Abstract
Cell-to-cell communication through ligand-receptor (LR) interactions can fundamentally shape the tumor microenvironment and immune responses, but the full spectrum of these interactions in anti-PD-1 therapy remains unexplored. We developed a predictive model for anti-PD-1 therapy responses incorporating 2,705 LR pairs across 121 melanoma samples. Using a random forest classifier, our model achieved robust accuracy in the training and test datasets as well as in two independent external validation cohorts. Feature importance analysis revealed nine key LR pairs with substantial predictive power, including both known immune checkpoint molecules and novel interaction pairs. The genes comprising these top-ranking pairs were significantly enriched in tumor-related pathways, particularly MAPK and PI3K/AKT signaling pathways. Importantly, our systematic LR profiling approach identified previously uncharacterized ligand-receptor interactions that may represent alternative therapeutic targets beyond the established PD-1/PD-L1 axis. These findings demonstrate the clinical utility of integrated LR expression analysis for predicting anti-PD-1 therapy responses and reveal potential novel biomarkers and combination therapy targets that could expand treatment options for immunotherapy-resistant patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10238-025-01875-w.
Keywords: Tumor immunotherapy, Anti-PD-1 therapy response, Machine Learning, Ligand-receptor interaction, Biomarker discovery
Introduction
Melanoma is the most aggressive form of skin cancer and is a significant global health concern. In the USA, it is the sixth most common cancer in both men and women [1]. Despite its relatively low incidence compared to other skin cancers, the high metastatic potential of melanoma makes it disproportionately deadly [1]. Melanoma is strongly associated with ultraviolet (UV) radiation exposure, which often contributes to its characteristically high tumor mutational burden (TMB).
Recent advances in cancer immunotherapy, particularly in immune checkpoint blockade targeting the programmed death-1 (PD-1) pathway, have revolutionized the treatment of advanced melanoma [2]. PD-1, an inhibitory receptor expressed on activated T cells, including CD8 + cytotoxic T lymphocytes (CTLs), plays a critical role in tumor-immune evasion [3]. Therapeutic use of PD-1 inhibitors such as pembrolizumab and nivolumab has demonstrated significant clinical benefits in patients with advanced disease [4] (Fig. 1A). However, response rates remain variable, with only 30–40% of patients achieving durable responses, highlighting the need for predictive biomarkers.
Fig. 1.
Mechanism of anti‐PD‐1 therapy and overview of the study design. A Schematic illustration of the anti‐PD‐1 therapeutic mechanism. PD‐1 blockade restores T cell function by preventing the interaction between PD‐1 on T cells and PD‐L1 on tumor cells, leading to enhanced anti‐tumor immunity. B Analytical workflow of the study. Individual heatmaps on the left show normalized gene expression levels for ml ligands and mr receptors across n patients. Green indicates low expression and red is high expression. LR pair expression matrix is visualized on a color scale from blue (low) to red (high)
The efficacy of anti-PD-1 therapy is intrinsically linked to a complex network of cellular interactions within the tumor microenvironment (TME). It comprises a highly heterogeneous group of cells, including inflammatory cells, immunosuppressive cells, cancer-associated fibroblasts, endothelial cells, adipocytes, pericytes, and tumor-associated macrophages [5]. Crucially, intricate signaling between these cellular components and tumor cells is mediated by diverse ligand-receptor (LR) interactions that collectively orchestrate immune responses.
For example, CXCL8 (interleukin [IL]-8) binds to CXCR1 and CXCR2, activating signaling pathways that drive tumor-associated inflammation, cellular reprogramming, epithelial-mesenchymal transition, and neovascularization [6, 7]. Similarly, the interaction between CD40 ligand (CD40L) on CD4 + T cells and CD40 on dendritic cells leads to the dendritic cell activation, subsequently stimulating CD8 + T cells [8, 9] [10]. These examples represent just a fraction of the thousands of potential LR interactions that may influence therapeutic outcomes.
Given the critical roles of LR interactions in modulating anti-tumor immunity, we hypothesized that comprehensive profiling of LR expression patterns could provide predictive power for anti-PD-1 therapy responses. The LR interactions could represent targetable cell-surface molecules that directly mediate tumor-immune communication and can serve as both biomarkers and therapeutic targets. Here, we developed a machine learning model that systematically analyzed 2705 LR pairs to predict treatment responses in melanoma patients (Fig. 1B). Beyond prediction, this approach enabled the discovery of previously uncharacterized LR interactions that may serve as novel therapeutic targets or combination therapy partners. Our findings suggest that integrated LR expression analysis can serve as a powerful tool for both predicting anti-PD-1 responses and identifying new avenues for immunotherapy development in melanoma.
