Abstract
Objective
To clarify the expression of microRNA-27a (miR-27a) and forkhead box protein O3 (FOXO3) in elderly patients with severe pneumonia complicated with acute respiratory distress syndrome (ARDS), and evaluate their relationship with disease severity and prognosis.
Methods
A total of 189 elderly patients with severe pneumonia were retrospectively analyzed, including 114 with ARDS (Group A) and 75 without ARDS (Group B). Seventy healthy individuals served as controls. Group A was further divided into mild (n=28), moderate (n=36), and severe (n=50) subgroups based on oxygenation index, and into survival (n=79) and death (n=35) subgroups based on 28-day outcome. Serum miR-27a and FOXO3 mRNA were measured by qRT-PCR. Their correlations with oxygenation index and prognosis were analyzed using Pearson, Spearman, logistic regression, and ROC curve methods.
Results
Serum miR-27a levels decreased and FOXO3 mRNA levels increased progressively from controls to Group B and Group A (F=77.352, 62.956, P<0.001). Within Group A, miR-27a declined and FOXO3 rose stepwise with increasing ARDS severity (F=83.597, 111.834, P<0.001). MiR-27a was negatively correlated with FOXO3 (r=−0.624, P<0.001), positively with oxygenation index (r=0.635, P<0.001), while FOXO3 showed the opposite pattern (r=−0.672, P<0.001). The 28-day mortality was 30.7%. Logistic regression identified age, prolonged ventilation, and high FOXO3 as risk factors, while higher oxygenation index and elevated miR-27a were protective. The AUCs for mortality prediction were 0.775 (miR-27a), 0.781 (FOXO3), and 0.867 (combined).
Conclusion
Reduced miR-27a and elevated FOXO3 are characteristic of elderly patients with severe pneumonia and ARDS, closely linked to disease severity and prognosis. Combined detection improves predictive accuracy, supporting their potential as biomarkers for survival evaluation.
Keywords: miR-27a, FOXO3, severe pneumonia, ARDS, elderly, prognosis
Introduction
Severe pneumonia is a major infectious disease in elderly patients, often characterized by rapid progression, multiple organ involvement, and poor prognosis.1,2 One of its most severe complications is acute respiratory distress syndrome (ARDS), which arises from diffuse alveolar-capillary damage and manifests as refractory hypoxemia, respiratory distress, and decreased lung compliance.3–5 Due to age-related immune decline, impaired pulmonary reserve, and high comorbidity burden, elderly patients are particularly vulnerable to developing ARDS after pneumonia, with mortality reaching 30–50%.6,7 Despite advances in supportive care and precision medicine approaches, early diagnosis and prognosis assessment of ARDS still rely mainly on clinical criteria, imaging, and arterial blood gases,8 which lack sensitivity and specificity. This underscores the urgent need for reliable molecular biomarkers to improve early identification, risk stratification, and clinical decision-making.
MicroRNAs (miRNAs) are endogenous, non-coding small RNAs that regulate gene expression at the post-transcriptional level by binding to the 3′-UTR of target mRNAs. They are involved in processes such as inflammation, oxidative stress, apoptosis, and immune regulation.9,10 miR-27a has been implicated in multiple inflammatory and malignant diseases, where it modulates immune signaling and cellular stress responses.11,12 In pulmonary studies, miR-27a has been linked to anti-inflammatory and antioxidant effects, suggesting its potential role as a biomarker of lung injury and prognosis.13
Forkhead box O3 (FOXO3), a transcription factor downstream of the PI3K/Akt pathway, plays a central role in regulating apoptosis, oxidative stress responses, and inflammatory mediator release.14,15 Previous reports indicate its involvement in lung tissue injury and ARDS pathogenesis.16,17 Importantly, FOXO3 is a validated target of miR-27a: downregulation of miR-27a leads to FOXO3 activation, thereby promoting inflammatory cascades and exacerbating tissue damage.18
However, evidence regarding the specific expression patterns of miR-27a and FOXO3 in elderly patients with severe pneumonia complicated with ARDS remains scarce. In particular, their correlation with oxygenation index and short-term prognosis has not been fully elucidated. Therefore, this study retrospectively analyzed elderly patients with severe pneumonia, aiming to (1) detect serum levels of miR-27a and FOXO3, (2) assess their relationship with ARDS severity and 28-day mortality, and (3) evaluate their predictive value as biomarkers for prognosis. These findings may provide a molecular basis for improved survival assessment and risk stratification in elderly ARDS patients.
Materials and Methods
Study Subjects
This was a retrospective observational study including a total of 189 elderly inpatients (aged ≥60 years) with severe pneumonia admitted to the intensive care unit of our hospital from February 2023 to October 2024. According to whether ARDS was present, patients were divided into two groups: Group A (n=114, with ARDS) and Group B (n=75, without ARDS). Additionally, 70 healthy volunteers undergoing physical examination at the physical examination center during the same period were selected as the healthy control group. Among the 114 patients in Group A, there were 70 males and 44 females, with an average age of (73.16±6.72) years; in Group B, there were 46 males and 29 females, with an average age of (74.11±6.98) years; in the control group, there were 41 males and 29 females, with an average age of (73.24±7.19) years. There were no statistically significant differences in gender and age among the three groups (P>0.05), indicating comparability.
