Abstract
The mechanism of epitope mimicry could have implications for the safety of vaccine development. OmpA is one of the most promising antigens of Acinetobacter baumannii. This property convinced us to investigate OmpA’s potential to trigger autoimmune responses. To this end, BLAST searches were performed using the OmpA sequence and its overlapping peptides to find identical peptides in the human proteome. These peptides were analyzed for their epitopic and HLA binding properties. The population coverage was then calculated for the peptides. OmpA showed high identity among numerous strains of A. baumannii and had no similar counterparts in the human proteome. Three OmpA peptides (TKNYDSKI, LSLARANS, and GQEAAAPA) shared identity and similarity with human proteins. Amongst, LSLARANS, which was found in Isthmin-1, the one with the highest potential to induce autoimmune responses was identified. LSLARANS was found in a B-cell positive assay of OmpA and acted as an HLA binder (such as HLA-A*03:01). Approximately 18% of the world’s population were determined to be more susceptible to probable A. baumannii-post-infection autoimmune disease, which could be rooted in OmpA similarity. Mutation sensitivity analyses indicated that the TKNYDSKI peptide is sensitive to engineering and modification. Given these circumstances, these peptides should be avoided in vaccine design efforts to reduce the risk of autoimmune responses.
Graphical Abstract
Keywords: A. baumannii, OmpA, Autoantibody, Epitope, Autoimmune disease, Vaccination
Introduction
Epitope mimicry is suggested as a mechanism to trigger autoimmune responses. These autoimmune responses could be associated with microbial infections and vaccinations [1]. Autoimmune responses could be deemed significant implications for the safety of vaccine candidates, which is considered one of the most controversial and pivotal criteria in vaccine design and development efforts [1, 2]. To date, several debates addressing this issue have emerged regarding commercial vaccines [1–7]. Adverse effects such as arthritis, dermatomyositis, Takayasu’s arteritis (TA), and reactive arthritis (ReA) have already been reported for the Bacillus Calmette-Guérin (BCG) vaccine [2]. Moreover, the Hepatitis B virus (HBV) vaccine is reported to be associated with autoimmune neuromuscular disorders (including optic neuritis, myelitis, and multiple sclerosis (MS)), Guillain-Barre syndrome (GBS), systemic lupus erythematosus (SLE), myopathy, antiphospholipid syndrome (APS), arthritis, and vasculitis [2]. It has been suggested that cross-reactivity between epitopes of HBV antigen (HBsAg) and vaccine ingredients is involved in the induction of autoimmune responses [3]. The Human papilloma virus (HPV) vaccine is another challenging vaccine for autoimmune responses, which is involved in several autoimmune diseases such as vasculitis, MS, GBS, Acute disseminated encephalomyelitis (ADEM), postural orthostatic tachycardia syndrome (POTS), Transverse Myelitis (TM), primary ovarian failure (POF), SLE, pancreatitis, Autoimmune hepatitis (AH), and immune thrombocytopenic purpura (ITP) [2]. In the case of SARS-CoV-2, elevated autoantibody serum levels (particularly IgG and IgA) have mostly been reported in patients with moderate or severe COVID-19 infection [8]. Higher levels of autoantibodies (mainly IgGs, mostly in severe COVID-19 patients) have been reported for the elderly compared with young COVID-19 patients [9]. Recently, it has been demonstrated that the proteome of SARS-CoV-2 shares identical peptides with the human proteome. It has been suggested that these peptides could be involved in the induction of post-infection and post-vaccination autoimmune responses [6].
Multi-drug-resistant (MDR) Acinetobacter baumannii strains have emerged as serious superbugs in hospitals [10, 11]. A. baumannii is placed among the six most dangerous microbes listed by the Infectious Diseases Society of America (IDSA) [12]. This pathogen has also been designated as a priority pathogen needing new antibiotics by the World Health Organization (WHO) [11]. Difficulty in clinical management [13] and high mortality rates (more than 70%) [14] have motivated the researchers to introduce more efficient antibiotics against A. baumannii-associated infections. However, failed efforts to introduce such commercial antibiotics [15] have encouraged researchers to pursue vaccination trials as an alternative strategy [16–18].
Among the several antigens that are evaluated for vaccination against A. baumannii [16–18], OmpA is one of the most progressed ones [19–28]. Outer membrane protein A is a member of unique integral membrane proteins, which has been extensively studied in Escherichia coli. This antigen has garnered a lot of attention due to its attributed key functions, potential for therapeutic targeting [29], and immunogenicity. OmpA is a major antigen of A. baumannii, which elicits specific antibodies in mice and humans. Active and passive immunization of mice with OmpA or anti-OmpA sera could develop protection against intravenous challenge with a lethal dose of extreme drug-resistant A. baumannii [27]. Mucosal immunization of mice with OmpA confers protection against MDR A. baumannii [25]. OmpA has also been administered in combination with other antigens. A combination of OmpA and a subunit of biofilm-associated protein (Bap) has shown 100% survival against A. baumannii ATCC 19,606 in a sepsis murine model, while the single OmpA showed less than 80% survival [30]. A combination of OmpA and BauA has reduced the bacterial load in the spleen and liver of immunized mice (in a sepsis model) [19]. Passive immunization of mice has also been investigated with specific egg yolk antibodies (IgYs), which were developed against OmpA, Omp34, and a combination of these antigens. The highest protection was conferred by anti-OmpA IgYs, while the lowest was for anti-OmpA + Omp34 IgYs [22, 23]. OmpA has also been investigated as a DNA vaccine platform. Administration of the OmpA DNA vaccine has developed partial protection against 5 × LD50 of A. baumannii LAC-4 in a murine pneumonia model. A DNA vaccine harboring both OmpA and Pal genes showed improved protection against the administered dose of bacteria [31].
Although OmpA has been vastly studied in animal models, no clinical trials have been reported for this antigen as well as other antigens of A. baumannii. Inadequate data about the safety of OmpA and other antigens could act as an obstacle to their clinical evaluations. Cytotoxicity and autoimmune responses are two aspects of vaccine safety that should be assessed for vaccine candidates before clinical examinations. Since OmpA is a cytotoxic protein, several approaches have been suggested to evade this drawback [24]. Based on a BLAST search, Luo et al. reported that OmpA shares minimal homology with human proteins while it is highly conserved across multiple clinical isolates [27]. However, to the best of our knowledge, no comprehensive reports are available on potential autoimmune responses triggered by OmpA. The existence of OmpA epitopes, which are identical to the sequence of epitopes from human proteins, has not been precisely investigated. Administration of OmpA as a vaccine candidate may trigger autoimmune responses due to epitope mimicry if the human proteins share identical epitopes with the OmpA sequence. In the current study, we aimed to delve into the safety of OmpA, which could trigger autoimmune responses via an in silico approach. Insights regarding the potentially autoimmune regions of OmpA would bring about more informed decisions in the design of peptide-based vaccines against A. baumannii.
Methods
Sequence retrieval and BLAST search
An overview of the workflow is provided as a graphical abstract (Fig. 1). The reference sequence of OmpA (accession No: WP_000777878.1) was obtained in FASTA format from the NCBI protein database. A BLAST search limited to A. baumannii (taxid: 470) was run on the OmpA protein sequence against a non-redundant protein database to evaluate the prevalence and conservancy of OmpA in this species. Hits showing identity and query coverage of ≥ 90% were included in the analysis. The selected identity and query coverage could retrieve hits with an overall >80% identity. Moreover, a tBLASTn limited to Homo sapiens (taxid: 9606) and mice (taxid: 10088) was also run on the sequence against the non-redundant protein database to find any potential homologous proteins in these organisms. Those results with an E-value of < 0.001 were considered significant hits. The “Expect Value” could represent the probability of the alignment occurrence by chance based on the size of the database searched. A higher “E-Value” threshold is less stringent than the BLAST. “E-Values” less than 0.001 could restrict the alignments shown to those of high quality [32].
Fig. 1.
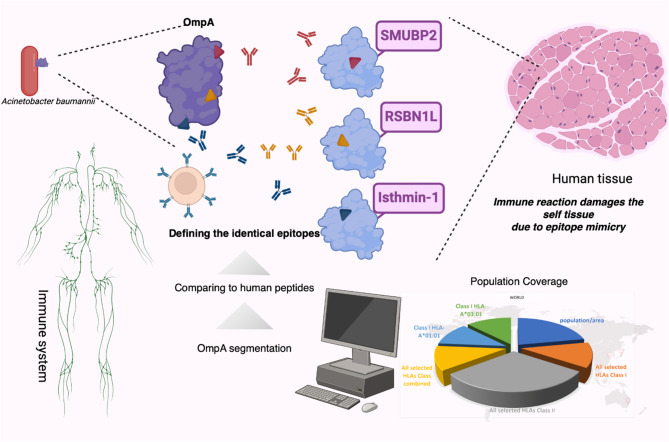
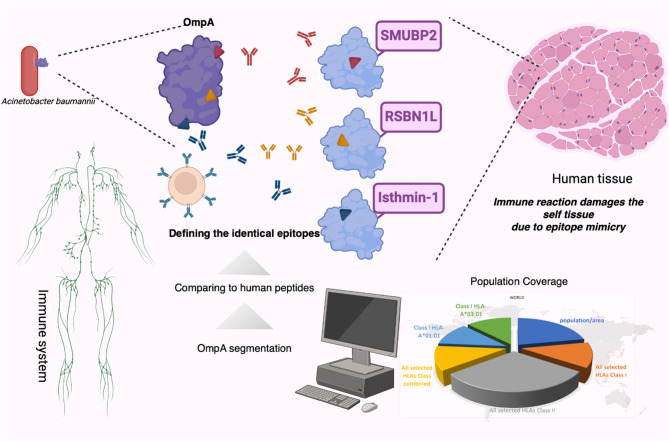
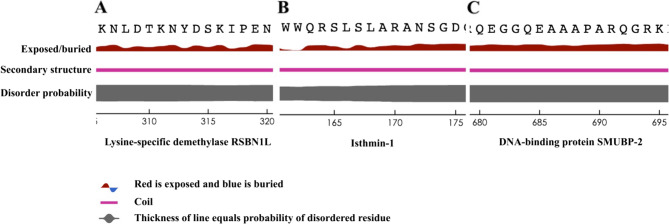
OmpA is a well-known antigen of a notorious pathogen, Acinetobacter baumannii. To investigate the safety of OmpA regarding the induction of autoimmune responses, the sequence was segmented into 8-meric peptides to find identical peptides within the human proteome. Similar experimentally confirmed and predicted epitopes and HLA binders were considered. The population coverage was calculated for the peptides and HLAs. Lysin-specific demethylase RSBN1L, Isthmin-1, and DNA-binding protein SUMBP2 are human proteins that are expressed in a wide array of body tissues. Three short peptides that are epitopes in OmpA can be found identically in three human proteins separately. About 18% of the world's population was predicted to be more susceptible to A. baumannii-post infection autoimmune disease related to OmpA. The Figure was drawn using the BioRender online server (BioRender.com)
A phylogenetic tree was constructed based on OmpA protein sequences of Gram-negative bacteria using NCBI BLAST [1] and iTOL visualization tools [2] at https://blast.ncbi.nlm.nih.gov and https://itol.embl.de, respectively. OmpA protein sequences were retrieved through BLAST searches against the NCBI clustered non-redundant protein database, in which A. baumannii sequences (taxid:470) were excluded. BLAST-based phylogenetic reconstruction employed pairwise alignment scores between query sequences and database hits. Phylogenetic trees were visualized using iTOL.