Materials and methods
Patient cohorts and data collection
RNA-seq data were collected from patients with melanoma prior to anti-PD-1 therapy (PRE-therapy). Response categories were consolidated into two groups: non-response (NR, including progressive disease [PD] and stable disease [SD]) and response (R, including complete response [CR], partial response [PR], and minor response [MR]). The training dataset from Liu et al. [11] comprises 121 samples (NR = 72, R = 49). Two independent cohorts were used for external validation: Riaz (GSE91061; NR = 39, R = 10) and Gide (PRJEB23709; NR = 11, R = 11) cohorts.
RNA-sequencing data processing
Raw FASTQ files were obtained using fastq-dl (v0.12.1) and quality controlled using Trimmomatic (v0.39). The sequence alignment was performed using HISAT2 (v2.2.1) against the GRCh38 genome. Gene expression was quantified using featureCounts (Subread v2.0.6) and Homo_sapiens.GRCh38.109. gtf as reference. The expression values were normalized to Transcripts Per Million (TPM).
LR pair information was obtained from CellTalkDB [12]. The LR pair expression was achieved by multiplying the normalized expression values of ligand and receptor genes, in a manner similar to previous studies, with modifications adapted for our datasets [13]. The gene expression values were normalized using TPM + 0.1, with 0.1, added to avoid negative infinity for zero TPM values, and multiplied by the LR pair information to represent LR interactions. The final values were scaled using min–max normalization before being used as the input for the model. Subsequently, the normalized ligand and receptor gene expression values were multiplied according to the LR pair information. In total, 2705 LR pairs were generated as input features.
Predictive model development
A random forest classification (RFC) model was initially developed using all available LR pairs from the Liu cohort, which was split into training (80%) and test (20%) sets. To determine whether a reduced feature set could achieve comparable performance, feature importance scores were calculated to identify key LR pairs. Additional RFC models were constructed using only features with importance scores > 0.05. The model performance was validated using the Riaz (49 samples) and Gide (22 samples) cohorts.
Cell type-specific expression analysis of top LR pairs
To elucidate the cellular sources of our top predictive LR pairs, single-cell RNA-seq (scRNA-seq) data with cell type annotation from melanoma patients (https://zenodo.org/records/14976008; GSE115978) were utilized. Expression levels of genes in the top 9 LR pairs were quantified expression across eight major cell types: CD8 + T cells, CD4 + T cells, B cells, NK cells, macrophages, cancer-associated fibroblasts, endothelial cells, and malignant cells. The percentage of cells expressing each gene within each cell type was calculated to determine predominant ligand- and receptor-expressing cell populations.
Differential expression and pathway analysis
Differentially expressed genes (DEG) analysis was analyzed using OmicVerse (v1.5.8). DEGs between responders and non-responders were identified using thresholds of q-value < 0.05 and |logFC|> 1. The results were visualized using Matplotlib (v3.6.3) and Seaborn (v0.13.2).
Enrichment analysis was conducted using GSEApy (v1.1.3), using KEGG_2021_Human gene sets. The results were visualized as bar plots ordered by increasing q-values.
Model comparison with tumor-immune dysfunction and exclusion (TIDE)
TIDE was performed using TIDEpy (v1.3.8), following established protocols [20]. The TIDE values were set at a default threshold of zero, and the prediction accuracies were compared with the performance of our model.
Results
LR pairs enable prediction of anti-PD-1 response
We developed an RFC model for anti-PD-1 therapy response based on the expression levels of 2705 LR pairs across 121 melanoma samples from the Liu cohort (Table 1).
Table 1.
