Abstract
In eukaryotes, the enzyme GDP-mannose pyrophosphorylase (GDPMP) is essential for the formation of GDP-mannose, the central activated mannose donor in glycosylation reactions. Deletion of its gene is lethal in fungi, most likely as a consequence of disrupted glycoconjugate biosynthesis. Furthermore, absence of GDPMP enzyme activity and the expected loss of all mannose-containing glycoconjugates have so far not been observed in any eukaryotic organism. In this study we have cloned and characterized the gene encoding GDPMP from the eukaryotic protozoan parasite Leishmania mexicana. We report the generation of GDPMP gene deletion mutants of this human pathogen that are devoid of detectable GDPMP activity and completely lack mannose-containing glycoproteins and glycolipids, such as lipophosphoglycan, proteophosphoglycans, glycosylphosphatidylinositol protein membrane anchors, glycoinositolphos pholipids and N-glycans. The loss of GDPMP renders the parasites unable to infect macrophages or mice, while gene addback restores virulence. Our study demonstrates that GDP-mannose biosynthesis is not essential for Leishmania viability in culture, but constitutes a virulence pathway in these human pathogens.
Keywords: GDP-mannose pyrophosphorylase/Leishmania/mannose/metabolism/virulence
Introduction
In eukaryotes, mannose (Man) is a key monosaccharide for the glycosylation of proteins and lipids. Man-containing glycoconjugates, such as protein N- and C-glycans, some O-glycans, glycosylphosphatidylinositol (GPI) protein membrane anchors, as well as some glycolipids, are considered to have a variety of important functions. These include the promotion of correct folding, solubility, stability and intracellular sorting of proteins. Further more, Man-containing glycans have been shown to be essential for the enzymatic, hormonal or receptor activity of many proteins, the formation of cell surface glycocalyces, extracellular matrices and protective cell walls, and they are also known to play major roles in cell–cell interactions. It is generally assumed that the biosynthesis of Man-containing glycoconjugates is of vital importance to eukaryotic organisms (summarized in Varki, 1999). In the absence of exogenous Man, eukaryotic cells synthesize this hexose by phosphomannose isomerase (PMI)-catalysed conversion of fructose (Frc)-6-PO4 to Man-6-PO4 (Smith et al., 1992). The consecutive action of phosphomannomutase (PMM) and GDP-mannose pyrophosphorylase (GDPMP) transforms Man-6-PO4 to GDP-Man (reviewed in Freeze and Aebi, 1999; see also Figure 1A), which is the critical metabolite of the Man activation pathway for glycoconjugate synthesis in eukaryotes. GDP-Man is utilized directly or indirectly as a Man donor for all mannosylation reactions, it is a precursor for GDP-fucose synthesis (Kaufman and Ginsburg, 1968) and acts as a substrate for ascorbic acid synthesis in plants (Wheeler et al., 1998). The crucial role of GDP-Man is supported by the finding that, in Saccharomyces cerevisiae and Candida albicans, deletion of the structural gene for GDPMP is lethal (Hashimoto et al., 1997; Warit et al., 2000), as are deletions of other genes of the Man pathway (Kepes and Schekman, 1988; Orlean et al., 1988). The absence of GDPMP activity has not yet been reported for any uni- or multicellular eukaryote, and the available data suggest that complete disruption of the Man pathway and the resulting absence of Man-containing glycoconjugates are incompatible with eukaryotic life.
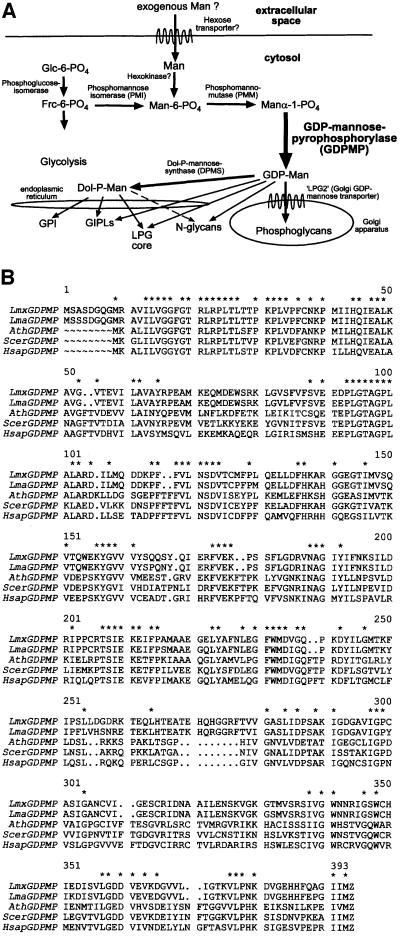
Fig. 1. (A) Putative Man activation pathways and glycoconjugate biosynthesis in L.mexicana. The indication of GDP-Man and Dol-P-Man as Man donors for the biosynthesis of different Leishmania glycoconjugates is based on earlier studies (Ilgoutz et al., 1999). (B) Alignment of L.mexicana GDPMP (LmxGDPMP) with GDPMP amino acid sequences from various organisms: L.major (LmaGDPMP); A.thaliana (AthGDPMP); S.cerevisiae (ScerGDPMP; Hashimoto et al., 1997); H.sapiens (HsapGDPMP). Amino acids conserved in GDPMP of all five species are indicated by asterisks.
Eukaryotic parasitic protozoa of the genus Leishmania are the causative agents of several human diseases ranging from self-healing skin ulcers to fatal visceral infections. These parasites exhibit a dimorphic life cycle comprising extracellular promastigotes that colonize the midgut of the sandfly vector and intracellular amastigotes that reside within the phagolysosomal compartment of mammalian macrophages (Alexander and Russell, 1992). Leishmania produce large amounts of unusual Man-rich cell surface-associated and secreted glycoconjugates, which include N-glycans, GPI anchors, O-phosphoglycosylated proteophosphoglycans (PPGs), lipophosphoglycan (LPG) and glycoinositolphospholipids (GIPLs), and whose biosynthesis depends directly or indirectly on the availability of GDP-Man (Figure 1A). A large number of studies suggest that glycoconjugate synthesis is required by Leishmania to resist the hostile conditions of their habitats as well as to maintain virulence (reviewed in Descoteaux and Turco, 1999; Ferguson, 1999; Ilg, 2000a), and it is expected that, like in yeast and humans, a functional Man pathway (and in particular, GDPMP), is of essential importance to these parasites (Ilgoutz et al., 1999).
In this study, we report for the first time GDPMP gene deletion mutants in a eukaryotic organism. Leishmania mexicana parasites lacking the GDPMP gene (ΔGDPMP) due to gene replacement are still viable in culture, but are completely unable to synthesize Man-containing glycoconjugates. However, Leishmania ΔGDPMP mutants have lost the capacity to infect macrophages and mice, which establishes GDPMP as a virulence factor.
