Abstract
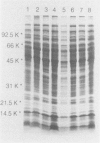
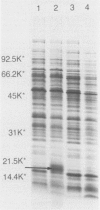
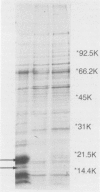
The whole-cell proteins of ten strains of Rhodococcus equi isolated from horses, pigs, or humans, including the type strain ATCC 6939, were examined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis. The protein profiles of seven different capsular serotypes and the type strain were very similar when bacteria were cultured under the same conditions. Protein profiles were largely unaffected by incubation at two temperatures (30 degrees C, 37 degrees C) or times (12 h, 48 h). There were generally minor differences in protein profiles between strains grown in different media (brain heart infusion, nutrient, minca broths, tryptic soy-blood agar) with the marked exception of a prominent diffuse 17.5 kd protein which was expressed in nutrient broth. This protein was not produced by the type strain and was lost on repeated passage in vitro (50th, 100th passage) in two of three other strains examined.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton M. D., Hughes K. L. Is Rhodococcus equi a soil organism? J Reprod Fertil Suppl. 1982;32:481–489. [PubMed] [Google Scholar]
- Bölin I., Portnoy D. A., Wolf-Watz H. Expression of the temperature-inducible outer membrane proteins of yersiniae. Infect Immun. 1985 Apr;48(1):234–240. doi: 10.1128/iai.48.1.234-240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirino-Trejo J. M., Prescott J. F. Antibody response of horses to Rhodococcus equi antigens. Can J Vet Res. 1987 Jul;51(3):301–305. [PMC free article] [PubMed] [Google Scholar]
- Elissalde G. S., Renshaw H. W., Walberg J. A. Corynebacterium equi: an interhost review with emphasis on the foal. Comp Immunol Microbiol Infect Dis. 1980;3(4):433–445. doi: 10.1016/0147-9571(80)90018-1. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. Electrophoretic heterogeneity and interstrain variation of the lipopolysaccharide of Haemophilus influenzae. J Infect Dis. 1983 Sep;148(3):492–499. doi: 10.1093/infdis/148.3.492. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linder R., Bernheimer A. W. Enzymatic oxidation of membrane cholesterol in relation to lysis of sheep erythrocytes by corynebacterial enzymes. Arch Biochem Biophys. 1982 Feb;213(2):395–404. doi: 10.1016/0003-9861(82)90565-3. [DOI] [PubMed] [Google Scholar]
- Martens R. J., Fiske R. A., Renshaw H. W. Experimental subacute foal pneumonia induced by aerosol administration of Corynebacterium equi. Equine Vet J. 1982 Apr;14(2):111–116. doi: 10.1111/j.2042-3306.1982.tb02359.x. [DOI] [PubMed] [Google Scholar]
- Phelps D. S. Electrophoretic transfer of proteins from fixed and stained gels. Anal Biochem. 1984 Sep;141(2):409–412. doi: 10.1016/0003-2697(84)90062-9. [DOI] [PubMed] [Google Scholar]
- Prescott J. F. Capsular serotypes of Corynebacterium equi. Can J Comp Med. 1981 Apr;45(2):130–134. [PMC free article] [PubMed] [Google Scholar]
- Prescott J. F., Markham R. J., Johnson J. A. Cellular and humoral immune response of foals to vaccination with Corynebacterium equi. Can J Comp Med. 1979 Oct;43(4):356–364. [PMC free article] [PubMed] [Google Scholar]
- Santos J. M., Verstreate D. R., Perera V. Y., Winter A. J. Outer membrane proteins from rough strains of four Brucella species. Infect Immun. 1984 Oct;46(1):188–194. doi: 10.1128/iai.46.1.188-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf-Watz H., Portnoy D. A., Bölin I., Falkow S. Transfer of the virulence plasmid of Yersinia pestis to Yersinia pseudotuberculosis. Infect Immun. 1985 Apr;48(1):241–243. doi: 10.1128/iai.48.1.241-243.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolcock J. B., Mutimer M. D. The capsules of Corynebacterium equi and Streptococcus equi. J Gen Microbiol. 1978 Nov;109(1):127–130. doi: 10.1099/00221287-109-1-127. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Wientjes F. B., Klaasen-Boor P. Production of K99 antigen by enterotoxigenic Escherichia coli strains of antigen groups o8, o9, o20, and o101 grown at different conditions. Infect Immun. 1980 Jan;27(1):216–221. doi: 10.1128/iai.27.1.216-221.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]