Abstract
Introduction and aims
Root canal sealers are indispensable in endodontic therapy, but the diffusion of their chemical components into periapical tissues may trigger osteoclast activation and bone resorption. This study aims to evaluate the pH values of 4 calcium silicate-based bioceramic sealers (C-root SP, iMTA SP, iRoot SP and NeoSEALER Flo) and their effects on the cell viability of osteoclast precursor cells, while further exploring their regulatory roles in receptor activator of nuclear factor kappa-B ligand (RANKL)-induced osteoclastogenesis in vitro.
Methods
After pH measurement of sealer extracts, the cell viability following sealer treatment was quantitatively assessed using the Cell Counting Kit-8 (CCK-8) assay, while tartrate-resistant acid phosphatase (TRAP) staining and phalloidin staining were employed to evaluate RANKL-induced osteoclast formation and fibrous actin (F-actin) ring formation, respectively. Furthermore, quantitative real-time PCR (qPCR) and Western blot analyses were conducted to examine the expression patterns of osteoclast-related genes and proteins.
Results
All sealer extracts exhibited alkaline properties. Low-concentration sealer extracts exhibited no significant impact on cell viability, whereas high-concentration extracts (50 mg/mL) displayed time-dependent cytotoxic effects. Notably, all tested sealers exhibited inhibitory effects on osteoclast differentiation, with NeoSEALER Flo demonstrating the most potent suppression. This was evidenced by a reduction in the formation of TRAP-positive multinucleated cells, disruption of F-actin ring organisation, and a coordinated downregulation of osteoclast-related gene and protein expression.
Conclusion
All tested sealers showed no obvious cytotoxicity at low concentrations and exhibited varying degrees of inhibitory effects on osteoclast formation and function. Among them, NeoSEALER Flo extracts showed the strongest inhibitory effect on osteoclasts.
Clinical Relevance
NeoSEALER Flo, a calcium silicate-based bioceramic sealer, effectively minimises cytotoxicity while inhibiting osteoclast formation and activity. This suggests that it may effectively prevent periapical tissue destruction and promote bone healing in apical periodontitis, highlighting its promising potential as a root canal obturation material.
Key Words: Bioceramic, Calcium silicate-based sealers, Osteoclast, Cytotoxicity, Root canal treatment
Introduction
Apical periodontitis represents a prevalent infectious disease characterised by microbial invasion and subsequent inflammatory destruction of the pulpal and periradicular tissues.1 Currently, endodontic therapy predominantly employs root canal treatment as the standard intervention. The obturation phase constitutes a critical step in root canal therapy, wherein a complete 3-dimensional sealing of the root canal system is achieved to effectively eliminate intraradicular microbial reservoirs and prevent recurrent infection.2 However, clinical observations demonstrate that sealer extrusion beyond the apical foramen remains a potential complication, wherein subsequent diffusion of bioactive components may affect the proliferation and function of periradicular cells.3 Therefore, the biocompatibility of root canal sealer materials is an essential consideration in their clinical selection and application for managing apical periodontitis.
The pathogenesis of apical periodontitis is characterised by disruption of the delicate balance between osteoclast-mediated bone resorption and osteoblast-mediated bone formation induced by inflammatory mediators.4 Osteoclasts, a group of multinucleated giant cells derived from the monocyte-macrophage lineage, are primarily regulated by 2 main molecular signals: macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-B ligand (RANKL).5 Among them, RANKL could regulate the differentiation and activity of osteoclasts by binding to the receptor activator for nuclear factor kappa-B (RANK) expressed on osteoclast surfaces,6 and this process is negatively regulated by osteopontin (OPG). Through the RANKL/RANK/OPG signalling axis, activation of downstream mediators such as TNF receptor associated factor 6 (TRAF6), nuclear factor kappa-B (NF-κB), and mitogen-activated protein kinase (MAPK) leads to the induction of key transcription factors, including nuclear factor of activated T cells 1 (NFATc1) and c-Fos, which in turn drive the transcription of osteoclast-specific genes, such as tartrate-resistant acid phosphatase (TRAP) and Cathepsin K (Ctsk). In apical periodontitis, elevated RANKL could promote excessive osteoclastogenesis, leading to periapical bone resorption and impaired healing.7 Therefore, targeting osteoclast formation and function is a key therapeutic strategy in the management of apical periodontitis.
Currently, calcium silicate-based bioceramic materials are the most widely used root canal sealers in clinical practice.8 Numerous studies have shown that these materials play a significant role in the antibacterial, anti-inflammatory and bone repair of periapical tissues by biological process including creating an alkaline environment, inhibiting the release of inflammatory factors, promoting osteogenesis and biomineralisation.9, 10, 11, 12, 13 In addition, these materials exhibit low cytotoxicity and good biocompatibility,14 and several of them have been proven to be capable of inhibiting osteoclast formation and function.3,15 Specifically, the components of bioceramic materials, such as silicate and calcium ions, can suppress osteoclastogenesis by inhibiting the RANKL/RANK/OPG signalling pathway, thereby downregulating TRAF6- and NF-κB-mediated signalling as well as the expression of transcription factors NFATc1 and c-Fos, thus reducing the expression of osteoclast functional genes. In addition, the local alkaline microenvironment created by these materials can attenuate bone resorption by neutralising the acidification induced by osteoclasts.16, 17, 18, 19 Among these sealers, iRoot SP has been extensively applied in both clinical practice and comparative research owing to its exceptional biocompatibility, and remarkable chemical stability and superior sealing capability.20,21 Additionally, C-root SP and i-MTA SP are commonly employed in clinical practice owing to their cost-efficient properties.8 NeoSEALER Flo, as a newly developed bioceramic sealer, exhibits enhanced mineralisation capacity and improved clinical sealing performance, indicating significant therapeutic potential.22,23 However, the direct effect of NeoSEALER Flo on osteoclastogenesis has remained to be defined. Furthermore, pH level, as a critical factor influencing cell viability and osteoclast function,15 should also be taken into account regarding the pH values resulting from the dissolution of the aforementioned sealer components.
