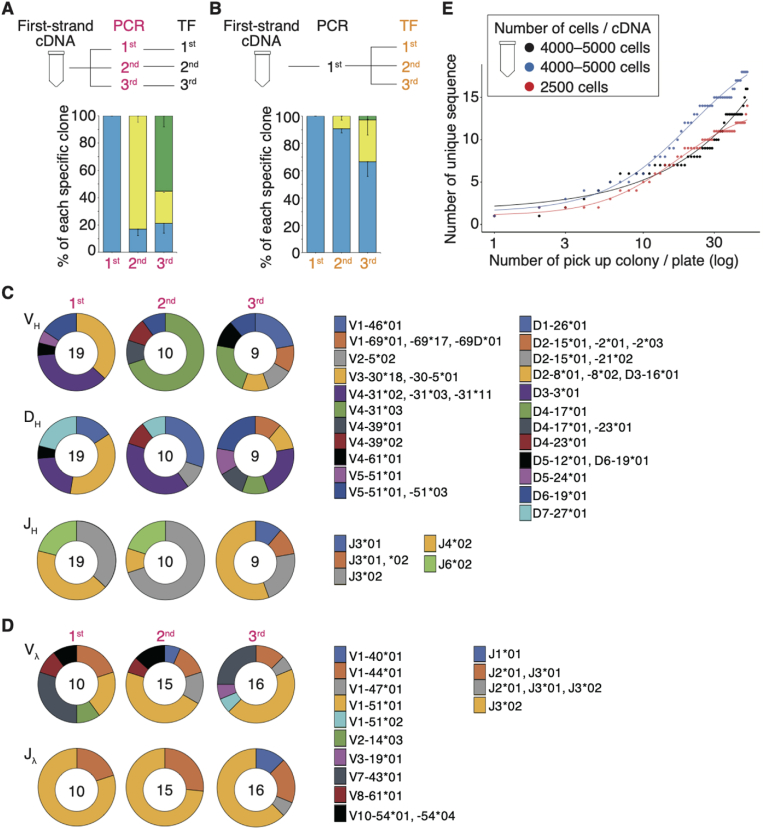
Random mutagenesis is one of the most common procedures for producing engineered antibodies using phage display technology [[32], [33], [34]]. These arbitrary mutations result in affinity maturation and high specificity. An error-prone DNA polymerase was not used in the SynCA method; however, it is possible that enhanced diversity arose during the Ig gene cloning process, such as gene amplification and transformation into a competent cell. We investigated which step, PCR or transformation, was more likely to enhance diversity. We performed three independent experiments for each PCR and transformation step using one cDNA (Fig. 2A and B). In the second independent PCR, the proportion of clones obtained in the first PCR was 17.1 ± 4.7 %. In the third independent PCR, the proportion of clones obtained in the first PCR was 21.2 ± 7.1 % and that in the second PCR was 23.6 ± 1.0 % (Fig. 2A). Conversely, in the independent transformation step using a single PCR product from one cDNA, the proportions of clones obtained in the first transformation were 90.8 ± 3.0 % and 66.7 ± 10.8 % in the second and third transformations, respectively (Fig. 2B). The proportion of clones obtained in the second transformation was 30.6 ± 11.1 % in the third transformation (Fig. 2B). The distribution of the heavy and light chain gene families in the third independent PCR is shown in Fig. 2C and D. These results suggest that the BCR repertoire diversity is more likely to increase during the PCR step than during transformation.
Fig. 2.
Increases in BCR repertoire diversity via the PCR step
(A and B) Graph showing the percentage of each specific clone in three independent experiments using the SynCA method. Three independent PCR steps were performed from a single first-strand cDNA, which was then transformed into competent cells to obtain specific clones in three experiments (A; n = 5). One PCR step was performed for a single first-strand cDNA, and then, three independent transformations were performed using the PCR product to obtain specific clones in three experiments (B; n = 4). Same colors depict identical clones.
(C and D) Pie charts showing the representative distribution of the heavy (C) and light (D) chain (IgGλ) gene families of three independent PCR steps in a moderate II patient infected with the WT strain. The number in each inner circle indicates the number of clones evaluated using the SynCA method. Same colors depict identical sequences.
(E) Graph showing the number of unique clones obtained when 50 colonies were sequentially picked up from a single plate (n = 3). Dots indicate individual clones. The inner box indicates the number of cells used to obtain cDNA during bulk sorting. A four-parameter log-logistic curve obtained using the drc package (v3.0.1) is shown in the graph.
Abbreviations: BCR, B cell receptor; PCR, polymerase chain reaction; SynCA, synthetic chimeric antibody method; WT, wild-type.
