Abstract
Ulcerative colitis (UC) involves immune dysregulation, barrier dysfunction, and dysbiosis, driving chronic inflammation and pain. Current treatments show limited efficacy and high toxicity. This study develops an engineered nanozyme, CO&MnOx@Hs (CMHs), synthesized via in-situ manganese oxide (MnOx) generation on halloysite nanotubes (Hs) with controlled encapsulation of the carbon monoxide (CO)-releasing molecule CORM-401 for gas therapy. CMHs selectively accumulate at inflamed UC sites, where MnOx scavenges reactive oxygen species (ROS) while CO exerts anti-inflammatory effects. These combined actions restore immune homeostasis, repair intestinal barrier, modulate gut microbiota, and alleviate inflammation-associated pain. In murine ulcerative colitis, CMHs outperform conventional treatments, demonstrating superior therapeutic efficacy. Mechanistic studies reveal that CMHs activate Nrf2/HO-1 for antioxidant effects while modulating PI3K-Akt and HIF-1α/LDHA pathways to promote M2 macrophage polarization and suppress NF-κB/TNF signaling. CMHs also fortify the intestinal barrier by mitigating bacterial invasion and ROS-induced damage while activating focal adhesion and ECM-receptor interaction. 16S ribosomal RNA sequencing further confirms CMHs' ability to remodel gut microbiota, reinforcing their immunomodulatory potential. Importantly, CMHs relieve chronic pain by reducing inflammation, inhibiting SP secretion and TACR1 expression, and suppressing TRPV1 channel activation and Ca2+ influx. These findings establish CMHs as a multi-targeted and highly effective therapeutic strategy for UC.
Keywords: Ulcerative colitis, Manganese oxide nanozyme, CO therapy, Gut mucosal immune homeostasis, Gut microbiota, Chronic pain, Intestinal barrier function
Graphical abstract
Highlights
-
•
An engineered nanozyme was fabricated by generating MnOx on halloysite nanotubes (Hs) and loading CORM-401 into their lumen.
-
•
CMHs selectively accumulate at inflamed UC sites for targeted therapy.
-
•
CMHs restore immune balance, repair intestinal barrier, and modulate microbiota.
-
•
CMHs alleviate chronic pain via inhibition of the SP/TACR1/TRPV1 pathway.
-
•
CMHs show superior efficacy to conventional therapies in murine colitis with low toxicity.
1. Introduction
Inflammatory bowel disease (IBD) is a chronic inflammatory condition of the gastrointestinal (GI) tract, comprising Crohn's disease and UC [1,2]. Patients often require lifelong management to prevent serious complications such as intestinal strictures, perforation, and colorectal cancer. The global prevalence of IBD continues to rise, with estimates projecting an increase from 660 to 790 cases per 100,000 people between 2015 and 2025, presenting growing challenges to healthcare systems worldwide [3]. Conventional treatments include aminosalicylates and corticosteroids, often supplemented with immunosuppressants such as azathioprine. More recently, biologic agents (e.g., anti-TNF antibodies) and targeted small molecules (e.g., JAK inhibitors) have become cornerstone therapies for maintaining long-term remission and mitigating disease progression in IBD [4,5]. However, these treatments are not without drawbacks: long-term use of 5-ASA and steroids is associated with hepatotoxicity and immunosuppression, while immunosuppressants increase susceptibility to infections and renal impairment [6]. Even biologics and targeted agents, despite their efficacy, may induce immune suppression, autoimmune reactions, or hepatic injury [7]. Consequently, there is an urgent need for novel therapies with superior efficacy and minimal safety risks.
The pathogenesis of UC remains incompletely understood, but oxidative stress and chronic inflammation are recognized as major contributors to its development and progression [8]. In UC, overproduction of ROS by immune cells—particularly neutrophils and macrophages—plays a central role [9]. Although ROS are essential in immune defense, their excessive accumulation leads to tissue damage [10]. When ROS levels overwhelm endogenous antioxidant capacity, oxidative stress occurs, causing damage to lipids, proteins, and DNA [11,12]. This impairs the intestinal barrier, increasing its permeability and allowing infiltration of pathogens and toxins that further amplify inflammation [13,14]. Simultaneously, ROS activate pro-inflammatory pathways such as NF-κB, upregulating the expression of cytokines and chemokines [15]. These mediators stimulate immune cells to produce additional ROS, thereby intensifying inflammation and tissue injury in the gut [16]. Oxidative stress and inflammation also disrupt the gut microbiota, promoting dysbiosis that exacerbates immune dysfunction [17]. Moreover, both ROS and inflammatory cytokines contribute to pain through neuronal damage and sensitization of nociceptive pathways [18,19]. This vicious feedback loop between oxidative stress and inflammation underscores the need for treatment strategies capable of simultaneously scavenging ROS and exerting potent anti-inflammatory effects. Such integrated therapies could restore mucosal immune homeostasis, rebalance the microbiome, strengthen barrier function, and reduce UC-related pain.
CO is an endogenous signaling molecule with notable therapeutic potential, owing to its cytoprotective, immunoregulatory, antioxidant, and anti-inflammatory properties [[20], [21], [22]]. Reportedly, CO level within the intestinal lumen of patients with UC was twofold higher than that of healthy volunteers [23], underscoring its critical role in the body's natural defense response. CO activates the Nrf2 (Nuclear factor erythroid 2-related factor 2) pathway, enhancing the expression of antioxidant enzymes and boosting the body's defense against oxidative stress [24]. Beyond its antioxidant effects, CO suppresses several pro-inflammatory pathways while activating anti-inflammatory ones [25]. Notably, CO can induce macrophage polarization from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype, offering additional benefits in chronic inflammation. Furthermore, CO aids in remodeling the gut microbiota by reducing oxidative stress and inflammation, promoting the growth of beneficial bacteria, particularly those producing short-chain fatty acids [26]. CO also provides pain relief by mitigating nerve damage induced by oxidative stress, inhibiting pro-inflammatory factors, enhancing the release of inhibitory neurotransmitters, and regulating key ion channels, all of which contribute to comprehensive pain management [27,28]. Given its broad therapeutic potential, CO therapy holds great therapeutic potential for UC. However, its gaseous nature makes controlled delivery highly challenging. Besides, its ROS scavenging effect relies primarily on the upregulation of antioxidant enzymes rather than direct neutralization; thus, its antioxidant activity remains limited.
Since the 2007 discovery of peroxidase-like activity in Fe3O4 nanoparticles, nanozyme research has progressed rapidly, especially in antioxidant therapy [[29], [30], [31]]. Numerous nanozymes—such as Prussian blue, MnFe2O4, CeO2, and IrO2 nanoparticles—have shown potent antioxidant capabilities [[32], [33], [34], [35]]. MnOx nanozymes, in particular, have garnered interest due to their dual superoxide dismutase (SOD) and catalase (CAT)-like activities [36], which are especially effective in the acidic microenvironment of UC lesions where MnOx exhibit enhanced ROS scavenging [37,38]. Despite their promise in alleviating oxidative stress, the anti-inflammatory performance of MnOx remains limited. Achieving their full therapeutic potential requires integrating antioxidant activity of MnOx with robust anti-inflammatory effects. Moreover, targeted delivery of MnOx to inflamed GI regions remains challenging; non-specific distribution may reduce efficacy and increase off-target risks. Thus, developing innovative strategies that enhance both the anti-inflammatory function and lesion-targeting specificity of MnOx is critical for advancing UC treatment. One promising approach combines CO gas therapy with MnOx, leveraging their complementary mechanisms to synergistically enhance antioxidant, anti-inflammatory, microbiota-modulating, and analgesic effects—offering a more comprehensive and effective treatment paradigm.
A key component of this innovative treatment strategy is the use of CORM-401, a unique CORMs [39]. CORM-401 remains stable under normal physiological conditions but rapidly releases CO in response to elevated ROS in pathological environments [40,41]. This ROS-responsive release makes CORM-401 ideal for UC treatment, as CO levels correspond to lesion severity. This enables precise, on-demand CO release, significantly enhancing therapeutic efficacy while minimizing off-target effects. To maximize therapeutic outcomes, a specialized delivery system is crucial to ensure the targeted delivery of both CORM-401 and MnOx to the inflamed colonic mucosa. Halloysite nanotubes (Hs), naturally occurring aluminosilicate clay minerals, offer an ideal carrier for this purpose [42]. With excellent biocompatibility, stability, intrinsic hemostatic, anti-diarrheal properties, Hs are well-suited for GI applications [43]. Their hollow tubular structure allows for the encapsulation of therapeutic agents, ensuring controlled and sustained release [44]. Additionally, the surface of Hs is rich in hydroxyl and alumina groups, facilitating electrostatic interactions that promote preferential accumulation within the positively charged, inflamed colonic tissues [45]. This targeted accumulation improves drug retention at the site of inflammation, enhancing the precision of drug delivery and boosting overall therapeutic efficacy in treating UC.
In this study, Hs served as a vehicle for the in-situ generation of MnOx on their surface and the controlled loading of CORM-401 within their tubular structure, resulting in an engineered nanozyme termed CMHs, which integrates the potent antioxidant capacity of MnOx with the strong anti-inflammatory action of CO. As illustrated in Scheme 1, following rectal administration, CMHs accumulate selectively in inflamed colonic regions. Here, MnOx scavenges ROS, while CO released from CORM-401 enhances antioxidant performance and delivers potent anti-inflammatory effects. These advanced features enable CMHs to restore mucosal immune homeostasis, repair the intestinal barrier, modulate gut microbiota, and reduce inflammation-associated pain. Both in vitro and in vivo studies confirmed the antioxidant and anti-inflammatory efficacy of CMHs, along with their ability to facilitate barrier repair and alleviate pain in a dextran sulfate sodium (DSS)-induced UC model. RNA sequencing indicated that CMHs activate the Nrf2/HO-1 pathway to exert antioxidant effects and modulate the PI3K-Akt, HIF-1α/LDHA, NF-κB, and TNF pathways to suppress inflammation and promote M2 macrophage polarization. Furthermore, CMHs strengthen the epithelial barrier by inhibiting bacterial invasion and ROS, while activating focal adhesion, ECM-receptor interaction, and PPAR signaling. They also alleviate pain through modulation of inflammatory mediators, suppression of SP secretion and TACR1 expression, and inhibition of TRPV1 activation and Ca2+ influx. 16S rRNA sequencing further demonstrated that CMHs positively reshape the gut microbiota, supporting therapeutic recovery. Overall, CMHs represent an innovative, effective, and safe nanotherapeutic strategy with strong potential for clinical application in UC.
Scheme 1.
Schematic illustration of the preparation of CMHs and their therapeutic mechanisms for remodeling gut immune homeostasis, restoring gut microbiota balance, repairing the intestinal barrier, and alleviating chronic inflammation-related pain. (1) Exhibits antioxidant activity by scavenging ROS through multi-enzyme activities such as CAT and SOD, and by activating the Nrf2/HO-1 pathway. It also demonstrates anti-inflammatory effects by activating the PI3K/AKT pathway and inhibiting key pathways, including p65 NF-κB and HIF-1-induced glycolysis. (2) Regulates gut microbiota by eliminating pathogenic bacteria and enhancing the richness and diversity of the gut microbiome. (3) Repairs the intestinal mucosal barrier by protecting IECs from ROS-induced oxidative apoptosis and bacterial invasion, and by activating focal adhesion, ECM-receptor interaction, and PPAR signaling pathways. (4) Alleviates pain via modulating inflammatory responses, inhibiting SP secretion and TACR1 expression, and suppressing TRPV1 channel activation and Ca2+ influx.
2. Results and discussion
2.1. Synthesis and characterization of CMHs
Effective UC treatments must simultaneously address oxidative stress, inflammation, and immune dysregulation. To meet this challenge, we synthesized CMHs, an engineered nanozyme constructed for ROS scavenging and controlled CO release at inflamed sites. As illustrated in Fig. 1A, CMHs were synthesized using Hs as a carrier. The synthesis process began with the preparation of amino-functionalized Hs (NHs). This was achieved by reacting the Si-O- groups of 3-aminopropyltriethylsilane (APTES) with the -SiOH groups on the surface of Hs, resulting in the formation of stable Si-O-Si bonds. Subsequently, KMnO4 was adsorbed onto NHs and reduced to MnOx under light, yielding MnOx@Hs (MHs). Finally, CORM-401 was encapsulated within the tubular structure of Hs to produce CMHs. Hs appeared as a distinct milky white suspension (Fig. 1B) with an average particle size of 541.9 nm and a polydispersity index (PDI) of 0.22. Amino modification was confirmed by changes in zeta potential and Fourier-transform infrared spectroscopy (FTIR). Unmodified Hs had a ζ potential of −42.6 mV, which shifted to +31.6 mV post-functionalization, indicating successful amino group introduction (Fig. 1E). FTIR spectra revealed new peaks at 1550 cm−1, 2930 cm−1, and 3459 cm−1, corresponding to C–N, N–H and C–H bending and stretching vibrations of APTES, further validating the modification (Fig. S1, Supporting Information) [46]. After MnOx deposition, the Hs solution turned brown, and the particle size increased to 680.6 nm (PDI = 0.26, Fig. 1C). The ζ potential decreased from +31.6 mV to −48.2 mV, confirming MnOx's successful in-situ growth. This shift from positive to negative charge suggests the replacement of amino groups with MnOx species, which introduce negatively charged oxygen-containing functional groups and stabilize the nanozyme in aqueous suspension. FTIR spectra also clearly confirmed the successful deposition of MnOx on NHs surface, as evidenced by the significant attenuation of the N-H bending vibration peak at 1550 cm−1 and the N-H stretching vibration peak at 3459 cm−1 of the amino group (Fig. S1, Supporting Information). To optimize MnOx content, different NHs:KMnO4 mass ratios (1:0.04, 1:0.08, 1:0.12) were tested. ICP-OES analysis revealed MnOx contents of 1.12 %, 2.41 %, and 6.48 %, respectively, with increasing KMnO4 amounts. Higher MnOx content enhanced antioxidant activity and also increased cytotoxicity.
Fig. 1.
Physicochemical characterization of CMHs. A) Flowchart depicting the synthesis process of CMHs. B-D) Particle size distribution and solution color images of Hs, MHs and CMHs. E) Zeta potential of Hs, NHs, MHs and CMHs. F-G) TEM images and elemental mapping of CMHs. H-I) Mn 2P XPS spectra of MHs and CMHs. J-K) ROS scavenging activities of O2·- and H2O2 by MHs and CMHs. L) CO release profiles of CMHs in SCF containing different concentrations of H2O2.
We next evaluated the cytotoxicity and antioxidant performance of MHs with different MnOx contents. As shown in Fig. S2 (Supporting Information), the viability of Caco-2 and RAW264.7 cells decreased progressively with increasing MHs concentration and MnOx content. Specifically, when the MHs concentration exceeded 100 μg/mL, cell viability dropped below 80 %. This is likely due to excessive ROS modulation, which disrupts redox balance and induces oxidative stress-related cytotoxicity. Therefore, a concentration of 100 μg/mL was selected for further evaluation of the impact of MnOx content on antioxidant performance. As shown in Fig. S3 (Supporting Information), the scavenging ability of MHs for hydrogen peroxide (H2O2) and superoxide anion (O2·-) increased with higher MnOx content. Higher MnOx content provides more active Mn3+/Mn4+ redox sites, thereby improving catalytic efficiency. MHs with MnOx contents of 1.12 % and 2.41 % exhibited relatively poor ROS scavenging efficiency compared to MHs with 6.48 % MnOx. Due to their superior ROS scavenging performance, MHs with 6.48 % MnOx were chosen as carriers for CORM-401 encapsulation at different weight ratios of CORM-401 to MHs (ranging from 0.025:1 to 0.1:1). After loading, the color of the MHs solution changed from brown to brown-green, and the particle size slightly decreased to 668 nm with a PDI of 0.21 (Fig. 1D). This color change confirmed the successful formation of CMHs. The UV–vis spectrum of CMHs showed a characteristic absorption peak at 370 nm (Fig. S4, Supporting Information), further confirming successful loading of CORM-401. ICP-OES analysis indicated that the loading content and loading efficiency of CORM-401 reached their optimal (3.09 % and 61.8 %, respectively) at a weight ratio of 0.05:1 (Table S1, Supporting Information). CMHs had a ζ potential of −42.0 mV, showing no significant change in the surface charge of the nanotubes (Fig. 1E). This property is beneficial for targeting and accumulation on the positively charged inflamed colon mucosal surface. Fig. 1F shows the surface morphology of Hs, MHs, and CMHs. Hs exhibits a smooth hollow tubular structure, while the surface of MHs becomes rough due to the uneven distribution of MnOx, resulting in a more complex surface topography. Encapsulation CORM-401 had minimal impact on the surface morphology of the nanotubes. Energy-dispersive spectroscopy (EDS) (Fig. S5, Supporting Information) and transmission electron microscopy (TEM) mapping confirmed the successful generation of MnOx on the Hs surface and the encapsulation of CORM-401 within the nanotubes. The Mn atoms were uniformly distributed across the CMHs’ surface, while the S atoms were predominantly located inside the nanotubes (Fig. 1G). XPS analysis revealed that MnOx grown on the Hs surface exists primarily in two oxidation states: Mn3+ and Mn4+, with a ratio of 27.14 % and 72.86 %, respectively (Fig. 1H). This distribution of oxidation states endows MnOx with strong oxidizing capabilities, significantly boosting its antioxidant activity. Further analysis of CMHs showed that the physical loading of CORM-401 did not significantly alter the oxidation states of the Mn elements (Fig. 1I).