Therefore, this study aimed to evaluate and compare the pH values of extracts from 4 available root canal sealers (C-root SP, i-MTA SP, iRoot SP, and NeoSEALER Flo), their effects on bone marrow-derived macrophages (BMMs) cell viability, as well as their roles in RANKL-mediated osteoclast differentiation and function, thereby providing references for clinical endodontic treatment.
Material and methods
Animals breeding, cell acquisition and culture
The experiment was approved and conducted under the guidance of the Ethics Committee for Animal Welfare of the Stomatological Hospital of Wuhan University (Approval number: S07924100D). A total of 15 eight-week-old male C57BL/6 mice were used in this study. All mice were housed under a 12-hour light/12-hour dark cycle and fed a standard rodent diet. BMMs were obtained and cultured as previously described.24 Briefly, BMMs were extracted from the femurs or tibias of eight-week-old male mice and cultured in α-minimal essential medium (α-MEM; Hyclone, Logan, USA) containing 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, USA), 10 ng/mL recombinant M-CSF (Peprotech, London, UK), 100 IU/mL penicillin and 100 μg/mL streptomycin (Hyclone, Logan, USA). The BMMs were maintained at 37°C with 5% CO2 and the medium was changed every 2 days. After 4 days of culture, the BMMs were lifted using 5 mM EDTA (Sigma-Aldrich) in PBS (Servicebio, Wuhan, China) for subsequent experiments.
Preparation of root canal sealer extracts and pH measurement
The following endodontic sealers were tested: C-root SP (C-Root Dental Medical, Beijing, China), i-MTA SP (Longly Biotechnology, Wuhan, China), iRoot SP (Innovative Bioceramix, Vancouver, Canada), and NeoSEALER Flo (Avalon Biomed, Houston, USA). The preparation of sealer extracts was performed according to the method described by Wu et al.25 Under sterile conditions, sealers were spread in cell culture dishes and placed at 37°C for 24 hours to allow them to dry. Subsequently, the dried sealer was dissolved in α-MEM to achieve a concentration of 200 mg/mL. The solution was then vigorously vortexed and stored at 37°C for 3 days to ensure complete dissolution of the soluble components. The liquid was collected and filtered through a 0.22 μm filter to obtain sterile sealer extracts.
Sealer extracts at a concentration of 200 mg/mL and α-MEM were used to prepare diluted sealer extracts at varying concentrations (0.2 mg/mL, 2 mg/mL, 5 mg/mL, 10 mg/mL, 20 mg/mL, and 50 mg/mL). The pH values of 4 sealer extracts (C-root SP, i-MTA SP, iRoot SP and NeoSEALER Flo) at each concentration were measured using an electronic pH meter (Lichen, Changsha, China). The pH meter was calibrated between each measurement.
Cell viability assay
BMMs were seeded at a density of 1.5 × 104 cells per well in 96-well plates and cultured with medium containing different concentrations (0.2 mg/mL, 2 mg/mL, 5 mg/mL, 10 mg/mL, 20 mg/mL, and 50 mg/mL) of sealer extracts (C-root SP, i-MTA SP, iRoot SP and NeoSEALER Flo). According to the manufacturer's instructions, the cell culture medium was replaced with α-MEM containing 10% Cell Counting Kit-8 (CCK-8) reagent (Dojindo, Kumamoto, Japan) for 24 or 48 hours. Cells were then incubated at 37°C with 5% CO2 for one hour. The absorbance was measured at 450 nm using a Thermomax microplate reader (Bio-Tek, Winooski, USA).
Since the concentration of 5 mg/mL did not show significant cytotoxicity compared with the control group (0 mg/mL) in any of the 4 sealer extracts, this concentration could ensure cell viability and achieve optimal stimulatory effects. Therefore, 5 mg/mL was used as the stimulation concentration for the sealer extracts in all subsequent experiments.
Osteoclast induction and sealer extracts stimulation
BMMs were seeded at a density of 1 × 104 cells per well in 96-well plates (for staining) or 3 × 105 cells per well in 6-well plates (for RNA or protein extraction). On the following day, the medium of the C-root SP, i-MTA SP, iRoot SP, and NeoSEALER Flo groups was replaced with α-MEM containing 5 mg/mL sealer extracts respectively and 10 ng/mL RANKL (R&D Systems, Minneapolis, USA), and cells were then incubated at 37°C with 5% CO2. Cells of the control group were cultured with medium containing no extracts or RANKL, while the RANKL group was treated with medium without sealer extracts but containing RANKL. The medium was changed every 2 days, and the cells were cultured for 5 days for subsequent experiments.
Osteoclastogenesis assay
After 5 days of osteoclast induction, the cells in 96-well plates were fixed with 4% polyformaldehyde (Servicebio) and washed with PBS. Tartrate-resistant acid phosphatase (TRAP) staining was performed using a leukocyte acid phosphatase kit (Sigma-Aldrich, 387A) according to the manufacturer's protocol. Multinucleated TRAP-positive cells with 3 or more nuclei were identified and counted as osteoclasts under a bright-field microscope.
Fibrous actin (F-actin) ring assay
The cells were washed with PBS and fixed with paraformaldehyde. They were then incubated with phalloidin (Sigma-Aldrich) labeled with fluorescein isothiocyanate (FITC) at 37°C for 30 minutes. Nuclei were stained with DAPI (Beyotime, Shanghai, China). F-actin ring formation was observed using a fluorescence microscope (Leica DMLS, Vienna, Austria), and quantification was performed using ImageJ software (NIH, Bethesda, USA).