To further evaluate the effect of PCR conditions, we performed three independent experiments for each PCR condition (i.e., the number of cycles and enzyme used). For three different cycle numbers, the proportion of clones obtained in 35 cycles was 46.0 ± 1.5 % in 45 cycles (Fig. S5A). In 55 cycles, the proportion of clones obtained in 35 cycles was 46.0 % ± 5.8 % and that obtained in 45 cycles was 25.0 ± 5.6 %. For three different PCR enzymes, the proportions of clones obtained for the primary enzyme (FX Neo), used in all cloning, were 27.8 ± 16.4 % and 36.7 ± 13.4 % when the second (Plus Neo) and third (Ex Taq) enzymes used, respectively (Fig. S5B). The proportion of clones obtained for the second enzyme was 3.3 ± 2.7 % when the third enzyme used. These proportions of unique clones were higher than those of three independent experiments for the transformation step (Fig. 2B).
Next, we investigated the CDR3 sequences when 50 colonies were picked from a 100 mm plate to evaluate the number of unique clones obtained by a single transformation. The number of unique CDR3 sequences obtained at once was drawn as a sigmoid curve following four-parameter log-logistic regression, implying a limit to the number of unique clones obtained in a single transformation (Fig. 2E). The maximum asymptote was larger when the number of sorted cells was increased (red line = 14.3; blue line = 22.1; and black line = 125.0). The Hill's slope of the sigmoid curve following four-parameter log-logistic regression was as follows: red line = −1.64; blue line = −1.47; and black line = −0.84. Interestingly, the slope of the curve may increase depending on the number of sorted cells used for cDNA synthesis.
4.3. IgG antibodies are reconstituted in the same and different donor-derived pairs using the SynCA method
Using the SynCA method, the original pair of heavy and light chains produced in the donor body remained unknown. The original repertoire of heavy and light chain pairs was identified using the single-cell sorting method, and the representative pairs of the two participants are shown in Fig. 3A and B. For example, the dominant V region, IGHV1-8, overlapped between the two participants. During the SynCA method, more than 10 clones of heavy and light (IgGλ/κ) chain genes were sequenced from a single cDNA, and arbitrary heavy and light chains were selected on the basis of relative abundance (Fig. 3C). We then reconstituted in vitro IgG antibodies using all pairs of heavy and light chains. Interestingly, the dominant V region, IGHV1-8, in the original repertoire showed little reconstitution (clone# G5-3 in Fig. 3C). Moreover, two clones with the same V region (IGHV5-51) showed different concentrations of reconstituted IgG (clone# G2-10 and G663-1 in Fig. 3C). These results suggest that antibody production was not only reconstituted in the same donor-derived pairs, but also in different donor-derived pairs. However, the rules governing in vitro reconstitution remain unclear.
Fig. 3.
Reconstitution of antibody production using the SynCA method
(A and B) Graph showing the frequencies of each V region for paired heavy (x-axes) and light (y-axes) chains in two moderate II patients infected with the WT strain. The color and size of the circles indicate the number of heavy and light chain pairs present in the antibody repertoires isolated via single-cell sorting.
(C) Mean IgG concentration in conditioned media of Expi293F cells transfected with arbitrary heavy and light chain pairs isolated from six donors (biological replicates = 2–6 per clone). The size of the circles corresponds to the relative abundance of clones present in each heavy and light chain. The color of the circles indicates identical donors.
Abbreviations: BCR, B cell receptor; V, variance; SynCA, synthetic chimeric antibody method; WT, wild-type; IgG, immunoglobulin G.
4.4. Autoantibody reactivity is present in antibodies derived from different donors using the SynCA method
Autoantibody reactivity increases in patients with infectious diseases even with antibodies produced in vivo [[15], [16], [17], [18]]. In the SynCA method, antibodies are produced using heavy and light chain pairs that are absent in vivo. We evaluated the autoantibody reactivity against 33 autoantigens in the same and different donor-derived antibodies using the SynCA method. Although no autoantibody reactivity was observed in the representative original antibody, some clones of the SynCA method showed reactivity to autoantigens such as myeloperoxidase, KRT8, dsDNA, and centromere protein B (Fig. S6A). The signal intensities of centromere protein B, KRT8, La/SS-B, Sm/Smith/snRNP core protein, and U1-snRNP70 kDa were significantly increased in the different donor-derived antibodies compared with those in the same donor-derived antibodies (P = 0.029, 0.001, 0.040, 0.040, and 0,029, respectively; Fig. S6B). These results indicate that autoantibody reactivity may be present in antibodies derived from different donors.