CMHs exhibit excellent storage stability in PBS at 4 °C, with no significant variations in particle size and zeta potential observed over a 28-day incubation period (Fig. S6, Supporting Information). They also demonstrate high stability in simulated colonic fluid (SCF), maintaining consistent particle size, and surface potential throughout 12 h of incubation at 37 °C (Fig. S7, Supporting Information). These results indicate that CMHs can maintain outstanding stability prior to reaching the colonic inflammatory site following rectal administration. The in vitro antioxidant performance of MHs and CMHs was evaluated using O2·- and H2O2 scavenging assays, as shown in Fig. 1J and K. The results demonstrated that as the concentration of MHs and CMHs increased from 50 ppm to 100 ppm, their scavenging efficiencies for O2·- and H2O2 improved significantly, exhibiting a concentration-dependent relationship. At 100 ppm, CMHs showed superior antioxidant performance compared to MHs, with scavenging rates for O2·- and H2O2 increasing to 41.7 % and 57.3 %, respectively, compared to 30.8 % and 40.1 % for MHs. This enhancement is likely attributable to the ROS-responsive release of CO by CORM-401, which synergistically eliminates additional ROS and further improves the overall antioxidant capacity. CMHs also exhibit remarkable scavenging capacity against ·OH, ABTS·+, and DPPH·, achieving scavenging rates of up to 58.5 %, 55.8 % and 41.9 %, respectively, at a concentration of 100 ppm (Fig. S8, Supporting Information). Notably, owing to the exceptional CAT- and SOD-like activities of MnOx, CMHs undergo progressive degradation in SCF containing 0.5 mM H2O2, mainly resulting from the detachment of MnOx from the Hs surface. This behavior was confirmed by a significant decrease in particle size and a concurrent increase in zeta potential observed for both CMHs and MHs after 8 h of incubation in SCF supplemented with 0.5 mM H2O2, whereas Hs maintained consistent particle size and zeta potential under the same incubation conditions (Fig. S9, Supporting Information).
Apart from confirming the excellent ROS scavenging ability of CMHs, we further monitored its real-time CO release in SCF supplemented with different concentrations of H2O2 (0, 0.1, 0.5, and 1 mM) using a CO detector. Under 0 mM H2O2 conditions, minimal CO was released over 1 h. However, with 0.1, 0.5, and 1 mM H2O2, the cumulative CO release after 1 h reached 1.30, 1.93, and 3.3 μmol, respectively (Fig. 1L). These results confirm that CORM-401 encapsulated in CMHs exhibits H2O2-responsive CO release behavior. The underlying mechanism involves a dynamic equilibrium under H2O2-free conditions, where CORM-401 maintains stability through reversible CO dissociation and rebinding to the Mn center. This labile coordination state permits competitive binding of H2O2, which subsequently oxidizes adjacent Mn centers. The oxidation process disrupts CO recapture and initiates electron transfer from Mn to the H2O2 ligand framework. Consequently, the weakening of Mn-CO backbonding facilitates complete liberation of residual CO [47]. The detailed CO release mechanism from CORM-401 is illustrated in Fig. S10 (Supporting Information).
2.2. Antioxidant and anti-inflammatory effects and mechanisms of CMHs
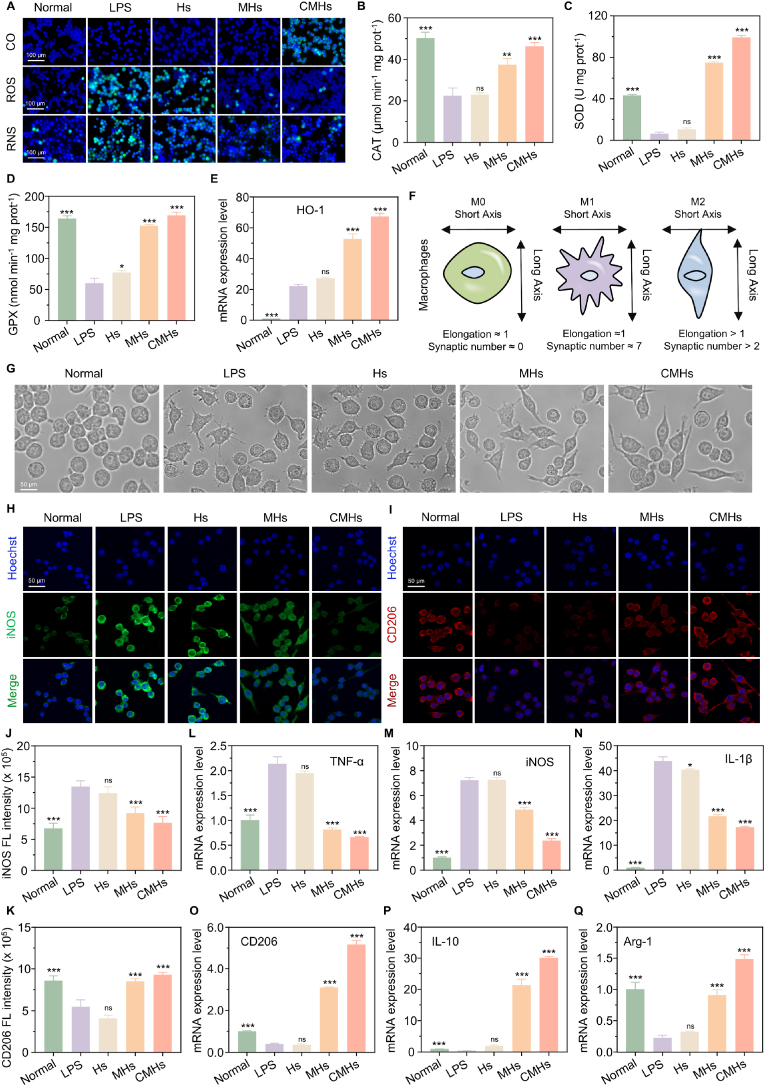
Before evaluating the antioxidant and anti-inflammatory activities of CMHs, we first assessed their biocompatibility. As shown in Fig. S11A–B (Supporting Information), after treatment with varying concentrations of CMHs for 24 h, the viability of RAW264.7 and Caco-2 cells remained above 80 % when the CMHs concentration was below 100 μg/mL. Live/dead staining confirmed minimal cell death at 100 μg/mL (CMHs-2 group), with only a small number of PI-positive cells (Fig. S11C, Supporting Information). Additionally, even at a concentration of up to 200 μg/mL (CMHs-3 group), the hemolysis rate remained below 1 % (Fig. S12, Supporting Information). This is well below the 5 % hemolytic activity threshold, confirming the excellent biocompatibility of CMHs. Next, we assessed CO generation in LPS-induced activated macrophages treated with CMHs using a CO probe system (PdCl2 + FL-CO-1). To evaluate the ability of CMHs to scavenge intracellular reactive species, we employed fluorescence probes such as DCFH-DA, ROS Green H2O2, and DAF-FM DA to detect ROS, H2O2, and reactive nitrogen species. As shown in Fig. 2A, CO generation was minimal in both normal macrophages and LPS-induced activated macrophages treated with PBS, Hs, and MHs. In contrast, CMHs-treated activated macrophages exhibited significant CO generation, indicating that CORM-401 encapsulated in CMHs can efficiently and rapidly release CO intracellularly. Additionally, PBS-treated activated macrophages exhibited strong fluorescence signals for ROS, RNS, and H2O2, confirming the substantial generation of these reactive species. Treatment with MHs, and particularly with CMHs, significantly reduced intracellular ROS, RNS, and H2O2 levels, as evidenced by a marked decrease in fluorescence signals (Fig. S13, Supporting Information). These results suggest that MnOx in MHs effectively scavenges ROS and RNS. Furthermore, CO release from CMHs depletes excess ROS and H2O2, amplifying their scavenging effects. The antioxidant activity of MHs and CMHs is mainly driven by the substantial upregulation of key antioxidant enzymes in macrophages, including CAT, SOD, glutathione peroxidase (GPX), and heme oxygenase-1 (HO-1). As shown in Fig. 2B–E, enzyme activity was significantly higher in MHs and CMHs treated macrophages than in PBS or Hs treated controls. Notably, CMHs treatment resulted in a 14.7-fold, 2.27-fold, 2.82-fold, and 3.03-fold increase in the activity of SOD, CAT, GPX, and HO-1, respectively.
Fig. 2.
In vitro antioxidant and anti-inflammatory activities of CMHs. A) Intracellular imaging showing CO release, ROS, and RNS scavenging in the CMHs group. B-D) Intracellular enzymatic activity levels of CAT, SOD, and GPX in the CMHs group. E) HO-1 mRNA expression in the CMHs group. F) Schematic illustration of a resting macrophage and an activated macrophage. G) Morphological changes of activated macrophages treated with CMHs. H-I) iNOS-positive and CD206-positive cells in the CMHs group. J-K) Corresponding intracellular fluorescence intensity of iNOS and CD206 in the CMHs group. L-Q) mRNA expression levels of TNF-α, iNOS, IL-1β, CD206, IL-10, and Arg-1 in the CMHs group. Statistical analyses were performed by comparing the LPS group with other groups (n = 3), with ∗p < 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, and ns indicating no significant difference.
Building on the confirmed antioxidant activity of CMHs, we next evaluated their anti-inflammatory effects. As shown in Fig. 2F and G, activated macrophages treated with pure culture medium exhibited the typical "sunflower" morphology, characterized by a multipolar shape with numerous radiating pseudopodia extending from the cell surface—hallmarks of the pro-inflammatory M1 macrophage phenotype [48]. In contrast, following treatment with CMHs, macrophages exhibited a more elongated morphology, indicative of M2 macrophage polarization. Quantitative analysis revealed that the maximum elongation rate of macrophages in the CMHs groups was 4.87, compared to just 1.07 in the LPS group (Fig. S14A, Supporting Information). Additionally, the number of protrusions in CMHs-treated cells was 1.67, which was 4.5 times lower than that in the LPS group (Fig. S14B, Supporting Information), further supporting the conclusion that CMHs promote M2 macrophage polarization. Immunofluorescence (IF) staining provided further confirmation of macrophage polarization (Fig. 2H and I). PBS-treated macrophages (LPS group) showed strong fluorescence after iNOS staining, indicating the dominance of the pro-inflammatory M1 phenotype. In contrast, CD206 staining exhibited relatively weak fluorescence, suggesting minimal presence of the anti-inflammatory M2 phenotype. On the other hand, CMHs-treated macrophages displayed significantly reduced fluorescence for iNOS and enhanced fluorescence for CD206, suggesting a shift towards the anti-inflammatory M2 phenotype. Quantitative analysis of fluorescence intensities for iNOS and CD206 in CMHs-treated macrophages is shown in Fig. 2J and K, respectively. RT-qPCR analysis further validated the anti-inflammatory activity of CMHs. As shown in Fig. 2L–N, the mRNA expression levels of the pro-inflammatory cytokines TNF-α, iNOS, and IL-1 in PBS-treated activated macrophages (LPS group) were 2.13, 7.22, and 43.80 times higher, respectively, compared to normal macrophages. However, following CMHs treatment, the expression levels of these cytokines decreased to 0.66, 2.37, and 17.33 times relative to normal macrophages. In addition to suppressing the expression of pro-inflammatory cytokines, CMHs treatment also significantly increased the expression of anti-inflammatory cytokines in activated macrophages. As shown in Fig. 2O–Q, the mRNA expression levels of CD206, IL-10, and Arg-1 in the CMHs-treated group were 5.17, 30.12, and 1.49 times higher, respectively, compared to the LPS group. These results highlight the strong anti-inflammatory potential of CMHs. Not only do they promote the polarization of M1 macrophages toward the M2 phenotype, but they also exert anti-inflammatory effects by inhibiting pro-inflammatory cytokines and promoting anti-inflammatory cytokines. Notably, CMHs exhibited significantly superior antioxidant and anti-inflammatory activities compared to MHs across all tested indicators. This suggests that the incorporation of CORM-401 significantly enhances the antioxidant and anti-inflammatory effects of MnOx positioning CMHs as a promising therapeutic agent for modulating inflammation and oxidative stress in diseases like UC.
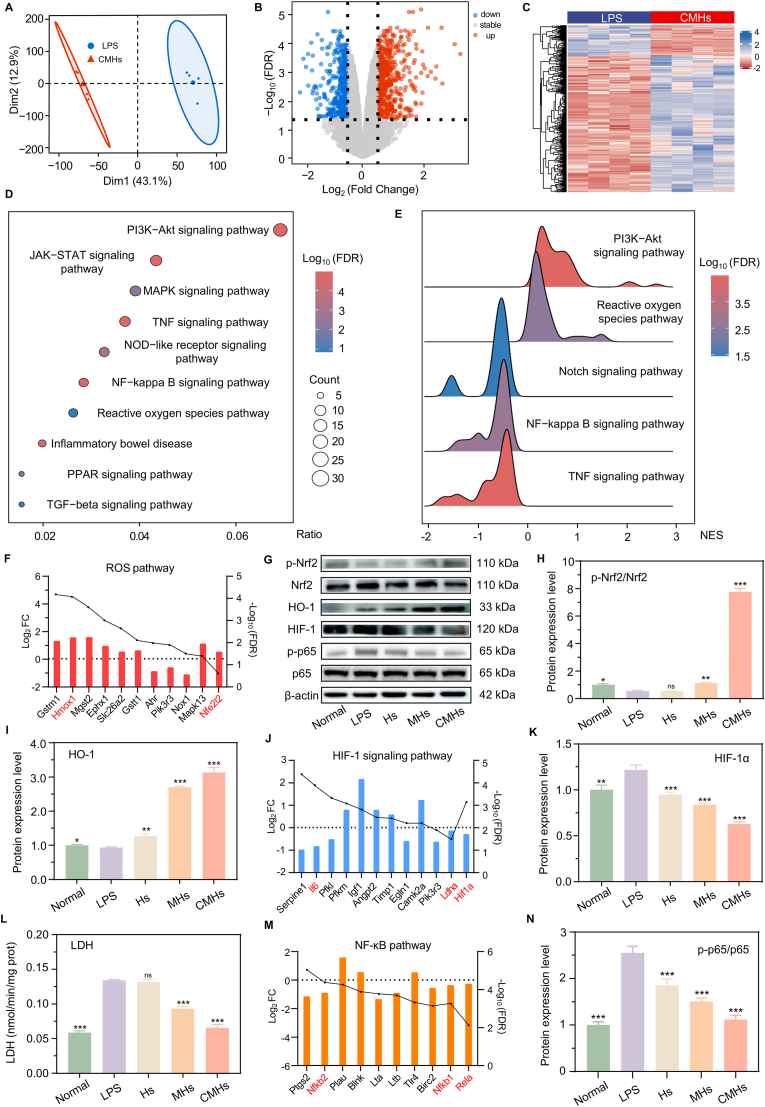
To further investigate the superior antioxidant and anti-inflammatory properties of CMHs, RNA-seq was performed on CMHs-treated activated macrophages. Principal component analysis (PCA) (Fig. 3A) revealed distinct transcriptome profiles between the CMHs-treated and LPS-treated groups, demonstrating good sample separation and suggesting substantial differences between the two treatment conditions. A total of 901 differentially expressed genes (DEGs) were identified (screening criteria: |Log2 FC| ≥ 0.5, FDR <0.05), with 521 DEGs upregulated and 380 DEGs downregulated (Fig. 3B-C). Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis highlighted that the DEGs were predominantly associated with inflammation and oxidative stress pathways, including the PI3K-Akt, TNF, HIF-1, NF-κB, and ROS pathways (Fig. 3D). Gene Set Enrichment Analysis (GSEA) further supported those findings, revealing that genes in the PI3K-Akt and ROS pathways were generally upregulated (NES >0), whereas genes in the Notch, NF-κB, and TNF pathways were downregulated (NES <0, Fig. 3E). The ROS pathway plays a critical role in regulating macrophage activation, polarization, and immune responses [49]. A key regulator of this pathway is Nrf2, which modulates the cellular response to oxidative stress. Upon activation, Nrf2 translocates to the nucleus and binds to the antioxidant response element (ARE), initiating the transcription of various antioxidant genes [24]. One of the most important targets of Nrf2 is HO-1, an essential antioxidant enzyme with both antioxidant and anti-inflammatory properties [50]. RNA-seq analysis revealed significant upregulation of both Nrf2 (Nfe2l2) and HO-1 (Hmox1) in CMHs-treated macrophages (Fig. 3F). Further validation through western blotting (WB) confirmed an increase in the protein levels of phosphorylated Nrf2 (p-Nrf2) and HO-1 (Fig. 3G–I), supporting the conclusion that CMHs exert antioxidant effects through the activation of the Nrf2/HO-1 signaling pathway. This activation enables CMHs to effectively scavenge ROS, thereby protecting macrophages from oxidative stress and modulating inflammatory responses.