Training, test, and validation datasets
| Cohort (size) | Response | Non-response | |||
|---|---|---|---|---|---|
| PR | MR | CR | PD | SD | |
| Liu (n = 121) | 31 | 2 | 16 | 56 | 16 |
| Gide (n = 22) | 8 | 3 | 9 | 2 | |
| Riaz (n = 49) | 7 | 3 | 23 | 16 | |
Partial response, PR; Minor response, MR; Complete response, CR; Progressive disease, PD; Stable disease, SD
To integrate the LR pair information, the normalized expression values of the ligand and receptor were multiplied by each LR pair. The Liu cohort was split into training and test datasets in an 8:2 ratio to construct a predictive model for anti-PD-1 therapy responsiveness. Using all 2705 LR pairs as input features, the model achieved accuracies of 0.885 and 0.800 for the training and test sets, respectively (Fig. 2A). The reliability of the model was also supported by a sensitivity of 0.800 and specificity of 0.800 for the test dataset. We also carried out the experiments using several other supervised classifiers in the same manners. However, the classification accuracies on the test dataset were significantly lower than RFC (0.503 for k-nearest neighbors; 0.568 for support vector machine; 0.611 for logistic regression; 0.616 for lasso regression).
Fig. 2.
LR pairs enable prediction of anti‐PD‐1 therapy response. A Predictive performance of the model trained using the complete set of LR pair features in both training (blue) and test (red) datasets. B Feature importance scores of the LR pairs. C Model performance using only the top 9 LR pairs (with feature importance scores ≥ 0.05) in both training (blue) and test (red) datasets
RFC provides the intrinsic capability to estimate feature importance scores, enabling us to identify the most predictive LR pairs in our model (Fig. 2B). LR pairs linked to cancer invasion and metastasis or involved in T-cell recruitment and immune response regulation were identified as the top-ranked features of importance [14–19]. Notably, WNT1-FZD5, CXCL9-DPP4, TGFB1-SMAD3, and FADD-FAS interact with anti-PD-1 therapy [20–23]. Moreover, MMP-9, FGFR3, and B2M have been implicated in responses to anti-PD-1 therapy, although their relationship with anti-PD-1 therapy response has not yet been thoroughly confirmed [24–26]. For instance, in neuroendocrine tumors, anti-MMP-9 agents has been shown to enhance the therapeutic efficacy of anti-PD-1 treatments [24].
To further determine the cellular sources of the identified LR pairs, we analyzed publicly available single-cell RNA-seq datasets from melanoma patients [27]. We examined expression levels for the LR pairs across eight annotated cell types including CD8 + T cells, CD4 + T cells, B cells, NK cells, macrophages, fibroblasts, endothelial cells, and malignant cells (Supplementary Fig. S1). For instance, WNT1 expression was most prominent in CD8 + T cells, whereas FZD5 expression was highest in endothelial cells, followed by NK cells and malignant cells. Previous studies have shown that WNT/FZD signaling promotes angiogenesis through endothelial cells [28, 29] and supports NK cell development and proliferation [30]. In malignant cells, WNT/FZD-mediated signaling has been reported to induce PD-L1 expression, decrease CD8 + T-cell abundance, and increase Treg infiltration [31]. These processes have been linked to enhanced resistance to anti-PD-(L)1 therapy in melanoma. We also applied SHAP (SHapley Additive exPlanations) as an another feature importance quantification method. The top features identified by SHAP were highly consistent with those determined by RFC’s feature importance scores (Supplementary Fig. S2).
Furthermore, we constructed a predictive model using only the top nine features with an importance score of > 0.05. Notably, the model incorporating the top nine LR pairs achieved training and testing accuracies of 0.885 and 0.760, respectively. In the training dataset, the top model achieved sensitivity and specificity of 0.897 and 0.877, respectively, whereas the test cohort showed sensitivity and specificity of 0.700 and 0.800, respectively (Fig. 2C). These results suggest that a limited set of LR pairs is sufficient to predict response to anti-PD-1 therapy, highlighting the potential biological significance of these specific molecular interactions.
External validation confirms robust performance of LR-based prediction model
To evaluate the generalizability of our model, we performed an external validation using two independent melanoma cohorts: Gide (n = 22) and Riaz (n = 49) (Table 2 and Supplementary Fig. S3). Our model incorporating all 2,705 LR pairs maintained consistent performance across both external datasets, achieving accuracies of 0.727 and 0.734 in the Gide and Riaz cohorts, respectively. In the Gide and Riaz cohort, the model achieved sensitivity, specificity of 0.727, 0.727 and 0.600, 0.769, respectively. These results support the robust predictive capability of this model in different patient populations.