4.5. Specific variable regions with heavy and light chains predict IgG reconstitution
Antibodies produced in the body are often not reconstituted in vitro [35], similar to our results (Fig. 3C). We aimed to evaluate potential signatures for predicting reconstitution to identify the determinants of IgG reconstitution. We first divided the 189 clones (including all clones shown in Fig. 3C) into four groups based on the human IgG concentration of conditioned media: non indicated a concentration of 10 ng/ml or less, low indicated a concentration of 10–598.2 ng/ml, middle indicated a concentration of 598.2–10501.4 ng/ml, and high indicated a concentration of 10501.4 ng/ml or more (see Methods). We then generated random forest classifiers, including antibody gene sequences of each region (VH, VDH, DH, DJH, CH, VL, VJL, JL, CL, and CDR3 regions), and donor features (origin, disease severity, SHM, and CDR3 length). The AUROC and 95 % CI obtained from 100 independent iterations were 0.942 (0.870–0.959), 1.000 (0.997–1.000), 1.000 (0.997–1.000), 0.919 (0.856–0.959), 0.948 (0.920–0.975), and 0.709 (0.642–0.760) in each predictive model (i.e., low vs. non, middle vs. non, high vs. non, middle vs. low, high vs. low, and high vs. middle, respectively; Fig. 4A). We found the DH region within CDR3 of the heavy chain to be an important parameter for predicting reconstitution (i.e., low vs. non, middle vs. non, and high vs. non; Fig. 4B). The JL region within CDR3 of the light chain was the most important parameter for predicting the concentration of reconstituted IgG (i.e., middle vs. low, high vs. low, and high vs. middle; Fig. 4C). The distribution of the DH and JL regions in each concentration-dependent group revealed specific nucleotide sequences in each non group (i.e., ATAGTGGCTACGA and ATTACTATGACAGCAGTG in the DH region and GTACA in the JL region; Fig. 4D and E). Although the relative abundances differed slightly, the nucleotide sequences overlapped between the middle and high groups in each region.
Fig. 4.
Prediction model for IgG reconstitution in the SynCA method
(A) Graphs showing AUROC curves for classifying IgG concentrations based on 20 rounds of 10-fold cross-validation. Based on IgG concentration (shown in Fig. 3C), the SynCA clones with heavy and light chain pairs were classified into four groups: non (n = 62) with a concentration of 10 ng/ml or less, low (n = 32) with a concentration ranging from 10 to 598.2 ng/ml (median), middle (n = 47) with a concentration ranging from 598.2 to 10501.4 ng/ml (75th percentile), and high (n = 48) with a concentration of 10501.4 ng/ml or more. The median and 95 % CI of AUROCs shown in the graph were obtained from 100 independent iterations of the random forest classifiers.
(B and C) The mean decrease in the Gini index, representing variable importance, was obtained from 100 independent iterations of the random forest classifier for determining the non and other groups (i.e., low vs. non, middle vs. non, and high vs. non; B) or determining low and middle, low and high, and middle and high groups (C).
(D and E) Pie chart showing the distribution of the DH (D) and JL (E) regions, the most variable importance (shown in panels B and C), in each IgG concentration-dependent group. Same colors depict identical antibody sequences.
(F) Confusion matrix showing the prediction quality of the classification for in vitro IgG reconstitution depending on the concentration. SynCA clones (n = 6 per category) were tested in three categories (i.e., non, low, and above middle) based on the distribution of the DH and JL regions (shown in panels D and E).
(F′) The table shows the prediction and test results for each heavy (DH) and light (JL) chain gene of the low group. Equal signs within the table indicate frequency levels among each group with overlapping nucleotide sequences.
Abbreviations: AUROC, area under the receiving operating characteristic curve; BCR, B cell receptor; V, variance; D, diversity; J, joining; SHM, somatic hypermutation; CDR3, complementarity-determining region 3; CI, confidence interval; SynCA, synthetic chimeric antibody; IgG, immunoglobulin G.
Finally, we validated the predictive capability for distinguishing concentration-dependent groups. We generated new clones in the non, low, and middle plus high (≥middle) groups using the SynCA method based on the most important parameters, the DH and JL regions (see Methods). The predictions designated as high confidence (i.e., low, middle, or high vs. non) did not contain mispredictions (Fig. 4F). Distinguishing the concentration-dependent group (≥middle) also exhibited 100 % prediction. However, the prediction of the low group contained four mispredictions belonging to the ≥middle group (Fig. 4F and F’). Thus, the nucleotide sequence information from the DH region may be a strong predictive signature for reconstituting IgG antibodies.