Sequence conservancy
To find conserved regions of OmpA in A. baumannii, a multiple sequence alignment was performed. All sequences with identity and query coverage of ≥ 90%, obtained from the BLAST search limited to A. baumannii (taxid: 470) in the previous step, were aligned together by PRALINE [33] at http://www.ibi.vu.nl/programs/pralinewww/. PRALINE alignment parameters were configured using a BLOSUM62 substitution matrix, a gap-opening penalty of 12, and a gap extension penalty of 1. Progressive alignment was performed with PSI-BLAST homology extension over three iterations at an E-value threshold of 0.01.
Similar epitopes and peptide screening
The sequence of mature OmpA [24] was segmented into overlapping 8- to 10-meric peptides offset by 1-amino-acid step size. To find identical peptides in the human proteome, the generated peptides were submitted to the peptide search tool of Uniprot (Release 2020_01 of 26-Feb-2020) [34] at https://www.uniprot.org/peptidesearch/. The search was restricted to the Homo sapiens proteome.
All structures of human proteins encompassing identical peptides were obtained from the Alpha Fold [35, 36] project by the relevant link provided at the UniProt knowledge base. The identical peptides were mapped onto the relevant structures.
Two approaches were followed to ascertain the surface accessibility of peptide sequences within human proteins. The first approach was a structure-based analysis. To this end, a meticulous examination of the structures of three distinct human proteins (containing the predicted peptides) was conducted. This investigation aimed to precisely determine the spatial placement of the predicted epitopes. The second approach was the sequence-based analysis of the peptides. In this regard, the surface accessibility of these peptides within the native protein sequences was assessed using Netsurf ver. 3 [37], accessible at https://services.healthtech.dtu.dk/services/NetSurfP-3.0/.
Conservancy of the identical peptides was assessed among other ESKAPE pathogens, viz. Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterobacter species. In this regard, the found identical peptides of human were submitted to the peptide search tool of UniProt [34] and the search was restricted to the proteome of these ESKAPE pathogens.
Potential immunogenicity of the peptides
The identical peptides to the human proteome served as queries for BLAST (with 90% similarity) against linear epitopes available at IEDB (http://www.iedb.org/). This analysis would help to find similar epitopes in positive assays. IEDB provides T cell, B cell, and MHC ligand assay results. Antigen, the origin of the antigen (organism), and MHC/HLA alleles were considered in T cell and MHC ligand assays. In B cell assays, the antigen and origin of the antigen were investigated.
The Mature OmpA sequence (23–356 aa), in which the signal peptide was removed, served as a query for NetMHCpan 4.1 [38] at https://services.healthtech.dtu.dk/service.php?NetMHCpan-4.1 and NetMHCIIpan 4.0 [38] at http://www.cbs.dtu.dk/services/NetMHCIIpan/ to predict potential HLA (classes I and II) binders. The NetMHCpan-4.1 nominates peptide binders to MHC class I using artificial neural networks (ANNs). Rank thresholds for strong and weak binding peptides are 0.5% and 2% respectively. Peptides with lower predicted values are assigned as binders. The NetMHCIIpan 4.0 nominates peptide binders to MHC class II using artificial neural networks (ANNs). Rank thresholds for strong and weak binding peptides predicted by NetMHCIIpan 4.0 are 2% and 10% respectively.
MHC/HLA alleles introduced by IEDB positive assays were selected for predictions. The selection of these alleles, which are experimentally introduced, enhances the validity of the prediction results. All peptide lengths (8–14 mer peptides) were selected for HLA class I predictions. In the case of NetMHCIIpan 4.0, the default peptide length (15 mer) was selected.
Characterization of the human antigens
Subcellular localization of human antigens harboring similar peptides to the OmpA was investigated. These data were obtained from UniProt or predicted by specialized tools. LocTree3 at https://rostlab.org/services/loctree3/ and BUSCA at http://busca.biocomp.unibo.it/ were invoked to predict the subcellular localization of unreviewed proteins in UniProt. Those proteins nominated as membrane proteins were subjected to topology analysis. These data were obtained from UniProt or were predicted by TOPCONS Ver. 2 (the topcons2 database has been updated to Pfam32.0) at https://topcons.cbr.su.se/pred/. Glycosylation of exposed human antigens was also analyzed. These data were obtained from UniProt. The detected human proteins were carefully viewed at the Human Protein Atlas [39] (Ver. 23; last update: 2023-06-19) to obtain further characterizations, such as the availability of specific antibodies against the corresponding protein and relevant regions.
Population coverage
The population coverage tool of IEDB at http://tools.iedb.org/population/ [40] was harnessed to perform population coverage analyses (Population datasets generously provided by Derek Middleton at The Allele Frequency Net Database [41]). Based on HLA frequencies, this tool could determine the population projected to respond to a specific epitope set.
HLA allele genotypic frequencies were obtained from the Allele Frequency database (http://www.allelefrequencies.net/). The Allele Frequency database currently contains allele frequencies for 115 nations and 21 distinct ethnic groups distributed over 16 distinct geographic regions. Furthermore, user-defined custom populations with specified allele frequencies can be defined by the users. It is possible to compute several population coverages at once and produce an average population coverage. Because T cell populations (HTL and CTL, respectively) respond differently to MHC class I and class II-restricted T cell epitopes, the program offers three options for calculation to support various coverage modes: (1) class I separate, (2) class II separate, and (3) class I and class II combined. The following is computed by the tool for each population coverage: (1) the minimum number of epitope hits/HLA combinations recognized by 90% of the population (PC90), (2) the average number of epitope hits/HLA combinations recognized by the population, and (3) the predicted population coverage.
The following estimates were made for the HLA allele frequencies for each of the population categories: The allelefrequencies.net database included the HLA allele frequencies and related information for various individual populations from studies conducted worldwide. The populations were arranged geographically, nationally, and ethnically into a hierarchy, and the data from the individual populations within each group were combined to estimate the allele frequencies for each merged population. For alleles (such as HLA-A*01:01) having a resolution of two sets of digits, the frequencies were approximated. To do this, the data for alleles (such as HLA-A*07 and HLA-A*09, which have a resolution of only one set of digits) with a resolution of fewer than two sets of digits were removed. however, the data for alleles with a resolution of more than two sets of digits were combined to two sets of digits. As an example, HLA-A*02:01:01, HLA-A*02:01:02, HLA-A*02:01:02:01, HLA-A*02:01:02:03, and so on were merged to HLA-A*02:01. Using the formula AF = a/2n, the allele frequencies were determined by estimating the total number of copies of alleles (a) and the number of individuals (n) in each group of populations. 3,245 allele frequencies (class I and class II) for the world, 16 regions, 21 ethnic groups, 115 nations, and ethnic groups broken down by nation are included in the final set.
Octameric OmpA peptides within predicted binders of selected HLAs, along with their corresponding HLAs (obtained from the “2.4 Potential immunogenicity of the peptides” section), served as input data. The population coverage of all peptides and their corresponding HLA molecules was also calculated. The analyses were conducted for all of the areas and populations provided by the server.
Measuring the mutability of regions in native OmpA
To assess the mutation resistance of particular positions within OmpA, the structural mutation sensitivity profile was evaluated using MaestroWeb [42], accessible at https://pbwww.services.came.sbg.ac.at/maestro/web. MaestroWeb generates a mutation sensitivity profile that depicts the predicted impact of mutations at each amino acid position within the protein sequence. This profile allows for the identification of “hotspots” – residues where mutations are likely to have a significant effect on stability – and “coldspots” – residues where mutations are predicted to be tolerated with minimal impact.
The input structure of OmpA was obtained from AlphaFold (An entirely redesigned version of AlphaFold, a neural network-based model, which was validated in the challenging 14th Critical Assessment of the Protein Structure Prediction (CASP14) [35]. The objective of this analysis was to ascertain whether the epitope positions hold the potential for strategic engineering aimed at eliminating sequence identity between identical epitopes and human proteins. MaestroWeb utilizes a consensus prediction approach, combining the predictions from multiple agent-based models. These models analyze the protein sequence and structural features to estimate the free energy change (ΔΔG) associated with a specific mutation. The server provides a confidence score (cpred) that reflects the agreement between the individual agent predictions. The suggested mutation with the lowest ΔΔG was rendered to each epitope. The obtained new epitope sequences were re-evaluated by the peptide search tool of UniProt to find identical peptides in the human proteome.