Table 2.
External validation (Gide and Riaz cohort) results of ligand-receptor (LR)-based prediction model
| All model | Top 9 model | |||
|---|---|---|---|---|
| Gide | Riaz | Gide | Riaz | |
| Accuracy | 0.727 | 0.734 | 0.682 | 0.714 |
| Sensitivity | 0.727 | 0.600 | 0.636 | 0.500 |
| Specificity | 0.727 | 0.769 | 0.727 | 0.769 |
| F1 score | 0.727 | 0.752 | 0.667 | 0.730 |
The model using only the top nine LR pairs demonstrated comparable stable performance in the external validation (Fig. 2C). This model achieved accuracies of 0.682 and 0.714 for the Gide and Riaz cohorts, respectively. The consistent performance of the model with a reduced number of features suggests that these key LR pairs would capture essential biological signals that determine anti-PD-1 therapy response. Additional performance metrics further supported the reliability of the model. In the Gide cohort, the top nine models achieved a sensitivity of 0.636 and a specificity of 0.727, whereas in the Riaz cohort, they showed a sensitivity of 0.500 and a specificity of 0.769. The area under the receiver operating characteristic curve (AUROC) was 0.814 and 0.709 for Riaz and Gide, respectively. These performances across independent patient populations verified the robust predictive power of the selected LR pairs.
Top-ranked LR pairs reveal tumor-related signaling pathways
To investigate the biological significance of these predictive features, LR pairs, we carried out Kyoto encyclopedia of genes and genomes (KEGG) pathway analysis for the top nine pairs (Fig. 3A and Supplementary Table S1). The results revealed a significant enrichment of signaling pathways associated with cancer progression.
Fig. 3.
Top-ranked LR pairs reveal tumor-related signatures. A KEGG pathway enrichment analysis of the top 9 LR pairs, highlighting their involvement in tumor-related signaling pathways. B DEG results from the Gide and Riaz cohorts. Red dots indicate upregulated genes, while blue dots represent downregulated genes. The key ligand and receptor genes were not significantly identified, emphasizing the value of LR integration
For example, the mitogen-activated protein kinase (MAPK) signaling pathway, one of the most well-characterized pathways in cancer biology, is involved in cell proliferation, invasion, migration, and metastasis. Notably, abnormal activation of this pathway has been observed in over 40% of patients with cancer. Therefore, MAPK inhibitors effectively suppress cancer growth.
The PI3K/AKT signaling pathway is among the most commonly overactivated intracellular pathways in multiple human cancers. The modulation of diverse downstream effectors promotes tumor initiation, proliferation, invasion, and metastasis.
Enrichment analysis validated the biological and clinical relevance of our model, demonstrating that the most predictive LR pairs were associated with pathways known to influence immune responses and cancer progression. These findings suggest that our model captures biologically meaningful signals that determine anti-PD-1 therapy outcomes.
Furthermore, to compare our approach with conventional methods, we performed DEG analysis in both the Gide and Riaz cohorts. None of the genes in the top nine predictive LR pairs were identified as differentially expressed genes (Fig. 3B). This finding demonstrates that our LR pair approach can identify potential biomarkers for anti-PD-1 therapy responses that cannot be identified by traditional DEG analysis.
LR-Based model outperforms previous prediction model
To validate our model against existing methods, we compared its performance with TIDE (Supplementary Fig. S4), a widely used computational method that predicts the immune checkpoint blockade response by modeling tumor-immune evasion mechanisms, using the validation datasets of the Gide and Riaz cohorts (Fig. 4).
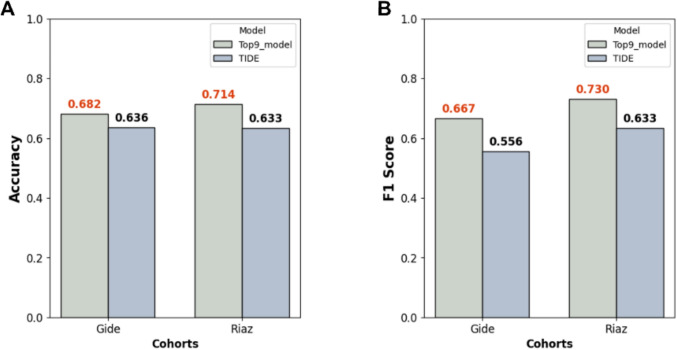
Fig. 4.