4.6. Diversity of neutralizing activities is enhanced using the SynCA method
Additionally, we evaluated the in vitro neutralizing activity of several SynCA clones derived from recovered participants infected with the WT strain before the appearance of variant strains (e.g., B. 1.1.7). We found a SynCA clone with higher (1.33-fold) neutralizing activity against the SARS-CoV2 Delta strain than the representative original antibody reconstituted in vitro (Fig. S7A). Neutralizing activities were significantly increased in the same donor-derived antibodies compared with those in different donor-derived antibodies (P = 0.048; Fig. S7B). These results suggest that the SynCA method enhances the diversity of neutralizing antibodies.
5. Discussion
The fundamental process involved in our technology is single cDNA synthesis derived from numerous Bmem pools obtained by bulk sorting and expansion of the antibody libraries of each heavy and light chain gene. The SynCA method identified more diverse antibody genes compared with single-cell sorting technology and predicted in vitro reconstitutions of IgG antibodies by nucleotide sequences of CDR3, specifically in the DH and JL regions. Thus, our antibody discovery technology, which enhances antibody diversity in two stages (i.e., bulk sorting and an arbitrary combination of heavy and light chain genes based on the predictive model), constructs libraries consisting of antibodies binding to specific antigens, which can potentially be helpful in developing therapeutic mAbs as well as antibodies for diagnosis and research.
Although antibody diversity in the human peripheral blood is estimated at 1016–1018 unique sequences, the frequency of shared clonotypes is greater than expected from V(D)J recombination [36]. During aging and infection, such as SARS-CoV2, clonal expansion of specific antibody sequences occurs, leading to a decline in the BCR repertoire diversity [37,38], including convergent responses to specific antigens [[39], [40], [41]]. Particularly with COVID-19, antigen-specific antibodies lacking neutralizing potency are present at high frequencies [[15], [16], [17], [18], [19]]. Indeed, a single-cell sorting analysis revealed no antibodies with high neutralizing activity in the convalescent sera with convergent antibody response to SARS-CoV2 [42]. Assuming that analyzing many antigen-specific B cells would be desirable, we hypothesized that by coupling the bulk sorting and random pairing of the heavy and light chains, constructing diverse antibody libraries more cost-effectively and rapidly than conventional single-cell sorting and phage display technologies would be possible.
Our SynCA method yielded unique clones that were completely different from those obtained by the single-cell sorting method in the same donor, implying that our technology can detect antibodies that either exist in small numbers or do not exist in the body. A rare population was obtained by performing single cDNA synthesis from approximately 50-fold as many Bmem as the single-cell sorting method. This hypothesis is consistent with the finding that the independent PCR step, including varying PCR conditions, was more important for increasing BCR repertoire diversity than the transformation step. Depending on the absolute copy number of each clone in a single cDNA, the clones that can be obtained using PCR may be biased [43]. Given that the major Bmem population in the single-cell sorting method was also the major population in the SynCA method, we expected that the frequency of clonotype sharing between the two methods would be high; however, the results were different. These results suggest that the major population in single-cell sorting represents the tip of the iceberg and may not reflect the actual major population of B cells in peripheral blood. Alternatively, the clones obtained by PCR using the SynCA method may be caused by an unknown mechanism that is independent of the copy number.
A cDNA with fewer numbers of sorted cells reaches the plateau in the number of unique clones of a single transformation earlier than those with more numbers of sorted cells. These results are consistent with previous studies that the absolute number of copies contained in cDNA causes bias in the clones obtained by the PCR step [43]. However, even with the same number of sorted cells, the maximum asymptotes of the sigmoid curve following four-parameter log-logistic regression varied greatly. Future studies that divide the number of sorted cells into broad ranges, considering donor biological factors, are necessary to understand the plateau. Although the number of sorted cells needs to be optimized taking into account donor biological factors, it is essential to obtain a single cDNA from numerous cells to generate diverse antibody libraries, including mAbs with high neutralizing potency. Importantly, unlike the single-cell method, our method has the advantage that the cost remains constant even if the number of sorted cells increases.