RNA extraction and quantitative real-time PCR
The original medium was removed, and the cells were washed with PBS. Total RNA was extracted using TRIzol reagent (Takara Bio, Shiga, Japan), and reverse transcription was performed using the SYBR Green One-Step qRT-PCR Kit (Beyotime). Quantitative polymerase chain reaction (qPCR) amplification was subsequently carried out using ChamQ Blue Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) and a QuantStudio 6 Flex instrument (Applied Biosystems, Foster City, USA). The Gapdh expression was used as a reference control to detect the expressions of Nfatc1, c-fos, c-src, Mmp9, Mmp14 and Ctsk. The primer sequences used in this study are shown in Table 1.
Table 1.
The primer sequences for qPCR.
| Gene | Forward primer (5’-3’) | Reverse primer (5’-3’) |
|---|---|---|
| Nfatc1 | GGTAACTCTGTCTTTCTAACCTTAAGCTC | GTGATGACCCCAGCATGCACCAGTCACAG |
| c-fos | CCAAGCGGAGACAGATCAACTT | TCCAGTTTTTCCTTCTCTTTCAGCAGAT |
| c-src | GAACCCGAGAGGGACCTTC | GAGGCAGTAGGCACCTTTTGT |
| Mmp9 | TCCAGTACCAAGACAAAGCCTA | TTGCACTGCACGGTTGAA |
| Mmp14 | TATGGTTTACAAGTGACAGGCA | AAACTTATCCGGAACACCACAG |
| Ctsk | CAGCAGAACGGAGGCATTGA | CCTTTGCCGTGGCGTTATAC |
| Gapdh | CTCCCACTCTTCCACCTTCG | TTGCTGTAGCCGTATTCATT |
Western blot
Cells were washed with PBS and then lysed in a lysis buffer containing protease and phosphatase inhibitors (Sigma-Aldrich). Protein concentration was determined using a Bicinchoninic Acid (BCA) protein assay kit (Applygen Technologies, Beijing, China). Protein denaturation, sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and Western blotting were performed according to the recommended protocols of manufacturers using SDS-PAGE loading buffer (Beyotime), a SDS-PAGE kit (Servicebio), PVDF membranes (Millipore, Billerica, USA), a rapid no-protein blocking solution (Servicebio), and a hypersensitive enhanced chemiluminescence (ECL) substrate (4A Biotech, Suzhou, China). Primary antibodies used were against NFATc1 (Santa Cruz Biotechnology, Dallas, USA, sc-7294), c-FOS (Cell Signalling Technology, Danvers, USA, #2250), MMP9 (Santa Cruz Biotechnology, sc-13520), MMP14 (Abcam, ab51074), CTSK (Santa Cruz Biotechnology, sc-48353), and β-actin (Proteintech, Wuhan, China, 66009-1-Ig). Secondary antibodies were species-specific antibodies (Cell Signalling Technology, and Proteintech) diluted according to their respective recommendations. The signals were imaged using the Odyssey imaging system (LI-COR Biosciences, Lincoln, USA), and visualised using the Image Studio 6.1 software (LI-COR Biosciences). ImageJ software was used to measure the grayscale values of the bands. The grayscale values of β-actin bands in each group were used as a normalisation control for the quantitative analysis of the Western blot.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 9.0 (GraphPad Software, Boston, USA). All values are presented as mean ± standard deviation (SD). After verifying data normality using the Shapiro-Wilk test and homogeneity of variance using the Brown-Forsythe test, differences between 2 groups were assessed using unpaired Student's t tests, while differences among multiple groups were evaluated using one-way ANOVA. A P value of <.05 was considered statistically significant.
Results
All sealer extracts exhibited alkaline properties, and demonstrated dose- and time-dependent inhibitory effects on BMMs viability
The pH values of the 4 sealer extracts (C-root SP, i-MTA SP, iRoot SP, and NeoSEALER Flo) at different concentrations (0.2 mg/mL, 2 mg/mL, 5 mg/mL, 10 mg/mL, 20 mg/mL, and 50 mg/mL) were measured. Overall, all extracts exhibited alkalinity, and their pH values increased with concentration (Figure 1A). At lower concentrations (≤ 5 mg/mL), the pH of the 4 extracts ranged from 7.00 to 9.00. At higher concentrations (≥ 10 mg/mL), the pH values gradually increased, and at 50 mg/mL, all extracts showed relatively strong alkaline properties (pH > 11.50). The pH ranking of the 4 sealer extracts was: C-root SP > iRoot SP > NeoSEALER Flo > iMTA SP.
Fig. 1.
Sealer extracts exhibited alkalinity and affected BMMs viability in dose- and time-dependent manners. (A) The pH values of C-root SP, iMTA SP, iRoot SP and NeoSEALER Flo extracts increased with concentration. n = 3. (B) The dose-dependent cytotoxic effects of C-root SP, iMTA SP, iRoot SP and NeoSEALER Flo extracts. n = 5, *p < .05, ⁎⁎p < .01, ⁎⁎⁎p < .001, ⁎⁎⁎⁎p < .0001 versus Control group (0 mg/mL) using one-way ANOVA with Dunnett’s post hoc test. (C) The time-dependent cytotoxic effects of C-root SP, iMTA SP, iRoot SP and NeoSEALER Flo extracts. n = 5, *p < .05, ⁎⁎p < .01, ⁎⁎⁎p < .001, ⁎⁎⁎⁎p < .0001 using unpaired Student's t test.
To evaluate the cytotoxicity of different sealers on osteoclast precursor cells, a CCK-8 assay was conducted to assess the effects of C-root SP, i-MTA SP, iRoot SP, and NeoSEALER Flo on the viability of BMMs. Overall, all sealers demonstrated a dose-dependent response: low concentrations exerted negligible effects on cell viability, whereas high concentrations exhibited cytotoxicity (Figure 1B). Specifically, C-root SP extract at concentrations ≤ 10 mg/mL showed no significant impact on BMM viability. In contrast, 20 and 50 mg/mL C-root SP significantly reduced viability at 24 hours and 48 hours. iMTA SP extract showed no significant cytotoxicity at any concentration after 24 hours. However, cell viability was significantly decreased in response to iMTA SP at 10, 20, and 50 mg/mL after 48 hours. iRoot SP extract did not affect cell viability at concentrations ≤ 20 mg/mL at 24 or 48h, while 50 mg/mL iRoot SP caused a significant reduction in cell viability. Moreover, the NeoSEALER Flo extract significantly reduced cell viability at both 20 and 50 mg/mL after 24 hours, and this cytotoxic effect persisted at 48 hours with the same concentrations. Overall, the effects of the 4 sealer extracts at varying concentrations on cell viability were correlated with their pH values.