Results
BLAST and sequence conservation: OmpA is highly prevalent and conserved in A. baumannii
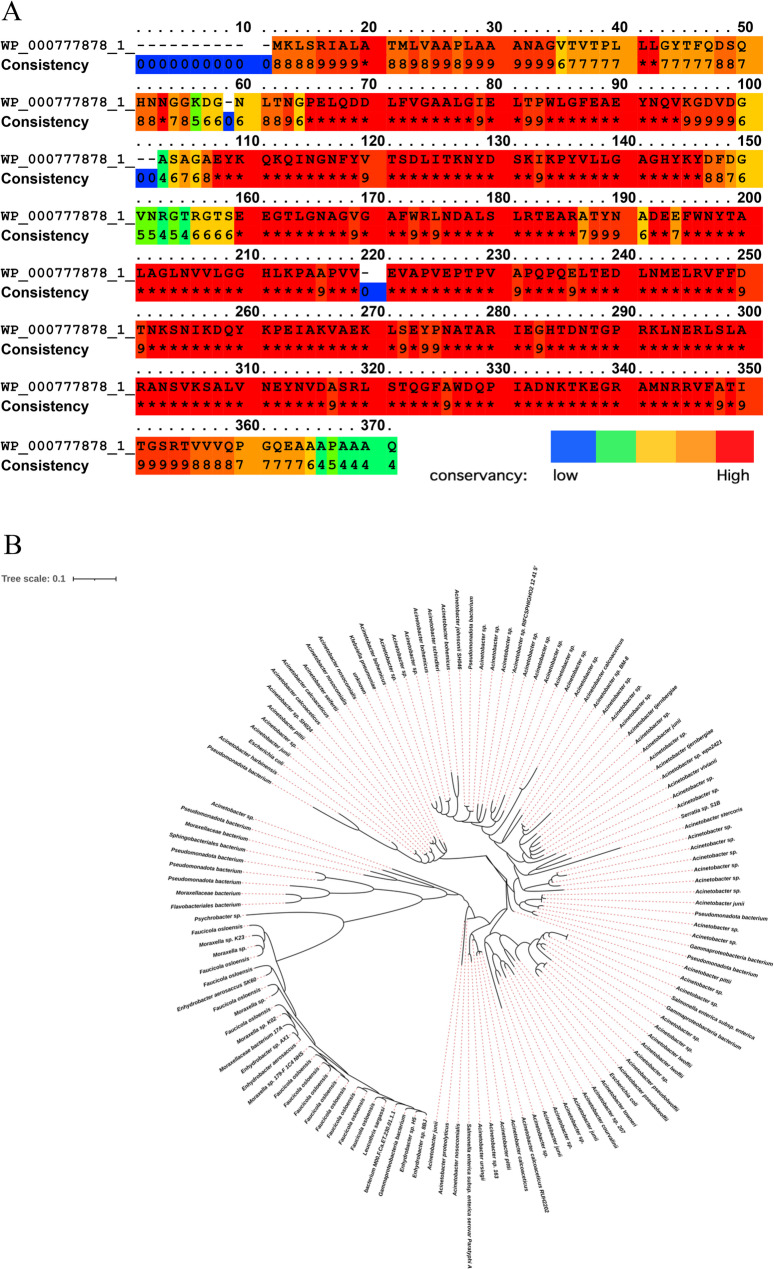
The prevalence and conservation of OmpA were evaluated in A. baumannii (taxid: 470) by a BLAST search. Twenty-nine accession numbers were found with identity and query coverage of ≥ 99% in A. baumannii. These hits covered more than 2020 strains of the species. Seventy accession numbers were found with identity and query coverage of ≥ 90% in A. baumannii. The E-value of these hits was 0.0. Pierce into the conservancy of OmpA in A. baumannii was carried out via a multiple sequence alignment in which all obtained sequences from a previous BLAST search (with identity and query coverage of ≥ 90%) were aligned. The alignment length was 371, and the sequence identity was 94% (Fig. 2). No hits were found in the BLAST search, which was limited to the Homo sapiens (taxid: 9606) and mice (taxid:10088).
Fig. 2.
OmpA sequence conservation. Sequence alignment of OmpA (A); Sequences with identity and query coverage of ≥ 90% in A. baumannii were aligned. The scoring scheme works from 0 for the least conserved alignment position, up to 10 (*) for the most conserved alignment position. The color key at the bottom right indicates the conservancy level of each position from blue (low) to red (high). Phylogenetic tree based on OmpA across Gram-negative bacteria (B); Circular phylogenetic tree reconstructed from BLAST-based alignments of OmpA sequences across 127 bacterial species representing 15 major taxonomic families. Enterobacteriaceae sequences (highlighted cluster, lower left quadrant) form a monophyletic group with compact branch lengths (mean = 0.023 ± 0.008). Three distinct OmpA clades are resolved: Enterobacteriaceae (compact cluster), Acinetobacter species (right quadrant), and diverse gram-negative families including Pseudomonadaceae, Moraxellaceae, Flavobacteriaceae, and Sphingomonadaceae (distributed throughout the tree). The tree scale bar represents 0.1 substitutions per site. Tree construction employed NCBI BLAST against clustered non-redundant protein databases with the iTOL visualization platform
Phylogenetic analysis revealed extensive OmpA distribution across gram-negative bacteria, with particular conservation within Enterobacteriaceae (Fig. 2B.). The reconstructed tree encompassed 127 bacterial species representing 15 major taxonomic families. Within Enterobacteriaceae, OmpA sequences clustered into a monophyletic group (mean of branch lengths = 0.023 ± 0.008).
Peptide screening: OmpA harbors three 8-meric peptides identical to the human proteome
The human proteome was searched for identical peptides using the peptide search tool of UniProt. No match was found for 10- and 9-meric peptides. However, 3 identical peptides (TKNYDSKI, LSLARANS, and GQEAAAPA) were found in the human proteome for 8-meric peptides. TKNYDSKI, LSLARANS, and GQEAAAPA were found in Lysine-specific demethylase RSBN1L, Isthmin-1 (ISM1), and DNA-binding protein SMUBP-2, respectively (Table 1).
Table 1.
Octameric peptides of OmpA, which are found in human proteins
| Peptide | Human protein | Length | Start position |
|---|---|---|---|
| TKNYDSKI | Lysine-specific demethylase RSBN1L | 846 | 310 |
| LSLARANS | Isthmin-1 | 464 | 166 |
| GQEAAAPA | DNA-binding protein SMUBP-2 | 993 | 683 |
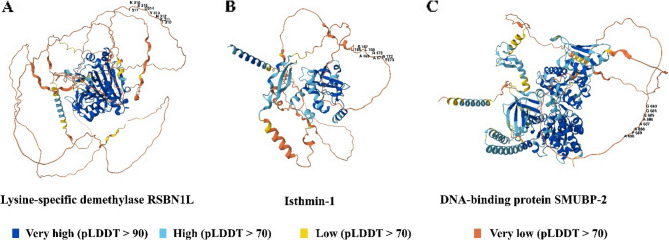
To pinpoint the exact location of the peptides within the native proteins, the structural information for each protein was procured from AlphaFold (Fig. 3). A thorough visual examination of the structures, coupled with detailed scrutiny of their structural constituents, indicated that all three identical peptides exist in the structurally disordered regions. This observation prompted the need for an assessment of the peptides’ surface accessibility. The ensuing analysis unveiled a notable elevation in the surface accessibility values for the regions encompassing the specified peptides (Fig. 4).
Fig. 3.
The position of the epitopes on the human protein. The structures are obtained from the Alphafold; all three structures are predictions. Cartoon ribbons illustrate the whole proteins; the epitopes are labeled and presented in red. A is lysis-specific demethylase RSBN1L (AF-Q6PCB5-F1) and the related epitope is TKNYDSKI; (B) is Isthemin-1 (AF-B1AKI9-F1) and the related epitope is LSLARANS; (C) is DNA-binding protein SMUBP-2 (AF-P38935-F1) and the related epitope is GQEAAAPA. The color scheme of structures is based on AlphaFold per-residue model confidence score, between 0 and 100. AlphaFold’s pLDDT (predicted Local Distance Difference Test) values provide an indication of the reliability of predicted protein structures at a per-residue level. High pLDDT values (typically above 70) suggest greater confidence in the predicted structure, indicating regions likely to be well-structured and reliable. Conversely, low pLDDT values suggest lower confidence, often correlating with regions that lack stable secondary or tertiary structures. The color key is shown below the figure
Fig. 4.
The surface accessibility profile of the epitopes within human native proteins. Only the sequences of the epitopes are presented for simplicity. A is lysis-specific demethylase RSBN1L and the related epitope is TKNYDSKI; (B) is Isthemin-1 and the related epitope is LSLARANS; (C) is DNA-binding protein SMUBP-2 and the related epitope is GQEAAAPA. No identical peptide was found among the proteomes of the ESKAPE pathogens
Immunogenicity of the peptides: the identical peptides were found in positive immunoassays
Similar linear epitopes with human proteins were found in positive assays via a BLAST analysis available at IEDB. Most of these epitopes shared six identical residues with the 3 identified peptides (Table 2). TKNYDSKI and LSLARANS were found in B-cell positive assays of OmpA (Table 2). TKNYDSKI, LSLARANS, and GQEAAAPA were matched to HLA binders from human proteins (Table 2). These proteins included the RNA polymerase II elongation factor ELL, Alpha/beta hydrolase domain-containing protein 14B, Myosin-9, Myosin-11, and an unknown protein eluted from human MHC allele (Table 2).
Table 2.