Previous studies have shown that in vitro reconstitution of functional IgG antibodies using mammalian cells, including cell-free systems, is not necessarily successful depending on the clone [35,44]. The present study did not observe IgG reconstitution in the original pairs of heavy and light chains. Folding and assembly processes are necessary for antibody secretion, especially a folded light chain is essential for the subsequent processes, including heavy chain folding and assembly [45,46]. Previous studies showing the association between the copy number of light chains and secretion rate support our results that the concentration of IgG in the culture medium was classified by the CDR3 of light chain genes, especially the JL region [35,47]. The SynCA clone using the nucleotide sequence that existed most frequently in the low group for both heavy and light chains showed 100 % prediction in validating the predictive capability for the low group. Conversely, the SynCA clones that used the light chain gene, mostly in the low group, and the heavy chain gene, mostly in the middle or high groups, showed misprediction. These results imply that the concentration of reconstituted IgG antibodies may depend not only on the JL region but also on the DH region. Additionally, two chimeric antibodies generated by shuffling different light chains, which have the same VL region but different JL regions, exhibited neutralizing activity against SARS-CoV-2 that differed by a 10-fold [22]. Structural analyses of the complex consisting of mAbs and RBD revealed the difference in the neutralizing activity resulting from the single substitution of a tryptophan for a tyrosine, making a stabilizing hydrophobic interaction. The JL region may be involved not only in reconstitution but also in determining neutralizing activity.
5.1. Limitations of the study
First, this study only used antigen-specific Bmem derived from recovered patients with COVID-19. However, the SynCA method does not include specialized processes or equipment and can be applied to B cells, including plasmablasts, in other diseases. Second, we used a high-fidelity PCR enzyme; however, it will be necessary to verify random mutagenesis during the PCR amplification by comparing the BCR repertoire diversities with and without PCR amplification [43]. Lastly, the validation of the neutralizing activity was insufficient. Further investigations of the neutralizing potency in purified SynCA clones using the live virus or pseudoviruses are needed to improve the SynCA method. Recent studies have predicted antibody–antigen binding affinity from sequencing information [[48], [49], [50], [51]]. Integrating data on the binding of SynCA clones and antigens into machine learning may enable more rapid reconstitution of antibodies with high neutralizing activity.
Resource availability
6.1. Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Chihiro Akazawa (c.akazawa.gt@juntendo.ac.jp).
6.2. Data and code availability
Clinical data, except those included in this article, are not available in a public repository or as supplementary material to protect the privacy and confidentiality of the study participants. Requests for clinical data and IgG gene sequences can be directed to the corresponding authors and will be reviewed by the Ethics Committee of the Juntendo University School of Medicine. All shared data were identified.
Author contributions
Conceptualization, D. H. and C. A.; investigation, D. H., R. O., A. I., and L. I.; formal analysis, D. H.; data curation, D. H., Y. M., M. H., H. M., Y. T., and T. N.; resources, Y. M., M. H., H. M., Y. T., and T. N.; software, D. H.; visualization, D. H.; writing – original draft, D. H.; writing – review and editing, D. H., Y. T., T. N., and C. A.; funding acquisition, D. H. and C. A.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgments
We would like to acknowledge all study participants. We are grateful to the entire COVID-19 team at Juntendo University Hospital and the Department of Research Support Utilizing Bioresource Bank Juntendo University Graduate School of Medicine for the use of their materials and facilities. We also thank Hiroaki Masuoka (Laboratory for Symbiotic Microbiome Sciences, RIKEN Center for Integrative Medical Sciences) for providing the original script for the randomforest package (v4.7−1.1) of the statistical programming language R version 4.3.3 (2024-02-29). This work was supported by the Japan Society for the Promotion of Science (JSPS)/Ministry of Education, Culture, Sports, Science, and Technology (MEXT), the KAKENHI Grant-in-Aid for Early-Career Scientists (grant numbers 21K15888 and 24K17820 to D.H.), and the YOKOYAMA Foundation for Clinical Pharmacology (#YRY-2121 to D.H.). We also acknowledge financial support from the ITOCHU Chemical Frontier Corporation, Japan, and Otsuka Holdings Co., Ltd., Japan. We also thank the Department of Intellectual Property and Technology Transfer at AMED for technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2025.102312.
Contributor Information
Daisuke Hisamatsu, Email: d.hisamatsu.ap@juntendo.ac.jp.
Chihiro Akazawa, Email: c.akazawa.gt@juntendo.ac.jp.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
No data was used for the research described in the article.