In addition to the dose-dependent effects, high-concentration sealer extracts also exhibited a time-dependent increase in cytotoxicity (Figure 1C). Notably, all sealers at 50 mg/mL induced significantly greater cytotoxicity in BMMs after 48 hours of exposure compared to 24 hours. Furthermore, BMMs treated with 5 mg/mL iMTA The SP extract showed a progressive decline in cell viability over time. No significant time-dependent changes in cell viability were observed in the remaining groups (iMTA SP at 0.2, 2, 10, 20 mg/mL, and the other 3 sealers at 0.2, 2, 5, 10, 20 mg/mL) between the 24- and 48-hour time points. Collectively, these findings suggest that low concentrations (≤ 5 mg/mL) of the tested sealers extracts (C-root SP, i-MTA SP, iRoot SP, and NeoSEALER Flo) do not markedly impair BMM viability, whereas higher concentrations (≥ 10 mg/mL) exert inhibitory effects. Taken together, the cytotoxicity of high-concentration extracts (50 mg/mL) appeared to increase with prolonged exposure.
In the above assessment of cell viability, the concentration of 5 mg/ml was identified as the maximum concentration of each sealer extract that did not affect cell viability after 24 and 48 hours of stimulation on BMMs. Therefore, in order to use the highest possible concentration of extract to achieve optimal stimulatory effects while ensuring no significant effects on cell viability, we selected 5 mg/ml as the stimulation concentration of each extract in subsequent experiments.
Sealer extracts inhibited RANKL-induced osteoclastogenesis and F-actin ring formation
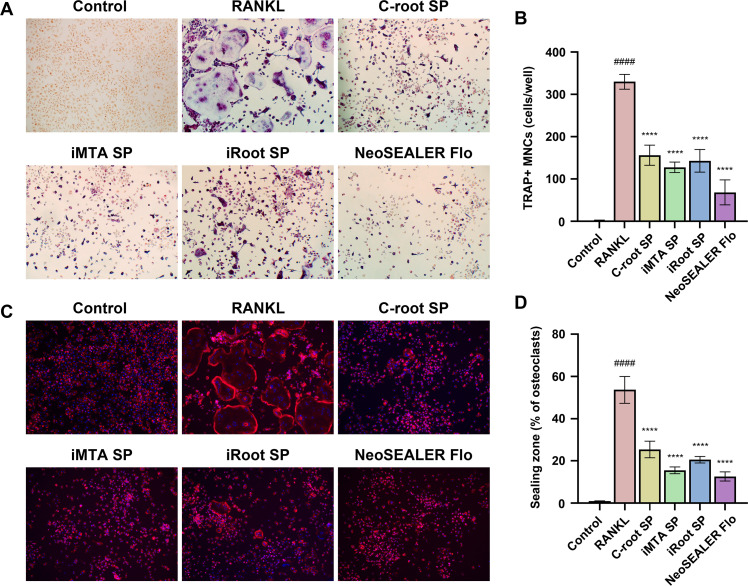
We next explored the potential of 4 tested sealers for osteoclast formation. Multinucleated TRAP-positive osteoclasts with 3 and more nuclei were observed in RANKL-induced BMMs (RANKL group) for 5 days, while no obvious TRAP-positive cell was found in the control group (Figure 2A and 2B). The numbers of osteoclasts in all sealer extract groups were significantly lower compared to the RANKL group. These results indicate that RANKL stimulation successfully induced osteoclast formation, whereas the addition of sealer extracts exerted an inhibitory effect on osteoclastogenesis. Among the tested sealer extracts, NeoSEALER Flo exhibited the strongest inhibitory effect on osteoclastogenesis, as evidenced by a significant reduction in the number of osteoclasts.
Fig. 2.
Sealer extracts inhibited RANKL-induced osteoclastogenesis and F-actin ring formation. (A) Representative images of osteoclasts in RANKL-induced BMMs by TRAP staining. Scale bar, 200μm, magnification, ×100. (B) The number of TRAP-positive multinucleated cells (TRAP+ MNCs) containing three or more nuclei per well, n = 5. (C) Representative pictures of F-Actin ring formation by phalloidin staining. Scale bar, 200 μm, magnification, ×100. (D) The percentage of osteoclasts with F-actin ring sealing zones among the total number of osteoclasts, n = 5. ####p < .0001 versus Control group using unpaired Student's t test, ⁎⁎⁎⁎p < .0001 versus RANKL group using one-way ANOVA with Dunnett’s post hoc test.
F-actin rings are cytoskeletal structures composed of filamentous actin that localise at the osteoclast-bone matrix interface, ensuring the efficient maintenance of a localised acidic environment and optimal enzymatic activity for bone degradation.26 F-actin rings serve as a reliable signature for evaluating osteoclast function and resorptive activity.26 We then performed phalloidin staining to investigate the impact of different sealer extracts on the formation of F-actin rings in osteoclasts (Fig. 2, Fig. 2). Consistent with the TRAP staining results, phalloidin staining demonstrated that the BMMs group did not form F-actin ring structures, while RANKL stimulation significantly induced typical F-actin ring assembly. However, the addition of sealer extracts markedly suppressed RANKL-induced F-actin ring assembly, with NeoSEALER Flo exhibiting the least F-actin formation, indicating a significant inhibitory effect on osteoclast function (Fig. 2, Fig. 2). Collectively, these results suggested that all tested extracts had inhibitory effects on RANKL-induced osteoclastogenesis and F-actin ring formation, while NeoSEALER Flo showed the most remarkable inhibition.