Experimentally validated epitopes sharing similarity with peptides found in the human proteome. Bold underlined letters show the identity of the octameric peptides
| Peptide | Validated Epitope | Type of epitope | Organism | Protein | MHC/HLA allele | Position |
|---|---|---|---|---|---|---|
| TKNYDSKI | LITKNYDSKIKPY | Linear B-cell | A. baumannii ATCC 17,978 | OmpA | - | 100–112 |
| TKNYDSKI | LSYDSKIWTTK | HLA binder | Homo sapiens | Unknown | HLA-A*01:01; HLA-A*03:01; HLA-B*07:02; HLA-B*27:05; HLA-C*02:02; HLA-C*07:02 | - |
| LSLARANS | PRKLNERLSLARA | Linear B-cell | A. baumannii ATCC 17,978 | OmpA | - | 265–277 |
| LSLARANS | EKETKALSLARALE | HLA binder | Homo sapiens |
Myosin-9 Myosin-11 |
HLA-DPA1*02:01/DPB1*10:01; HLA-DPA1*02:01/DPB1*09:01; HLA-DPA1*02:01/DPB1*14:01; HLA-DPA1*02:01/DPB1*17:01; HLA-DRB1*07:01 |
1473–1486 1480–1494 |
| GQEAAAPA | EAAAPAPTV | HLA binder | Homo sapiens | RNA polymerase II elongation factor ELL | HLA-B*51:01 | 370–378 |
| GQEAAAPA | LGHSKEAAAPAPIG | HLA binder | Homo sapiens | Alpha/beta hydrolase domain-containing protein 14B | HLA-DPA1*02:01/DPB1*14:01; HLA-DPA1*02:01/DPB1*10:01 | 72–85 |
HLA class I and II binders of OmpA were predicted by two online tools. HLA-A*01:01, HLA-A*03:01, HLA-B*07:02, HLA-B*27:05, HLA-B*51:01, HLA-C*02:02, and HLA-C*07:02 were selected for the prediction of HLA class I binders of OmpA. HLA-DPA1*02:01/DPB1*14:01, HLA-DPA1*02:01/DPB1*10:01, HLA-DPA1*02:01/DPB1*09:01, HLA-DPA1*02:01/DPB1*17:01, and HLA-DRB1*07:01 were selected for the prediction of HLA class II binders of OmpA. TKNYDSKI was found in a weak 12-meric binder of HLA-A*01:01. LSLARANS was found in weak 10- and 11-meric binders of HLA-A*03:01. This peptide was also found in 14 weak 15-meric binders of HLA-DPA1*02:01/DPB1*09:01, HLA-DPA1*02:01/DPB1*10:01, HLA-DPA1*02:01/DPB1*14:01, and HLA-DPA1*02:01/DPB1*17:01. Five weak or strong binders of HLA-DRB1*07:01 also encompassing LSLARANS. None of the predicted binders harbors GQEAAAPA (Table 3).
Table 3.
Predicted HLA binders within the OmpA sequence harboring identical peptides with the human proteome. HLA class I binders predicted by NetMHCpan 4.1 and HLA class II binders predicted by NetMHCIIpan 4.0. SB: strong binder; and WB: weak binder
| 8-meric peptide | Found binder peptide in OmpA | HLA allele | Strength of binder | Length |
|---|---|---|---|---|
| TKNYDSKI | ITKNYDSKIKPY | HLA-A*01:01 | WB | 12 meric |
| LSLARANS | RLSLARANSVK | HLA-A*03:01 | WB | 11 meric |
| LSLARANS | LSLARANSVK | HLA-A*03:01 | WB | 10 meric |
| LSLARANS | KLNERLSLARANSVK | HLA-DPA1*02:01/DPB1*09:01 | WB | 15 meric |
| LSLARANS | LNERLSLARANSVKS | HLA-DPA1*02:01/DPB1*09:01 | WB | 15 meric |
| LSLARANS | NERLSLARANSVKSA | HLA-DPA1*02:01/DPB1*09:01 | WB | 15 meric |
| LSLARANS | LNERLSLARANSVKS | HLA-DPA1*02:01/DPB1*10:01 | WB | 15 meric |
| LSLARANS | NERLSLARANSVKSA | HLA-DPA1*02:01/DPB1*10:01 | WB | 15 meric |
| LSLARANS | KLNERLSLARANSVK | HLA-DPA1*02:01/DPB1*10:01 | WB | 15 meric |
| LSLARANS | RKLNERLSLARANSV | HLA-DPA1*02:01/DPB1*10:01 | WB | 15 meric |
| LSLARANS | KLNERLSLARANSVK | HLA-DPA1*02:01/DPB1*14:01 | WB | 15 meric |
| LSLARANS | LNERLSLARANSVKS | HLA-DPA1*02:01/DPB1*14:01 | WB | 15 meric |
| LSLARANS | NERLSLARANSVKSA | HLA-DPA1*02:01/DPB1*14:01 | WB | 15 meric |
| LSLARANS | RKLNERLSLARANSV | HLA-DPA1*02:01/DPB1*17:01 | WB | 15 meric |
| LSLARANS | KLNERLSLARANSVK | HLA-DPA1*02:01/DPB1*17:01 | WB | 15 meric |
| LSLARANS | LNERLSLARANSVKS | HLA-DPA1*02:01/DPB1*17:01 | WB | 15 meric |
| LSLARANS | NERLSLARANSVKSA | HLA-DPA1*02:01/DPB1*17:01 | WB | 15 meric |
| LSLARANS | RKLNERLSLARANSV | HLA-DRB1*07:01 | WB | 15 meric |
| LSLARANS | KLNERLSLARANSVK | HLA-DRB1*07:01 | SB | 15 meric |
| LSLARANS | LNERLSLARANSVKS | HLA-DRB1*07:01 | SB | 15 meric |
| LSLARANS | NERLSLARANSVKSA | HLA-DRB1*07:01 | SB | 15 meric |
| LSLARANS | ERLSLARANSVKSAL | HLA-DRB1*07:01 | WB | 15 meric |
The human antigen characterization: localization, topology, glycosylation, and antibody interaction
No glycosylation site was found within the identical sequences of human proteins. Isthmin-1 was an extracellular or secreted protein. None of the remaining human proteins was assigned as extracellular or secreted proteins (Table 4). As per the information provided by the Human Protein Atlas [43] (https://www.proteinatlas.org/), RSBN1L (ENSG00000187257-RSBN1L) is expressed in a wide array of tissues and encompasses an antigenic segment (length: 107, from 267 to 373) that reacts with two specific antibodies (HPA020406 and HPA020413). Notably, the identified peptide (TKNYDSKI) falls within this region (from 310 to 317). This antibody assay data signifies that the specified region is positioned on the surface of the RSBN1L protein and possesses antigenic characteristics. No data is available for antibody interaction in the Human Protein Atlas regarding Isthmin-1 (ENSG00000101230-ISM1). This protein is primarily located in thyroid glandular cells. Regarding the DNA-binding protein SMUBP2 (ENSG00000132740-IGHMBP2), although antibody interactions are documented, none of the reported binding sites encompass the associated peptide.
Table 4.
Length and subcellular localization of human proteins sharing similarity/identity with octameric peptides of OmpA. Bold, underlined letters show the identity of the octameric peptides
| Peptide/Identity | Position | Human protein | Length | Subcellular localization | Source/tool |
|---|---|---|---|---|---|
| TKNYDSKI | 310–317 | Lysine-specific demethylase RSBN1L | 846 | Nucleus | UniProt Annotation |
| LSLARANS | 106–173 | Isthmin-1 | 464 | Secreted | UniProt Annotation |
| LSLARANS | 1479–1484 | Myosin-9 | 1960 |
Cytoplasm Cytoplasmic vesicle Cytoskeleton |
UniProt Annotation |
| LSLARANS | 1486–1491 | Myosin-11 | 1972 | Thick filament | UniProt Annotation |
| GQEAAAPA | 683–690 | DNA-binding protein SMUBP-2 | 993 |
Cell projection Cytoplasm Nucleus |
UniProt Annotation |
| GQEAAAPA | 282–287 | RNA polymerase II elongation factor ELL | 621 | Nucleus | UniProt Annotation |
| GQEAAAPA | 77–82 | Alpha/beta hydrolase domain-containing protein 14B | 210 |
Cytoplasm Nucleus |
UniProt Annotation |
Population coverage
The world population coverage of human-identical HLA binders of OmpA was calculated by population coverage analyses. The overall world population coverage of TKNYDSKI and LSLARANS as binders of HLA-A*01:01, HLA-A*03:01, HLA-DPA1*02:01/DPB1*09:01, HLA-DPA1*02:01/DPB1*10:01, HLA-DPA1*02:01/DPB1*14:01, HLA-DPA1*02:01/DPB1*17:01 or HLA-DRB1*07:01 was 44.85% (as combined classes of HLAs). Among the selected alleles, only HLA-A*01:01, HLA-A*03:01, and HLA-DRB1*07:01 were available by the IEDB population coverage tool. Therefore, the remaining HLAs were not included in the calculation. The highest overall population coverage was for Europe with 59.51%, while the lowest was for Southeast Asia with 9.43% (Table 5). The world population coverage of TKNYDSKI as a binder of HLA-A*01:01 was 17.34%. The world population coverage of LSLARANS as a binder of HLA-A*03:01 and HLA-DRB1*07:01 was 16.81% and 18.23% respectively (Table 5).
Table 5.