LR-based model Outperforms an existing predictive model. Comparison of predictive performance using between Our top 9 model and TIDE, evaluated using A accuracy and B F1 score
TIDE analysis yielded accuracies of 0.636 and 0.633 and F1 scores of 0.556 and 0.633 in the Gide and Riaz cohorts, respectively, whereas Our LR-based model using the top nine features achieved accuracies of 0.682 and 0.667 and F1 scores of 0.714 and 0.730, respectively. The higher predictive accuracy of our model across both independent cohorts suggests that LR pair expression patterns may capture additional biological signals relevant to anti-PD-1 response predictions.
Discussion
Anti-PD-1 therapy has revolutionized melanoma treatment; however, its application is complicated by potential immune-related adverse events and varying response rates among patients. This variability underscores the critical need for reliable response prediction methods before treatment initiation. Our study addresses this challenge by developing a comprehensive predictive model based on LR pair expression patterns, offering new insights into immunotherapy response prediction while simultaneously revealing novel therapeutic opportunities.
LR interactions play fundamental roles in cellular communication within the TME. These interactions govern crucial biological processes including growth, migration, survival, and differentiation, while orchestrating complex immune responses through interconnected signaling networks. By systematically analyzing 2705 LR pairs, our model captured the intricate molecular interactions that collectively determine anti-PD-1 therapy responses, moving beyond the limitations of single-gene biomarkers.
Our study yields several key findings. First, we demonstrated that a focused set of LR pairs could effectively predict therapy response, with Our model achieving robust performance across independent cohorts. When we compared RFC with several conventional machine learning methods given Our high-dimensional setting with 2705 features but limited samples, RFC clearly outperformed other methods. Notably, the model using only the top nine LR pairs performed well, suggesting that the therapeutic response may be governed by a core set of critical molecular interactions rather than widespread transcriptional changes. Typically, random forest feature importance scores can exhibit potential biases [32]. But we additionally performed SHAP analysis, and these results were consistent with our initial feature selection, confirming the robustness of our identified LR pairs. Our approach was further validated through a comparison with the previous representative immunotherapy response prediction tool TIDE, in which our model showed consistently better performance across the two independent cohorts.
Beyond the predictive capabilities, our systematic LR profiling identified previously uncharacterized interaction pairs that may represent promising therapeutic targets. Notably, the PD-1/PD-L1 pair did not rank among the top features while several novel LR pairs were emerged with a predictive power. In fact, previous studies have shown that PD-L1 expression showed limited accuracy in predicting the immunotherapy response, supporting the need for more comprehensive biomarkers [33, 34]. Our finding suggests that the newly identified LR interactions may better reflect the overall immune competence and communication capacity of the tumor microenvironment. Moreover, they can highlight potential opportunities for combination therapies or alternative treatment strategies for patients who fail to respond to current anti-PD-1 therapy. The identification of these novel interactions opens new avenues for drug development, potentially expanding the therapeutic options for melanoma treatment.
The biological relevance of our findings is further supported by pathway enrichment analyses, which revealed significant enrichment of tumor-related pathways, particularly MAPK and PI3K/AKT signaling pathways, among genes comprising our top LR pairs. This mechanistic insight not only validates our computational approach but also provides a rational foundation for understanding why specific LR interaction patterns predict treatment outcomes.
While our findings are promising, this study has several limitations. First, the limited sample sizes could constrain statistical power. Even though our model showed reliable performance using two independent cohort even with the limited sample size, larger prospective cohorts are needed to improve the generalizability. Furthermore, clinical implementation will require validation in larger, prospective cohorts with diverse patient populations to confirm these findings.
Second, our bulk RNA-seq approaches, while clinically practical, cannot capture several biological aspects including post-translational modifications, receptor trafficking dynamics, spatial proximity, and so on. Further analyses incorporating single-cell transcriptomics and spatial transcriptomics are needed to better understand their functional roles in intercellular communication. Moreover, future integration with proteomics would provide more precise quantification of functional interactions.