Fig. 3.
Antioxidant and anti-inflammatory mechanisms of CMHs. A) PCA analysis of samples from the LPS and CMHs groups. B) DEGs and C) volcano plots for the LPS and CMHs groups. D) KEGG pathway enrichment and E) GSEA of samples from the LPS and CMHs groups. F J M) Expression levels of key genes significantly altered in the ROS, HIF-1, and NF-κB pathways. G H I K N) Protein expression levels of p-Nrf2, Nrf2, HO-1, HIF-1, p-p65, and p65 in the CMHs group. L) LDH expression levels in the CMHs group. Statistical analyses were performed by comparing the LPS group with other groups (n = 3), with ∗p < 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, and ns indicating no significant difference.
The PI3K-Akt pathway is a key anti-inflammatory pathway that promotes macrophage polarization toward the M2 phenotype and downregulates inflammatory cytokine expression [51]. Gene expression levels related to this pathway, shown in Fig. S15 (Supporting Information), suggest its activation in CMHs-treated macrophages. In contrast, the HIF-1 signaling pathway plays a pivotal role in promoting M1 macrophage polarization through metabolic reprogramming and gene regulation. Under hypoxic conditions, HIF-1α is stabilized and translocates to the nucleus, where it activates the transcription of genes involved in glycolysis, such as LDHA, and alters mitochondrial metabolism [52]. This metabolic shift drives macrophage polarization toward the M1 inflammatory phenotype. Additionally, HIF-1α signaling modulates the expression of key transcription factors, including NF-κB and TNF, amplifying the pro-inflammatory gene expression program and enhancing the macrophage's immune response [53]. As shown in Fig. 3J, transcriptomic analysis revealed that the expression of HIF-1α and LDHA were downregulated in the CMHs-treated group. WB analysis revealed a significant decrease in HIF-1α protein levels (Fig. 3K), while LDH assay results confirmed reduced LDHA expression (Fig. 3L). These findings collectively suggest that the HIF-1α/LDHA signaling pathway was suppressed in the CMHs-treated group. Following this suppression, the NF-κB and TNF signaling pathways were also effectively inhibited, as evidenced by the downregulation of Nfkb1, Nfkb2, Rela (p65), and TNF in the transcriptomic data (Fig. 3M and Fig. S16, Supporting Information). Further validation via WB revealed a decrease in p-p65 protein levels (Fig. 3N). In summary, RNA-seq and in vitro experiments confirm that CMHs exert anti-inflammatory effects by remodeling the metabolic processes of activated macrophages. This occurs through PI3K-Akt pathway activation, HIF-1α/LDHA pathway suppression, macrophage M2 polarization, and NF-κB and TNF pathway inhibition. Notably, although RNA-seq and KEGG/GSEA analyses revealed that CMHs are involved in antioxidant and anti-inflammatory-related pathways, the causal links among these mechanisms remain incompletely established. In particular, the observed connections between CO release, Nrf2/HO-1 activation, HIF-1α/LDHA suppression, and M2 macrophage polarization are correlative and have not been directly validated. The causal relationships among these mechanisms represent a key focus that will be systematically investigated in our follow-up studies.
2.3. Intestinal barrier repair effects and mechanisms of CMHs
Given the superior antioxidant and anti-inflammatory properties of CMHs, we further investigated their potential to repair intestinal epithelial barrier integrity. To achieve this, we established an H2O2-induced oxidative damage model and compared the transcriptomic profiles of Caco-2 cells treated with both H2O2 and CMHs to those treated with H2O2 alone [54]. Caco-2 cells, derived from human colon cancer, are commonly used as a model for intestinal epithelial cells due to their ability to spontaneously differentiate and form structures that closely resemble the intestinal epithelium in vitro [55]. As such, they are widely employed in studies focused on intestinal epithelial barrier function. Using PCA, we reduced the matrix data from the H2O2 and CMHs-treated group into two dimensions. The results revealed distinct separation between the treatment groups, indicating strong sample independence (Fig. 4A). A total of 459 DEGs were identified (screening criteria: |Log2 FC| ≥ 0.5, FDR <0.05), with 249 upregulated and 210 downregulated, as shown in the volcano plot and heatmap (Fig. 4B and C). KEGG enrichment analysis revealed that DEGs after CMHs treatment were associated with several pathways, including bacterial invasion of epithelial cells, ROS signaling, focal adhesion, ECM-receptor interaction, PI3K-Akt, TGF-β, and PPAR signaling pathways (Fig. 4D). Additionally, GSEA indicated that pathways such as bacterial invasion of epithelial cells and ROS signaling were generally downregulated (NES <0) in the CMHs-treated group, while focal adhesion, ECM-receptor interaction, and the PPAR signaling pathway were generally upregulated (NES >0) (Fig. 4E). It is well known that bacterial-mediated activation of pathways involved in bacterial invasion of epithelial cells, along with the ROS pathway, can directly or indirectly disrupt the intestinal epithelial barrier through various mechanisms [56]. This disruption leads to inflammation and oxidative damage, further exacerbating the progression of UC. Bacterial invasion disrupts tight junctions, triggers inflammatory responses, and alters the microbiota balance, ultimately compromising barrier function [57]. Concurrently, ROS increases intestinal barrier permeability by inducing oxidative damage, modulating the expression of tight junction proteins (TJPs), and promoting inflammation, which facilitates the translocation of pathogens and harmful substances across the barrier [58]. Therefore, based on the results of KEGG and GSEA, the downregulation of both the bacterial invasion of epithelial cells and ROS pathways in the CMHs-treated group suggests that CMHs have a protective effect against bacterial invasion and ROS-induced disruption of the epithelial barrier. Meanwhile, we also observed that the focal adhesion, ECM-receptor interaction and PPAR signaling pathways were generally upregulated in the CMHs-treated groups. These pathways play crucial roles in regulating intestinal homeostasis, barrier function, cell proliferation, differentiation, and damage repair in the intestinal epithelium [59,60]. Specifically: 1) Focal adhesion is a crucial structural component that mediates cell attachment to the extracellular matrix (ECM) via adhesion molecules like integrins. It plays a pivotal role in regulating the dynamic stability of TJPs, thereby maintaining epithelial barrier integrity and protecting against damage. 2) The ECM-receptor interaction pathway provides structural support through integrins, which help maintain cell adhesion and tissue integrity. It also regulates the expression of tight junction and adhesion junction proteins, enhancing epithelial barrier function; 3) The PPAR pathway maintains epithelial barrier integrity by regulating the expression of TJPs. It is activated after intestinal injury to promote epithelial cell proliferation and migration, accelerating barrier repair. The roles of these pathways are closely tied to the maintenance of intestinal epithelial homeostasis, repair, and functional regulation, all of which are important for the onset and treatment of UC. Based on our RNA-seq results, we conclude that CMHs can repair intestinal epithelial barrier integrity through multiple effective mechanisms.
Fig. 4.
Effectiveness of CMHs in repairing the intestinal mucosal barrier and its underlying mechanism. A) PCA analysis of samples from the H2O2 and CMHs groups; B) DEGs and C) volcano plots for the H2O2 and CMHs groups. D) KEGG pathway enrichment and E) GSEA for samples from the H2O2 and CMHs groups. F) Cell viability, G) EdU staining, H) PI staining, and I) scratch wound healing assays of Caco-2 cells treated with various conditions. Intracellular expression levels of J) ZO-1, K) Occludin, and L) Claudin-1 in Caco-2 cells following treatment with various conditions. Statistical analyses were performed by comparing the H2O2 group with other groups (n = 3), with ∗p < 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, and ns indicating no significant difference.
To further validate the above findings, we assessed whether CMHs could protect Caco-2 cells from H2O2-induced apoptosis and promote their proliferation, migration, and expression of TJPs. As shown in Fig. S17 (Supporting Information), increasing the H2O2 concentration to 500 μM drastically reduced Caco-2 cell viability to 59 %, indicating significant toxicity from the high H2O2 concentration. However, after treatment with CMHs, cell viability significantly increased to 96 %, nearly returning to the normal level of untreated Caco-2 cells (Fig. 4F). Following the confirmation that CMHs significantly improved cell viability, we further explored their anti-apoptotic and pro-proliferative effects. As shown in Fig. 4G–H and Fig. S18A–B (Supporting Information), treatment with 500 μM H2O2 significantly increased the number of PI-positive cells and decreased the number of EdU-positive cells, confirming the inhibitory effect of H2O2 on cell proliferation and its pro-apoptotic effects, which were accompanied by noticeable nuclear shrinkage. However, after treatment with MHs and CMHs, the number of apoptotic cells significantly decreased, and cell proliferation significantly increased. Notably, in the CMHs-treated group, both apoptosis and cell proliferation nearly returned to normal levels, suggesting that CMHs have a significant protective effect against ROS-induced cell apoptosis. Additionally, as shown in Fig. 4I, CMHs significantly enhanced the migration ability of Caco-2 cells. Compared to the H2O2 group, the CMHs group exhibited a significantly smaller scratch area, and after 48 h of incubation, the scratch was nearly completely healed. In addition to inhibiting apoptosis and promoting cell proliferation and migration, CMHs also significantly increased the expression levels of various TJPs in Caco-2 cells. As shown in Fig. 4J–L, the expression levels of ZO-1, Occludin, and Claudin-1 in the H2O2 group were 0.67, 0.52, and 1.24, respectively, After CMHs treatment, these levels significantly increased to 1.02, 1.05, and 3.64, respectively, which were much higher than those in the H2O2 and MHs groups. In summary, CMHs demonstrate robust mucosal repair effects by inhibiting ROS-induced cell apoptosis, promoting cell proliferation and migration, and enhancing tight junction protein expression. Further analysis revealed that CMHs treatment effectively scavenged mitochondrial ROS in Caco-2 cells (Fig. S19, Supporting Information), thereby preserving mitochondrial membrane integrity (Fig. S20, Supporting Information) and supporting key mitochondrial functional aspects—including electron transport chain activity, ATP synthesis efficiency, and mitochondrial adaptive capacity and damage resistance (Fig. S21, Supporting Information). Collectively, these findings further highlight the ability of CMHs to restore intestinal barrier integrity through mitochondrial protection and enhanced epithelial regeneration, underscoring their therapeutic potential for treating intestinal barrier dysfunction.
2.4. Therapeutic effects of CMHs in the DSS-induced UC mouse model
Building on the excellent in vitro performance of CMHs—including their potent antioxidant and anti-inflammatory activities, as well as their capacity to promote intestinal mucosal barrier repair—we further established a DSS-induced UC mouse model to evaluate their in vivo therapeutic potential (Fig. 5A). Prior to assessing therapeutic outcomes, we employed an in vivo imaging system to investigate the colonic targeting and adhesive capabilities of Cy5.5-labeled CMHs (Cy5.5-CMHs). Fluorescence imaging revealed markedly prolonged retention of CMHs in inflamed colonic lesions than in healthy tissue (Fig. 5B), and subsequent ex vivo quantification confirmed significantly higher fluorescence intensity in colitic mice relative to normal controls (Fig. 5C). This selective accumulation likely arises from electrostatic attraction between negatively charged CMHs and positively charged glycoproteins on the damaged mucosal surface [61,62]. This conclusion is further supported by the notably prolonged retention of the negatively charged Cy5.5-CMHs compared to the positively charged Cy5.5-labeled Hs (Cy5.5-Hs) (Fig. S22, Supporting Information).
Fig. 5.
Therapeutic efficacy of CMHs in mice with DSS-induced UC. A) Experimental scheme. B) Time-dependent biodistribution of Cy5.5-CMHs in the colon after rectal administration. C) Corresponding fluorescence intensity. D) Body weight changes, E) DAI scores, F-G) Colonic length, H) Spleen index, and I) Colonic MPO levels at the end of treatment. J) Representative H&E-stained colon sections and K) corresponding histological scores on day 11. Statistical analyses were performed by comparing the CMHs group with other groups (n = 4), with ∗p < 0.05, ∗∗∗p ≤ 0.001, and ns indicating no significant difference.
We next evaluated the therapeutic efficacy of CMHs by monitoring body weight change, colon length, spleen index, disease activity index (DAI), histological score, and myeloperoxidase (MPO) activity. Body weight in the Normal group increased steadily, whereas DSS and Hs groups exhibited progressive weight loss. Although MHs partially mitigated this loss, CMHs proved most effective, with weight recovery beginning on day 9 (Fig. 5D). Consistent with this, the CMHs group maintained the lowest DAI throughout the treatment period—registering 1.62 on day 11 compared to 2.88 (DSS), 2.66 (Hs), and 2.10 (MHs) (Fig. 5E)—highlighting its superior symptom alleviation. Ex vivo analyses further corroborated these findings: colon-length shortening averaged 40 % in the DSS group, 37 % with Hs, and 22 % with MHs, but was limited to only 10.2 % with CMHs (Fig. 5F and G). Similarly, spleen index increases were also most effectively suppressed by CMHs treatment (Fig. 5H and Fig. S23, Supporting Information), underscoring the protective effect of CMHs on colon architecture and systemic inflammatory response. Additionally, MPO activity—a key indicator of neutrophil infiltration—was significantly reduced in the CMHs group, returning to levels comparable to those in healthy mice (Fig. 5I). In line with the histological scoring criteria for murine intestinal inflammation proposed by Erben et al. [63], H&E staining of colonic tissue further confirmed the therapeutic efficacy of CMHs. The treatment group exhibited reduced immune cell infiltration, preserved goblet cells, and minimal crypt destruction. The histological damage score in the CMHs group was only 3.3, significantly lower than the 17.6 observed in the DSS group (Fig. 5J and K). Taken together, these results clearly demonstrate the robust therapeutic efficacy of CMHs in alleviating UC. Notably, all evaluated efficacy indicators—including body weight change, DAI, colon length, spleen index, MPO activity, and histopathological scores—consistently indicated that CMHs elicited significantly stronger therapeutic effects than free CORM-401 (Fig. S24, Supporting Information). This superiority can be largely attributed to the poor aqueous solubility of CORM-401, which impedes its accumulation and retention at the inflamed colon following rectal administration, leading to inadequate local bioavailability and therapeutic coverage. Moreover, the lack of active targeting and short residence time further limit tissue interaction, resulting in compromised CO release, anti-inflammatory activity, and cytoprotection. These findings emphasize the key advantages of the CMHs-based delivery system: by enhancing solubility, improving targetability, and prolonging local retention, CMHs effectively overcome the inherent limitations of free CORM-401, thereby fully harnessing the therapeutic potential of CO and producing a synergistic treatment effect in UC. Furthermore, CMHs also outperformed 5-ASA in overall therapeutic outcome, suggesting superior anti-inflammatory and tissue repair capabilities—a promising indicator for clinical translation. Importantly, CMHs exhibited excellent biocompatibility throughout the study. Minimal hemoccult positivity was observed during treatment (Fig. S25, Supporting Information), and plasma COHb levels remained stable after three administrations of CMHs, indicating no signs of systemic CO poisoning (Fig. S26, Supporting Information). Key hematological parameters associated with CO toxicity—such as white blood cell count, neutrophil count, hemoglobin concentration, and red blood cell count—all remained within normal ranges, with no significant tissue damage detected (Fig. S27, Supporting Information). Additionally, no adverse effects were observed on liver and kidney function (Fig. S28, Supporting Information) and intestinal smooth muscle function (Fig. S29, Supporting Information). Finally, Mn ion concentrations in the colon and major organs of CMHs-treated UC mice showed no significant accumulation compared to healthy controls (Fig. S30, Supporting Information).