The sample size was estimated based on a preliminary analysis of 40 patients in our institution, where the difference in serum miR-27a expression between ARDS and non-ARDS patients was approximately 0.8 standard deviations. Using a two-sided α=0.05 and power (1–β)=0.80, the minimum required sample size per group was calculated as 64 cases. Considering potential dropouts and missing data, the final enrollment exceeded this requirement, ensuring adequate statistical power for group comparisons.
This study was approved by the Liberation Army General Hospital Medical Ethics Committee (Approval No.: 2024ZZLS12) and conducted in strict accordance with the ethical principles of the Declaration of Helsinki. All participants provided informed consent and signed relevant informed consent forms.
Inclusion and Exclusion Criteria
Inclusion criteria: (1) Age ≥60 years, regardless of gender; (2) First diagnosis of severe pneumonia or severe pneumonia with ARDS; (3) Diagnosis of severe pneumonia meets the criteria of the “Chinese Expert Consensus on Clinical Practice of Severe Pneumonia in Emergency Medicine”;19 (4) Diagnosis of ARDS conforms to the “Berlin Definition of Acute Respiratory Distress Syndrome”;20 (5) Complete and reliable clinical data available for analysis.
Exclusion criteria: (1) Combined with malignant tumors, tuberculosis, HIV infection, or other immunodeficiency diseases; (2) Severe hepatic or renal failure, or heart failure; (3) Complicated with pulmonary tuberculosis, COPD, congenital lung dysplasia, or other pulmonary diseases; (4) History of immunosuppressant or hormone therapy within the past 6 months; (5) Complicated with severe infection at other sites or multiple organ failure; (6) Incomplete test data or improper specimen storage; (7) Pregnant or lactating women; (8) Considered unsuitable for inclusion by the researchers, such as those with psychiatric disorders or cognitive impairment who cannot cooperate with the study procedures.
Clinical Data
The following clinical data were collected for all enrolled patients: (1) Demographic data: gender; age; body mass index (BMI); smoking history; drinking history; living alone status; (2) Pneumonia-related information: type of pneumonia (community-acquired pneumonia/hospital-acquired pneumonia); whether complicated with underlying pulmonary diseases (eg, COPD, bronchiectasis, interstitial lung disease, etc).; (3) Underlying diseases: hypertension; diabetes; coronary heart disease; chronic liver disease; (4) Laboratory test indicators (within 24 h of admission): white blood cell count (WBC); C-reactive protein (CRP); procalcitonin (PCT); serum creatinine (Scr); blood urea nitrogen (BUN); (5) Mechanical ventilation: duration of mechanical ventilation.
All clinical data were extracted from the standardized hospital electronic medical record system, and only baseline information within 24 hours of admission was included to minimize bias. Data collection was independently performed by two trained researchers in a blinded manner, and any inconsistent entries were rechecked by a third investigator to ensure accuracy and reliability.
Grouping Method
Patients in Group A (with ARDS) were further subdivided according to the oxygenation index [arterial oxygen partial pressure (PaO₂) / fraction of inspired oxygen (FiO₂)] after admission: Mild ARDS subgroup: PaO₂/FiO₂ between 200–300 mmHg (n=28); Moderate ARDS subgroup: PaO₂/FiO₂ between 100–200 mmHg (n=36); Severe ARDS subgroup: PaO₂/FiO₂ <100 mmHg (n=50). Meanwhile, Group A patients were also stratified by their 28-day outcome into: Survival subgroup: patients who survived within 28 days (n=79); Death subgroup: patients who died within 28 days (n=35).