Sealer extracts inhibited osteoclast-related gene and protein expression
To evaluate the impact of different sealer extracts on the function of osteoclasts, we assessed the mRNA and protein expression levels of osteoclast-related genes (Nfatc1, c-fos, c-src, Mmp9, Mmp14 and Ctsk) through qPCR and Western Blot analyses (Figure 3). Overall, all 4 sealer extracts exhibited varying degrees of inhibitory effects on osteoclast-specific gene expression. Specifically, C-Root SP and iMTA SP extracts significantly downregulated the mRNA expression of c-Fos, c-Src, Mmp9, and Ctsk. iRoot SP extract demonstrated broader inhibitory effects, significantly suppressing the expression of Nfatc1, c-Fos, c-Src, Mmp9, and Ctsk. Notably, NeoSEALER Flo extract exerted the most extensive inhibition, significantly reducing the expression of all analysed osteoclast-related genes (Nfatc1, c-Fos, c-Src, Mmp9, Mmp14, and Ctsk).
Fig. 3.
Sealer extracts inhibit osteoclast-related gene expression. Relative mRNA expression of Nfatc1, c-fos, c-src, Mmp9, Mmp14 and Ctsk in osteoclasts treated with positive control (RANKL), C-root SP, iMTA SP, iRoot SP and NeoSEALER Flo. n = 3. ns not significant, *p < .05, ⁎⁎p < .01, ⁎⁎⁎p < .001, ⁎⁎⁎⁎p < .0001 versus RANKL group using one-way ANOVA with Dunnett’s post hoc test.
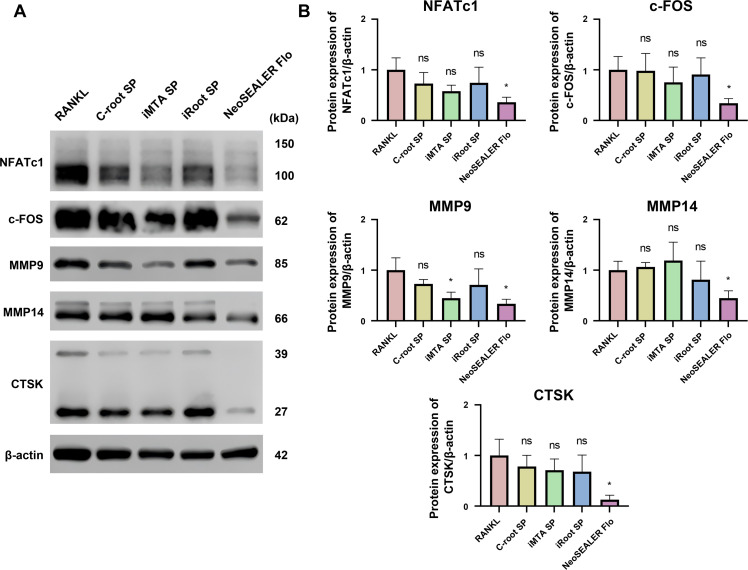
Consistent with the qPCR results, Western blot analysis confirmed that all four sealers suppressed the expression of osteoclast-associated proteins to varying extents (Figure 4A). Among them, the NeoSEALER Flo group exhibited the most potent inhibitory effect, markedly reducing the protein levels of NFATc1, c-FOS, MMP9, MMP14, and CTSK (Figure 4B). Collectively, these findings indicate that all tested sealer extracts impede osteoclast differentiation and function at both the transcriptional and translational levels, with NeoSEALER Flo demonstrating the most pronounced inhibitory effect.
Fig. 4.
Sealer extracts inhibit osteoclast-related protein expression. (A) Western blot of NFATc1, c-FOS, MMP9, MMP14 and CTSK expression in RANKL-induced osteoclasts treated with C-root SP, iMTA SP, iRoot SP and NeoSEALER Flo in BMMs. (B) Western blot results quantification, n = 3. ns not significant, *p < .05 versus RANKL group using one-way ANOVA with Dunnett’s post hoc test.
Discussion
Root canal treatment primarily aims to eliminate bacterial infection, with root canal sealers playing an indispensable role by establishing an apical biological seal to facilitate periapical lesion healing. While the ideal obturation requires complete confinement of the sealer within the root canal system,27 the inherent flow characteristics of injectable sealers may lead to material extrusion beyond the apical foramen or chemical release into periapical tissues during clinical procedures.28 This process inevitably results in sealer components interacting with periapical tissues, directly affecting cellular activity and potentially causing cytotoxic effects and functional impairment.29 In recent years, calcium silicate-based bioceramic materials have gained widespread clinical application.30,31 Our study evaluated the pH values of extracts from 4 different sealers (C-root SP, iMTA SP, iRoot SP and NeoSEALER Flo), their effects on the viability of BMMs and their impact on osteoclast formation and function.
The pH values of dissolved root canal obturation sealers are a critical factor to consider in clinical applications, as an alkaline environment not only exhibits antibacterial properties but also influences the activity and function of periapical cells. In this study, to simulate the condition for in-vitro cell culture, we first measured the pH values of sealer extracts at varying concentrations after dilution with cell culture medium. Overall, all 4 extracts were alkaline, with pH values ranging from 7.00 to 9.00 at low concentrations, which is consistent with previous reports.15 Subtle differences in the alkalinity levels among the 4 sealer extracts were observed, which can be directly attributed to factors such as material composition, solubility, and ion release.32 Therefore, these characteristics of the 4 sealers warrant further investigation.