References
- 1.Pedrioli A., Oxenius A. Single B cell technologies for monoclonal antibody discovery. Trends Immunol. 2021;42:1143–1158. doi: 10.1016/j.it.2021.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Regeneron Pharmaceuticals I. 2020. Regeneron's REGN‐COV2 Antibody Cocktail Reduced Viral Levels and Improved Symptoms in Non‐Hospitalized COVID‐19 Patients. [Google Scholar]
- 3.Walker L., Kaku C.I. Google Patents; 2025. Sars-cov2 Antibodies and Uses Thereof. [Google Scholar]
- 4.Krasner J. 2023. Aerium Therapeutics Advances next Generation Antibodies to Protect Immunocompromised Persons Against COVID-19. [Google Scholar]
- 5.Francica J., Cai Y., Diallo S., Rosenthal K., Ren K., Flores D., Dippel A., Wu Y., Chen X., Novick S. 2023. The SARS-CoV-2 Monoclonal Antibody AZD3152 Potently Neutralises Historical and Currently Circulating Variants; pp. 15–18. [Google Scholar]
- 6.Webber C., Beavon R., Thomas S., Chang L., Cohen T., Perez J. Trial in progress: a phase I/III, randomised, modified double-blind, placebo-and active-controlled pre-exposure prophylaxis study of the SARS-CoV-2–neutralising antibody AZD3152 (SUPERNOVA) Copenhagen, Denmark. 2023:15–18. [Google Scholar]
- 7.Focosi D., Franchini M., Casadevall A., Maggi F. An update on the anti-spike monoclonal antibody pipeline for SARS-CoV-2. Clin. Microbiol. Infection. 2024;30:999–1006. doi: 10.1016/j.cmi.2024.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Casadevall A., Focosi D. Lessons from the use of monoclonal antibodies to SARS-CoV-2 spike protein during the COVID-19 pandemic. Annu. Rev. Med. 2025;76:1–12. doi: 10.1146/annurev-med-061323-073837. [DOI] [PubMed] [Google Scholar]
- 9.Hansen J., Baum A., Pascal K.E., Russo V., Giordano S., Wloga E., Fulton B.O., Yan Y., Koon K., Patel K. Studies in humanized mice and convalescent humans yield a SARS-CoV-2 antibody cocktail. Science. 2020;369:1010–1014. doi: 10.1126/science.abd0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wec A.Z., Wrapp D., Herbert A.S., Maurer D.P., Haslwanter D., Sakharkar M., Jangra R.K., Dieterle M.E., Lilov A., Huang D. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science. 2020;369:731–736. doi: 10.1126/science.abc7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rappazzo C.G., Tse L.V., Kaku C.I., Wrapp D., Sakharkar M., Huang D., Deveau L.M., Yockachonis T.J., Herbert A.S., Battles M.B. Broad and potent activity against SARS-like viruses by an engineered human monoclonal antibody. Science. 2021;371:823–829. doi: 10.1126/science.abf4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iketani S., Liu L., Guo Y., Liu L., Chan J.F.-W., Huang Y., Wang M., Luo Y., Yu J., Chu H. Antibody evasion properties of SARS-CoV-2 omicron sublineages. Nature. 2022;604:553–556. doi: 10.1038/s41586-022-04594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosen L.E., Tortorici M.A., De Marco A., Pinto D., Foreman W.B., Taylor A.L., Park Y.-J., Bohan D., Rietz T., Errico J.M. A potent pan-sarbecovirus neutralizing antibody resilient to epitope diversification. Cell. 2024;187:7196–7213. e7126. doi: 10.1016/j.cell.2024.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Addetia A., Piccoli L., Case J.B., Park Y.-J., Beltramello M., Guarino B., Dang H., de Melo G.D., Pinto D., Sprouse K., et al. Neutralization, effector function and immune imprinting of omicron variants. Nature. 2023;621:592–601. doi: 10.1038/s41586-023-06487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bastard P., Rosen L.B., Zhang Q., Michailidis E., Hoffmann H.-H., Zhang Y., Dorgham K., Philippot Q., Rosain J., Béziat V., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q., Bastard P., Bolze A., Jouanguy E., Zhang S.-Y., Cobat A., Notarangelo L.D., Su H.C., Abel L., Casanova J.-L. Life-threatening COVID-19: defective interferons unleash excessive inflammation. Méd. Sur. 2020;1:14–20. doi: 10.1016/j.medj.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang E.Y., Mao T., Klein J., Dai Y., Huck J.D., Jaycox J.R., Liu F., Zhou T., Israelow B., Wong P. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- 18.van der Wijst M.G.P., Vazquez S.E., Hartoularos G.C., Bastard P., Grant T., Bueno R., Lee D.S., Greenland J.R., Sun Y., Perez R., et al. Type I interferon autoantibodies are associated with systemic immune alterations in patients with COVID-19. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abh2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y., Soh W.T., Kishikawa J.I., Hirose M., Nakayama E.E., Li S., Sasai M., Suzuki T., Tada A., Arakawa A., et al. An infectivity-enhancing site on the SARS-CoV-2 spike protein targeted by antibodies. Cell. 2021;184:3452–3466 e3418. doi: 10.1016/j.cell.2021.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan M., West B.R., Foreman W.B., Powers C., Feng Z., Taylor A.L., Yu X., Wang G., Walker L., Starr T.N., et al. Structural and functional analysis of VYD222: a broadly neutralizing antibody against SARS-CoV-2 variants. bioRxiv. 2025 doi: 10.1101/2025.08.28.672883. 2025.2008.2028.672883. [DOI] [Google Scholar]