Detection of Serum miR-27a and FOXO3 mRNA
All enrolled patients underwent collection of a fasting early-morning venous blood sample (5 mL) within 24 hours of admission. Blood was placed in anticoagulant-free centrifuge tubes, left to clot at room temperature for 30 minutes, and then centrifuged at 3000 rpm for 10 minutes to isolate serum. The serum was immediately aliquoted into RNase-free centrifuge tubes (free of RNA contamination) and stored at –80°C in an ultra-low temperature freezer to ensure the quality and stability for subsequent RNA extraction. To detect the expression levels of miR-27a and FOXO3 mRNA in serum, qRT-PCR (quantitative real-time polymerase chain reaction) was used. The detection process included three main steps: total RNA extraction, reverse transcription, and real-time PCR amplification, detailed as follows: (1) Total RNA extraction: According to the instructions of the RNA extraction kit produced by Nanjing Vazyme Biotech Co., Ltd. (Product No.: RC112-01), total RNA was extracted from frozen serum. Strict RNase-free procedures were followed throughout. The concentration and purity of RNA samples were assessed using a NanoDrop™ UV spectrophotometer, and samples with A260/A280 between 1.8 and 2.1 were considered qualified. (2) Reverse transcription: RNA samples that passed quality assessment were reverse-transcribed into cDNA using the reverse transcription kit provided by Nanjing Saihongrui Biotech Co., Ltd. (Product No.: DV807A). Specific stem-loop primers were used for miRNA reverse transcription with U6 small nuclear RNA as the internal control, while mRNA reverse transcription was performed using a mixed system of Oligo(dT) and random primers, with GAPDH as the reference gene.(3) Real-time quantitative PCR amplification: The PCR reaction system was constructed based on the kit provided by Yeasen Biotechnology (Shanghai) Co., Ltd. (Product No.: 11203ES03). The total volume per reaction was 20 μL, including: 2× Master Mix buffer 10 μL, forward primer 0.5 μL, reverse primer 0.5 μL, cDNA template 2.0 μL, and RNase-free DEPC-treated water to 20 μL. Amplification was performed on an ABI 7500 real-time PCR system. Reaction conditions were: Pre-denaturation: 95°C for 30 seconds, 1 cycle; Amplification: 95°C for 5 seconds and 60°C for 40 seconds, 40 cycles; Melting curve: fluorescence signals were acquired from 65°C to 95°C, with 0.5°C increments to verify amplification specificity. (4) Data analysis and quality control: Fluorescence signal data were collected and calculated using the instrument’s built-in software. All raw amplification plots and melting curves were manually reviewed to confirm specificity. Samples with ambiguous amplification results were repeated to ensure reproducibility. Relative expression levels of miR-27a and FOXO3 mRNA were calculated using the 2–ΔΔCt method, with U6 and GAPDH as internal controls for normalization, respectively. All primers were designed based on published sequences, verified in the NCBI database, and synthesized with quality certification by Wuhan GeneCreate Bioengineering Co., Ltd., and the primer sequences are shown in Table 1.
Table 1.
Primer Sequence Information
| Gene | Primer Sequence |
|---|---|
| miR-27a | F: 5′-AAGGAGCCCCACGAGAAAAA-3′ |
| R: 5′-ACCGAACTTGCATTGATTCC-3′ | |
| U6 | F: 5′-CTCGCTTCGGCAGCACA-3′ |
| R: 5′-AACGCTTCACGAATTTGCGT-3′ | |
| FOXO3 | F: 5′-ACACTCCAGCTGGGTCCCTGA-3′ |
| R: 5′-TGTCGTGGAGTCGGCAATTC-3’ |
Statistical Analysis
Statistical analysis was performed using SPSS 26.0 software, and figures were generated using GraphPad Prism 9.0. Continuous variables conforming to normal distribution were expressed as (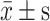 ); comparisons between two groups were made using the t-test, and comparisons among multiple groups were performed using analysis of variance (ANOVA). Categorical data were expressed as counts (n) and percentages (%), and comparisons were made using the χ²-test. Correlation analysis was performed using Pearson or Spearman correlation methods. Multivariate analysis used a binary logistic regression model to identify independent risk factors for 28-day mortality. ROC curves were plotted to compare the predictive performance of miR-27a, FOXO3, and their combination for patient mortality. Differences in AUC were compared using the Z-test. The significance level was set at α=0.05, with P<0.05 considered statistically significant.
); comparisons between two groups were made using the t-test, and comparisons among multiple groups were performed using analysis of variance (ANOVA). Categorical data were expressed as counts (n) and percentages (%), and comparisons were made using the χ²-test. Correlation analysis was performed using Pearson or Spearman correlation methods. Multivariate analysis used a binary logistic regression model to identify independent risk factors for 28-day mortality. ROC curves were plotted to compare the predictive performance of miR-27a, FOXO3, and their combination for patient mortality. Differences in AUC were compared using the Z-test. The significance level was set at α=0.05, with P<0.05 considered statistically significant.
Results
Comparison of Serum miR-27a and FOXO3 mRNA Levels Among the Three Groups
Serum miR-27a levels were significantly higher in Group B than in Group A, and further elevated in the control group compared with Group B. Conversely, FOXO3 mRNA levels were significantly lower in Group B than in Group A, and further reduced in the control group (F=77.352, 62.956, P<0.001), as shown in Figure 1. This indicates that compared with the control group, serum miR-27a levels were downregulated while FOXO3 mRNA levels were upregulated in the disease groups.
Figure 1.
Comparison of serum miR-27a and FOXO3 mRNA levels among the three groups.
Notes: *P<0.05 vs Group A; #P<0.05 vs Group B. All comparisons are made with reference to the control group.