2.5. In vivo remodeling of colonic immune response and intestinal barrier integrity
Fig. 5 illustrates the significant therapeutic effects of CMHs in treating UC, primarily attributed to their potent antioxidant, anti-inflammatory, and intestinal barrier repair properties. To substantiate this hypothesis, we systematically assessed the therapeutic effects of CMHs on these functions in a UC mouse model (Fig. 6A). Additionally, we explored their roles in gut microbiota modulation and pain regulation through 16S rRNA sequencing of mouse feces and RNA transcriptome sequencing of colon tissues. ROS scavenging in inflamed regions was assessed using the L-012 fluorescence probe. The DSS group showed significantly elevated ROS signals, indicating high ROS concentrations in the inflamed tissues. Treatments with MHs reduced ROS levels, but CMHs treatment restored ROS levels to near-normal (Fig. 6B and C). The frozen section results of colonic tissues further confirmed the excellent ROS scavenging effect of CMHs at the UC site (Fig. S31, Supporting Information). Measurements of antioxidant enzyme activity (GPX, CAT, and SOD) in colon tissues confirmed these effects. While MHs increased enzyme activity compared to the DSS and Hs groups, CMHs demonstrated superior antioxidant activity (Fig. 6D–F). This enhanced efficacy is attributed to the CO release from CMHs (Fig. S32, Supporting Information), which activates endogenous antioxidant pathways and boosts the activity of MnOx. The anti-inflammatory potential of CMHs was confirmed through IF, WB, and ELISA analyses. IF staining revealed a marked reduction in iNOS-positive M1 macrophages and a significant enrichment of CD206-positive M2 macrophages in the CMHs group (Fig. 6G–H and Fig. S33, Supporting Information). Furthermore, the significantly reduced protein expression of TNF-α, along with the remarkably enhanced expression of IL-10 in colon tissues from CMHs-treated mice (Fig. S34, Supporting Information), further demonstrated that CMHs promote macrophage polarization from the M1 to the M2 phenotype. Pro-inflammatory cytokines IL-6 and TNF-α were significantly downregulated in the CMHs group compared to the DSS and other treatment groups (Fig. 6I and J). In contrast, anti-inflammatory cytokines IL-4 and IL-10 were markedly elevated, further highlighting CMHs' role in inflammation modulation (Fig. 6K and L). These findings underscore CMHs' dual role in suppressing inflammation and regulating macrophage polarization, positioning them as a potent anti-inflammatory therapy. The combined antioxidant and anti-inflammatory properties of CMHs also contributed to protecting the intestinal barrier from oxidative and inflammatory damage. Elevated FITC-Dex levels in the peripheral blood of DSS-treated mice indicated severe intestinal barrier dysfunction (Fig. 6M). Treatments with MHs reduced FITC-Dex levels, while CMHs nearly restored permeability to normal levels, demonstrating superior barrier protection. IF analysis of colon tissues showed significant upregulation of TJPs Claudin-5, ZO-1, and Occludin-1 in the CMHs group (Fig. 6N and Fig. S35, Supporting Information). These proteins are crucial for maintaining intestinal barrier integrity, further confirming CMHs' efficacy in preserving mucosal barrier function. In summary, CMHs exhibit robust antioxidant, anti-inflammatory, and intestinal barrier repair effects, all of which are critical for maintaining colonic mucosal immune homeostasis and improving intestinal function. CO release from CMHs amplifies therapeutic efficacy by boosting antioxidant activity, regulating inflammatory mediators, and promoting macrophage polarization. Notably, across all evaluated efficacy indicators—including ROS scavenging, regulation of antioxidant enzyme activity, anti-inflammatory effects, and intestinal barrier repair—CMHs consistently elicited markedly superior therapeutic outcomes compared to both 5-ASA and free CORM-401 (Fig. S36, Supporting Information), further underscoring their comprehensive and enhanced functionality in UC treatment. Together, these properties position CMHs as a promising novel therapeutic agent for the management of UC.
Fig. 6.
In vivo antioxidant, anti-inflammatory, and mucosal barrier repair effects of CMHs. A) Experimental scheme. B–C) ROS elimination in colon tissue. D-F) Antioxidant enzyme levels in colon tissue. G-H) iNOS-positive and CD206-positive cells in colon tissues. I-L) Inflammatory cytokine expression levels in colon tissues. M) FITC concentration in the peripheral blood of mice. N) Expression levels of TJPs in colon tissue. In C-F, and M, comparisons were made between the CMHs group and other groups (n = 3). In I-L, comparisons were made between the DSS group and other groups (n = 3), ∗p < 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, and ns indicating no significant difference.
2.6. In vivo modulation of gut microbiota composition and function
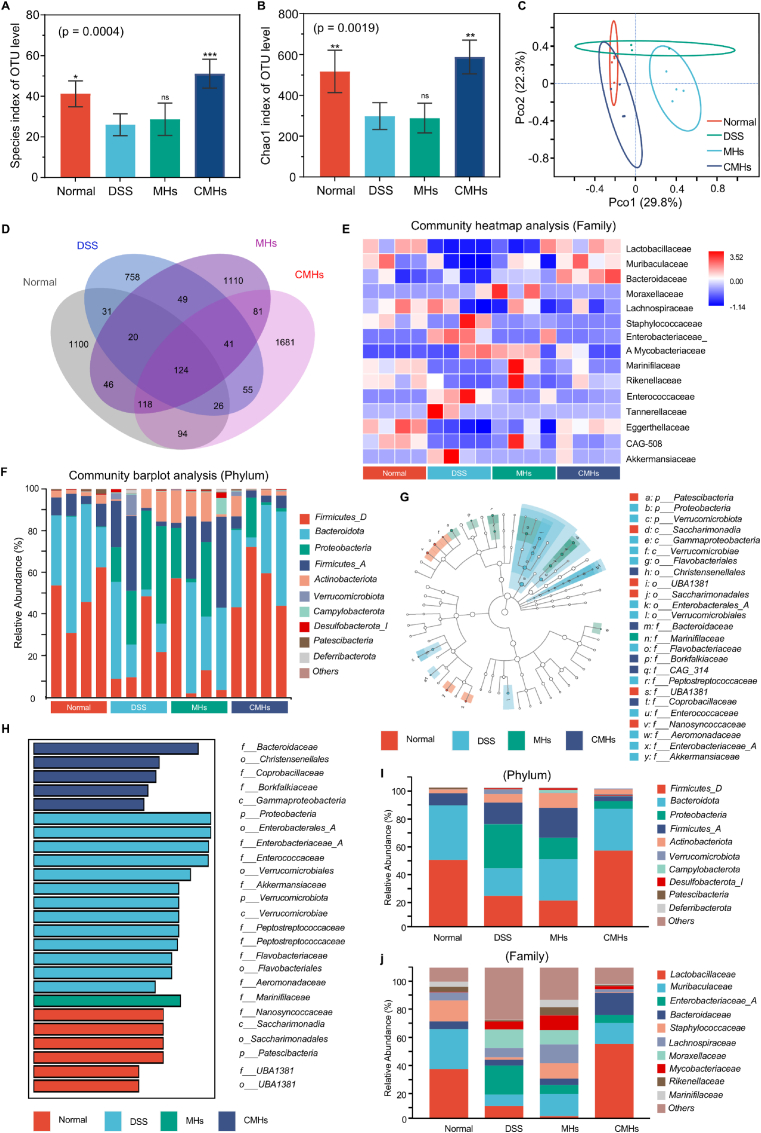
The gut microbiota and the intestinal mucosal barrier share a critical, interdependent relationship essential for maintaining overall health [64]. The gut microbiota regulates immune responses, preserves intestinal barrier integrity, and protects the host from pathogenic invasions [65]. Dysbiosis, or disruptions in gut microbiota composition, compromises the intestinal barrier and increases permeability, commonly referred to as "leaky gut." This condition facilitates the translocation of pathogens, toxins, and inflammatory molecules across the intestinal epithelium, triggering systemic inflammation [66]. Given CMHs' potent antioxidant, anti-inflammatory, and mucosal repair properties observed in vivo, we explored their effects on gut microbiota regulation using 16S rRNA gene sequencing. Chao1 and Observed Species metrics, commonly employed to assess microbial species richness, revealed significant differences between the control and DSS groups (Fig. 7A and B). These results confirm that DSS-induced UC markedly reduced gut microbiota diversity and species richness. MHs treatment showed limited improvement, as no significant differences were observed between the MHs and DSS groups. In contrast, the CMHs group exhibited no significant differences compared to the control group, indicating a protective effect of CMHs on microbiota diversity and a near-complete restoration to healthy levels. β-diversity analysis using principal coordinates analysis (PCoA) further revealed distinct community composition clusters among the control, DSS, and treatment groups (Fig. 7C). While the DSS group showed significant shifts away from the control group, the treatment groups, particularly the CMHs group, displayed community structures closer to the control group. These findings indicate successful remodeling of gut microbiota composition by CMHs. Further evidence from Venn diagrams (Fig. 7D) confirmed increased species richness in the CMHs-treated group. Heatmaps and bar charts provided a detailed visualization of the microbiome composition at the family and phylum levels, showcasing the restructuring effect of CMHs (Fig. 7E and F). Linear discriminant analysis effect size (LEfSe) analysis identified taxa with differential enrichment across groups, highlighting dominant microbial populations and their taxonomic levels (Fig. 7G and H). The following changes in microbial composition were observed (Fig. 7I–J and Fig. S37, Supporting Information): 1) The overall microbiome profile of the CMHs group closely resembled that of the control group, underscoring the effectiveness of CMHs in restoring gut microbiota balance. 2) CMHs treatment significantly reduced the abundance of Enterobacteriaceae_A and Proteobacteria, two pathogenic taxa commonly elevated in patients with UC. These reductions are indicative of a decreased pathogenic bacterial burden. Besides, the abundance of Aeromonadaceae [67] and Enterococcaceae [68], which are linked to ROS accumulation and mucosal injury, were also significantly reduced after CMHs treatment. 3) The CMHs group exhibited a notable increase in Firmicutes_D, Bacteroidaceae, Lactobacillaceae, Muribaculaceae, and Bacteroidota. These beneficial taxa contribute to a healthy intestinal microenvironment by inhibiting harmful microbial growth through competitive exclusion and modulating immune responses. Moreover, CMHs treatment also significantly increased the abundance of Ruminococcaceae, another important probiotic associated with short-chain fatty acid production and anti-inflammatory effects [69]. Taken together, these results indicate that CMHs not only restore probiotics but also suppress pathogenic taxa, highlighting the superior advantage of combining CO therapy with nanozymes in remodeling the gut microbiota and alleviating UC.
Fig. 7.
16S rRNA sequencing analysis of the gut microbiome regulated by CMHs in mice with DSS-induced UC. A-B) α-diversity analysis, including the Observed Species index and Chao1 index. C) β-diversity analysis via PCoA. D) Venn diagram illustrating differences in microbiota composition between treatment groups. E) Heatmap of microbial composition at the family level. F) Histogram showing microbial community composition at the phylum level. G) Cladogram highlighting microbial species with significant differences. H) LDA identifying the most abundant genera in different groups. I-J) Significantly altered microbial communities at the family and phylum levels. Statistical analyses were performed by comparing the DSS group with other groups (n = 4), with ∗p < 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, and ns indicating no significant difference.
To further explore whether these compositional changes translated into functional alterations, we conducted PICRUSt2-based functional prediction, simultaneously referencing KEGG and MetaCyc databases for annotation. The KEGG analysis results in Fig. S38 (Supporting Information) revealed that, compared with controls, DSS-induced UC mice exhibited profound functional impairments at the microbial level. Specifically, core metabolic pathways such as pantothenate and CoA biosynthesis and pyruvate metabolism were markedly suppressed, reflecting a decline in the capacity for energy generation and coenzyme turnover. These deficiencies are consistent with a dysbiotic state in which beneficial microbial activities are compromised. In parallel, DSS treatment significantly enriched inflammation- and pathogen-associated functions, including nitrogen metabolism, flagellar assembly, and bacterial invasion of epithelial cells, highlighting an unfavorable shift toward virulence and pro-inflammatory microbial traits. Importantly, CMHs treatment largely reversed these abnormalities. The restoration of pantothenate and CoA biosynthesis and pyruvate metabolism suggests that CMHs help re-establish fundamental microbial metabolic activities that are essential for host–microbiota homeostasis. Notably, we also detected the pathway “pyruvate fermentation to butanoate” in Fig. S39 (Supporting Information), which represents a canonical route for butyrate synthesis. Butyrate is a key SCFA that serves as the primary energy source for colonocytes, reinforces epithelial barrier integrity, and exerts potent anti-inflammatory effects by modulating NF-κB signaling and promoting Treg differentiation [70]. The recovery of this pathway suggests that CMHs not only remodel gut microbial community structure but also enhance microbial functional capacity for SCFA production, thereby contributing to the improvement of the intestinal microenvironment and the alleviation of UC. This further indicates that CMHs may enhance the potential for SCFA production at the functional level. However, it should be noted that PICRUSt2 results are computational predictions, and direct metabolite measurements will be necessary in future studies to validate these functional changes and confirm their contribution to the therapeutic effects of CMHs. In summary, CMHs treatment significantly alleviates DSS-induced gut microbiota disorders by reducing the abundance of pathogenic bacteria, increasing probiotic populations, and restoring the intestinal microecological balance. These findings underscore the potential of CMHs in modulating gut microbiota composition and maintaining intestinal homeostasis, which is key to their therapeutic efficacy in UC.
2.7. In vivo alleviation of chronic inflammation-related pain
Over 50 % of patients with UC experience moderate to severe pain during active disease phases, and approximately 20 %–30 % continue to suffer from chronic pain even during periods of remission [71]. This highlights that pain is not only a common symptom of UC but also a significant factor that profoundly impacts patients' quality of life throughout the disease course. Chronic intestinal inflammation, disruption of the intestinal mucosal barrier, and dysbiosis of the gut microbiota have been identified as key factors contributing to UC-related pain [72]. Given our compelling evidence that CMHs remodel intestinal mucosal immunity, restore intestinal barrier, and modulate the gut microbiota, we hypothesize that CMHs may also exert analgesic effects. Pain is known to cause substantial suppression of feeding and drinking behaviors in animal models [73]. In line with this, during our evaluation of therapeutic effects in the DSS-induced UC mouse model, we observed a marked reduction in both drinking and feeding behaviors in the diseased mice (DSS group). As shown in Fig. 8A and B, water and food intake progressively declined as disease severity increased, reflecting a significant suppression of essential physiological functions. In contrast, mice treated with CMHs exhibited varying degrees of recovery in both water and food intake, suggesting that CMHs treatment may alleviate pain and restore normal physiological functions in DSS-induced UC mice. Additionally, using the abdominal withdrawal reflex (AWR) scoring method as reported previously [74], we further evaluated the effect of CMHs on pain sensitivity in UC mice. AWR is widely utilized in the literature and is considered a classic experimental method for assessing visceral organ pain. As shown in Video S1-S3 (Supporting Information) and Fig. 8C, colonic distention progressively worsened with increasing PBS injection volume. Mice in the Normal group remained calm, showing only slight abdominal withdrawal after the injection of 400 μL PBS, with an AWR score of 1.2. In contrast, the behavioral response of DSS group mice significantly intensified after the injection of only 200 μL PBS, exhibiting noticeable abdominal withdrawal, increased peristalsis, and other typical pain responses. The AWR scores of DSS mice were significantly higher than those of the other groups throughout the experiment. Notably, CMHs-treated UC mice showed some abdominal withdrawal and increased peristalsis after the injection of 400 μL PBS, but the overall response was significantly weaker than that of the DSS group, with an AWR score of only 2.1. Taken together, these findings suggest that CMHs not only modulate the underlying pathophysiology of UC but also positively influence the pain-associated behavioral changes in the disease.
Fig. 8.
Mechanisms of pain modulation by CMHs in mice with DSS-induced UC. A-B) Food and water intake in mice. C) AWR scores of normal mice and UC mice-treated PBS or CMHs after administration of different volumes of PBS. D) Volcano plot and E) DEGs plots for the DSS and CMHs groups. F) Histogram of GO enrichment analysis. G) KEGG pathway enrichment analysis and H) GSEA for samples from the H2O2 and CMHs groups. I) Expression levels of key genes significantly downregulated in the neuroactive ligand-receptor interaction pathway. J) TRPV1-positive cells and K) TACR1-positive cells in colon tissue.