Results
Isolation of the L.mexicana LmxGDPMP gene
For the cloning of the Leishmania GDPMP gene, a degenerate PCR primer pair was constructed from the partially conserved peptide sequences PMILHQIE and WMDVGQPKDY/F (Figure 1B). PCR was performed using L.mexicana genomic DNA as a template. The resulting PCR product was sequenced and an open reading frame (ORF) was identified with high homology to known GDPMPs (data not shown). The digoxigenin (DIG)-labeled PCR fragment was used to screen a λ-DashII library of genomic L.mexicana DNA. Sequencing of an LmxGDPMP gene-containing DNA fragment revealed an ORF of 1266 bp (Figure 2A) encoding a protein of ∼46.4 kDa (Figures 1B and 3A), which showed between 50 and 54% identity to GDPMPs from other eukaryotes like S.cerevisiae, Arabidopsis thaliana and Homo sapiens, and 92.4% identity to a gene annotated as being a potential GDPMP, which has been identified in the Leishmania major genome project (Figure 1B). Southern blot analysis of L.mexicana genomic DNA digested with different restriction enzymes and hybridized with an ORF probe suggests that LmxGDPMP is a single-copy gene (Figure 2C and data not shown). Northern blotting and RT–PCR analysis suggest that LmxGDPMP mRNA is present in both parasite life stages, but is more abundant in the forms occurring in the mammalian host, the amastigotes. However, immunoblots of total parasite cell lysates probed with affinity-purified antibodies raised against recombinant L.mexicana GDPMP indicate equal abundance of this enzyme in both life stages (Figure 3D). Leishmania mexicana GDPMP activity is largely (>90%) soluble after disruption of promastigotes followed by ultracentrifugation (data not shown), which is in agreement with immunoblotting studies on membrane fractions (Figure 3E). Immunofluorescence experiments on permeabilized promastigotes using anti-LmxGDPMP serum yielded a diffuse signal throughout the cell body, which suggests a cytoplasmic localization of the enzyme (Figure 5C).
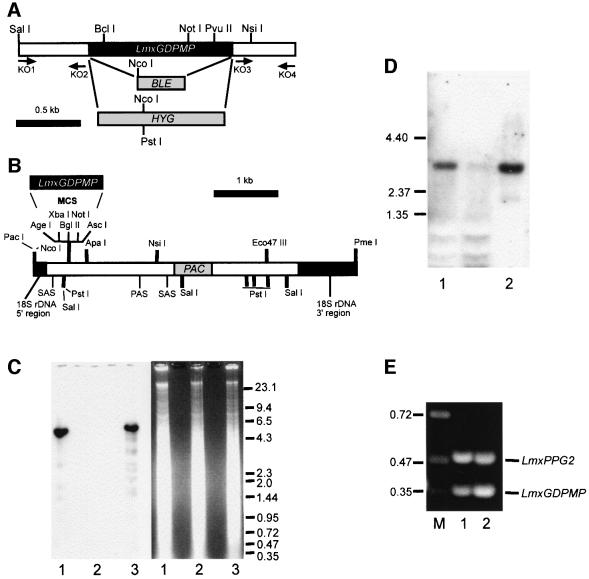
Fig. 2. Targeted gene replacement and gene addback of the LmxGDPMP alleles and analysis of mRNA expression. (A) Restriction maps of the LmxGDPMP locus. The resistance genes BLE and HYG and the primer binding sites (KO1–4) used for the construction of gene deletion cassettes are indicated. (B) Restriction map of the gene addback cassette for genetic rescue of the L.mexicana ΔGDPMP mutant. (C) Southern blot analysis of NcoI–PstI-digested chromosomal DNA (10 µg) from L.mexicana wild type (lane 1), a ΔGDPMP mutant (lane 2) and a ΔGDPMP + cRIBLmxGDPMP gene addback mutant (lane 3). DNA was separated on an ethidium bromide-containing 0.7% agarose gel (right panel), blotted onto a nylon membrane and incubated with a DIG-labeled LmxGDPMP ORF probe (left panel). The sizes of DNA standards are indicated in kilobases. (D) Northern blot analysis of total RNA (10 µg) isolated from L.mexicana log-phase promastigotes (lane 1) and amastigotes (lane 2). RNA was separated on a 0.7% formaldehyde-containing agarose gel, blotted onto a nylon membrane and incubated with a DIG-labeled LmxGDPMP ORF probe. The sizes of RNA standards are indicated in kilobases. (E) Amplification of LmxGDPMP cDNA from L.mexicana log-phase promastigote (lane 1) and amastigote (lane 2) by RT–PCR from total RNA. The loading was normalized to the co-amplified cDNA fragment derived from the LmxPPG2 gene whose mRNA is roughly equally abundant in L.mexicana promastigotes and amastigotes. The sizes of DNA standards are indicated in kilobases.
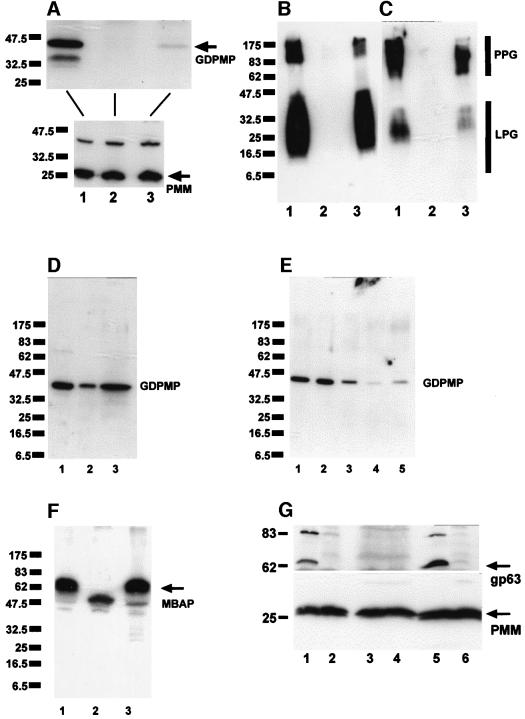
Fig. 3. SDS–PAGE/immunoblotting of L.mexicana total cell lysates. (A–C) Lane 1, WT; lane 2, ΔGDPMP; lane 3 ΔGDPMP + cRIBLmxGDPMP. Each lane was loaded with 1 × 107 promastigotes (∼40 µg of protein). (A) Blot probed with affinity-purified rabbit anti-L.mexicana GDPMP antibodies (upper panel) and an identically loaded blot was probed with affinity-purified rabbit anti-L.mexicana PMM antibodies (lower panel) as a loading control. The same or identically loaded blots were then stripped and probed with mAb LT6 (anti-6-Galβ1-4Manα1-PO4-) (B) and mAb LT17 [anti 6-(Glcβ1-3)Galβ1-4Manα1-PO4-] (C). (D) SDS–PAGE/immunoblotting of total cell lysates of L.mexicana promastigotes (lane 1, 2.5 × 106 parasites, corresponding to ∼10 µg of protein) and lesion-derived amastigotes (lane 2, 2.5 × 106 parasites, corresponding to ∼3.5 µg of protein; and lane 3, 7 × 106 parasites, corresponding to ∼10 µg of protein). The blots were probed with affinity-purified rabbit anti-L.mexicana GDPMP antibodies. (E) SDS–PAGE/immunoblotting of total cell lysates of L.mexicana promastigotes fractionated by ultracentrifugation. Lane 1, total cell lysate of 2.5 × 106 parasites, corresponding to ∼10 µg of protein; lane 2, first ultracentrifugation supernatant; lane 3, first ultracentrifugation pellet; lane 4, second ultracentrifugation supernatant; lane 5, second ultracentrifugation pellet. Equivalent sample volumes were loaded and the blots were probed with affinity-purified rabbit anti-L.mexicana GDPMP antibodies. (F) SDS–PAGE/immunoblotting of promastigote lysates (2 × 107 parasites, corresponding to ∼80 µg of protein) from WT (lane 1), ΔGDPMP (lane 2) and ΔGDPMP + cRIBLmxGDPMP (lane 3) probed with affinity-purified rabbit anti-L.mexicana MBAP antibodies. (G) SDS–PAGE/immunoblotting of promastigote lysates with (lanes 1, 3 and 5) or without (lanes 2, 4 and 6) prior GPI-PLC treatment. Lanes 1 and 2, WT; lanes 3 and 4, ΔGDPMP; lanes 5 and 6, ΔGDPMP + cRIBLmxGDPMP. The blot was probed with rabbit anti-CRD antibodies (upper panel). Equal loading was confirmed by stripping and reprobing with rabbit anti-PMM antibodies (lower panel).