Population coverage of HLAs for which OmpA-derived peptides with identity/similarity to the human proteome are as their binders
| population/area | All selected HLAs | Class I | Class II | |||
|---|---|---|---|---|---|---|
| Class I | Class II | Class combined | HLA-A*01:01 | HLA-A*03:01 | HLA-DRB1*07:01 | |
| Central Africa | 14.95% | 13.23% | 26.2% | 2.84% | 12.29% | 13.23% |
| Central America | 0.0% | 11.22% | 11.22% | --- | --- | 11.22% |
| East Africa | 18.29% | 8.99% | 25.63% | 11.24% | 7.49% | 8.99% |
| East Asia | 4.01% | 7.47% | 11.18% | 2.55% | 1.47% | 7.47% |
| Europe | 47.02% | 23.58% | 59.51% | 25.67% | 25.05% | 23.58% |
| North Africa | 22.46% | 25.63% | 42.33% | 14.18% | 8.96% | 25.63% |
| North America | 24.94% | 19.38% | 39.48% | 12.72% | 13.11% | 19.38% |
| Northeast Asia | 6.44% | 8.26% | 14.17% | 3.44% | 3.05% | 8.26% |
| Oceania | 15.18% | 1.87% | 16.77% | 11.38% | 4.04% | 1.87% |
| South Africa | 22.06% | 0.0% | 22.06% | 13.83% | 8.89% | --- |
| South America | 12.21% | 9.31% | 20.38% | 6.03% | 6.37% | 9.31% |
| South Asia | 25.22% | 28.49% | 46.52% | 13.8% | 12.33% | 28.49% |
| Southeast Asia | 3.27% | 6.37% | 9.43% | 2.1% | 1.18% | 6.37% |
| Southwest Asia | 23.1% | 11.93% | 32.27% | 14.66% | 9.15% | 11.93% |
| West Africa | 16.42% | 8.42% | 23.46% | 7.31% | 9.48% | 8.42% |
| West Indies | 29.22% | 18.09% | 42.02% | 14.43% | 16.05% | 18.09% |
| World | 32.55% | 18.23% | 44.85% | 17.34% | 16.81% | 18.23% |
Mutation sensitivity profile
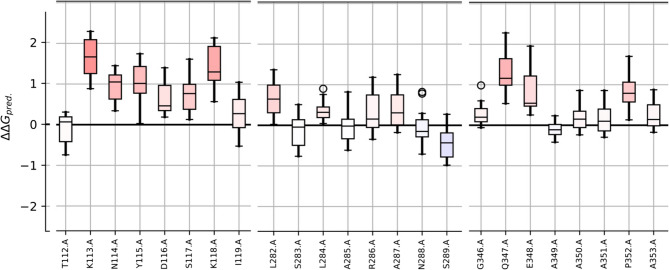
To predict a protein’s susceptibility to mutations, a mutation sensitivity profile of OmpA was conducted. The intention behind this analysis was to determine the potential of identical epitopes to human proteins for epitope engineering. The results from the mutation sensitivity profile indicate that the TKNYDSKI peptide cannot be readily modified to decrease its immunogenicity or eliminate its peptide identity. However, the two other peptides exhibit higher resistance to mutation (Fig. 5). This suggests that these two peptides can potentially be modified to minimize sequence identity. This adaptation could be undertaken if one aims to employ OmpA as a vaccine candidate without excluding these specific sequences. The best mutations for each residue of the epitopes are provided in Table 6.
Fig. 5.
The mutation sensitivity profile of OmpA. The x-axis is amino acid and the y-axis is the ΔΔG (the change in the change in Gibbs free energy (double changes intended)). Each bar is colored from blue (resistant to mutation) to red (sensitive to mutation). For simplicity, just the position of the epitopes is presented
Table 6.
The best mutations for each residue of the epitopes. The amino acid of the epitope, its position in the source sequence, and its substitute are presented. ΔΔG is presented for each substitution; the negative values indicate the stabilizing mutations. For each prediction, a confidence score typically between 0 to 1 (the higher the better) is provided
| TKNYDSKI | LSLARANS | GQEAAAPA | ||||||
|---|---|---|---|---|---|---|---|---|
| Mutant | ΔΔG_pred | Confidence | Mutant | ΔΔG_pred | Confidence | Mutant | ΔΔG_pred | Confidence |
| T112{D} | −0.741 | 0.966 | L282{W} | 0.022 | 0.944 | G346{Q} | −0.060 | 0.899 |
| K113{W} | 0.886 | 0.818 | S283{W} | −0.762 | 0.925 | Q347{R} | 0.537 | 0.788 |
| N114{E} | 0.342 | 0.830 | L284{M} | 0.045 | 0.896 | E348{R} | 0.254 | 0.823 |
| Y115{W} | 0.027 | 0.916 | A285{M} | −0.613 | 0.968 | A349{E} | −0.418 | 0.969 |
| D116{Y} | 0.187 | 0.857 | R286{M} | −0.344 | 0.856 | A350{E} | −0.237 | 0.909 |
| S117{Q} | 0.128 | 0.853 | A287{W} | −0.177 | 0.950 | A351{Q} | −0.296 | 0.922 |
| K118{W} | 0.568 | 0.762 | N288{W} | −0.709 | 0.906 | P352{W} | 0.133 | 0.870 |
| I119{W} | −0.528 | 0.925 | S289{L} | −0.981 | 0.863 | A353{S} | −0.173 | 0.893 |
The mutated epitopes were DKNYDSKI, LSLARANL, and GQEEAAPA. No identical peptide was found in the human proteome for the new mutated peptides.
Discussion
Several studies have demonstrated that infectious diseases can lead to post-infection autoimmunity [44–48]. Homology between human proteins and microbial antigens could result in cross-reactivity and consequently, break self-tolerance [1]. Moreover, the induction of autoimmune responses by currently employed vaccines remains a concerning issue [5]. The H1N1 pandemic in 2009 and the recent COVID-19 pandemic required the administration of millions of vaccine doses to combat these infectious diseases. Available reports revealed an increased incidence of autoimmune diseases following the massive vaccine administration for these pandemics [1, 5–7, 49]. This increase was attributed to the genetic susceptibility of populations and epitope mimicry between the viral proteins and human proteome [1, 5, 6]. The existence of shared epitopes between the sequences of human proteins and the proteins used for vaccine development could lead to two different immunological scenarios. Tolerance could occur against this shared peptide as an undesired effect for a strong vaccine candidate. As an alternative consequence, autoimmunity could be developed as a side effect of vaccine administration.
OmpA is a conserved pivotal virulence factor of A. baumannii [50]. It is one of the most abundant antigens of A. baumannii, which could [51] elicit antibodies during infection [27, 52]. The conducted BLAST and alignment of the obtained sequences in A. baumannii were in line with previous studies concerning the prevalence and conservation. Hence, the effect of OmpA-based vaccines and immunological outcomes could be generalized to most strains. Since it could be generalized to vast strains of the pathogen, the conservation of sequences could be considered a desired property for vaccine designs. However, it could be a disadvantage for unfavorable properties such as toxicity, allergenicity, as well as autoimmune responses. Moreover, this protein has been assigned as one of the most promising antigens for active and passive immunizations against A. baumannii [22, 23, 25, 27] without significant homology to the human proteome [27]. The global BLAST of the OmpA protein sequence showed no significant similarity with human proteins. This observation is in line with the previous study [27]. However, the employed strategy for BLAST analyses seems to be inappropriate to identify the minimum sequence similarities, which are sufficient for cross-reactivity. Theoretically, the existence of short identical peptides could be sufficient to trigger autoimmune responses [53]. These epitopes could be cryptic in a global protein BLAST search. Hence, more robust approaches should be harnessed to unveil these cryptic peptides. An in silico study has recently been conducted to investigate the possible molecular mimicry between the S. typhi and human host proteins. In this study, the most homologous proteins between the proteomes of the pathogen and the human were considered for the search [54]. Hence, proteins harboring short (e.g., 8-meric) peptides have been neglected. To compensate for this negligence, in the current study, OmpA was segmented into overlapping 8- to 10-meric peptides to find identical peptides within the human proteome. Only the 8-meric peptides matched with the human proteome. This is the shortest length of the HLA class I binder, which could serve as a B-cell epitope as well. This length of peptides was also considered to evaluate the safety of the SARS-CoV-2 proteome for the induction of autoimmune responses [6]. It should be noted that peptides, which are almost identical (but are not the same), could be sufficient for potential pathogenicity [1, 53]. Reports for higher pathogenicity of almost identical peptides are available [53, 55]. Hence, the existence of 8-meric identical peptides within longer HLA binders could exert deleterious effects.
The SARS-CoV-2 spike glycoprotein, which is about 4-fold larger than mature OmpA in length, contains two octapeptides identical to the human proteome [6], while mature OmpA encompasses three identical octapeptides to the human proteome. In comparison to the HPV16 proteome, which has only two identical octapeptides of the human proteome [56], OmpA harbors higher numbers of identical peptides. Since the number of identical octapeptides between OmpA and the human proteome is higher than the HPV16 proteome and spike glycoprotein of SARS-CoV-2, mature OmpA could be more deleterious regarding the induction of ADs.
Finding the OmpA peptides in positive experimental assays (T cell, B cell, and MHC ligands) could assure the elicitation of the immune responses against these peptides. The identical 8-meric peptides of OmpA (TKNYDSKI, LSLARANS, and GQEAAAPA) were matched to epitopes from A. baumannii, humans, and other organisms. Although identical octapeptides were only found for TKNYDSKI in a B-cell assay of OmpA, the LSLARANS shared 6-meric identical peptides with a validated B-cell epitope of OmpA. It should be noted that most B-cell epitopes are 5–22 amino acids in length [57]. Hence, anti-TKNYDSKI and LSLARANS antibodies could be able to recognize the identical peptides in human proteins. Availability of anti-RSBN1L antibodies recognizing a region including TKNYDSKI, flanking this peptide to be antigenic within the human protein. All three identical peptides were predicted to be surface accessible in the relevant human proteins, which could be exposed to the specific antibodies and B-cell receptors. However, it should be noted that these predictions are based on AI-predicted structures and they may be associated with low confidence. AI-based tools such as AlphaFold may perform poorly regarding the local flexibility of the predicted structures, and conclusions about these regions should be taken with caution. It is essential to consider the pLDDT scores when assessing peptide solvent exposure, as low-confidence regions may not accurately represent the true conformational state of the protein. It has been shown that there is no relationship between pLDDT and the B-factors of experimental structures. This means that AI-based models are unable to provide direct information on the local flexibility of the predicted structures. This limitation highlights the need for experimental data to confirm predictions. Although this challenge lies ahead of AI-based structural bioinformatics, AI can still contribute significantly to protein flexibility and dynamics studies. Machine learning has been used to construct force fields using large amounts of data from traditional molecular dynamics methods. Recent research groups have developed coarse-grained molecular potentials using artificial neural networks, allowing faster simulations than traditional methods. This could revolutionize protein flexibility research [58].
In addition to the identity and similarity of epitopes, other risk factors are involved in the etiology of autoimmune responses. Among the pool of various HLAs distributed in the human population, some of them, such as A*24, A*68, B*08, B*15, B*27, B*42, B*51, and DRB1, are involved in the augmentation of susceptibility to ADs [53, 59–62]. Peptides found within binders of HLAs, which are involved in autoimmune diseases and share sequence identity with experimentally validated B-cell epitopes, could be considered the most deleterious peptides.