Comparison of Serum miR-27a and FOXO3 mRNA Levels in Patients with Different Severities of Severe Pneumonia with ARDS
In patients with severe pneumonia and ARDS, serum miR-27a levels decreased progressively from the mild to moderate and severe subgroups, whereas FOXO3 mRNA levels increased in the same order (F=83.597, 111.834, P<0.001), as shown in Figure 2. These subgroups all belong to the disease group and reflect different severity classifications.
Figure 2.
Comparison of serum miR-27a and FOXO3 mRNA levels in patients with different severities of severe pneumonia with ARDS.
Notes: aP<0.05 vs mild subgroup; bP<0.05 vs moderate subgroup. All comparisons are relative within the disease group.
Correlation Between Serum miR-27a and FOXO3 mRNA Levels
Pearson correlation analysis showed that serum miR-27a and FOXO3 mRNA levels in elderly patients with severe pneumonia and ARDS were negatively correlated (r=–0.624, P<0.001), as shown in Figure 3.
Figure 3.
Scatter plot of correlation between serum miR-27a and FOXO3 mRNA levels.
Spearman correlation analysis showed that the oxygenation index (mild=3, moderate=2, severe=1) was positively correlated with serum miR-27a levels (r=0.635, P<0.001), and negatively correlated with FOXO3 mRNA levels (r=–0.672, P<0.001), as shown in Figure 4. The oxygenation index was expressed in mmHg to ensure clarity.
Figure 4.
Scatter plot of correlation between serum miR-27a, FOXO3 mRNA levels and oxygenation index (mmHg).
Comparison of Clinical Data in Patients with Different Prognoses of Severe Pneumonia with ARDS
The 28-day mortality rate in elderly patients with severe pneumonia and ARDS was 30.70% (35/114). The death subgroup had higher age, CRP, mechanical ventilation time, and FOXO3 mRNA levels, and lower oxygenation index and miR-27a levels compared to the survival subgroup (P<0.05). There were no statistically significant differences in other data (P>0.05), as shown in Table 2.
Table 2.
Comparison of Clinical Data in Patients with Different Prognoses of Severe Pneumonia with ARDS
| Clinical Data | Prognosis | t/x² | P | |
|---|---|---|---|---|
| Survival Subgroup (n=79) | Death Subgroup (n=35) | |||
| Male | 48 (60.76) | 21 (60.00) | 0.005 | 0.938 |
| Age (years) | 71.93±6.57 | 76.59±6.32 | 3.533 | <0.001 |
| BMI (kg/m²) | 22.74±2.41 | 22.53±2.49 | 0.424 | 0.671 |
| Smoking history | 29 (36.71) | 15 (42.86) | 0.386 | 0.533 |
| Alcohol history | 13 (16.46) | 9 (25.71) | 1.335 | 0.249 |
| Living alone | 22 (27.85) | 11 (31.43) | 0.151 | 0.697 |
| CAP | 60 (75.95) | 23 (65.71) | 1.283 | 0.257 |
| Lung comorbidities | 45 (56.96) | 22 (62.86) | 0.347 | 0.555 |
| Hypertension | 26 (32.91) | 14 (40.00) | 0.535 | 0.464 |
| Diabetes | 13 (16.46) | 8 (22.86) | 0.661 | 0.416 |
| Coronary heart disease | 20 (25.32) | 11 (31.43) | 0.457 | 0.498 |
| Chronic liver disease | 11 (13.92) | 7 (20.00) | 0.673 | 0.411 |
| WBC (×109/L) | 12.67±2.55 | 13.54±2.59 | 1.672 | 0.097 |
| CRP (mg/L) | 122.43±57.68 | 149.21±50.46 | 2.372 | 0.019 |
| PCT (μg/L) | 4.57±2.14 | 5.32±2.49 | 1.640 | 0.103 |
| Scr (μmol/L) | 84.82±26.15 | 93.49±25.07 | 1.653 | 0.101 |
| BUN (mmol/L) | 10.84±3.47 | 12.15±3.78 | 1.808 | 0.073 |
| Mechanical ventilation time (days) | 3.26±0.91 | 5.34±1.15 | 10.357 | <0.001 |
| Oxygenation index (mmHg) | 165.43±70.98 | 71.86±21.77 | 7.624 | <0.001 |
| miR-27a | 0.93±0.15 | 0.75±0.14 | 6.029 | <0.001 |
| FOXO3 mRNA | 1.14±0.18 | 1.31±0.16 | 4.806 | <0.001 |
Multivariate Logistic Regression Analysis of Prognostic Factors in Elderly Patients with Severe Pneumonia and ARDS
Taking prognosis (survival=0, death=1) as the dependent variable, possible influencing factors from Table 1 were assigned as independent variables (see Table 3). A multivariate logistic regression model was established. Results showed that increased age, prolonged mechanical ventilation time, and elevated FOXO3 mRNA were independent risk factors, while increased oxygenation index and miR-27a levels were independent protective factors, as shown in Table 4.
Table 3.