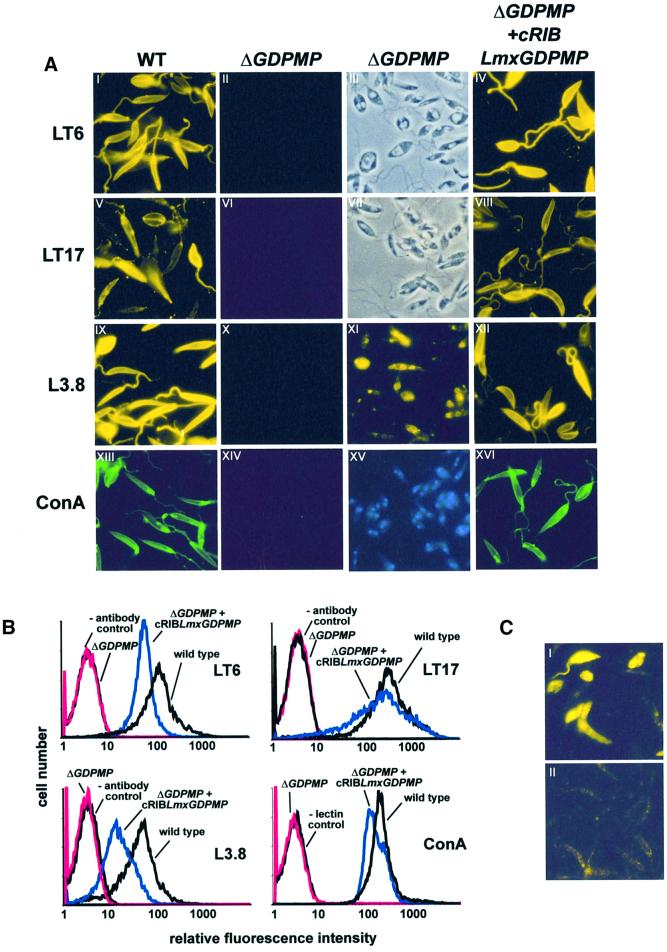
Fig. 5. Immuno-/lectin fluorescence microscopy and FACS analysis of Leishmania WT and mutant promastigotes. (A) Immuno-/lectin fluorescence microscopy of fixed L.mexicana promastigotes. The cells were not permeabilized, except for XI. The parasite lines and the mAbs/lectins used are indicated by the labeling of columns and rows. Exposure times within rows (A) are identical, except for XI, which is ∼20× overexposed compared with IX. III and VII in (A) are phase-contrast light microscopy images of II and VI, respectively. (B) FACS analysis of live L.mexicana promastigotes. The parasite lines and the mAbs/lectins used are indicated in each panel. (C) IFM of saponin-permeabilized L.mexicana WT promastigotes labeled with rabbit anti-GDPMP serum (1:200) (I) and pre-immune serum (1:200) (II). Exposure times were identical in I and II.
Targeted gene replacement of LmxGDPMP
Deletion of the single-copy GDPMP gene in S.cerevisiae or C.albicans is lethal, and it is generally assumed that this is also the case in other eukaryotes (Hashimoto et al., 1997; Warit et al., 2000). However, when two rounds of targeted LmxGDPMP gene replacement using the antibiotic resistance markers HYG and BLE were performed on L.mexicana (Figure 2A), remarkably, several clones were obtained that lacked both alleles of the LmxGDPMP ORF (Figure 2C, lane 2), but were viable in standard culture medium and showed only a mild growth defect compared with wild-type parasites (Figure 4H). The absence of the gene products in L.mexicana ΔGDPMP:: HYG ΔGDPMP::BLE (ΔGDPMP) was confirmed by immunoblots of total cell lysates (Figure 3A, lane 2). GDPMP activity was detected in the soluble fraction of L.mexicana wild-type promastigote total-cell lysate, but the complexity of the GDPMP assay system results in strong GDP-Man-independent background reactions, probably due to metabolites and/or enzymes present in crude extracts. Therefore, for comparison of GDPMP activity in wild-type and ΔGDPMP promastigotes, concentrated ultracentrifugation supernatants were fractionated by Superose 6 gel filtration to remove interfering compounds from GDPMP fractions. Equivalent loading of the Superose 6 column was confirmed by measuring the enzyme activity of UDP-glucose pyrophosphorylase (UDPGP) in both chromatography runs (Figure 4A). While in the wild-type fractions a prominent GDPMP activity peak eluting between 240 and 300 kDa was observed, no GDPMP activity was detected in the ΔGDPMP mutant (Figure 4A).
Fig. 4. (A) Superose 6 gel filtration of soluble fractions from L.mexicana wild type (WT) and ΔGDPMP promastigote lysates. The enzymatic activities of GDPMP and UDPGP were determined in each fraction. The elution positions of size standards are indicated by arrows. (B) High-pH anion-exchange HPLC of 2 M TFA hydrolysates of L.mexicana WT and ΔGDPMP promastigote membranes. The hexose fraction of equal amounts of cells (∼1 × 108) was loaded onto a Carbo-Pac PA10 column. (C) SDS–PAGE/fluorography of delipidated total promastigote lysates from [3H]myo-inositol-labeled L.mexicana WT (lane 1), L.mexicana ΔGDPMP (lane 2) and L.mexicana ΔGDPMP + cRIBLmxGDPMP (lane 3). Each lane was loaded with 2.5 × 107 delipidated promastigotes labeled overnight with [3H]myo-inositol. The positions of LPG and the major GPI-anchored surface metalloproteinase gp63 are indicated by a bar and an arrow, respectively. (D–G) SDS–PAGE/immunoblot of L.mexicana culture supernatants. Loading was normalized to SAP activity (2 mU). (D and E) Lane 1, WT; lane 2, ΔGDPMP. (F) Lane 1, WT; lane 2, ΔGDPMP; lane 3, ΔGDPM + cRIBLmxGDPMP. (G) Lane 1, WT; lane 2, ΔPMI; lane 3, ΔPMI + 20 µM Man; lane 4, ΔPMI + 200 µM Man; lane 5, ΔGDPMP; lane 6, ΔGDPMP + 200 µM Man; lane 7, ΔGDPMP + 2 mM Man; lane 8, WT. (H) Growth curves of L.mexicana WT and of L.mexicana ΔGDPMP in the presence of various Man concentrations. (D), (F) and (G) were probed with affinity-purified rabbit anti-L.mexicana GDPMP antibodies, while (E) was probed with mAb L7.25. The molecular masses and relative positions of standard proteins are indicated on the gels and blots in (C–G).