To elucidate the pain-regulatory mechanism of CMHs in UC mice, we performed RNA-seq analysis on colon tissues from DSS-induced UC mice and CMHs-treated mice. PCA revealed a significant transcriptional difference between the DSS and CMHs groups (Fig. S40, Supporting Information). A total of 2242 DEGs were identified (screening criteria: |Log2 FC| ≥ 0.5, FDR <0.05), with 673 upregulated and 1579 downregulated, as shown in the volcano plot and heatmap (Fig. 8D and E). Gene Ontology (GO) enrichment analysis revealed significant enrichment in terms related to neural signal transmission, including biological processes such as regulation of membrane potential, regulation of metal ion transport, and regulation of synapse structure or activity (Fig. 8F). It also identified molecular functions such as metal ion transmembrane transporter activity, monoatomic ion channel activity, and gated channel activity, along with cellular components including the postsynaptic membrane, neuron to neuron synapse, transmembrane transporter complex, and distal axon. These findings suggest that CMHs may regulate neural transmission through multiple mechanisms, particularly by regulating specific ion channels to control neuronal excitability. Although the mechanisms of chronic pain in IBD are not fully understood, studies suggest that inflammatory infiltration and altered sensory processing contribute to peripheral and central sensitization [75]. Further KEGG enrichment analysis revealed significant enrichment of neuroactive ligand-receptor interaction among the DEGs, particularly in the calcium signaling pathway, glutamatergic synapse, and cGMP-PKG signaling pathway (Fig. 8G). These findings further support our hypothesis that CMHs alleviate UC-related pain by modulating neural transmission. To confirm this, we performed GSEA enrichment analysis on the neural transmission-related pathways identified in the KEGG analysis (Fig. 8H). The results indicated that all four pathways were significantly downregulated after CMHs treatment, reinforcing the idea that CMHs inhibit pain transmission by suppressing neuroactive ligand-receptor interactions. Pain signal transduction involves the activation of glutamate receptors, and inhibiting genes related to glutamatergic synapse activity (Fig. S41, Supporting Information) can help block the transmission of pain signals [76]. Additionally, the cGMP-PKG signaling pathway plays a crucial role in the formation and maintenance of chronic pain, which can facilitate pain signal transmission and central sensitization by regulating neuronal excitability, thereby enhancing pain perception [77]. Following CMHs treatment, the cGMP-PKG signaling pathway was significantly downregulated, and the expression of multiple guanylate cyclase subunits, including Gucy1a1 and Gucy1b1, was notably reduced (Fig. S42, Supporting Information). Noteworthy, calcium channels play a critical role in pain development under pathological conditions, with Cacna1e (Cav2.3) identified as a key mediator of nociception, contributing to pain processing and sensitivity [78]. In the CMHs-treated group, the calcium signaling pathway was also significantly downregulated, and the expression of several calcium channels, including Cacna1e and Cacna1d, was significantly reduced (Fig. S43, Supporting Information). These findings suggest that CMHs may exert analgesic effects by inhibiting glutamatergic synapse activity, the cGMP-PKG signaling pathway, and Ca2+ channel activity.
The increased activity of acute inflammatory cells leads to visceral hyperalgesia, which is closely associated with elevated levels of Substance P (SP) [79]. Notably, SP binding to TACR1 activates downstream G protein-coupled receptor signaling, indirectly regulating Ca2+ channels (such as TRPV1), leading to Ca2+ influx. This process exacerbates pain perception and the transmission of pain signals, while further promoting the release of SP [80]. To evaluate the expression of nociception-related receptors, we further extracted genes involved in neuroactive ligand-receptor interactions and selected the top 26 genes with the most significant downregulation (Fig. 8I). Heatmap analysis showed marked downregulation of several receptors, including TACR1 and TACR2. Further IF staining of colonic tissue confirmed these findings, showing that the expression of both TACR1 and TRPV1 was significantly reduced (Fig. 8J and K). In summary, this study demonstrates that CMHs have significant potential to alleviate UC-related pain through multiple mechanisms, including downregulating pain-related pathways (e.g., glutamatergic synapse, cGMP-PKG, and calcium signaling) and modulating nociceptive receptors like TACR1 and TRPV1, offering a potential therapeutic approach for pain management in UC.
Given that Ca2+ influx plays a central role in pain perception, especially in chronic pain and inflammatory pain, by enhancing neuronal excitability, promoting synaptic transmission, activating pain-related signaling pathways, and inducing central sensitization, and our animal tissue transcriptomic results have confirmed that CO can significantly inhibit the activation of calcium signaling pathways, we further established a co-culture system of BV-2 microglial cells and PC-12 neuronal cells as an in vitro inflammatory pain model. Using this model, we evaluated whether CMHs could protect PC-12 cells from inflammation-mediated damage, inhibit SP secretion, downregulate TACR1 expression, and indirectly inhibit the activation of TRPV1 channels, thereby suppressing calcium ion influx and regulating pain. As shown in Fig. 9A, LPS-activated BV-2 cells significantly reduced the viability of PC-12 cells in the co-culture system to 76.6 %, indicating that the inflammatory factors secreted by BV-2 cells exert significant neurotoxic effects on neuronal cells. After CMHs treatment, the viability of PC-12 cells recovered to 86.6 %, demonstrating that CMHs effectively protect neuronal cells from inflammatory factor-induced damage. Further analysis revealed that CMHs significantly suppressed the expression of inflammatory factors in BV-2 cells. As shown in Fig. 9B and C, the expression levels of IL-1β and IL-6 in the CMHs-treated group were reduced by 2.1-fold and 2.8-fold, respectively, compared to the LPS group, indicating that CMHs alleviate neuronal damage by modulating inflammatory responses. Notably, CMHs also significantly inhibited the secretion of SP. As shown in Fig. 9D, LPS treatment markedly promoted the expression of SP in PC-12 cells, while CMHs treatment reduced the expression level of SP to 0.65-fold of the LPS group, nearly restoring it to normal levels. This suggests that CMHs effectively suppress LPS-induced overexpression of SP, thereby alleviating SP-mediated pain and inflammatory responses.
Fig. 9.
In vitro evaluation of CMHs efficacy in modulating LPS-induced inflammatory nociception. A) Viability of PC-12 cells treated with CMHs. B-C) Expression levels of IL-1β and IL-6. D) Expression levels of SP. E-F) TACR1-positive cells in the CMHs-treated group. G-H) Protein expression levels of TRPV1 in the CMHs-treated group. I-J) Intracellular Ca2+ imaging in CMHs-treated cells. K) Schematic illustration of the pain-regulation mechanisms of CMHs. Statistical analyses were performed by comparing the LPS group with other groups (n = 3), with ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001.
IF staining results further demonstrated that LPS treatment significantly activated the overexpression of TACR1 on the surface of PC-12 cells. In contrast, CMHs treatment significantly inhibited TACR1 expression, with fluorescence intensity reduced by approximately 0.59-fold compared to the LPS group (Fig. 9E and F), indicating that CMHs effectively suppress TACR1 expression. Additionally, we investigated the effects of CMHs on Ca2+ channels on the surface of PC-12 cells using WB analysis, with the TRPV1 agonist capsaicin (CAP) and antagonist capsazepine (CZP) as controls. As shown in Fig. 9G–H and Fig. S44 (Supporting Information), TRPV1 protein expression was significantly increased in the LPS and CAP treatment groups, indicating the activation of Ca2+ channels. In contrast, TRPV1 protein expression in the CMHs and CZP treatment groups decreased to 0.67-fold and 0.46-fold of the LPS group, respectively, demonstrating that both CMHs and CZP significantly inhibit the opening of Ca2+ channels. More importantly, CMHs treatment significantly suppressed Ca2+ influx. As shown in Fig. 9I and J, the Fluo-4 probe in PC-12 cells of the LPS group exhibited strong green fluorescence, indicating a significant increase in Ca2+ influx. In contrast, the fluorescence intensity of Fluo-4 in the CMHs-treated group was significantly reduced, decreasing by approximately 0.6-fold compared to the LPS group, suggesting that CMHs effectively inhibit Ca2+ influx, thereby reducing cell depolarization and pain signal transmission. Combining the results of Fig. 8, Fig. 9, it is concluded that CMHs can exert notable analgesic effects by modulating inflammatory responses, inhibiting SP secretion and TACR1 expression, and suppressing TRPV1 channel activation and Ca2+ influx (Fig. 9K). These actions collectively regulate neural transmission pathways and reduce pain perception.
3. Conclusion
This study introduces an engineered nanozyme (CMHs) combining MnOx and CO gas therapy, achieved through the in-situ generation of MnOx on Hs surfaces and controlled encapsulation of CORM-401 within the Hs tubular structure. CMHs selectively accumulate at UC sites, where MnOx scavenges ROS and CO enhances antioxidant and anti-inflammatory effects. These advanced features enable CMHs to restore immune homeostasis in the colonic mucosa, repair the intestinal barrier, modulate the gut microbiota, and alleviate inflammation-related pain. In vitro and in vivo experiments confirmed the superior therapeutic efficacy of CMHs, particularly in treating DSS-induced UC in mouse models. RNA-seq revealed that CMHs exert antioxidant effects via activation of the Nrf2/HO-1 signaling pathway and reduce inflammation through PI3K-Akt pathway activation, suppression of the HIF-1α/LDHA pathway, promotion of macrophage M2 polarization, and inhibition of NF-κB and TNF pathways. Additionally, CMHs repair the intestinal epithelial barrier by inhibiting bacterial invasion and ROS, while activating focal adhesion, ECM-receptor interaction, and PPAR signaling pathways. 16S rRNA sequencing further confirmed that CMHs effectively regulated the gut microbiota, contributing to the therapeutic outcomes. Notably, this study is the first to report that CMHs alleviate pain by downregulating pain-related pathways (e.g., glutamatergic synapse, cGMP-PKG, and calcium signaling) and modulating nociceptive receptors like TRPV1 and TACR1. Overall, this study establishes CMHs as an innovative, highly effective, and safe therapeutic strategy for UC with strong potential for clinical application.
4. Experimental section
4.1. Materials
CORM-401 was synthesized in the laboratory. Hs were procured from Guangzhou Runwo Materials Technology Co. KMnO4 and APTES were purchased from Aladdin (China), and toluene from Shanghai Titan Technology Co. The ROS DCFH-DA probe and Cy5.5-NHS were obtained from Solaibio (USA), while the ROS Green H2O2 probe was purchased from Shanghai Maokang Biotech Co. (China). The BCA protein assay kit, 5-ASA and Fluo-4 AM were acquired from Beyotime (China). FITC-Dextran and CAP were purchased from MedChemExpress (Shanghai, China). CPZ was obtained from Meilunbio (China) Calcein-AM/PI double staining kit, Hoechst 33342, Cell Counting Kit-8, and NO DAF-FM DA were sourced from Nanjing Keygen Biotech Co. (China). Kits for detecting CAT, SOD, GPX and LDH were supplied by Grace Biotech Co. Cell Light™ EdU Apollo® 567 Imaging Kit were from Guangzhou Ribobio (China). ELISA kits for mouse iNOS, IL-1β, IL-6, TNF-α, IL-10, Arg-1, CD206, and MPO, along with antibodies against TRPV1, HO-1, Nrf-2, p-Nrf-2 were obtained from Proteintech (USA). The Rat SP ELISA Kit was sourced from Elabscience (China). The anti-HIF-1α antibody was obtained from Cell Signaling Technology (USA), while the anti-TACR1 antibody was sourced from Hangzhou HUABIO (China). Hydroxyl radical scavenging activity assay kits, and antibodies against iNOS and CD206, were supplied by Beijing Bioss (China). L-012 sodium salt and protease inhibitor cocktail were obtained from APEBio (USA). The mouse COHb ELISA kit was purchased from Beijing Meimian Biotech (China). RAW264.7 mouse macrophages, human Caco-2 cells, BV-2 microglial cells, and PC-12 neuronal cells were provided by the ATCC (USA).
4.2. Synthesis of MHs
Under a nitrogen atmosphere, 1 g of Hs was dispersed in 100 mL of toluene, and 30 mL of APTES was subsequently added dropwise. The mixture was stirred magnetically at 90 °C for 12 h. The resulting precipitate was then collected by centrifugation, washed three times with ethanol and deionized water, and freeze-dried to obtain NHs. Subsequently, a total of 100 mg of NHs was dispersed in 2 mL of 1 mol/L NaOH solution and sonicated for 10 min to achieve a uniform dispersion. Next, 2 mL of an aqueous KMnO4 solution (12.5 mg/mL) was added dropwise. The mixture was then vortexed for 5 min and irradiated under visible light at 90 °C for 4 h. The precipitate was collected via centrifugation, thoroughly washed with deionized water until reaching a neutral pH, and then freeze-dried to obtain MHs. The structure of MHs was analyzed using FT-IR, XPS, and UV–Vis. The MnOx content was quantified by ICP-OES.
4.3. Preparation of CMHs
To begin, 10 mg of MHs was dispersed in 2 mL of deionized water. Subsequently, 1 mL of an alkaline aqueous solution of CORM-401 (10 mg/mL, pH = 8) was added dropwise to the mixture. The reaction was stirred at room temperature in the dark for 24 h. Afterward, the precipitate was collected by centrifugation, washed with deionized water, and freeze-dried to yield a green-brown powder, labeled CMHs. Dynamic light scattering (DLS) using a NanoTM 90 instrument was employed to analyze the particle size and zeta potential of the CMHs. SEM (SU80l0, Hitachi, Japan), TEM (JEM-2100 Plus) and EDS were employed to examine the surface morphology and to map the distribution of CORM-401 and MnOx on the CMHs. The loading efficiency CORM-401 was quantified using ICP-OES. The scavenging capabilities of CMHs (50 and 100 ppm) against O2·-, H2O2, ·OH, ABTS·+, and DPPH· were evaluated according to the manufacturers' instructions. The storage stability of CMHs was assessed by incubating CMHs (1 mg/mL in PBS, pH 7.4) at 4 °C. The particle size and zeta potential of the solutions were measured at 0, 1, 2, 3, and 4 weeks [81]. The stability of CMHs in SCF was evaluated by incubating CMHs (1 mg/mL) in SCF at 37 °C. The particle size and zeta potential were measured at 1, 3, 6, 9, and 12 h. The degradation behavior of CMHs in SCF was further examined by incubating CMHs (1 mg/mL) in SCF supplement with 500 μM H2O2 at 37 °C, with particle size and zeta potential measured at 2, 4, and 8 h.
4.4. CO release profile of CMHs
CO release from CMHs was determined using a custom-designed detection system. A 2 mL solution of CMHs (12 mg/mL in SCF) was transferred into a vial, which was then placed inside a sealed cylindrical glass container fitted with a CO detector (BH-90, Henan Baoshian Electronic Technology Co.). The system was sealed, and the release of CO was initiated by injecting different concentrations of H2O2 (0, 0.1, 0.5, or 1 mM) into the CMHs solution through a rubber stopper at the top of the glass container. The CO concentration (ppm) was monitored and recorded in real time, which facilitated the calculation of the total CO released.
4.5. Cell viability
In this study, RAW264.7 and Caco-2 cells were cultured using macrophage and Caco-2-specific culture media provided by Wuhan Pricella Bio. ‘Normal macrophages’ refers to RAW264.7 cells that were not treated with LPS, whereas ‘activated macrophages’ refers to RAW264.7 cells that were stimulated with LPS (5 μg/mL) for 24 h. The cytotoxicity of CMHs on RAW264.7 and Caco-2 cells was evaluated using the CCK-8 assay and live/dead staining. Specifically, RAW264.7 and Caco-2 cells were inoculated at a density of 1 × 104 cells/well in a 96-well plate and maintained overnight. The culture medium was refreshed with fresh medium containing different concentrations of CMHs (6.13–200 μg/mL) and incubated for 24 h. After washing the cells with PBS, they were incubated with Calcein-AM (5 μM) and propidium iodide (PI, 5 μM) and visualized using an inverted fluorescence microscope (Axio Observer 3 ZEISS) to assess cell viability. For the CCK-8 assay, the cells were initially washed with PBS, followed by incubation with 10 μL of CCK-8 solution for 1 h. Absorbance at 450 nm was then measured using a microplate reader to evaluate cell viability. In both the CCK-8 and live/dead staining experiments, cells treated with PBS, Hs (100 μg/mL), or MHs (100 μg/mL) were used as control groups.
4.6. Intracellular CO imaging
Intracellular CO production was monitored using a CO probe system (PdCl2+FL-CO-1). In summary, activated macrophages (2 × 105 cells per dish) were plated in culture dishes and kept for 24 h. The cells were then exposed to DMEM medium containing 50 or 100 μg/mL of CMHs for 4 h. After discarding the medium, the cells were maintained for 30 min in serum-free DMEM supplemented with 0.5 mL of 5 μM Hoechst 33342, 5 μM PdCl2, and 5 μM FL-CO-1. The medium was harvested, and the fluorescence intensity of the probe system in the supernatant was quantified using a microplate reader (Biotech, Epoch 2, USA) [25]. Afterward, the cells were rinsed with PBS and examined using a confocal laser scanning microscope (CLSM) to evaluate the generation of intracellular CO.