A. baumannii is an extracellular pathogen against which specific antibodies could be sufficient to develop protection [12, 19, 22, 25, 27, 63–71]. Therefore, humoral responses are critical in vaccine designs against this pathogen. Although most of the immunization trials focused on active and passive immunization with protein antigens, some reports have been published about the administration of OmpA as a DNA vaccine against A. baumannii [31, 72, 73]. However, the potential risk of DNA vaccines to develop ADs should be considered. This vaccination platform could be exploited to elicit cytotoxic T cell (CTL) responses and is appropriate against intracellular pathogens such as Mycobacterium tuberculosis, Listeria monocytogenes, and viral infections in which CTLs and HLA class I play pivotal roles [74, 75]. The existence of HLA class I binders, which are identical to the human proteome, increases the risk of DNA vaccination for autoimmune responses. Thus, in addition to autoantibodies and HLA class II-associated autoreactive responses, HLA class I-associated autoreactive responses could be triggered by the DNA vaccine platform. TKNYDSKI is identical to a peptide within human Lysine-specific demethylase and could be found in a 12-meric binder of HLA-A*01:01. Population coverage analyses indicated that 17.34% of the world population could be at risk of CTL responses against Lysine-specific demethylase by administration of a DNA vaccine, which encodes OmpA. LSLARANS was found in 10- and 11-meric binders of HLA-A*03:01. Population coverage analyses indicated that 16.81% of the world population could be at risk of CTL responses against Isthmin-1 by administration of DNA vaccine, which encodes OmpA. Since CTL responses are not the main defense mechanism against A. baumannii, only the alleles found in positive assays were analyzed. The obtained results are sufficient to unveil the potential risk of the OmpA-encoding DNA vaccine to induce autoimmune responses. However, more comprehensive studies could be conducted to predict binders of various HLA class I alleles in OmpA. Moreover, the DNA vaccine platform could introduce glycosylation patterns rather than those that occur for native OmpA expressed by bacteria. Hence, this platform of vaccine is not suggested against A. baumannii.
The TKNYDSKI peptide was found within the Lysine-specific demethylase RSBN1L. Moreover, this peptide was also found within an experimentally validated B-cell epitope of OmpA. Hence, antibodies raised against this epitope of OmpA could recognize TKNYDSKI within Lysine-specific demethylase RSBN1L; however, this human protein is localized in the nucleus. Therefore, it could be inaccessible to antibodies, which reduces the risk of autoimmune responses. In most cases, intracellular antigens are not directly involved in pathogenesis owing to their inaccessibility to autoantibodies. However, it has been demonstrated that immune complex formation, immunological activation, and other processes could induce autoantibody-mediated damage to intracellular targets [76]. In addition, it is not clear whether antibodies specific to TKNYDSKI could be triggered during human infection. This peptide is not topologically exposed to antibodies in the context of native OmpA, which is expressed by A. baumannii [24]. TKNYDSKI also shared a 5-meric identity with an experimental binder of various class I HLAs, including HLA-A*01:01. Although TKNYDSKI was found in a predicted 12-meric binder of HLA-A*01:01, since A. baumannii is known as an extracellular pathogen, presentation of its potential HLA class I binders in human infections had not been demonstrated. This issue is also applicable to candidates of protein subunit vaccine. However, this binder of HLA-A*01:01 could be presented by the administration of OmpA as a DNA vaccine. This peptide is highly conserved among various strains of A. baumannii, and the expected outcome of infection could be generalized to almost all strains of the pathogen. No reports have suggested this peptide to be protective; hence, it could be removed or engineered in vaccine-candidate designs. However, the mutation sensitivity profile suggested that engineering/mutation of residues within the TKNYDSKI peptide could disturb the native structure of OmpA. Hence, this issue could be considered in structural vaccine design.
The LSLARANS peptide was found within Isthmin-1. Moreover, this peptide shared 6-meric identity with an experimentally validated B-cell epitope of OmpA. The identical 6-meric sequence also exists within an HLA binder from Myosin-9 and Myosin-11. Hence, antibodies raised against this epitope of OmpA could probably recognize LSLARANS within Isthmin-1, Myosin-9, and Myosin-11. In contrast to Lysine-specific demethylase RSBN1L, Isthmin-1 is a secreted anti-angiogenic human protein that could be readily accessible to antibodies. This protein contributes to endothelial cell apoptosis [77, 78]. Isthmin-1, which is recently known as an adipokine (released by adipose tissue), could enhance the absorption of glucose by adipose tissue, while inhibiting the synthesis of hepatic lipids. It also has anti-inflammatory properties, most likely by inhibiting the activation of NF-κB and the generation of inflammatory cytokines and chemokines [79]. Antibodies recognizing this protein could probably block its functions and result in diseases. It is not clear whether antibodies specific to LSLARANS could be triggered during human infection. LSLARANS was found in predicted 10- and 11-meric binders of HLA-A*03:01. These binders of HLA-A*03:01 could be presented by the administration of OmpA as a DNA vaccine. LSLARANS also shared 6-meric (75%) identity with an experimental binder of various class II HLAs, including HLA-DRB1*07:01. This HLA class II allele is associated with ADs such as limbic encephalitis (LE) with antibody against leucine-rich glioma-inactivated 1 (anti-LGI1 Ab) [59] and Vitiligo [80]. LSLARANS was also found in predicted 15-meric binders of HLA-DRB1*07:01 derived from OmpA. Thus, it could be deleterious when OmpA is administered as a protein subunit vaccine candidate. Similar to TKNYDSKI, this peptide is also highly conserved among various strains of A. baumannii, and the expected outcome of infection could be generalized to almost all strains of the pathogen. This peptide is not reported to be protective; hence, it could be removed or engineered in the design of vaccine candidates.
The sequence of the GQEAAAPA peptide was found within the SMUBP-2 DNA-binding protein, which is not exposed to antibodies. No similarity was detected between this peptide and the validated B-cell epitopes of OmpA. In addition, despite sharing 6-meric identity with experimental binders of some HLA classes I and II, it was not found in predicted OmpA-derived HLA binders. This peptide is not conserved among various strains of A. baumannii. Previous vaccine design studies suggested that this peptide could be removed from the sequence of the final vaccine candidate.
Although no reliable report has been published on the role of A. baumannii infections in AD induction, our results revealed that it would be considered in future studies. Based on our results, about 18% of the world’s population is more susceptible to A. baumannii-post infection ADs. Among the different regions of the world population, South Asia, North Africa, and European population are the most susceptible, respectively.
To the best of our knowledge, this is the first study about inducing autoimmune responses by an antigen of A. baumannii; no relevant clinical or animal model study is available to indicate reactivity of OmpA antibodies or T cell response from A. baumannii. Several reasons could be suggested for this limitation. The studies on vaccines against A. baumannii started in 2010 [81, 82], while in the case of Group A Streptococcus vaccines, more than 50 years [45, 83, 84]. A. baumannii is an opportunistic nosocomial pathogen that mostly causes infection in immunocompromised individuals. Immune responses in this population against the infection are weaker than healthy individuals. Hence, the occurrence of autoimmune responses in the population with weakened immune systems could be less than healthy peoples post-infection. In infection, the host immune system recognizes a wide pool of antigens and epitopes; amongst them, identical and similar epitopes to the host proteome constitute a small proportion. So, it would be expected that a vaccine candidate designed and developed based on subunit/multi-epitope peptide and protein could be more deleterious if it contains identical and similar epitopes to the host proteome. This situation could break the tolerance. In addition, due to high mortality of the pathogen, survived individuals to be followed up could be less than expected. The genetic and HLA susceptibility of the infected individuals should also be considered.
It had been hypothesized that bacterial co-infections by Staphylococci, Streptococci, A. baumannii, Escherichia coli, and Klebsiella in severe COVID-19 could cause coagulopathies. These phenomena could occur via molecular mimicry of blood proteins with both viral and bacterial antigens, in which virus-induced autoimmunity is enhanced by bacterial antigens [85]. In a recent study, A. baumannii was among the most commonly detected bacteria in the culture of autoimmune disease (AID)-related ulcers [86]. The current study is a pure in silico study, and the findings are theoretical; therefore, this limitation should be complemented with further future wet-lab experiments to be validated.
Conclusion
Although OmpA is known as one of the most promising immunogens against A. baumannii, it could not serve as a safe vaccine candidate without fair consideration of its cytotoxicity and potential for induction of autoimmune responses. Subunit antigens harboring TKNYDSKI, LSLARANS, and GQEAAAPA peptides should be cautiously dealt with. Among these peptides, LSLARANS is endowed with the most deleterious properties. In novel recombinant vaccine designs, these peptides could be removed or engineered within the final vaccine candidate. However, in structural vaccine approaches, it should be noted that mutations in the TKNYDSKI residues could disturb the OmpA structure. DNA vaccine platform could elevate the risk of autoimmune responses. This platform of vaccine is not suitable for being administered against A. baumannii. Future studies could be focused on an epitope-based vaccine in which deleterious peptides and regions are removed. In addition, it seems that A. baumannii infections could trigger autoimmune responses. It could be strongly suggested that patient who are exposed to this pathogen should be under surveillance for autoantibodies. Experimental studies could be designed and conducted to confirm the obtained findings of the current study.
Acknowledgements
The authors wish to thank the Applied Microbiology Research Center of Baqiyatallah University of Medical Sciences, Tehran-Iran for supporting this work.
Clinical trial number
Not applicable.
Declaration of AI use
The authors confirm that no generative AI tools were used in the design, execution, or interpretation of this study. All experimental data, statistical analyses, and manuscript preparation were conducted through traditional human-led methods. AI-based software was used only for routine tasks such as language editing, and all outputs were critically reviewed and validated by the authors.
Authors’ contributions
AJ conceptualized the study. All authors were involved in the data analysis and manuscript preparation.