Absence of Man-containing glycoproteins and glycolipids in L.mexicana ΔGDPMP
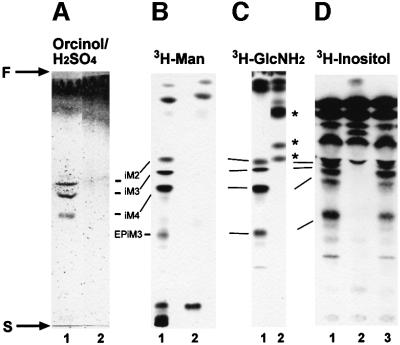
Earlier studies have suggested that in L.mexicana, as in other eukaryotes, GDP-Man is directly or indirectly the sole Man donor for glycoconjugate synthesis (Mengeling et al., 1997; Ilgoutz et al., 1999; Moss et al., 1999 and references therein; see Figure 1A). In agreement with the expected inability of the GDPMP-deficient mutant to synthesize GDP-Man, expression of all known lipid- and protein-bound Man-containing glycoconjugates was found to be defective in L.mexicana ΔGDPMP: (i) LPG and phosphoglycans on PPGs were absent, as judged by the lack of specific bands on immunoblots of total cell lysates probed with the anti-phosphoglycan repeat monoclonal antibodies (mAbs) LT6 and LT17, even after extensive overexposure (Figure 3B and C); the complete absence of surface and flagellar pocket signals in immunofluorescence experiments (Figure 5A, compare I and II, V and VI) and of fluorescence-activated cell sorter (FACS) signals in labelings of live cells with LT6 and LT17 (Figure 5B); the lack of detectable LPG in metabolic [3H]inositol labelings; the absence of LPG in attempted purifications by a standard protocol (data not shown and McConville et al., 1990); and the complete lack of binding sites for anti-phosphoglycan cap mAb L7.25 (Figure 4D and E) and other anti-phosphoglycan mAbs (not shown) on the normally highly phosphoglycosylated secreted acid phosphatase (SAP). (ii) ΔGDPMP promastigotes did not express the normally dominant GPI-anchored surface protein gp63 on their surface (Figure 5A, IX and X), although it could be detected within permeabilized cells, particularly in the perinuclear region, which suggested its retention in the endoplasmic reticulum (Figure 5A, XI). Furthermore, the cross-reactive determinant (CRD), which is indicative of GPI-anchored proteins in many eukaryotes, including L.mexicana (Zamze et al., 1988; Ilg et al., 1993), was not present in ΔGDPMP promastigote total cell lysates after GPI-phospholipase C (GPI-PLC) digestion (Figure 3G, lanes 3 and 4), and no [3H]inositol-labeled gp63 was detected after metabolic labeling (Figure 4C), suggesting that protein GPI anchor synthesis is severely downregulated in the mutant parasites. (iii) Evidence for a defect in N-glycosylation was obtained by immunoblots of L.mexicana wild type and ΔGDPMP total cell lysates, where a mobility shift of 15–20 kDa for the normally heavily N-glycosylated, but neither GPI-anchored nor phosphoglycosylated, membrane-bound acid phosphatase (MBAP) (Menz et al., 1991; Wiese et al., 1996) was observed in the mutant (Figure 3F, lanes 1 and 2), which is indicative of the loss of N-glycans in this molecule. (iv) The Man-containing GIPLs are undetectable in L.mexicana ΔGDPMP in high-performance thin-layer chromatography (HPTLC)-separated total lipids either by orcinol staining or by fluorography after metabolic labeling with [3H]Man, [3H]GlcNH2 and [3H]myo-inositol (Figure 6). (v) ΔGDPMP promastigotes also did not show any signal, in fluorescence microscopy or FACS analysis, with concanavalin A, a lectin that strongly binds to α-Man residues present in L.mexicana N-glycans, LPG, PPGs and GIPLs (Ilgoutz et al., 1999; Ilg, 2000a,b; Ilg et al., 2001) (Figure 5A, XIII and XIV, and B). In contrast to the situation in L.mexicana ΔPMI promastigotes that lack PMI activity (Garami and Ilg, 2001), the growth delay and the profound lipid and protein glycosylation defects in the ΔGDPMP mutant could not be rescued by addition of Man to the culture medium (Figure 4F and G, and data not shown). Finally, hexose analysis of trifluoroacetic acid (TFA)-hydrolyzed promastigote membranes by high-pH anion-exchange high-pressure liquid chromatography (HPLC) showed that Man is absent (<1%) from macromolecules of the L.mexicana ΔGDPMP mutant compared with wild-type parasites (Figure 4B), a result confirmed by hexose analysis using gas chromatography–mass spectrometry (<0.5% of wild-type levels; T.Ilg, A.Mehlert and M.A.J.Ferguson, unpublished results). The profound glycosylation defects in L.mexicana ΔGDPMP promastigotes were caused by the absence of the GDPMP gene. LmxGDPMP addback, either by gene integration into an rRNA gene locus (Figure 2B and C) or by introduction of episomal gene copies (pX, not shown), reconstituted the synthesis of all glycoconjugates investigated in this study (Figure 3A–C and F, lanes 3; Figure 3G, lanes 5 and 6; Figure 5A, IV, VIII, XII, XVI and B), although the level of enzyme re-expression was only ∼10–20% of the wild-type levels (Figure 3A and data not shown). The reason for this lower level of GDPMP re-expression is not known. Possibly upstream or downstream elements of Lmx GDPMP not present in the DNA constructs are required for optimal enzyme production.
Fig. 6. Silica gel 60 HPTLC analysis of the predominant promastigote glycolipids of L.mexicana in WT (lane 1), ΔGDPMP (lane 2) and ΔGDPMP + cRIBLmxGDPMP (lane 3) parasites. (A) Total lipids from 2 × 108 promastigotes visualized by orcinol/H2SO4 spraying. (B) Fluorography of total lipids from 2.5 × 107 [3H]Man-labeled promastigotes (∼100 000 c.p.m.). (C) Fluorography of total lipids from 5 × 106 [3H]GlcNH2-labeled promastigotes (∼100 000 c.p.m.). (D) Fluorography of total lipids from 5 × 106 [3H]myo-inositol-labeled promastigotes (∼100 000 c.p.m.). The positions of the abundant L.mexicana GIPLs (McConville et al., 1993) are indicated by the bars, and the start and front of the TLCs are marked by S and F, respectively. Asterisks mark new [3H]GlcNH2-labeled compounds accumulating in the ΔGDPMP mutant.
Loss of virulence in L.mexicana ΔGDPMP mutants
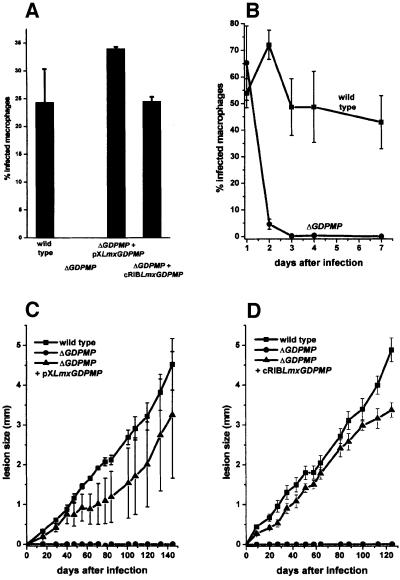
Leishmania mexicana ΔGDPMP promastigotes were totally unable to establish an infection in cultured macrophages (Figure 7A). This inability was not due to a lack of attachment to and invasion of host cells, where the ΔGDPMP mutant proved to be as efficient as L.mexicana wild type (Figure 7B, 1 day after infection), but, as demonstrated by time course experiments of macrophage infections, the ΔGDPMP parasites were rapidly killed by the host cells, whereas wild-type parasites survived (Figure 7B) and proliferated (data not shown). Furthermore, L.mexicana ΔGDPMP promastigotes proved to be completely avirulent to Balb/c mice, even at a high parasite dose (1 × 107/mouse) (Figure 7C and D). Furthermore, repeated attempts to re-isolate ΔGDPMP parasites from the site of injection, the draining lymph nodes or the spleen of challenged animals were unsuccessful, which suggests the absence of persistent parasites. Virulence of ΔGDPMP mutants to macrophages and mice could be completely restored by the LmxGDPMP gene expressed from an episome or integrated into the rRNA locus (Figure 7A, C and D).