4.7. In vitro anti-oxidant of CMHs
The antioxidant activity of CMHs was evaluated by monitoring the scavenging of intracellular ROS, H2O2, and NO using DCFH-DA, the ROS Green H2O2 probe, and DAF-FM DA probes, respectively. Activated macrophages (2 × 105 cells/dish) were plated in culture dishes and cultured for 12 h. Afterward, the cells were exposed to DMEM medium containing 100 μg/mL CMHs and incubated over an additional 12 h. Following incubation, the medium was discarded, and the cells were treated with 300 μL of PBS containing Hoechst and 5 μM of DCFH-DA, DCF, or DAF-FM DA probes in DMEM medium for 30 min. The supernatant was collected, and the fluorescence intensity of the probes in the supernatant was measured using a multi-mode microplate reader [25]. Simultaneously, the cells were washed with PBS, and the intracellular distribution of the fluorescent probes was observed using an inverted fluorescence microscope. The excitation and emission wavelengths were as follows: 644 nm and 665 nm for DCFH-DA, 490 nm and 514 nm for DCF, and 500 nm and 515 nm for DAF-FM DA. The antioxidant activity of CMHs was assessed by measuring the levels of CAT, SOD, and GPX determined by their specific assay kits. Briefly, activated macrophages (1 × 107 cells/dish) were plated into 8 × 8 cm culture dishes and cultured for 12 h. Following incubation, the cells exposed to DMEM containing 100 μg/mL CMHs and cultured for a further 24 h. Following the treatment, the culture medium was discarded, and the cells were lysed via ultrasonication on ice (300 W, 5 s on/25 s off cycles) for 30 min. The lysates were centrifuged, and the supernatant was harvested for further analysis. Protein concentrations were quantified with a BCA protein assay kit. To assess the levels of CAT, SOD, and GPX activity, aliquots of the supernatant were mixed with specific reagents as per the manufacturer's guidelines. Absorbance was assessed at 510 nm for CAT, 450 nm for SOD, and at 412 nm for GPX analysis with a microplate reader. Enzyme activities were calculated using the provided formulas and expressed as follows: CAT: μmol/min/mg protein (μmol min−1 mg−1); SOD: U/mg protein (U mg−1); GPX: nmol/min/mg protein (nmol min−1 mg−1); Control groups included cells treated with PBS, Hs (100 μg/mL), or MHs (100 μg/mL), and these were used for comparison in all assays. To assess the effects of CMHs treatment on HO-1 expression in activated macrophages, cells (1 × 106 cells/well) were plated in 6-well plates and cultured for 24 h. The cells were then exposed to DMEM containing CMHs (100 μg/mL) for another 24 h. After the treatment, the cells were collected for further analysis. The expression levels of HO-1s mRNA were quantified using RT-qPCR. WB was employed to quantify the expression levels of proteins involved in the ROS signaling pathway, including HO-1, Nrf2, and p-Nrf2, to explore the mechanisms by which CMHs modulate ROS-related signaling pathways. For RT-qPCR, mRNA was isolated from the lysed cell samples, followed by reverse transcription and amplification. Quantification was performed using a real-time PCR system. The primer sequences used are shown in the Supporting Information (Table S2). In the WB analysis, cells were homogenized in RIPA buffer supplemented with protease inhibitors to extract proteins. Protein concentrations were quantified through a BCA protein assay kit. The samples were subjected to gel electrophoresis, membrane transfer, blocking, and incubation with a primary antibody against HO-1. Protein bands were detected with an enhanced chemiluminescence (ECL) kit, and imaging was carried out using a gel imaging system. Protein band intensity was analyzed via ImageJ software, with β-actin serving as an internal control for normalization. Control groups included cells treated with PBS, Hs (100 μg/mL), or MHs (100 μg/mL).
4.8. In vitro anti-inflammatory activity of CMHs
To assess the effects of CMHs treatment on macrophage phenotypes, activated macrophages (5 × 105 cells/well) were plated into confocal dish wells and maintained for 12 h. The cells were then exposed to DMEM containing CMHs (100 μg/mL) for an extra 24 h. After treatment, cell morphology was examined using an inverted fluorescence microscope, and parameters such as cell elongation and synapse count were quantitatively assessed. Additionally, activated macrophages treated with CMHs were immune-stained with antibodies against iNOS and CD206, and observed using a laser confocal microscope [25]. The mRNA expression levels of iNOS and CD206 were quantitatively assessed by RT-qPCR following CMHs treatment. Furthermore, the mRNA expression levels of TNF-α, IL-1β, Arg-1, and IL-10 were similarly quantified. WB was used to quantitatively assess the expression levels of inflammatory pathway-related proteins (p65, p-p65, and HIF-1α), to investigate the mechanisms through which CMHs regulate inflammatory signaling pathways. Control groups included untreated activated macrophages, normal macrophages, and activated macrophages treated with Hs (100 μg/mL) or MHs (100 μg/mL) [82]. The specific primers used for RT-qPCR are listed in the Supporting Information (Table S2).
4.9. In vitro intestinal mucosal barrier repair performance of CMHs
To evaluate the protective effects of CMHs on colon epithelial cells, Caco-2 cells (2 × 104 cells/well) were placed in 96-well plates and incubated for 24 h. The cells were then treated with DMEM containing H2O2 (500 μM) for a subsequent 24 h. After this, the cells were further incubated in fresh DMEM containing CMHs (100 μg/mL) for 24 h. After treatment, the cells were collected and stained with PI to assess apoptosis. The procedure involved fixing the collected cells with 4 % paraformaldehyde (PFA), followed by the sequential addition of PI working solution and Hoechst 33342 solution (5 μg/mL). Finally, cell morphology was examined using an inverted fluorescence microscope. To evaluate the proliferative effect of CMHs on colon epithelial cells, Caco-2 cells were cultured and treated as described above. After treatment, the cells were cultured in fresh DMEM containing 10 mM EdU for 6 h. Following this, the cells were fixed with 4 % PFA, permeabilized, and stained with Hoechst 33342 (5 μg/mL). Cell proliferation was then observed using an inverted microscope. To evaluate the effect of CMHs on the scratch healing of colon epithelial cells, Caco-2 cells (5 × 104 cells/well) were seeded into a 12-well plate with a three-well Ibidi culture insert and incubated for 24 h at 37 °C. Following the removal of the insert, a 0.5 mm wide scratch was made on the cell monolayer. The cells were then exposed to DMEM containing H2O2 (500 μM) for 24 h. After this, the cells were further incubated in fresh DMEM containing CMHs (100 μg/mL) for an additional 24 h. Cell migration and scratch healing were observed at 0 h and 24 h using an inverted microscope. Finally, the cells were stained with crystal violet and observed under the microscope. The fluorescence intensity and width of the scratch were quantified using ImageJ software to assess healing. The mRNA expression levels of three TJPs (including Claudin-1, Occludin, ZO-1) were quantified by RT-qPCR after treatment with CMHs. The specific primers used for RT-qPCR are listed in the Supporting Information (Table S3). To evaluate the effects of CMHs treatment on mitochondrial function in Caco2 cells, the cells were first treated as described above. The culture medium was then removed, and the cells were incubated with 500 μL of PBS containing 10 μM JC-1 or Hoechst dye and 5 μM Mito-SOX probe for 30 min. The experimental procedure was adapted from the study by Song et al. [83]. After incubation, the cells were washed with PBS, and the intracellular distribution of the probes was observed under an inverted fluorescence microscope. The excitation and emission wavelengths for each probe were set as follows: JC-1 monomers at 514 nm/527 nm, JC-1 aggregates at 585 nm/590 nm, and Mito-SOX at 540 nm/670 nm. Additionally, the mRNA expression levels of three mitochondria-encoded genes (MTCO1, MTND1, and ATP6) were quantified using RT‒qPCR. The specific primers used for RT-qPCR are provided in the Supporting Information (Table S4, Supporting Information). The following control groups were used for the six experiments described above: 1) Caco-2 cells incubated in normal DMEM; 2) Caco-2 cells incubated in DMEM supplemented with 500 μM H2O2; and 3) Caco-2 cells cultured in DMEM supplemented with 500 μM H2O2 and exposed to Hs (100 μg/mL) or MHs (100 μg/mL).
4.10. In vitro anti-oxidant, anti-inflammatory, and mucosal barrier repair mechanisms of CMHs
To investigate the antioxidant, anti-inflammatory, and mucosal barrier repair mechanisms of CMHs, activated macrophages treated with CMHs and Caco-2 cells pre-exposed to H2O2 followed by CMHs treatment were harvested for RNA sequencing. Total RNA was isolated from the cells using TRIzol reagent. The extracted RNA was quantified and its purity assessed using a Nanodrop 2000 spectrophotometer. RNA purity was evaluated by measuring the A260/A280 and A260/A230 ratios, with ideal values of approximately 2.0 and 2.0–2.2, respectively, to ensure the absence of protein or other contaminants. Additionally, RNA integrity was evaluated via an Agilent 5300 Bioanalyzer, and the RNA Integrity Number (RIN) was determined. A RIN value greater than 7 was considered acceptable for subsequent RNA-seq analysis. This study utilized R software (version 4.3.1) to analyze raw count data, employing various R packages for data analysis and visualization. First, the Limma package was applied to normalize the raw counts, ensuring comparability across samples. The normalized data were then used to generate a principal component analysis (PCA) plot, which provided insights into sample variability and potential grouping patterns. Differentially expressed genes (DEGs) were identified based on predefined criteria, with statistical significance and biological relevance determining their importance. A volcano plot was created to visually represent the upregulated and downregulated DEGs, highlighting their distribution and relationships. To further visualize gene expression, a heatmap was generated using the pheatmap package in R, offering detailed view of gene expression patterns across samples. The heatmap offered a more detailed insight into the differences between samples, with color coding emphasizing gene expression levels. Finally, GO and KEGG pathway enrichment analyses were performed on the DEGs using the clusterProfiler package. These analyses revealed the biological processes, cellular components, molecular functions, and signaling pathways related to the DEGs, aiding in the understanding of the underlying biological mechanisms.
4.11. Evaluation of the therapeutic effect of CMHs on DSS-induced UC in mice
Animal experiments were carried out following the ethical guidelines established by the Animal Ethics Committee of Wenzhou Medical University (Approval No: wydw 2024-0269). Female BALB/c mice, aged 6–8 weeks, were purchased from Beijing SPF Biotech Co. (China) and given a one-week acclimatization period before the experiments commenced. UC was induced by providing 3 % (w/v) DSS (MP Biomedicals, Solon, OH, USA) in the drinking water for a period of 7 days. To assess the specific adhesion of CMHs to inflamed colonic tissue, three normal mice and three DSS-induced UC mice were randomly selected. Following a 24-h fasting period, 150 μL of Cy5.5-labeled CMHs (1 mg/mL) was administered rectally. The distribution of Cy5.5-CMHs in the GI tract of both UC and normal mice was monitored at 1, 3, 6, 9, and 12 h via an in vivo imaging system (IVIS Lumina XRMS Series III, PerkinElmer) [84]. At the conclusion of the experiment, the mice were euthanized, and intestinal tissues were harvested for the quantitative assessment of Cy5.5-CMHs fluorescence intensity. To confirm the electrostatic interaction between negatively charged Cy5.5-CMHs and positively charged glycoproteins on the damaged mucosal surface, 150 μL of Cy5.5-Hs (1 mg/mL) and Cy5.5-labeled CMHs (1 mg/mL) were administered rectally. The mice were euthanized at 3, 6, and 9 h after administration, and colonic tissues were collected. The tissues were homogenized, and the supernatant was collected to measure the retention rates of Cy5.5-CMHs and Cy5.5-Hs.
To evaluate the therapeutic effects of CMHs, Mice with DSS-induced UC were randomly assigned to five groups (n = 6 per group). Each group received 150 μL rectal administration of either PBS, 5-ASA (100 μg/mL), CORM-401 (46.35 μg/mL), Hs (1.5 mg/mL), MHs (1.5 mg/mL), or CMHs (1.5 mg/mL) every other day for 11 days. Throughout the treatment, the mice had access to sterile water containing 3 % (w/v) DSS. The control group consisted of seven healthy mice. Daily monitoring was conducted for body weight changes and fecal characteristics, including stool consistency and the presence of blood. DAI was calculated based on the following parameters: Weight change: 0 points (no weight loss); 1 point (≤5 % weight loss); 2 points (6–10 % weight loss); 3 points (11–20 % weight loss); 4 points (≥20 % weight loss); Stool consistency: 0 points (normal); 2 points (loose stools); 4 points (diarrhea); Fecal blood: 0 points (no blood); 2 points (occult blood); 4 points (visible blood) [85]. On day 11, 100 μL of the L-012 probe (5 μg/mL) was injected intraperitoneally into the mice. The presence of residual ROS in the inflamed colon was then monitored using the IVIS Lumina XRMS Series III imaging system. On day 11, mice were orally gavaged with FITC-Dextran (5 mg/100 g, Mw: 3500) to evaluate the ability of CMHs to repair the colonic mucosal barrier. After 4 h, orbital blood samples were collected, and the fluorescence intensity of FITC-Dextran in the serum was measured using a multi-mode microplate reader. The excitation and emission wavelengths for FITC were set to 494 nm and 525 nm, respectively. Plasma levels of FITC-Dextran were quantified using a pre-established standard curve. After the treatment period, all mice were euthanized, and their colons were excised and measured for colon length. For histopathological analysis, colon sections were washed with cold PBS, fixed in formalin, embedded in paraffin, sectioned, and stained with H&E. The levels of ROS and CO in the colon tissues were analyzed via a cryosectioning technique. Histological scoring was then performed according to established criteria to evaluate inflammation and damage in the colon. To further evaluate the antioxidant effects of CMHs, a 2 cm segment of colon tissue was homogenized in 2 mL of cold PBS, followed by centrifugation at 10,000 rpm for 10 min at 4 °C. The activities of CAT, SOD, and GPX were measured using corresponding assay kits. Additionally, for cytokine analysis, homogenized colon tissue was processed similarly, and the levels of IL-4, TNF-α, IL-6, IL-10, and MPO were quantified by ELISA kits. For IF staining, colon sections were incubated with anti-iNOS and anti-CD206 antibodies. After appropriate washing and incubation, the sections were analyzed under a laser confocal microscope to observe the expression of these markers. The protein expression levels of IL-10 and TNF-α in colon tissues were quantitatively analyzed by WB. To evaluate the biosafety of CMHs, orbital blood samples were collected 6 h after administration, and plasma was separated by centrifugation. COHb levels in the plasma were assessed using an ELISA kit. Blood samples were collected for hematological analysis, including WBC, NEUT, HGB, and RBC counts, as well as for biochemical assays of liver function (AST and ALT) and renal function (BUN) using commercial kits according to the manufacturer's instructions [86,87]. To evaluate the effects of CMHs on colonic smooth muscle function, IF was performed by staining the sections with an α-SMA antibody. After the initial 11-day treatment, mice were maintained under standard husbandry conditions for an additional 17 days. Subsequently, the animals were euthanized, and colon tissues along with other major organs were harvested for ICP-OES analysis to quantify Mn ion accumulation in critical organs.
To evaluate the analgesic effects of CMHs, three CMHs-treated UC mice and three PBS-treated UC mice were selected. The behavior of each mouse was monitored daily at fixed time points, and their food and water intake were precisely recorded over an 11-day period to assess behavioral responses. To more accurately evaluate the pain-relieving effect of CMHs in colitits mice, we employed the AWR scoring method, as reported by Yang, with minor modifications [74]. Both healthy mice and those with DSS-induced UC were fasted for 18 h before the experiment but were allowed continuous access to water. Prior to the procedure, the mice were lightly anesthetized using 3 % ether to ensure they were in a state of mild anesthesia. A catheter, lubricated with corkscrew, was inserted through the mouse's anus and advanced to the rectum. One end of the catheter was connected to a gas bag. The catheter was then secured at the base of the tail with adhesive tape, and the mouse was placed flat on a black base plate. After the mice had acclimated and were fully calm, 100 μL, 200 μL, 300 μL, and 400 μL of PBS were slowly injected into the airbag in four separate injections, with a 5-min interval between each. The airbag was maintained in a distended state for 5 s after each injection. The mice were observed for signs of abdominal retraction reflex, after which the injected PBS was withdrawn. The mice were scored based on their responses, and the degree of visceral sensitivity was determined according to the AWR scoring level. (The AWR scale is as follows: 0: No apparent behavioral change. 1: No movement or only simple head movement. 2: Contraction of the abdominal wall muscles without lifting off table. 3: Contraction of the abdominal wall muscles with movement off the table or visible straightening of the body. 4: Arching of the abdominal wall, often accompanied by bowing of the body and pelvis.)