Third, the previous known biomarkers such as tumor mutational burden (TMB) and PD-L1 expression, and immunohistochemistry (IHC) have demonstrated predictive value for immunotherapy response, but our model uniquely used the LR pairs. But this information also can offer critical insights by directly capturing intercellular communication within the tumor microenvironment, a key factor in anti-tumor immunity. In addition, our pathway enrichment analysis suggests that the LR expression patterns can effectively reflect relevant intracellular signaling states. Future studies should explore integrating various factors, including TMB, IFN-γ signatures, immune cytolytic activity scores, PD-L1 expression, and IHC to develop more comprehensive predictive models.
Fourth, experimental validation remains essential. Co-culture experiments could test whether modulating specific LR interactions affects T cell activation and tumor killing, distinguishing predictive biomarkers from functional mediators and potentially revealing new therapeutic targets.
Finally, in this study, we focused on melanoma due to data availability and high immunotherapy response rates. However, our framework could extend to other malignancies and might provide particular values for cancers with limited biomarker options. For example, recent evidence of Notch signaling and PD-1/PD-L1 pathway interactions in aggressive tumor like pancreatic sarcomatoid carcinoma [35] suggests that comprehensive LR analysis could reveal targetable dependencies beyond classical checkpoints, potentially guiding novel combination strategies for these challenging tumors.
In summary, our results have significant implications for both research and clinical practice. The success of our integrated LR approach suggests that considering molecular interactions rather than individual gene expression alone may provide a more accurate prediction of therapeutic response. In particular, the identification of nine key LR pairs enables development of targeted, cost-effective diagnostic panels feasible for clinical use. Unlike other comprehensive omics profiling methods, a focused qPCR or targeted RNA-seq panel could be standardized across laboratories and implemented at reasonable cost. Moreover, the robust performance of our model with top-ranking LR pairs highlight the possibility of developing targeted and cost-effective diagnostic tools. Furthermore, the identification of key markers will help in patient stratification and potential therapeutic targeting in combination with anti-PD-1 therapy.
In conclusion, our study demonstrates that the integrated analysis of LR pair expression patterns provides a powerful approach for predicting the response to anti-PD-1 therapy in patients with melanoma. These findings represent a significant step forward in personalizing immunotherapy and improving patient outcomes of melanoma treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (grant numbers RS-2021-NR061451 and RS-2022-NR067277) and Ministry of Education (grant number RS-2021-NR060140).
Author contributions
JKR designed and supervised the project; SYS, DYN, and HL carried out the experiments and analyzed the data; SYS and JKR wrote the manuscript.
Funding
National Research Foundation of Korea, RS-2021-NR061451, RS-2022-NR067277 and RS-2021-NR060140.
Data availability
All the source codes and datasets for the current study are available in https://github.com/seoseyeon/anti-PD-1_response_ML.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sahni S, Valecha G, and Sahni A. Role of anti-PD-1 antibodies in advanced melanoma: the era of immunotherapy. Cureus. 2018;10(12). [DOI] [PMC free article] [PubMed]
- 2.Kuzmanovszki D, et al. Anti-PD-1 monotherapy in advanced melanoma—real-world data from a 77-month-long retrospective observational study. Biomedicines. 2022;10(7):1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J, et al. PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy. Front Pharmacol. 2021;12:731798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang AC, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol. 2022;23(5):660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baghban R, et al. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun Signal. 2020;18:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asokan S and Bandapalli OR. CXCL8 signaling in the tumor microenvironment. Tumor Microenvironment: the role of chemokines–Part B, 2021; p. 25–39. [DOI] [PubMed]
- 7.Han Z-J, et al. Roles of the CXCL8-CXCR1/2 axis in the tumor microenvironment and immunotherapy. Molecules. 2021;27(1):137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoniades C, et al. The CD40/CD40 ligand system: linking inflammation with atherothrombosis. J Am Coll Cardiol. 2009;54(8):669–77. [DOI] [PubMed] [Google Scholar]
- 9.Bullock TN. CD40 stimulation as a molecular adjuvant for cancer vaccines and other immunotherapies. Cell Mol Immunol. 2022;19(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alexandroff AB, et al. Role for CD40–CD40 ligand interactions in the immune response to solid tumours. Mol Immunol. 2000;37(9):515–26. [DOI] [PubMed] [Google Scholar]