Extensive evidence confirms that osteoclast precursor cells possess the capacity to undergo osteoclast differentiation in vitro when stimulated by M-CSF and RANKL.33 Therefore, we focus on evaluating the influence of root canal sealer extracts on osteoclast precursor cell viability. The ISO standard establishes cytotoxicity as cell viability below 70%,34 a threshold which none of the tested sealers exceed at concentrations below 20 mg/mL in our study, though cytotoxic effects emerge at 50 mg/mL. Previous studies have consistently demonstrated the superior cytocompatibility of bioceramic sealers relative to conventional epoxy resin-based formulations, an advantageous property potentially mediated through calcium ion release and its associated cytoprotective mechanisms.35 The present results demonstrate preserved cell viability at clinically relevant concentrations for all tested formulations (C-root SP, iMTA SP, iRoot SP, and NeoSEALER Flo), while revealing dose-dependent cytotoxic responses at elevated concentrations (50 mg/mL) that are further exacerbated by prolonged exposure duration. Importantly, the evaluated sealers exhibit comparable cytotoxicity profiles, with their constituent materials demonstrating favorable biosafety characteristics and overall biocompatibility within therapeutic concentration ranges.
Given the critical role of osteoclast-mediated bone resorption in periapical periodontitis, research on the effects of root canal sealers on osteoclast differentiation and function holds significant clinical relevance. The healing process of apical periodontitis involves a critical regulatory mechanism where upregulated osteoclast activity is directly associated with the control of lesion expansion. Osteoclasts are multinucleated cells derived from hematopoietic progenitor cells present in both bone marrow and peripheral circulation, undergoing subsequent fusion and terminal differentiation at bone surfaces.36 Osteoclastogenesis represents a sophisticated biological process governed by intricate cytokine networks and paracrine signalling pathways, with M-CSF and RANKL serving as the principal molecular mediators capable of inducing complete osteoclastogenesis in vitro.36 When osteoclast differentiation is initiated, cytoskeletal remodelling in mature osteoclasts will be facilitated. The osteoclast cytoskeleton exhibits a distinctive architecture, characterised by the formation of a gasket-like structure known as the F-actin ring or sealing zone.37 This specialised structure effectively isolates the bone resorptive microenvironment from the surrounding extracellular space, thus acting as a critical determinant of bone-resorbing capacity of osteoclasts.38 Although previous studies have investigated the effects of certain calcium silicate-based bioceramic sealers on osteoclastogenesis,3 no available study to date has evaluated the 4 specific sealers examined in our research (C-root SP, iMTA SP, iRoot SP, and NeoSEALER Flo). In the present study, we demonstrate that NeoSEALER Flo exerts the most pronounced inhibitory effect on osteoclastogenesis among the tested sealers, suggesting a good clinical application prospect, especially in the case of bone resorption. Overall, the anti-osteoclastic effects of these calcium silicate-based bioceramic sealers hold great promise for the healing of apical periodontitis, as they can effectively prevent the progression of periapical bone destruction.39 At the same time, their well-demonstrated osteogenic and pro-mineralisation properties can promote alveolar bone regeneration and facilitate local bone remodelling. In the long term, such modulation of the osteoclast–osteoblast balance will effectively contribute to the reduction and healing of periapical lesions, increase apical bone density and bone repair, and ultimately enhance the success rate of treatment.40
Previous studies have demonstrated that the binding of M-CSF to its receptor on osteoclasts could activate a signalling complex composed of kinases such as c-SRC, thereby promoting the spreading of osteoclasts and the formation of F-actin rings.38 Meanwhile, RANKL could activate signalling pathways mediated by transcription factors such as NFATc1 and c-FOS, both of which play pivotal roles in osteoclast differentiation.32,41 Enzymes secreted by osteoclasts (such as MMP9, MMP14, CTSK and TRAP) could be activated by these cytokines and signalling pathways, collectively contributing to bone resorption.24,42 In this study, we evaluated the effects of 4 calcium silicate-based bioceramic sealers on osteoclastogenesis and osteoclastic activity by detecting their impacts on the expression of the factors above (NFATc1, c-FOS, c-SRC, MMP9, MMP14, CTSK and TRAP), as well as the formation of F-actin rings. Our findings demonstrate that all 4 tested sealers significantly inhibit osteoclastogenesis and osteoclastic activity to varying degrees, with NeoSEALER Flo exhibiting the most pronounced suppressive effects. This conclusion is supported by quantitative evidence showing substantial reductions in TRAP-positive multinucleated cell formation, impaired F-actin ring assembly, and downregulation of key osteoclast-specific markers at both gene and protein expression levels. The observed variations in inhibitory efficacy among different sealers appear to correlate with their distinct physicochemical characteristics, particularly their silicon ion release profiles during bioactive dissolution processes.3,43 These findings not only confirm the anti-osteoclastic properties of calcium silicate-based bioceramic sealers but also emphasise the necessity for further systematic investigations under simulated periapical microenvironment conditions to better understand their therapeutic mechanisms. However, this study has several limitations. Since we only employed murine BMMs in vitro, further in-vivo studies are required to validate our conclusions. In addition, data obtained from murine cells may not fully correspond to the human clinical situation. Consequently, the long-term biological effects of these sealers require further comprehensive investigation.
Conclusions
In summary, our findings indicate that while the 4 evaluated calcium silicate-based sealers (C-root SP, iMTA SP, iRoot SP, and NeoSEALER Flo) demonstrate alkaline properties, maintain BMM viability at clinically relevant concentrations, and exhibit significant dose- and time-dependent cytotoxic effects at elevated concentrations. All 4 sealers display measurable suppression of osteoclast differentiation and function, with NeoSEALER Flo showing the most marked inhibitory activity. These conclusions offer clinically relevant guidance for evidence-based sealer selection in root canal obturation.
Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.
Author contributions
Zhuo Chen: Data curation; Formal analysis; Methodology; Writing – original draft. Xiaoyue Sun: Data curation; Methodology; Funding acquisition; Writing – review and editing. Zijun Wang: Data curation; Methodology; Writing – review and editing. Zhengrui Chang: Methodology. Lingxin Zhu: Conceptualisation; Funding acquisition; Resources; Supervision; Writing – review and editing. Li Wang: Conceptualisation; Project administration; Resources; Supervision; Writing – review and editing.