Fig. 7. Analysis of macrophage and mouse infections by L.mexicana WT, ΔGDPMP and LmxGDPMP gene addback mutants. (A and B) Infection of peritoneal macrophages by L.mexicana WT, ΔGDPMP, ΔGDPMP + pXLmxGDPMP and ΔGDPMP + cRIBLmxGDPMP. Peritoneal macrophages were infected at a ratio of two stationary phase promastigotes per cell. The percentage of infected macrophages (sample size 300) was counted 6 days (A) or at day 1, 2, 3, 4 and 7 (B) after the infection. The standard errors of duplicate experiments are indicated. In in vivo infection experiments, Balb/c mice were challenged with 1 × 107 L.mexicana promastigotes in the right hind footpad. The swelling caused by L.mexicana WT, ΔGDPMP and ΔGDPMP + pXLmxGDPMP (C), and ΔGDPMP + cRIBLmxGDPMP (D) was recorded. The infection experiments were performed in quadruplicates and the standard errors are indicated.
Discussion
Mutations that lead to a complete block in the Man activation pathway are invariably lethal in fungi, because N-glycosylation, O-mannosylation and GPI protein membrane anchor synthesis are essential processes in these organisms (Kapteyn et al., 1999; Knauer and Lehle, 1999; Strahl-Bolsinger et al., 1999). In mammalian cell lines, only dolicholphosphate mannose synthase (DPMS) mutants are known, which lack GPI protein membrane anchors and the outer Man residues in N-glycans, but still synthesize and transfer Man5GlcNAc2 to proteins (Chapman et al., 1980; Stoll et al., 1982; Sugiyama et al., 1991). Complete lack of any of the other Man pathway enzymes has never been observed in mammals and is expected to be lethal, mainly because a basal level of N-glycosylation appears to be essential in multicellular organisms (Stanley and Ioffe, 1995; Knauer and Lehle, 1999). This work is the first demonstration that a eukaryotic organism exists in which the complete disruption of the Man activation pathway and the concomitant loss of all known Man-containing glycoconjugates are compatible with viability in culture. Leishmania mexicana ΔGDPMP down-regulates expression of LPG, PPGs, Man-containing GIPLs, GPI-anchored proteins and Man-containing N-glycans to levels undetectable by a variety of methods, including immunoblotting, immunofluorescence microscopy (IFM), FACS analysis, metabolic labelings with [3H]Man, [3H]GlcNH2 and [3H]myo-inositol, and hexose analysis by two different methods. GDPMP activity is not detectable, and there is no evidence at present for the existence of an alternative pathway for GDP-Man synthesis. If such a hypothetical pathway exists in L.mexicana, it is extremely inefficient.
In agreement with the results of this study, we have succeeded in generating L.mexicana gene deletion mutants for all other known enzymes and transporters of the L.mexicana Man biosynthesis and activation pathway, which include the PMI (Garami and Ilg, 2001), PMM, DPMS (A.Garami, A.Mehlert and T.Ilg, submitted) and the GDP-mannose transporter LPG2 (Descoteaux et al., 1995; Ilg et al., 2001) (Figure 1A). These results suggest that, again in contrast to other eukaryotes (Kapteyn et al., 1999; Knauer and Lehle, 1999; Strahl-Bolsinger et al., 1999), in L.mexicana none of the mannosyltransferases involved in glycoconjugate synthesis is expected to be essential for viability in culture. Therefore, L.mexicana could be used as a unique model system for investigation of the biosynthesis and function of N-glycans, GPI protein anchors, phosphoglycans and GIPLs by a reverse genetics approach.
Deletion of the gene encoding UDP-GlcNAc:Dol- P-GlcNAc-1-P transferase has been reported to be lethal in several fungi, mammals and L.mexicana (Chen et al., 1999; Knauer and Lehle, 1999), indicating that a complete lack of N-glycosylation is not tolerated by eukaryotes. Therefore, the question arises whether ΔGDPMP parasites possibly transfer (GlcNAc)1–2 from a truncated N-glycan precursor [e.g. Dol-PP-(GlcNAc)1–2] to their proteins. The latter process has been observed in in vitro N-glycosyl ation assays, but has so far not been detected in living organisms due to the lethality of the mutations required for this phenotype (Knauer and Lehle, 1999).
While the simultaneous absence of LPG, PPGs, GPI-anchored proteins as well as Man-containing GIPLs and N-glycans does not grossly affect viability in culture, it appears likely that the capacity of L.mexicana ΔGDPMP to infect sandflies will be strongly impaired, as parasites with less severe glycosylation defects were unable to colonize these vector insects (Sacks et al., 2000). Based on the observation that deletion of the lmxDPMS gene is apparently lethal, it has been suggested recently that Man-containing GIPLs are essential for L.mexicana viability in culture (Ilgoutz et al., 1999). The results of the current study and our recent success in generating ΔDPMS mutants by double targeted gene replacement (A.Garami, A.Mehlert and T.Ilg, submitted) argue against this hypothesis. However, it will be important to determine whether Man-free GIPL biosynthetic intermediates like GlcNH2- phosphatidylinositol accumulate in ΔGDPMP mutants, as suggested by our radioactive metabolic labeling experiments with [3H]GlcNH2 (Figure 6C), and whether these truncated GIPLs are displayed on the parasite cell surface.
Our observation that a lack of GDPMP (and the consequent absence of Man-containing glycoconjugates) leads to complete loss of L.mexicana virulence to macrophages and mice may be medically relevant. To our knowledge, a compound is considered a virulence factor if it is not essential for viability of a pathogen but is indispensable for its infectivity to the respective host organism. By this definition, GDPMP is a virulence factor. Our results define the Man metabolism of Leishmania as a virulence pathway, and suggest that the enzyme GDPMP may be a potential target for the design of new anti-Leishmania drugs.
Materials and methods
Parasite culture and experimental infections of mice and peritoneal macrophages
Promastigotes of the L.mexicana wild-type (WT) strain MNYC/BZ/62/M379 and derived mutants were grown at 27°C in semi-defined medium 79 (SDM) supplemented with 4% heat-inactivated fetal calf serum (iFCS) as described previously (Ilg et al., 1993). Infection of mice with 1 × 107 stationary phase promastigotes and infection of mouse peritoneal macrophages were performed as outlined earlier (Ilg, 2000b). Growth curves of L.mexicana WT and mutants were obtained by seeding SDM/4% iFCS with 2 × 106 promastigotes and counting the parasite numbers at 8–24 h intervals. In some experiments, the medium was supplemented with various concentrations of Man (20 µM–2 mM).