Variable Assignment Table
| Independent Variable | Assignment Method |
|---|---|
| Age | Original value |
| CRP | Original value |
| Mechanical ventilation time | Original value |
| Oxygenation index | Original value |
| miR-27a | Original value |
| FOXO3 mRNA | Original value |
Table 4.
Multivariate Logistic Regression Analysis of Prognostic Factors in Elderly Patients with Severe Pneumonia and ARDS
| Factor | β | SE | Wald | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age increase | 0.187 | 0.082 | 5.713 | <0.05 | 1.204 | 1.031–1.409 |
| CRP increase | 0.015 | 0.009 | 1.944 | >0.05 | 1.017 | 0.992–1.034 |
| Prolonged ventilation | 0.441 | 0.225 | 4.011 | <0.05 | 1.539 | 1.008–2.375 |
| Oxygenation index increase | −0.032 | 0.010 | 11.573 | <0.05 | 0.969 | 0.954–0.991 |
| miR-27a increase | −0.106 | 0.037 | 8.972 | <0.05 | 0.905 | 0.847–0.969 |
| FOXO3 mRNA increase | 0.101 | 0.033 | 10.084 | <0.05 | 1.113 | 1.038–1.179 |
Predictive Value of miR-27a, FOXO3 mRNA, and Their Combination for Mortality in Elderly Patients with Severe Pneumonia and ARDS
The AUCs for serum miR-27a, FOXO3 mRNA, and their combination in predicting mortality in elderly patients with severe pneumonia and ARDS were 0.775, 0.781, and 0.867, respectively. The combined AUC was superior to each single index (Z_combined–miR-27a=2.557, P<0.05; Z_combined–FOXO3 mRNA=2.974, P<0.05), as shown in Table 5 and Figure 5.
Table 5.
Predictive Value of miR-27a, FOXO3 mRNA, and Their Combination for Mortality in Elderly Patients with Severe Pneumonia and ARDS
| Marker | Cutoff Value | AUC | 95% CI | Sensitivity (%) | Specificity (%) | Youden Index |
|---|---|---|---|---|---|---|
| miR-27a | 0.91 | 0.775 | 0.691–0.854 | 90.42 | 50.76 | 0.441 |
| FOXO3 mRNA | 1.24 | 0.781 | 0.699–0.860 | 71.86 | 73.43 | 0.455 |
| Combination | - | 0.867 | 0.804–0.932 | 84.78 | 80.09 | 0.646 |
Figure 5.
ROC curves for predictive value of miR-27a, FOXO3 mRNA, and their combination in elderly patients with severe pneumonia and ARDS.
Discussion
This study focused on elderly patients with severe pneumonia complicated by ARDS, systematically analyzing the relationship between serum miR-27a and FOXO3 mRNA expression levels and disease severity and prognosis. Compared with healthy controls and patients with severe pneumonia without ARDS, ARDS patients showed significantly decreased serum miR-27a levels and markedly increased FOXO3 mRNA levels. Moreover, across different oxygenation index strata, miR-27a levels progressively declined with worsening ARDS severity, whereas FOXO3 mRNA levels increased stepwise relative to less severe subgroups. These findings indicate that these alterations are evident not only when compared with non-ARDS populations but also dynamically vary with disease progression, suggesting that both markers are closely associated with the pathophysiology of ARDS.
The observed inverse correlation between miR-27a and FOXO3 mRNA highlights a potential regulatory axis, in which miR-27a may play a protective role while FOXO3 promotes tissue damage. Previous studies21,22 have confirmed that miR-27a regulates inflammatory, apoptotic, and oxidative stress pathways and plays crucial roles in various pulmonary diseases, including asthma, pulmonary fibrosis, and infections. Mechanistically, miR-27a may inhibit the release of pro-inflammatory mediators, limit oxidative damage, and reduce apoptosis by modulating NF-κB, TGF-β, and PI3K/Akt signaling pathways.23–25 Downregulation of miR-27a weakens these protective effects, thereby amplifying inflammatory cascades. Conversely, persistent activation of FOXO3 exacerbates oxidative stress, induces mitochondrial dysfunction, and promotes immune imbalance.26,27
Our results are consistent with the study by Lv et al,28 who reported that downregulation of miR-27a aggravated alveolar injury in a murine ARDS model, whereas miR-27a mimic intervention effectively alleviated inflammation and tissue damage. FOXO3, on the other hand, is recognized as a transcription factor that promotes oxidative stress responses and cellular senescence. Wu et al29 demonstrated that inhibition of FOXO3 could reduce alveolar epithelial apoptosis and preserve lung function. Together, these studies support the hypothesis that an imbalance between miR-27a and FOXO3 signaling contributes to the pathogenesis and progression of ARDS. Recent evidence also indicates that FOXO3 can influence macrophage polarization and T-cell differentiation, leading to immune dysregulation and impaired tissue repair.30,31 These processes collectively create a vicious cycle of lung injury and inadequate repair, which aligns with the clinical features of refractory hypoxemia in elderly ARDS patients.