- 21.Xie J., Ding C., He J., Zhang Y., Ni S., Zhang X., Chen Q., Wang J., Huang L., He H. Novel monoclonal antibodies and recombined antibodies against variant SARS-CoV-2. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.715464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dejnirattisai W., Zhou D., Ginn H.M., Duyvesteyn H.M., Supasa P., Case J.B., Zhao Y., Walter T.S., Mentzer A.J., Liu C. The antigenic anatomy of SARS-CoV-2 receptor binding domain. Cell. 2021;184:2183–2200. e2122. doi: 10.1016/j.cell.2021.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kramer R.A., Marissen W.E., Goudsmit J., Visser T.J., Clijsters‐Van der Horst M., Bakker A.Q., de Jong M., Jongeneelen M., Thijsse S., Backus H.H. The human antibody repertoire specific for rabies virus glycoprotein as selected from immune libraries. Eur. J. Immunol. 2005;35:2131–2145. doi: 10.1002/eji.200526134. [DOI] [PubMed] [Google Scholar]
- 24.Throsby M., van den Brink E., Jongeneelen M., Poon L.L., Alard P., Cornelissen L., Bakker A., Cox F., van Deventer E., Guan Y. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One. 2008;3 doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clackson T., Hoogenboom H.R., Griffiths A.D., Winter G. Making antibody fragments using phage display libraries. Nature. 1991;352:624–628. doi: 10.1038/352624a0. [DOI] [PubMed] [Google Scholar]
- 26.Hisamatsu D., Ikeda A., Ito L., Matsushita Y., Hiki M., Mori H., Tabe Y., Naito T., Akazawa C. Longitudinal analyses after COVID-19 recovery or prolonged infection reveal unique immunological signatures after repeated vaccinations. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadras V., Bongiovanni R. Use of lorenz curves and gini coefficients to assess yield inequality within paddocks. Field Crops Res. 2004;90:303–310. [Google Scholar]
- 28.Shifrut E., Baruch K., Gal H., Ndifon W., Deczkowska A., Schwartz M., Friedman N. CD4+ T cell-receptor repertoire diversity is compromised in the spleen but not in the bone marrow of aged mice due to private and sporadic clonal expansions. Front. Immunol. 2013;4:379. doi: 10.3389/fimmu.2013.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas P.G., Handel A., Doherty P.C., La Gruta N.L. Ecological analysis of antigen-specific CTL repertoires defines the relationship between naive and immune T-cell populations. Proc. Natl. Acad. Sci. 2013;110:1839–1844. doi: 10.1073/pnas.1222149110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiller T., Meffre E., Yurasov S., Tsuiji M., Nussenzweig M.C., Wardemann H. Efficient generation of monoclonal antibodies from single human B cells by single cell RT-PCR and expression vector cloning. J. Immunol. Methods. 2008;329:112–124. doi: 10.1016/j.jim.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouckaert R.R., Frank E. Springer; 2004. Evaluating the Replicability of Significance Tests for Comparing Learning Algorithms; pp. 3–12. [Google Scholar]
- 32.Ledsgaard L., Ljungars A., Rimbault C., Sorensen C.V., Tulika T., Wade J., Wouters Y., McCafferty J., Laustsen A.H. Advances in antibody phage display technology. Drug Discov. Today. 2022;27:2151–2169. doi: 10.1016/j.drudis.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Daugherty P.S., Chen G., Iverson B.L., Georgiou G. Quantitative analysis of the effect of the mutation frequency on the affinity maturation of single chain Fv antibodies. Proc. Natl. Acad. Sci. U. S. A. 2000;97:2029–2034. doi: 10.1073/pnas.030527597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sioud M. Phage display libraries: from binders to targeted drug delivery and human therapeutics. Mol. Biotechnol. 2019;61:286–303. doi: 10.1007/s12033-019-00156-8. [DOI] [PubMed] [Google Scholar]
- 35.Bhoskar P., Belongia B., Smith R., Yoon S., Carter T., Xu J. Free light chain content in culture media reflects recombinant monoclonal antibody productivity and quality. Biotechnol. Prog. 2013;29:1131–1139. doi: 10.1002/btpr.1767. [DOI] [PubMed] [Google Scholar]