- 11.Liu D, et al. Integrative molecular and clinical modeling of clinical outcomes to PD1 blockade in patients with metastatic melanoma. Nat Med. 2019;25(12):1916–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shao X, et al. New avenues for systematically inferring cell-cell communication: through single-cell transcriptomics data. Protein Cell. 2020;11(12):866–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vahid MR, et al. Disir: fast and robust method to identify ligand–receptor interactions at subunit level from single-cell RNA-sequencing data. NAR Genom Bioinform. 2023;5(1):lqad030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jing Y, et al. TLR9 exerts an oncogenic role in promoting osteosarcoma progression depending on the regulation of NF-κB signaling pathway. Biol Pharm Bull. 2022;45(12):1733–42. [DOI] [PubMed] [Google Scholar]
- 15.Ravindranath MH, et al. Diversity in the HLA-I recognition of HLA-F monoclonal antibodies: HLA-F or HLA-Ib monospecific, HLA-E or HLA-G bispecific antibodies with or without HLA-Ia reactivity. Antibodies. 2024;13(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ko S-B, et al. Functional role of the Frizzled linker domain in the Wnt signaling pathway. Commun Biol. 2022;5(1):421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong D, et al. FZD5 prevents epithelial-mesenchymal transition in gastric cancer. Cell Commun Signal. 2021;19:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou X, O’Leary HA, Broxmeyer HE. Implications of DPP4 modification of proteins that regulate stem/progenitor and more mature cell types. Blood, Journal Am Soc Hematol. 2013;122(2):161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng M, et al. EFNA3 as a predictor of clinical prognosis and immune checkpoint therapy efficacy in patients with lung adenocarcinoma. Cancer Cell Int. 2021;21:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He M, et al. Upregulated FADD is associated with poor prognosis, immune exhaustion, tumor malignancy, and immunotherapy resistance in patients with lung adenocarcinoma. Front Oncol. 2023;13:1228889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park BV, et al. TGFβ1-mediated SMAD3 enhances PD-1 expression on antigen-specific T cells in cancer. Cancer Discov. 2016;6(12):1366–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grasso CS, et al. Conserved interferon-γ signaling drives clinical response to immune checkpoint blockade therapy in melanoma. Cancer cell. 2020;38(4):500-515e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.House IG, et al. Macrophage-derived CXCL9 and CXCL10 are required for antitumor immune responses following immune checkpoint blockade. Clin Cancer Res. 2020;26(2):487–504. [DOI] [PubMed] [Google Scholar]
- 24.Juric V, et al. MMP-9 inhibition promotes anti-tumor immunity through disruption of biochemical and physical barriers to T-cell trafficking to tumors. PLoS One. 2018;13(11):e0207255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St-Laurent M-P, Black PC. Re: FGFR inhibition augments anti-PD-1 efficacy in murine FGFR3-mutant bladder cancer by abrogating immunosuppression. Eur Urol. 2024. 10.1016/j.eururo.2024.03.031. [DOI] [PubMed] [Google Scholar]
- 26.Torrejon DY, et al. Antitumor immune responses in B2M-deficient cancers. Cancer Immunol Res. 2023;11(12):1642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ru B, et al. Estimation of cell lineages in tumors from spatial transcriptomics data. Nat Commun. 2023;14(1):568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandt MM, et al. Endothelial loss of Fzd5 stimulates PKC/Ets1-mediated transcription of Angpt2 and Flt1. Angiogenesis. 2018;21(4):805–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman AC, Hughes CC. Macrophages and angiogenesis: a role for Wnt signaling. Vasc Cell. 2012;4(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haseeb M, et al. Wnt signaling in the regulation of immune cell and cancer therapeutics. Cells. 2019;8(11):1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mortezaee K. WNT/β-catenin regulatory roles on PD-(L) 1 and immunotherapy responses. Clin Exp Med. 2024;24(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strobl C, et al. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics. 2007;8(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis AA, Patel VG. The role of PD-L1 expression as a predictive biomarker: an analysis of all US food and drug administration (FDA) approvals of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grossman JE, et al. Is PD-L1 a consistent biomarker for anti-PD-1 therapy? The model of balstilimab in a virally-driven tumor. Oncogene. 2021;40(8):1393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silvestris N, et al. PD-L1 and Notch as novel biomarkers in pancreatic sarcomatoid carcinoma: a pilot study. Expert Opin Ther Targets. 2021;25(11):1007–16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the source codes and datasets for the current study are available in https://github.com/seoseyeon/anti-PD-1_response_ML.