Cloning of the L.mexicana LmxGDPMP gene, generation of gene knockout and gene addback mutants, heterologous expression of GDPMP and generation of antibodies
DNA techniques were performed as described previously (Ilg et al., 1999). A 568 bp fragment of the L.mexicana GDPMP gene (Lmx GDPMP) was obtained from L.mexicana genomic DNA by PCR using the degenerate primers CC(A/G/C/T)ATGAT(A/C/T)(CT)T(A/G/C/T)CA(C/T)CA(A/G)(AG)T(A/G/C/T)GA and A(AG)TC(CT)TT(A/G/C/T)GG(A/G/C/T)CC(A/G/C/T)AC(AG)TCCATCCA, which were derived from the conserved S.cerevisiae (DDBJ/EMBL/GenBank accession No. U24437) and C.albicans (DDBJ/EMBL/GenBank AF030299) GDPMP peptide sequences PMILHQIE and WMDVGQPKDY/F. The PCR product was subcloned into pGEM-T (Promega) and sequenced. The DIG-labeled PCR product was used to screen a λ-DashII library (Wiese et al., 1996) derived from genomic L.mexicana DNA. Positive clones were subcloned into pBSK+ (Stratagene) or pGEM-5z (Promega) and sequenced on both strands by the dideoxy chain termination method using an ALFexpress automated sequencer (Amersham-Pharmacia) as described earlier (Ilg et al., 1999). The ORF corresponding to LmxGDPMP was identified by homology to known GDPMP genes in the database and by determination of the spliced leader site (Ilg et al., 1999). The sequence data for the LmxGDPMP-containing genomic DNA fragment have been submitted to the EMBL database under accession No. AJ292039. Double targeted gene replacement was performed by PCR amplification of the 5′-untranslated region (5′-UTR) of LmxGDPMP using the primers KO1 (AATGCGGCCGCGTTAACGACTCAACCAAAATG) and KO2 (AGTACTAGTCTTGTCGGTTGAGATAGAG), and by amplification of the 3′-UTR of LmxGDPMP using the primers KO3 (AGTACTAGTAGATCTTGCTGCATCGACGGGG) and KO4 (CTTAAGCTTGTTAACAGAAGGGAGATGGG). The NotI–SpeI-cut LmxGDPMP 5′-UTR PCR DNA fragment, the BglII–HindIII-cut LmxGDPMP 3′-UTR PCR DNA fragment and a SpeI–BamHI DNA fragment containing a hygromycin phosphotransferase gene (HYG) (Cruz et al., 1991) were ligated consecutively into pBSK+. For the second LmxGDPMP gene replacement cassette, a SpeI–BamHI fragment encoding bleomycin binding protein gene (BLE) was used (Ilg, 2000b). The HYG- and BLE-containing LmxGDPMP gene replacement cassettes were excised from the plasmids by HpaI digestion, and transfected into L.mexicana promastigotes as previously described (Ilg et al., 1999). Selection on 96-well microtiter plates and analysis of positive clones were performed as outlined earlier (Ilg, 2000b). LmxGDPMP 5′-UTR DNA and ORF probes were generated either by PCR using a PCR-DIG labeling kit (Roche) or by random priming (DIG-high prime; Roche). For gene addback and heterologous expression studies, the ORF of LmxGDPMP was amplified from an LmxGDPMP gene-containing plasmid using the primers GDPMPORF1 (ACCGGTACCGCGGATGTCTGCATCCGATGGCCAGG) and GDPMPORF2 (CCGCGGTACCCGGGTTACATGATGATCCCAGCCTGC), and cloned into pGEM-T (Promega). The accuracy of the cloned PCR amplicon was checked by sequencing. Episomal gene addback was achieved by cloning the SacII–XmaI-cut LmxGDPMP ORF into pX (LeBowitz et al., 1990) and transfection of L.mexicana ΔGDPMP promastigotes with this construct as described earlier (Ilg et al., 1999). Transfectants were selected by growth in SDM/4% iFCS containing 10–50 µg/ml G418 (Roche). Alternatively, the LmxGDPMP gene was expressed under the control of the rRNA promoter by first cloning it into pRIB (Garami and Ilg, 2001). The LmxGDPMP ORF (see above) was excised with AgeI and XmaI, and ligated into AgeI-cut pRIB, yielding pRIBLmxGDPMP. For chromosomal integration into the ribosomal locus of L.mexicana, the integration cassette was excised by digestion with PacI and PmeI (Figure 2B), gel purified and transfected into L.mexicana. Recombinant clones were isolated by limiting dilution on 96-well plates in SDM medium containing 20 µg/ml hygromycin, 2.5 µg/ml bleomycin and 20 µM puromycin. Isolation of total RNA from L.mexicana promastigotes and mouse lesion-derived amastigotes, as well as northern blot analysis, were performed as described earlier (Göpfert et al., 1999). RT–PCR was performed with the Titan one tube system (Roche) using the primer pairs CCGTACTCGTTTTTTCAGCAGCAAC/AGTGGAGCGGTAAAGTGAACTTCTC for reverse transcription/amplification of the control LmxPPG2 mRNAs (Göpfert et al., 1999) and TCACCAAATTGAGGCATTGAAGGCG/ATCTGGTAACTCTGCTGCGAGTAGA for the LmxGDPMP mRNAs.
High-level expression of L.mexicana GDPMP in Escherichia coli M15 as inclusion bodies was achieved by cloning the KpnI–XmaI-cut LmxGDPMP PCR fragment (primers: GDPMPORF1/GDPMPORF2) into pQE31, followed by transformation of the bacteria. Inclusion bodies were solubilized in 8 M urea, and denatured GDPMP was purified by Ni-NTA–agarose chromatography as described by the manufacturer (Qiagen). Rabbits were immunized with 200 µg of purified recombinant protein, which was dissolved in 8 M urea, 50 mM NaH2PO4 pH 4.8 and emulsified with 50% (v/v) complete Freund’s adjuvant for primary immunizations and with 50% incomplete Freund’s adjuvant (v/v) for all subsequent booster immunizations. Serum was obtained 10–14 days after each booster immunization. The antiserum was affinity purified on recombinant protein that had been electrotransferred to PVDF membranes after discontinuous SDS–PAGE, as described earlier (Ilg et al., 1999).
Analytical procedures
Production of SDS-cell lysates, discontinuous SDS–PAGE, immunoblotting using the mAbs LT6, LT17 and L7.25 {directed against [PO4-6Galβ1-4Manα1-]x, [PO4-6(Glcβ1-3)Galβ1-4Manα1-]x (x = unknown) and [Manα1-2]0-2Manα1-PO4, respectively} (Ilg et al., 1993), affinity-purified rabbit anti-L.mexicana SAP antibodies (Garami and Ilg, 2001), anti-L.mexicana MBAP antibodies (Wiese et al., 1996), anti-CRD antibodies (Oxford Glycosystems), anti-L.mexicana PMM antibodies (A.Garami, A.Mehlert and T.Ilg, submitted) and anti-L.mexicana GMP antibodies (this study) as well as acid phosphatase enzyme assays were all performed as described earlier (Ilg, 2000b). Stripping of antibodies from immunoblots for reprobing was performed by three 15 min washes in 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 8 M urea, 100 mM 2-mercaptoethanol at 65°C. GPI-PLC digestion of Leishmania total cell lysates was performed as described previously (Ilg et al., 1999). Total lipids from washed L.mexicana promastigotes were obtained by two extractions with CHCl3/CH3OH/H2O (4:8:3). HPTLC (Silica60; Merck, Germany) of total lipids was performed as described earlier (McConville et al., 1993) using the solvent CHCl3/CH3OH/1 M NH4OH (10:10:3). Glycolipids on HPTLC plates were selectively stained by orcinol/H2SO4 spraying. Leishmania mexicana promastigotes were metabolically labeled by incubating 5 × 107 cells/ml overnight at 27°C with either 10 µCi/ml [3H]myo-inositol, 20 µCi/ml [3H]GlcNH2 or 50 µCi/ml 2-[3H]Man (Hartmann Analytics) in myo-inositol-, Glc/GlcNH2- or Glc/Man-free SDM medium, respectively. In labelings with [3H]myo-inositol and [3H]GlcNH2, the lipid extracts were further purified by 1-butanol/H2O phase separation (McConville et al., 1993). Radioactively labeled lipids of the 1-butanol phase were separated by HPTLC and detected by spraying with 3H-EnHance (Dupont), followed by fluorography. [3H]myo-inositol-labeled delipidated cells were incubated with benzonuclease to cleave nucleic acids (Ilg et al., 1999) and then separated by SDS-PAGE. Labeled compounds in acrylamide gels were detected by immersion of the polyacrylamide gel in Amplify™ (Amersham-Pharmacia), followed by drying and fluorography.