At the end of the experiment, colon tissue samples were collected and incubated with anti-TRPV1 and anti-TACR1 antibodies for IF staining. After appropriate washing and incubation, tissue sections were analyzed using a laser confocal microscope to evaluate the expression of these markers. To evaluate the pain regulation mechanisms of CMHs, mice were euthanized after treatment, and colonic tissue was collected. The tissues were immediately snap-frozen in liquid nitrogen to preserve sample integrity. Total RNA was then extracted from the colonic tissue sections using TRIzol reagent, according to the manufacturer's protocol. The extracted RNA was subjected to transcriptomic sequencing, and the data were analyzed using the previously described methods.
In this study, fresh fecal samples were collected from five groups of mice: a healthy control group, a DSS-induced UC model without treatment, and three treatment groups receiving Hs, MHs, and CMHs, respectively. The samples were promptly snap-frozen in liquid nitrogen post-surgery and stored at −80 °C to maintain stability. Total genomic DNA was extracted using the E.Z.N.A. Soil DNA Kit. The quality and concentration of the extracted DNA were assessed through agarose gel electrophoresis and a NanoDrop 2000 spectrophotometer, ensuring it was suitable for subsequent sequencing. Specific primers (338F and 806R) were employed to amplify the V3-V4 region of the 16S rRNA gene through PCR using an ABI GeneAmp 9700 thermal cycler. This amplification generated crucial sequence data for microbiome analysis, enabling the assessment of gut microbiota composition and differences among the groups. The Illumina MiSeq sequencing platform was utilized to analyze microbiome composition, adhering to the standard procedures provided by Majorbio Bio-Pharm Technology Ltd. For the evaluation of microbiota structure, UPARSE software (version 7.1) was applied to group operational taxonomic units (OTUs) at a 97 % similarity level, reducing redundant data and enhancing the accuracy of the analysis. OUT classification was verified using the RDP classifier version 2.2 against the Silva database. Subsequent analysis and visualization were performed on the Piseno BioCloud platform (https://www.genescloud.cn/home).
4.12. Exploring the mechanisms of CMHs in pain relief
BV-2 and PC-12 cells were cultured using microglial cells- and neuronal cells-specific culture media provided by Wuhan Pricella Bio. To elucidate the pain-alleviating mechanism of CMHs, an in vitro co-culture system was established using a 6-well Transwell plate. PC-12 cells (5 × 105 cells/well) were seeded in the lower chamber, while BV-2 cells (3 × 105 cells/well) were inoculated in the upper chamber. The cells were cultured separately for 24 h. Subsequently, the upper chamber containing BV-2 cells was placed onto the lower chamber containing PC-12 cells for co-culture. The BV-2 cells in the upper chamber were stimulated with lipopolysaccharide (LPS, 5 μg/mL) to induce inflammation, while the cells were exposed to fresh DMEM medium containing CMHs (100 μg/mL). The co-culture system was incubated for 24 h at 37 °C. The viability of PC-12 cells was evaluated using the CCK-8 assay. After 24 h of co-culture, the cells were washed with PBS, and 10 % CCK-8 solution was added to each well. The cells were incubated at 37 °C for 1 h, and the absorbance at 450 nm was measured using a microplate reader. To assess the anti-inflammatory effects of CMHs, the supernatant from BV-2 cells was collected after co-culture. The expression levels of pro-inflammatory cytokines, IL-1β and IL-6, were measured using ELISA. The expression levels of SP in the supernatant of PC-12 cells were also measured using an ELISA kit. For IF staining of TACR1 and intracellular Ca2+ imaging, cell coverslips were first placed in the lower chamber of the Transwell. PC-12 cells were then seeded and treated as previously described. After treatment, the cells were washed with PBS and fixed with 4 % paraformaldehyde. IF staining was performed using a TACR1 antibody, and fluorescence labeling was carried out with the Fluo-4 Ca2+ probe. Finally, the cells were analyzed using a CLSM. To assess the inhibition of Ca2+ channels on the cell membrane of PC-12 cells, the cells from the lower chamber were collected, and WB analysis was performed to evaluate the expression level of the TRPV1 protein.
4.13. Statistical analysis
Results are expressed as the mean ± standard deviation (SD). All data represent at least three independent experiments. Statistical analysis was conducted using unpaired t-tests and one-way analysis of variance (ANOVA) as applicable. Data were analyzed using Prism software. A p-value of less than 0.05 was considered statistically significant (∗p < 0.05), with additional significance levels indicated as ∗∗p ≤ 0.01 and ∗∗∗p ≤ 0.001.
CRediT authorship contribution statement
He Zhang: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Linsa Zhou: Software, Resources, Methodology, Investigation. Qiang Zhou: Visualization, Validation, Software, Resources. Huiyun Wen: Project administration. Hongyang Lu: Software, Resources, Methodology. Jiayu Li: Methodology, Investigation. Li Yu: Methodology, Investigation. Hongyi Li: Methodology, Investigation. Yinci Zhu: Validation, Software. Mengzhu Xu: Supervision, Methodology. Bin Lu: Methodology. Chengge Shi: Visualization, Software. Yanmei Zhang: Resources, Methodology. Xiaowen Hu: Supervision, Project administration. Quazi T.H. Shubhra: Writing – review & editing, Conceptualization. Xiaosong He: Funding acquisition. Xiaojun Cai: Writing – review & editing, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Ethics approval and consent to participate
Animal experiments were carried out following the ethical guidelines established by the Animal Ethics Committee of Wenzhou Medical University (Approval No: wydw 2024-0269) and strictly followed the ethical and welfare requirements of the Animal Experiment Center of Wenzhou Institute of Biomaterials and Engineering during experiments.
Data availability statement
The data that support the findings of this study are available in the supplementary material of this article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (82572425 and 82272150).
Footnotes
Peer review under the responsibility of editorial board of Bioactive Materials.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2025.09.043.
Contributor Information
Huiyun Wen, Email: huiyunwen@nwu.edu.cn.
Xiaosong He, Email: hw990421@wmu.edu.cn.
Xiaojun Cai, Email: cxj520118@njtech.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Dolinger M., Torres J., Vermeire S. Crohn's disease. Lancet. 2024;403(10432):1177–1191. doi: 10.1016/S0140-6736(23)02586-2. [DOI] [PubMed] [Google Scholar]
- 2.Pabla B.S., Schwartz D.A. Assessing severity of disease in patients with ulcerative colitis. Clin. North Am. 2020;49(4):671–688. doi: 10.1016/j.gtc.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan G.G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015;12(12):720–727. doi: 10.1038/nrgastro.2015.150. [DOI] [PubMed] [Google Scholar]
- 4.Stallmach A., Hagel S., Bruns T. Adverse effects of biologics used for treating IBD. Best Pract. Res. Clin. Gastroenterol. 2010;24(2):167–182. doi: 10.1016/j.bpg.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Hindryckx P., Vande Casteele N., Novak G., Khanna R., D'Haens G., Sandborn W.J., Danese S., Jairath V., Feagan B.G. The expanding therapeutic armamentarium for inflammatory bowel disease: how to choose the right drug[s] for our patients? J. Crohns Colitis. 2018;12(1):105–119. doi: 10.1093/ecco-jcc/jjx117. [DOI] [PubMed] [Google Scholar]
- 6.Ferretti F., Cannatelli R., Monico M.C., Maconi G., Ardizzone S. An update on current pharmacotherapeutic options for the treatment of ulcerative colitis. J. Clin. Med. 2022;11(9):2302. doi: 10.3390/jcm11092302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adami G., Saag K.G., Chapurlat R.D., Guanabens N., Haugeberg G., Lems W.F., Matijevic R., Peel N., Poddubnyy D., Geusens P. Balancing benefits and risks in the era of biologics. Ther. Adv. Musculoskelet. Dis. 2019;11 doi: 10.1177/1759720X19883973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell E.L., Colgan S.P. Control and dysregulation of redox signalling in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2019;16(2):106–120. doi: 10.1038/s41575-018-0079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni D., Jiang D., Kutyreff C.J., Lai J., Yan Y., Barnhart T.E., Yu B., Im H.-J., Kang L., Cho S.Y., Liu Z., Huang P., Engle J.W., Cai W. Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat. Commun. 2018;9(1):5421. doi: 10.1038/s41467-018-07890-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang Y., Xu L., Dong J., Yuan X., Ye J., Fan Y., Liu B., Xie J., Ji X. Programmed microalgae-gel promotes chronic wound healing in diabetes. Nat. Commun. 2024;15(1):1042. doi: 10.1038/s41467-024-45101-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Ma S., Chang W., Yu W., Zhang L. Nanozymes targeting mitochondrial repair in disease treatment. J. Biotechnol. 2024;394:57–72. doi: 10.1016/j.jbiotec.2024.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Hong L., Chen G., Cai Z., Liu H., Zhang C., Wang F., Xiao Z., Zhong J., Wang L., Wang Z., Cui W. Balancing microthrombosis and inflammation via injectable protein hydrogel for inflammatory bowel disease. Adv. Sci. 2022;9(20) doi: 10.1002/advs.202200281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brioukhanov A.L., Netrusov A.I. Aerotolerance of strictly anaerobic microorganisms and factors of defense against oxidative stress: a review. Appl. Biochem. Microbiol. 2007;43:567–582. [PubMed] [Google Scholar]
- 14.Yang J., Ye J., Li R., Li R., Liu X., Han J., Yang Y., Ran N., Yuan M., Zhang Z., Chong W., Ji X. Nanozyme-functionalized microalgal biohybrid microrobots in inflammatory bowel disease treatment. Biomaterials. 2025;319 doi: 10.1016/j.biomaterials.2025.123231. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y.-H., Shangguan W.-J., Zhao Z.-J., Zhou F.-C., Liu H.-T., Liang Z.-H., Song J., Shao J. Naringin inhibits apoptosis induced by cyclic stretch in rat annular cells and partially attenuates disc degeneration by inhibiting the ROS/NF-κB pathway. Oxid. Med. Cell. Longev. 2022;2022 doi: 10.1155/2022/6179444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan M.J., Liu Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21(1):103–115. doi: 10.1038/cr.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao Y., Xue B., Lessing D.J., Chu W. Probiotics-loaded selenium nanoparticles effectively alleviated DSS-Induced colitis by scavenging ROS and remodeling intestinal innate immune and microbiota. Adv. Ther. 2024;7(11) [Google Scholar]
- 18.Wang C., Wu L., Zhou R., Song C., Chen P., Huang S., Ali Khan A., Lu D., Hu Y., Chen L. Integration of microbiota and metabolomics reveals the analgesic mechanisms of emodin against neuropathic pain. Int. Immunopharmacol. 2023;125 doi: 10.1016/j.intimp.2023.111170. Pt A. [DOI] [PubMed] [Google Scholar]
- 19.Ruan Y., Ling J., Ye F., Cheng N., Wu F., Tang Z., Cheng X., Liu H. Paeoniflorin alleviates CFA-induced inflammatory pain by inhibiting TRPV1 and succinate/SUCNR1-HIF-1α/NLPR3 pathway. Int. Immunopharmacol. 2021;101 doi: 10.1016/j.intimp.2021.108364. Pt B. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J., Cao X., Wen H., Shubhra Q.T.H., Hu X., He X., Cai X. Integrative gas-based therapeutics for cancer treatment: recent advances and multi-therapy innovations. Coord. Chem. Rev. 2025;539 [Google Scholar]
- 21.Cai X., Tian J., Zhu J., Chen J., Li L., Yang C., Chen J., Chen D. Photodynamic and photothermal co-driven CO-enhanced multi-mode synergistic antibacterial nanoplatform to effectively fight against biofilm infections. Chem. Eng. J. 2021;426 [Google Scholar]
- 22.Ma W., Chen X., Fu L., Zhu J., Fan M., Chen J., Yang C., Yang G., Wu L., Mao G., Yang X., Mou X., Gu Z., Cai X. Ultra-efficient antibacterial system based on photodynamic therapy and co gas therapy for synergistic antibacterial and ablation biofilms. ACS Appl. Mater. Interfaces. 2020;12(20):22479–22491. doi: 10.1021/acsami.0c01967. [DOI] [PubMed] [Google Scholar]
- 23.Bansal S., Liu D., Mao Q., Bauer N., Wang B. Carbon monoxide as a potential therapeutic agent: a molecular analysis of its safety profiles. J. Med. Chem. 2024;67(12):9789–9815. doi: 10.1021/acs.jmedchem.4c00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu H., Zhou Q., Li J., Xu S., Yu L., Zhu Y., Zhang H., Shi C., Zuo T., Xu M., Su M., Zhang Y., Hu R., Shubhra Q.T.H., Deng H., Hu X., Cai X. CO-releasing polyoxometalates nanozyme with gut mucosal immunity and microbiota homeostasis remodeling effects for restoring intestinal barrier integrity. Adv. Sci. 2025;12(17) doi: 10.1002/advs.202500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng Y., Fan M., Zhou Q., Chen D., Jin T., Mu Z., Li L., Chen J., Qiu D., Zhang Y., Pan Y., Shen X., Cai X. Reactive oxygen species-activated co versatile nanomedicine with innate gut immune and microbiome remodeling effects for treating inflammatory bowel disease. Adv. Funct. Mater. 2023;33(49) [Google Scholar]
- 26.Zhang X., Yuan Z., Wu J., He Y., Lu G., Zhang D., Zhao Y., Wu R., Lv Y., Cai K., He S. An orally-administered nanotherapeutics with carbon monoxide supplying for inflammatory bowel disease therapy by scavenging oxidative stress and restoring gut immune homeostasis. ACS Nano. 2023;17(21):21116–21133. doi: 10.1021/acsnano.3c04819. [DOI] [PubMed] [Google Scholar]
- 27.Kaczara P., Proniewski B., Lovejoy C., Kus K., Motterlini R., Abramov A.Y., Chlopicki S. CORM-401 induces calcium signalling, no increase and activation of pentose phosphate pathway in endothelial cells. FEBS J. 2018;285(7):1346–1358. doi: 10.1111/febs.14411. [DOI] [PubMed] [Google Scholar]
- 28.Hervera A., Leánez S., Negrete R., Motterlini R., Pol O. Carbon monoxide reduces neuropathic pain and spinal microglial activation by inhibiting nitric oxide synthesis in mice. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0043693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao L., Zhuang J., Nie L., Zhang J., Zhang Y., Gu N., Wang T., Feng J., Yang D., Perrett S., Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007;2(9):577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 30.Gao L., Wei H., Dong S., Yan X. Nanozymes. Adv. Mater. 2024;36(10) doi: 10.1002/adma.202305249. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F., Kang Y., Feng L., Xi G., Chen W., Kong N., Tao W., Luan T., Koo S., Ji X. Infected wound repair with an ultrasound-enhanced nanozyme hydrogel scaffold. Mater. Horiz. 2023;10(12):5474–5483. doi: 10.1039/d3mh01054f. [DOI] [PubMed] [Google Scholar]
- 32.Feng K., Wang Z., Wang S., Wang G., Dong H., He H., Wu H., Ma M., Gao X., Zhang Y. Elucidating the catalytic mechanism of prussian blue nanozymes with self-increasing catalytic activity. Nat. Commun. 2024;15(1):5908. doi: 10.1038/s41467-024-50344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim J., Kim H.Y., Song S.Y., Go S.-h., Sohn H.S., Baik S., Soh M., Kim K., Kim D., Kim H.-C., Lee N., Kim B.-S., Hyeon T. Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano. 2019;13(3):3206–3217. doi: 10.1021/acsnano.8b08785. [DOI] [PubMed] [Google Scholar]