Conflict of interest
None disclosed.
Funding
This work was supported by the NSFC 82370914, the Fundamental Research Funds for the Central Universities 2042024YXA010, the International Science and Technology Cooperation Project of Hubei Province 2024EHA062 (Lingxin Zhu), and the NSFC 82201042 (Xiaoyue Sun).
Contributor Information
Lingxin Zhu, Email: lingxin.zhu@whu.edu.cn.
Li Wang, Email: dentist-wang@whu.edu.cn.
REFERENCES
- 1.Galler K.M., Weber M., Korkmaz Y., Widbiller M., Feuerer M. Inflammatory response mechanisms of the dentine-pulp complex and the periapical tissues. Int J Mol Sci. 2021;22(3):1480. doi: 10.3390/ijms22031480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf M., Küpper K., Reimann S., Bourauel C., Frentzen M. 3D analyses of interface voids in root canals filled with different sealer materials in combination with warm gutta-percha technique. Clin Oral Investig. 2014;18(1):155–161. doi: 10.1007/s00784-013-0970-y. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigues C., Costa-Rodrigues J., Capelas J.A., Fernandes M.H. Long-term dose- and time-dependent effects of endodontic sealers in human in vitro osteoclastogenesis. J Endod. 2013;39(6):833–838. doi: 10.1016/j.joen.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Li Y., Ling J., Jiang Q. Inflammasomes in alveolar bone loss. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pereira M., Petretto E., Gordon S., Bassett J.H.D., Williams G.R., Behmoaras J. Common signalling pathways in macrophage and osteoclast multinucleation. J Cell Sci. 2018;131(11) doi: 10.1242/jcs.216267. [DOI] [PubMed] [Google Scholar]
- 6.Vernal R., Dezerega A., Dutzan N., et al. RANKL in human periapical granuloma: possible involvement in periapical bone destruction. Oral Dis. 2006;12(3):283–289. doi: 10.1111/j.1601-0825.2005.01191.x. [DOI] [PubMed] [Google Scholar]
- 7.Belibasakis G.N., Rechenberg D.K., Zehnder M. The receptor activator of NF-κB ligand-osteoprotegerin system in pulpal and periapical disease. Int Endod J. 2013;46(2):99–111. doi: 10.1111/j.1365-2591.2012.02105.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhou G., Zhao Y., Cai L., et al. Cytotoxicity and cell migration evaluation of a strontium silicate-based root canal sealer on stem cells from rat apical papilla: an in vitro study. BMC Oral Health. 2024;24(1):1023. doi: 10.1186/s12903-024-04774-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-García S., Sanz J.L., Murcia L., et al. Assessment of the anti-inflammatory and biological properties of Bioroot Flow: a novel bioceramic sealer. Tissue Cell. 2024;88 doi: 10.1016/j.tice.2024.102391. [DOI] [PubMed] [Google Scholar]
- 10.Yan Y., Li Y., Chi Y., Ji M., Shen Y., Zou L. A comparative study of biological properties of three root canal sealers. Clin Oral Investig. 2023;28(1):11. doi: 10.1007/s00784-023-05402-7. [DOI] [PubMed] [Google Scholar]
- 11.Xue K., Hu G., Wu L., et al. The bioceramic sealer iRoot SP promotes osteogenic differentiation of human stem cells from apical papilla via miR-141-3p/SPAG9/MAPK signalling pathway. Int Endod J. 2023;56(10):1241–1253. doi: 10.1111/iej.13948. [DOI] [PubMed] [Google Scholar]
- 12.Jing Y., Gong T., Duan C., Wang H., Zhang C., Neelakantan P. In vitro cytocompatibility and osteogenic potential of calcium silicate-based dental cements in a root canal-filling model. J Int Med Res. 2020;48(4) doi: 10.1177/0300060519894801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tez BÇ, Eliaçık B.B.K., Taşlı P.N., Yılmaz H., Şahin F. Biocompatibility and cytotoxicity of pulp-capping materials on DPSCs, with marker mRNA expressions. Int Dent J. 2024;74(5):1064–1077. doi: 10.1016/j.identj.2024.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X., Ge X., Fu W., Zhang Z., Xiao K., Lv H. Effects of novel nanoparticulate bioceramic endodontic material on human dental pulp stem cells in vitro. Int Dent J. 2024;74(3):482–491. doi: 10.1016/j.identj.2023.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosatto C.M.P., Souza G.L., Ferraz D.C., Silva M.J.B., Tanomaru Filho M., Moura C.C.G. Physicochemical properties and osteoclastogenesis for three premixed calcium silicate-based sealers post set. Braz Oral Res. 2022;36:e065. doi: 10.1590/1807-3107bor-2022.vol36.0065. [DOI] [PubMed] [Google Scholar]
- 16.Hung C.J., Kao C.T., Chen Y.J., Shie M.Y., Huang TH. Antiosteoclastogenic activity of silicate-based materials antagonizing receptor activator for nuclear factor kappaB ligand-induced osteoclast differentiation of murine marcophages. J Endod. 2013;39(12):1557–1561. doi: 10.1016/j.joen.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J., Zhu L., Yan P., Peng B. Effect of BioAggregate on receptor activator of nuclear factor-kappa B ligand-induced osteoclastogenesis from murine macrophage cell line in vitro. J Endod. 2015;41(8):1265–1271. doi: 10.1016/j.joen.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Tian J., Qi W., Zhang Y., et al. Bioaggregate inhibits osteoclast differentiation, fusion, and bone resorption in vitro. J Endod. 2015;41(9):1500–1506. doi: 10.1016/j.joen.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Silva R.A.B., Borges A.T.N., Hernandéz-Gatón P., et al. Histopathological, histoenzymological, immunohistochemical and immunofluorescence analysis of tissue response to sealing materials after furcation perforation. Int Endod J. 2019;52(10):1489–1500. doi: 10.1111/iej.13145. [DOI] [PubMed] [Google Scholar]