Clinically, our study found that the 28-day mortality rate among elderly ARDS patients reached 30.70%, higher than that reported for general ARDS populations,32 reflecting age-related vulnerability and the influence of comorbidities. Notably, multivariate logistic regression analysis indicated that elevated FOXO3 levels were an independent risk factor, whereas miR-27a and oxygenation index served as independent protective factors. ROC curve analysis showed that combined detection of these two markers achieved an AUC of 0.867, outperforming individual markers and providing a practical approach for risk stratification. Zhao et al33 similarly demonstrated that multi-marker combined detection significantly improves prognostic prediction in ARDS patients. Therefore, this study expands the ARDS biomarker panel in elderly patients and validates the clinical utility of miR-27a and FOXO3.
In terms of novelty, this study has three main contributions. First, it is the first to combine the detection of miR-27a and FOXO3 mRNA in elderly ARDS patients, integrating molecular mechanisms with clinical prognostic assessment. Second, the inclusion of a relatively large cohort with stratification across ARDS severity enhances the clinical representativeness and reliability of the findings. Third, by focusing on elderly patients—a subgroup with poor outcomes that is often underrepresented in biomarker studies—this work fills a critical gap in ARDS research. These findings not only enrich current understanding but also provide a foundation for future therapeutic strategies targeting the miR-27a/FOXO3 signaling pathway.
However, several limitations should be acknowledged. First, as a single-center retrospective study, selection bias cannot be excluded, and multicenter prospective cohort studies are needed for validation. Second, only serum levels were assessed, lacking mechanistic validation in bronchoalveolar lavage fluid, lung tissue, or animal models. Moreover, miR-27a may regulate multiple targets beyond FOXO3, and FOXO3 may be influenced by other miRNAs or upstream signals; therefore, causal relationships remain to be confirmed. Functional experiments and multi-omics approaches could provide deeper insights into these interactions.
In conclusion, this study demonstrates that downregulated serum miR-27a and upregulated FOXO3 mRNA are closely associated with ARDS severity and short-term prognosis in elderly patients with severe pneumonia. Combined detection of these markers enhances predictive accuracy, providing a novel molecular basis for early identification, risk assessment, and potential therapeutic intervention. Future studies should integrate mechanistic validation and dynamic longitudinal monitoring to establish causal roles and explore their feasibility as intervention targets, ultimately advancing personalized management of ARDS.
Conclusion
The results of this study indicate that, compared with healthy controls and elderly patients with severe pneumonia without ARDS, serum miR-27a levels are significantly decreased, whereas FOXO3 mRNA levels are significantly increased in elderly patients with severe pneumonia complicated by ARDS. Within ARDS subgroups stratified by oxygenation index, miR-27a levels progressively decreased from mild to moderate to severe ARDS, while FOXO3 mRNA levels increased stepwise, highlighting their close association with disease severity. Elevated miR-27a may act as a protective factor, whereas elevated FOXO3 mRNA serves as an independent risk factor for poor short-term outcomes. Combined detection of these two biomarkers provides higher predictive efficacy for 28-day mortality than either marker alone, underscoring their potential utility for early risk stratification and clinical intervention. These findings suggest that the imbalance between miR-27a and FOXO3 is not only involved in the pathogenesis of ARDS but also has practical implications as prognostic biomarkers. Future studies should further investigate the molecular mechanisms underlying miR-27a regulation of FOXO3 and related downstream signaling pathways and validate their clinical utility in elderly ARDS populations through multicenter, large-sample prospective studies.