- 34.Feng Y., Luo X., Li Z., Fan X., Wang Y., He R.-R., Liu M. A ferroptosis-targeting ceria anchored halloysite as orally drug delivery system for radiation colitis therapy. Nat. Commun. 2023;14(1):5083. doi: 10.1038/s41467-023-40794-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu S., Nan F., Zhang K., Hao W., Shi D., Li Y., Deng W., Jarhen N., Li K., Xiao Y., Li J., Lin X. A novel metal-organic framework encapsulated iridium oxide nanozyme enhanced antisense oligonucleotide combo for osteoarthritis synergistic therapy. Aggregate. 2024;5(6) [Google Scholar]
- 36.Wu A., Li M., Chen Y., Zhang W., Li H., Chen J., Gu K., Wang X. Multienzyme active manganese oxide alleviates acute liver injury by mimicking redox regulatory system and inhibiting ferroptosis. Adv. Healthc. Mater. 2024;13(11) doi: 10.1002/adhm.202302556. [DOI] [PubMed] [Google Scholar]
- 37.Gao F.L., Wu J., Gao H.Q., Hu X.Y., Liu L.H., Midgley A.C., Liu Q.Q., Sun Z.Y., Liu Y.J., Ding D., Wang Y.M., Kong D.L., Huang X.L. Hypoxia-tropic nanozymes as oxygen generators for tumor-favoring theranostics. Biomaterials. 2020;230 doi: 10.1016/j.biomaterials.2019.119635. [DOI] [PubMed] [Google Scholar]
- 38.Han J., Ye J., Shi J., Fan Y., Yuan X., Li R., Niu G., Abubakar M., Kang Y., Ji X. A programmable oral nanomotor microcapsule for the treatment of inflammatory bowel disease. Adv. Funct. Mater. 2025;35(3) [Google Scholar]
- 39.Crook S.H., Mann B.E., Meijer A.J., Adams H., Sawle P., Scapens D., Motterlini R. [Mn(CO)4{S2CNMe(CH2CO2H)}], a new water-soluble CO-releasing molecule. Dalton Trans. 2011;40(16):4230–4235. doi: 10.1039/c1dt10125k. [DOI] [PubMed] [Google Scholar]
- 40.Fayad-Kobeissi S., Ratovonantenaina J., Dabiré H., Wilson J.L., Rodriguez A.M., Berdeaux A., Dubois-Randé J.L., Mann B.E., Motterlini R., Foresti R. Vascular and angiogenic activities of CORM-401, an oxidant-sensitive CO-releasing molecule. Biochem. Pharmacol. 2016;102:64–77. doi: 10.1016/j.bcp.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 41.Wilson J.L., Bouillaud F., Almeida A.S., Vieira H.L., Ouidja M.O., Dubois-Randé J.L., Foresti R., Motterlini R. Carbon monoxide reverses the metabolic adaptation of microglia cells to an inflammatory stimulus. Free Radic. Biol. Med. 2017;104:311–323. doi: 10.1016/j.freeradbiomed.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Lin X., Zhang D., Chen X., Liu M. Self-cross-linked oxidized sodium alginate/gelatin/halloysite hydrogel as injectable, adhesive, antibacterial dressing for hemostasis. ACS Sustain. Chem. Eng. 2024;12(31):11739–11753. [Google Scholar]
- 43.Cai L., Li N., Zhang Y., Gu H., Zhu Y. Microfluidics-derived microcarrier systems for oral delivery. Biomed. Tech. 2023;1:30–38. [Google Scholar]
- 44.Murugesan S., Scheibel T. Copolymer/clay nanocomposites for biomedical applications. Adv. Funct. Mater. 2020;30(17) [Google Scholar]
- 45.Homayun B., Choi H.-J. Halloysite nanotube-embedded microparticles for intestine-targeted co-delivery of biopharmaceuticals. Int. J. Pharm. 2020;579 doi: 10.1016/j.ijpharm.2020.119152. [DOI] [PubMed] [Google Scholar]
- 46.Fidecka K., Giacoboni J., Picconi P., Vago R., Licandro E. Quantification of amino groups on halloysite surfaces using the Fmoc-method. RSC Adv. 2020;10(24):13944–13948. doi: 10.1039/d0ra01994a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu L., Cai X., Zhu H., Li J., Shi D., Su D., Yue D., Gu Z. PDT-driven highly efficient intracellular delivery and controlled release of CO in combination with sufficient singlet oxygen production for synergistic anticancer therapy. Adv. Funct. Mater. 2018;28(41) [Google Scholar]
- 48.Chen J., Mu Z., Chen D., Huang C., Jin T., Li L., Zeng Y., Zhou Q., Zhang Y., Mao H., Deng H., Shen X., Yang H., Cai X. H2S-releasing versatile hydrogel dressing with potent antimicrobial, anti-inflammatory, epithelialization and angiogenic capabilities for diabetic wound healing. Chem. Eng. J. 2023;469 [Google Scholar]
- 49.Zhang Y., Choksi S., Chen K., Pobezinskaya Y., Linnoila I., Liu Z.-G. ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 2013;23(7):898–914. doi: 10.1038/cr.2013.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang P., Geng J., Gao J., Zhao H., Li J., Shi Y., Yang B., Xiao C., Linghu Y., Sun X., Chen X., Hong L., Qin F., Li X., Yu J.-S., You H., Yuan Z., Zhou D., Johnson R.L., Chen L. Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 2019;10(1):755. doi: 10.1038/s41467-019-08680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vergadi E., Ieronymaki E., Lyroni K., Vaporidi K., Tsatsanis C. Akt signaling pathway in macrophage activation and M1/M2 polarization. J. Immunol. 2017;198(3):1006–1014. doi: 10.4049/jimmunol.1601515. [DOI] [PubMed] [Google Scholar]
- 52.Pan J., Zhang L., Li D., Li Y., Lu M., Hu Y., Sun B., Zhang Z., Li C. Hypoxia-inducible factor-1: regulatory mechanisms and drug therapy in myocardial infarction. Eur. J. Pharmacol. 2024;963 doi: 10.1016/j.ejphar.2023.176277. [DOI] [PubMed] [Google Scholar]
- 53.Görlach A., Bonello S. The cross-talk between NF-κB and HIF-1: further evidence for a significant liaison. Biochem. J. 2008;412(3):e17–e19. doi: 10.1042/BJ20080920. [DOI] [PubMed] [Google Scholar]
- 54.Yang R., Hui Q., Jiang Q., Liu S., Zhang H., Wu J., Lin F., O K., Yang C. Effect of manitoba-grown red-osier dogwood extracts on recovering caco-2 cells from H2O2-induced oxidative damage. Antioxidants. 2019;8(8):250. doi: 10.3390/antiox8080250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahman S., Ghiboub M., Donkers J.M., van de Steeg E., van Tol E.A.F., Hakvoort T.B.M., de Jonge W.J. The progress of intestinal epithelial models from cell lines to gut-on-chip. Int. J. Mol. Sci. 2021;22(24) doi: 10.3390/ijms222413472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inczefi O., Bacsur P., Resál T., Keresztes C., Molnár T. The influence of nutrition on intestinal permeability and the microbiome in health and disease. Front. Nutr. 2022;9 doi: 10.3389/fnut.2022.718710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gong Y., Liu Z., Zhou P., Li J., Miao Y.-B. Biomimetic nanocarriers harnessing microbial metabolites usher the path for brain disease therapy. Nano TransMed. 2023;2(4) [Google Scholar]
- 58.Liu H., Cai Z., Wang F., Ruan H., Zhang C., Zhong J., Wang Z., Cui W. Platelet membrane fragment self-assembled oral hydrogel microspheres for restoring intestinal microvascular injury. Adv. Funct. Mater. 2023;33(33) [Google Scholar]
- 59.Stolfi C., Maresca C., Monteleone G., Laudisi F. Implication of intestinal barrier dysfunction in gut dysbiosis and diseases. Biomedicines. 2022;10(2):289. doi: 10.3390/biomedicines10020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhai Z., Zhang Y., Liang X., Li J., Chen Z., Zhang J., Li W., Wang T., He Q., Li F., Meng Q., Cao J., Su Z., Chang Y., Chen X., Hong A. Acesulfame potassium triggers inflammatory bowel disease via the inhibition of focal adhesion pathway. J. Hazard. Mater. 2024;476 doi: 10.1016/j.jhazmat.2024.134901. [DOI] [PubMed] [Google Scholar]
- 61.Tirosh B., Khatib N., Barenholz Y., Nissan A., Rubinstein A. Transferrin as a luminal target for negatively charged liposomes in the inflamed colonic mucosa. Mol. Pharm. 2009;6(4):1083–1091. doi: 10.1021/mp9000926. [DOI] [PubMed] [Google Scholar]
- 62.Canny G., Levy O., Furuta G.T., Narravula-Alipati S., Sisson R.B., Serhan C.N., Colgan S.P. Lipid mediator-induced expression of bactericidal/permeability-increasing protein (BPI) in human mucosal epithelia. Proc. Natl. Acad. Sci. USA. 2002;99(6):3902–3907. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erben U., Loddenkemper C., Doerfel K., Spieckermann S., Haller D., Heimesaat M.M., Zeitz M., Siegmund B., Kühl A.A. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int. J. Clin. Exp. Pathol. 2014;7(8):4557–4576. [PMC free article] [PubMed] [Google Scholar]
- 64.Ma J., Piao X., Mahfuz S., Long S., Wang J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022;9:159–174. doi: 10.1016/j.aninu.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li J., Song J., Deng Z., Yang J., Wang X., Gao B., Zhu Y., Yang M., Long D., Luo X., Zhang M., Zhang M., Li R. Robust reactive oxygen species modulator hitchhiking yeast microcapsules for colitis alleviation by trilogically intestinal microenvironment renovation. Bioact. Mater. 2024;36:203–220. doi: 10.1016/j.bioactmat.2024.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou A., Yuan Y., Yang M., Huang Y., Li X., Li S., Yang S., Tang B. Crosstalk between the gut microbiota and epithelial cells under physiological and infectious conditions. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.832672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Naidovski N., Chong S.K.T., Liu F., Riordan S.M., Wehrhahn M.C., Yuwono C., Zhang L. Human macrophage response to the emerging enteric pathogen aeromonas veronii: inflammation, apoptosis, and downregulation of histones. Virulence. 2025;16(1) doi: 10.1080/21505594.2024.2440554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li C., Zhang P., Xie Y., Wang S., Guo M., Wei X., Zhang K., Cao D., Zhou R., Wang S., Song X., Zhu S., Pan W. Enterococcus-derived tyramine hijacks α2A-adrenergic receptor in intestinal stem cells to exacerbate colitis. Cell Host Microbe. 2024;32(6):950–963.e8. doi: 10.1016/j.chom.2024.04.020. [DOI] [PubMed] [Google Scholar]
- 69.Hu S., Bourgonje A.R., Gacesa R., Jansen B.H., Björk J.R., Bangma A., Hidding I.J., van Dullemen H.M., Visschedijk M.C., Faber K.N., Dijkstra G., Harmsen H.J.M., Festen E.A.M., Vich Vila A., Spekhorst L.M., Weersma R.K. Mucosal host-microbe interactions associate with clinical phenotypes in inflammatory bowel disease. Nat. Commun. 2024;15(1):1470. doi: 10.1038/s41467-024-45855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen G., Ran X., Li B., Li Y., He D., Huang B., Fu S., Liu J., Wang W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine. 2018;30:317–325. doi: 10.1016/j.ebiom.2018.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wils P., Caron B., D'Amico F., Danese S., Peyrin-Biroulet L. Abdominal pain in inflammatory bowel diseases: a clinical challenge. J. Clin. Med. 2022;11(15):4269. doi: 10.3390/jcm11154269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Basso L., Bourreille A., Dietrich G. Intestinal inflammation and pain management. Curr. Opin. Pharmacol. 2015;25:50–55. doi: 10.1016/j.coph.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 73.Cortright D.N., Matson D.J., Broom D.C. New frontiers in assessing pain and analgesia in laboratory animals. Expet Opin. Drug Discov. 2008;3(9):1099–1108. doi: 10.1517/17460441.3.9.1099. [DOI] [PubMed] [Google Scholar]
- 74.Yang D., Jacobson A., Meerschaert K.A., Sifakis J.J., Wu M., Chen X., Yang T., Zhou Y., Anekal P.V., Rucker R.A., Sharma D., Sontheimer-Phelps A., Wu G.S., Deng L., Anderson M.D., Choi S., Neel D., Lee N., Kasper D.L., Jabri B., Huh J.R., Johansson M., Thiagarajah J.R., Riesenfeld S.J., Chiu I.M. Nociceptor neurons direct goblet cells via a CGRP-RAMP1 axis to drive mucus production and gut barrier protection. Cell. 2022;185(22):4190–4205.e25. doi: 10.1016/j.cell.2022.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arzamendi M.J., Habibyan Y.B., Defaye M., Shute A., Baggio C.H., Chan R., Ohland C., Bihan D.G., Lewis I.A., Sharkey K.A., McCoy K.D., Altier C., Geuking M.B., Nasser Y. Sex-specific post-inflammatory dysbiosis mediates chronic visceral pain in colitis. Gut Microbes. 2024;16(1) doi: 10.1080/19490976.2024.2409207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wozniak K.M., Rojas C., Wu Y., Slusher B.S. The role of glutamate signaling in pain processes and its regulation by GCP II inhibition. Curr. Med. Chem. 2012;19(9):1323–1334. doi: 10.2174/092986712799462630. [DOI] [PubMed] [Google Scholar]
- 77.Li D.-Y., Gao S.-J., Sun J., Zhang L.-Q., Wu J.-Y., Song F.-H., Liu D.-Q., Zhou Y.-Q., Mei W. Targeting the nitric oxide/cGMP signaling pathway to treat chronic pain. Neural. Regen. Res. 2023;18(5):996–1003. doi: 10.4103/1673-5374.355748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weiss N., Zamponi G.W. Genetic t-type calcium channelopathies. J. Med. Genet. 2020;57(1):1–10. doi: 10.1136/jmedgenet-2019-106163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Verma-Gandhu M., Verdu E.F., Bercik P., Blennerhassett P.A., Al-Mutawaly N., Ghia J.-E., Collins S.M. Visceral pain perception is determined by the duration of colitis and associated neuropeptide expression in the mouse. Gut. 2007;56(3):358–364. doi: 10.1136/gut.2006.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li F., Yang W., Jiang H., Guo C., Huang A.J.W., Hu H., Liu Q. TRPV1 activity and substance P release are required for corneal cold nociception. Nat. Commun. 2019;10(1):5678. doi: 10.1038/s41467-019-13536-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zou J., Jiang K., Chen Y., Ma Y., Xia C., Ding W., Yao M., Lin Y., Chen Y., Zhao Y., Gao F. Tofacitinib citrate coordination-based Dual-Responsive/Scavenge nanoplatform toward regulate colonic inflammatory microenvironment for relieving colitis. Adv. Healthc. Mater. 2024;13(30) doi: 10.1002/adhm.202401869. [DOI] [PubMed] [Google Scholar]
- 82.Yang G., Fan M., Zhu J., Ling C., Wu L., Zhang X., Zhang M., Li J., Yao Q., Gu Z., Cai X. A multifunctional anti-inflammatory drug that can specifically target activated macrophages, massively deplete intracellular H2O2, and produce large amounts CO for a highly efficient treatment of osteoarthritis. Biomaterials. 2020;255 doi: 10.1016/j.biomaterials.2020.120155. [DOI] [PubMed] [Google Scholar]
- 83.Song X., Huang Q., Yang Y., Ma L., Liu W., Ou C., Chen Q., Zhao T., Xiao Z., Wang M., Jiang Y., Yang Y., Zhang J., Nan Y., Wu W., Ai K. Efficient therapy of inflammatory bowel disease (IBD) with highly specific and durable targeted Ta2C modified with chondroitin sulfate (TACS) Adv. Mater. 2023;35(36) doi: 10.1002/adma.202301585. [DOI] [PubMed] [Google Scholar]
- 84.Fu W., Huang Z., Li W., Xu L., Yang M., Ma Y., Liu H., Qian H., Wang W. Copper–luteolin nanocomplexes for mediating multifaceted regulation of oxidative stress, intestinal barrier, and gut microbiota in inflammatory bowel disease. Bioact. Mater. 2025;46:118–133. doi: 10.1016/j.bioactmat.2024.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang R., Chen T., Wang Q., Yuan X.-M., Duan Z.-L., Feng Z.-Y., Ding Y., Bu F., Shi G.-P., Chen Y.-G. Total flavone of abelmoschus manihot ameliorates stress-induced microbial alterations drive intestinal barrier injury in DSS colitis. Drug Des. Dev. Ther. 2021;15:2999–3016. doi: 10.2147/DDDT.S313150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang Q., Yang Y., Zhu Y., Chen Q., Zhao T., Xiao Z., Wang M., Song X., Jiang Y., Yang Y., Zhang J., Xiao Y., Nan Y., Wu W., Ai K. Oral metal-free melanin nanozymes for natural and durable targeted treatment of inflammatory bowel disease (IBD) Small. 2023;19(19) doi: 10.1002/smll.202207350. [DOI] [PubMed] [Google Scholar]
- 87.Lv Y., Ren M., Yao M., Zou J., Fang S., Wang Y., Lan M., Zhao Y., Gao F. Colon-specific delivery of methotrexate using hyaluronic acid modified pH-responsive nanocarrier for the therapy of colitis in mice. Int. J. Pharm. 2023;635 doi: 10.1016/j.ijpharm.2023.122741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article.