For hexose analysis, 5 × 108 promastigotes were washed three times with phosphate-buffered saline, and lysed by resuspension in 1 ml of H2O and sonication. After centrifugation at 10 000 g for 30 min, the membrane-containing pellet was resuspended in 300 µl of 2 M TFA and hydrolysed for 2.5 h at 100°C. After evaporation of the 2 M TFA, the samples were resuspended in 1 ml of H2O, delipidated by passage through a Sep-Pac C18 column (Waters) and lyophilized. The hexoses of samples equivalent to 1 × 108 promastigotes were analysed by high-pH anion-exchange HPLC using a Carbo-Pac PA10 column equilibrated in 20 mM NaOH at a flow rate of 1 ml/min and pulsed amperometric detection.
Enzyme assays and partial purification of L.mexicana GDPMP
Enzyme assays were performed at room temperature in 1 ml of 50 mM triethylamine–HCl pH 7.0, 0.1 mM EDTA, 2.5 mM MgCl2 and 0.1% bovine serum albumin. For GDPMP assays, this buffer was supplemented with 0.5 mM NADP+ (Roche), 1 mM 2-mercaptoethanol, 1 mM glucose, 1 mM ADP, 1 mM Na-pyrophosphate, 0.5 U/ml nucleoside diphosphate kinase (Sigma), 1 U/ml hexokinase (Sigma) and 2 U/ml glucose-6- phosphate dehydrogenase (Roche). After addition of sample (2.5–20 µl), the rate of background reactions, as indicated by the increase in absorbance at 340 nm, was recorded for 10 min. The specific GDPMP assay was initiated by the addition of GDP-Man to a final concentration of 100 µM and recorded for 2–5 min. UDPGP was measured in assay buffer with the addition of 0.5 mM NADP+ (Roche), 1 mM 2-mercaptoethanol, 1 mM Na-pyrophosphate, 0.5 mM UDP-Glc, 1 U/ml phosphoglucomutase (Roche) and 2 U/ml glucose-6-phosphate dehydrogenase. UDPGP assays were started by the addition of 2.5–10 µl of cell lysates or column fractions and the absorbance at 340 nm was recorded for 1–10 min. One unit of enzyme activity is defined as the amount of enzyme converting 1 µmol substrate/min into the respective product.
To generate L.mexicana total cell lysates, 2 × 1010 promastigotes were resuspended in 8 ml of cold homogenization buffer (50 mM triethylamine–HCl pH 7.0, 150 mM NaCl, 1 mM 2-mercaptoethanol, 5 mM O-phenanthroline, 20 µg/ml leupeptin, 0.5 mM phenylmethylsulfonyl fluoride), sonicated on ice and centrifuged at 100 000 g for 30 min. The ultracentrifugation supernatant containing GDPMP and UDPGP was concentrated to ∼1 ml by Centricon 10 ultrafiltration, and 200 µl aliquots were loaded onto a Superose 6 column (Amersham Pharmacia) pre-equilibrated with 50 mM triethylamine–HCl pH 7.0, 150 mM NaCl, 1 mM 2-mercaptoethanol, eluted in the same buffer at 0.5 ml/min, and collected with a fraction size of 0.5 ml. The Superose 6 column was calibrated with the size markers dextran blue (2000 kDa), thyroglobulin (667 kDa), catalase (240 kDa), ovalbumin (43 kDa), cytochrome c (12.5 kDa) and CTP as marker for the total liquid volume (Vt) of the column. The preparation of promastigote lysates, soluble fractions and washed membranes for SDS–PAGE/immunoblot analysis was performed as described earlier (Ilg et al., 1999).
IFM and FACS of Leishmania promastigotes and infected macrophages
IFM and FACS studies on Leishmania promastigotes and infected macrophages were performed as described previously (Stierhof et al., 1994; Ilg, 2000b) using 4′,6-diamidino-2-phenylindole staining and the mAbs (Ilg et al., 1993) LT6, L7.25 and LT17 (see above), mAb L3.8 directed against a polypeptide epitope of L.mexicana leishmanolysin/gp63, and the biotinylated lectins concanavalin A and ricin120 (Sigma). The mAbs were diluted 1:2–1:10 (hybridoma supernatant) or 1:500–1:2000 (ascites fluid) and the lectins were used at 10 µg/ml. Bound mAbs and the biotinylated lectin were detected by incubation with Cy3-labeled goat anti-mouse IgG/IgM (1:250; Dianova) and fluorescein isothiocyanate-labeled streptavidin (1:250; Sigma), respectively.
Acknowledgments
Acknowledgements
The authors would like to thank Suzanne Gokool and Peter Overath for helpful comments on the manuscript, and Dorothee Harbecke and Monika Demar for expert technical assistance.
References
- Alexander J. and Russell,D.G. (1992) The interaction of Leishmania species with macrophages. Adv. Parasitol., 31, 175–254. [DOI] [PubMed] [Google Scholar]
- Chapman A., Fujimoto,K. and Kornfeld,S. (1980) The primary glycosylation defect in class E Thy-1-negative mutant mouse lymphoma cells is an inability to synthesize dolichol-P-mannose. J. Biol. Chem., 255, 4441–4446. [PubMed] [Google Scholar]
- Chen D.Q., Lu,H. and Chang,K.P. (1999) Replacement of Leishmania N-acetylglucosamine-1-phosphate transferase gene requires episomal rescue. Mol. Biochem. Parasitol., 100, 223–227. [DOI] [PubMed] [Google Scholar]
- Cruz A., Coburn,C.M. and Beverley,S.M (1991) Double targeted gene replacement for creating null mutants. Proc. Natl Acad. Sci. USA, 88, 7170–7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoteaux A. and Turco,S.J. (1999) Glycoconjugates in Leishmania infectivity. Biochim. Biophys. Acta, 1455, 341–352. [DOI] [PubMed] [Google Scholar]
- Descoteaux A., Luo,Y., Turco,S.J. and Beverley,S.M. (1995) A specialized pathway affecting virulence glycoconjugates of Leishmania. Science, 269, 1869–1872. [DOI] [PubMed] [Google Scholar]
- Ferguson M.A.J. (1999) The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors and the contributions of trypanosome research. J. Cell Sci., 112, 2799–2809. [DOI] [PubMed] [Google Scholar]
- Freeze H. and Aebi,M. (1999) Molecular basis of carbohydrate-deficient glycoprotein syndromes type I with normal phosphomannomutase activity. Biochim. Biophys. Acta, 1455, 167–178. [DOI] [PubMed] [Google Scholar]
- Garami A. and Ilg,T. (2001) The role of phosphomannose isomerase in Leishmania mexicana glycoconjugate synthesis and virulence. J. Biol. Chem., 276, 6566–6575. [DOI] [PubMed] [Google Scholar]
- Göpfert U., Goehring,N., Klein,C. and Ilg,T. (1999) Proteophos phoglycans of Leishmania mexicana. Molecular cloning and characterization of the Leishmania mexicana ppg2 gene encoding the proteophosphoglycans aPPG and pPPG2 that are secreted by amastigotes and promastigotes. Biochem. J., 344, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto H., Sakakibara,A., Yamasaki,M. and Yoda,K. (1997) Saccharomyces cerevisiae VIG9 encodes GDP-mannose pyrophosphorylase, which is essential for protein glycosylation. J. Biol. Chem., 272, 16308–16314. [DOI] [PubMed] [Google Scholar]