Funding Statement
Project of National Clinical Research Center for Geriatric Diseases, Research on Diagnosis, Treatment and Comprehensive Prevention and Control Measures of Multidrug Resistant Pathogenic Bacteria Infections in Elderly Patients (Project Number: NCRCG-PLAGH-DX-2024002) 2. Military Health Care Project: Research on the Clinical Application Value of the Combined Evaluation Method of Pepsin, amylase and Lipid Cells in Tracheal Aspirates for the Diagnosis of Airway Aspiration (Project Number: 21BJZ24).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Xu Y, Wang XY. [Clinical characters and influencing factors of perioperative pneumonia in elderly patients with hip fractures aged ≥80 years at a tertiary hospital in Beijing City]. Zhonghua Yu Fang Yi Xue Za Zhi. 2025;59(6):916–924. doi: 10.3760/cma.j.cn112150-20250330-00257 Wolof [DOI] [PubMed] [Google Scholar]
- 2.Kang M, Li J, Wan Q, et al. [Factors influencing the choice of endotracheal intubation and mechanical ventilation in patients with acute respiratory distress syndrome caused by viral pneumonia]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022;34(6):586–591. doi: 10.3760/cma.j.cn121430-20220607-00549 Dutch [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Li Y, Li H, et al. [Acute respiratory distress syndrome caused by severe respiratory infectious diseases: clinical significance and solution of maintaining artificial airway closure]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2025;37(3):221–224. doi: 10.3760/cma.j.cn121430-20240506-00404 Dutch [DOI] [PubMed] [Google Scholar]
- 4.Tang R, Tang W, Wang D. [Predictive value of machine learning for in-hospital mortality for trauma-induced acute respiratory distress syndrome patients: an analysis using the data from MIMIC III]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2022;34(3):260–264. doi: 10.3760/cma.j.cn121430-20211117-01741 Dutch [DOI] [PubMed] [Google Scholar]
- 5.Liu YN, Ma XC. [The precision medicine for the treatment of acute respiratory distress syndrome is based on identification of phenotypes]. Zhonghua Yi Xue Za Zhi. 2024;104(15):1253–1257. doi: 10.3760/cma.j.cn112137-20230928-00603 Danish [DOI] [PubMed] [Google Scholar]
- 6.Wu J, Xiao H, Li X, et al. [Evaluation value of sequential organ failure assessment score for predicting the prognosis of patients with acute respiratory distress syndrome due to severe pneumonia]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2021;33(9):1057–1062. doi: 10.3760/cma.j.cn121430-20210115-00076 Dutch [DOI] [PubMed] [Google Scholar]
- 7.Zhang HL, Shang Y. [The value of point-of-care ultrasound in the diagnosis and management of acute respiratory distress syndrome]. Zhonghua Yi Xue Za Zhi. 2024;104(15):1225–1229. doi: 10.3760/cma.j.cn112137-20230906-00407 Danish [DOI] [PubMed] [Google Scholar]
- 8.Chalmers S, Khawaja A, Wieruszewski PM, et al. Diagnosis and treatment of acute pulmonary inflammation in critically ill patients: the role of inflammatory biomarkers. World J Crit Care Med. 2019;8(5):59–71. doi: 10.5492/wjccm.v8.i5.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian F, Ying H, Liao S, et al. lncRNA SNHG14 promotes the proliferation, migration, and invasion of thyroid tumour cells by regulating miR-93-5p. Zygote. 2022;30(2):183–193. doi: 10.1017/S0967199421000319 [DOI] [PubMed] [Google Scholar]
- 10.Xing J, Chen LP, Yu WJ. [Non-small cell lung cancer-derived exosomal circular RNA circEZH2 activates fibroblasts by regulating the miR-495-3p / TPD52 axis and NF-κB pathway]. Zhonghua Zhong Liu Za Zhi. 2024;46(12):1176–1186. doi: 10.3760/cma.j.cn112152-20230717-00015 Polish [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Liu R, Dang X, et al. [Experimental study on improvement of osteonecrosis of femoral head with exosomes derived from miR-27a-overexpressing vascular endothelial cells]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2021;35(3):356–365. doi: 10.7507/1002-1892.202011026 Danish [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie E, Lin M, Sun Z, et al. Serum miR-27a is a biomarker for the prognosis of non-small cell lung cancer patients receiving chemotherapy. Transl Cancer Res. 2021;10(7):3458–3469. doi: 10.21037/tcr-20-3276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan X, Wang J, Qin T, et al. Exosome miR-27a-3p secreted from adipocytes targets ICOS to promote antitumor immunity in lung adenocarcinoma. Thorac Cancer. 2020;11(6):1453–1464. doi: 10.1111/1759-7714.13411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruan P, Zheng Y, Dong Z, et al. [Research progress in the regulation of autophagy and mitochondrial homeostasis by AMPK signaling channels]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2024;36(4):425–429. doi: 10.3760/cma.j.cn121430-20230302-00132 Dutch [DOI] [PubMed] [Google Scholar]
- 15.Yang F, Zhang H, Zhuo M, et al. [Effects of Peiminine on biological behavior and chemotherapy resistance of ovarian cancer by regulating the FOXO3-FOXM1 signaling axis]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2024;40(11):976–982. Wolof [PubMed] [Google Scholar]