Funding
This research received no specific grant from any funding agency.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rojas M, Restrepo-Jiménez P, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, et al. Molecular mimicry and autoimmunity. J Autoimmun. 2018;95:100–23. [DOI] [PubMed] [Google Scholar]
- 2.Guimarães LE, Baker B, Perricone C, Shoenfeld Y. Vaccines, adjuvants and autoimmunity. Pharmacol Res. 2015;100:190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perricone C, Shoenfeld Y. Hepatitis B vaccination and undifferentiated connective tissue disease: another brick in the wall of the autoimmune/inflammatory syndrome induced by adjuvants (Asia). J Clin Rheumatol. 2013;19(5):231–3. [DOI] [PubMed] [Google Scholar]
- 4.Bragazzi NL, Watad A, Amital H, Shoenfeld Y. Debate on vaccines and autoimmunity: do not attack the author, yet discuss it methodologically. Vaccine. 2017;35(42):5522–6. [DOI] [PubMed] [Google Scholar]
- 5.Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fath MK, Jahangiri A, Ganji M, Sefid F, Payandeh Z, Hashemi ZS et al. SARS-CoV-2 proteome harbors peptides which are able to trigger autoimmunity responses: implications for infection, vaccination, and population coverage. Front Immunol. 2021;12. [DOI] [PMC free article] [PubMed]
- 7.Chen C-C, Chen C-J. New-onset inflammatory arthritis after COVID-19 vaccination: a systematic review. Int J Rheum Dis. 2023;26(2):267–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fonseca DLM, Filgueiras IS, Marques AH, Vojdani E, Halpert G, Ostrinski Y et al. SARS-CoV-2 infection induces the production of autoantibodies in severe COVID-19 patients in an age-dependent manner. MedRxiv. 2022:2022.12.04.22282902.
- 9.Baiocchi GC, Vojdani A, Rosenberg AZ, Vojdani E, Halpert G, Ostrinski Y et al. Autoantibodies linked to autoimmune diseases associate with COVID-19 outcomes. MedRxiv. 2022:2022.02.17.22271057.
- 10.Perez F, Bonomo RA. Vaccines for acinetobacter baumannii: thinking out of the box. Vaccine. 2014;32(22):2537–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27. [DOI] [PubMed] [Google Scholar]
- 12.Huang W, Yao Y, Wang S, Xia Y, Yang X, Long Q, et al. Immunization with a 22-kDa outer membrane protein elicits protective immunity to multidrug-resistant acinetobacter baumannii. Sci Rep. 2016;6:20724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassetti M, Labate L, Russo C, Vena A, Giacobbe DR. Therapeutic options for difficult-to-treat acinetobacter baumannii infections: a 2020 perspective. Expert Opin Pharmacother. 2021;22(2):167–77. [DOI] [PubMed] [Google Scholar]
- 14.Ballouz T, Aridi J, Afif C, Irani J, Lakis C, Nasreddine R, et al. Risk Factors, clinical Presentation, and outcome of acinetobacter baumannii bacteremia. Front Cell Infect Microbiol. 2017;7:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isler B, Doi Y, Bonomo RA, Paterson DL. New treatment options against Carbapenem-Resistant < i > Acinetobacter baumannii Infections. Antimicrob Agents Chemother. 2019;63(1):e01110–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan YC, Lahiri C. Promising Acinetobacter baumannii vaccine candidates and drug targets in recent years. Front Immunol. 2022;13:900509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh R, Capalash N, Sharma P. Vaccine development to control the rising scourge of antibiotic-resistant acinetobacter baumannii: a systematic review. 3 Biotech. 2022;12(3):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McConnell MJ, Martín-Galiano AJ. Designing Multi-Antigen vaccines against acinetobacter baumannii using systemic approaches. Front Immunol. 2021;12:666742. [DOI] [PMC free article] [PubMed]
- 19.Tamehri M, Rasooli I, Pishgahi M, Jahangiri A, Ramezanalizadeh F, Langroodi SRB. Combination of BauA and OmpA elicit Immunoprotection against acinetobacter baumannii in a murine sepsis model. Microb Pathog. 2022;173:105874. [DOI] [PubMed]
- 20.Rahbar MR, Mubarak SM, Hessami A, Khalesi B, Pourzardosht N, Khalili S, et al. A unique antigen against SARS-CoV-2, acinetobacter baumannii, and Pseudomonas aeruginosa. Sci Rep. 2022;12(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fereshteh S, Ajdary S, Sepehr A, Bolourchi N, Barzi SM, Jouriani FH, et al. Immunization with Recombinant DcaP-like protein and AbOmpA revealed protections against sepsis infection of multi-drug resistant acinetobacter baumannii ST2Pas in a C57BL/6 mouse model. Microb Pathog. 2022;174:105882. [DOI] [PubMed]
- 22.Jahangiri A, Owlia P, Rasooli I, Salimian J, Derakhshanifar E, Aghajani Z, et al. Specific egg yolk immunoglobulin as a promising non-antibiotic biotherapeutic product against Acinetobacter baumannii pneumonia infection. Sci Rep. 2021;11(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahangiri A, Owlia P, Rasooli I, Salimian J, Derakhshanifar E, Naghipour Erami A, et al. Specific egg yolk antibodies (IgY) confer protection against acinetobacter baumannii in a murine pneumonia model. J Appl Microbiol. 2019;126(2):624–32. [DOI] [PubMed] [Google Scholar]
- 24.Jahangiri A, Rasooli I, Owlia P, Fooladi AAI, Salimian J. In silico design of an immunogen against Acinetobacter baumannii based on a novel model for native structure of outer membrane protein A. Microb Pathog. 2017;105:201–10. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Yang T, Cao J, Sun J, Dai W, Zhang L. Mucosal immunization with purified OmpA elicited protective immunity against infections caused by multidrug-resistant Acinetobacter baumannii. Microb Pathog. 2016;96:20–5. [DOI] [PubMed] [Google Scholar]
- 26.Lin L, Tan B, Pantapalangkoor P, Ho T, Hujer AM, Taracila MA, et al. Acinetobacter baumannii rOmpA vaccine dose alters immune polarization and immunodominant epitopes. Vaccine. 2013;31(2):313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo G, Lin L, Ibrahim AS, Baquir B, Pantapalangkoor P, Bonomo RA, et al. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS ONE. 2012;7(1):e29446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hessami A, Mogharari Z, Rahim F, Khalesi B, Nassrullah OJ, Rahbar MR, et al. In silico design of a novel hybrid epitope-based antigen harboring highly exposed immunogenic peptides of BamA, OmpA, and Omp34 against Acinetobacter baumannii. Int Immunopharmacol. 2024;142:113066. [DOI] [PubMed] [Google Scholar]
- 29.Nie D, Hu Y, Chen Z, Li M, Hou Z, Luo X, et al. Outer membrane protein A (OmpA) as a potential therapeutic target for acinetobacter baumannii infection. J Biomed Sci. 2020;27(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badmasti F, Ajdary S, Bouzari S, Fooladi AAI, Shahcheraghi F, Siadat SD. Immunological evaluation of OMV (PagL) + Bap (1-487aa) and AbOmpA (8-346aa) + Bap (1-487aa) as vaccine candidates against acinetobacter baumannii sepsis infection. Mol Immunol. 2015;67(2):552–8. [DOI] [PubMed] [Google Scholar]
- 31.Lei L, Yang F, Zou J, Jing H, Zhang J, Xu W, et al. DNA vaccine encoding OmpA and Pal from acinetobacter baumannii efficiently protects mice against pulmonary infection. Mol Biol Rep. 2019;46(5):5397–408. [DOI] [PubMed] [Google Scholar]
- 32.Wheeler D, Bhagwat M. BLAST quickstart: example-driven web. -based BLAST tutorial: Springer; 2008. [PMC free article] [PubMed] [Google Scholar]
- 33.Pirovano W, Feenstra KA, Heringa J. PRALINE™: a strategy for improved multiple alignment of transmembrane proteins. Bioinformatics. 2008;24(4):492–7. [DOI] [PubMed] [Google Scholar]
- 34.Consortium TU. UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res. 2018;47(D1):D506-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jumper J, Evans R, Pritzel A, Green T, Figurnov M, Ronneberger O, et al. Highly accurate protein structure prediction with alphafold. Nature. 2021;596(7873):583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jumper J, Hassabis D. Protein structure predictions to atomic accuracy with alphafold. Nat Methods. 2022;19(1):11–2. [DOI] [PubMed] [Google Scholar]
- 37.Høie MH, Kiehl EN, Petersen B, Nielsen M, Winther O, Nielsen H, et al. NetSurfP-3.0: accurate and fast prediction of protein structural features by protein Language models and deep learning. Nucleic Acids Res. 2022;50(W1):W510–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynisson B, Alvarez B, Paul S, Peters B, Nielsen M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 2020;48(W1):W449-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karlsson M, Zhang C, Méar L, Zhong W, Digre A, Katona B, et al. A single–cell type transcriptomics map of human tissues. Sci Adv. 2021;7(31):eabh2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bui H-H, Sidney J, Dinh K, Southwood S, Newman MJ, Sette A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinformatics. 2006;7:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Galarza Faviel F, McCabe A, Santos Eduardo J, Md, Jones J, Takeshita L, Ortega-Rivera Nestor D, et al. Allele frequency net database (AFND) 2020 update: gold-standard data classification, open access genotype data and new query tools. Nucleic Acids Res. 2019;48(D1):D783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laimer J, Hiebl-Flach J, Lengauer D, Lackner P. Maestroweb: a web server for structure-based protein stability prediction. Bioinformatics. 2016;32(9):1414–6. [DOI] [PubMed] [Google Scholar]