- 36.Briney B., Inderbitzin A., Joyce C., Burton D.R. Commonality despite exceptional diversity in the baseline human antibody repertoire. Nature. 2019;566:393–397. doi: 10.1038/s41586-019-0879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang C., Liu Y., Xu L.T., Jackson K.J., Roskin K.M., Pham T.D., Laserson J., Marshall E.L., Seo K., Lee J.Y., et al. Effects of aging, cytomegalovirus infection, and EBV infection on human B cell repertoires. J. Immunol. 2014;192:603–611. doi: 10.4049/jimmunol.1301384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monzo C., Gkioni L., Beyer A., Valenzano D.R., Gronke S., Partridge L. Dietary restriction mitigates the age-associated decline in mouse B cell receptor repertoire diversity. Cell Rep. 2023;42 doi: 10.1016/j.celrep.2023.112722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galson J.D., Schaetzle S., Bashford-Rogers R.J.M., Raybould M.I.J., Kovaltsuk A., Kilpatrick G.J., Minter R., Finch D.K., Dias J., James L.K., et al. Deep sequencing of B cell receptor repertoires from COVID-19 patients reveals strong convergent immune signatures. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.605170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nielsen S.C.A., Yang F., Jackson K.J.L., Hoh R.A., Roltgen K., Jean G.H., Stevens B.A., Lee J.Y., Rustagi A., Rogers A.J., et al. Human B cell clonal expansion and convergent antibody responses to SARS-CoV-2. Cell Host Microbe. 2020;28:516–525 e515. doi: 10.1016/j.chom.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong P., Gautam A., Windsor I.W., Travers M., Chen Y., Garcia N., Whiteman N.B., McKay L.G.A., Storm N., Malsick L.E., et al. Memory B cell repertoire for recognition of evolving SARS-CoV-2 spike. Cell. 2021;184:4969–4980 e4915. doi: 10.1016/j.cell.2021.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi Z., Zhang Q., Yan H., Yang Y., Wang P., Zhang Y., Deng Z., Yu M., Zhou W., Wang Q., et al. More than one antibody of individual B cells revealed by single-cell immune profiling. Cell Discov. 2019;5:64. doi: 10.1038/s41421-019-0137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ojima-Kato T., Hashimura D., Kojima T., Minabe S., Nakano H. In vitro generation of rabbit Anti-Listeria monocytogenes monoclonal antibody using single cell based RT-PCR linked cell-free expression systems. J. Immunol. Methods. 2015;427:58–65. doi: 10.1016/j.jim.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Leitzgen K., Knittler M.R., Haas I.G. Assembly of immunoglobulin light chains as a prerequisite for secretion. A model for oligomerization-dependent subunit folding. J. Biol. Chem. 1997;272:3117–3123. doi: 10.1074/jbc.272.5.3117. [DOI] [PubMed] [Google Scholar]
- 46.Lee Y.-K., Brewer J.W., Hellman R., Hendershot L.M. BiP and immunoglobulin light chain cooperate to control the folding of heavy chain and ensure the fidelity of immunoglobulin assembly. Mol. Biol. Cell. 1999;10:2209–2219. doi: 10.1091/mbc.10.7.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borth N., Strutzenberger K., Kunert R., Steinfellner W., Katinger H. Analysis of changes during subclone development and ageing of human antibody-producing heterohybridoma cells by northern blot and flow cytometry. J. Biotechnol. 1999;67:57–66. doi: 10.1016/s0168-1656(98)00162-x. [DOI] [PubMed] [Google Scholar]
- 48.Rube H.T., Rastogi C., Feng S., Kribelbauer J.F., Li A., Becerra B., Melo L.A.N., Do B.V., Li X., Adam H.H., et al. Prediction of protein-ligand binding affinity from sequencing data with interpretable machine learning. Nat. Biotechnol. 2022;40:1520–1527. doi: 10.1038/s41587-022-01307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee I., Nam H. Sequence-based prediction of protein binding regions and drug-target interactions. J. Cheminf. 2022;14:5. doi: 10.1186/s13321-022-00584-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bisarad P., Kelbauskas L., Singh A., Taguchi A.T., Trenchevska O., Woodbury N.W. Predicting monoclonal antibody binding sequences from a sparse sampling of all possible sequences. Commun. Biol. 2024;7:979. doi: 10.1038/s42003-024-06650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Azulay A., Cohen-Lavi L., Friedman L.M., McGargill M.A., Hertz T. Mapping antibody footprints using binding profiles. Cell Rep Methods. 2023;3 doi: 10.1016/j.crmeth.2023.100566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Clinical data, except those included in this article, are not available in a public repository or as supplementary material to protect the privacy and confidentiality of the study participants. Requests for clinical data and IgG gene sequences can be directed to the corresponding authors and will be reviewed by the Ethics Committee of the Juntendo University School of Medicine. All shared data were identified.
No data was used for the research described in the article.