- 20.Donnermeyer D., Bürklein S., Dammaschke T., Schäfer E. Endodontic sealers based on calcium silicates: a systematic review. Odontology. 2019;107(4):421–436. doi: 10.1007/s10266-018-0400-3. [DOI] [PubMed] [Google Scholar]
- 21.Jafari F., Jafari S. Composition and physicochemical properties of calcium silicate based sealers: a review article. J Clin Exp Dent. 2017;9(10):e1249–e1255. doi: 10.4317/jced.54103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lepure C., Walsh R.M., Attar S., Turner C.L., Crawford J., Jalali P. Clinical outcomes of nonsurgical root canal treatment using NeoSealer Flo and Endosequence BC sealer: a retrospective analysis with short-term follow-up. Clin Oral Investig. 2024;28(11):598. doi: 10.1007/s00784-024-05995-7. [DOI] [PubMed] [Google Scholar]
- 23.Zamparini F., Prati C., Taddei P., Spinelli A., Di Foggia M., Gandolfi MG. Chemical-physical properties and bioactivity of new premixed calcium silicate-bioceramic root canal sealers. Int J Mol Sci. 2022;23(22) doi: 10.3390/ijms232213914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L., Tang Y., Li X.Y., et al. Osteoclast-mediated bone resorption is controlled by a compensatory network of secreted and membrane-tethered metalloproteinases. Sci Transl Med. 2020;12(529):eaaw6143. doi: 10.1126/scitranslmed.aaw6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu X., Yan M., Lu J., et al. iRoot SP promotes osteo/odontogenesis of bone marrow mesenchymal stem cells via activation of NF-κB and MAPK signaling pathways. Stem Cells Int. 2020;2020 doi: 10.1155/2020/6673467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Z.H., Wu J.J., Guo D.Y., et al. Physiological functions of podosomes: From structure and function to therapy implications in osteoclast biology of bone resorption. Ageing Res Rev. 2023;85 doi: 10.1016/j.arr.2023.101842. [DOI] [PubMed] [Google Scholar]
- 27.Baraba A., Zelježić D., Kopjar N., Mladinić M., Anić I., Miletić I. Evaluation of cytotoxic and genotoxic effects of two resin-based root-canal sealers and their components on human leucocytes in vitro. Int Endod J. 2011;44(7):652–661. doi: 10.1111/j.1365-2591.2011.01869.x. [DOI] [PubMed] [Google Scholar]
- 28.Alsubait S.A., Al Ajlan R., Mitwalli H., et al. Cytotoxicity of different concentrations of three root canal sealers on human mesenchymal stem cells. Biomolecules. 2018;8(3):68. doi: 10.3390/biom8030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hauman C.H., Love RM. Biocompatibility of dental materials used in contemporary endodontic therapy: a review. Part 2. Root-canal-filling materials. Int Endod J. 2003;36(3):147–160. doi: 10.1046/j.1365-2591.2003.00637.x. [DOI] [PubMed] [Google Scholar]
- 30.Estivalet M.S., de Araújo L.P., Immich F., et al. Bioactivity potential of bioceramic-based root canal sealers: a scoping review. Life (Basel) 2022;12(11):1853. doi: 10.3390/life12111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura T., Chen L., Tsumano N., et al. Biocompatibility of a high-plasticity, calcium silicate-based, ready-to-use material. Materials (Basel) 2020;13(21):4770. doi: 10.3390/ma13214770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva Almeida L.H., Moraes R.R., Morgental R.D., Pappen F.G. Are premixed calcium silicate-based endodontic sealers comparable to conventional materials? A systematic review of in vitro studies. J Endod. 2017;43(4):527–535. doi: 10.1016/j.joen.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 34.Souza L.C., Neves G.S.T., Kirkpatrick T., Letra A., Silva R. Physicochemical and biological properties of AH plus bioceramic. J Endod. 2023;49(1):69–76. doi: 10.1016/j.joen.2022.10.009. [DOI] [PubMed] [Google Scholar]
- 35.Lee B.N., Hong J.U., Kim S.M., et al. Anti-inflammatory and osteogenic effects of calcium silicate-based root canal sealers. J Endod. 2019;45(1):73–78. doi: 10.1016/j.joen.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Park-Min KH. Mechanisms involved in normal and pathological osteoclastogenesis. Cell Mol Life Sci. 2018;75(14):2519–2528. doi: 10.1007/s00018-018-2817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee BS. Myosins in osteoclast formation and function. Biomolecules. 2018;8(4):157. doi: 10.3390/biom8040157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teitelbaum SL. The osteoclast and its unique cytoskeleton. Ann N Y Acad Sci. 2011;1240:14–17. doi: 10.1111/j.1749-6632.2011.06283.x. [DOI] [PubMed] [Google Scholar]
- 39.Ricucci D., Siqueira J.F., Jr, Loghin S., Lin L.M. Repair of extensive apical root resorption associated with apical periodontitis: radiographic and histologic observations after 25 years. J Endod. 2014;40(8):1268–1274. doi: 10.1016/j.joen.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Li Y., Ling J., Jiang Q. Inflammasomes in alveolar bone loss. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.691013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takayanagi H., Kim S., Koga T., et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 42.Boyle W.J., Simonet W.S., Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 43.Ramaswamy Y., Wu C., Van Hummel A., Combes V., Grau G., Zreiqat H. The responses of osteoblasts, osteoclasts and endothelial cells to zirconium modified calcium-silicate-based ceramic. Biomaterials. 2008;29(33):4392–4402. doi: 10.1016/j.biomaterials.2008.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting this study's findings are available from the corresponding author upon reasonable request.