- 43.Digre A, Lindskog C. The human protein atlas—spatial localization of the human proteome in health and disease. Protein Sci. 2021;30(1):218–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parikh NS, Merkler AE, Iadecola C. Inflammation, autoimmunity, infection, and stroke. Stroke. 2020;51(3):711–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunningham MW. Molecular mimicry, autoimmunity, and infection: the cross-reactive antigens of group A streptococci and their sequelae. Microbiol Spectr. 2019;7(4):7.4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smatti MK, Cyprian FS, Nasrallah GK, Al Thani AA, Almishal RO, Yassine HM. Viruses and autoimmunity: a review on the potential interaction and molecular mechanisms. Viruses. 2019;11(8):762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hussein HM, Rahal EA. The role of viral infections in the development of autoimmune diseases. Crit Rev Microbiol. 2019;45(4):394–412. [DOI] [PubMed] [Google Scholar]
- 48.Yazdanpanah N, Rezaei N. Autoimmune complications of COVID-19. J Med Virol. 2022;94(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Xu Z, Wang P, Li X-M, Shuai Z-W, Ye D-Q, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165(4):386–401. [DOI] [PubMed] [Google Scholar]
- 50.Nie D, Hu Y, Chen Z, Li M, Hou Z, Luo X, et al. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J Biomed Sci. 2020;27(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Uppalapati SR, Sett A, Pathania R. The outer membrane proteins OmpA, CarO, and OprD of Acinetobacter baumannii confer a two-pronged defense in facilitating its success as a potent human pathogen. Front Microbiol. 2020;11:589234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fajardo Bonin R, Chapeaurouge A, Perales J, Silva JG, do Nascimento HJ, D’Alincourt Carvalho Assef AP, et al. Identification of Immunogenic proteins of the bacterium acinetobacter baumannii using a proteomic approach. PROTEOMICS-Clinical Appl. 2014;8(11–12):916–23. [DOI] [PubMed] [Google Scholar]
- 53.Dendrou CA, Petersen J, Rossjohn J, Fugger L. HLA variation and disease. Nat Rev Immunol. 2018;18(5):325. [DOI] [PubMed] [Google Scholar]
- 54.Rahman N, Begum S, Khan A, Afridi SG, Sahibzada MUK, Atwah B, et al. An insight in Salmonella Typhi associated autoimmunity candidates’ prediction by molecular mimicry. Comput Biol Med. 2022;148:105865. [DOI] [PubMed] [Google Scholar]
- 55.Ehser J, Holdener M, Christen S, Bayer M, Pfeilschifter JM, Hintermann E, et al. Molecular mimicry rather than identity breaks T-cell tolerance in the CYP2D6 mouse model for human autoimmune hepatitis. J Autoimmun. 2013;42:39–49. [DOI] [PubMed] [Google Scholar]
- 56.Kanduc D. Quantifying the possible cross-reactivity risk of an HPV16 vaccine. J Exp Ther Oncol. 2009;8(1):65–76. [PubMed] [Google Scholar]
- 57.Singh H, Ansari HR, Raghava GP. Improved method for linear B-cell epitope prediction using antigen’s primary sequence. PLoS ONE. 2013;8(5):e62216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carugo O. pLDDT values in AlphaFold2 protein models are unrelated to globular protein local flexibility. Crystals. 2023;13(11):1560. [Google Scholar]
- 59.Muñiz-Castrillo S, Vogrig A, Honnorat J. Associations between HLA and autoimmune neurological diseases with autoantibodies. Autoimmun Highlights. 2020;11(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Y, Li S, Huang R, Zhang Z, Petersen F, Zheng J, et al. Comprehensive meta-analysis reveals an association of the HLA-DRB1* 1602 allele with autoimmune diseases mediated predominantly by autoantibodies. Autoimmun Rev. 2020;19(6):102532. [DOI] [PubMed] [Google Scholar]
- 61.Elfishawi MM, Elgengehy F, Mossallam G, Elfishawi S, Alfishawy M, Gad A, et al. HLA class I in Egyptian patients with Behçet’s disease: new association with susceptibility, protection, presentation and severity of manifestations. Immunol Invest. 2019;48(2):121–9. [DOI] [PubMed] [Google Scholar]
- 62.Dashti N, Mahmoudi M, Aslani S, Jamshidi A. HLA-B* 27 subtypes and their implications in the pathogenesis of ankylosing spondylitis. Gene. 2018;670:15–21. [DOI] [PubMed] [Google Scholar]
- 63.Ranjbar A, Rasooli I, Jahangiri A, Ramezanalizadeh F. Specific egg yolk antibody raised to biofilm associated protein (Bap) is protective against murine pneumonia caused by acinetobacter baumannii. Sci Rep. 2022;12(1):12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moghaddam MM, Rasooli I, Ghaini MH, Jahangiri A, Ramezanalizadeh F, Tootkleh RG. Immunoprotective characterization of egg yolk immunoglobulin raised to loop 3 of outer membrane protein 34 (Omp34) in a murine model against acinetobacter baumannii. Mol Immunol. 2022;149:87–93. [DOI] [PubMed] [Google Scholar]
- 65.Akbari Z, Rasooli I, Ghaini MH, Chaudhuri S, Andisi VF, Jahangiri A, et al. BauA and Omp34 surface loops trigger protective antibodies against Acinetobacter baumannii in a murine sepsis model. Int Immunopharmacol. 2022;108:108731. [DOI] [PubMed] [Google Scholar]
- 66.Erami AN, Rasooli I, Jahangiri A, Astaneh SDA. Anti-Omp34 antibodies protect against acinetobacter baumannii in a murine sepsis model. Microb Pathog. 2021;161:105291. [DOI] [PubMed]
- 67.Eslam ED, Astaneh SDA, Rasooli I, Nazarian S, Jahangiri A. Passive immunization with chitosan-loaded biofilm-associated protein against Acinetobacter baumannii murine infection model. Gene Rep. 2020;20:100708. [Google Scholar]
- 68.Singh R, Capalash N, Sharma P. Immunoprotective potential of BamA, the outer membrane protein assembly factor, against MDR acinetobacter baumannii. Sci Rep. 2017;7(1):12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nielsen TB, Pantapalangkoor P, Luna BM, Bruhn KW, Yan J, Dekitani K, et al. Monoclonal antibody protects against acinetobacter baumannii infection by enhancing bacterial clearance and evading sepsis. J Infect Dis. 2017;216(4):489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh R, Garg N, Shukla G, Capalash N, Sharma P. Immunoprotective efficacy of acinetobacter baumannii outer membrane protein, FilF, predicted in silico as a potential vaccine candidate. Front Microbiol. 2016;7:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang W, Wang S, Yao Y, Xia Y, Yang X, Long Q, et al. OmpW is a potential target for eliciting protective immunity against acinetobacter baumannii infections. Vaccine. 2015;33(36):4479–85. [DOI] [PubMed] [Google Scholar]
- 72.Ansari H, Doosti A, Kargar M, Bijanzadeh M, Jaafarinia M. Cloning of OmpA gene from acinetobacter baumannii into the eukaryotic expression vector pBudCE4. 1 as DNA vaccine. Indian J Microbiol. 2018;58(2):174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hosseinnezhad-Lazarjani E, Doosti A, Sharifzadeh A. Novel csuC-DNA nanovaccine based on chitosan candidate vaccine against infection with Acinetobacter baumannii. Vaccine. 2023;41(13):2170–83. [DOI] [PubMed] [Google Scholar]
- 74.Sefidi-Heris Y, Jahangiri A, Mokhtarzadeh A, Shahbazi M-A, Khalili S, Baradaran B, et al. Recent progress in the design of DNA vaccines against tuberculosis. Drug Discovery Today. 2020;25(11):1971–87. [DOI] [PubMed]
- 75.Jahangiri A, Rasooli I, Gargari SLM, Owlia P, Rahbar MR, Amani J, et al. An in Silico DNA vaccine against Listeria monocytogenes. Vaccine. 2011;29(40):6948–58. [DOI] [PubMed] [Google Scholar]
- 76.Burbelo PD, Iadarola MJ, Keller JM, Warner BM. Autoantibodies targeting intracellular and extracellular proteins in autoimmunity. Front Immunol. 2021;12:548469. [DOI] [PMC free article] [PubMed]
- 77.Pera EM, Kim JI, Martinez SL, Brechner M, Li S-Y, Wessely O, et al. Isthmin is a novel secreted protein expressed as part of the Fgf-8 synexpression group in the xenopus midbrain–hindbrain organizer. Mech Dev. 2002;116(1):169–72. [DOI] [PubMed] [Google Scholar]
- 78.Sahiri V, Caron J, Roger E, Desterke C, Ghachem K, Mohamadou I, et al. The angiogenesis inhibitor isthmin-1 (ISM1) is overexpressed in experimental models of glomerulopathy and impairs the viability of podocytes. Int J Mol Sci. 2023;24(3):2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kirichenko TV, Markina YV, Bogatyreva AI, Tolstik TV, Varaeva YR, Starodubova AV. The role of adipokines in inflammatory mechanisms of obesity. Int J Mol Sci. 2022;23(23):14982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Singh A, Sharma P, Kar HK, Sharma VK, Tembhre MK, Gupta S, et al. HLA alleles and Amino-Acid signatures of the Peptide-Binding pockets of HLA molecules in vitiligo. J Invest Dermatology. 2012;132(1):124–34. [DOI] [PubMed] [Google Scholar]
- 81.Rahbar MR, Rasooli I, Gargari SLM, Amani J, Fattahian Y. In silico analysis of antibody triggering biofilm associated protein in acinetobacter baumannii. J Theor Biol. 2010;266(2):275–90. [DOI] [PubMed] [Google Scholar]
- 82.McConnell MJ, Pachón J. Active and passive immunization against Acinetobacter baumannii using an inactivated whole cell vaccine. Vaccine. 2010;29(1):1–5. [DOI] [PubMed] [Google Scholar]
- 83.Fox EN. M proteins of group A streptococci. Bacteriol Rev. 1974;38(1):57–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fox EN, Waldman RH, Wittner MK, Mauceri AA, Dorfman A. Protective study with a group A streptococcal M protein vaccine. Infectivity challenge of human volunteers. J Clin Invest. 1973;52(8):1885–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Root-Bernstein R. COVID‐19 coagulopathies: human blood proteins mimic SARS‐CoV‐2 virus, vaccine proteins and bacterial co‐infections inducing autoimmunity: combinations of bacteria and SARS‐CoV‐2 synergize to induce autoantibodies targeting cardiolipin, cardiolipin‐binding proteins, platelet factor 4, prothrombin, and coagulation factors. Bioessays. 2021;43(12):2100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei M, Xia D, Takashi E, Qiu Y, Huang L, Sun Z et al. Clarification of the clinical characteristics of autoimmune Disease-Related ulcers to improve treatment outcomes: A retrospective study. Int J Low Extrem Wounds. 2025;24(3):605-610. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.