- Ilg T. (2000a) Proteophosphoglycans of Leishmania. Parasitol. Today, 16, 489–497. [DOI] [PubMed] [Google Scholar]
- Ilg T. (2000b) Lipophosphoglycan is not required for infection of macrophages or mice by Leishmania mexicana. EMBO J., 19, 1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg T., Harbecke,D., Wiese,M. and Overath,P. (1993) Monoclonal antibodies directed against Leishmania secreted acid phosphatase and lipophosphoglycan. Partial characterization of private and public epitopes. Eur. J. Biochem., 217, 603–615. [DOI] [PubMed] [Google Scholar]
- Ilg T., Montgomory,J., Stierhof,Y.-D. and Handman,E. (1999) Molecular cloning and characterization of a novel repeat-containing L. major gene, ppg1, that encodes a membrane-associated form of proteo phosphoglycan with a putative glycosylphosphatidylinositol anchor. J. Biol. Chem., 274, 31410–31420. [DOI] [PubMed] [Google Scholar]
- Ilg T., Demar,M. and Harbecke,D. (2001) Phosphoglycan repeat-deficient Leishmania mexicana parasites remain infectious to macrophages and mice. J. Biol. Chem., 276, 4988–4997. [DOI] [PubMed] [Google Scholar]
- Ilgoutz S.C., Zawadzki,J.L., Ralton,J.E. and McConville,M.J. (1999) Evidence that free GPI glycolipids are essential for growth of Leishmania mexicana. EMBO J., 18, 2746–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapteyn J.C., Van Den Ende,H. and Klis,F.M. (1999) The contribution of cell wall proteins to the organization of the yeast cell wall. Biochim. Biophys. Acta, 1426, 373–383. [DOI] [PubMed] [Google Scholar]
- Kaufman R.L. and Ginsburg,V. (1968) The metabolism of l-fucose by HeLa cells. Exp. Cell Res., 50, 127–132. [DOI] [PubMed] [Google Scholar]
- Kepes F. and Schekman,R. (1988) The yeast SEC53 gene encodes phosphomannomutase. J. Biol. Chem., 263, 9155–9161. [PubMed] [Google Scholar]
- Knauer R. and Lehle,L. (1999) The oligosaccharidyltransferase complex from yeast. Biochim. Biophys. Acta, 1426, 259–273. [DOI] [PubMed] [Google Scholar]
- LeBowitz J.H., Coburn,C.M., McMahon-Pratt,D. and Beverley,S.M. (1990) Development of a stable Leishmania expression vector and application to the study of parasite surface antigen genes. Proc. Natl Acad. Sci. USA, 87, 9736–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConville M.J., Thomas-Oates,J., Ferguson,M.A.J. and Homans,S.W. (1990) Structure of the lipophosphoglycan from Leishmania major. J. Biol. Chem., 265, 19611–19623. [PubMed] [Google Scholar]
- McConville M.J., Collidge,T.A., Ferguson,M.A.J. and Schneider,P. (1993) The glycoinositol phospholipids of Leishmania mexicana promastigotes. Evidence for the presence of three distinct pathways of glycolipid biosynthesis. J. Biol. Chem., 268, 15595–15604. [PubMed] [Google Scholar]
- Mengeling B.J., Beverley,S.M. and Turco,S.J. (1997) Designing glycoconjugate biosynthesis for an insidious intent: phosphoglycan assembly in Leishmania parasites. Glycobiology, 7, 873–880. [DOI] [PubMed] [Google Scholar]
- Menz B., Winter,G., Ilg,T., Lottspeich,F. and Overath,P. (1991) Purification and characterization of a membrane-bound acid phosphatase of Leishmania mexicana. Mol. Biochem. Parasitol., 47, 101–108. [DOI] [PubMed] [Google Scholar]
- Moss J.M., Reid,G., Mullin,K.A., Zawadzki,J.L., Simpson,R.J. and McConville,M.J. (1999) Characterization of a novel GDP-mannose: serine-protein mannose-1-phosphotransferase from Leishmania mexicana. J. Biol. Chem., 274, 6678–6688. [DOI] [PubMed] [Google Scholar]
- Orlean P., Albright,C. and Robbins,P.W. (1988) Cloning and sequencing of the yeast gene for dolichol phosphate mannose synthase, an essential protein. J. Biol. Chem., 263, 17499–17507. [PubMed] [Google Scholar]
- Sacks D.L., Modi,G., Rowton,E., Späth,G., Epstein,L., Turco,S.J. and Beverley,S.M. (2000) The role of phosphoglycans in Leishmania– sandfly interactions. Proc. Natl Acad. Sci. USA, 97, 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.J., Proudfoot,A., Friedli,L., Klig,L.S., Paravic,G. and Payton,M.A. (1992) PMI40, an intron-containing gene required for early steps in yeast mannosylation. Mol. Cell. Biol., 12, 2924–2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. and Ioffe,E. (1995) Glycosyltransferase mutants: key to new insights in glycobiology. FASEB J., 9, 1436–1444. [DOI] [PubMed] [Google Scholar]
- Stierhof Y.-D., Ilg,T., Russell,D.G., Hohenberg,H. and Overath,P. (1994) Characterization of polymer release from the flagellar pocket of Leishmania mexicana promastigotes. J. Cell Biol., 125, 321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll J., Robbins,A.R. and Krag,S.S. (1982) Mutant of Chinese hamster ovary cells with altered mannose 6-phosphate receptor activity is unable to synthesize mannosylphosphoryldolichol. Proc. Natl Acad. Sci. USA, 79, 2296–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahl-Bolsinger S., Gentzsch,M. and Tanner,W. (1999) Protein O-mannosylation. Biochim. Biophys. Acta, 1426, 297–307. [DOI] [PubMed] [Google Scholar]
- Sugiyama E., DeGasperi,R., Urakaze,M., Chang,H.M., Thomas,L.J., Hyman,R., Warren,C.D. and Yeh,E.T. (1991) Identification of defects in glycosylphosphatidylinositol anchor biosynthesis in the Thy-1 expression mutants. J. Biol. Chem., 266, 12119–12122. [PubMed] [Google Scholar]
- Varki A. (1999) Exploring the biological roles of glycans. In Varki,A., Cummings,R., Esko,J., Freeze,H., Hart,G. and Marth,J. (eds), Essentials ofGlycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 57–68. [Google Scholar]
- Warit S., Zhang,N., Short,A., Walmsley,R.M., Oliver,S.G. and Stateva,L.I. (2000) Glycosylation deficiency phenotypes resulting from depletion of GDP-mannose pyrophosphorylase in two yeast species. Mol. Microbiol., 36, 1156–1166. [DOI] [PubMed] [Google Scholar]
- Wheeler G.L., Jones,M.A. and Smirnoff,N. (1998) The biosynthetic pathway of vitamin C in higher plants. Nature, 393, 365–369. [DOI] [PubMed] [Google Scholar]
- Wiese M., Berger,O., Stierhof,Y.-D., Wolfram,M., Fuchs,M. and Overath,P. (1996) Gene cloning and cellular localization of a membrane-bound acid phosphatase of Leishmania mexicana. Mol. Biochem. Parasitol., 82, 153–165. [DOI] [PubMed] [Google Scholar]
- Zamze S.E., Ferguson,M.A., Collins,R., Dwek,R.A. and Rademacher,T.W. (1988) Characterization of the cross-reacting determinant (CRD) of the glycosyl-phosphatidylinositol membrane anchor of Trypano soma brucei variant surface glycoprotein. Eur. J. Biochem., 176, 527–534. [DOI] [PubMed] [Google Scholar]