- 16.Tao Y, Li -L-L, Liu S-H, et al. [FOXO3a signaling pathway in prostate cancer: progress in studies]. Zhonghua Nan Ke Xue. 2020;26(8):745–750. Basque [PubMed] [Google Scholar]
- 17.Wang Y, Liang H-X, Zhang C-M, et al. FOXO3/TRIM22 axis abated the antitumor effect of gemcitabine in non-small cell lung cancer via autophagy induction. Transl Cancer Res. 2020;9(2):937–948. doi: 10.21037/tcr.2019.12.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Xia J, Hu Q, et al. Long non‑coding RNA XIST promotes cerebral ischemia/reperfusion injury by modulating miR‑27a‑3p/FOXO3 signaling. Mol Med Rep. 2021;24(2):566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shang WF, Chen DC. [Prone positioning ventilation therapy in acute respiratory distress syndrome: knowns and unknowns in clinical efficacy]. Zhonghua Yi Xue Za Zhi. 2024;104(15):1236–1241. doi: 10.3760/cma.j.cn112137-20231012-00720 Danish [DOI] [PubMed] [Google Scholar]
- 20.Yuan XY, Liu L, Qiu HB. [New 2023 global definition of acute respiratory distress syndrome: progress and limitation]. Zhonghua Yi Xue Za Zhi. 2024;104(15):1216–1220. doi: 10.3760/cma.j.cn112137-20231016-00770 Danish [DOI] [PubMed] [Google Scholar]
- 21.Dong M, Wang X, Li T, et al. miR-27a-3p alleviates lung transplantation-induced bronchiolitis obliterans syndrome (BOS) via suppressing Smad-mediated myofibroblast differentiation and TLR4-induced dendritic cells maturation. Transpl Immunol. 2023;78:101806. doi: 10.1016/j.trim.2023.101806 [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Jiang R, Hu X, et al. CircGSAP alleviates pulmonary microvascular endothelial cells dysfunction in pulmonary hypertension via regulating miR-27a-3p/BMPR2 axis. Respir Res. 2022;23(1):322. doi: 10.1186/s12931-022-02248-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abd-Elhakim YM, Mohamed AA-R, Khamis T, et al. Alleviative effects of green-fabricated zinc oxide nanoparticles on acrylamide-induced oxidative and inflammatory reactions in the rat stomach via modulating gastric neuroactive substances and the MiR-27a-5p/ROS/NF-κB axis. Tissue Cell. 2024;91:102574. doi: 10.1016/j.tice.2024.102574 [DOI] [PubMed] [Google Scholar]
- 24.Ong J, Faiz A, Timens W, et al. Marked TGF-β-regulated miRNA expression changes in both COPD and control lung fibroblasts. Sci Rep. 2019;9(1):18214. doi: 10.1038/s41598-019-54728-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao XR, Zhang Z, Gao M, et al. MicroRNA-27a-3p aggravates renal ischemia/reperfusion injury by promoting oxidative stress via targeting growth factor receptor-bound protein 2. Pharmacol Res. 2020;155:104718. doi: 10.1016/j.phrs.2020.104718 [DOI] [PubMed] [Google Scholar]
- 26.Shen J, Wang H, Wang J-S, et al. [The relationship between MicroRNA expression profiling in imatinib-resistant cell line K562/G and potential mechanism through FOXO3/Bcl-6 signaling pathway]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2022;30(1):107–112. doi: 10.19746/j.cnki.issn.1009-2137.2022.01.017 Hausa [DOI] [PubMed] [Google Scholar]
- 27.Artham S, Gao F, Verma A, et al. Endothelial stromelysin1 regulation by the forkhead box-O transcription factors is crucial in the exudative phase of acute lung injury. Pharmacol Res. 2019;141:249–263. doi: 10.1016/j.phrs.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lv X, Zhang X-Y, Zhang Q, et al. lncRNA NEAT1 aggravates sepsis-induced lung injury by regulating the miR-27a/PTEN axis. Lab Invest. 2021;101(10):1371–1381. doi: 10.1038/s41374-021-00620-7 [DOI] [PubMed] [Google Scholar]
- 29.Wu Z, Wang Y, Lu S, et al. SIRT3 alleviates sepsis-induced acute lung injury by inhibiting pyroptosis via regulating the deacetylation of FoxO3a. Pulm Pharmacol Ther. 2023;82:102244. doi: 10.1016/j.pupt.2023.102244 [DOI] [PubMed] [Google Scholar]
- 30.Duan XH, Li H, Lyu Y, et al. Regulation of baicalin on growth of extranodal NK/T cell lymphoma cells through FOXO3/CCL22 signaling pathway. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2023;31(3):730–738. doi: 10.19746/j.cnki.issn.1009-2137.2023.03.017 [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Wang Z. Oroxylin-A attenuates taurocholate-induced lung injury via NF-κB pathway suppression. Clin Mol Epidemiol. 2025;2:5. doi: 10.53964/cme.2025005 [DOI] [Google Scholar]
- 32.Wang HF, Hu WH, Song QW, et al. [Clinical study on the relationship between the exosomes in bronchoalveolar lavage fluid and plasma and the severity of lung injury and outcome in early acute respiratory distress syndrome patients]. Zhonghua Yi Xue Za Zhi. 2022;102(13):935–941. doi: 10.3760/cma.j.cn112137-20211105-02448 Danish [DOI] [PubMed] [Google Scholar]
- 33.Zhao C, Li Y, Wang Q, et al. Establishment of risk prediction nomograph model for sepsis related acute respiratory distress syndrome. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2023;35(7):714–718. doi: 10.3760/cma.j.cn121430-20230215-00088 [DOI] [PubMed] [Google Scholar]







